Introduction
The dual-specificity phosphatase enzyme belongs to
the protein tyrosine phosphatase (PTP)/dual specificity phosphatase
(DSP) family. It is involved in numerous basic physiological
activities, including cell signaling, chondrocyte growth and other
cell metabolic processes. The majority of DSPs play a key role in
the regulation of mitotic signaling and cell cycle control
following external stimulation (1–5).
The activity of mitogen-activated protein kinase (MAPK) is
suppressed following its dephosphorylation by dual specificity
phosphatase 1 (DUSP1). A previous study demonstrated that DUSP1 not
only inhibits the activity of MAPK3/1, but also regulates the
expression of its CG subunit (6).
Nyati et al (7) reported
that telangiectasias caused by radioactive mutation downregulates
the function of phosphor-extracellular signal-regulated kinase
(p-ERK) and accordingly enhances the activity of DUSP1. Wang et
al (8) found that DUSP1
expression was induced by the abnormal expression of the
transcription factor, E2F-1, thereby providing a link between E2F-1
and MAPK. During septicaemia, DUSP1 minimizes damage to the liver
(as well as other organs) by suppressing the levels of
pro-inflammatory cytokines (9).
DUSP1 has also been shown to inhibit the growth of liver cancer
cells through the regulation of ERK (10), and its expression has been shown
to be regulated by cancer related genes, such as p53 (11). It has also been previously
demonstrated however, that the level of DUSP1 in the blood
correlates with post-operative morbidity and that high levels of
DUSP1 cause prolonged hospitalization following coronary artery
bypass grafting (12). Cancer
cells are characterized by uncontrolled cell division, primarily
due to the continued activation of MAPK. Since DUSP1 phosphorylates
MAPK, it has the potential to modulate cancer cell proliferation.
However, to the best of our knowledge, this has not been
investigated to date due to the difficulty in expressing the
protein with biological activity.
The baculovirus expression system has recently been
identified as a superior tool compared with the Escherichia coli
(E. coli) system due to its simple operation procedures, the
high expression of co-factors and the close-to-natural function of
the expressed proteins (13).
Similarly, the Bac-to-Bac expression system, a recently developed
easy and rapid research tool, has been adopted by certain
researchers (14,15). In the present study, we used the
baculovirus system to produce the biologically active DUSP1 protein
and we subsequently examined its effects on cancer cells following
incubation with DUSP1 protein.
Materials and methods
Materials
The template DUSP1 gene was obtained from
Open Biosystems/Thermo Fisher Scientific Inc. (Rockford, IL, USA);
The BamHI and HindIII restriction enzymes were
purchased from New England Biolabs (Ipswich, MA, USA); Taq DNA
polymerase and T4 ligase were purchased from Takara Bio Inc.
(Tokyo, Japan). The plasmid isolation kit, Cellfectin®
reagent, the Sf9 cell line, the pFastBac1 cloning kit, the ECL kit
and growth media (Grace’s insect medium, DMEM and fetal bovine
serum) were all purchased from Invitrogen (Carlsbad, CA, USA). The
anti-His tag antibody was from Tiangen Biotech (Beijing, China;
Cat. no. 168-10481); the p-ERK antibody was obtained from Cell
Signaling Technology Inc. (Danvers, MA, USA; Cat. no. 2371); the
HRP-anti-mouse IgG Fc was purchased from Sigma-Aldrich Inc. (St.
Louis, MO, USA; Cat. no. 63134). All other reagents and instruments
were provided by ProMab Biotechnologies Inc. (Richmond, VA, USA;
Cat. no. 23173).
Cell culture
Sf9 insect cells were cultured in Grace’s insect
medium containing 10% fetal bovine serum at 37°C without
CO2 in Petri dishes. The cells cultures were subculured
at a ratio of 1:5 (cells:medium) and the medium was renewed every 2
to 3 days. The cells in suspension were cultured in Grace’s insect
medium containing 1% F-68 in a sterile flask and incubated on a
shaking table at a speed of 120 rpm. Cell density was kept at
1–2×106 cells/ml. Subcultures were prepared twice each
week.
Cancer cell lines (A549 human lung cancer cells,
HepG2 human hepatoma cells, PC-2 human pancreatic cancer cells,
HeLa human cervical carcinoma cells, MCF-7 human breast cancer
cells and GC-7901 human gastric cancer cells) were cultured in the
Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal
bovine serum (FBS) and 0.05 μg/ml kanamycin at 37°C and 5%
atmospheric CO2. Subcultivation was conducted by
transferring 20% of monolayer cells to new growth medium every 2 to
3 days. To examine the effect of DUSP1, the cells were seeded into
6-well plates at a density of 1×106 cells/well or on
96-well plates at a density of 1×105 cells/well. Cell
monolayers were incubated for 24 h before the experiment was
conducted.
Primer design and PCR amplification
Based on the sequence of the DUSP1 template,
PCR amplification was performed with Taq DNA polymerase using the
following primers: upstream,
5′-CTCGGATCCACCATGGTCATGGAAGTGGGCACCCT-3′; and downstream,
5′-CTCAAGCTTAGTGGTGATGGTGATGATGGCAGCTGGGAGAGGTCGTAAT-3′. Following
amplification, a BamHI restriction site was added in front
of the upstream primer, and a HindIII restriction site and
termination codon were added after the downstream primer. PCR was
performed under the following conditions: pre-denaturation at 94°C
for 3 min; 35 cycles of denaturation at 94°C for 45 sec, annealing
at 55°C for 45 sec and extension at 72°C for 5 min; final extension
at 72°C for 7 min. PCR products were separated on a 1% agarose gel
and the target fragment was recovered by gel elution.
