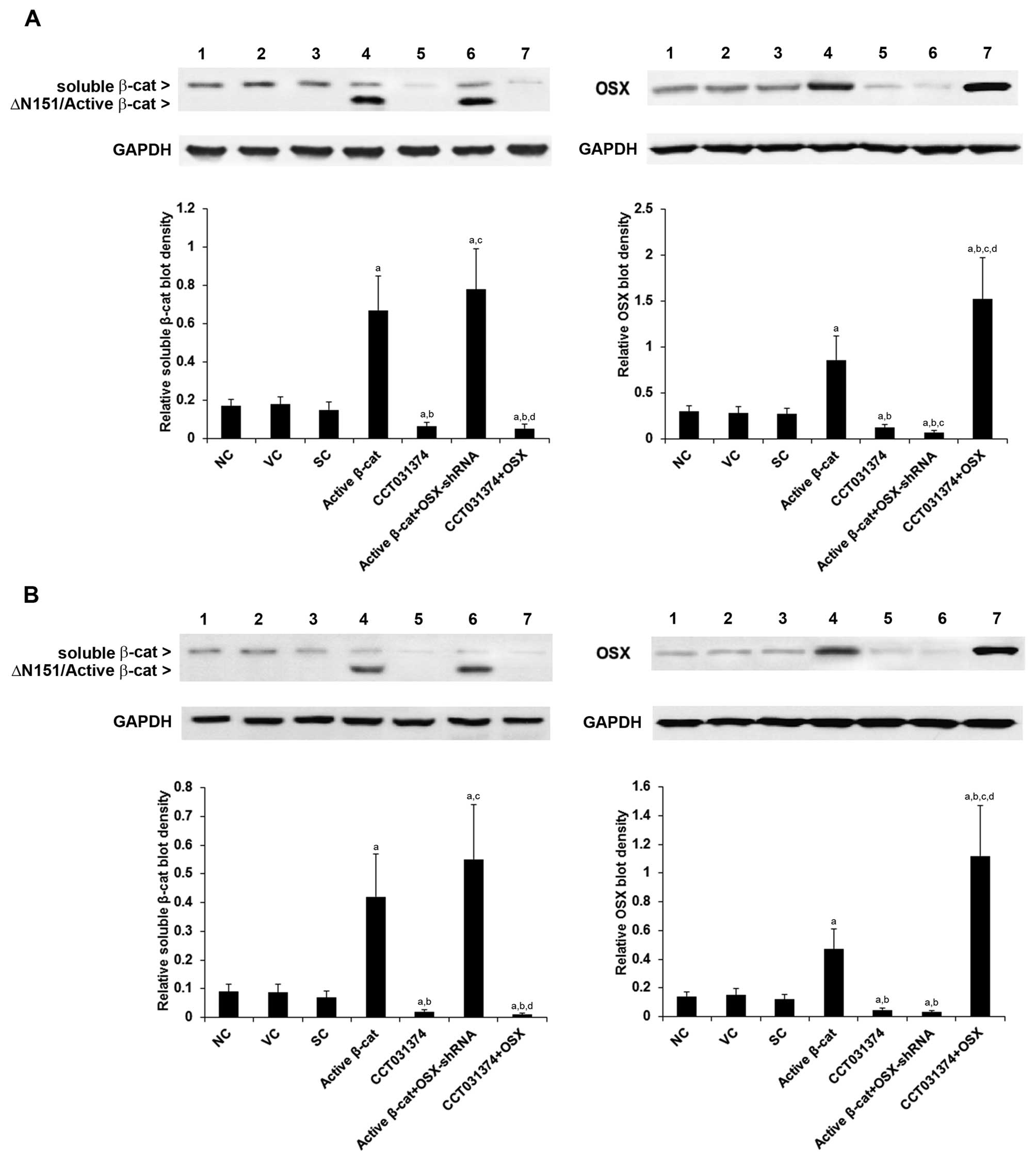
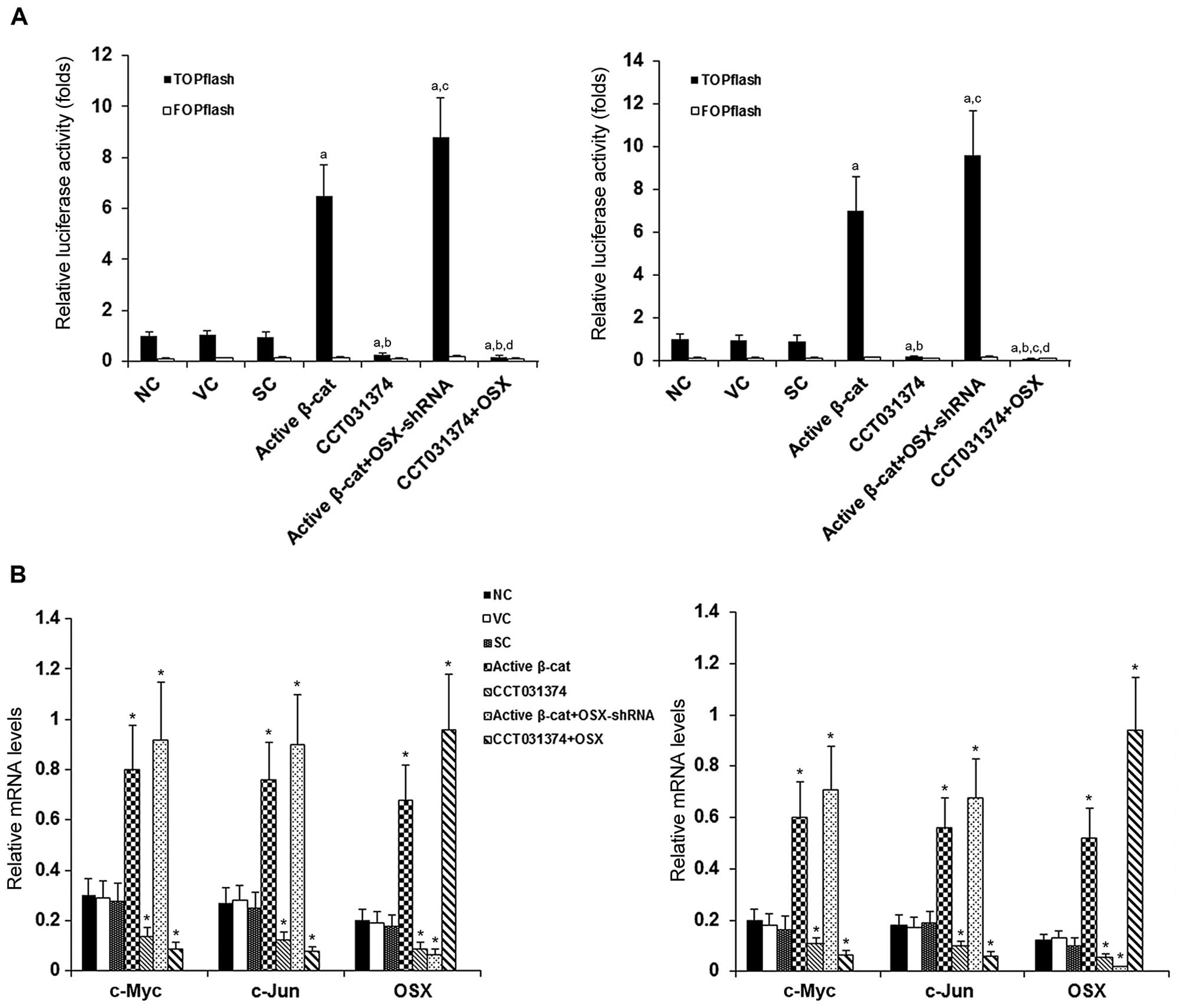
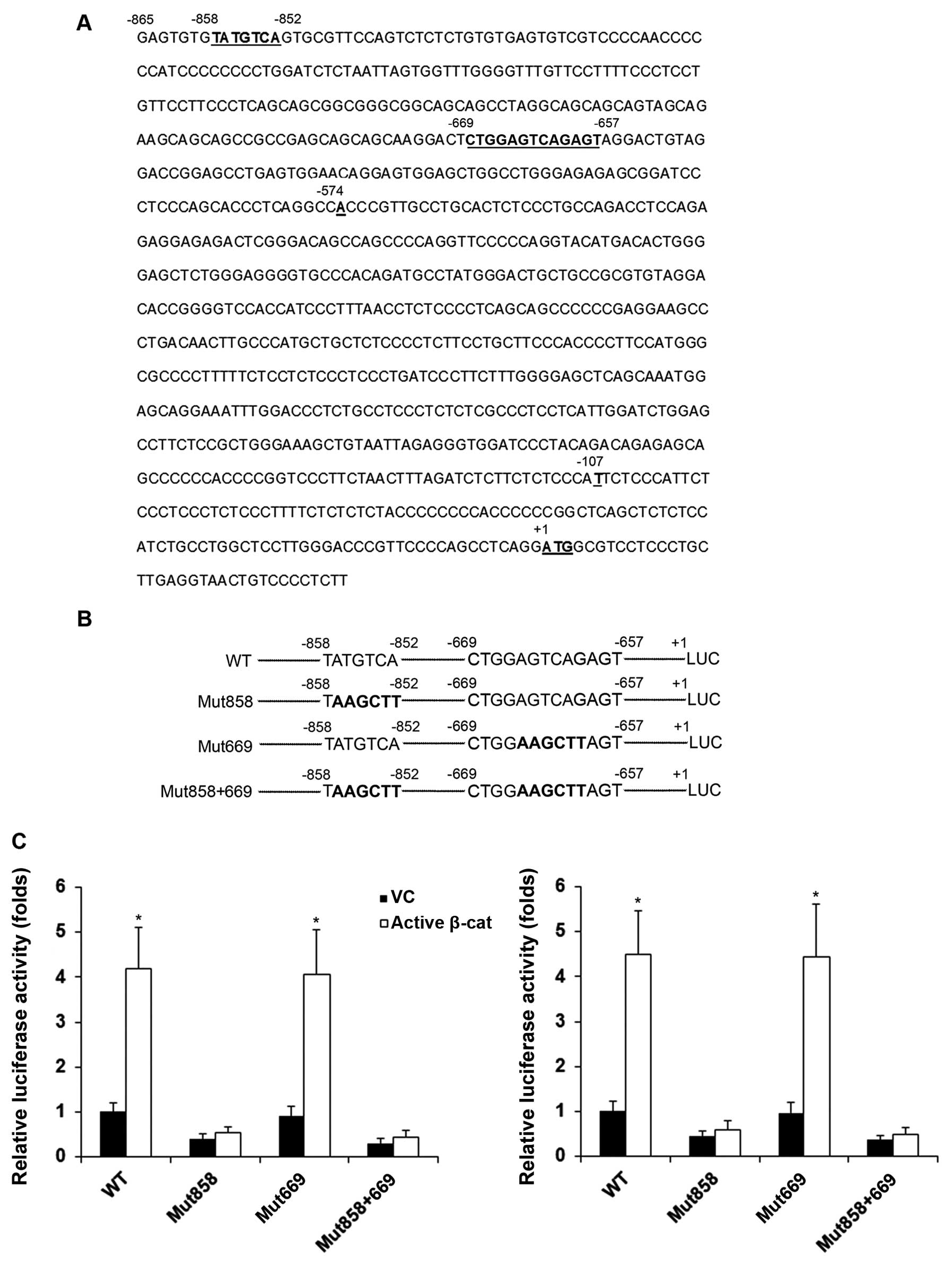
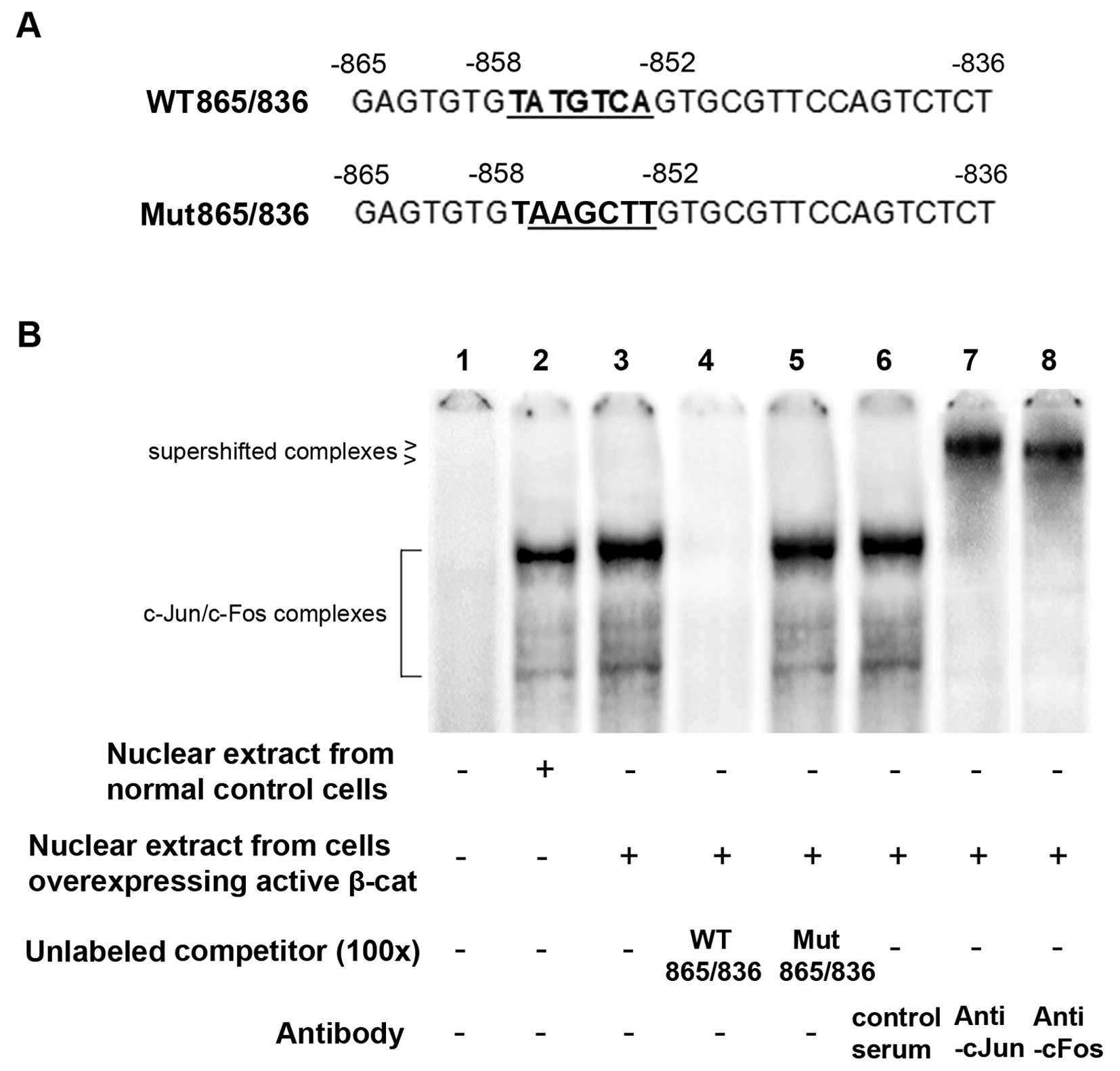
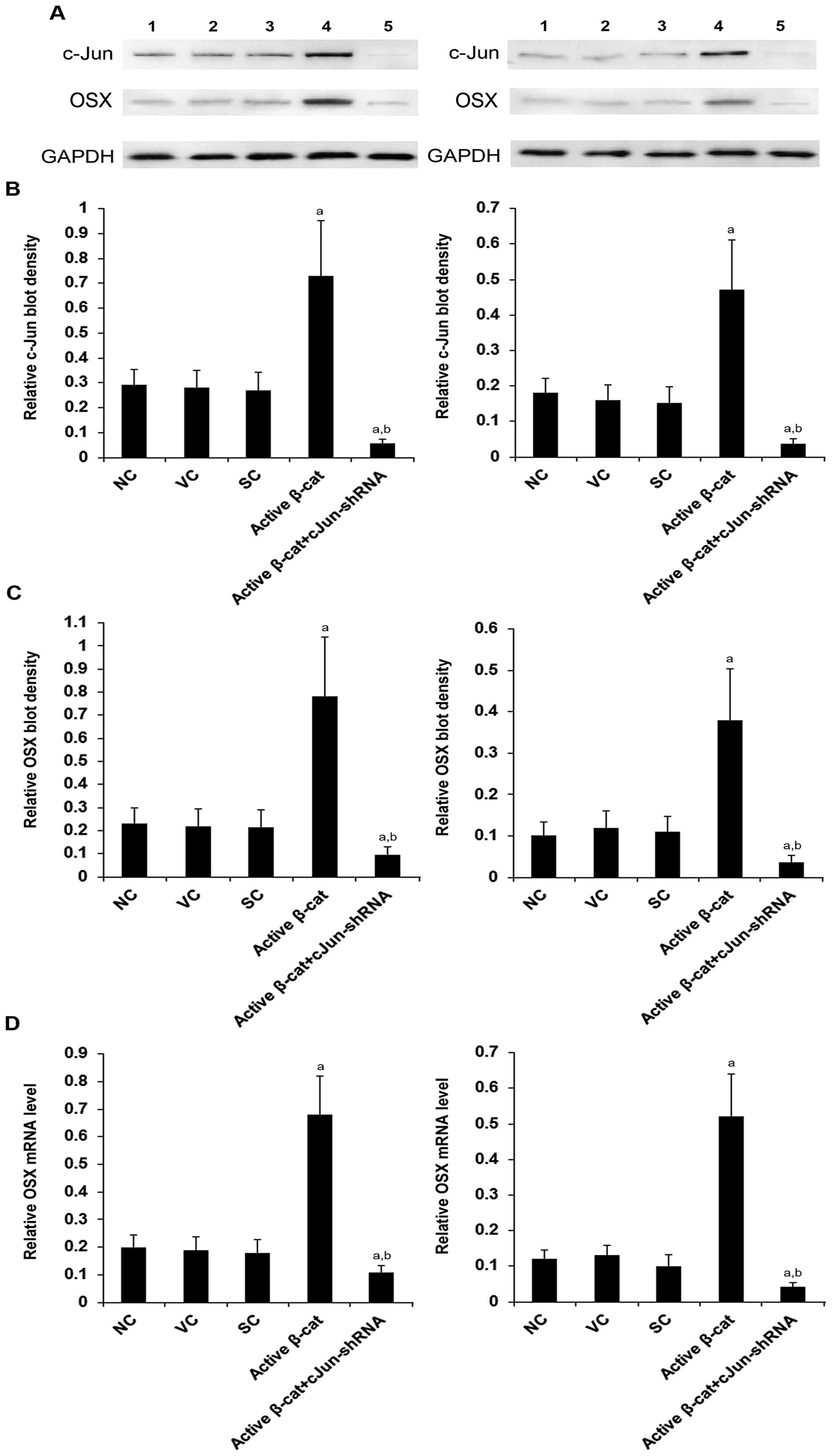
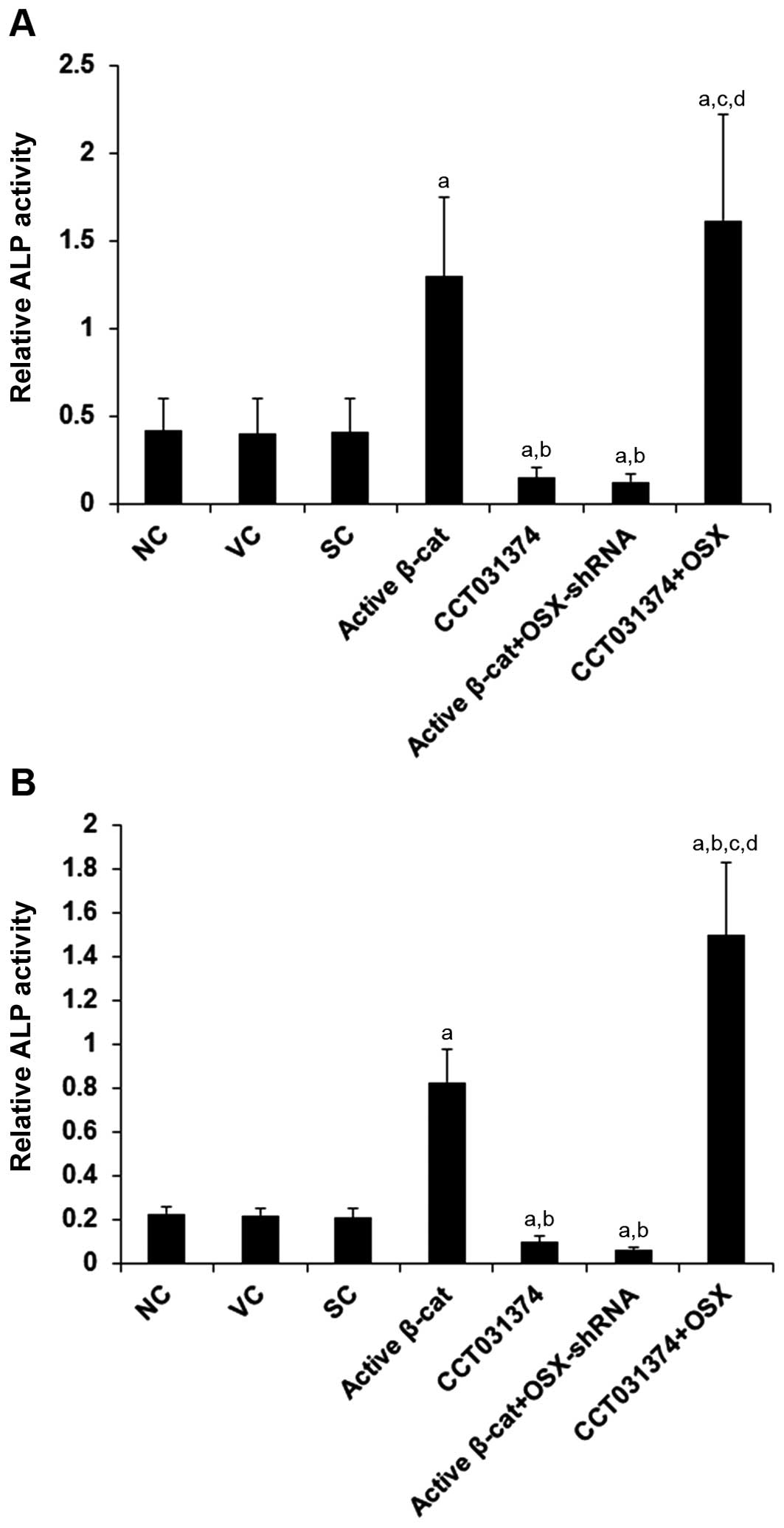
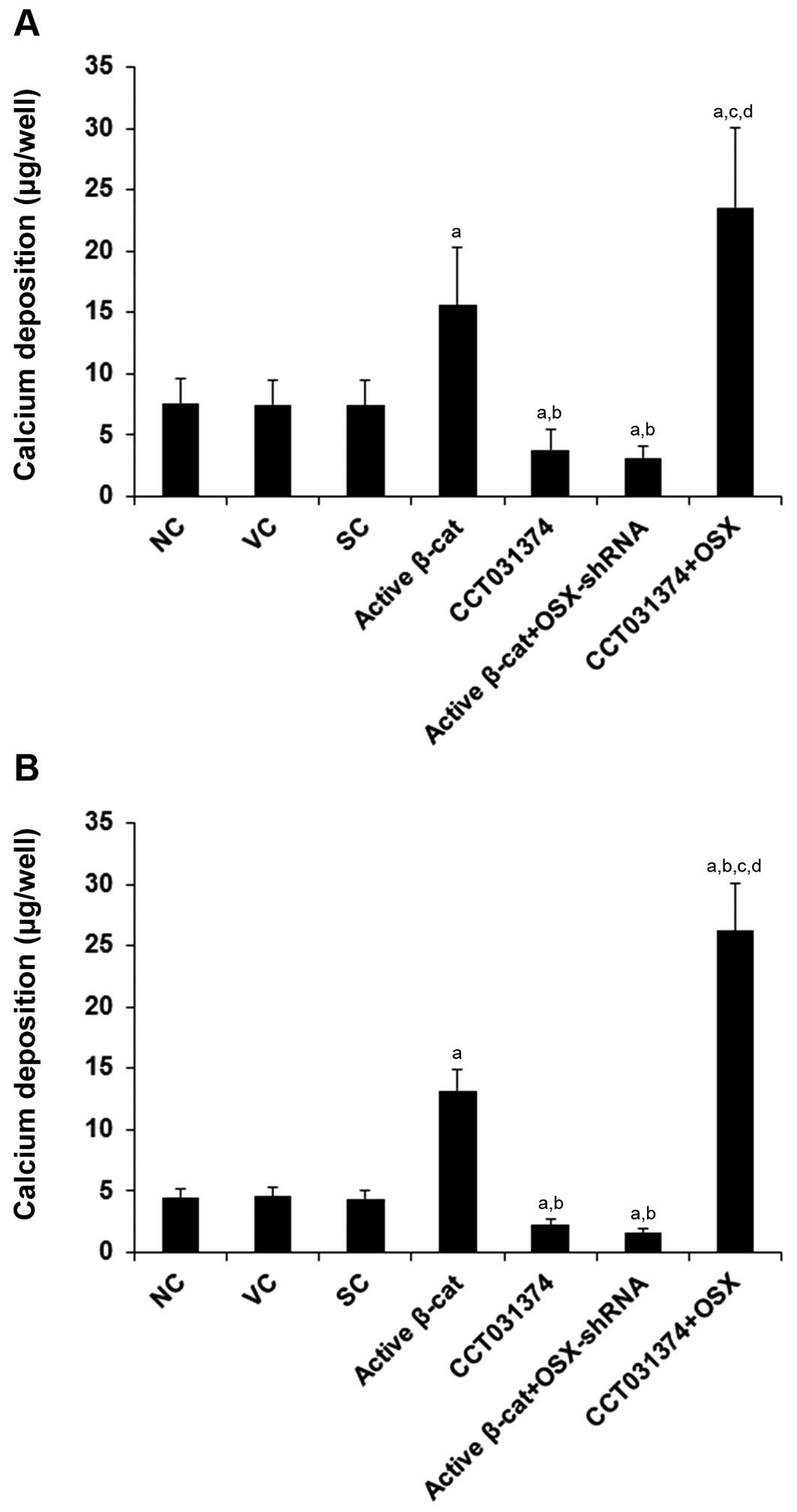
|
1
|
Zhou X, Zhang Z, Feng JQ, Dusevich VM,
