Introduction
Osteosarcoma is the most common primary malignant
bone tumor that predominantly affects children and adolescents
(1). Although traditional
therapies such as chemotherapy, surgery and radiotherapy have been
developed, the 5-year survival rates of osteosarcoma patients
remain at only 60–70% (2).
Invasion and metastasis are responses leading to mortality of
osteosarcoma patients. The metastasis of osteosarcoma is mainly
hematogenous, and often occurs in the distal organs, such as lungs
(3). In many cases, osteosarcomas
have already metastasized at the time of diagnosis (4). Therefore, identification of the
molecular mechanisms underlying osteosarcoma invasion and
metastasis, and improvement of new clinical approaches for the
diagnosis and therapy of osteosarcoma is crucial.
Accumulating evidence suggests that the levels of
many inflammatory cytokines are increased in the tumor
micro-environment, and these inflammatory cytokines play crucial
roles in the progression of cancer, including cell growth, invasion
and metastasis (5,6). Interleukin-32 (IL-32) is a type of
inflammatory cytokine that is mainly produced by T-, natural
killer, epithelial cells and monocytes after stimulation by IL-2,
IL-18 or IFN-γ (7,8). The IL-32 gene is located on
human chromosome 16p13.3, and has six splice variants, IL-32α,
IL-32β, IL-32γ, IL-32δ, IL-32ε and IL-32ζ (9). Although the receptor for IL-32
remains to be determined, IL-32 has been found to stimulate TNF-α,
IL-1β and IL-8 production and thus act as a pro-inflammatory
mediator in inflammatory diseases and tumor (7,10).
IL-32 expression is increased in malignant esophageal tissues
(11), and overexpression of
IL-32 is reversely associated with 5-year recurrence-free,
disease-specific and overall survival rates in localized clear cell
renal cell carcinoma (12).
However, the role of IL-32 in osteosarcoma progression remains to
be elucidated. In the present study, we focused on the effects of
IL-32 on cell invasion and motility, and aimed to determine the
molecular mechanisms of IL-32 in osteosarcoma cells.
Materials and methods
Reagents and cell culture
Recombinant IL-32 was purchased from R&D Systems
(Minneapolis, MN, USA). AKT selective inhibitor LY294002 was
purchased from Calbiochem (San Diego, CA, USA). Antibody of IL-32
was obtained from Abcam (Cambridge, UK). Antibodies of AKT and
phosphorylated AKT were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Antibody of β-actin was obtained from
Sigma Aldrich (St. Louis, MO, USA). The MG-63 human osteosarcoma
cell line was purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA). Cells were incubated in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) and maintained in 5% CO2 at 37°C in a
humidified incubator.
Invasion assay
Cell invasion ability was assessed using an invasion
assay. Briefly, a 24-well Transwell plate was purchased from Costar
(Corning, NY, USA) and the upper chambers were coated with Matrigel
prior to use. The cells were adjusted to a concentration of
5×105/ml and then treated with different concentrations
of IL-32 (0, 50, 100 and 200 ng/ml). Subsequently, 200 μl of
cell suspension was added into the upper chambers, and 500
μl DMEM supplemented with 20% FBS was added into the lower
chambers. After 18 h, the cells that invaded through the
Matrigel-coated filters were fixed and stained with crystal violet.
The invaded cells were counted under a microscope at a
magnification of ×200.
Wound-healing assay
Cell motility was assessed by a scratch
wound-healing assay. The cells were seeded in a 6-well plate,
cultured until confluent and then treated with or without IL-32
(100 ng/ml). The cell layer was wounded by a sterile tip and the
spread of wound closure was observed after 18 h under a microscope
at a magnification of ×100.
