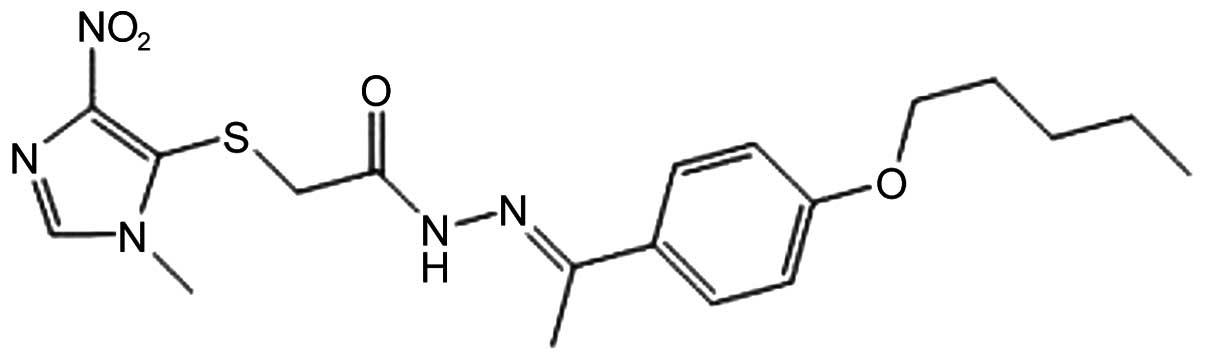
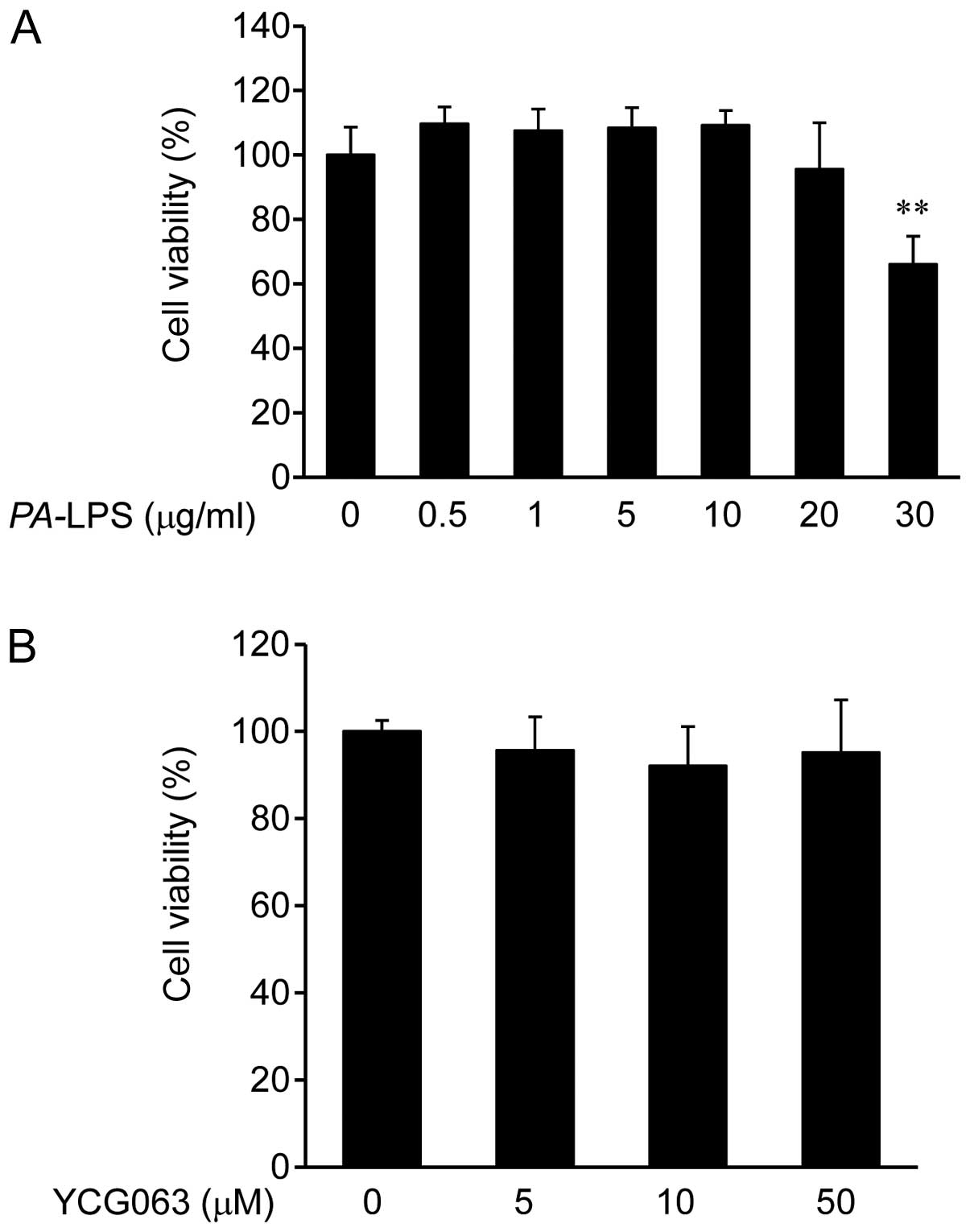
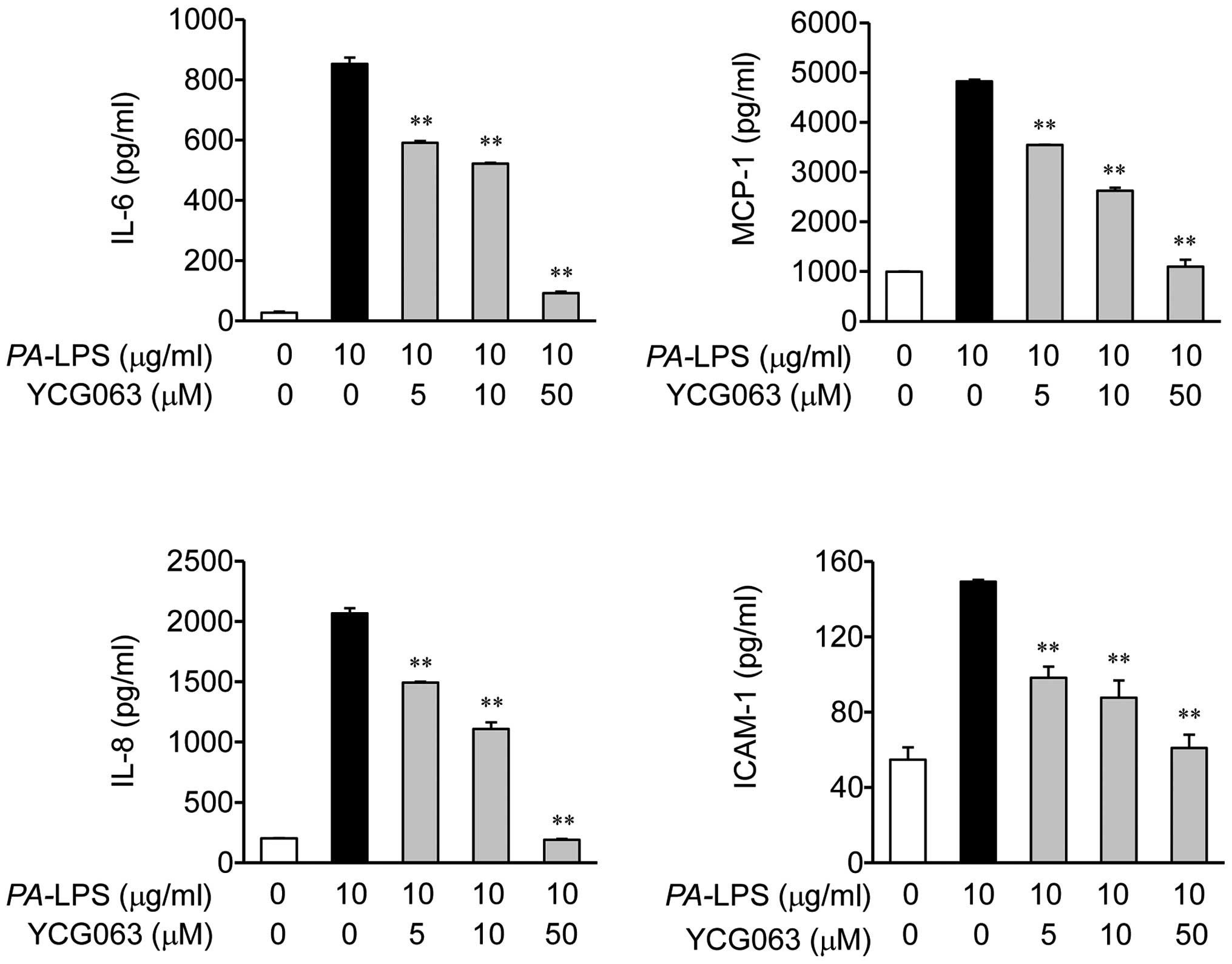
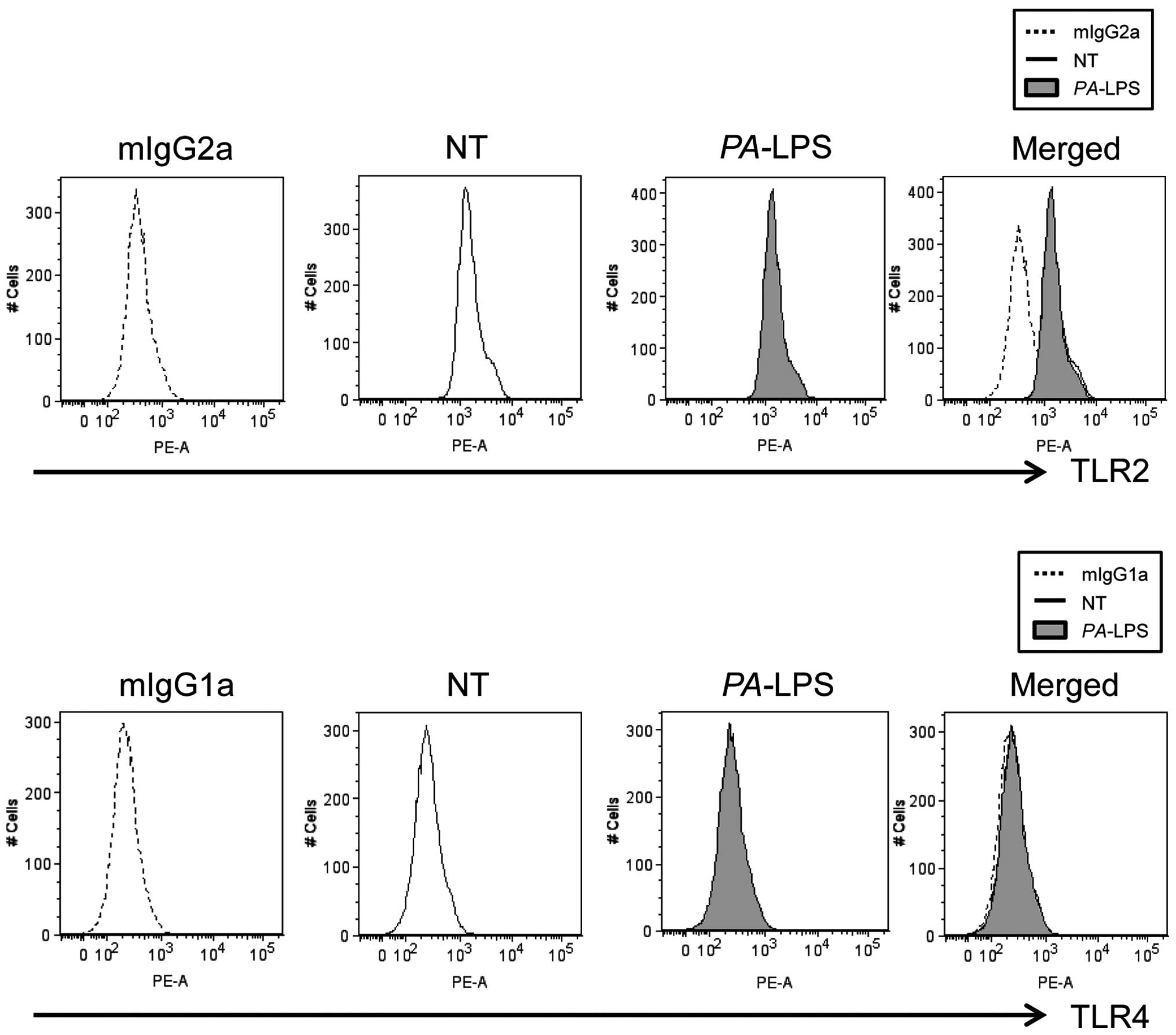
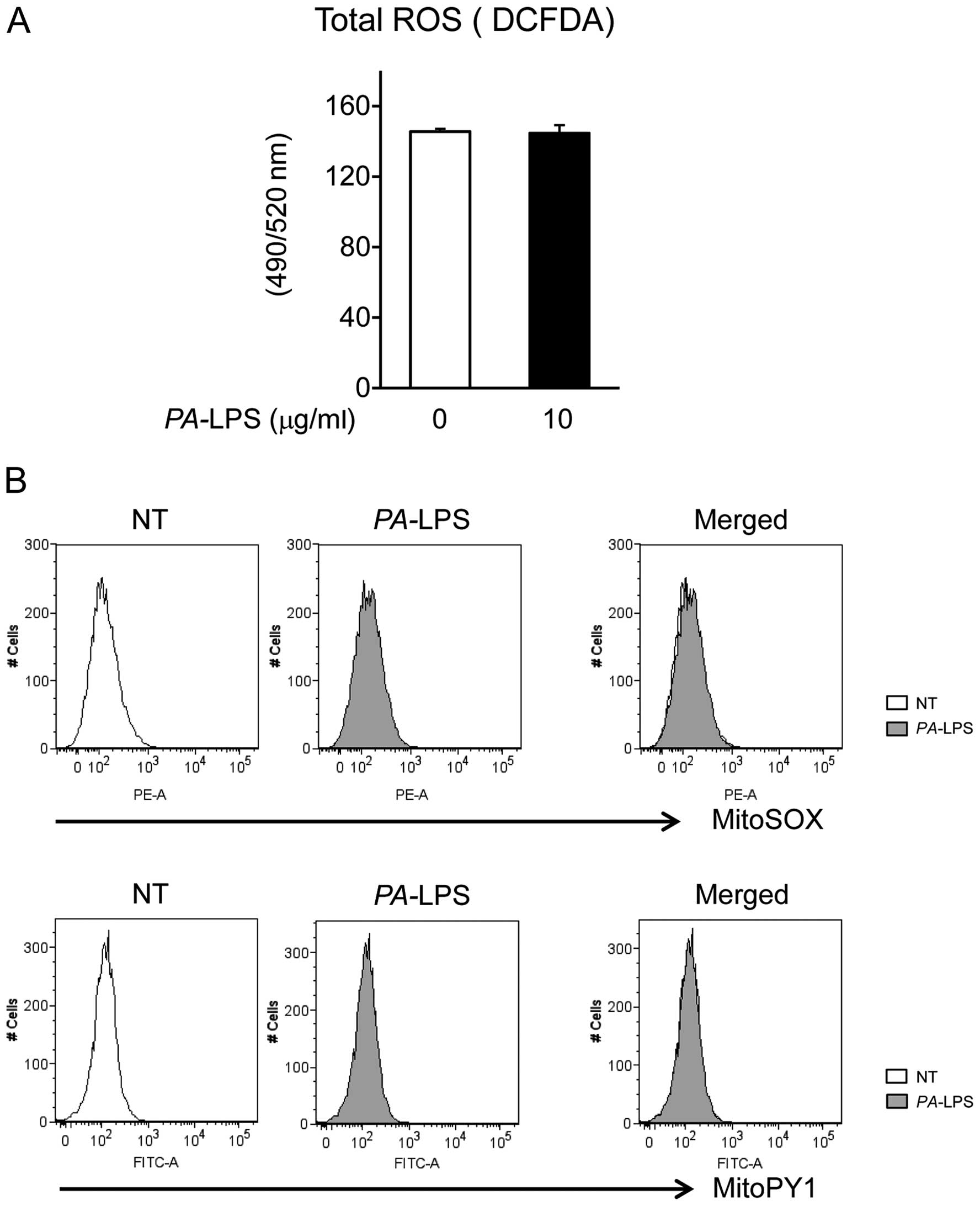
|
1
|
Bertino JS Jr: Impact of antibiotic
