Introduction
Endometriosis, which is a benign and
estrogen-dependent disease, is the most common chronic inflammatory
gynecological disease in women of reproductive age, and it is
mainly caused by the ectopic presence and growth of normal
endometrial cells in the pelvic cavity instead of the uterine
cavity (1,2). The morbidity associated with
endometriosis is approximately 10% in women of child-bearing age,
and this increases to 20–30% in women with subfertility and to
40–60% in women with dysmenorrhea (1–3).
The most common symptoms are severe dysmenorrhea, dyspareunia,
pelvic pain and infertility (4),
which markedly reduce the quality of life of affected women.
The goal of current medical treatments is to inhibit
the effects of estrogen on ectopic implants through the suppression
of the production of ovarian estrogen through oral contraceptives,
aromatase inhibitors, androgenic agents and gonadotropin-releasing
hormone analogues (1,2,5,6).
Anti-estrogen hormonal therapies can be prescribed only for a short
period of time (6–9 months) due to the undesirable side-effects,
such as bone density loss, pseudomenopause, hot flushes, mood
swings, an increased risk of uterine and ovarian cancers, and
compromised pregnancy, which profoundly affect the quality of life
and emotional and physical wellbeing of patients with endometriosis
(1,2,5,6).
Surprisingly, the disease re-establishes at a rate of approximately
50–60% within a year, after the cessation of anti-estrogen therapy
(5,6). Hence, the discovery of a
non-estrogen or non-steroidal therapeutic target, such as histone
modification, which controls the growth and survival of endometrial
stromal cells (ESCs), is urgently required for the treatment of
endometriosis.
Epigenetics, a relatively new field of study,
focuses on investigating the stable inheritance of phenotypes of
cells and organisms which occur without changes in the DNA sequence
or DNA content (7). Epigenetic
phenotypes can be conferred through nuclear processes, such as DNA
methylation and chromatin modifications (e.g., acetylation,
biotinylation, isomerization, methylation, phosphorylation,
ribosylation, sumoylation and ubiquitination of histones), and they
underlie the regulation of all genome functions, including gene
expression, DNA replication and genome stability (8,9).
Accumulating evidence indicates that several epigenetic aberrations
are involved in the pathogenesis of endometriosis (10–12).
Histone modification is the main mechanism of
epigenetics, and it serves to regulate gene expression following
transcription without altering the sequence of the silenced genes.
Modifications to histones at N-terminal histone tails, which
protrude from the nucleosomes, have been recognized as markers of
genes undergoing epigenetic abnormality in diseases (13). At least 8 patterns of histone
modification have been identified, and acetylation has been the
most intensively studied (14).
Histone acetylation is a reversible situation that can either
disrupt chromosomal contacts or affect non-histone protein
interactions with chromatin, thus altering chromatin structure and
gene expression (15). Histone
acetyltransferases (HATs) and histone deacetylases (HDACs)
acetylate and deacetylate lysine residues on the N-terminal region
of histone proteins, which regulate the access of transcriptional
factors to DNA and consequently regulate gene expression. Histone
deacetylases inhibitors (HDACis) appear to significantly promote
histone acetylation (11).
Several HDACis have been identified, such as trichostatin A (TSA),
sodium butyrate (NaB) and valproic acid (VPA) (16). However, the effects of histone
acetylation on the CYP19 gene have not yet been fully
investigated.
The CYP19 gene encodes P450 aromatase, and
cyclo-oxegenase-2 (COX2) increases the expression of P450 aromatase
(17–19). P450 aromatase catalyzes the final
steps in the biosynthesis of estrogen from androgens in two ways:
i) from androstenedione to estrone and ii) from testosterone to
estradiol (20). Thus, the
aromatase cytochrome P450 is involved in the final and
rate-limiting step of estrogen synthesis and is associated with
circulating estrogen levels (21). Studies have indicated that
aromatase activity does not occur in endometrial tissue from women
without uterine diseases. By contrast, endometrial lesions express
aromatase, which is accompanied by increased mRNA levels, and
aromatase enzyme activity is detectable in endometriosis (18,22). The enzyme aromatase P450 is
aberrantly expressed in patients with endometriosis, and this
results in the production of estrogen in endometrial lesions.
Furthermore, estrogen promotes the secretion of several
inflammatory cytokines and growth factors, which contributes to the
progression of endometriosis and stimulates estrogen production
(23).
In the present study, we first identified normal
endometrial stromal cells (NESCs) and ESCs. We then promoted
histone acetylation in the ESCs through VPA. Subsequently, we
measured cell viability, proliferation and apoptosis. Finally, ChIP
assay, reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis were performed to investigate
the effects of histone acetylation on CYP19 expression. The
results demonstrated that histone acetylation inhibited the
viability and proliferation of the ESCs whilst promoting apoptosis,
possibly by downregulating CYP19 expression. This discovery
may prove to be of use in the targeted therapy of
endometriosis.
Materials and methods
Cell culture and identification
Human NESCs and ESCs were kindly donated by the
School of Medicine, Shanghai Jiao Tong University (Shanghai,
China). The ESCs and NESCs were cultured in DMEM/F12 (HyClone,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 mg/ml streptomycin (Sigma-Aldrich, St.
Louis, MO, USA) in an atmosphere of 5% CO2 at 37°C. In
the monolayer culture, and as previously described, the cells were
identified by immunofluorescence staining using vimentin,
cytokeratin (24) and prolactin
(PRL) (25).
Immunofluorescence staining
After the third passage and when the ESCs reached
approximately 80% confluence, they were fixed in 4%
paraformaldehyde on ice. They were then washed 3 times with
phosphate-buffered saline (PBS) and blocked with 10% horse serum.
Subsequently, the samples were incubated overnight with primary
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
including mouse monoclonal vimentin (sc-6260), mouse monoclonal
cytokeratin (sc-57004) and goat polyclonal PRL (sc-7805) at 4°C.
After the cells had been again washed 3 times, the cell slides were
incubated at room temperature with the goat anti-mouse (A-11017)
and rabbit anti-goat (A-11078) secondary antibodies (Invitrogen,
Carlsbad, CA, USA) for 30 min. Images were captured using a laser
confocal microscope (FV1000; Olympus, Tokyo, Japan).
