The cornea is the protective ocular surface, and is
transparent to enable the transmission of light. Chemical burns can
damage this barrier (1), and in
addition to corneal injury and eyelid burns, are risk factors for
ocular complications, including ulcers, scars and
neovascularization (NV) (2,3).
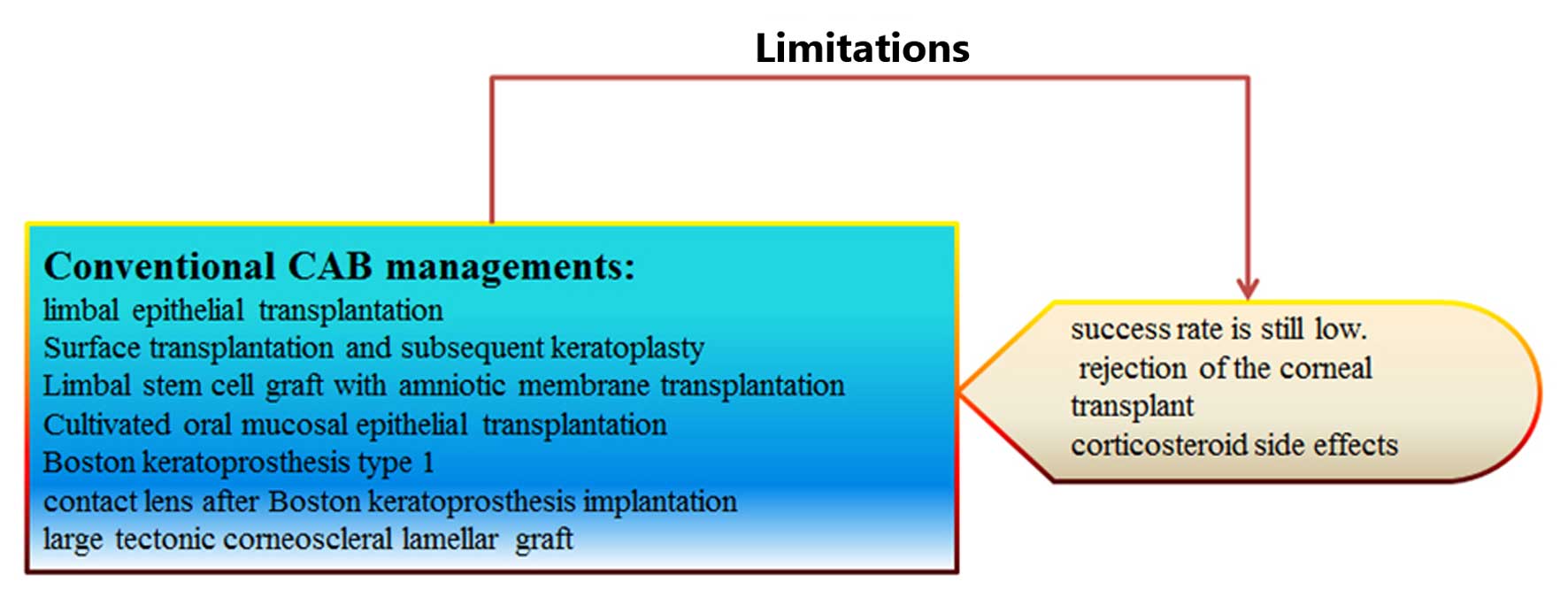
Several potential interventional strategies, including limbal stem
cells, amniotic membranes and corneal transplantations have been
demonstrated to have some success in clinical outcomes. There are
numerous risk factors and molecular markers for the progression of
ocular chemical burns. Improvements in the knowledge of the novel
biomarkers associated with the inflammation, angiogenesis and
fibrosis of ocular chemical injuries have contributed to the
development of novel therapeutics. Chemical burns can be divided
into alkali and acid burns, with corneal alkali burns (CAB)
frequently resulting in a greater severity of injury (4). Peroxisome proliferator-activated
receptor (PPAR) controls the regulation of genes through the
activation of nuclear receptors, and plays a role in the control of
a variety of inflammatory, angiogenic and fibrotic physiological
processes (5). This reviews
covers the key aspects associated with biomarker research into the
pathological process of CAB, and analyzes the potential therapeutic
role of PPAR agonists in the treatment of CAB. The processes of
cytokine production, chemotaxis, inflammatory and immune responses,
signal transduction, matrix metalloproteinase (MMP) production and
vascular factors in CAB are summarized, and the potential
application of PPAR agonists as treatments to control lesion
severity in CAB are also discussed.
Stem cells potentiate regeneration due to their
ability to differentiate into multiple cell lineages. The most
common sources of stem cells for clinical use are embryonic, adult
and induced (6). Surface
transplantation and subsequent keratoplasty can result in good
visual function following ocular injury (7). Limbal stem cell grafts with amniotic
membrane transplantation or simple limbal epithelial
transplantation may additionally be used to restore vision and
reduce symptoms in cases with limbal stem cell deficiency following
chemical burns (8–12). Cultivated oral mucosal epithelial
transplantation has been indicated to enable the complete
epithelialization of persistent corneal epithelial defects, and
stabilize the ocular surface in patients with severe ocular surface
disease (13). The Boston
keratoprosthesis type I is an effective artificial cornea and aids
in the recovery from advanced ocular surface disease, and has been
shown to result in a significant increase in eyesight (14). Additionally, Boston
keratoprosthesis implantation may reduce the risk of
post-keratoplasty complications by the wearing of contact lenses
(15). Alternatively, a large
tectonic corneoscleral lamellar graft represents a good treatment
method (16). These methods can
treat a selection of clinical applications and present some
benefits; however, they require further study.
Scarring has been attributed to the proliferation of
inflammatory cells and fibroblasts during burn wound healing. The
irregular remodeling of matrix structures may lead to scar
formation. Stem cell immunomodulation has been indicated to address
pathological scarring (17–19). Limbal transplantation is a
standard procedure to restore ocular surface disorders and,
considering the shortage of corneal donors, is a viable alternative
treatment strategy; however, the success rate remains low (20). Additionally, the separation and
purification rates of limbal cells and the efficiency of migration
require further investigation, and the therapeutic efficacy and
safety of limbal transplantation should be clarified (21). Furthermore, the rejection rate of
keratoplasty is high in cases of CAB, and the number of suitable
donor corneas available is not sufficient to meet the demands
(22,23). Corticosteriods are the predominant
current treatment, and treat the inflammation associated with
corneal NV (CNV); however, they can result in side-effects, such as
cataracts and increased intraocular pressure (24). Further studies are required to
understand stem cell applications targeting NV and the inflammatory
and fibrotic processes associated with CAB (Fig. 1).
A number of topical therapeutics against NV or
inflammation associated with CAB are under investigation (25). Some of these potential topical
therapeutics under investigation are aloe vera, prospero homeobox 1
short interfering RNA, Rho-associated protein kinase inhibitors
(AMA0526), 0.5% ketorolac tromethamine, keratinocyte growth
factor-2, omentum, protein phosphatase magnesium dependent-1 and
melatonin, and may potentially be used for the treatment of CAB in
clinical practice (26–33). Subconjunctival bevacizumab
injection may be considered as a secondary treatment for CNV caused
by chemical injuries that are not responsive to conventional
steroid therapy (34,35). These topical therapies may be
effective treatments for severe cases of CAB, although further
studies may be required to fully determine this.
PPARs belong to a nuclear receptor superfamily that
includes steroid, thyroid hormone, vitamin D and retinoid
receptors. PPAR-γ is activated by transcription factors and plays
an important role in the regulation of cell proliferation and
inflammation (36,37). PPAR suppresses inflammatory
cytokines, proteolytic enzymes, adhesion molecules, chemotactic and
atherogenic factors (38–40). Transforming growth factor (TGF) β1
has been shown to transdifferentiate keratocytes to myofibroblasts
involved in the repair of the corneal epithelium, and stromal and
corneal scar formation in CAB, by regulating monocytes,
macrophages, vascular endothelial growth factor (VEGF), neutrophils
and monocyte/macrophage chemotactic protein-1 (32,41). In a previous study, the expression
of the PPAR-γ gene was shown to induce anti-inflammatory and
anti-fibrogenic responses in an alkali-burned mouse cornea.
Additionally, PPAR-γ gene expression suppressed TGFβ1 and MMP
expression in macrophages, indicating a potentially effective
strategy for the treatment of CAB (3). PPAR-γ expression has been reported
to increase with the infiltration of numerous inflammatory cells in
the pathological process of CAB. As previously demonstrated,
treatment with an ophthalmic solution of a PPAR-γ agonist
suppressed the expression levels of interleukin (IL)-1β, IL-6,
IL-8, monocyte chemoattractant protein-1 (MCP-1), tumor necrosis
factor-α (TNF-α), TGFβ1 and VEGF-A in corneal inflammation induced
by an alkali burn. An ophthalmic solution of the PPAR-γ agonist may
provide a novel treatment strategy with useful clinical
applications for corneal inflammation and wound healing (42). Burns induce the activation of an
inflammatory cascade and wound progression. The PPAR-γ agonsist,
rosiglitazone, reduces the percentage of unburned skin interspaces
that progress to full necrosis in a rat model and prevent
burn-induced organ damage. Therefore, the PPAR-γ agonists hold
potential for clinical application (36,43). In this review, the potential role
of PPAR-γ agonists in the treatment of CAB and the underlying
molecular mechanisms are discussed.
