Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease characterized by chronic synovitis and bone erosions. The
massive infiltration of inflammatory cells as well as multiple
cytokines in the synovium contribute to the progression of
synovitis, which plays a key role in the destruction of bone.
Osteoclast formation and differentiation have been described in the
inflamed synovial membrane, where they derive from precursors of
the monocyte/macrophage lineage (1). Macrophages are central effectors of
synovitis, mainly acting through the release of cytokines [such as
tumor necrosis factor-α (TNF)-α and interleukin (IL)-1 and IL-6] to
induce osteoclastogenesis (2).
Some proinflammatory cytokines, such as TNF-α, or IL-1β provided by
activated CD4+ T cells and synoviocytes may induce
osteoclastogenesis directly or act indirectly on osteoclastogenesis
by enhancing the release of receptor activator of nuclear factor-κB
ligand (RANKL) from other cells (3,4).
Agents capable of regulating these proinflammatory cytokines may
prevent disease progression, and are therefore suitable candidates
for use in the treatment of RA.
IL-21 is a recently discovered cytokine produced by
activated CD4+ T lymphocytes and follicular helper T
(Tfh) cells (5,6). Functional IL-21 receptor (IL-21R) is
broadly expressed on immune cells and some non-immune cells
including fibroblasts and epithelial cells (7). One study showed that the
CD4+ T cells of patients with RA expressed significantly
higher levels of IL-21R than those from patients with
osteoarthritis (8).
Correspondingly, IL-21 exerts pleiotropic effects on a broad range
of cell types; IL-21 regulates the proliferation of T cells, the
proliferation and differentiation of B cells, and the activation
and expansion of natural killer cells (9). It also promotes the secretion of
matrix metalloproteinases in fibroblasts (10). It has been demonstrated that IL-21
may play a role in many autoimmune diseases, including RA (9).
Serum levels of IL-21 and IL-23 positively correlate
with disease activity and radiologic changes in RA (11). The blockade of IL-21 signaling
pathways with an IL-21R Fc fusion protein (IL-21R.Fc) reduces the
production of inflammatory cytokines and attenuates the progression
of arthritis in animal models of collagen-induced arthritis (CIA)
(12). It has been demonstrated
that IL-21 enhances RANKL expression in CD4+ T cells and
fibroblast-like synoviocytes (FLSs) from patients with RA, and
IL-21 has osteoclastogenic potential in the presence of low dose
RANKL and macrophage colony-stimulating factor (M-CSF), both in
humans with RA and in mice with CIA (13). These results show that IL-21 may
play a role in synovitis and bone erosion in RA.
It remains unclear whether IL-21 promotes
osteoclastogenesis directly in the absence of RANKL. To determine
the direct effect of IL-21 on osteoclastogenesis, we evaluated the
osteoclastogenic potential of IL-21 on RAW264.7 cells, using
tartrate-resistant acid phosphatase (TRAP) staining for osteoclasts
and quantitative assays to measure the expression of osteoclastic
markers. The results demonstrated that IL-21 induces the
osteoclastogenesis of RAW264.7 cells independently of RANKL through
the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway.
IL-21 also showed osteogenic potency in the peripheral blood
mononuclear cells (PBMCs) isolated from patients with RA. Taken
together, these findings suggest that IL-21 may be a novel
therapeutic target for the treatment of RA.
Materials and methods
Antibodies and reagents
Phycoerythrin-conjugated rat monoclonal anti-mouse
RANK antibody (12-6612) and the isotypic antibody,
phycoerythrin-conjugated rat IgG2b (15-4815), were purchased from
eBioscience, Inc. (San Diego, CA, USA). Anti-phospho-AKT (Ser473)
(9271), anti-AKT (9272), anti-phospho-p44/42 ERK (Thr202/Tyr204)
(4370), anti-ERK (4695), and anti-signal transducer and activator
of transcription 3 (STAT3) (4904) antibodies were obtained from
Cell Signaling Technology, Inc. (Beverly, MA, USA). The antibody
against mouse phospho-STAT3 (Ser727) (sc-135649) was purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-RANK
(ab200369), anti-calcitonin receptor (CTR) (ab11042) and
anti-IL-21R (ab5980) and anti-β-actin (ab8229) antibodies were
obtained from Abcam (Cambridge, UK). Horseradish
peroxidase-conjugated secondary antibodies were purchased from
LI-COR Biosciences (Lincoln, Nebraska, USA). Recombinant IL-21,
RANKL and M-CSF were obtained from Peprotech, Inc. (Rocky Hill, NJ,
USA). AG490 [Janus kinase 2 (JAK2)/STAT3 inhibitor], LY294002
(PI3K/AKT inhibitor), and PD98059 (ERK inhibitor) were supplied by
Selleck Chemicals (Houston, TX, USA).
Induction of osteoclastogenesis by IL-21
in RAW264.7 cells
RAW264.7 cells, a murine macrophage cell line, were
obtained from the Cell Center of Peking Union Medical College
(Beijing, China). The cells were cultured in high-glucose
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
(v/v) inactivated fetal bovine serum (FBS), 100 U/ml penicillin and
100 µg/ml streptomycin (all from Gibco, Grand Island, NY,
USA) in a 37°C incubator containing a 5% CO2-enriched
atmosphere. The RAW264.7 cells were further cultured onto 24-well
plates (5×104 cells/well) in the presence of 5 ng/ml
recombinant M-CSF, with or without 10 ng/ml soluble RANKL (sRANKL),
or various concentrations of IL-21 (0, 1, 10, 20 and 40 ng/ml) for
5 days in order to generate osteoclasts. Osteoprotegerin (OPG;
Peprotech, Rocky Hill, NJ, USA) (20 and 100 ng/ml) was added to the
culture with M-CSF and IL-21 to eliminate the osteoclastogenic
effects of endogenous RANKL. Prior to the addition of IL-21 (20
ng/ml) into the culture, various signaling pathway inhibitors
(AG490 50 µM, LY294002 10 µM and PD98059 20
µM) were incubated with RAW264.7 cells for 30 min.
Osteoclast formation was determined after 5 days of culture. The
phosphorylation of the signaling proteins was detected by western
blot analysis precisely 0, 5, 15, 30, 45, 60, 90 and 120 min after
initiating stimulus with IL-21 (20 ng/ml).
