Introduction
Epidural fibrosis (EF) ranks as a common
complication of laminectomy, a common type of surgery utilized to
treat spinal diseases, including lumbar disc herniation, lumbar
(low back) spinal stenosis and other lumbar disorders (1,2).
Extensive scar adhesion between the dural mater and surrounding
muscles may contribute to the formation of EF and subsequently
cause post-operative pain recurrence after laminectomy or
discectomy. Large therapeutic schedules are utilized to prevent
epidural scar adhesion. Accordingly, the reduction of
scar-triggered epidural fibrosis is crucial for the treatment of
spinal diseases.
Scar formation and adhesion often lead to negative
effects on outcome after laminectomy. Previous findings have
demonstrated that fibroblast hyperplasia is critical for epidural
fibrosis as it plays important roles in the generation of the
fibrotic matrix during scar formation (3,4).
As a highly mechano sensitive cell-type, fibroblasts transform into
profibrotic myofibroblasts to enhance the expression of α-smooth
muscle actin (α-SMA) and extracellular matrix (ECM) proteins, which
play a vital role in wound repair and scar formation (5,6).
Accumulating evidence has indicated that blocking fibroblast
proliferation with 10-hydroxycamptothecin (HCPT) significantly
reduces the degree of epidural adhesion after laminectomy,
indicating a potential strategy for the improvement of the surgical
curative effect (3).
The connective tissue growth factor/cysteine-rich
61/nephroblastoma overexpressed (CCN) family comprises six highly
conserved cysteine rich proteins. Mounting evidence suggests the
critical roles of CCN family in various cell processes including
proliferation and adhesion, homeostasis, osteoblast
differentiation, wound repair, inflammation, fibrosis and
tumorigenesis (7–9). Of these members, connective tissue
growth factor (CTGF)/CCN2 acts as an ECM protein and is associated
with wound healing and scar formation (10,11). The downregulation of CCN2
expression with its specific anti-sense oligonucleotides
significantly decreases myofibroblast numbers, collagen formation
and limits scar hypertrophy (10).
CCN5 (also known as rCop-1, Wisp-2 and CTGF-l) is
another key member of the CCN5 family and is located on chromosome
20q12-q13.1. Unlike other members, CCN5 is absent in the cysteine
knot (CT) containing a carboxyl-terminal domain. Previous studies
have demonstrated a growth inhibition effect of CCN5 on smooth
muscle cell proliferation and motility (12). Furthermore, an inhibitory effect
of CCN5 on CCN2-mediated fibrogenesis has been identified,
suggesting a novel anti-fibrotic function of CCN5 (13). CCN2 and CCN5 were found to be
upregulated during the development of cardiac hypertrophy. However,
CCN2 expression accelerates cardiac fibrosis and hypertrophy,
whereas CCN5 exerted anti-hypertrophic and -fibrotic effects,
indicating that CCN5 antagonizes CCN2 function during the
development of cardiac hypertrophy and fibrosis (14). Moreover, a negative regulatory
function of CCN5 on α-SMA and collagen I expression was confirmed
(15). Additionally, CCN5
repressed transforming growth factor-β (TGF-β) signaling pathway,
which plays a pivotal role in scar adhesion and epidural fibrosis
after laminectomy (16). However,
CCN5 function in epidural fibrosis and its underlying mechanism
remain unclear.
In the present study, the expression levels of CCN5
analyzed in laminectomized rats. The isolated fibroblasts were
employed in order to explore the proliferative and fibrotic effect
of CCN5. Furthermore, the underlying mechanisms were
investigated.
Materials and methods
Reagents
Unless stated otherwise, all the substances were
purchased from Gibco (Grand Island, NY, USA). The primary
antibodies against CCN2 (ab6992) were purchased from Abcam
(Cambridge, MA, USA). Anti-CCN5 antibodies (sc-12010) were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Anti-phospho-Smad6 and Smad6 antibodies (9519) were purchased
from Cell Signaling Technology (Beverly, MA, USA). Rabbit
anti-α-SMA polyclonal antibody (ab5694) was purchased from Abcam
(Cambridge, UK). Rabbit polyclonal antibody raised against COL1A1
was obtained from Abnova Corp. (Taipei, Taiwan).
Animal models
Twenty-four healthy 12-week-old male Lewis rats were
provided by the Laboratory Animal Center of Xi’an Jiaotong
University and included in this study. The rat laminectomy models
were performed as previously described (4). Animal experiments were undertaken
follwing the approval of the Institutional Animal Care and Use
Committee. Briefly, the rats were anaesthetized with sodium
pentobarbital (50 mg/kg). Prior to laminectomy, all the animals
were shaved in the area near the first lumbar vertebra (L1) and the
third lumbar vertebra (L2). The exposed skin was sterilized,
followed by a midline skin incision. The dura mater of the L1
vertebrae was exposed after removing the spinous processes. A total
laminectomy at L2 vertebra was performed by a rongeur. Fourteen
days later, the rats were sacrificed, the scar and the surrounding
normal tissues were collected for subsequent analysis.
Fibroblast isolation and culture in
vitro
The primary fibroblasts were obtained from the tail
skin of rats. The protocol conformed to the guidelines of the
Institutional Animal Care and Use Committee. Following enzymatic
digestion, the isolated cells were incubated in Dulbecco’s modified
essential medium (DMEM) (Life Technologies, Gaithersburg, MD, USA)
containing 10% fetal bovine serum (FBS) (Invitrogen, Grand Island,
NY, USA), 100 µg/ml streptomycin and penicillin. The cells
were stimulated with TGF-β1 (10 ng/ml) (Sigma, St. Louis, MO, USA)
for 12 h and cultured in a humidified atmosphere at 37°C with 5%
CO2. Fibroblasts from passages 4 to 6 were used in the
subsequent study.
Lentivirus construction and
infection
To construct the recombinant lentiviral vector
carrying CCN5, the full-length CCN5 cDNA was amplified with its
specific primers (sense, 5′-GCCGCGTGGGACACGGTGACATGAGG-3′,
containing MluI restriction enzyme site; 5′-CGGTCGACCAGTTGGCCTTAGAAAGC-3′,
containing SalI restriction enzyme site). Following
digestion with MluI and SalI restriction enzymes, the
CCN5 cDNA was ligated into the lentivirus plasmid pWPT-GFP (Toyobo,
Tokyo, Japan) to construct the recombinant pWPT-CCN5 plasmids,
which contained the green fluorescent protein (GFP) and were
digested with MluI and SalI restriction enzymes. The
293 cells were then co-transfected with the lentivirus plasmids
pWPT-CCN5, packaging vectors of pCMV-VSV-G and pCMV-dR8.91
(Clontech, Saint-Germain-en-Laye, France) using
Lipofectamine® 2000 Reagent (Invitrogen-Life
Technologies, Carlsbad, CA, USA) at 37°C, 5% CO2 for 12
h. The cultured medium was collected and filtered. The cultured
fibroblasts were then infected with the collected LV-CCN5
adenovirus. The virus was amplified and purified. Virus titers were
determined by p24 ELISA kit (Cell Biolabs, Inc., San Diego, CA,
USA), and then stored at −80°C for use. The vectors were used as a
negative control.