Plasmid construction
The PCR fragment of DUSP1 was double digested
by BamHI and HindIII at 37°C for 2 h before the
target fragment was gel purified on a 1% agarose gel. The
recombinant plasmid, pFastBac1-DUSP1, was constructed by inserting
the DUSP1 fragment into the pFastBac1 vector at the
BamHI and HindIII sites, and then transformed into
E. coli DH10Bac cells by incubation at 42°C for 90 sec
followed by 30 min on ice incubation. The transformed cells were
subsequently recovered in 500 ml LB medium by shaking and
incubation at 37°C for 4 h. Positive colonies were screened by
plating the recovered cells (2, 20 and 200 μl) on LB agar
plates containing gentamicin, kanamycin, tetracycline, X-gal and
IPTG and incubation at 37°C until the appearance of blue or white
colonies. A single white clony was picked up and transferred to a
new LB agar plate by streaking and incubation for 72 h.
Subsequently, a well separated white colony from this plate was
used to inoculate 10 ml of liquid LB medium by shaking and
incubation for 14 h. Subsequently, the cells were collected for
isolation and confirmation of the recombinant bacmid DNA.
Transfection and western blot
analysis
When the density of the Sf9 cells reached 70–80% in
the 6-well culture plate, the recombinant bacmid DNA was
transfected into the Sf9 cells using the Cellfectin reagent. If
obvious signs of infection were observed under the microscope
(Olympus IX70; Olympus, Tokyo, Japan) 5 days after transfection,
the supernatant and the cells were collected separately. The
supernatant medium contained the P1 generation of the virus. The
collected cells were rapidly washed with phosphate-buffer and
sonicated on ice in SDS loading buffer (62.5 mM Tris-HCl with pH
6.8, 10% glycerol, 50 mM DTT, 2% SDS and 0.025% bromphenol blue) 3
