1. Introduction
O'Dowd et al firstly discovered a type of
receptor protein APJ related to angiotensin type 1 receptor (AT1)
in 1993. This was a new G protein-coupled receptor (GPCR) family
termed 'orphan receptors', as the relevant endogenous ligand was
not found at that time (1). In
1998, Tatemoto et al (2)
extracted the endogenous ligand of APJ from the secretions of
cattle stomach tissues, and named apelin (APJ endogenous ligand).
Recent research has found that ELABELA may be the new endogenous
agonist of APJ, and that it plays an important role in cardiac
development (3). The amino acid
sequence of apelin is similar to that of angiotensin II (Ang II),
while APJ and AT1 in the hydrophobic transmembrane region have a
homology of 40–50%, which suggests that apelin/APJ may be
self-contained and affects the relevant biological function
independently (4,5).
Apelin/APJ has been shown to be expressed in various
tissues and cells in the human body, particularly in vascular
endothelial cells (ECs) and adipose tissue (6,7).
Studies have found that apelin mRNA is expressed in many tissues in
rats, such as the heart, kidneys, adipose tissue and the central
nervous system [Pope et al (8) and Medhurst et al (9)], which suggests that apelin/APJ can
affect the pathophysiological function of the cardiovascular
system. The apelin/APJ system has a variety of biological effects.
In the cardiovascular system, apelin/APJ can strengthen cardiac
contractility, relax blood vessels and lower blood pressure
(10,11). In addition, apelin/APJ can
regulate gastrointestinal function and insulin sensitivity, and can
promote cell proliferation, migration and angiogenesis, thus
regulating immune function (12–16).
Oxidative stress is a condition in which the normal
dynamic homeostasis has been overwhelmed by the excessive
accumulation of reactive oxygen species (ROS). Oxidative stress is
considered to be one of the underlying mechanisms of inflammatory
diseases. There are strong links between apelin/APJ and oxidative
stress. Apelin is able to suppress the production and release of
ROS in adipocytes (17), and to
alleviate oxidative stress in cardiomyocytes (18). By contrast, Li et al
(19) found that apelin-13 can
promote the generation of ROS in vascular smooth muscle cells
(VSMCs). Consequently, this review summarizes the role of apelin in
regulating oxidative stress-related inflammatory diseases.
Moreover, drugs targeting apelin/APJ are recommended, thus
providing a novel therapeutic target for oxidative stress-related
inflammatory diseases.
2. Apelin induces atherosclerosis by
promoting oxidative stress
As discussed below, studies have shown that the
development of atherosclerosis (AS) is closely related to VSMC
proliferation and the injury of vascular ECs. Moreover, the injury
of vascular ECs results in monocytes (MCs) adhering to the ECs, as
well as in oxidative stress and inflammation.
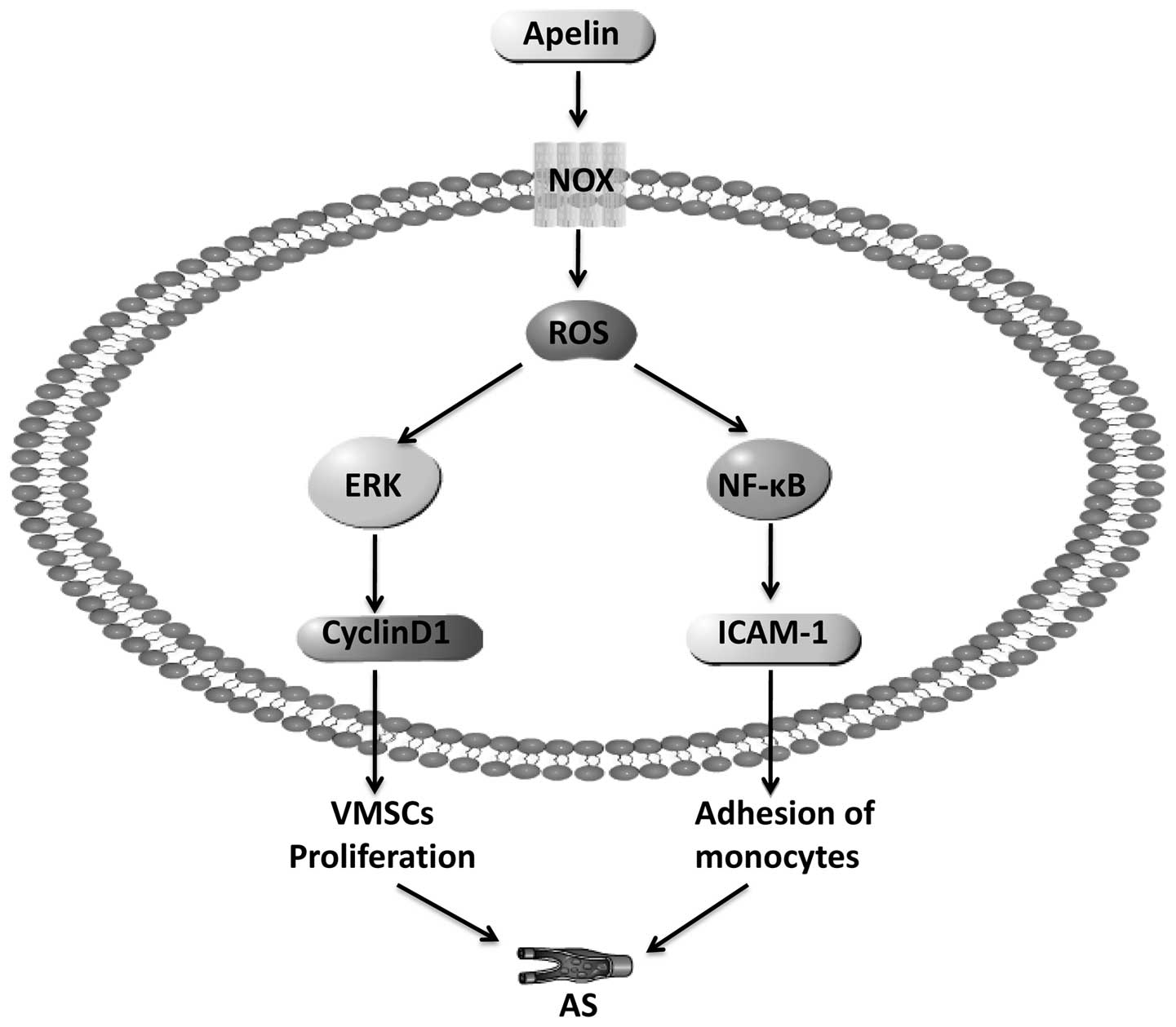
Apelin is highly expressed in ECs and VSMCs
(20–22). Our laboratory first discovered and
reported that the phosphorylation of phosphatidylinositol 3-kinase
(PI3K) was stimulated by apelin-13 after binding to APJ, which
thereby activated Akt and its downstream signaling molecule,
extracellular regulated protein kinase 1/2 (ERK1/2), then promoted
the synthesis of cyclin D1, and ultimately induced the
proliferation of VMSCs (23,24). Studies have found that apelin may
induce the expression of nuclear factor-κB (NF-κB)/JNK which may
then induce intercellular adhesion molecule-1 (ICAM-1), vascular
cell adhesion molecule-1 (VCAM-1) and monocyte chemoattractant
protein-1 (MCP-1) expression, leading to MCs adhering to human
umbilical vein endothelial cells (HUVECs) (25,26).
Oxidative stress and inflammation play a key role in
the pathogenesis of AS. Lassègue and Clempus (27) found that NADPH oxidase (NOX) is
the main enzyme which generates ROS in blood vessels; ROS produced
by the enzyme are involved in the process of occurrence and
development of AS. ROS can inhibit the activity of endothelial
nitric oxide synthase (eNOS) in cells in order to blunt the
production of nitric oxide (NO), while promoting the proliferation
of VSMCs and thrombus formation, ultimately leading to or
aggravating endothelial dysfunction (28,29). The increase in ROS production can
promote the expression of a variety of inflammatory factors and
adhesion molecules in ECs, such as MCP-1 and ICAM-1, VCAM-1, tumor
necrosis factor-α (TNF-α) and interleukin (IL)-1. However,
increased inflammatory cytokine production can also promote the
increased production of ROS (30). Therefore, oxidative stress
promotes the development of AS by regulating the production of
ROS.
Li et al (19) found that apelin-13 promotes the
increased expression of NOX4, and promotes the generation of ROS.
When NOX4 was inhibited, ROS generation and VSMC proliferation
induced by apelin-13 were also inhibited. The phosphorylation of
ERK was decreased, indicating that NOX4, as the upstream signaling
molecule of ERK, is involved in the promoting effects of apelin-13
on the proliferation of VSMCs. In another study, Li et al
(31) also found that apelin-13
promoted the proliferation of VMSCs by activating the
ERK-Jagged-1/Notch3-cyclin D1 pathway. Li et al found that
apelin-13 promoted the expression of NOX4 and the increased
generation of ROS in VSMCs (19),
and Lu et al found that apelin-APJ induced ICAM-1, VCAM-1
and MCP-1 expression through the NF-κB/JNK signaling pathway
(26). Therefore, we speculated
that apelin and oxidative stress may be involved in the development
of AS through the NOX4-ROS-NF-κB/ERK signaling pathway (Fig. 1).