Transfection with Smad6 siRNA
To silence Smad6 levels in fibroblasts, the specific
siRNA fragments of Smad6 and control siRNA were designed as
previously described (17) and
synthesized by Takara. For transfection, the cells were seeded into
6-well plates and grown to 40-50% confluence. Subsequently, 2
µg/ml Smad6 siRNA or control siRNA was transfected into the
cells using Lipofectamine 2000 (Invitrogen-Life Technologies)
according to the manufacturer’s instructions. The transfection
efficiency was analyzed by western blotting. Data are reported as
the mean of three or four distinct experiments.
RNA extraction and real-time polymerase
chain reaction (PCR)
Total RNA was extracted using TRIzol reagent
according to the manufacturer’s instructions (Biostar, Shanghai,
China). Approximately 5 µg of RNA was reverse transcribed to
synthesize the first-strand cDNA using the cDNA Synthesis kit
(Fermentas, St. Leon-Rot, Germany). Real-time PCR was performed at
a final volume of 20 µl which consisted of 10 µl
SYBR® Premix Ex Taq™ II, 10 µmol/l specific
primers, 4 µl of DNA, and H2O. The specific
primers used were: CCN2, 5′-TAG CAAGAGCTGGGTGTGTG-3′ (sense) and
5′-TTCACTTGC CACAAGCTGTC-3′ (antisense); CCN5, 5′-TTAGCACTTGTG
GTGGCTTG-3′ (sense) and 5′-CCATTGAGAGAAGGCAG AGG-3′ (antisense);
collagen type I, α1 (COL1A1), 5′-ATCAGCCCAAACCCCAAGGAGA-3′ (sense)
and 5′-CGCAGGAAG GTCAGCTGGATAG-3′ (antisense). The above mRNA
levels were normalized to β-actin. All the samples were performed
in triplicate.
Western blot analysis
After lysis with RIPA lysis buffer (100 mM NaCl, 50
mM Tris-HCl pH 7.5, 1% Triton X-100, 1 mM EDTA, 10 mM
β-glycerophosphate, 2 mM sodium vanadate and protease inhibitor),
total protein extracts were analyzed by the BCA protein assay
(Pierce, Rockford, IL, USA). Then, 200 µg protein was
separated by SDS-PAGE and transferred to a PVDF membrane. After
blocking with 5% non-fat milk, the membrane was incubated with the
primary antibodies against CCN2, CCN5, Smad-6, p-Smad6, α-SMA and
COL1A1, followed by incubation with the corresponding secondary
antibodies to horseradish peroxidase (HRP). The proteins were
detected with enhanced chemiluminescence (ECL; Amersham Pharmacia
Biotech, Piscataway, NJ, USA) and normalized with β-actin.
[3H]-Tzhymidine incorporation
assay ([3H]-TdR)
The cells were seeded in 24-well plates with the
density of 4×104 cells/well. The cells were cultured in
DMEM medium for 24 h, followed by the serum-starved incubation for
2 h with the indicated treatments. After treatment with
[3H]-thymidine (Sigma) for 6 h, the cells were washed
three times with ice-cold normal saline. Trichloroacetic acid (10%)
was added for a further 30-min incubation at 4°C. The liquid
scintillation counter (Beckman Coulter, Fullerton, CA, USA) was
introduced to evaluate cell proliferation by detecting the
radioactivity.
MTT assays
The MTT assay was performed to evaluate cell
viability. Briefly, the cells were seeded in 24-well plates with a
density of 1×105 cells/well. After preconditioning with
the above indicated treatments, 20 µl MTT reagent (Sigma)
was added for 6 h at 37°C, followed by treatment with 200 µl
isopropanol to dissolve formazan production. Cell viability was
then evaluated by analyzing the absorbance of MTT at 590 nm using a
micro-ELISA reader (Bio-Rad, Hercules, CA, USA). The samples were
performed in triplicate and the results were presented as the
percentage of growth inhibition.
Measurement of collagen protein
Following treatment as described above, the total
soluble collagen in culture supernatants was detected using a
Sircol Assay kit (Biocolor, Belfast, Northern Ireland, UK),
according to the manufacturer’s instructions.
Statistical analysis
Data are shown as the means ± SD from at least three
experiments. The Student’s t-test was used to assess the
statistical significance differences in multiple comparisons. Data
were analyzed using SPSS 11.0 software. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression levels of CCN5 and CCN2 in
scar tissue after laminectomy
CCN5 exerted an opposing function in CCN2-induced
cardiac hypertrophy and fibrosis (14). However, its roles in
scar-triggered epidural fibrosis remain to be determined.