times (sonication for 3 sec and rest for 3 sec). The lysates were
stored at -20°C until further analysis. Western blot analysis was
carried out according to Molecular Cloning: A Laboratory Manual
(16).
Expression of DUSP1 protein and sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)
The amplification of the viruses was carried out
according to the Invitrogen protocol. Briefly, the P1 generation of
the virus was used to infect the Sf9 cells. Following lysis of the
majority of the cells, the supernatant was collected as the P2
generation, which was then used to inoculate 100 ml of the Sf9
cells at a density of 2×106 cells/ml. The mixture was
incubated for 72 h at 27°C before the cells were harvested to
collect the DUPS1 protein. The harvested cell pellet was
resuspended in PBS, sonicated on ice for 99 cycles (sonication for
10 sec, rest for 10 sec) and centrifuged at 12,500 rpm for 25 min.
The supernatant was collected and purified by affinity
chromatography using a nickel column. The recombinant protein was
eluted by gradient imidazole solutions. For SDS-PAGE, 40 μl
of total protein were added to 10 μl 5X loading buffer and
boiled for 5 min before the mixture was loaded onto a
polyacrylamide gel.
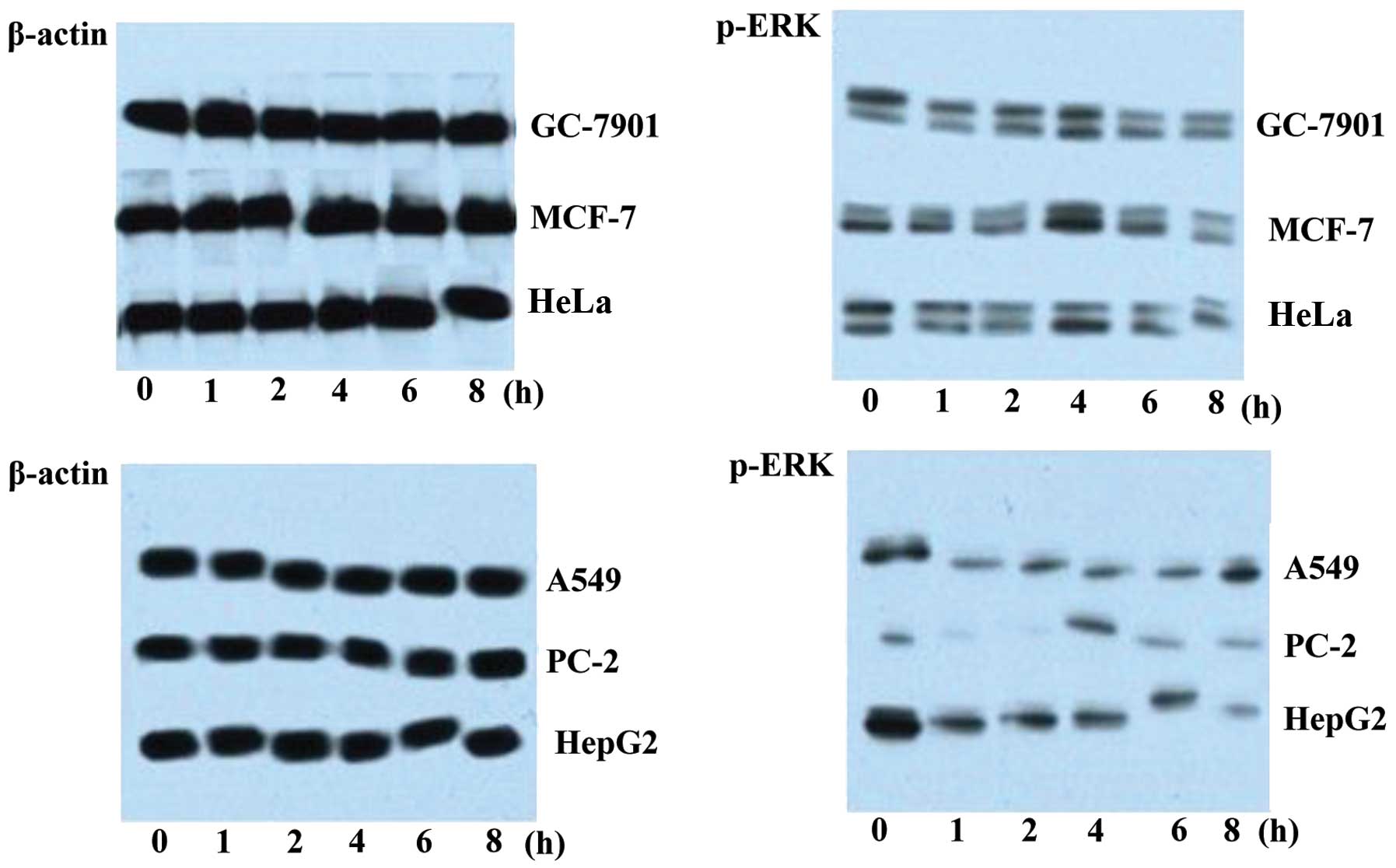
Western blot analysis of p-ERK expression
in 6 cancer cell lines
Six cancer cell lines (HepG2, PC-2, HeLa, MCF-7,
A549 and GC-7901) were cultured in 6-well plates separately before
1 μg/ml purified DUSP1 protein was added into each well.
Each cell line was incubated with DUSP1 for various periods of time
(0, 1, 2, 4, 6 and 8 h). Thereafter, the cells were harvested and
lysed in lysis buffer. For western blot analysis of p-ERK, lysate
from each cell type at each time point was loaded onto a gel for
SDS-PAGE (20 μl/well). Following resolution, the proteins
