1. Introduction
Curcumin is the principal constituent of turmeric
i.e., the ground rhizomes of Curcuma longa, which contains
two other curcuminoids: desmethoxycurcumin and
bis-desmethoxycurcumin (1).
Turmeric is widely used as a spice mostly in Asian countries.
However, it is also used to treat acne, psoriasis, dermatitis and
rash. It should be stressed that traditionally, turmeric was
suspended in whole milk or buttermilk that dissolved it in fat
fractions and/or stabilized curcumin (2). Over the past few decades,
preclinical and clinical studies have revealed that curcumin is
active against variety of diseases, such as cancer and pulmonary
diseases, as well as neurological, liver, metabolic, autoimmune and
cardiovascular diseases, and numerous other chronic ailments
(3–6). Over 116 clinical studies on curcumin
in humans were registered with the US National Institutes of Health
in 2015 encomapssing a number of conditions, such as cancer,
cognitive disorders, gastrointestinal diseases and psychiatric
conditions. In humans, the administration of curcumin at up to 12 g
per day has not been found to exert any toxic effects (7–10).
One of the puzzling questions is how curcumin can be
so effective in the treatment of diseases, since it has a very low
water solubility and bioavailability. For example, the oral dose of
8 g/day in humans translates to low nanogram levels of circulating
curcumin in plasma (only 22–41 ng/ml) (11,12). Moreover, curcumin is not stable
under various conditions, such as aqueous phosphate buffer or
serum-free medium at 37°C, degrading to the bioactive compounds,
including ferulic acid, feruloylmethane and vanillin, which may be
responsible for its biological activities rather than curcumin
itself (12,13).
In view of the very low bioavailability of curcumin
as observed in clinical studies, the role of the degradation or
condensation products should be taken into consideration when
evaluating the activity of curcumin in various diseases.
2. Physico-chemical properties of
curcumin
Curcumin (IUPAC name:
(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione)
is, practically insoluble in water at a neutral and lower pH, but
is soluble in acetone, dichloromethane, methanol, ethanol, alkali
and oils. The water solubility of curcumin may be increased by its
incorporation into various surfactants, such as sodium dodecyl
sulfate, polysaccharides, polyethylene glycol and cyclodextrins, as
well as others (13,14). In addition, in aqueous solutions
and at an alkaline pH, the acidic phenol group in curcumin
dissociates its hydrogen, forming the phenolate ion(s) that render
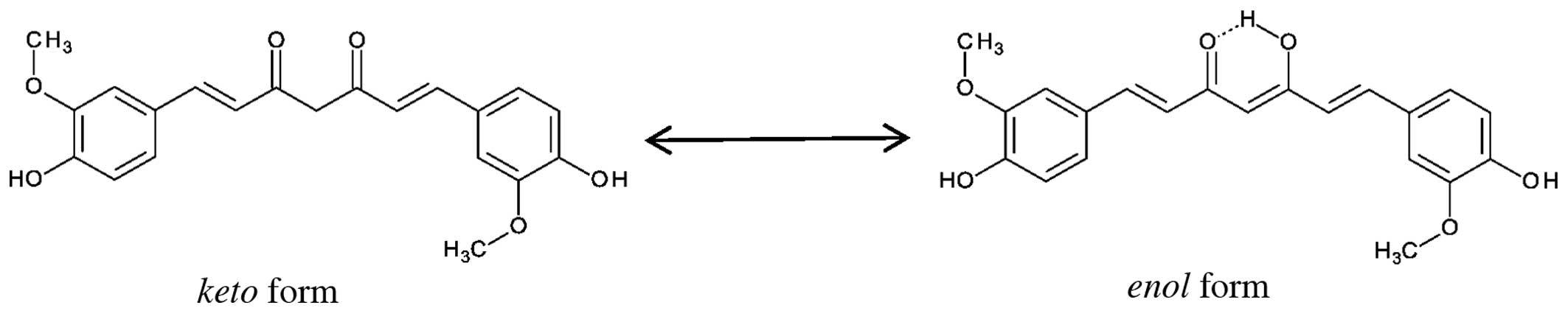
the solubility of curcumin in water somewhat possible (15–18). Curcumin is a natural polyphenol
that is responsible for the yellow color of turmeric and exhibits
keto-enol tautomerism (Fig.
1). The enol form is more energetically stable in the
solid phase and, depending on the solvent, up to 95% can be in the
enol form (1). Three
reactive functional groups, namely diketone moiety and two phenolic
groups determine the activity of curcumin. The biologically
important chemical reactions of curcumin are the following: the
hydrogen donation leading to oxidation, reversible and irreversible
nucleophilic addition (Michael reaction), hydrolysis, degradation
and enzymatic reactions (19).
In a previous review article, Agrawal and Mishra
analyzed studies (years 1815–2009) on curcumin and 728 curcumin
analogs (20). This very large
group of compounds was tested for pharmacological properties and
mostly on anticancer activity on different cell lines. Some analogs
have been shown to exhibit antioxidant, anti-mutagenic and anti-HIV
activities (21), as well as
anti-angiogenic anti-malaria and anti-tuberculosis activities
(7) or anti-inflammatory
activities [cyclooxygenases (COX) inhibitors]. Based on a
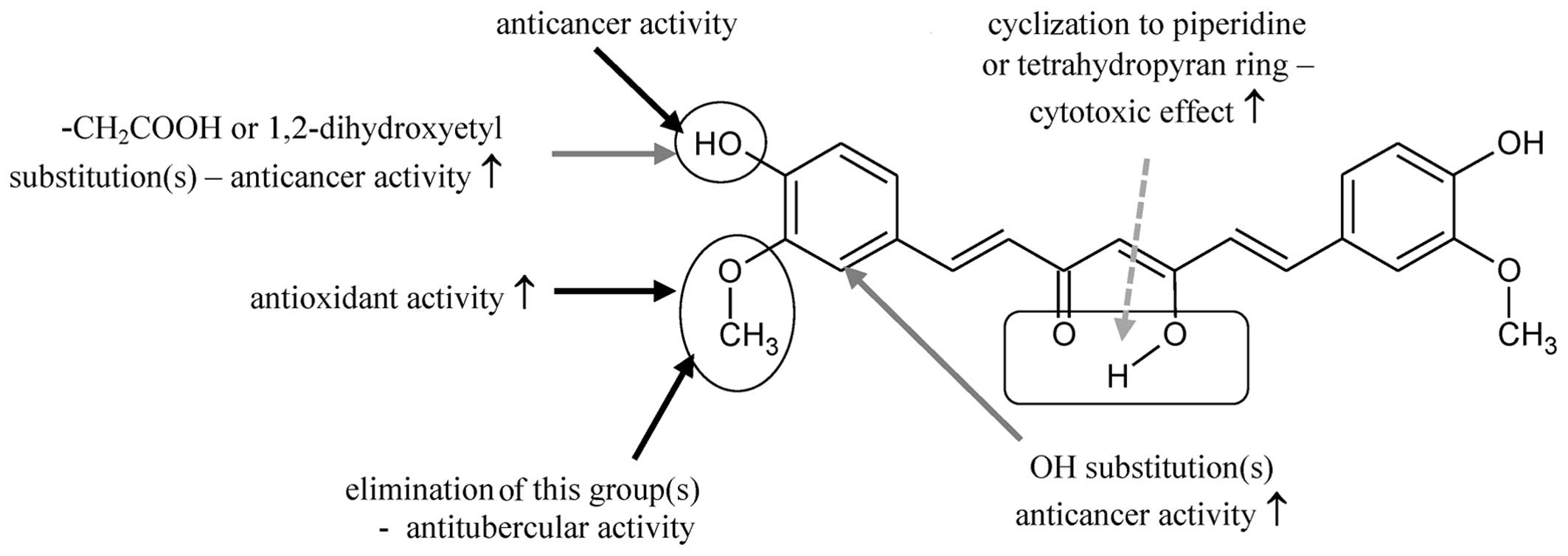
literature search, the authors concluded the following (Fig. 2): the anticancer properties of
curcuminoids depend on the presence of OH groups in the phenolic
ring (entries 4 and 4′). These groups are an electron donor to free
radicals. The methoxy group at position 3 and 3′ increases the
antioxidant properties of curcuminoids; substitution in the 2 and
2′ positions increases all activities than the unsubstituted
analogs; cyclization in the central part of the compound and the
introduction of heteroatoms (oxygen and nitrogen) leads to the
formation of compounds with enhanced antitumor and anti-angiogenic
activities; attaching solubilizing groups to the OH group in
position 4 and 4′ is responsible for the cytotoxicity of
curcuminoids; the elimination of one of the methoxy group reveals
the effect of tuberculosis (7);
conversion of methoxy groups to hydroxyl increases the anti-HIV
activity (21).
