Introduction
Lung cancer is the main leading cause of
cancer-related fatality worldwide, with a rapidly increasing rate
in China and other Asian countries (1–4).
Chemotherapy is currently the most frequently used treatment for
lung cancer and other types of cancer. However, while this method
of treatment kills cancer cells, it also destroys some normal
cells. Thus, the identification of novel natural compounds with low
toxicity and high selectivity for killing cancer cells is a
significant area in cancer research (5), and several natural products have
been used as alternative treatments for cancer (6,7).
Curcumin, a natural and crystalline compound
isolated from the plant Curcuma longa, has been widely
studied for its anti-inflammatory, antiangiogenic, antioxidant and
anticancer effects in Chinese systems of medicine (8,9).
Additionally, previous studies have shown that curcumin exhibits
antiproliferative and anticarcinogenic properties in a wide variety
of cell lines and animals (10,11). Previous studies have demonstrated
that curcumin inhibits the growth and apoptosis of human A549 lung
adenocarcinoma cells (12–14).
However, the mechanisms of curcumin-induced apoptosis via oxidative
stress remain unclear.
The physiological status of the reactive oxygen
species (ROS) levels can regulate cell proliferation: When
intracellular ROS levels are above a certain threshold, ROS inhibit
the cell cycle, leading to increased cell apoptosis and necrosis.
In the tumor cells it often maintains a higher state of oxidation,
with higher levels of oxygen free radicals and lower levels of the
antioxidant enzyme activity. This higher oxidation state can
activate certain transcription factors and associated genes, such
as NF-κB and API, thus, ensuring the survival,
proliferation and migration of tumor cells. Certain research has
shown that the antitumor function of curcumin occurs by regulation
of the intracellular redox state (15,16). In tumor cells, low concentrations
of curcumin inhibit cell proliferation, with no induction of
apoptosis. However, intracellular ROS are slightly decreased and
the level of superoxide dismutase (SOD) is elevated, which causes
lipid peroxidation in the presence of other factors to suppress
malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE). In high
concentrations of curcumin, cellular ROS declines rapidly and the
SOD level increases, along with a significant increase of apoptosis
products (17,18).
Heat-shock proteins (HSP) are necessary proteins
under physiological conditions that are upregulated in response to
stress (particularly heat stress). Higher expression of HSP70 was
identified in tumor cells compared with normal cells and was
closely associated with histological types of lung cancer and
prognosis (19). High expression
of HSP70 may be required to maintain the stability of tumor cells
through regulation of gene expression and immune response (20). Under conditions of oxidative
stress, ionizing radiation and heat-shock stress, HSP70 expression
increases, and subsequently participates in the process of the
B-cell lymphoma 2 (Bcl-2) family to regulate tumor cell apoptosis
(21). HSP70, as a molecular
chaperone, changes the conformation of the Bcl-2-associated X
protein (Bax) gene to prevent cellular apoptosis. Upon exposure to
HSP70 inhibitors, the tumor cells undergo apoptosis (22).
These findings suggest that HSPs are closely
associated with ROS in the process of tumor cell apoptosis.
Following the disruption of the high expression level of ROS and
HSP70 in the tumor cells, the cells undergo apoptosis. Although
numerous studies have confirmed that curcumin induces apoptosis via
the mitochondrial pathway (23,24), the signaling pathways for the
ROS-mediated mitochondrial apoptotic cell death triggered by
curcumin still remain unclear, particularly in lung cancer
cells.
In the present study, the molecular mechanisms of
the effects of curcumin on the induction of apoptosis of the human
A549 non-small cell lung cancer cell line were examined. The data
indicate that the initiation of curcumin-induced apoptotic
signaling involves decreased ROS, HSP70 and mitochondrial membrane
potential (MMP). These findings aid in elucidating the mechanisms
of curcumin-induced apoptosis and may contribute to the development
of a novel drug based on curcumin alone or in combination
therapies.
Materials and methods
Reagents and cell lines
Curcumin (C21H20O6)
was purchased from Sigma Chemical (St. Louis, MO, USA). Propidium
iodide (PI) and Annexin V-fluorescein isothiocyanate (FITC) were
purchased from BD Pharmingen (Minneapolis, MN, USA). HSP70, Bcl-2,
Bax, caspase-3, caspase-9, cleaved caspase-3, cleaved caspase-9,
total c-Jun N-terminal kinase (JNK), p38 and extracellular
signal-regulated kinase (ERK), and phosphorylated JNK, p38 and ERK
were purchased from Cell Signaling Technology (Danvers, MA, USA).
U0126 (ERK inhibitor), SB203580 (p38 inhibitor) and SP600125 (JNK
inhibitor) were from Sigma Chemical. Dichloro-dihydro-fluorescein
diacetate (DCFH-DA) was obtained from Beyotime Institute of
Biotechnology (Jiangsu, China). MDA and 4-HNE were obtained from
Nanjing Jiancheng Bioengineering Institute (Jiangsu, China).
MitoTracker was obtained from Molecular Probes (Eugene, OR, USA).
Fetal bovine serum (FBS), RPMI-1640 and penicillin-streptomycin
were obtained from Thermo Fisher Scientific (Waltham, MA, USA).
A549 human lung carcinoma cells were obtained from the Shanghai
Cell Center of Chinese Academy of Sciences.
Cell culture
The A549 cell line was cultured in RPMI-1640
supplemented with 10% FBS and was grown in a humidified atmosphere
with 5% CO2 at 37°C.
Cell growth inhibition assay
Cell proliferation was measured by cell counting and
MTT assays. Cells were seeded in 96-well plates with
2×104 cells/well. After 24 h, the cells were incubated
with different concentrations of curcumin for different times.
Following incubation, MTT solution at 20 µl/well [5 mg/ml in
phosphate-buffered saline (PBS)] was added and cells were incubated
for an additional 5 h. The medium was aspirated and replaced with
150 µl/well dimethyl sulfoxide (DMSO) to dissolve the
formazan salt. The color intensity of the formazan solution, which
reflects the cell growth condition, was measured at 570 nm using a
microplate reader (BioTek Instruments, Winooski, VT, USA). In the
presence of antioxidants, the cells were washed with PBS and
evaluated by the MTT assay.
Flow cytometry
For detection of apoptosis by fluorescence-activated
cell sorting, ~25×104 cells/ml in 6-cm plates were
treated with various concentrations of curcumin. The cells were
harvested and used for FITC-conjugated Annexin V and PI staining
for 5 min at room temperature. The stained cells were analyzed by
flow cytometry (BD Biosciences, Minneapolis, MN, USA) to determine
the percentages of apoptotic cells.
Electron microscopy
A549 cells were trypsinized and fixed in ice-cold
2.5% electron microscopy grade glutaraldehyde in PBS (pH 7.3). The
specimens were rinsed with PBS, post-fixed in 1% osmium tetroxide
with 0.1% potassium ferricyanide, dehydrated through a graded
series of ethanol (30–90%), and embedded in Epon. Semi-thin (300
nm) sections were cut using a Reichart Ultracut, stained with 0.5%
toluidine blue, and examined under a light microscope (Olympus,
Tokyo, Japan). Ultra-thin sections (65 nm) were stained with 2%
uranyl acetate and Reynold's lead citrate, and examined on a
transmission electron microscope (magnification, ×5,000).
Measurement of MMP
A549 cells were seeded in 6-well plates with
30×104 cells/well. After 24-h incubation, cells were
treated with curcumin (20 µM). After 24 h, cells were
incubated with 5 µM JC-1 fluorescent dye for 30 min at 37°C
in the dark, and were subsequently washed with PBS. MMP was
evaluated qualitatively under a fluorescence microscope (Olympus
IX81) using a 568 nm filter.
Immunofluorescence analysis
Cells were incubated with 10 nM MitoTracker Green
for 1 h. Cells were subsequently cytospinned on glass slides,
washed twice with PBS, fixed with 4% para-formaldehyde for 10 min,
permeabilized with 0.3% Triton for 10 min and incubated with PBS
containing 10% bovine serum albumin (BSA) for 1 h at room
temperature. Primary antibodies diluted in PBS containing 1% BSA
(1/200 for anti-cytochrome c antibody; Cell Signaling
Technology) were incubated with cells at 4°C overnight. Cells were
washed and incubated with the appropriate fluorescent secondary
antibody. Cells were washed twice in PBS and stained with
4′,6-diamidino-2-phenylindole. Fluorescent images were obtained on
a Zeiss Axioplan microscope (Carl Zeiss AG, Oberkochen, Germany)
and 10 sections of each preparation were scanned.