were electrotransferred onto nitrocellulose membranes at a constant
current (300 mA/70 min) and on ice. The membranes were then blocked
in TBST containing 5% dried fat-free milk for 2 h at room
temperature, followed by 2 h of incubation at room temperature with
the primary antibody (p-ERK antibody, 1:500; or β-actin antibody,
1:4×104). The membranes were subsequently washed 3 times
in TBST and incubated for 1 h at room temperature with HRP-labeled
secondary antibody (1:2×104). Following extensive
washes, the signals were visualized using the ECL kit according to
the manufacturer’s instructions.
Analysis of the growth of HeLa cells
When the cells reached 40–50% confluency in
monolayer in the 96-well plate, DUSP1 protein was added to each
well (apart from the blank control) at a final concentration of 1
μg/ml. The cells were then incubated for various periods of
time (0, 12, 24, 36, 48, 60, 72, 84 and 96 h) before they were
collected to count the cell number. Quadruplets were performed for
each data point and the average value was used to plot the growth
curve.
Results
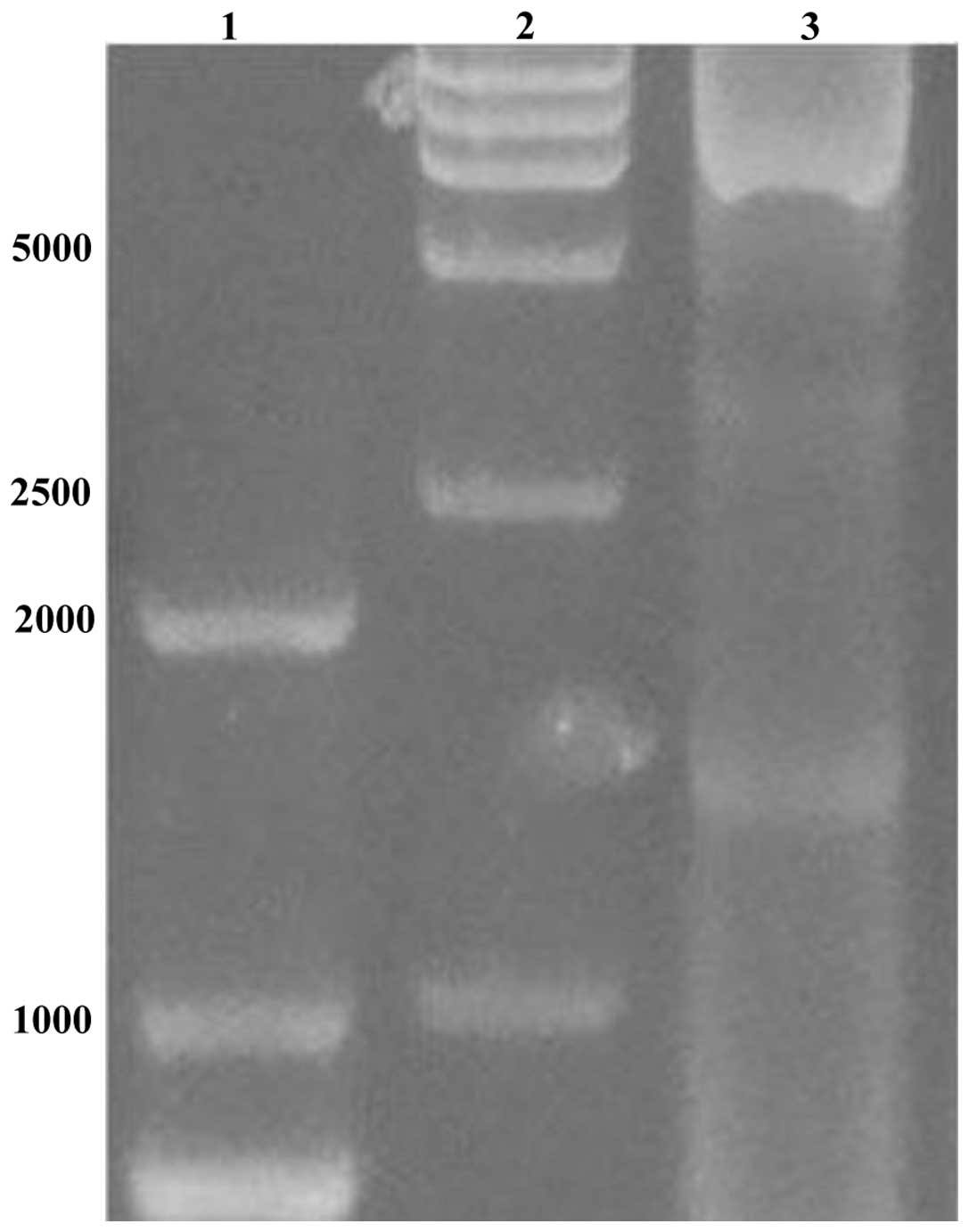
Amplification of the target fragment
The amplified PCR product (Fig. 1) was verified by agarose gel
electrophoresis. The segment size was in line with
expectations.
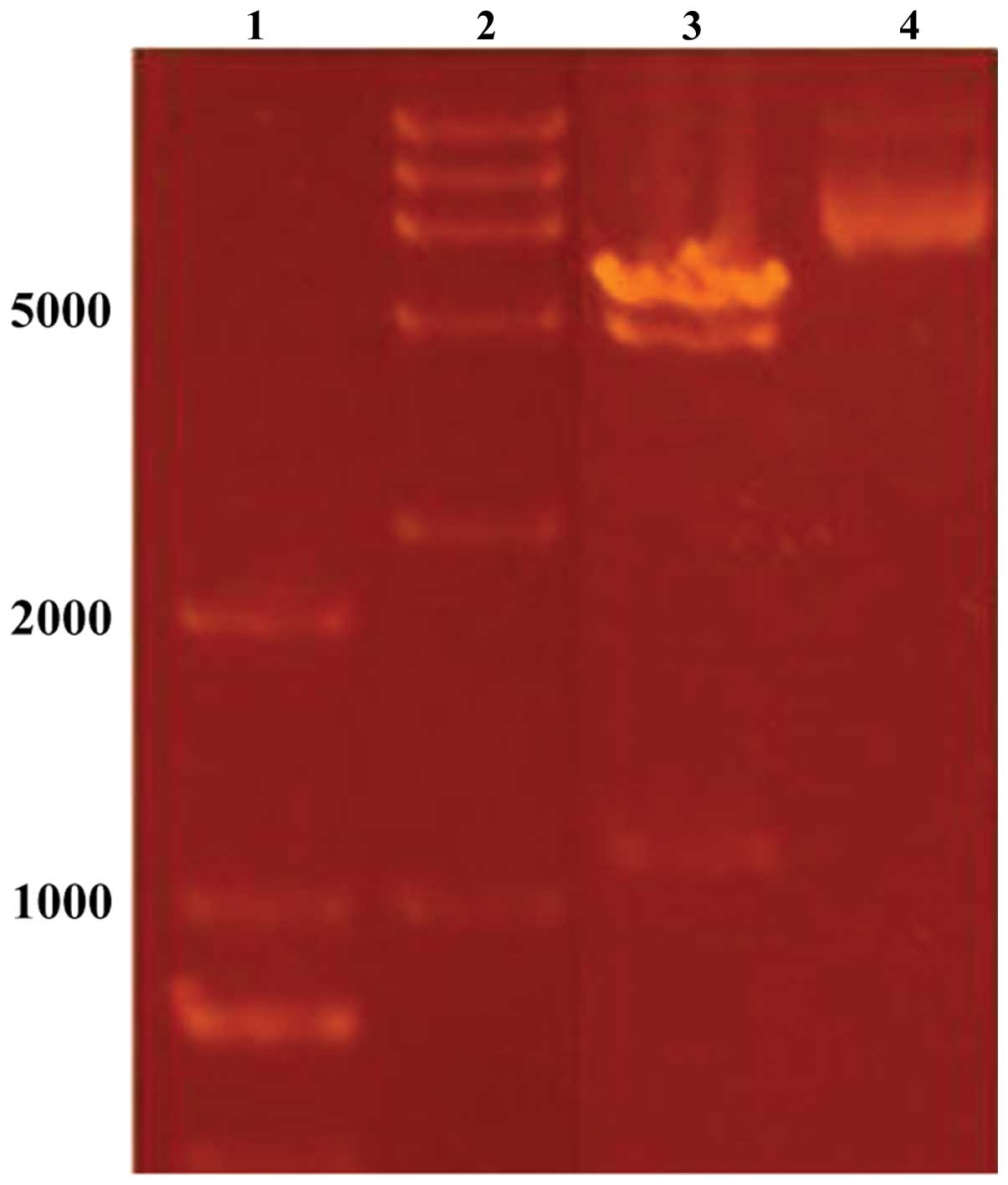
Plasmid constructs
The DUSP1 gene was inserted at the Bacmid
locus of the pFastBac1 vector. Since the α-complementarity was
broken, the bacterium bearing the resulting plasmid construct
produced white colonies on solid agar plates containing gentamicin
(Gm), kanamycin (Kan), tetracycline (Tet) and X-gal. Plasmids
isolated from these white colonies were double digested with
BamHI and HindIII, and the resultant fragment
produced the expected size of the DUSP1 gene (Fig. 2) when analyzed on an agarose gel.