resistance in the management of ocular infections: The role of
current and future antibiotics. Clin Ophthalmol. 3:507–521. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Amri MS: Endogenous endophthalmitis
secondary to pyogenic liver abscess. Int J Diabet Mellitus. 2:646.
2010. View Article : Google Scholar
|
|
3
|
Callegan MC, Engelbert M, Parke DW II,
Jett BD and Gilmore MS: Bacterial endophthalmitis: Epidemiology,
therapeutics, and bacterium-host interactions. Clin Microbiol Rev.
15:111–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
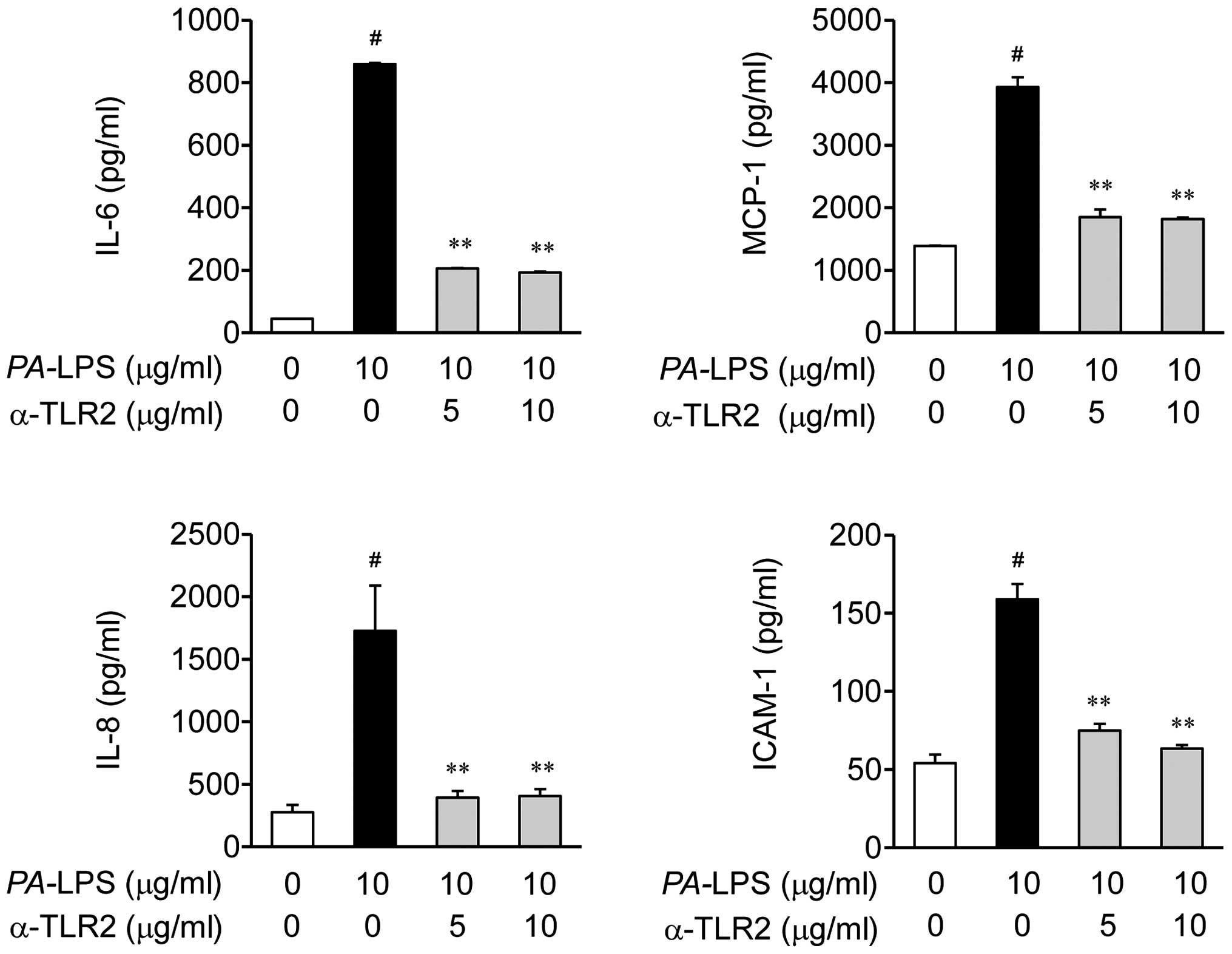
Obritsch MD, Fish DN, MacLaren R and Jung
R: Nosocomial infections due to multidrug-resistant Pseudomonas
aeruginosa: Epidemiology and treatment options. Pharmacotherapy.