PPAR-γ ligands are divided into endogenous (9, 13
and 15-hydroxyoctadecadienoic acid) and synthetic (pioglitazone,
troglitazone, rosiglitazone, liglitazone and TZD18) compounds
(44). PPAR isoforms (PPAR-γ,
PPAR-α and PPAR-β/δ) have been shown to exhibit anti-inflammatory
and immunomodulatory properties. PPARs may represent a novel target
in the treatment of inflammatory and vascular diseases (45). Pioglitazone may inhibit corneal
fibroblast migration and reduce corneal fibroblast-induced collagen
contraction in the corneal wound healing process (46). Previous studies have supported the
anti-inflammtory, anti-angiogenic and anti-fibrotic functions of
PPAR-γ.
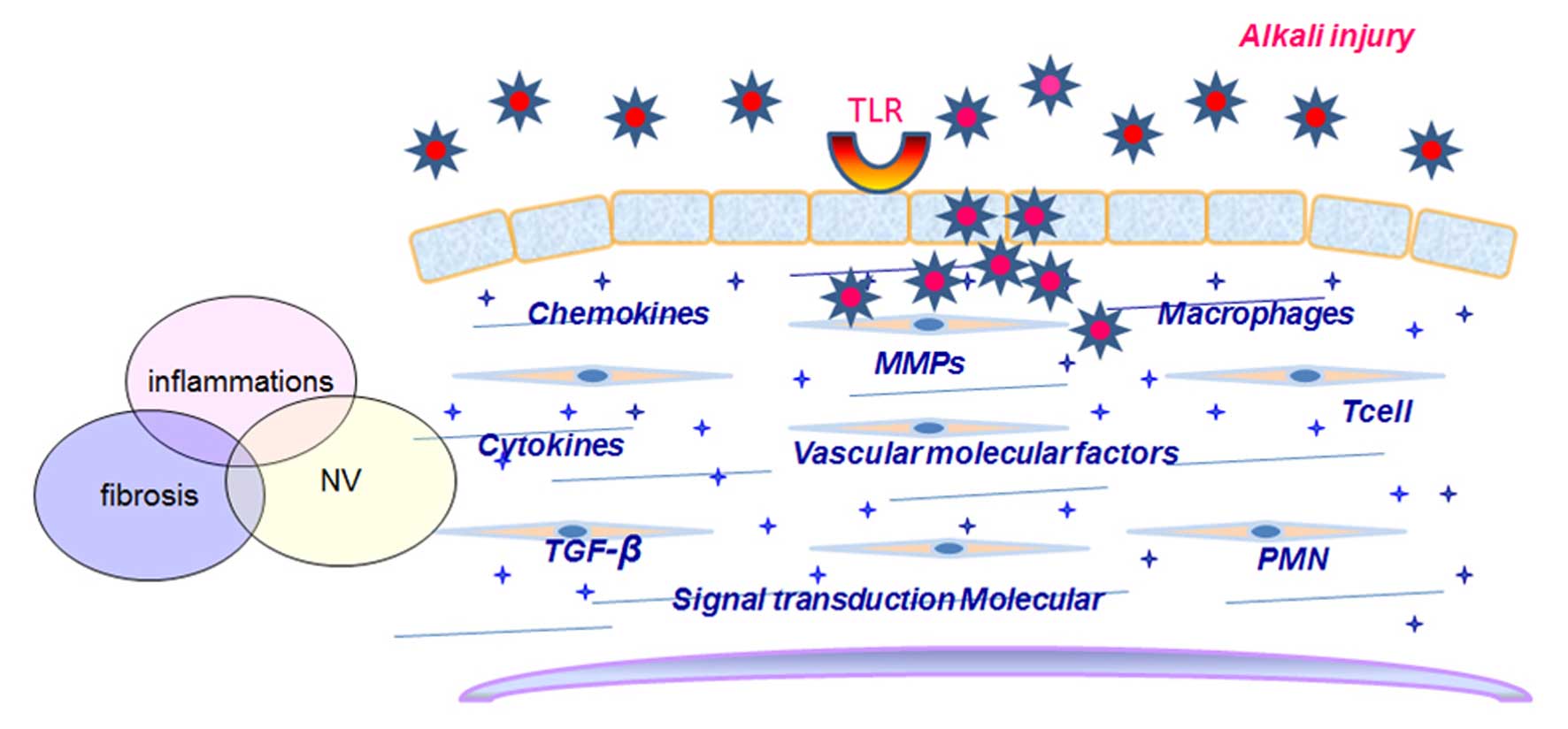
Toll-like receptors (TLRs) play key roles in innate
immune responses. PPAR-γ gene silencing affects genes involved in
the innate immune process (47).
Injury primes the innate immune system for enhanced TLR-2- and
TLR-4-mediated responses, and suggests that increasing TLR activity
may contribute to the progression of systemic inflammation
following severe injury (48).
Previously, Th1-activated macrophages were considered a key
cellular defense against intracellular pathogens. However, more
recently, Th2-activated macrophages have been indicated to be
involved in repair and tissue regeneration via the modulation of
PPARs in immunological inflammation, and this may lead to new
therapeutic approaches (49–51). Dendritic cells (DCs) from burned
skin notably express low levels of human leukocyte antigen-antigen
D related and TLR-4 immediately following cell isolation. In the
post-burn period, the ability of skin DCs to respond to bacterial
stimuli is impaired. These alterations in DCs may contribute to
impaired host defenses against bacteria, leading to post-burn
infection (52). Burns are
associated with γδ T-cell activation at the injury site, which
initiates the infiltration of the wound with large numbers of αβ
T-cells that may facilitate the transition from the inflammatory to
the proliferative phase of healing (53). Burns and TLRs are associated with
the induction of the innate immune system, with a greater number of
TLR-2-induced Kupffer cells (KCs) and macrophage inflammatory
protein (MIP)-1β production post-injury, whereas the levels of
IL-6, IL-10 and MIP-1β and the number of KCs are greater following
TLR-4-induced activation following burns. TLR-mediated inflammatory
responses have been reported to be augmented post-burn by the
induction of inflammatory mediators (54). TLR-5 is normally present on the
superficial cells of the conjunctival epithelium, and may be
upregulated following chemical burns (55). TLR activates the innate immune
system to recognize antigens and induce the production of
inflammatory cytokines and chemokines (56,57). The TLR-related genes, heat-shock
70kDa protein (HSP)A1A, Harvey rat sarcoma viral oncogene homolog,
mitogen-activated protein kinase (MAPK) kinase 3, Toll interacting
protein, v-rel avian reticuloendotheliosis viral oncogene homolog
A, FBJ murine osteosarcoma viral oncogene homolog and TLR-1 have
been observed to be reduced in the primary epidermal keratinocytes
of patients with severe burns, and restoring the expression of
these genes may improve clinical outcomes (58). High levels of cytokines promote
collagen degradation, the apoptosis of keratinocytes and vascular
compromise. Local inflammation induced by severe burns can clear
cellular debris, protect against microbial agents and induce cell
growth and proliferation (58,59). The reduction of the activation and
recruitment of macrophages may be a potential therapeutic strategy
for the corneal scarring of alkali-burned ocular surfaces (60). Agonists of TLR-4, 1/2 and -5
suppress the activity of PPAR-α and PPAR-γ in astrocytes (61). PPAR-β/δ expression is regulated in
TLR agonist-stimulated astrocytes via the regulation of the
pro-inflammatory genes. p38, MAP2K1/2, MAPK2/3 and c-Jun N-terminal
kinase (JNK) (62). The PPAR-α
agonist, WY14643, has been shown to significantly reduce amylase,
lipase and myeloperoxidase activity, and IL-6, intercellular
adhesion molecule-1, and TLR-2 and 4 levels (63). PPAR-γ inhibits interferon (IFN)-β
production in TLR3- and 4-stimulated macrophages by preventing
interferon regulatory factor 3 binding to the IFN-β promoter
(64). Treatment with
rosiglitazone was previously shown to result in higher levels of
PPARγ and a reduction in serum inflammatory cytokine levels, and
the levels of TLR2/4 and nuclear factor-κB (NF-κB) activity in
aortic tissues. These biological functions of rosiglitazone in
P. gingivalis-accelerated atherosclerosis were shown to be
dependent upon the inhibition of the inflammatory response and the
TLR/NF-κB signaling pathway (65). PPAR-γ and TGF-β can enhance
regulatory T cell (Treg) generation, providing a potential
therapeutic strategy for the treatment of inflammatory and
autoimmune diseases (66). PPAR-γ
restores the abnormal immune gene expression of p38MAPK, activating
transcription factor-2, MAPK-activated protein kinase 2 and HSP27
in T-cell mediated immune responses in vivo (67). Cell types in the innate and
adaptive immune system, including neutrophils, macrophages, mast
cells, B cells and T cells, have all been implicated to play a role
in burn-induced immunology (68).
