Introduction
As sugar-binding proteins, lectins are highly
specific for their sugar moieties and ability to agglutinate cells
(1). Lectins are widely
distributed in microorganisms, viruses and animals that are capable
of mediating a series of biological recognition events (2–4).
Lectins from plants have been well studied, showing severe
pro-inflammatory effects in vivo and in vitro
(5–7).
Certain plant lectins are highly toxic in clinical
and animal experiments, accompanied by neutrophil migration at
inflammatory sites. Neutrophil migration into inflamed tissues is
an important hallmark of acute inflammation, and resident
macrophages have a critical role in triggering immune response and
in inducing neutrophil infiltration at the early stage of damage
(8–14). Inflammatory response is a complex
phenomenon that involves vascular and cellular events. In cellular
events, lymphocyte recirculation depends on the response to a cell
surface signal (15–17).
Arum maculatum agglutinin, a plant lectin,
exhibits pro-inflammatory activity and induces neutrophil migration
by stimulating resident macrophages (18). Furthermore, our previous study
revealed that certain lectins from plants in the Araceae family
also had such pro-inflammatory effects, and that a lectin purified
from Arisaema erubescens (Wall.) Schott induced rat paw
edema and neutrophil migration, possibly via the release of
inflammatory mediators from macrophages (19).
Pinellia ternata (PT) (Araceae) has been
widely used as a traditional Chinese medicine to treat excessive
phlegm and emesis. The raw material has a throat-irritating
toxicity, which can be alleviated by being immersed in alum
solution or boiled for a long time. Such toxicity is associated
with PT lectin (PTL) (20).
PTL was isolated to continue the study on the
pro-inflammatory effects of lectins from the Araceae family. In
addition, the pro-inflammatory effects of PTL on the activation of
macrophages, and the possible involvement of PTL-stimulated
resident macrophages in neutrophil recruitment were evaluated,
aiming to understand the toxicity mechanism of PTL and to provide
evidence for detoxification of PT.
Materials and methods
Animals
Male Institute of Cancer Research (ICR) mice
obtained from the animal facilities of Nanjing Medical University
(Nanjing, Jiangsu, China) were housed (n=5) in a
temperature-controlled room and received water and food ad
libitum. Animal welfare and experimental procedures were
strictly in accordance with the Guide for the Care and Use of the
Laboratory Animals (US National Research Council, 1996) and the
associated ethics regulations of Nanjing University of Chinese
Medicine (Nanjing, Jiangsu, China).
Plant material
The tubers of PT were collected from Jiangsu
Province (China) with the help of Taizhou Gaogang Pieces Factory
Co., Ltd., (Jiangsu, China) on May 25, 2012. The fresh plant was
identified by Professor Chungen Wang (Nanjing University of Chinese
Medicine).
Apparatus, chemicals and reagents
Mini protean cell and semi-dry transfer cell was
supplied by Bio-Rad Laboratories (Hercules, CA, USA). HiPrep™
Phenyl FF, HiTrap™ Q FF, HiTrap™ desalting and AKTA purifier were
supplied by GE Healthcare (Uppsala, Sweden). Scanning electron
microscope (SEM) was supplied by Nikon (Tokyo, Japan). Fluorescence
microplate reader was supplied by BioTek Instruments, Inc.
(Winooski, VT, USA). RPMI-1640 was from Gibco (Waltham, MA, USA).
FCS was from Sijiqing (Hangzhou, China). Tumor necrosis factor-α
(TNF-α), interleukin (IL)-1β and IL-6 ELISA kits were from
eBioscience (San Diego, CA, USA). Transwell 24-well sets and 12-
and 48-well plates were from Corning Inc. (Corning, NY, USA).
Dextran T-500 and Percoll were from Pharmacia (Piscataway, NJ,
USA). 2′,7′-Dichlorofluorescein-diacetate (DCFH-DA) was from
Nanjing Jiancheng Chemical Industrial Co., Ltd. (Nanjing, China).
Anti-GAPDH antibody (ab9485) and anti-NF-κB p65 (ab16502)
antibodies were from Abcam (Cambridge, UK). Horseradish peroxidase
(HRP)-labeled secondary antibody was from Hangzhou Lianke Biology
Technology Ltd. (Hangzhou, China). The ECL chemiluminescence
detection kit was from Millipore (Burlington, MA, USA). Distilled
water was produced in the EPED Superpure water purifying system
(Nanjing EPED Science and Technology Development Co., Nanjing,
China). All the reagents were at least analytical grade.
Extraction and purification of PTL
Fresh tubers of PT weighing 50 g were washed
thoroughly with tap water and subsequently with distilled water.
The combined roots and tubers were separated from the stems and
leaves prior to crushing using a juice extractor to extract the
juice and centrifugation for 20 min at 4°C. The clear supernatant
was dissolved with saturated
(NH4)2SO4 (pH 7.0) and was
centrifuged for 30 min at 4°C. The precipitate was fully dissolved
with 0.6 mol/l (NH4)2SO4 (pH 7.0)
and centrifuged at a high speed. The resulting supernatant obtained
was applied to a HiPrep™ Phenyl FF (10 ml) column. The mobile phase
was a 0.6-0 mol/l (NH4)2SO4
gradient at a flow rate of 1.4 ml/min. The main peak was collected
and applied to a column of HiTrap™Q FF. The column was eluted with
a gradient of 0–0.4 mol/l NaCl at a flow rate of 2.5 ml/min.
Finally, the main peak was desalted with 0.02 mol/l Tris-HCl (pH
8.0) buffer for lectin purification and subsequently
freeze-dried.