3. Alkaline degradation and autoxidation of
curcumin
Wang et al (13) incubated curcumin in 0.1 M
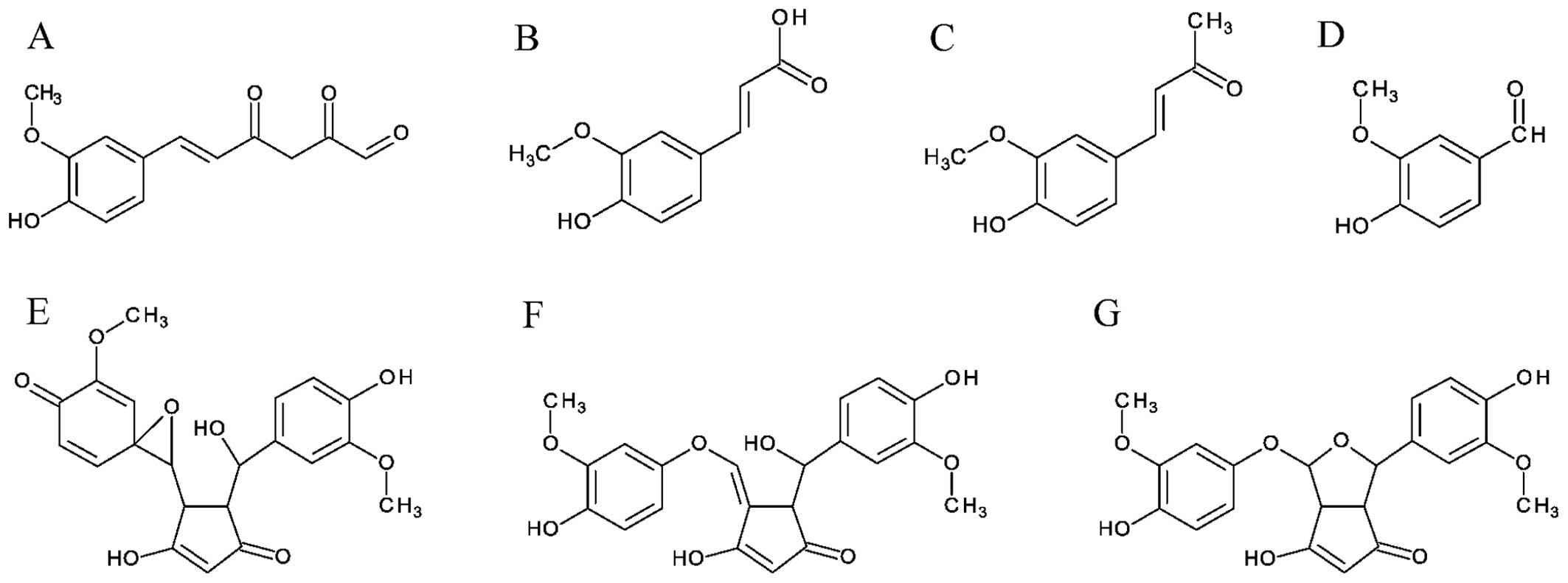
phosphate buffer, pH 7.2 at 37°C, and found that 90% was degraded
in 30 min. Trans-6-(4-hydroxy-3-methoxyphenyl)-2,4-dioxo-5-hexenal,
vanillin, ferulic acid and feruloyl methane (Fig. 3A–D) were identified as degradation
products (13). This is a
plausible explanation of the biological activity of curcumin, since
the degradation products have better aqueous solubility as
reflected by their respective logP values: 1.42 for ferulic acid
and 1.09 for vanillin, lower than the keto and enol
form of curcumin, which are respectively 2.56 and 2.17 (12). Moreover, it has been reported that
ferulic acid inhibits COX-1 and -2 and suppresses the activation of
nuclear factor-κB (NF-κB), which are known to be important targets
in the prevention of cancer development (12,22,23). Vanillin as well can inhibit COX-2
gene expression and NF-κB activation (12,24).
Shen and Ji (12),
in a comprehensive review of curcumin degradation, described the
curcumin-mediated inhibition of xanthine oxidase that is involved
in the pathogenesis of many diseases. The authors described
molecular modeling, demonstrating that all degradation products can
enter into the binding pocket of an enzyme. Surprisingly, curcumin
itself failed to efficiently fit within the binding pocket of
xanthine oxidase and only entered the binding pocket with low
binding affinity (12). This is
consistent with the experimental findings that the degradation
products (ferulic acid, vanillin, ferulic acid and feruloyl
methane), rather than curcumin itself can inhibit xanthine oxidase
(12,25,26).
In a previous study, Gordon and Schneider
demonstrated that the cleavage of the heptadienedione chain,
resulting in vanillin, ferulic acid and feruloylmethane as
products, was not the prevailing degradation reaction (27). Rather, they proposed that the
degradation of curcumin is a spontaneous autoxidation, free
radical-driven incorporation of oxygen and that the major product
of this process is a bicyclopentadione (15,27). It has been reported that different
product profiles of curcumin autoxidation reactions are dependent
on time. In reactions between 20–45 min, the chromatograms exhibit
peaks, indicating spiroepoxide and vinylether as major products
(Fig. 3E–G), and dihydroxy,
ketohydroxy and hemiketal cyclopentadiones as minor products.
Degradation between 30 min and 4 h also produces the
bicyclopentadiones as major products and, several unidentified
chemicals. When autoxidation is longer than 4 h, bicyclopentadione
is detected as well (28,29).