Burn injury disrupts the immune system, resulting in the marked
suppression of the immune response. The mononuclear phagocyte
system (MPS) is a critical component of the innate immune response,
and is able to initiate an adaptive immune response. Severe burns
inhibits the functions of DCs, monocytes and macrophages. The MPS
in the pathophysiology of severe burns will guarantee a more
rational immunotherapy for patients with severe burns (69). These results collectively suggest
that PPAR-α, -γ and -β/δ are likely mediators of TLR activation in
transducing inflammation in CAB pathologies; however, the relative
immune mechanisms require clarification. The molecular mechanisms
of CAB are summarized in Fig.
2.
As an anti-TNF-α monoclonal antibody, topical
infliximab has been reported to significantly reduce corneal
perforation, leukocyte infiltration, cluster of differentiation
(CD)45+ cell infiltration and fibrosis in the eyelids.
The topical application of infliximab may be useful in the
treatment of ocular diseases (70). Topically applied IL-1 receptor
antagonist (IL-1ra) may suppress corneal inflammation and promote
recovery following CAB. All cytokine/chemokine levels, in
particular IL-6 and IL-10, have been shown to be significantly
reduced in IL-1ra-treated eyes, with the opposite effect observed
in IL-1ra knockout mice (71–74). The treatment of inflammation with
minimal infiltrating cells and normal levels of IL-1α and IL-1β may
accelerate the healing of CAB (75). A reduction in IL-6 and TGF-β1
expression has been indicated to protect the cornea from chemical
damage (76). In addition, the
inhibition of inflammation and NV has been reported to play a
significant role in preventing corneal angiogenesis and
inflammation in alkali-burned corneal beds, which results in higher
allograft survival rates (77).
Furthermore, in a CAB model, the infiltrated polymorphonuclear
leukocytes and the mRNA expression of VEGF receptor 1 and 2, basic
fibroblast growth factor, IL-1β, IL-6, MMP-2, -9 and -13, in
addition to the protein expression levels of VEGFR2, IL-1β, IL-6
and MMP-2 and -9, were upregulated in the corneas. The suppression
of CNV, inflammatory cytokines and MMPs aids in reducing the damage
associated with CAB (78). Human
peripheral blood mononuclear cells and inflammatory cytokines can
be stimulated by chemically injured keratocytes. MMP-9 and
macrophage migration inhibitory factor levels have been reported to
be higher in burn injury (79).
CD4 and CD44 (memory) CD8 T cells have been found to be
significantly increased, in addition to TLR-4, post-burn injury,
and functional T cell responses have additionally been
demonstrated. Complex adaptive immune responses have been reported
in burn injury (80); however,
this differs in the process of CAB. IFN-γ and CD4 were not detected
in rat corneas following alkali burns, indicating that cytokines
were induced in the cornea by burn injury without a specific
immunological stimulus (81). To
inhibit excessive inflammatory damage, particular anti-inflammatory
agents may be applied for the treatment of alkali burns. PPAR-γ
agonists are a good candidate for anti-inflammatory activity in
preventing TNF-α damage (82).
Pioglitazone therapy has been demonstrated to suppress the mRNA
levels of the inflammatory cytokines monocyte, MCP-1, IL-1 and
IL-6, produced by macrophages in the cerebral arteries (83). PPAR-γ represents an appealing
strategy for decreasing inflammation and improving the healing of
chronic injuries, and PPAR-γ in inflammatory cells may be a
potential therapeutic target (84,85). Pioglitazone has been shown to
exert anti-inflammatory effects on acute gouty arthritis by
inhibiting the expression of TNF-α and IFN-γ (86). Notably, there is anti-inflammatory
therapeutic potential for the treatment of Alzheimer's disease,
dental implants and lipid inflammation processes through the PPAR-γ
pathway (47,87,88). PPAR-γ modulates macrophage and T
cell-mediated inflammation. Reductions in the levels of PPAR-γ in T
cells have been shown to result in an increased expression of
adhesion molecules and pro-inflammatory cytokines (IL-6 and IL-1β),
and to modulate Treg recruitment (89). Thus, PPAR-γ agonists are effective
in controlling inflammation-related damage and inhibiting cytokines
and chemokines, suggesting their therapeutic potential in the
treatment of CAB.
Pathological conditions including infection, trauma
and loss of the limbal stem cell barrier can lead to CNV formation,
from the limbal area to the vascular cornea (90). NV is mediated by cellular and
molecular factors, such as VEGF and pigment epithelium-derived
factor (PEDF), which play roles in the development of NV (91). Corneal transparency is essential
for maintaining good visual acuity, and NV in CAB forms the basis
of multiple visual pathologies that may result in blindness.
However, CNV formations respond poorly to current therapies.
Therefore, potential anti-angiogenic topical treatments against CNV
resulting from alkali burns have been investigated in in
vitro studies and clinical trials (25,92–95). The suppression of VEGF and
placental growth factor levels in the cornea in a mouse model of
alkali burns was observed to significantly inhibit NV growth and
the regression of established vessels (96). PPAR-γ agonists are potent
inhibitors of NV and show potential for the treatment of
inflammatory vasculoproliferative diseases (97–100). Rosiglitazone has been shown to
protect vascular endothelial cells by reducing the expression of
the chemerin receptor, ChemR23 (101). Thiazolidinediones (TZDs) inhibit
retinal and choroidal NV by suppressing tube formation in human
umbilical vein endothelial cells (HUVECs). In addition, TZDs may
inhibit VEGF induced non-inflammatory NV in vivo (102). PEDF is a potent anti-angiogenic
factor and can induce endothelial cell apoptosis, and can inhibit
angiogenesis by augmenting PPAR-γ expression in ischemic heart
tissue (103). Therefore, PPAR-γ
may be a useful target in the prevention and treatment of vascular
inflammatory diseases.
Corneal fibrosis can result in visual impairment and
blindness. Alkali burned corneas were observed to exhibit obvious
interfibrillar distances with greater levels of the fibrotic marker
α-smooth muscle actin (αSMA) (104). The TGFβ-induced differentiation
of corneal fibroblasts to myofibroblasts could be prevented
(105). The level of
inflammation and scarring/fibrosis has been observed to increase
during healing in injured tissue in a model of CAB. The prognosis
of CAB is dependent upon ocular surface inflammation, and the
scarring and fibrosis of the cornea and eyelid (70,106). PPAR-γ possess strong
anti-fibrotic properties in the cornea and several other types of
tissue, with PPAR-γ ligands blocking αSMA induction (107). A number of studies have
demonstrated that treatment with ophthalmic solutions of PPAR-γ
agonists reduced the fibrotic reaction in the early phase post-CAB
and in additional fibrotic pathologies (106,108,109).
PPAR-γ is an important modulator of lipid metabolism
during inflammation, via the inhibition of the expression of
proinflammatory molecules (110). NF-κB is activated and
translocates to the nucleus where it controls the expression of a
large number of target genes, which are involved in the regulation
of inflammation and innate and adaptive immune responses (111). Telomeric repeat binding factor
was discovered as a modulator that regulates NF-κB signaling. The
inhibition of repeat binding factor may lead to the design of
specific inhibitors of NF-κB for the treatment of ocular injuries
(112). The effects of SN50, an
inhibitor of NF-κB, were reported to be dependent on TNF-α/JNK
signaling in a mouse model of CAB, with the topical application of
SN50 shown to be effective in treating CAB (113). PPAR-γ has been indicated to be
the predominant pathway involved in the inhibition of IL-1β-induced
inflammation [nitric oxide and prostaglandin E2 production, in
addition to inducible nitric oxide synthase and cyclooxygenase 2
(COX-2) expression, NF-κB and MAPK activation (114).
TC14012 [a chemokine (C-X-C motif) receptor 4
(CXCR4) antagonist and CXCR7 agonist] has been reported to
initially enhance alkali burn-induced CNV, then reduce CNV in later
stages. In addition to CXCR4, CXCR7 has been implicated in the
pathogenesis of CNV (115).