25:1353–1364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eifrig CW, Scott IU, Flynn HW Jr and
Miller D: Endophthalmitis caused by Pseudomonas aeruginosa.
Ophthalmology. 110:1714–1717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren Y, Xie Y, Jiang G, Fan J, Yeung J, Li
W, Tam PK and Savill J: Apoptotic cells protect mice against
lipopolysaccharide-induced shock. J Immunol. 180:4978–4985. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vallejo-Garcia JL, Asencio-Duran M,
Pastora-Salvador N, Vinciguerra P and Romano MR: Role of
inflammation in endo-phthalmitis. Mediators Inflamm.
2012:1960942012. View Article : Google Scholar
|
|
8
|
Campochiaro PA: Ocular neovascularization.
J Mol Med Berl. 91:311–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonilha VL: Age and disease-related
structural changes in the retinal pigment epithelium. Clin
Ophthalmol. 2:413–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arranz-Valsero I, Schulze U,
Contreras-Ruiz L, García-Posadas L, López-García A, Paulsen F and
Diebold Y: Involvement of corneal epithelial cells in the Th17
response in an in vitro bacterial inflammation model. Mol Vis.
19:85–99. 2013.PubMed/NCBI
|
|
11
|
Gallucci RM, Simeonova PP, Matheson JM,
Kommineni C, Guriel JL, Sugawara T and Luster MI: Impaired
cutaneous wound healing in interleukin-6-deficient and
immunosuppressed mice. FASEB J. 14:2525–2531. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gallucci RM, Sugawara T, Yucesoy B,
Berryann K, Simeonova PP, Matheson JM and Luster MI: Interleukin-6
treatment augments cutaneous wound healing in immunosup-pressed
mice. J Interferon Cytokine Res. 21:603–609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Biswas PS, Banerjee K, Kinchington PR and
Rouse BT: Involvement of IL-6 in the paracrine production of VEGF
in ocular HSV-1 infection. Exp Eye Res. 82:46–54. 2006. View Article : Google Scholar
|
|
14
|
De Vos AF, Hoekzema R and Kijlstra A:
Cytokines and uveitis, a review. Curr Eye Res. 11:581–597. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fenton RR, Molesworth-Kenyon S, Oakes JE
and Lausch RN: Linkage of IL-6 with neutrophil chemoattractant
expression in virus-induced ocular inflammation. Invest Ophthalmol
Vis Sci. 43:737–743. 2002.PubMed/NCBI
|
|
16
|
Cole N, Bao S, Willcox M and Husband AJ:
Expression of interleukin-6 in the cornea in response to infection
with different strains of Pseudomonas aeruginosa. Infect Immun.
67:2497–2502. 1999.PubMed/NCBI
|
|
17
|
Youker K, Smith CW, Anderson DC, Miller D,
Michael LH, Rossen RD and Entman ML: Neutrophil adherence to
isolated adult cardiac myocytes. Induction by cardiac lymph
collected during ischemia and reperfusion. J Clin Invest.
89:602–609. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Shaw SK, Ma S, Yang L, Luscinskas
FW and Parkos CA: Regulation of leukocyte transmigration: Cell
surface interactions and signaling events. J Immunol. 172:7–13.
2004. View Article : Google Scholar
|
|
19
|
Tacke F and Randolph GJ: Migratory fate
and differentiation of blood monocyte subsets. Immunobiology.
211:609–618. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Riper G, Siciliano S, Fischer PA,
Meurer R, Springer MS and Rosen H: Characterization and species
distribution of high affinity GTP-coupled receptors for human
rantes and monocyte chemoattractant protein 1. J Exp Med.
