Introduction
The prevalence of diabetes mellitus continues to
increase worldwide, with diabetic retinopathy (DR) remaining a
leading cause of vision loss in several countries. The pathogenesis
of DR is multifactorial and affects all cell types in the retina
(1). Although hyperglycaemia is
recognised as a symptom and a complication of diabetes, the precise
molecular mechanisms affected during hyperglycaemic conditions are
not yet well understood. In previous studies, glyoxal and
methylglyoxal were defined as toxic metabolites and their levels
were proven to increase under hyperglycaemic conditions (2). Glyoxal and methylglyoxal promote the
formation of advanced glycation end products (AGEs) to produce
oxidative stress, and they can cause various types of cellular
damage (3). As one factor
responsible for the generation of reactive oxygen species (ROS),
AGEs have been implicated in the pathogenesis of DR (4–6),
cataractogenesis (7) and other
diabetic complications (8,9).
Under hyperglycaemic conditions, ROS increase vascular permeability
and induce retinal ischaemia, which are common pathways in the
development and progression of DR (5,6,10).
The addition of O-linked N-acetylglucosamine to intracellular
proteins (O-GlcNAcylation) is an inducible and a reversible process
and it occurs in both the cytoplasm and mitochondria. It involves
the post-translational modification of serine (Ser)/threonine (Thr)
residues, in the presence of O-GlcNAc transferase (OGT) and
O-GlcNAcase (OGA) (11). Studies
on several transformed cell lines have reported that high glucose
levels increase O-linked β-N-acetyl glucosamine (O-GlcNAc) levels
through a number of different mechanisms, which are possibly
important in the pathogenesis of diabetes (12,13). O-GlcNAc levels increase in
response to hyperglycaemia or glyoxal-induced stress; this
mechanism is associated with increased cell survival (14–16). The protective effects of
O-GlcNAcylation and the association between O-GlcNAcylation and
apoptosis have been previously investigated (17,18). To the best of our knowledge
however, there is no study available to date on the association
between O-GlcNAcylation and cell apoptosis in DR. Thus, as the
association between O-GlcNAcylation and cellular function is
complex and poorly understood as regards DR, in the present study,
we exposed human retinal microvascular endothelial cells (HRECs) to
glyoxal to establish the detrimental effects of ROS and treated
them with PUGNAc in order to ascertain whether O-GlcNAcylation
prevents cellular injury through its effects on ROS generation.
Materials and methods
Reagents
The following reagents were purchased: CTD110.6
antibody (sc-59623) was purchased from Covance Inc. (Princeton, NJ,
USA); antibodies against caspase-3 (#9665) and β-actin (#8457) were
purchased from Cell Signaling Technology (Danvers, MA, USA);
anti-von Willebrand factor (vWF; ab6994) was purchased from Abcam
(Cambridge, MA, USA);
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimid-azolyl-carbocyanine
iodide (JC-1) and 2′,7′dichlorofluorescein diacetate (DCFH-DA) were
purchased from the Beyotime Institute of Biotechnology (Haimen,
China); PUGNAc, N-acetyl cysteine (NAC) and glyoxal were all
obtained from Sigma-Aldrich (St. Louis, MO, USA); the Annexin
V-FITC Apoptosis detection kit was purchased from BD Biosciences
(San Diego, CA, USA); fetal bovine serum (FBS) and trypsin-EDTA
were purchased from Gibco (Carlsbad, CA, USA); extracellular matrix
(ECM), endothelial cell growth supplements (ECGS) (X100) and
penicillin/streptomycin were purchased from Sciencell Research
Laboratories (Carlsbad, CA, USA); plastic tissue culture flasks
were obtained from Costar (Cambridge, MA, USA); and the
Immobilon-NC transfer membrane was purchased from Millipore
(Billerica, MA, USA); the real-time PCR system was obtained from
Promega, (Madison, WI, USA); CCK-8 was purchased from Dojindo
Laboratories (Kumamoto, Japan); and a Leica laser scanning
microscope from Leica Microsystems (Mannheim, Germany) was used for
scanning.
HREC culture
The HRECs were acquired from Cell Systems Corp.
(CSC; Kirkland, WA, USA). Using immunofluorescence staining, we
confirmed that the cells expressed vWF, which was demonstrated by
green particles in the cytoplasm (data not shown); the cells used
in the experiments were within 5 passages of authentication. The
cells were grown in ECM supplemented with 5% FBS, ECGS 100 U/ml and
1% penicillin/streptomycin. The cells were incubated at 37°C in a
5% CO2 incubator. The culture medium was replaced every
3 days, and after reaching confluence, the cells were passaged with
a 0.25% solution of trypsin-EDTA. As regards treatment, the HRECs
were divided into 4 groups as follows: HRECs treated with normal
glucose (5 mM; normal control), HRECs treated with glyoxal (500
µM to produce oxidative stress), glyoxal-treated HRECs also
treated with 200 µM PUGNAc (to increase levels of O-GlcNAc),
and glyoxal-treated HRECs infected with O-GlcNAc transferase (OGT)
siRNA (to decrease the levels of O-GlcNAc). After the HRECs were
treated with PUGNAc (an inhibitor of OGA) and transfected with
siRNA against OGT in serum-free DMEM for 12 h, they were cultured
with glyoxal for a further 24 h.
Western blot analysis
Following the appropriate treatment and rinsing with
cold phosphate-buffered saline (PBS), the HRECs were scraped into
lysis buffer containing a protease inhibitor cocktail. Equal
amounts (30 µg) of protein from the cell extracts were
separated on a 10% acrylamide gel and subsequently transferred
electrophoretically onto polyvinylidene fluoride transfer membranes
at 200 mA for 1.5 h. After blocking in PBS (10 mM Tris-HCl buffer,
pH 8.0, 150 mM NaCl) containing 5% (w/v) BSA for 1 h at room
temperature, the membranes were incubated overnight at 4°C with
primary rabbit monoclonal antibody against anti-O-GlcNAc (CTD110.6;
Covance) and anti-β actin antibody (Cell Signaling Technology) (all
antibodies were used at a 1:1,000 dilution). The blots were washed
with PBS-T (0.1% Tween-20 in PBS) 3 times prior to incubation with
the secondary antibody (horseradish peroxidase-conjugated goat
anti-mouse IgG, 1:5,000; Pierce Biotechnology, Rockford, IL, USA)
for 1 h at room temperature. The hybridised membrane was washed in
PBS-T buffer and visualised using Odyssey (LI-COR Biosciences,
Lincoln, NE, USA). The optical density of each band was determined
by Quantity One software (Bio-Rad).
siRNA transfection and treatment with
PUGNAc followed by treatment wth glyoxal
Total protein O-GlcNAcylation was inhibited with the
use of siRNA tarteging OGT (OGT siRNA) and was enhanced with the
use of PUGNAc. siRNA targeting OGT was purchased from Shanghai
GenePharma Co., Ltd., (Shanghai, China) as previously described
(13). The HRECs were seeded at
5×105 in 6-well plates in ECM and incubated with
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) and siRNA in 0.5
ml of serum-free medium for 20 min. The siRNA-Lipofectamine 2000
complex was added to the cells in 1.5 ml of serum-free medium and
maintained for 6 h. Another cell group was incubated with 200
µM PUGNAc for 12 h without serum. All the treated cells were
then maintained for 24 h, co-cultured with 500 µM glyoxal.