Treatment with VPA
To enhance the acetylation of histones, the ESCs
were digested and plated in 10-cm dishes (Corning, Inc., Tewksbury,
MA, USA), followed by incubation for 24 h with VPA (8 mM;
Sigma-Aldrich). Untreated cells served as controls.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed using a Pierce Agarose ChIP
kit (Pierce Biotechnology, Rockford, IL, USA) according to the
manufacturer's instructions. The immunoprecipitated DNA was
subjected to PCR analysis of the promoter regions of the
CYP19 gene. The sequences of the primers for the ChIP assay
were as follows: CYP19 sense, 5′-AGT AA CACA GAA CAG TTG
CA-3′ and antisense, 5′-TCC AGA CTC GCA TGA ATT CTC CGT A-3′, and
the product was 188 bp. The PCR conditions were as follows: 94°C
for 10 min, followed by 35 cycles of 94°C for 30 sec, 55°C for 30
sec, and 72°C for 30 sec, with a final extension at 72°C for 10
min, using a PCR amplification kit (Takara Biotechnology, Dalian,
China). The PCR products were analyzed by 2% agarose gel
electrophoresis.
Transfection with small interfering RNA
(siRNA)
The siRNA was synthesized by GenaPharma Co.
(Shanghai, China). Briefly, 5 µl of siRNA (CYP19
siRNA, sc-41498; Santa Cruz Biotechnology, Inc.), 10 µl of
Lipofectamine 2000 (Invitrogen) and 245 µl of non-serum DMEM
were mixed, followed by incubation for 30 min at room temperature.
The mixtures were then equally distributed into the 6-well cultured
cells followed by incubation at 37°C for transfection into the
cells. Cells transfected with the control siRNA (sc-37007; Santa
Cruz Biotechnology, Inc.) instead of the CYP19 siRNA were
used as the negative control.
MTT assay
Cell viability was evaluated by MTT assay. Briefly,
the cells were digested and re-seeded into evenly 96-well plates.
Subsequently, 20 µl MTT solution (5 mg/ml) were added to the
medium, and the cells were incubated for 4 h at 37°C. The mixtures
were then centrifuged at 8,000 rpm for 15 min and the supernatant
was discarded. The formazan crystals were dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich). The absorbance of the samples was
measured at 490 nm using an EnVision® Multilabel Reader
(PerkinElmer, Waltham, MA, USA).
5-Bromo-2-deoxyuridine (BrdU) assay
The proliferation of the ESCs was investigated by
BrdU staining using BrdU enzyme-linked immunosorbent assay (ELISA)
kit (colorimetric; Roche Diagnostics GmbH, Penzberg, Germany).
Briefly, 5×103 cells/well were seeded into 96-well
plates. When the cells grew to a confluence of 50%, the medium was
discarded and fresh BrdU-medium was added. Subsequently, the cells
were incubated in 5% CO2 at 37°C for 60 min. After being
washed with PBS and fixed with 70% ethanol, the cells were
incubated with mouse monoclonal BrdU antibody (sc-32323 FITC; Santa
Cruz Biotechnology, Inc.). The cells were observed and counted in
randomly selected fields under a fluorescence microscope (Olympus).
Data were presented as percentages of BrdU-positive cells vs. the
total cell percentage.
Hoechst staining and terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) assay
For Hoechst staining, 1 ml of Hoechst 33258 staining
solution (10 µg/ml; Beyotime, Nanjing, China) was added to
the medium, and the cells were incubated at room temperature for 5
min. Subsequently, after the Hoechst medium was discarded and the
cells were washed with PBS, fluorescence mounting liquid (Beyotime)
was added. The apoptotic rate was calculated as the number of
apoptotic cells/total number of cells x100%. For the TUNEL, the
cells were fixed with 4% paraformaldehyde for 10 min at room
temperature and permeabilized with ice-cold 0.1% Triton X-100 for
10 min. Cell apoptosis was evaluated using a TUNEL Apoptosis
Detection kit (Merck Millipore, Billerica, MA, USA) following the
manufacturer's instructions. All cells were observed under a
fluorescence microscope (Olympus). The positive cells were counted
in randomly selected fields, and the number was averaged for
further analysis.
RT-qPCR
Total RNA was isolated using TRIzol reagent
(Invitrogen). Reverse transcription of the RNA (1 mg) into
complementary DNA (cDNA) was conducted using the QuantiTect Reverse
Transcription kit (Qiagen, Courtaboeuf, France). Subsequently,
quantitative PCR (qPCR_ was performed using the QuantiTect
SYBR-Green RT-PCR kit (Qiagen) with the StepOnePlus™ Real-Time PCR
system (Life Technologies, Rockville, MD, USA). The CYP19
mRNA levels were measured by RT-qPCR. β-actin served as a reference
gene. All samples were tested in triplicate. Data were analyzed
using the comparative threshold cycle method
(2ΔΔCt).
Western blot analyisis
The cells were digested using 1%
trypsin-ethylenediaminetetraacetic acid (EDTA) and harvested.
Subsequently, the cell lysates were centrifuged, and then mixed
with radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich).
The mixture was occasionally shaken on ice to release the protein.
The protein concentration was determined using Bio-Rad Protein
Analysis kits (Bio-Rad, Hercules, CA, USA). Target proteins were
isolated and transferred onto polyvinylidene difluoride (PVDF)
membranes by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The transferred proteins were incubated
with primary antibodies (all from Santa Cruz Biotechnology, Inc.),
including goat polyclonal acetylated histone H3 (Lys9, sc-8655;
diluted at 1:1,000, v/v), rabbit polyclonal acetylated histone H4
(Lys12, sc-8661-R; 1:1,000), goat polyclonal CYP19
(sc-14245; 1:500) and rabbit polyclonal β-actin (sc-130656;
1:1,000) antibodies. The PVDF membranes were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibody (diluted
at 1:5,000, v/v; Santa Cruz Biotechnology, Inc.), followed by
treatment with chemiluminescence substrate (Pierce Biotechnology).
The blots were observed and photographed using an ImageQuant LAS
4000 biomolecular imager (GE Healthcare, Piscataway, NJ, USA), and
densitometric analysis was carried out using Quantity One software
(Bio-Rad).
Statistical analysis
Data are presented as the means ± SD of at least 3
independent experiments. The differences between 2 groups were
examined using the Student's t-test, while the differences between
3 or more groups were compared by one-way analysis of variance
(ANOVA). Values of P<0.05 and P<0.01 were considered to
indicate statistically significant differences.
