Introduction
It is well known that tyrosine hydroxylase (TH), a
rate-limiting enzyme for the synthesis of catecholamines (CAs), is
expressed in neurons and endocrine cells. Over the years, studies
have demonstrated that immunocytes can synthesize and secrete CAs,
including dopamine (DA), norepinephrine (NE) and epinephrine (E)
(1–6). Therefore, the role of CAs is likely
to be more complex than previously thought. Endogenous CAs in
immunocompetent cells modulate many functions of the immune system
(7,8). We have also previously demonstrated
that lymphocytes express TH, and that the three types of CAs exist
in lymphocytes (9,10). Subsequently, we demonstrated that
lymphocyte-derived endogenous CAs inhibit lymphocyte proliferation
and interleukin (IL)-2 production, and accelerate lymphocyte
apoptosis (11,12). However, the role of endogenous CAs
synthesized and secreted by lymphocytes in immunomodulation and the
relevant mechanisms require further investigation.
Classically, CD4+ T cells can be
functionally differentiated into T helper (Th)1 cells and Th2
cells. Th1 cells are mainly involved in the cellular immune
response through the secretion of cytokines, such as IL-2,
interferon-γ (IFN-γ) and tumor necrosis factor (TNF). Th2 cells
mainly mediate the humoral immune response and exert
anti-inflammatory effects through the secretion of IL-4, IL-5 and
IL-10 (13). It has been reported
that a high expression of CD26 (also known as dipeptidyl peptidase
IV) correlates with the production of Th1-like cytokines (14), whereas Del Prete et al
(15) suggested that human
CD4+ Th2 cell clones express a higher amount of CD30, a
member of the TNF receptor superfamily. Moreover, since there is a
fine regulation of Th1- and Th2-type immune responses to maintain
the normal immune balance in the body (16), a Th1/Th2 imbalance is associated
with the onset and progression of a number of autoimmune diseases,
microbial infection and tumors (17–20). It has been reported that exogenous
CAs can inhibit the secretion of pro-inflammatory cytokines and
promote the release of anti-inflammatory cytokines (21). Although there is recent
pharmacological evidence indicating that lymphocyte-derived CAs
induce a shift in the Th1/Th2 balance toward Th2 polarization, that
and α1-adrenoceptors (ARs) and β2-ARs are
involved in mediating this effect (22,23), less is known about the role of the
AR-coupled signaling pathway.
The present study was therefore undertaken to
further explore the effects of lymphocyte-derived CAs on the
differentiation and function of Th cells by inducing the
overexpression of the TH gene in lymphocytes. We also aimed to
elucidate the possible signaling pathway mediating these effects.
These findings may provide a better understanding of the role of
endogenous CAs in the neuroimmune network and may lead to
advancements in the prevention and treatment of some autoimmune
diseases.
Materials and methods
Cell separation and culture
The mesenteric lymph nodes of ICR mice (Center of
Experimental Animals, Nantong University, Nantong, China) were
harvested by celiotomy and single cell suspensions were obtained by
gently squeezing the lymph nodes. The cells were then washed twice
and resuspended in RPMI-1640 medium supplemented with 10%
heat-inactivated calf serum, 2.5×10−2 M HEPES,
1×10−3 M sodium pyruvate, 5×10−5 M
mercaptoethanol and antibiotics (100 U/ml penicillin, 100 U/ml
streptomycin), at a final concentration of 1×106
cells/ml. Concanavalin A (Con A; 5 µg/ml) was added to the
suspensions in the presence or absence of NE to induce cell
proliferation. The cultures were then incubated at 37°C in a moist
atmosphere with 5% CO2 for 48 h. The animal experiments
carried out in this study were in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals and were approved by the Institutional Animal Care and Use
Committee of Nantong University.
Construction of recombinant plasmid
The pEGFP-N1 plasmid was kindly provided by the
Department of Immunology of School of Medicine, Nantong University.
Standard molecular biology techniques were used for the
construction of the pEGFP-N1-TH recombinant plasmid. The PCR
product of mouse TH fragment was restricted and inserted between
the XhoI and HindIII restriction sites in the
pEGFP-N1-basic vector. The forward and reverse primers used to
amplify this fragment were 5′-CCCAAGCTTATGCCCACCCCCAGCGCCTC-3′ and
5′-CCGCTCGAGTTAGCTAATGGCACTCAGTG-3′, respectively (NM_009377.1).
The plasmid was verified by restriction enzyme digestion by
XhoI and HindIII and DNA sequencing.
Transfection with TH overexpression
vector
The constructed plasmid, pEGFP-N1-TH (pEGFP-N1-TH
group), or the empty vector, pEGFP-N1 (mock group), were
transfected into the lymphocytes using nucleofection technology
(Amaxa Biosystems, Koln, Germany) according to the manufacturer's
instructions. Briefly, following incubation with Con A for 48 h,
the lymphocytes were resuspended in 100 µl of T cell
nucleofector solution. The plasmid (4 µg) was added to 100
µl of 5×106 lymphocyte suspension. The mixtures
were subsequently transferred to an electroporation cuvette with
aluminum electrodes and placed in the nucleofection device (Amaxa
Biosystems). The nucleofection of these cells were accomplished
using the X-001 program, and the samples were immediately
transferred to 12-well plates containing 2 ml pre-warmed medium.
The transfection efficiency was determined by the
fluorescence-positive cells under a fluorescence microscope (Leica,
Wetzlar, Germany). The number of GFP-labeled cells was
approximately 50–70% (data not shown).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the lymph node cells
which had been transfected and incubated for 48 h, with TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. This process was followed by reverse
transcription into cDNA using M-MLV reverse transcriptase (Promega,
Madison, WI, USA). Real-time PCR for the detection of TH was
performed in a Rotor-Gene 3000 Real-Time Cycler (Corbett Research,
Sydney, Australia) and the detection was made by measuring the
binding of the fluorescence dye SYBR-Green I (Molecular Probe,
Invitrogen, Eugene, OR, USA) to double-stranded DNA. Each 20
µl of reaction mixture contained 2 µl of cDNA, 2
µl PCR buffer, 3.0 mM MgCl2, 0.2 mM of each dNTP,
0.2 µM of each pair of oligonucleotide primers, and 1 unit
TaqDNA polymerase. The sequences of oligonucleotide primers for the
amplification of specific TH gene (130 bp, NM_009377.1) were
5′-CGGAAGCTGATTGCAGAGAT-3′ (sense) and 5′-GGGTAGCATAGAGGCCCTTC-3′
(antisense). The reaction procedures were as follows: an initial
step at 95°C for 5 min, 40 cycles of 94°C for 15 sec, 60°C for 20
sec and 72°C for 20 sec. Analysis was performed by the standard
curve method. To verify the specificity of the amplification
reaction, melting curve analysis was performed. The relative
expression level of TH was expressed as a ratio relative to the
value of β-actin, the housekeeping gene. The primer sequences for
the β-actin gene (218 bp, NM_007393) were 5′-CTGTCCCTGTATGCCTCTG-3′
(sense) and 5′-ATGTCACGCACGATTTCC-3′ (antisense).
