Introduction
A glioma is one of the most common intracranial
tumors which is characterized by invasive growth, high risk of
relapse and cellular atypia (1).
Current treatments which aim to improve the survival of patients
with glioma include surgery, radiotherapy and chemotherapy
(2). However, all of these
treatments have not yet achieved significant outcomes. The
prognosis of gliomas is far from satisfactory as it has a median
survival time of only 14.6 months and a 5-year survival rate
<9.8% (3). With the current
developments in immunohistochemistry, molecular biology, genetic
testing and treatment, many cytokines involved with the genesis and
development of glioma have been identified, which has provided a
new direction in the diagnosis and treatment of gliomas (4).
Histone methylation is an important
post-translational modification which affects many biochemical
processes including chromatin formation, translational regulation
and DNA damage repair (5). It has
been confirmed that the methylation of multiple histone H3 lysine
residues may occur. Among them, the methylation of H3K4, H3K36,
H3K79 is associated with transcriptional activation, and the
methylation of H3K9 and H3K27 plays a role transcriptional
repression (6). To date, dozens
of histone demethylases have been identified (6). These known histone demethylases are
divided into two categories: one represented by lysine-specific
histone demethylase 1 (LSD1) which requires flavin adenine
dinucleotide as cofactor in the event of a catalytic reaction; the
other comprises histone demethylases containing a Jmjc domain which
require Fe(II) and α-ketoglutarate in the demethylation process
(6).
Jumonji AT-rich interactive domain 1B (JARID1B)
belongs to the second category and it specifically removes the
trimethyl modification of H3K4 in order to inhibit the
transcription of the corresponding genes (7,8).
Previous studies have suggested that the JARID1B protein plays a
vital role in the development of breast cancer and prostate cancer,
and it is therefore considered to be an important drug target
protein (8–11). Although JARID1B expression has
been studied in some types of cancer, little is known about the
expression of JARID1B in glioma and its function in
tumorigenesis.
In the present study, we examined the roles of
JARID1B in the genesis of glioma. We demonstrated that the
expression of JARID1B was elevated in the majority of the human
glioma tissues examined. The overexpression of JARID1B
significantly enhanced cell proliferation, migration and sphere
formation in vitro. JARID1B overexpression also increased
the tumorigenicity of cells in a nude mouse xenograft model of
glioma. Moreover, JARID1B increased the protein levels of
phosphorylated (p-)Smad2. It also increased the expression of
octamer-binding transcription factor 4 (Oct4); nestin; BMI1
proto-oncogene, polycomb ring finger (Bmi-1) and CD133. Conversely,
JARID1B knockdown by short hairpin RNA (shRNA) inhibited the
proliferation and migration of glioma cells as well as sphere
formation. Moreover, the activation of p-Smad2 contributes to
JARID1B-induced oncogenic activities. These findings suggest that
high JARID1B expression is involved in the pathogenesis of
glioma.
Materials and methods
Samples, cells and antibodies
All experiments described in the present study were
performed after obtaining informed consent and were in accordance
with an Institutional Review Board protocol approved by the
Partners Human Research Committee at Dalian Medical University
(Dalian, China). The human normal brain tissue samples and glioma
tissue samples were provided by the Department of Neurosurgery at
the Second Affiliated Hospital of Dalian Medical University
(Dalian, China) between 2010 and 2012. The human glioma cell lines,
U87, T98G, SW1088 and SW1783, were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). The human glioma cell
lines, U251 (#09063001) and U373 (#08061901) were obtained from
Sigma (St. Louis, MO, USA). The cells were maintained in Minimum
Essential Medium (MEM) supplemented with 10% fetal bovine serum
(FBS) (both from Invitrogen, Carlsbad, CA, USA). Mouse monoclonal
JARID1B (ab198884), p-Smad2 (ab53100), Smad2 (ab119907),
transforming growth factor-β1 (TGF-β1; ab92486), Oct4 (ab181557),
nestin (ab11306) and Bmi-1 (ab85688) antibodies were purchased from
Abcam (Cambridge, MA, USA). Mouse monoclonal β-actin antibody was
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
LY364947, an inhibitor of TGF-βRI, was obtained from Selleckchem
(Houston, TX, USA).
Survival analysis
In order to determine the association between
JARID1B and patient survival, Kaplan-Meier survival analysis was
performed. All the carcinoma tissues which were used for
immunohistochemical analysis were randomly collected from glioma
tissue samples. Follow-up data were summarized at the end of March
2014, with a median observation time of 759 days.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the glioma tissues and
the cell lines using TRIzol reagent (Invitrogen). Reverse
transcription was performed using the ThermoScript RT-PCR system
(Invitrogen). HotStart PCR conditions were set as follows: 45 sec
at 94°C, 30 sec at 55°C, and 1 min at 72°C for 28–30 cycles (for
JARID1B) or 26 cycles [for glyceraldehyde 3-phosphate dehydrogenase
(GAPDH)]. The following primers were used: JARID1B sense,
5′-AGAGTTTCATCCCCAAAGACAA-3′ and antisense,
5′-AGTTCAGGCAGTAGGCAAAGTC-3′; GAPDH sense,
5′-TGCCTCCTGCACCACCAACT-3′ and antisense, 5′-CCC
GTTCAGCTCAGGGATGA-3′.
Western blot analysis
The samples and the cells were solubilized in
radioimmunoprecipitation assay lysis buffer [50 mmol/l Tris-HCl (pH
7.4), 1% NP-40, 0.25%Na-deoxycholate, 150 mmol/l NaCl, 1 mmol/l
EDTA, 1 mmol/l phenylmethylsulfonyl fluoride, 1 mg/ml each of
aprotinin, leupeptin and pepstatin, 1 mmol/l
Na3VO4, 1 mmol/l NaF]. The supernatants,
which contained the whole-cell protein extracts, were obtained
following centrifugation of the cell lysates at 10,000 × g for 10
min at 4°C. The protein samples (20 µg) were loaded on a
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) gel (5% stacking gel and 12% separating gel). The
proteins were then transferred to polyvinylidene difluoride
membranes (Millipore, Bedford, MA, USA). The membranes were first
probed with a primary antibody and then with a secondary antibody.
The bound antibody was detected by enhanced chemiluminescence
detection reagents (Amersham Bioscience, Piscataway, NJ, USA)
according to the manufacturer's instructions. The band intensity
was quantified with the use of ImageQuant software (Molecular
Dynamics, Sunnyvale, CA, USA).
Plasmid construction
JARID1B human cDNA was cloned as previously reported
(12). The full-length cDNAs were
subcloned into the multiple cloning sites of the pBabe plasmid, to
form the pBabe-JARID1B expression plasmids. shRNA targeting JARID1B
(sense,
5′-TCGACGCGAATCTCTCTTTGGCAAGTTCAAGAGACTTGCCAAAGAGAGATTCGTTTTTTGGAAT-3′
and antisense,
5′-CTAGATTCCAAAAAACGAATCTCTCTTTGGCAAGTCTCTTGAACTTGCCAAAGAGAGATTCGCG-3′)
was initially inserted into the SalI and XbaI sites
of pSuper plasmid, forming the pSuper-shJARID1B plasmids. The pBabe
and pSper plasmids were obtained from Addgene (Cambridge, MA,
USA).
Generation of stable cell lines
The U251 cells were transfected with the pBabe or
pBabe-JARID1B plasmid using the Lipofectamine 2000 (Invitrogen)
according to the manufacturer's instructions. The SW1783 cells were
transfected with the pSuper or pSuper-shJARID1B plasmid using
Lipofectamine 2000. Stable transfectants were obtained after
selection with puromycin (10 µg/ml; Invitrogen) for 2 weeks.
The mRNA and protein expression of JARID1B in the stable cell lines
were analyzed by RT-PCR and western blot analysis,
respectively.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
An MTT assay was performed in order to determine the
effect of overexpression or knockdown of JARID1B on the
proliferation of glioma cells. Five thousand cells were seeded into
a 96-well plate. The cells were cultured in 100 µl growth
medium. At various time-points, 20 µl of sterile MTT dye (5
mg/ml; Sigma) was added, followed by incubation at 37°C for 4 h.
The MTT solution was then replaced with dimethyl sulfoxide (DMSO)
(200 µl) and thoroughly mixed for 30 min. The absorbance at
570 nm was measured using a microplate reader (Spectra Max 340;
Molecular Devices, Sunnyvale, CA, USA) with background subtraction
at 660 nm.
Wound healing assay
Equal numbers of different cells were seeded into
6-well tissue culture plates. When 90% confluence was reached, a
single wound was created by gently removing the attached cells
using a sterile plastic pipette tip. Debris was removed by washing
the cells with serum-free medium. The migration of the cells into
the wounded area was observed and noted at different time-points.