Naturally occurring polyphenols have been shown to
act with topoisomerase II, increasing the levels of topoisomerase
II-mediated DNA cleavage. Topoisomerase poisons are used in
anticancer and antibacterial therapies. Thus, Ketron et al
(30) investigated whether
curcumin, its structurally related degradation products (vanillin,
ferulic acid and feruloylmethane) and its oxidative metabolites
exert any effects on the DNA cleavage of human topoisomerase IIα
and IIβ. Curcumin, bicyclopentadione, vanillin, ferulic acid and
feruloylmethane were shown to have no effect on DNA cleavage.
However, intermediates of the curcumin oxidation pathway increased
the level of DNA cleavage by both enzymes ~4–5-fold. Moreover,
under conditions that promote oxidation, curcumin enhanced
topoisomerase II-mediated DNA cleavage even further (30).
Gordon et al (28) also demonstrated that the product
of curcumin oxidation, a stable spiroepoxide, was able to poison
recombinant human topoisomerase IIα and that this process was
significantly increased in the presence of potassium ferricyanide,
indicating that oxidative conversion was needed to achieve full DNA
cleavage activity. They concluded that oxidative metabolites may be
responsible for the biological effects of curcumin (28).
4. Photodegradation of curcumin
It is common knowledge that turmeric stains can be
removed by exposure to sunlight. This is due to the fact that
curcumin absorbs strongly in the visible wavelength range, making
it predisposed to degradation and modification in daylight and
artificial lighting. The photodegradation of curcumin takes place
in solid state, as well as in different organic solvents (14,18,31–34). However, the composition,
degradation kinetics and the relative abundance of the degradation
products differ, depending on the physical state of the compound
and the conditions.
Previously, the photochemical degradation of solid
state curcumin exposed to sunlight for 120 h yielded vanillin
(34%), ferulic aldehyde (0.5%), ferulic acid (0.5%), vanillic acid
(0.5%) and three unidentified compounds. The photodegradation of
dissolved curcumin depends on the solvent and wavelength. When
curcumin was dissolved in isopropanol and irradiated for 4 h at
400–510 nm, then similar products as in the case of
light-irradiated crystalline curcumin were observed, such us
vanillin, vanillic acid and ferulic acid, in addition to aldehyde
4-vinylguaiacol (34).
Exposure to visible light inflicts more degradation
than UV light; the irradiation of curcumin in 254-nm in methanol
has been shown to produce three unspecified degradation products,
whereas irradiation with daylight produces five unspecified
degradation chemicals products (31). The exposure of curcumin to visible
light is solvent-dependent. Irradiation with light (400–750-nm) for
4 h was shown to be associated with cyclization at one of the
o-methoxyphenyl groups, producing
7-hydroxy-1-[(2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoyl]-6-methoxy-naphthalen-2(1H)-one
in isopropanol, methanol and chloroform, but not in acetonitrile
and ethyl acetate (32,34). Galer and Šket irradiated
acetonitrile solution of curcumin by light (350 nm) and found that
90% of all formed products included 3,5-dimethoxybenzaldehyde,
3,5-dimethoxybenzoic acid and Z and E isomers of
3-(3,5-dimethoxyphenyl)propenoic acid (35).
It has also been reported that the photodegradation
of curcumin involves the formation of the excited states and
generation of singlet oxygen that is responsible for the
photobiological and photodynamic activity of curcumin (19,33). Thus, it was postulated that the
degradation of curcumin following photoexcitation must proceed
though the triplet excited state of curcumin (19). Curcumin is photoactivated by blue
light (420–480 nm) that has limited tissue penetration. That
property makes curcumin an ideal surface antibacterial agent for
oral or skin disinfection, particularly for antibiotic-resistant
bacterial strains as it does not affect healthy tissue (36–38).
An interesting observation was previously made when
studying the interaction of curcumin with lipoxygenase (LOX) by a
single-crystal X-ray analysis, showing the complex Enz:Fe-O-O-R
with the curcumin degradation product instead, identified as
4-hydroperoxy-2-methoxyphenol bound to the enzyme's iron cofactor.
Irradiation by X-ray is known to produce free radicals, but
curcumin itself is stable under such conditions. LOX is a very good
biocatalyst stimulating many reactions, neither of which would lead
to the observed product. Thus, it was obvious that the X-ray
radiation, LOX and curcumin properties together were responsible
for the curcumin transformation to this peroxide, which converts it
into 2-methoxycyclohexa-2,5-diene-1,4-dione (39). While the enzyme in that experiment
was of plant origin, humans do have six LOXs, four of which (5-,
12S-, 12R-LOX and eLOX-3, [for comparison see (40)] have a highly similar structure of
the enzymatic active site. X-rays radiation used for curative
purposes in humans often causes severe side-effects, including
inflammatory responses caused by various eicosanoids produced by
oxygenases: COX and LOX and cytochrome P450 (CYP450). It may be
worth exploring whether and how curcumin may be utilized during
radiation therapy to improve the treatment outcomes and the comfort
of patients.
5. Curcumin complexes with metals
Curcumin can form complexes with transition metals
to protect against degradation in the treatment of Alzheimer's
disease (41,42). Several curcumin complexes with
metals (Cu, Mn, V, Ga and In) have been synthesized and evaluated
for their biological activity (43–49). However, all these metallocomplexes
have been synthetized under high temperature conditions, reflux at
100°C in the presence of different organic solvents for 3 h. Zebib
et al (50) synthesized
curcumin complexes with divalent ions of Zn2+,
Cu2+, Mg2+ and Se2+, in
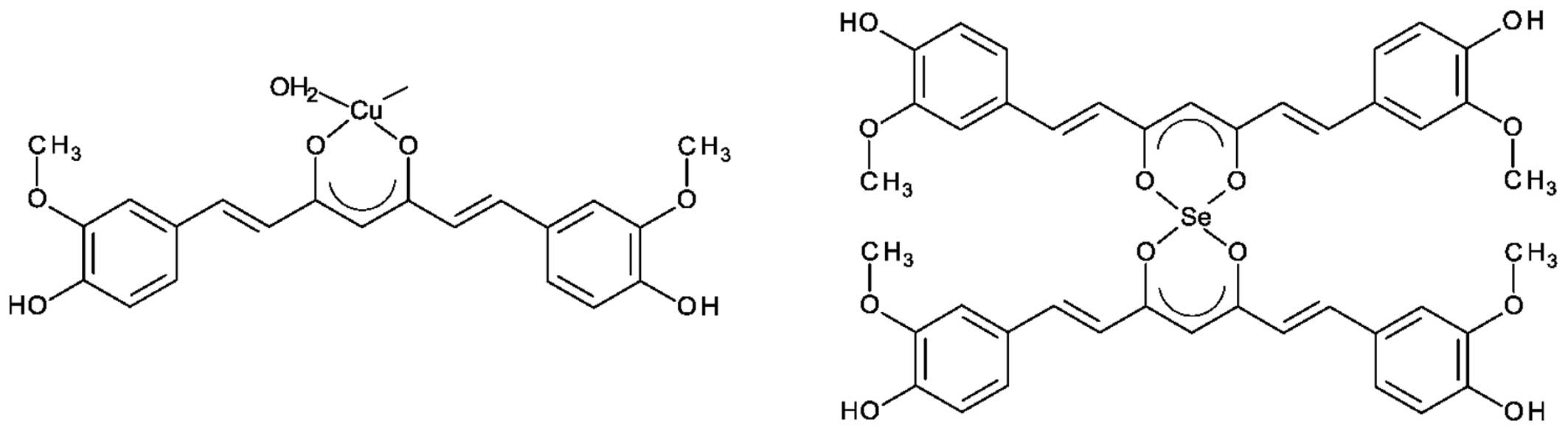
glycerol/water solution and room temperature (50) (Fig.
