Introduction
Currently, osteoarthritis (OA) is a significant
clinical issue worldwide, and this situation is expected to worsen
with the growth of aging populations (1,2).
OA, also known as degenerative arthritis, degenerative joint
disease, or osteoarthrosis, is a type of chronic and degenerative
joint disease characterized by an increase in protease activity,
which results in the degradation of critical extracellular matrix
(ECM) proteins as well as pain which results in disabilities
(1,2).
In addition to mechanical and genetic factors,
chronic and excessive production of inflammatory cytokines and
mediators is an important characteristic of OA (2,3).
Of the inflammatory cytokines, interleukin-1β (IL-1β) is considered
to be one of the most potent catabolic factors in arthritis
(3–5). IL-1β plays a pivotal role in the
destruction of the cartilage matrix by upregulating the production
of proteases, such as matrix metal-loproteinases (MMPs) and
zinc-dependent endopeptidases, which are specifically controlled by
the tissue inhibitors of matrix metalloproteinases (TIMPs)
(3,6). In healthy cartilage, there is
homeostasis between MMPs and TIMPs, which is disturbed in OA
(2,6). In the joint cartilage, MMPs are
synthesized and secreted by the residing chondrocytes, particularly
under arthritic conditions (6,7).
Of the various MMPs, collagenases such as MMP-1, -8 and -13, which
are also known as collagenase-1, -2 and -3, are of particular
importance, as they are elevated in joint disorders and closely
related to pathological conditions, such as joint inflammation and
joint degenerative diseases (6).
Thus, reducing the activity of collagenases may have beneficial
results, in the form of chondroprotection against pathological
conditions such as OA.
It is also well documented that the expression of
collagenases in chondrocytes is regulated by the activation of
mitogen-activated protein kinase (MAPK) family members, including
c-Jun N-terminal kinase (JNK), p38 MAPK, and extracellular
signal-regulated kinase (ERK), and several transcription factors,
including nuclear factor-κB (NF-κB), which is known to be activated
in human chondrocytes (8–10). In addition, IL-1β induces
excessive inducible nitric oxide (NO) synthase (iNOS) and
cyclooxygenase-2 (COX-2) expression in chondrocytes, leading to the
elevated production of NO and prostaglandin E2
(PGE2), which accelerate cartilage degradation by
inhibiting proteoglycan biosynthesis (11,12). Accordingly, NO and
PGE2, which are found in high levels in the synovial
fluid of OA patients, are also regarded as beneficial therapeutic
targets in the treatment of OA; thus, non-steroidal
anti-inflammatory drugs (NSAIDs) have been used for treatment for
several years. However, adverse effects, particularly
gastrointestinal diseases and cardiovascular risks, are commonly
associated with these agents (13,14). Therefore, there is currently
considerable interest in the development of new drugs from natural
sources, which can be safely used for the prolonged treatment of
OA.
For thousands of years, herbal medicines have been
used safely and effectively for alleviating and treating various
diseases in many countries; there is increasing interest in the
pharmacological activity of traditional herbs that are widely used
in traditional medicine, and numerous studies support their
potential clinical benefits for diseases that are difficult to
treat (15,16). Among them, Morus alba L.,
or white mulberry, a deciduous tree that belongs to the Moraceae
family, is widely distributed in Asia (16). In particular, Mori folium, the
leaves of Morus alba L., is one of the most widely consumed
medicinal herbs in Asia, including Korea, and medications made from
Mori folium have been found to be useful in the treatment of
metabolic disorders, such as diabetes, hyperlipidemia, and high
blood pressure (17–19). Previous research has shown that
Mori folium exerts diverse pharmacological effects, including
anti-microbial (20),
anti-inflammatory (21),
antioxidant (22), antitumor
(23,24), anti-atherosclerotic (25), anti-obesity (17,26), anti-hypotensive (25), and anti-hypoglycemic effects
(21), and has the potential to
protect the liver (25,27). To the best of our knowledge, no
study has been conducted to date on the potential chondroprotective
effects and cellular action mechanisms of Mori folium. In this
study, as a part of our research on novel biologically active
substances for the prevention and treatment of OA from traditional
medicinal resources, we investigated the potential of Mori folium
water extract (MF) on the production of MMPs and inflammatory
mediators in IL-1β-stimulated SW1353 human chondrocyte cells.
Materials and methods
Preparation of MF
The leaves of Morus alba L., Mori folium,
were obtained from Bio-Port Korea, Inc. (Busan, Korea) and
authenticated by Professor Su Hyun Hong, Department of
Biochemistry, Dongeui University College of Korean Medicine (Busan,
Korea). The dried leaves were subsequently cut into small pieces,
ground into a fine powder, and then boiled with distilled water (50
g/500 ml) for 3 h. The extract was filtered through Whatman no. 3
filter paper (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) twice
in order to remove any insoluble materials, and the filtrate was
lyophilized and then crushed into a thin powder. The extracts (MF)
were dissolved in a 100 mg/ml concentration with distilled water,
and the stock solution was then diluted with Dulbecco's modified
Eagle's medium (Gibco-BRL, Grand Island, NY, USA) to the desired
concentration prior to use.
Cell culture
SW1353 chondrocytes were purchased from the American
Type Culture Collection (Manassas, VA, USA). The cells were
cultured in Dulbecco's modified Eagle's medium (Gibco-BRL) which
contained 10% v/v fetal bovine serum, 100 U/ml penicillin, and 100
mg/ml streptomycin in the presence or absence of MF at 37°C in
humidified air with 5% CO2.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability was measured using an MTT assay.
Briefly, SW1353 cells were seeded in 6-well plates at a density of
2×105 cells/well. After incubation for 24 h, the cells
were treated with various concentrations of the MF or 40 ng/ml
IL-1β (R&D Systems, Inc., Minneapolis, MN, USA) individually or
were pretreated with different concentrations of MF for 1 h before
the IL-1β treatment. After 24 h, the medium was removed, and the
MTT working solution (0.5 mg/ml; Sigma-Aldrich Chemical Co.) was
then added to the culture plates and incubated continuously at 37°C
for 2 h. Subsequently, the culture supernatant was removed from the
wells, and dimethyl sulfoxide (DMSO; Sigma-Aldrich Chemical Co.)
was added to dissolve the formazan crystals. After shaking, the
absorbance of each well was measured at 540 nm with a microplate
reader (Dynatech Laboratories, Chantilly VA, USA), and the results
were expressed as cell viability relative to the untreated control,
which was considered 100% viable.
Enzyme-linked immunosorbent assay
(ELISA)
The inhibitory effects of MF on the production of
MMPs (MMP-1, -2, -3 and -13) and PGE2 were examined
using commercial ELISA kits (purchased from R&D Systems, Inc.).
Briefly, cells were treated with IL-1β (40 ng/ml) only or
alternatively were pretreated with various concentrations of MF for
1 h before IL-1β treatment. After 24 h, the concentrations of the
MMPs and PGE2 in the culture medium were determined
using selective ELISA kits, according to the manufacturer's
instructions.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the cultured cells was isolated using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer's instructions. cDNA was synthesized
from 1 µg total RNA using AccuPower® RT PreMix
(Bioneer, Daejeon, Korea) containing moloney murine leukemia virus
reverse transcriptase. RT-generated cDNA was amplified by PCR using
selected primers (Table I), which
were purchased from Bioneer. After amplification, the PCR reactions
were electrophoresed in 1% agarose gels and visualized using
ethidium bromide (EtBr; Sigma-Aldrich Chemical Co.) staining. In a
parallel experiment, glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was used as an internal control.
 | Table ISequences of the primer pairs
employed in the RT-qPCR reactions. |
Table I
Sequences of the primer pairs
employed in the RT-qPCR reactions.
