Introduction
Parkinson's disease (PD) is a common and chronic
neurode-generative disorder caused by the selective and progressive
loss of dopaminergic neurons in the substantia nigra, leading to a
depletion of the dopamine neurotransmitter in the striatum
(1). While the etiology remains
unclear, environmental toxins such as
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; a lipophilic
molecule that rapidly crosses the blood-brain barrier), have been
suggested to be involved in the pathogenesis of PD (2–5).
Having crossed the barrier, it is oxidized in the brain to its
toxic metabolite, 1-methyl-4-phenylpyridinium (MPP+), by
monoamine oxidase type B (MAO-B) (6). MPP+ then enters the
dopaminergic neurons via the dopamine transporter and is
transported to the mitochondria where it causes the inhibition of
mitochondrial respiration and energy depletion, by interacting with
the respiration complex I (7),
leading to reactive oxygen species (ROS) production (8–11).
ROS production is widely recognized as a major initiator triggering
sequential events leading to the degeneration of dopaminergic
neurons (12–15). Postmortem studies of the brains of
patients with PD have shown increased levels of 4-hydroxy-2-nonenal
(HNE), a by-product of lipid peroxidation (16,17) and carbonyl modifications of
soluble proteins (18),
supporting the involvement of oxidative damage in dopaminergic
neuron degeneration. The well-known parkinsonism inducers, MPTP,
rotenone and 6-hydroxydopamine (6-OHDA) have been shown to cause
ROS production and the degeneration of dopaminergic neurons in
animal models, further supporting the involvement of oxidative
stress in the pathogenesis of PD (19–23). The molecular mechanisms
responsible for the gradual loss of dopaminergic neurons under
conditions of oxidative stress are not yet fully understood.
However, DNA damage-mediated cell death has been
suggested to be associated with neuronal cell death in PD (24). This is supported by analyses
showing selective increases in levels of DNA and RNA oxidation
products, 8-hydroxy-deoxyguanosine and 8-hydroxy-guanosine, in the
substantia nigra during postmortem studies of brains affected by PD
(25,26). Oxidative DNA damage has also been
observed in the brains tissue of mice exposed to MPTP and other
neuronal toxins that induce a PD-like pathology (27). Proliferating cell nuclear antigen
(PCNA) is a well known determinant of DNA biological function,
including DNA replication and repair, as well as cell cycle control
(28,29), and thus plays a crucial role in
maintaining in the integrity of the genome, as well as cell
survival. Previous studies have shown that PCNA plays a role in the
repair of DNA damage under conditions of oxidative stress (30,31). In this study, we examined the
changes in the levels of this protein in MPP+-stimulated
PC12 cells, in order to identify potential causes of dopaminergic
neuron degeneration and to elucidate the underlying molecular
mechanisms. Our results demonstrated that MPP+ induced
the loss of cell viability and the apoptosis of dopaminergic
neuronal cells, in a time- and dose-dependent manner.
MPP+ also decreased PCNA protein expression, and this
was accompanied by the impairment of PC12 cells, suggesting a
correlation between the levels of this protein and damage to PC12
cells under conditions of oxidative stress. Notably,
MPP+ induced the significant upregulation of p53
expression, which is an upstream modulator of PCNA and has been
recognized as a key contributor responsible for dopaminergic
neuronal cell death in mouse models of MPTP-induced PD (32,33). Overall, these findings indicate
that PCNA may play a crucial role in oxidative stress-induced
damage to dopaminergic neurons, thus, providing a therapeutic
target for molecular-based strategies in the treatment of PD.
Materials and methods
Drugs and chemicals
All reagents and chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise.
Cell culture
The rat adrenal pheochromocytoma cell line, PC12,
was purchased from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were maintained in high glucose
Dulbecco's modified Eagle's medium (DMEM) supplemented with 100
U/ml of penicillin, 100 µg/ml of streptomycin, 4 mM
L-glutamine and 10% inactivated fetal serum, (Gibco, Grand Island,
NY, USA). The cultures were maintained in a 5% CO2
incubator at 37°C.
Cell viability assay
The viability of the PC12 cells was evaluated using
the modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltet-razolium
bromide (MTT) assay. MTT is reduced by metabolically active cells
to form blue formazan crystals. The PC12 cells were plated on
96-well plates at a density of 30,000 cells/cm2 and
incubated for 24 h. To assess the toxicity of MPP+ to
the PC12 cells, the cells were exposed to various concentrations of
MPP+ (0.5, 1 and 2 mM). MTT solution (5 mg/ml) was then
added to each well and the cells were incubated for 4 h. The
culture medium was removed, and the formazan crystals were
dissolved in dimethyl sulfoxide (DMSO). The absorbance of the
colored solution was measured at 570 nm using a microplate reader
(Epoch; BioTek, Winooski, VT, USA). The results are expressed as a
percentage of the absorbance of the control culture wells (cells
not exposed to MPP+). The experiment was repeated 3
times.
Nuclear staining assay
Morphological changes in the cell nuclei induced by
MPP+ were evaluated using acridine orange/ethidium
bromide (AO/EB) staining. The PC12 cells were plated on 6-well
plates at a density of 30,000 cells/cm2 and incubated in
DMEM for 24 h. Following exposure to 1 mM MPP+ for 48 h,
the cells were washed and resuspended in phosphate-buffered saline
(PBS) followed by the addition of AO/EB (final concentration 1
µg/ml). The cells were then examined under a fluorescence
microscope (IX71; Olympus Corp., Tokyo, Japan). Living cells with
intact structures were stained green, whereas early apoptotic cells
exhibited condensed green nuclei, and late apoptotic cells
exhibited condensed red-orange chromatin. At least 300 cells were
randomly observed and the number of apoptotic cells is expressed as
a percentage of the total number of cells counted.
DNA fragmentation assay
DNA denaturation in the apoptotic cells was
determined by a single-stranded DNA (ssDNA) assay using an ssDNA
apoptosis enzyme-linked immunosorbent assay (ELISA) kit (Chemicon
International, Temecula, CA, USA), according to the manufacturer's
instructions. This procedure is based on the ability of a
monoclonal antibody to detect ssDNA in apoptotic cells but not in
necrotic cells. The cells at a concentration of 30,000
cells/cm2 were cultured for 24 h, followed by treatment
with various concentrations (0.5, 1, 2 mM) of MPP+.
