Introduction
Lung cancer still remains the most common type of
cancer and the leading cause of cancer-related mortality worldwide,
particularly in China (1,2). Although there have been recent
advances in surgical resection and chemoradiation therapy, the
prognosis for patients with lung cancer, particularly non-small
cell lung cancer (NSCLC), remains poor, with a 5-year survival rate
of no more than 15%, which has not improved over the past years
(3). The lack of an effective
biomarker for the early screening and detection of NSCLC is the
reason for that the majority of NSCLC cases are diagnosed at
advanced stages, which leads directly to a high mortality rate in
patients with this type of cancer. Therefore, the identification of
non-invasive biomarkers is urgently required to complement and
advance current diagnostic and prognostic tools for NSCLC.
MicroRNAs (miRNAs or miRs) are endogenously
expressed non-coding RNA molecules that cause mRNA translation,
inhibition or degradation via direct base-pairing interactions,
which mainly repress the expression of several putative targets
post-transcriptionally (4).
Moreover, previous research has demonstrated that miRNAs are
abnormally expressed in various types of cancer, and act as
oncogenes or tumor-suppressor genes to regulate a wide range of
biological processes, such as tumorigenesis, differentiation,
proliferation, apoptosis, tumor invasion and metastasis (5,6).
The dysregulation of specific miRNAs can also serve as an effective
biomarker for predicting prognosis in patients with various types
of cancer, including NSCLC (7–9).
More importantly, certain studies have indicated that circulating
miRNAs are stable enough to be detected in serum and plasma
(10,11), and thus may be promising
non-invasive biomarkers for the early detection and prognosis of
cancers. Indeed, previous studies have demonstrated that miRNAs in
plasma may be utilized as novel diagnostic or prognostic biomarkers
in various types of cancer, including colorectal cancer (12), prostate cancer (13), breast cancer (14) and gastric cancer (15).
Of these aberrantly expressed miRNAs, 4 common
miRNAs (miR-124, miR-204, miR-146 and miR-106a) have been shown to
be associated with tumor-related biological processes, and are
dysregulated in patients with cancer, compared to healthy
individuals. miR-204 is located at chromosome 9q21.12, and recent
studies have found that it is downregulated in gastric cancer
(16), breast cancer (17), renal cell carcinoma (18), and NSCLC (19). Functionally, the overexpression of
miR-204 in cancer cells inhibits migration and proliferation, and
promotes apoptosis (20–23). Moreover, it has also been reported
that miR-124, miR-146 and miR-106a act as oncogenic or
tumor-suppressor genes which are involved in tumor progression.
However, to the best of our knowledge, no previous research to date
has focused on the correlation between the expression levels of
miR-124, miR-204, miR-146 and miR-106a in plasma and NSCLC.
Therefore, we conducted this study to evaluate whether these 4
miRNAs could serve as useful biomarkers in the screening of
NSCLC.
For the purposes of our study, we examined previous
studies and found that the plasma levels of 4 miRNAs (miR-124,
miR-146, miR-204 and miR-106a) appear not to have been studied in
relation to NSCLC. Thus, we first measured the expression levels of
these miRNAs in plasma samples from patients with NSCLC (training
phase). Of these tested miRNAs, only miR-204 was found to be
dysregulated in the patients with NSCLC, and thus it was selected
for further analysis. In the validation phase, we evaluated the
expression levels of miR-204 in patients with NSCLC (n=126) and
healthy controls (n=50) to determine whether a correlation exists
between its expression and the clinicopathological parameters of
patients with NSCLC. We also compared the diagnostic sensitivity
and specificity of plasma miR-204 levels with those of conventional
NSCLC biomarkers, such as carbohydrate antigen 19-9 (CA19-9) and
carcinoembryonic antigen (CEA) in blood.
Subjects and methods
Subjects and tissue samples
The study population consisted of 151 plasma samples
obtained from patients with NSCLC who underwent primary surgical
resection at the Department of Thoracic Surgery, Zhongshan Hospital
of Fudan University (Shanghai, China), between January 2009 and May
2011. We obtained blood samples from patients prior to surgery. In
addition, 15 cases were randomly selected for collecting blood
samples post-operatively. Patients who had received pre-operative
chemo- or radiotherapy were excluded from this study. Clinical
information on histological type, tumor stage and lymph node
involvement was obtained from medical records. Patients who had
stopped smoking for over 2 years were defined as former smokers.
All patients were regularly followed-up post-surgery though
clinical visits or through telephone communications. Two surgical
pathologists performed histological evaluations of the tumors. All
cases of cancer were staged according to the guidelines of the
tumor- node-metastasis (TNM) classification of the Union for
International Cancer Control (UICC). A total of 75 age- and
gender-matched healthy controls were also selected, including
smokers and non-smokers, but with no history of tumors.
This study was approved by the Ethics Committee of
Zhongshan Hospital of Fudan University. All patients provided
written informed consent indicating their willingness to donate
their blood for scientific research.
Study design
This study was divided into 2 phases, the training
phase and validation phase. In the training phase, we selected 25
patients with early-stage NSCLC and 25 age- and gender-matched
healthy controls to compare the expression profiles of these 4
miRNAs between patients with NSCLC and healthy individuals.
According to the training set results, we found that some of the
miRNAs exhibited statistically significant differences in
expression, and we thus conducted a validation experiment to
further examine the diagnostic and prognostic performance of these
miRNAs. In the validation set, plasma samples were collected from
126 patients with NSCLC and 50 healthy controls.