4). They found that all complexes were stable in water at pH
6.5 up to 30 h at 37°C. All complexes rapidly decomposed by
demetallization at acidic pH 2 and greatly decreased at higher pH
10. At pH 7.0, in phosphate buffer, curcumin was degraded after 1
h, while <5% of complexes were degraded. The authors estimated
that the stability of curcumin metal complexes at pH 7 was ~20-fold
greater than that of curcumin alone (50).
John et al (49) synthetized four synthetic
curcuminoids and their Cu2+ metallocomplexes. Using L929
mouse fibrosarcoma cells, they found that the concentration
required for the 50% inhibition of cell growth was ~10 µg/ml
for curcuminoids, but only 1 µg/ml for their copper
counterparts. Moreover, they observed a significant reduction
(p<0.001) in tumor volume in mice treated with copper chelates
of curcuminoids (49). Mei et
al (51) investigated the
anti-ulcerogenic effects of a Zn-curcumin chelate in mice.
Treatment with Zn-curcumin reduced gastric lesions in a
dose-dependent manner (12, 24 and 48 mg/kg) in comparison with the
control group (51). In a
different study from the same group, the effects of Zn-curcumin on
hemorheological alterations, oxidative stress and liver injury in a
rat model of acute alcoholism were investigated. They found that
the oral dose of Zn-chelated curcumin prevented the alcohol-induced
increase in malondialdehyde (MDA) levels in serum and the reduction
in glutathione levels and superoxide dismutase (SOD) activity.
Furthermore the Zn-curcumin complex inhibited ethanol-induced liver
injury. In addition, this curcumin derivative reduced the
alcohol-induced elevation of blood viscosity, plasma viscosity,
erythrocyte aggregation index and hematocrit. In all of these
experiments Zn-curcumin was found to be more effective than
curcumin (52).
In another study, Refat (53) synthesized curcumin complexes with
Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) and
tested the antibacterial and antifungal activity. Only the cobalt
[Co(II)]-curcumin complex exhibited antibacterial activity against
three bacterial strains (Bacillus subtilis, Pseudomonas
aeruginosa and Staphylococcus aureus) (53).
6. Interaction of enzymes with curcumin
Curcumin interacts with very large number of
proteins, such us albumin (54),
Ca2+-ATPase of the sarcoplasmic reticulum (55,56), Ca2+-dependent protein
kinase (CDPK) (57), COX-2
(58,59), LOX (39,60), LOX-5 (61), pp60c-src tyrosine kinase (62,63), PKC (63), xanthine oxidase (64,65) and many others (66). Dr Duke's Phytochemical and
Ethnobotanical Database (https://phytochem.nal.usda.gov/phytochem/search)
provides a long list of curcumin anti- and pro-health properties.
It is known as an inhibitor of the oxygenases 5- and 12-LOX, COX-2
and CYP450, but an inducer of lipase which is up in the arachidonic
acid pathway. It can inhibit protein kinase C (PKC), protein
tyrosine kinase (PTK), IKB-kinase and IKK involved in NF-κB
signaling pathway, tumor necrosis factor (TNF), topoisomerase I and
II, vascular endothelial growth factor (VEGF), sortase A and
ornithine decarboxylase. It promotes maltase and sucroses, and
glutathione synthetase activity, while it suppresses amyloid β
activity. This list, probably far from complete, shows that
curcumin has a very broad range of activities and that its impact
would depend on dose and environment.
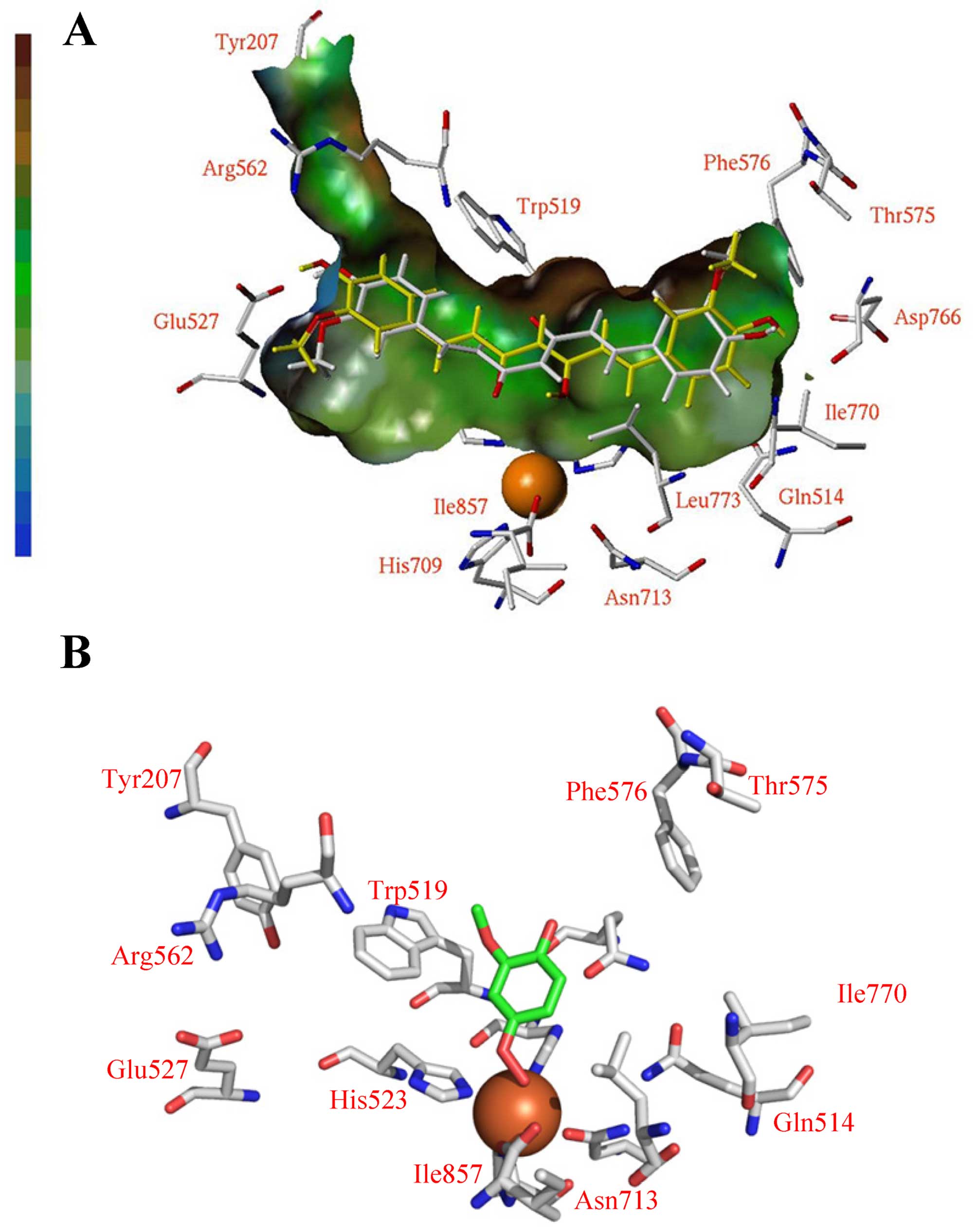
Computational molecular modeling methods have shown
that curcumin can bind into the central active pocket of soybean
LOX-3 lipoxygnase (39). However,
the solved X-ray structure of LOX shows the photodegradation
product of curcumin (Fig. 5).