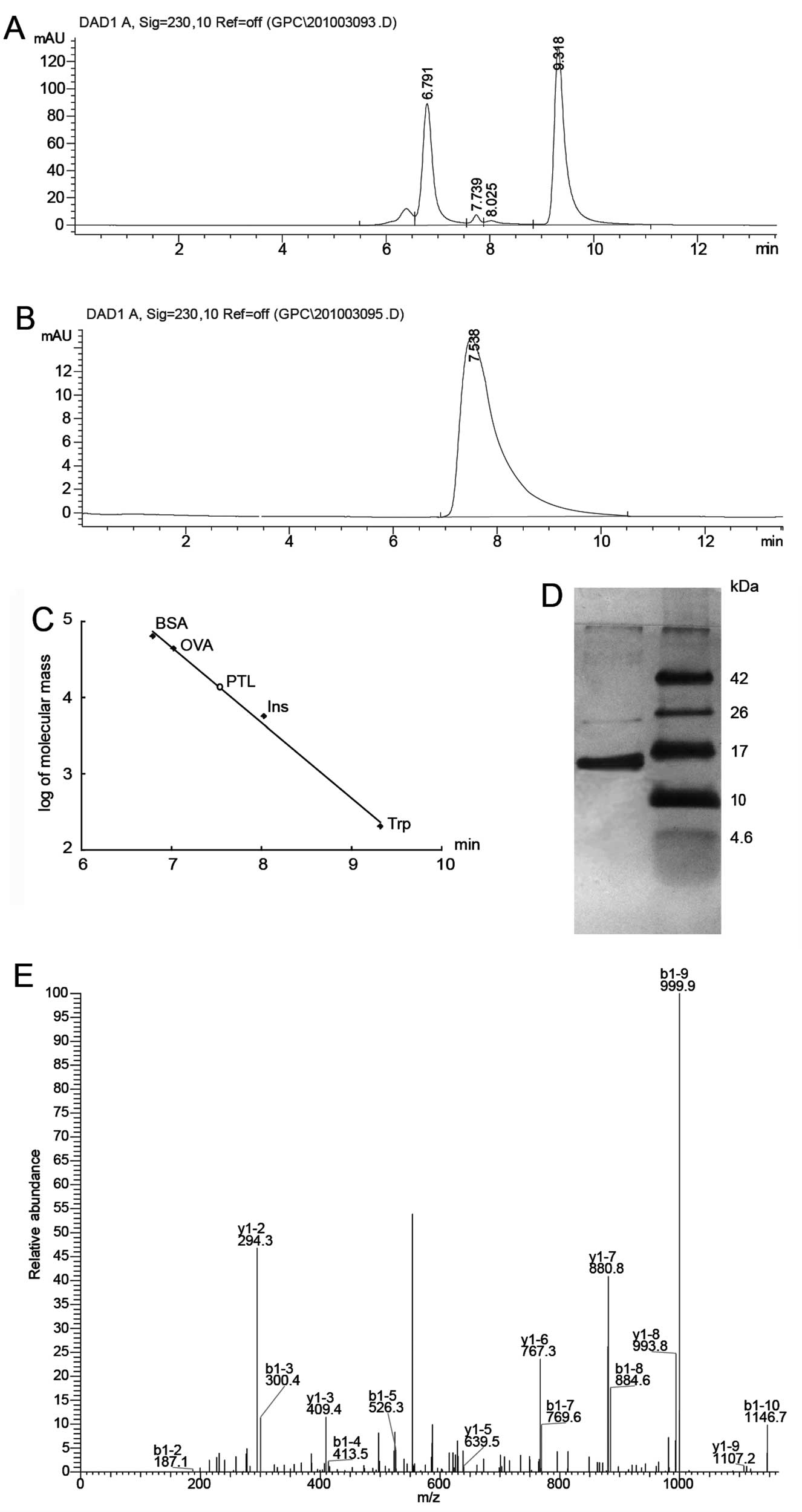
Size exclusion
chromatography-high-performance liquid chromatography (HPLC), 1D
SDS-PAGE and ingel digestion
Purified lectin preparation was checked on an
Agilent 1200 system equipped with a DAD detector using an Agilent
Zorbax GF-450 column (9.4×250 mm). The mobile phase was 0.1 mol/l
phosphate buffer at a flow rate of 1.5 ml/min. PTL was also
subjected to SDS-PAGE (pH 8.3), using a 15% (w/v) acrylamide slab
gel for subunit molecular mass determination. The sample was heated
for 5 min in a boiling water bath. The gel was stained with silver
nitrate. Molecular weights of the standard marker proteins were
matched with that of the sample protein (4.6–42.0 kDa) to determine
the subunit molecular weight of PTL.
The 12-kDa protein band was destained with 400
µl of 30% acetonitrile in 100 mM
NH4HCO3, reduced with 10 µl of 100 mM
dithiothreitol at 56°C for 30 min, dehydrated with 100 µl of
acetonitrile, alkylated with 30 µl of 200 mM iodoacetamide
at room temperature in the dark for 20 min, dehydrated with 100
µl of acetonitrile, and subsequently dried. In-gel tryptic
digestion was carried out at 37°C for 20 h using a 10.0 ng/ml
solution of sequencing grade-modified trypsin (Promega, Madison,
WI, USA). Tryptic peptides resulting from the digestion were
extracted with 100 µl of 60% acetonitrile in 0.1% formic
acid for 15 min. The extraction was repeated 3 times. The extracted
peptides were dried and reconstituted with 5% acetonitrile and 0.1%
formic acid in water.
LC-mass spectrometry (MS)/MS and database
search
The extracted peptides were analyzed using an LC-LTQ
system (LTQ VELOS, Thermo Finnigan, San Jose, CA, USA). Briefly,
peptides were first enriched on a reverse-phase trap column (Zorbax
300SB-C18 peptide traps; Agilent Technologies,
Wilmington, DE, USA) and subsequently eluted to an analytical
column (RP-C18, 0.15 × 150 mm; Column Technologies Inc.,
Lombard, IL, USA). The flow rate of the pump was 0.15 ml/min. The
mobile phases used for the reverse phase were (A) 0.1% formic acid
in 84% ACN and (B) 0.1% formic acid in water, pH 3.0. The gradient
started at 4% solvent A, where it was held for 4 min, went linearly
to 50% solvent A in 15 min, and subsequently went linearly to 100%
solvent A in 3 min. An electrospray ionization mass spectrometer
was used for peptide detection. The positive-ion mode was employed
and the spray voltage was set at 3.2 kV. The spray temperature was
set at 200°C for peptides. Collision energy was set automatically
by the LTQ system. The mass spectrometer was set as one full MS
scan followed by 10 MS/MS scans on the most intense ions. Protein
identification using MS/MS raw data was performed with the BioWorks
Browser 3.3 (University of Washington, Seattle, WA, USA; licensed
to Thermo Finnigan) searching program against the UniProt Arecaceae
protein database. The protein identification criteria that were
used were based on Delta CN (≥0.1) and Xcorr (one charge ≥1.9, two
charges ≥2.2, three charges ≥3.75).
Isolation of mouse peritoneal
macrophages
Animals were sacrificed and sterilized in 75%
alcohol. Each mouse was injected intraperitoneally with 4 ml of
cold phosphate-buffered saline (PBS) and massaged softly for 3 min.
Harvested peritoneal fluid was centrifuged at 70 × g for 4 min and
the precipitate was dissolved and adjusted to 1×106
cells/ml with RPMI-1640 containing 10% fetal bovine serum (FBS).
Peritoneal cells were subsequently cultured in a 48-well plate and
washed 3 times with cold PBS after incubation at 37°C for 2 h.
Macrophage purity was examined with non-specific esterase staining
as >98%. The viability of cells was >99%, as determined by
the trypan blue exclusion test.
Isolation of human venous blood
neutrophils
Blood from healthy volunteers (5 ml) was drawn into
vacuum blood collection tubes containing EDTA-2Na. The blood was
mixed with cold PBS and Dextran T-500 (1.5:1.5:1, v/v/v) at room
temperature until sedimentation of the red blood cells. The
supernatant was collected 30 min later and centrifuged at 110 × g
for 10 min. The precipitate was subsequently centrifuged together
with 75% Percoll and 60% Percoll at 250 × g for 20 min. Following
centrifugation, neutrophils were purified from the blood cells.
This procedure usually produced a cell fraction containing over 99%
neutrophils, as determined by quick Giemsa staining. The trypan
blue exclusion test showed that the viability of cells was >99%
(21).