Western blot analysis
Total protein was extracted from the lymph node
cells which were transfected with the plasmid pEGFP-N1-TH or
treated with NE (10−5 M; Sigma-Aldrich, St. Louis, MO,
USA) for 48 h. The cells were homogenized in lysis buffer and the
supernatants were collected by centrifugation at 4°C at 12,000 × g
for 15 min. The supernatants containing 20 µg of total
cellular protein were mixed with loading buffer and boiled for 10
min. The proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto PVDF membranes (Pall, Port Washington, NY, USA)
using a wet transfer apparatus. After blocking non-specific binding
with 5% (w/v) non-fat dry milk, the membranes were probed with
mouse monoclonal antibodies specific for TH (MAB318, 1:500;
Millipore Co., Billerica, MA, USA), or with rabbit monoclonal
antibodies specific for p38 mitogen-activated protein kinase (MAPK;
4511P, 1:1,000) and p-CREB (9198S, 1:1,000) (both from Cell
Signaling Technology Inc., Beverly, MA, USA) at 4°C overnight,
followed by incubation with a fluorescent-conjugated affinity
purified goat anti-mouse IgG (610-130-121) or goat anti-rabbit IgG
(611-132-122) (1:5,000; Rockland Immunochemicals Inc.,
Gilbertsville, PA, USA) for 1 h at room temperature and
visualization by a Odyssey laser scanning system (LI-COR
Biosciences, Lincoln, NE, USA). The blots were reprobed with
monoclonal mouse anti-β-actin antibody (ab6276, 1:1,000;
Sigma-Aldrich) to confirm equal protein loading. The molecular
weight and relative quantity of the protein bands were determined
by an image analysis system (Odyssey 3.0 software).
High performance liquid chromatography
with electrochemical detection (HPLC-ED) assay of CAs
For the detection of CAs in the cultured lymphocytes
which were transfected with the TH overexpression vector, the
cultures were centrifuged and the cell pellet was resuspended in
0.15 ml of 0.1 N perchloric acid and disrupted by ultrasonication.
The mixture was then supplemented with 0.15 ml of 0.8 N perchloric
acid and centrifuged for 15 min at 12,000 × g at 4°C. The
supernatants were recovered, filtered and stored at −80°C until
analysis. For the detection of CAs in the supernatants of the
lymphocyte cultures, the culture supernatants were collected after
the cultures were centrifuged. To prevent electrochemical
interference in the HPLC-ED by unidentified compounds, CAs in the
cell supernatants were subjected to alumina-extraction. Briefly, 2
ml of cell supernatant were added to 40 mg of alumina, and 2 ml of
0.5 M Tris buffer, and gently shaken for 15 min at room temperature
in the dark. The samples were centrifuged at 1,500 x g for 5 min.
The supernatant was discarded and the alumina was washed 3 times
with cold deionized water. Finally, CAs was eluted into 0.5 ml of
0.5 M perchloric acid by continuously vortexing for 10 min. Each
extract of 20 µl was injected into a HPLC apparatus equipped
with a reverse-phase column (Altantis™ dC18, 5 µm, 100 Å;
Waters, Milford, MA, USA) to quantify NE, E and DA. The mobile
phase was pumped at a flow rate of 1.0 ml/min. NE, E and DA in the
samples was quantified by using the peak areas of a standard
curve.
Intracellular cytokine staining
Intracellular cytokine staining was quantified as
previously described (24) with
modifications. In brief, following 48 h of transfection with TH
overexpression vector, the lymphocytes were stimulated with phorbol
12-myristate 13-acetate (PMA, 50 ng/ml) and ionomycin (1 µM)
(both from Sigma-Aldrich) in the presence of GolgiStop (BD
Pharmingen, San Diego, CA, USA) at the concentration recommended by
the manufacturer for 5 h. Following stimulation, the cells were
harvested and stained with fluorescein isothiocyanate
(FITC)-conjugated anti-CD4 (eBioscience, San Diego, CA, USA) and
with phycoerythrin (PE)-conjugated anti-IFN-γ or PE-labeled
anti-IL-4 antibodies (all from BD Phamingen) following fixation and
permeabilization. The stained cells were analyzed using a
FACSCalibur flow cytometer with CellQuest software (BD Biosciences,
San Jose, CA, USA).
Three-color flow cytometry
Briefly, the cells, having been transfected and
incubated for 48 h, were harvested and washed twice with ice-cold
phosphate-buffered saline (PBS) containing 1% bovine serum albumin
and subsequently 106 cells/test were incubated with
anti-CD4-FITC antibody for 30 min on ice. After washing, the cells
were treated with peridinin chlorophyll protein complex with
cyanin-5.5 (PerCP-Cy5.5)-conjugated anti-CD26 or PE-conjugated
anti-CD30 antibodies (all antibodies were from eBioscience) for a
further 30 min on ice. After final washing, the expression of cell
surface markers was analyzed on a FACSCalibur flow cytometer.
Cytometric bead array (CBA)
immunoassay
Following transfection with TH overexpression
vector, the supernatants of the lymphocyte cultures were collected
for cytokine measurements. A mouse Th1/Th2 cytokine CBA kit (BD
Biosciences) was used to measure the IL-2, IFN-γ, TNF, IL-4 and
IL-5 levels. The procedure was carried out according to the
manufacturer's instructions. Briefly, 50 µl of test samples
and the provided standard cytokines were added to the premixed
capture beads. After the addition of 50 µl of a mixture of
PE-conjugated antibodies against the cytokines, the mixture was
incubated for 2 h in the dark at room temperature. The mixture was
then washed and centrifuged at 500 × g for 5 min and the pellet was
resuspended in 300 µl of wash buffer. The flow cytometer (BD
Pharmingen) was calibrated with setup beads and 5,000 events were
acquired for each sample. The results were analyzed using BD CBA
analysis software (BD Biosciences).
Cell proliferation assay
MTT assay was used to measure cell proliferation
quantitatively. The lymphocytes were incubated with 5 µg/ml
Con A in the absence or presence of NE (10−5 or
10−6 M) at 37°C in a moist atmosphere with 5%
CO2 for 48 h. The MTT (Sigma-Aldrich) solution of 5
mg/ml was added to the cultures (10 µl MTT solution/100
µl medium) and the cells were cultured for an additional 4
h. Subsequently, the cells were lysed using dimethylsulfoxide
(Sigma-Aldrich). When the formanzan crystals were completely
dissolved, the optical density (OD) was measured at 570 nm using a
microplate reader (Bio-Tek, Winooski, VT, USA).