That was based on the size, volume and position of the unoccupied
(Fo-Fc) difference map (39,60).
7. Changes in the body
Interestingly, curcumin has is metabolized
differently in the mammalian body depending on the type of
administration (oral, intravenous or intraperitoneal). Curcumin
administered orally undergoes glucuronidation and sulfation to the
glucuronide of hexahydrocurcumin (67). However, when administered
intravenously or intraperitoneally, it undergoes a reduction that
leads to the formation of tetrahydrocurcumin and hexahydrocurcumin,
the two major metabolites of curcumin in body fluids, organs and
cells (68), and two minor
metabolites: octahydrocurcumin and dihydrocurcumin. Curcumin is
poorly absorbed from the gastrointestinal tract after oral intake,
with extremely low concentrations being detected only in bile,
urine (67,69) and blood plasma (70). Furthermore, in available tissue
samples obtained during surgery from patients administered high
doses of curcumin, either no curcumin, or very low concentrations
of curcumin conjugates were detected (71). Despite the lower bioavailability,
in clinical investigations, curcumin has been shown to have
therapeutic potential in various human diseases. The main activity
of curcumin seems to be connected with its modulatory activity of
cell signaling pathways and transcription factors, such as NF-κB,
activator protein 1 (AP-1) and mitogen-activated protein kinase
(MAPK) (72), and its suppressive
effects on the expression of inflammatory cytokines, such as
interleukin-6 (IL-6), IL-1β, TNF-α, matrix metalloproteinase
(MMP)-2 and MMP-9 (73).
Periodontitis, as an imbalance between biofilm and
immune host reaction, is an opportunistic and chronic infection in
which the above-mentioned cell signaling pathways regulating the
expression of inflammatory mediators have become promising
therapeutic targets (74). The
oral cavity can be easily used to observe the reactions of tissue
to curcumin in vitro and in vivo. In many animal
studies, the antioxidant (75)
and anti-inflammatory (76), as
well as the angiogenic and wound-healing effects of curcumin, by
increasing the number of fibroblasts and promoting collagen
synthesis (77), or by modulating
urokinase plasminogen activator (uPA) expression (78), have been demonstrated.
Interestingly, curcumin does not prevent alveolar bone resorption
in vivo in rats (76), but
inhibits RANKL-induced osteoclastogenesis in vitro (79). Guimarães et al also
observed the lack of inhibition of bone resorption in an
experimental model of lipopolysacharide (LPS)-induced periodontal
disease (76), which is
surprising, keeping in mind that curcumin was shown to
significantly inhibit Porphyromonas gingivalis LPS-induced
TNF-α and IL-1β production (80).
Curcumin has also exhibited exhibited bactericidal activity against
Porphyromonas gingivalis, Prevotella intermedia,
Fusobacterium nucleatum and capnocytophaga at a
minimal inhibitory concentration (MIC) of 1 mg/ml by the local
application into periodontal pockets in humans (81). The results of the
benzoyl-DL-arginine-naphthylamide (BANA) test, which verifyied the
presence of Tannerella forsythia, Treponema
denticola, Porphyromonas gingivalis and
capnocytophaga species, was similar for 1% curcumin solution
and 0.2% chlorhexidine gluconate in subgingival irrigation. The
only difference was the earlier re-colonization by periopathogens
places treated with curcumin (82). Curcumin exhibits a local activity
in the chemopreventive therapy of premalignant lesions for oral
cancer. In an in vitro model consisting of primary cultures
of normal epithelial cells, cell lines derived from dysplastic
leukoplakia and squamous cell carcinoma cells, curcumin was equally
effective for all cell types tested and blocked the cells in the
S/G2M phase of the cell cycle (83). The local effectiveness of curcumin
was higher in combination with(−)-epigallocatechin-3-gallate (EGCG)
(83). This suggests some
limitations of curcumin even in local treatment.
Numerous approaches have been undertaken to improve
the poor solubility, rapid metabolic disposition and the lack of
systemic bioavailability of curcumin. One of the possibilities is
the use of curcumin metabolites or chemically modified curcumin.
Unlike curcumin, tetrahydrocurcumin is stable in phosphate buffer
and in saline at various pH values and is easy absorbed through the
gastrointestinal tract (84). An
in vitro investigation revealed that treatment with
tetrahydrocurcumin reduced fibrosarcoma cell (HT1080) adhesion to
the extracellular matrix and laminin, and the secretion of MMPs
(MMP-2 and MMP-9) and uPA (85).
It has also been shown to affect the migration and proliferation of
gingival fibroblast cells (86).
Chemically modified curcumin with a carbonyl substituent at the C-4
position exhibits better solubility, serum albumin-binding activity
and enhanced zinc-binding characteristics (87). 4-Metoxycarbonyl curcumin,
administered orally, was shown to inhibit MMP-9 and MMP-13 activity
more effectively than curcumin (by 2–7-fold). It also demonstrates
greater therapeutic activity, based on its inhibitory effect on
MMPs and pro-inflamatory mediators [TNF-α, IL-1β, monocyte
chemoattractant protein-1 (MCP-1), IL-6 and prostaglandin (PGE)-2]
(87). In another study,
phenylamino carbonyl curcumin exerted inhibitory effects on MMPs in
rats with LPS-induced periodontitis; however, unlike curcumin, it
significantly reduced alveolar bone loss (88). The bioavailability of curcumin
extremely was shown to increase when combined with piperine (20
mg/kg) in rats and humans with no side-effects (89). Promising results were also
obtained in a recent study which used mucoadhesive nanoparticles
loaded with curcumin as a new approach with which to deliver
curcumin for the local treatment of oral cancer (90).
8. Conclusion
Clearly curcumin exhibits a variety of health
benefits; however, proof that the curcumin molecule itself is
responsible for these effects seems to be illusive. This is due to
the poor bioavailability, instability and strong reactivity of
curcumin. An additional complicating factor is the fact that
curcumin metabolizes differently depending on the type of
administration. The ability of curcumin to undergo degradation or
condensation strongly suggests that research should also focus on
the health benefits of these molecules rather than only on curcumin
itself.