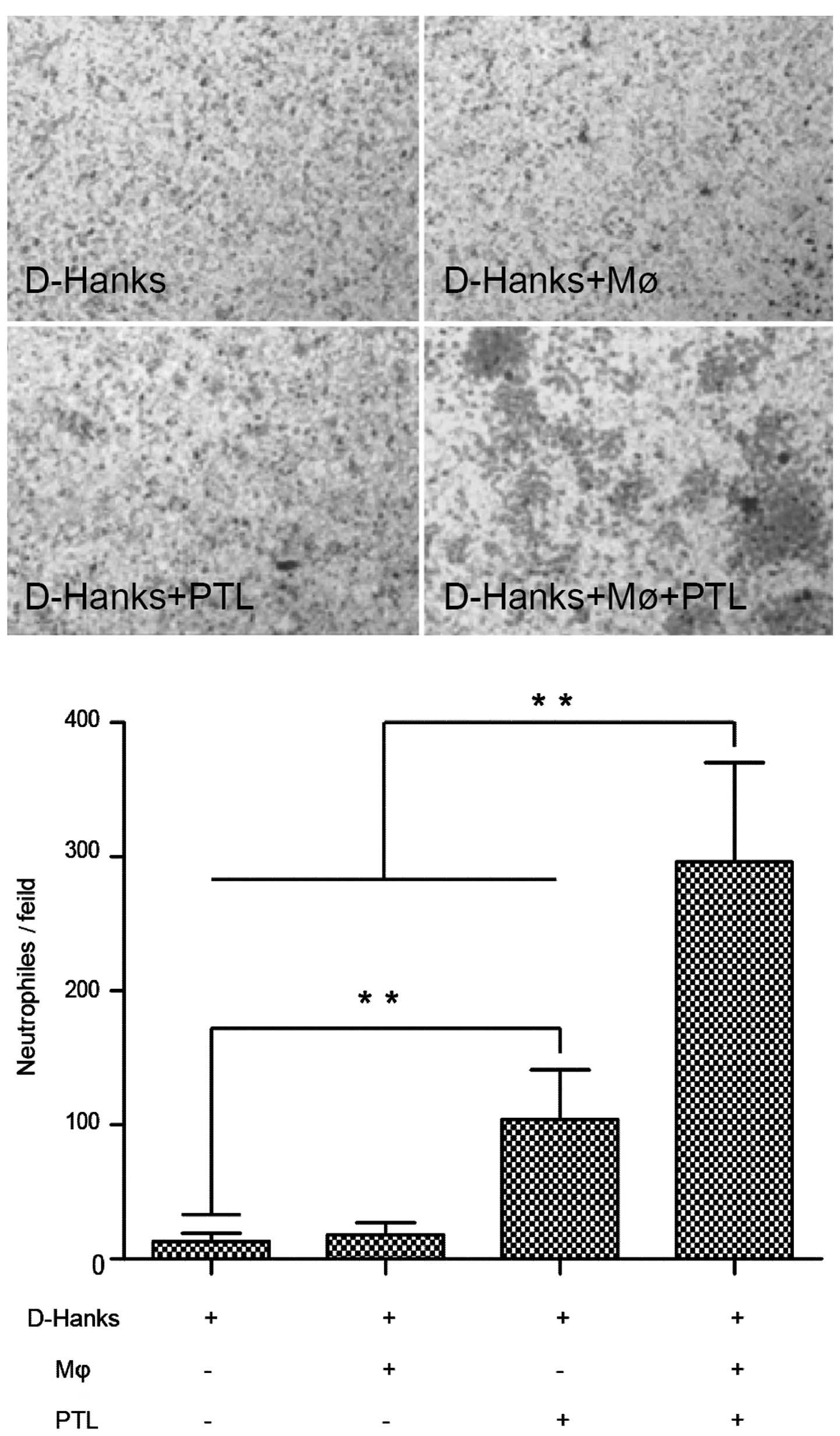
Neutrophil chemotaxis in vitro is induced
by macrophages stimulated with PTL
To confirm that macrophages activated by PTL were
involved in the recruitment of neutrophils, an in vitro
chemotaxis experiment was conducted as follows: 100 µl of
the neutrophil suspension (106 cells/ml) was placed in
the upper wells of a 24-well modified Corning Transwell set
equipped with a polycarbonate filter (8 µm pore size); in
the lower wells, the protocol was set up either with D-Hanks or PTL
(50 µg/ml in D-Hanks)-treated fresh medium, or with D-Hanks
or PTL (50 µg/ml in D-Hanks)-treated pre-cultured
macrophages (106 cells/ml).
Following Transwell plate incubation for 1.5 h at
37°C in 5% CO2, the cells remaining in the upper wells
were removed using cotton swabs and those retained on the lower
side of the filter were stained with Giemsa staining. The number of
neutrophils reaching the lower side of the filter was counted in 5
random fields using an electron microscope (objective, ×40) for
each set of wells.
Induction of the cytokines released from
macrophage stimulated by different doses of PTL
Mouse macrophages were harvested with RPMI-1640,
cultured in plastic dishes (48-wells of 500 µl capacity),
washed and replaced with 450 µl/well fresh RPMI-1640
containing 10% FBS prior to the experiment. The protocol for the
assay of TNF-α and IL-1β was conducted using 6 groups (3
wells/group) of macrophages treated as follows: Group 1, 50
µl/well PBS alone; and groups 2–6, 50 µl/well PTL
(25, 50, 100, 200 and 400 µg/ml). The protocol for the assay
of IL-6 was conducted using 8 groups (3 wells/group) of macrophages
treated as follows: Group 1, 50 µl/well PBS alone; and
groups 2–6, 50 µl/well PTL (6.25, 12.5, 25, 50, 100, 200 and
400 µg/ml). After 3 h of incubation, all the supernatants
were harvested and stored at −80°C. The levels of TNF-α, IL-1β,
IL-6 and nitric oxide (NO) released by macrophages were measured
using ELISA kits according to the manufacturer's instructions.
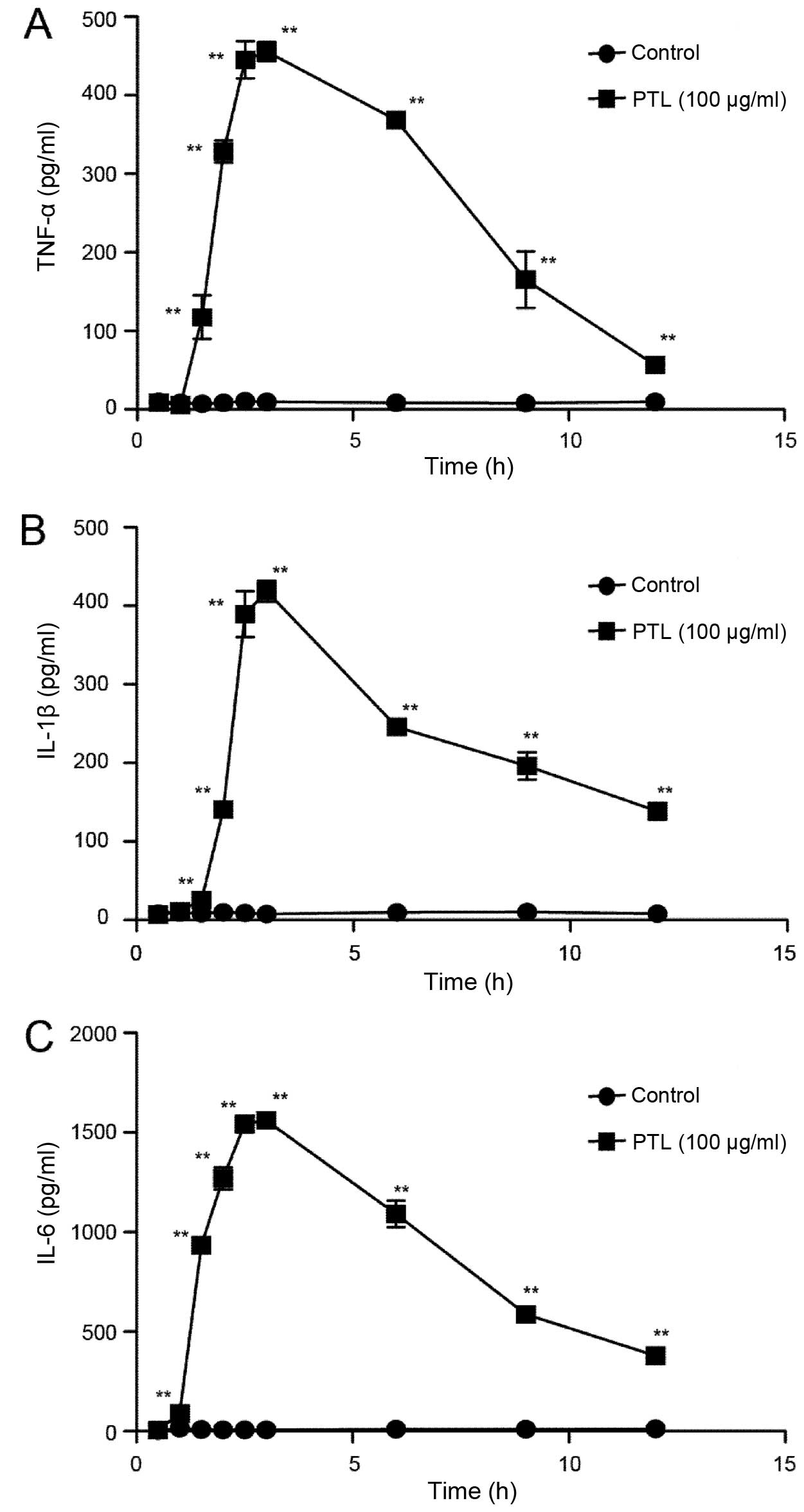
Time curves of the cytokines released
from macrophages stimulated by PTL
Mouse macrophages were harvested with RPMI-1640,
cultured in plastic dishes (48-wells of 500 µl capacity),
washed and replaced with 450 µl/well fresh RPMI-1640
containing 10% FBS prior to the experiment. The protocol was
conducted using 18 groups (3 wells/group) of macrophages treated
half with 50 µl of 100 µg/ml PTL and the others with
50 µl of PBS as control groups. The incubating times were as
follows: 0.5, 1, 1.5, 2, 2.5, 3, 6, 9 and 12 h. After the
incubation, all the supernatants were harvested and stored at
−80°C. The levels of TNF-α, IL-1β, IL-6 and NO released by
macrophages were measured using the ELISA kits according to the
manufacturer's instructions.