Migration and extended protrusion of cells from the border of the
wound were visualized and images were captured using an inverted
microscope (Olympus DP27).
Assays of cell invasion and
migration
The invasion of cells was measured in Transwell
inserts (6.5 mm; Costar, Manassas, VA, USA) coated with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA) containing polycarbonate
filters with 8-µm pores as described previously (13–15). The inserts were coated with 50
µl of 1 mg/ml Matrigel matrix according to the
manufacturer's instructions. The cells (2×105) in 200
µl of serum-free medium were plated in the upper chamber
whereas 600 µl of medium with 10% FBS was added to the lower
well. After a 24 h incubation, the top cells were removed and the
number of cells on the bottom surface of the Transwell insert was
counted. The cells that had migrated to the lower surface of the
membrane were fixed in 4% paraformaldehyde and stained with 0.5%
crystal violet (Sigma). For each membrane, five random fields were
counted at x10 magnification. The mean was calculated and the data
are presented as the means ± SD from three independent experiments
performed in triplicate. The migration assay was similar to the
Matrigel invasion assay except that the Transwell insert was not
coated with Matrigel.
Sphere formation assay
Mammosphere culture was performed as previously
described by Sun et al with slight modifications (14). Briefly, single-cell suspensions
were plated in ultralow attachment 96-well plates (Costar) at
different densities of viable cells. The cells were grown in a
serum-free mammary epithelial growth medium (MEGM), supplemented
with 1:50 B27 (Invitrogen), 20 ng/ml epidermal growth factor (EGF),
20 ng/ml basic fibroblast growth factor (bFGF) (BD Biosciences) and
10 µg/ml heparin (Sigma). The numbers of spheroids were
counted after 7–10 days.
Immunohistochemical staining
Paraffin-embedded sections were deparaffinized,
blocked, and incubated with 1:200 anti-JARID1B antibody at 4°C
overnight. Horseradish peroxidase-conjugated secondary antibody
(1:500) was then added and further incubated for 1 h at room
temperature. The sections were developed using a
3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate kit
(Thermo Fisher Scientific, Waltham, MA, USA) at room temperature
for 1–5 min and then counterstained with hematoxylin (Sigma).
In vivo tumor formation assay
U251-pBabe and U251-pBabe-JARID1B cells at their
exponential growth phase were harvested and washed twice in
phosphate-buffered saline (PBS). The cells were resuspended in PBS
at a density of 5×107 cells/ml. The cell suspension (0.1
ml; 5×106 cells) was subcutaneously injected into the
right flank of 5- to 6-week-old female BALB/c-nu/nu mice
(n=3/group). The mice were purchased from the Shanghai Slac
Laboratory Animal Co. Ltd and maintained in microisolator cages.