Both apelin and oxidative stress can regulate the
development of AS; we thus hypothesized that there is an
association between apelin and oxidative stress. In 2007, Hashimoto
et al reported that AS plaques and NOX were markedly
decreased in the vascular tissue of APJ receptor and apolipoprotein
E (apoE) double knockout mice; thus, the authors suggested that the
APJ receptor is involved in the development of AS through oxidative
stress (32). In 2009, Leeper
et al (33) demonstrated
that the apelin/APJ system is a regulatory factor of vascular
oxidative stress. In a rat model of AS induced by a
high-cholesterol diet (32),
apelin/APJ was shown to regulate oxidative stress in vascular
tissue, suggesting that apelin is a key factor in the process of AS
caused by a high cholesterol diet; APJ deficiency can protect blood
vessels from generating oxidative stress-related AS, and can also
prevent oxidative stress-induced AS (34). In conclusion, apelin and oxidative
stress can promote the development of AS, but a deficiency in APJ
can prevent the development of AS due to oxidative stress.
3. Apelin induces hypertension by regulating
oxidative stress
Apelin can regulate angiogenesis and adjust blood
pressure through different mechanisms. A previous study
demonstrated the anti-hypertensive effects of apelin; the
administration of apelin-13 to male Wistar rats via intravenous
injection reduced systolic and diastolic blood pressure temporarily
(35). The main mechanism
responsible for the reducing effect of apelin on blood pressure is
through eNOS phosphorylation, promoting the release of NO, and
thereby promoting endothelial-dependent vasodilation. As previously
demonstrated, following an intra-peritoneal injection of apelin-12,
apelin-13 and apelin-36 to anesthetized rats, the mean arterial
pressure decreased, and the results revealed that the
anti-hypertensive effects of apelin-12 were the most prominent.
Following the exogenous apelin-12 injection into mice, the nitrite
levels in plasma transiently increased. However, in rats treated
with the eNOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME),
prior to the injection of apelin-12, no significant change in blood
pressure was observed; thus the anti-hypertensive effects of apelin
rapidly diminished (10,36). Japp et al (37) found that the acute administration
of apelin to humans leads to human peripheral vascular and coronary
vasodilation and increases cardiac output. In APJ-deficient mice
injected with apelin, there was no significant change in blood
pressure (10), suggesting that
apelin needs to develop the function of vasodilation and lower
blood pressure through its APJ receptors. Katugampola et al
(20) found that in the saphenous
vein in vitro, apelin combined with the APJ receptor in
vascular smooth muscle, and stimulated myosin light chain to induce
vascular contraction, indicating that apelin may directly constrict
vascular smooth muscle; however, only in the intact endothelium
there was a vasodilatory effect when combining apelin with
endothelial APJ. The apelin/APJ system is involved in the
regulation of blood pressure through a variety of mechanisms, and
has a certain connection with the condition that depends on whether
the endothelium is intact or not.
The occurrence of eNOS uncoupling is caused by
oxidative stress (38).
L-arginine (L-Arg) as the substrate generated by eNOS in
vivo and tetrahydrobiopterin (BH4) as the co-factor in short
supply, may lead to eNOS dysfunction and the production of
O2−, but not NO, so as to promote the
development of hypertension and the occurrence of vascular
complications. ROS are superoxide anions, and can result in the
inactivation of NO, which is an endothelium-derived relaxing
factor, further leading to detrimental effects on vasodilation,
leading to endothelial dysfunction, and finally giving rise to
hypertension (39). Thus,
oxidative stress is involved in the occurrence of hypertension and
has a close association with endothelial dysfunction.
The incidence of hypertension is closely associated
with a significant increase in ROS generation in the human body.
The elevated levels of ROS may be associated with the occurrence of
oxidative stress, directly resulting in vascular injury and a
series of inflammatory reactions, finally causing hypertension.
Thus, the aforementioned apelin-13 can promote the increased
expression of NOX4, and can also promote the generation of ROS. The
NADH/NADPH oxidative system of the vascular wall can be regulated
by Ang II, which can markedly increase the generation of
O2− and H2O2, causing
vascular injury, increased tension and increased resistance,
resulting in vascular remodeling, and ultimately in elevated blood
pressure. Moreover, in the pathogenesis of hypertension, the renin
angiotensin system (RAS) plays an important role. Ang II is known
as one of the strongest vasoconstrictor substances, when acting on
vascular smooth muscle, it can cause the arteriole contracts of the
whole body to increase arterial pressure; under physiological and
pathological conditions, apelin/APJ has completely opposite effects
to the function of RAS; thus, under such conditions, apelin has an
antagonistic effect to that of Ang II (40,41). In conclusion, it is suggested that
apelin can increase hypertension by regulating the NOX4-Ang II
pathway. Moreover, apelin can also inhibit the oxidative stress
which is involved in the occurrence of hypertension, possibly
through the eNOS/NO pathway (Fig.
2).
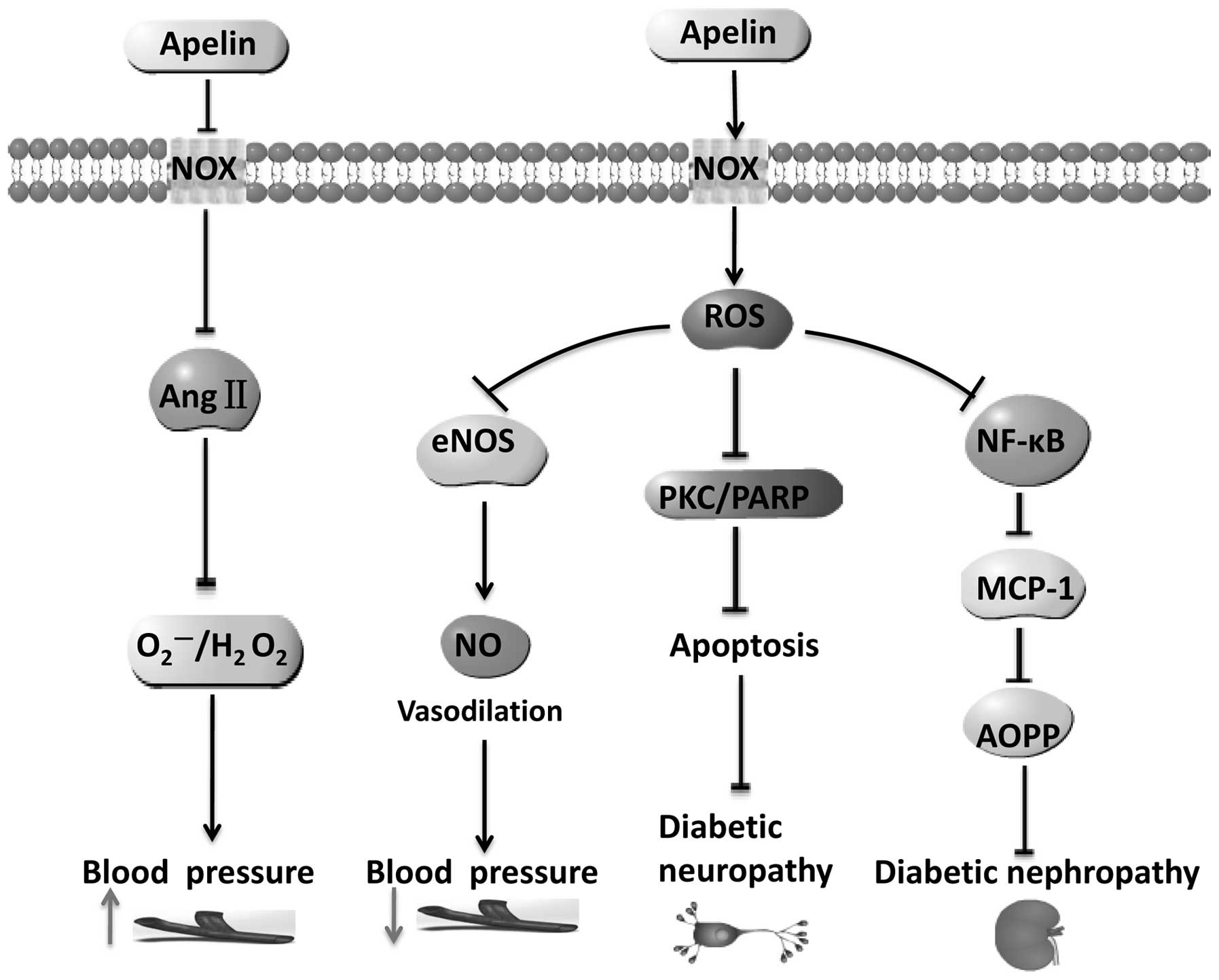 | Figure 2Apelin induces ROS generation,
participating in the occurrence of hypertension and
diabetes-related microvascular complications. ↓, promotion; ⊥,
inhibition; ↑ (gray), up; ↓ (gray), down. ROS, reactive oxygen
species; eNOS, endothelial nitric oxide synthase; PKC, protein
kinase C; PARP, poly ADP-ribose polymerase; MCP-1, monocyte
chemoattractant protein-1; AOPP, advanced oxidation protein
products. |
4. Apelin, oxidative stress and diabetes
with associated microvascular complications
Some research results have indicated that apelin has
a close association with diabetic microvascular complications, such
as diabetic nephropathy (DN), diabetic retinopathy (DR) and
pathological changes of the nervous system. Oxidative stress is
also thought to be one of the underlying mechanisms of diabetic
microvascular complications (42).