Determination of ROS, SOD, MDA and 4-HNE
levels
The effects of curcumin on intracellular ROS
generation were evaluated by DCFH-DA fluorescence assay. In brief,
A549 cells were co-incubated with different concentrations of
curcumin for various times, and were subsequently incubated with
DCFH-DA at a final concentration of 10 µM at 37°C for 30
min. Cells were harvested, washed three times with PBS and were
suspended in PBS (1×106 cells/ml), and the fluorescence
intensity was measured on a fluorescence reader (BD Biosciences,
Franklin Lakes, NJ, USA). Relative DCF fluorescence intensity of
treated cells was expressed as percentage of the control (as
100%).
SOD activity was determined by specific assay kits
purchased from Beyotime Institute of Biotechnology, according to
the manufacturer's instructions. In brief, following curcumin
treatment, cells were lysed in cell lysis buffer. Cell lysates (100
µg/well) were evaluated for SOD activity, and SOD activity
was expressed as a percentage of the control.
MDA activity was measured by the assay kit (Nanjing
Jiancheng Bioengineering Institute) and performed according to the
manufacturer's instructions. Briefly, curcumin-treated cells were
harvested and lysed in cell lysis buffer and MDA activity was
evaluated. MDA activity was determined by comparison with the
standard curve prepared from predetermined MDA standards.
4-HNE, a biomarker of oxidative stress, was examined
using an ELISA kit (Nanjing Jiancheng Bioengineering Institute).
Briefly, 4-HNE protein adducts present in the sample or standard
were probed with the 4-HNE primary antibody, followed by incubation
with a horseradish peroxidase-conjugated secondary antibody. The
4-HNE protein adduct content in the unknown sample was determined
by comparison with the standard curve prepared from predetermined
HNE-BSA standards.
Western blot analysis
Cells were seeded in 60-mm dishes and incubated with
different concentrations of curcumin for different times. Proteins
were extracted in lysis buffer, supplemented with 1 mM
phenylmethylsulfonyl fluoride, protease inhibitor and phosphatase
inhibitors. Total proteins (25 µg) were electrophoresed on
10% polyacrylamide gels and transferred to nitrocellulose
membranes. Membranes were blocked with 5% dried skimmed milk in
Tris-buffered saline/Tween-20 buffer for 1 h at room temperature.
The membranes were incubated with primary antibodies at 1:1,000
dilution in 5% non-fat milk overnight at 4°C, and secondary
antibodies (1:5,000) were subsequently incubated for 2 h at room
temperature. Protein bands were visualized with the enhanced
chemiluminescence method. The results were corrected for protein
loading by normalization for β-actin expression and quantified by
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
All data are presented as mean ± standard deviation.
Statistical analysis of the results was performed by one-way
analysis of variance followed by the SPSS statistical package (SPSS
13.0 for Windows; SPSS, Inc., Chicago, IL, USA). For all the
statistical analyses, P<0.05 or P<0.01 were considered to
indicate a statistically significant difference.
Results
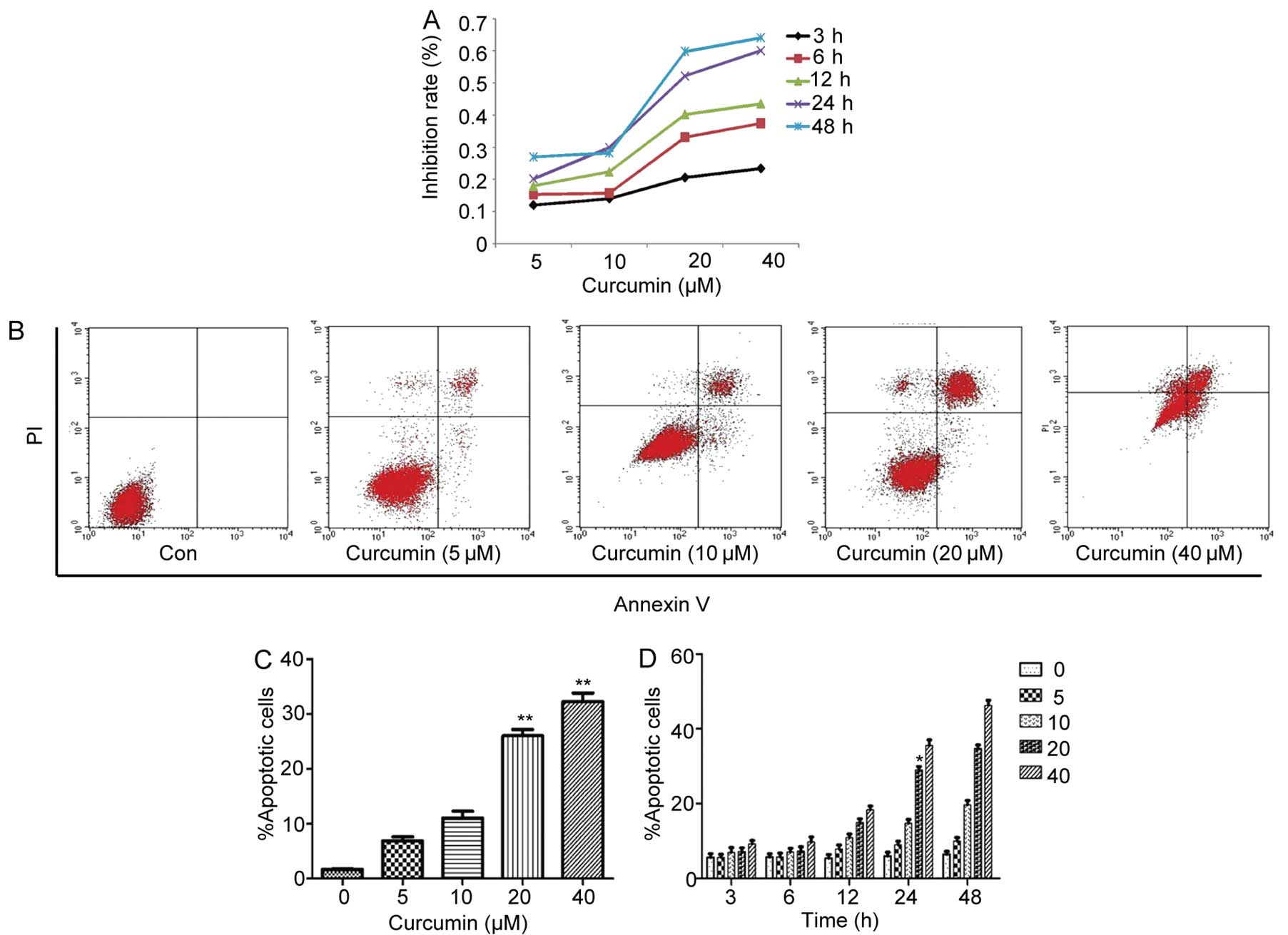
Curcumin inhibits cell growth of A549
cells
To evaluate the growth inhibitory effects of
curcumin, A549 cells were treated with curcumin for various times
and viable cells were measured by the MTT assay. A decrease in cell
viability was observed in response to curcumin treatment in a time-
and dose-dependent manner (Fig.
1A).
Curcumin induces the apoptosis of A549
cells
To determine whether the growth inhibition by
curcumin was associated with apoptosis, the degree of apoptosis was
examined by PI and Annexin V staining through flow cytometric
analysis. As shown in Fig. 1B and
C, curcumin caused a significant inhibition of cell
proliferation in a concentration-dependent manner. When cells were
treated with curcumin (5–40 µM) for 24 h, the proportion of
AV+/PI− (apoptotic cells) was increased from
1.65% of control cells to 26.58% of the group treated with 20
µM curcumin (P<0.05), the 40 µM curcumin group was
increased by ~30.63% (P<0.05). In addition, the percentage of
apoptotic cells increased in a time- and dose-dependent manner. As
shown in Fig. 1D, no clear
difference in the number of AV+/PI−
(apoptotic cells) was observed under relatively short incubation
conditions (3 and 6 h). After 12-h treatment, however, the
proportion of apoptotic cells slowly increased. Apoptotic cells
were significantly elevated in 20 µM curcumin-treated cells,
from 5.7% in untreated cells to 29.06% after 24-h treatment. These
results suggest that curcumin-induced apoptosis has a dose- and
time-dependent association.