RNA interference (RNAi) assay
Small-interfering RNA (siRNA) was designed and
obtained from Shanghai GeneChem (Shanghai, China). Using
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA), MG-63
cells were transfected with an IL-32 siRNA
(5′-GCUCACUCCUCUACUUGAA-3′) or a scramble control siRNA
(5′-UGGUUUACAUGUUUUCUGA-3′). The cells were then incubated for 48 h
and the knockdown efficiency was assessed by western blot
analysis.
Western blot analysis
Cell lysates were extracted by RIPA lysis buffer
supplemented with protease and phosphatase inhibitors (Applygen
Technologies, Inc., Beijing, China), and then the BCA method was
used to measure the concentration of total protein. An equal amount
of protein was separated by SDS-PAGE gels and then transferred onto
a PVDF membrane. The membrane was then blocked for 1 h in TBST
containing 5% BSA, and subsequently immunoblotted with primary
antibody overnight at 4°C. After washing with TBST, the membrane
was incubated for 1 h with secondary antibody. Finally, the bands
were visualized via chemiluminescence using an ECL Detection kit
(Applygen Technologies).
RT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen), as per the manufacturer’s instructions. Reverse
transcription was performed to obtain cDNA using the RevertAid
First Strand cDNA synthesis kit (Fermentas, Burlington, Ontario,
Canada). Subsequently, 2 μg of cDNA was amplified with the
primers of matrix metalloproteinase (MMP)-13 (F,
5′-ACTGAGAGGCTCCGAGAAATG-3′ and R, 5′-GAACCCCGCATCTTGGCTT-3′); and
β-actin (F, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and R,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′) using a SYBR-Green PCR kit
(Applied Biosystems, Carlsbad, CA, USA). RT-PCR analysis was
performed using the following cycle parameters: 10 min at 95°C, and
then 40 cycles of 15 sec at 95°C and 1 min at 60°C. β-actin was
used as internal control and the relative expression of MMP-13 was
determined by the 2−ΔΔCt method.
ELISA
The cells were incubated with or without IL-32 (100
ng/ml) for 18 h. The cell supernatant was collected and stored at
−80°C until the ELISA was performed. The MMP-13 protein level in
the cell supernatant was assessed by the MMP-13 ELISA kit
(Millipore, Billerica, MA, USA), according to the manufacturer’s
instructions.
Statistical analysis
The experiments were performed at least three times.
Data were presented as mean ± standard error of the mean. The data
were analyzed using SPSS software (version 18.0). Comparisons
between any two groups were assessed by the Student’s t-test and
comparisons among multiple groups were assessed by one-way analysis
of variance (ANOVA). P<0.05 was considered to indicate a
statistically significant result.
Results
IL-32 promotes the invasion and motility
of osteosarcoma cells
In order to investigate the role of IL-32 in the
invasion of osteosarcoma cells, MG-63 cells were stimulated with
different concentrations of IL-32 (0, 50, 100 and 200 ng/ml) and
then an invasion assay was performed. The results showed that IL-32
stimulation promoted the invasion of MG-63 cells in a
dose-dependent manner (Fig. 1A).
Furthermore, the effect of IL-32 on cell motility was determined by
the wound-healing assay. Notaby, the results showed that IL-32
stimulation led to a significant increase in MG-63 cell motility
(Fig. 1B). These results
indicated that IL-32 stimulation enhances the invasion and motility
of osteosarcoma cells.
