Introduction
Osteoarthritis (OA) is the most common type of
arthritis in adults throughout the world, and is characterized by
the progressive degeneration of articular cartilage including
chondrocyte loss and degradation of the extracellular matrix (ECM)
(1,2). Although the etiology and pathology
of OA is not completely elucidated, the degradation and destruction
of type II collagen caused by matrix metalloproteinase-13 (MMP-13)
is considered the central feature in the occurrence and development
of OA (3,4). MMP-13 is therefore a target for
therapeutic intervention that potentially reduces the development
and progression of OA.
Pro-inflammatory cytokines secreted by chondrocytes
contribute to the progression of OA, of which interleukin-1β
(IL-1β) plays a key role in the pathogenesis of OA by suppressing
the synthesis of type II collagen and aggrecan, the important
constituents of cartilage matrix (5–7).
Furthermore, in the development of OA, IL-1β upregulated
MMP-mediated cartilage degradation by activating a diverse spectrum
of signaling cascades in human chondrocytes or inhibiting the
expression of the tissue inhibitor of MMPs in arthritic joints
(3,8,9).
It has been reported that the gene expression profiles, especially
MMP expression in response to IL-1β treatment, are similar between
the human SW1353 chondrosarcoma cell line and primary human adult
articular chondrocytes (10–12).
Previous studies demonstrated the potent
anti-inflammatory property of ω-3 polyunsaturated fatty acids (n-3
PUFAs) in chronic inflammatory disorders, such as periodontal
disease (PD) and inflammatory bowel disease (IBD) (13–15). Docosahexenoic acid (DHA), a
long-chain n-3 PUFA, has been used to treat different types of
inflammatory diseases. Recent studies have shown that treatment
with n-3 PUFAs, especially DHA, can decrease the levels of
pro-inflammatory markers, and increase the levels of
anti-inflammatory markers, as well as improve outcomes in diseases,
including rheumatoid arthritis (RA) and cardiovascular disease
(16,17). Therefore, increasing the dietary
level of DHA in patients with arthritis for attenuation of pain and
inflammation in their joints is recommended (18). Additionally n-3 PUFAs, such as DHA
and eicosapentaenoic acid (EPA), downregulate the transcription of
various important proteins associated with the pathology of OA,
including a disintegrin and metalloproteinase with thrombospondin
motifs (ADAMTS) family, such as ADAMTS-4 and ADAMTS-5, and other
genes including MMP-3, cyclooxygenase-2, IL-1α, IL-1β and tumor
necrosis factor-α (TNF-α) (19).
However, the underlying mechanism by which DHA reduces MMP-13
expression in OA remains to be elucidated.
The aim of the present study was to investigate the
inhibitory effects of DHA on OA development, using IL-1β-stimulated
SW1353 chondrosarcoma cells, and a rat adjuvant-induced arthritis
(AIA) model, and examine the related mechanisms.
Materials and methods
Reagents and cell line
DHA and recombinant human IL-1β were purchased from
Sigma-Aldrich (St. Louis, MO, USA). DHA was dissolved in dehydrated
alcohol and then diluted to 100 µg/ml using Dulbecco's
Modified Eagle's Medium (DMEM; Gibco Life Technologies, Carlsbad,
CA, USA). Stock solutions of DHA (100 µg/ml) and IL-1β (100
ng/ml) in DMEM were stored at −20°C. Prior to treatment, the
solutions were diluted to the required concentrations.
The human SW1353 chondrosarcoma cell line (Cell
Applications, Inc., San Diego, CA, USA) was grown in monolayer at
37°C, in a humidified atmosphere under 5% CO2, with DMEM
supplemented with 10% (v/v) FBS and 100 U/ml
penicillin-streptomycin solution. Prior to the treatment, SW1353
cells were starved for 24 h with serum-free DMED medium (containing
100 U/ml penicillin-streptomycin), and then pretreated with various
concentrations of DHA as required for 1 h, followed by the addition
of IL-1β (5 ng/ml) to culture medium in the presence of DHA for
another 24 h.
Flow cytometry
The number of apoptotic cells was determined by flow
cytometric analysis. After DHA treatment for 24 h, the SW1353 cells
were collected and resuspended at a density of 1×106
cells/ml in 1X binding buffer (Sigma, St. Louis, MO, USA). The
cells were simultaneously stained with fluorescein
isothiocyanate-annexin V and propidium iodide included in an
Apoptosis Detection kit I (BD Biosciences, San Jose, CA, USA), and
then examined by FACSAria™ II flow cytometer (BD Biosciences),
according to the manufacturer's instructions.
MTT assay
The cytotoxicity of DHA (0–100 µg/ml) in
SW1353 cells was evaluated using an MTT assay. SW1353 cells were
cultured in 96-well plates at a density of 1×104
cells/well and treated with various concentrations of DHA for 24 h.
At the endpoint of treatment,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphe-nyltetrazolium bromide (MTT)
reagent (Sigma) was added to each well at the final concentration
of 5 mg/ml for another 4 h incubation. The supernatants in each
well were removed, and insoluble formazan crystals were dissolved
in dimethylsul-phoxide (Sigma). The absorbance of the colored
solution was measured at 570 nm using an enzyme-labeled meter
(Thermo Fisher Scientific, Waltham, MA, USA).
ELISA
SW1353 cells were pretreated with DHA (3.125, 6.25,
12.5, 25 and 50 µg/ml) as mentioned earlier. The level of
MMP-13 protein in the culture supernatant, which was secreted from
SW1353 cells, was determined by ELISA kits (Abcam, Cambridge, UK)
according to the manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted using TRIzol
reagent (Dingguo Changsheng Biotechnology Co., Ltd., Beijing,
China), according to the manufacturer's instructions. Purified RNA
was dissolved in diethylpyrocarbonate-treated water, and stored at
−80°C prior to use. The first-strand cDNA was synthesized using 1
µg of total RNA using the PrimeScript RT reagent kit
(TianGen Biotech, Beijing, China). PCR amplification was carried
out using specific primers as indicated in Table I, the SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd., Dalian, China) and reagents of the StepOne
Real-Time PCR System (Applied Biosystems, Foster City, CA, USA).
Typical profiles for PCR were denaturation at 95°C for 30 sec,
followed by annealing at 95°C for 15 sec and extension at 60°C for
32 sec for total 40 cycles. GAPDH was used as an endogenous control
in PCR. The mRNA level of MMP-13 was normalized to that of GAPDH.
The data from three independent experiments, were analyzed using
the ΔΔCt method and compared with the control group.
 | Table IPrimers used in polymerase chain
reaction. |
Table I
Primers used in polymerase chain
reaction.
| Target | Primer sequence
(5′→3′) |
|---|
| MMP-13 | F:
5′-CAGAACATCATCCCTGCCTCT-3′ |
| R:
5′-GCCCATCAAATGGGTAGAAG-3′ |
| GAPDH | F:
5′-CAGAACATCATCCCTGCCTCT-3′ |
| R:
5′-GCTTGACAAAGTGGTCGTTGAG-3′ |
Western blotting
Proteins were extracted from cells, using radio
immunoprecipitation assay buffer plus 1% protease inhibitor
cocktail (Roche Molecular Diagnostics, Pleasanton, CA, USA, USA).