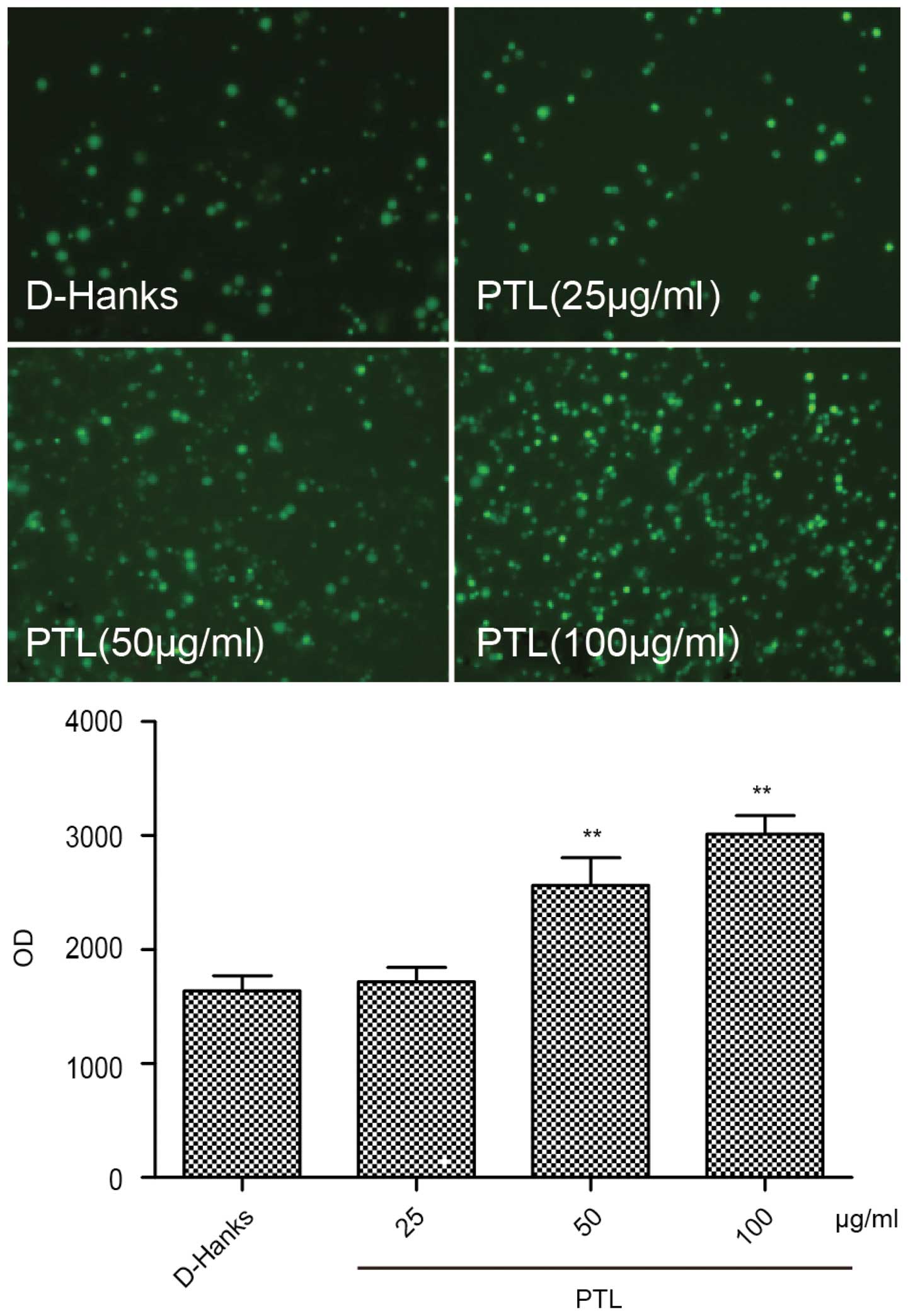
Measurement of intracellular reactive
oxygen species (ROS) in macrophages stimulated by PTL
Intracellular ROS production was measured using
DCFH-DA (22). DCFH-DA penetrates
into cells and is hydrolyzed by intracellular esterase to the
non-fluorescent DCFH, which can be rapidly oxidized to the highly
fluorescent 2,7-dichlorofluorescein (DCF) in the presence of ROS.
Peritoneal macrophages were seeded at 1×106 cells/well
in 12-well plates. PTL was dissolved and diluted in D-Hanks. Mouse
macrophages were harvested with RPMI-1640, cultured in plastic
dishes (48-wells of 500 µl capacity), washed and replaced
with 450 µl/well fresh RPMI-1640 containing 10% FBS prior to
the experiment. Peritoneal macrophages were seeded at
1×106 cells/well in 12-well plates. The protocol was
conducted using 4 groups (3 wells per group) of macrophages treated
as follows: Group 1, 50 µl/well D-Hanks alone; and groups
2–4, 50 µl/well PTL (25, 50 and 100 µg/ml). Following
stimulation and incubation with or without different treatments for
1 h, the cells were treated with 4 µM DCFH-DA at 37°C for 20
min and examined using a fluorescence microscope (Nikon, Tokyo,
Japan) with an emission wavelength of 598 nm. The fluorescence
intensity was measured with a fluorescence microplate reader with
an excitation wavelength of 420 nm and an emission wavelength of
560 nm.
Western blotting of nuclear factor-κB
(NF-κB) p65 in cytoplasm and nucleus of macrophages stimulated by
PTL
To confirm that pro-inflammation induced by PTL was
associated with the NF-κB signaling pathway, the content of p65 in
the cytoplasm and nucleus was analyzed by western blot analysis,
which was conducted using 4 groups (3 wells/group) of macrophages
treated as follows: Group 1, 50 µl/well PBS alone; and
groups 2–4, 50 µl/well PTL (12.5, 25 and 50 µg/ml).
