Introduction
Osteosarcoma (OS), the most common primary malignant
solid bone tumor, is the second leading cause of cancer-related
fatality in children and young adults (1–3).
OS are high-grade aggressive tumors that comprise ~20% of all
primary bone cancers and 2.4% of all malignancies in pediatric
patients (4,5). Currently, although several
conventional therapies are offered for the clinical treatment of
OS, such as radiation, chemotherapy, surgical resection, or
combinations of chemotherapy and radiation, therapeutic outcomes
for OS are not satisfactory (6).
Evidence suggests that the therapeutic options for OS have not
improved over the past four decades (7,8);
thus, novel approaches to OS are required. Efforts to identify new
genes and signaling pathways that affect tumor progress have
suggested that the aplasia Ras homologue member I (ARHI) gene (also
known as DIRAS3) has a crucial role in numerous types of
cancer, such as breast cancer (9,10),
ovarian cancer (11,12), hepatocellular carcinoma (13), lung cancer (14) and glioma (15). Additionally, it has been reported
that the ARHI protein is downregulated in multiple myeloma
endothelial cells and is involved in proliferation and signal
transduction in these cells (16). However, the specific functions of
ARHI in the progression of OS remain unclear.
ARHI, a newly discovered, maternally endogenous
imprinted tumor-suppressor gene, is a 26-kDa small GTP-binding
protein-encoding gene that is located on human chromosome 1p31
(17). Although ARHI is a member
of the Ras superfamily and shares 60% homology with Ras and Rap, it
has been identified as a tumor-suppressor gene that suppresses
tumor cell growth (18). ARHI has
attracted increasing attention in tumor research since evidence was
reported indicating that ARHI could inhibit tumor cell
proliferation (19). Accumulating
evidence has shown that ARHI has an important role in the
occurrence, metastasis, invasion and development of cancers
(20–22). In addition, numerous types of
cancer have low or absent levels of ARHI (23,24). Increased expression of ARHI in
pancreatic cancer cells can inhibit cell proliferation by acting on
cell cycle genes (19,21,25). It is now well established that
ARHI is most highly expressed in normal breast and ovarian tissues,
whereas the ARHI level in breast and ovarian cancer is
significantly reduced (17,26). Another study also demonstrated
that high levels of ARHI inhibit tumor growth and angiogenesis in
hepatocellular carcinoma (27).
Although ARHI has a number of positive effects in
numerous types of tumor, it remains to be established whether ARHI
is expressed in OS cells, and whether ARHI overexpression is
associated with cell growth and apoptosis. The present study shows
for the first time that overexpression of the ARHI gene induced by
cellular transfection significantly curbs the growth of OS cells
and induces cell apoptosis. ARHI overexpression inhibits OS cell
proliferation and induces cell apoptosis through the
phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway.
Additionally, the study provides evidence that ARHI could serve as
a target for OS and upregulation of ARHI may be a useful method in
treating OS in humans.
Materials and methods
Antibodies
Mouse anti-ARHI monoclonal antibody (ab45768), mouse
anti-β-actin monoclonal antibody (ab6276) and horseradish
peroxidase (HRP)-conjugated rabbit anti-mouse immunoglobulin G
(IgG; ab6728) were all obtained from Abcam (Cambridge, MA,
USA).
Cell culture and transfection
Three human OS cell lines, MG-63, U2OS and SAOS-2,
were used in the study. All the cell lines were obtained from the
American Type Culture Collection (Rockville, MD, USA). All the OS
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
Mediatech, Inc., Herndon, VA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco-BRL, Gaithersburg, MD, USA) in a
humidified atmosphere at 37°C supplemented with 5% CO2.
The human osteoblast precursor cell line hFOB1.19 was cultured in
DMEM/F12 medium supplemented with 10% FBS at 37°C supplemented with
5% CO2. Cells in the exponential growth phase were used
in the study. Cell transfection was performed with either human
ARHI cDNA (pcDNA3.1-ARHI) or the control vector pcDNA3.1 using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer's instructions. The cells
transfected with pcDNA3.1-ARHI were harvested and measured by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis.
Cell viability
MG-63, U2OS and SAOS-2 cell viability was assessed
by the trypan blue exclusion assay, as previously described with
minor modifications (28).
Briefly, transfected cells and control cells were seeded at
2×104 cells/well on 96-well plates using DMEM and were
incubated for different periods of time (0, 24, 48, 72 and 96 h).
Cells were subsequently stained with trypan blue (40 µl; Sigma, St.
Louis, MO, USA) and counted using a light microscope.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT)
MG-63 cells were transfected as described above and
seeded at 2×104 cells/well on 24-well plates. After
culturing for 72 h, the live cells were measured using a cell
proliferation MTT kit (Chemicon International, Inc., Temecula, CA,
USA) by measuring MTT cleavage of active mitochondria, according to
the manufacturer's instructions.
Flow cytometric analysis of
apoptosis
MG-63 cell apoptosis was analyzed using flow
cytometry (Beckman Coulter, Inc., Brea, CA, USA). Briefly,
2×104 cells/well were seeded in 96-well plates. Cells
were incubated with Annexin V and propidium iodide (PI) for 20 min
at room temperature. Apoptotic MG-63 cells were subsequently
detected by flow cytometry.
Total RNA extraction and RT-qPCR
The RNA extraction and RT-qPCR procedure were
performed as previously described (29,30). Briefly, total RNA was extracted
from OS cells using the TRIzol® reagent (Invitrogen Life
Technologies) according to the manufacturer's instructions. RT-qPCR
was carried out using the SYBR-Green ReadyMix on an ABI PRISM 7000
Sequence Detection system (both from Applied Biosystems, Foster
City, CA, USA). The primer sequences (13) used for RT-qPCR were as follows:
ARHI forward, 5′-CAGCTGGTTTCTTACCACGTAT-3′ and reverse,
5′-GCACAAGTTCTCCCACACTTAG-3′; and β-actin forward,
5′-TCACCCACACTGTGCCCATCTACGA-3′ and reverse,
5′-CAGCGGAACCGCTCATTGCCAATGG-3′. The primers used were all
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Relative
expression of the ARHI gene was calculated using the
2−ΔΔCT method (31).