Laminectomized rats were used to determine the expression levels of
CCN5 and CCN2 in scar tissue after laminectomy. RT-PCR analysis
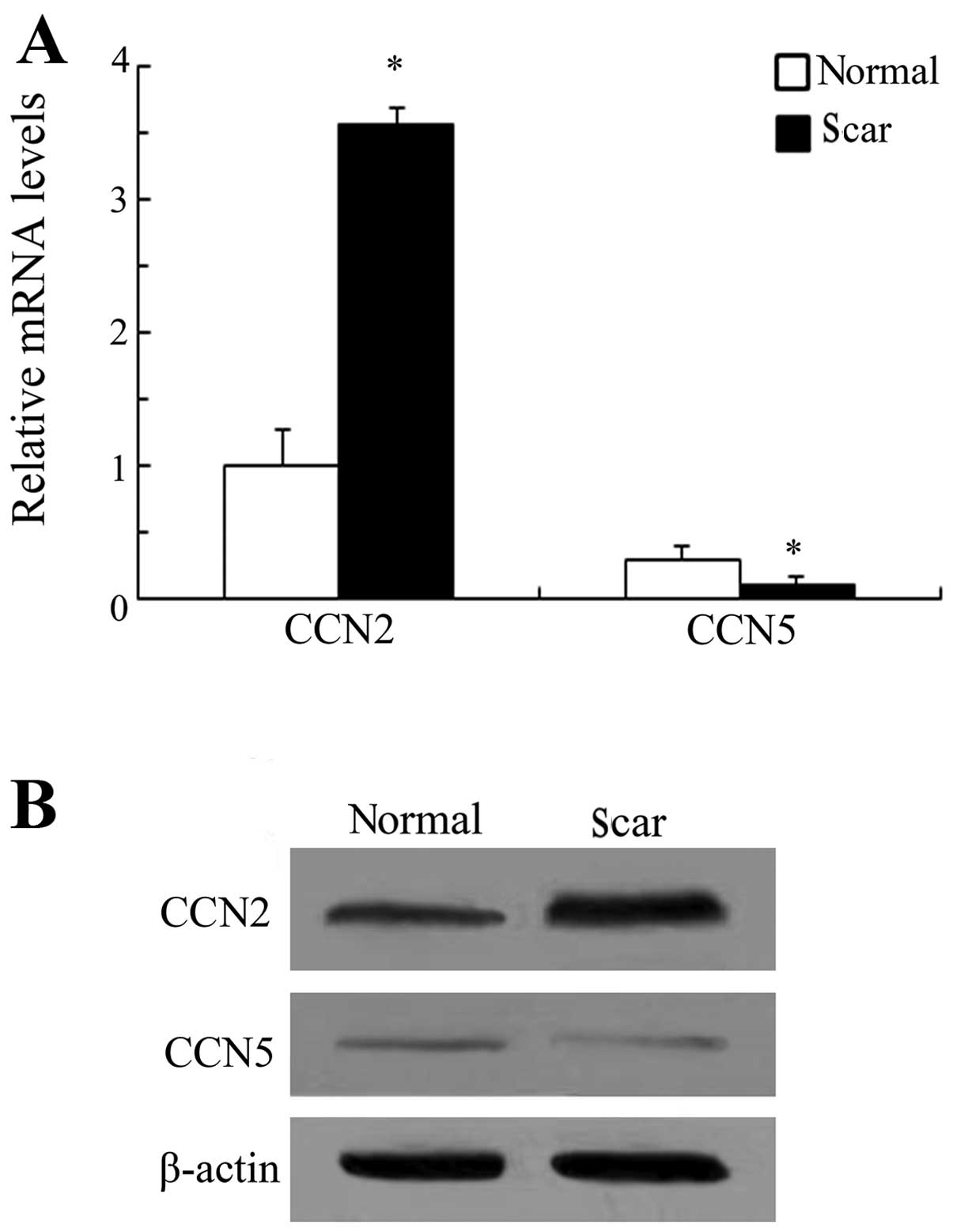
confirmed an obvious upregulation of CCN2 in scar tissues as
compared to the surrounding normal tissues (Fig. 1A). A similar upregulation in CCN2
protein levels was demonstrated by western blotting (Fig. 1B). However, CCN5 expression levels
were significantly downregulated during scar formation following
laminectomy. CCN2 has been believed to act as a positive regulator
of fibrogenesis, scar formation and wound repair (11). Therefore, these results suggest
that CCN5 elicits potential anti-fibrotic effects during the
development of scar formation based on opposing expression levels
to CCN2 in scar tissues.
rCCN5 transfection enhances CCN5
expression in primary fibroblasts
Fibroblasts are essential for epidural fibrosis due
to their function in fibrotic matrix formation during scar
formation (3,4). To investigate the effect of CCN5 on
scar formation, the recombinant lentiviral vector-carrying CCN5
(LV-CCN5) was constructed and transfected into the isolated primary
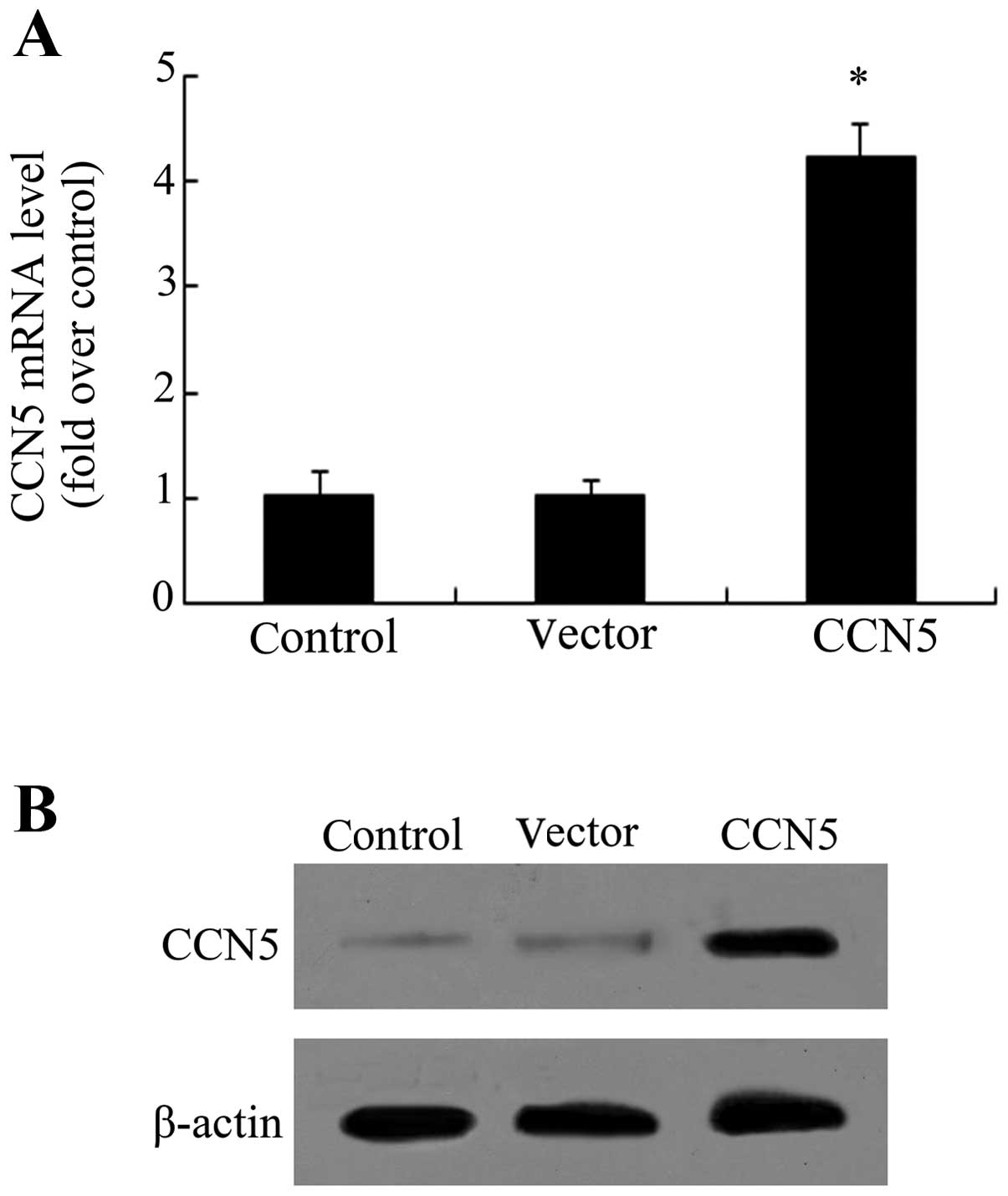
fibroblasts. As shown in Fig. 2A,
an ~4.1-fold increase in CCN5 mRNA levels was observed when the
cells were transfected with LV-CCN5. Furthermore, western blot
analysis confirmed the obvious upregulation of CCN5 protein
following LV-CCN5 transfection, compared with the control and
vector-treated groups (Fig. 2B),
indicating that a stable overexpression system of CCN5 had been
successfully constructed.