Results
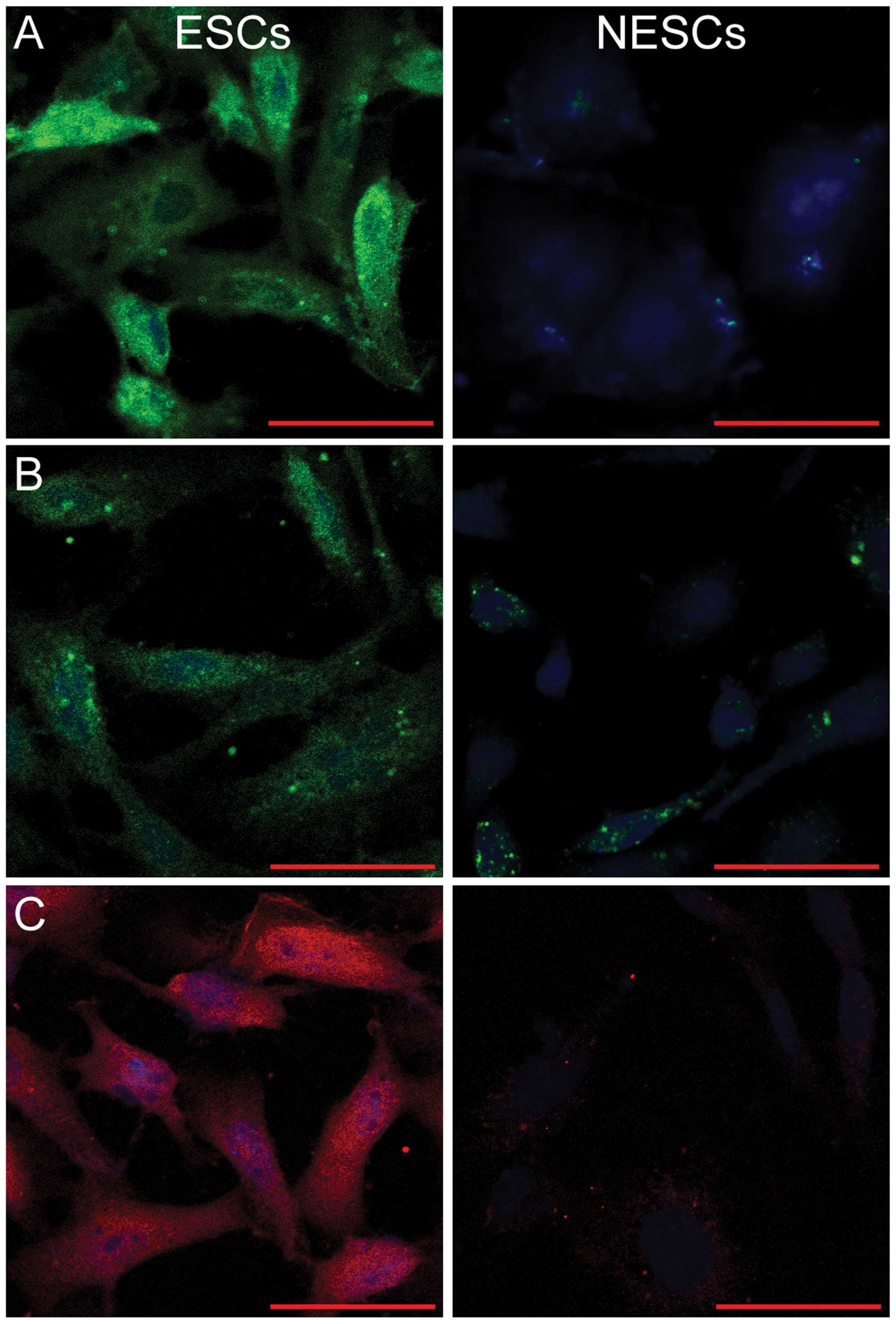
ESCs are positive for vimentin,
cytokeratin and PRL, whereas NESCs are not
As ESCs are characterized by the positive expression
of vimentin, cytokeratin and PRL (26), both the ESCs and NESCs were
subjected to immunofluorescence staining for the detection of the
expression of these markers. As shown in Fig. 1, fluorescence staining for
vimentin, cytokeratin and PRL was visible in the cytoplasm of the
ESCs, but was absent in the NESCs (Fig. 1).
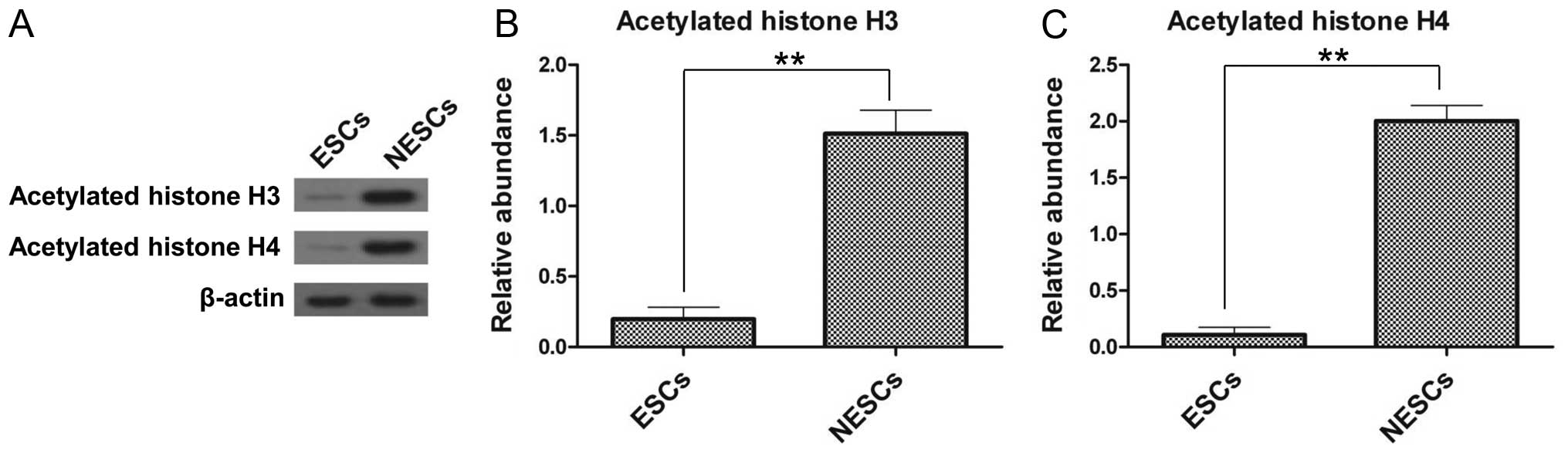
Histone acetylation is decreased in
ESCs
The differences in the expression of acetylated
histones between the ESCs and NESCs were further examined by
western blot analysis. The results revealed that the ESCs had a
significantly lower expression of acetylated histone H3 and H4
compared with the NESCs (Fig.