| Gene name | Sequence |
|---|
| MMP-1 | |
| Sense |
5′-CTG-TTC-AGG-GAC-AGA-ATG-TGC-3′ |
| Antisense |
5′-TTG-GAC-TCA-CAC-CAT-GTG-TT-3′ |
| MMP-2 | |
| Sense |
5′-GTC-AGT-GAG-AAG-GAA-GTG-GAC-TCT-3′ |
| Antisense |
5′-ATG-TTC-TTC-TCT-GTG-ACC-CAG-TC-3′ |
| MMP-3 | |
| Sense |
5′-TGC-GTG-GCA-GTT-TGC-TCA-GCC-3′ |
| Antisense |
5′-GAA-TGT-GAG-TGG-AGT-CAC-CTC-3′ |
| MMP-13 | |
| Sense |
5′-GGC-TCC-GAG-AAA-TGC-AGT-CTT-TCT-T-3′ |
| Antisense |
5′-ATC-AAA-TGG-GTA-GAA-GTC-GCC-ATG-C-3′ |
| iNOS | |
| Sense |
5′-GTG-AGG-ATC-AAA-AAC-TGG-GG-3′ |
| Antisense |
5′-ACC-TGC-AGG-TTG-GAC-CAC-3′ |
| COX-2 | |
| Sense |
5′-TCA-GCC-ACG-CAG-CAA-ATC-CT-3′ |
| Antisense |
5′-GTG-ATC-TGG-ATG-TCA-CG-3′ |
| GAPDH | |
| Sense | 5′-CGG AGT CAA CGG
ATT TGG TCG TAT-3′ |
| Antisense | 5′-AGC CTT CTC CAT
GGT GGT GAA GAC-3′ |
Protein extraction and western blot
analysis
For total protein extraction, the cells were
harvested and lysed in lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM
NaCl, 5 mM ethylene-diaminetetraacetic acid (EDTA), 1% Nonidet-P40
(NP-40), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 5 mM
dithiothreitol (DTT)] for 1 h. In a parallel experiment, nuclear
and cytosolic proteins were prepared using nuclear extraction
reagents (Pierce, Rockford, IL, USA), according to the
manufacturer's instructions. The insoluble materials were discarded
by centrifugation at 13,000 x g for 20 min at 4°C. Protein
concentration was measured using a Bio-Rad protein assay kit
(Bio-Rad Laboratories, Hercules, CA, USA), according to the
manufacturer's instructions. For the western blot analysis,
equivalent amounts of proteins were separated by electrophoresis on
sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and
transferred to nitrocellulose membranes (Schleicher and Schuell,
Keene, NH, USA). After blocking with 5% skimmed milk, the membranes
were incubated with protein specific antibodies [MMP-1 (1:1,000;
ab52631, rabbit monoclonal; Abcam, Cambridge, UK), MMP-2 (1:1,000;
SC-10736, rabbit polyclonal; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), MMP-3 (1:1,000; ab52915, rabbit monoclonal; Abcam),
MMP-13 (1:1,000; ab51072, rabbit monoclonal; Abcam), iNOS (1:500;
SC-7271, mouse monoclonal, Santa Cruz Biotechnology, Inc.), COX-2
(1:500; SC-19999, mouse monoclonal; Santa Cruz Biotechnology,
Inc.), NF-κB p65 (1:500; SC-109, rabbit polyclonal; Santa Cruz
Biotechnology, Inc.), IκB-α (1:500; SC-371, rabbit polyclonal;
Santa Cruz Biotechnology, Inc.), ERK (1:1,000; SC-154, rabbit
polyclonal; Santa Cruz Biotechnology, Inc.), p-ERK (1:500; #9106S,
mouse monoclonal; Cell Signaling Technology, Inc., Danvers, MA,
USA), p38 (1:1,000; SC-535, rabbit polyclonal; Santa Cruz
Biotechnology, Inc.), p-p38 (1:500; #9211S, rabbit polyclonal; Cell
Signaling Technology, Inc.), JNK (1:1,000; #9252S, rabbit
polyclonal; Cell Signaling Technology, Inc.), p-JNK (1:500; #9255S,
mouse monoclonal; Cell Signaling Technology, Inc.), Lamin B (1:500;
SC-6216, goat polyclonal; Santa Cruz Biotechnology, Inc.) and
β-actin (1:1,000; sc-1616, goat polyclonal; Santa Cruz
Biotechnology, Inc.)] for 1 h, subsequently incubated with
appropriate enzyme-linked secondary antibodies [mouse IgG,
HRP-linked whole antibody (NA931) and rabbit IgG, HRP-linked whole
antibody (NA934); Amersham Corp., Arlington Heights, IL, USA], and
visualized using an enhanced chemiluminescence (ECL) solution
(Amersham Corp.), according to the manufacturer's instructions. The
primary antibodies were purchased from Santa Cruz Biotechnology,
Inc. and Abcam.
Measurement of NO production
The concentration of NO in the culture supernatants
was determined by measuring nitrite, a stable oxidation product of
nitric oxide, using Griess reagent (Sigma-Aldrich Chemical Co.).
The cell culture conditions were the same as those used for the
ELISA. Following incubation with IL-1β and/or MF for 24 h, 100
µl of each culture supernatant was mixed with an equal
volume of Griess reagent for 10 min at room temperature.
Subsequently, absorbance was measured at 540 nm with a microplate
reader, and nitrite production was determined by NaNO2
standard curve, as previously described (28).
Immunofluorescence staining
For the detection of the translocation of NF-κB p65,
the SW1353 cells were grown on glass coverslips in 6-well plates
for 24 h. Cells were pretreated with MF (800 µg/ml) for 1 h
prior to stimulation with IL-1β (40 ng/ml). Following incubation
for 30 min, the cells were washed twice with phosphate-buffered
saline (PBS), fixed with 3.7% paraformaldehyde, treated with 0.2%
Triton X-100, and subsequently blocked with 2% bovine serum
albumin. The cells were sequentially incubated with an anti-NF-κB
p65 antibody (SC-109; Santa Cruz Biotechnology, Inc.) and
fluorescein isothiocyanate-conjugated donkey anti-rabbit
immunoglobulin G (IgG; Cat. no. 711-001-003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA). After washing with PBS,
the nuclei were stained with 4′6-diamidino-2-phenylindole (DAPI;
Sigma-Aldrich Chemical Co.), and fluorescence was visualized using
a fluorescence microscope (Carl Zeiss, Jena, Germany), as
previously described (29).
Statistical analysis
The values are expressed as the means ± SD. A
Student's t-test was used to evaluate the differences between the
samples treated with IL-1β only and the samples treated with IL-1β
and MF. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
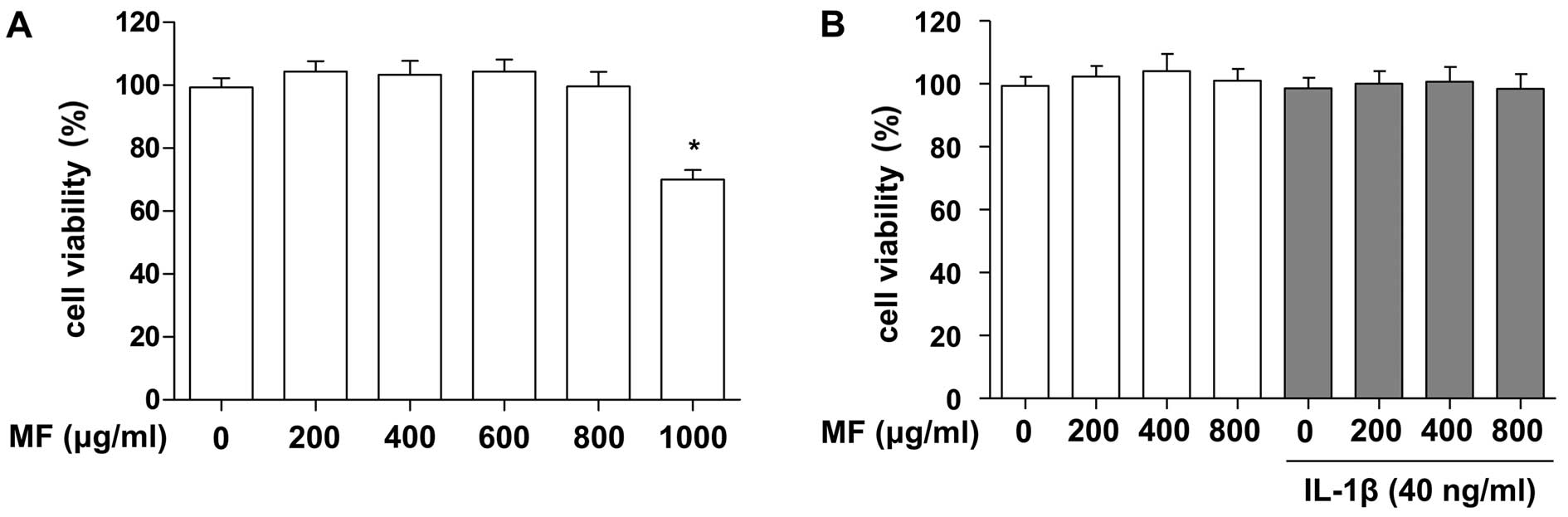
Effects of MF on the viability of SW1353
chondrocytes
To evaluate whether or not MF exerted cytotoxic
effects on SW1353 cells, the cells were treated with various
concentrations of MF for 24 h. In the MTT assays, we noted that MF
concentrations up to 800 µg/ml did not induce cytotoxicity
in the SW1353 cells, whereas 1,000 µg/ml MF significantly
reduced viability (approximately 70%; Fig. 1A). In a subsequent experiment,
when we administered MF to IL-1β-treated SW1353 cells at
concentrations below 800 µg/ml, no adverse effects on cell
viability were noted (Fig. 1B).