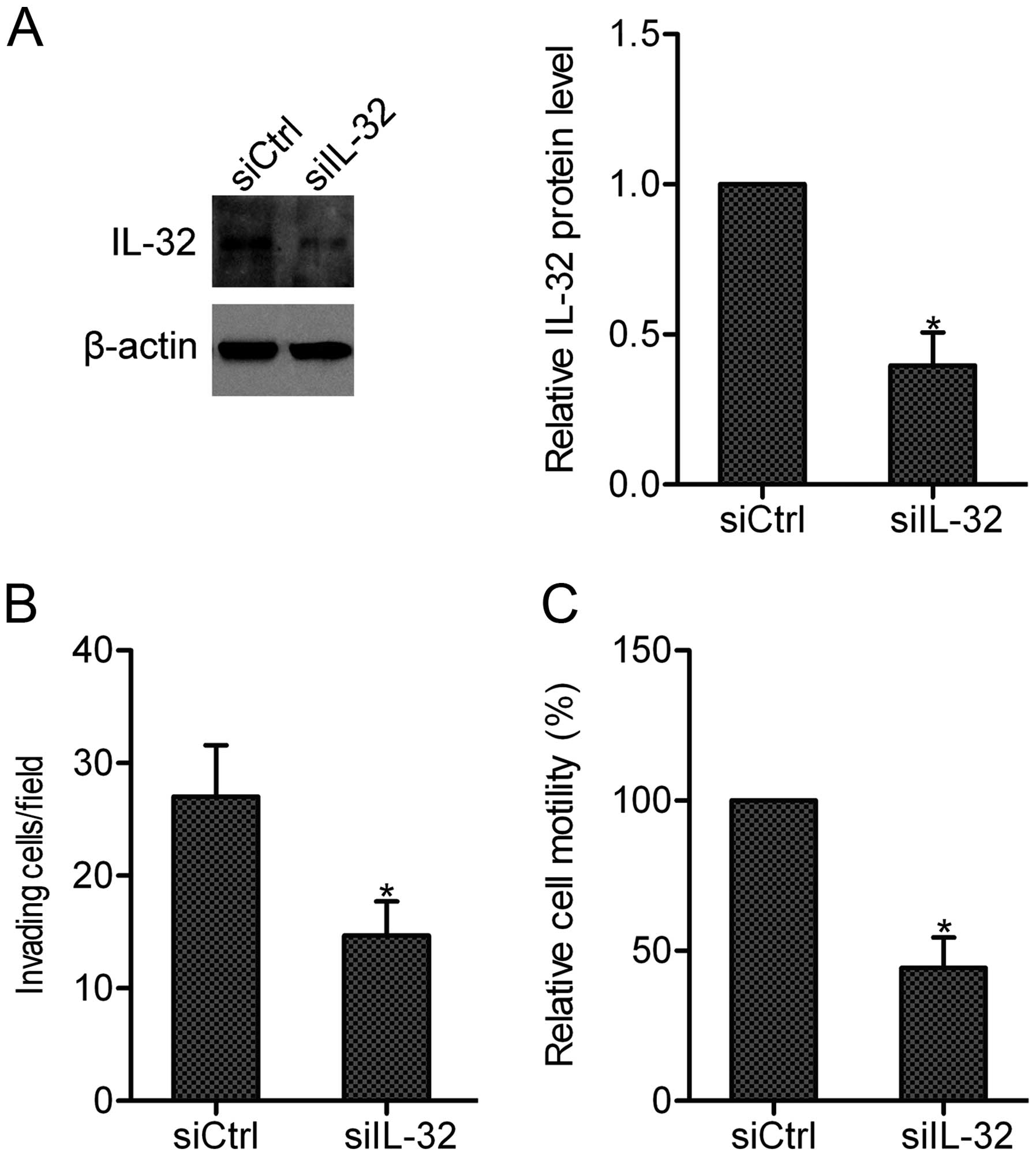
Knockdown of IL-32 suppressed the
invasion and motility of osteosarcoma cells
To examine whether endogenous IL-32 can affect the
invasion and motility of osteosarcoma cells, we silenced the
expression of IL-32 in MG-63 cells by siRNA. Western blot analysis
revealed that the endogenous expression of IL-32 was efficiently
repressed by siRNA IL-32 (Fig.
2A). Resutls of the invasion and wound-healing assays showed
that knockdown of IL-32 markedly inhibited the invasion and
motility abilities of MG-63 cells, supporting the hypothesis that
IL-32 is important in osteosarcoma cell invasion and motility
(Fig. 2B and C).