Granulocyte-colony stimulating factor (G-CSF) post-traumatic gene
expression activates innate immune responses and suppresses
adaptive immune responses. The G-CSF signal transducer and
activator of transcription axis has been indicated to be a key
protective mechanism post-injury in reducing the risk of infection
(116). PPAR-γ reduces the
expression levels of pro-inflammatory chemokines, including
chemokine (C-C motif) ligand 20, CXC ligand (CXCL)2, CXCL3 and
chemokine (C-X3-C motif) ligand 1 (CX3CL1) in colon tissues. It has
been shown that increasing the transcriptional activity of PPAR-γ
can modulate inflammatory signaling pathways, suggesting a novel
target for therapeutic agents (117). The investigation of inflammatory
markers in vascular disorders reveals augmented levels of
circulating cytokines and chemokines among carriers of classic risk
factors for atherosclerosis. Dysregulation of the PPAR signaling
pathway may explain the association of IL-8/12 and very low density
lipoprotein (VLDL)-c in the promotion of dysglycemia (118). The PPAR signaling pathway was
shown to be important in the modulation of inflammatory factors,
including MCP-1, TNF-α, IL-1 and IL-6, COX-2, nicotinamide adenine
dinucleotide phosphate, protein kinase C, vascular cell adhesion
molecule-1, NF-κB and monocyte expressions in HUVECs. The
inhibition of the PPAR pathway in endothelial inflammation suggests
a potential role of PPAR agonists in the treatment of vascular
inflammation (119,120). Rosiglitazone has been shown to
suppress angiogenesis by downregulating the expression of CXCR4 in
a dose-, time- and PPAR-γ-dependent manner (121). Regulating the expression of
MCP-1 and activating the 5′ AMP-activated protein kinase-sirtuin
1-PPAR signaling pathway may be a novel therapeutic agent for
atherosclerosis (122). PPAR-γ
has been indicated to regulate hypoxia/reoxygenation-stimulated
IL-8 production in U937 cells (123). PPAR-γ serves an inhibitory role
in hepatic injury by downregulating the local expression of
proinflammatory cytokines, chemokines and adhesion molecules
following reperfusion (124,125).
Keratocytes are able to directly degrade type I
collagen and create stromal spaces, promoting CNV through VEGF
induced MMP-13 expression (126). The inhibition of alkali
burn-induced CNV in mice may be possible via reductions in the
production of the angiogenic factors, inflammatory cytokines and
MMPs involved in the angiogenic response (78,127,128). MMP-12 may disintegrate certain
components of the extracellular matrix (ECM) released following
severe alkali burn, which may be involved in ECM remodeling
(129). Inhibiting alkali
burn-induced CNV by accelerating corneal wound healing and by
reducing the production of angiogenic factors, inflammatory
cytokines and MMPs may be a potential therapeutic strategy
(29,125,130–133). PPAR-γ agonists are able to
affect proliferation, differentiation, apoptosis and inflammation
in different cell types. PPAR-γ ligands were able to inhibit K562
and HL-60 cell adhesion to ECM proteins by inhibiting the
expression of MMP-2 and -9 (134). PPAR-γ agonists have been shown
to inhibit macrophage infiltration, the expression of TNF-α and
MMP-9 in aortic tissue, thus may be used as anti-inflammatory
agents in cardiovascular fields (135). Degradation of the epithelial
basement membrane in burned cornea in vivo was reversed by
an MMP inhibitor (136),
additionally; MMP inhibitors have been shown to block the
progression of alkali burns to ulceration (137). These data may
indicate that PPAR-γ agonists are a potential strategy for
preventing CAB progression.
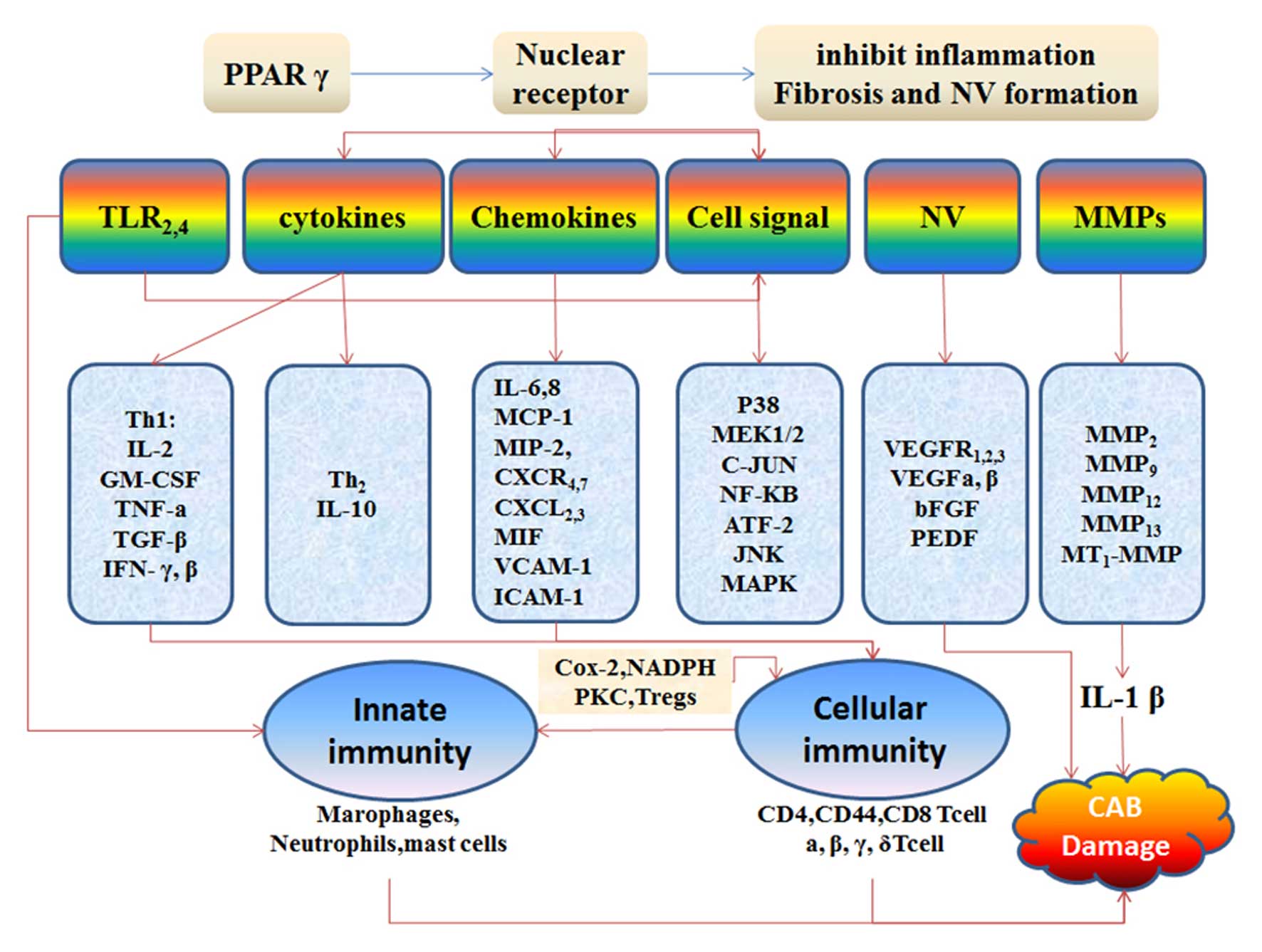
Taken together, the evidence suggests that PPAR-γ
may lessen NV, inflammation and scarring. However, additional
studies are necessary to evaluate the potential therapeutic effects
of PPAR-γ in ocular NV, tissue inflammation and the resultant
fibrosis following burn injury (Fig.
3).
The present study was supported by the Natural
Science Foundation of China (grant no. 81300727) and Jilin
University Basic Scientific Research Operating Expenses Fund
(Research Fund of the Bethune B Plan of Jilin University; grant no.
2012230).
|
1
|
Leong YY and Tong L: Barrier function in
the ocular surface: From conventional paradigms to new
opportunities. Ocul Surf. 13:103–109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cabalag MS, Wasiak J, Syed Q, Paul E, Hall
AJ and Cleland H: Early and late complications of ocular burn
injuries. J Plast Reconstr Aesthet Surg. 68:356–361. 2015.
View Article : Google Scholar
|
|
3
|
Saika S, Yamanaka O, Okada Y, Miyamoto T,
Kitano A, Flanders KC, Ohnishi Y, Nakajima Y, Kao WW and Ikeda K:
Effect of overexpression of PPARgamma on the healing process of
corneal alkali burn in mice. Am J Physiol Cell Physiol.
293:C75–C86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pargament JM, Armenia J and Nerad JA:
Physical and chemical injuries to eyes and eyelids. Clin Dermatol.
33:234–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang S, Gu H and Hu N: Role of Peroxisome
Proliferator-Activated Receptor γ in Ocular Diseases. J Ophthalmol.
2015:2754352015. View Article : Google Scholar
|
|
6
|
Hsu CC, Peng CH, Hung KH, Lee YY, Lin TC,
Jang SF, Liu JH, Chen YT, Woung LC, Wang CY, et al: Stem cell
therapy for corneal regeneration medicine and contemporary
nanomedicine for corneal disorders. Cell Transplant. 24:1915–1930.
2015. View Article : Google Scholar
|
|
7
|
Mittal V, Jain R, Mittal R, Vashist U and
Narang P: Successful management of severe unilateral chemical burns
in children using simple limbal epithelial transplantation (SLET).