TRAP staining
The RAW264.7 cells were plated at a density of
5×104 cells/well in 24-well plates for 12 h, and then
treated with the indicated compounds for an additional 5 days. The
supernatants were removed, and the cells were washed twice with
phosphate-buffered saline (PBS). Paraformaldehyde (4%) was added to
the cells for 20 min at room temperature and then thoroughly
removed with deionized water. Naphthol AS-BI phosphate and tartrate
solution (Sigma-Aldrich, St Louis, MO, USA) were added to the cells
for 30 min at 37°C, followed by counterstaining with a hematoxylin
solution (Sigma-Aldrich). Osteoclasts were determined to be
TRAP-positively stained, multinucleated (three or more nuclei)
cells and counted under a light microscope (original magnification,
×100).
Immunocytochemistry
The RAW264.7 cells were cultured in 24-well plates
in the presence of M-CSF, with or without sRANKL, or various
concentrations of IL-21 for 5 days. The supernatants were removed,
and the cells were fixed in paraformaldehyde (4%) for 20 min at
room temperature. The cells were depleted of endogenous peroxidase
activity with 3% H2O2 for 15 minutes, blocked
with secondary antibody serum for 20 min, and incubated with
anti-CTR antibody at 4°C overnight. The samples were incubated with
an HRP-conjugated secondary antibody at 37°C for 30 minutes
followed by 3,3′-diaminobenzidine (Dako, Glostrup, Denmark). The
cells were counterstained with haematoxylin, and the images of the
samples were captured using a photomicroscope (original
magnification, ×100).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions. Two micrograms of total RNA from each sample was
reverse transcribed using Superscript II Reverse Transcriptase
(Invitrogen). The reverse transcription reaction was performed at
42°C for 50 min, and then at 70°C for 15 min. PCR amplification was
performed on a Bio-Rad iQ5 Real-Time PCR system (Berkely, CA, USA)
using SYBR-Green dye (Promega Corp., Madison, WI, USA). The melting
curve temperatures of each PCR gene were all 60°C. The following
primers were used: GAPDH forward, 5′-AAATGGTGAAGGTCGGTGTG-3′ and
reverse, 5′-TGAAGGGGTCGTTGATGG-3′; CTR forward,
5′-TGGCGACTATCTACTGCTTCTG-3′ and reverse,
5′-GTTGTTGCTGATTGGAGGATTC-3′; RANK forward,
5′-GTCTCATCGTTCTGCTCCTCTT-3′ and reverse,
5′-AACTGCTTTTTGAGCCAGGAC-3′; and cathepsin K forward,
5′-GTTGTATGTATAACGCCACGGC-3′ and reverse,
5′-CTTTCTCGTTCCCCACAGGA-3. The fold changes compared with the
control were calculated using the formula 2−ΔΔCt.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen) according to the manufacturer's instructions
and 1 µg of total RNA from each sample was reverse
transcribed using Superscript II Reverse Transcriptase
(Invitrogen). The reverse transcription reaction was performed at
42°C for 50 min, and then at 70°C for 15 min. PCR amplification was
performed by denaturation at 94°C for 30 sec, annealing at 60°C for
30 sec, and extension at 72°C for 30 sec using Takara Ex Taq
(Takara Bio, Inc., Otsu, Japan). The amplified PCR products were
separated on a 1.5% agarose gel. The following primers were used:
IL-21R forward, 5′-GGCTGCCTTACTCCTGCTG-3′ and reverse,
5′-TCATCTTGCCAGGTGAGACTG-3′; and TRAP forward,
5′-AACTTCCCCAGCCCTTACTACC-3′ and reverse,
5′-AACTGCTTTTTGAGCCAGGAC-3′; The primer sequences for GAPDH, RANK,
CTR and cathepsin K were the same as those used for RT-qPCR.
Western blot analysis
The RAW264.7 cells were lysed in protein extraction
solution. The lysates were centrifuged at 12,000 × g for 15 min at
4°C to remove the cell debris. The protein concentration of the
extract was determined using the BCA protein assay kit (Beijing
ComWin Biotech Co., Ltd., Beijing, China). Equal amounts of protein
were separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride (PVDF)
membranes (Immobilon-P; Millipore, Billerica, MA, USA) and blocked
with 5% skim milk in 0.05% Tween-20 and Tris-buffered saline (TBST)
at room temperature for 2 h. The membranes were then incubated with
various primary antibodies, which were diluted in 5% bovine serum
albumin (BSA)-TBST at 4°C with gentle shaking overnight. After
washing, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibody at room temperature for 1
h. The blots were developed using an enhanced chemiluminescence
detection kit (Pierce Biotechnology, Inc., Rockford, IL, USA).
Bone resorption analysis
The RAW264.7 macrophages prepared using the methods
described above were cultured in 96-well plates with thin slices of
femoral cow bone (Nordic Bioscience, Copenhagen, Denmark). The
RAW264.7 cells were further cultured in the presence of 5 ng/ml
recombinant M-CSF, with or without 10 ng/ml sRANKL, or IL-21 (20
ng/ml) in order to generate osteoclasts. After 7 days, the cells on
the bone slice were removed, and the resorption pits on the bone
slices were observed under a scanning electronic microscope
(S-4800; Hitachi, Tokyo, Japan).
Induction of osteoclastogenesis by IL-21
in RA
The present study was approved by the Ethics
Committee of Peking University Third Hospital (Beijing, China).
Informed consent for the use of human mononuclear cells were
obtained from all patients. Peripheral blood was obtained from 5
patients with RA (2 male, 3 female; mean age, 41.14±11.07 years).
PBMCs were isolated from buffy coats by density-gradient
centrifugation using Ficoll-Hypaque (Life Technologies, Grand
Island, NY, USA). The cells were washed three times with sterile
PBS and resuspended in RPMI-1640 (Life Technologies) supplemented
with 10% FBS, then seeded onto 24-well plates at a density of
1×106 cells/well and incubated at 37°C for 2 h to
separate the floating and adherent cells. The adherent cells were
washed with sterile PBS and cultured with M-CSF (50 ng/ml), M-CSF +
RANKL (100 ng/ml), or various concentrations of IL-21 (0, 1, 10, 50
and 100 ng/ml). On day 7, TRAP-positive multinucleated cells were
identified using an acid phosphatase kit (Sigma-Aldrich), according
to the manufacturer's instructions. To examine the
osteoclast-togenetic effect of IL-21 in the presence of T
lymphocytes, the floating cells were not removed when adding M-CSF
(50 ng/ml), M-CSF + RANKL (100 ng/ml), or IL-21 (0, 1, 10, 50 and
100 ng/ml) in order to generate osteoclasts.
Statistical analysis
Statistical analysis was performed using the SPSS
17.0 statistical software package (SPSS, Inc., Chicago, IL, USA)
and GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA,
USA). All quantitative data is expressed as the means ± standard
error of the mean (SEM) for each condition. For comparisons of
multiple groups, a one-way analysis of variance (ANOVA) followed by
a Scheffe's post hoc test was performed. P-values <0.05 were
considered to indicate a statistically significant difference.