IL-32 stimulation induces the activation
of AKT
Multiple signaling pathways have been observed to be
activated by IL-32 (13). In the
present study, we studied whether IL-32 stimulation affected the
activation of AKT in osteosarcoma cells. The results showed that
IL-32 (100 ng/ml) induced the activation of AKT in a time-dependent
manner, with peak activation occurring at 30 min (Fig. 3), indicating that IL-32 induces
the activation of AKT in osteosarcoma cells.
IL-32 increases the expression and
secretion of MMP-13
Since the MMPs are essential for the invasion and
metastasis of tumor, we assessed whether IL-32 affected MMPs
expression in osteosarcoma cells. MG-63 cells were stimulated with
or without IL-32 (100 ng/ml) for 12 or 18 h, and the mRNA levels of
MMP-2, MMP-9 and MMP-13 were detected by RT-PCR. The results showed
that the mRNA expression of MMP-13 in MG-63 cells was markedly
increased after IL-32 stimulation (Fig. 4A). Thus, we further examined the
effect of IL-32 on MMP-13 secretion in MG-63 cells. ELISA showed
that IL-32 stimulation resulted in increased protein secretion of
MMP-13 in MG-63 cells (Fig. 4B).
These results suggested that IL-32 stimulates the expression and
secretion of MMP-13 in osteosarcoma cells.
AKT pathway is involved in IL-32-enhanced
cell invasion and motility
To detect the role of AKT pathway in IL-32-mediated
invasion and motility, LY294002, a selective AKT inhibitor, was
added to MG-63 cells prior to IL-32 stimulation. The results showed
that IL-32 stimulated the invasion and motility in the DMSO-treated
group. However, after inhibition of AKT by LY294002, the invasion
and motility abilities of MG-63 cells were markedly suppressed,
suggesting the involvement of AKT pathway in IL-32-enhanced
invasion and motility in osteosarcoma cells (Fig. 5).
IL-32 regulates MMP-13 expression and
secretion via the AKT pathway
We verified the effect of AKT activation the
IL-32-regulated MMP-13 production. RT-PCR and the ELISA showed that
when AKT activation was blocked by LY294002 prior to IL-32
stimulation, the mRNA expression and protein secretion of MMP-13
induced by IL-32 stimulation were significantly inhibited (Fig. 6), suggesting that IL-32
upregulates MMP-13 expression and secretion dependent on AKT
activation.
Discussion
As a member of the inflammatory cytokines, IL-32 has
been found to be invovled in the progression of cancer. Previous
findings have shown that IL-32 exerts antitumor activity by
inhibiting cell growth and inducing cell apoptosis in various types
of cancer, such as colon cancer and hepatocellular carcinoma
(14,15). However, IL-32 is able to promote
the tumorigenesis of colon cancer cells (16), and stimulate the angiogenesis of
endothelial cells (17). IL-32
has been shown to be associated with the invasion and metastasis of
gastric and lung cancer (18,19). Moreover, experimental data have
demonstrated that IL-32 stimulates the migration of breast cancer
cells (20), and increases the
invasion and metastasis of gastric and lung cancer cells (21,22), indicating that IL-32 is a crucial
mediator for tumor invasion and metastasis. IL-32 acts as a potent
modulator of osteoclastogenesis (23); however, little is known concerning
the effect of IL-32 on osteosarcoma. In the present study, we found
that IL-32 stimulation dose-dependently increased the invasion and
motility of osteosarcoma cells, and knockdown of endogenous IL-32
by siRNA significantly inhibited osteosarcoma cell invasion and
motility. These results strongly support the hypothesis that IL-32
contributes to the invasion and motility of osteosarcoma cells.
Although the receptor for IL-32 is not well
established, studies have proven that IL-32 stimulation leads to
the activation of multiple signaling pathways, including ERK1/2,
p38 and nuclear factor (NF)-κB pathways (24,25). It is reported that IL-32 treatment
induces a massive activation of AKT in osteoclast (23). Previous findings have shown that
IL-32 stimulates the activation of AKT in gastric cancer cells
(21). Similarly, we found that
IL-32 stimulation time-dependently induced the activation of AKT.