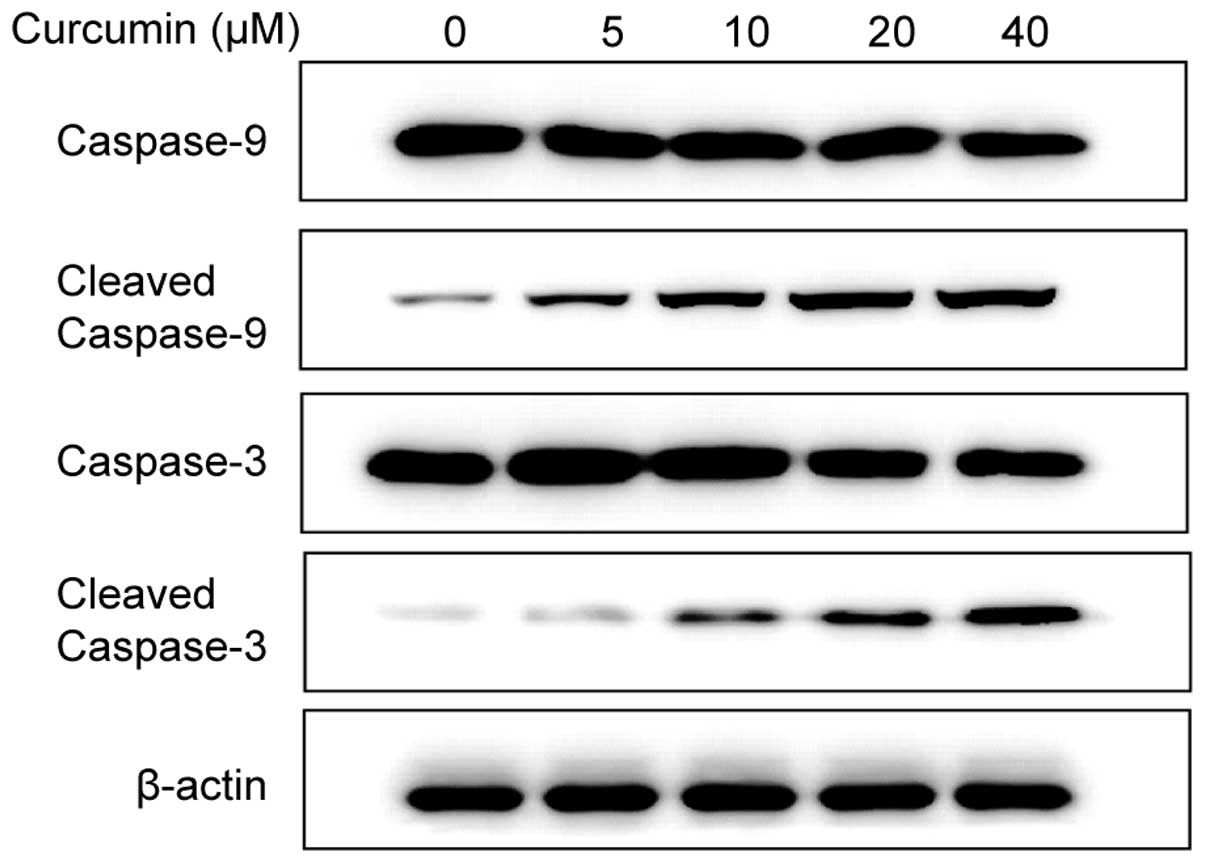
Involvement of caspase activation in the
curcumin-induced apoptotic effect
Caspase activation is a significant event in the
proteolytic cascade elicited by apoptotic stimuli, particularly
caspase-3, which is an effector caspase that has a critical role in
cell death induced by a variety of stimuli. To determine the
mechanism by which curcumin treatment trigger A549 cell apoptosis,
the protein levels of cleaved caspase-9 and -3 were examined by
western blot analyses. The result suggested that caspase-9 and -3
were activated in a dose-dependent manner in curcumin-treated cells
(Fig. 2).
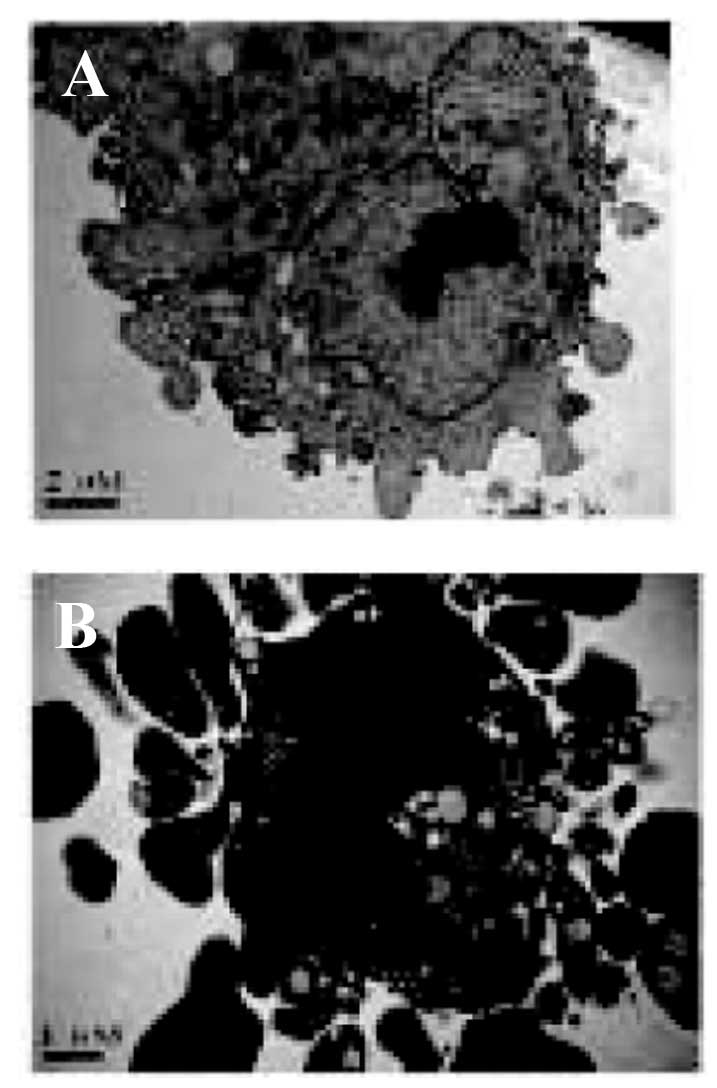
Curcumin causes changes to the
morphological features of apoptosis
The morphological characteristics of A549 cells
treated with curcumin were further examined by electron microscopy.
As shown in Fig. 3, the control
cells exhibited an intact nuclear structure, while cells treated
with curcumin showed morphological changes that are characteristic
of apoptosis, including disappearance of mitochondrial cristae,
cell shrinkage, chromatin aggregation containing a half-moon of
condensed chromatin, and appearance of membrane blebbing and
numerous apoptotic bodies.