Therefore, concentrations of MF up to 800 µg/ml were used in
the remaining experiments.
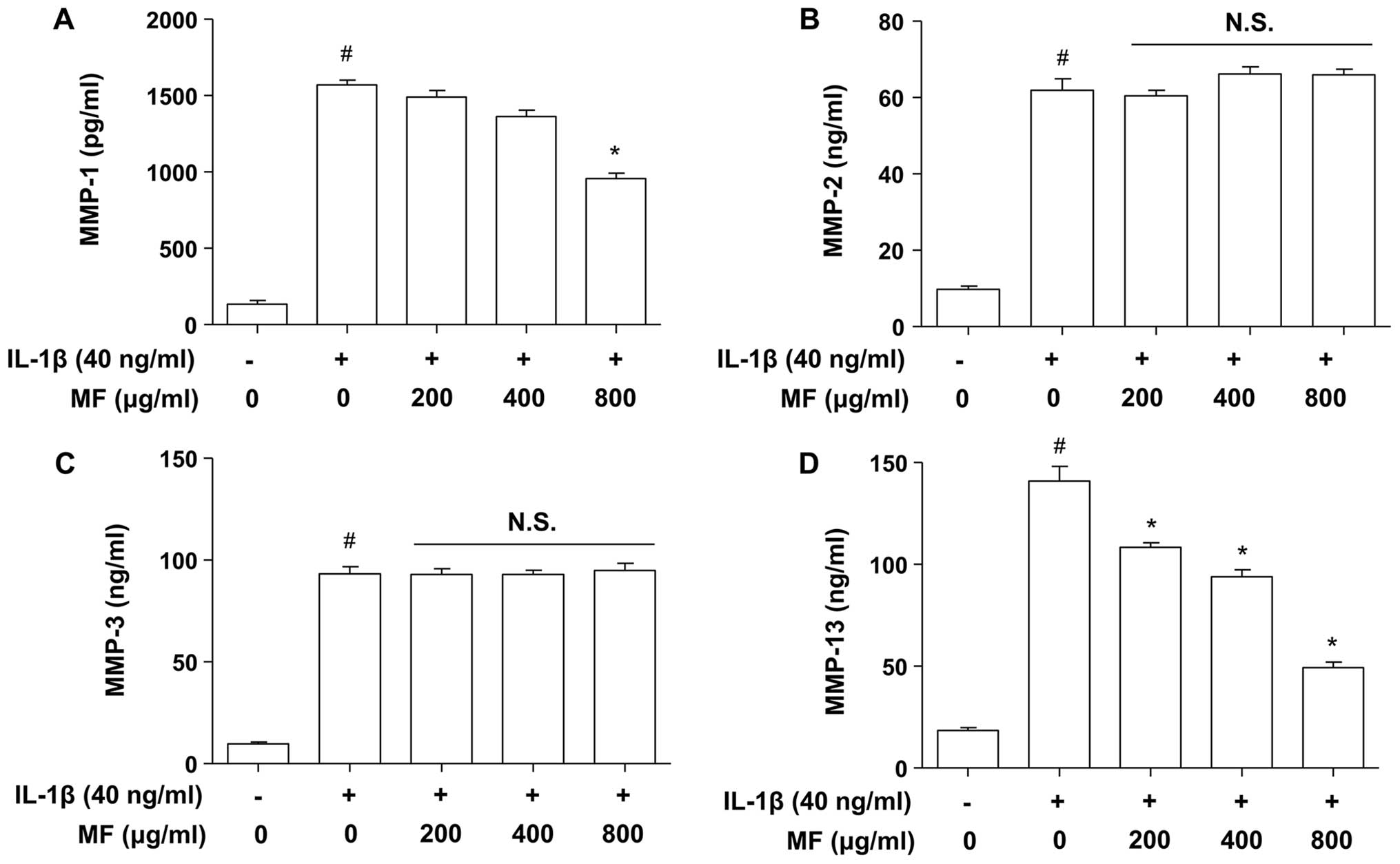
Suppressive effects of MF on
IL-1β-induced production of MMP-1 and -13 in SW1353
chondrocytes
Previous studies have suggested that various MMPs
are involved in both physiological collagen turnover in articular
cartilage and matrix degradation in OA cartilage (2,6).
Therefore, to examine the inhibitory effect of MF on IL-1β-induced
MMP production, SW1353 cells were stimulated with IL-1β (40 ng/ml)
for 24 h in the presence and absence of MF, and subsequently the
release of MMPs from SW1353 cells was detected in the culture
supernatants using ELISA. As also reported previously (30,31), increased release of MMPs (MMP-1,
-2, -3 and -13) was detected in the culture supernatants after
stimulation with IL-1β (Fig. 2);
however, pretreatment of the SW1353 cells with MF dose-dependently
reduced the production of IL-1β-stimulated collagenases MMP-1 and
-13, but not MMP-2 (gelatinase A) or -3 (a stromelysin), and levels
were comparable to those found in cells not treated with MF.