On the 3rd day, the cells were prepared for the assessment of ROS
production, and for use in western blot analysis, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
CCK-8 assay and JC-1 staining.
Assessment of ROS production
Changes in intracellular ROS levels were determined
by measuring the oxidative conversion of cell permeable DCFH-DA to
green fluorescent 2′,7′dichlorofluorescein (DCF). DCFH-DA is widely
used to detect the generation of ROS and to assess overall
oxidative stress in toxicological phenomena. DCFH-DA is able to
diffuse through the cell membrane, and it is enzymatically
hydrolysed by intracellular esterases to produce non-fluorescent
DCFH. To evaluate the generation of ROS, DCFH-DA dissolved in DMSO
was added to the cell cultures at a final concentration of 5
µM, for 20 min. The cells were lysed with 400 mM NaOH. The
total fluorescence intensity of each well was quantified using a
fluorescence multi-well plate reader (Synergy 2; BioTek
Instruments, Inc., Winooski, VT, USA) with excitation and emission
wavelengths of 485 and 530 nm, respectively. Total protein
concentrations were determined using bicinchoninic acid (BCA)
protein assay kits (Pierce Biotechnology). For quantification, ROS
levels were assessed by determining the fluorescence
intensity/protein concentration. ROS levels were also assessed
using fluorescence microscopy to observe the changes in DCF
fluorescence.
Cell viability assay
The HRECs were seeded in 96-well plates (3,000
cells/well) and grown for 24 h in medium supplemented with 5% FBS,
at 37°C in 5% CO2. The cells were then treated as
described above. In order to confirm that the damage from ROS was
induced by glyoxal, before co-culturing with 500 µM glyoxal,
one group of cells was treated with NAC (5 mM). Subsequently, 10
µl of CCK-8 solution {containing WST-8
[2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazolium,
monosodium salt was added to each well of the plate (total medium
100 µl/well) and the cells were incubated at 37°C. After 4 h
of incubation, the absorbance was measured using a multi-well plate
reader at a wavelength of 450 (650 nm reference wavelength). The
effects of O-GlcNAcylation on oxidative stress-induced damage to
the HRECs were then determined. In order to confirm that
O-GlcNAcylation protects the HRECs from releasing ROS, the treated
HRECs (siRNA transfection, treatment with PUGNAc and NAC) were
exposed to 200 µM hydrogen peroxide
(H2O2) for 2 h; the viability of the HRECs
was then assessed by CCK-8 assay as already described above.
RT-qPCR for the measurement of superoxide
dismutase (SOD) and glutathione peroxidase (GPX) mRNA
expression
Total RNA was extracted using TRIzol reagent
(Invitrogen). The synthesis of the cDNA was performed using 1
µg of total RNA with reverse transcription reagents (Takara
Bio, Inc., Shiga, Japan) and qPCR was carried out on the Bio-Rad
iQ5 Optical System in 20 µl TaqMan Gene Expression Master
Mix (Promega) using 200 ng cDNA. Human primer sets were ordered and
used according to the manufacturer's instructions. The human
β-actin gene was used as an endogenous reference to control for the
independent expression of sample-to-sample variability. The
relative expression of the target genes was normalised by dividing
the target Ct value by the endogenous Ct values. The primer
sequences that were tested in the present study were as follows:
human SOD sense, 5′-GCAATGTGACTGCTGACAAAGAT-3′ and antisense,
5′-ATTACACCACAAGCCAAACGACT-3′; human GPX sense,
5′-ACTCTCTCGTTTCCTTTCTGTTGCT-3′ and antisense,
5′-CTCTTCGTTCTTGGCGTTCTCC-3′; and human β-actin sense,
5′-ATGTCACGCACGATTTCCC-3′ and antisense,
5′-GAGACCTTCAACACCCCAGC-3′.
Annexin V and PI double staining by flow
cytometry
The HRECs were incubated with 200 µM PUGNAc
or infected with siRNA for 12 h without serum. All the treated
cells were then cultured for 24 h with 500 µM glyoxal. As
the positive control, the cells treated with PUGNAc and infected
with siRNA were incubated with H2O2 (100
µM) for 12 h. The cells were resuspended in Annexin V
binding buffer (BD Biosciences) at a concentration of
1×106 cells/ml. Annexin V-FITC (BD Biosciences) was then
added followed by incubation for 15 min in the dark in a 100
µl cell suspension. PI was then spiked into 400 µl
Annexin V binding buffer and added immediately to the cell
suspension, and subsequently analysed on a FACScan flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Assessment of mitochondrial membrane
potential
Mitochondrial membrane potential was examined by
staining with JC-1, a lipophilic, cationic dye that exhibits a
fluorescence emission shift upon aggregation from 530 nm (green) to
590 nm (red). In healthy cells with a high mitochondrial membrane
potential, JC-1 enters the mitochondrial matrix in a
potential-dependent manner and forms aggregates. Staining was
performed using 2.5 µM JC-1 at 37°C for 15 min. Following
staining, the cells were rinsed 3 times with PBS. Images were
captured using an inverted fluorescence microscope (Leica
Microsystems) at excitation/emission wavelengths of 530/590 nm. The
fluorescence intensity was analysed using Image-Pro Plus v6.0 image
analysis software.
Statistical analysis
All experiments were performed with 3 biological
replicates. The statistical signifiance of the differences was
determined using one-way analysis of variance followed by Dunnett's
multiple comparison test. Values are presented as the means ±
standard deviation and a value of P<0.05 was considered to
indicate a statistically significant difference.