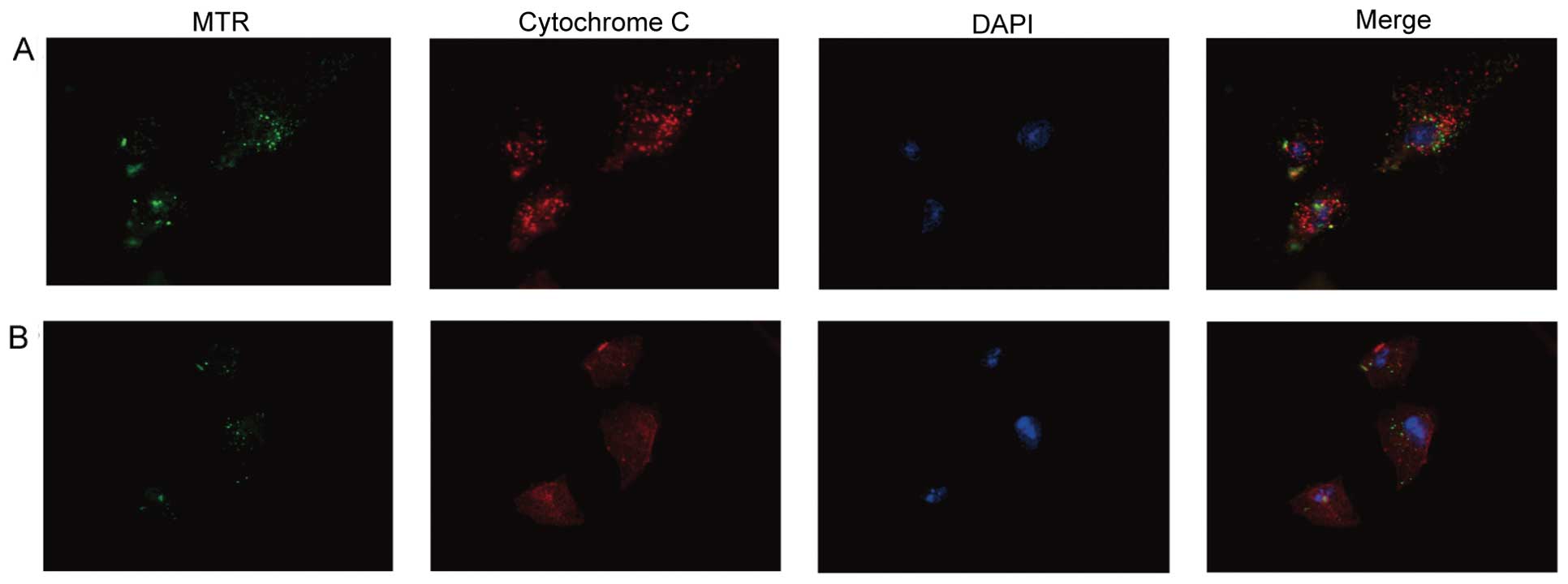
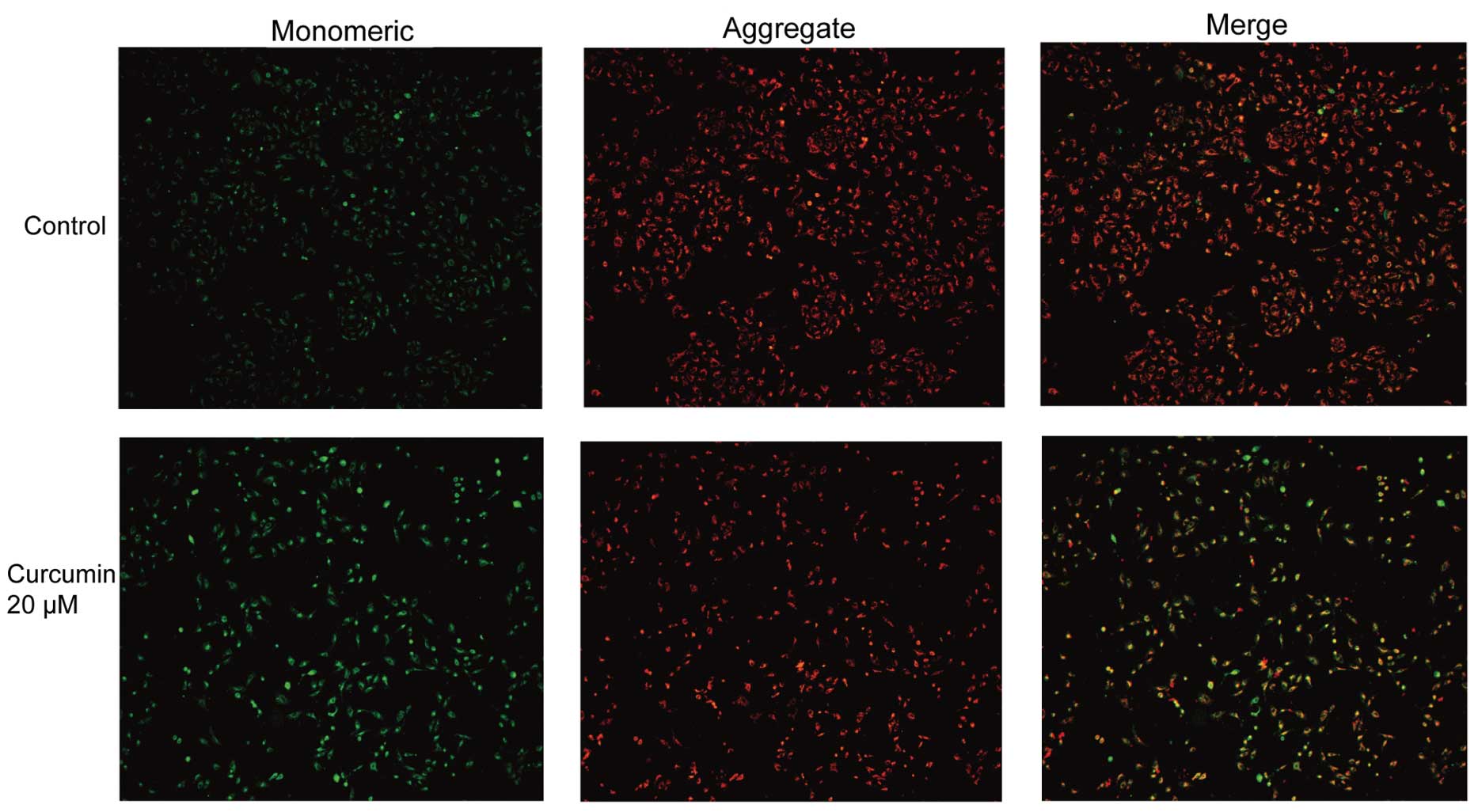
Curcumin induces cytochrome c
translocation
Cytochrome c release from mitochondria is a
critical event promoting the intrinsic death pathway by apoptotic
protease activating factor 1 (Apaf-1)-mediated caspase-3 activation
and apoptosis. The effects of curcumin on cytochrome c
translocation were subsequently examined. In DMSO-treated control
A549 cells, cytochrome c exhibited punctate cytoplasmic
staining in agreement with its localization in mitochondria, as
evidenced by yellow-orange staining from merging with MitoTracker
Green staining (Fig. 4A). Upon
treatment with curcumin, the yellow-orange staining was markedly
abolished. A large fraction of curcumin-treated cells exhibited red
fluorescence, indicating cytochrome c release from the
mitochondria into the cytosol (Fig.
4B). These data suggested that curcumin treatment of A549 cells
increased the level of cytochrome c in the cytosol with a
concomitant decreased level of cytochrome c in the
mitochondria.
Effects of curcumin on MMP
To further confirm the observations regarding the
induction of apoptosis of A549 cells by curcumin, A549 cells were
exposed to 5–40 µM curcumin for 24 h and were subsequently
stained with JC-1, which is used to assess the integrity of the
MMP. Cellular mitochondria are stained red when their membranes are
intact and polarized, and exhibit green fluorescence when the
membranes are depolarized. Fluorescence microscopic evaluation of
JC-1-stained control cells showed heterogeneous staining of the
cytoplasm with red and green fluorescence coexisting in the same
cell (Fig. 5). This staining was
consistent with mitochondrial localization. In the control group,
the red fluorescence was distributed throughout the cytoplasm.
Cells treated with curcumin showed marked changes in MMP, such as
the disappearance of red fluorescence and increase of green
fluorescence in the majority of cells. These data indicate that
curcumin induces loss of MMP.