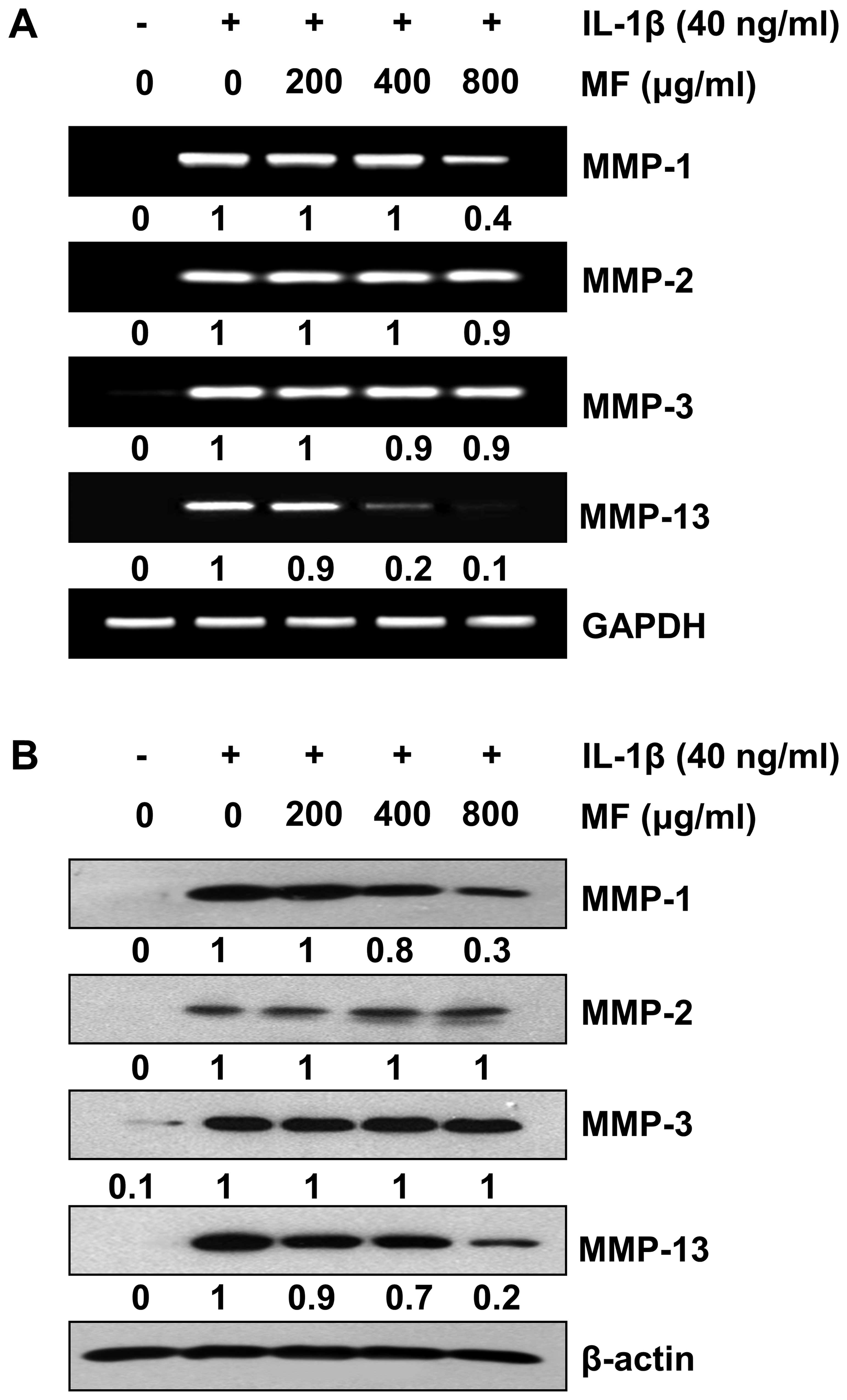
Inhibition of IL-1β-induced expression of
MMP-1 and -13 by MF in SW1353 chondrocytes
To evaluate the effects of MF on IL-1β-induced
expression of MMPs in SW1353 cells, cells were treated with IL-1β
alone or with different concentrations of MF for 24 h. RT-qPCR and
western blot analysis revealed that the levels of all MMPs in the
SW1353 cells treated with IL-1β alone markedly increased compared
to the levels detected in the untreated cells (Fig. 3); however, pretreatment with MF
was found to inhibit the increase in MMP-1 and -13 but not MMP-2
and -3 caused by treatment with IL-1β in a concentration-dependent
manner. These results suggest that MF is a potent inhibitor of
IL-1β-mediated MMP-1 and -13 transcription in SW1353 cells, whereas
it appears not to interfere with MMP-2 and -3 gene expression.
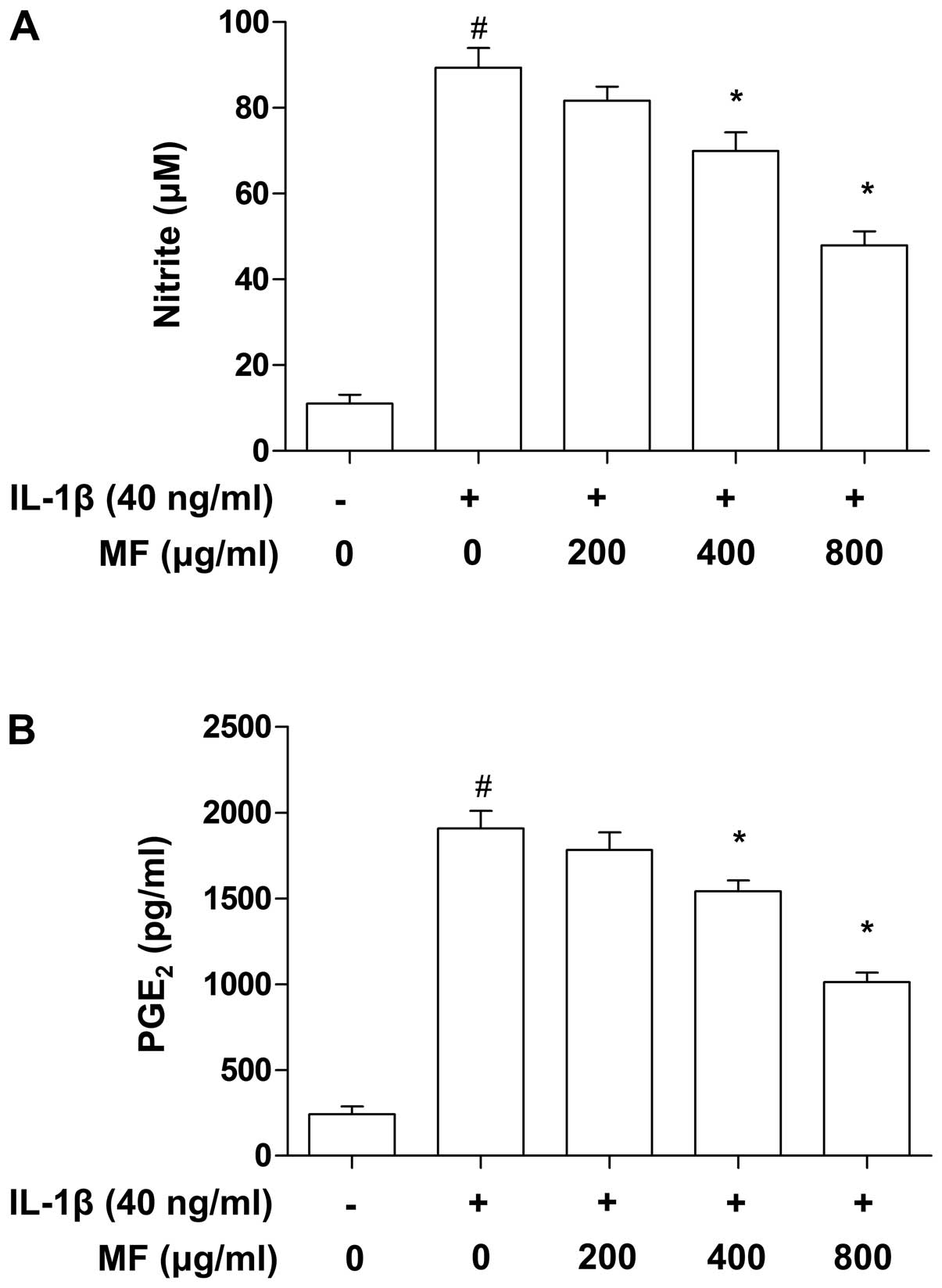
Attenuation of IL-1β-induced NO and
PGE2 production by MF in SW1353 chondrocytes
It is well-known that inflammatory mediators, such
as NO and PGE2, play key roles in the progression of
cartilage destruction in OA (32,33), and thus the effects of MF on the
level of NO and PGE2 in IL-1β-stimulated SW1353 cells
were examined in this study. To verify the effects of MF on the
levels of NO and PGE2 produced by SW1353 cells,
supernatants of treated cell cultures were collected and subjected
to Griess reagent and ELISA, respectively. Consistent with previous
reports (34,35), treating SW1353 cells with IL-1β
alone markedly elevated the levels of NO and PGE2
compared to the control group (Fig.
4); however, MF considerably abrogated the release of NO and
PGE2 in IL-1β-treated SW1353 cells in a
concentration-dependent manner at a range of 200–800
µg/ml.