Tumor volume (mm3) was measured every 7 days in two
dimensions using a caliper and was calculated as 0.4 × (short
length)2 × long length. The mice were humanely
euthanized by carbon dioxide when the subcutaneous tumors reached
15 mm in diameter. After euthanasia, the tumors were removed from
the mice and weighed. This experiment was conducted according to
international Guidelines for the Care and Use of Laboratory
Animals, and was approved by the Institutional Animal Care and Use
Committee of Dalian Medical University (Dalian, China) (approval
no. DMU201507).
Statistical analysis
Experimental data are presented as the means ±
standard deviation (SD). The results from different treatment
groups were compared using a two-tailed Student's t-test. P<0.05
was considered to indicate a statistically significant difference.
Statistical analysis was performed using SPSSn Win11.0 software
(SPSS, Inc., Chicago, IL, USA).
Results
JARID1B is overexpressed in human glioma
samples
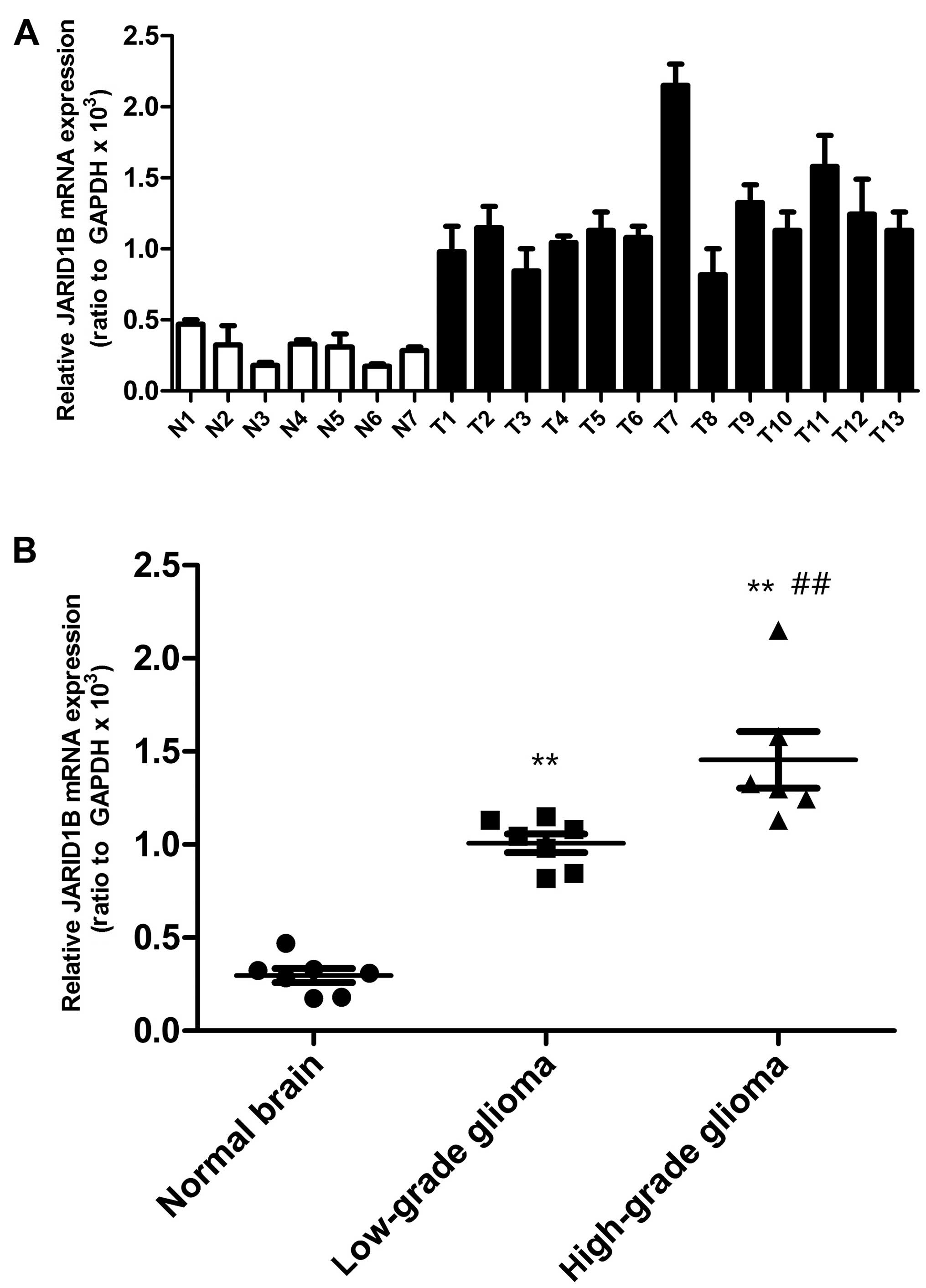
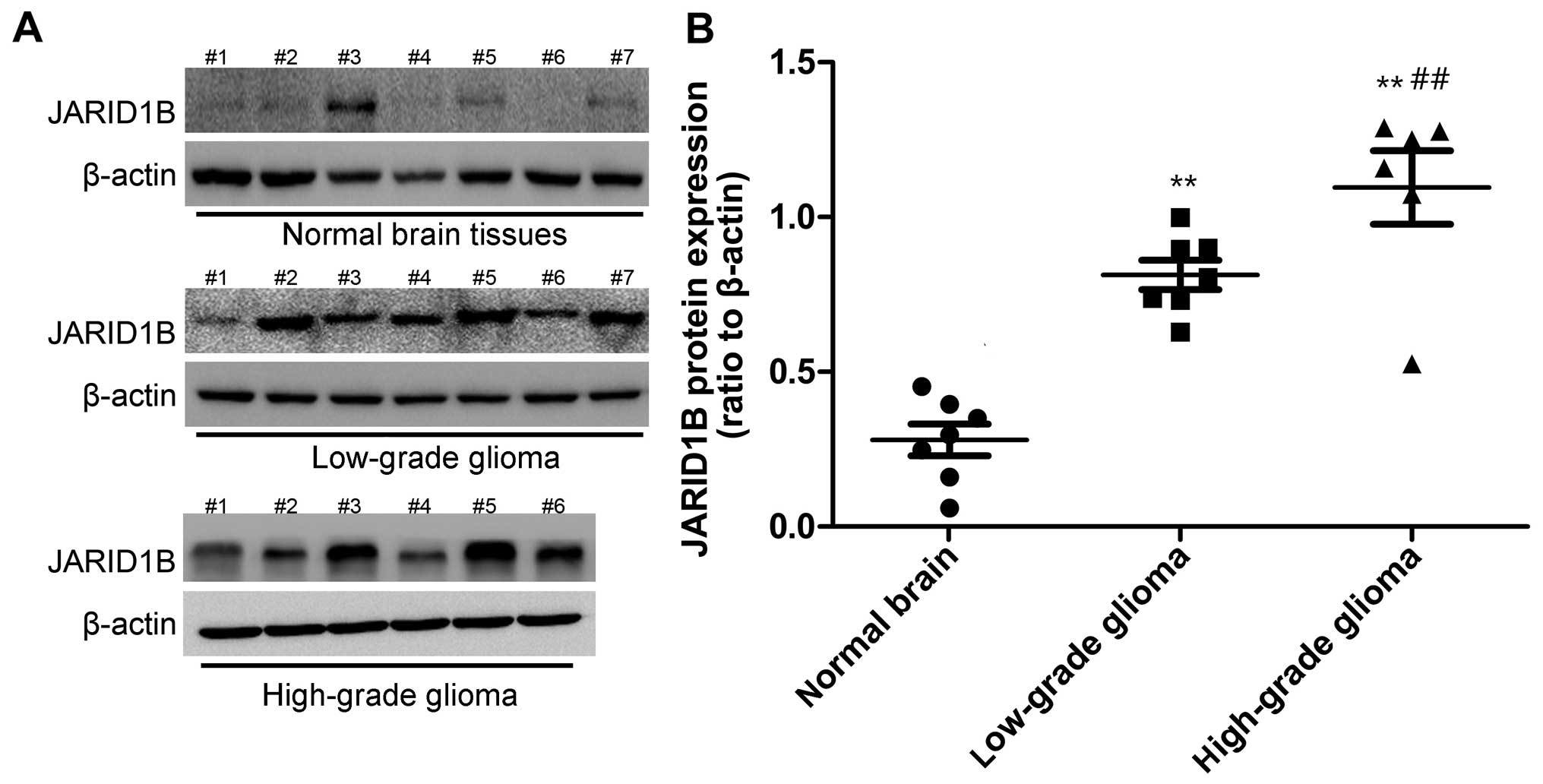
Firstly, we detected the mRNA and protein levels of
JARID1B in the seven normal brain samples and 13 glioma samples.
The expression of JARID1B in normal brain samples, seven low-grade
glioma samples (grades I–III) and six high-grade glioma tissue
samples (grade IV) was analyzed by RT-PCR (Fig. 1) and western blot analysis
(Fig. 2), respectively. Compared
with the normal brain tissues, we found that the mRNA and protein
expression of JARID1B was upregulated in seven out of seven (100%)
low-grade samples and six out of six (100%) high-grade glioma
samples. Furthermore, we also found that the expression level of
JARID1B was significantly higher in high-grade primary gliomas than
in low-grade gliomas (Figs. 1 and
2).
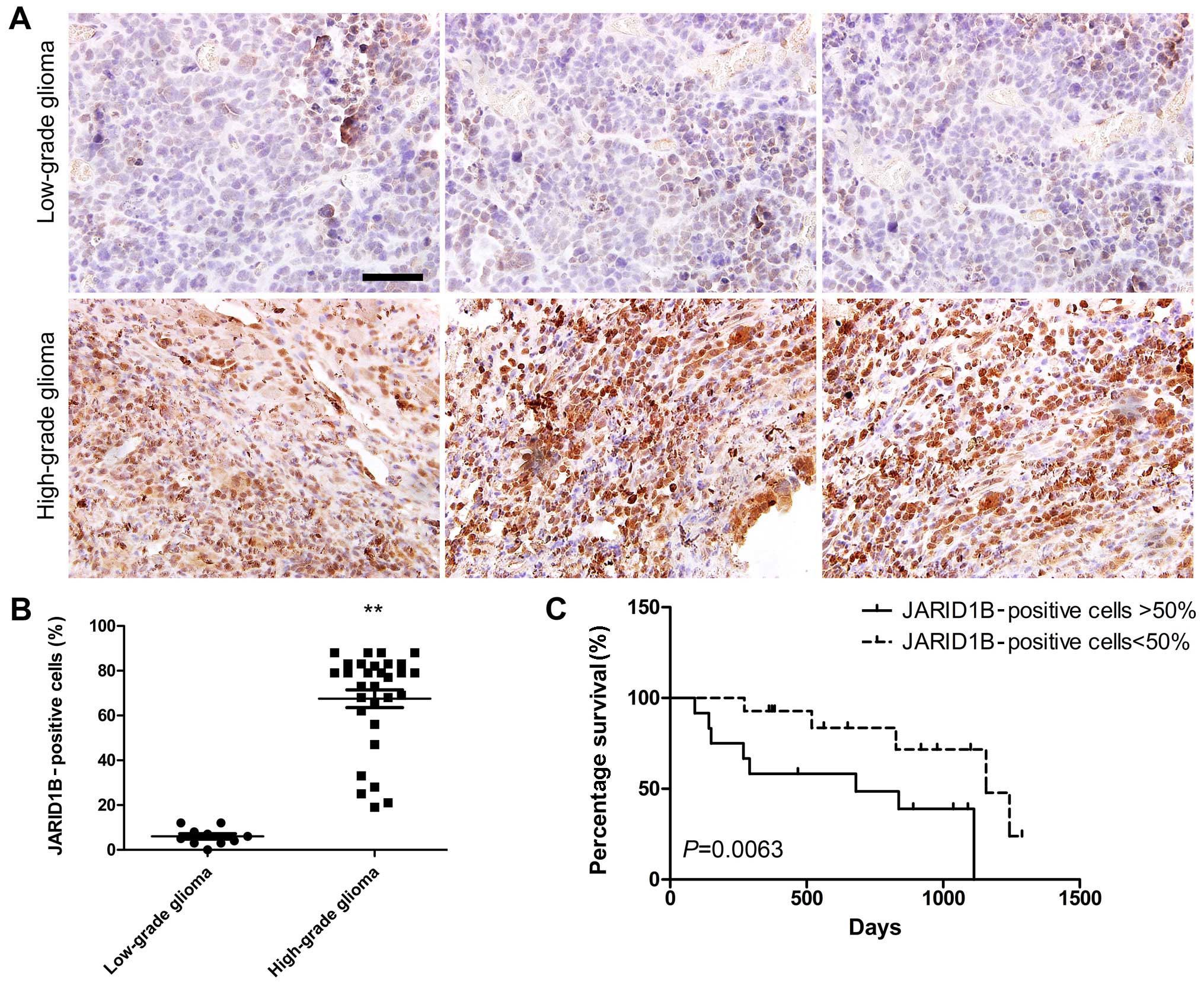
Secondly, we performed immunohistochemical staining
in order to analyze the expression of JARID1B in more samples
(low-grade glioma, 10; high-grade glioma, 30). The results showed
that JARID1B protein was expressed in all human glioma samples. The
protein was localized in the nuclei of the tumor cells (Fig. 3A). The nuclei of the majority of
the high-grade glioma cells (Fig.
3A) were more intensely stained compared with those of the
low-grade tumors (Fig. 3A). The
ratio of JARID1B-positive cells in the high-grade glioma samples
was higher than that in the low-grade glioma samples (Fig. 3B). Survival analysis revealed
significantly better survival for glioma patients with low
expression of JARID1B (<50%) than for glioma patients with high
expression of JARID1B (>50%) (Fig.
3C). These results suggest that JARID1B expression in gliomas
is not simply a reflection of the expression pattern of the tumor
cells of origin nor a consequence of therapeutic agents but rather
represents an aberrant gene activation event associated with tumor
progression.