Results
IL-21 enhances RANK expression in
RAW264.7 cells
RANK and RANKL are known to be the prototype
mediators of osteoclastogenesis (14). A previous study has shown that
IL-21 enhances RANKL expression in cultured mixed joint cells and
CD4+ T cells from mice with CIA (13). Herein, we examined whether IL-21
induced the expression of RANK in RAW264.7 cells. As shown in
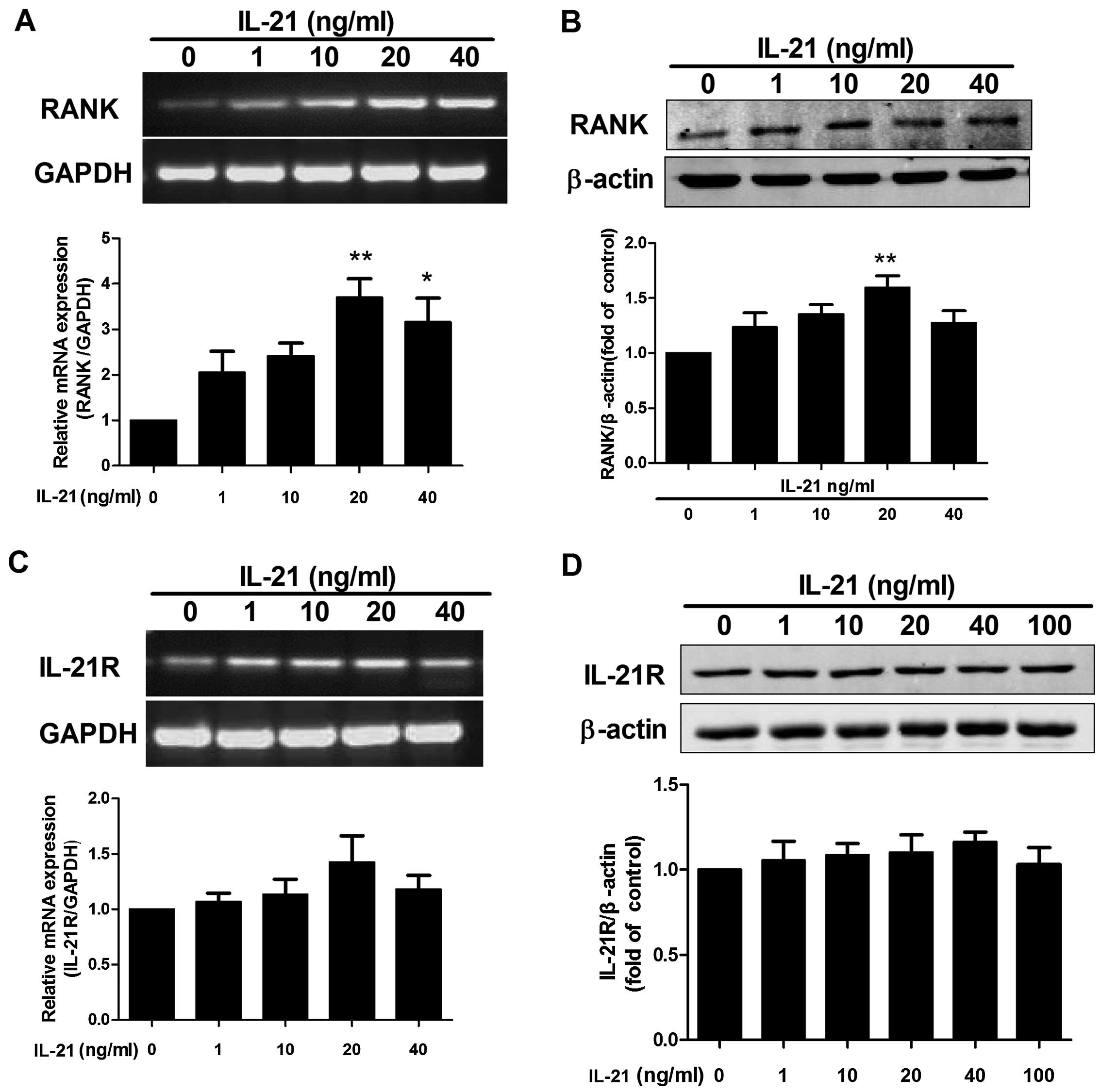
Fig. 1A, the RT-PCR results
showed that the mRNA expression of RANK was increased by
stimulation with IL-21 (20 ng/ml). Western blot analysis confirmed
that IL-21 upregulated the expression of RANK (Fig. 1B). We also examined whether IL-21
induced IL-21R expression in RAW264.7 cells. As shown in Fig. 1C and D, IL-21 had no significant
effect on IL-21R expression.