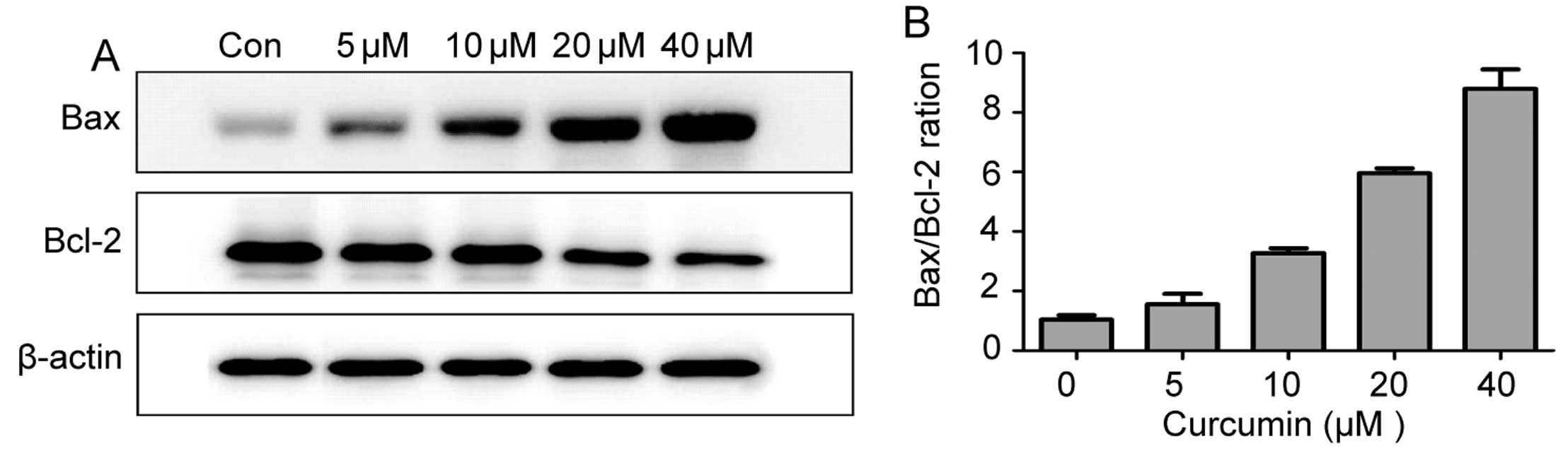
Effect of curcumin on the expression of
the Bcl-2 family of proteins
To further investigate the mechanisms underlying
curcumin-induced apoptosis in lung cancer cells, the apoptotic
proteins Bcl-2 and Bax were analyzed by western blot analysis. As
shown in Fig. 6A, the
proapoptotic protein Bax was increased following treatment with
curcumin for 24 h, whereas antiapoptotic protein Bcl-2 levels
decreased. Therefore, an increase in the Bax/Bcl-2 ratio may be
involved in apoptosis induced by curcumin (Fig. 6B).
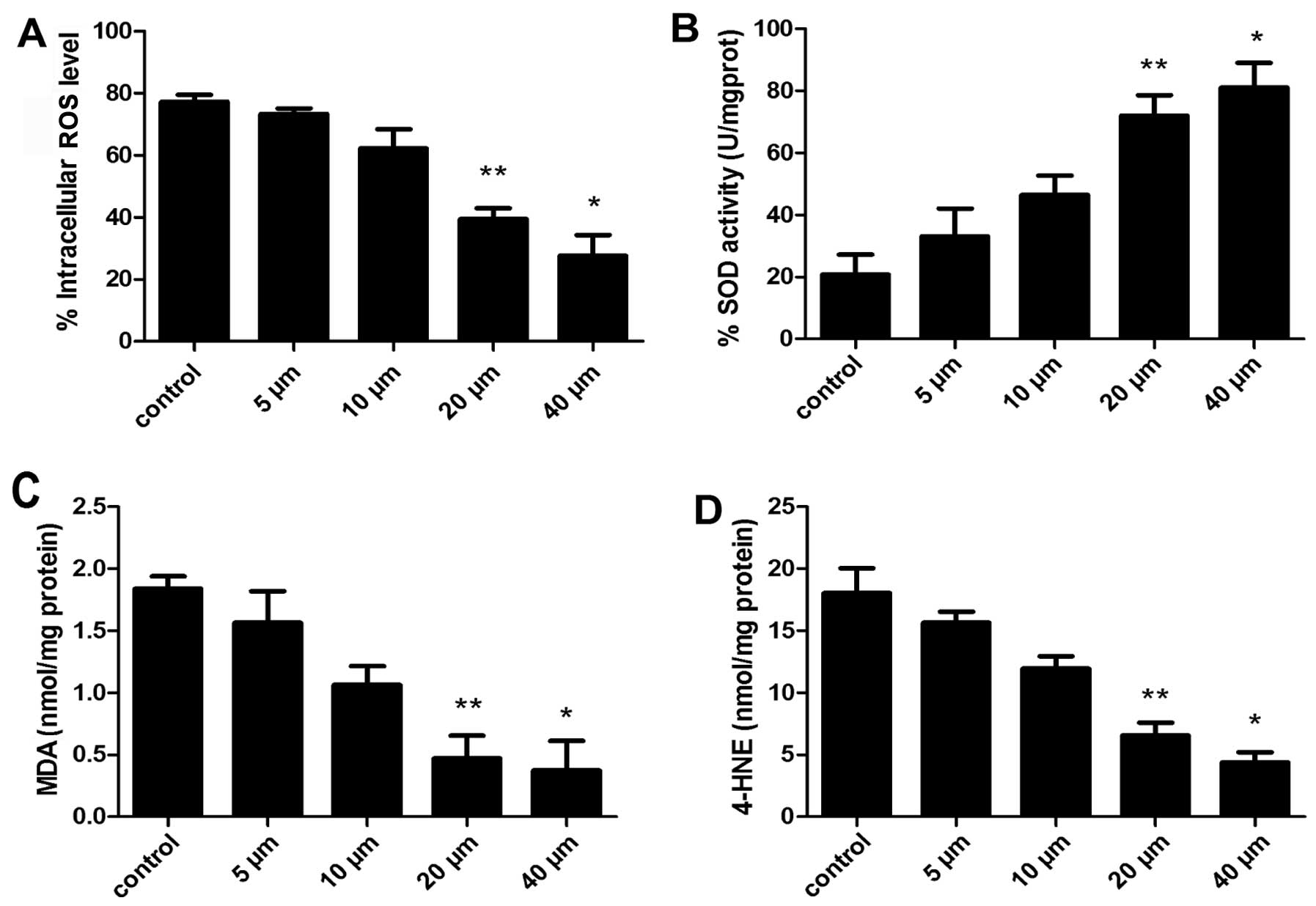
Effects of curcumin on the levels of
intracellular ROS, SOD, MDA and 4-HNE
ROS are important signaling molecules that have a
role in gene expression, proliferation and apoptosis, as well as
oxygen sensing in various cell types. To explore the possible
mechanisms by which curcumin induced the apoptosis of A549 cells,
cells were exposed to 5–40 µM curcumin and ROS activity was
evaluated using a DCF kit. Changes in DCF fluorescence were
detected by flow cytometric analysis. The proportion of cells with
lower fluorescence intensity was decreased in cells exposed to 20
µM curcumin for 24 h (Fig.
7A), indicating that curcumin significantly decreased the level
of ROS in a dose-dependent manner.
Subsequently, the effects of curcumin were examined
on SOD levels. As shown in Fig.
7B, exposure of A549 cells to 5–40 µM curcumin for 24 h
had a significant increase of intracellular SOD levels compared
with the control following 20 and 40 µM treatment
(P<0.05, P<0.01, respectively).
To evaluate the role of oxidative stress on
mitochondrial function, the effect of lipid peroxidation (MDA and
4-HNE) induced by curcumin in A549 cells was evaluated. After 24 h
of exposure to curcumin, the degree of lipid peroxidation was
determined by measuring MDA in cells. As illustrated in Fig. 7C, curcumin treatment reduced MDA
levels below baseline values compared with the control cells
(P<0.05).
Another biomarker of oxidative stress, the lipid
peroxidation marker 4-HNE (14,28), was examined. As shown in Fig. 7D, A549 cells treated with 5–40
µM curcumin for 24 h had significantly lower levels of 4-HNE
protein adducts (P<0.05).