Specifically, phosphorylated proteins were extracted using a
phosphorylated protein extraction kit (Roche Molecular
Diagnostics). The protein samples were separated by 12% sodium
dodecyl sulphate-polyacrylamide gel electrophoresis and transferred
onto polyvinylidene fluoride membranes. The membranes were then
blocked with 5% non-fat milk dissolved in 1% TBST buffer and probed
with primary antibodies against MMP-13 (polyclonal antibody, 1:500;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), p-p38 (monoclonal
antibody, 1:1,000; Santa Cruz Biotechnology, Inc.), cleaved PARP
(polyclonal antibody, 1:500; Santa Cruz Biotechnology, Inc.),
caspase-3 (polyclonal antibody, 1:500; Santa Cruz Biotechnology,
Inc.) and β-actin (monoclonal antibody 1:5,000; Santa Cruz
Biotechnology, Inc.) on a shaker at 4°C overnight. After washing
with 1% TBST, the blots were incubated with appropriate diluted
horseradish peroxidase (HRP)-labeled secondary antibodies for 1 h
at room temperature. Signals were visualized by using enhanced
chemiluminescence (Beyotime Institute of Biotechnology, Shanghai,
China). ChemiDoc XRS+ (Bio-Rad, Berkeley, CA, USA) was used to
quantify the protein bands. Data from three independent experiments
were calculated as the ratio of the gray values of MMP-13
protein/gray values of β-actin.
Animals and husbandry
Twenty adult male Sprague-Dawley (SD) rats (280–300
g, 8-week-old) were supplied by the Beijing Friendship Hospital
Laboratory Animal Center. The rats were randomly divided into the
normal control, AIA, DHA and inhibitor groups. The rats were kept
in separate cages (5 rats per cage), at a temperature of 22–23°C
and a relative humidity of 40–60%. The rats were allowed free
access to distilled water and food and allowed to acclimatize for
one day prior to use. Procedures were approved by the Animal Ethics
Committee of Beijing Friendship Hospital, Capital Medical
University.
Food preparation and administration of SD
rats
The rats in the normal control and AIA groups were
fed the AIN-93G diet, while those in the DHA group were fed the
AIN-93G diet containing DHA (50 g/kg) (20) and the inhibitor group on the
AIN-93G diet containing SB203580 (10 mg/kg). Food was supplied by
the China-Japan Food Research Center, China Agricultural
University. Complete Freund's adjuvant (CFA) was purchased from
Sigma-Aldrich and dissolved in sterile paraffin oil (5 mg/ml). The
normal control group received an injection of 0.3 ml of paraffin
oil in the left hind paw, while other groups received a
subcutaneous injection of 0.3 ml of CFA to induce experimental AIA,
at day 0.
Assessment of clinical parameters in
experimental animals
The clinical parameters were assessed, including
body weight, paw volume, and articular index (AI) score (range 0–4;
0 for no swelling or erythema, 1 for slight swelling and/or
erythema, 2 for low-to-moderate edema, 3 for pronounced edema with
limited use of the joint, and 4 for excessive edema with joint
rigidity) (21). AI scores were
recorded for each knee joint by the same observer, who was blinded
to the treatment in the 4 groups. Paw volume was measured by a
plethysmometer (MK-101P; NatureGene Corp., Beijing, China).
Parameters were recorded on days 0, 5, 10, 15, 20, 30, 40 and
45.
Histological examination and
immunohistochemistry (IHC)
At day 45th, the rats were sacrificed to collect
their hind knee joints. Portions of the joints were fixed in 10%
neutral-buffered formalin. After 5 days of fixation, the sections
were decalcified using a decalcifying solution, containing 24.4%
formic acid, and 0.5 N sodium hydroxide for 5 days, in which
decalcifying solution was changed daily. The median joint parts
were then longitudinally trimmed and embedded in paraffin,
sectioned (4 µm), and stained with hematoxylin and eosin
(H&E) and Safranin-Orange-fast green (Safranin-O). Histological
features of specimens were graded using a modified Mankin's score
(22). For IHC, the sections were
incubated with primary antibody against MMP-13 (polyclonal, 1:50),
and secondary biotinylated goat anti-rabbit immunoglobulin G (IgG).
The positive signals were visualized with HRP/avidin-biotin
complex, using 3,3′-diaminobenzidine tetrahydrochloride as the
chromogen (Dako, Carpinteria, CA, USA). Negative control in IHC was
made by incubating with an isotype of IgG. MMP-13-positive
chondrocytes were determined by counting the number of positive
cells in IHC and then dividing this number by the total number of
chondrocytes visualized by hematoxylin counterstaining.
Statistical analysis
Data from three independent experiments were
presented as the mean ± standard errors of means. Statistical
significance of the results among each group was determined using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference. Statistical analysis was
performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
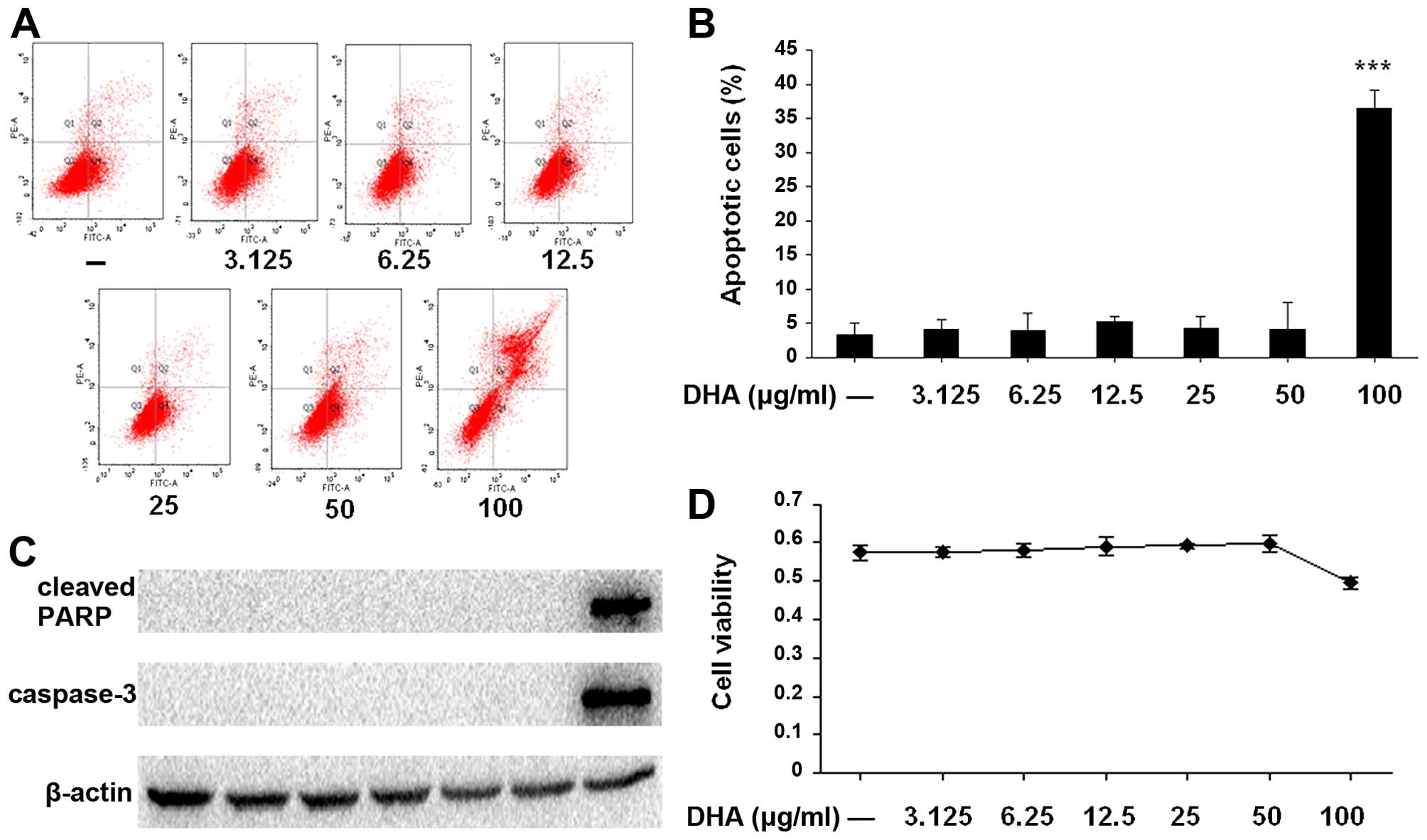
Safe concentration range of DHA in
treatment of SW1353 cells is 0–50 µg/ml in vitro
The cytotoxicity of DHA in the treatment of SW1353
cells at different concentration of DHA (3.125, 6.25, 12.5, 25, 50
and 100 µg/ml) was assessed by flow cytometry and MTT assay.