CCN5 overexpression inhibits cell
viability and proliferation
TGF-β1 is known to be critical for scar-triggered
epidural fibrosis following laminectomy due to its important role
in the development of fibrosis (16). Based on the stable expression of
CCN5 in fibroblasts, cell viability and proliferation in response
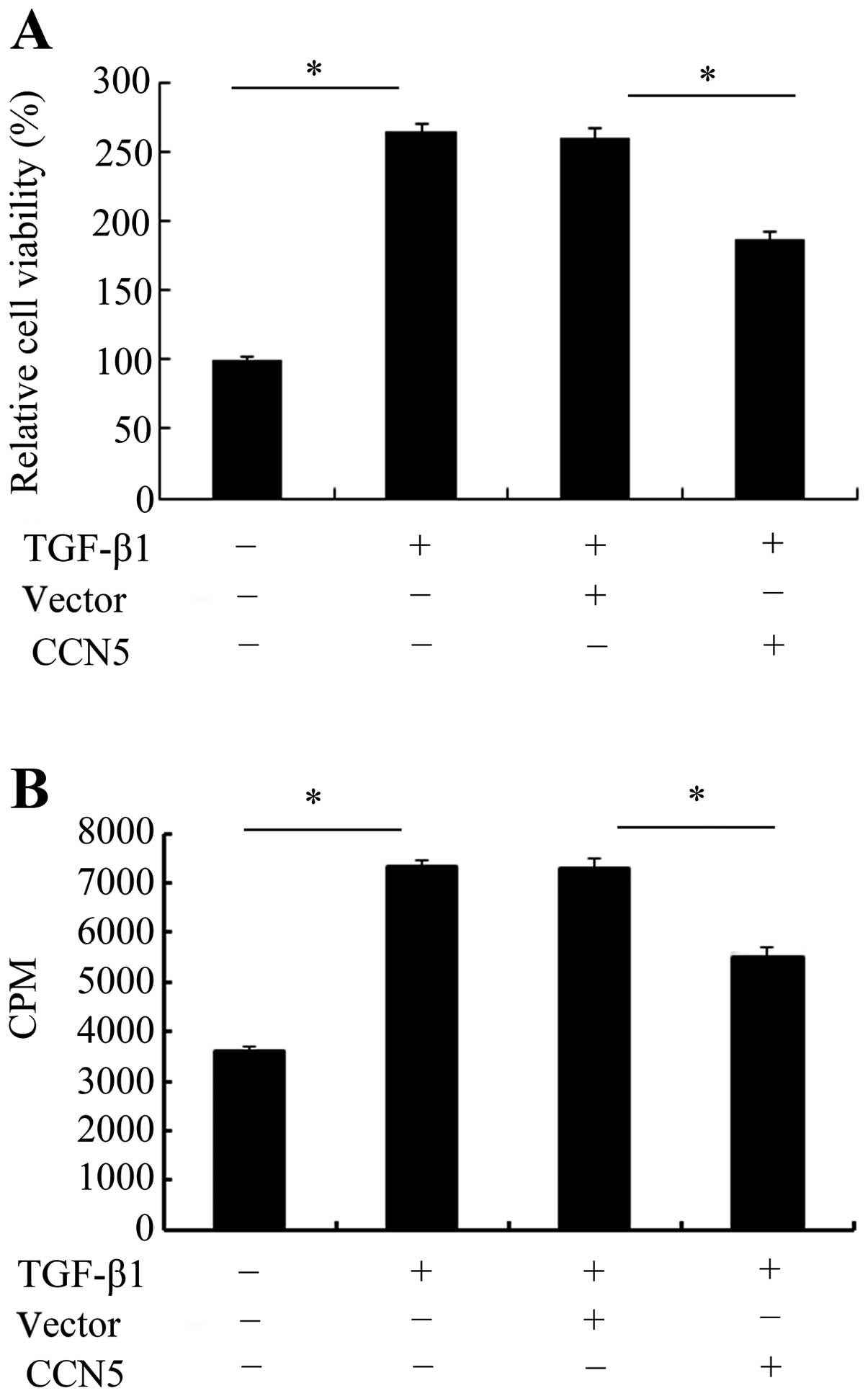
to TGF-β1 was investigated. An MTT assay was used to assess the
effect of CCN5 on cell viability. As shown in Fig. 3A, TGF-β1 stimulation exhibited a
2.5-fold increase in cell viability. However, this increase was
markedly attenuated when CCN5 was overexpressed in fibroblasts.
Further [3H]-TdR analysis confirmed that TGF-β1
treatment induced cell proliferation and resulted in an ~2.0-fold
increase in cell numbers (Fig.
3B). Elevated CCN5 expression decreased TGF-β1-induced cell
proliferation. These results suggested that CCN5 exhibits a
negative inhibitory function during the TGF-β1-induced process of
fibrosis by inhibiting fibroblast cell viability and
proliferation.
Elevated CCN5 expression reduces the
profibrotic phenotype of fibroblasts induced by TGF-β1
To assess the function of CCN5 during the
development of scar formation, the anti-fibrotic effect of CCN5 was
examined. α-SMA is believed to be a biochemical marker of
myofibroblasts transformed from fibroblasts, which result in the
excessive accumulation of fibrotic tissue and subsequent scar
formation and adhesion (18).