Plasma preparation and RNA
extraction
Plasma samples were obtained by centrifugation of
the peripheral blood (3 ml, collected in EDTA-K2 anti-coagulant
tubes) at 3,000 rpm for 20 min and at 12,000 rpm for 10 min at 4°C.
The plasma samples were aliquoted and stored in fresh tubes at
−80°C for the further analysis.
Total RNA (including miRNAs) from plasma samples was
extracted using the miRNeasy mini kit (Qiagen Inc., Valencia, CA,
USA) according to the manufacturer's instructions, and dissolved in
20 µl RNase-free water. The concentration and quality of the
isolated RNA were assessed on a NanoDrop ND-1000 Spectrophotometer
(NanoDrop Products, Wilmington, DE, USA). RNA samples were stored
at −80°C until further use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The reverse transcription reaction was conducted
using a TaqMan microRNA Reverse Transcription kit (Applied
Biosystems, Foster City, CA, USA) in a 15 µl solution
consisting of 5 µl RNA template, 1.5 µl 10X reverse
transcription buffer, 1 µl MultiScribe reverse
transcriptase, 0.15 µl of 100 mM dNTPs, 0.19 µl of
RNase inhibitor (20 U/µl), 1 µl of gene-specific
primer, and supplemented with nuclease-free water. For the
synthesis of complementary DNA (cDNA), the reaction mixtures were
incubated at 16°C for 30 min, at 42°C for 30 min, and at 85°C for
15 min and then held at 4°C.
RT-qPCR was used to quantify the expression levels
of the miRNAs. Each amplification reaction was carried out in a
final volume of 20 µl containing 2 µl of the cDNA
(100 ng), 10 µl of TaqMan 2X Universal PCR Master Mix with
No AmpErase UNG (Applied Biosystems), 1 µl of gene-specific
primer, and 7 µl of nuclease-free water. The ABI Prism 7300
RT-qPCR system (Applied Biosystems) was used to detect the plasma
miRNA levels. The PCR conditions consisted of an initial polymerase
activation step at 95°C for 10 min, 46 cycles of 95°C for 15 sec
and 60°C for 1 min. Each reaction was performed in triplicate. The
expression levels of the miRNAs in plasma were normalized to the
levels of small nuclear RNU6B (U6) and were calculated using the
2−ΔΔCt method, as previously described (24).
Measurement of CA19-9 and
carcinoembryonic levels in serum
Serum levels of CA19-9 and CEA are generally used in
the diagnosis of many types of cancer, including NSCLC (25). In this study, serum CA19-9 and CEA
levels in patients with NSCLC and healthy individuals were measured
by chemiluminescent enzyme immunoassays (Fujirebio, Inc., Tokyo,
Japan) according to the manufacturer's instructions. In brief, 50
µl of antigen and 50 µl of antibody were added to the
microwell and thoroughly mixed for 30 sec. Subsequently, 5 washings