Following 24 h of incubation, the staining of ssDNA was performed,
and ssDNA fragmentation was determined by measuring the absorbance
at a wavelength of 405 nm using a microplate reader (Epoch;
BioTek).
Measurement of oxidative stress
Oxidative stress was measured in the PC12 cells
using 2′-7′-dichlorofluorescein diacetate (DCFH-DA) based on the
ROS-dependent oxidation of DCFH-DA to fluorescent
dichlorofluorescein (DCF). DCFH-DA easily crosses the membrane into
cells and is converted into non-fluorescent dichlorofluorescein
(DCFH) by intracellular esterase. DCFH is then oxidized into highly
fluorescent DCF by intracellular ROS, thereby the density of
fluorescence reflects an overall index of oxidative activity.
Following exposure, the cells were incubated in bovine serum
albumin (BSA)-free DMEM with DCFH-DA at a final concentration of 20
µM for 30 min at 37°C. Thereafter, the cells in each group
were analyzed by flow cytometry using the FL1 flow cytometer
detection channels (BD Biosciences, San Jose, CA, USA). The
excitation wavelength was 485 nm and the reading was performed at
530 nm.
Western blot analysis
Following exposure to MPP+, the PC12
cells were harvested and lysed with cell lysis solution containing
4% sodium dodecyl sulfate (SDS), 2 mM EDTA, 50 mM Tris-HCl (pH
6.8). Equal amounts of protein were loaded onto a 12%
SDS-polyacrylamide gel. Following electrophoretic separation, the
gels were transferred onto PVDF membranes (Amersham Biosciences,
Uppsala, Sweden). The membranes were subsequently incubated in
Tris-buffered saline/Tween-20 (TBST) buffer supplemented with 5%
fat-free milk for 1 h. The membranes were then blotted with mouse
monoclonal anti-rat PCNA antibodies (Cat. no. 610664; BD
Biosciences) and mouse monoclonal anti-rat p53 antibodies (Cat. no.
554157; BD Biosciences), and horseradish peroxidase-conjugated
anti-mouse secondary antibodies (Cat. no. R-21455; Pierce
Biotechnology, Inc., Rockford, IL, USA) were used as the secondary
antidodies. β-actin was used as an internal control.
Statistic analysis
Data are expressed as the means ± SEM. Statistical
analysis was performed using one-way analysis of variance (ANOVA)
or a Student's t-test. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
MPP+ induces the loss of the
viability of PC12 cells
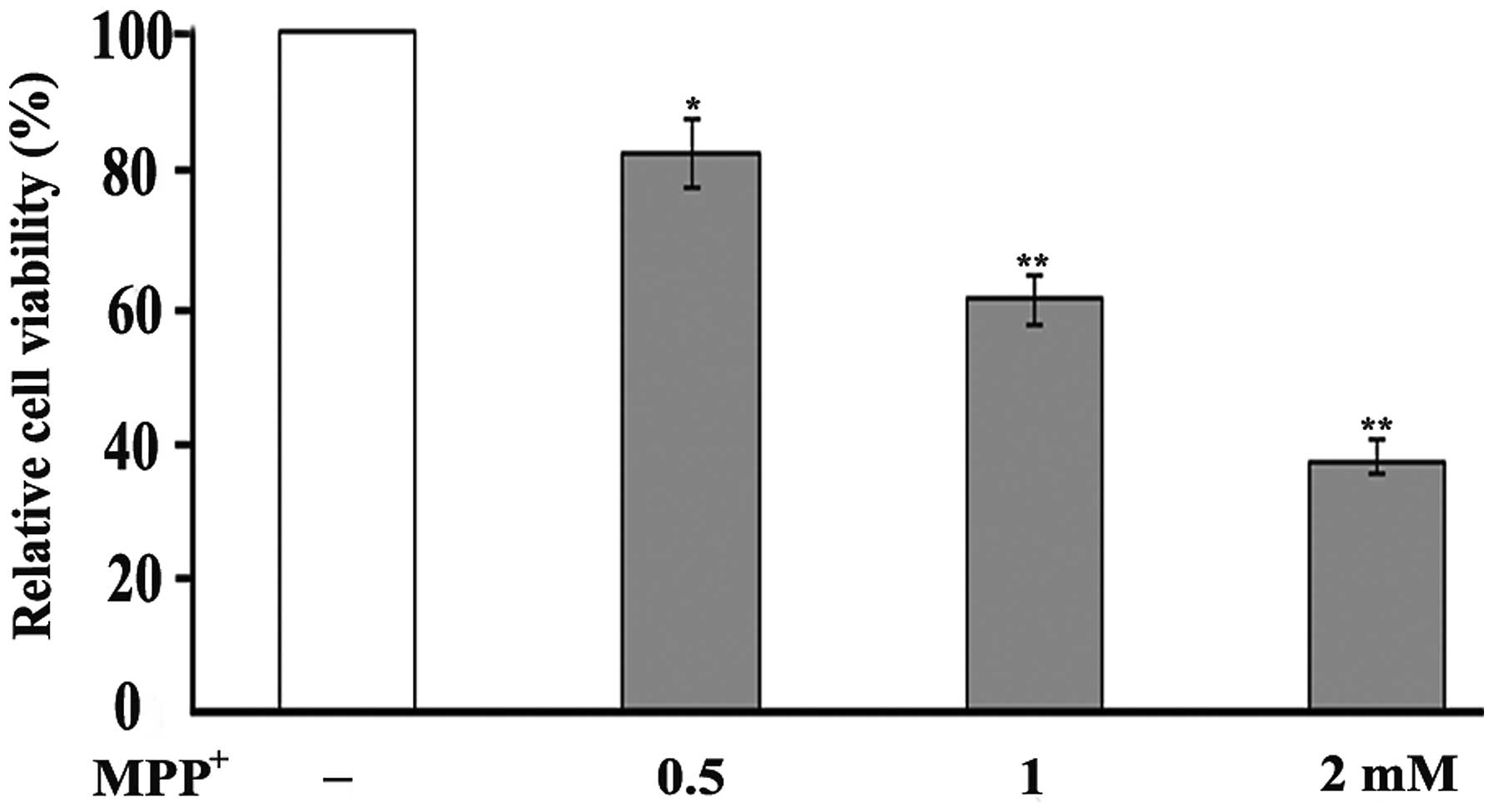
The oxidative damage induced by MPP+ to
the PC12 cells was examined by MTT assay, a colorimetric assay used
for measuring the activity of mitochondrial dehydrogenases in
metabolically active cells. The measurements revealed a decrease in
cell viability following the exposure of PC12 cells to
MPP+ in a dose-dependent manner. Following 48 h of
exposure to 0.5 mM MPP+, cell viability was reduced to
82% of the control, while exposure to 1 and 2 mM MPP+
decreased cell viability to 61 and 37% of the control, respectively
(Fig. 1).