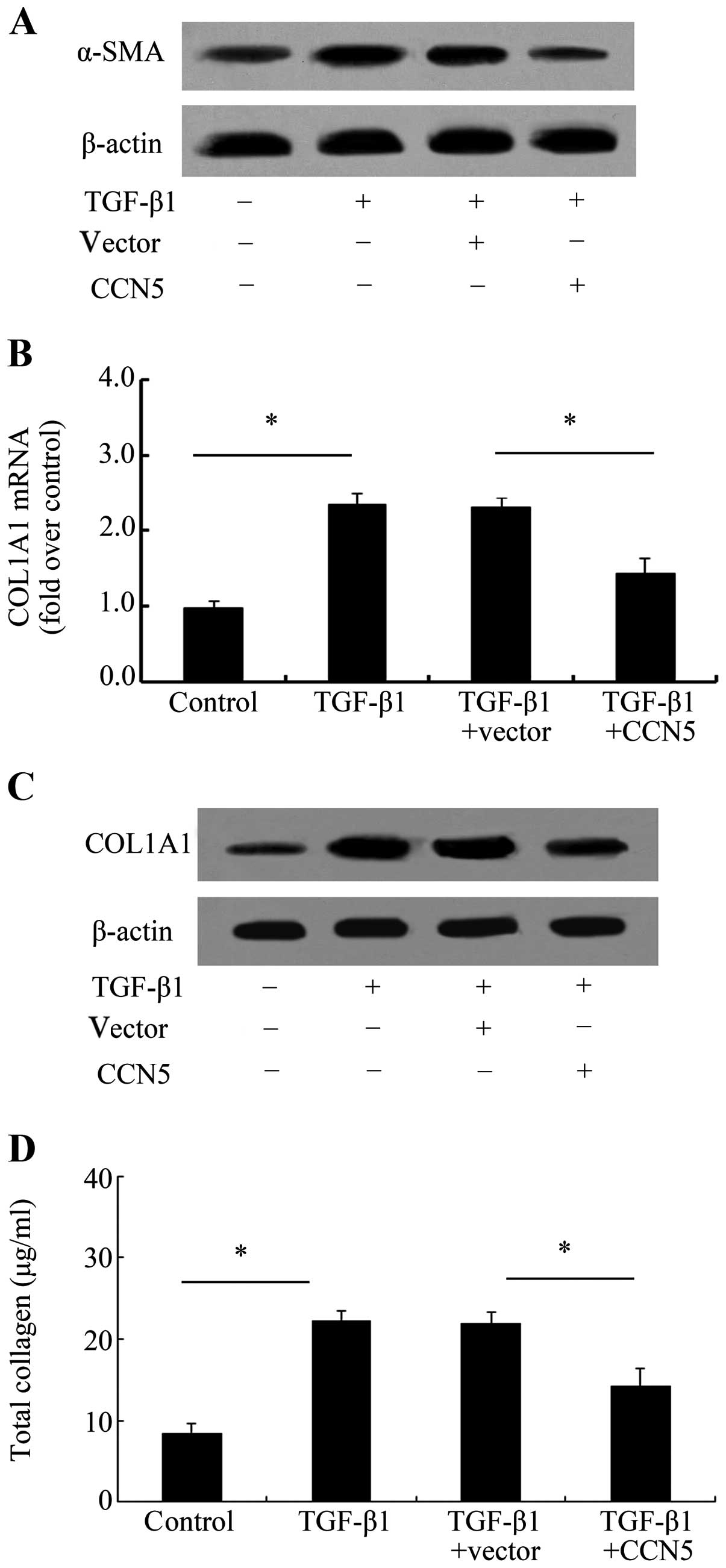
Therefore, we assessed the effect of CCN5 on α-SMA and the results
confirmed that CCN5 overexpression markedly decreased α-SMA levels
induced by TGF-β1 (Fig. 4A),
suggesting that CCN5 attenuated the transformation of fibroblasts
into profibrotic myofibroblasts. COL1A1 is a major ECM protein that
is able to induce collagen I formation, which is the most abundant
product of fibrosis. Further analysis exhibited a notable
upregulation in COL1A1 mRNA levels (Fig. 4B) and protein levels (Fig. 4C) after TGF-β1 stimulation.
However, this increase was obviously attenuated when
preconditioning with LV-CCN5 transfection. Simultaneously, CCN5
over expression also markedly downregulated the total collagen
concentration (Fig. 4D),
indicating that CCN5 antagonized TGF-β1-induced profibrotic
phenotype of fibroblasts.
CCN5 attenuates TGF-β1-induced CCN2
expression
CCN2 is overexpressed in tissue repair and human
diseases characterized by excessive scarring and fibrosis allowing
it to be induced by TGF-β1 thereby enhancing the progressive
fibrotic response to scar tissue formation (11,19). Based on the opposing expression
levels of CCN5 and CCN2 in scar tissues, we analyzed the
correlation between CCN5 and CCN2 during the fibrotic process of
fibroblasts. TGF-β1 treatment induced an obvious upregulation of
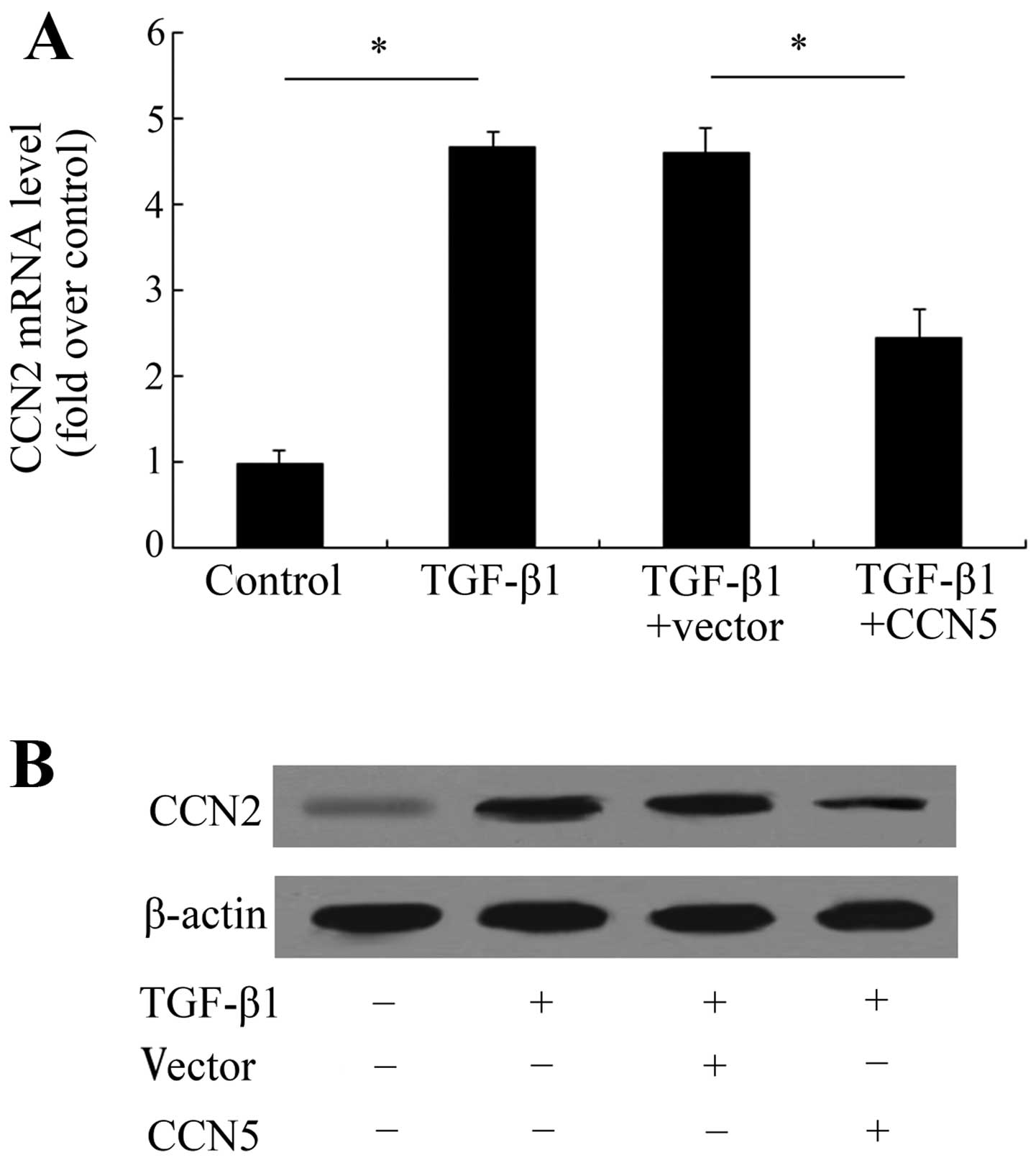
CCN2 mRNA levels (Fig. 5A).