Immunoassay for cAMP content
Following incubation with NE (10−5 M) for
48 h, the lymphocytes were washed with cold PBS, adjusted to a
concentration of 1×107 cells/ml, treated with 1 N HCl at
room temperature for 10 min and centrifuged at 600 × g for 10 min
at 4°C. The supernatants were neutralized with 1 N NaOH. Cell
lysate was used to measure cAMP levels using the cAMP immunoassay
kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturers' instructions. Briefly, after washing with wash
buffer, the streptavidin-coated microplate was incubated with
biotinylated primary antibody to cAMP for 1 h at room temperature.
After washing, cAMP conjugated to horseradish peroxidase was added
to the wells immediately. The cell lysates or standards were then
added to the wells and incubated for 2 h at room temperature.
Tetramethylbenzidine with hydrogen peroxide was added to each well
after washing, followed by incubation for 30 min at room
temperature away from light. The action was terminated with stop
solution. The absorbance was determined with a microplate reader
(Bio-Tek) at 450 nm. The cAMP concentrations were calculated by
comparing with a curve derived from the standards provided with
each kit.
Statistical analysis
Data are expressed as the means ± standard deviation
(means ± SD). Statistical analysis was performed with the
Statistical Package for Social Science (SPSS 16.0). The data were
subjected to one-way analysis of variance (ANOVA), followed by the
Student-Newman-Keul's test to compare the data of all groups
between each other. Differences were considered statistically
significant at p<0.05.
Results
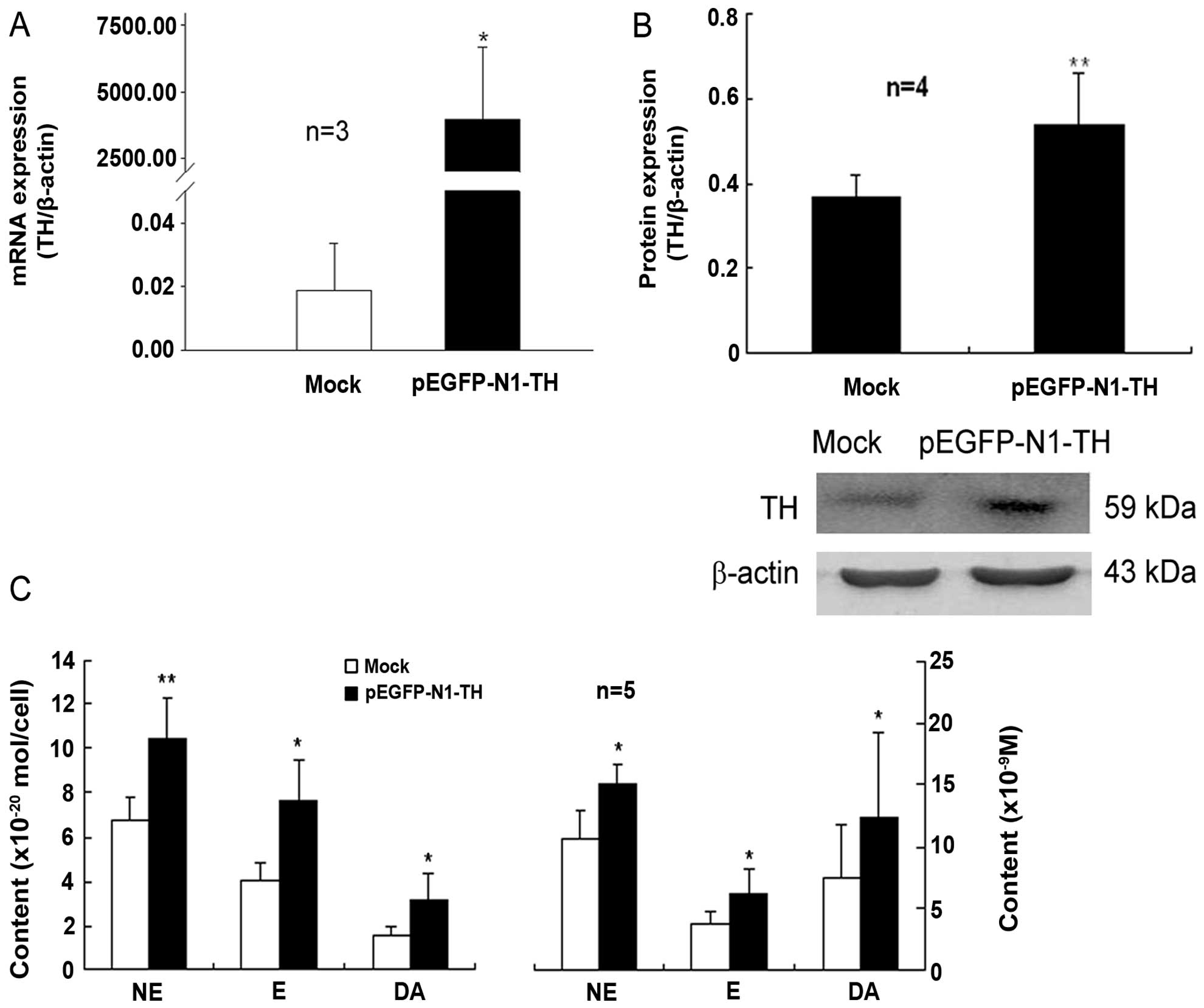
Transfection of lymphocytes with TH gene
overexpression plasmid increases TH expression and the CA content
in these cells
The plasmid, pEGFP-N1-TH, was constructed and then
transfected into lymphocytes that had been activated by Con A. The
mRNA and protein expression of TH in the lymphocytes transfected
with the TH overexpression plasmid was markedly upregulated
compared to that of the mock-transfected control cells (Fig. 1A and B). Moreover, the contents of
intracellular and supernatant CAs, including NE, E and DA in the
Con A-activated lymphocytes were significantly increased by TH gene
overexpression compared with those of the control cells
(mock-transfected cells) (Fig.
1C). These data indicated that transfection with the TH gene
overexpression plasmid led to an evident enhancement of TH
expression and CA synthesis in the Con A-activated lymphocytes.