2A). The expression of acetylated histone H3 and H4 in the
NESCs was 7.9-fold (Fig. 2B) and
18.2-fold (Fig. 2C) greater,
respectively, compared with that in the ESCs. These results confirm
the differences between ESCs and NESCs, and the following
experiments were conducted using the ESC model.
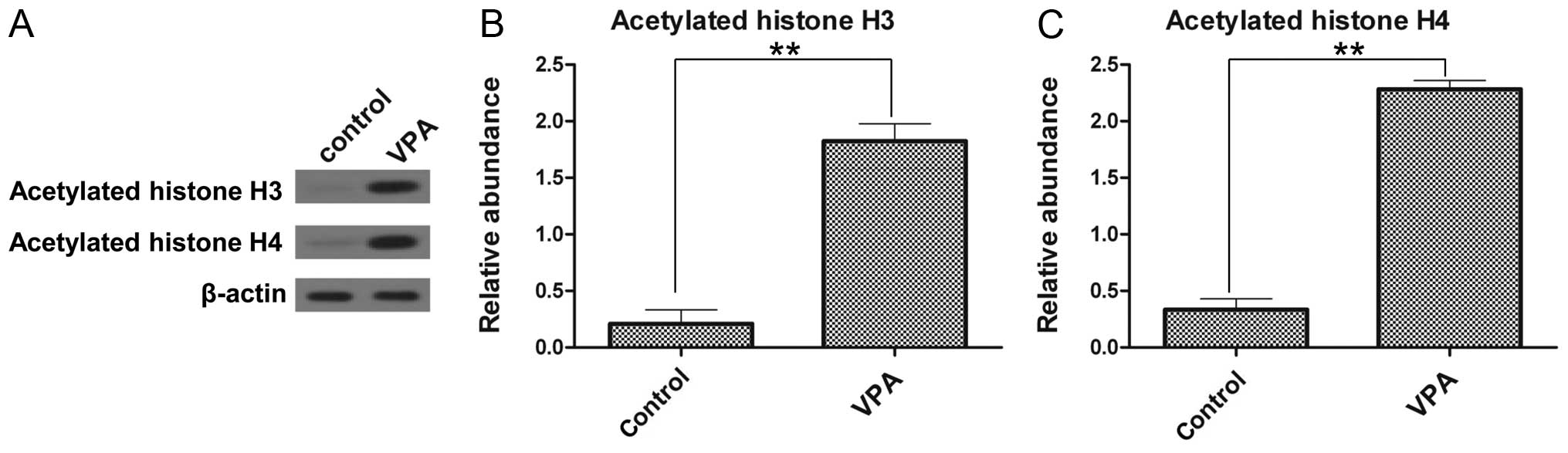
VPA enhances the acetylation of histone
H3 and H4 in the ESCs
To investigate whether VPA alters the acetylation of
histones in ESCs, we evaluated the global acetylation of histone H3
and H4 by western blot analysis. Low levels of acetylated histone
H3 and H4 were detected in the control group (Fig. 3). By contrast, the levels of
acetylated histone H3 and acetylated histone H4 in the ESCs were
significantly increased by treatment with VPA. The levels of
acetylated histone H3 and H4 in the VPA-treated ESCs were 8.7-fold
(Fig. 3B) and 6.9-fold (Fig. 3C) greater, respectively, compared
with those of the control group, thus demonstrating that VPA
promotes histone acetylation.
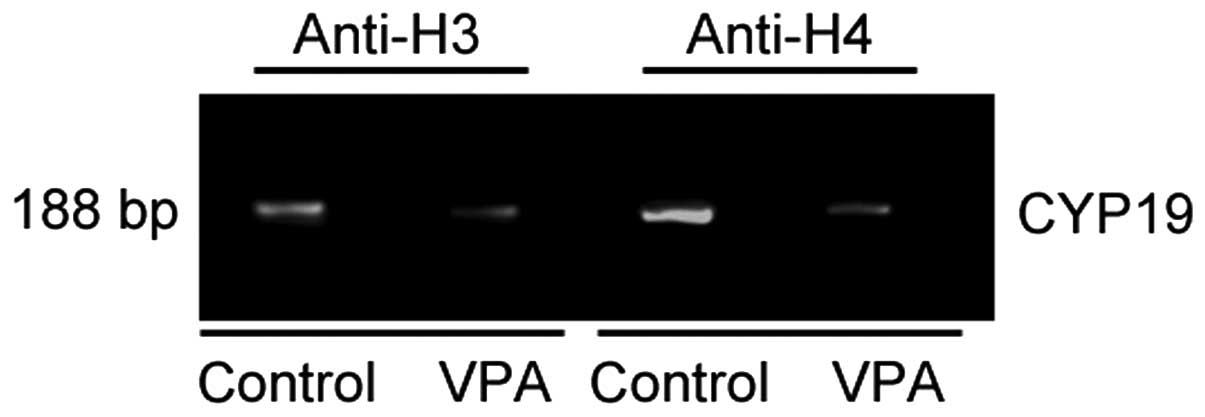
Histone acetylation is reduced in the
promoter region of the CYP19 gene by VPA
To examine the effects of VPA on the acetylation of
the promoter region of the CYP19 gene in ESCs, ChIP assay
with antibodies against acetylated histone H3 and acetylated
histone H4 was performed. The immunoprecipitated DNA from the
untreated and VPA-treated ESCs was isolated and subjected to PCR
using primers for the promoter regions of the CYP19 gene,
and a 188-bp fragment was amplified (Fig. 4). VPA significantly decreased the
amounts of PCR product, suggesting that the acetylation of histone
H3 and H4 was reduced in the promoter region of the CYP19
gene in the VPA-treated ESCs (Fig.
4).