Further assay showed that CCN5 expression significantly attenuated
this increase in CCN2 mRNA from 4.59- to 2.45-fold. The protein
levels of CCN2 were also decreased following LV-CCN5 treatment,
compared with the TGF-β1-treated group (Fig. 5B). These data indicate that CCN5
may exert anti-fibrotic effects by inhibiting the activation of the
CCN2 pathway.
CCN5 functions in TGF-β1-induced
proliferation and profibrotic phenotype through Smad6-CCN2
pathway
To clarify the underlying mechanisms involved in the
CCN5-induced anti-fibrotic function, we investigated Smad6
signaling due to the critical role it plays in blocking
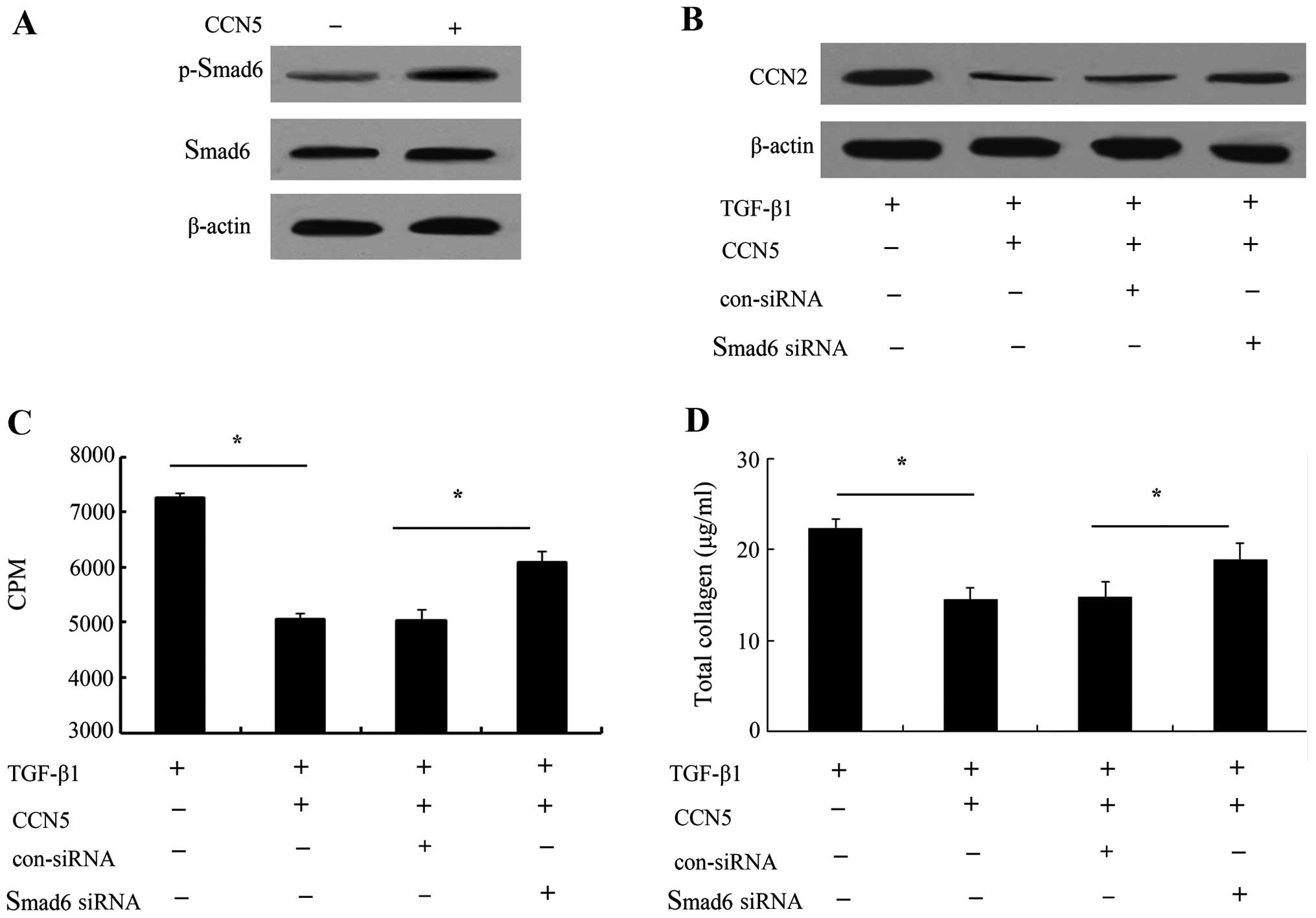
TGF-β1-triggered other Smad signaling (20,21). As shown in Fig. 6A, the elevated CCN5 expression
markedly increased the phosphorylation levels of Smad6. When CCN5
expression was upregulated, CCN2 protein levels induced by TGF-β1
were markedly inhibited. However, silencing Smad6 signaling
obviously ameliorated this inhibitory effect on CCN2 levels,
suggesting that CCN5 was able to abrogate TGF-β1-induced CCN2
expression levels by Smad6 signaling (Fig. 6B). Furthermore, the Smad6 pathway
was blocked with its specific siRNA, and cell proliferation was
markedly increased in the CCN5 and TGF-β1-treated groups compared
with the control group (Fig. 6C).