Results
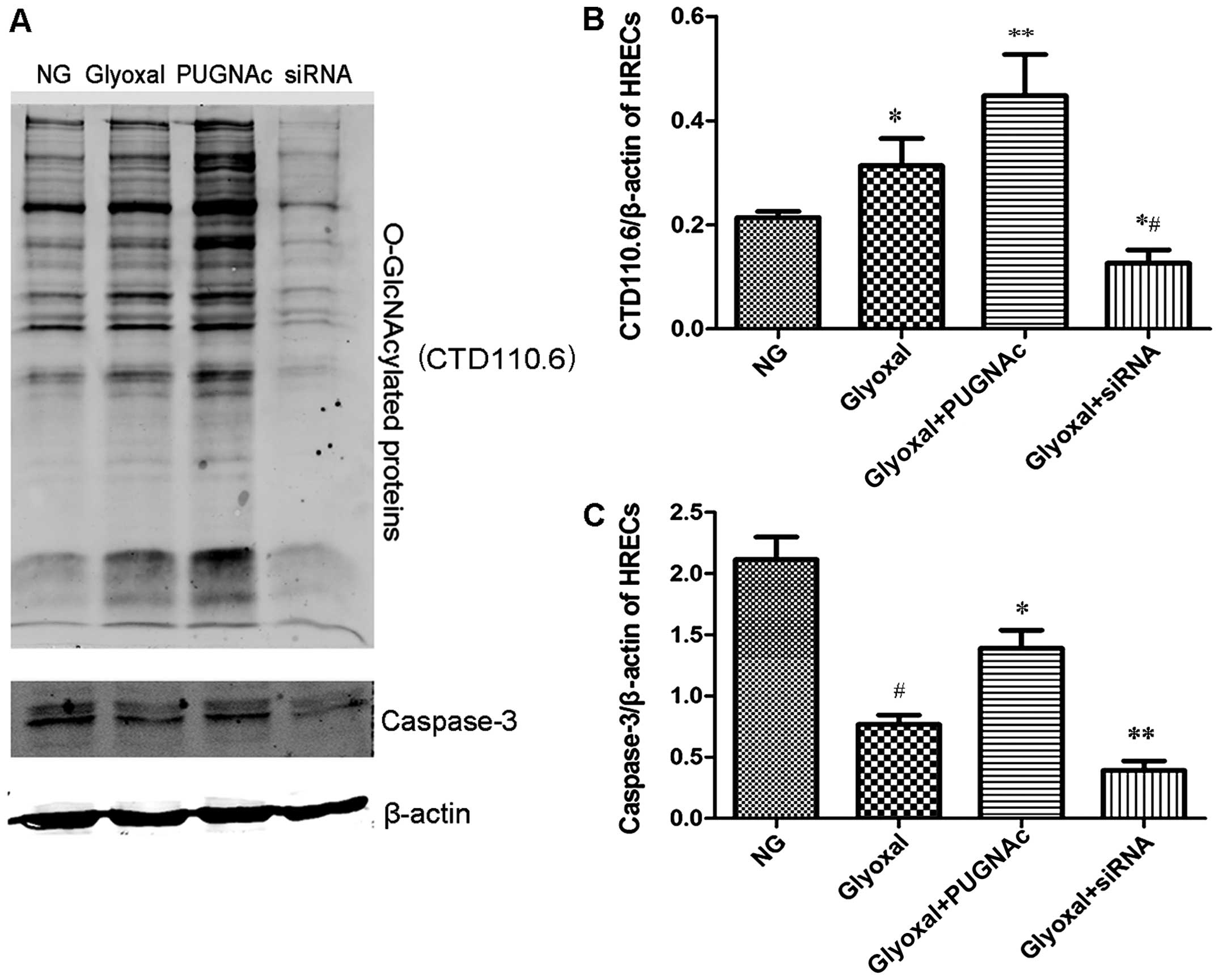
O-GlcNAcylation and caspase-3 levels in
HRECs
The total levels of O-GlcNAc were determined by
western blot analysis using CTD110.6, as described in the Materials
and methods (Fig. 1A). The
O-GlcNAc levels increased in the glyoxal-treated HRECs compared
with the normal glucose-treated HRECs (Fig. 1B, P<0.05). PUGNAc, as an OGA
inhibitor, significantly increased the O-GlcNAc levels (Fig. 1B, P<0.01), while transfection
with OGT siRNA decreased the levels of O-GlcNAc in the presence of
glyoxal (Fig. 1B, P<0.01). Our
results also demonstrated that the expression level of total
caspase-3 decreased in the presence of glyoxal. The augmentation of
O-GlcNAcylation increased the expression of total caspase-3,
whereas the attenuation of O-GlcNAcylation (by siRNA) decreased the
expression of total caspase-3 (Fig.
1C, P<0.01). These results clearly indicated that glyoxal
increased the total levels of O-GlcNAc and that the augmentation of
O-GlcNAcylation protected the HRECs from glyoxal-induced damage.
Cleaved caspase-3 is a critical executioner of apoptosis. The
activation of caspase-3 requires the proteolysis of total caspase-3
into cleaved caspase-3. The level of total caspase-3 increased,
which indicates that the level of cleaved caspase-3 decreased
(19,37,38). The augmentation of O-GlcNAcylation
increased the level of total caspase-3 and decreased the
proteolysis of total caspase-3, which indicated that the
augmentation of O-GlcNAcylation reduced the level of cleaved
caspase-3 and cell apoptosis; thus, our results suggest that the
augmentation of O-GlcNAcylation protects the HRECs from
glyoxal-induced damage.
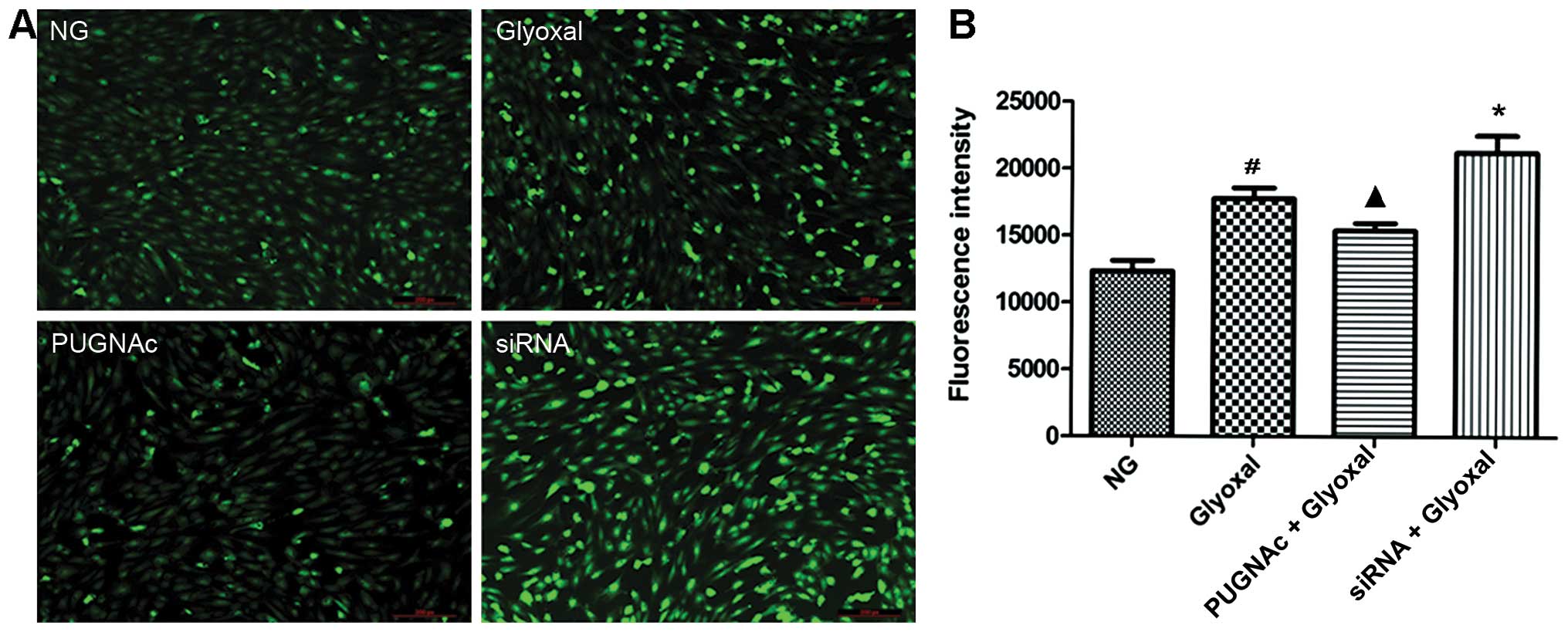
Effects of O-GlcNAcylation on
glyoxal-induced ROS generation
ROS are important contributors to AGE-induced injury
and they are important in the development of DR (20–23). Images captured by fluorescence
microscopy confirmed a higher level of ROS in vitro in the
presence of glyoxal, as shown by the strong bright green
fluorescence in the representative image (Fig. 2A). Our data confirmed that the ROS
levels were higher in the glyoxal-treated HRECs compared with the
normal glucose-treated HRECs (Fig.