After 0.5 h of incubation, the culture supernatant was discarded,
and the macrophages were washed with PBS buffer. Subsequently, the
cytoplasmic and nuclear proteins of the macrophages were extracted
(23). The cytoplasmic and
nuclear protein solutions were denatured by boiling at 100°C for 5
min, mixed evenly with 5X bromophenol blue loading buffer, and were
loaded (10 µl for each lane) onto 10% SDS polyacrylamide gel
followed by electroblotting onto nitrocellulose membrane. Following
blocking of the non-specific binding with 5% non-fat milk (prepared
in TBS containing 0.1% Tween-20) for 3 h, the membrane was applied
with antibodies against p65 and GAPDH, followed by incubation with
HRP-conjugated anti-rabbit antibody. Subsequently, the protein was
detected using an ECL chemiluminescence detection kit and cultured
for 5 min in the dark. Densitometry was performed using the
software Quantity One (Biorad).
SEM analysis of surface characteristic
changes of macrophages stimulated by PTL
Mouse macrophages were harvested as indicated. For
SEM, the cells were cultured on the sterile slides placed in 6-well
plastic plates. The assay was performed with 5 groups (3
wells/group) of macrophages treated with 500 µl of 6.25,
12.5, 50 and 100 µg/ml PTL. All the supernatants were
harvested following incubation for 0.5, 1 and 3 h. Each slide was
washed with cold PBS 3 times and was fixed with 2.5% glutaraldehyde
overnight. Sample slides were prepared through sequential
dehydration in ethanol (30, 50, 70, 80, 90 and 100%). The sample
slides were subsequently coated with gold-palladium prior to SEM
observation. The morphological changes of macrophages were
evaluated by capturing images focusing on a single macrophage at
(magnification, ×3,000–5,000).
Statistical analysis
The data are presented as mean ± standard error of
the mean and compared using t-test by SPSS (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Extraction, purification and
identification of PTL
Hydrophobic interaction, ion exchange and desalting
chromatographic steps were applied to purify PTL. The crude protein
extract was eluted by hydrophobic interaction. The main peak was
eluted with a linear gradient of NaCl (0–0.4 mol/l). A single peak
on HPLC and a single band of ~13 kDa on SDS-PAGE (Fig. 1) were observed, suggesting that
the isolated PTL was quite pure. Gel electrophoresis was combined
with LC-MS/MS for the proteomic analysis of PTL. The 13-kDa band
was subjected to in-gel trypsin digestion. The peptides extracted
from each band were loaded to LC-MS/MS for protein identification.
Through a protein database search, the 13-kDa band protein was
identified as PTL, with the molecule matching those previously
reported (24).
Effects of PTL on neutrophil chemotaxis
mediated by macrophages
In order to investigate the involvement of
macrophages in neutrophil infiltration induced by PTL treatment,
neutrophil migration assay was used with a 24-well Transwell set.
Compared to the control (D-Hanks) group, significantly more
neutrophils were retained on the lower surface of the filter
following treatment with PTL (PTL+MΦ+D-Hanks).
Macrophages untreated with PTL (D-Hanks+MΦ) and the
group in which PTL did not coexist with macrophages (D-Hanks+PTL)
did not show any neutrophil chemotaxis (Fig. 2), therefore, PTL-stimulated
neutrophil chemotaxis was mediated by macrophages.
Induction of the cytokines released from
macrophages activated by different doses of PTL
Peritoneal macrophages were treated with indicated
doses of PTL and the levels of released cytokines, such as TNF-α,
IL-1β and IL-6, were measured. PTL activated macrophages to release
TNF-α, IL-1β and IL-6 in a dose-dependent manner. Compared to the
control group (PBS), the levels of TNF-α, IL-1β and IL-6
significantly increased following exposure of the macrophages to
PTL (50, 100, 200 and 400 µg/ml) for 3 h (Fig. 3). Notably, IL-6 was more prone to
induction compared with TNF-α and IL-1β.
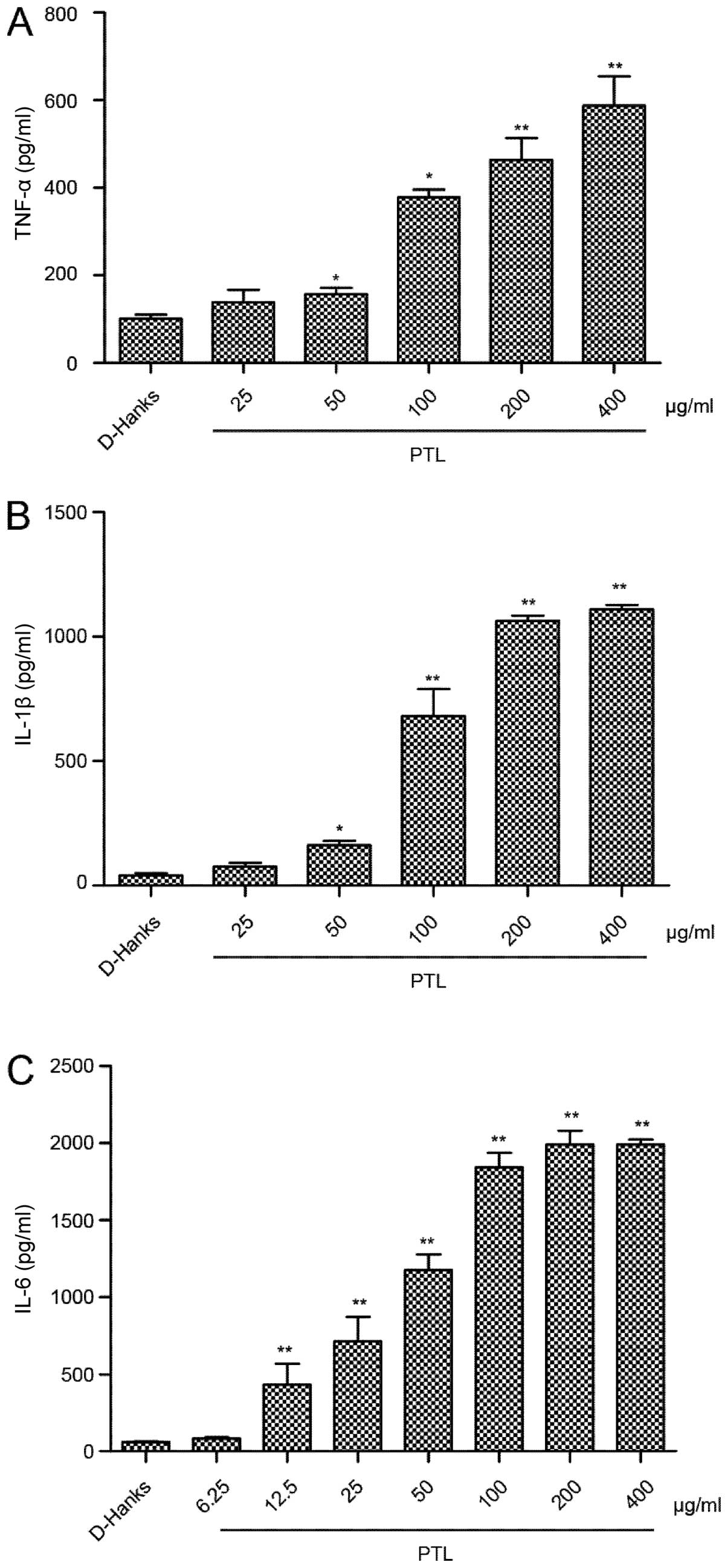 | Figure 3Induction of the cytokines released
from macrophages activated by different doses of PTL. Cultured
mouse resident macrophages were treated with PTL for 3 h. PTL (50,
100, 200 and 400 µg/ml) induced (A) TNF-α and (B) IL-1β
dose-dependently in the supernatant. The level of (C) IL-6 was also
dose dependent (PTL: 12.5, 25, 50, 100, 200 and 400 µg/ml).