Glomerular capillary damage directly leads to the
occurrence of DN; the increase in free radicals and oxidative
stress is one of the causes of DN (43). Furthermore, the inflammatory
pathway plays an important role in the pathogenesis of DN.
8-Hydroxy-2-deoxyguanosine (8-OHdG) is the specific and main
product of DNA oxidative damage, and advanced oxidation protein
products (AOPP) reflects the protein damage caused by oxidative
stress. The levels of AOPP in plasma and 8-OHdG in the urine of
patients with DN with large amounts of albumin in urine were
significantly higher than those of the patinets with normal albumin
urine levels (44). The data
suggest that oxidative stress promotes the development of DN.
Hyperglycemia activates NF-κB through ROS to promote the expression
of MCP-1; the expression of MCP-1 in renal tissue is a marker of
the inflammatory occurrence (45,46).
Studies have shown that the subcutaneous injection
of apelin can be effective in preventing the occurrence of renal
enlargement, reducing the expression of MCP-1 and VCAM-1, and
inhibiting the activation of NF-κB (47). Apelin can also restore the levels
of antioxidant enzymes in the kidneys of diabetic mice, reduce
oxidative stress, and prevent damage to kidneys from oxidative
stress (48). Thus, apelin may be
a therapeutic target in DN by exerting anti-oxidantive effects
(Fig. 2).
An important reason for the occurrence of diabetic
neuropathy is the increased formation of advanced glycation
end-products caused by oxidative stress, promoting the apoptosis of
nerve cells mediated through the phosphoinositide/protein kinase C
(PKC) system and DNA repair enzyme poly ADP-ribose polymerase
(PARP), NF-κB and damage sensory fibers (49,50). Studies have demonstrated that
apelin inhibits the activation of NF-κB (47), and thus protects nerve cells. In
cortical neurons, apelin can also inhibit the generation of ROS
(51). Thus, apelin can inhibit
oxidative stress to prevent the occurrence of diabetic
neuropathy.
DR is the leading cause of human blindness. The
close associatoin between vascular endothelial growth factor (VEGF)
and proliferative DR is considered to be the most important
mechanism responsible for DR (52). Although the inhibition of VEGF may
reduce retinal neogenesis, it cannot completely inhibit the
formation of neovascularization and the proliferation of retinal
cells caused by ischemia. Studies have shown that the mRNA levels
of apelin, APJ and VEGF are all increased in the vascular tissue
membrane in proliferative DR (53), and that VEGF is involved. It has
been proven that apelin promotes the expression of VEGF (54). Saint-Geniez et al (55) found that apelin/APJ is involved in
retinal angiogenesis in mice, and has a unique expression pattern.
Thus, the function of apelin in promoting retinal neogenesis is
considered to be one of the causes of DR. Studies have indicated
that oxidative stress has a significant association with the
occurrence of DR. The enhancement of free radical activity and the
decrease in antioxidant capacity are the important mechanisms
responsible for DR. Increased levels of oxygen free radical in
vivo leads to an increase in retinal cell apoptosis, as oxygen
free radicals can activate the caspase family through the
mitochondrial pathway, leading to the increased concentration of
intracellular Ca2+ in retinal cells, and inducing
apoptosis through the activation of NF-κB (56,57). Thus, apelin and oxidative stress
are involved in the occurrence of DR, and the inhibition of the
apelin/APJ system in the eyes may be an effective method to prevent
DR (Fig. 2).
5. Apelin regulates oxidative stress by
inhibiting ischemia-reperfusion injury
During the process of ischemia-reperfusion injury,
superoxide anion (O2−·) synthesis occurs. In
addition to superoxide anion, ROS also play an important role in
ischemia-reperfusion injury. It has been suggested that oxidative
stress may have a definite association with ischemia-reperfusion
injury. The main mechanisms of the regulation of
ischemia-reperfusion injury by oxidative stress are the functional
inactivation of intracellular DNA, RNA proteins and other
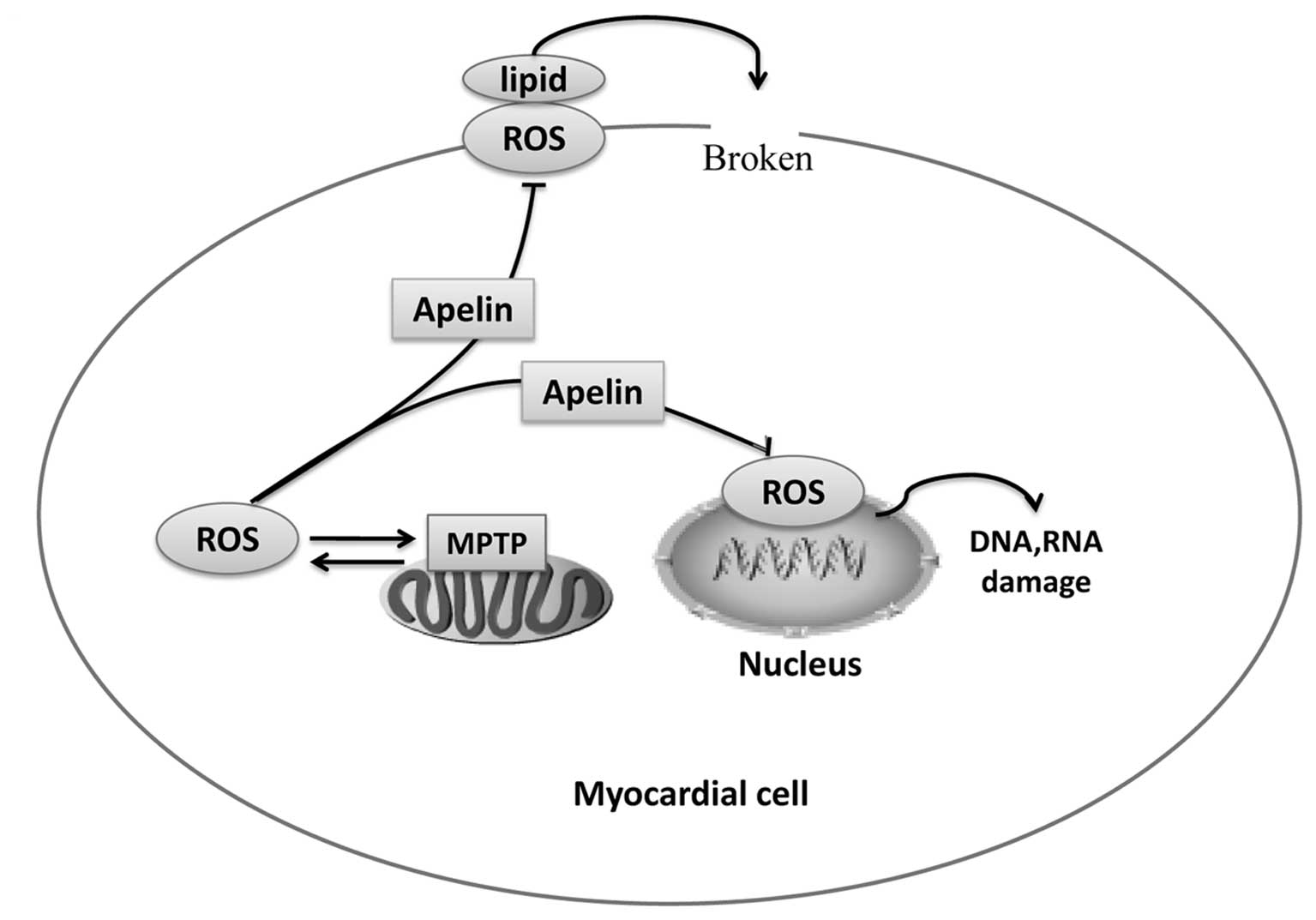
substances so as to lead to cell dysfunction (58–60), mainly through ROS: the overload of
ROS promotes mitochondrial permeability transition pore (MPTP)
opening, which in turn increases the release of mitochondrial ROS,
to form a vicious positive feedback loop between ROS activation and
ROS release. ROS, in combination with lipids can damage cell
membranes and organelle membranes in myocardial cells, reducing
membrane fluidity and permeability; ROS can also attack the genetic
material to cause the cross linking or breaking of DNA and RNA,
affecting gene expression in myocardial cells (Fig. 3).
During the process of ischemia-reperfusion, apelin
can protect myocardial cells against oxidative stress, and reduce
the damage to myocardial cells (Fig.
3). Zeng et al (61)
found that ROS levels in myocardial cells increased following
exposure to hypoxia, and the addition of apelin to the medium 30
min prior to exposure to hypoxia effectively reduced the levels of
ROS. It has been shown that apelin can activate eNOS and enhance NO
release through reperfusion injury salvage kinase (RISK), and may
thus inhibit mitochondrial oxidative damage and lipid peroxidation
(62). All the above-mentioned
results strongly suggest that apelin plays an antagonistic role in
the process of ischemia-reperfusion, thus exerting a myocardial
protective effect.