were performed, after which 50 µl of chemiluminescence
substrate were added, and the mixture was incubated at room
temperature in the dark.
Statistical analysis
The SPSS 16.0 software package (SPSS Inc., Chicago,
IL, USA) and GraphPad Prism 5.0 statistical software (GraphPad
Software Inc., La Jolla, CA, USA) were utilized for all statistical
analyses. The expression levels of miRNAs were calculated based on
the 2−ΔΔCt method, and were analyzed as the means ±
standard deviation (SD). The independent two-sample Student's
t-test was used to analyze the correlations between miR-204
expression and the clinicopathological variables of the patients.
The Kruskal-Wallis test was performed to compare the expression of
miR-204 in different types of lung cancer. Receiver-operating
characteristic (ROC) curves of miR-204, CA19-9 and CEA were
generated to differentiate the patients with NSCLC from the healthy
controls. The optimal cut-off value for diagnosis was obtained
using Youden's Index (sensitivity + specificity −1 is maximal), as
previously described (26). The
Kaplan-Meier method was used for survival analysis, and the
differences in survival curves were compared using the log-rank
test. Cox regression analysis was used to estimate the univariate
and multivariate hazard ratios for prognosis. All p-values shown
are two-sided, and a p-value <0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miRNAs in the training
set
Of the 25 patients with NSCLC in the training set, 8
patients had stage I and 17 had stage II NSCLC, 12 had
adenocarcinoma, 8 had squamous cell carcinoma and 5 had another
subtype of NSCLC (Table I). A
total of 25 healthy individuals was selected as the normal
controls. The age and gender of the healthy controls were matched
with those of the patients with NSCLC. Firstly, we measured the
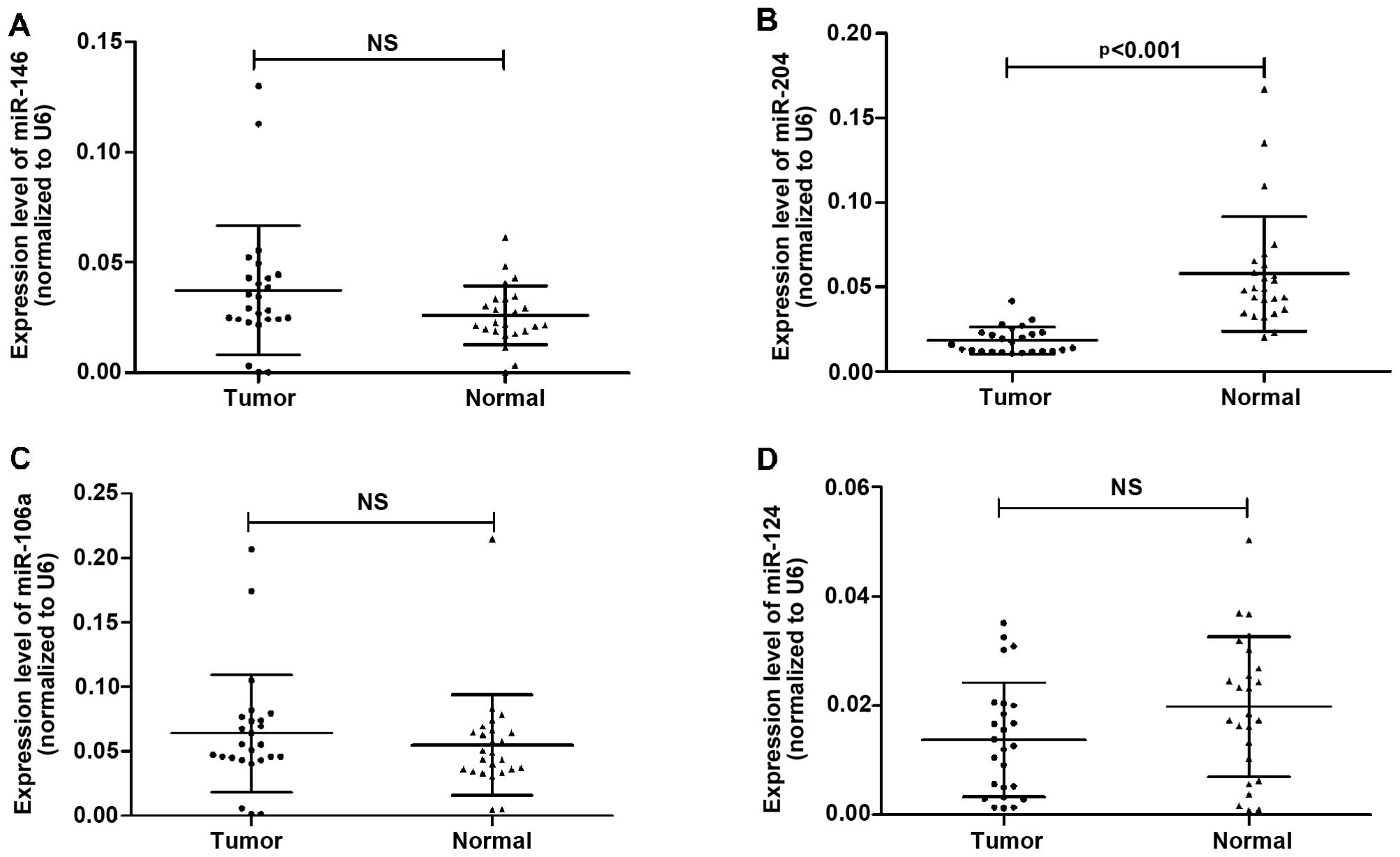
expression levels of miR-124, miR-146, miR-106a and miR-204 in
plasma from 25 patients with NSCLC and 25 healthy controls. We
observed a significant difference in the levels of miR-204 in
plasma between the patients with NSCLC and the healthy controls
(0.018±0.008 in tumors vs. 0.057±0.034 in controls, p<0.001),
whereas the difference in the expression levels of miR-146
(0.037±0.029 in tumors vs. 0.026±0.013 in controls), miR-106a
(0.064±0.045 in tumors vs. 0.055±0.039 in controls) and miR-124
(0.014±0.010 in tumors vs. 0.019±0.013 in controls) in plasma
between the patients with NSCLC and the controls were not deemed
statistically significant (Fig.
1). Thus, we selected miR-204 for further analysis.
 | Table ICharacteristics of patients in
training set. |
Table I
Characteristics of patients in
training set.