The level of β-actin mRNA was used as the internal control.
Western blot analysis
ARHI protein expression in OS cells was determined
according to a previously described method with minor modifications
(15). Total protein was isolated
from each group of OS cells using radioimmunoprecipitation assay
lysis buffer, and the protein concentration was determined using a
bicinchoninic acid assay kit (Beyotime, Jiangsu, China). Protein
samples (20 µg/lane) were separated by 10% SDS-PAGE and
electrophoretically transferred to nitrocellulose membranes. After
blocking overnight at 4°C with 5% skimmed milk, the resulting blots
were first probed with mouse anti-ARHI antibody (1:1,000), and
subsequently with HRP-conjugated rabbit anti-mouse IgG (1:4,000),
followed by detection using the ECL reagent (Boehringer Mannheim
GmbH, Mannheim, Germany).
Small interfering RNA (siRNA)
transfection
OS MG-63 cells with the ARHI protein were
transfected with ARHI siRNA (siARHI) or the control siRNA (siMock)
using the DharmaFECT 4 Transfection reagent (GE Dharmacon,
Lafayette, CO, USA), as previously described (20,32). Briefly, 100 nM of the siRNA
mixture with transfection reagents was co-incubated at room
temperature for 20 min. Subsequently, the mixture was added to
MG-63 cells for 48 h. The cells were harvested and the measurement
of mRNA and protein expression was performed by RT-qPCR and western
blot analysis, respectively. siARHI- and siMock-transfected cells
were used for further studies.
Caspase-3 activity assay
The activity of caspase-3 in OS MG-63 cells was
assayed using the Caspase-Glo® 3/7 assay (Promega Corp.,
Madison, WI, USA) according to the manufacturer's instructions
(33). Briefly, MG-63 cells were
seeded in 24-well plates and were subsequently treated with the
Caspase-Glo® 3/7 reagent (50 ml) for 30 min.
Luminescence was measured on a TECAN GENios Pro microplate reader.
Caspase activity was measured after 72 h for cells treated with
Ad-ARHI and after 48 h for cells treated with siARHI.
Statistical analysis
The data were analyzed using SPSS 13.0 statistical
analysis software (SPSS, Inc., Chicago, IL, USA). The differences
between two groups were compared by Dunnett's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
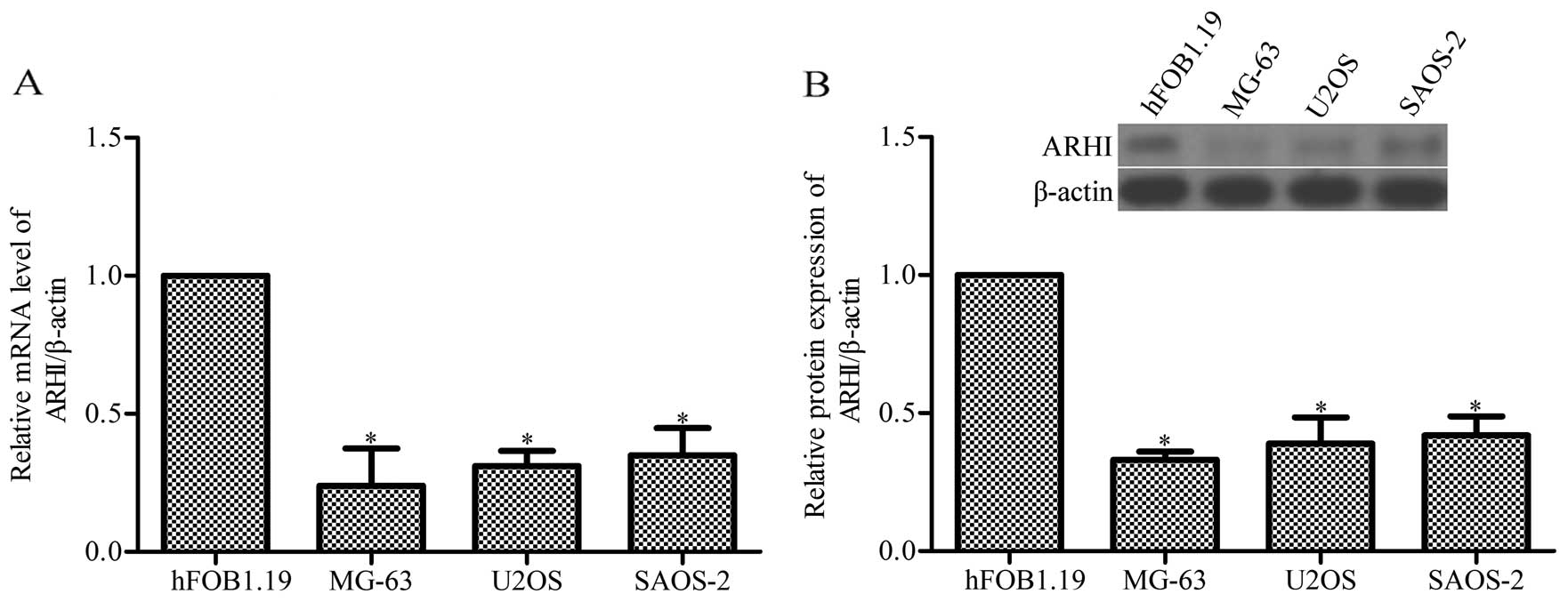
ARHI mRNA and protein expression is
markedly decreased in OS cell lines
The evidence indicates that the expression of ARHI
is reduced in numerous types of cancer and a high level of ARHI
inhibits tumor growth (13);
however, the level and the function of ARHI in OS remain unclear.