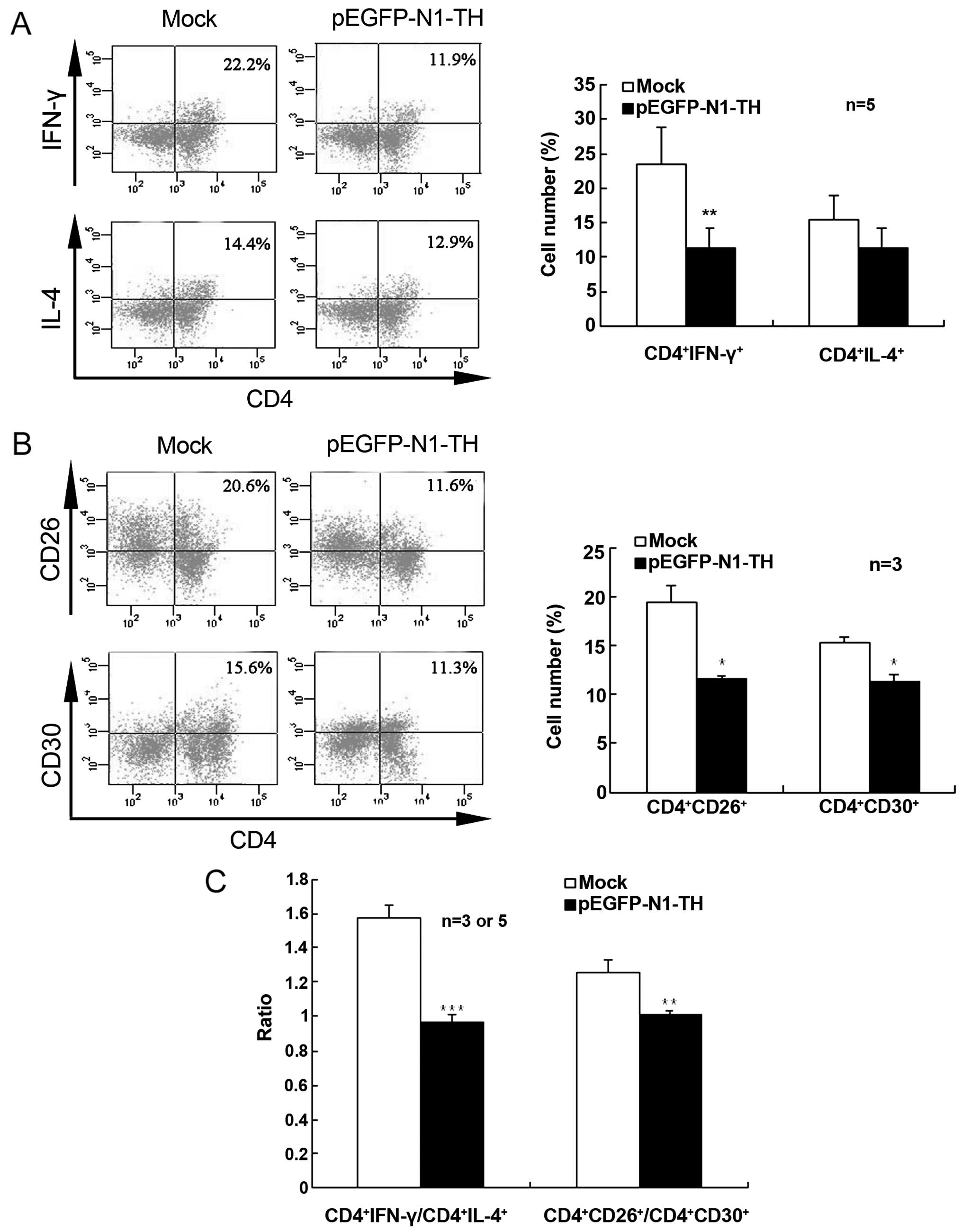
Effect of TH overexpression in
lymphocytes on Th1/Th2 differentiation
To further confirm that CAs regulate Th1/Th2
differentiation in vitro, we examined IFN-γ- and
IL-4-producing CD4+ T cells in Con A-activated
lymphocytes transfected with TH overexpression vector using
intracellular cytokine staining. TH gene overexpression in
lymphocytes markedly decreased the percentage of IFN-γ-producing
CD4+ T cells when compared with the mock-transfected
cells, indicating that CAs suppressed Th1 cell differentiation
(Fig. 2A). However, TH
overexpression did not significantly alter the percentage of
IL-4-producing CD4+ cells. Therefore, the ratio of
CD4+IFN-γ+/CD4+IL-4+
was notably lower in the lymphocytes transfected with the TH gene
overexpression vector than in the mock-transfected control cells
(Fig. 2C).
Furthermore, TH gene overexpression in the
lymphocytes markedly decreased both the percentage of
CD4+CD26+ T cells and the percentage of
CD4+CD30+ T cells (Fig. 2B). The ratio of
CD4+CD26+/CD4+CD30+ was
also significantly lower in the lymphocytes transfected with the TH
gene overexpression vector than in the mock-transfected control
cells (Fig. 2C). These results
indicated that an increase in the CA content promoted a shift in
Th1/Th2 differentiation toward Th2.
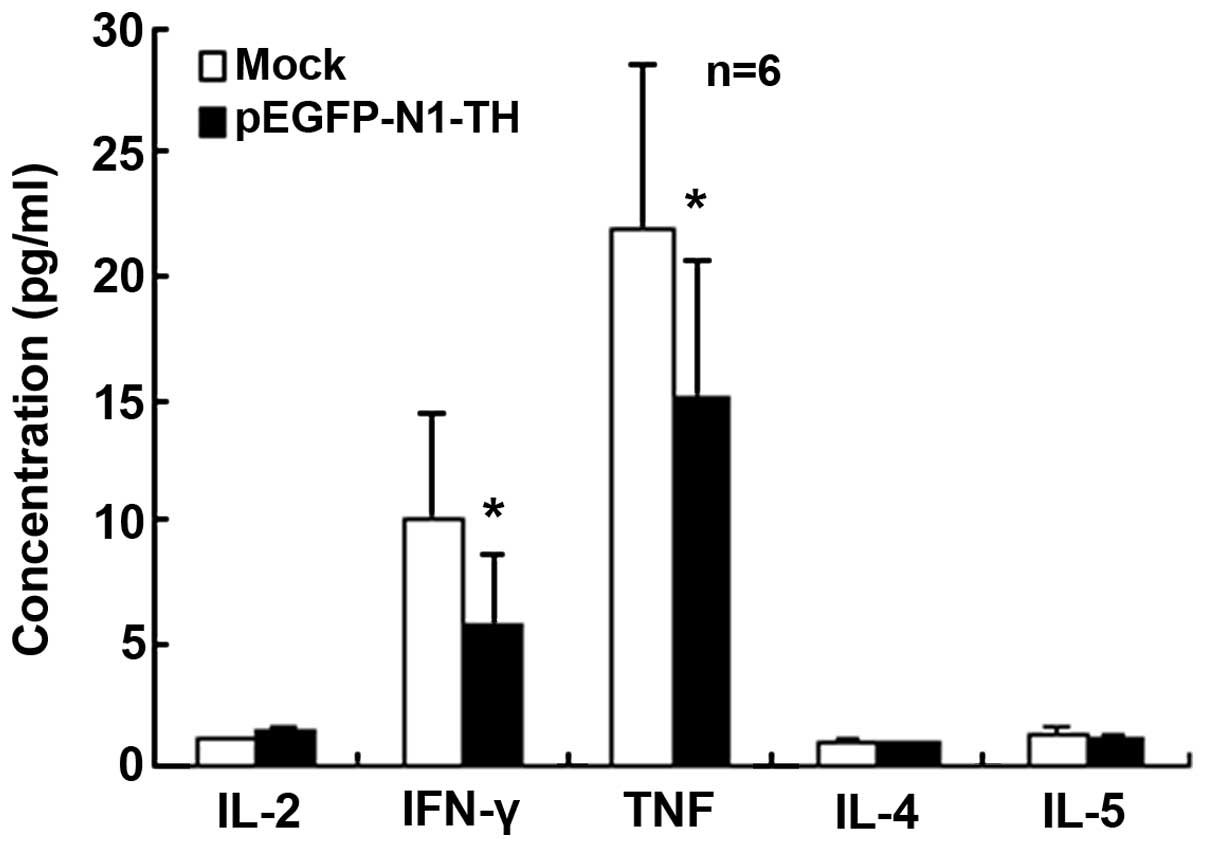
TH gene overexpression in lymphocytes
reduces Th1 cytokine secretion
To determine the effects of TH overexpression in
lymphocytes on Th cell function, cytokines relevant to the
different Th subsets were quantified in the supernatant.