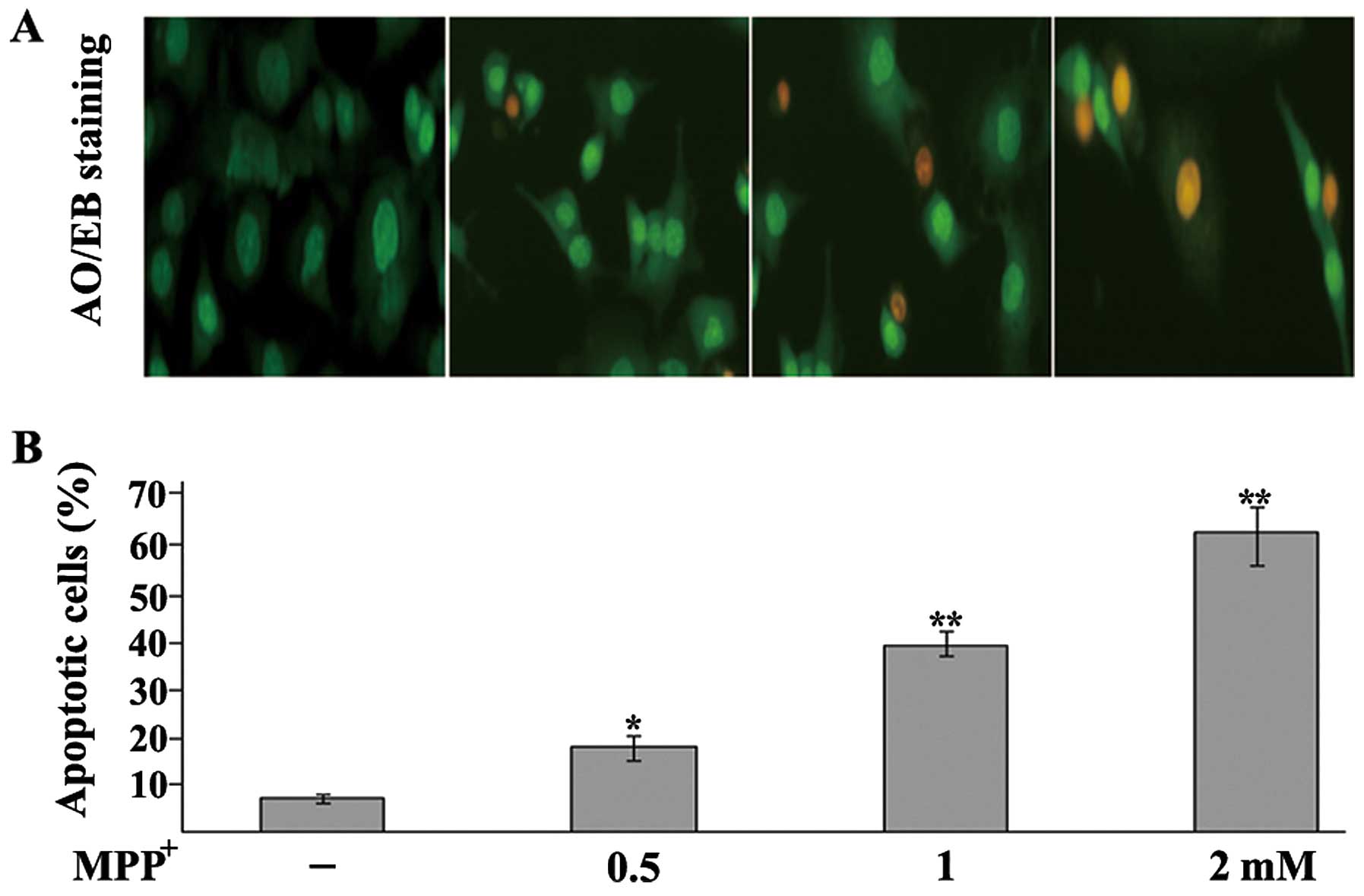
MPP+ induces the apoptosis of
dopaminergic neurons
To examine the MPP+-induced apoptosis of
the PC12 cells, an AO/EB staining assay and a DNA fragmentation
assay were performed. Apoptosis is a process of programmed cell
death characterized by a series of distinct nuclear morphological
changes, which can be detected by AO/EB staining. Exposure to
MPP+ significantly increased the percentage of apoptotic
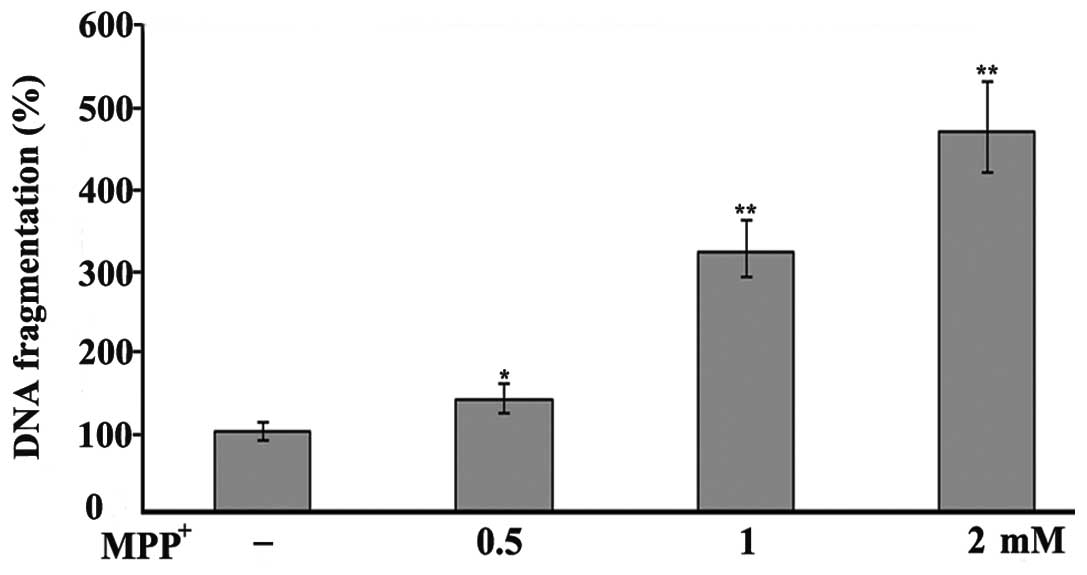
cells in a concentration-dependent manner (Fig. 2). To further examine the toxic
effects of MPP+ on the PC12 cells, DNA fragmentation was
investigated by an ssDNA assay. The results revealed an increase in
DNA fragmentation following exposure to MPP+ (Fig. 3), thus incating that
MPP+ is toxic to PC12 cells.