2B, P<0.01). Both the inhibition of OGT (by PUGNAc) and OGA
(by siRNA) significantly altered the ROS levels. Glyoxal-induced
ROS production was attenuated by increased O-GlcNAcylation
(P<0.05), while OGT siRNA increased the generation of ROS
(P<0.05). These data indicated that the augmentation of
O-GlcNAcylation decreased the oxidative stress induced by
glyoxal.
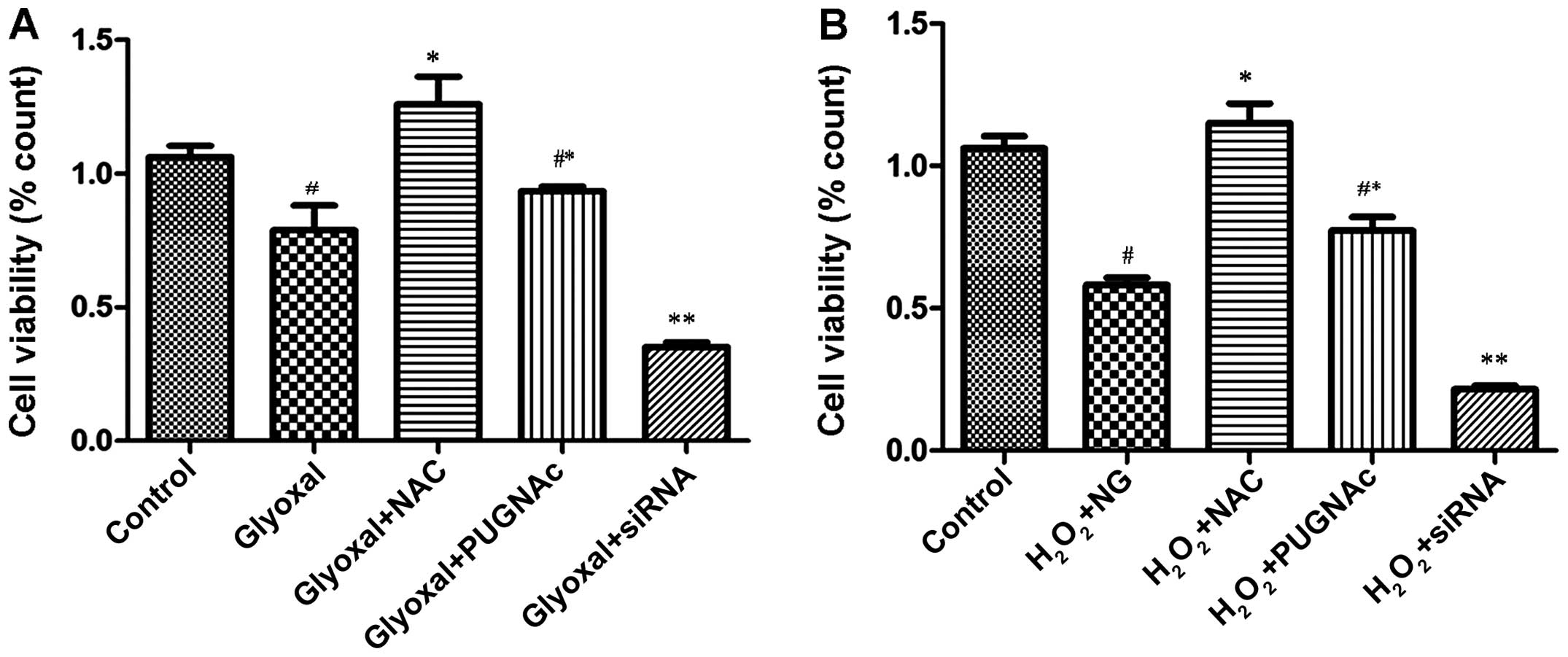
Effects of O-GlcNAcylation on the
viability of HRECs
We examined the effects of O-GlcNAcylation on the
viability of HRECs exposed to the different treatments. Our
statistical analysis revealed that there were significant
differences between some of the treatment groups. The cell
viability of the glyoxal-treated group was significantly lower
compared with that of the control group (normal glucose;
P<0.005) (Fig. 3A). The
viability of the PUGNAc-treated cells was higher compared with that
of the glyoxal group, although the difference was not significant
(P>0.05); however, the viability of the OGT siRNA-treated group
was significantly lower (P<0.0001). Following treatment with
NAC, the viability of the glyoxal-treated group increased
significantly, indicating that O-GlcNAcylation protected the cells
by attenuating oxidative stress. To confirm that the reduction of
oxidative stress was a contributory mechanism involved in the
protective effects of O-GlcNAcylation, the treated HRECs were
exposed to H2O2 (200 µM) for 2 h. The
viability of the HRECs was then assessed. As shown in Fig. 3B, the decrease in cell viability
induced by H2O2 was mitigated by the OGA
inhibitor (PUGNAc) and aggravated by OGT siRNA (Fig. 3B, P<0.0001). These findings
indicated that O-GlcNAcylation was associated with changes in cell
viability. O-GlcNAcylation reduced the vulnerability of the HRECs
to H2O2, and protected the cells by
attenuating oxidative stress.