The gene sequence was further confirmed through sequencing of the
recombinant plasmid.
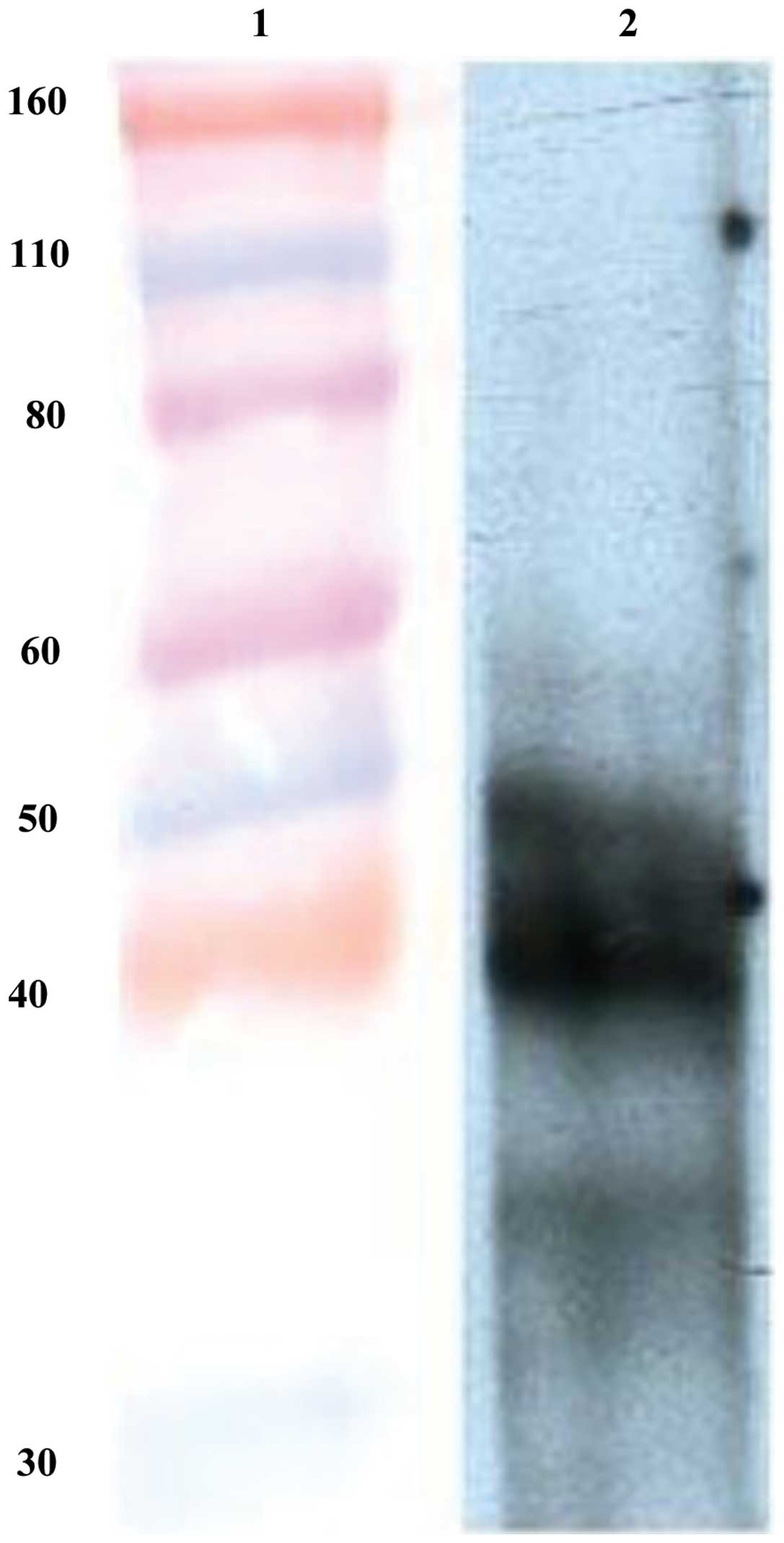
Western blot analysis
Five days after transfection of the recombinant
plasmid, the Sf9 cells in the middle of 6-well culture plate
appeared darker than the ones on the edge when observed under an
inverted microscope. These darker cells showing apparent
co-transfection were harvested and lysed for western blot analysis.