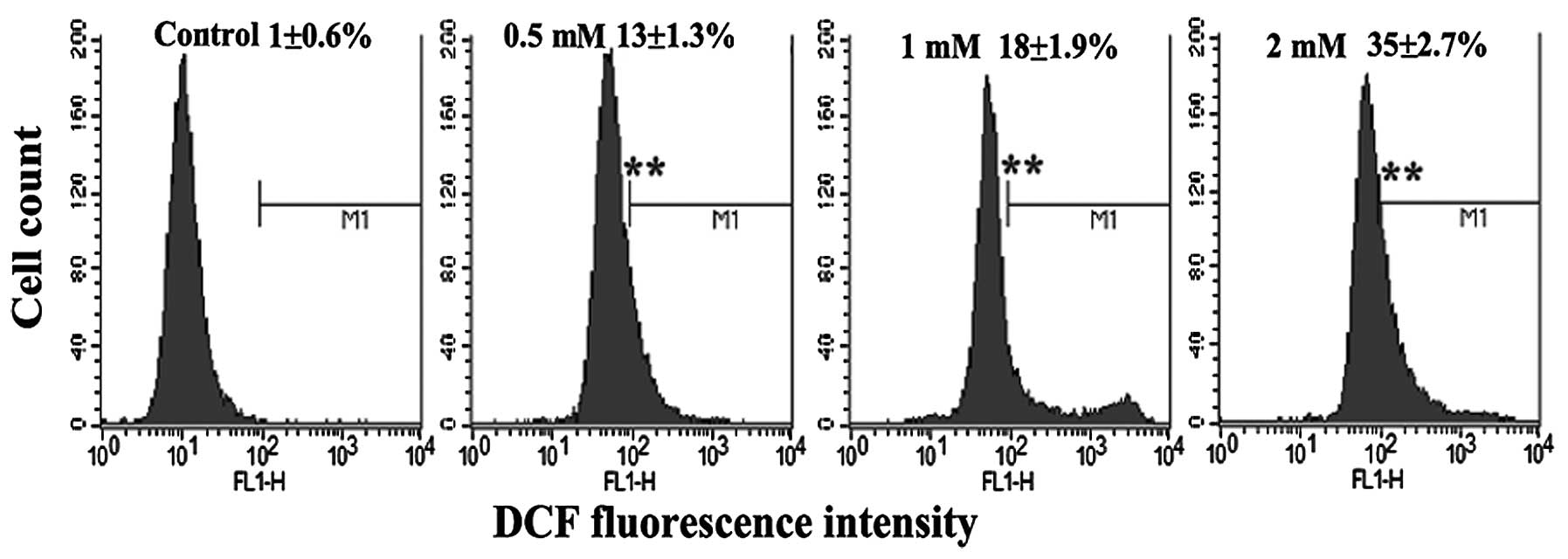
MPP+ induces the production of
ROS
To determine whether MPP+-induced damage
is mediated by oxidative damage in PC12 cells, the level of ROS
production was evaluated by flow cytometry with DCFH-DA. DCFH-DA is
a stable compound that easily diffuses into cells where it is
converted into DCFH by intracellular esterase. DCFH is then trapped
within cells and oxidized to highly fluorescent DCF by
intracellular ROS; thereby, the intensity of the fluorescence
produced by DCF may reflect an intracellular oxidative state.
Exposure to MPP+ induced a significant increase in DCFH
oxidation in the PC12 cells (Fig.
4), which supports the hypothesis that oxidative damage is
involved in the degeneration of dopaminergic neurons.
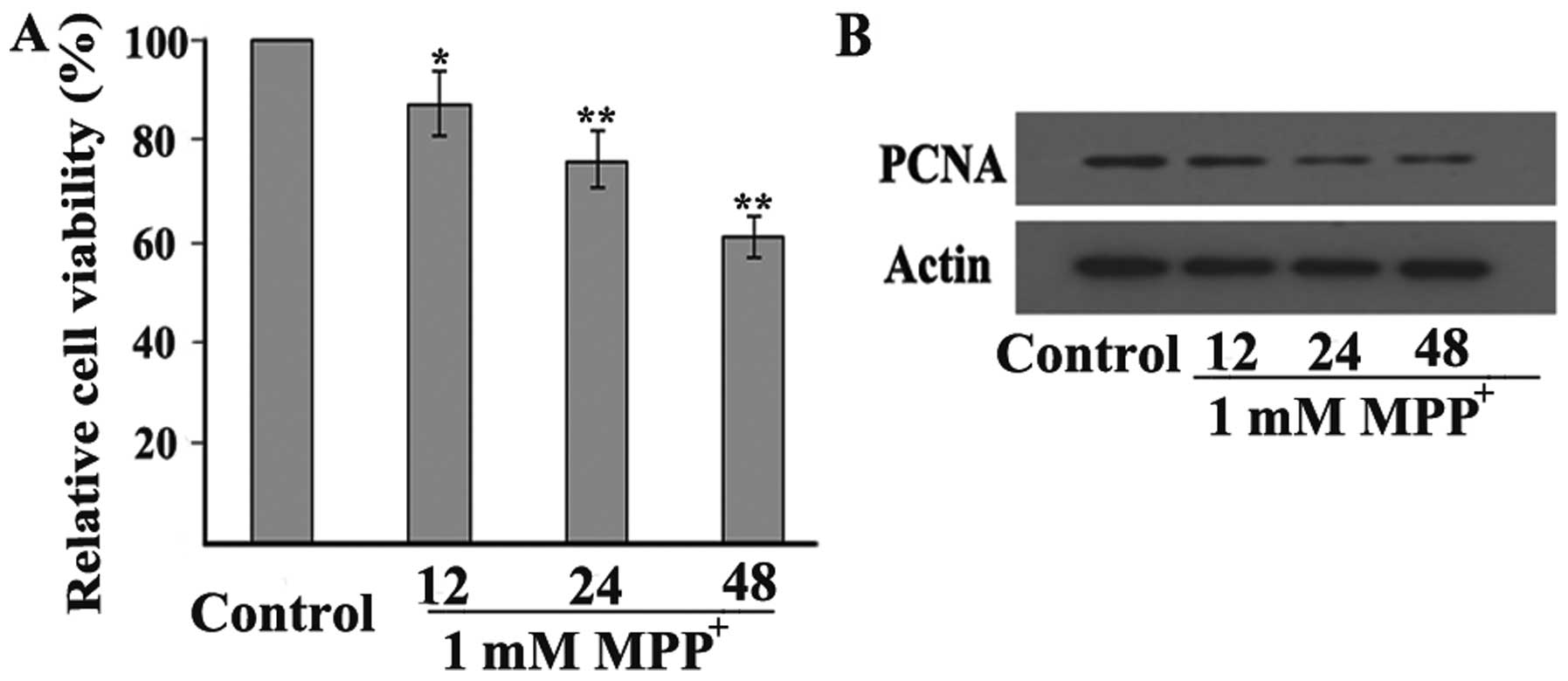
MPP+ decreases PCNA expression
in dopaminergic neuronal cells
To determine whether PCNA is involved in
dopaminergic neuronal cell death under conditions of oxidative
stress, the expression levels of PCNA were measured in a cellular
model of MPP+-induced PD. Firstly, we examined whether
MPP+ induced toxicity to the PC12 cells in a
time-dependent manner. The PC12 cells were exposed to 1 mM
MPP+, and then cell viability was determined after 12,
24 and 48 h by an MTT assay. The results revealed that exposure of
the PC12 cells to 1 mM MPP+ for 12 h caused cell
viability to decrease to 87% of the control, whereas exposure for
24 and 48 h decreased cell viability to 77 and 61% of the control,
respectively (Fig. 5A). In
addition, exposure to 1 mM MPP+ reduced PCNA protein
expression in the PC12 cells. Consistent with the changes observed
in cell viability, PCNA expression was decreased from 12 h and
further decreased until 48 h following exposure to 1 mM
MPP+ in the PC12 cells (Fig. 5B), thus indicating that PCNA is