JARID1B facilitates the proliferation of
glioma cells
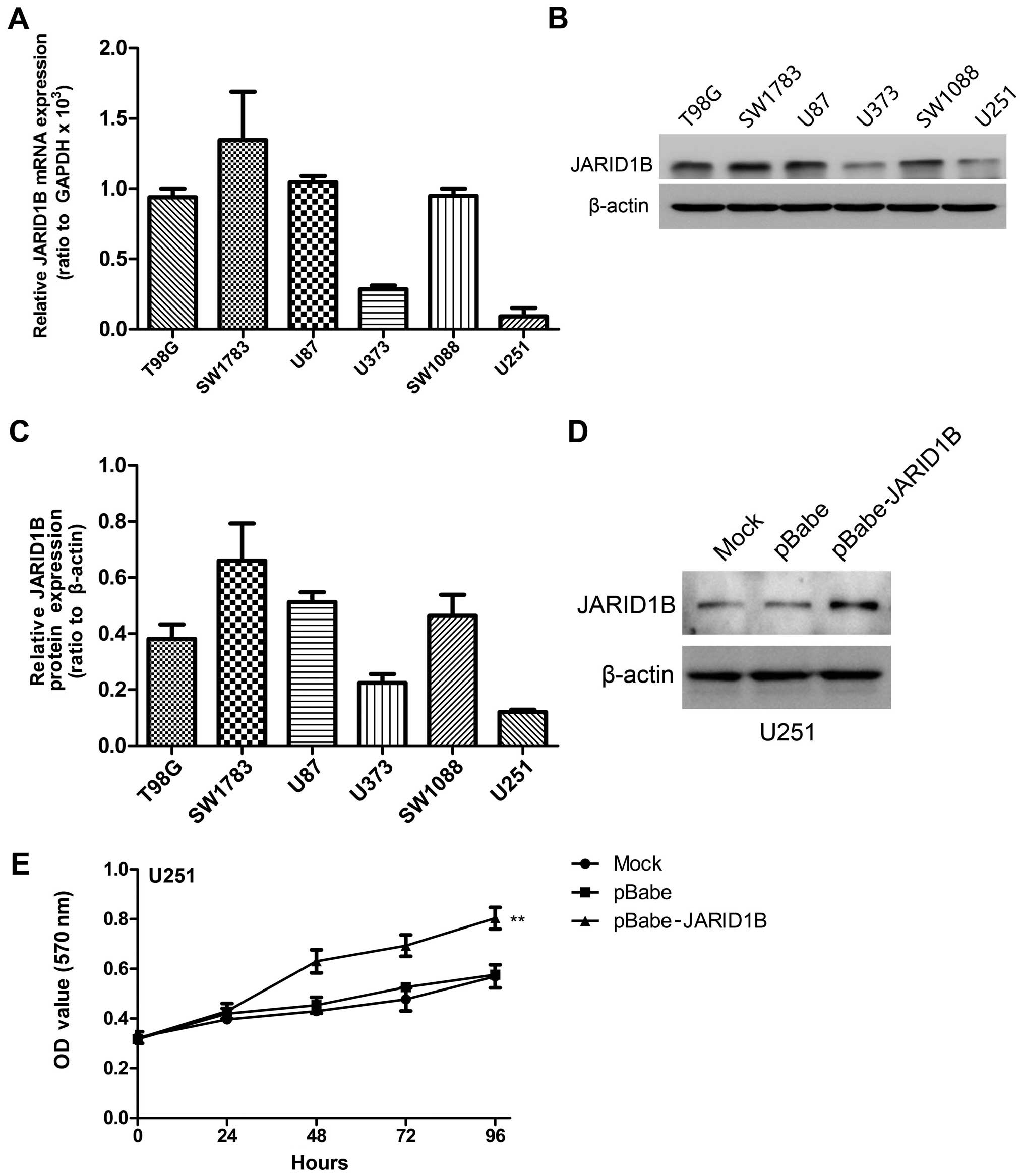
We detected the mRNA and protein expression levels
of JARID1B in the six glioma cell lines by RT-PCR and western blot
analysis, respectively (Fig.
4A–C). The results revealed high levels of JARID1B expression
in four grade IV glioblastoma cell lines (T98G, SW1783, U87 and
SW1088). By contrast, the other two low-grade glioma cell lines
(U373 and U251) expressed low levels of JARID1B.
To demonstrate the functional role of JARID1B in
glioma, we established a glioma cell clone that stably
overexpressed JARID1B protein (pBabe JARID1B) and transfected U251
cells with pBabe JARID1B in order to examine the effect on cell
proliferation. The U251 cell line was selected for use in this
experiment as a result of having the lowest expression of JARID1B
in the six glioma cell lines. Following selection with puromycin,
JARID1B expression was evaluated by western blot analysis (Fig. 4D). A high level of JARID1B was
expressed in the U251-pBabe-JARID1B cells, whereas there was lower
expression of JARID1B in the control cells transfected with the
empty pBabe plasmid (U251-pBabe) and mock U251 cells. An MTT assay
was performed to detect proliferation and the results revealed that
U251-pBabe-JARID1B displayed higher proliferation rates than the
control cells (Fig. 4E).
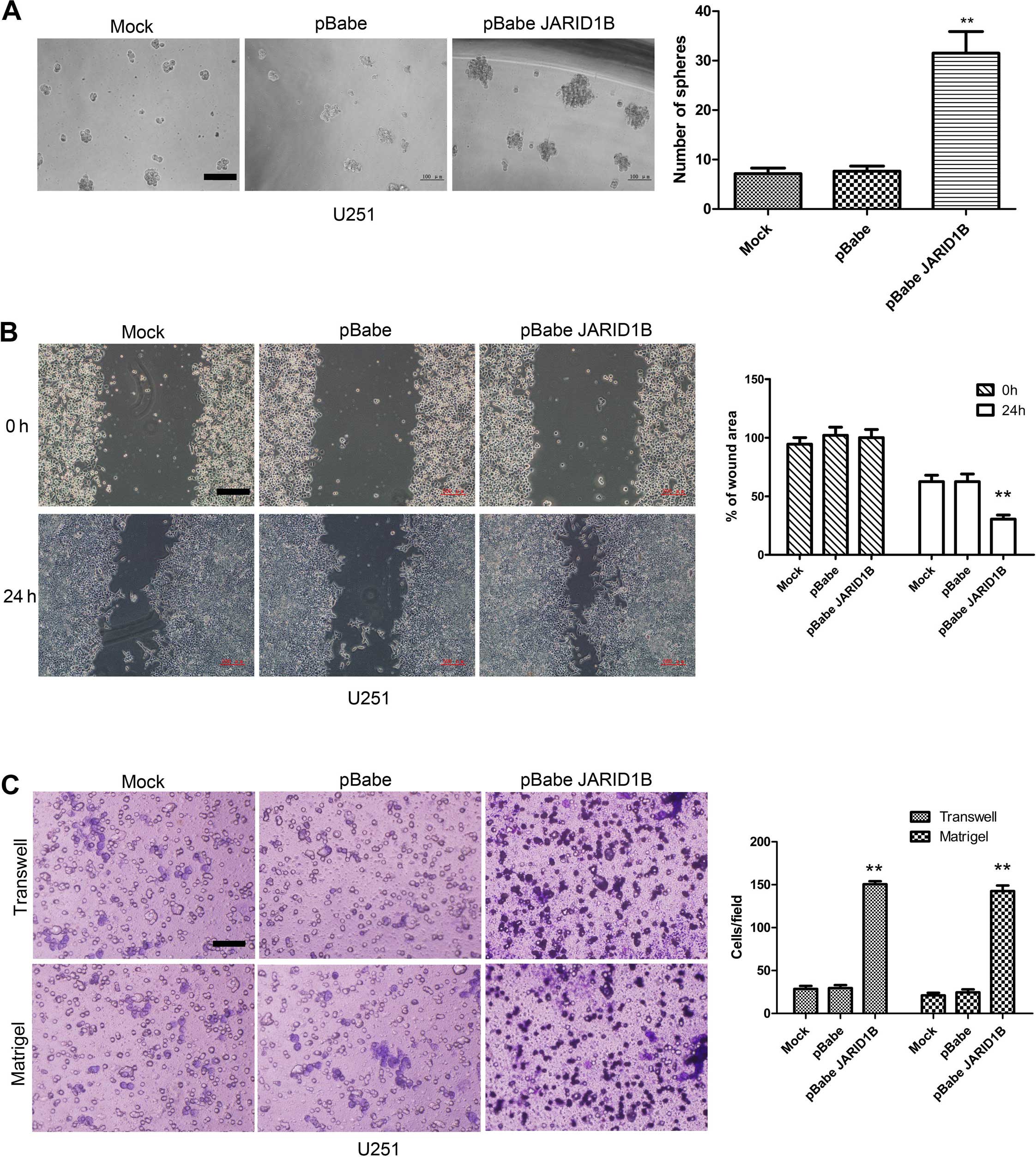
JARID1B enhances sphere formation as well
as the migratory and invasive capacities of glioma cells
To explore the role of JARID1B in cellular
transformation, the ability of the JARID1B-expressing U251 cells to
form spheres was analyzed. The parent U251 cells and U251-pBabe
cells formed only a few spheres (Fig.