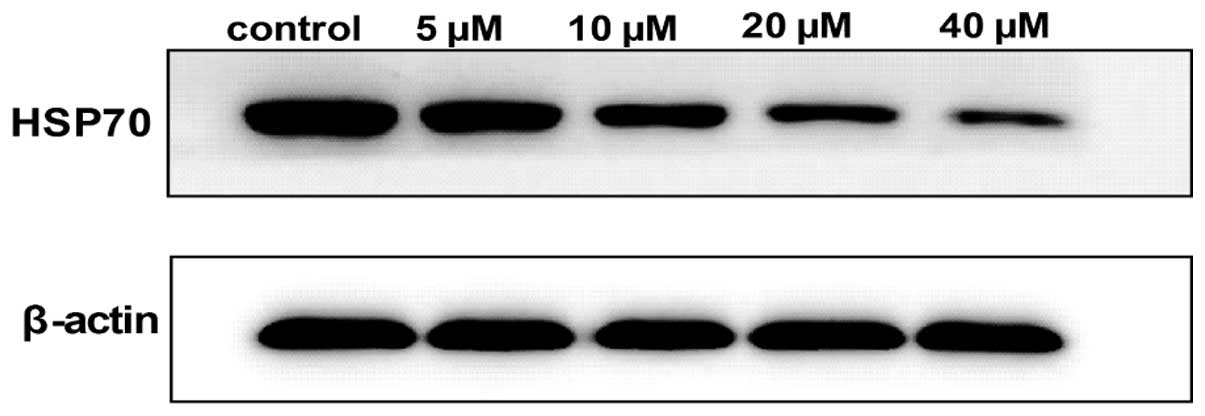
Roles of HSP70 in curcumin-induced cell
apoptosis
The conclusion drawn thus far is that
curcumin-induced A549 cell apoptosis has a relative association
with oxidative stress. To further investigate whether oxidative
stress was involved in the curcumin-induced cell death, the level
of HSP70 was analyzed by western blot analysis. A549 cells were
exposed to various concentrations of curcumin (5–40 µM) for
24 h, and the cells were lysed and analyzed for the expression of
proteins as indicated. Notably, as shown in Fig. 8, the expression of HSP70 was
inhibited in a dose-dependent manner.
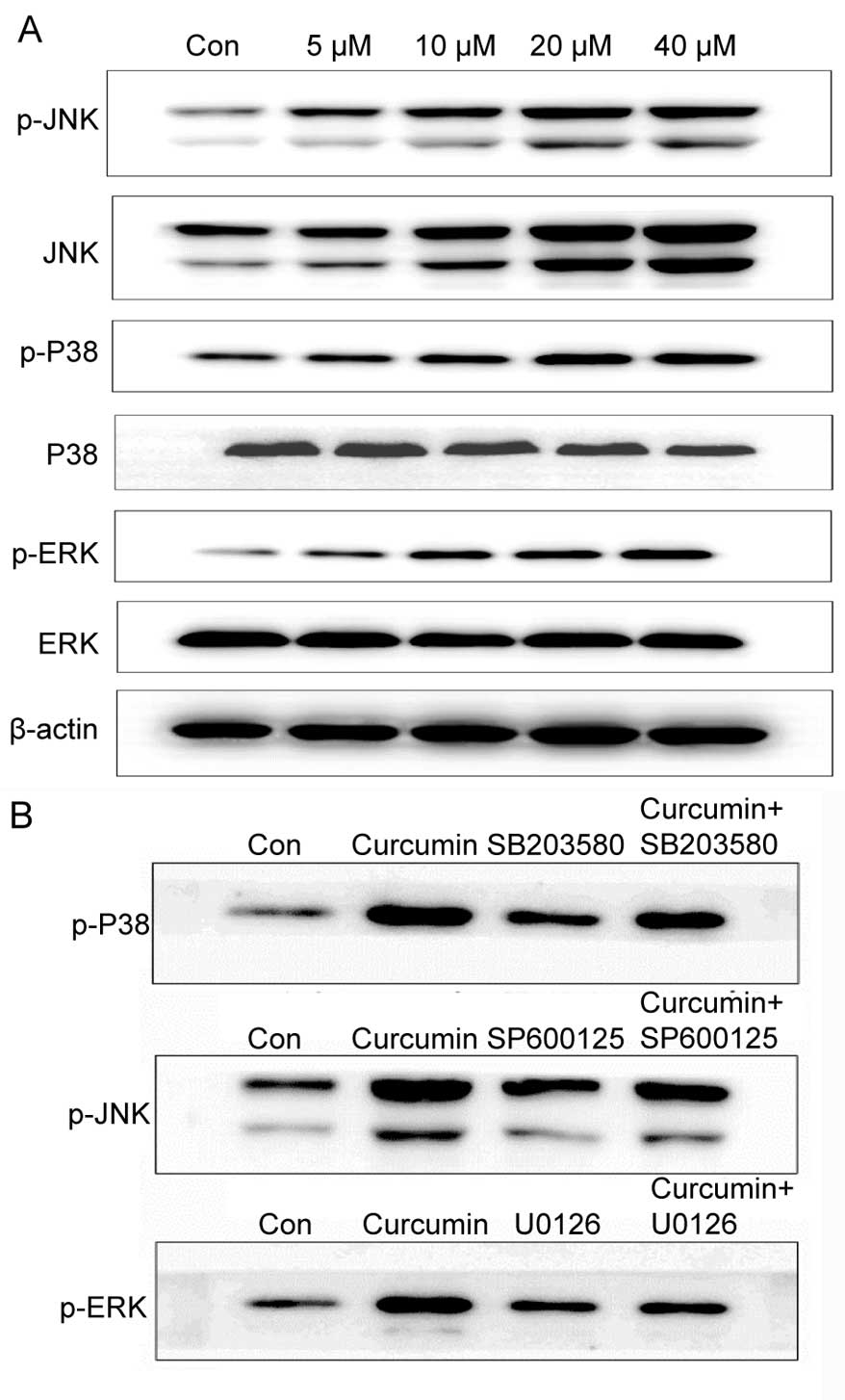
Role of MAPK signaling in
curcumin-induced cell death
MAPK pathways have a vital role in signal
transduction of extracellular stimuli to the nucleus and subsequent
activation of gene transcription. The MAPK signaling cascade can be
activated by oxidative stress, which affects cell proliferation and
apoptosis. When the redox balance is disturbed, HSP70 activates JNK
phosphorylation, and the MAPK signaling pathway is activated,
triggering a series of downstream signal responses. To examine
whether curcumin-mediated apoptosis is involved in the MAPK
signaling pathways, the levels of total and phosphorylated ERK, p38
and JNK protein were examined in A549 cells treated with 5–40
µM curcumin for 24 h by western blot analysis. With the
increase of concentrations of curcumin, the activation of
phosphorylated ERK, JNK and p38 proteins was also enhanced,
whereas, total ERK, p38 and JNK protein had no significant changes
(Fig. 9A). These data suggest
that the MAPK signaling pathway is involved in curcumin-mediated
apoptosis. To investigate whether these kinases were required for
curcumin-induced A549 cell apoptosis, the effects of MAPK
inhibitors on cellular apoptosis and MAPK activation were examined.
Cells were preincubated with U0126 (ERK inhibitor), SB203580 (P38
inhibitor) or SP600125 (JNK inhibitor) for 1 h, and subsequently
exposed to 20 µM curcumin for 24 h. As shown in Fig. 9B, p38 phosphorylation was reduced
in cells pretreated for 1 h with 20 µM SB203580. Similarly,
JNK phosphorylation was also reduced in cells pretreated with 20
µM SP600125 (Fig. 9B).
However, ERK phosphorylation was increased in cells pretreated for
1 h with 20 µM U0126. These results demonstrate that
curcumin induces the activation of p38, JNK and ERK, indicating
that the MAPK pathway is involved in curcumin-induced apoptosis of
A549 cells.
Discussion
Multiple studies have shown that curcumin promotes
anti-tumor effects by induction of apoptosis (25,26). However, the proapoptotic role of
curcumin and underlying mechanism remains unknown. In the present
study, curcumin not only inhibited cell proliferation of A549 cells
by MTT assay, but also induced apoptosis in a dose- and
time-dependent manner. A concentration of 20 µM curcumin
exhibited significant growth inhibition in A549 cells. As evidence
of apoptosis, cells treated with 20 µM curcumin showed cell
shrinkage with numerous apoptotic bodies compared with untreated
cells as observed by electron microscopy.