involved in the MPP+-induced degeneration of
dopaminergic neurons.
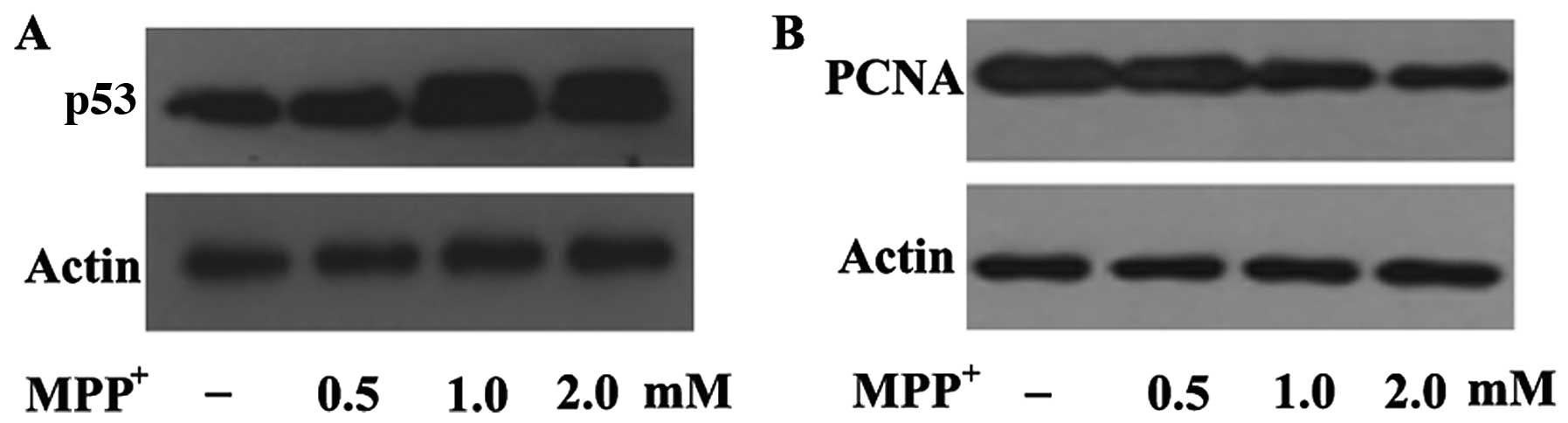
MPP+ increases the epression
of p53 in dopaminergic neurons
In order to elucidate the mechanisms through which
MPP+ decreases PCNA expression in
MPP+-exposed PC12 cells, we examined a well-known PCNA
upstream regulator, p53. p53 has been suggested to play a pivotal
role in dopaminergic neuronal cell death in a mouse model of
MPTP-induced PD (33,34), and transcriptional activation is
the principal mechanism through which PCNA expression is regulated.
Our results revealed that p53 expression was upregulated following
exposure to 0.5 mM MPP+ and further upregulated
following exposure to 1 and 2 mM MPP+ in the PC12 cells
(Fig. 6A). In contrast to p53
expression, PCNA expression was decreased in a dose-dependent
manner following exposure to the indicated concentrations of
MPP+ (Fig. 6B),
suggesting a negative correlation between p53 and PCNA expression
under conditions of oxidative stress. This further indicates that
MPP+-induced oxidative damage is mediated by the
downregulation of PCNA through the p53 pathway in a cellular model
of PD.
Discussion
In this study, we demonsrated that the pleio tropic
protein PCNA is involved in the damage to dopaminergic neurons in
neurodegenerative conditions, and that the downregulated expression
of this protein may be mediated by the p53 signaling pathway. PCNA
is an essential protein involved in DNA replication and repair
(35), and the dysregulation of
its expression may aggravate oxidative stress-induced DNA damage, a
central event involved in the neuronal cell death in PD (25). The downregulation of PCNA is at
least partly responsible for the DNA damage-mediated death of
dopaminergic neurons, thus providing a potential target for the
molecular-based therapeutic management of PD.