5A). By comparison, the U251 cells expressing pBabe-JARID1B
showed a significant increase in the number of spheres formed. To
determine whether the overexpression of JARID1B affects cell
migratory and invasive capacities, the wound healing assay,
Transwell assay and Matrigel assay were performed using the
established U251 stable transfectants and its parent cells. The
results showed that U251-pBabe-JARID1B cells exhibited marked
increases in cell migratory activity compared to the control
U251-pBabe cells and the parent cells, suggesting that JARID1B may
also enhance the rate of migration of glioma cells (Fig. 5B). This result was confirmed by
the findings of the Transwell assay (Fig. 5C). Furthermore, U251-pBabe-JARID1B
cells exhibited a greater degree of invasion through Matrigel
(Fig. 5C).
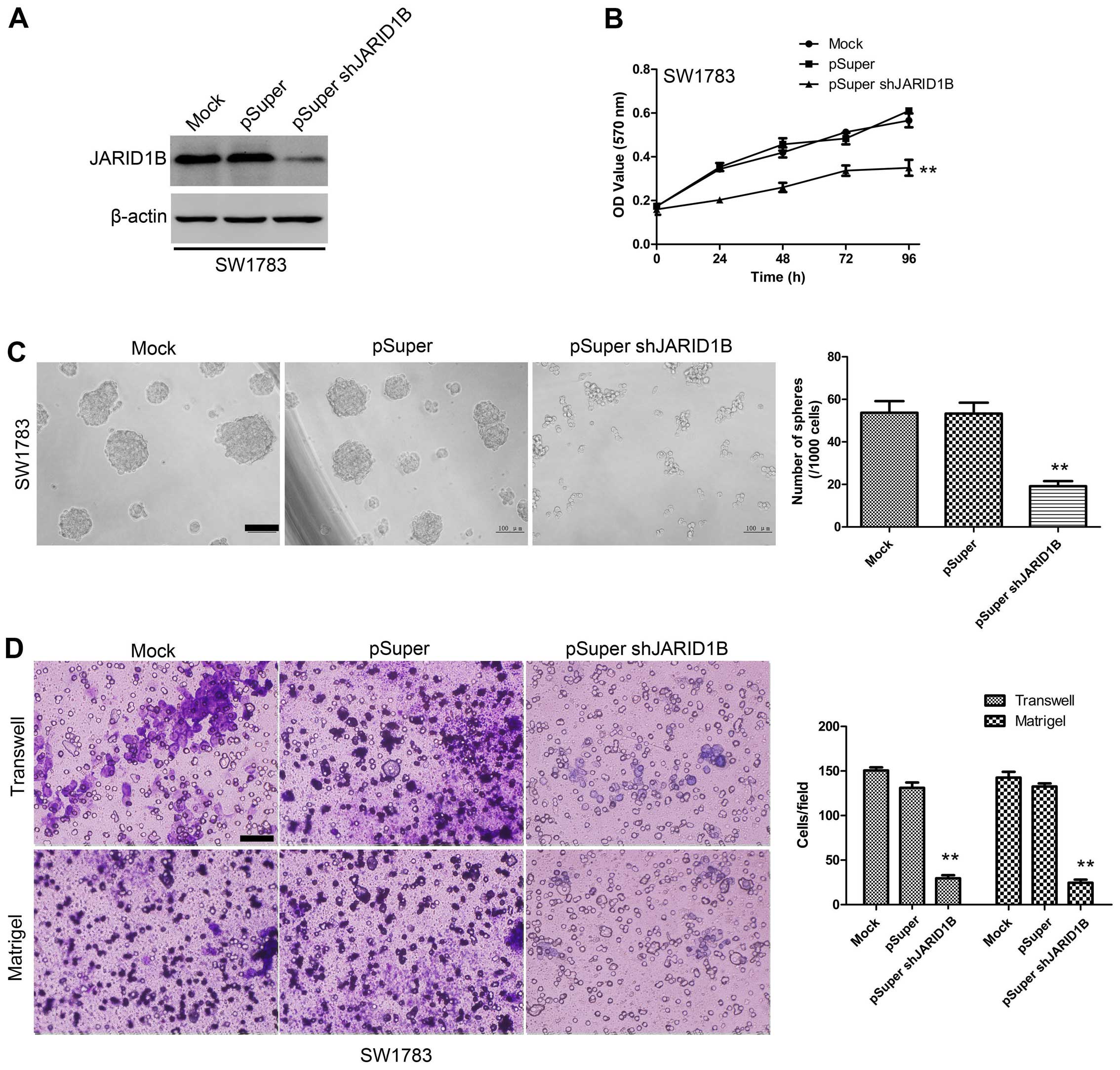
Suppression of JARID1B inhibits the
proliferation, migration and invasiveness of glioma cells as well
as sphere formation
To examine the effect of JARID1B knockdown on the
proliferation, migration and invasiveness of glioma cells as well
as sphere formation, the SW1783 human glioma cell line, a highly
tumorigenic cell line commonly used in glioma research, was
transfected with pSuper-shJARID1B or the control (pSuper). As shown
by western blot analysis (Fig.
6A), JARID1B protein expression levels were significantly
suppressed in the cells transfected with pSuper-shJARID1B. The
results of the MTT assay also revealed that the suppression of
JARID1B was associated with a decrease in cell proliferation
(Fig. 6B). The sphere formation
assay showed that the suppression of JARID1B significantly
decreased the number of spheres formed (Fig. 6C). As indicated by the Transwell
assay and Matrigel assay, knockdown of JARID1B expression
significantly inhibited the migration and invasiveness of glioma
cell (Fig. 6D). These results
provide further evidence to indicate that JARID1B is involved in
the proliferation and migration of glioma cells.
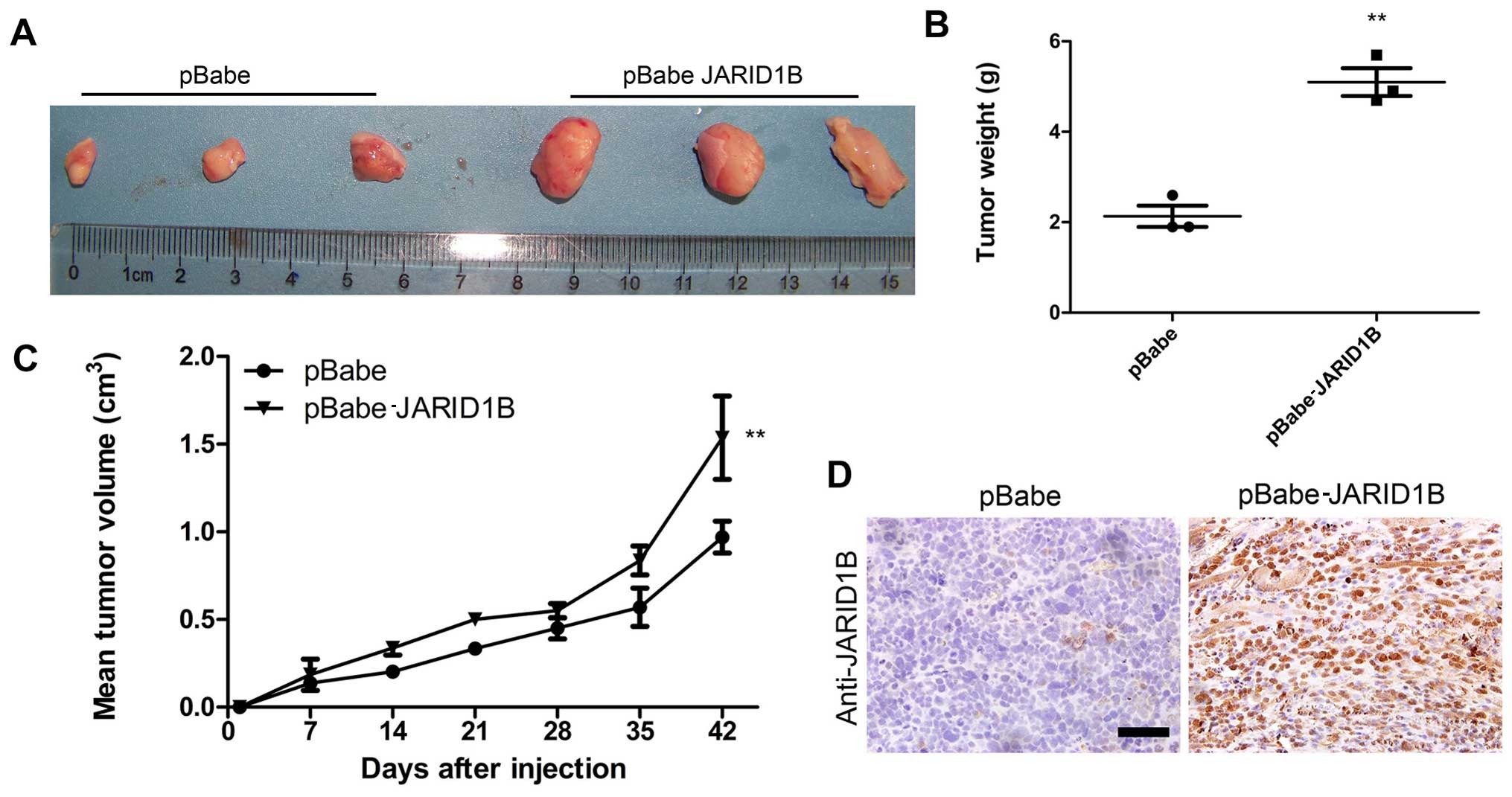
JARID1B promotes tumorigenesis in glioma
cells in vivo
To examine the function of JARID1B in vivo in
glioma carcinogenesis, a xenograft model of glioma was established
by implanting U251-pBabe and U251-pBabe-JARID1B cells
subcutaneously into the right flanks of nude mice. The tumor size
was continuously monitored weekly. The tumors of the mice injected
with U251-pBabe-JARID1B cells were significantly larger than those
of the control mice injected with U251-pBabe cells 2 weeks after
tumor cell injection (Fig. 7A).