Whether ARHI also has a role in OS cell growth was determined. As a
result of the RT-qPCR and western blot analysis on the three OS
cell lines, MG-63, U2OS and SAOS-2, the data show that the
expression of ARHI was markedly decreased compared with the human
osteoblast precursor hFOB1.19 (P<0.05; Fig. 1).
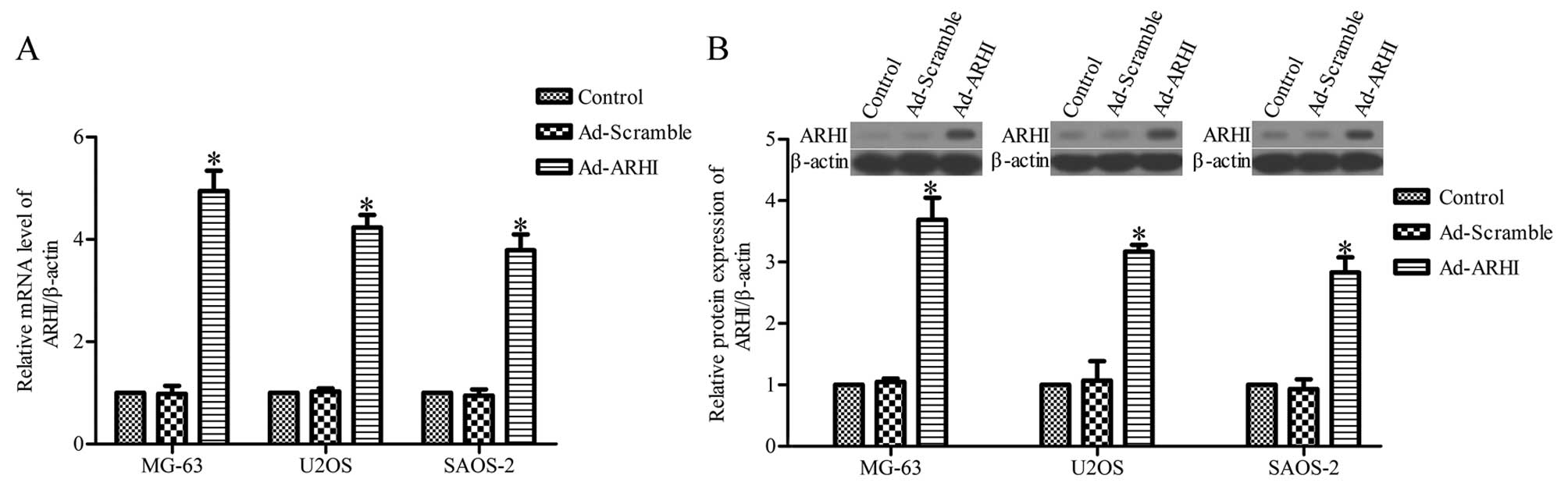
Overexpression of ARHI inhibits cell
viability and proliferation
In order to assess the effects of ARHI on OS cell
viability and cell growth, stable upregulation of ARHI in the OS
cell lines, MG-63, U2OS and SAOS-2, using pcDNA3.1-ARHI was
constructed. RT-qPCR and western blot analysis results showed that
the level of ARHI mRNA and protein in the three OS cell lines
transfected with Ad-ARHI were much higher compared with the control
group 72 h later (P<0.05) (Fig. 2A
and B), whereas ARHI expression was not significantly different
between the Ad-Mock group and the control group (P>0.05)
(Fig. 2A and B). The mRNA and
protein expression were also upregulated in MG-63, U2OS and SAOS-2
cells transfected with pcDNA3.1-ARHI for 24, 48 and 96 h (data not
shown).
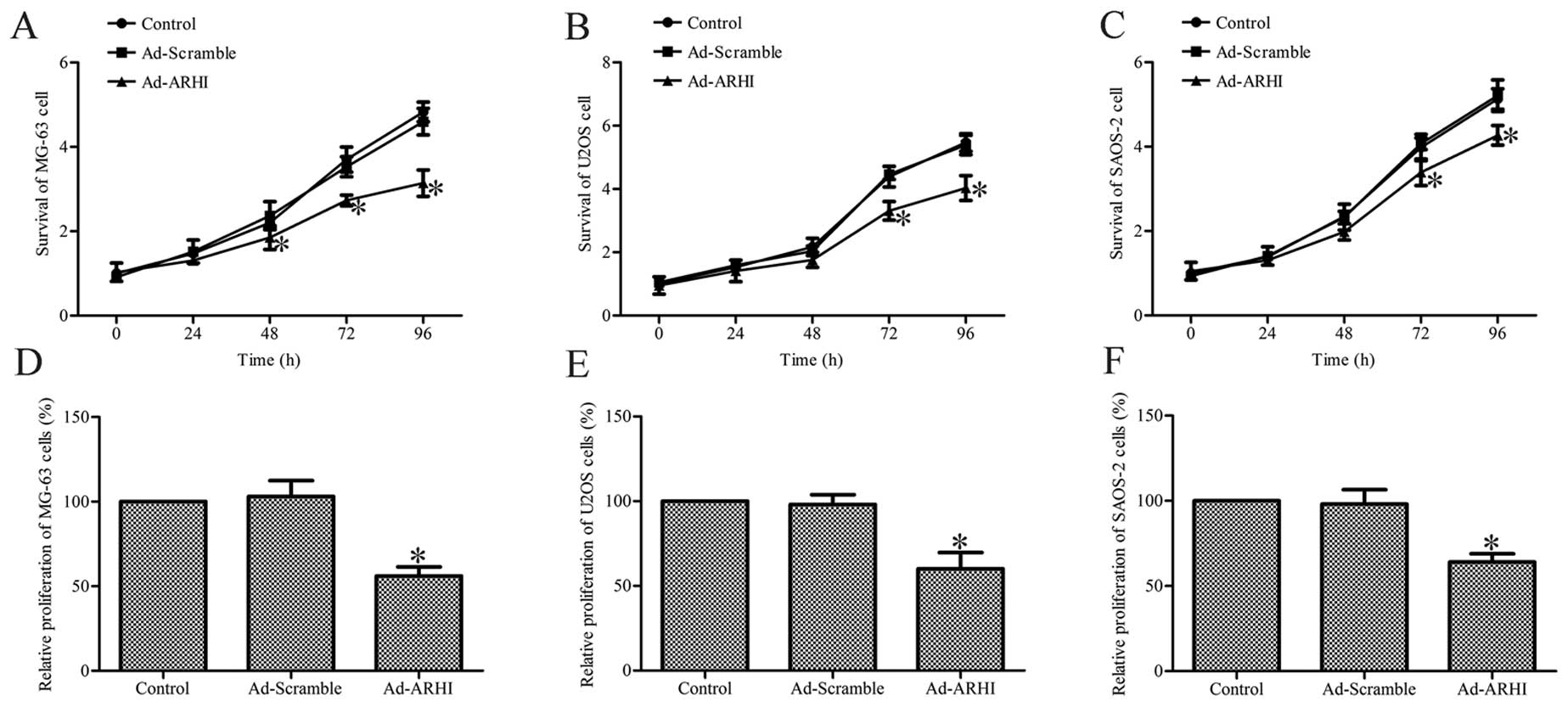
Overexpression of ARHI inhibits cell
viability and proliferation
To determine the effect of ARHI overexpression on
the viability of OS cells, the trypan blue exclusion assay was
performed on all the groups at various post-transfection