Cytokine levels were measured by ELISA. Results are mean ± standard
error of the mean (n=3). *P<0.05,
**P<0.01 compared to phosphate-buffered saline
(t-test). PTL, Pinellia ternata lectin; TNF, tumor necrosis
factor; IL, interleukin. |
Time curves of the cytokines released
from macrophages activated by PTL
To confirm whether PTL could cause acute
inflammation in animal models, macrophages were treated by PTL for
different periods of time and the levels of cytokines, TNF-α, IL-1β
and IL-6, were measured. As shown in Fig. 4, the levels of TNF-α and IL-1β
significantly increased 1.5 h after stimulation compared to the
control. The levels of TNF-α and IL-1β reached maxima after 3 h of
treatment with PTL, and subsequently gradually decreased (Fig. 4A and B). The time curve of IL-6
changed in a similar manner. In addition, PTL significantly
increased the level of IL-6 starting from 1 h (Fig. 4C). Therefore, PTL may cause
inflammation in macrophages at an early stage.
Effects of PTL on ROS overproduction by
macrophages
To evaluate the effects of PTL on ROS production,
macrophages were stimulated with different doses of PTL (25, 50 and
100 µg/ml) for 1 h and observed under an inverted
fluorescence microscope following the addition of a fluorescent
probe, DCFH-DA. The fluorescence intensity was measured with a
fluorescence microplate reader. Compared with the D-Hanks group,
elevating the PTL dose significantly increased the intracellular
ROS contents (Fig. 5). Thus, PTL
stimulated macrophages to undergo intense oxidative stress,
releasing considerable ROS eventually.
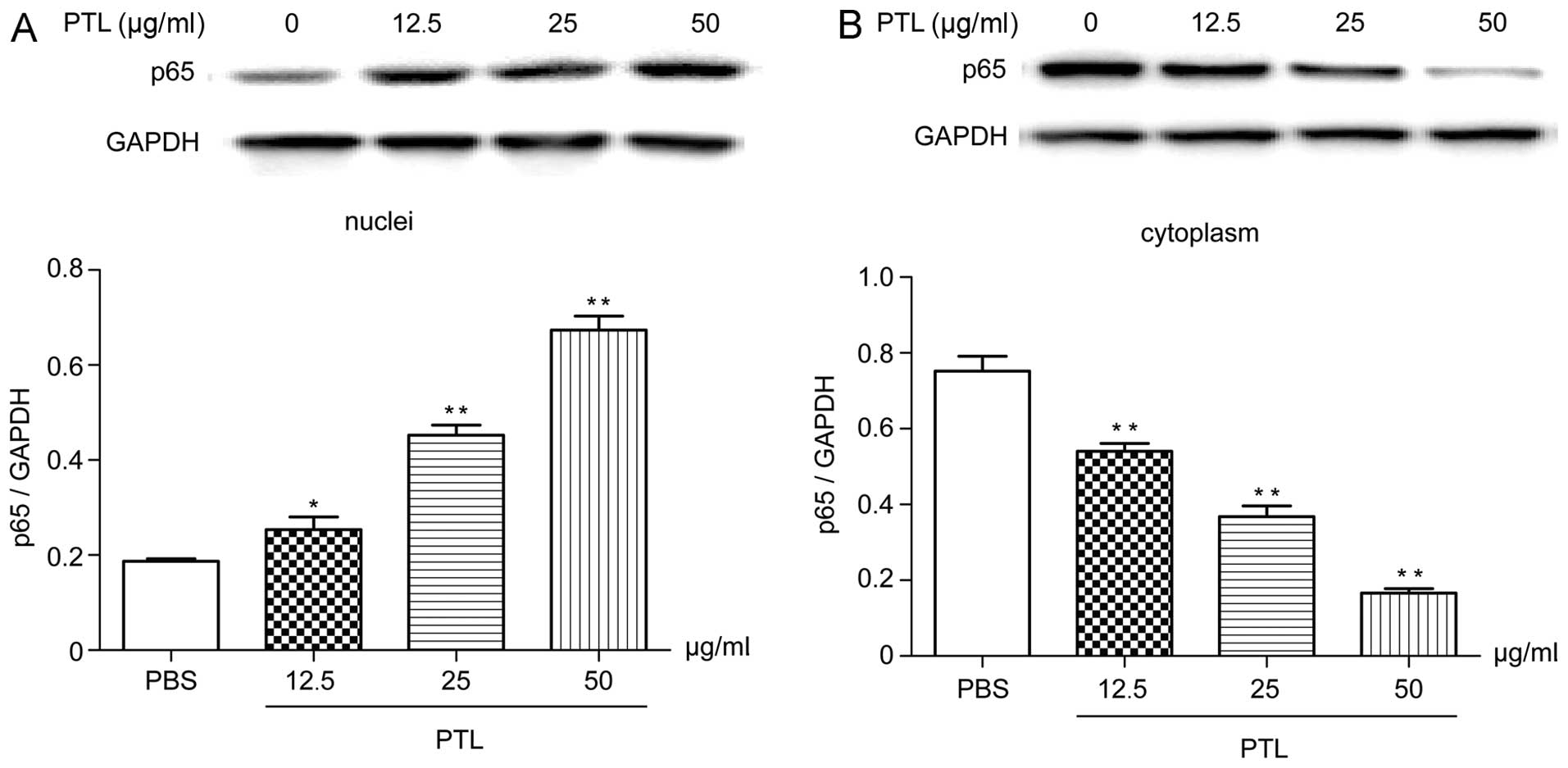
Western blotting of NF-κB p65 in
cytoplasm and the nucleus
Following stimulation with PTL (5, 12.5 and 25
µg/ml) for 0.5 h, the content of p65 in the macrophage
cytoplasm reduced compared with that of the blank group (Fig. 6B), however, this content in the
macrophage nucleus markedly increased (Fig. 6A), all showing evident dose-effect
associations. Therefore, PTL stimulation caused transfer of p65
from the macrophage cytoplasm to the nucleus, which suggested that
the inflammation induced by PTL-stimulated macrophage was
associated with the NF-κB signaling pathway.