As shown in Fig. 1, when SW1353
cells were treated with DHA at a dose of 100 µg/ml, cell
viability significantly decreased and the number of apoptotic cells
was higher, as compared to other doses ranging from 3.125 to 50
µg/ml, in which DHA did not present cytotoxic effects.
Western blot analysis also showed that the levels of cleaved PARP
and caspase-3 were markedly increased with the dose of 100
µg/ml DHA. Thus, 0–50 µg/ml of DHA was used in the
subsequent experiments.
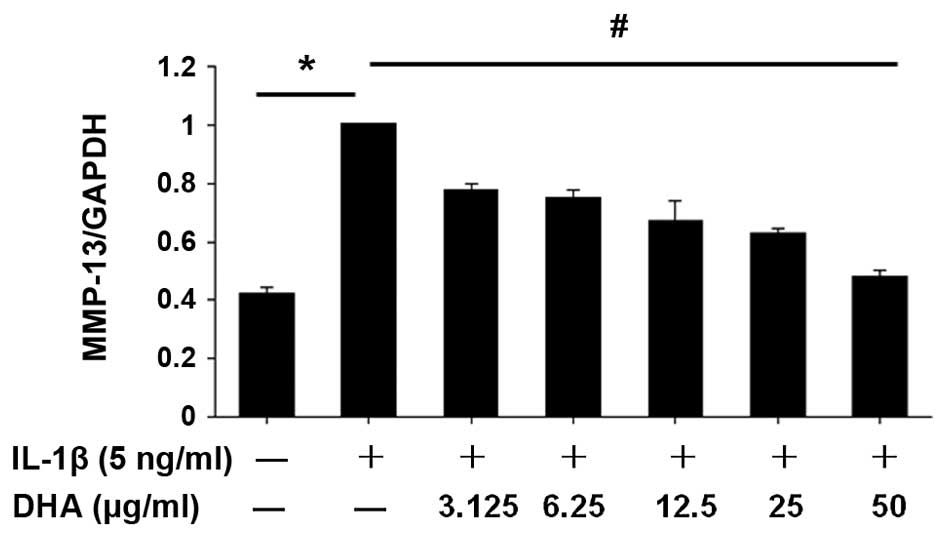
DHA suppresses the mRNA expression of
MMP-13 in IL-1β-stimulated SW1353 cells
SW1353 cells were treated with a series of
concentrations of DHA (0, 3.125, 6.25, 12.5, 25 and 50
µg/ml) for 1 h, followed by stimulation of IL-1β (5 ng/ml)
i.e., co-incubation with IL-1β and DHA for 24 h. Total RNA was then
isolated from the harvested cells. The mRNA expression level of
MMP-13 in DHA-treated cells was measured by RT-qPCR. As shown in
Fig. 2, IL-1β-enhanced MMP-13
expression in SW1353 cells was inhibited by DHA in a dose-dependent
manner.
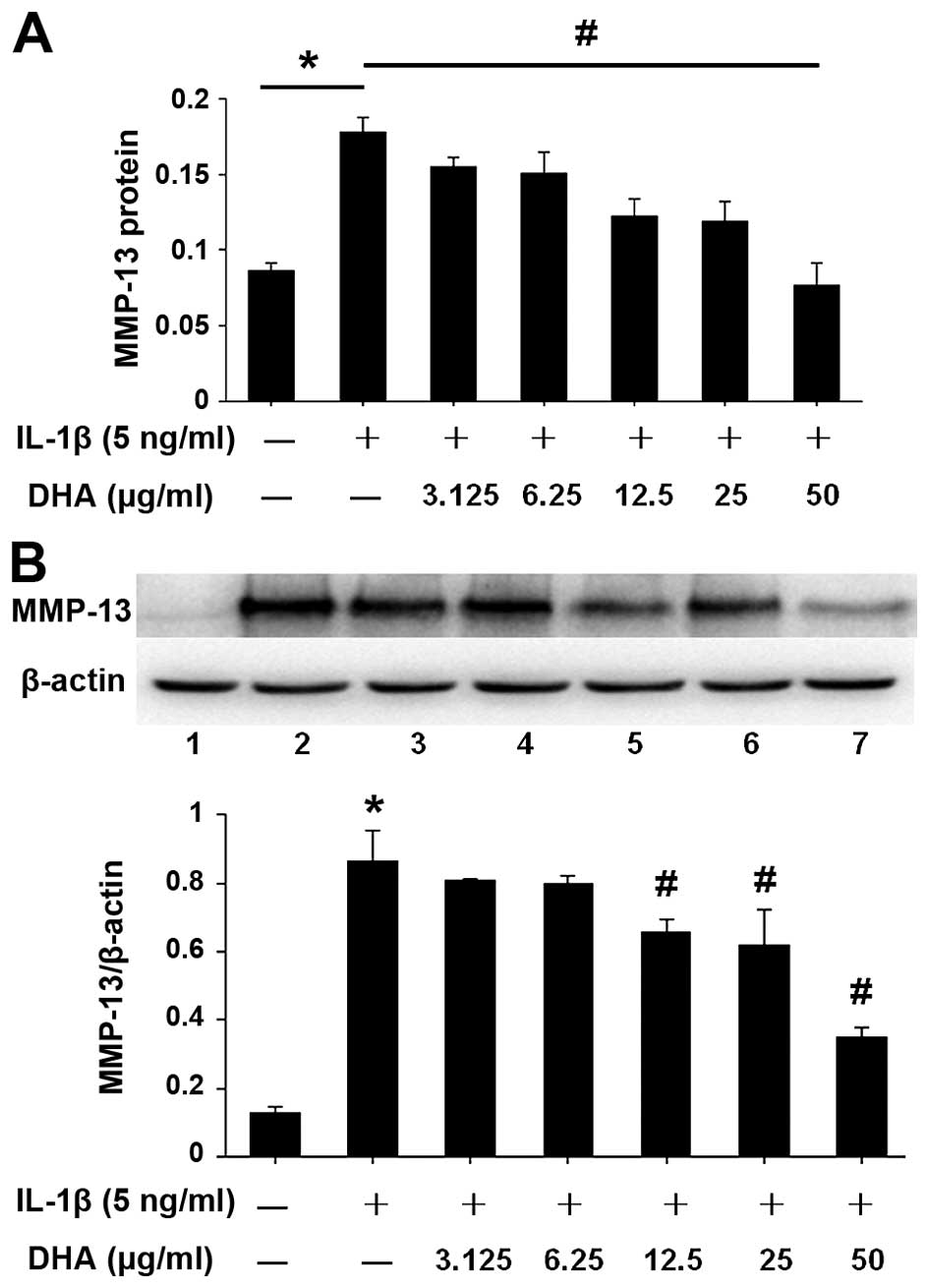
DHA inhibits MMP-13 protein expression in
IL-1β-stimulated SW1353 cells
To evaluate the inhibitory effect of DHA on MMP-13
protein expression, DHA pretreatment and co-incubation with IL-1β
were performed in SW1353 cells, under the same conditions as those
for Fig. 2. Following treatment,
protein was extracted from cell pellets and MMP-13 expression was
analyzed by western blotting (Fig.