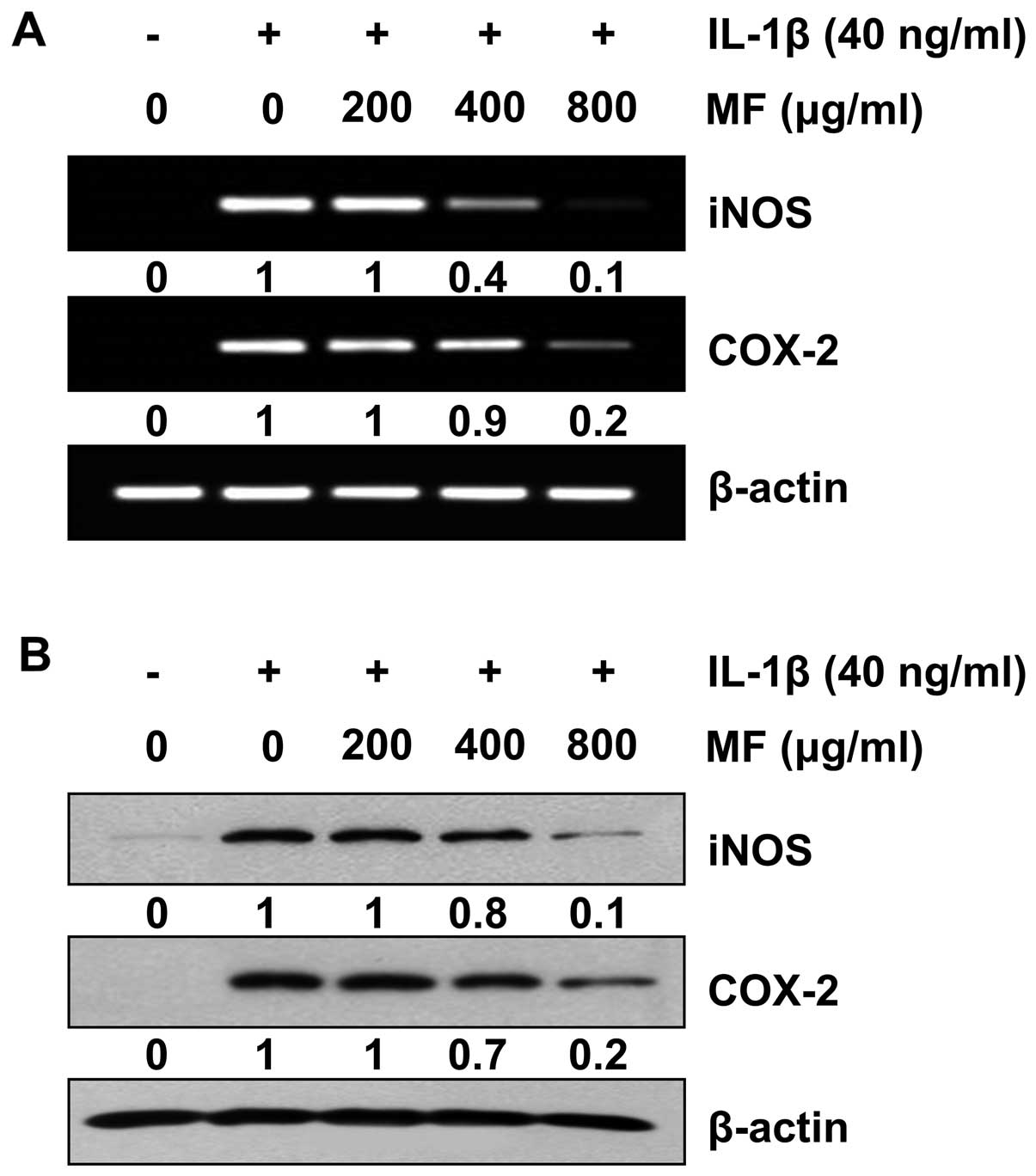
Reduction of IL-1β-induced iNOS and COX-2
expression by MF in SW1353 chondrocytes
Subsequently, RT-qPCR and western blot analysis were
performed to determine whether or not the inhibition of NO and
PGE2 production by MF in the IL-1β-stimulated SW1353
cells was associated with decreased levels of iNOS and COX-2
expression. The results indicate that the IL-1β-induced increase in
the iNOS and COX-2 mRNA levels was reversed by MF in a
concentration-dependent manner (Fig.
5A). In a parallel experiment, the elevated protein levels of
iNOS and COX-2 resulting from stimulation with IL-1β were also
decreased following pretreatment with MF (Fig. 5B). These results indicate that the
reduced expression of iNOS and COX-2 at the transcriptional levels
contributed to the inhibitory effect of MF on IL-1β-induced NO and
PGE2 production.
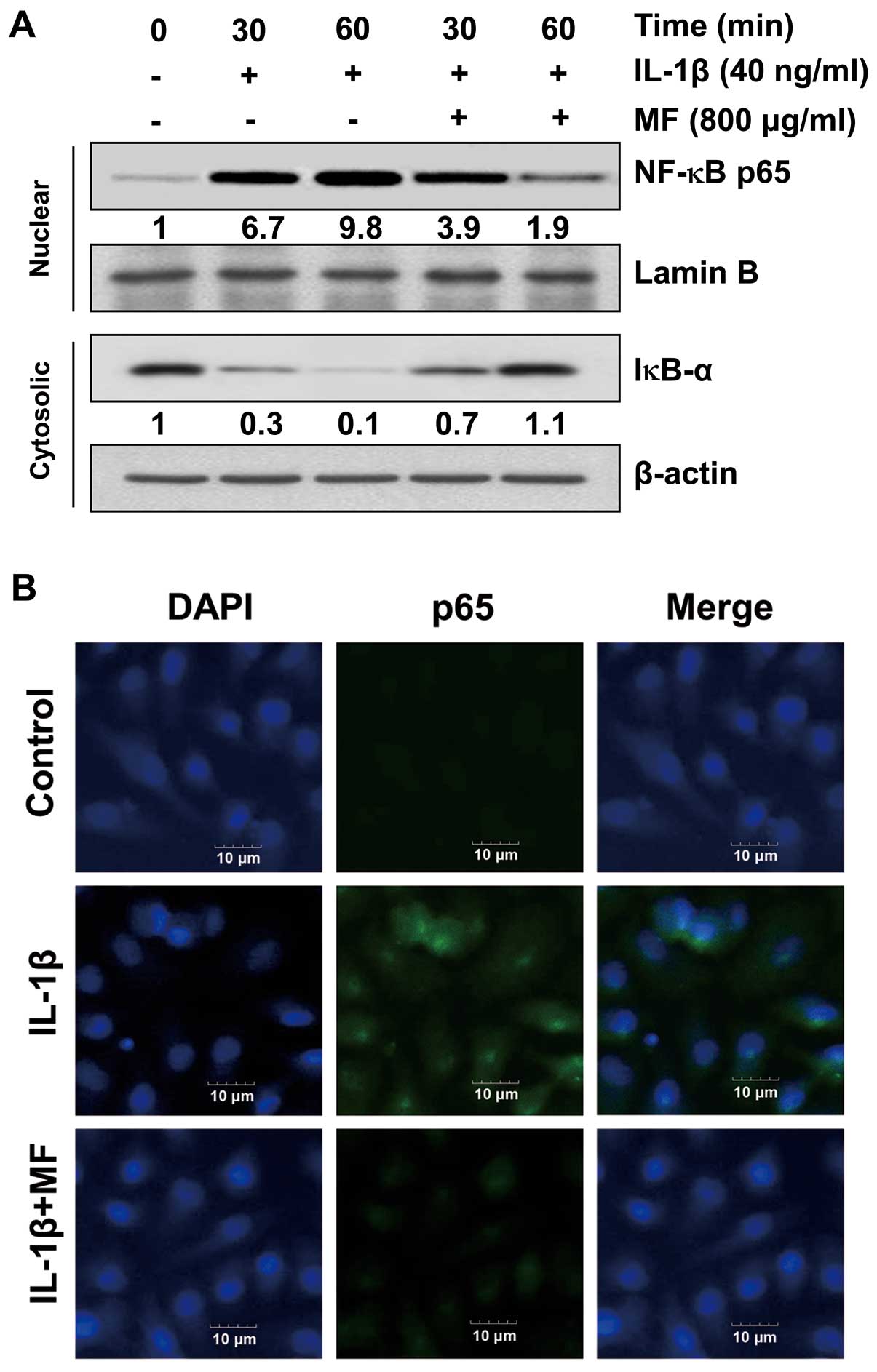
Effect of MF on IL-1β-induced nuclear
translocation of NF-κB in SW1353 chondrocytes
Previous studies have reported that IL-1β-induced
NF-κB activation is involved in upregulating MMPs, iNOS and COX-2
transcriptional activities (36,37). Therefore, we determined whether or
not the inhibitory effect of MF on the IL-1β-induced activation of
MMPs, iNOS and COX-2 was mediated by the suppression of NF-κB
signaling by measuring the nuclear translocation of NF-κB. Western
blot analyses revealed that treatment with IL-1β enhanced the
nuclear accumulation of NF-κB proteins within 30 min, concomitantly
with the degradation of IκB-α in the cytosol (Fig. 6A); however, pretreatment with MF
reduced the IL-1β-induced nuclear accumulation of NF-κB and the
degradation of IκB-α compared to those in cells treated with IL-1β
alone. The immunofluorescence images also revealed that the nuclear
translocation of NF-κB p65 was strongly induced after the
stimulation of cells with IL-1β, and the shift in NF-κB p65 to the
nucleus was completely abolished after pretreating the cells with
MF (Fig. 6B). These findings
indicate that MF inhibits IL-1β-induced NF-κB activation in SW1353
cells through the suppression of IκB degradation and the nuclear
translocation of NF-κB.