IL-21 induces osteoclastogenesis in
RAW264.7 cells directly
To examine whether IL-21 has osteoclastogenic
potential without RANKL, RAW264.7 cells were stimulated with IL-21
and M-CSF, in the presence or absence of RANKL. The results showed
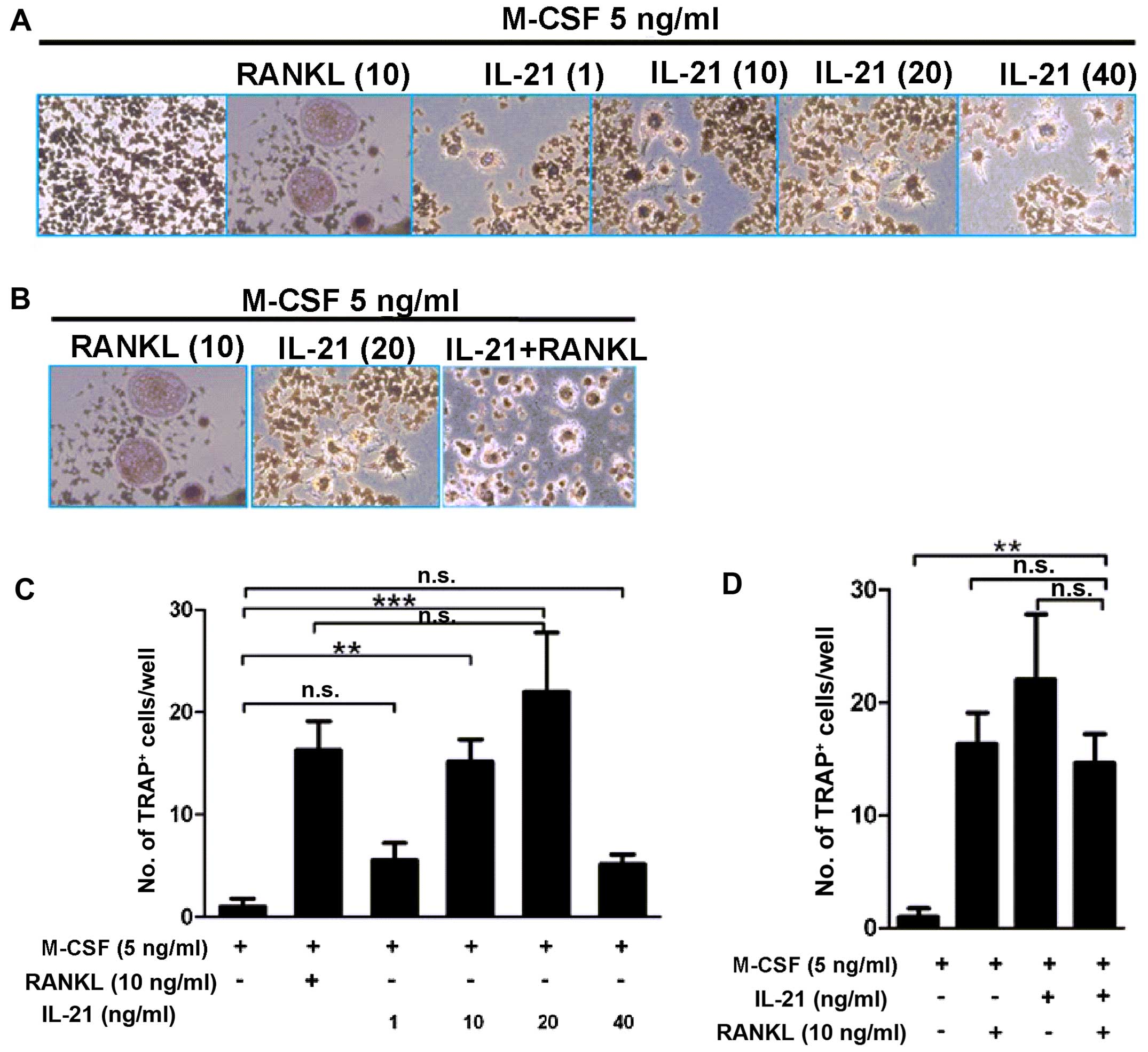
that IL-21 induced osteoclastogenesis. As shown in Fig. 2, the IL-21 10 ng/ml group
(15.17±3.79/well, P<0.01) and 20 ng/ml group (22±10.04/well,
P<0.01) showed osteoclast formation with more TRAP-positive
cells than the negative control group (1±1.32/well). By contrast,
IL-21 plus RANKL (14.67±4.37/well) did not show any advantages in
promoting osteoclastogenesis compared with IL-21 (22±10.04/well,
P=0.241) and RANKL (16.33±4.8/well, P>0.05) single
stimulation.
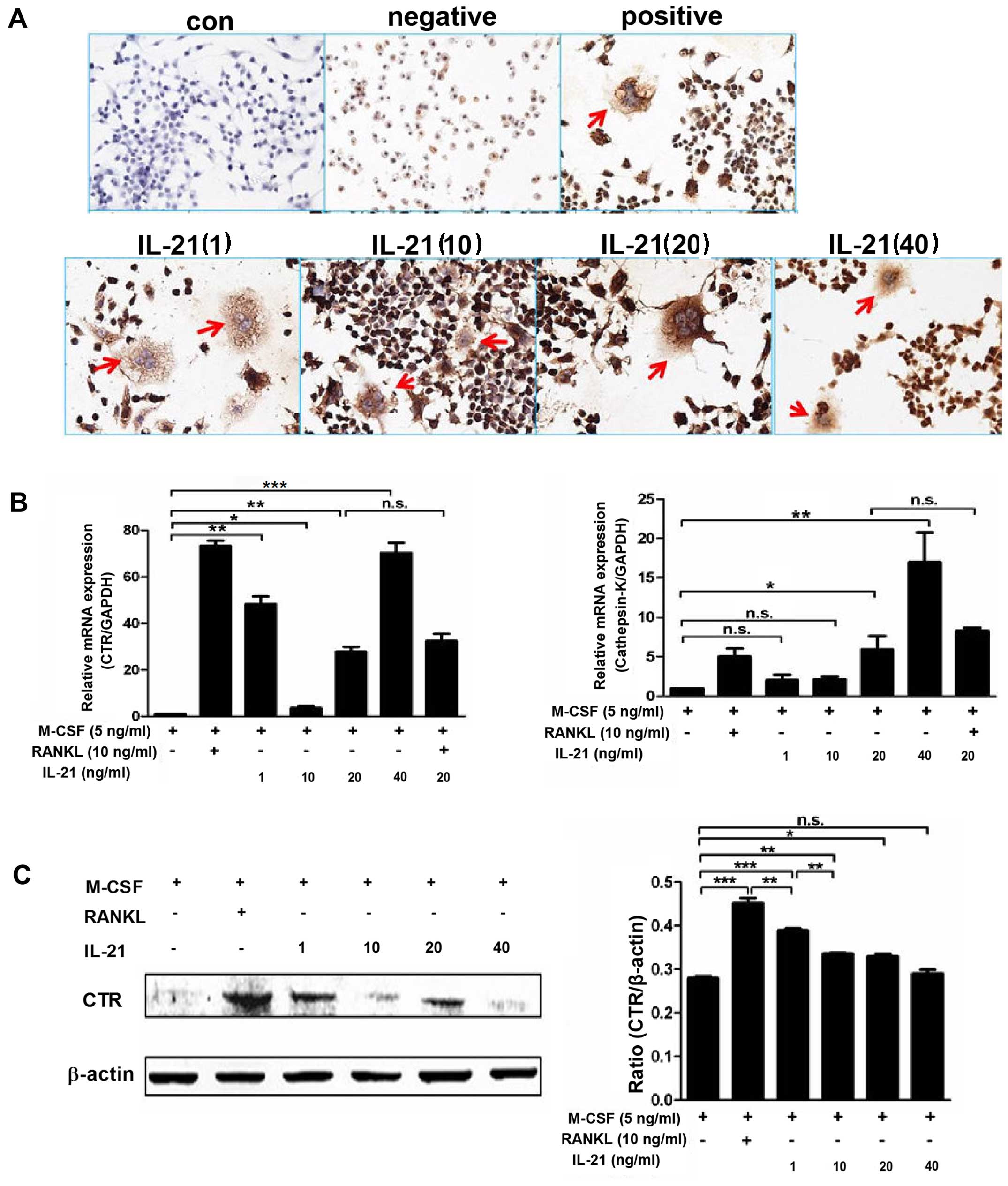
Immunocytochemistry revealed higher positive
expression of CTR in the IL-21 group (Fig. 3A) than in the control group.