References
|
1
|
Manolova Y, Deneva V, Antonov L, Drakalska
E, Momekova D and Lambov N: The effect of the water on the curcumin
tautomerism: A quantitative approach. Spectrochim Acta A Mol Biomol
Spectrosc. 132:815–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fu S, Shen Z, Ajlouni S, Ng K, Sanguansri
L and Augustin MA: Interactions of buttermilk with curcuminoids.
Food Chem. 149:47–53. 2014. View Article : Google Scholar
|
|
3
|
Gostner J, Ciardi C, Becker K, Fuchs D and
Sucher R: Immunoregulatory impact of food antioxidants. Curr Pharm
Des. 20:840–849. 2014. View Article : Google Scholar
|
|
4
|
Gupta SC, Kismali G and Aggarwal BB:
Curcumin, a component of turmeric: From farm to pharmacy.
Biofactors. 39:2–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maruta H: Herbal therapeutics that block
the oncogenic kinase PAK1: A practical approach towards
PAK1-dependent diseases and longevity. Phytother Res. 28:656–672.
2014. View
Article : Google Scholar
|
|
6
|
Srinivasan K: Antioxidant potential of
spices and their active constituents. Crit Rev Food Sci Nutr.
54:352–372. 2014. View Article : Google Scholar
|
|
7
|
Current clinical trials on curcumin. US
National Institutes of Health, Clinical Trial Registry; June. 2015.
2015
|
|
8
|
Bar-Sela G, Epelbaum R and Schaffer M:
Curcumin as an anticancer agent: Review of the gap between basic
and clinical applications. Curr Med Chem. 17:190–197. 2010.
View Article : Google Scholar
|
|
9
|
Chainani-Wu N: Safety and
anti-inflammatory activity of curcumin: A component of tumeric
(Curcuma longa). J Altern Complement Med. 9:161–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as 'Curecumin': From kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008. View Article : Google Scholar
|
|
11
|
Dhillon N, Aggarwal BB, Newman RA, Wolff
RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V and Kurzrock
R: Phase II trial of curcumin in patients with advanced pancreatic
cancer. Clin Cancer Res. 14:4491–4499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen L and Ji HF: Contribution of
degradation products to the anticancer activity of curcumin. Clin
Cancer Res. 15:7108author reply 7108–7109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS,
Hsieh CY and Lin JK: Stability of curcumin in buffer solutions and
characterization of its degradation products. J Pharm Biomed Anal.
15:1867–1876. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tønnesen HH: Solubility, chemical and
photochemical stability of curcumin in surfactant solutions.
Studies of curcumin and curcuminoids, XXVIII. Pharmazie.
57:820–824. 2002.
|
|
15
|
Schneider C, Gordon ON, Edwards RL and
Luis PB: Degradation of Curcumin: From Mechanism to Biological
Implications. J Agric Food Chem. 63:7606–7614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Metzler M, Pfeiffer E, Schulz SI and Dempe
JS: Curcumin uptake and metabolism. Biofactors. 39:14–20. 2013.
View Article : Google Scholar
|
|
17
|
Mohanty C and Sahoo SK: The in vitro
stability and in vivo pharmacokinetics of curcumin prepared as an
aqueous nanoparticulate formulation. Biomaterials. 31:6597–6611.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tønnesen HH and Karlsen J: Studies on
curcumin and curcuminoids. VI Kinetics of curcumin degradation in
aqueous solution. Z Lebensm Unters Forsch. 180:402–404. 1985.
View Article : Google Scholar
|
|
19
|
Priyadarsini KI: The chemistry of
curcumin: From extraction to therapeutic agent. Molecules.
19:20091–20112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agrawal DK and Mishra PK: Curcumin and its
analogues: Potential anticancer agents. Med Res Rev. 30:818–860.
2010.
|
|
21
|
Mazumder A, Neamati N, Sunder S, Schulz J,
Pertz H, Eich E and Pommier Y: Curcumin analogs with altered
potencies against HIV-1 integrase as probes for biochemical
mechanisms of drug action. J Med Chem. 40:3057–3063. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jayaprakasam B, Vanisree M, Zhang Y,
Dewitt DL and Nair MG: Impact of alkyl esters of caffeic and
ferulic acids on tumor cell proliferation, cyclooxygenase enzyme,
and lipid peroxidation. J Agric Food Chem. 54:5375–5381. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung KJ, Go EK, Kim JY, Yu BP and Chung
HY: Suppression of age-related renal changes in NF-kappaB and its
target gene expression by dietary ferulate. J Nutr Biochem.
20:378–388. 2009. View Article : Google Scholar
|
|
24
|
Murakami Y, Hirata A, Ito S, Shoji M,
Tanaka S, Yasui T, Machino M and Fujisawa S: Re-evaluation of
cyclooxygenase-2-inhibiting activity of vanillin and guaiacol in
macrophages stimulated with lipopolysaccharide. Anticancer Res.
27:801–807. 2007.PubMed/NCBI
|
|
25
|
Chang YC, Lee FW, Chen CS, Huang ST, Tsai
SH, Huang SH and Lin CM: Structure-activity relationship of C6-C3
phenylpropanoids on xanthine oxidase-inhibiting and free
radical-scavenging activities. Free Radic Biol Med. 43:1541–1551.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen L and Ji HF: Insights into the
inhibition of xanthine oxidase by curcumin. Bioorg Med Chem Lett.
19:5990–5993. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gordon ON and Schneider C: Vanillin and
ferulic acid: Not the major degradation products of curcumin.
Trends Mol Med. 18:361–363; author reply 363–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gordon ON, Luis PB, Ashley RE, Osheroff N
and Schneider C: Oxidative transformation of demethoxy- and
bisdemethoxycurcumin: Products, mechanism of formation, and
poisoning of human topoisomerase IIα. Chem Res Toxicol. 28:989–996.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Griesser M, Pistis V, Suzuki T, Tejera N,
Pratt DA and Schneider C: Autoxidative and cyclooxygenase-2
catalyzed transformation of the dietary chemopreventive agent
curcumin. J Biol Chem. 286:1114–1124. 2011. View Article : Google Scholar :
|
|
30
|
Ketron AC, Gordon ON, Schneider C and
Osheroff N: Oxidative metabolites of curcumin poison human type II
topoisomerases. Biochemistry. 52:221–227. 2013. View Article : Google Scholar :
|
|
31
|
Ansari MJ, Ahmad S, Kohli K, Ali J and
Khar RK: Stability-indicating HPTLC determination of curcumin in
bulk drug and pharmaceutical formulations. J Pharm Biomed Anal.
39:132–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heger M, van Golen RF, Broekgaarden M and
Michel MC: The molecular basis for the pharmacokinetics and
pharmacodynamics of curcumin and its metabolites in relation to
cancer. Pharmacol Rev. 66:222–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tønnesen HH, de Vries H, Karlsen J and
Beijersbergen van Henegouwen G: Studies on curcumin and
curcuminoids. IX: Investigation of the photobiological activity of
curcumin using bacterial indicator systems. J Pharm Sci.