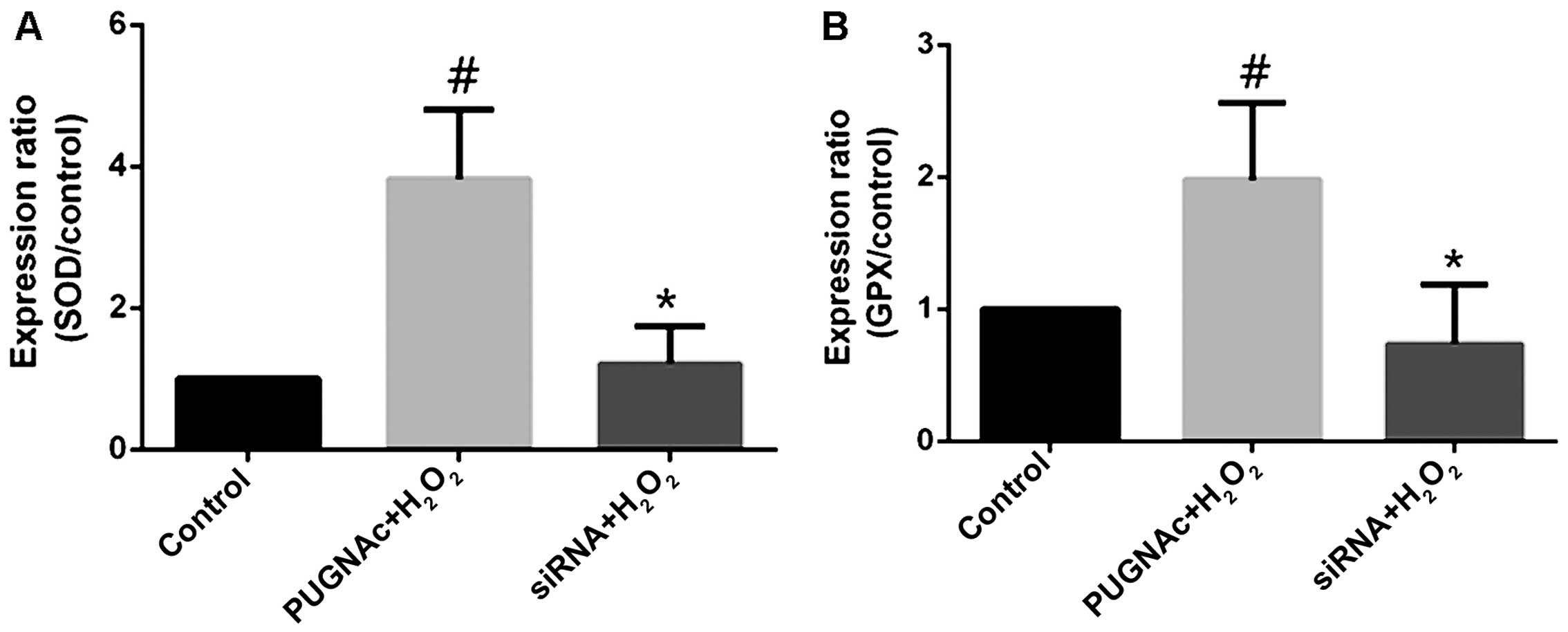
Effects of O-GlcNAcylation on the levels
of antioxidant enzymes
The HRECs treated with PUGNAc or OGT siRNA were
subjected to oxidative stress (with H2O2),
and the mRNA levels of antioxidant enzymes were measured by
RT-qPCR. The inhibition of OGA with PUGNAc increased the SOD mRNA
levels (Fig. 4A, P<0.05),
while transfection with OGT siRNA decreased the SOD mRNA levels
(not significant compared to the normal glucose-treated group,
P>0.05; Fig. 4A). Although
changes in GPX mRNA levels occurred in the presence of PUGNAc or
OGT siRNA, these changes were not significant compared to the
normal glucose-treated group. The trend indicated however, that
PUGNAc increased the GPX mRNA levels, while OGT siRNA decreased the
GPX mRNA levels (Fig. 4B,
P>0.05).
Effects of O-GlcNAcylation on the
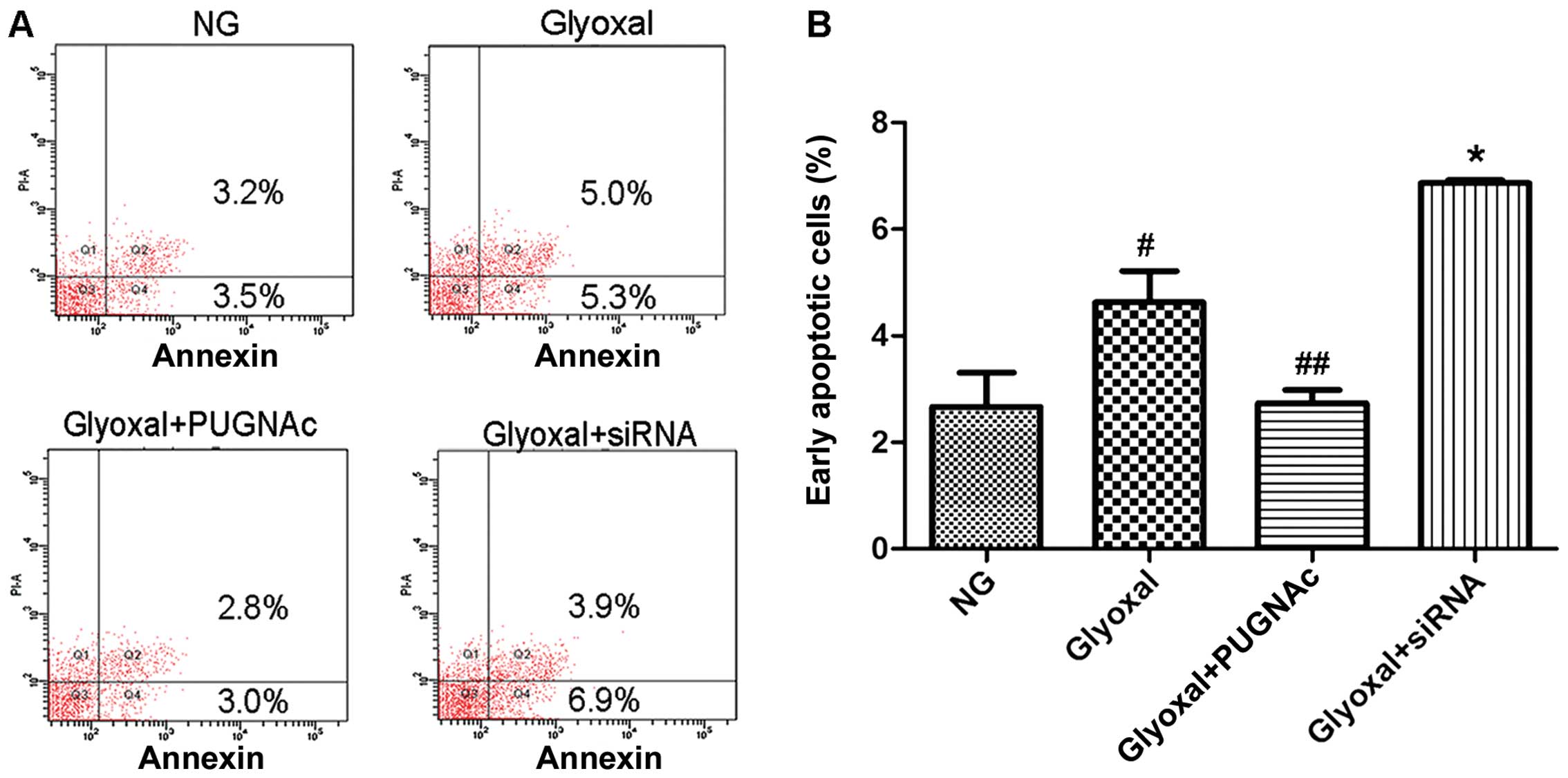
apoptosis of HRECs
O-GlcNAcylation protected the cells by attenuating
oxidative stress; thus, the effects of O-GlcNAcylation on the
apoptosis of HRECs were also investigated. Treatment with normal
glucose resulted in 3.5% apoptotic cells, which increased to 5.3%
following treatment with glyoxal (Fig. 5, P<0.005). While glyoxal
treatment alone led to apoptosis, the combination of glyoxal and
PUGNAc resulted in fewer apoptotic cells compared with glyoxal
treatment alone (Fig. 5,
P<0.001). The decrease in O-GlcNAcylation (by siRNA)
significantly increased the apoptotic rate of the HRECs (Fig. 5, P<0.001). Taking these results
into account together with the results of western blot analysis, we
hypotheiszed that O-GlcNAcylation plays a protective role against
cellular apoptosis.