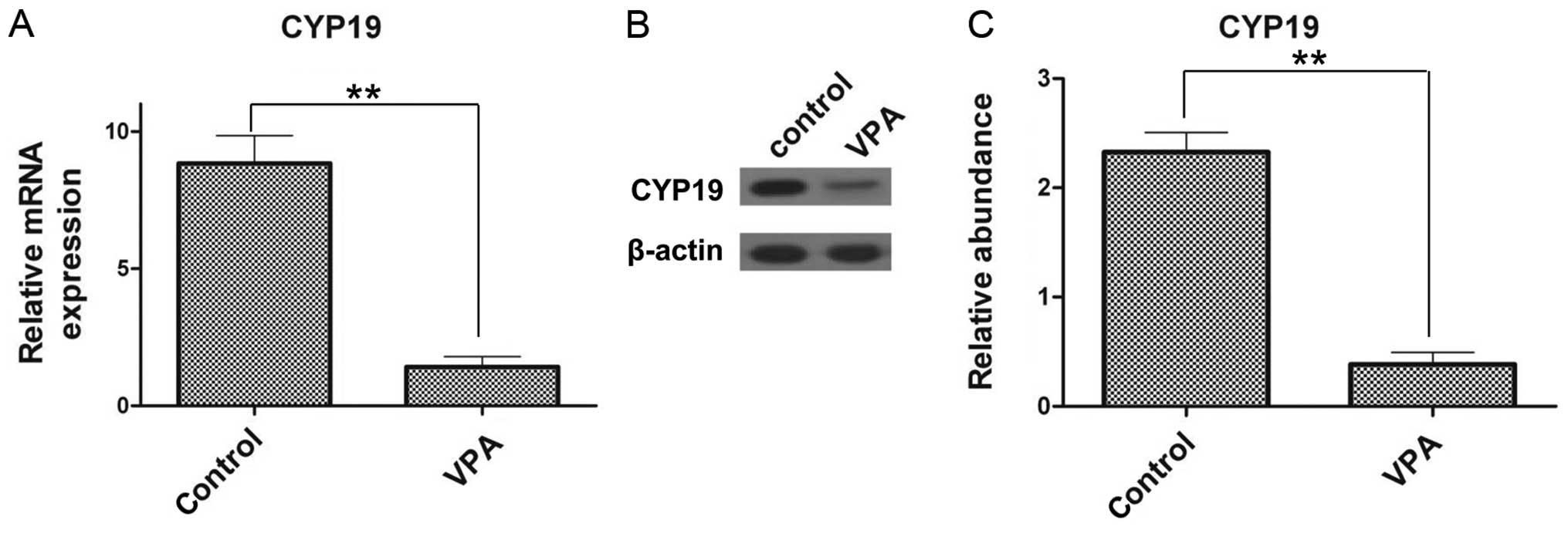
CYP19 expression is suppressed by
VPA
To investigate the expression of CYP19 at the
mRNA and protein level following treatment with VPA, RT-qPCR and
western blot analysis were performed. The relative mRNA expression
of CYP19 in the VPA-treated ESCs (1.42±0.38) was
significantly decreased compared with that of the control group
(8.83±1.02; Fig. 5A). Similarly,
at the protein level, CYP19 expression was inhibited by VPA
(Fig. 5B) and a 6.1-fold decrease
was noted, compared with the control group (Fig. 5C). These results confirm that VPA
inhibits CYP19 expression.
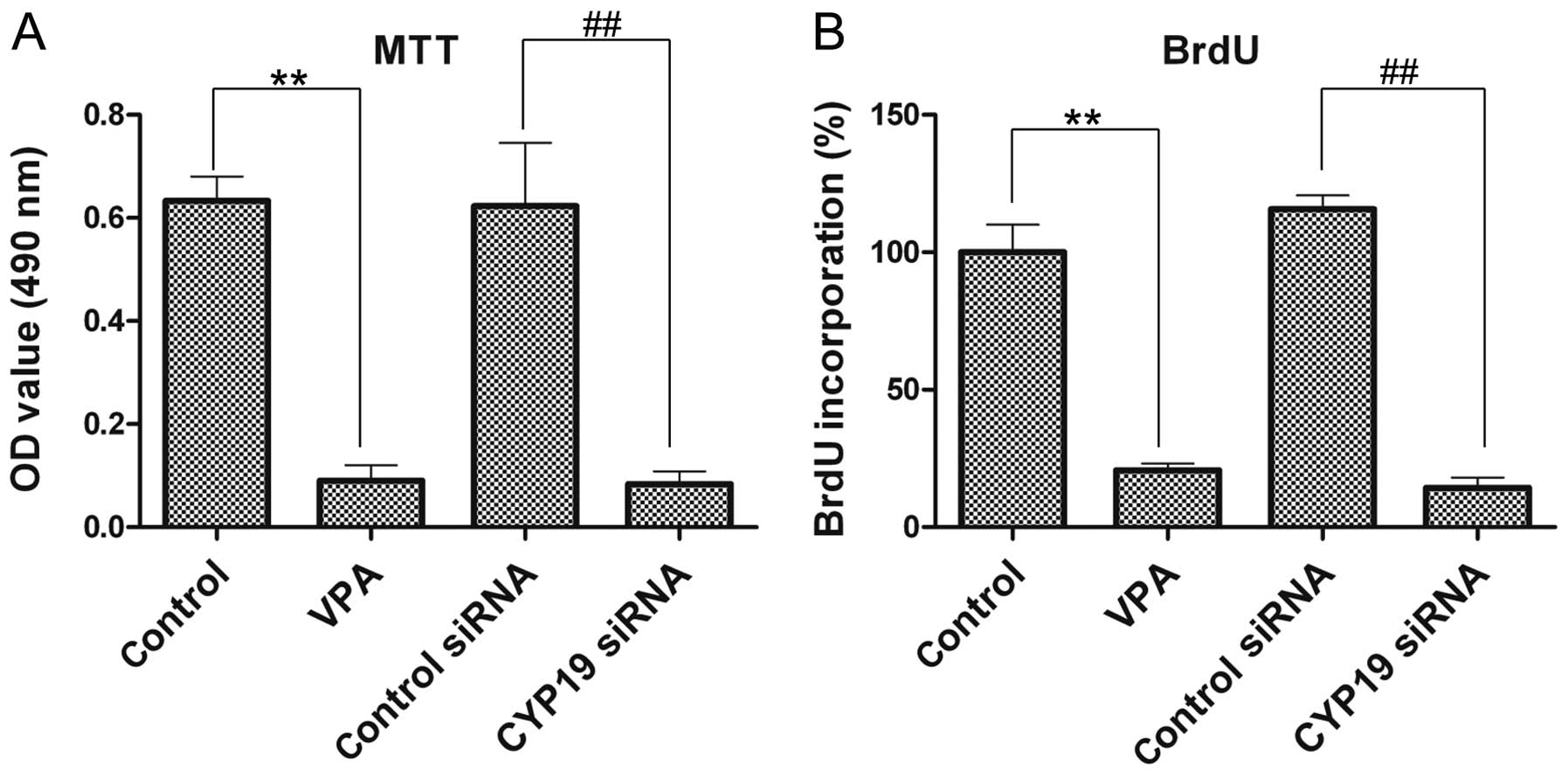
VPA and CYP19 knockout co-inhibit the
viability and proliferation of ESCs
The effects of VPA and CYP19 on the viability
and proliferation of the ESCs were investigated by MTT assay and
BrdU assay, respectively. The viability of the VPA-treated ESCs was
significantly decreased compared with that of the control group
(Fig. 6A). VPA significantly
inhibited BrdU incorporation into the ESCs. BrdU incorporation
decreased to 20.7±2.52% relative to the control group following
treatment with VPA at 8 mM (Fig.