PD is a common neurodegenerative movement disorder,
clinically characterized by rigidity, resting tremor, bradykinesia
and postural instability, caused by the degeneration and death of
dopaminergic neurons in the pars compacta of the substantia nigra
(36). Although the cellular and
molecular mechanisms underlying the loss of dopaminergic neurons in
PD remain unclear, accumulating evidence indicates that increased
levels of oxidative stress play a crucial role in triggering a
programmed cell death cascade, involved in the pathogenesis of PD
(24,37). Oxidative damage is a pathological
event responsible for a number of human diseases, including
cardiovascular, metabolic, inflammatory and neurodegenerative
diseases, as well as cancer (25,27,38–41). Dopaminergic neurons are more prone
to oxidative damage due to high levels of lipids, iron as well as
dopamine metabolism (42–51). Oxidative stress is mainly elicited
by the excessive production of ROS, including hydrogen peroxide
(H2O2), superoxide anion and hydroxyl radical
(52–54). The overproduction of ROS damages
nucleic acids, including DNA and RNA, finally causing cell death.
This pathological mechanism is thought to be at least partly
responsible for the death of dopaminergic neurons in PD. Postmortem
studies on PD-affected brains and on brain tissue from mice exposed
to MPTP and other neuronal toxins that induce a PD-like pathology
have shown increased oxidative DNA damage, selectively targeting
dopaminergic neurons of substantia nigra pars compacta (25,27), strongly implicating DNA
damage-induced cell death as a causative factor of PD. DNA is the
most important determinant of cell survival and death. Replication
and repair are required for DNA integrity, since DNA is frequently
subjected to damage by endogenous and environmental toxic agents
(55). Under pathological
conditions, numerous mechanisms are involved in DNA repair to
protect against DNA damage (56,57). PCNA is an essential protein in DNA
replication, and its function was originally described as the
auxiliary protein of DNA polymerases (35). However, PCNA has also been shown
to affect multiple vital cellular processes, including chromatin
remodeling, DNA repair and cell cycle control (35,58). PCNA has no intrinsic enzymatic
activity, and its complex role in cells depends on its capacity to
regulate other proteins. PCNA interacts with a wide range of
enzymes and regulatory proteins, such as cyclin-dependent kinases
(CDKs) (59) or the CDK inhibitor
p21/waf1 (60), which allows this
protein to modulate a wide range of biological functions. In
differentiated neutrophils, for example, it was found that
cytoplasmic PCNA sequesters procaspases and prevents their
activation, promoting the cell survival (61). Additionally, PCNA plays a crucial
role in the repair of DNA damage under conditions of oxidative
stress (30,31). In this study, we found that the
neurotoxin, MPP+, a well-established inducer of
parkinsonism-like symptoms in humans and primates, induced an
increase in ROS productino and in the number of apoptotic
dopaminergic neurons, supporting the involvement of oxidative
stress in the pathogenesis of PD. Importantly, exposure to
MPP+ also decreased the expression level of PCNA in a
time- and dose-dependent manner, suggesting the involvement of PCNA
in MPP+-induced neuronal toxicity in PD. The
downregulation of this protein may aggravate DNA damage under
pathological conditions due to the crucial role of PCNA in
maintaining DNA integrity against various insults including
oxidative damage. However, the mechanisms responsible for this
change in PCNA expression in a cellular model of
MPP+-induced PD remain unclear.
The transcription factor p53 modulates a set of
target genes that are involved in a wide range of cellular
processes, including cell cycle progression, DNA repair, apoptosis
and cellular stress responses (62–65). p53-dependent apoptosis in neuronal
cells is mainly mediated by DNA damage (66,67). The overproduction of ROS activates
p53, leading to further DNA damage under conditions of oxidative
stress. It is well known that p53 is an upstream inducer of PCNA.
The interaction of p53 with the PCNA promoter, the specific
sequence for the p53 binding site, regulates the production of this
protein. Higher concentrations of wild-type p53 inhibit the PCNA
promoter and reduce PCNA expression (68,69). Evidence has indicated that p53 is
upregulated and plays a pivotal role in dopaminergic neuronal cell
death in mouse models of MPTP-induced PD (33,34). It has been demonstrated that p53
inhibitors are highly effective in reducing damage to dopaminergic
neurons and in preserving motor function in a mouse model of PD
(33). Consistent with these
reports, our results demonstrated that MPP+
significantly increased p53 expression in dopaminergic neuronal
cells, supporting the involvement of p53 in the pathogenesis of PD.
In addition, a decrease in PCNA expression was also observed in the
cells exposed to MPP+, and this expression pattern is in
contrast to that of p53 expression, suggesting a negative
correlation between p53 and PCNA expression under conditions of
oxidative stress. Taken together, these findings suggest that a
PCNA-dependent apoptotic pathway is a potential molecular mechanism
that is involved in neuronal cell death in PD, and the p53
signaling pathway is also implicated in this process.
In the present study, we present evidence that
MPP+-induced oxidative damage is mediated by the
downregulation of PCNA through the p53 pathway in a cellular model
of PD. The cellular and molecular mechanisms responsible for the
effects of PCNA on dopaminergic neurons require further
elucidation, and may provide a potential and efficient therapeutic
target for molecular-based strategies for the treatment of PD.
Abbreviations:
|
PD
|
Parkinson's disease
|
|
ROS
|
reactive oxygen species
|
|
MPP+
|
1-methyl-4-phenylpyridinium
|
|
MPTP
|
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
DCF
|
2′7′-dichlorodihydrofluorescein
|
References
|
1
|
Forno LS: Neuropathology of Parkinson's
disease. J Neuropathol Exp Neurol. 55:259–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Langston JW and Irwin I: MPTP: Current
concepts and controversies. Clin Neuropharmacol. 9:485–507. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kopin IJ and Markey SP: MPTP toxicity:
implications for research in Parkinson's disease. Annu Rev
Neurosci. 11:81–96. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heikkila RE, Sieber BA, Manzino L and
Sonsalla PK: Some features of the nigrostriatal dopaminergic
neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in
the mouse. Mol Chem Neuropathol. 10:171–183. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calon F, Lavertu N, Lemieux AM, Morissette
M, Goulet M, Grondin R, Blanchet PJ, Bédard PJ and Di Paolo T:
Effect of MPTP-induced denervation on basal ganglia GABA(B)
receptors: correlation with dopamine concentrations and dopamine
transporter. Synapse. 40:225–234. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chiba K, Trevor A and Castagnoli N Jr:
Metabolism of the neurotoxic tertiary amine, MPTP, by brain
monoamine oxidase. Biochem Biophys Res Commun. 120:574–578. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Javitch JA, D'Amato RJ, Strittmatter SM
and Snyder SH: Parkinsonism-inducing neurotoxin,
N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: uptake of the
metabolite N-methyl-4-phenylpyridine by dopamine neurons explains
selective toxicity. Proc Natl Acad Sci USA. 82:2173–2177. 1985.