time-points. The results show that Ad-AHRI-expressing MG-63, U2OS
and SAOS-2 cell viability was significantly lower compared with the
respective cells in the Ad-Mock and control groups (P<0.05;
Fig. 3A–C).
Subsequently, the effect of ARHI overexpression for
72 h on the growth of MG-63, U2OS and SAOS-2 cells was determined
by the MTT method. The results indicate that the growth of
Ad-AHRI-expressing MG-63, U2OS and SAOS-2 cells was markedly
decreased compared to the respective cells in the control groups
(P<0.05; Fig. 3D–F). However,
no significant effects were observed in cells treated with Ad-Mock
(Fig. 3D–F). These results
suggest that ARHI overexpression significantly inhibits the
viability and growth of OS cells.
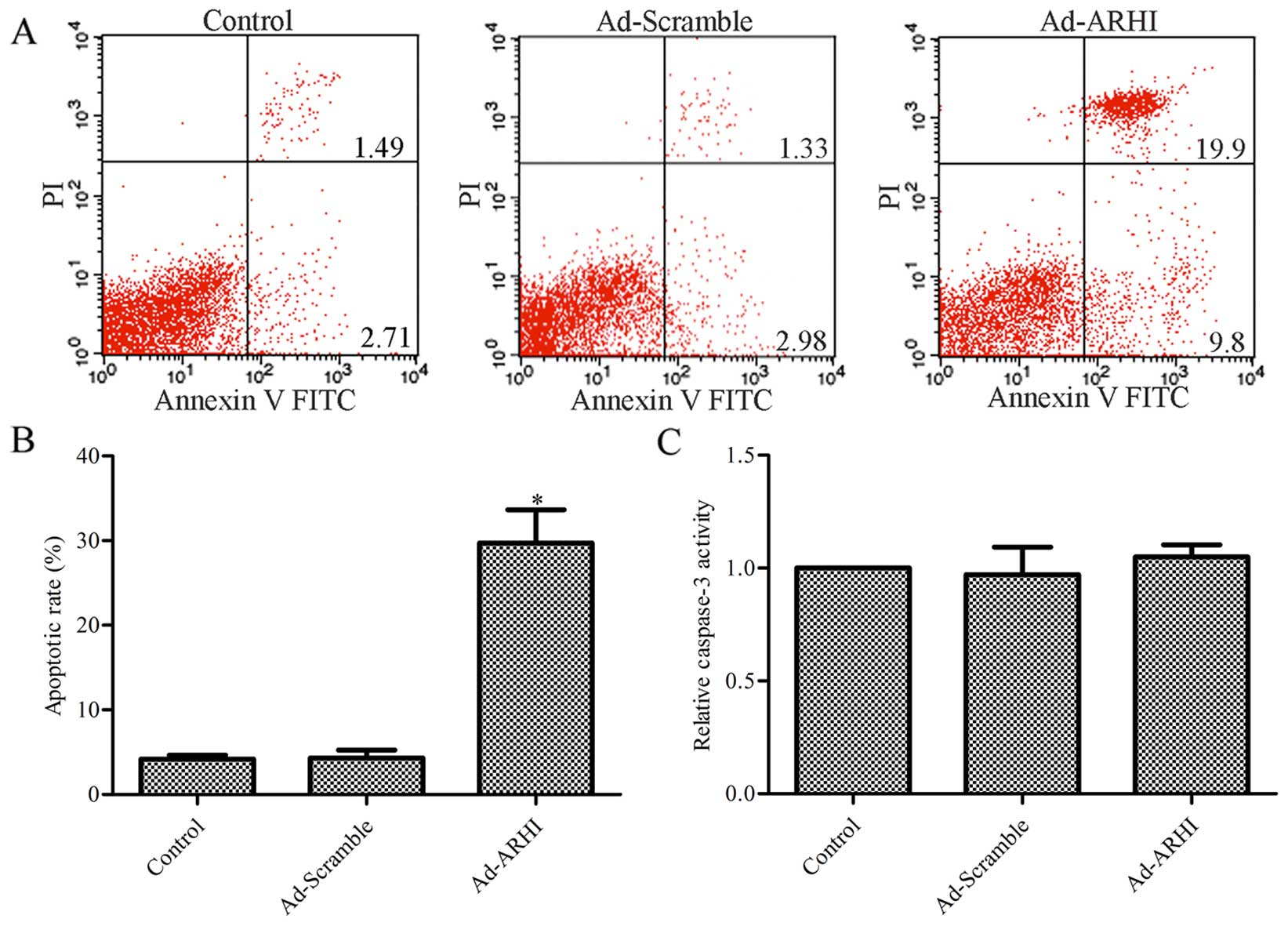
Effect of ARHI overexpression on MG-63
cell apoptosis
To further determine the mechanism by which ARHI
contributes to reduced survival in OS cells, the effect of ARHI on
MG-63 cell apoptosis was assessed by flow cytometric analysis. The
results indicate that the rate of apoptosis was significantly
higher with ARHI overexpression compared with the control group
(P<0.05; Fig. 4A and B),
whereas there was no evident difference between the Ad-Mock group
and the control group (P>0.05; Fig. 4A and B). A total of 4.2% apoptotic
cells in the control group, and 4.3 and 29.7% apoptotic cells were
observed in the Ad-ARHI and Ad-Mock group, respectively.