6. Apelin regulates oxidative stress,
promoting tumor formation and growth
With the continuous progress in relevant research,
the function of apelin and oxidative stress in tumors has attracted
increasing attention. In 2007, Sorli et al (63) firstly reported that hypoxia caused
by the hypermorphosis of tumor cells can promote the expression
level of apelin. The hypoxia-induced elevation in apelin expression
is most likely mediated by hypoxia inducible factors (HIFs)
(64). Under a hypoxic
environment, increased levels of ROS induce the expression of HIF
in cancer stem cells (CSCs) (65). ROS are also closely related to
tumorigenesis, and thus it is hypothesized that oxidative stress
induced by ROS is closely associated with tumorigenesis and may be
regulated by apelin. Studies have indicated the involvement of
oxidative stress in the formation and development in bladder
cancer, and there are observed changes in the activity of
transcription factors, such as hypoxia-inducible factor HIF-1α
(66). Raina et al
(67) evaluated the efficacy of
grape seed extract (GSE) in bladder cancer and found that
GSE-generated oxidative stress induced marked programmed cell death
in human bladder cancer cells.
Although ROS are involved in tumorigenesis, they
also play a role in the treatment of tumors (68), and the mechanisms involved include
DNA damage induced by ROS. More importantly, lipid peroxidation
induced by ROS and damage to DNA or proteins can cause or promote
the development of tumors. Naturally, oxidative stress can
stimulate tumorigenesis and growth, but can also inhibit tumor
progression. Apelin can inhibit the generation of ROS, which
suggests that apelin suppresses tumorigenesis by regulating
oxidative stress. However, studies have found that cancer stem
cells, as a type of cell with the characteristics of stem cells in
tumor tissues, can resist the inhibitory effects of oxidative
stress caused by radiotherapy or chemotherapy (69–71). There is evidence that compared
with ordinary tumor cells, there are fewer oxidation products of
DNA, stronger resistance to oxidative stress, and higher activation
level of the antioxidant stress system, existing in cancer stem
cells (71).
The above-mentioned studies all demonstrate that
apelin and oxidative stress can cause or accelerate tumorigenesis
and growth; apelin and ROS are possible targets for the diagnosis
and treatment of tumors, and for the prediction of prognosis.
However, the specific molecular mechanisms of action of apelin and
oxidative stress require further investigation.
7. Apelin protects the central nervous
system by stimulating ROS production
A recent study demonstrated that apelin-36 reduced
the cerebral infarct volume and promoted long-term functional
recovery following hypoxic/ischemic (H/I) brain injury. The
mechanisms responsible for the protective effects of apelin-36
against H/I brain injury involved a decrease in the levels of
cleaved caspase-3 and Bax. Apelin-36 also increased the expression
level of phosphorylated Akt; however, with the use of a specific
PI3K inhibitor, the levels of phosphorylated Akt decreased, and the
protective effects of apelin-36 against apoptosis were attenuated
(72). This suggests that the
PI3K/Akt pathway is at least partly involved in the anti-apoptotic
effects of apelin-36. Thus, apelin-36 may be a promising
therapeutic agent in the treatment of ischemic brain injury. As
apelin can promote the phosphorylation of PI3K/Akt and simulate the
expression of NOX-ROS, PI3K/Akt may thus also be associated with
ROS. Silva et al (73)
demonstrated that ROS mediate the phosphatase and tensin homolog
(PTEN)-independent activation of the PI3K/Akt pathway in some T
cell acute lymphoblastic leukemia (T-ALL) cells. Min et al
suggested that ROS may activate PI3K/Akt and Nrf2 signaling
(74). Thus, it can be
hypothesized that apelin induces ROS production, activates
PI3K/Akt, and ultimately, exerts anti-apoptotic and neroprotective
effects.
Apelin-13 can also protect against serum
deprivation-induced apoptosis, as apelin-13 not only blocks typical
apoptosis, but also exerts inhibitory effects on the NMDA-induced
increase in intracellular Ca2+ levels and excitotoxicity
(51). The study of Khaksari
et al (75) demonstrated
that apelin-13 protects the brain against ischemia-reperfusion
injury and cerebral edema. In addition, apelin-13 attenuates
traumatic brain injury (TBI)-induced brain damage by suppressing
autophagy (76). Autophagy may be
involved in the protective effects of apelin-13 against damage to
neurons in TBI. Another study found that apelin expectedly had a
neuroprotective effect combined with VEGF, but not alone (77). It is clear that apelin, which
activates the PI3K/Akt signaling pathway, enhances angiogenesis
induced by VEGF (78). All the
above-mentioned data indicate that apelin can protect the brain
against ischemia-reperfusion injury, and the mechanisms involved
may be associated with the activation of the PI3K/Akt pathway and
ROS.
8. Apelin inhibits hypertension in
pre-eclampsia by regulating oxidative stress
Pre-eclampsia is a hypertensive disorder and a
complication of some pregnancies. Studies have shown that apelin is
also closely associated with normal pregnancies and pre-eclampsia.
Compared with normal pregnancies, the RNA and protein level of
apelin are significantly decreased in pre-eclamptic placentas
(79,80). The above findings suggest that the
reduced expression of apelin may be associated with preeclampsia
and the apelinergic system plays an important role in
pre-eclampsia-induced hypertensive maternal disorders. A study on
pregnancy-induced hypertension (PIH) indicated that apelin/APJ was
poorly stained in the ECs of early-onset PIH placentas, thus
reflecting poor fetal growth (81).
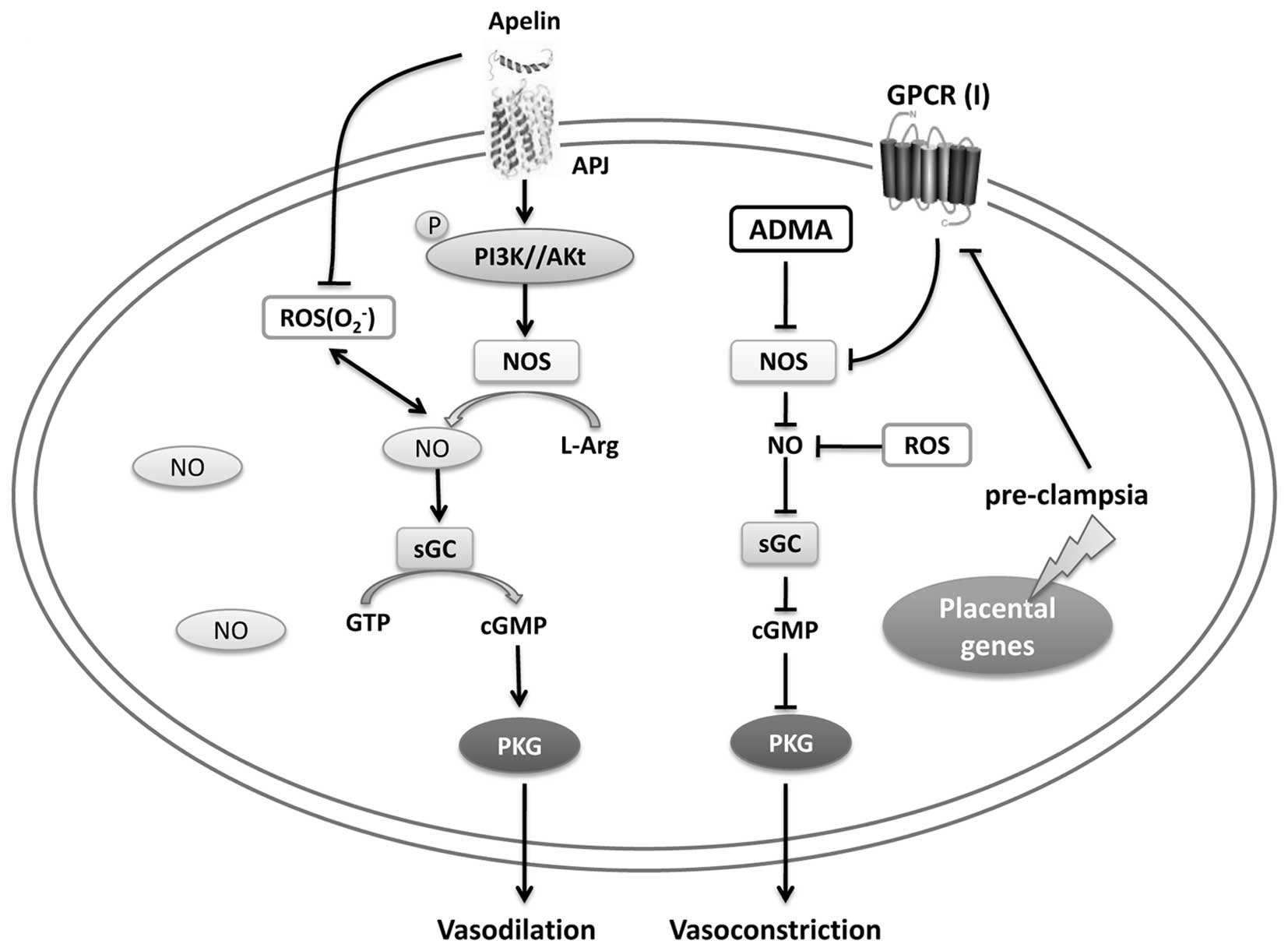
It has been shown that NO signaling is important for
placental function, and in particular for the maintenance of
vascular tone. In the normal placenta, the release of NO depends on
the availability of L-Arg, and when L-Arg bioavailability is
reduced, the synthesis of NO is inhibited, leading to rapid NO
degradation by ROS, for example O2−.