Br J Ophthalmol. 2015:3071792015.
|
|
8
|
Movahedan A, Genereux BM, Darvish-Zargar
M, Shah KJ and Holland EJ: Long-term management of severe ocular
surface injury due to methamphetamine production accidents. Cornea.
34:433–437. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kafle PA, Singh SK, Sarkar I and Surin L:
Amniotic membrane transplantation with and without limbal stem cell
transplantation in chemical eye injury. Nepal J Ophthalmol.
7:52–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scholz SL, Thomasen H, Hestermann K,
Dekowski D, Steuhl KP and Meller D: Long-term results of autologous
transplantation of limbal epithelium cultivated ex vivo for limbal
stem cell deficiency. Ophthalmologe. 113:321–329. 2016.In German.
View Article : Google Scholar
|
|
11
|
Almaliotis D, Koliakos G, Papakonstantinou
E, Komnenou A, Thomas A, Petrakis S, Nakos I, Gounari E and
Karampatakis V: Mesenchymal stem cells improve healing of the
cornea after alkali injury. Graefes Arch Clin Exp Ophthalmol.
253:1121–1135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Holan V, Trosan P, Cejka C, Javorkova E,
Zajicova A, Hermankova B, Chudickova M and Cejkova J: Comparative
Study of the Therapeutic Potential of Mesenchymal Stem Cells and
Limbal Epithelial Stem Cells for Ocular Surface Reconstruction.
Stem Cells Transl Med. 4:1052–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sotozono C, Inatomi T, Nakamura T, Koizumi
N, Yokoi N, Ueta M, Matsuyama K, Kaneda H, Fukushima M and
Kinoshita S: Cultivated oral mucosal epithelial transplantation for
persistent epithelial defect in severe ocular surface diseases with
acute inflammatory activity. Acta Ophthalmol. 92:e447–e453. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudnisky CJ, Belin MW, Guo R and Ciolino
JB: Boston Type 1 Keratoprosthesis Study Group: Visual Acuity
Outcomes of the Boston Keratoprosthesis Type 1: Multicenter Study
Results. Am J Ophthalmol. 162:89–98. 2016. View Article : Google Scholar
|
|
15
|
Kammerdiener LL, Speiser JL, Aquavella JV,
Harissi-Dagher M, Dohlman CH, Chodosh J and Ciolino JB: Protective
effect of soft contact lenses after Boston keratoprosthesis. Br J
Ophthalmol. 100:549–552. 2016. View Article : Google Scholar
|
|
16
|
Iyer G, Srinivasan B, Rishi E, Rishi P,
Agarwal S and Subramanian N: Large lamellar corneoscleral grafts:
Tectonic role in initial management of severe ocular chemical
injuries. Eur J Ophthalmol. 26:12–17. 2016. View Article : Google Scholar
|
|
17
|
Prockop DJ: Inflammation, fibrosis, and
modulation of the process by mesenchymal stem/stromal cells. Matrix
Biol. 51:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi Y, Jiang D, Sindrilaru A, Stegemann A,
Schatz S, Treiber N, Rojewski M, Schrezenmeier H, Vander Beken S,
Wlaschek M, et al: TSG-6 released from intradermally injected
mesenchymal stem cells accelerates wound healing and reduces tissue
fibrosis in murine full-thickness skin wounds. J Invest Dermatol.
134:526–537. 2014. View Article : Google Scholar
|
|
19
|
Prockop DJ and Oh JY: Mesenchymal
stem/stromal cells (MSCs): Role as guardians of inflammation. Mol
Ther. 20:14–20. 2012. View Article : Google Scholar :
|
|
20
|
Moreira PB, Magalhães RS, Pereira NC,
Oliveira LA and Sousa LB: Limbal transplantation at a tertiary
hospital in Brazil: A retrospective study. Arq Bras Oftalmol.
78:207–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schimke MM, Marozin S and Lepperdinger G:
Patient-Specific age: The other side of the coin in advanced
mesenchymal stem cell therapy. Front Physiol. 6:3622015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamm V, Hara H, Mammen A, Dhaliwal D and
Cooper DK: Corneal blindness and xenotransplantation.
Xenotransplantation. 21:99–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heindl LM and Cursiefen C: Split-cornea
transplantation-a novel concept to reduce corneal donor shortage.
Klin Monbl Augenheilkd. 229:608–614. 2012.In German. PubMed/NCBI
|
|
24
|
Li X, Zhou Q, Hanus J, Anderson C, Zhang
H, Dellinger M, Brekken R and Wang S: Inhibition of multiple
pathogenic pathways by histone deacetylase inhibitor SAHA in a
corneal alkali-burn injury model. Mol Pharm. 10:307–318. 2013.
View Article : Google Scholar :
|
|
25
|
Bakunowicz-Łazarczyk A and Urban B:
Assessment of therapeutic options for reducing alkali burn-induced
corneal neovascularization and inflammation. Adv Med Sci.
61:101–112. 2016. View Article : Google Scholar
|
|
26
|
Atiba A, Wasfy T, Abdo W, Ghoneim A, Kamal
T and Shukry M: Aloe vera gel facilitates re-epithelialization of
corneal alkali burn in normal and diabetic rats. Clin Ophthalmol.
9:2019–2026. 2015.PubMed/NCBI
|
|
27
|
Rho CR, Choi JS, Seo M, Lee SK and Joo CK:
Inhibition of lymphangiogenesis and hemangiogenesis in corneal
inflammation by subconjunctival Prox1 siRNA injection in rats.
Invest Ophthalmol Vis Sci. 56:5871–5879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sijnave D, Van Bergen T, Castermans K,
Kindt N, Vandewalle E, Stassen JM, Moons L and Stalmans I:
Inhibition of Rho-associated kinase prevents pathological wound
healing and neovascularization after corneal trauma. Cornea.
34:1120–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lima TB, Ribeiro AP, Conceição LF,
Bandarra M, Manrique WG and Laus JL: Ketorolac eye drops reduce
inflammation and delay re-epithelization in response to corneal
alkali burn in rabbits, without affecting iNOS or MMP-9. Arq Bras
Oftalmol. 78:67–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai J, Dou G, Zheng L, Yang T, Jia X, Tang
L, Huang Y, Wu W, Li X and Wang X: Pharmacokinetics of topically
applied recombinant human keratinocyte growth factor-2 in
alkali-burned and intact rabbit eye. Exp Eye Res. 136:93–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shadmani A, Kazemi K, Khalili MR and
Eghtedari M: Omental transposition in treatment of severe ocular
surface alkaline burn: An experimental study. Med Hypothesis Discov
Innov Ophthalmol. 3:57–61. 2014.
|
|
32
|
Dvashi Z, Sar Shalom H, Shohat M, Ben-Meir
D, Ferber S, Satchi-Fainaro R, Ashery-Padan R, Rosner M, Solomon AS
and Lavi S: Protein phosphatase magnesium dependent 1A governs the
wound healing-inflammation-angiogenesis cross talk on injury. Am J
Pathol. 184:2936–2950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crooke A, Guzman-Aranguez A, Mediero A,
Alarma-Estrany P, Carracedo G, Pelaez T, Peral A and Pintor J:
Effect of melatonin and analogues on corneal wound healing:
Involvement of Mt2 melatonin receptor. Curr Eye Res. 40:56–65.
2015. View Article : Google Scholar
|
|
34
|
Iannetti L, Abbouda A, Fabiani C, Zito R
and Campanella M: Treatment of corneal neovascularization in ocular
chemical injury with an off-label use of subconjunctival
bevacizumab: A case report. J Med Case Reports. 7:1992013.
View Article : Google Scholar
|
|
35
|
Ozdemir O, Altintas O, Altintas L, Ozkan
B, Akdag C and Yüksel N: Comparison of the effects of
subconjunctival and topical anti-VEGF therapy (bevacizumab) on
experimental corneal neovascularization. Arq Bras Oftalmol.
77:209–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taira BR, Singer AJ, McClain SA, Lin F,
Rooney J, Zimmerman T and Clark RA: Rosiglitazone, a PPAR-gamma
ligand, reduces burn progression in rats. J Burn Care Res.
30:499–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pershadsingh HA and Moore DM: PPARgamma
Agonists: Potential as Therapeutics for Neovascular Retinopathies.
PPAR Res. 2008:1642732008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gelman L, Fruchart JC and Auwerx J: An
update on the mechanisms of action of the peroxisome
proliferator-activated receptors (PPARs) and their roles in
inflammation and cancer. Cell Mol Life Sci. 55:932–943. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chinetti G, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors and inflammation: From
basic science to clinical applications. Int J Obes Relat Metab
Disord. 27(Suppl 3): S41–S45. 2003. View Article : Google Scholar
|
|
40
|
Kostadinova R, Wahli W and Michalik L:
PPARs in diseases: Control mechanisms of inflammation. Curr Med
Chem. 12:2995–3009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen M, Matsuda H, Wang L, Watanabe T,
Kimura MT, Igarashi J, Wang X, Sakimoto T, Fukuda N, Sawa M, et al:
Pretranscriptional regulation of Tgf-β1 by PI polyamide prevents
scarring and accelerates wound healing of the cornea after exposure
to alkali. Mol Ther. 18:519–527. 2010. View Article : Google Scholar
|
|
42
|
Uchiyama M, Shimizu A, Masuda Y, Nagasaka
S, Fukuda Y and Takahashi H: An ophthalmic solution of a peroxisome
proliferator-activated receptor gamma agonist prevents corneal
inflammation in a rat alkali burn model. Mol Vis. 19:2135–2150.