Specifically, IL-2, TNF, IFN-γ, IL-4 and IL-5 were quantified. No
significant differences were observed in the concentrations of
IL-2, IL-4 and IL-5 (Fig. 3),
whereas the concentrations of IFN-γ and TNF were significantly
decreased in the culture supernatants of lymphocytes transfected
with the TH overexpression vector compared with those of the
mock-transfected control cells (Fig.
3). These data indicated that TH gene overexpression in
lymphocytes reduced Th1 cytokine secretion.
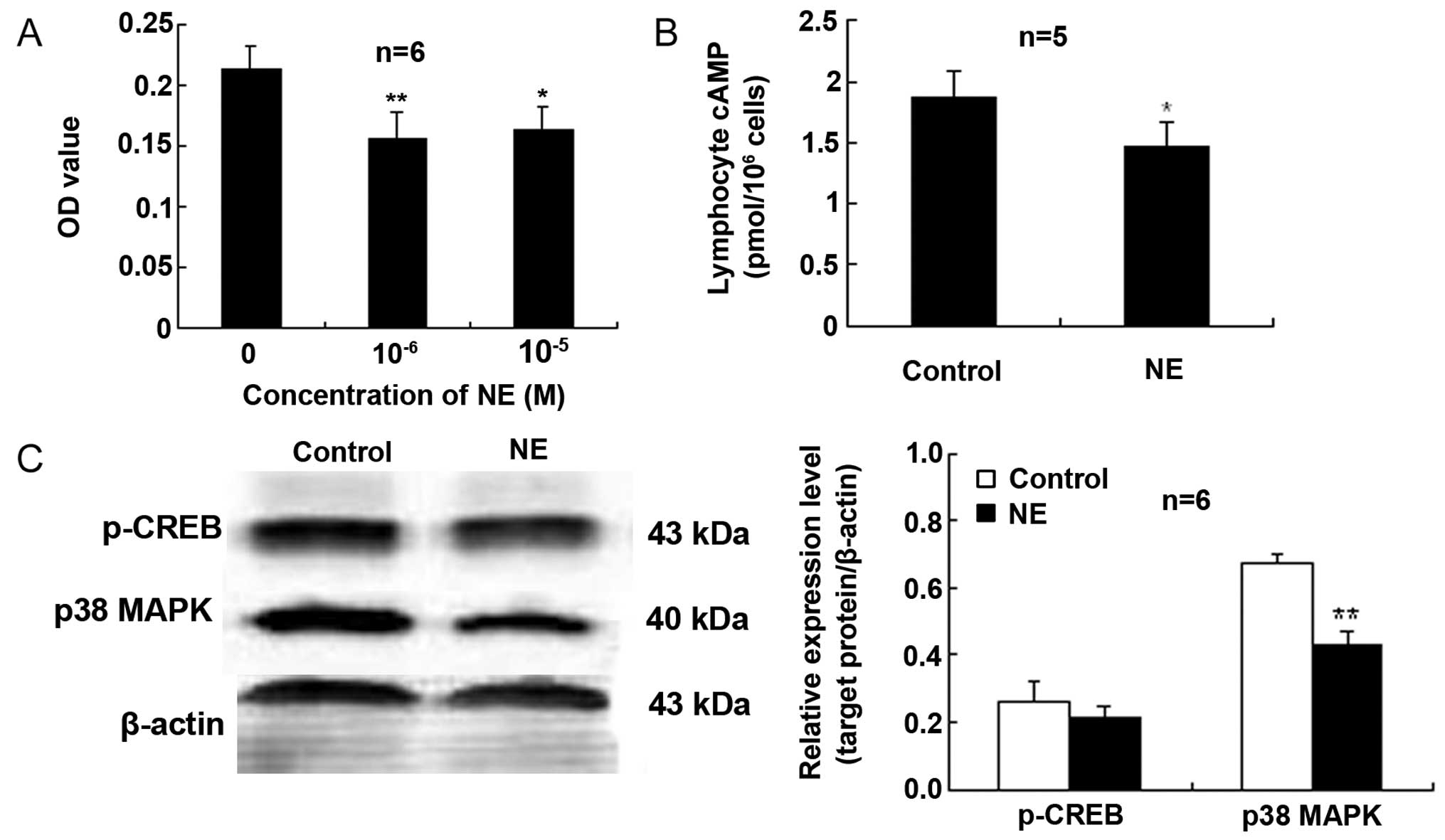
NE inhibits proliferation and
downregulates the cAMP levels and p38 MAPK expression in
lymphocytes activated by Con A
The MTT OD values of the lymphocytes treated with NE
at 10−6 or 10−5 M were markedly reduced when
compared with those of the control cells not treated with NE
(Fig. 4A). These findings
indicate an attenuating effect of NE on Con A-induced lymphocyte
proliferation.
To further determine the signal transduction pathway
involved in the effects of CAs on lymphocytes, intracellular cAMP
was measured by immunoassay. As shown in Fig. 4B, in the Con A-stimulated
lymphocytes exposed to NE (10−5 M), the content of cAMP
was lower than that of the control cells.
Exposure of the Con A-stimulated lymphocytes to NE
(10−5 M) elicited a reduction in the expression of p38
MAPK compared to the control (Fig. 4C
and D). However, NE did not significantly affect CREB
activation (Ser133 phosphorylation, p-CREB) compared with the
control (Fig. 4).