Downstream NO in smooth muscle cells during pregnancy, then
activates the NO sensitive soluble form, soluble guanosine cyclase
(sGC), which is responsible for cGMP generation, then activates
cGMP-sensitive protein kinase G (PKG), and subsequently promotes
vasodilation (82). Consistent
with laboratory research results, apelin promotes the
phosphorylation of PI3K/Akt, and then activates eNOS, inducing the
generation of NO. However, in pre-eclampsia, the expression of a
group of GPCRs (group I) is reduced, and the L-Arg availability for
NOS may be further minimized, which leads to the aberrant and rapid
NO degradation by ROS, subsequently, inducing vasoconstriction
through the NO-cGMP-PKG pathway. Moreover, apelin inhibits ROS
formation. Above all, apelin regulates oxidative stress, inhibiting
hypertension through NO-cGMP pathway in pre-eclampsia (Fig. 4).
9. Apelin regulates oxidative stress in the
occurrence of other inflammatory diseases
In intestinal inflammation, a variety of
inflammatory cytokines can upregulate apelin expression by
activating the Jak/STAT3 pathway; apelin upregulation is able to
promote the proliferation of epithelial cells (64,83). The incidence of inflammatory bowel
disease is closely related to the oxidative stress generated by
intestinal epithelia. The incidence of oxidative stress can cause
intestinal epithelial cell damage; ROS can induce the activation of
a variety of signaling pathways to promote intestinal epithelial
cell apoptosis, including the PI3K pathway, which plays an
important role in this process (84,85). The Jak/STAT3 and PI3K/Akt pathways
are closely associated with the IL-6 receptor family; thus, it can
be hypothesized that apelin/oxidative stress promotes the
occurrence and development of intestinal inflammation, and may be
related to the IL-6 receptor family.
Septicopyemia is a syndrome of systemic inflammatory
response caused by infection. Research has shown that apelin
expression is closely related to septicopyemia. During the
occurrence of septicopyemia, the levels of apelin expression in
serum are not only significantly higher compared with the levels
prior to the occurence of septicopyemia; however, the increase in
apelin expression can antagonize myocardial cell injury from
septicopyemia (86–88). Oxidative stress and inflammation
are closely related to septicopyemia (89). During the course of septicopyemia,
the electron transfer of mitochondrial respiratory chain can
produce ROS which then leads to oxidative stress (90). The electron transfer causes
myocardial damage directly; oxidative stress can also result in
cell death (91). However, in
this process, lipid peroxidation is significantly increased.
Apelin, as a new-type peptide, has a protective function, and can
attenuate damage to heart tissue and reduce lipid peroxidation
(92). Therefore, apelin can be
used for the diagnosis and treatment of septicopyemia as a
promising therapeutic agent (93), and can prevent myocardial injury
caused by oxidative stress in the process of septicopyemia.
10. Drugs targeting apelin/APJ
The above-mentioned research results demonstrate
that the apelin/APJ system plays an important role in the process
of occurrence and development of various diseases (AS,
hypertension, diabetes, etc.). Therefore, the apelin/APJ system has
become one of the potential drug targets (94), and drugs targeting aplelin/APJ may
apply to the treatment of several diseases.
Recent studies have reported small molecule agonists
and antagonists targeting APJ. E339-3D6 was the firstly reported
non-peptide agonist of APJ receptor which can inhibit the release
of antidiuretic hormone of water-dependent induction in rats and
reduce arterial blood pressure (95). ML233, as another small molecule
compound ligand of APJ, can selectively inhibit AT1 receptors,
which can promote vasoconstriction through phospholipase C
(96). Both E339-3D6 and ML233
can inhibit the release of forskolin-activated rennin induced by
the cAMP pathway, which can play an important role in the
development of hypertension (97).
In addition, studies on APJ receptor antagonists
have also made rapid progress.
4-Oxo-6-((pyrimidin-2-ylthio)methyl)-4H-pyran-3-yl4-nitrobenzoate
(ML221) is the first APJ receptor antagonists to be found,
primarily as tool drug used to mark APJ receptor (98); later, ALX40-4C as another APJ
receptor antagonists has also been found. ALX40-4C is the APJ and
CXCR4 receptor antagonists containing nine arginine residues; the
drug by combing with APJ receptor can inhibit ligand-induced
intracellular calcium mobilization and receptor internalization, in
order to achieve the effect of lowering blood pressure (99). Above the small molecules, a number
of peptides based on apelin-13 has led to development of more
potent and stable analogs targeted APJ (100). As showed in the review of Cao
et al (94), the analogs
of apelin may directly reduce blood pressure or by activating
Akt-eNOS/NO pathway.
Apelin could regulate the oxidative stress-linked
inflammation diseases, so the drugs targeted apelin may apply to
the treatment the diseases. Studies showed that crude drug puerarin
could downregulated expression of apelin and exerted a protective
effect on renal hypertension (101), suggesting that puerarin may cure
oxidative stress-linked blood pressure. In conclusion, every drugs
targeted APJ and apelin, they play an important role in the
research of pharmacological effect on Apelin/APJ system, and also
become the reliable tools to explore the system on the development
mechanism of oxidative stress-mediated disease.
11. Conclusions and future prospects
Studies have indicated that the apelin/APJ system
plays a certain role in inflammation-related diseases (Table I). Apelin/APJ regulates oxidative
stress in the occurrence of inflammation-related diseasesl;
however, this process is intricate. Thus, further research into
this matter is required.
 | Table IFunction of the apelin/APJ system in
inflammation-related diseases. |
Table I
Function of the apelin/APJ system in
inflammation-related diseases.
| Disease types | Experiment
models | Treatment | Pathway | Function |
Authors/(Refs.) |
|---|
| AS | VSMCs | Exogenous
apelin | ERK1/2,
PI3K/Akt | Exacerbation | Li et al
(23)
Liu et al (24) |
| AS | HUVECs | Exogenous
apelin | NF-κB/JNK | Exacerbation | Lu et al
(26) |
| Hypertension | Wistar rats | Exogenous
apelin | eNOS/NO | Inhibition | Lee et al
(35) |
| DN | Ove26 mice | Exogenous
apelin | NF-κB | Inhibition | Day et al
(47) |
| DR | Diabetic rats | Exogenous
apelin | VEGF | Exacerbation | Lu et al
(54) |
| I/R injury | Rat heart
models | Exogenous
apelin | ROS |
Cardioprotection | Zeng et al
(61) |
| Tumor | TS/A cells | Apelin gene | Gaseous
hypoxia | Exacerbation | Sorli et al
(63) |
| H/I brain
injury | H/I brain injury
models | Exogenous
apelin | PI3K/Akt |
Neuroprotection | Gu et al
(72) |
| Intestinal
inflammation | Rat ileum | Enteric apelin | Jak/STAT3 | Exacerbation | Han et al
(83) |
| Pyemia | Male rats | Exogenous
apelin | Inhibit MCP-1 and
IL-8 |
Cardioprotection | Pan et al
(88) |
At a first glance, the effects of apelin and
oxidative stress are similar. On the one hand, vascular endothelial
shear stress, oxidative stress, inflammation and other factors can
alter apelin expression; on the other hand, apelin has an impact on
insulin sensitivity and cardiovascular function, exerting
anti-inflammatory and antioxidant effects, acting as an adipokine
(102). In addition, the
overproduction of ROS can lead to a series of inflammatory
reactions, and apelin can regulate the production of ROS; thus,
there is a close link between apelin, oxidative stress and
inflammation.
In addition, apelin regulates oxidative
stress-related inflammatory diseases. However, there are still
certain contradictions and doubts. For example, the assumption that
apelin inhibits ROS is opposite to the conclusion made by our
research group; apelin and oxidative stress have an opposite effect
on hypertension; the effects of apelin and oxidative stress on
tumorigenesis are not yet fully elucidated; apelin and oxidative
stress promote the occurrence and development of intestinal
inflammation, and this is related to the IL-6 receptor family.
Thus, these contradictory effects may be caused by the existence of
multiple isoforms of apelin (103), the activation of diverse
signaling pathways and different stimuli/factors. For example,
pyr-apelin-13 can reduce renal enlargement and inflammation, and
improve albumin urine levels in DN (47); however, there are also studies
showing that the angiogenic effects of apelin may hinder or blunt
its beneficial effects on albumin urine levels (104). Thus, the regulatory effects of
apelin regulates on oxidative stress-related diseases warrant
further investigation.
Finally, the apelin/APJ system is abundantly found,
which suggests that apelin is a critical factor in the occurrence
of various diseases. The apelin/APJ system has a number of
biological functions; for instance, apelin has been shown to exert
cardioprotective effects in renal ischemia-reperfusion injury
(105). In cerebral
ischemia-reperfusion, both apelin-13 and apelin-36 can protect
neurons and inhibit damage induced by inflammation by activating
the PI3K/Akt pathway (72,106,107).
Furthermore, small molecular compounds may be developed to target
the apelin/APJ system for the treatment of inflammation-related
diseases.
In summary, in this review, we discussed the related
functions of the apelin/APJ system, and its effects on oxidative
stress and inflammation-related diseases, and provide a new
theoretical foundation for the study of the occurrence and
development of oxidative stress-related inflammatory diseases. The
apelin/APJ system functions as a double-edged sword in regulating
oxidative stress-related inflammatory diseases. With the continuous
progress made by research, an increasing number of apelin/APJ
functions are being discovered. Thus, the apelin/APJ system may
prove to be a novel therapeutic target in inflammation-related
diseases. Further research is warranted however, in order to
further elucidate the different apelin subtypes and their related
functions. Consequently, the relevant mechanisms of action of
apelin/APJ and its role in oxidative stress or its antioxidant
effects in inflammation-related diseases need to be further
explored in future studies.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81270420 and 81470434),
the Hunan Province Cooperative innovation Center for Molecular
Target New Drug Study [Hunan Provincial Education Department
document (approval no. 2014-405)] the Hunan Provincial Natural
Science Foundation of China (no. 14JJ3102), the project funded by
China Postdoctoral Science Foundation (no. 2014M560647).