76:371–373. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tønnesen HH, Karlsen J and van Henegouwen
GB: Studies on curcumin and curcuminoids. VIII Photochemical
stability of curcumin. Z Lebensm Unters Forsch. 183:116–122. 1986.
View Article : Google Scholar
|
|
35
|
Galer P and Šket B: Photodegradation of
methoxy substituted curcuminoids. Acta Chim Slov. 62:346–353. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leite DP, Paolillo FR, Parmesano TN,
Fontana CR and Bagnato VS: Effects of photodynamic therapy with
blue light and curcumin as mouth rinse for oral disinfection: A
randomized controlled trial. Photomed Laser Surg. 32:627–632. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mahdi Z, Habiboallh G, Mahbobeh NN, Mina
ZJ, Majid Z and Nooshin A: Lethal effect of blue light-activated
hydrogen peroxide, curcumin and erythrosine as potential oral
photosensitizers on the viability of Porphyromonas gingivalis and
Fusobacterium nucleatum. Laser Ther. 24:103–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin R and Hamblin MR: Antimicrobial
photosensitizers: Drug discovery under the spotlight. Curr Med
Chem. 22:2159–2185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Skrzypczak-Jankun E, Zhou K, McCabe NP,
Selman SH and Jankun J: Structure of curcumin in complex with
lipoxygenase and its significance in cancer. Int J Mol Med.
12:17–24. 2003.PubMed/NCBI
|
|
40
|
Jankun J, Doerks T, Aleem AM,
Lysiak-Szydłowska W and Skrzypczak-Jankun E: Do human lipoxygenases
have a PDZ regulatory domain? Curr Mol Med. 8:768–773. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ji HF and Zhang HY: Multipotent natural
agents to combat Alzheimer's disease. Functional spectrum and
structural features. Acta Pharmacol Sin. 29:143–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang HY: One-compound-multiple-targets
strategy to combat Alzheimer's disease. FEBS Lett. 579:5260–5264.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barik A, Mishra B, Kunwar A, Kadam RM,
Shen L, Dutta S, Padhye S, Satpati AK, Zhang HY and Indira
Priyadarsini K: Comparative study of copper(II)-curcumin complexes
as superoxide dismutase mimics and free radical scavengers. Eur J
Med Chem. 42:431–439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barik A, Mishra B, Shen L, Mohan H, Kadam
RM, Dutta S, Zhang HY and Priyadarsini KI: Evaluation of a new
copper(II)-curcumin complex as superoxide dismutase mimic and its
free radical reactions. Free Radic Biol Med. 39:811–822. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eybl V, Kotyzová D, Lesetický L, Bludovská
M and Koutenský J: The influence of curcumin and manganese complex
of curcumin on cadmium-induced oxidative damage and trace elements
status in tissues of mice. J Appl Toxicol. 26:207–212. 2006.
View Article : Google Scholar
|
|
46
|
Fouladvand M, Barazesh A and Tahmasebi R:
Evaluation of in vitro antileishmanial activity of curcumin and its
derivatives 'gallium curcumin, indium curcumin and diacethyle
curcumin'. Eur Rev Med Pharmacol Sci. 17:3306–3308. 2013.
|
|
47
|
Gaurav C, Goutam R, Rohan KN, Sweta KT,
Abhay CS and Amit GK: (Copper-curcumin) beta-cyclodextrin vaginal
gel: delivering a novel metal-herbal approach for the development
of topical contraception prophylaxis. Eur J Pharm Sci. 65:183–191.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
John VD, Kuttan G and Krishnankutty K:
Anti-tumour studies of metal chelates of synthetic curcuminoids. J
Exp Clin Cancer Res. 21:219–224. 2002.PubMed/NCBI
|
|
50
|
Zebib B, Mouloungui Z and Noirot V:
Stabilization of curcumin by complexation with divalent cations in
glycerol/water system. Bioinorg Chem Appl. 2010:2927602010.
View Article : Google Scholar :
|
|
51
|
Mei X, Xu D and Xu S, Zheng Y and Xu S:
Gastroprotective and antidepressant effects of a new
zinc(II)-curcumin complex in rodent models of gastric ulcer and
depression induced by stresses. Pharmacol Biochem Behav. 99:66–74.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yu C, Mei XT, Zheng YP and Xu DH:
Zn(II)-curcumin protects against hemorheological alterations,
oxidative stress and liver injury in a rat model of acute
alcoholism. Environ Toxicol Pharmacol. 37:729–737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Refat MS: Synthesis and characterization
of ligational behavior of curcumin drug towards some transition
metal ions: Chelation effect on their thermal stability and
biological activity. Spectrochim Acta A Mol Biomol Spectrosc.
105:326–337. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barik A, Priyadarsini KI and Mohan H:
Photophysical studies on binding of curcumin to bovine serum
albumins. Photochem Photobiol. 77:597–603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wootton LL and Michelangeli F: The effects
of the phenylalanine 256 to valine mutation on the sensitivity of
sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA)
Ca2+ pump isoforms 1, 2, and 3 to thapsigargin and other
inhibitors. J Biol Chem. 281:6970–6976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Logan-Smith MJ, Lockyer PJ, East JM and
Lee AG: Curcumin, a molecule that inhibits the
Ca2+-ATPase of sarcoplasmic reticulum but increases the
rate of accumulation of Ca2+. J Biol Chem.
276:46905–46911. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hasmeda M and Polya GM: Inhibition of
cyclic AMP-dependent protein kinase by curcumin. Phytochemistry.
42:599–605. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jung KT and Lim KJ: Curcumin, COX-2, and
Protein p300/CBP. Korean J Pain. 27:365–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bukhari SN, Lauro G, Jantan I, Bifulco G
and Amjad MW: Pharmacological evaluation and docking studies of
α,β-unsaturated carbonyl based synthetic compounds as inhibitors of
secretory phospholipase A2, cyclooxygenases,
lipoxygenase and proinflammatory cytokines. Bioorg Med Chem.
22:4151–4161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Skrzypczak-Jankun E, McCabe NP, Selman SH
and Jankun J: Curcumin inhibits lipoxygenase by binding to its
central cavity: Theoretical and X-ray evidence. Int J Mol Med.
6:521–526. 2000.PubMed/NCBI
|
|
61
|
Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang
S, Lee MJ and Yang CS: Modulation of arachidonic acid metabolism by
curcumin and related beta-diketone derivatives: Effects on
cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxy-genase.
Carcinogenesis. 25:1671–1679. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Heng MC: Signaling pathways targeted by
curcumin in acute and chronic injury: Burns and photo-damaged skin.
Int J Dermatol. 52:531–543. 2013. View Article : Google Scholar
|
|
63
|
Reddy S and Aggarwal BB: Curcumin is a
non-competitive and selective inhibitor of phosphorylase kinase.
FEBS Lett. 341:19–22. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Manikandan P, Sumitra M, Aishwarya S,
Manohar BM, Lokanadam B and Puvanakrishnan R: Curcumin modulates
free radical quenching in myocardial ischaemia in rats. Int J
Biochem Cell Biol. 36:1967–1980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lin JK and Shih CA: Inhibitory effect of
curcumin on xanthine dehydrogenase/oxidase induced by
phorbol-12-myristate-13-acetate in NIH3T3 cells. Carcinogenesis.