Subsequently, the activity of caspase-3 was determined in the
Ad-ARHI, Ad-Mock and control groups by the Caspase-Glo®
3/7 assay. As shown in Fig. 4C,
the activity of caspase-3 had no significantly change in the
Ad-ARHI group compared with the Ad-Mock and control groups
(P>0.05).
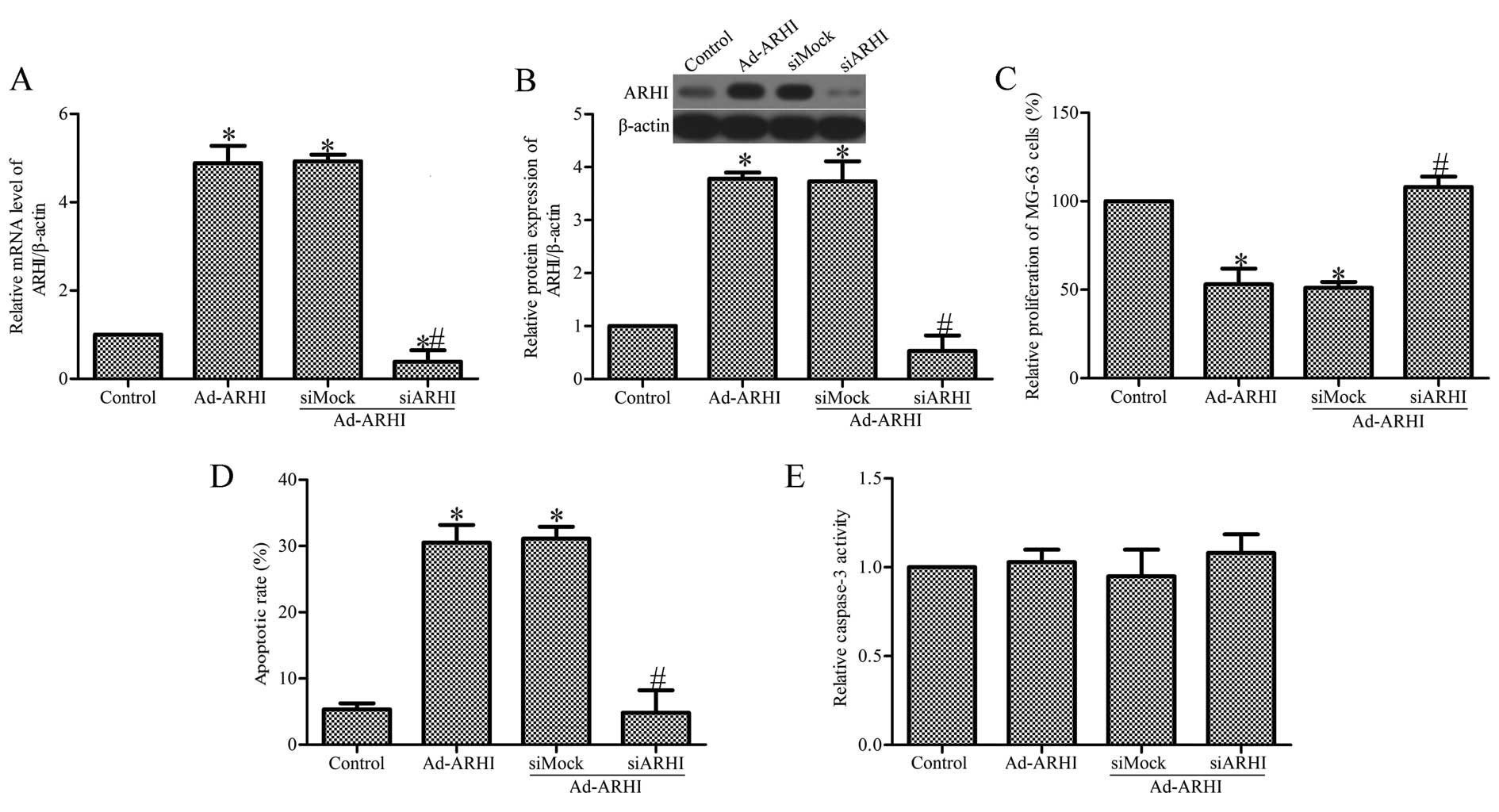
Specific inhibition of ARHI expression by
ARHI-specific siRNA
To further examine the role of ARHI in OS cell
growth, Ad-AHRI-expressing MG-63 cells were transfected with siARHI
or control siRNA (siMock). RT-qPCR and western blot analysis showed
that the mRNA and protein expression of ARHI in the
siARHI-transfected cells was markedly lower compared with the
siMock and control groups (Fig. 5A
and B). Transfection of siARHI evidently suppressed the
proliferation of MG-63 cells (Fig.