177:851–856. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carr MW, Roth SJ, Luther E, Rose SS and
Springer TA: Monocyte chemoattractant protein 1 acts as a
T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 91:3652–3656.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawashima A, Suzuki T, Nishihara F,
Kobayashi T, Takaku Y, Nakagome K, Soma T, Hagiwara K, Kanazawa M
and Nagata M: Effect of formoterol on eosinophil trans-basement
membrane migration induced by interleukin-8-stimulated neutrophils.
Int Arch Allergy Immunol. 161(Suppl 2): 10–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen YJ, Huang YS, Chen JT, Chen YH, Tai
MC, Chen CL and Liang CM: Protective effects of glucosamine on
oxidative-stress and ischemia/reperfusion-induced retinal injury.
Invest Ophthalmol Vis Sci. 56:1506–1516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KH, Park JY, Jung HJ and Kwon HJ:
Identification and biological activities of a new antiangiogenic
small molecule that suppresses mitochondrial reactive oxygen
species. Biochem Biophys Res Commun. 404:541–545. 2011. View Article : Google Scholar
|
|
25
|
Meng Z, Yan C, Deng Q, Gao DF and Niu XL:
Curcumin inhibits LPS-induced inflammation in rat vascular smooth
muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways.
Acta Pharmacol Sin. 34:901–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Novosad BD, Astley RA and Callegan MC:
Role of Toll-like receptor (TLR) 2 in experimental Bacillus cereus
endo-phthalmitis. PLoS One. 6:e286192011. View Article : Google Scholar
|
|
27
|
Schwarz JM and Bilbo SD: LPS elicits a
much larger and broader inflammatory response than Escherichia coli
infection within the hippocampus of neonatal rats. Neurosci Lett.
497:110–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vogel SN, Fitzgerald KA and Fenton MJ:
TLRs: Differential adapter utilization by toll-like receptors
mediates TLR-specific patterns of gene expression. Mol Interv.
3:466–477. 2003. View Article : Google Scholar
|
|
31
|
McGuire VA, Gray A, Monk CE, Santos SG,
Lee K, Aubareda A, Crowe J, Ronkina N, Schwermann J, Batty IH, et
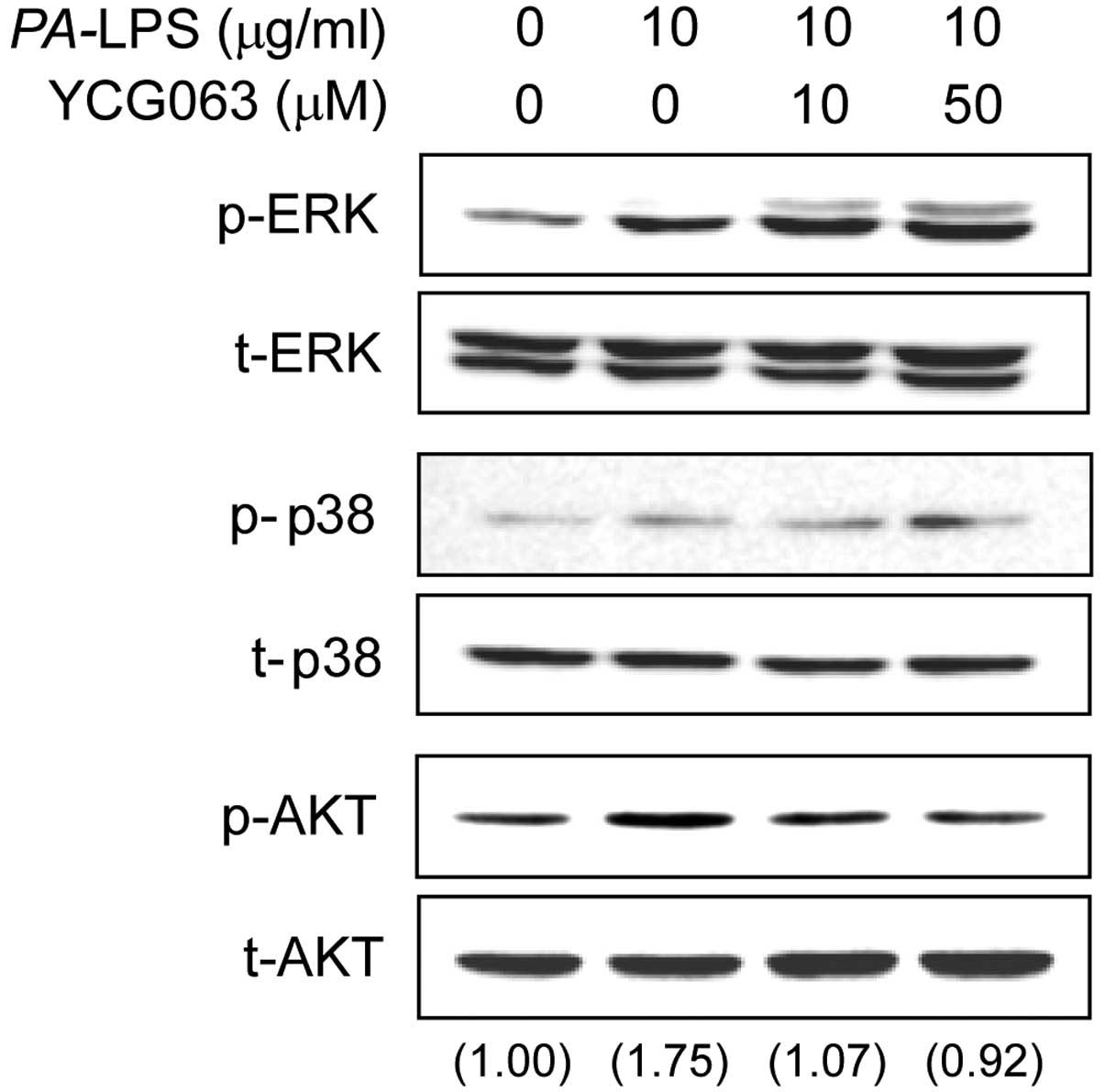
al: Cross talk between the Akt and p38α pathways in macrophages
downstream of Toll-like receptor signaling. Mol Cell Biol.