| Characteristics | No. of patients
(n=25) |
|---|
| Gender |
| Male | 16 |
| Female | 9 |
| Age (years) |
| <60 | 10 |
| ≥60 | 15 |
| Smoking
history |
| Never, former | 9 |
| Current | 16 |
| Histological
type |
|
Adenocarcinoma | 12 |
| Squamous cell
carcinoma | 8 |
| Others | 5 |
| TNM staging |
| I | 8 |
| II | 17 |
| Lymph node
metastasis |
| No | 9 |
| Yes | 16 |
Diagnostic performance of plasma miR-204
for NSCLC
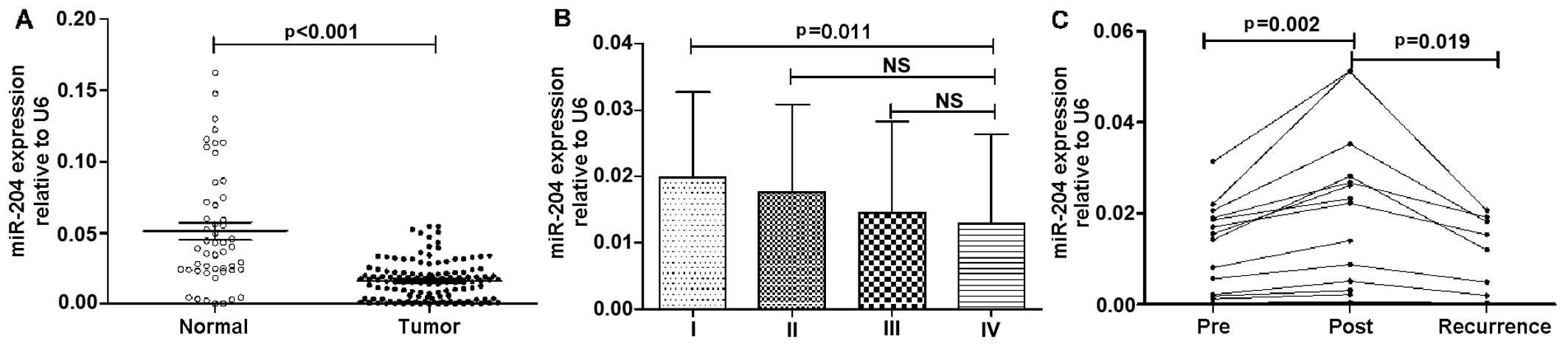
In the validation set, the plasma levels of miR-204
in 126 patients with NSCLC and 50 healthy controls (patient
characteristics in the validation set are shown in Table II) were measured by RT-qPCR. The
expression levels of miR-204 in plasma were significantly decreased
in the patients with NSCLC compared to the healthy controls
(0.016±0.013 vs. 0.051±0.041, p<0.001) (Fig. 2A). In addition, as shown in
Fig. 2B, the plasma levels of
miR-204 in the patients with stage IV NSCLC were much lower than
those in the patients with stage I NSCLC (p=0.011), whereas no
significant differences were observed between the patients with
stage II and IV of the disease, or between those with stage III and
IV of the disease (p>0.05). Moreover, the expression levels of
miR-204 in plasma were found to be re-decreased in 8 patients who
developed post-operative recurrence of distant metastasis during
follow-up examinations (Fig.
2C).
 | Table IIPatient clinicopathological
characteristics and correlation with plasma miR-204 expression
levels. |
Table II
Patient clinicopathological
characteristics and correlation with plasma miR-204 expression
levels.
|
Characteristics | No. of patients
n=126 | miR-204 expression
(means ± SD) | p-value |
|---|
| Gender | | | 0.346a |
| Female | 45 | 0.015±0.012 | |
| Male | 81 | 0.017±0.014 | |
| Age (years) | | | 0.186a |
| <60 | 61 | 0.018±0.014 | |
| ≥60 | 65 | 0.015±0.013 | |
| Smoking
condition | | | 0.858a |
| Former/never | 47 | 0.017±0.013 | |
| Current | 79 | 0.016±0.014 | |
| Histological
type | | | 0.359b |
|
Adenocarcinoma | 63 | 0.017±0.013 | |
| Squamous cell
carcinoma | 53 | 0.015±0.014 | |
| Other | 10 | 0.019±0.011 | |
|
Differentiation | | | 0.157a |
| Well/moderate | 86 | 0.017±0.013 | |
| Poor | 40 | 0.014±0.014 | |
| TNM stage | | | 0.009a |
| I, II | 66 | 0.019±0.013 | |
| III, IV | 60 | 0.014±0.014 | |
| Lymph node
metastasis | | | 0.432a |
| N0 | 77 | 0.017±0.013 | |
| N1-3 | 49 | 0.016±0.014 | |
| Distant
metastasis | | | 0.048a |
| No | 97 | 0.017±0.013 | |
| Yes | 29 | 0.013±0.014 | |
| Recurrence | | | 0.189a |
| No | 111 | 0.017±0.014 | |
| Yes | 15 | 0.012±0.010 | |
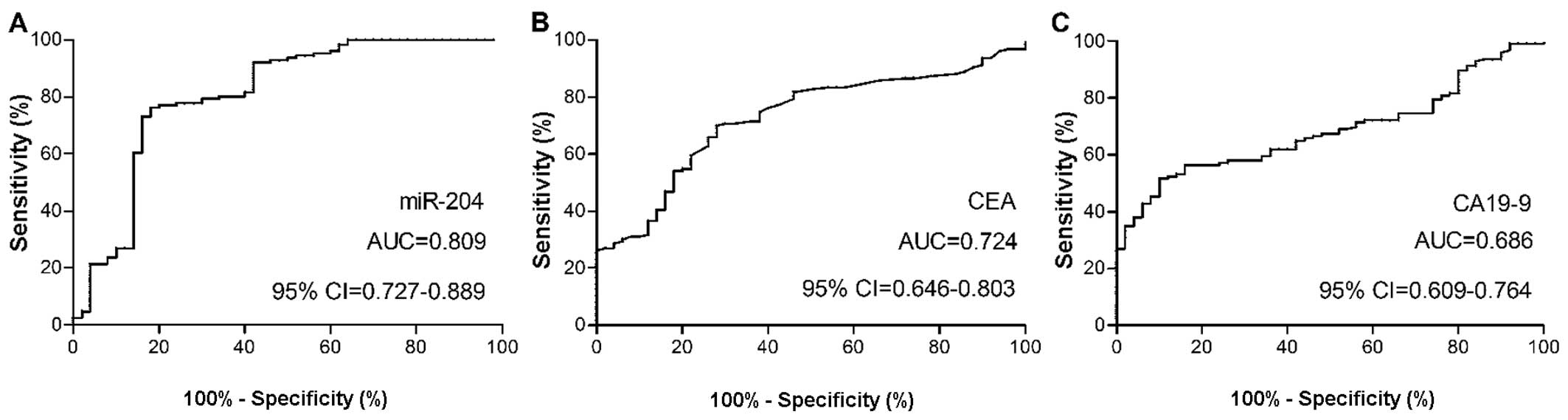
In order to evaluate the diagnostic capabilities of
the plasma levels of miR-204, and serum levels of CEA and CA19-9
for NSCLC, ROC curve analysis was performed. The optimal cut-off
value for plasma miR-204 levels in the patients with NSCLC was
0.023, which provided a sensitivity of 76% and a specificity of
82%, with an area under the curve (AUC) value of 0.809, which was
used for differentiating the patients with NSCLC from the healthy
controls. Two conventional NSCLC markers, CEA and CA19-9, were also
measured in all subjects. At a cut-off value of 5.6 for the CEA
expression levels, the optimal sensitivity and specificity were 70
and 72%, respectively. At a cut-off value of 51.9 for the CA19-9
expression levels, the optimal sensitivity and specificity were 57
and 84%, respectively. The AUC for the plasma level of plasma
miR-204 was significantly larger than that for CEA (0.724) or
CA19-9 (0.686) (Fig. 3),
indicating that the plasma miR-204 levels are superior to the serum
levels of CEA or CA19-9 for the detection of NSCLC.