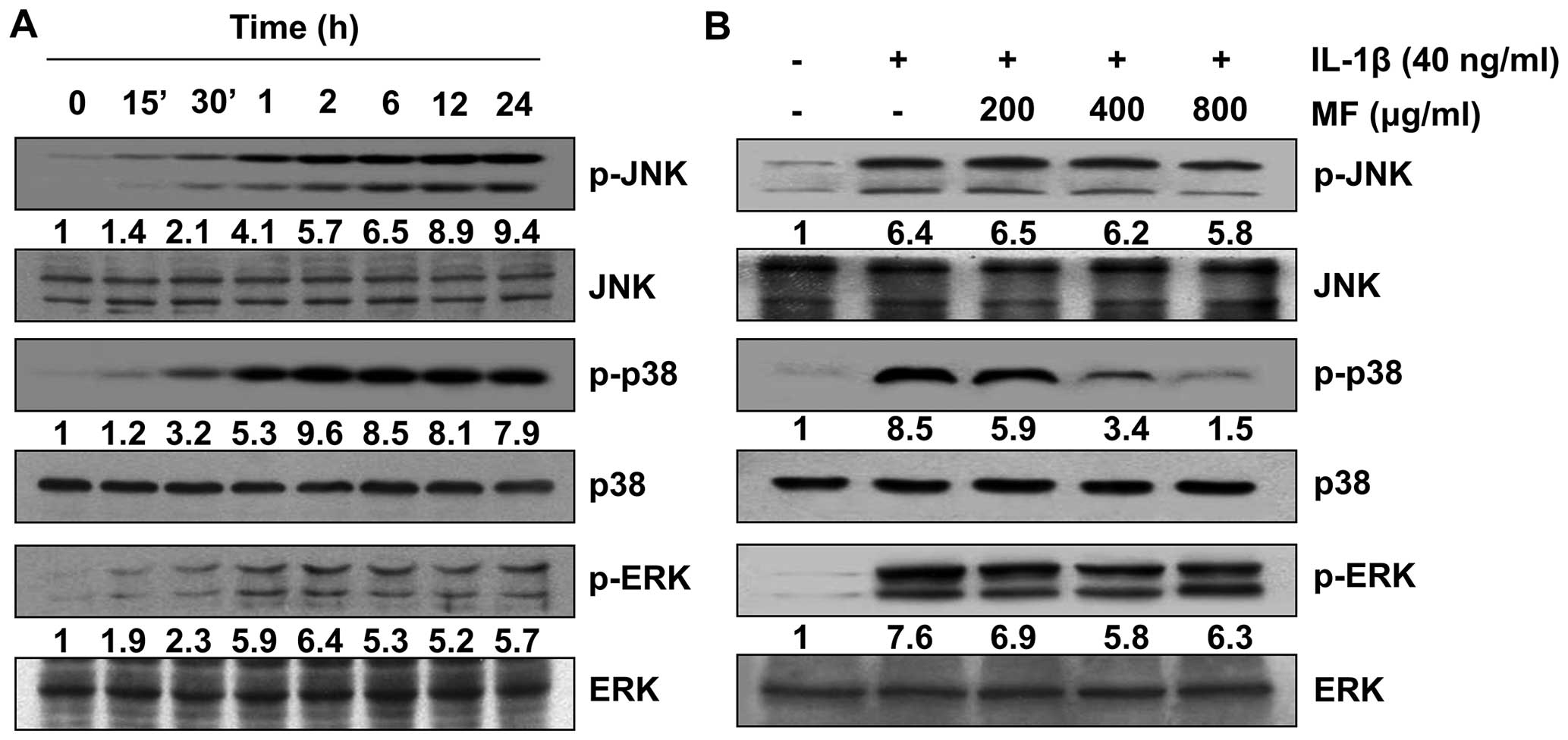
Inhibition of IL-1β-induced activation of
p38 MAPK by MF in SW1353 chondrocytes
As it is well-known that the MAPK pathway is
involved in IL-1β-induced MMP and inflammatory mediator expression
(10,38), we subsequently investigated
whether or not the inhibitory effect of MF on their expressions was
mediated by MAPK signaling cascades. Western blot analysis
confirmed that IL-1β treatment enhanced the phosphorylation of JNK,
p38 MAPK and ERK without markedly affecting their total protein
levels (Fig. 7A). Therefore, the
effect of MF on IL-1β-induced phosphorylation of MAPKs in SW1353
cells was evaluated. The results reveal that pretreatment with MF
significantly reduced the IL-1β- stimulated activation of p38 MAPK
to near the control levels in a concentration-dependent manner, but
it had no such effect on the activity of JNK and ERK (Fig. 7B). The results suggest that the
suppression of IL-1β-induced p38 MAPK activation by MF is
responsible for the observed condroprotective effect of MF on
IL-1β-stimulated SW1353 cells.
Discussion
In this study, we investigated whether or not MF
inhibited the release and expression of MMPs and inflammatory
mediators induced by a representative pro-inflammatory cytokine,
IL-1β, in SW1353 chondrocytes. As the data demonstrated,
pretreating SW1353 cells with MF effectively suppressed the
IL-1β-mediated release of MMP-1, -3, NO and PGE2, as
indicated by the attenuation of their corresponding gene
expressions, at least in part via blocking NF-κB and p38 MAPK
activation.
It is well-known that OA, a heterogeneous and
complicated joint disorder, is characterized by the destruction of
articular cartilage due to an imbalance between the biosynthesis
and degradation of the ECM (1,8).
Since MMPs are usually abundantly expressed in joint disorders, and
the effect of these proteolytic enzymes is largely due to their
ability to degrade the ECM, it is widely accepted that MMPs
represent promising pharmacological targets for the treatment of OA
(2,6). In particular, collagenases including
MMP-1, -8 and -13 provide a suitable microenvironment for the
development and progression of OA; moreover, they specifically
degrade type II collagen and proteoglycans through other MMPs in
the ECM of cartilage (6,9). Of the MMPs, MMP-1 is expressed
ubiquitously and is found in a broad range of normal tissue cells,
including chondrocytes, whereas MMP-13 is more closely linked to
the degradation of type II collagen than MMP-1 or -8 and has long
been regarded as a key mediator of cartilage degradation in joint
disorders (6,9). In the present study, the OA
microenvironment was mimicked by cultured SW1353 cells stimulated
with IL-1β. As indicated in the Results, the production of MMP-1
and -13 were significantly promoted in SW1353 cells after
stimulation with IL-1β, and MF inhibited MMP-13 release more than
MMP-1. Conversely, MF pretreatment effectively suppressed
IL-1β-stimulated MMP-1 and MMP-13 release in SW1353 cells by
downregulating the overexpression of MMP-1 and -13 but not MMP-2
and -3. Thus, it is likely that treatment with MF has a significant
impact on cartilage homeostasis, as it consistently inhibited the
IL-1β-induced upregulation of MMP-1 and -13 at the transcriptional
level in SW1353 chondrocytes.
In addition to examining the roles of MMPs in OA,
elevated levels of inflammatory mediators, such as NO and
PGE2, have been previously observed in the cartilage and
serum of OA patients (39,40).
IL-1β-mediated overproduction of NO has also been reported to act
as an important inflammatory mediator that plays a critical role in
the pathogenesis of OA through inducing chondrocyte and synoviocyte
deaths (41). IL-1β also
stimulates the expression of COX-2 to increase the synthesis of
PGE2, which is responsible for PGE2 and is
implicated in bone resorption and joint pain in OA (42). Moreover, both NO and
PGE2 are capable of upregulating the production of MMPs
and other inflammatory cytokines (43,44). Therefore, the inhibition of the
production of IL-1β-induced inflammatory mediators has been shown
to be useful in the treatment of OA. In the present study, we
demonstrated that MMP-1 and -13 expression was suppressed by MF,
and pretreatment with MF significantly decreased IL-1β-induced NO
and PGE2 production by attenuating the expression of
their upstream molecules, iNOS and COX-2, on both mRNA and protein
levels. These observations indicate that the expression of iNOS and
COX-2 was regulated by MF at the transcriptional level and also
that MF exerts anti-inflammatory effects on IL-1β-induced
inflammation in chondrocytes.