Abbreviations:
|
ROS
|
reactive oxygen species
|
|
VSMCs
|
vascular smooth muscle cells
|
|
ECs
|
endothelial cells
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
VCAM-1
|
vascular cell adhesion molecule-1
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
NOX
|
NADPH oxidase
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
TNF-α
|
tumor necrosis factor-α
|
|
L-NAME
|
NG-nitro-L-arginine methyl ester
|
|
DN
|
diabetic nephropathy
|
|
DR
|
diabetic retinopathy
|
|
8-OHdG
|
8-hydroxy-2-deoxyguanosine
|
|
AOPP
|
advanced oxidation protein
products
|
|
PKC
|
protein kinase C
|
|
PARP
|
poly ADP-ribose polymerase
|
|
VEGF
|
vascular endothelial growth factor
|
|
MPTP
|
mitochondrial permeability transition
pore
|
|
RISK
|
reperfusion injury salvage kinase
|
|
HIFs
|
hypoxia inducible factors
|
|
PTEN
|
phosphatase and tensin homolog
|
|
TBI
|
traumatic brain injury
|
|
sGC
|
soluble guanosine cyclase
|
References
|
1
|
O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui
LC, Kennedy JL, Shi X, Petronis A, George SR and Nguyen T: A human
gene that shows identity with the gene encoding the angiotensin
receptor is located on chromosome 11. Gene. 136:355–360. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie F, Lv D and Chen L: ELABELA: A novel
hormone in cardiac development acting as a new endogenous ligand
for the APJ receptor. Acta Biochim Biophys Sin (Shanghai).
46:620–622. 2014. View Article : Google Scholar
|
|
4
|
Lee DK, Cheng R, Nguyen T, Fan T,
Kariyawasam AP, Liu Y, Osmond DH, George SR and O'Dowd BF:
Characterization of apelin, the ligand for the APJ receptor. J
Neurochem. 74:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saavedra JM, Correa FM, Seltzer A, Pinto
JE, Viglione P and Tsutsumi K: Enhanced angiotensin converting
enzyme binding in arteries from spontaneously hypertensive rats. J
Hypertens. 10:1353–1359. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choe W, Albright A, Sulcove J, Jaffer S,
Hesselgesser J, Lavi E, Crino P and Kolson DL: Functional
expression of the seven-transmembrane HIV-1 co-receptor APJ in
neural cells. J Neurovirol. 6(Suppl 1): S61–S69. 2000.PubMed/NCBI
|
|
7
|
Habata Y, Fujii R, Hosoya M, Fukusumi S,
Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa
T, et al: Apelin, the natural ligand of the orphan receptor APJ, is
abundantly secreted in the colostrum. Biochim Biophys Acta.
1452:25–35. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pope GR, Roberts EM, Lolait SJ and
O'Carroll AM: Central and peripheral apelin receptor distribution
in the mouse: species differences with rat. Peptides. 33:139–148.
2012. View Article : Google Scholar :
|
|
9
|
Medhurst AD, Jennings CA, Robbins MJ,
Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G,
Bolaky JE, et al: Pharmacological and immunohistochemical
characterization of the APJ receptor and its endogenous ligand
apelin. J Neurochem. 84:1162–1172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishida J, Hashimoto T, Hashimoto Y,
Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R,
Shiota N, et al: Regulatory roles for APJ, a seven-transmembrane
receptor related to angiotensin-type 1 receptor in blood pressure
in vivo. J Biol Chem. 279:26274–26279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szokodi I, Tavi P, Földes G,
Voutilainen-Myllylä S, Ilves M, Tokola H, Pikkarainen S, Piuhola J,
Rysä J, Tóth M and Ruskoaho H: Apelin, the novel endogenous ligand
of the orphan receptor APJ, regulates cardiac contractility. Circ
Res. 91:434–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Anini Y, Wei W, Qi X, OCarroll AM,
Mochizuki T, Wang HQ, Hellmich MR, Englander EW and Greeley GH Jr:
Apelin, a new enteric peptide: localization in the gastrointestinal
tract, ontogeny, and stimulation of gastric cell proliferation and
of cholecystokinin secretion. Endocrinology. 145:1342–1348. 2004.
View Article : Google Scholar
|
|
13
|
Castan-Laurell I, Dray C, Attane C, Duparc
T, Knauf C and Valet P: Apelin, diabetes, and obesity. Endocrine.
40:1–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv D, Li L, Lu Q, Li Y, Xie F, Li H, Cao
J, Liu M, Wu D, He L and Chen LX: PAK1-cofilin phosphorylation
mediates human lung adenocarcinoma cells migration induced by
apelin-13. Clin Exp Pharmacol Physiol. In Press.
|
|
15
|
Tiani C, Garcia-Pras E, Mejias M, de
Gottardi A, Berzigotti A, Bosch J and Fernandez M: Apelin signaling
modulates splanchnic angiogenesis and portosystemic collateral
vessel formation in rats with portal hypertension. J Hepatol.
50:296–305. 2009. View Article : Google Scholar
|
|
16
|
Adam F, Khatib AM, Lopez JJ, Vatier C,
Turpin S, Muscat A, Soulet F, Aries A, Jardin I, Bobe R, et al:
Apelin: an antithrombotic factor that inhibits platelet function.
Blood. 127:908–920. 2016. View Article : Google Scholar
|
|
17
|
Than A, Zhang X, Leow MK, Poh CL, Chong SK
and Chen P: Apelin attenuates oxidative stress in human adipocytes.
J Biol Chem. 289:3763–3774. 2014. View Article : Google Scholar :
|
|
18
|
Foussal C, Lairez O, Calise D, Pathak A,
Guilbeau-Frugier C, Valet P, Parini A and Kunduzova O: Activation
of catalase by apelin prevents oxidative stress-linked cardiac
hypertrophy. FEBS Lett. 584:2363–2370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Li F, Li F, Mao X, Yang L, Huang H,
Guo Y, Chen L and Li J: NOX4-derived reactive oxygen species drive
apelin-13-induced vascular smooth muscle cell proliferation via the
ERK pathway. Int J Pept Res Ther. 17:307–315. 2011. View Article : Google Scholar
|
|
20
|
Katugampola SD, Maguire JJ, Matthewson SR
and Davenport AP: [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand
for localizing the APJ orphan receptor in human and rat tissues
with evidence for a vasoconstrictor role in man. Br J Pharmacol.
132:1255–1260. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Falco M, De Luca L, Onori N, Cavallotti
I, Artigiano F, Esposito V, De Luca B, Laforgia V, Groeger AM and
De Luca A: Apelin expression in normal human tissues. In Vivo.
16:333–336. 2002.PubMed/NCBI
|
|
22
|
Kleinz MJ and Davenport AP:
Immunocytochemical localization of the endogenous vasoactive
peptide apelin to human vascular and endocardial endothelial cells.
Regul Pept. 118:119–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li F, Li L, Qin X, Pan W, Feng F, Chen F,
Zhu B, Liao D, Tanowitz H, Albanese C and Chen L: Apelin-induced
vascular smooth muscle cell proliferation: the regulation of cyclin
D1. Front Biosci. 13:3786–3792. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Su T, Li F, Li L, Qin X, Pan W,
Feng F, Chen F, Liao D and Chen L: PI3K/Akt signaling transduction
pathway is involved in rat vascular smooth muscle cell
proliferation induced by apelin-13. Acta Biochim Biophys Sin
(Shanghai). 42:396–402. 2010. View Article : Google Scholar
|
|
25
|
Mao XH, Tao S, Zhang XHui, Li F, Qin XP,
Liao DF, Li LF and Chen LX: Apelin-13 promotes monocyte adhesion to
human umbilical vein endothelial cell mediated by
phosphatidylinositol 3-kinase signaling pathway. Prog Biochem
Biophys. 38:1162–1170. 2011. View Article : Google Scholar
|
|
26
|
Lu Y, Zhu X, Liang GX, Cui RR, Liu Y, Wu
SS, Liang QH, Liu GY, Jiang Y, Liao XB, et al: Apelin-APJ induces
ICAM-1, VCAM-1 and MCP-1 expression via NF-κB/JNK signal pathway in
human umbilical vein endothelial cells. Amino Acids. 43:2125–2136.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lassègue B and Clempus RE: Vascular
NAD(P)H oxidases: Specific features, expression, and regulation. Am
J Physiol Regul Integr Comp Physiol. 285:R277–R297. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Potdar S and Kavdia M: NO/peroxynitrite
dynamics of high glucose-exposed HUVECs: Chemiluminescent
measurement and computational model. Microvasc Res. 78:191–198.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cohen RA and Tong X: Vascular oxidative
stress: The common link in hypertensive and diabetic vascular
disease. J Cardiovasc Pharmacol. 55:308–316. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang JH, Li YN, Qi JS and Jia XX:
Peroxynitrite-induced protein nitration is responsible for renal
mitochondrial damage in diabetic rat. J Endocrinol Invest.