Correlation between plasma miR-204 and
clinicopathological characteristics
We evaluated the correlation of plasma miR-204 with
clinicopathological parameters, including gender, age, TNM stage
and histological type. These data are summarized in Table II. The low level of plasma
miR-204 was found to significantly correlated with TNM stage
(p=0.009) and distant metastasis (p=0.048), whereas no significant
correlation between miR-204 and gender, age, differentiation,
histological type and lymph node involvement was observed.
Expression of miR-204 in plasma samples
in relation to prognosis
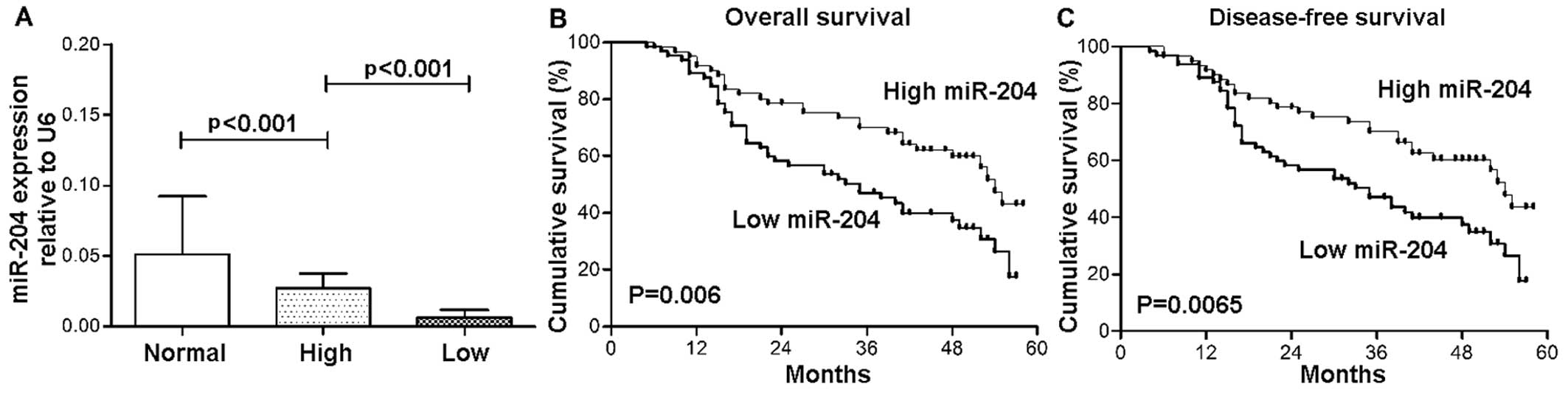
The patients were divided into 2 groups according to
the median relative expression levels of miR-204: the low and high
expression group. As shown in Fig.
4, the overall survival (OS) and disease-free survival (DFS) of
the patients with lower plasma levels of miR-204 were poorer than
those of patients with higher levels of miR-204 [lower (median
survival = 32 months) vs. higher (median survival = 43 months) for
OS (p=0.006), and lower (30 months) vs. higher (40 months) for DFS
(p=0.0065), respectively].
We also carried out univariate and multivariate
analyses in order to examine the prognostic significance of the
clinicopathological characteristics and miR-204 expression. Using
Cox regression analysis, we found that a higher TNM stage (III and
IV), positivity for distant metastasis, recurrence and a low
miR-204 expression significantly correlated with a relative risk of
mortality of 4.311, 12.942, 2.757 and 1.936, respectively (Table III). All these prognostic
factors were confirmed by multivariate analysis. These data
revealed that the lower expression of miR-204 in plasma [hazard
ratio (HR), 1.712; 95% confidence interval (CI), 1.038–2.825;
p=0.035], a higher TNM stage (HR, 2.215; 95% CI, 1.207–4.063;
p=0.010), the presence of distant metastasis (HR, 8.086; 95% CI,
4.176–15.657; p=0.000) and recurrence (HR, 2.329; 95% CI,
1.251–4.334; p=0.008) were independently associated with a
significantly increased risk of mortality (Table III).