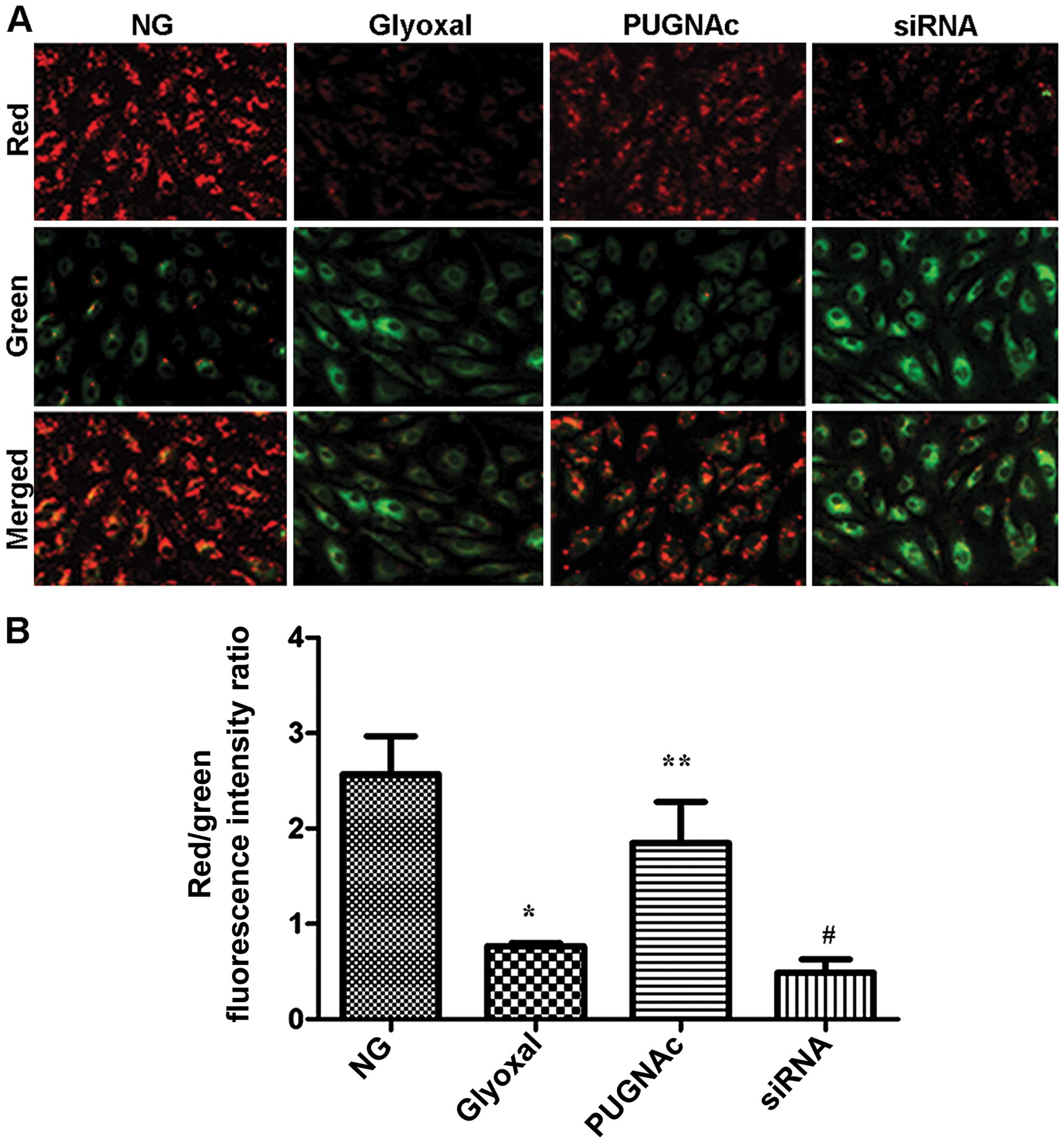
Effects of O-GlcNAcylation on
mitochondrial membrane potential
As an indicator of early apoptosis, mitochondrial
membrane potential was assessed. The effects of O-GlcNAcylation on
mitochondrial membrane potential were determined using a JC-1
probe. As shown in Fig. 6, there
was a significant increase in green fluorescence in the cells
exposed to glyoxal (Fig. 6,
P<0.001). Treatment with PUGNAc attenuated the changes in
mitochondrial membrane potential induced by glyoxal, as indicated
by a decrease in green fluorescence and the restoration of red
fluorescence (Fig. 6, P<0.01).
However, transfection with siRNA and treatment with glyoxyl
abolished the effects of O-GlcNAcylation on mitochondrial membrane
potential insignificantly (Fig.
6, P>0.05). These results suggested that the augmentation of
O-GlcNAcylation markedly suppressed the glyoxal-induced collapse of
mitochondrial membrane potential in HRECs.
Discussion
DR is a serious complication of diabetes, which can
lead to blindness (24).
O-GlcNAcylation is important in the pathogenesis of diabetes
(25,26), and recent studies have suggested
that it participates in the pathogenesis of DR (12,13). Glucose metabolism through the
hexosamine biosynthesis pathway (HBP) leads to the formation of
uridine 5′-diphos-phate-N-acetylglucosamine (UDP-GlcNAc) (8), which serves as the substrate for
post-translational modification by OGT and OGA (27,28). ROS, one of the major causes of
cellular stress, are associated with the development of DR
(6,29,30). During the early stages of DR, high
glucose levels lead to retina hypoxia (31), which causes increased
intracellular ROS levels. A previous study demonstrated that
hypoxia-inducible factor (HIF)-1α expression levels increased
significantly in the vitreous fluid of surgically treated eyes with
proliferative DR (32), which
also verified the existence of hypoxia, oxidative stress and
vascular endothelial growth factor (33). Other studies on O-GlcNAcylation
have indicated that when cells are subjected to diverse types of
stress (including oxidative stress), the levels of O-GlcNAcylation
increase (15). In our previous
study on DR, we demonstrated that the levels of O-GlcNAc increased
in vitro and in vivo (13). While O-GlcNAcylation has been
demonstrated to participate in the process of diabetic
complications, the cytoprotective effects have been confirmed in
cardiac cells (17). On the other
hand, O-GlcNAcylation has also been shown to be part of a mechanism
for the regulation of nuclear apoptosis in T cells (18). However, the exact mechanisms
responsible for controlling O-GlcNAcylation that occurs in DR
remain unclear. In the present study, we aimed to establish a
direct association between O-GlcNAcylation and ROS production in
glyoxal-damaged HRECs. AGEs or glyoxal-regulated O-GlcNAc
modifications increase the apoptosis of HRECs, suggesting that
O-GlcNAcylation is an additional mechanism through which cells
sense and respond to stress (34). In addition, the mechanisms
responsible for increasing O-GlcNAcylation in response to stress
may be the result of increasing pools of UDP-GlcNAc induced by
glucose uptake (34). Another
mechanism of increased O-GlcNAcylation is that oxidative stress
activates the HBP (35), which
has been shown to be related to cell protection in some models
(36). The reduction of oxidative
stress may be a contributory mechanism involved in the protective
effects on HRECs, indicating decreased apoptosis and increased cell
viability. It is known that the capillaries lined with endothelial
cells are responsible for maintaining the blood retinal barrier,
and the loss of endothelial cells by apoptosis is one of the most
important reasons for the development of DR. While O-GlcNAcylation
is involved in the protective effects on HRECs, it may be important
in the developmental process of DR.