33:140–146. 2010. View Article : Google Scholar
|
|
31
|
Li L, Li L, Xie F, Zhang Z, Guo Y, Tang G,
Lv D, Lu Q, Chen L and Li J: Jagged-1/Notch3 signaling transduction
pathway is involved in apelin-13-induced vascular smooth muscle
cells proliferation. Acta Biochim Biophys Sin (Shanghai).
45:875–881. 2013. View Article : Google Scholar
|
|
32
|
Hashimoto T, Kihara M, Imai N, Yoshida S,
Shimoyamada H, Yasuzaki H, Ishida J, Toya Y, Kiuchi Y, Hirawa N, et
al: Requirement of apelin-apelin receptor system for oxidative
stress-linked atherosclerosis. Am J Pathol. 171:1705–1712. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leeper NJ, Tedesco MM, Kojima Y, Schultz
GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL and Quertermous T:
Apelin prevents aortic aneurysm formation by inhibiting macrophage
inflammation. Am J Physiol Heart Circ Physiol. 296:H1329–H1335.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lv D, Li H and Chen L: Apelin and APJ, a
novel critical factor and therapeutic target for atherosclerosis.
Acta Biochim Biophys Sin (Shanghai). 45:527–533. 2013. View Article : Google Scholar
|
|
35
|
Lee DK, Saldivia VR, Nguyen T, Cheng R,
George SR and O'Dowd BF: Modification of the terminal residue of
apelin-13 antagonizes its hypotensive action. Endocrinology.
146:231–236. 2005. View Article : Google Scholar
|
|
36
|
Tatemoto K, Takayama K, Zou MX, Kumaki I,
Zhang W, Kumano K and Fujimiya M: The novel peptide apelin lowers
blood pressure via a nitric oxide-dependent mechanism. Regul Pept.
99:87–92. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Japp AG, Cruden NL, Barnes G, van Gemeren
N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan
AD and Newby DE: Acute cardiovascular effects of apelin in humans:
Potential role in patients with chronic heart failure. Circulation.
121:1818–1827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dikalova AE, Góngora MC, Harrison DG,
Lambeth JD, Dikalov S and Griendling KK: Upregulation of Nox1 in
vascular smooth muscle leads to impaired endothelium-dependent
relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol.
299:H673–H679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ghiadoni L, Taddei S and Virdis A:
Hypertension and endothelial dysfunction: Therapeutic approach.
Curr Vasc Pharmacol. 10:42–60. 2012. View Article : Google Scholar
|
|
40
|
Siddiquee K, Hampton J, Khan S, Zadory D,
Gleaves L, Vaughan DE and Smith LH: Apelin protects against
angiotensin II-induced cardiovascular fibrosis and decreases
plasminogen activator inhibitor type-1 production. J Hypertens.
29:724–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun X, Iida S, Yoshikawa A, Senbonmatsu R,
Imanaka K, Maruyama K, Nishimura S, Inagami T and Senbonmatsu T:
Non-activated APJ suppresses the angiotensin II type 1 receptor,
whereas apelin-activated APJ acts conversely. Hypertens Res.
34:701–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ryu S, Ornoy A, Samuni A, Zangen S and
Kohen R: Oxidative stress in Cohen diabetic rat model by
high-sucrose, low-copper diet: Inducing pancreatic damage and
diabetes. Metabolism. 57:1253–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kitada M, Kume S, Imaizumi N and Koya D:
Resveratrol improves oxidative stress and protects against diabetic
nephropathy through normalization of Mn-SOD dysfunction in
AMPK/SIRT1-independent pathway. Diabetes. 60:634–643. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee SH, Nam BY, Kang EW, Han SH, Li JJ,
Kim H, Kim SH, Kwak SJ, Park JT, Chang TI, et al: Effects of an
oral adsorbent on oxidative stress and fibronectin expression in
experimental diabetic nephropathy. Nephrol Dial Transplant.
25:2134–2141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ha H, Yu MR, Choi YJ, Kitamura M and Lee
HB: Role of high glucose-induced nuclear factor-kappaB activation
in monocyte chemoattractant protein-1 expression by mesangial
cells. J Am Soc Nephrol. 13:894–902. 2002.PubMed/NCBI
|
|
46
|
Morii T, Fujita H, Narita T, Shimotomai T,
Fujishima H, Yoshioka N, Imai H, Kakei M and Ito S: Association of
monocyte chemoattractant protein-1 with renal tubular damage in
diabetic nephropathy. J Diabetes Complications. 17:11–15. 2003.
View Article : Google Scholar
|
|
47
|
Day RT, Cavaglieri RC and Feliers D:
Apelin retards the progression of diabetic nephropathy. Am J
Physiol Renal Physiol. 304:F788–F800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nishida M, Okumura Y, Oka T, Toiyama K,
Ozawa S, Itoi T and Hamaoka K: The role of apelin on the
alleviative effect of Angiotensin receptor blocker in unilateral
ureteral obstruction-induced renal fibrosis. Nephron Extra.
2:39–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cameron NE and Cotter MA: Pro-inflammatory
mechanisms in diabetic neuropathy: Focus on the nuclear factor
kappa B pathway. Curr Drug Targets. 9:60–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ganesh Yerra V, Negi G, Sharma SS and
Kumar A: Potential therapeutic effects of the simultaneous
targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy.
Redox Biol. 1:394–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zeng XJ, Yu SP, Zhang L and Wei L:
Neuroprotective effect of the endogenous neural peptide apelin in
cultured mouse cortical neurons. Exp Cell Res. 316:1773–1783. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Simó R, Carrasco E, García-Ramírez M and
Hernández C: Angiogenic and antiangiogenic factors in proliferative
diabetic retinopathy. Curr Diabetes Rev. 2:71–98. 2006. View Article : Google Scholar
|
|
53
|
Tao Y, Lu Q, Jiang YR, Qian J, Wang JY,
Gao L and Jonas JB: Apelin in plasma and vitreous and in
fibrovascular retinal membranes of patients with proliferative
diabetic retinopathy. Invest Ophthalmol Vis Sci. 51:4237–4242.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lu Q, Feng J and Jiang YR: The role of
apelin in the retina of diabetic rats. PLoS One. 8:e697032013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saint-Geniez M, Masri B, Malecaze F,
Knibiehler B and Audigier Y: Expression of the murine msr/apj
receptor and its ligand apelin is upregulated during formation of
the retinal vessels. Mech Dev. 110:183–186. 2002. View Article : Google Scholar
|
|
56
|
Cain K, Bratton SB and Cohen GM: The
Apaf-1 apoptosome: A large caspase-activating complex. Biochimie.
84:203–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Matsushita H, Morishita R, Nata T, Aoki M,
Nakagami H, Taniyama Y, Yamamoto K, Higaki J, Yasufumi K and
Ogihara T: Hypoxia-induced endothelial apoptosis through nuclear
factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: In vivo
evidence of the importance of NF-kappaB in endothelial cell
regulation. Circ Res. 86:974–981. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Di Stilo A, Chegaev K, Lazzarato L,
Fruttero R, Gasco A, Rastaldo R and Cappello S: Effects of nitric
oxide donor antioxidants containing the phenol vitamin E
substructure and a furoxan moiety on ischemia/reperfusion injury.
Arzneimittelforschung. 59:111–116. 2009.PubMed/NCBI
|
|
59
|
Rastaldo R, Cappello S, Folino A, Di Stilo
A, Chegaev K, Tritto I, Pagliaro P and Losano G: Low concentrations
of an nitric oxide-donor combined with a liposoluble antioxidant
compound enhance protection against reperfusion injury in isolated
rat hearts. J Physiol Pharmacol. 61:21–27. 2010.PubMed/NCBI
|
|
60
|
Chen Z, Li T and Zhang B: Morphine
postconditioning protects against reperfusion injury in the
isolated rat hearts. J Surg Res. 145:287–294. 2008. View Article : Google Scholar
|
|
61
|
Zeng XJ, Zhang LK, Wang HX, Lu LQ, Ma LQ
and Tang CS: Apelin protects heart against ischemia/reperfusion
injury in rat. Peptides. 30:1144–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Simpkin JC, Yellon DM, Davidson SM, Lim
SY, Wynne AM and Smith CC: Apelin-13 and apelin-36 exhibit direct
cardioprotective activity against ischemia-reperfusion injury.
Basic Res Cardiol. 102:518–528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sorli SC, Le Gonidec S, Knibiehler B and
Audigier Y: Apelin is a potent activator of tumour neoangiogenesis.
Oncogene. 26:7692–7699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Han S, Wang G, Qi X, Lee HM, Englander EW
and Greeley GH Jr: A possible role for hypoxia-induced apelin
expression in enteric cell proliferation. Am J Physiol Regul Integr
Comp Physiol. 294:R1832–R1839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu J and Wang Z: Increased oxidative
stress as a selective anticancer therapy. Oxid Med Cell Longev.
2015:2943032015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sawicka E, Lisowska A, Kowal P and Długosz
A: The role of oxidative stress in bladder cancer. Postepy Hig Med
Dosw (Online). 69:744–752. 2015.In Polish. View Article : Google Scholar
|
|
67
|
Raina K, Tyagi A, Kumar D, Agarwal R and
Agarwal C: Role of oxidative stress in cytotoxicity of grape seed
extract in human bladder cancer cells. Food Chem Toxicol.
61:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tong L, Chuang CC, Wu S and Zuo L:
Reactive oxygen species in redox cancer therapy. Cancer Lett.
367:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shipitsin M and Polyak K: The cancer stem
cell hypothesis: In search of definitions, markers, and relevance.