5C). In addition, transfection of siARHI also significantly
inhibited MG-63 cell apoptosis (Fig.
5D). To study the effect of siARHI on the activity of caspase-3
in Ad-AHRI-expressing MG-63 cells, the Caspase-Glo® 3/7
assay was performed. The results indicate that transfection with
siARHI did not affect caspase-3 activity. There was also no clear
effect in the siMock group (Fig.
5E).
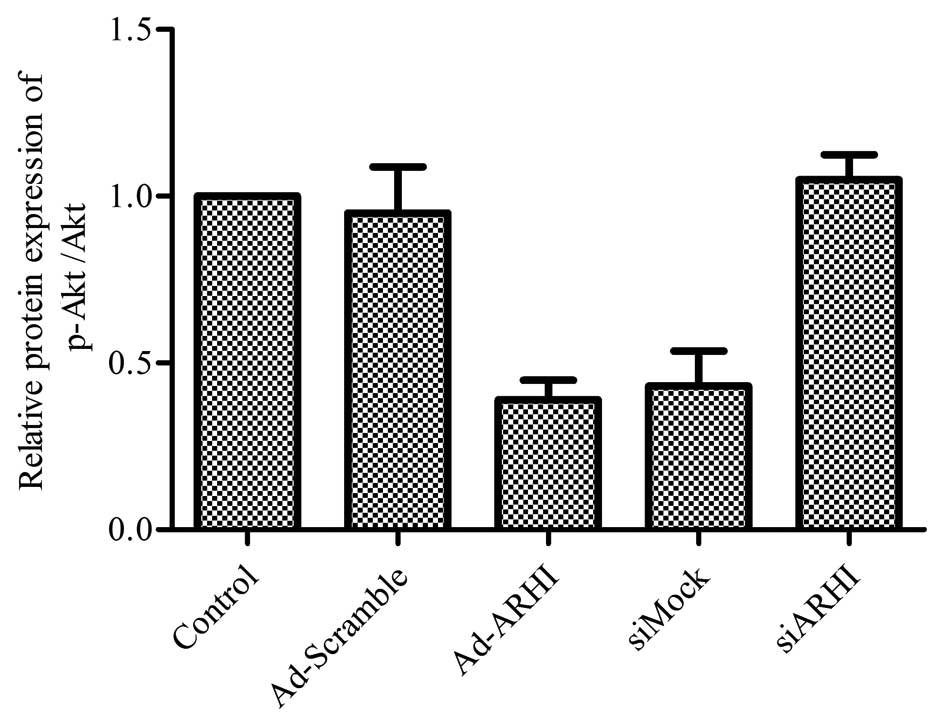
Overexpression of ARHI is directly
associated with the inhibition of the cell survival pathway
PI3K/Akt
Evidence shows that the PI3K/Akt signaling pathway
is closely correlated with cancer cell biology, including OS
(34,35). In addition, Lu et al
(36) showed that ARHI inhibits
the PI3K/Akt signaling pathway in ovarian cancer cells. Thus, the
effects of ARHI overexpression on the PI3K/Akt signaling cascade in
MG-63 cells were examined. The results suggest that after Ad-ARHI
was transfected into the OS cell line MG-63 for 72 h, the
expression level of p-Akt was markedly reduced in the Ad-ARHI group
when compared with the levels in the Ad-Mock and control groups
(Fig. 6). Notably, ablating the
level of ARHI by siARHI markedly increased the activity of p-Akt
compared with the Ad-ARHI group; however, the control siRNA showed
no apparent difference (Fig.
6).
Discussion
Emerging studies have revealed that the
identification of the specific target gene involved in
tumorigenesis could provide valuable insight into the diagnosis and
therapy of human malignancies (37). ARHI, a newly discovered,
maternally imprinted tumor-suppressor gene, has been previously
demonstrated to have an impact on growth, apoptosis, invasion,
metastasis and tumor development in numerous types of cancers
(26,38). ARHI has been found to be reduced
or absent in numerous tumors, while high expression of ARHI results
in decreased cell proliferation, mainly through the induction of
cell cycle-related genes (23,26). The present study indicates that
ARHI expression was reduced in the OS cell lines MG-63, U2OS and
SAOS-2 compared to the human osteoblast precursor hFOB1.19 cell
line (Fig. 1). ARHI
overexpression was also shown to suppress cell viability and
proliferation in these three OS cell lines (Fig. 2). In addition, ablating the
expression of ARHI by siARHI significantly inhibited the growth of
MG-63 cells (Fig. 4). Taken
together, these results suggest that ARHI may act as a
tumor-suppressor gene with an important role in the progression of
OS. However, its roles in vivo require further study.
Increasing evidence has suggested that a high level
of ARHI could lead to cancer cell apoptosis (14). It has been shown in other studies
that significant overexpression of ARHI using an adenovirus vector
induces caspase-independent apoptosis in ovarian and breast cancer
cells (39). In the present
study, Annexin V and PI staining indicated that the ARHI-expressing
group had a significantly higher incidence of apoptosis compared
with the Ad-Mock and control groups, and there was no apparent
difference between the latter two groups (Fig. 3). In addition, knockdown of ARHI
markedly suppressed MG-63 cell apoptosis in the ARHI-expressing
group (Fig. 4). The effect of
ARHI overexpression on the activity of caspase-3 was measured by
the Caspase-Glo® 3/7 assay. The results revealed that
there was no clear difference between the Ad-ARHI, Ad-Mock and
control groups; these results are consistent with those reported in
the literature (39). These
results indicate that ARHI-induced MG-63 cell apoptosis is
independent of the caspase pathway; however, the specific mechanism
of ARHI-induced apoptosis requires further study.