The AKT pathway is known to be essential for tumor invasion and
metastasis (26). In the present
study, inhibition of the AKT pathway markedly attenuated
IL-32-enhanced osteosarcoma cell invasion and motility, indicating
that IL-32 may promote the invasion and motility of osteosarcoma
cells by activating the AKT pathway.
MMP-13 is an important member of the MMP family, and
plays a pivotal role in tumor cell invasion and metastasis
processes via the degradation of the extracellular matrix (ECM)
(27). In the present study, we
showed that IL-32 upregulated the expression and secretion of
MMP-13 in osteosarcoma cells. In addition, we found that the AKT
pathway was required for IL-32-mediated MMP-13 upregulation.
Previous studies have reported the involvement of MMP-13 in the
regulation of osteosarcoma progression (28–30). Therefore, it is possible that AKT
pathway-mediated MMP-13 upregulation participates in IL-32-promoted
osteosarcoma cell invasion and motility.
In summary, the present study has demonstrated that
IL-32 is capable of promoting the invasion and motility in osteo
sarcoma cells. The activation of AKT and subsequent upregulation of
MMP-13 production contributes to the biological functions of IL-32.
Thus, IL-32 acts as a potential therapeutic target for
osteosarcoma.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81260274).
References
|
1
|
Heare T, Hensley MA and Dell’Orfano S:
Bone tumors: osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr.
21:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: a
review. Sarcoma. 2011:9592482011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kakhki VR, Anvari K, Sadeghi R, Mahmoudian
AS and Torabian-Kakhki M: Pattern and distribution of bone
metastases in common malignant tumors. Nucl Med Rev Cent East Eur.
16:66–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu PK, Chen WM, Chen CF, Lee OK, Haung CK
and Chen TH: Primary osteogenic sarcoma with pulmonary metastasis:
clinical results and prognostic factors in 91 patients. Jpn J Clin
Oncol. 39:514–522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheu BC, Chang WC, Cheng CY, Lin HH, Chang
DY and Huang SC: Cytokine regulation networks in the cancer
microenvironment. Front Biosci. 13:6255–6268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilson J and Balkwill F: The role of
cytokines in the epithelial cancer microenvironment. Semin Cancer
Biol. 12:113–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SH, Han SY, Azam T, Yoon DY and
Dinarello CA: Interleukin-32: a cytokine and inducer of TNFalpha.
Immunity. 22:131–142. 2005.PubMed/NCBI
|
|
8
|
Netea MG, Azam T, Ferwerda G, et al: IL-32
synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2
ligands for IL-1beta and IL-6 production through a caspase
1-dependent mechanism. Proc Natl Acad Sci USA. 102:16309–16314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi JD, Bae SY, Hong JW, et al:
Identification of the most active interleukin-32 isoform.
Immunology. 126:535–542. 2009. View Article : Google Scholar :
|
|
10
|
Yagi Y, Andoh A, Imaeda H, et al:
Interleukin-32α expression in human colonic subepithelial
myofibroblasts. Int J Mol Med. 27:263–268. 2011.
|
|
11
|
Yousif NG, Al-Amran FG, Hadi N, Lee J and
Adrienne J: Expression of IL-32 modulates NF-kappaB and p38 MAP
kinase pathways in human esophageal cancer. Cytokine. 61:223–227.
2013. View Article : Google Scholar
|
|
12
|
Lee HJ, Liang ZL, Huang SM, et al:
Overexpression of IL-32 is a novel prognostic factor in patients
with localized clear cell renal cell carcinoma. Oncol Lett.
3:490–496. 2012.PubMed/NCBI
|
|
13
|
Joosten LA, Heinhuis B, Netea MG and
Dinarello CA: Novel insights into the biology of interleukin-32.