 | Table IIICox proportional regression analysis
for assessing the correlation of miR-204 expression with the
prognosis of patients with non-small cell lung cancer. |
Table III
Cox proportional regression analysis
for assessing the correlation of miR-204 expression with the
prognosis of patients with non-small cell lung cancer.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| Age (<60,
≥60) | 0.962 | 0.602-1.539 | 0.872 | | | |
| Gender (fema) | 1.106 | 0.689-1.775 | 0.678 | | | |
| Distant metastasis
(no, yes)le, male) | 1.006 | 0.611-1.659 | 0.980 | | | |
| Smoking history
(former/never, current) | 1.095 | 0.667-1.797 | 0.720 | | | |
| Histological type
(AC, SCC) | 1.253 | 0.880-1.786 | 0.211 | | | |
| Differentiation
(well/moderate, poor) | 1.138 | 0.676-1.716 | 0.805 | | | |
| TNM stage (I/II,
III/IV) | 4.311 | 2.575-7.219 | 0.000 | 2.215 | 1.207-4.063 | 0.010 |
| Lymph node
metastasis (N0, N1-3 | 12.942 | 7.351-22.788 | 0.000 | 8.086 | 4.176-15.657 | 0.000 |
| Recurrence (no,
yes) | 2.757 | 1.490-5.100 | 0.001 | 2.329 | 1.251-4.334 | 0.008 |
| miR-204 level
(high, low) | 1.936 | 1.193-3.143 | 0.008 | 1.712 | 1.038-2.825 | 0.035 |
Discussion
miRNAs are generally defined as regulatory
molecules, and studies have indicated that the abnormal expression
of miRNAs is associated with various types of cancer. More
importantly, miRNAs have been found to be stably expressed in
bodily fluids, including serum, plasma, urine, and saliva (10,27–30), thus providing a promising strategy
for the identification of miRNAs for use as a less invasive and
more convenient tool that can be used in diagnosis, prognosis or
treatment monitoring. However, the majority of studies have focused
on the examination of miRNA levels in tissue specimens (7), and only a limited number have
examined the practicability of plasma miRNAs as biomarkers
(13,14). As is already known, early
detection is the most effective method of reducing the high
mortality rate of patients with NSCLC. Thus, in this study, we
investigated potential miRNAs for use as biomarkers for the
diagnosis of NSCLC.
In the present study, we initially set out to
analyze the plasma levels of 4 miRNAs, including miR-124, miR-204,
miR-106a and miR-146 in patients with NSCLC and healthy controls
based on 50 participants (training phase). These miRNAs have been
reported to act as oncogenes or tumor-suppressors, and have been
shown to be associated with several critical biological processes,
including migration, invasion, apoptosis and proliferation
(5,6). However, these selected miRNAs
exhibited no significant differences in expression between the
patients with NSCLC and the healthy controls, with the exception of
miR-204. Thus, we subsequently measured the expression levels of
miR-204 in plasma samples from a large cohort of patients with
NSCLC and evaluated the diagnostic capabilities and prognostic
value of miR-204 in these patients. To the best of our knowledge,
this study is the first study which used plasma samples to evaluate
the predictive value of miR-204 as a potential biomarker for
NSCLC.
As a tumor suppressor, miR-204 has been reported to
be markedly decreased in malignant tissues, including gastric
cancer (16), breast cancer
(17), renal cell carcinoma
(18), and NSCLC (19). Researchers firstly identified
miR-204 as a novel, non-invasive biomarker for endometrioid
endometrial cancer diagnosis by using genome-wide serum miRNA
expression profiling (31). In
the present study, we also noted a significantly decreased level of
miR-204 in plasma from patients with NSCLC compared with healthy
controls. Our findings were consistent with those of previous
studies and suggested that the downregulation of miR-204 is
involved in the initiation and progression of NSCLC. Functionally,
the loss of miR-204 promotes cancer cell migration and invasion by
regulating target genes (19,20). miR-204 plays a role in the direct
regulation of epithelial-to-mesenchymal transition (EMT) through
its targeting of SMAD4 (32). The
overexpression of miR-204 has been shown to inhibit cancer cell
proliferation in gastric and colorectal cancer (21,33). Thus, the findings of these
above-mentioned previous studies, as well as those from our study
support the tumor-suppressor role of miR-204 in cancer.
Certain serum markers have been widely used in the
diagnosis of cancer, such as CEA and CA19-9. Therefore, we compared
the diagnostic capabilities of miR-204 with those of conventional
tumor markers. Based on ROC curve analysis, we found that miR-204
was suitable for differentiating patients with NSCLC from healthy
individuals, with a sensitivity and specificity of 76 and 82%,
respectively. The AUC value for plasma miR-204 was 0.809, which was
higher than the AUC values obtained with CEA and CA19-9. Our
results suggest that plasma miR-204 is a good diagnostic marker and
a more reliable alternative for the diagnosis of NSCLC.
miR-204 has previously been shown to be
downregulated in tissue specimens from patients with NSCLC
(19,34). However, whether plasma levels of
miR-204 are associated with prognosis, OS and DFS in patients with
NSCLC remains unclear. In this study, we found that the plasma
levels of miR-204 were significantly upregulated in plasma
collected post-operatively compared to that in plasma collected
pre-operatively from the same patients, and was then downregulated
again in post-operative recurrent cases. Our results suggest that
miR-204 may be used to monitor and evaluate the tumor dynamics of
NSCLC. Moreover, we observed a stepwise decreased expression of
plasma miR-204 with the increasing tumor stage. In addition, we
analyzed the prognostic role of the expression of miR-204 in
plasmas from patients with NSCLC. We found that a low expression of
miR-204 in plasma samples correlated with an advanced clinical
stage and distant metastasis in NSCLC. This result was in
accordance with that of previous studies n breast cancer (17), nasopharyngeal carcinoma (35) and endometrial cancer (36). These results suggest that miR-204
in plasma isa potential marker which reflects cancer
progression.