Discussion
Previous studies, including those from our
laboratory, have shown that lymphocytes express TH and synthesize
and secrete CAs (1,9). Antigen or mitogen stimulation can
increase the synthesis and secretion of CAs by lymphocytes
(9,10,25,26). Therefore, in the present study,
Con A was used to activate lymphocytes and to increase the basal
levels of CAs. We found that TH overexpression elevated the
expression of TH and the synthesis of CAs in the Con A-activated
lymphocytes. These results reveal that the process of CA synthesis
catalyzed by TH also exists in lymphocytes and show the
effectiveness of TH gene overexpression on the elevation of CA
synthesis.
The overexpression of the TH gene in lymphocytes
induced a decrease in the percentage of IFN-γ-producing
CD4+ T cells and in the ratio of
CD4+IFN-γ+/CD4+IL-4+,
and in the percentages of CD4+CD26+ and
CD4+CD30+ cells and the ratio of
CD4+CD26+/CD4+CD30+
cells. It is well known that IFN-γ-producing and IL-4-producing
CD4+ T cells represent Th1 and Th2 cells, respectively.
In IFN-γ-producing CD4+ T cells, CD26 expression is
higher (14), whereas in T cells
producing Th2-type cytokines, CD30 expression is increased
(15). Accordingly, our present
results strongly indicate that TH gene overexpression suppresses
Th1 differentiation, but enhances Th2 differentiation. It has been
reported that Th1 cells exert their effects via the production of
cytokines, such as IL-2 and IFN-γ, and Th2 cells implement their
functions via the production of cytokines, such as IL-4 and IL-10
(13). The present study
demonstrated that the concentrations of IFN-γ and TNF in the
culture supernatants of Con A-activated lymphocytes transfected
with the TH gene overexpression vector were significantly lower
than those of the control, although no significant differences were
observed in the IL-2, IL-4 and IL-5 concentrations. The
concentrations of cytokines in culture supernatants represent
secreted levels by lymphocytes. Thus, the reduction of Th1-type
cytokines, IFN-γ and TNF, indicates an attenuation of Th1 function.
These results reveal that TH gene overexpression in lymphocytes
promotes a functional bias towards Th2 cells, suggesting that
lymphocyte-derived CAs can promote Th cell function towards Th2
polarization. The present data extend our recent findings showing
that α-methyl-p-tyrosine (α-MT), an inhibitor of TH, upregulates
the expression of T-bet and IFN-γ, but downregulates the expression
of GATA-3 and IL-4 in lymphocytes and that pargyline, an inhibitor
of monoamine oxydase that degrades CAs, has the opposite effects to
those of α-MT (22), supporting
the viewpoint that lymphocyte-endogenous CAs regulate the Th1/Th2
balance and promote Th2 polarization. It has been reported that CAs
derived from neurons and endocrine cells suppress the cellular
immunity and shift the Th1/Th2 cytokine balance towards the Th2
profile (21,27–29). These effects of exogenously added
CAs are similar to the present findings of endogenous CAs from
lymphocytes. Therefore, we suggest that the effects of exogenous
CAs also probably represent an action of endogenous CAs, as they
can be secreted out of lymphocytes into extracellular fluid to
exert their modulatory effects through autocrine/paracrine pathways
(25,30,31). Furthermore, our present findings
of the role of lymphocyte-derived CAs in the modulation of the
Th1/Th2 balance extend the knowledge on neuroimmunomodulation by
CAs. The immunoregulation by endogenous CAs derived from
lymphocytes is probably more important than that by exogenous CAs
arising from neurons or endocrine cells, as lymphocyte-derived
endogenous CAs have closer and more direct contact with immune
cells.
In addition, the present study demonstrated that TH
overexpression promoted CA synthesis in Con A-activated
lymphocytes, not only at the intracellular level, but also in the
supernatant; among the three types of CAs, NE was the most
effective. To confirm that the influence of TH overexpression on
the function of lymphocytes may be related to NE, our studies
employed NE to explore the effects and reveal the possible
mechanisms. The effect that NE inhibited the proliferation of
lymphocytes is similar to the present finding that TH
overexpression attenuated the differentiation and function of Th1
cells. Recently, we demonstrated that the CA effect of promoting
Th2 polarization was mediated by α1-AR and
β2-AR, but not by α2-AR or β1-AR
by using respective AR antagonists (23), suggesting that both
α1-AR and β2-AR are involved in the role of
CAs. Canonically, the stimulation of α1-AR activates the
PLC-PKC signaling pathway and the stimulation of β2-AR
activates the cAMP-PKA signaling pathway. In addition, it has been
reported that NE stimulation of the βAR/Gs/PKA pathway activates
p38 MAPK and leads to changes in T cell dynamics (32), suggesting that p38 MAPK is
involved in β-AR signaling. Dissimilarly, the present results show
that NE simulation of lymphocytes decreases cAMP/p38 MAPK
signaling. This inconsistency may be explained by a mutual
regulation between α1-AR and β2-AR. It has
been presented that PKC and PKA may cause the phosphorylation of
β2-AR, which results in the receptor desensitization and
represents a negative feedback loop (33–36). For example, the stimulation of
α1-AR causes the phosphorylation of β2-AR in
the human prostate, most likely by G protein-coupled receptor
kinase 2, which may result in the desensitization of
β2-AR and the enhancement of α1-AR (37). On the other hand, the
phosphorylation of β-AR decreases its affinity for Gs, whereas it
increases Gi binding (38,39).
Therefore, the reduced cAMP/p38 MAPK signaling in lymphocytes by NE
in this study suggests a possibility of negative regulation of
β2-AR signaling by α1-AR activation.
The Th1/Th2 balance is crucial to maintain the
functional homeostasis of the immune system. Once the balance is
broken, it leads to the onset and progression of autoimmune
diseases. A number of autoimmune diseases, such as rheumatoid
arthritis (RA), experimental autoimmune encephalomyelitis (EAE),
autoimmune thyroiditis and systemic lupus erythematosus, have been
confirmed to be associated with a Th1/Th2 imbalance. In most states
of these diseases, the balance is skewed towards Th1 cells and an
excess production of pro-inflammatory cytokines, whereas the
production of anti-inflammatory cytokines is deficient (40–43). Studies have proven that polarizing
autoimmune Th1 cells toward Th2 directions (44,45) leads to the suppression of the
clinical and pathological manifestations of EAE. Accordingly, the
shifts of Th1/Th2 balance towards Th2 polarization by
lymphocyte-derived CAs presented in this study imply the possible
way, through which CAs alleviate autoimmune diseases. Moreover, the
present results, together with those of our recent study (23), imply that the cAMP/p38 MAPK
signaling pathway may be implicated in mediating Th cell
differentiation and function. Therefore, the signal transduction
mechanisms of CAs related to Th differentiation and function
provides a basis for the therapeutic strategy of autoimmune
diseases.