Western blot analysis showed (Fig.
3C) that RANKL markedly induced CTR expression. IL-21 induced a
moderate effect on CTR expression compared with RANKL. Low dose
IL-21 (1 ng/ml) stimulated the expression of CTR in a manner which
was not dose-dependent. In the absence of RANKL or RANKL-producing
cells, IL-21 enhanced the expression of CTR directly, and the
number of multinucleated cells and CTR+ osteoclasts was
increased. RT-qPCR also verified the increased mRNA expression of
CTR and cathepsin K in the IL-21 group compared with that in the
negative group (Fig. 3B).
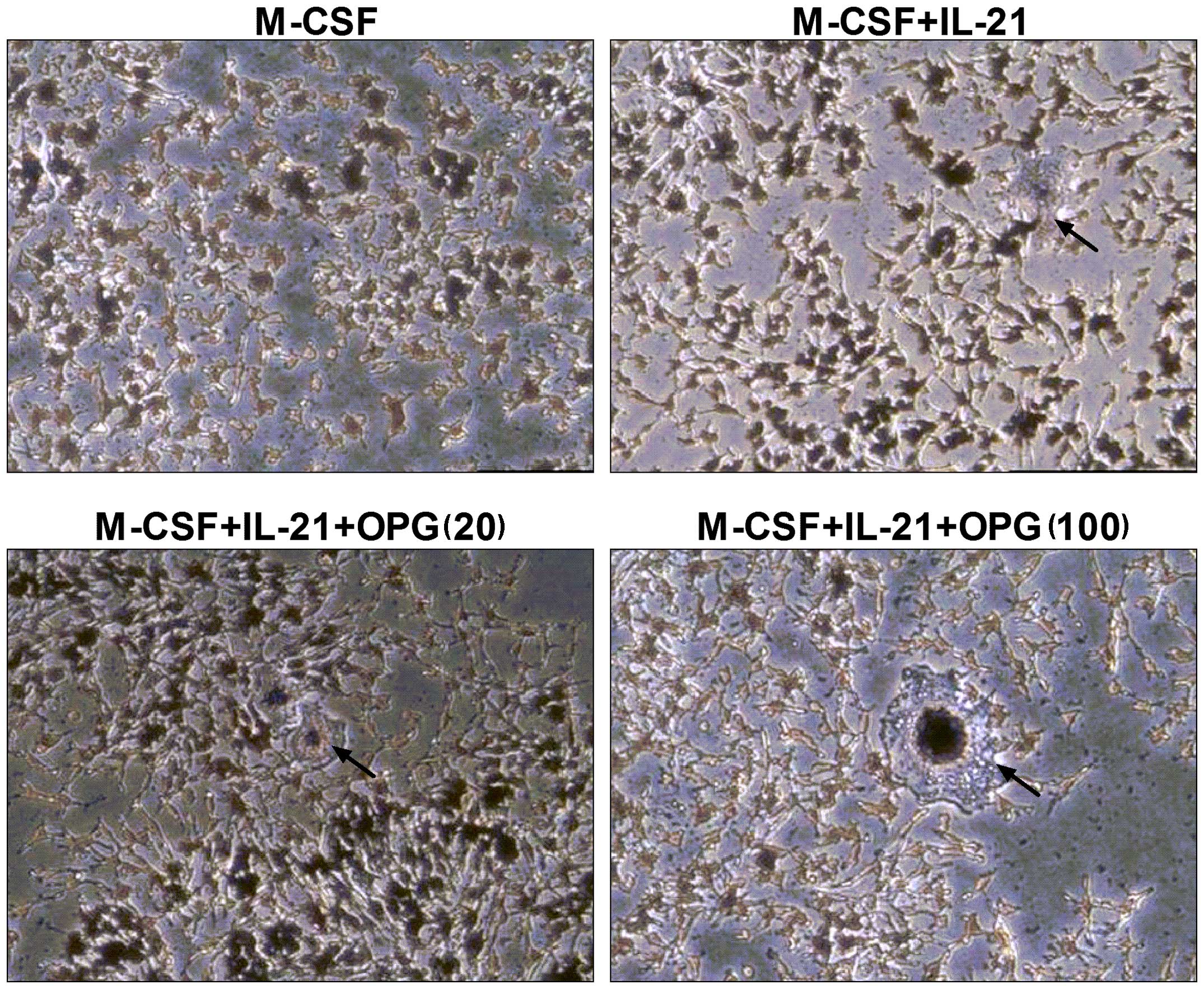
In order to eliminate the osteoclastogenic effects
of endogenous RANKL, OPG was added to the culture system. The
results showed that OPG had no effect on IL-21-induced
osteoclastogenesis (Fig. 4). The
results suggested that the effect of IL-21 on the stimulation of
osteoclast differentiation was unaffected by endogenous RANKL.
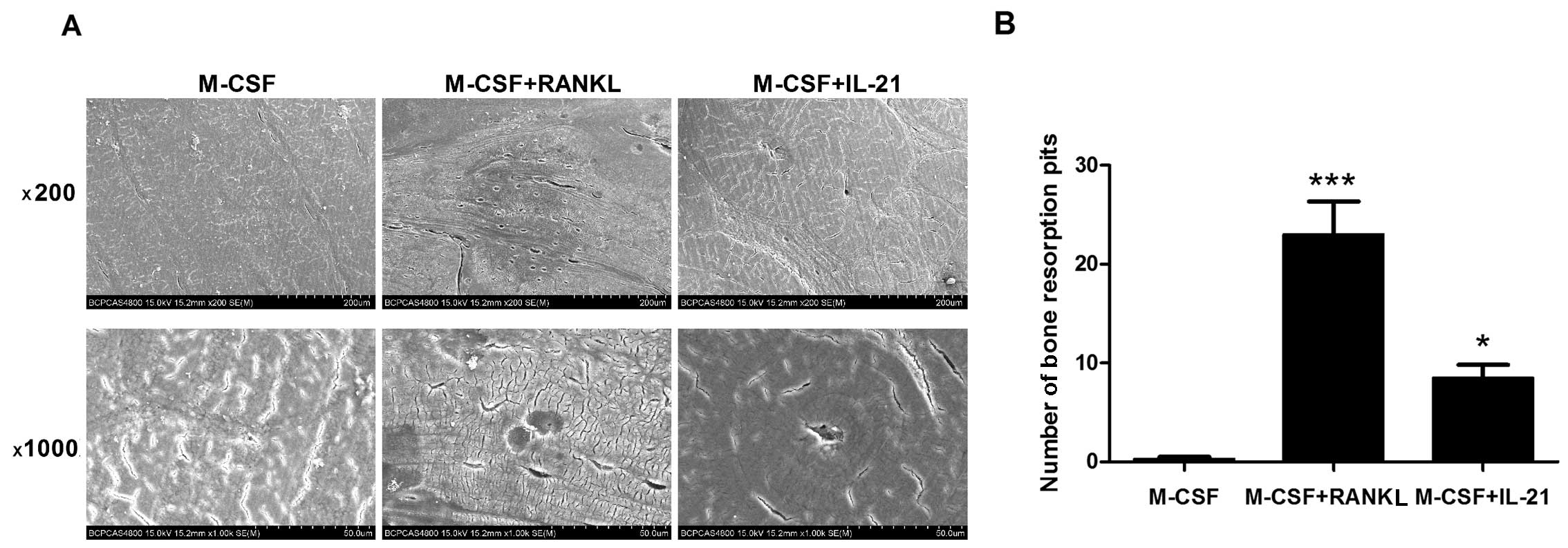
The scanning electron microscope images showed a
number of resorption pits on the bone slices cultured with RANKL.