2013.PubMed/NCBI
|
|
43
|
Sener G, Sehirli AO, Gedik N and Dülger
GA: Rosiglitazone, a PPAR-gamma ligand, protects against
burn-induced oxidative injury of remote organs. Burns. 33:587–593.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pershadsingh HA, Benson SC, Marshall,
Kurtz TW, Pravenec M, King JC, Stopa EG and Famiglietti EV: Ocular
diseases and peroxisome proliferator-activated receptor-γ (PPAR-γ)
in mammalian eye. Soc Neurosci Abstr. 25:21931999.
|
|
45
|
Balachandar S and Katyal A: Peroxisome
proliferator activating receptor (PPAR) in cerebral malaria (CM): A
novel target for an additional therapy. Eur J Clin Microbiol Infect
Dis. 30:483–498. 2011. View Article : Google Scholar
|
|
46
|
Pan H, Chen J, Xu J, Chen M and Ma R:
Antifibrotic effect by activation of peroxisome
proliferator-activated receptor-γ in corneal fibroblasts. Mol Vis.
15:2279–2286. 2009.PubMed/NCBI
|
|
47
|
Kaul D, Anand PK and Khanna A: Functional
genomics of PPAR-gamma in human immunomodulatory cells. Mol Cell
Biochem. 290:211–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Paterson HM, Murphy TJ, Purcell EJ,
Shelley O, Kriynovich SJ, Lien E, Mannick JA and Lederer JA: Injury
primes the innate immune system for enhanced Toll-like receptor
reactivity. J Immunol. 171:1473–1483. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bashir S, Sharma Y, Elahi A and Khan F:
Macrophage polarization: The link between inflammation and related
diseases. Inflamm Res. 65:1–11. 2016. View Article : Google Scholar
|
|
50
|
Valvis SM, Waithman J, Wood FM, Fear MW
and Fear VS: The Immune Response to Skin Trauma Is Dependent on the
Etiology of Injury in a Mouse Model of Burn and Excision. J Invest
Dermatol. 135:2119–2128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fletcher HA, Keyser A, Bowmaker M, Sayles
PC, Kaplan G, Hussey G, Hill AV and Hanekom WA: Transcriptional
profiling of mycobacterial antigen-induced responses in infants
vaccinated with BCG at birth. BMC Med Genomics. 2:102009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
D'Arpa N, D'Amelio L, Accardo-Palumbo A,
Pileri D, Mogavero R, Amato G, Napoli B, Alessandro G, Lombardo C
and Conte F: Skin dendritic cells in burn patients. Ann Burns Fire
Disasters. 22:175–178. 2009.PubMed/NCBI
|
|
53
|
Rani M, Zhang Q, Scherer MR, Cap AP and
Schwacha MG: Activated skin γδ T-cells regulate T-cell infiltration
of the wound site after burn. Innate Immun. 21:140–150. 2015.
View Article : Google Scholar
|
|
54
|
Schwacha MG, Zhang Q, Rani M, Craig T and
Oppeltz RF: Burn enhances toll-like receptor induced responses by
circulating leukocytes. Int J Clin Exp Med. 5:136–144.
2012.PubMed/NCBI
|
|
55
|
Yamada K, Ueta M, Sotozono C, Yokoi N,
Inatomi T and Kinoshita S: Upregulation of Toll-like receptor 5
expression in the conjunctival epithelium of various human ocular
surface diseases. Br J Ophthalmol. 98:1116–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
West AP, Koblansky AA and Ghosh S:
Recognition and signaling by toll-like receptors. Annu Rev Cell Dev
Biol. 22:409–437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Drage MG, Pecora ND, Hise AG, Febbraio M,
Silverstein RL, Golenbock DT, Boom WH and Harding CV: TLR2 and its
co-receptors determine responses of macrophages and dendritic cells
to lipoproteins of Mycobacterium tuberculosis. Cell Immunol.
258:29–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cornick SM, Noronha SA, Noronha SM,
Cezillo MV, Ferreira LM and Gragnani A: Toll like receptors gene
expression of human keratinocytes cultured of severe burn injury.
Acta Cir Bras. 29(Suppl 3): 33–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shupp JW, Nasabzadeh TJ, Rosenthal DS,
Jordan MH, Fidler P and Jeng JC: A review of the local
pathophysiologic bases of burn wound progression. J Burn Care Res.
31:849–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kitano A, Okada Y, Yamanka O, Shirai K,
Mohan RR and Saika S: Therapeutic potential of trichostatin A to
control inflammatory and fibrogenic disorders of the ocular
surface. Mol Vis. 16:2964–2973. 2010.
|
|
61
|
Chistyakov DV, Aleshin SE, Astakhova AA,
Sergeeva MG and Reiser G: Regulation of peroxisome
proliferator-activated receptors (PPAR) a and -γ of rat brain
astrocytes in the course of activation by toll-like receptor
agonists. J Neurochem. 134:113–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chistyakov DV, Aleshin S, Sergeeva MG and
Reiser G: Regulation of peroxisome proliferator-activated receptor
β/δ expression and activity levels by toll-like receptor agonists
and MAP kinase inhibitors in rat astrocytes. J Neurochem.
130:563–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ding JL, Zhou ZG, Zhou XY, Zhou B, Wang L,
Wang R, Zhan L, Sun XF and Li Y: Attenuation of acute pancreatitis
by peroxisome proliferator-activated receptor-α in rats: The effect
on Toll-like receptor signaling pathways. Pancreas. 42:114–122.
2013. View Article : Google Scholar
|
|
64
|
Zhao W, Wang L, Zhang M, Wang P, Zhang L,
Yuan C, Qi J, Qiao Y, Kuo PC and Gao C: Peroxisome
proliferator-activated receptor gamma negatively regulates IFN-beta
production in Toll-like receptor (TLR) 3- and TLR4-stimulated
macrophages by preventing interferon regulatory factor 3 binding to
the IFN-beta promoter. J Biol Chem. 286:5519–5528. 2011. View Article : Google Scholar
|
|
65
|
Pan S, Lei L, Chen S, Li H and Yan F:
Rosiglitazone impedes Porphyromonas gingivalis-accelerated
atherosclerosis by down-regulating the TLR/NF-κB signaling pathway
in atherosclerotic mice. Int Immunopharmacol. 23:701–708. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lian M, Luo W, Sui Y, Li Z and Hua J:
Dietary n-3 PUFA protects mice from Con A induced liver injury by
modulating regulatory T cells and PPAR-γ expression. PLoS One.
10:e01327412015. View Article : Google Scholar
|
|
67
|
Li T, Wang W, Zhao JH, Zhou X, Li YM and
Chen H: Pseudolaric acid B inhibits T-cell mediated immune response
in vivo via p38MAPK signal cascades and PPARγ activation. Life Sci.
121:88–96. 2015. View Article : Google Scholar
|
|
68
|
Kraft CT, Agarwal S, Ranganathan K, Wong
VW, Loder S, Li J, Delano MJ and Levi B: Trauma-induced heterotopic
bone formation and the role of the immune system: A review. J
Trauma Acute Care Surg. 80:156–165. 2016. View Article : Google Scholar
|
|
69
|
Xiu F and Jeschke MG: Perturbed
mononuclear phagocyte system in severely burned and septic
patients. Shock. 40:81–88. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ferrari G, Bignami F, Giacomini C,
Franchini S and Rama P: Safety and efficacy of topical infliximab
in a mouse model of ocular surface scarring. Invest Ophthalmol Vis
Sci. 54:1680–1688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yamada J, Dana MR, Sotozono C and
Kinoshita S: Local suppression of IL-1 by receptor antagonist in
the rat model of corneal alkali injury. Exp Eye Res. 76:161–167.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sotozono C, He J, Matsumoto Y, Kita M,
Imanishi J and Kinoshita S: Cytokine expression in the
alkali-burned cornea. Curr Eye Res. 16:670–676. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lu P, Li L, Liu G, Zhang X and Mukaida N:
Enhanced experimental corneal neovascularization along with
aberrant angiogenic factor expression in the absence of IL-1
receptor antagonist. Invest Ophthalmol Vis Sci. 50:4761–4768. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sakimoto T, Yamada A, Kanno H and Sawa M:
Upregulation of tumor necrosis factor receptor 1 and TNF-alpha
converting enzyme during corneal wound healing. Jpn J Ophthalmol.