View Article : Google Scholar
|
|
8
|
Akaneya Y, Takahashi M and Hatanaka H:
Involvement of free radicals in MPP+ neurotoxicity
against rat dopaminergic neurons in culture. Neurosci Lett.
193:53–56. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jenner P: Oxidative mechanisms in nigral
cell death in Parkinson's disease. Mov Disord. 13(Suppl 1): 24–34.
1998.PubMed/NCBI
|
|
10
|
Przedborski S and Vila M: The
1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine mouse model: a tool
to explore the pathogenesis of Parkinson's disease. Ann N Y Acad
Sci. 991:189–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Segura Aguilar J and Kostrzewa RM:
Neurotoxins and neurotoxic species implicated in neurodegeneration.
Neurotox Res. 6:615–630. 2004. View Article : Google Scholar
|
|
12
|
Schapira AH and Jenner P: Etiology and
pathogenesis of Parkinson's disease. Mov Disord. 26:1049–1055.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu J and Chu CT: Mitochondrial
dysfunction in Parkinson's disease. J Alzheimers Dis. 20(Suppl 2):
S325–S334. 2010.PubMed/NCBI
|
|
14
|
Parker WD Jr, Parks JK and Swerdlow RH:
Complex I deficiency in Parkinson's disease frontal cortex. Brain
Res. 1189:215–218. 2008. View Article : Google Scholar
|
|
15
|
Jenner P and Olanow CW: The pathogenesis
of cell death in Parkinson's disease. Neurology. 66(Suppl 4):
S24–S36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jenner P: Oxidative stress in Parkinson's
disease. Ann Neurol. 53(Suppl 3): S26–S38. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoritaka A, Hattori N, Uchida K, Tanaka M,
Stadtman ER and Mizuno Y: Immunohistochemical detection of
4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl
Acad Sci USA. 93:2696–2701. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Floor E and Wetzel MG: Increased protein
oxidation in human substantia nigra pars compacta in comparison
with basal ganglia and prefrontal cortex measured with an improved
dinitrophenyl-hydrazine assay. J Neurochem. 70:268–275. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Callio J, Oury TD and Chu CT: Manganese
superoxide dismutase protects against 6-hydroxydopamine injury in
mouse brains. J Biol Chem. 280:18536–18542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vila M and Przedborski S: Targeting
programmed cell death in neurodegenerative diseases. Nat Rev
Neurosci. 4:365–375. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perier C, Bové J, Vila M and Przedborski
S: The rotenone model of Parkinson's disease. Trends Neurosci.
26:345–346. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun SY, An CN and Pu XP: DJ-1 protein
protects dopaminergic neurons against 6-OHDA/MG-132-induced
neurotoxicity in rats. Brain Res Bull. 88:609–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heikkila RE, Hess A and Duvoisin RC:
Dopaminergic neurotoxicity of
1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science.
224:1451–1453. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar
|
|
25
|
Alam ZI, Jenner A, Daniel SE, Lees AJ,
Cairns N, Marsden CD, Jenner P and Halliwell B: Oxidative DNA
damage in the parkinsonian brain: an apparent selective increase in
8-hydroxyguanine levels in substantia nigra. J Neurochem.
69:1196–1203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Perry G, Smith MA, Robertson D,
Olson SJ, Graham DG and Montine TJ: Parkinson's disease is
associated with oxidative damage to cytoplasmic DNA and RNA in
substantia nigra neurons. Am J Pathol. 154:1423–1429. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mandir AS, Przedborski S, Jackson-Lewis V,
Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella
DB, Dawson VL and Dawson TM: Poly(ADP-ribose) polymerase activation
mediates 1-methyl-4-phenyl-1, 2,3,6-tetra-hydropyridine
(MPTP)-induced parkinsonism. Proc Natl Acad Sci USA. 96:5774–5779.
1999. View Article : Google Scholar
|
|
28
|
Tan CK, Castillo C, So AG and Downey KM:
An auxiliary protein for DNA polymerase-delta from fetal calf
thymus. J Biol Chem. 261:12310–12316. 1986.PubMed/NCBI
|
|
29
|
Prelich G, Tan CK, Kostura M, Mathews MB,
So AG, Downey KM and Stillman B: Functional identity of
proliferating cell nuclear antigen and a DNA polymerase-delta
auxiliary protein. Nature. 326:517–520. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burkovics P, Hajdú I, Szukacsov V, Unk I
and Haracska L: Role of PCNA-dependent stimulation of
3′-phosphodiesterase and 3′-5′ exonuclease activities of human Ape2
in repair of oxidative DNA damage. Nucleic Acids Res. 37:4247–4255.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Amoroso A, Concia L, Maggio C, Raynaud C,
Bergounioux C, Crespan E, Cella R and Maga G: Oxidative DNA damage
bypass in Arabidopsis thaliana requires DNA polymerase λ and
proliferating cell nuclear antigen 2. Plant Cell. 23:806–822. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hirata H and Cadet JL: p53-knockout mice
are protected against the long-term effects of methamphetamine on
dopaminergic terminals and cell bodies. J Neurochem. 69:780–790.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z,
Oyler J, Cutler RG, Cadet JL, Greig NH and Mattson MP: p53
inhibitors preserve dopamine neurons and motor function in
experimental parkinsonism. Ann Neurol. 52:597–606. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Trimmer PA, Smith TS, Jung AB and Bennett
JP Jr: Dopamine neurons from transgenic mice with a knockout of the
p53 gene resist MPTP neurotoxicity. Neurodegeneration. 5:233–239.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moldovan GL, Pfander B and Jentsch S:
PCNA, the maestro of the replication fork. Cell. 129:665–679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Samii A, Nutt JG and Ransom BR:
Parkinson's disease. Lancet. 363:1783–1793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li DW, Yao M, Dong YH, Tang MN, Chen W, Li
GR and Sun BQ: Guanosine exerts neuroprotective effects by
reversing mitochondrial dysfunction in a cellular model of
Parkinson's disease. Int J Mol Med. 34:1358–1364. 2014.PubMed/NCBI
|
|
38
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: a radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martinon F: Signaling by ROS drives
inflammasome activation. Eur J Immunol. 40:616–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhat AH, Dar KB, Anees S, Zargar MA,
Masood A, Sofi MA and Ganie SA: Oxidative stress, mitochondrial
dysfunction and neurodegenerative diseases; a mechanistic insight.