It has previously been reported that the low
expression of miR-204 correlates with a poor prognosis in several
types of cancer (17,19,21,37). Thus, in the present study we
further analyzed the association of plasma levels of miR-204 with
patient clinical outcomes. Similarly, we found that low plasma
levels of miR-204 correlated with poorer survival by using the
log-rank test, and univariate and multivariate analyses, indicating
that miR-204 is an independent prognostic factor for survival.
These are surprising novel data which are important for our current
understanding of NSCLC. Moreover, Yin et al found that
restoring miR-204 expression promoted tumor sensitivity to
chemotherapy in colorectal cancer patients (21). miR-204 targeted Bcl-2 messenger
RNA and increased the responsiveness of gastric cancer cells to
5-fluorouracil and oxaliplatin treatment (38). If these findings are further
determined in NSCLC patients, miR-204 may thus have the potential
to assist in the treatment of patients with NSCLC and reduce the
mortality rate in patients with this disease.
In conclusion, in the present study, we demonstrated
that miR-204 in plasma from patients with NSCLC was downregu-lated.
Thus, we suggest that the plasma levels of miR-204 are more
reliable than the levels of CEA and CA19-9 for diagnosis. More
importantly, the decreased expression of miR-204 in plasma is
likely a promising marker for predicting poor survival in patients
with NSCLC. Further investigations with larger sample sizes and
other types of cancer are warranted be in order to evaluate the
possibility of using plasma miR-204 as a non-invasive marker for
cancer detection and clinical outcome prediction.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu YL and Zhou Q: Clinical trials and
biomarker research on lung cancer in China. Expert Opin Ther
Targets. 16(Suppl 1): S45–S50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dykxhoorn DM: MicroRNAs and metastasis:
little RNAs go a long way. Cancer Res. 70:6401–6406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garzon R, Fabbri M, Cimmino A, Calin GA
and Croce CM: MicroRNA expression and function in cancer. Trends
Mol Med. 12:580–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
7
|
Li W, Wang Y, Zhang Q, Tang L, Liu X, Dai
Y, Xiao L, Huang S, Chen L, Guo Z, Lu J and Yuan K: MicroRNA-486 as
a biomarker for early diagnosis and recurrence of non-small cell
lung cancer. PLoS One. 10:e01342202015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mavridis K, Gueugnon F, Petit-Courty A,
Courty Y, Barascu A, Guyetant S and Scorilas A: The oncomiR miR-197
is a novel prognostic indicator for non-small cell lung cancer
patients. Br J Cancer. 112:1527–1535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tejero R, Navarro A, Campayo M, Viñolas N,
Marrades RM, Cordeiro A, Ruíz-Martínez M, Santasusagna S, Molins L,
Ramirez J and Monzó M: miR-141 and miR-200c as markers of overall
survival in early stage non-small cell lung cancer adenocarcinoma.
PLoS One. 9:e1018992014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patnaik SK, Mallick R and Yendamuri S:
Detection of microRNAs in dried serum blots. Anal Biochem.
407:147–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang X, Zeng Z, Hou Y, Yuan T, Gao C, Jia
W, Yi X and Liu M: MicroRNA-92a as a potential biomarker in
diagnosis of colorectal cancer: a systematic review and
meta-analysis. PLoS One. 9:e887452014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen J, Hruby GW, McKiernan JM, Gurvich I,
Lipsky MJ, Benson MC and Santella RM: Dysregulation of circulating
microRNAs and prediction of aggressive prostate cancer. Prostate.
72:1469–1477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ng EK, Li R, Shin VY, Jin HC, Leung CP, Ma
ES, Pang R, Chua D, Chu KM, Law WL, et al: Circulating microRNAs as
specific biomarkers for breast cancer detection. PLoS One.
8:e531412013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gorur A, Balci Fidanci S, Dogruer Unal N,
Ayaz L, Akbayir S, Yildirim Yaroglu H, Dirlik M, Serin MS and Tamer
L: Determination of plasma microRNA for early detection of gastric
cancer. Mol Biol Rep. 40:2091–2096. 2013. View Article : Google Scholar
|
|
16
|
Zhang B, Yin Y, Hu Y, Zhang J, Bian Z,
Song M, Hua D and Huang Z: MicroRNA-204-5p inhibits gastric cancer
cell proliferation by downregulating USP47 and RAB22A. Med Oncol.
32:3312015. View Article : Google Scholar
|
|
17
|
Li W, Jin X, Zhang Q, Zhang G, Deng X and
Ma L: Decreased expression of miR-204 is associated with poor
prognosis in patients with breast cancer. Int J Clin Exp Pathol.
7:3287–3292. 2014.PubMed/NCBI
|
|
18
|
Munari E, Marchionni L, Chitre A, Hayashi
M, Martignoni G, Brunelli M, Gobbo S, Argani P, Allaf M, Hoque MO
and Netto GJ: Clear cell papillary renal cell carcinoma: micro-RNA
expression profiling and comparison with clear cell renal cell
carcinoma and papillary renal cell carcinoma. Hum Pathol.