Numerous studies have established that ARHI induces
tumor cell apoptosis, and excessive autophagy is closely associated
with the PI3K/Akt signaling pathway (12,36). In addition, the cell survival
pathway PI3K/Akt has been implicated in tumor cell growth (40). In the present study, the findings
indicate that overexpression of ARHI upregulated the expression of
p-Akt in OS MG-63 cells, whereas knockdown of ARHI reduced the
expression of p-Akt in these cells, suggesting that ARHI may be
indicated in the progression of OS via regulation of the PI3K/Akt
pathway.
Taken together, there is a low level of ARHI in the
OS cell lines. Overexpression of ARHI inhibited OS cell growth,
induced cell apoptosis and suppressed PI3K/Akt signaling pathway
activation. These results suggest that upregulation of ARHI
expression is correlated with OS cell growth inhibition through
inhibition of the PI3K/Akt pathway, and knockdown of ARHI promotes
the growth of OS cells, suggesting that upregulation of ARHI may
serve as a novel potential therapeutic target for the prevention
and treatment of OS.
Abbreviations:
|
ARHI
|
aplasia Ras homologue member I
|
|
OS
|
osteosarcoma
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
Acknowledgments
Financial support was provided by the Science and
Technology Department of Gansu Province Natural Science Foundation
Program (1208RJZA272).
References
|
1
|
Li HX, Meng QP, Liu W, Li YG, Zhang HM,
Bao FC, Song LL and Li HJ: IMPDH2 mediate radioresistance and
chemoresistance in osteosarcoma cells. Eur Rev Med Pharmacol Sci.
18:3038–3044. 2014.PubMed/NCBI
|
|
2
|
Huh WW, Holsinger FC, Levy A, Palla FS and
Anderson PM: Osteosarcoma of the jaw in children and young adults.
Head Neck. 34:981–984. 2012. View Article : Google Scholar
|
|
3
|
Scholten DJ II, Timmer CM, Peacock JD,
Pelle DW, Williams BO and Steensma MR: Down regulation of Wnt
signaling mitigates hypoxia-induced chemoresistance in human
osteosarcoma cells. PLoS One. 9:e1114312014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mirabello L, Troisi RJ and Savage SA:
International osteo-sarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
6
|
Shang HS, Chang JB, Lin JH, Lin JP, Hsu
SC, Liu CM, Liu JY, Wu PP, Lu HF, Au MK, et al: Deguelin inhibits
the migration and invasion of U-2 OS human osteosarcoma cells via
the inhibition of matrix metalloproteinase-2/-9 in vitro.
Molecules. 19:16588–16608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuijjer ML, Hogendoorn PC and
Cleton-Jansen AM: Genome-wide analyses on high-grade osteosarcoma:
Making sense of a genomically most unstable tumor. Int J Cancer.
133:2512–2521. 2013.PubMed/NCBI
|
|
8
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuo X, Qin Y, Zhang X, Ning Q, Shao S, Luo
M, Yuan N, Huang S and Zhao X: Breast cancer cells are arrested at
different phases of the cell cycle following the re-expression of
ARHI. Oncol Rep. 31:2358–2364. 2014.PubMed/NCBI
|
|
10
|
Li Y, Liu M, Zhang Y, Han C, You J, Yang
J, Cao C and Jiao S: Effects of ARHI on breast cancer cell
biological behavior regulated by microRNA-221. Tumour Biol.
34:3545–3554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu Y, Chen J, Pang B, Li C, Zhao J and
Shen K: EZH2-induced H3K27me3 is associated with epigenetic
repression of the ARHI tumor-suppressor gene in ovarian cancer.
Cell Biochem Biophys. 71:105–112. 2015. View Article : Google Scholar
|
|
12
|
Li J, Cui G, Sun L, Wang SJ, Tian S, Guan
Z, Fan WS, Yan ZF, Yang YZ, You YQ, et al: ARHI overexpression
induces epithelial ovarian cancer cell apoptosis and excessive
autophagy. Int J Gynecol Cancer. 24:437–443. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Lin Y, Li L, Qing D, Teng XM,
Zhang YL, Hu X, Hu Y, Yang P and Han ZG: ARHI, as a novel
suppressor of cell growth and downregulated in human hepatocellular
carcinoma, could contribute to hepatocarcinogenesis. Mol Carcinog.
48:130–140. 2009. View
Article : Google Scholar
|
|
14
|
Wu X, Liang L, Dong L, Yu Z and Fu X:
Effect of ARHI on lung cancer cell proliferation, apoptosis and
invasion in vitro. Mol Biol Rep. 40:2671–2678. 2013. View Article : Google Scholar
|
|
15
|
Chen J, Shi S, Yang W and Chen C:
Over-expression of ARHI decreases tumor growth, migration, and
invasion in human glioma. Med Oncol. 31:8462014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ria R, Todoerti K, Berardi S, Coluccia AM,
De Luisi A, Mattioli M, Ronchetti D, Morabito F, Guarini A,
Petrucci MT, et al: Gene expression profiling of bone marrow
endothelial cells in patients with multiple myeloma. Clin Cancer
Res. 15:5369–5378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Badgwell DB, Lu Z, Le K, Gao F, Yang M,
Suh GK, Bao JJ, Das P, Andreeff M, Chen W, et al: The
tumor-suppressor gene ARHI (DIRAS3) suppresses ovarian cancer cell
migration through inhibition of the Stat3 and FAK/Rho signaling
pathways. Oncogene. 31:68–79. 2012. View Article : Google Scholar
|
|
18
|
Yu Y, Xu F, Peng H, Fang X, Zhao S, Li Y,
Cuevas B, Kuo WL, Gray JW, Siciliano M, et al: NOEY2 (ARHI), an
imprinted putative tumor suppressor gene in ovarian and breast
carcinomas. Proc Natl Acad Sci USA. 96:214–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prager GW, Poettler M, Unseld M and
Zielinski CC: Angiogenesis in cancer: anti-VEGF escape mechanisms.