3B). The level of MMP-13 in the supernatant was also measured,
using the ELISA kit (Fig. 3A).
The results showed that the expression level of MMP-13 protein in
each group was consistent with the mRNA expression level detected
by PCR. DHA treatment reduced the protein expression of MMP-13 in
IL-1β-stimulated SW1353 cells. DHA-mediated inhibition was
dose-dependent and 50 µg/ml DHA showed the strongest
inhibitory effect (Fig. 3).
Inhibitory effect of DHA on MMP-13
expression via a p38 mitogen-activated protein kinase
(MAPK)-dependent mechanism
Previous findings showed that the MAPK signaling
pathway was involved in cartilage proliferation, apoptosis and
differentiation (23). IL-1
induced activation of the key enzymes in the MAPK signaling
pathway, such as p38, c-Jun N-terminal kinase (JNK) and
extracellular signal-regulated kinase (ERK) (23–25). IL-1 also upregulated MMP
expression to promote the degradation of articular cartilage. To
identify the signaling pathway and related enzyme(s) involved in
the inhibitory effects of DHA, SW1353 cells were pretreated with
DHA at different concentrations (0, 3.125, 6.25, 12.5, 25 and 50
µg/ml) for 1 h, and then stimulated with IL-1β (5 ng/ml) for
24 h. The level of p-p38 in the treated cells was examined by
western blotting. As shown in Fig.
4A, the p-p38 level was decreased by DHA treatment in a
dose-dependent manner. Furthermore, SW1353 cells were pretreated 1
h with DHA (50 µg/ml) alone or SB203580 (10 µM)
alone, a p38-specific inhibitor (Sigma, San Antonio, TX, USA), or
both. The cells were stimulated with 5 ng/ml IL-1β for 24 h. The
levels of p-p38 and MMP-13 in the treated cells were examined by
western blot analysis (Fig. 4B and
C), which showed the levels of p-p38 and MMP-13 proteins were
significantly reduced, as compared to that in the IL-1β only
group.
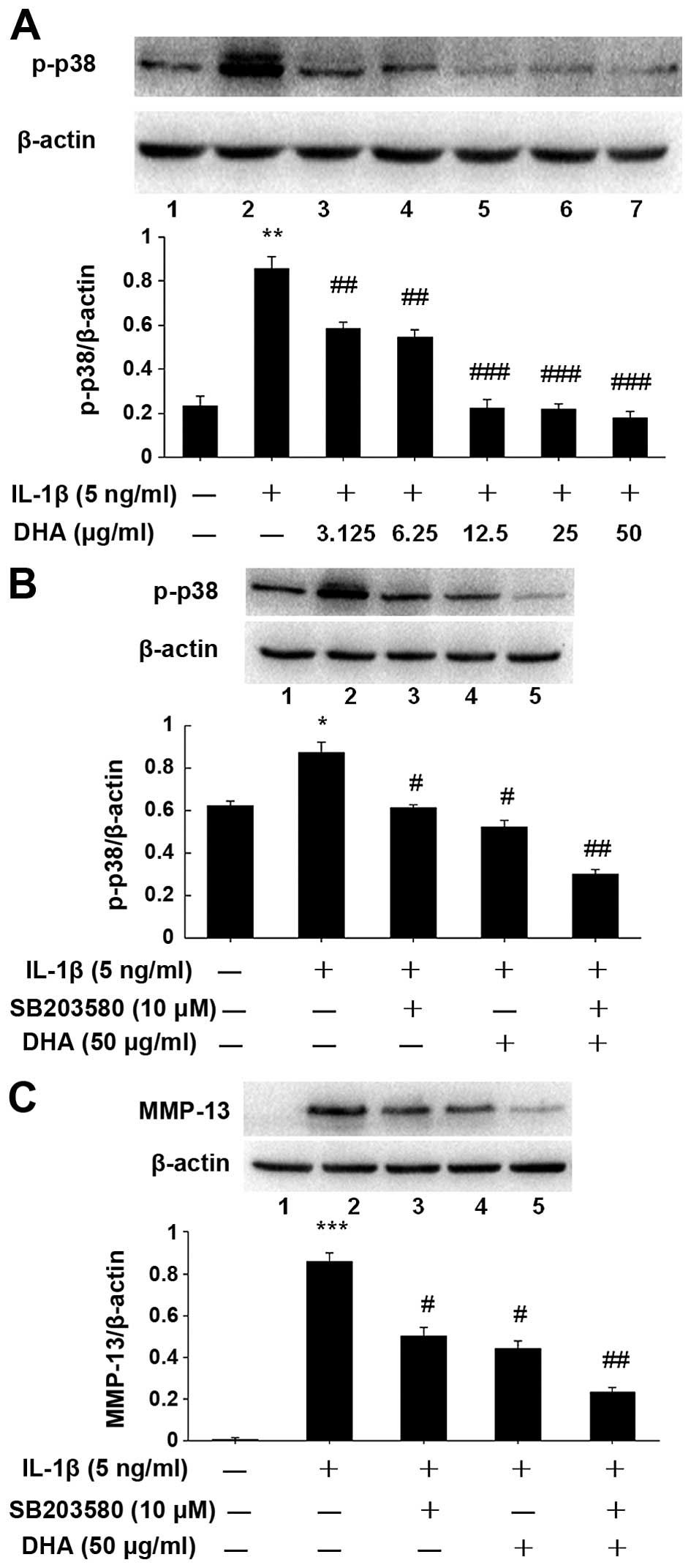 | Figure 4Inhibitory effect of docosahexenoic
acid (DHA) on matrix metal-loproteinase-13 (MMP-13) expression via
a p38 mitogen-activated protein kinase (MAPK)-dependent mechanism.