52:393–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pattamatta U, Willcox M, Stapleton F and
Garrett Q: Bovine lactoferrin promotes corneal wound healing and
suppresses IL-1 expression in alkali wounded mouse cornea. Curr Eye
Res. 38:1110–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shin YJ, Hyon JY, Choi WS, Yi K, Chung ES,
Chung TY and Wee WR: Chemical injury-induced corneal opacity and
neovascularization reduced by rapamycin via TGF-β1/ERK pathways
regulation. Invest Ophthalmol Vis Sci. 54:4452–4458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ling S, Li W, Liu L, Zhou H, Wang T, Ye H,
Liang L and Yuan J: Allograft survival enhancement using
doxycycline in alkali-burned mouse corneas. Acta Ophthalmol.
91:e369–e378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Xiao O, Xie ZL, Lin BW, Yin XF, Pi RB and
Zhou SY: Minocycline inhibits alkali burn-induced corneal
neovascularization in mice. PLoS One. 7:e418582012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jeon HS, Yi K, Chung TY, Hyon JY, Wee WR
and Shin YJ: Chemically injured keratocytes induce cytokine release
by human peripheral mononuclear cells. Cytokine. 59:280–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cairns B, Maile R, Barnes CM, Frelinger JA
and Meyer AA: Increased Toll-like receptor 4 expression on T cells
may be a mechanism for enhanced T cell response late after burn
injury. J Trauma. 61:293–298; discussion 298–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Planck SR, Rich LF, Ansel JC, Huang XN and
Rosenbaum JT: Trauma and alkali burns induce distinct patterns of
cytokine gene expression in the rat cornea. Ocul Immunol Inflamm.
5:95–100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
De Nuccio C, Bernardo A, Cruciani C, De
Simone R, Visentin S and Minghetti L: Peroxisome proliferator
activated receptor-γ agonists protect oligodendrocyte progenitors
against tumor necrosis factor-alpha-induced damage: Effects on
mitochondrial functions and differentiation. Exp Neurol.
271:506–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shimada K, Furukawa H, Wada K, Korai M,
Wei Y, Tada Y, Kuwabara A, Shikata F, Kitazato KT, Nagahiro S, et
al: Protective Role of Peroxisome Proliferator-Activated Receptor-γ
in the Development of Intracranial Aneurysm Rupture. Stroke.
46:1664–1672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mirza RE, Fang MM, Novak ML, Urao N, Sui
A, Ennis WJ and Koh TJ: Macrophage PPARγ and impaired wound healing
in type 2 diabetes. J Pathol. 236:433–444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lan LF, Zheng L, Yang X, Ji XT, Fan YH and
Zeng JS: Peroxisome proliferator-activated receptor-γ agonist
pioglitazone ameliorates white matter lesion and cognitive
impairment in hypertensive rats. CNS Neurosci Ther. 21:410–416.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang RC and Jiang DM: PPAR-γ agonist
pioglitazone affects rat gouty arthritis by regulating cytokines.
Genet Mol Res. 13:6577–6581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cheng Y, Dong Z and Liu S: β-Caryophyllene
ameliorates the Alzheimer-like phenotype in APP/PS1 mice through
CB2 receptor activation and the PPARγ pathway. Pharmacology.
94:1–12. 2014. View Article : Google Scholar
|
|
88
|
Bhattarai G, Lee YH and Yi HK: Peroxisome
proliferator activated receptor gamma loaded dental implant
improves osteogenesis of rat mandible. J Biomed Mater Res B Appl
Biomater. 103:587–595. 2015. View Article : Google Scholar
|
|
89
|
Guri AJ, Mohapatra SK, Horne WT II,
Hontecillas R and Bassaganya-Riera J: The role of T cell PPAR γ in
mice with experimental inflammatory bowel disease. BMC
Gastroenterol. 10:602010. View Article : Google Scholar
|
|
90
|
Amparo F, Sadrai Z, Jin Y,
Alfonso-Bartolozzi B, Wang H, Shikari H, Ciolino JB, Chodosh J,
Jurkunas U, Schaumberg DA, et al: Safety and efficacy of the
multitargeted receptor kinase inhibitor pazopanib in the treatment
of corneal neovascularization. Invest Ophthalmol Vis Sci.
54:537–544. 2013. View Article : Google Scholar :
|
|
91
|
Huang X, Han Y, Shao Y and Yi JL: Efficacy
of the nucleotide-binding oligomerzation domain 1 inhibitor
Nodinhibit-1 on corneal alkali burns in rats. Int J Ophthalmol.
8:860–865. 2015.PubMed/NCBI
|
|
92
|
Lee CM, Jung WK, Na G, Lee DS, Park SG,
Seo SK, Yang JW, Yea SS, Lee YM, Park WS, et al: Inhibitory effects
of the platelet-activating factor receptor antagonists, CV-3988 and
Ginkgolide B, on alkali burn-induced corneal neovascularization.
Cutan Ocul Toxicol. 34:53–60. 2015. View Article : Google Scholar
|
|
93
|
Giacomini C, Ferrari G, Bignami F and Rama
P: Alkali burn versus suture-induced corneal neovascularization in
C57BL/6 mice: An overview of two common animal models of corneal
neovascularization. Exp Eye Res. 121:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bignami F, Giacomini C, Lorusso A, Aramini
A, Rama P and Ferrari G: NK1 receptor antagonists as a new
treatment for corneal neovascularization. Invest Ophthalmol Vis
Sci. 55:6783–6794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Koenig Y, Bock F, Kruse FE, Stock K and
Cursiefen C: Angioregressive pretreatment of mature corneal blood
vessels before keratoplasty: Fine-needle vessel coagulation
combined with anti-VEGFs. Cornea. 31:887–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhou AY, Bai YJ, Zhao M, Yu WZ and Li XX:
KH902, a recombinant human VEGF receptor fusion protein, reduced
the level of placental growth factor in alkali burn induced-corneal
neovascularization. Ophthalmic Res. 50:180–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Xin X, Yang S, Kowalski J and Gerritsen
ME: Peroxisome proliferator-activated receptor gamma ligands are
potent inhibitors of angiogenesis in vitro and in vivo. J Biol
Chem. 274:9116–9121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Vucic E, Dickson SD, Calcagno C, Rudd JH,
Moshier E, Hayashi K, Mounessa JS, Roytman M, Moon MJ, Lin J, et
al: Pioglitazone modulates vascular inflammation in atherosclerotic
rabbits noninvasive assessment with FDG-PET-CT and dynamic
contrast-enhanced MR imaging. JACC Cardiovasc Imaging. 4:1100–1109.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Usui T, Sugisaki K, Iriyama A, Yokoo S,
Yamagami S, Nagai N, Ishida S and Amano S: Inhibition of corneal
neovascularization by blocking the angiotensin II type 1 receptor.
Invest Ophthalmol Vis Sci. 49:4370–4376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Panigrahy D, Kaipainen A, Huang S,
Butterfield CE, Barnés CM, Fannon M, Laforme AM, Chaponis DM,
Folkman J and Kieran MW: PPARalpha agonist fenofibrate suppresses
tumor growth through direct and indirect angiogenesis inhibition.
Proc Natl Acad Sci USA. 105:985–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Hao F, Mu JW, Zhang HJ, Kuang HY, Yu QX,
Bai MM and Meng P: Damage to vascular endothelial cells by high
insulin levels is associated with increased expression of ChemR23,
and attenuated by PPAR-gamma agonist, rosiglitazone. Neuro
Endocrinol Lett. 36:59–66. 2015.PubMed/NCBI
|
|
102
|
Sarayba MA, Li L, Tungsiripat T, Liu NH,
Sweet PM, Patel AJ, Osann KE, Chittiboyina A, Benson SC,
Pershadsingh HA and Chuck RS: Inhibition of corneal
neovascularization by a peroxisome proliferator-activated
receptor-gamma ligand. Exp Eye Res. 80:435–442. 2005. View Article : Google Scholar : PubMed/NCBI
Exp Eye Res. 80:435–442. 2005. View Article : Google Scholar
|
|
103
|
Zhang H, Wei T, Jiang X, Li Z, Cui H, Pan
J, Zhuang W, Sun T, Liu Z, Zhang Z and Dong H: PEDF and 34-mer
inhibit angiogenesis in the heart by inducing tip cells apoptosis
via up-regulating PPAR-γ to increase surface FasL. Apoptosis.
21:60–68. 2016. View Article : Google Scholar
|
|
104
|
Gronkiewicz KM, Giuliano EA, Kuroki K,
Bunyak F, Sharma A, Teixeira LB, Hamm CW and Mohan RR: Development
of a novel in vivo corneal fibrosis model in the dog. Exp Eye Res.
143:75–88. 2016. View Article : Google Scholar
|
|
105
|
Donnelly KS, Giuliano EA, Sharm A and
Mohan RR: Suberoylanilide hydroxamic acid (vorinostat): Its role on
equine corneal fibrosis and matrix metalloproteinase activity. Vet
Ophthalmol. 17(Suppl 1): 61–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhou Q, Yang L, Qu M, Wang Y, Chen P, Wang
Y and Shi W: Role of senescent fibroblasts on alkali-induced
corneal neovascularization. J Cell Physiol. 227:1148–1156. 2012.