The average weight of tumors from the mice injected with
U251-pBabe-JARID1B cells was 5 g while that of the control mice
injected with U251-pBabe cells was 2 g (Fig. 7B). Apparently, the tumor growth
rate in the mice injected with U251-pBabe-JARID1B cells was greater
than that in the control mice and this was confirmed by measuring
the mean tumor volume 42 days after injection (Fig. 7C). Furthermore, JARID1B expression
in the xenograft was confirmed by immunohistochemical analysis
(Fig. 7D).
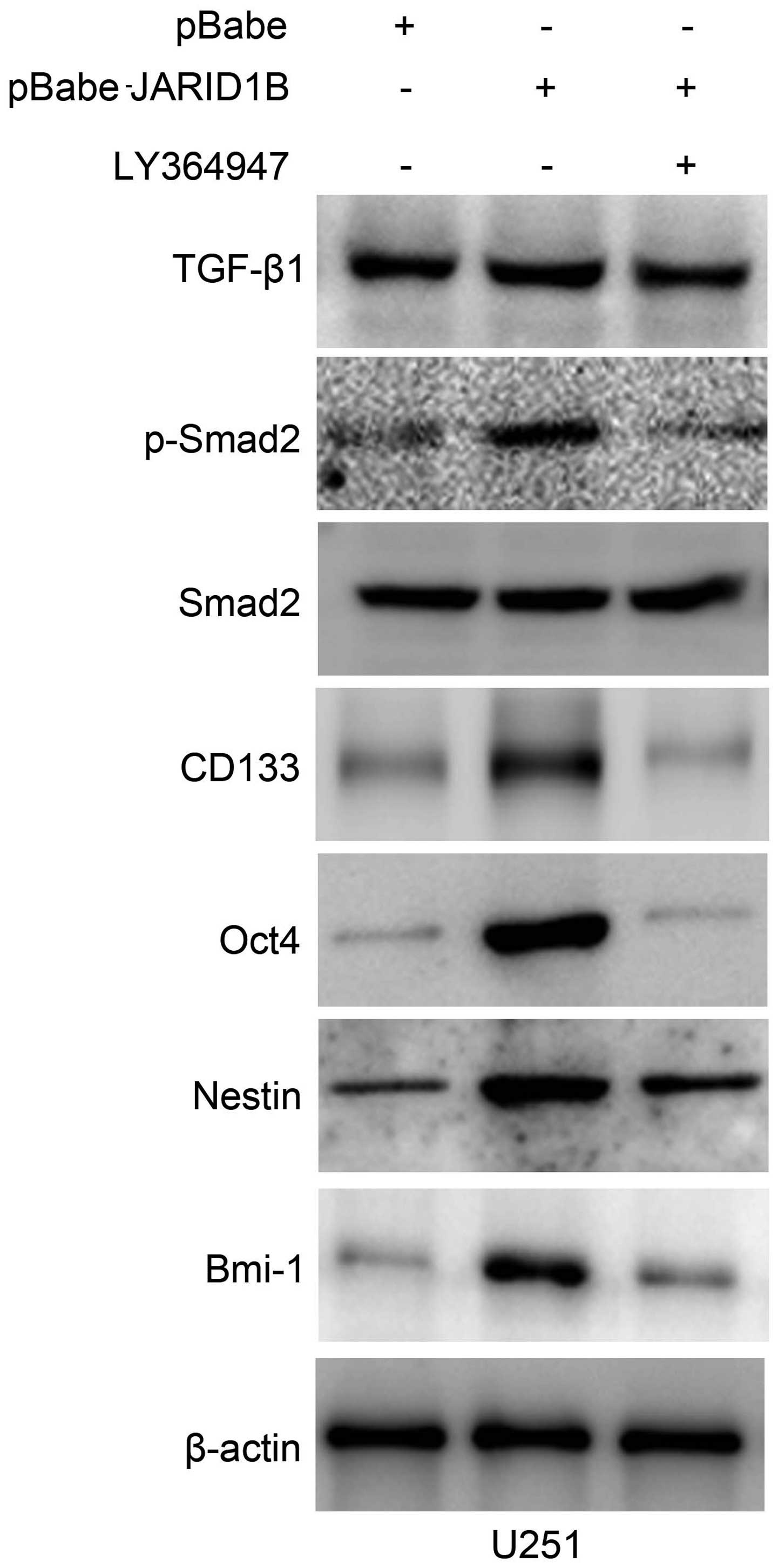
Activation of p-Smad2 contributes to
JARID1B-induced oncogenic activities
The overexpression of JARID1B in the U251 cells
significantly increased the expression of p-Smad2 (Fig. 8A). In comparison, the expression
of TGF-β and Smad2 were unchanged. Significantly, we also found
that the expression of cancer stem cell-related markers, namely
CD133, Oct4, nestin and Bmi-1 were induced by JARID1B in the
U251-pBabe-JARID1B cells, compared with that in the parental cells
and U251-pBabe cells (Fig. 8A).