(A) SW1353 cells were incubated for 24 h with DHA and interleukin
(IL)-1β. The level of p-p38 in treated cells was determined by
western blot analysis. The dose-dependent reduction of
phosphorylation of p38 was consistent with that of MMP-13 protein
expression in DHA-treated cells. (B and C) SW1353 cells were
pretreated 1 h with SB203580 (10 µM), a specific inhibitor
of p38, or DHA (50 µg/ml), or both, and stimulated with
IL-1β (5 ng/ml) for 24 h. Western blotting results revealed that
DHA attenuates IL-1β-enhanced MMP-13 expression via the p38 MAPK
pathway by inhibiting phosphorylation of p38. In addition, the
inhibitory effect of DHA on MMP-13 expression was similar to that
presented by SB203580 (p>0.05). The blots were normalized to
β-actin. *P<0.05, **P<0.01,
***P<0.001, compared with non-treatment control,
#P<0.05, ##P<0.01, compared with IL-1β
alone group. |
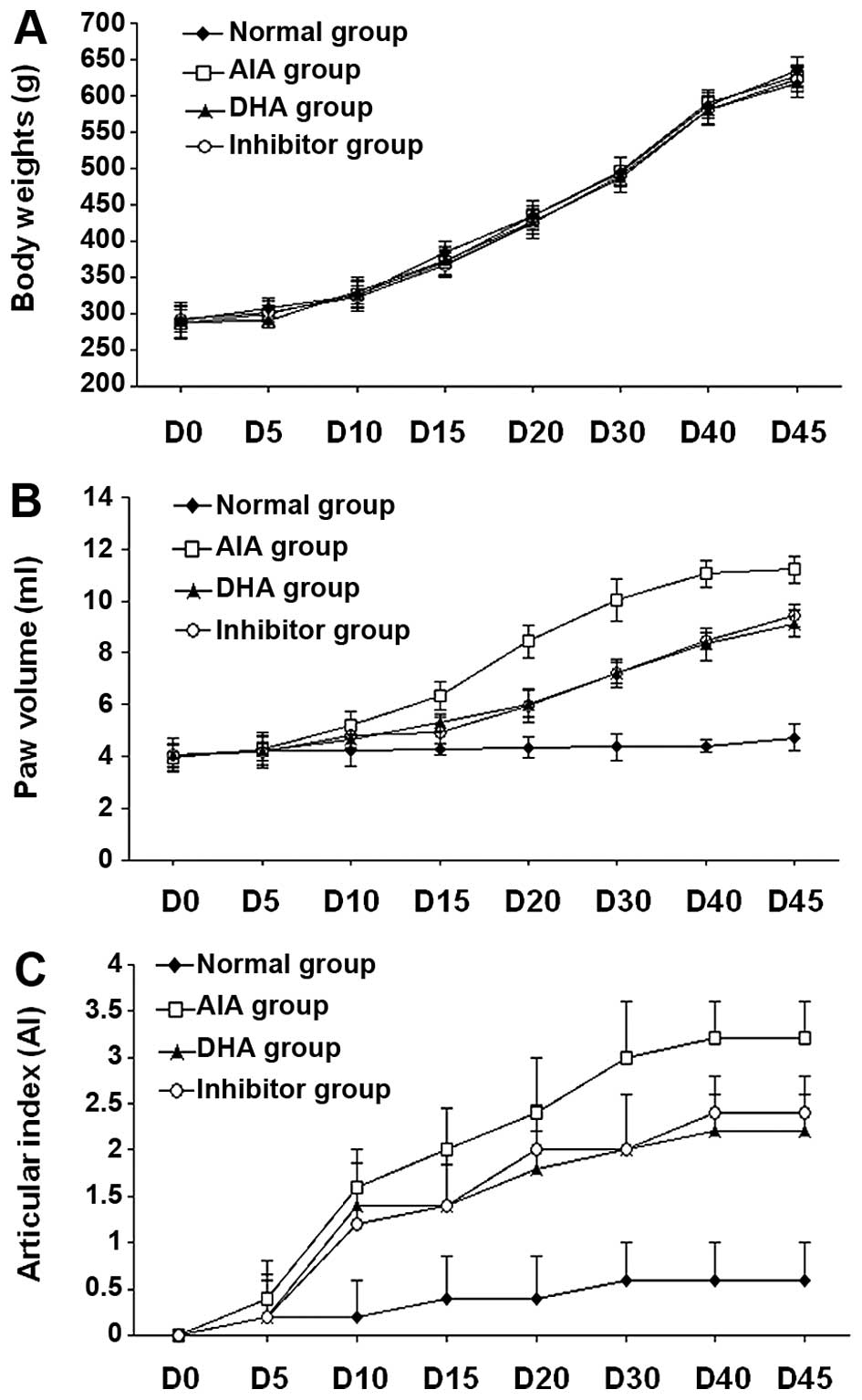
DHA treatment improves the clinical
parameters in a rat model of AIA
After 5 days of CFA injection, the animals began to
show symptoms and signs of clinical inflammation in hind paws and
knee joints. The first manifestation of disease was edema of the
ankle joints, followed by involvement of the metatarsal and
interphalangeal joints. The typical time course of arthritis
progression is shown in Fig. 5B and
C and the degree of lesion was assessed by the paw volume and
arthritis index (AI). In the present study, no significant changes
in body weight (loss or gain) were found in the DHA or inhibitor
group, as compared to the normal control and AIA groups. As shown
in Fig. 5A, changes in body
weight in all the rats were within the normal range of age-matched
rats. Changes of the paw volume and AI score were observed on day
5, albeit there were no significant differences between the groups
(P>0.05). At 10 days after injection of CFA, the parameters
showed statistical difference between the CFA treatment and normal
groups (P<0.05), and remained as such throughout the duration of
the experiment. Improvement of paw volume and AI score was evident
in the DHA and inhibitor groups, as compared to the AIA group
(P<0.05). However, there was no statistically significant
difference between the DHA and inhibitor groups (P>0.05).
DHA treatment ameliorates cartilage
damage in experimental animals through the suppression of MMP-13
expression
Histopathological examination of H&E-stained
sections found that in the normal control group, articular
cartilage was characterized by a smooth, polished and regular
surface (Fig. 6Aa), whereas, in
the AIA group we found a matt, unpolished, moderate wear and tear
surface and reduction in chondrocytes. In addition, we observed
that in the middle to deep zone of cartilage, there were multifocal
vertical micro-cracks (Fig. 6Ab).
Cartilage lesions associated with OA were also observed in the DHA
and inhibitor treatment groups, albeit the severity thereof was
less than that in the AIA group (Fig.