33:4152–4165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung WK, Lee DY, Park C, et al: Cilostazol
is anti-inflammatory in BV2 microglial cells by inactivating
nuclear factor-kappaB and inhibiting mitogen-activated protein
kinases. Br J Pharmacol. 159:1274–1285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
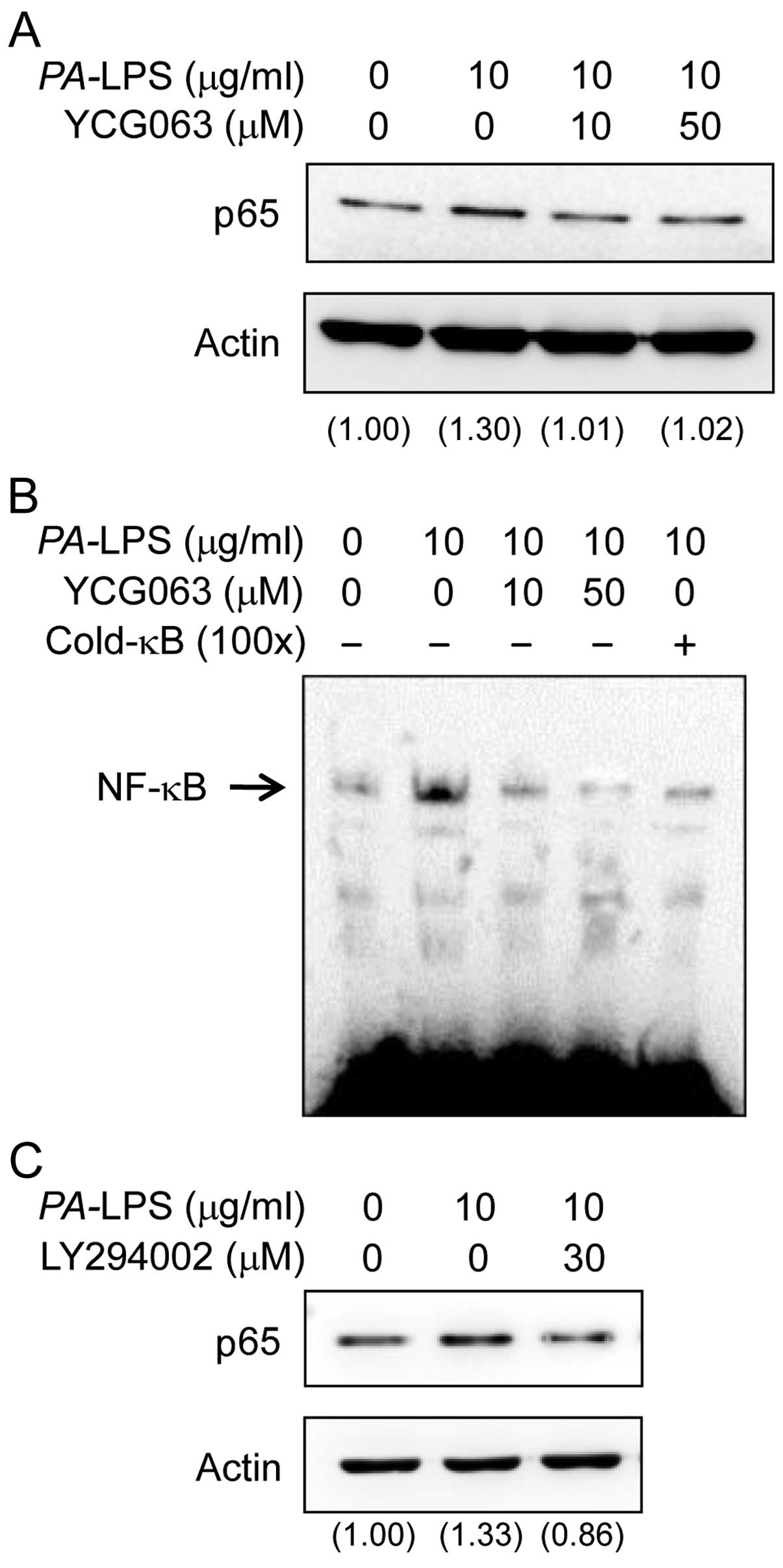
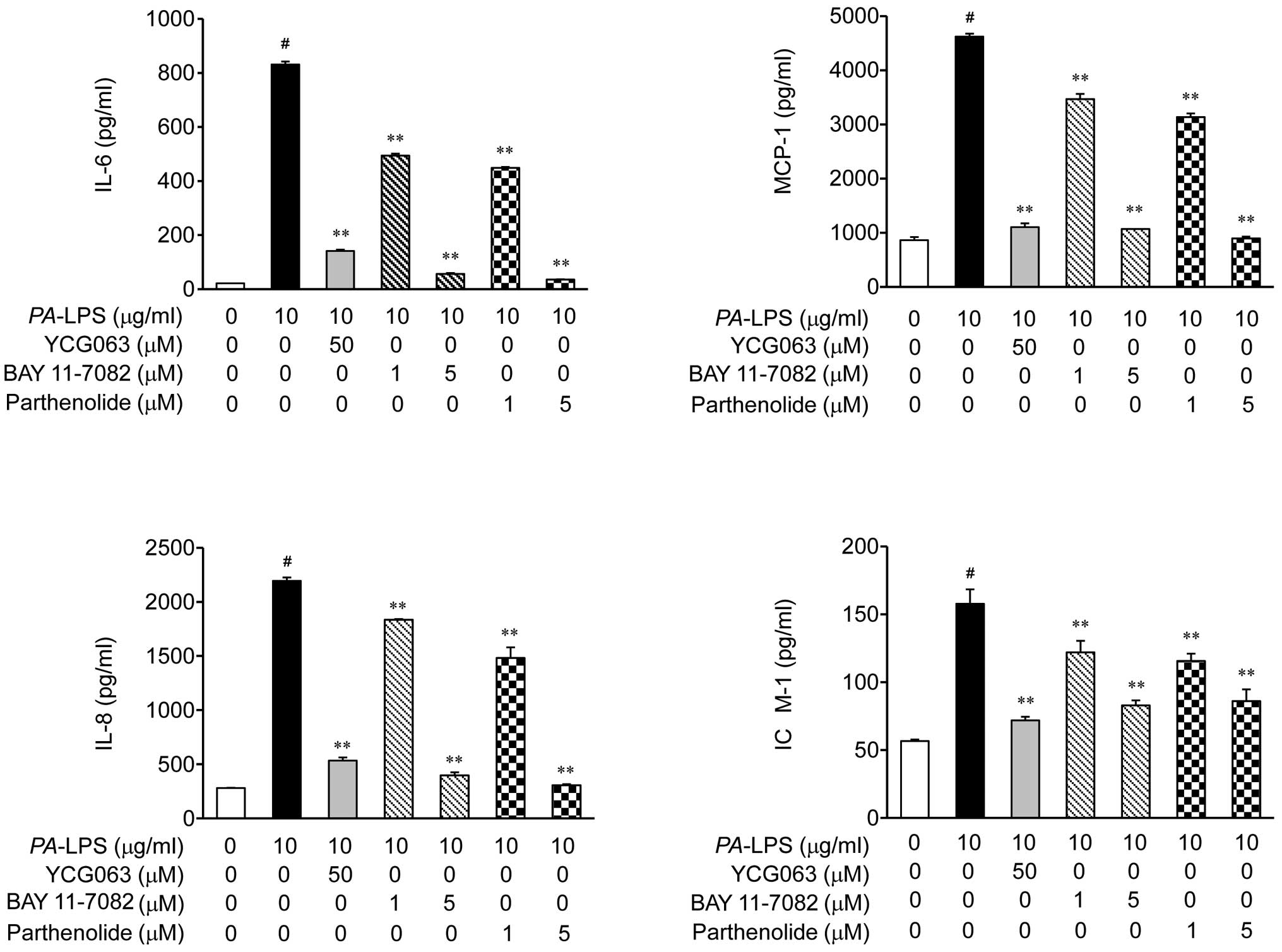
Dąbek J, Kułach A and Gąsior Z: Nuclear
factor kappa-light-chain-enhancer of activated B cells (NF-κB): a
new potential therapeutic target in atherosclerosis? Pharmacol Rep.
62:778–783. 2010. View Article : Google Scholar
|
|
34
|
Chen BC, Wu WT, Ho FM and Lin WW:
Inhibition of interleukin-1beta-induced NF-kappa B activation by
calcium/calmodulin-dependent protein kinase kinase occurs through
Akt activation associated with interleukin-1 receptor-associated
kinase phosphorylation and uncoupling of MyD88. J Biol Chem.
277:24169–24179. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Yan F, Zhou H, Lin X, Wu Y, Chen C,
Zhou N, Chen Z, Li JD and Shen H: P. aeruginosa
lipopolysaccharide-induced MUC5AC and CLCA3 expression is partly
through Duox1 in vitro and in vivo. PLoS One. 8:e639452013.
View Article : Google Scholar : PubMed/NCBI
|