Two general pathways are involved in apoptosis,
including the death receptor-mediated extrinsic and
mitochondria-mediated intrinsic pathways (27). The mitochondria-mediated apoptotic
pathway is characterized by loss of MMP and release of cytochrome
c from mitochondria into the cytoplasm (28). In the present study, the
mitochondrial-specific cationic dye, JC-1, was used to confirm the
loss of MMP upon exposure to curcumin, and an immunofluorescence
assay showed cytochrome c translocation, occurring in a
dose-dependent manner. Therefore, curcumin induced the apoptosis of
A549 cells through mitochondria-mediated apoptotic pathways.
Oxidative stress is a physiological state in the
body, and when the body suffers from a variety of harmful
stimulation, highly reactive molecules, such as active oxygen free
radicals (ROS) and reactive nitrogen free radicals, are generated
in large quantities and the redox balance is broken. These changes
induced apoptosis and even tissue damage through the mitochondria
signaling pathway, endoplasmic reticulum stress pathway and death
receptor pathway (29–31). Oxidative stress is closely
associated with cell apoptosis. The physiological state of the ROS
level participates in the regulation of cell proliferation: When
intracellular ROS levels are above a certain threshold, ROS inhibit
the cell cycle, leading to DNA rupture, cell apoptosis and
necrosis. A higher state of oxidation is often maintained in the
tumor cells, this higher oxidation state activate certain
transcription factors and associated genes, such as NF-κB
and API, thus, ensuring the survival, proliferation and
migration of tumor cells. In the present study, curcumin affected
intracellular oxidative stress, decreased ROS and increased SOD
activity, further reducing the generation of MDA and 4-HNE
(32). These changes trigger the
intrinsic apoptotic pathway: HSP70 expression is restrained, the
proapoptotic protein Bax and antiapoptotic protein Bcl-2 are
activated, MMP is lost, cytochrome c is released into the
cytoplasm, the caspase-9/3 classical apoptotic pathway is
activated, and eventually cells die (33,34). When cells were pretreated with the
HSP70 inhibitor, the indicators of the redox state did not show any
change, however, MMP, cytochrome c and the Bax/Bcl-2 ratio
were significantly changed, and cells were prevented from
undergoing cell apoptosis. Accordingly, oxidative stress is the
upstream signal of HSP70, and the Bcl-2 family is the downstream
point of HSP70, and subsequently it activates cellular apoptosis
via the mitochondrial signaling pathway.
HSPs are a group of proteins that can rapidly
respond to any physical changes, including high temperature, metal,
drugs, poisoning and oxidative stress (35). HSP70 is one of the most important
members of the HSP family, and is closely associated with tumor
cell apoptosis (36). HSP70 has a
high expression level in tumor cells (particularly malignant tumor
cells), and has an important role of antitumor cell apoptosis
through participating in the process of gene regulation and immune
response. Certain studies have shown that HSP70 is expressed highly
under stress conditions, such as heat shock and oxidative stress
and subsequently activate the Bc1-2 family protein, compete Apaf-1
with caspase-9, thus, causing the inactivation of caspase-9, which
affects the cytoplasm of the caspase cascade and inhibits
apoptosis. The study by Stankiewicz et al (37) suggested that HSP70 induced tumor
cells apoptosis mainly by blocking heat stress, inhibiting the
activity of Bax and protecting the release of apoptotic initiation
factors from mitochondria. Therefore, the anti-apoptosis function
of HSP70 is indicated by regulation of the Bcl-2 family activity
(38). In the present study,
curcumin regulated the intracellular redox state to suppress the
activity of HSP70, thus, inducing cells apoptosis through
activating the mitochondrial apoptotic pathways.
The MAPK signaling pathways are important signal
transduction pathways in eukaryotic cells that mediate signal
transduction from the cell surface to the nucleus. These pathways
are closely associated with cell proliferation, survival,
differentiation, apoptosis and other physiological processes
(39). There are three groups of
MAPK pathways: The ERK1/2 MAPK, P38MAPK and JNK/SAPK MAPK families.
The ERK1/2 MAPK family is mainly associated with cell
differentiation and proliferation (40). The JNK and P38 family mainly
participate in the regulation of cell apoptosis (41,42). Certain studies showed that excess
ROS can interfere with a variety of signaling pathways, and all the
MAP kinases exhibit sensitivity to ROS and oxidizing agents
(43,44). The MAPK signaling cascade can be
activated by oxidative stress, which affects cell proliferation and
apoptosis. Numerous studies have stated that HSP70 mediated cell
apoptosis through inhibition of JNK phosphorylation. When the redox
balance is disturbed, HSP70 activates JNK phosphorylation, and the
MAPK signaling pathway is activated, triggering a series of
downstream signal responses (45). In the present study,
phosphorylated JNK and p38 were increased in response to curcumin,
whereas ERK was reduced in a dose-dependent manner. The present
results showed that curcumin induced the activation of p38, JNK and
ERK, and the MAPK signaling pathway was involved in the induction
of apoptosis of A549 cells.
In conclusion, the present findings indicated that
curcumin inhibited the proliferation of A549 cells by inducing
apoptosis in a dose- and time-dependent manner. Additionally,
curcumin induced apoptosis in A549 cells via oxidative
stress-dependent mitochondria and MAPK signaling pathways. These
results suggest the potential of curcumin as a promising candidate
for lung cancer therapy.
Acknowledgments
The present study was supported by a grant from the
Laboratory of Cancer Epigenetics, Biomedical Research Center, Sir
Run Run Shaw Hospital, School of Medicine, Zhejiang University and
Zhejiang Provincial Natural Science Foundation of China (grant no.
64212006). The authors would like to thank the Laboratory of Cancer
Epigenetics, Biomedical Research Center, Sir Runrun Shaw Hospital,
School of Medicine, Zhejiang University and Zhejiang Provincial Key
Laboratory of Gastroenterology for providing the experimental
facilities, instruments and guidance.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: Globocan 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Chiang CJ, Chen YC, Chen CJ, You SL and
Lai MS; Taiwan Cancer Registry Task Force: Cancer trends in Taiwan.
Jpn J Clin Oncol. 40:897–904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the human
development index (2008–2030): a population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conforti F and Menichini F: Phenolic
compounds from plants as nitric oxide production inhibitors. Curr
Med Chem. 18:1137–1145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Satia JA, Littman A and Slatore CG:
Associations of herbal and specialty supplements with lung and
colorectal cancer risk in the VITamins and Lifestyle study. Cancer
Epidemiol Biomarkers Prev. 18:1419–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cassileth BR, Deng GE and Gomez JE:
Complementary therapies and integrative oncology in lung cancer:
ACCP evidence-based clinical practice guidelines (2nd edition).
Chest. 132(3 Suppl): 340S–354S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guzman-Villanueva D, El-Sherbiny IM,
Herrera-Ruiz D and Smyth HD: Design and in vitro evaluation of a
new nanomicroparticulate system for enhanced aqueous-phase
solubility of curcumin. Biomed Res Int. 2013:7247632013. View Article : Google Scholar
|
|
9
|
Xu P, Yao Y, Guo P, Wang T, Yang B and
Zhang Z: Curcumin protects rat heart mitochondria against
anoxia-reoxygenation induced oxida tive injury. Can J Physiol
Pharmacol. 91:715–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CC, Sureshbabul M, Chen HW, Lin YS,
Lee JY, Hong QS, Yang YC and Yu SL: Curcumin suppresses metastasis
via Sp-1, FAK inhibition, and E-cadherin upregulation in colorectal
cancer. Evid Based Complement Alternat Med.