Transl Lung Cancer Res. 1:14–25. 2011.
|
|
20
|
Zou CF, Jia L, Jin H, Yao M, Zhao N, Huan
J, Lu Z, Bast RC Jr, Feng Y and Yu Y: Re-expression of ARHI
(DIRAS3) induces autophagy in breast cancer cells and enhances the
inhibitory effect of paclitaxel. BMC Cancer. 11:222011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Méndez M, Custodio A and Provencio M: New
molecular targeted therapies for advanced non-small-cell lung
cancer. J Thorac Dis. 3:30–56. 2011.
|
|
22
|
Waltering KK, Helenius MA, Sahu B, Manni
V, Linja MJ, Jänne OA and Visakorpi T: Increased expression of
androgen receptor sensitizes prostate cancer cells to low levels of
androgens. Cancer Res. 69:8141–8149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin D, Cui F, Bu Q and Yan C: The
expression and clinical significance of GTP-binding RAS-like 3
(ARHI) and microRNA 221 and 222 in prostate cancer. J Int Med Res.
39:1870–1875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen MY, Liao WS, Lu Z, Bornmann WG,
Hennessey V, Washington MN, Rosner GL, Yu Y, Ahmed AA and Bast RC
Jr: Decitabine and suberoylanilide hydroxamic acid (SAHA) inhibit
growth of ovarian cancer cell lines and xenografts while inducing
expression of imprinted tumor suppressor genes, apoptosis, G2/M
arrest, and autophagy. Cancer. 117:4424–4438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu X, Qian J, Yu Y, Yang H and Li J:
Expression of the tumor suppressor ARHI inhibits the growth of
pancreatic cancer cells by inducing G1 cell cycle arrest. Oncol
Rep. 22:635–640. 2009.PubMed/NCBI
|
|
26
|
Janssen EA, Øvestad IT, Skaland I, Søiland
H, Gudlaugsson E, Kjellevold KH, Nysted A, Søreide JA and Baak JP:
LOH at 1p31 (ARHI) and proliferation in lymph node-negative breast
cancer. Cell Oncol. 31:335–343. 2009.PubMed/NCBI
|
|
27
|
Zhao X, Li J, Zhuo J and Cai L:
Reexpression of ARHI inhibits tumor growth and angiogenesis and
impairs the mTOR/VEGF pathway in hepatocellular carcinoma. Biochem
Biophys Res Commun. 403:417–421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Visser S and Yang X: Identification of
LATS transcriptional targets in HeLa cells using whole human genome
oligonucleotide microarray. Gene. 449:22–29. 2010. View Article : Google Scholar
|
|
29
|
Li L, Luo J, Wang B, Wang D, Xie X, Yuan
L, Guo J, Xi S, Gao J, Lin X, et al: Microrna-124 targets
flotillin-1 to regulate proliferation and migration in breast
cancer. Mol Cancer. 12:1632013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Yuan L, Luo J, Gao J, Guo J and Xie
X: MiR-34a inhibits proliferation and migration of breast cancer
through down-regulation of Bcl-2 and SIRT1. Clin Exp Med.
13:109–117. 2013. View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
32
|
Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare
S, Kondo S, Kondo Y, Yu Y, Mills GB, et al: The tumor suppressor
gene ARHI regulates autophagy and tumor dormancy in human ovarian
cancer cells. J Clin Invest. 118:3917–3929. 2008.PubMed/NCBI
|
|
33
|
Jovicic A, Zaldivar Jolissaint JF, Moser
R, Silva Santos MF and Luthi-Carter R: MicroRNA-22 (miR-22)
overexpression is neuroprotective via general anti-apoptotic
effects and may also target specific Huntington's disease-related
mechanisms. PLoS One. 8:e542222013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou Y, Zhu LB, Peng AF, Wang TF, Long XH,
Gao S, Zhou RP and Liu ZL: LY294002 inhibits the malignant
phenotype of osteosarcoma cells by modulating the
phosphatidylinositol 3-kinase/Akt/fatty acid synthase signaling
pathway in vitro. Mol Med Rep. 11:1352–1357. 2015.
|
|
35
|
Zhang Y, Yang CQ, Gao Y, Wang C, Zhang CL
and Zhou XH: Knockdown of CXCR7 inhibits proliferation and invasion
of osteosarcoma cells through inhibition of the PI3K/Akt and
β-arrestin pathways. Oncol Rep. 32:965–972. 2014.PubMed/NCBI
|
|
36
|
Lu Z, Yang H, Sutton MN, Yang M, Clarke
CH, Liao WS and Bast RC Jr: ARHI (DIRAS3) induces autophagy in
ovarian cancer cells by downregulating the epidermal growth factor
receptor, inhibiting PI3K and Ras/MAP signaling and activating the
FOXo3a-mediated induction of Rab7. Cell Death Differ. 21:1275–1289.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao Y, Luo LH, Li S and Yang C: miR-17
inhibitor suppressed osteosarcoma tumor growth and metastasis via
increasing PTEN expression. Biochem Biophys Res Commun.
444:230–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Papadimitriou K, Ardavanis A and
Kountourakis P: Neoadjuvant therapy for locally advanced breast
cancer: Focus on chemotherapy and biological targeted treatments'
armamentarium. J Thorac Dis. 2:160–170. 2010.PubMed/NCBI
|
|
39
|
Bao J-J, Le X-F, Wang R-Y, Yuan J, Wang L,
Atkinson EN, LaPushin R, Andreeff M, Fang B, Yu Y, et al:
Reexpression of the tumor suppressor gene ARHI induces apoptosis in
ovarian and breast cancer cells through a caspase-independent
calpain-dependent pathway. Cancer Res. 62:7264–7272.
2002.PubMed/NCBI
|
|
40
|
Yu P, Ye L, Wang H, Du G, Zhang J, Zhang J
and Tian J: NSK-01105 inhibits proliferation and induces apoptosis
of prostate cancer cells by blocking the Raf/MEK/ERK and
PI3K/Akt/mTOR signal pathways. Tumour Biol. 15:152014.
|