Inversely, the knockdown of JARID1B in the SW1783 cells
significantly decreased the expression of p-Smad2, CD133, Oct4,
nestin and Bmi-1 (Fig. 8B)
whereas it did not affect the expression of TGF-β and Smad2. As for
p-Smad2, a well-known regulator of cancer stem cells, we examined
whether the activation of p-Smad2 contributes to JARID1B-induced
oncogenic activities. The suppression of p-Smad2 receptor signaling
using the inhibitor LY364947 suppressed Smad2 phosphorylation,
without affecting the expression of Smad2 in the U251-pBabe-JARID1B
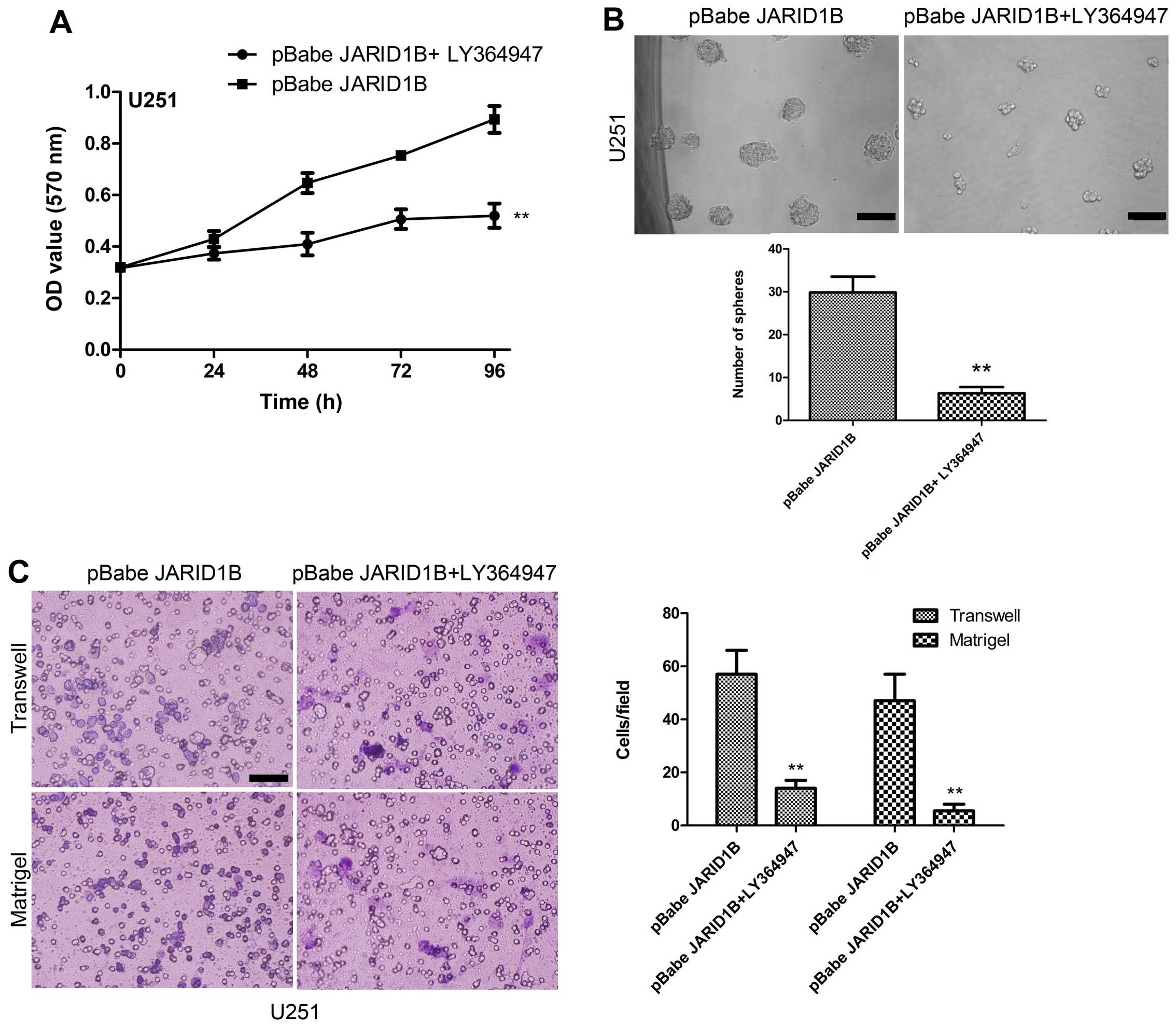
cells (Fig. 9). Treatment with
LY364947, an inhibitor of p-Smad2 receptor signaling, suppressed
the expression of CD133, Oct4, nestin and Bmi-1 in the
U251-pBabe-JARID1B cells (Fig.
9). The suppression of p-Smad2 signaling also reduced the
migration, invasiveness, proliferation of U251-pBabe-JARID1B cells
as well as sphere formation (Fig.
10). This indicates that the induction of p-Smad2 expression by
JARID1B activates a critical signaling pathway implicated in the
formation of gliomas.
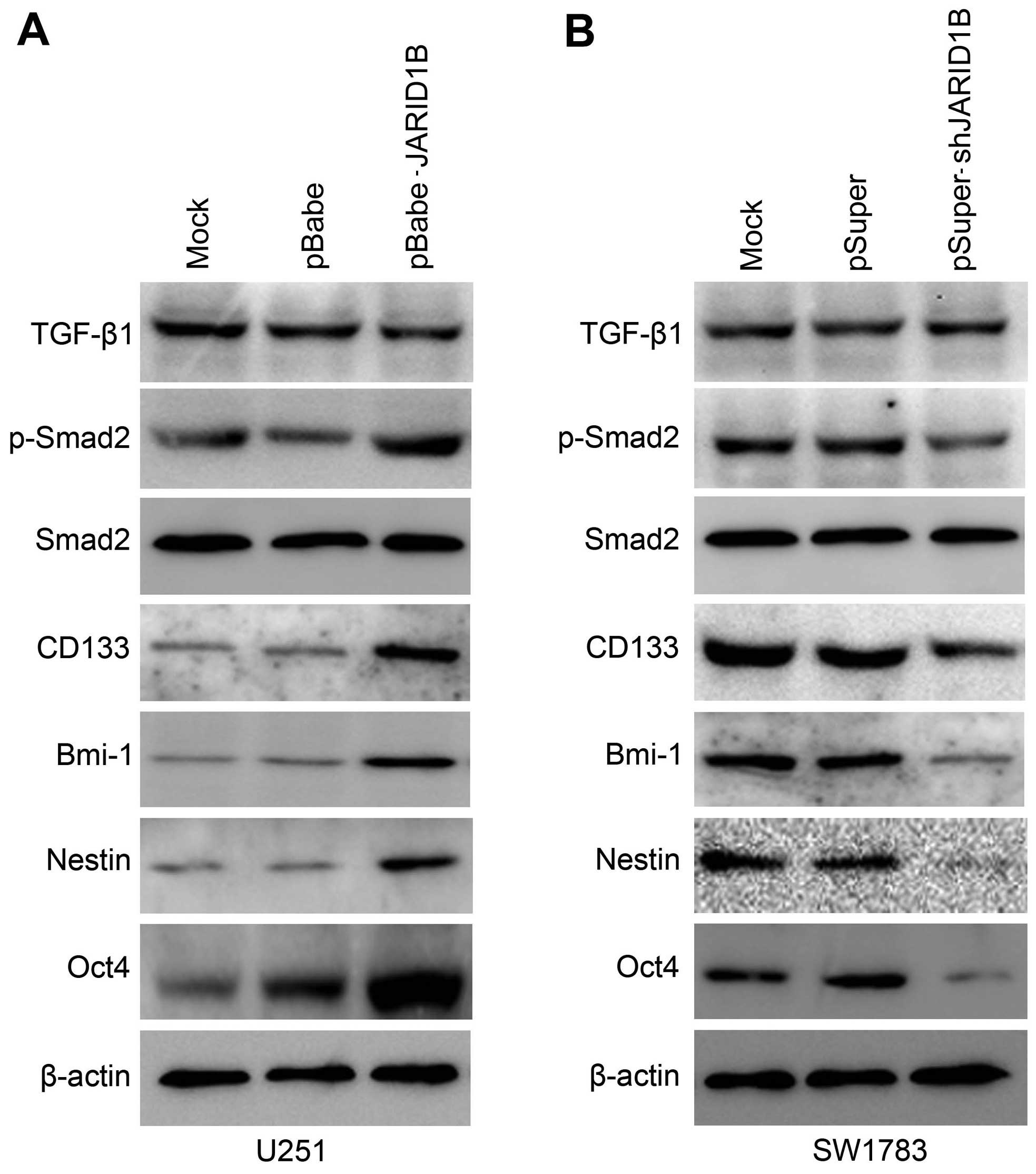 | Figure 8Jumonji AT-rich interactive domain 1B
(JARID1B) upregulates the expression of phosphorylated (p-)SMAD
family member 2 (Smad2) and cancer stem cell-related markers in
glioma cells. (A) Western blot analysis of transforming growth
factor-β1 (TGF-β1), p-Smad2, Smad2, CD133, octamer-binding
transcription factor 4 (Oct4), nestin and BMI1 proto-oncogene,
polycomb ring finger (Bmi-1) protein levels in U251-pBabe-JARID1B
and its control cells. (B) Western blot analysis of TGF-β1,
p-Smad2, Smad2, CD133, Oct4, nestin and Bmi-1 protein levels in
SW1783-pSuper-shJARID1B and its control cells. β-actin was used as
the internal control for western blot analysis. |
Discussion
In the present study, we examined the role of
JARID1B in glioma. Compared with the normal brain tissues, we
detected raised levels of JARID1B in the majority of the glioma
tissues. It was found that the overexpression of JARID1B promoted
the proliferation, migration and invasiveness of glioma cells as
well as sphere formation, and accelerated tumor growth in nude mice
in vivo. Conversely, the knockdown of JARID1B suppressed the
proliferation, migration and invasiveness of glioma cells as well
as sphere formation. We also demonstrated that the function of
JARID1B was associated with p-Smad2. Taken together, these findings
suggest that JARID1B is involved in gliomagenesis.
JARID1B belongs to the family of histone
demethylases, and it is capable of specifically removing the
trimethyl modification of H3K4 in order to inhibit the
transcription of the corresponding genes (16–18). Previous studies have suggested
that JARID1B protein plays a vital role in the genesis of breast
cancer and prostate cancer, and it is therefore considered to be an
important drug target protein (8–10).
Although the expression of JARID1B has been studied in some types
of cancer, little is known about the expression of JARID1B in
gliomas and its function in tumorigenesis. Thus, we examined the
expression of JARID1B in glioma. We found that compared with the
normal brain tissues, JARID1B was overexpressed in the majority of
the high-grade glioma tissues examined.