Of note, the inhibitory function of CCN5 on collagen production was
notably decreased (Fig. 6D).
These results suggested that the Smad6-CCN2 pathway was involved in
the anti-fibrotic function of CCN5.
Discussion
Postoperative epidural fibrosis, the development of
dense and thick scar tissue adjacent to the dura mater after lumbar
laminectomy and discectomy, causes compression and stretching of
the nerve root and results in persistent back and leg pain
(22,23). Although multifactorial factors are
involved in the process of failed spine surgery, epidural fibrosis
is ranked as the critical contributor to unfavorable clinical
outcomes and recurring symptoms (24,25). Given the pivotal roles of scar
formation in epidural fibrosis, the prevention of scar formation
has received more attention in order to improve spine surgery. In
this study, we constructed the post-laminectomy rat models and
found an obvious downregulation of CCN5 in scar tissues, suggesting
a potentially important role of CCN5 during the development of scar
formation and subsequent epidural fibrosis.
Fibrosis often occurs in response to wound repair by
excessive scar formation following stimulation such as surgery
(1). During this process,
fibroblasts are believed to be the prominent participators
(3,5). Their proliferation and migration
into the wound may contribute to wound repair. Scar is considered
to be created by fibroblast proliferation, when inhibiting
proliferation, the degree of epidural adhesion was significantly
attenuated after laminectomy (3).
The TGF-β superfamily is known to exert a number of functions in
various physiological processes, including embryonic development,
wound repair and cell proliferation (16,26). TGF-β1 ranks as a crucial
profibrotic protein and is associated with fibroblast chemotaxis,
prolife ration, and ECM deposition (27,28). In this study, we successfully
constructed CCN5-ovexpressed cell models. Further analysis revealed
that CCN5 upregulation in fibroblasts abrogated cell viability in
response to TGF-β1. Consistent with these results, TGF-β1-elevated
cell proliferation was significantly attenuated, indicating an
important role of CCN5 in fibroblast cell survival and
proliferation.
The fibroblasts involved in scarring and contraction
are myofibroblasts, which are specialized contractile fibroblasts.
It is widely accepted that fibroblasts can convert into profibrotic
myofibroblasts following stimulation, which are proven to be
increased within fibrotic lesions and contribute to excessive scar
formation (29). TGF-β1 is a
central regulator for fibrotic response. Its stimulation can
transform fibroblasts into myofibroblasts and promotes the
synthesis and deposition of ECM, which plays an important role in
wound repair and scar formation (28). To clarify the effect of CCN5
during scar development, we analyzed CCN5 function in the
anti-fibrotic process. In this study, TGF-β1 triggered an increase
in α-SMA and collagen type I expression. Following transfection
with LV-CCN5, the upregulation of α-SMA, a specific marker of
myofibroblasts, was markedly decreased. Furthermore, CCN5
overexpression reduced the COL1A1 expression levels, which induced
collagen I formation, the most abundant product of fibrosis
(30). Of note, CCN5 upregulation
obviously attenuated TGF-β1-induced total collagen content.
Therefore, the results confirm a potential anti-fibrotic effect of
CCN5 during the development of EF.
CCN2 is known to be an important profibrotic
mediator and is overexpressed in various fibrotic diseases. It
often acts as a downstream effector of TGF-β signaling to promote
scar tissue production, while suppressing its expression prevents a
progressive fibrotic response to TGF-β (11). Furthermore, blocking CCN2 levels
with its specific antisense oligonucleotides significantly
downregulate myofibroblast numbers and collagen formation, and
limits scar hypertrophy (10).
Previous findings have shown an opposing role of CCN5 and CCN2
during cardiac fibrosis (14). In
this study, we observed an obvious upregulation of CCN2 and
downregulation of CCN5 in scar tissues following laminectomy.
Further analysis confirmed that CCN5 overexpression markedly
decreased CCN2 expression levels induced by TGF-β1 stimulation,
indicating a potential anti-fibrosis role of CCN5 by CCN2
signaling.
TGF-β1 is considered a key mediator in fibrosis
progression by activating its downstream Smad signaling pathway.
The Smad family has been confirmed to be involved in various
fibrotic diseases (31-33). Unlike other Smad members, Smad6
can prevent the phosphorylation of Smad members and then act as a
negative feedback regulator of the TGF-β superfamily-mediated
pathway (21). Previous findings
have shown that blocking CCN5 expression increases Smad6 levels
(34). Thus, to explore the
underlying mechanism involved in the CCN5-induced anti-fibrotic
role, Smad6 signaling was analyzed. The results showed that CCN5
upregulation enhanced the phosphorylation of Smad6. When blocking
Smad6 with its specific siRNA, the inhibitory effect of CCN5 on
CCN2 levels induced by TGF-β1 was notably ameliorated. Smad6 siRNA
transfection obviously restored CCN5-inhibited cell proliferation
and collagen contents.
In conclusion, this study demonstrated the marked
downregulation in CCN5 levels in scar tissues after laminectomy. In
this study, CCN5 overexpression significantly abrogated fibroblast
proliferation, viability and the profibrotic phenotype of
fibroblasts through Smad6-CCN2 signaling, which may contribute to
scar formation. Therefore, our findings provide prominent insight
into the degree to which CCN5 exerts a potential anti-fibrotic
effect during the development of epidural fibrosis. Future studies
are required to prove the effect and precise mechanisms of action
of CCN5 in ameliorating epidural fibrosis following laminectomy
in vivo.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 30972556), the Science and
Technology Research and Development of Shaanxi province (no.
2011K14-08-02)
References
|
1
|
Bottegaro NB, Kos J, Pirkic B, et al:
Reduction of epidural fibrosis after laminectomy in rabbits by
omental free graft. Vet Med. 58:25–31. 2013.
|
|
2
|
Guo JD, Hou SX, Li L, et al: Laminectomy
and extraction of nucleus pulposus for treatment of lumbar disc
herniation: effect evaluation of over 10-year-followed-up. Zhongguo
Gu Shang. 26:24–28. 2013.In Chinese. PubMed/NCBI
|
|
3
|
Sun Y, Wang L, Sun S, Liu B, Wu N and Cao
X: The effect of 10-hydroxycamptothecine in preventing fibroblast
proliferation and epidural scar adhesion after laminectomy in rats.