In the IL-21 groups, a few resorption pits were observed (Fig. 5). The results indicated that IL-21
had direct osteoclastogenic potential without the facilitation of
RANKL, although the effect was mild.
IL-21 induces osteoclastogenesis of
RAW264.7 cells through the PI3K/AKT signaling pathway
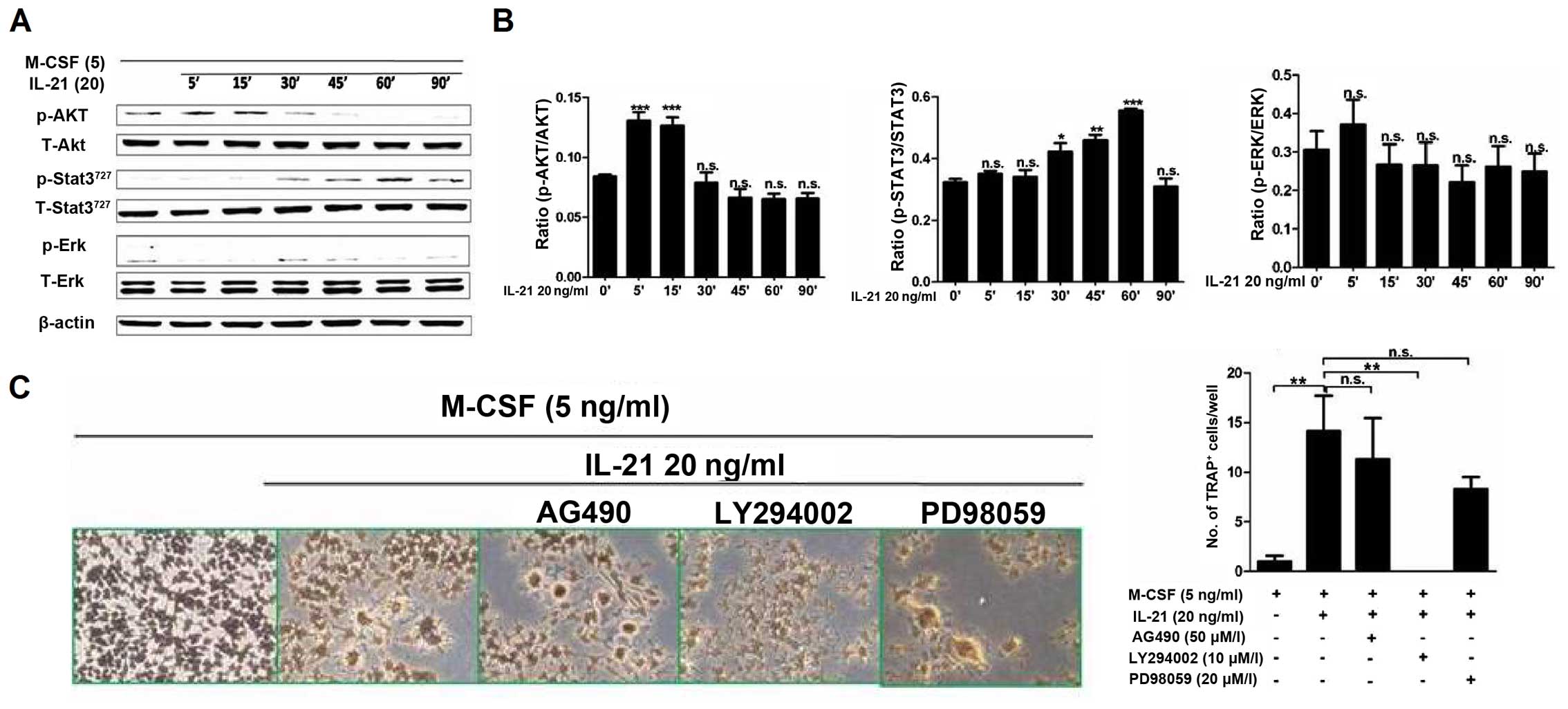
IL-21 activates several signaling pathways,
including the JAK1 and STAT3 pathway, the mitogen-activated protein
kinase (MAPK) pathway, and the PI3K signaling pathway (9). To determine the intracellular
mechanisms involved in IL-21-induced osteoclastogenesis, we
evaluated the effect of IL-21 on the PI3K/AKT, STAT3 and ERK1/2
pathways. We stimulated RAW264.7 cells with IL-21 (20 ng/ml) for 5,
15, 30, 45, 60, 90 and 120 min. Western blot analysis revealed that
(Fig. 6A and B) the
phosphorylation of AKT and STAT3 were significantly induced by
IL-21. Phosphorylated (p-)AKT was highly activated at 5–15 min
after IL-21 (20 ng/ml) stimulation and p-STAT3 levels were
increased at 30–60 min of IL-21 stimulation. There was no
phosphorylation of ERK.