6B). Furthermore, we also noted that CYP19 siRNA
inhibited the viability (Fig. 6A)
and proliferation (Fig. 6B) of
the ESCs compared with the control siRNA group and even enhanced
the inhibitory effects of VPA.
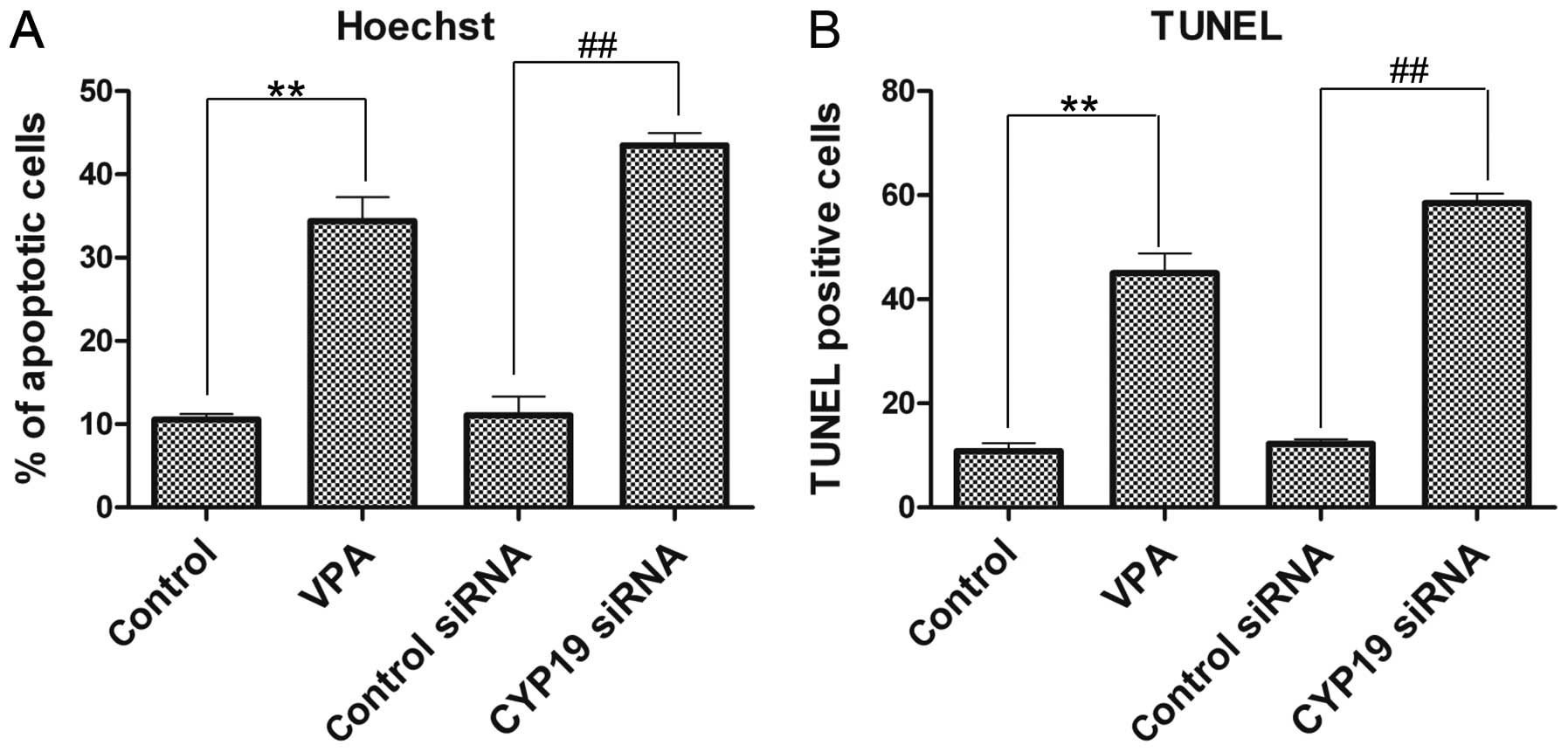
VPA and CYP19 co-induce the apoptosis of
ESCs
To investigate the effects of VPA and CYP19
siRNA on ESC apoptosis, Hoechst staining and TUNEL assay were
performed. The percentage of apoptotic cells in the VPA-treated
group increased to 34.4±2.89%, and in the CYP19 siRNA group,
it increased to 43.5±1.49%, which was a significant increase
compared with the control and control siRNA groups, respectively
(Fig. 7A). Similarly, the results
from TUNEL assay results also revealed a sudden increase in the
number of TUNEL-positive cells in both the VPA- and CYP19
siRNA-treated groups (Fig.
7B).
Discussion
This study is, to the best of our knowledge, the
first to examine histone acetylation, endometriosis and
CYP19 gene expression. This findings of this study may will
help us to gain a better understanding of the development and
treatment of endometriosis. We demonstrated in this study that VPA
not only inhibited ESC viability and proliferation, and induced the
apoptosis of ESCs, but also inhibited CYP19 expression,
which is the encoding gene of P450 aromatase, and all of these
factors promoted estrogen synthesis. Excessive estrogen production
is necessary for the development of endometriosis. Hence, this
study discovered a combined therapeutic target from the cellular
level and histone modification at the gene level.