In conclusion, TH gene overexpression in lymphocytes
by transfection results in an upregulation of TH expression and an
elevation of CA synthesis. Simultaneously, TH gene overexpression
facilitates a shift in Th cell differentiation and function towards
Th2 cell polarization. These findings further confirm that
lymphocyte-derived CAs regulate the differentiation and function of
Th cells, promoting a shift in the Th1/Th2 balance towards Th2
predominance. Furthermore, NE inhibited Con A-induced lymphocyte
proliferation and decreased both the cAMP level and p38 MAPK
expression in lymphocytes. Accordingly, we hypothesize that the
cAMP/p38 MAPK signaling pathway may be implicated in mediating the
differentiation and function of Th cells induced by the
lymphocyte-derived CAs.
Acknowledgments
This study was supported by grant nos. 81271323 and
31371182 from the National Natural Science Foundation of China, no.
09B14 from the Natural Science Foundation of Nantong University,
KYZZ-0355 from the Postgraduate Science-Technology Innovation
Program of Jiangsu Educational Committee, and a project funded by
the Priority Academic Program Development (PAPD) of Jiangsu Higher
Education Institutions.
References
|
1
|
Bergquist J, Tarkowski A, Ekman R and
Ewing A: Discovery of endogenous catecholamines in lymphocytes and
evidence for catecholamine regulation of lymphocyte function via an
autocrine loop. Proc Natl Acad Sci USA. 91:12912–12916. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Josefsson E, Bergquist J, Ekman R and
Tarkowski A: Catecholamines are synthesized by mouse lymphocytes
and regulate function of these cells by induction of apoptosis.
Immunology. 88:140–146. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Musso NR, Brenci S, Setti M, Indiveri F
and Lotti G: Catecholamine content and in vitro catecholamine
synthesis in peripheral human lymphocytes. J Clin Endocrinol Metab.
81:3553–3557. 1996.PubMed/NCBI
|
|
4
|
Marino F, Cosentino M, Bombelli R, Ferrari
M, Lecchini S and Frigo G: Endogenous catecholamine synthesis,
metabolism storage, and uptake in human peripheral blood
mononuclear cells. Exp Hematol. 27:489–495. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cosentino M, Marino F, Bombelli R, Ferrari
M, Lecchini S and Frigo G: Endogenous catecholamine synthesis,
metabolism, storage and uptake in human neutrophils. Life Sci.
64:975–981. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cosentino M, Bombelli R, Ferrari M, Marino
F, Rasini E, Maestroni GJ, Conti A, Boveri M, Lecchini S and Frigo
G: HPLC-ED measurement of endogenous catecholamines in human immune
cells and hematopoietic cell lines. Life Sci. 68:283–295. 2000.
View Article : Google Scholar
|
|
7
|
Cosentino M, Fietta AM, Ferrari M, Rasini
E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F and
Lecchini S: Human CD4+CD25+ regulatory T
cells selectively express tyrosine hydroxylase and contain
endogenous catecholamines subserving an autocrine/paracrine
inhibitory functional loop. Blood. 109:632–642. 2007. View Article : Google Scholar
|
|
8
|
Cosentino M, Marino F and Lecchini S:
Resistance of naturally occurring regulatory T cells toward
oxidative stress: Possible link with intracellular catecholamine
content and implications for cancer therapy. Blood. 114:487–489.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu YH, Peng YP, Jiang JM and Wang JJ:
Expression of tyrosine hydroxylase in lymphocytes and effect of
endogenous catecholamines on lymphocyte function.
Neuroimmunomodulation. 11:75–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu YH, Cheng C, Dai L and Peng YP: Effect
of endogenous catecholamines in lymphocytes on lymphocyte function.
J Neuroimmunol. 167:45–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng YP, Qiu YH, Jiang JL and Wang JJ:
Effect of catecholamines on IL-2 production and NK cytotoxicity of
rats in vitro. Acta Pharmacol Sin. 25:1354–1360. 2004.PubMed/NCBI
|
|
12
|
Jiang JL, Peng YP, Qiu YH and Wang JJ:
Effect of endogenous catecholamines on apoptosis of Con A-activated
lymphocytes of rats. J Neuroimmunol. 192:79–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
D'Ambrosio D, Iellem A, Colantonio L,
Clissi B, Pardi R and Sinigaglia F: Localization of Th-cell subsets
in inflammation: Differential thresholds for extravasation of Th1
and Th2 cells. Immunol Today. 21:183–186. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Willheim M, Ebner C, Baier K, Kern W,
Schrattbauer K, Thien R, Kraft D, Breiteneder H, Reinisch W and
Scheiner O: Cell surface characterization of T lymphocytes and
allergen-specific T cell clones: Correlation of CD26 expression
with T(H1) subsets. J Allergy Clin Immunol. 100:348–355. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del Prete G, De Carli M, Almerigogna F,
Daniel CK, D'Elios MM, Zancuoghi G, Vinante F, Pizzolo G and
Romagnani S: Preferential expression of CD30 by human
CD4+ T cells producing Th2-type cytokines. FASEB J.
9:81–86. 1995.PubMed/NCBI
|
|
16
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
17
|
Simon AK, Seipelt E and Sieper J:
Divergent T-cell cytokine patterns in inflammatory arthritis. Proc
Natl Acad Sci USA. 91:8562–8566. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dolff S, Bijl M, Huitema MG, Limburg PC,
Kallenberg CG and Abdulahad WH: Disturbed Th1, Th2, Th17 and T(reg)
balance in patients with systemic lupus erythematosus. Clin
Immunol. 141:197–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borgogni E, Sarchielli E, Sottili M,
Santarlasci V, Cosmi L, Gelmini S, Lombardi A, Cantini G, Perigli
G, Luconi M, et al: Elocalcitol inhibits inflammatory responses in
human thyroid cells and T cells. Endocrinology. 149:3626–3634.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bleotu C, Chifiriuc MC, Grigore R, Grancea
C, Popescu CR, Anton G and Cernescu C: Investigation of Th1/Th2
cytokine profiles in patients with laryngo-pharyngeal, HPV-positive
cancers. Eur Arch Otorhinolaryngol. 270:711–718. 2013. View Article : Google Scholar
|
|
21
|
Salicrú AN, Sams CF and Marshall GD:
Cooperative effects of corticosteroids and catecholamines upon
immune deviation of the type-1/type-2 cytokine balance in favor of
type-2 expression in human peripheral blood mononuclear cells.
Brain Behav Immun. 21:913–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang HW, Tang JL, Han XH, Peng YP and Qiu
YH: Lymphocyte-derived catecholamines induce a shift of Th1/Th2
balance toward Th2 polarization. Neuroimmunomodulation. 20:1–8.