To examine the intracellular signaling pathways
mediating the induction of osteoclast differentiation by IL-21,
RAW264.7 cells were pre-treated with the signaling pathway
inhibitors AG490 (JAK-2/STAT-3 inhibitor; 50 µM), LY294002
(PI3K/AKT inhibitor; 10 µM), and PD98059 (ERK inhibitor; 20
µM) for 30 min, and then cultured with IL-21 (20 ng/ml) for
5 days. The PI3K/AKT pathway inhibitor LY294002 significantly
suppressed IL-21-induced osteoclastogenesis. As shown in Fig. 6C, no TRAP+ cells were
found in the LY294002 group. No significant differences in the
number of TRAP+ cells were observed among the
IL-21+AG490 (11.33±7.11/well, P>0.05), IL-21+PD98059
(8.33±2.02/well, P>0.05), and IL-21 (20 ng/ml) groups. The mRNA
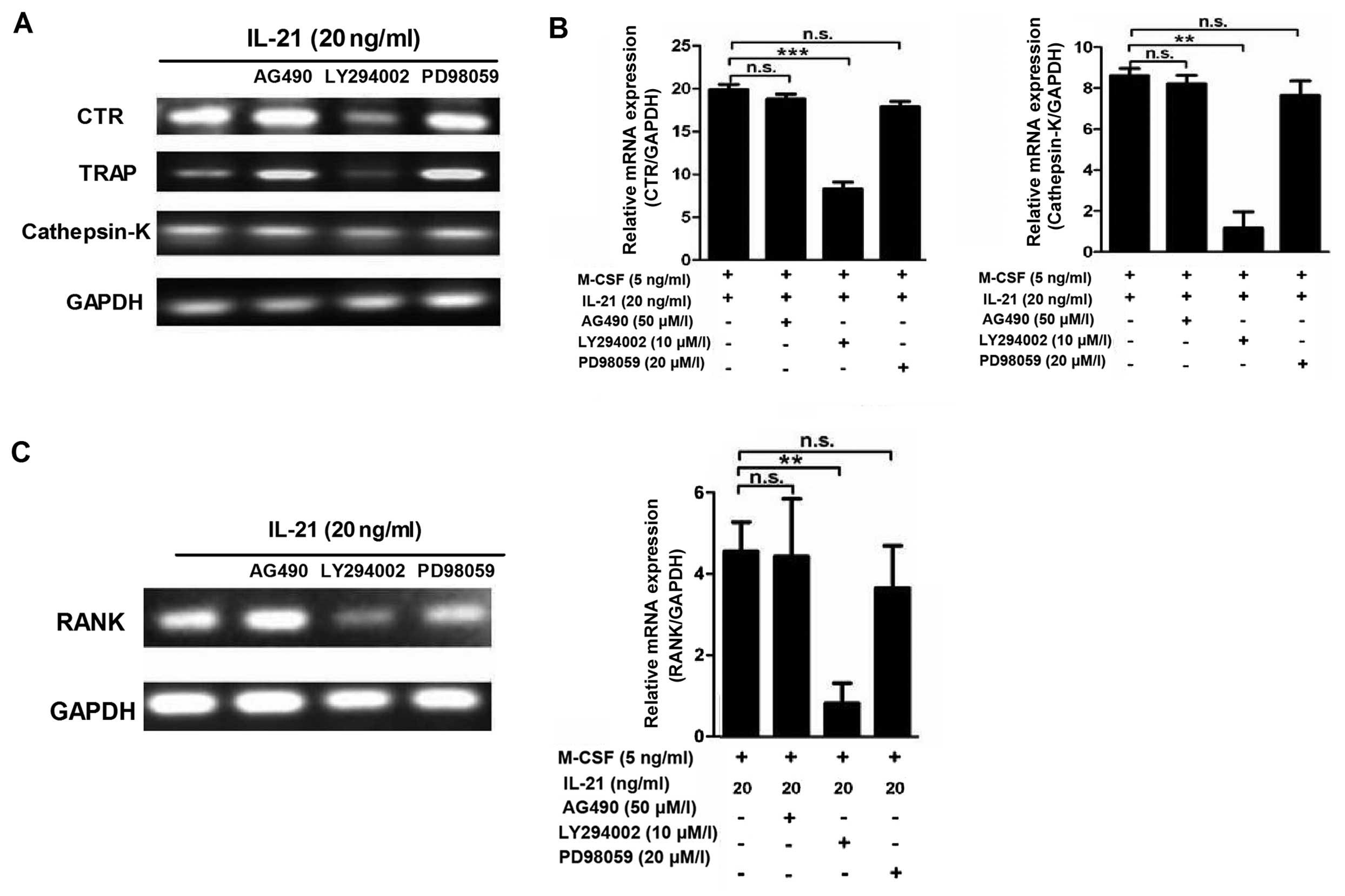
expression of CTR, TRAP and cathepsin K were also decreased in the
LY294002 group (Fig. 7A and B).
The RAW264.7 cells were pre-treated with signaling pathway
inhibitors (AG490 50 µM, LY294002 10 µM and PD98059
20 µM) for 30 min prior to adding IL-21. RANK expression was
significantly inhibited by the PI3K/AKT signal pathway inhibitor
LY294002 (Fig. 7C). These results
showed that IL-21 may promote osteoclastogenesis through the
PI3K/AKT signaling pathway.
Osteoclastogenesis is induced by IL-21 in
RA
PBMCs obtained from patients with RA were cultured
with IL-21 to induce osteoclastogenesis. IL-21 induced osteoclast
formation in the PBMCs isolated from patients with RA, showing
similar results to the cell line (Fig. 8A). With the existence of T cells
in the culture, IL-21 induced more TRAP+ cells in the 10
ng/ml group (10±4.97/well, P=0.019) and 50 ng/ml group
(21±8.46/well, P<0.01) than the negative control (0.8±0.57/well)
(Fig. 8A). Without
RANKL-providing T cells, IL-21 50 ng/ml (14.38±3.22/well,
P<0.01) also showed osteoclastogenic activity, compared with
negative control group (1.38±1.03/well) (Fig. 8C). IL-21 did not enhance the
osteoclastogenic effect of RANKL (Fig. 8B and D).