6Ac and Ad). In the present study, cartilage thickness was
measured by a photomicrograph of the sagittal section of condylus
femoris. The results showed that cartilage thickness was
significantly decreased in the AIA group, as compared to that in
the normal group (P<0.05). By contrastt, in the DHA and
inhibitor groups, cartilage thickness was markedly increased, as
compared to that in the AIA group (P<0.05, Fig. 6B). In 3 CFA-treated groups, we
observed the sections with moderate to severe lack of Safranin-O
staining in the superficial and middle zones of cartilage (Fig. 6C). In addition, an irregular
chondrocyte arrangement was found in the AIA group (Fig. 6Cb). The severity of cartilage
destruction was also scaled using the Mankin score. There was a
significant increase in the Mankin score in the AIA group, in the
comparison of all the groups (P<0.05, vs. normal or vs. both the
DHA and inhibitor groups, Fig.
6D), indicating that the treatment of AIA rats with DHA or p38
MAPK inhibitor can decrease the Mankin score, while there was no
statistically significant difference of the Mankin score between
the DHA and inhibitor groups. In addition, the level of MMP-13
expression was evaluated by immunohistochemical staining (Fig. 6E), by which MMP-13 expressed cells
were moderately brown-stained. The results showed that MMP-13
expression was elevated in the articular cartilage of AIA rats,
especially in the superficial to middle zone. Compared with the
other 3 groups, the percentage of MMP-13-positive cells in the AIA
group was significantly increased (p<0.05). The percentage of
MMP-13-positive cells in the DHA and inhibitor groups was
10.21±2.43 and 11.35±2.05%, respectively, both of which were
significantly lower than that in the AIA group (24.5±3.25%)
(P<0.05, Fig. 6F). The
histopathological and IHC examinations confirmed that DHA treatment
can ameliorate cartilage destruction in experimental animals by
inhibiting MMP-13 expression.
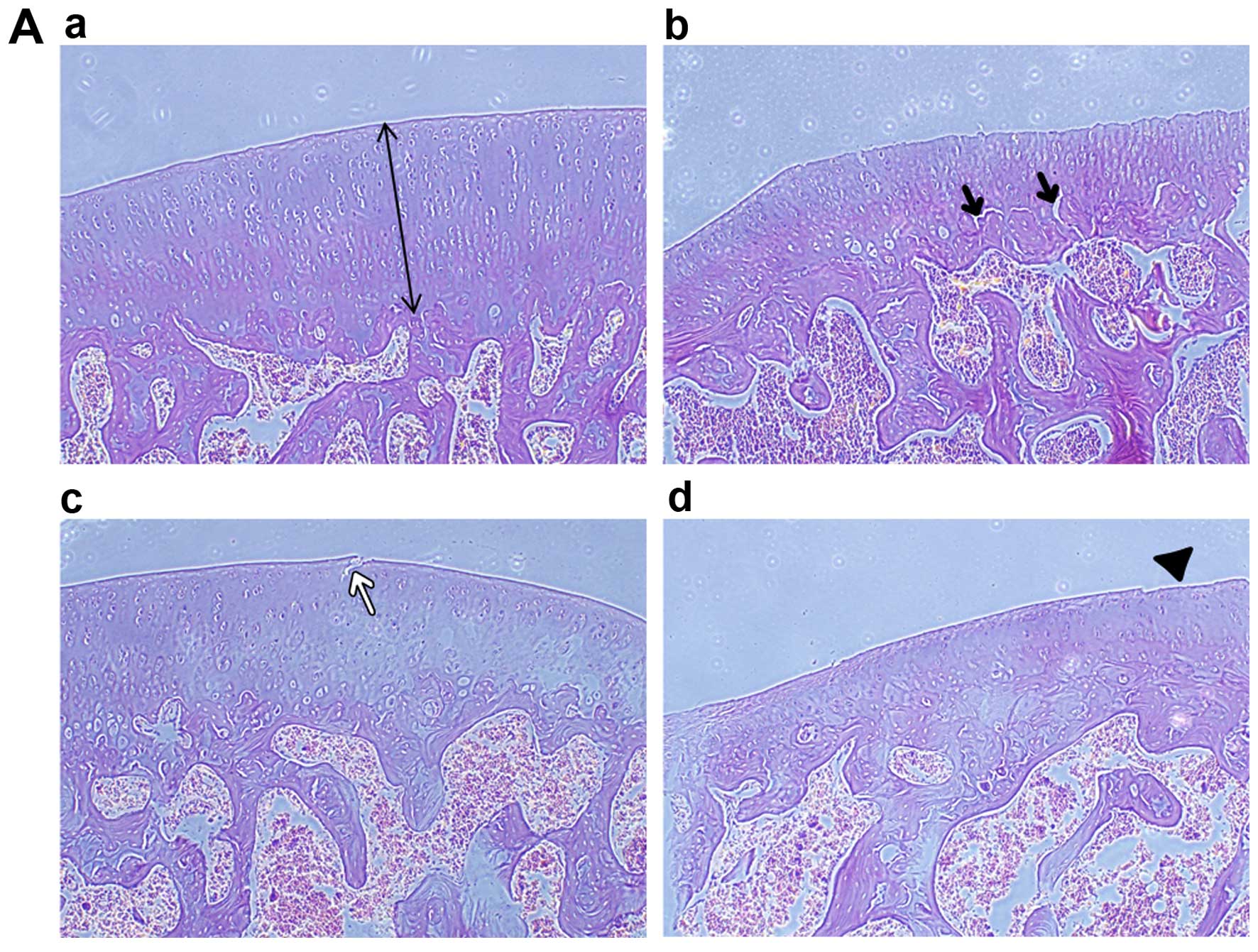 | Figure 6Histopathological and IFC
examinations of articular cartilage of experimental rats. (Aa)
Normal architecture of articular cartilage of rat condylus femoris.
The thickness of cartilage was marked in photomicrograph. (Ab) A
matt, unpolished, moderate wear and tear surface and reduction of
chondrocytes is evident in the adjuvant-induced arthritis (AIA)
group. Vertical micro-cracks are marked (black arrows). (Ac and Ad)
Cartilage lesions are evident in the docosahexenoic acid (DHA) and
inhibitor groups, but the severity in those groups was less than
that in the AIA group, as indicated by the white arrow and
arrow-head, hematoxylin and eosin staining (magnification, ×100).
(C) Compared with the normal group, the sections from the other 3
groups were found with moderate to severe lack of Safranin-O
staining, particularly in the superficial and middle zones. Of
note, there was an irregular chondrocyte arrangement in the AIA
group (Fig. Cb), compared with that of the DHA and inhibitor groups
(magnification, ×100). (B and D) Cartilage thickness and the Mankin
score were assessed. DHA and the inhibitor treatment increased the
cartilage thickness and decreased the Mankin score significantly,
as compared with those in the AIA group. (E and F) In the DHA and
inhibitor groups, the percentage of matrix metalloproteinase-13
positive cells was less than that in the AIA group
(#P<0.05), but more than that in the normal group
(*P<0.05), according to the photomicrograph of
immunohistochemical staining (magnification, ×200). |
Discussion
In the present study, we demonstrated that DHA
inhibited MMP-13 expression in IL-1β-stimulated SW1353 cells and
retarded cartilage destruction in the rat model of AIA. Our in
vitro and in vivo studies also demonstrated that the
inhibitory effect of DHA occurs via the p38 MAPK-dependent
mechanism, which is associated with the upregulation of MMP-13
expression in OA.