2013. View Article : Google Scholar
|
|
23
|
Huang HW, Fang XX, Wang XQ, Peng YP and
Qiu YH: Regulation of differentiation and function of helper T
cells by lymphocyte-derived catecholamines via α1- and
β2-adrenoceptors. Neuroimmunomodulation. 22:138–151.
2015. View Article : Google Scholar
|
|
24
|
Switzer KC, Fan YY, Wang N, McMurray DN
and Chapkin RS: Dietary n-3 polyunsaturated fatty acids promote
activation-induced cell death in Th1-polarized murine
CD4+ T-cells. J Lipid Res. 45:1482–1492. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cosentino M, Marino F, Bombelli R, Ferrari
M, Rasini E, Lecchini S and Frigo G: Stimulation with
phytohaemagglutinin induces the synthesis of catecholamines in
human peripheral blood mononuclear cells: Role of protein kinase C
and contribution of intracellular calcium. J Neuroimmunol.
125:125–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cosentino M, Zaffaroni M, Marino F,
Bombelli R, Ferrari M, Rasini E, Lecchini S, Ghezzi A and Frigo G:
Catecholamine production and tyrosine hydroxylase expression in
peripheral blood mononuclear cells from multiple sclerosis
patients: Effect of cell stimulation and possible relevance for
activation-induced apoptosis. J Neuroimmunol. 133:233–240. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elenkov IJ, Papanicolaou DA, Wilder RL and
Chrousos GP: Modulatory effects of glucocorticoids and
catecholamines on human interleukin-12 and interleukin-10
production: Clinical implications. Proc Assoc Am Physicians.
108:374–381. 1996.PubMed/NCBI
|
|
28
|
Takayanagi Y, Osawa S, Ikuma M, Takagaki
K, Zhang J, Hamaya Y, Yamada T, Sugimoto M, Furuta T, Miyajima H
and Sugimoto K: Norepinephrine suppresses IFN-γ and TNF-α
production by murine intestinal intraepithelial lymphocytes via the
β1 adrenoceptor. J Neuroimmunol. 245:66–74. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Loza MJ, Peters SP, Foster S, Khan IU and
Penn RB: beta-Agonist enhances type 2 T-cell survival and
accumulation. J Allergy Clin Immunol. 119:235–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Musso NR, Brenci S, Indiveri F and Lotti
G: Acetylcholine-induced, calcium-dependent norepinephrine outflow
from peripheral human lymphocytes. J Neuroimmunol. 87:82–87. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Engler KL, Rudd ML, Ryan JJ, Stewart JK
and Fischer-Stenger K: Autocrine actions of macrophage-derived
catecholamines on interleukin-1 β. J Neuroimmunol. 160:87–91. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lajevic MD, Suleiman S, Cohen RL and
Chambers DA: Activation of p38 mitogen-activated protein kinase by
norepinephrine in T-lineage cells. Immunology. 132:197–208. 2011.
View Article : Google Scholar :
|
|
33
|
Oostendorp J, Obels PP, Terpstra AR,
Nelemans SA and Zaagsma J: Modulation of beta2- and
beta3-adrenoceptor-mediated relaxation of rat oesophagus smooth
muscle by protein kinase C. Eur J Pharmacol. 495:75–81. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Boterman M, Smits SR, Meurs H and Zaagsma
J: Protein kinase C potentiates homologous desensitization of the
beta2-adrenoceptor in bovine tracheal smooth muscle. Eur J
Pharmacol. 529:151–156. 2006. View Article : Google Scholar
|
|
35
|
Tran TM, Friedman J, Qunaibi E, Baameur F,
Moore RH and Clark RB: Characterization of agonist stimulation of
cAMP-dependent protein kinase and G protein-coupled receptor kinase
phosphorylation of the beta2-adrenergic receptor using
phosphoserine-specific antibodies. Mol Pharmacol. 65:196–206. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Violin JD, DiPilato LM, Yildirim N, Elston
TC, Zhang J and Lefkowitz RJ: beta2-adrenergic receptor signaling
and desensitization elucidated by quantitative modeling of
real-time cAMP dynamics. J Biol Chem. 283:2949–2961. 2008.
View Article : Google Scholar
|
|
37
|
Hennenberg M, Strittmatter F, Walther S,
Hedlund P, Andersson KE, Stief CG, Schlenker B and Gratzke C:
α1-adrenoceptor activation induces phosphorylation of
β2-adrenoceptors in human prostate tissue. BJU Int.
108:922–928. 2011.PubMed/NCBI
|
|
38
|
Daaka Y, Luttrell LM and Lefkowitz RJ:
Switching of the coupling of the beta2-adrenergic receptor to
different G proteins by protein kinase A. Nature. 390:88–91. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zamah AM, Delahunty M, Luttrell LM and
Lefkowitz RJ: Protein kinase A-mediated phosphorylation of the beta
2-adrenergic receptor regulates its coupling to Gs and Gi.
Demonstration in a reconstituted system. J Biol Chem.
277:31249–31256. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Horwitz DA, Gray JD, Behrendsen SC, Kubin
M, Rengaraju M, Ohtsuka K and Trinchieri G: Decreased production of
interleukin-12 and other Th1-type cytokines in patients with
recent-onset systemic lupus erythematosus. Arthritis Rheum.
41:838–844. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakashima H, Akahoshi M and Masutani K:
Th1/Th2 balance of SLE patients with lupus nephritis. Rinsho Byori.
54:706–713. 2006.PubMed/NCBI
|
|
42
|
Ruschpler P and Stiehl P: Shift in Th1
(IL-2 and IFN-gamma) and Th2 (IL-10 and IL-4) cytokine mRNA balance
within two new histological main-types of rheumatoid-arthritis
(RA). Cell Mol Biol (Noisy-le-grand). 48:285–293. 2002.
|
|
43
|
Fuse K, Kodama M, Ito M, Okura Y, Kato K,
Hanawa H, Aoki S and Aizawa Y: Polarity of helper T cell subsets
represents disease nature and clinical course of experimental
autoimmune myocarditis in rats. Clin Exp Immunol. 134:403–408.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garren H, Ruiz PJ, Watkins TA, Fontoura P,
Nguyen LT, Estline ER, Hirschberg DL and Steinman L: Combination of
gene delivery and DNA vaccination to protect from and reverse Th1
autoimmune disease via deviation to the Th2 pathway. Immunity.
15:15–22. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Miyamoto K, Miyake S and Yamamura T: A
synthetic glycolipid prevents autoimmune encephalomyelitis by
inducing TH2 bias of natural killer T cells. Nature. 413:531–534.
2001. View Article : Google Scholar : PubMed/NCBI
|