15:1717–1721. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Aggarwal BB, Surh J and Shishodia S: The
Molecular Targets and Therapeutic Uses of Curcumin in Health and
Disease. Springer; New York, NY: 2007, View Article : Google Scholar
|
|
67
|
Asai A and Miyazawa T: Occurrence of
orally administered curcuminoid as glucuronide and
glucuronide/sulfate conjugates in rat plasma. Life Sci.
67:2785–2793. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Holder GM, Plummer JL and Ryan AJ: The
metabolism and excretion of curcumin
(1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in
the rat. Xenobiotica; the fate of foreign compounds in biological
systems. 8:761–768. 1978. View Article : Google Scholar
|
|
69
|
Wahlström B and Blennow G: A study on the
fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh).
43:86–92. 1978. View Article : Google Scholar
|
|
70
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Garcea G, Jones DJ, Singh R, Dennison AR,
Farmer PB, Sharma RA, Steward WP, Gescher AJ and Berry DP:
Detection of curcumin and its metabolites in hepatic tissue and
portal blood of patients following oral administration. Br J
Cancer. 90:1011–1015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kim GY, Kim KH, Lee SH, Yoon MS, Lee HJ,
Moon DO, Lee CM, Ahn SC, Park YC and Park YM: Curcumin inhibits
immunostimulatory function of dendritic cells: MAPKs and
translocation of NF-kappa B as potential targets. J Immunol.
174:8116–8124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mun SH, Kim HS, Kim JW, Ko NY, Kim K, Lee
BY, Kim B, Won HS, Shin HS, Han JW, et al: Oral administration of
curcumin suppresses production of matrix metalloproteinase (MMP)-1
and MMP-3 to ameliorate collagen-induced arthritis: Inhibition of
the PKCdelta/JNK/c-Jun pathway. J Pharmacol Sci. 111:13–21. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kirkwood KL, Cirelli JA, Rogers JE and
Giannobile WV: Novel host response therapeutic approaches to treat
periodontal diseases. Periodontol 2000. 43:294–315. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mahakunakorn P, Tohda M, Murakami Y,
Matsumoto K, Watanabe H and Vajaragupta O: Cytoprotective and
cytotoxic effects of curcumin: Dual action on H2O2-induced
oxidative cell damage in NG108-15 cells. Biol Pharm Bull.
26:725–728. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Guimarães MR, Coimbra LS, de Aquino SG,
Spolidorio LC, Kirkwood KL and Rossa C Jr: Potent anti-inflammatory
effects of systemically administered curcumin modulate periodontal
disease in vivo. J Periodontal Res. 46:269–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jagetia GC and Rajanikant GK: Curcumin
treatment enhances the repair and regeneration of wounds in mice
exposed to hemibody gamma-irradiation. Plast Reconstr Surg.
115:515–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Madhyastha R, Madhyastha H, Nakajima Y,
Omura S and Maruyama M: Curcumin facilitates fibrinolysis and
cellular migration during wound healing by modulating urokinase
plasminogen activator expression. Pathophysiol Haemost Thromb.
37:59–66. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Oh S, Kyung TW and Choi HS: Curcumin
inhibits osteoclastogenesis by decreasing receptor activator of
nuclear factor-kappaB ligand (RANKL) in bone marrow stromal cells.
Mol Cells. 26:486–489. 2008.PubMed/NCBI
|
|
80
|
Singh R, Chandra R, Bose M and Luthra MP:
Antibacterial activity of curcumin longa rhizome extract on
periopathogenic bacteria. Curr Sci. 83:737–740. 2002.
|
|
81
|
Bhatia M, Urolagin SS, Pentyala KB,
Urolagin SB, Menaka KB and Bhoi S: Novel therapeutic approach for
the treatment of periodontitis by curcumin. J Clin Diagn Res.
8:ZC65–ZC69. 2014.
|
|
82
|
Gottumukkala SN, Koneru S, Mannem S and
Mandalapu N: Effectiveness of sub gingival irrigation of an
indigenous 1% curcumin solution on clinical and microbiological
parameters in chronic periodontitis patients: A pilot randomized
clinical trial. Contemp Clin Dent. 4:186–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Khafif A, Schantz SP, Chou TC, Edelstein D
and Sacks PG: Quantitation of chemopreventive synergism between
(−)-epigal-locatechin-3-gallate and curcumin in normal,
premalignant and malignant human oral epithelial cells.
Carcinogenesis. 19:419–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pari L and Murugan P: Tetrahydrocurcumin:
Effect on chloroquine-mediated oxidative damage in rat kidney.
Basic Clin Pharmacol Toxicol. 99:329–334. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yodkeeree S, Garbisa S and Limtrakul P:
Tetrahydrocurcumin inhibits HT1080 cell migration and invasion via
downregulation of MMPs and uPA. Acta Pharmacol Sin. 29:853–860.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
San Miguel SM, Opperman LA, Allen EP,
Zielinski J and Svoboda KK: Bioactive antioxidant mixtures promote
proliferation and migration on human oral fibroblasts. Arch Oral
Biol. 56:812–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Gu Y, Lee HM, Napolitano N, Clemens M,
Zhang Y, Sorsa T, Zhang Y, Johnson F and Golub LM:
4-methoxycarbonyl curcumin: A unique inhibitor of both inflammatory
mediators and periodontal inflammation. Mediators Inflamm.
2013:3297402013. View Article : Google Scholar
|
|
88
|
Elburki MS, Rossa C, Guimaraes MR,
Goodenough M, Lee HM, Curylofo FA, Zhang Y, Johnson F and Golub LM:
A novel chemically modified curcumin reduces severity of
experimental periodontal disease in rats: Initial observations.
Mediators Inflamm. 2014:9594712014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shoba G, Joy D, Joseph T, Majeed M,
Rajendran R and Srinivas PS: Influence of piperine on the
pharmacokinetics of curcumin in animals and human volunteers.
Planta Med. 64:353–356. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mazzarino L, Loch-Neckel G, Bubniak LS,
Mazzucco S, Santos-Silva MC, Borsali R and Lemos-Senna E:
Curcumin-loaded chitosan-coated nanoparticles as a new approach for
the local treatment of oral cavity cancer. J Nanosci Nanotechnol.
15:781–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gordon ON, Luis PB, Sintim HO and
Schneider C: Unraveling curcumin degradation: Autoxidation proceeds
through spiroepoxide and vinylether intermediates en route to the
main bicyclopentadione. J Biol Chem. 290:4817–4828. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kaźmierkiewicz R, Czaplewski C and
Ciarkowski J: Elucidation of neurophysin/bioligand interactions
from molecular modeling. Acta Biochim Pol. 44:453–466. 1997.
|
|
93
|
Zhang YL, Tropsha A, McPhail AT and Lee
KH: Antitumor agents. 152 In vitro inhibitory activity of etoposide
derivative NPF against human tumor cell lines and a study of its
conformation by X-ray crystallography, molecular modeling, and NMR
spectroscopy. J Med Chem. 37:1460–1464. 1994. View Article : Google Scholar : PubMed/NCBI
|