Sinha K, Zhang H, Darnay BG and de Crombrugghe B: Multiple
functions of Osterix are required for bone growth and homeostasis
in postnatal mice. Proc Natl Acad Sci USA. 107:12919–12924. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodda SJ and McMahon AP: Distinct roles
for Hedgehog and canonical Wnt signaling in specification,
differentiation and maintenance of osteoblast progenitors.
Development. 133:3231–3244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang C, Cho K, Huang Y, Lyons JP, Zhou X,
Sinha K, McCrea PD and de Crombrugghe B: Inhibition of Wnt
signaling by the osteoblast-specific transcription factor Osterix.
Proc Natl Acad Sci USA. 105:6936–6941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Timpson NJ, Tobias JH, Richards JB,
Soranzo N, Duncan EL, Sims AM, Whittaker P, Kumanduri V, Zhai G,
Glaser B, et al: Common variants in the region around Osterix are
associated with bone mineral density and growth in childhood. Hum
Mol Genet. 18:1510–1517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Styrkarsdottir U, Halldorsson BV,
Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T,
Jonsdottir T, Saemundsdottir J, Snorradóttir S, Center JR, et al:
New sequence variants associated with bone mineral density. Nat
Genet. 41:15–17. 2009. View
Article : Google Scholar
|
|
6
|
Chesire DR and Isaacs WB: Beta-catenin
signaling in prostate cancer: an early perspective. Endocr Relat
Cancer. 10:537–560. 2003. View Article : Google Scholar
|
|
7
|
Holmen SL, Zylstra CR, Mukherjee A, Sigler
RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL and Williams BO:
Essential role of beta-catenin in postnatal bone acquisition. J
Biol Chem. 280:21162–21168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y and Jiang YG: Podocalyxin promotes
glioblastoma multiforme cell invasion and proliferation via
β-catenin signaling. PLoS One. 9:e1113432014. View Article : Google Scholar
|
|
9
|
Cawthorn WP, Heyd F, Hegyi K and Sethi JK:
Tumour necrosis factor-alpha inhibits adipogenesis via a
beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ.