It has previously been noted that NF-κB is the most
important transcription factor that regulates the expression of
MMPs, COX-2, and iNOS in OA (9,38).
Despite the previously demonstrated function of the activator
protein-1 in regulating MMP expression (45,46), data demonstrating the
NF-κB-mediated induction of MMPs, in particular MMP-1 and -13,
indicate that NF-κB is a potential therapeutic target in OA
(37,47). Normally, NF-κB is located in the
cytoplasm in an inactive form in which the heterodimer is bound to
IκB inhibitor proteins. Stimulation with inflammatory molecules,
such as IL-1β, leads to phosphorylation and the subsequent
degradation of IκB-α, which leads to the nuclear translocation of
NF-κB and the promotion of the regulation of transcription of
response genes (48,49). Therefore, previous research has
focused on the inhibition of MMP and inflammatory mediator
expression by blocking the mobilization of NF-κB into the nucleus
in chondrocytes. In addition, the high-level expression of MMPs,
iNOS, and COX-2 in arthritic joints results from the activation of
a tightly regulated and synchronized signaling cascade activated by
IL-1β and involving the MAPKs signaling pathway (10,38). MAPKs are activated through the
phosphorylation of specific tyrosine and threonine residues by the
upstream kinases in response to inflammatory signals (10,11,38). In the present study, it was
demonstrated that IL-1β enhances the degradation of IκB-α and
enhances the translocation of NF-κB p65 from the cytoplasm to the
nucleus; however, pretreatment with MF effectively attenuated the
IL-1β-induced degradation of IκB-α and the nuclear translocation of
NF-κB p65 in SW1353 cells. It was also observed that following
stimulation of SW1353 cells with IL-1β, the activation of the JNK,
p39 MAPK and ERK signaling pathways was evident. Interestingly, it
was found that MF selectively inhibited IL-1β-induced p38 MAPK
activation without markedly affecting ERK and JNK activation.
Although other signaling pathways also play an important role in
the regulation of OA-related gene expression, the results of our
study suggest that the attenuation of NF-κB and p38 activity partly
accounts for the inhibitory effects of MF on the gene expression of
MMP-1, MMP-13, iNOS and COX-2. As previously mentioned, traditional
herbal medicines have become one of the most important alternative
treatments for OA. At present, the wider public is also more aware
of the significance of prophylactic medications which complement
traditional treatments or prevent OA. Therefore, we consider our
water extract of Mori folium to be a useful nutritional supplement
for OA therapy. In the future, we will further characterize the
active compounds from our extract and examined its
chondroprotective effect against OA and investigate the molecular
mechanisms responsible in detail.
In conclusion, we have demonstrated in the present
study that MF effectively suppresses the IL-1β-induced expression
of MMP-1, -13, iNOS and COX-2 in SW1353 cells, all of which play a
pivotal role in the progression of OA. Furthermore, we have also
shown that this effect is mediated, at least in part, through the
regulation of NF-κB and p38 signaling pathways. Our findings
suggest that MF merits consideration as a therapeutic agent in the
treatment and prevention of OA. Therefore, an in-depth study to
define the bioavailability of active agent(s) in MF which are
capable of exerting cartilage and chondroprotective effects in
vivo is warranted.
Acknowledgments
This study was supported by the High Value-added
Food Technology Development Program (314043-3), Ministry of
Agriculture, Food and Rural Affairs, Republic of Korea.
References
|
1
|
Hardingham T: Extracellular matrix and
pathogenic mechanisms in osteoarthritis. Curr Rheumatol Rep.
10:30–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilusz RE, Sanchez-Adams J and Guilak F:
The structure and function of the pericellular matrix of articular
cartilage. Matrix Biol. 39:25–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bian Q, Wang YJ, Liu SF and Li YP:
Osteoarthritis: genetic factors, animal models, mechanisms, and
therapies. Front Biosci (Elite Ed). 4:74–100. 2012. View Article : Google Scholar
|
|
4
|
Daheshia M and Yao JQ: The interleukin
1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol.
35:2306–2312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang HS, Park IY, Kim DW, Choi SY, Jung
YO and Kim HA: PEP-1-FK506BP12 inhibits matrix metalloproteinase
expression in human articular chondrocytes and in a mouse
carrageenan-induced arthritis model. BMB Rep. 48:407–412. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar
|
|
7
|
Hadler-Olsen E, Fadnes B, Sylte I,
Uhlin-Hansen L and Winberg JO: Regulation of matrix
metalloproteinase activity in health and disease. FEBS J.
278:28–45. 2011. View Article : Google Scholar
|
|
8
|
Berenbaum F: Signaling transduction:
target in osteoarthritis. Curr Opin Rheumatol. 16:616–622. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vincenti MP and Brinckerhoff CE:
Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in
arthritis: integration of complex signaling pathways for the
recruitment of gene-specific transcription factors. Arthritis Res.
4:157–164. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sondergaard BC, Schultz N, Madsen SH,
Bay-Jensen AC, Kassem M and Karsdal MA: MAPKs are essential
upstream signaling pathways in proteolytic cartilage degradation -
divergence in pathways leading to aggrecanase and MMP-mediated
articular cartilage degradation. Osteoarthritis Cartilage.
18:279–288. 2010. View Article : Google Scholar
|
|
11
|
Mengshol JA, Vincenti MP, Coon CI,
Barchowsky A and Brinckerhoff CE: Interleukin-1 induction of
collagenase 3 (matrix metalloproteinase 13) gene expression in
chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear
factor kappaB: differential regulation of collagenase 1 and
collagenase 3. Arthritis Rheum. 43:801–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Studer R, Jaffurs D, Stefanovic-Racic M,
Robbins PD and Evans CH: Nitric oxide in osteoarthritis.
Osteoarthritis Cartilage. 7:377–379. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farkouh ME, Greenberg JD, Jeger RV,
Ramanathan K, Verheugt FW, Chesebro JH, Kirshner H, Hochman JS, Lay
CL, Ruland S, et al: Cardiovascular outcomes in high risk patients
with osteoarthritis treated with ibuprofen, naproxen or
lumiracoxib. Ann Rheum Dis. 66:764–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herndon CM: Topical delivery of
nonsteroidal anti-inflammatory drugs for osteoarthritis. J Pain
Palliat Care Pharmacother. 26:18–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mobasheri A: Intersection of inflammation
and herbal medicine in the treatment of osteoarthritis. Curr
Rheumatol Rep. 14:604–616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wei S, Liu T, Pang J, Gao N, Ding
D, Duan T, Cao Y, Zheng Y and Zhan H: Effectiveness, medication
patterns, and adverse events of traditional chinese herbal patches
for osteoarthritis: a systematic review. Evid Based Complement
Alternat Med. 2014:3431762014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ann JY, Eo H and Lim Y: Mulberry leaves
(Morus alba L.) ameliorate obesity-induced hepatic lipogenesis,
fibrosis, and oxidative stress in high-fat diet-fed mice. Genes
Nutr. 10:462015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jo SP, Kim JK and Lim YH:
Antihyperlipidemic effects of stilbenoids isolated from Morus alba
in rats fed a high-cholesterol diet. Food Chem Toxicol. 65:213–218.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HG, Jeong HU, Park G, Kim H, Lim Y and
Oh MS: Mori folium and mori fructus mixture attenuates high-fat
diet-induced cognitive deficits in mice. Based Complement Alternat
Med. 2015:3794182015.
|
|
20
|
Tirupathi RG, Suresh BK, Ujwal KJ, Sujana
P, Raoa AV and Sreedhar AS: Anti-microbial principles of selected
remedial plants from Southern India. Asian Pac J Trop Biomed.