The result revealed that DUSP1 was successfully expressed in the
Sf9 cells (Fig. 3).
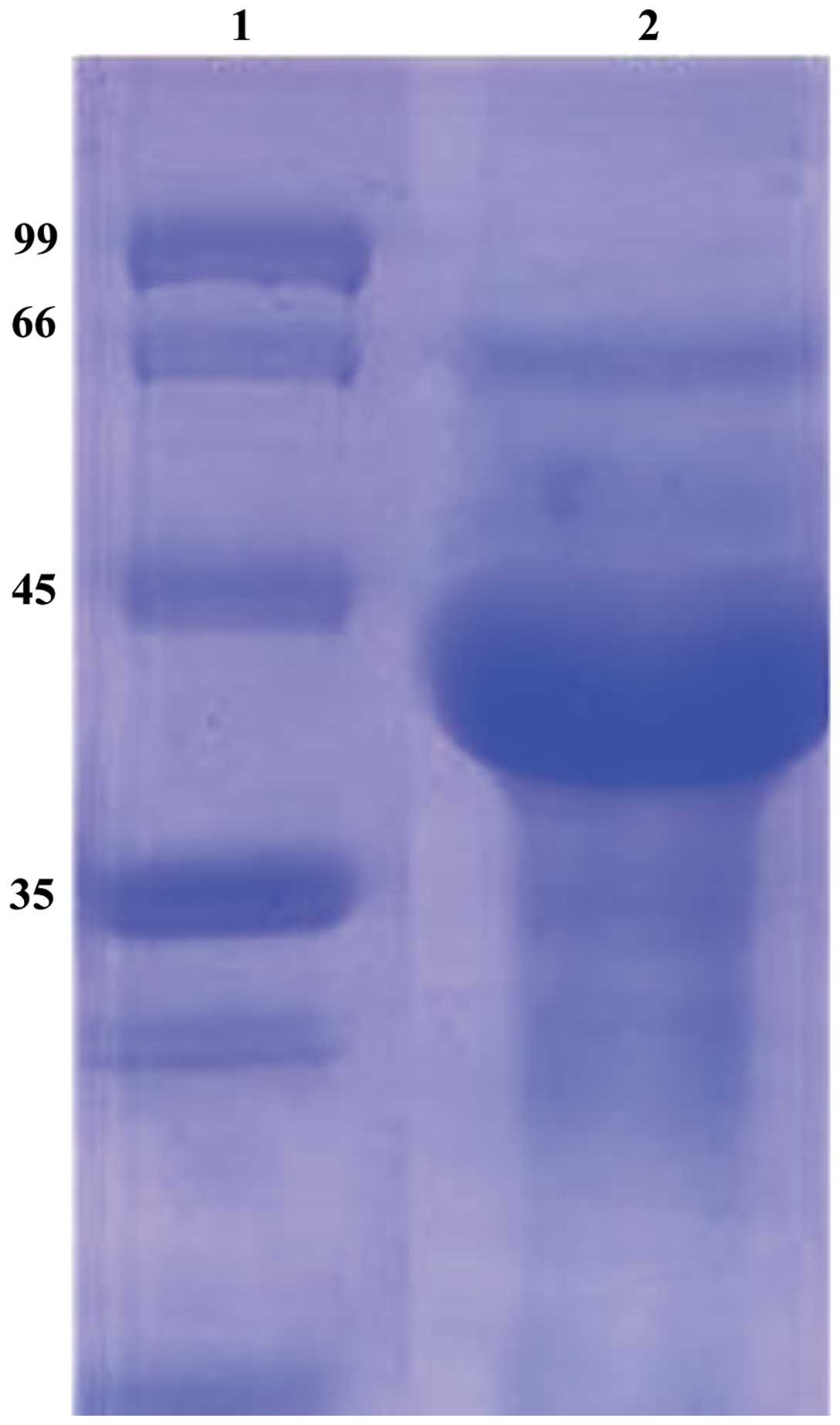
SDS-PAGE
The lysate of Sf9 cells containing DUSP1 protein was
purified by Ni-affinity chromatography. The purified products were
examined by SDS-PAGE. A prominent band with the identical size of
DUSP1 was found in the lysate (Fig.
4).
Effect of DUSP1 on the level of p-ERK in
cancer cells
The expression of p-ERK in the 6 different cancer
cell lines as analyzed by western blot analysis following exposure
to DUSP1 for various periods of time (Fig. 5). Compared to the β-actin level
which remained constant following exposure to DUSP1, the amount of
p-ERK markedly decreased after 1 h, indicating that DUSP1
suppressed the expression of p-ERK in all 6 cancer cell lines.