The results obtained with the fluorescence
multi-well plate reader revealed that either augmented (by PUGNAc)
or diminished (by OGT siRNA) O-GlcNAcylation significantly altered
the baseline ROS levels, induced by glyoxal. Enhanced
O-GlcNAcylation was shown to reduce ROS generation in the presence
of glyoxal, while the aggravation of glyoxal-induced ROS generation
was observed following transfection with OGT siRNA. Thus, it was
concluded that O-GlcNAcylation was one of the regulatory factors
that adjusted HREC function by manipulating ROS production. To
evaluate the protective effects of O-GlcNAcylation on HRECs, we
measured caspase-3 activity, and performed CCK-8 assay, and Annexin
V and PI double staining, and measured mitochondrial membrane
potential. Total caspase-3 activity was markedly decreased in the
HRECs following exposure to glyoxal for 24 h, which indicates that
the level of cleaved caspase-3 had increased (37,38). The level of total caspase-3
increased when the cells were treated with PUGNAc compared with the
group of HRECs treated with glyoxal. However, transfection with OGT
siRNA reversed the effects of PUGNAc. We also found that both the
augmentation and decrease of O-GlcNAcylation significantly altered
cell viability in the presence of glyoxal. Following culture with
H2O2, the protective effects of
O-GlcNAcylation on cell viability were more obvious. The changes in
cell viability were consistent with the results of early apoptosis
in HRECs. Taking these results into account together with the
results of western blot anlaysis, we hypothesised that the
protective effects of O-GlcNAcylation on HRECs were achieved by
manipulating ROS production, as shown by decreased ROS generation
following treatment with PUGNAc and the aggravation of oxidative
stress following transfection with OGT siRNA. Our data confirmed
that O-GlcNAcylation attenuated ROS generation by upregulating the
activity of the antioxidant enzymes, SOD and GPX. Moreover, as a
regulator affecting the transcription of the oxidative stress
responsive enzymes, catalase and MnSOD (SOD2), FoxO1 has been shown
to be O-GlcNAcylation-modified (39). It is known that the mitochondria
are a critical target of O-GlcNAcylation-mediated cytoprotection
(40), which is associated with
ROS production and Ca2+ channels within the mitochondria
(41). Nagy et al
demonstrated that by enhancing O-GlcNAcylation with glucosamine
treatment, Ca2+ overload was blocked in neonatal
cardiomyocytes (42). However,
the mechanisms through which O-GlcNAcylation attenuates
Ca2+ overload under conditions of acute stress remain
unknown. It was considered that a mitochondrial OGT isoform existed
that interacted with the mitochondrial Ca2+ uniporter,
which was related to Ca2+ uptake (43). It has been demonstrated that the
augmentation of O-GlcNAcylation attenuates mitochondrial
permeability transition pore (mPTP) formation, while diminished
O-GlcNAcylation increases mPTP formation (43). These data support our findings
demonstrating that enhanced O-GlcNAcylation prevents the collapse
of the mitochondrial membrane potential, while diminished
O-GlcNAcylation sensitises the cells to the loss of mitochondrial
membrane potential. Based on the present data, the destruction of
antioxidant enzymes and mitochondrial membrane potential are the
reasons for ROS production; therefore, it is possible that
O-GlcNAcylation mitigates ROS formation by protecting antioxidant
enzymes and maintaining mitochondrial membrane potential. As ROS
are involved in cell apoptosis, it can be concluded that
O-GlcNAcylation prevents the apoptosis of HRECs by attenuating
oxidative stress.
The present study provides strong evidence regarding
the possible contribution of increased O-GlcNAcylation to HREC
protection, and thus, it can be concluded that the decreased ROS
generation is one of the mechanisms through which O-GlcNAcylation
reduces the apoptosis of HRECs. Understanding the molecular
mechanisms involving O-GlcNAcylation and ROS in HRECs may help to
determine the effects of O-GlcNAcylation early on in the
development of DR. Thus, regulating O-GlcNAcylation may provide an
alternative target in the treatment of DR.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China Grants (nos. 81271029
and 81300772), the Specialized Research Fund Q2 for the Doctoral
Program of Higher Education (20130072120053), and the Science and
Technology Commission of Shanghai Q3 (11Jc1409900).
References
|
1
|
Cheung N, Mitchell P and Wong TY: Diabetic
retinopathy. Lancet. 376:124–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shangari N, Bruce WR, Poon R and O'Brien
PJ: Toxicity of glyoxals - role of oxidative stress, metabolic
detoxification and thiamine deficiency. Biochem Soc Trans.
31:1390–1393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li SY, Sigmon VK, Babcock SA and Ren J:
Advanced glycation endproduct induces ROS accumulation, apoptosis,
MAP kinase activation and nuclear O-GlcNAcylation in human cardiac
myocytes. Life Sci. 80:1051–1056. 2007. View Article : Google Scholar
|
|
4
|
Al-Mesallamy HO, Hammad LN, El-Mamoun TA
and Khalil BM: Role of advanced glycation end product receptors in
the pathogenesis of diabetic retinopathy. J Diabetes Complications.
25:168–174. 2011. View Article : Google Scholar
|
|
5
|
Mohamed IN, Soliman SA, Alhusban A,
Matragoon S, Pillai BA, Elmarkaby AA and El-Remessy AB: Diabetes
exacerbates retinal oxidative stress, inflammation, and
microvascular degeneration in spontaneously hypertensive rats. Mol
Vis. 18:1457–1466. 2012.PubMed/NCBI
|
|
6
|
Santos JM, Mohammad G, Zhong Q and Kowluru
RA: Diabetic retinopathy, superoxide damage and antioxidants. Curr
Pharm Biotechnol. 12:352–361. 2011. View Article : Google Scholar :
|
|
7
|
Scalbert P and Birlouez-Aragon I:
Relationship between lens protein glycation and membrane structure
in human cataract. Exp Eye Res. 56:335–340. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buse MG: Hexosamines, insulin resistance,
and the complications of diabetes: current status. Am J Physiol
Endocrinol Metab. 290:E1–E8. 2006. View Article : Google Scholar
|
|
9
|
Younessi P and Yoonessi A: Advanced
glycation end-products and their receptor-mediated roles:
inflammation and oxidative stress. Iran J Med Sci. 36:154–166.
2011.PubMed/NCBI
|
|
10
|
Fernandes R, Hosoya K and Pereira P:
Reactive oxygen species downregulate glucose transport system in
retinal endothelial cells. Am J Physiol Cell Physiol.
300:C927–C936. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hart GW: Dynamic O-linked glycosylation of
nuclear and cytoskeletal proteins. Annu Rev Biochem. 66:315–335.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gurel Z, Sieg KM, Shallow KD, Sorenson CM
and Sheibani N: Retinal O-linked N-acetylglucosamine protein
modifications: implications for postnatal retinal vascularization
and the pathogenesis of diabetic retinopathy. Mol Vis.