Biomed Pharmacother. 74:101–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He F and Zuo L: Redox roles of reactive
oxygen species in cardiovascular diseases. Int J Mol Sci.
16:27770–27780. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lotharius J and Brundin P: Pathogenesis of
Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat
Rev Neurosci. 3:932–942. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Montine KS, Quinn JF, Zhang J, Fessel JP,
Roberts LJ II, Morrow JD and Montine TJ: Isoprostanes and related
products of lipid peroxidation in neurodegenerative diseases. Chem
Phys Lipids. 128:117–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagatsu T and Sawada M: Molecular
mechanism of the relation of monoamine oxidase B and its inhibitors
to Parkinson's disease: possible implications of glial cells. J
Neural Transm Suppl. 71:53–65. 2006. View Article : Google Scholar
|
|
45
|
Kumar MJ and Andersen JK: Perspectives on
MAO-B in aging and neurological disease: where do we go from here?
Mol Neurobiol. 30:77–89. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Norris EH, Giasson BI, Hodara R, Xu S,
Trojanowski JQ, Ischiropoulos H and Lee VM: Reversible inhibition
of alpha-synuclein fibrillization by dopaminochrome-mediated
conformational alterations. J Biol Chem. 280:21212–21219. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zecca L, Wilms H, Geick S, Claasen JH,
Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G,
Sievers J and Lucius R: Human neuromelanin induces
neuroinflammation and neurodegeneration in the rat substantia
nigra: implications for Parkinson's disease. Acta Neuropathol.
116:47–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sadrzadeh SM and Saffari Y: Iron and brain
disorders. Am J Clin Pathol. 121(Suppl): S64–S70. 2004.PubMed/NCBI
|
|
49
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Núñez MT, Urrutia P, Mena N, Aguirre P,
Tapia V and Salazar J: Iron toxicity in neurodegeneration.
Biometals. 25:761–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lan J and Jiang DH: Desferrioxamine and
vitamin E protect against iron and MPTP-induced neurodegeneration
in mice. J Neural Transm. 104:469–481. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fang J, Seki T and Maeda H: Therapeutic
strategies by modulating oxygen stress in cancer and inflammation.
Adv Drug Deliv Rev. 61:290–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fruehauf JP and Meyskens FL Jr: Reactive
oxygen species: a breath of life or death? Clin Cancer Res.
13:789–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Day BJ: Catalytic antioxidants: A radical
approach to new therapeutics. Drug Discov Today. 9:557–566. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nikitaki Z, Hellweg CE, Georgakilas AG and
Ravanat JL: Stress-induced DNA damage biomarkers: applications and
limitations. Front Chem. 3:352015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Aziz K, Nowsheen S, Pantelias G, Iliakis
G, Gorgoulis VG and Georgakilas AG: Targeting DNA damage and
repair: embracing the pharmacological era for successful cancer
therapy. Pharmacol Ther. 133:334–350. 2012. View Article : Google Scholar
|
|
57
|
Smolarz B, Wilczyński J and Nowakowska D:
DNA repair mechanisms and human cytomegalovirus (HCMV) infection.
Folia Microbiol (Praha). 60:199–209. 2015. View Article : Google Scholar
|
|
58
|
Mailand N, Gibbs-Seymour I and
Bekker-Jensen S: Regulation of PCNA-protein interactions for genome
stability. Nat Rev Mol Cell Biol. 14:269–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Koundrioukoff S, Jónsson ZO, Hasan S, de
Jong RN, van der Vliet PC, Hottiger MO and Hübscher U: A direct
interaction between proliferating cell nuclear antigen (PCNA) and
Cdk2 targets PCNA-interacting proteins for phosphorylation. J Biol
Chem. 275:22882–22887. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Waga S, Hannon GJ, Beach D and Stillman B:
The p21 inhibitor of cyclin-dependent kinases controls DNA
replication by interaction with PCNA. Nature. 369:574–578. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Witko-Sarsat V, Mocek J, Bouayad D,
Tamassia N, Ribeil JA, Candalh C, Davezac N, Reuter N, Mouthon L,
Hermine O, et al: Proliferating cell nuclear antigen acts as a
cytoplasmic platform controlling human neutrophil survival. J Exp
Med. 207:2631–2645. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Polyak K, Xia Y, Zweier JL, Kinzler KW and
Vogelstein B: A model for p53-induced apoptosis. Nature.
389:300–305. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mirza A, Wu Q, Wang L, McClanahan T,
Bishop WR, Gheyas F, Ding W, Hutchins B, Hockenberry T, Kirschmeier
P, et al: Global transcriptional program of p53 target genes during
the process of apoptosis and cell cycle progression. Oncogene.
22:3645–3654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao R, Gish K, Murphy M, Yin Y, Notterman
D, Hoffman WH, Tom E, Mack DH and Levine AJ: Analysis of
p53-regulated gene expression patterns using oligonucleotide
arrays. Genes Dev. 14:981–993. 2000.PubMed/NCBI
|
|
66
|
Chipuk JE and Green DR: Dissecting
p53-dependent apoptosis. Cell Death Differ. 13:994–1002. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Culmsee C and Mattson MP: p53 in neuronal
apoptosis. Biochem Biophys Res Commun. 331:761–777. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Morris GF, Bischoff JR and Mathews MB:
Transcriptional activation of the human proliferating-cell nuclear
antigen promoter by p53. Proc Natl Acad Sci USA. 93:895–899. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shivakumar CV, Brown DR, Deb S and Deb SP:
Wild-type human p53 transactivates the human proliferating cell
nuclear antigen promoter. Mol Cell Biol. 15:6785–6793. 1995z.
View Article : Google Scholar
|