Eur J Pharmacol. 593:44–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang C, Kong X, Ning G, et al: All-trans
retinoic acid prevents epidural fibrosis through NF-κB signaling
pathway in post-laminectomy rats. Neuropharmacology. 79:275–281.
2014. View Article : Google Scholar
|
|
5
|
Ray S, Ju X, Sun H, Finnerty CC, Herndon
DN and Brasier AR: The IL-6 trans-signaling-STAT3 pathway mediates
ECM and cellular proliferation in fibroblasts from hypertrophic
scar. J Invest Dermatol. 133:1212–1220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jun JI and Lau LF: The matricellular
protein CCN1 induces fibroblast senescence and restricts fibrosis
in cutaneous wound healing. Nat Cell Biol. 12:676–685. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kular L, Pakradouni J, Kitabgi P, Laurent
M and Martinerie C: The CCN family: a new class of inflammation
modulators? Biochimie. 93:377–388. 2011. View Article : Google Scholar
|
|
8
|
Chen PC, Cheng HC, Yang SF, Lin CW and
Tang CH: The CCN family proteins: modulators of bone development
and novel targets in bone-associated tumors. Biomed Res Int.
2014:4370962014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Minhas U, Martin TA, Ruge F, Harding KG
and Jiang WG: Pattern of expression of CCN family members Cyr61,
CTGF and NOV in human acute and chronic wounds. Exp Ther Med.
2:641–645. 2011.PubMed/NCBI
|
|
10
|
Sisco M, Kryger ZB, O’Shaughnessy KD, et
al: Antisense inhibition of connective tissue growth factor
(CTGF/CCN2) mRNA limits hypertrophic scarring without affecting
wound healing in vivo. Wound Repair Regen. 16:661–673. 2008.
View Article : Google Scholar
|
|
11
|
Shi-Wen X, Leask A and Abraham D:
Regulation and function of connective tissue growth factor/CCN2 in
tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev.
19:133–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lake AC, Bialik A, Walsh K and Castellot
JJ Jr: CCN5 is a growth arrest-specific gene that regulates smooth
muscle cell proliferation and motility. Am J Pathol. 162:219–231.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leask A: Yin and Yang Part Deux: CCN5
inhibits the pro-fibrotic effects of CCN2. J Cell Commun Signal.
4:155–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon PO, Lee MA, Cha H, et al: The
opposing effects of CCN2 and CCN5 on the development of cardiac
hypertrophy and fibrosis. J Mol Cell Cardiol. 49:294–303. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang CY, Xie X, Gao DS and Zhang CX:
Effects of CCN5 overexpression on the expression of alpha-SMA and
collagen I in hepatic stellate cells and its mechanism. Zhongguo
Ying Yong Sheng Li Xue Za Zhi. 29:411–415. 2013.In Chinese.
|
|
16
|
Penn JW, Grobbelaar AO and Rolfe KJ: The
role of the TGF-β family in wound healing, burns and scarring: a
review. Int J Burns Trauma. 2:18–28. 2012.
|
|
17
|
Ichijo T, Voutetakis A, Cotrim AP, et al:
The Smad6-histone deacetylase 3 complex silences the
transcriptional activity of the glucocorticoid receptor: Potential
clinical implications. J Biol Chem. 280:42067–42077. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koźma EM, Wisowski G, Kusz D and Olczyk K:
The role of decorin and biglycan dermatan sulfate chain(s) in
fibrosis-affected fascia. Glycobiology. 21:1301–1316. 2011.
View Article : Google Scholar
|
|
19
|
Wang Q, Usinger W, Nichols B, et al:
Cooperative interaction of CTGF and TGF-β in animal models of
fibrotic disease. Fibrogenesis Tissue Repair. 4:42011. View Article : Google Scholar
|
|
20
|
Imamura T, Takase M, Nishihara A, et al:
Smad6 inhibits signalling by the TGF-beta superfamily. Nature.
389:622–626. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amankwah EK, Thompson RC, Nabors LB, et
al: SWI/SNF gene variants and glioma risk and outcome. Cancer
Epidemiol. 37:162–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kasimcan MO, Bakar B, Aktaş S, Alhan A and
Yilmaz M: Effectiveness of the biophysical barriers on the
peridural fibrosis of a postlaminectomy rat model: an experimental
research. Injury. 42:778–781. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karatay M, Celik H, Koktekir E, et al:
Role of tenoxicam in the prevention of postlaminectomy peridural
fibrosis in rats. J Neurol Sci. 30:559–565. 2013.
|
|
24
|
Zeinalizadeh M, Miri SM, Ardalan FA, et
al: Reduction of epidural fibrosis and dural adhesions after lamina
reconstruction by absorbable cement: an experimental study. Spine
J. 14:113–118. 2014. View Article : Google Scholar
|
|
25
|
Bosscher HA and Heavner JE: Incidence and
severity of epidural fibrosis after back surgery: an endoscopic
study. Pain Practice. 10:18–24. 2010. View Article : Google Scholar
|
|
26
|
Massagué J: TGF-β signaling in development
and disease. FEBS Lett. 586:18332012. View Article : Google Scholar
|
|
27
|
Zhang C, Kong X, Zhou H, et al: An
experimental novel study: Angelica sinensis prevents epidural
fibrosis in laminectomy rats via downregulation of hydroxyproline,
IL-6, and TGF-β1. Evid Based Complement Alternat Med.
2013:2918142013.
|
|
28
|
Kohta M, Kohmura E and Yamashita T:
Inhibition of TGF-beta1 promotes functional recovery after spinal
cord injury. Neurosci Res. 65:393–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gabbiani G: The myofibroblast in wound
healing and fibrocontractive diseases. J Pathol. 200:500–503. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rujitanaroj PO, Jao B, Yang J, et al:
Controlling fibrous capsule formation through long-term
downregulation of collagen type I (COL1A1) expression by
nanofiber-mediated siRNA gene silencing. Acta Biomater.
9:4513–4524. 2013. View Article : Google Scholar
|
|
31
|
Ding N, Yu RT, Subramaniam N, et al: A
vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic
response. Cell. 153:601–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu X, Hu H and Yin JQ: Therapeutic
strategies against TGF-beta signaling pathway in hepatic fibrosis.
Liver Int. 26:8–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar
|
|
34
|
Sabbah M, Prunier C, Ferrand N, et al:
CCN5, a novel transcriptional repressor of the transforming growth
factor β signaling pathway. Mol Cell Biol. 31:1459–1469. 2011.
View Article : Google Scholar : PubMed/NCBI
|