14:1361–1373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun P, Xiong H, Kim TH, Ren B and Zhang Z:
Positive inter-regulation between beta-catenin/T cell factor-4
signaling and endothelin-1 signaling potentiates proliferation and
survival of prostate cancer cells. Mol Pharmacol. 69:520–531. 2006.
View Article : Google Scholar
|
|
11
|
Messeguer X, Escudero R, Farré D, Núñez O,
Martínez J and Albà MM: PROMO: detection of known transcription
regulatory elements using species-tailored searches.
Bioinformatics. 18:333–334. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Farré D, Roset R, Huerta M, Adsuara JE,
Roselló L, Albà MM and Messeguer X: Identification of patterns in
biological sequences at the ALGGEN server: PROMO and MALGEN.
Nucleic Acids Res. 31:3651–3653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Zhou M, Brand J and Huang L:
Inflammation activates the interferon signaling pathways in taste
bud cells. J Neurosci. 27:10703–10713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnson DR, Levanat S and Bale AE: Direct
molecular analysis of archival tumor tissue for loss of
heterozygosity. Biotechniques. 19:190–192. 1995.PubMed/NCBI
|
|
15
|
Baylan N, Bhat S, Ditto M, Lawrence JG,
Lecka-Czernik B and Yildirim-Ayan E: Polycaprolactone nanofiber
interspersed collagen type-I scaffold for bone regeneration: a
unique injectable osteogenic scaffold. Biomed Mater. 8(045011)2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stratford EW, Daffinrud J, Munthe E,
Castro R, Waaler J, Krauss S and Myklebost O: The
tankyrase-specific inhibitor JW74 affects cell cycle progression
and induces apoptosis and differentiation in osteosarcoma cell
lines. Cancer Med. 3:36–46. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferreira E, Porter RM, Wehling N,
O'Sullivan RP, Liu F, Boskey A, Estok DM, Harris MB, Vrahas MS,
Evans CH and Wells JW: Inflammatory cytokines induce a unique
mineralizing phenotype in mesenchymal stem cells derived from human
bone marrow. J Biol Chem. 288:29494–29505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ewan K, Pajak B, Stubbs M, Todd H, Barbeau
O, Quevedo C, Botfield H, Young R, Ruddle R, Samuel L, et al: A
useful approach to identify novel small-molecule inhibitors of
Wnt-dependent transcription. Cancer Res. 70:5963–5973. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sikavitsas VI, Bancroft GN, Holtorf HL,
Jansen JA and Mikos AG: Mineralized matrix deposition by marrow
stromal osteoblasts in 3D perfusion culture increases with
increasing fluid shear forces. Proc Natl Acad Sci USA.
100:14683–14688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dias NJ and Selcer KW: Steroid sulfatase
mediated growth Sof human MG-63 pre-osteoblastic cells. Steroids.
88:77–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HK, Kim MG and Leem KH: Collagen
hydrolysates increased osteogenic gene expressions via a MAPK
signaling pathway in MG-63 human osteoblasts. Food Funct.
5:573–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vallet S, Pozzi S, Patel K, Vaghela N,
Fulciniti MT, Veiby P, Hideshima T, Santo L, Cirstea D, Scadden DT,
et al: A novel role for CCL3 (MIP-1α) in myeloma-induced bone
disease via osteocalcin downregulation and inhibition of osteoblast
function. Leukemia. 25:1174–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halazonetis TD, Georgopoulos K, Greenberg
ME and Leder P: c-Jun dimerizes with itself and with c-Fos, forming
complexes of different DNA binding affinities. Cell. 55:917–924.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stein GS and Lian JB: Molecular mechanisms
mediating developmental and hormone-regulated expression of genes
in osteoblasts: an integrated relationship of cell growth and
differentiation. Cellular and molecular biology of bone. Noda M:
Academic Press; Tokyo: pp. 47–95. 1993
|
|
25
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|