Furthermore, we found that the overexpression of
JARID1B increased the proliferation of glioblastoma cells and
promoted tumorigenesis in vitro and in vivo, whereas
the knockdown of JARID1B expression by shRNA produced an inhibitory
effect on tumorigenesis. Notably, we found that the overexpression
of JARID1B enhanced cell migration and invasion, whereas the
knockdown of JARID1B produced an opposite effect. To the best of
our knowledge, this is the first study to demonstrate that JARID1B
regulates the proliferation and migratory abilities of glioma
cells.
p-Smad2 is a member of the TGF-β superfamily which
is involved in signal transduction (19). Signal pathways regulated by TGF-β
include the Smad-dependent and Smad-independent pathways (20). TGF-β1/Smad is a classic signal
transduction pathway. Its basic signal transduction process is:
TGF-β extracellularly conjoins TβRII to activate TβR, then TβRI
phosphorylates Smad2/3, and finally p-Smad2/3 in combination with
Smad4 enters the cell nucleus and binds to DNA in order to regulate
the transcription of many genes (20). p-Smad2 signaling is involved in
regulating the proliferation, differentiation and survival/or
apoptosis of many types of cells, including glioma cells (21,22). The increased expression of p-Smad2
correlates with the degree of malignancy of human gliomas (23). p-Smad2 may contribute to tumor
pathogenesis by directly supporting tumor growth, the self-renewal
of glioma initiating stem cells and inhibiting antitumor immunity
(21). Therefore, we hypothesized
that JARID1B may also modulate p-Smad2 expression in glioma cells.
In this study, we found that the overexpression of JARID1B
significantly increased the expression of p-Smad2 whereas the
knockdown of JARID1B produced the opposite effect. We also found
that JARID1B regulated the expression of cancer stem cell-related
markers, namely CD133, nestin, Oct4 and Bmi-1. Suppression of the
p-Smad2 signaling pathway by LY364947 inhibited the inductive
effect of JARID1B on the expression of the cancer stem cell-related
markers. Moreover, the suppression of p-Smad2 signaling also
reduced the migration, invasiveness and proliferation of
U251-pBabe-JARID1B cells as well as sphere formation. Thus,
aberrant JARID1B expression in gliomas may enhance the oncogenic
effects of the activated p-Smad2 signaling pathway. However,
further investigation of the mechanisms involved in the expression
profile of other genes is warranted.
In conclusion, we have demonstrated for the first
time, to the best of our knowledge, that JARID1B is overexpressed
in glioma tissues, and the overexpression of JARID1B increased the
growth and migration of glioma cells in vitro and enhanced
glioma tumorigenesis in vivo. Taken together, these findings
suggest that JARID1B is potentially an important molecular target
for the design of novel anti-glioma therapies.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81301168).
References
|
1
|
Opocher E, Kremer LC, Da Dalt L, van de
Wetering MD, Viscardi E, Caron HN and Perilongo G: Prognostic
factors for progression of childhood optic pathway glioma: a
systematic review. Eur J Cancer. 42:1807–1816. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weiler M and Wick W: Molecular predictors
of outcome in low-grade glioma. Curr Opin Neurol. 25:767–773. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen R, Ravindra VM, Cohen AL, Jensen RL,
Salzman KL, Prescot AP and Colman H: Molecular features assisting
in diagnosis, surgery, and treatment decision making in low-grade
gliomas. Neurosurg Focus. 38:E22015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seward DJ, Cubberley G, Kim S, Schonewald
M, Zhang L, Tripet B and Bentley DL: Demethylation of trimethylated
histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat Struct Mol
Biol. 14:240–242. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wynder C, Stalker L and Doughty ML: Role
of H3K4 demethylases in complex neurodevelopmental diseases.
Epigenomics. 2:407–418. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto S, Wu Z, Russnes HG, Takagi S,
Peluffo G, Vaske C, Zhao X, Moen Vollan HK, Maruyama R, Ekram MB,
et al: JARID1B is a luminal lineage-driving oncogene in breast
cancer. Cancer Cell. 25:762–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang Y, Zhu Z, Han G, Ye X, Xu B, Peng Z,
Ma Y, Yu Y, Lin H, Chen AP and Chen CD: JARID1B is a histone H3
lysine 4 demethylase up-regulated in prostate cancer. Proc Natl
Acad Sci USA. 104:19226–19231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Q, Shi L, Gui B, Yu W, Wang J, Zhang D,
Han X, Yao Z and Shang Y: Binding of the JmjC demethylase JARID1B
to LSD1/NuRD suppresses angiogenesis and metastasis in breast
cancer cells by repressing chemokine CCL14. Cancer Res.
71:6899–6908. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitra D, Das PM, Huynh FC and Jones FE:
Jumonji/ARID1 B (JARID1B) protein promotes breast tumor cell cycle
progression through epigenetic repression of microRNA let-7e. J
Biol Chem. 286:40531–40535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Catchpole S, Spencer-Dene B, Hall D,
Santangelo S, Rosewell I, Guenatri M, Beatson R, Scibetta AG,
Burchell JM and Taylor-Papadimitriou J: PLU-1/JARID1B/KDM5B is
required for embryonic survival and contributes to cell
proliferation in the mammary gland and in ER+ breast
cancer cells. Int J Oncol. 38:1267–1277. 2011.PubMed/NCBI
|
|
12
|
Wang Y, Wen M, Kwon Y, Xu Y, Liu Y, Zhang
P, He X, Wang Q, Huang Y, Jen KY, et al: CUL4A induces
epithelial-mesenchymal transition and promotes cancer metastasis by
regulating ZEB1 expression. Cancer Res. 74:520–531. 2014.
View Article : Google Scholar :
|
|
13
|
Wang Y, Zhang P, Liu Z, Wang Q, Wen M,
Wang Y, Yuan H, Mao JH and Wei G: CUL4A overexpression enhances
lung tumor growth and sensitizes lung cancer cells to erlotinib via
transcriptional regulation of EGFR. Mol Cancer. 13:2522014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G
and Wei J: Estrogen promotes stemness and invasiveness of
ER-positive breast cancer cells through Gli1 activation. Mol
Cancer. 13:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Q, Wang Y, Zhang Y, Zhang Y and Xiao
W: Involvement of urokinase in cigarette smoke extract-induced
epithelial-mesenchymal transition in human small airway epithelial
cells. Lab Invest. 95:469–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kano Y, Konno M, Ohta K, Haraguchi N,
Nishikawa S, Kagawa Y, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T,
et al: Jumonji/Arid1b (Jarid1b) protein modulates human esophageal
cancer cell growth. Mol Clin Oncol. 1:753–757. 2013.
|
|
17
|
Roesch A, Vultur A, Bogeski I, Wang H,
Zimmermann KM, Speicher D, Körbel C, Laschke MW, Gimotty PA,
Philipp SE, et al: Overcoming intrinsic multidrug resistance in
melanoma by blocking the mitochondrial respiratory chain of
slow-cycling JARID1B(high) cells. Cancer Cell. 23:811–825. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albert M, Schmitz SU, Kooistra SM,
Malatesta M, Morales Torres C, Rekling JC, Johansen JV, Abarrategui
I and Helin K: The histone demethylase Jarid1b ensures faithful
mouse development by protecting developmental genes from aberrant
H3K4me3. PLoS Genet. 9:e10034612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parvani JG, Taylor MA and Schiemann WP:
Noncanonical TGF-β signaling during mammary tumorigenesis. J
Mammary Gland Biol Neoplasia. 16:127–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HM, Haraguchi N, Ishii H, Ohkuma M,
Okano M, Mimori K, Eguchi H, Yamamoto H, Nagano H, Sekimoto M, et
al: Increased CD13 expression reduces reactive oxygen species,
promoting survival of liver cancer stem cells via an
epithelial-mesenchymal transition-like phenomenon. Ann Surg Oncol.
19(Suppl 3): S539–S548. 2012. View Article : Google Scholar
|
|
22
|
Grzmil M, Morin P Jr, Lino MM, Merlo A,
Frank S, Wang Y, Moncayo G and Hemmings BA: MAP kinase-interacting
kinase 1 regulates SMAD2-dependent TGF-β signaling pathway in human
glioblastoma. Cancer Res. 71:2392–2402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun J, Liu SZ, Lin Y, Cao XP and Liu JM:
TGF-β promotes glioma cell growth via activating Nodal expression
through Smad and ERK1/2 pathways. Biochem Biophys Res Commun.
443:1066–1072. 2014. View Article : Google Scholar
|