Cartilage destruction caused by degradation of type
II collagen is the hallmark of OA and determines the irreversible
progression of OA (26). Previous
findings have shown the aberrant expression of MMPs is crucial in
the destruction of the articular cartilage (3,27).
In patients with OA, several pro-inflammatory cytokines are
secreted by inflamed synovium and chondrocytes in response to
inflammation, which induce overexpression of MMPs (6,28,29). MMPs belong to a large family of
collagenolytic enzymes that regulate a variety of functions in
articular cartilage, including the turnover, catabolism and
degradation of the ECM. Among the members of MMPs, MMP-13 is
thought to be the primary collagenase in OA, because MMP-13 is 5-
to 10-fold more effective at degrading type II collagen than other
MMPs. Furthermore, it has been found that MMP-13 is mainly
localized in the deeper layers of cartilage (30). In the present study, we have
demonstrated that in an AIA rat model, MMP-13 expression was
upregulated in the middle zone in arthritic rat cartilage, albeit
this aberrant expression of MMP-13 can be attenuated by DHA
treatment. Furthermore, in the experimental OA model of MMP
knock-out mice, a decrease in cartilage destruction and osteophyte
formation was found, as compared with the wild-type mice after the
induction of knee OA (31,32).
Thus, an optimal agent that can attenuate over-expressed MMP-13 and
has fewer side effects is a promising therapeutic intervention for
the treatment of OA.
Accumulated evidence has shown that n-3 PUFAs
possess anti-inflammatory properties in certain inflammatory
disorders. For example, n-3 PUFAs retard the progression of chronic
glomerulonephritis, relieving the symptoms of RA and IBD (15,18,33). The results demonstrate that
DHA-treated arthritic rats had mild clinical symptoms, as indicated
by the improvement of inflammatory parameters, including smaller
paw volume and decreased arthritic index. Inclusion of fish or fish
oil which are rich sources of DHA in diet, have been shown to
inhibit the production of prostaglandin E2, and reduce the level of
leukotriene B4, a powerful inducer of leukocytechemotaxis and
adherence that cause inflammation (13,34,35). Furthermore, the expression of
pro-inflammatory genes, including IL-1β, TNF-α, IL-6, are
significantly suppressed in macrophages that are pretreated with
DHA/EPA mixture (33). In a
marine model of arthritis, inflammation and joint destruction are
reduced by prophylactic treatment with DHA (36). Subsequently, we examined the
effect of DHA on MMP-13 expression in vitro and in
vivo, using an IL-1β-stimulated human chondrosarcoma cell line
and an AIA rat model. The findings show that DHA treatment potently
downregulates MMP-13 expression at the mRNA and protein levels in
chondrosarcoma cells and reduces the number of MMP-13-positive
cells in animals with arthritis. Previous studies on human or
animal studies have shown that at either low or high dose, the side
effects of n-3 PUFAs were minimal (37,38). In the present study, we found that
the safe concentration of DHA treatment in SW1353 cells was <50
µg/ml, whereas >50 µg/ml, decreased cell viability
and increased apoptosis in DHA-treated cells, as indicated by flow
cytometry and MTT assay.
The MAPK signaling pathway is involved in the
catabolic responses of cartilage cells. Additionally, IL-1β-induced
MMP gene expression is mediated by activated MAPK pathway (25,39), which consists of a group of
serine/threonine protein kinases for cellular signal transduction,
including p38, JNK and ERK1/2. Under inflammatory conditions,
specific tyrosine and threonine residues of MAPKs are
phosphorylated by their upstream kinases, leading to their
activation. Using human SW1353 chondrosarcoma cells as a model, we
evaluated the effect of DHA on p38, JNK and ERK1/2 activation in
response to IL-1β stimulation. The results showed that DHA
selectively blocked IL-1β-induced p38 activation, rather than the
activation of JNK or ERK. Therefore, the DHA-mediated inhibition of
p38 phosphorylation contributes to the mechanism associated with
the chondroprotective effect of DHA in the IL-1β-stimulated
chondrocyte and AIA rat model. Since the involvement of other
signaling pathways, such as nuclear factor-κB in the regulation of
OA-related gene expression has also been reported by other studies
(40,41), additional studies are needed to
elucidate the precise mechanisms underlying the inhibition of
MMP-13 by DHA.
The results of the present study have shown that DHA
ameliorates cartilage degeneration from OA via the inactivation of
p38MAPK, based on the data from IL-1β-stimulated SW1353
chondrosarcoma cells and an AIA rat model. Thus, DHA is a promising
therapeutic agent for the prevention and treatment of OA.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 31171672). We would like
to thank Wentong Xue of the China Agricultural University, for
providing animal feed supply.
References
|
1
|
Moyer RF, Ratneswaran A, Beier F and
Birmingham TB: Osteoarthritis year in review 2014: mechanics -
basic and clinical studies in osteoarthritis. Osteoarthritis
Cartilage. 22:1989–2002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fibel KH, Hillstrom HJ and Halpern BC:
State-of-the-Art management of knee osteoarthritis. World J Clin
Cases. 3:89–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Troeberg L and Nagase H: Proteases
involved in cartilage matrix degradation in osteoarthritis. Biochim
Biophys Acta. 1824:133–145. 2012. View Article : Google Scholar
|
|
4
|
Takaishi H, Kimura T, Dalal S, Okada Y and
D'Armiento J: Joint diseases and matrix metalloproteinases: A role
for MMP-13. Curr Pharm Biotechnol. 9:47–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mabey T and Honsawek S: Cytokines as
biochemical markers for knee osteoarthritis. World J Orthop.
6:95–105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar
|
|
7
|
Goldring MB and Otero M: Inflammation in
osteoarthritis. Curr Opin Rheumatol. 23:471–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garvican ER, Vaughan-Thomas A, Redmond C,
Gabriel N and Clegg PD: MMP-mediated collagen breakdown induced by
activated protein C in equine cartilage is reduced by
corticosteroids. J Orthop Res. 28:370–378. 2010.
|
|
9
|
Houard X, Goldring MB and Berenbaum F:
Homeostatic mechanisms in articular cartilage and role of
inflammation in osteoarthritis. Curr Rheumatol Rep. 15(375)2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi J, Schmitt-Talbot E, DiMattia DA and
Dullea RG: The differential effects of IL-1 and TNF-alpha on
proinflammatory cytokine and matrix metalloproteinase expression in
human chondrosarcoma cells. Inflamm Res. 53:377–389. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gebauer M, Saas J, Sohler F, Haag J, Soder
S, Pieper M, Bartnik E, Beninga J, Zimmer R and Aigner T:
Comparison of the chondrosarcoma cell line SW1353 with primary
human adult articular chondrocytes with regard to their gene
expression profile and reactivity to IL-1beta. Osteoarthritis
Cartilage. 13:697–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tetsunaga T, Nishida K, Furumatsu T,
Naruse K, Hirohata S, Yoshida A, Saito T and Ozaki T: Regulation of
mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2
transcriptional factor in SW1353 chondrocyte-like cells.