Effect of DUSP1 on the proliferation rate
of cancer cells
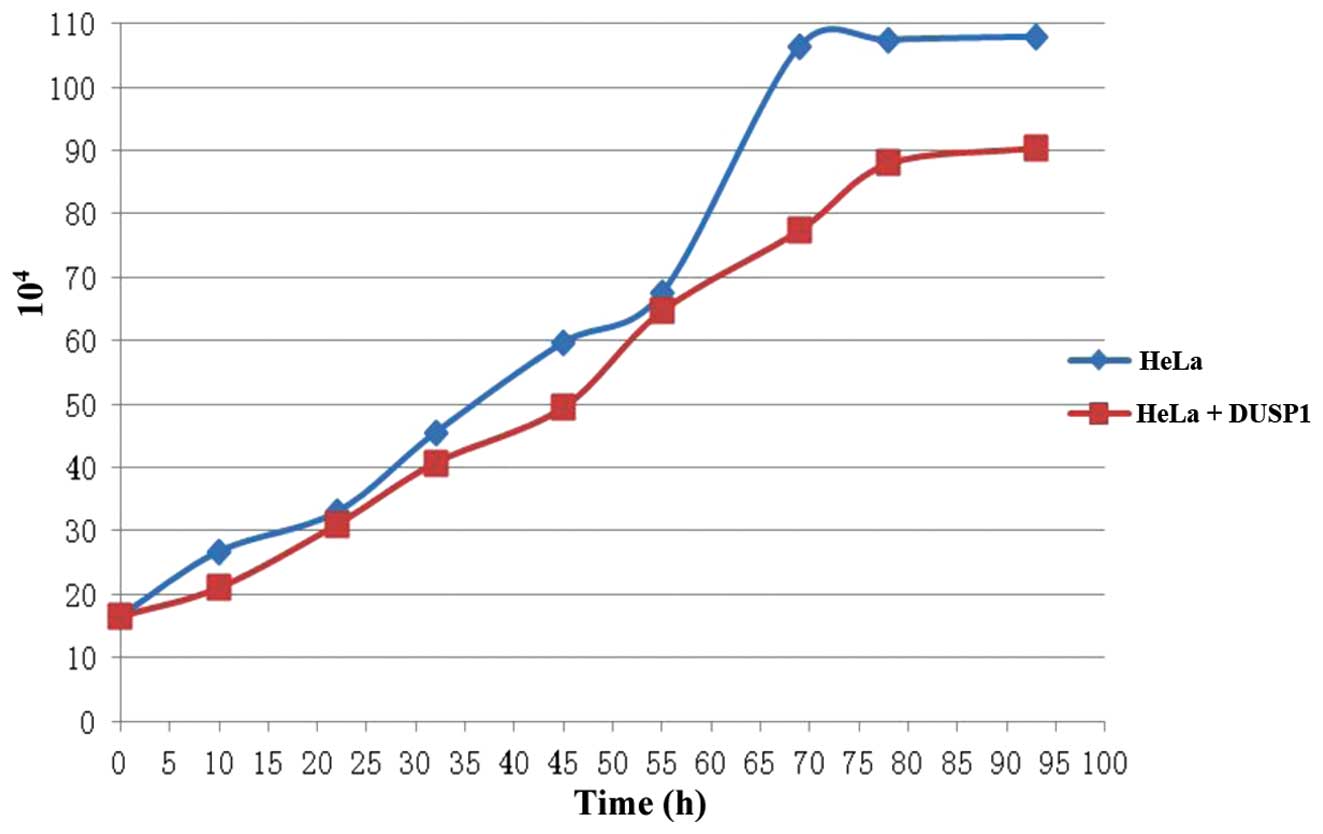
As shown in Table
I and Fig. 6, DUSP1 affected
the proliferation rate of the cancer cells. For example, the HeLa
cells showed a much smoother growth curve when exposed to DUSP1,
lacking the apparent pattern of the exponential phase. The
capability of binary fission was also reduced when the cells were
examined under a microscope, suggesting that DUSP1 suppressed the
proliferation of the cancer cells.
 | Table IEffect of DUSP1 on the growth rate of
HeLa cells. |
Table I
Effect of DUSP1 on the growth rate of
HeLa cells.
| Time (h) | 0 | 12 | 24 | 36 | 48 | 60 | 72 | 84 | 96 |
|---|
| HeLa
(104) | 16.5 | 26.5 | 33 | 45.5 | 50.75 | 67.5 | 106.5 | 107.5 | 108 |
| HeLa + DUSP1
(104) | 16.5 | 21 | 31 | 40.75 | 49.5 | 64.7 | 77.5 | 88 | 90.5 |
Discussion
MAPKs, including ERK, JNK and p38, play critical
roles in the regulation of cell growth, differentiation and the
control of cellular responses to cytokines and stress (17). The MAPK-ERK signaling pathway
plays an important role in cell metabolism and has attracted
widespread attention in recent years (18). The prototype of this family, DUSP1
(encoding the protein MKP-1), regulates the activation of ERK, JNK
and p38, and the alteration of DUSP1 expression has been associated
with malignant progression in a various types of cancer (19). DUSP1 protein suppresses the
activity of MAPK-ERK through dephosphorylation. Since DUSP1 also
dephosphorylates p-ERK, the mechanisms through which DUSP1
modulates the MAPK-ERK pathway are not yet clear. Therefore, a
better understanding of the specific mechanisms responsible for the
regulation of DUSP1 by p-ERK and the functional consequences of
this regulation is required (20).
The baculovirus protein expression system is a newly
discovered superior post-translational modification system compared
to the traditional E. coli expression system, and it
produces large quantities of protein with near-natural protein
functions. In this study, we expressed DUSP1 using this improved
expression tool and demonstrated that DUSP1 suppressed the
proliferation of HeLa cells, possibly through the regulation of the
MAPK-ERK signaling pathway. In addition to the possibility of the
regulation of DUSP1 by replication-independent mechanisms in HeLa
cells, it is plausible that DUSP1 expression is regulated by other
replication-dependent mechanisms (21). Overall, our data demonstrate that
DUSP1 inhibits the proliferation of human cervical cancer cells and
provide valuable resources which will consitute the basis of
further investigations regarding the factors responsible for the
proliferation of cancer cells.