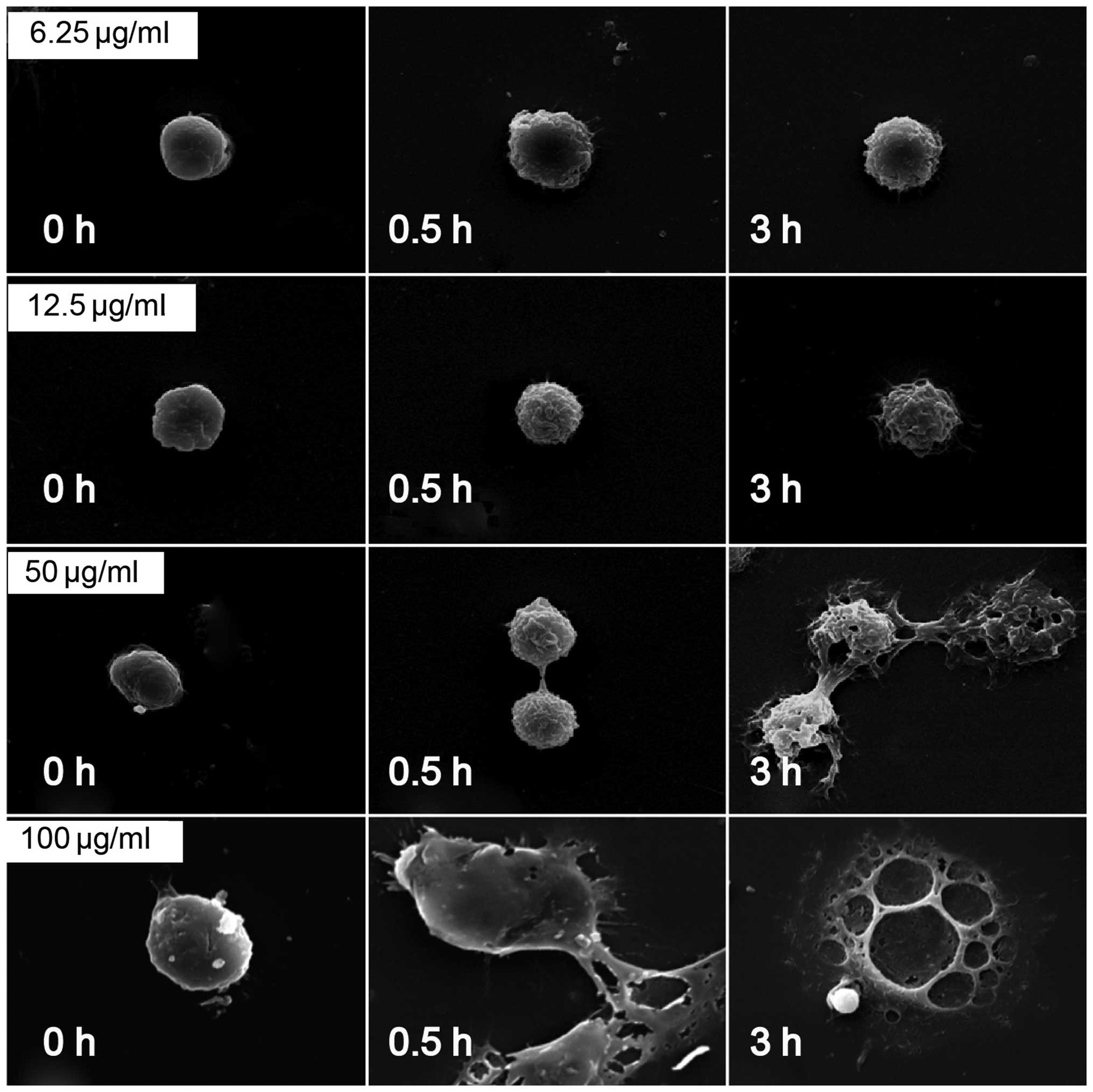
SEM analysis of surface characteristic
changes of macrophages stimulated by PTL
The morphological changes of macrophages following
treatment with PTL were observed using SEM. As shown in Fig. 4, the mouse peritoneal macrophages
were normal at 0 h of PTL treatment. From 0.5 h of treatment, 6.25,
12.5 and 50 µg/ml PTL caused apparent wrinkles on the
macrophage surface and more pseudopodia, accompanied by clear
morphological changes. At 3 h, 50 and 100 µg/ml PTL
evidently damaged or even destructed the macrophage surface
(Fig. 7). Therefore, PTL at
appropriate doses could induce the activation of macrophages, and
at a high dose induced cell death.
Discussion
The toxicity of lectins from plants has been widely
reported in clinical and animal experiments. Despite numerous
trials aiming to discover the mechanism of neutrophil infiltration
involved in inflammation progression, the role of resident
macrophages remains unclear.
Resident macrophages dominantly control such
processes by targeting the stimuli, phagocytizing and producing
chemo-tactic cytokines (25,26). In the acute inflammatory response,
neutrophil migration from venous blood to the affected sites
involves complex processes between neutrophils, endothelial cells,
macrophages and other immune cells.
Plant lectins, particularly monocot mannose-binding
lectins, have shown pro-inflammatory effects. Vatairea
macrocarpa lectin (VML), Pisum arvense lectin (PAL),
Helianthus tuberosus lectin (HTL) and Dioclea
rostrata lectin (DRL) induced acute inflammation through
neutrophils migration in vivo, and injecting culture
supernatants of DRA-stimulated macrophages in vivo
significantly induced the release of TNF-α, IL-1β and NO in the
mouse peritoneal fluid (5,6,12–14,27).
In our previous study, Arisaema erubescens lectin, which was
derived from a plant in the Arisaema family, induced rat paw edema
and in vivo neutrophil migration, possibly by releasing
inflammatory mediators from macrophages (19).
In the present study, PTL stimulated chemotactic
activity of neutrophils under the mediation of macrophages, and it
also significantly elevated the production of cytokines (TNF-α,
IL-1β and IL-6) dose- and time-dependently, suggesting that PTL
stimulated macrophages to induce inflammation. In addition, PTL
also dose-dependently induced intracellular ROS. An intermediate
amount of ROS triggers an inflammatory response through the
activation of NF-κB (28). The
present data showed that PTL caused transfer of p65 from the
macrophage cytoplasm to the nucleus, which indicated that the NF-κB
signaling pathway was activated. Therefore, PTL induced ROS
overproduction and activated the NF-κB signaling pathway that
subsequently released numerous pro-inflammatory cytokines,
consistent with other results in the present study.
A high level of ROS also contributed to TNF-induced
necrotic cell death in certain experiments (29,30). In general, cell death includes two
classic ways, apoptosis and necrosis, and the latter is described
as a morphologically distinct form of cell death, which has
traditionally been regarded as passive and unregulated (31,32). In the present study, the
PTL-induced cell damage was more similar to necrosis, rather than
apoptosis, as indicated by the morphological changes. The SEM
images showed that increasing the dose of PTL caused more
pseudopodia, swelling and finally destructed the cell membrane as
typical necrosis. Therefore, PTL at a high dose induced cell death
possibly by overproducing ROS, and necrosis also triggered
inflammation. Release of pro-inflammatory cytokines and neutrophil
migration may be ascribed to ROS overproduction-induced activation
of the NF-κB signaling pathway and necrosis.
PTL can induce inflammation, as well as cell death,
and the latter aggravates the former. P. ternata has
significant toxicity through the penetration of raphides. The
findings of the present study provide valuable evidence for
revealing the toxicity mechanism of P. ternata.
In conclusion, PTL showed pro-inflammatory activity
in vitro, which induced neutrophil migration and
pro-inflammatory cytokines release. PTL-stimulated macrophages
exhausted the pro-inflammation effect, which was possibly
associated with ROS overproduction, activating the NF-κB signaling
pathway and necrosis-like damage on mouse peritoneal macrophages.
These findings can be used to improve the understanding of the
mechanisms involved in the inflammatory response of PTL.
Acknowledgments
The present study was supported by the National
Nature Science Fund of China (grant no. 81173549), the Specialized
Research Fund for the Doctoral Program of Higher Eucation (grant
no. 20113237120010) and a project funded by the Priority Academic
Program Development of Jiangsu Higher Education Institutions
(PAPD). For other helpful assistance, the authors would like to
thank Nanjing University of Chinese Medicine.