Osteoarthritis Cartilage. 19:222–232. 2011. View Article : Google Scholar
|
|
13
|
Tabbaa M, Golubic M, Roizen MF and
Bernstein AM: Docosahexaenoic acid, inflammation, and bacterial
dysbiosis in relation to periodontal disease, inflammatory bowel
disease, and the metabolic syndrome. Nutrients. 5:3299–3310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raphael W and Sordillo LM: Dietary
polyunsaturated fatty acids and inflammation: The role of
phospholipid biosynthesis. Int J Mol Sci. 14:21167–21188. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caplan MS and Jilling T: The role of
polyunsaturated fatty acid supplementation in intestinal
inflammation and neonatal necrotizing enterocolitis. Lipids.
36:1053–1057. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baker KR, Matthan NR, Lichtenstein AH, Niu
J, Guermazi A, Roemer F, Grainger A, Nevitt MC, Clancy M, Lewis CE,
et al: Association of plasma n-6 and n-3 polyunsaturated fatty
acids with synovitis in the knee: the MOST study. Osteoarthritis
Cartilage. 20:382–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishijima M, Kaneko H and Kaneko K: The
evolving role of biomarkers for osteoarthritis. Ther Adv
Musculoskelet Dis. 6:144–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Calder PC: Polyunsaturated fatty acids,
inflammation, and immunity. Lipids. 36:1007–1024. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zainal Z, Longman AJ, Hurst S, Duggan K,
Caterson B, Hughes CE and Harwood JL: Relative efficacies of
omega-3 polyunsaturated fatty acids in reducing expression of key
proteins in a model system for studying osteoarthritis.
Osteoarthritis Cartilage. 17:896–905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmitt D, Tran N, Peach J, Bauter M and
Marone P: Toxicologic evaluation of DHA-rich algal oil:
Genotoxicity, acute and subchronic toxicity in rats. Food Chem
Toxicol. 50:3567–3576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shahrara S, Proudfoot AE, Woods JM, Ruth
JH, Amin MA, Park CC, Haas CS, Pope RM, Haines GK, Zha YY, et al:
Amelioration of rat adjuvant-induced arthritis by Met-RANTES.
Arthritis Rheum. 52:1907–1919. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iannitti T, Elhensheri M, Bingöl AO and
Palmieri B: Preliminary histopathological study of intra-articular
injection of a novel highly cross-linked hyaluronic acid in a
rabbit model of knee osteoarthritis. J Mol Histol. 44:191–201.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saklatvala J: Inflammatory signaling in
cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use
of inhibitors for research into pathogenesis and therapy of
osteoarthritis. Curr Drug Targets. 8:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao SC, Yin HB, Liu HX and Sui YH:
Research progress on MAPK signal pathway in the pathogenesis of
osteoarthritis. Zhongguo Gu Shang. 27:441–444. 2014.In Chinese.
PubMed/NCBI
|
|
25
|
Amir M, Somakala K and Ali S: p38 MAP
kinase inhibitors as anti inflammatory agents. Mini Rev Med Chem.
13:2082–2096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okubo M and Okada Y: Destruction of the
articular cartilage in osteoarthritis. Clin Calcium. 23:1705–1713.
2013.In Japanese. PubMed/NCBI
|
|
27
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: an overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar
|
|
28
|
Liu-Bryan R: Synovium and the innate
inflammatory network in osteoarthritis progression. Curr Rheumatol
Rep. 15(323)2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fernandes JC, Martel-Pelletier J and
Pelletier JP: The role of cytokines in osteoarthritis
pathophysiology. Biorheology. 39:237–246. 2002.PubMed/NCBI
|
|
30
|
Fernandes JC, Martel-Pelletier J,
Lascau-Coman V, Moldovan F, Jovanovic D, Raynauld JP and Pelletier
JP: Collagenase-1 and collagenase-3 synthesis in normal and early
experimental osteoarthritic canine cartilage: An
immunohistochemical study. J Rheumatol. 25:1585–1594.
1998.PubMed/NCBI
|
|
31
|
Mudgett JS, Hutchinson NI, Chartrain NA,
Forsyth AJ, McDonnell J, Singer II, Bayne EK, Flanagan J, Kawka D,
Shen CF, et al: Susceptibility of stromelysin 1-deficient mice to
collagen-induced arthritis and cartilage destruction. Arthritis
Rheum. 41:110–121. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inada M, Wang Y, Byrne MH, Rahman MU,
Miyaura C, López-Otín C and Krane SM: Critical roles for
collagenase-3 (Mmp13) in development of growth plate cartilage and
in endo-chondral ossification. Proc Natl Acad Sci USA.
101:17192–17197. 2004. View Article : Google Scholar
|
|
33
|
Xue B, Yang Z, Wang X and Shi H: Omega-3
polyunsaturated fatty acids antagonize macrophage inflammation via
activation of AMPK/SIRT1 pathway. PLoS One. 7:e459902012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caughey GE, Mantzioris E, Gibson RA,
Cleland LG and James MJ: The effect on human tumor necrosis factor
alpha and interleukin 1 beta production of diets enriched in n-3
fatty acids from vegetable oil or fish oil. Am J Clin Nutr.
63:116–122. 1996.PubMed/NCBI
|
|
35
|
Trebble TM, Wootton SA, Miles EA, Mullee
M, Arden NK, Ballinger AB, Stroud MA, Burdge GC and Calder PC:
Prostaglandin E2 production and T cell function after fish-oil
supplementation: Response to antioxidant cosupplementation. Am J
Clin Nutr. 78:376–382. 2003.PubMed/NCBI
|
|
36
|
Olson MV, Liu YC, Dangi B, Paul Zimmer J,
Salem N Jr and Nauroth JM: Docosahexaenoic acid reduces
inflammation and joint destruction in mice with collagen-induced
arthritis. Inflamm Res. 62:1003–1013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sydenham E, Dangour AD and Lim WS: Omega 3
fatty acid for the prevention of cognitive decline and dementia.
Cochrane Database Syst Rev. 6:CD0053792012.PubMed/NCBI
|
|
38
|
Schulzke SM, Patole S and Simmer K:
Long-chain polyunsaturated fatty acid supplementation in preterm
infants. Cochrane Database Syst Rev. 1:CD0003752008.PubMed/NCBI
|
|
39
|
Geng Y, Valbracht J and Lotz M: Selective
activation of the mitogen-activated protein kinase subgroups c-Jun
NH2 terminal kinase and p38 by IL-1 and TNF in human articular
chon-drocytes. J Clin Invest. 98:2425–2430. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mengshol JA, Vincenti MP, Coon CI,
Barchowsky A and Brinckerhoff CE: Interleukin-1 induction of
collagenase 3 (matrix metalloproteinase 13) gene expression in
chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear
factor kappaB: Differential regulation of collagenase 1 and
collagenase 3. Arthritis Rheum. 43:801–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marcu KB, Otero M, Olivotto E, Borzi RM
and Goldring MB: NF-kappaB signaling: multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|