45:1130–1138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi L, Zhang B, Sun X, Lu S, Liu Z, Liu Y,
Li H, Wang L, Wang X and Zhao C: MiR-204 inhibits human NSCLC
metastasis through suppression of NUAK1. Br J Cancer.
111:2316–2327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imam JS, Plyler JR, Bansal H, Prajapati S,
Bansal S, Rebeles J, Chen HI, Chang YF, Panneerdoss S, Zoghi B, et
al: Genomic loss of tumor suppressor miRNA-204 promotes cancer cell
migration and invasion by activating AKT/mTOR/Rac1 signaling and
actin reorganization. PLoS One. 7:e523972012. View Article : Google Scholar
|
|
21
|
Yin Y, Zhang B, Wang W, Fei B, Quan C,
Zhang J, Song M, Bian Z, Wang Q, Ni S, et al: miR-204-5p inhibits
proliferation and invasion and enhances chemotherapeutic
sensitivity of colorectal cancer cells by downregulating RAB22A.
Clin Cancer Res. 20:6187–6199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu X, Zeng Y, Wu S, Zhong J, Wang Y and Xu
J: MiR-204, down-regulated in retinoblastoma, regulates
proliferation and invasion of human retinoblastoma cells by
targeting CyclinD2 and MMP-9. FEBS Lett. 589:645–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li G, Luna C, Qiu J, Epstein DL and
Gonzalez P: Role of miR-204 in the regulation of apoptosis,
endoplasmic reticulum stress response, and inflammation in human
trabecular meshwork cells. Invest Ophthalmol Vis Sci. 52:2999–3007.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Niklinski J, Furman M, Laudanski J and
Kozlowski M: Prognostic value of pretreatment CEA, SCC-Ag and CA
19-9 levels in sera of patients with non-small cell lung cancer.
Eur J Cancer Prev. 1:401–406. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruopp MD, Perkins NJ, Whitcomb BW and
Schisterman EF: Youden Index and optimal cut-point estimated from
observations affected by a lower limit of detection. Biom J.
50:419–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schwarzenbach H, Nishida N, Calin GA and
Pantel K: Clinical relevance of circulating cell-free microRNAs in
cancer. Nat Rev Clin Oncol. 11:145–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang K, Peng X, Luo J and Gou D:
Identification of circulating miRNA biomarkers based on global
quantitative real-time PCR profiling. J Anim Sci Biotechnol.
3:42012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Zhang KY, Liu SM and Sen S:
Tumor-associated circulating microRNAs as biomarkers of cancer.
Molecules. 19:1912–1938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cortez MA, Bueso-Ramos C, Ferdin J,
Lopez-Berestein G, Sood AK and Calin GA: MicroRNAs in body fluids -
the mix of hormones and biomarkers. Nat Rev Clin Oncol. 8:467–477.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia W, Wu Y, Zhang Q, Gao G, Zhang C and
Xiang Y: Identification of four serum microRNAs from a genome-wide
serum microRNA expression profile as potential non-invasive
biomarkers for endometrioid endometrial cancer. Oncol Lett.
6:261–267. 2013.PubMed/NCBI
|
|
32
|
Wang Y, Li W, Zang X, Chen N, Liu T,
Tsonis PA and Huang Y: MicroRNA-204-5p regulates
epithelial-to-mesenchymal transition during human posterior capsule
opacification by targeting SMAD4. Invest Ophthalmol Vis Sci.
54:323–332. 2013. View Article : Google Scholar
|
|
33
|
Zhou X, Li L, Su J and Zhang G: Decreased
miR-204 in H pylori- associated gastric cancer promotes cancer cell
proliferation and invasion by targeting SOX4. PLoS One.
9:e1014572014. View Article : Google Scholar
|
|
34
|
Xia Y, Zhu Y, Ma T, Pan C, Wang J, He Z,
Li Z, Qi X and Chen Y: miR-204 functions as a tumor suppressor by
regulating SIX1 in NSCLC. FEBS Lett. 588:3703–3712. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma L, Deng X, Wu M, Zhang G and Huang J:
Down-regulation of miRNA-204 by LMP-1 enhances CDC42 activity and
facilitates invasion of EBV-associated nasopharyngeal carcinoma
cells. FEBS Lett. 588:1562–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bao W, Wang HH, Tian FJ, He XY, Qiu MT,
Wang JY, Zhang HJ, Wang LH and Wan XP: A TrkB-STAT3-miR-204-5p
regulatory circuitry controls proliferation and invasion of
endometrial carcinoma cells. Mol Cancer. 12:1552013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ryan J, Tivnan A, Fay J, Bryan K, Meehan
M, Creevey L, Lynch J, Bray IM, O'Meara A, Tracey L, et al:
MicroRNA-204 increases sensitivity of neuroblastoma cells to
cisplatin and is associated with a favourable clinical outcome. Br
J Cancer. 107:967–976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sacconi A, Biagioni F, Canu V, Mori F, Di
Benedetto A, Lorenzon L, Ercolani C, Di Agostino S, Cambria AM,
Germoni S, et al: miR-204 targets Bcl-2 expression and enhances
responsiveness of gastric cancer. Cell Death Dis. 3:e4232012.
View Article : Google Scholar : PubMed/NCBI
|