References
|
1
|
Peumans WJ and Van Damme EJ: Lectins as
plant defense proteins. Plant Physiol. 109:347–352. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moreira RA, Ainouz IL, De Oliveira JT and
Cavada BS: Plant lectins, chemical and biological aspects. Mem Inst
Oswaldo Cruz. 86(Suppl 2): 211–218. 1991. View Article : Google Scholar
|
|
3
|
Cavada BS, Santos CF, Grangeiro TB, Nunes
EP, Sales PV, Ramos RL, De Sousa FA, Crisostomo CV and Calvete JJ:
Purification and characterization of a lectin from seeds of
Vatairea macrocarpa Duke. Phytochemistry. 49:675–680. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gabius HJ and Gabius S: Glycosciences:
Status and perspectives. Wiley-VCH; New York: pp. 6592002
|
|
5
|
Alencar VB, Brito GA, Alencar NM, Assreuy
AM, Pinto VP, Teixeira EH, Souza EP, Debray H, Ribeiro RA and
Cavada BS: Helianthus tuberosus agglutinin directly induces
neutrophil migration, which can be modulated/inhibited by resident
mast cells. Biochem Cell Biol. 83:659–666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mota MR, Criddle DN, Alencar NM, Gomes RC,
Meireles AV, Santi-Gadelha T, Gadelha CA, Oliveira CC, Benevides
RG, Cavada BS, et al: Modulation of acute inflammation by a
chitin-binding lectin from Araucaria angustifolia seeds via mast
cells. Naunyn Schmiedebergs Arch Pharmacol. 374:1–10. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santos-de-Oliveira R, Dias-Baruffi M,
Thomaz SM, Beltramini LM and Roque-Barreira MC: A neutrophil
migration-inducing lectin from Artocarpus integrifolia. J Immunol.
153:1798–1807. 1994.PubMed/NCBI
|
|
8
|
Assreuy AM, Shibuya MD, Martins GJ, De
Souza ML, Cavada BS, Moreira RA, Oliveira JT, Ribeiro RA and Flores
CA: Anti-inflammatory effect of glucose-mannose binding lectins
isolated from Brazilian beans. Mediators Inflamm. 6:201–210. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alencar NM, Teixeira EH, Assreuy AM,
Cavada BS, Flores CA and Ribeiro RA: Leguminous lectins as tools
for studying the role of sugar residues in leukocyte recruitment.
Mediators Inflamm. 8:107–113. 1999. View Article : Google Scholar
|
|
10
|
Cavada BS, Barbosa T, Arruda S, Grangeiro
TB and Barral-Netto M: Revisiting proteus: Do minor changes in
lectin structure matter in biological activity? Lessons from and
potential biotechnological uses of the Diocleinae subtribe lectins.
Curr Protein Pept Sci. 2:123–135. 2001. View Article : Google Scholar
|
|
11
|
Alencar NM, Assreuy AM, Alencar VB, Melo
SC, Ramos MV, Cavada BS, Cunha FQ and Ribeiro RA: The
galactose-binding lectin from Vatairea macrocarpa seeds induces in
vivo neutrophil migration by indirect mechanism. Int J Biochem Cell
Biol. 35:1674–1681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Assreuy AM, Alencar NM, Cavada BS,
Rocha-Filho DR, Feitosa RF, Cunha FQ, Calvete JJ and Ribeiro RA:
Porcine spermadhesin PSP-I/PSP-II stimulates macrophages to release
a neutrophil chemotactic substance: Modulation by mast cells. Biol
Reprod. 68:1836–1841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alencar NM, Assreuy AM, Criddle DN, Souza
EP, Soares PM, Havt A, Aragão KS, Bezerra DP, Ribeiro RA and Cavada
BS: Vatairea macrocarpa lectin induces paw edema with leukocyte
infiltration. Protein Pept Lett. 11:195–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alencar VB, Assreuy AM, Alencar NM,
Meireles AV, Mota MR, Aragão KS, Cajazeiras JB, Nagano CS, Brito
GA, Silva LI, et al: Lectin of Pisum arvense seeds induces in vivo
and in vitro neutrophil migration. J Pharm Pharmacol. 57:375–381.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McEver RP: Leukocyte-endothelial cell
interactions. Curr Opin Cell Biol. 4:840–849. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cronstein BN and Weissmann G: The adhesion
molecules of inflammation. Arthritis Rheum. 36:147–157. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malik AB and Lo SK: Vascular endothelial
adhesion molecules and tissue inflammation. Pharmacol Rev.
48:213–229. 1996.PubMed/NCBI
|
|
18
|
Alencar NM, Assreuy AM, Havt A, Benevides
RG, de Moura TR, de Sousa RB, Ribeiro RA, Cunha FQ and Cavada BS:
Vatairea macrocarpa (Leguminosae) lectin activates cultured
macrophages to release chemotactic mediators. Naunyn Schmiedebergs
Arch Pharmacol. 374:275–282. 2007. View Article : Google Scholar
|
|
19
|
Liu XQ, Wu H, Yu HL, Zhao TF, Pan YZ and
Shi RJ: Purification of a lectin from Arisaema erubescens (Wall.)
Schott and its pro-inflammatory effects. Molecules. 16:9480–9494.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu HL, Zhu FG and Wu G: Toxic proteins on
Raphides from Pinellia ternata and Pinellia pedatisecta. Chin J
Tradit Chin Med Pharm. 26:1037–1042. 2011.
|
|
21
|
Sawant KV, Cho H, Lyons M, Ly LH and
McMurray DN: Guinea pig neutrophil-macrophage interactions during
infection with Mycobacterium tuberculosis. Microbes Infect.
12:828–837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sánchez-Fidalgo S, da Silva MS, Cárdeno A,
Aparicio-Soto M, Salvador MJ, Frankland Sawaya AC, Souza-Brito AR
and de la Lastra CA: Abarema cochliacarpos reduces LPS-induced
inflammatory response in murine peritoneal macrophages regulating
ROS-MAPK signal pathway. J Ethnopharmacol. 149:140–147. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim MK, Chung SW, Kim DH, Kim JM, Lee EK,
Kim JY, Ha YM, Kim YH, No JK, Chung HS, et al: Modulation of
age-related NF-kappaB activation by dietary zingerone via MAPK
pathway. Exp Gerontol. 45:419–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shangary S, Singh J, Kamboj SS, Kamboj KK
and Sandhu RS: Purification and properties of four monocot lectins
from the family Araceae. Phytochemistry. 40:449–455. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anstead GM, Chandrasekar B, Zhang Q and
Melby PC: Multinutrient undernutrition dysregulates the resident
macrophage proinflammatory cytokine network, nuclear factor-kappaB
activation, and nitric oxide production. J Leukoc Biol. 74:982–991.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silva MT: When two is better than one:
Macrophages and neutrophils work in concert in innate immunity as
complementary and cooperative partners of a myeloid phagocyte
system. J Leukoc Biol. 87:93–106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alencar VB, Alencar NM, Assreuy AM, Mota
ML, Brito GA, Aragão KS, Bittencourt FS, Pinto VP, Debray H,
Ribeiro RA, et al: Pro-inflammatory effect of Arum maculatum lectin
and role of resident cells. Int J Biochem Cell Biol. 37:1805–1814.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gloire G, Legrand-Poels S and Piette J:
NF-kappaB activation by reactive oxygen species: Fifteen years
later. Biochem Pharmacol. 72:1493–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim YS, Morgan MJ, Choksi S and Liu ZG:
TNF-induced activation of the Nox1 NADPH oxidase and its role in
the induction of necrotic cell death. Mol Cell. 26:675–687. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han J, Zhong CQ and Zhang DW: Programmed
necrosis: Backup to and competitor with apoptosis in the immune
system. Nat Immunol. 12:1143–1149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kung G, Konstantinidis K and Kitsis RN:
Programmed necrosis, not apoptosis, in the heart. Circ Res.
108:1017–1036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCall K: Genetic control of necrosis -
another type of programmed cell death. Curr Opin Cell Biol.
22:882–888. 2010. View Article : Google Scholar : PubMed/NCBI
|