View Article : Google Scholar
|
|
107
|
Jeon KI, Phipps RP, Sime PJ and Huxlin KR:
Inhibitory effects of PPARγ ligands on TGF-β1-induced CTGF
expression in cat corneal fibroblasts. Exp Eye Res. 138:52–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yoon YS, Kim SY, Kim MJ, Lim JH, Cho MS
and Kang JL: PPARγ activation following apoptotic cell instillation
promotes resolution of lung inflammation and fibrosis via
regulation of efferocytosis and proresolving cytokines. Mucosal
Immunol. 8:1031–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Luo H, Zhu H, Zhou B, Xiao X and Zuo X:
MicroRNA-130b regulates scleroderma fibrosis by targeting
peroxisome proliferator-activated receptor γ. Mod Rheumatol.
25:595–602. 2015. View Article : Google Scholar
|
|
110
|
Zoccal KF, Paula-Silva FW, Bitencourt CS,
Sorgi CA, Bordon KC, Arantes EC and Faccioli LH: PPAR-γ activation
by Tityus serrulatus venom regulates lipid body formation and lipid
mediator production. Toxicon. 93:90–97. 2015. View Article : Google Scholar
|
|
111
|
Wang C, Zeng L, Zhang T, Liu J and Wang W:
Tenuigenin prevents IL-1β-induced inflammation in human
osteoarthritis chondrocytes by suppressing pi3k/akt/nf-κb signaling
pathway. Inflammation. 39:807–812. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Poon MW, Yan L, Jiang D, Qin P, Tse HF,
Wong IY, Wong DS, Tergaonkar V and Lian Q: Inhibition of RAP1
enhances corneal recovery following alkali injury. Invest
Ophthalmol Vis Sci. 56:711–721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Saika S, Miyamoto T, Yamanaka O, Kato T,
Ohnishi Y, Flanders KC, Ikeda K, Nakajima Y, Kao WW, Sato M, et al:
Therapeutic effect of topical administration of SN50, an inhibitor
of nuclear factor-κB, in treatment of corneal alkali burns in mice.
Am J Pathol. 166:1393–1403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ma Z, Piao T, Wang Y and Liu J: Astragalin
inhibits IL-1β-induced inflammatory mediators production in human
osteoarthritis chondrocyte by inhibiting nf-κb and MAPK activation.
Int Immunopharmacol. 25:83–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Shen M, Yuan F, Jin J and Yuan Y: The
effect of TC14012 on alkali burn-induced corneal neovascularization
in mice. Ophthalmic Res. 52:17–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Gardner JC, Noel JG, Nikolaidis NM, Karns
R, Aronow BJ, Ogle CK and McCormack FX: G-CSF drives a
posttraumatic immune program that protects the host from infection.
J Immunol. 192:2405–2417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Choo J, Lee Y, Yan XJ, Noh TH, Kim SJ, Son
S, Pothoulakis C, Moon HR, Jung JH and Im E: A Novel Peroxisome
Proliferator-activated Receptor (PPAR)γ Agonist 2-Hydroxyethyl
5-chloro-4,5-didehydrojasmonate Exerts Anti-Inflammatory Effects in
Colitis. J Biol Chem. 290:25609–25619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Pires AS, Souza VC, Paula RS, Toledo JO,
Lins TC, Moraes CF, Córdova C, Pereira RW and Nóbrega OT:
Pro-inflammatory cytokines correlate with classical risk factors
for atherosclerosis in the admixed Brazilian older women. Arch
Gerontol Geriatr. 60:142–146. 2015. View Article : Google Scholar
|
|
119
|
Zhang F, Sun D, Chen J, Guan N, Huo X and
Xi H: Simvastatin attenuates angiotensin II-induced inflammation
and oxidative stress in human mesangial cells. Mol Med Rep.
11:1246–1251. 2015.
|
|
120
|
Xu S, Song H, Huang M, Wang K, Xu C and
Xie L: Telmisartan inhibits the proinflammatory effects of
homocysteine on human endothelial cells through activation of the
peroxisome proliferator-activated receptor-δ pathway. Int J Mol
Med. 34:828–834. 2014.PubMed/NCBI
|
|
121
|
Qin L, Gong C, Chen AM, Guo FJ, Xu F, Ren
Y and Liao H: Peroxisome proliferator-activated receptor γ agonist
rosiglitazone inhibits migration and invasion of prostate cancer
cells through inhibition of the CXCR4/CXCL12 axis. Mol Med Rep.
10:695–700. 2014.PubMed/NCBI
|
|
122
|
Dong W, Wang X, Bi S, Pan Z, Liu S, Yu H,
Lu H, Lin X, Wang X, Ma T and Zhang W: Inhibitory effects of
resveratrol on foam cell formation are mediated through monocyte
chemotactic protein-1 and lipid metabolism-related proteins. Int J
Mol Med. 33:1161–1168. 2014.PubMed/NCBI
|
|
123
|
Higashihara H, Kokura S, Imamoto E, Ueda
M, Naito Y, Yoshida N and Yoshikawa T: Hypoxia-reoxygenation
enhances interleukin-8 production from U937 human monocytic cells.
Redox Rep. 9:365–369. 2004. View Article : Google Scholar
|
|
124
|
Akahori T, Sho M, Hamada K, Suzaki Y,
Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H and Nakajima
Y: Importance of peroxisome proliferator-activated receptor-gamma
in hepatic ischemia/reperfusion injury in mice. J Hepatol.
47:784–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sakimoto T and Ishimori A:
Anti-inflammatory effect of topical administration of tofacitinib
on corneal inflammation. Exp Eye Res. 145:110–117. 2016. View Article : Google Scholar
|
|
126
|
Ma J, Zhou D, Fan M, Wang H, Huang C,
Zhang Z, Wu Y, Li W, Chen Y and Liu Z: Keratocytes create stromal
spaces to promote corneal neovascularization via MMP13 expression.
Invest Ophthalmol Vis Sci. 55:6691–6703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang H, Li C and Baciu PC: Expression of
integrins and MMPs during alkaline-burn-induced corneal
angiogenesis. Invest Ophthalmol Vis Sci. 43:955–962.
2002.PubMed/NCBI
|
|
128
|
Yang JW, Lee SM, Oh KH, Park SG, Choi IW
and Seo SK: Effects of topical chondrocyte-derived extracellular
matrix treatment on corneal wound healing, following an alkali burn
injury. Mol Med Rep. 11:461–467. 2015.
|
|
129
|
Iwanami H, Ishizaki M, Fukuda Y and
Takahashi H: Expression of matrix metalloproteinases (MMP)-12 by
myofibroblasts during alkali-burned corneal wound healing. Curr Eye
Res. 34:207–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Bian F, Pelegrino FS, Tukler Henriksson
JT, Pflugfelder SC, Volpe EA, Li DQ and de Paiva CS: Differential
Effects of Dexamethasone and Doxycycline on Inflammation and MMP
Production in Murine Alkali-Burned Corneas Associated with Dry Eye.
Ocul Surf. 14:242–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yang SJ, Jo H, Kim KA, Ahn HR, Kang SW and
Jung SH: Diospyros kaki Extract Inhibits Alkali Burn-Induced
Corneal Neovascularization. J Med Food. 19:106–109. 2016.
View Article : Google Scholar
|
|
132
|
Ke Y, Wu Y, Cui X, Liu X, Yu M, Yang C and
Li X: Polysaccharide hydrogel combined with mesenchymal stem cells
promotes the healing of corneal alkali burn in rats. PLoS One.
10:e01197252015. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Liu J, Lu H, Huang R, Lin D, Wu X, Lin Q,
Wu X, Zheng J, Pan X, Peng J, et al: Peroxisome proliferator
activated receptor-gamma ligands induced cell growth inhibition and
its influence on matrix metalloproteinase activity in human myeloid
leukemia cells. Cancer Chemother Pharmacol. 56:400–408. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Motoki T, Kurobe H, Hirata Y, Nakayama T,
Kinoshita H, Rocco KA, Sogabe H, Hori T, Sata M and Kitagawa T:
PPAR-γ agonist attenuates inflammation in aortic aneurysm patients.
Gen Thorac Cardiovasc Surg. 63:565–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kato T, Saika S and Ohnishi Y: Effects of
the matrix metalloproteinase inhibitor GM6001 on the destruction
and alteration of epithelial basement membrane during the healing
of post-alkali burn in rabbit cornea. Jpn J Ophthalmol. 50:90–95.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Fini ME, Cui TY, Mouldovan A, Grobelny D,
Galardy RE and Fisher SJ: An inhibitor of the matrix
metalloproteinase synthesized by rabbit corneal epithelium. Invest
Ophthalmol Vis Sci. 32:2997–3001. 1991.PubMed/NCBI
|