Local remodeling and changes in chromatin the
nucleosome play a key role in the regulation of gene expression
(27). One of the most important
mechanisms in chromatin remodeling is protein acetylation (28). The acetylation levels of histone
are controlled by the balance between HATs and HDACs. HATs transfer
acetyl groups from acetyl-CoA to lysine residues in the N-terminal
region of histones (29).
Conversely, HDACs remove the acetyl groups and thus restore the
positive charge on lysine residues (16). As demonstrated in this study
(Fig. 3), HDACis (VPA in this
case) significantly promoted the acetylation of histone H3 and
H4.
Prior to this study, several theories were proposed
to explain the development of endometriosis, such as coelomic
metaplasia (Mayer's theory), vascular and lymphatic metastasis
(Halban's theory), the embryonal rest theory and the
stem/progenitor cell theory (30,31). The exact pathogenesis of
endometriosis remains unknown; however, retrograde menstruation,
proposed by Sampson (32) in
1927, is still considered the most widely accepted theory (33). According to this theory,
endometrial tissues travel from the uterine cavity to the pelvic
cavity through the fallopian tubes during menstrual shedding,
adhere to the cavity wall, invade the extracellular matrix (ECM),
proliferate, and form endometrial lesions (32). It is worth noting that the
incidence of retrograde menstruation is similar in women with and
without endometriosis, and thus the pathogenesis seems to involve a
multifactorial mechanism, which includes functionally different
endometrial tissue in addition to altered immunity and other
molecular abnormalities (34).
Due to the high morbidity in women with dysmenorrhea or other
gynecological diseases, and the fact that endometriosis is an
estrogen-dependent disease (19),
we hypothesized that the estrogen level was one of the
'multifactorial mechanisms' and thus was closely related to the
development of endometriosis.
High estrogen production is a consistently observed
endocrine feature of endometriosis (19,21,35). The disease develops in women of
reproductive age and regresses after menopause, suggesting that it
is estrogen-dependent in nature. The hormone-dependent nature of
the disease has prompted research on local estrogen production,
with a major focus on the expression of cytochrome P450 aromatase
(36). It has been demonstrated
that the increased expression of CYP19 aromatase occurs in
ectopically located endometrial lesions, particularly in ovarian
endometriomas (37). CYP19
aromatase expression has also been detected in the eutopic
endometrium of women with other uterine diseases, such as leiomyoma
and adenomyosis (38). Hence, in
this study, we focused on the CYP19 gene in order to
investigate its association with histone acetylation.
In the present study, we found that the acetylation
of histone H3 and H4 in the promoter region of the CYP19
gene was inhibited by VPA-induced histone acetylation, and thus the
expression of CYP19 in the ESCs was inhibited. This seems a
paradoxical result. However, it has been proven previously that
histone suppresses gene expression (39). HDACis, such as VPA, play a key
role in promoting the acetylation of histones closely folded with
DNA, and is defined as a whole as epigenetic (11,27); by contrast, VPA has a limited
effect on the histones of specific genes, such as CYP19. As
a result, the balance between HATs and HDACs was disrupted and the
effects of HDACs remained present in histone H3 and H4 of the
CYP19 gene, leading to deacetylation. Inhibition of
CYP19 expression may contribute to the decreased synthesis
of P450 aromatase, which is the rate-limiting enzyme for the
pathway from androgen to estrogen, and therefore limits the
synthesis of excessive estrogen. This observation may aid in the
treatment of endometriosis. As previously reported, the acetylation
of histones may contribute to the better treatment of endometriosis
(11,12,27) and aromatase inhibitors (AIs) may
lead to a more effective treatment of endometriosis (34,35). Hence, according to our results,
VPA not only promote histone acetylation, but also acts as an AI to
inhibit CYP19 gene expression, which assists with the
treatment of endometriosis. VPA inhibited ESC survival and induced
apoptosis, and we proved that the silencing of CYP19 had the
same effects on the ESCs as VPA treatment. This indicates that VPA
plays a key role in mediating CYP19 gene expression.
In conclusion, in this study, we combined the study
of histone acetylation, endometriosis and the CYP19 gene.
Our results provide a better and more specific therapeutic method
for the treatment of endometriosis.
Abbreviations:
|
VPA
|
valproic acid
|
|
HATs
|
histone acetyltransferases
|
|
HDACs
|
histone deacetylases
|
|
HDACis
|
histone deacetylase inhibitors
|
|
ESCs
|
endometriotal stromal cells
|
|
NESCs
|
normal endometrial stromal cells
|
Acknowledgments
The present study was supported by the Shanghai
Natural Science Fund (no. 12ZR1438000).
References
|
1
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Falcone T and Lebovic DI: Clinical
management of endometriosis. Obstet Gynecol. 118:691–705. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arya P and Shaw R: Endometriosis: Current
thinking. Curr Obstet Gynaecol. 15:191–198. 2005. View Article : Google Scholar
|
|
5
|
Guo SW and Olive DL: Two unsuccessful
clinical trials on endometriosis and a few lessons learned. Gynecol
Obstet Invest. 64:24–35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kyama CM, Mihalyi A, Simsa P, Mwenda JM,
Tomassetti C, Meuleman C and D'Hooghe TM: Non-steroidal targets in
the diagnosis and treatment of endometriosis. Curr Med Chem.
15:1006–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldberg AD, Allis CD and Bernstein E:
Epigenetics: a landscape takes shape. Cell. 128:635–638. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaenisch R and Bird A: Epigenetic
regulation of gene expression: how the genome integrates intrinsic
and environmental signals. Nat Genet. 33(Suppl): 245–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turner BM: Cellular memory and the histone
code. Cell. 111:285–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abe W, Nasu K, Nakada C, Kawano Y,
Moriyama M and Narahara H: miR-196b targets c-myc and Bcl-2
expression, inhibits proliferation and induces apoptosis in
endometriotic stromal cells. Hum Reprod. 28:750–761. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawano Y, Nasu K, Li H, Tsuno A, Abe W,
Takai N and Narahara H: Application of the histone deacetylase
inhibitors for the treatment of endometriosis: histone
modifications as pathogenesis and novel therapeutic target. Hum
Reprod. 26:2486–2498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nasu K, Kawano Y, Tsukamoto Y, Takano M,
Takai N, Li H, Furukawa Y, Abe W, Moriyama M and Narahara H:
Aberrant DNA methylation status of endometriosis: epigenetics as
the pathogenesis, biomarker and therapeutic target. J Obstet
Gynaecol Res. 37:683–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huan Y, Wu Z, Zhang J, Zhu J, Liu Z and
Song X: Epigenetic modification agents improve gene-specific
methylation reprogramming in porcine cloned embryos. PLoS One.