2013:5416952013.PubMed/NCBI
|
|
11
|
Ahn JC, Kang JW, Shin JI and Chung PS:
Combination treatment with photodynamic therapy and curcumin
induces mitochondria-dependent apoptosis in AMC-HN3 cells. Int J
Oncol. 41:2184–2190. 2012.PubMed/NCBI
|
|
12
|
Chen QY, Wu LJ, Wu YQ, Lu GH, Zhan JW,
Jiang ZY, Yan J and Zhou JY: Molecular mechanism of trifluoperazine
induces apoptosis in human A549 lung adenocarcinoma cell lines. Mol
Med Rep. 2:811–817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Qi HW and Wu CG: Research of
anti-proliferation of curcumin on A549 human lung cancer cells and
its mechanism. Zhong Yao Cai. 27:923–927. 2004.In Chinese.
|
|
14
|
Tian DZ, Zhu H and Liang YJ: Effects and
mechanisms of curcuminon apoptosis of lung adenocarcinoma A549
cells. Chin J Clin Pharmacol. 15:8–10. 2006.
|
|
15
|
Chen QY and Wang YY: Curcumin induces
apoptosis in human lung adenocarcinoma A549 cells through a
reactive oxygen species-dependent mitochondrial signaling pathway.
Oncol Rep. 23:397–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li PM, Li YL, Liu B and Wang WJ: Curcumin
inhibits MHCC97H liver cancer cells by activating ROS/TLR-4/caspase
signaling pathway. Asian Pac J Cancer Prev. 5:2329–2334. 2014.
View Article : Google Scholar
|
|
17
|
Gopal PK, Paul M and Paul S: Curcumin
induces caspase mediated apoptosis in JURKAT cells by disrupting
the redox balance. Asian Pac J Cancer Prev. 15:93–100. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaushik G, Kaushik T and Yadav SK:
Curcumin sensitizes lung adenocarcinoma cells to apoptosis via
intracellular redox status mediated pathway. Indian J Exp Biol.
50:853–861. 2012.
|
|
19
|
Fuenzalida K, Quintanilla R, Ramos P,
Piderit D, Fuentealba RA, Martinez G, Inestrosa NC and Bronfman M:
Peroxisome Proliferator-activated receptor gamma upregulates the
Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial
stabilization and protection against oxidative stress and
apoptosis. J Biol Chem. 282:37006–37010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XH, Qin Y, Hu MH and Xie Y: Dendritic
cells pulsed with hsp70-peptide complexes derived from human
hepatocellular carcinoma induce specific anti-tumor immune
responses. World J Gastroenterol. 11:5614–5620. 2005.PubMed/NCBI
|
|
21
|
Arya R, Mallik M and Lakhotia SC: Heat
shock genes-integating cell survival and death. J Biosci.
32:595–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stankiewicz AR, Lachapelle G, Foo CP,
Radicioni SM and Mosser DD: Hsp70 inhibits heat-induced apoptosis
upstream of mitochondria by preventing Bax translocation. J Biol
Chem. 280:38729–38739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mishra S, Kapoor N, Mubarak Ali A,
Pardhasaradhi BV, Kumari AL, Khar A and Misra K: Differential
apoptotic and redoxregulatory activities of curcumin and its
derivatives. Free Radic Biol Med. 38:1353–1360. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Skommer J, Wlodkowic D and Pelkonen J:
Cellular foundation of curcumin induced apoptosis in follicular
lymphoma cell lines. Exp Hematol. 34:463–474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu DJ, Chen XW, Wang JZ and Ju YL:
Proteomic analysis identifies proteins associated with
curcumin-enhancing efficacy of irinotecan-induced apoptosis of
colorectal cancer LOVO cell. Int J Clin Exp Pathol. 7:1–15.
2013.
|
|
26
|
Shehzad A, Lee J and Lee YS: Curcumin in
various cancers. Biofactors. 39:56–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lv ZD, Liu XP and Zhao WJ: Curcumin
induces apoptosis in breast cancer cells and inhibits tumor growth
in vitro and in vivo. Int J Clin Exp Pathol. 7:2818–2824.
2014.PubMed/NCBI
|
|
28
|
Xue X, Yu JL, Sun DQ, Kong F, Qu XJ, Zou
W, Wu J and Wang RM: Curcumin induces apoptosis in SGC-7901 gastric
adenocarcinoma cells via regulation of mitochondrial signaling
pathways. Asian Pac J Cancer Prev. 15:3987–3992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siddique YH, Naz F and Jyoti S: Effect of
curcumin on lifespan, activity pattern, oxidative stress, and
apoptosis in the brains of transgenic Drosophila model of
Parkinson's disease. Biomed Res Int. 2014:6069282014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fuenzalida K, Quintanilla R, Ramos P,
Piderit D, Fuentealba RA, Martinez G, Inestrosa NC and Bronfman M:
Peroxisome proliferator-activated receptor gamma up-regulates the
Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial
stabilization and protection against oxidative stress and
apoptosis. J Biol Chem. 282:37006–37015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wall NR, Mohamm RM and AI-Katib AM:
Bax:Bcl-2 ratio modulation by bryostatin 1 and novel antitubulin
agents is important for susceptibility to drug induced apoptosis in
the human early pre-B acute lymphoblastic leukemia cell line, Reh.
Leuk Res. 23:881–888. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su JY, Lai HQ and Chen JP: Natural
borneol, a monoterpenoid compound, potentiates
selenocystine-induced apoptosis in human hepatocellular carcinoma
cells by enhancement of cellular uptake and activation of
ROS-mediated DNA damage. PLoS One. 8:635–642. 2013.
|
|
33
|
Heath-Engel HM and Shore GC: Mitochondrial
membrane dynamics, cristae remodelling and apoptosis. Biochim
Biophys Acta. 1763:549–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang SK, Cha SH and Jeon HG:
Curcumin-induced histone hypoacetylation enhances
caspase-3-dependent glioma cell death and neurogenesis of neural
progenitor cells. Stem Cells. 5:165–174. 2006. View Article : Google Scholar
|
|
35
|
Westerheide SD and Morimoto RI: Heat shock
response modulators as therapeutic tools for diseases of protein
conformation. J Biol Chem. 280:33097–33100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang YG, Wang YM and Li QF: Expression
significance of HLA-DR antigen and heat shock protein 70 in
hepatocellular carcinoma. World J Gastroenterol. 9:11392001.
|
|
37
|
Stankiewicz AR, Livingstone AM, Mohseni N
and Mosser DD: Regulation of heat-induced apoptosis by Mcl-1
degradation and its inhibition by Hsp70. Cell Death Differ.
16:638–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schmitt E, Maingret L, Puig PE, Rerole AL,
Ghiringhelli F, Hammann A, Solary E, Kroemer G and Garrido C: Heat
shock protein 70 neutralization exerts potent antitumor effects in
animal models of colon cancer and melanoma. Cancer Res.
66:4191–4197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Poddar R and Paul S: Novel crosstalk
between ERK MAPK and p38 MAPK leads to homocysteine-NMDA receptor
mediated neuronal cell death. J Neurochem. 124:558–570. 2013.
View Article : Google Scholar :
|
|
40
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
down-regulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rane MJ, Song Y, Jin S, Barati MT, Wu R,
Kausar H, Tan Y, Wang Y, Zhou G, Klein JB, et al: Interplay between
Akt and p38 MAPK pathways in the regulation of renal tubular cell
apoptosis associated with diabetic nephropathy. Am J Physiol Renal
Physiol. 298:F49–F61. 2010. View Article : Google Scholar :
|
|
42
|
Tarapore RS, Yang Y and Katz JP: Restoring
KLF5 in esophageal squamous cell cancer cells activates the JNK
pathway leading to apoptosis and reduced cell survival. Neoplasia.
15:472–480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Son Y, Cheong YK and Kim NH:
Mitogen-activated protein kinases and reactive oxygen species: how
can ROS activate MAPK pathways? J Signal Transduct.
2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Z, Jiang H, Wan Y, Bi C and Yuan Y:
H2O2-induced secretion of tumor necrosis
factor-α evokes apoptosis of cardiac myocytes through reactive
oxygen species-dependent activation of p38 MAPK. Cytotechnology.
64:65–73. 2012. View Article : Google Scholar :
|
|
45
|
Ha YJ, Seul HJ and Lee JR: Ligation of
CD40 receptor in human B lymphocytes triggers the 5-lipoxygenase
pathway to produce reactive oxygen species and activate p38 MAPK.
Exp Mol Med. 43:101–110. 2011. View Article : Google Scholar : PubMed/NCBI
|