1:298–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qin J, Fan M, He J, Wu XD, Peng LY, Su J,
Cheng X, Li Y, Kong LM, Li RT and Zhao Q: New cytotoxic and
anti-inflammatory compounds isolated from Morus alba L. Nat Prod
Res. 29:1711–1718. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan MA, Rahman AA, Islam S, Khandokhar P,
Parvin S, Islam MB, Hossain M, Rashid M, Sadik G, Nasrin S, et al:
A comparative study on the antioxidant activity of methanolic
extracts from different parts of Morus alba L. (Moraceae). BMC Res
Notes. 6:242013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deepa M, Sureshkumar T, Satheeshkumar PK
and Priya S: Antioxidant rich Morus alba leaf extract induces
apoptosis in human colon and breast cancer cells by the
downregulation of nitric oxide produced by inducible nitric oxide
synthase. Nutr Cancer. 65:305–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deepa M and Priya S: Purification and
characterization of a novel anti-proliferative lectin from Morus
alba L. leaves. Protein Pept Lett. 19:839–845. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Enkhmaa B, Shiwaku K, Katsube T, Kitajima
K, Anuurad E, Yamasaki M and Yamane Y: Mulberry (Morus alba L.)
leaves and their major flavonol quercetin 3-(6-malonylglucoside)
attenuate atherosclerotic lesion development in LDL
receptor-deficient mice. J Nutr. 135:729–734. 2005.PubMed/NCBI
|
|
26
|
Sugimoto M, Arai H, Tamura Y, Murayama T,
Khaengkhan P, Nishio T, Ono K, Ariyasu H, Akamizu T, Ueda Y, et al:
Mulberry leaf ameliorates the expression profile of adipocytokines
by inhibiting oxidative stress in white adipose tissue in db/db
mice. Atherosclerosis. 204:388–394. 2009. View Article : Google Scholar
|
|
27
|
Lee YJ, Choi DH, Kim EJ, Kim HY, Kwon TO,
Kang DG and Lee HS: Hypotensive, hypolipidemic, and vascular
protective effects of Morus alba L. in rats fed an atherogenic
diet. Am J Chin Med. 39:39–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee H, Pyo MJ, Bae SK, Heo Y, Kim CG, Kang
C and Kim E: Improved Therapeutic Profiles of PLA2-Free Bee Venom
Prepared by Ultrafiltration Method. Toxicol Res. 31:33–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim BH, Oh I, Kim JH, Jeon JE, Jeon B,
Shin J and Kim TY: Anti-inflammatory activity of compounds isolated
from Astragalus sinicus L. in cytokine-induced keratinocytes and
skin. Exp Mol Med. 46:e872014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gebauer M, Saas J, Sohler F, Haag J, Söder
S, Pieper M, Bartnik E, Beninga J, Zimmer R and Aigner T:
Comparison of the chondrosarcoma cell line SW1353 with primary
human adult articular chondrocytes with regard to their gene
expression profile and reactivity to IL-1beta. Osteoarthritis
Cartilage. 13:697–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Toegel S, Wu SQ, Otero M, Goldring MB,
Leelapornpisid P, Chiari C, Kolb A, Unger FM, Windhager R and
Viernstein H: Caesalpinia sappan extract inhibits IL1β-mediated
overexpression of matrix metalloproteinases in human chondrocytes.
Genes Nutr. 7:307–318. 2012. View Article : Google Scholar :
|
|
32
|
Torzilli PA, Tehrany AM, Grigiene R and
Young E: Effects of misoprostol and prostaglandin E2 on
proteoglycan biosynthesis and loss in unloaded and loaded articular
cartilage explants. Prostaglandins. 52:157–173. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amin AR, Attur M and Abramson SB: Nitric
oxide synthase and cyclooxygenases: distribution, regulation, and
intervention in arthritis. Curr Opin Rheumatol. 11:202–209. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wada Y, Shimada K, Sugimoto K, Kimura T
and Ushiyama S: Novel p38 mitogen-activated protein kinase
inhibitor R-130823 protects cartilage by downregulating matrix
metal-loproteinase-1,-13 and prostaglandin E2 production
in human chondrocytes. Int Immunopharmacol. 6:144–155. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park SJ, Cheon EJ and Kim HA: MicroRNA-558
regulates the expression of cyclooxygenase-2 and IL-1β-induced
catabolic effects in human articular chondrocytes. Osteoarthritis
Cartilage. 21:981–989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu YC, Jayakumar T, Duann YF, Chou YC,
Hsieh CY, Yu SY, Sheu JR and Hsiao G: Chondroprotective role of
sesamol by inhibiting MMPs expression via retaining NF-κB signaling
in activated SW1353 cells. J Agric Food Chem. 59:4969–4978. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rigoglou S and Papavassiliou AG: The NF-κB
signalling pathway in osteoarthritis. Int J Biochem Cell Biol.
45:2580–2584. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liacini A, Sylvester J, Li WQ, Huang W,
Dehnade F, Ahmad M and Zafarullah M: Induction of matrix
metalloproteinase-13 gene expression by TNF-alpha is mediated by
MAP kinases, AP-1, and NF-kappaB transcription factors in articular
chondrocytes. Exp Cell Res. 288:208–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salvatierra J, Escames G, Hernandez P,
Cantero J, Crespo E, Leon J, Salvatierra D, Acuña-Castroviejo D and
Vives F: Cartilage and serum levels of nitric oxide in patients
with hip osteoarthritis. J Rheumatol. 26:2015–2017. 1999.PubMed/NCBI
|
|
40
|
Miyaura C, Inada M, Suzawa T, Sugimoto Y,
Ushikubi F, Ichikawa A, Narumiya S and Suda T: Impaired bone
resorption to prostaglandin E2 in prostaglandin E
receptor EP4-knockout mice. J Biol Chem. 275:19819–19823. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen WP, Wang YL, Tang JL, Hu PF, Bao JP
and Wu LD: Morin inhibits interleukin-1β-induced nitric oxide and
prostaglandin E2 production in human chondrocytes. Int
Immunopharmacol. 12:447–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Graham S, Gamie Z, Polyzois I, Narvani AA,
Tzafetta K and Tsiridis E, Helioti M, Mantalaris A and Tsiridis E:
Prostaglandin EP2 and EP4 receptor agonists in bone formation and
bone healing: in vivo and in vitro evidence. Expert Opin Investig
Drugs. 18:746–766. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sasaki K, Hattori T, Fujisawa T, Takahashi
K, Inoue H and Takigawa M: Nitric oxide mediates
interleukin-1-induced gene expression of matrix metalloproteinases
and basic fibroblast growth factor in cultured rabbit articular
chondrocytes. J Biochem. 123:431–439. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tung JT, Arnold CE, Alexander LH,
Yuzbasiyan-Gurkan V, Venta PJ, Richardson DW and Caron JP:
Evaluation of the influence of prostaglandin E2 on
recombinant equine interleukin-1beta-stimulated matrix
metalloproteinases 1, 3, and 13 and tissue inhibitor of matrix
metalloproteinase 1 expression in equine chondrocyte cultures. Am J
Vet Res. 63:987–993. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barchowsky A, Frleta D and Vincenti MP:
Integration of the NF-kappaB and mitogen-activated protein
kinase/AP-1 pathways at the collagenase-1 promoter: divergence of
IL-1 and TNF-dependent signal transduction in rabbit primary
synovial fibroblasts. Cytokine. 12:1469–1479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
White LA and Brinckerhoff CE: Two
activator protein-1 elements in the matrix metalloproteinase-1
promoter have different effects on transcription and bind Jun D,
c-Fos, and Fra-2. Matrix Biol. 14:715–725. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Itthiarbha A, Phitak T, Sanyacharernkul S,
Pothacharoen P, Pompimon W and Kongtawelert P: Polyoxypregnane
glycoside from Dregea volubilis extract inhibits IL-1β-induced
expression of matrix metalloproteinase via activation of NF-κB in
human chondrocytes. In Vitro Cell Dev Biol Anim. 48:43–53. 2012.
View Article : Google Scholar
|
|
49
|
Sunaga T, Oh N, Hosoya K, Takagi S and
Okumura M: Inhibitory effects of pentosan polysulfate sodium on
MAP-kinase pathway and NF-κB nuclear translocation in canine
chondrocytes in vitro. J Vet Med Sci. 74:707–711. 2012. View Article : Google Scholar : PubMed/NCBI
|