Lab Invest. 88:459–463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Shi X, Zhang Y, Zheng J and Pan J:
Reactive oxygen species in cancer stem cells. Antioxid Redox
Signal. 16:1215–1228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gu Q, Zhai L, Feng X, Chen J, Miao Z, Ren
L, Qian X, Yu J, Li Y, Xu X, et al: Apelin-36, a potent peptide,
protects against ischemic brain injury by activating the PI3K/Akt
pathway. Neurochem Int. 63:535–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Silva A, Yunes JA, Cardoso BA, Martins LR,
Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA and Barata
JT: PTEN posttranslational inactivation and hyperactivation of the
PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin
Invest. 3762–3774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Min KJ, Lee JT, Joe EH and Kwon TK: An
IκBα phosphorylation inhibitor induces heme oxygenase-1(HO-1)
expression through the activation of reactive oxygen species
(ROS)-Nrf2-ARE signaling and ROS-PI3K/Akt signaling in an
NF-κB-independent mechanism. Cell Signal. 23:1505–1513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Khaksari M, Aboutaleb N, Nasirinezhad F,
Vakili A and Madjd Z: Apelin-13 protects the brain against ischemic
reperfusion injury and cerebral edema in a transient model of focal
cerebral ischemia. J Mol Neurosci. 48:201–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bao HJ, Zhang L, Han WC and Dai DK:
Apelin-13 attenuates traumatic brain injury-induced damage by
suppressing autophagy. Neurochem Res. 40:89–97. 2015. View Article : Google Scholar
|
|
77
|
Kasai A, Kinjo T, Ishihara R, Sakai I,
Ishimaru Y, Yoshioka Y, Yamamuro A, Ishige K, Ito Y and Maeda S:
Apelin deficiency accelerates the progression of amyotrophic
lateral sclerosis. PLoS One. 6:e239682011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kidoya H, Ueno M, Yamada Y, Mochizuki N,
Nakata M, Yano T, Fujii R and Takakura N: Spatial and temporal role
of the apelin/APJ system in the caliber size regulation of blood
vessels during angiogenesis. EMBO J. 27:522–534. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Inuzuka H, Nishizawa H, Inagaki A, Suzuki
M, Ota S, Miyamura H, Miyazaki J, Sekiya T, Kurahashi H and Udagawa
Y: Decreased expression of apelin in placentas from severe
pre-eclampsia patients. Hypertens Pregnancy. 32:410–421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bortoff KD, Qiu C, Runyon S, Williams MA
and Maitra R: Decreased maternal plasma apelin concentrations in
preeclampsia. Hypertens Pregnancy. 31:398–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Furuya M, Okuda M, Usui H, Takenouchi T,
Kami D, Nozawa A, Shozu M, Umezawa A, Takahashi T and Aoki I:
Expression of angiotensin II receptor-like 1 in the placentas of
pregnancy-induced hypertension. Int J Gynecol Pathol. 31:227–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Vatish M, Randeva HS and Grammatopoulos
DK: Hormonal regulation of placental nitric oxide and pathogenesis
of pre-eclampsia. Trends Mol Med. 12:223–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Han S, Wang G, Qi X, Englander EW and
Greeley GH Jr: Involvement of a Stat3 binding site in
inflammation-induced enteric apelin expression. Am J Physiol
Gastrointest Liver Physiol. 295:G1068–G1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cai X, Chen X, Wang X, Xu C, Guo Q, Zhu L,
Zhu S and Xu J: Pre-protective effect of lipoic acid on injury
induced by H2O2 in IPEC-J2 cells. Mol Cell
Biochem. 378:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Baregamian N, Song J, Jeschke MG, Evers BM
and Chung DH: IGF-1 protects intestinal epithelial cells from
oxidative stress-induced apoptosis. J Surg Res. 136:31–37. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gad GI, Ismail RI, El-Masry SA and Gouda
HR: Serum apelin in early-onset neonatal sepsis: Is it diagnostic?
J Neonatal Perinatal Med. 7:207–212. 2014.PubMed/NCBI
|
|
87
|
Lesur O, Roussy JF, Chagnon F, Gallo-Payet
N, Dumaine R, Sarret P, Chraibi A, Chouinard L and Hogue B: Proven
infection-related sepsis induces a differential stress response
early after ICU admission. Crit Care. 14:R1312010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Pan CS, Teng X, Zhang J, Cai Y, Zhao J, Wu
W, Wang X, Tang CS and Qi YF: Apelin antagonizes myocardial
impairment in sepsis. J Card Fail. 16:609–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Mertens K, Lowes DA, Webster NR, Talib J,
Hall L, Davies MJ, Beattie JH and Galley HF: Low zinc and selenium
concentrations in sepsis are associated with oxidative damage and
inflammation. Br J Anaesth. 114:990–999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bar-Or D, Carrick MM, Mains CW, Rael LT,
Slone D and Brody EN: Sepsis, oxidative stress, and hypoxia: Are
there clues to better treatment? Redox Rep. 20:193–197. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rastaldo R, Cappello S, Folino A and
Losano G: Effect of apelin-apelin receptor system in postischaemic
myocardial protection: a pharmacological postconditioning tool?
Antioxid Redox Signal. 14:909–922. 2011. View Article : Google Scholar
|
|
92
|
Azizi Y, Faghihi M, Imani A, Roghani M and
Nazari A: Post-infarct treatment with [Pyr1]-apelin-13 reduces
myocardial damage through reduction of oxidative injury and nitric
oxide enhancement in the rat model of myocardial infarction.
Peptides. 46:76–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen XY, Liu XM, Feng LL and Tang CS:
Changes and clinical significance of serum Apelin in patients with
severe sepsis and septic shock. Zhongguo Yi Xue Ke Xue Yuan Xue
Bao. 30:131–135. 2008.In Chinese. PubMed/NCBI
|
|
94
|
Cao J, Li H and Chen L: Targeting drugs to
APJ receptor: The prospect of treatment of hypertension and other
cardiovascular diseases. Curr Drug Targets. 16:148–155. 2015.
View Article : Google Scholar
|
|
95
|
Iturrioz X, Alvear-Perez R, De Mota N,
Franchet C, Guillier F, Leroux V, Dabire H, Le Jouan M, Chabane H,
Gerbier R, et al: Identification and pharmacological properties of
E339-3D6, the first nonpeptidic apelin receptor agonist. FASEB J.
24:1506–1517. 2010. View Article : Google Scholar
|
|
96
|
Khan P, Maloney PR, Hedrick M, Gosalia P,
Milewski M, Li L, Roth GP, Sergienko E, Suyama E, Sugarman E, et
al: Functional Agonists of the Apelin (APJ) Receptor. Probe Reports
from the NIH Molecular Libraries Program [Internet]. Last Update.
Dec 12–2011
|
|
97
|
Mendez M: Renin release: Role of SNAREs.
Am J Physiol Regul Integr Comp Physiol. 307:R484–R486. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Maloney PR, Khan P, Hedrick M, Gosalia P,
Milewski M, Li L, Roth GP, Sergienko E, Suyama E, Sugarman E, et
al: Discovery of 4-oxo-6-((pyrimidin-2-ylthio)methyl)-4H-pyran-3-yl
4-nitro-benzoate (ML221) as a functional antagonist of the apelin
(APJ) receptor. Bioorg Med Chem Lett. 22:6656–6660. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhou N, Fang J, Acheampong E, Mukhtar M
and Pomerantz RJ: Binding of ALX40-4C to APJ, a CNS-based receptor,
inhibits its utilization as a co-receptor by HIV-1. Virology.
312:196–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang Y, Maitra R, Harris DL, Dhungana S,
Snyder R and Runyon SP: Identifying structural determinants of
potency for analogs of apelin-13: Integration of C-terminal
truncation with structure-activity. Bioorg Med Chem. 22:2992–2997.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jin G, Yang P, Gong Y, Fan X, Tang J and
Lin J: Effects of puerarin on expression of apelin and its receptor
of 2K1C renal hypertension rats. Zhongguo Zhong Yao Za Zhi.
34:3263–3267. 2009.In Chinese.
|
|
102
|
Wu D, He L and Chen L: Apelin/APJ system:
A promising therapy target for hypertension. Mol Biol Rep.
41:6691–6703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
El Messari S, Iturrioz X, Fassot C, De
Mota N, Roesch D and Llorens-Cortes C: Functional dissociation of
apelin receptor signaling and endocytosis: Implications for the
effects of apelin on arterial blood pressure. J Neurochem.
90:1290–1301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang BH, Wang W, Wang H, Yin J and Zeng
XJ: Promoting effects of the adipokine, apelin, on diabetic
nephropathy. PLoS One. 8:e604572013. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chen H, Wan D, Wang L, Peng A, Xiao H,
Petersen RB, Liu C, Zheng L and Huang K: Apelin protects against
acute renal injury by inhibiting TGF-β1. Biochim Biophys Acta.
1852:1278–1287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yang Y, Zhang X, Cui H, Zhang C, Zhu C and
Li L: Apelin-13 protects the brain against ischemia/reperfusion
injury through activating PI3K/Akt and ERK1/2 signaling pathways.
Neurosci Lett. 568:44–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Xin Q, Cheng B, Pan Y, Liu H, Yang C, Chen
J and Bai B: Neuroprotective effects of apelin-13 on experimental
ischemic stroke through suppression of inflammation. Peptides.
63:55–62. 2015. View Article : Google Scholar
|