Acknowledgments
The present study was supported by the Ministry of
Agriculture ‘948’ project (2013-Z58).
References
|
1
|
Hammer M, Mages J, Dietrich H, et al: Dual
specific city hosphatase 1 (DUSP1) regulates a subset of
LPS-induced genes and protects mice from lethal endotoxin shock. J
Exp Med. 1:15–20. 2006. View Article : Google Scholar
|
|
2
|
Moncho-Amor V, Ibañez de Cáceres I,
Bandres E, et al: DUSP1/MKP1 promotes angiogenesis, invasion and
metastasis in non-small-cell lung cancer. Oncogene. 30:668–678.
2011. View Article : Google Scholar
|
|
3
|
Chen CC, Hardy DB and Mendelson CR:
Progesterone receptor inhibits proliferation of human breast cancer
cells via induction of MAPK phosphatase 1 (MKP-1/DUSP1). J Biol
Chem. 286:43091–43102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu C, Shi Y, Du Y, et al:
Dual-specificity phosphatase1 DUSP1 protects over activation of
hypoxia-inducible factor 1 through inactivating ERK MAPK. Exp Cell
Res. 309:410–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu W, Pew T, Zou M, Pang D and Conzen SD:
Glucocorticoid receptor-induced mapk phosphatase-1(MPK-1)
expression inhibits paclitaxel-associated MAPK activation and
contributes to breast cancer cell survival. J Biol Chem.
280:4117–4124. 2005. View Article : Google Scholar
|
|
6
|
Purwana IN, Kanasaki H, Mijiddorj T, Oride
A and Miyazaki K: Induction of dual-specificity phosphatase 1
(DUSP1) by pulsatile gonadotropin-releasing hormone stimulation:
role for gonadotropin subunit expression in mouse pituitary LbetaT2
cells. Biol Reprod. 84:996–1004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nyati MK, Feng FY, Maheshwari D, et al:
Ataxia telangiectasia mutated down-regulates phosphor-extracellular
signal-regulated kinase 1/2 via activation of MKP-1 in response to
radiation. Cancer Res. 66:11554–11559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Yin DP, Liu YX, Baer R and Yin Y:
Dual specificity phosphatase 1/CL100 is a direct transcriptional
target of E2F-1 in the apoptotic response to oxidative stress.
Cancer Res. 67:6737–6744. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jacob A, Rajan D, Pathickal B, et al: The
inhibitory effect of ghrelin on sepsis-induced inflammation is
mediated by the MAPK phosphatase-1. Int J Mol Med. 25:159–164.
2010.
|
|
10
|
Calvisi DF, Pinna F, Meloni F, et al:
Dual-specificity phosphatase 1 ubiquitination in extracellular
signal-regulated kinase-mediated control of growth in human
hepatocellular carcinoma. Cancer Res. 68:4191–4200. 2008.
View Article : Google Scholar
|
|
11
|
Liu YX, Wang J, Guo J, et al: DUSP1 is
controlled by p53 during the cellular response to oxidative stress.
Mol Cancer Res. 6:624–633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hägg S, Alserius T, Noori P, et al: Blood
levels of dual-specificity phosphatase-1 independently predict risk
for post-operative morbidities causing prolonged hospitalization
after coronary artery bypass grafting. Int J Mol Med. 27:851–857.
2011.PubMed/NCBI
|
|
13
|
Gómez-Sebastián S, Nuñez MC, Garaicoechea
L, Alvarado C, Mozgovoj M, Lasa R, Kahl A, Wigdorovitz A, Parreño V
and Escribano JM: Rotavirus A-specific single-domain antibodies
produced in baculovirus-infected insect larvae are protective in
vivo. BMC Biotechnol. 12:592012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farinha-Arcieri LE, Porchia BM, Carromeu
C, Simabuco FM, et al: Expression and purification of a recombinant
adenovirus fiber knob in a baculovirus system. Intervirology.
51:189–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cha HJ, Dalal NG, Vakharia VN and Bentley
WE: Expression and purification of human interleukin-2 simplified
as a fusion with green fluorescent protein in suspended Sf-9 insect
cells. J Biotechnol. 69:9–17. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maniatis T: Molecular cloning: a
laboratory manual. Cold Spring Harbor Laboratory Press; New York:
pp. 403–410. 1992
|
|
17
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fey D, Croucher DR, Kolch W and Kholodenko
BN: Crosstalk and signaling switches in mitogen-activated protein
kinase cascades. Front Physiol. 3:3552012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu GS: Role of mitogen-activated protein
kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev.
26:579–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keyse SM: Dual-specificity MAP kinase
phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 27:253–261.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin Z, Dai L, Defee M, Findlay VJ, Watson
DK, Toole BP, Cameron J, Peruzzi F, Kirkwood K and Parsons C:
Kaposi’s sarcoma-associated herpesvirus suppression of DUSP1
facilitates cellular pathogenesis following de novo infection. J
Virol. 87:621–635. 2013. View Article : Google Scholar :
|