19:1047–1059. 2013.PubMed/NCBI
|
|
13
|
Xu C, Liu G, Liu X and Wang F:
O-GlcNAcylation under hypoxic conditions and its effects on the
blood-retinal barrier in diabetic retinopathy. Int J Mol Med.
33:624–632. 2014.
|
|
14
|
Hart GW, Slawson C, Ramirez-Correa G and
Lagerlof O: Cross talk between O-GlcNAcylation and phosphorylation:
Roles in signaling, transcription, and chronic disease. Annu Rev
Biochem. 80:825–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zachara NE, O'Donnell N, Cheung WD, Mercer
JJ, Marth JD and Hart GW: Dynamic O-GlcNAc modification of
nucleocytoplasmic proteins in response to stress. A survival
response of mammalian cells. J Biol Chem. 279:30133–30142. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ngoh GA, Facundo HT, Zafir A and Jones SP:
O-GlcNAc signaling in the cardiovascular system. Circ Res.
107:171–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zafir A, Readnower R, Long BW, McCracken
J, Aird A, Alvarez A, Cummins TD, Li Q, Hill BG, Bhatnagar A, et
al: Protein O-GlcNAcylation is a novel cytoprotective signal in
cardiac stem cells. Stem Cells. 31:765–775. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson B, Opimba M and Bernier J:
Implications of the O-GlcNAc modification in the regulation of
nuclear apoptosis in T cells. Biochim Biophys Acta. 1840:191–198.
2014. View Article : Google Scholar
|
|
19
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: the proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar
|
|
20
|
Kowluru RA and Chan PS: Oxidative stress
and diabetic retinopathy. Exp Diabetes Res. 2007:436032007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brownlee M: The pathobiology of diabetic
complications: a unifying mechanism. Diabetes. 54:1615–1625. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caldwell RB, Bartoli M, Behzadian MA,
El-Remessy AE, Al-Shabrawey M, Platt DH, Liou GI and Caldwell RW:
Vascular endothelial growth factor and diabetic retinopathy: role
of oxidative stress. Curr Drug Targets. 6:511–524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kanwar M, Chan PS, Kern TS and Kowluru RA:
Oxidative damage in the retinal mitochondria of diabetic mice:
possible protection by superoxide dismutase. Invest Ophthalmol Vis
Sci. 48:3805–3811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tarr JM, Kaul K, Chopra M, Kohner EM and
Chibber R: Pathophysiology of diabetic retinopathy. ISRN
Ophthalmol. 2013:3435602013. View Article : Google Scholar
|
|
25
|
Slawson C, Housley MP and Hart GW:
O-GlcNAc cycling: how a single sugar post-translational
modification is changing the way we think about signaling networks.
J Cell Biochem. 97:71–83. 2006. View Article : Google Scholar
|
|
26
|
Ma J and Hart GW: Protein O-GlcNAcylation
in diabetes and diabetic complications. Expert Rev Proteomics.
10:365–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang X, Ongusaha PP, Miles PD, Havstad JC,
Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ and
Evans RM: Phosphoinositide signalling links O-GlcNAc transferase to
insulin resistance. Nature. 451:964–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanover JA, Krause MW and Love DC: The
hexosamine signaling pathway: O-GlcNAc cycling in feast or famine.
Biochim Biophys Acta. 1800:80–95. 2010. View Article : Google Scholar :
|
|
29
|
Sun J, Xu Y, Sun S, Sun Y and Wang X:
Intermittent high glucose enhances cell proliferation and VEGF
expression in retinal endothelial cells: the role of mitochondrial
reactive oxygen species. Mol Cell Biochem. 343:27–35. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Shabrawey M and Smith S: Prediction of
diabetic retinopathy: role of oxidative stress and relevance of
apoptotic biomarkers. EPMA J. 1:56–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kennedy A and Frank RN: The influence of
glucose concentration and hypoxia on VEGF secretion by cultured
retinal cells. Curr Eye Res. 36:168–177. 2011. View Article : Google Scholar
|
|
32
|
Loukovaara S, Koivunen P, Inglés M,
Escobar J, Vento M and Andersson S: Elevated protein carbonyl and
HIF-1α levels in eyes with proliferative diabetic retinopathy. Acta
Ophthalmol. 92:323–327. 2014. View Article : Google Scholar
|
|
33
|
Izuta H, Matsunaga N, Shimazawa M,
Sugiyama T, Ikeda T and Hara H: Proliferative diabetic retinopathy
and relations among antioxidant activity, oxidative stress, and
VEGF in the vitreous body. Mol Vis. 16:130–136. 2010.PubMed/NCBI
|
|
34
|
Moley KH and Mueckler MM: Glucose
transport and apoptosis. Apoptosis. 5:99–105. 2000. View Article : Google Scholar
|
|
35
|
Du XL, Edelstein D, Rossetti L, Fantus IG,
Goldberg H, Ziyadeh F, Wu J and Brownlee M: Hyperglycemia-induced
mitochondrial superoxide overproduction activates the hexosamine
pathway and induces plasminogen activator inhibitor-1 expression by
increasing Sp1 glycosylation. Proc Natl Acad Sci USA.
97:12222–12226. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pang Y, Hunton DL, Bounelis P and Marchase
RB: Hyperglycemia inhibits capacitative calcium entry and
hypertrophy in neonatal cardiomyocytes. Diabetes. 51:3461–3467.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao L, Yan X, Shi J, Ren F, Liu L, Sun S
and Shan B: Ethanol extract of Forsythia suspensa root induces
apoptosis of esophageal carcinoma cells via the mitochondrial
apoptotic pathway. Mol Med Rep. 11:871–880. 2015.
|
|
38
|
Watanabe J, Nakamachi T, Ohtaki H,
Naganuma A, Shioda S and Nakajo S: Low dose of methylmercury (MeHg)
exposure induces caspase mediated-apoptosis in cultured neural
progenitor cells. J Toxicol Sci. 38:931–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Housley MP, Udeshi ND, Rodgers JT,
Shabanowitz J, Puigserver P, Hunt DF and Hart GW: A
PGC-1alpha-O-GlcNAc transferase complex regulates FoxO
transcription factor activity in response to glucose. J Biol Chem.
284:5148–5157. 2009. View Article : Google Scholar :
|
|
40
|
O'Rourke B: Mitochondrial ion channels.
Annu Rev Physiol. 69:19–49. 2007. View Article : Google Scholar
|
|
41
|
Feissner RF, Skalska J, Gaum WE and Sheu
SS: Crosstalk signaling between mitochondrial Ca2+ and
ROS. Front Biosci (Landmark Ed). 14:1197–1218. 2009. View Article : Google Scholar
|
|
42
|
Nagy T, Champattanachai V, Marchase RB and
Chatham JC: Glucosamine inhibits angiotensin II-induced cytoplasmic
Ca2+ elevation in neonatal cardiomyocytes via
protein-associated O-linked N-acetylglucosamine. Am J Physiol Cell
Physiol. 290:C57–C65. 2006. View Article : Google Scholar
|
|
43
|
Ngoh GA, Watson LJ, Facundo HT and Jones
SP: Augmented O-GlcNAc signaling attenuates oxidative stress and
calcium overload in cardiomyocytes. Amino Acids. 40:895–911. 2011.
View Article : Google Scholar :
|