Cell Mol Life Sci. 70:3883–3892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang YH, Park MY, Yoon DY, et al:
Dysregulation of over-expressed IL-32alpha in hepatocellular
carcinoma suppresses cell growth and induces apoptosis through
inactivation of NF-kappaB and Bcl-2. Cancer Lett. 318:226–233.
2012. View Article : Google Scholar
|
|
15
|
Oh JH, Cho MC, Kim JH, et al: IL-32gamma
inhibits cancer cell growth through inactivation of NF-kappaB and
STAT3 signals. Oncogene. 30:3345–3359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang CJ, Chien Y, Lu KH, et al:
Oct4-related cytokine effects regulate tumorigenic properties of
colorectal cancer cells. Biochem Biophys Res Commun. 415:245–251.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nold-Petry CA, Rudloff I, Baumer Y, et al:
IL-32 promotes angiogenesis. J Immunol. 192:589–602. 2014.
View Article : Google Scholar :
|
|
18
|
Ishigami S, Arigami T, Uchikado Y, et al:
IL-32 expression is an independent prognostic marker for gastric
cancer. Med Oncol. 30:4722013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sorrentino C and Di Carlo E: Expression of
IL-32 in human lung cancer is related to the histotype and
metastatic phenotype. Am J Respir Crit Care Med. 180:769–779. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JS, Choi SY, Lee JH, et al:
Interleukin-32beta stimulates migration of MDA-MB-231 and MCF-7
cells via the VEGF-STAT3 signaling pathway. Cell Oncol (Dordr).
36:493–503. 2013. View Article : Google Scholar
|
|
21
|
Tsai CY, Wang CS, Tsai MM, et al:
Interleukin-32 increases human gastric cancer cell invasion
associated with tumor progression and metastasis. Clin Cancer Res.
20:2276–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng Q, Li S, Zhou Y, et al:
Interleukin-32 contributes to invasion and metastasis of primary
lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases
2 and 9 expression. Cytokine. 65:24–32. 2014. View Article : Google Scholar
|
|
23
|
Mabilleau G and Sabokbar A: Interleukin-32
promotes osteoclast differentiation but not osteoclast activation.
PLoS One. 4:e41732009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho KS, Park SH, Joo SH, Kim SH and Shin
CY: The effects of IL-32 on the inflammatory activation of cultured
rat primary astrocytes. Biochem Biophys Res Commun. 402:48–53.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Felaco P, Castellani ML, De Lutiis MA, et
al: IL-32: a newly-discovered proinflammatory cytokine. J Biol
Regul Homeost Agents. 23:141–147. 2009.PubMed/NCBI
|
|
26
|
Brader S and Eccles SA: Phosphoinositide
3-kinase signalling pathways in tumor progression, invasion and
angiogenesis. Tumori. 90:2–8. 2004.PubMed/NCBI
|
|
27
|
Balbin M, Pendas AM, Uria JA, Jimenez MG,
Freije JP and Lopez-Otin C: Expression and regulation of
collagenase-3 (MMP-13) in human malignant tumors. APMIS. 107:45–53.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ye Z, Jingzhong L, Yangbo L, Lei C and
Jiandong Y: Propofol inhibits proliferation and invasion of
osteosarcoma cells by regulation of microRNA-143 expression. Oncol
Res. 21:201–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Osaki M, Takeshita F, Sugimoto Y, et al:
MicroRNA-143 regulates human osteosarcoma metastasis by regulating
matrix metalloprotease-13 expression. Mol Ther. 19:1123–1130. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma O, Cai WW, Zender L, et al: MMP13,
Birc2 (cIAP1), and Birc3 (cIAP2), amplified on chromosome 9,
collaborate with p53 deficiency in mouse osteosarcoma progression.
Cancer Res. 69:2559–2567. 2009. View Article : Google Scholar : PubMed/NCBI
|