10:e01298032015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiaomeng X, Ming Z, Jiezhi M and Xiaoling
F: Aberrant histone acetylation and methylation levels in woman
with endometriosis. Arch Gynecol Obstet. 287:487–494. 2013.
View Article : Google Scholar
|
|
15
|
Vialou V: Histone acetylation, gene
regulation and depression. Med Sci (Paris). 26:465–467. 2010.In
French. View Article : Google Scholar
|
|
16
|
de Ruijter AJ, van Gennip AH, Caron HN,
Kemp S and van Kuilenburg AB: Histone deacetylases (HDACs):
characterization of the classical HDAC family. Biochem J.
370:737–749. 2003. View Article : Google Scholar
|
|
17
|
Attar E, Tokunaga H, Imir G, Yilmaz MB,
Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB and
Bulun SE: Prostaglandin E2 via steroidogenic factor-1 coordinately
regulates transcription of steroidogenic genes necessary for
estrogen synthesis in endometriosis. J Clin Endocrinol Metab.
94:623–631. 2009. View Article : Google Scholar :
|
|
18
|
Bulun SE, Fang Z, Imir G, Gurates B,
Tamura M, Yilmaz B, Langoi D, Amin S, Yang S and Deb S: Aromatase
and endometriosis. Semin Reprod Med. 22:45–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bulun SE, Yang S, Fang Z, Gurates B,
Tamura M and Sebastian S: Estrogen production and metabolism in
endometriosis. Ann NY Acad Sci. 955:75–88. 396–406. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Lu X, Wang D, Qu W, Li W, Xu X,
Huang Q, Han X and Lv J: CYP19 gene variant confers susceptibility
to endometriosis-associated infertility in Chinese women. Exp Mol
Med. 46:e1032014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gruber CJ, Tschugguel W, Schneeberger C
and Huber JC: Production and actions of estrogens. N Engl J Med.
346:340–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitawaki J, Kusuki I, Koshiba H, Tsukamoto
K, Fushiki S and Honjo H: Detection of aromatase cytochrome P-450
in endometrial biopsy specimens as a diagnostic test for
endometriosis. Fertil Steril. 72:1100–1106. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ferrero S, Remorgida V, Maganza C,
Venturini PL, Salvatore S, Papaleo E, Candiani M and Leone Roberti
Maggiore U: Aromatase and endometriosis: estrogens play a role. Ann
NY Acad Sci. 1317:17–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishida M, Nasu K, Fukuda J, Kawano Y,
Narahara H and Miyakawa I: Down-regulation of interleukin-1
receptor type 1 expression causes the dysregulated expression of
CXC chemokines in endometriotic stromal cells: a possible mechanism
for the altered immunological functions in endometriosis. J Clin
Endocrinol Metab. 89:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang F, Zou Y, Wang H, Cao J and Yin T:
In vitro apoptosis effects of GnRHII on endometrial stromal cells
from patients with endometriosis. Int J Clin Exp Pathol.
6:1603–1609. 2013.PubMed/NCBI
|
|
26
|
Delbandi AA, Mahmoudi M, Shervin A, Akbari
E, Jeddi-Tehrani M, Sankian M, Kazemnejad S and Zarnani AH: Eutopic
and ectopic stromal cells from patients with endometriosis exhibit
differential invasive, adhesive, and proliferative behavior. Fertil
Steril. 100:761–769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takai N, Desmond JC, Kumagai T, Gui D,
Said JW, Whittaker S, Miyakawa I and Koeffler HP: Histone
deacetylase inhibitors have a profound antigrowth activity in
endometrial cancer cells. Clin Cancer Res. 10:1141–1149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Strahl BD and Allis CD: The language of
covalent histone modifications. Nature. 403:41–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kai K, Nasu K, Kawano Y, Aoyagi Y,
Tsukamoto Y, Hijiya N, Abe W, Okamoto M, Moriyama M and Narahara H:
Death receptor 6 is epigenetically silenced by histone
deacetylation in endometriosis and promotes the pathogenesis of
endometriosis. Am J Reprod Immunol. 70:485–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amer S: Endometriosis. Obstetrics,
Gynaecol Reprod Med. 18:126–133. 2008. View Article : Google Scholar
|
|
31
|
Sasson IE and Taylor HS: Stem cells and
the pathogenesis of endometriosis. Ann NY Acad Sci. 1127:106–115.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sampson JA: Metastatic or embolic
endometriosis, due to the menstrual dissemination of endometrial
tissue into the venous circulation. Am J Pathol. 3:93–110.
1431927.PubMed/NCBI
|
|
33
|
Burney RO and Giudice LC: Pathogenesis and
pathophysiology of endometriosis. Fertil Steril. 98:511–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abu Hashim H: Potential role of aromatase
inhibitors in the treatment of endometriosis. Int J Womens Health.
6:671–680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pavone ME and Bulun SE: Aromatase
inhibitors for the treatment of endometriosis. Fertil Steril.
98:1370–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rhee HS, Oh SH, Ko BJ, Han DM, Jeon BH,
Park H, Moon HB and Kim WS: Expression of 3beta-hydroxysteroid
dehydrogenase and P450 side chain cleavage enzyme in the human
uterine endometrium. Exp Mol Med. 35:160–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bukulmez O, Hardy DB, Carr BR, Auchus RJ,
Toloubeydokhti T, Word RA and Mendelson CR: Androstenedione
up-regulation of endometrial aromatase expression via local
conversion to estrogen: potential relevance to the pathogenesis of
endometriosis. J Clin Endocrinol Metab. 93:3471–3477. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wölfler MM, Nagele F, Kolbus A, Seidl S,
Schneider B, Huber JC and Tschugguel W: A predictive model for
endometriosis. Hum Reprod. 20:1702–1708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Verdone L, Caserta M and Di Mauro E: Role
of histone acetylation in the control of gene expression. Biochem
Cell Biol. 83:344–353. 2005. View Article : Google Scholar : PubMed/NCBI
|