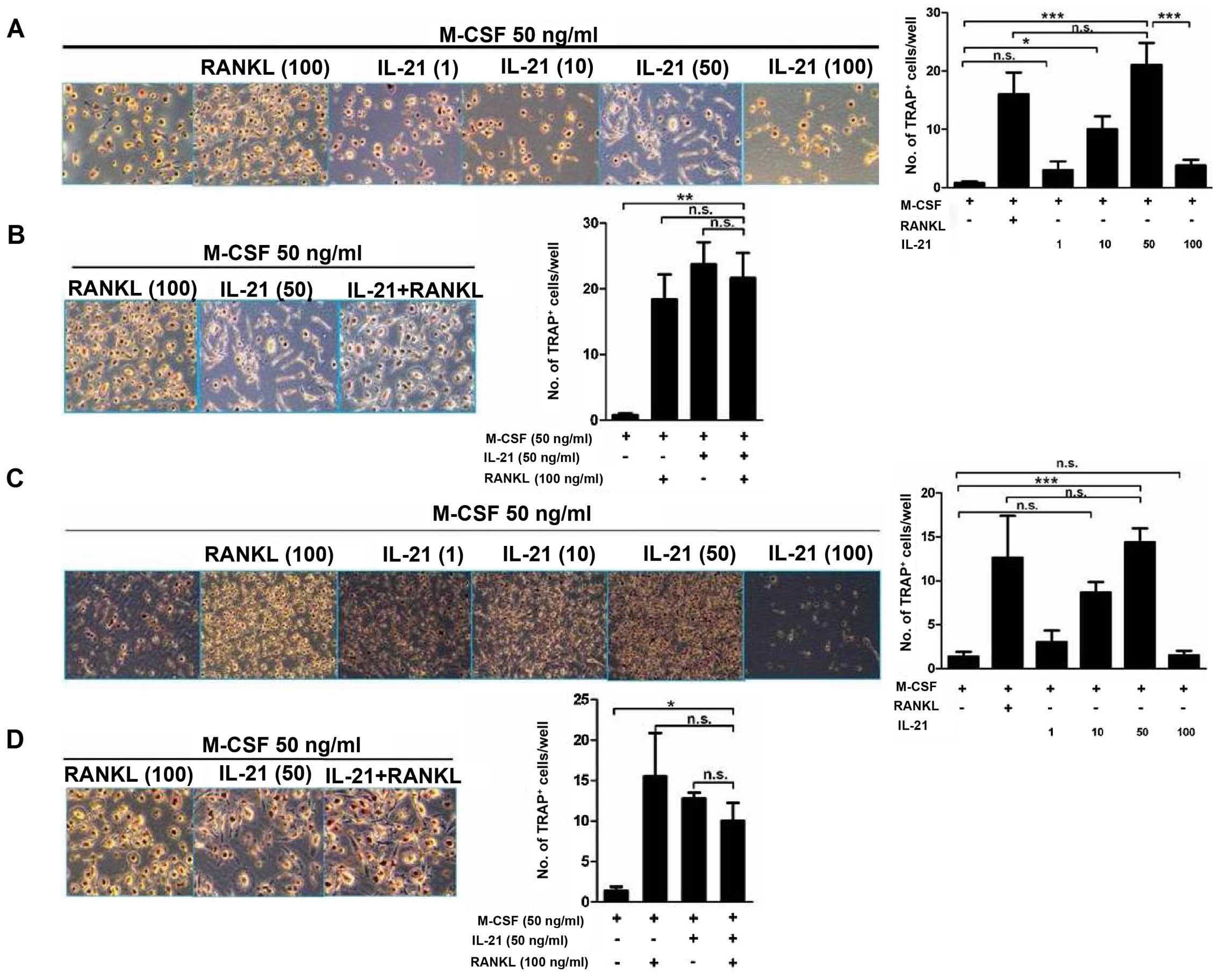 | Figure 8Osteoclastogenesis is induced by
interleukin-21 (IL-21) in peripheral blood mononuclear cells
(PBMCs) isolated from patients with rheumatoid arthritis (RA). (A)
PBMCs obtained from patients with RA were cultured in 24-well
plates at a density of 1×106 cells/well in the presence
of T cells, and then macrophage colony-stimulating factor (M-CSF)
(50 ng/ml), M-CSF + receptor activator of nuclear factor-κB ligand
(RANKL) (100 ng/ml), or IL-21 (1, 10, 50 and 100 ng/ml) were added
for 7 days in order to generate osteoclasts. Tartrate-resistant
acid phosphatase (TRAP)-positive cells were identified using an
acid phosphatase kit. (B) PBMCs were co-cultured with T cells in
the presence of M-CSF (50 ng/ml), M-CSF + RANKL (100 ng/ml), or
IL-21 (50 ng/ml) for 7 days. TRAP-positive cells were identified
using an acid phosphatase kit. (C) PBMCs were cultured with M-CSF
(50 ng/ml), M-CSF + RANKL (100 ng/ml), or IL-21 (1, 10, 50 and 100
ng/ml) for 7 days. TRAP-positive cells were identified using an
acid phosphatase kit. (D) PBMCs were cultured with M-CSF (50
ng/ml), M-CSF + RANKL (100 ng/ml), or IL-21 (50 ng/ml) for 7 days.
TRAP-positive cells were identified using an acid phosphatase kit.
Original magnification, ×100. Data are expressed as the means ± SEM
of three samples. *P<0.05, **P<0.01,
***P<0.001. |
Discussion
IL-21 is a four-helix-bundle cytokine produced by
activated CD4+ T cells, activated natural killer T cells
and Tfh cells. IL-21 binds to the cell surface receptor IL-21R, a
member of the class I cytokine receptor family, specifically to the
γc subunit which is shared with IL-2, IL-4, IL-7, IL-9, IL-13 and
IL-15 receptors (15), leading to
the activation of members of the JAK family of protein tyrosine
kinases and STAT molecules. In some cell types, IL-21 also
activates members of the MAPK family (16). In addition, IL-21R signaling may
also lead to activation of both the MAPK and PI3K pathways, which
are important for IL-21-mediated cell proliferation (17). IL-21 may play important roles in
regulating the function of multiple types of immune and non-immune
cells.
IL-21 driven tissue damage has been demonstrated in
some T cell-mediated diseases, including RA. IL-21 was demonstrated
to participate in T cell activation and synovial inflammation in RA
(8). Young et al
demonstrated that the blockade of IL-21 with IL-21R.Fc ameliorated
clinical disease activity in animal models of CIA (12). Blocking IL-21 by IL-21R.Fc
effectively ameliorated CIA by suppressing Th17 and antibody
production (18).
IL-21R-deficiency protects against severe inflammation and joint
destruction in antigen-induced arthritis and chronic streptococcal
cell wall-induced arthritis (19). Kwok et al found that IL-21
enhanced in vitro osteoclastogenesis by inducing RANKL
expression in CD4+ T cells and FLSs (13). The results indicate that IL-21 may
also be involved in the bone erosion associated with RA. Whether
IL-21 has a direct osteolastogenic role remains to be
clarified.
In this study, we demonstrated that IL-21 promoted
osteoclastogenesis in RAW264.7 cells in the absence of RANKL. IL-21
promoted osteoclast formation as demonstrated by increased numbers
of TRAP-positive cells and increased CTR protein expression. Bone
resorption analysis showed the presence of resorption pits in the
IL-21 group, although the numbers of resorption pits was fewer than
those of the RANKL group. These findings suggest that IL-21 has
direct osteoclastogenic potential in the absence of RANKL, although
IL-21 is not as potent as RANKL. The findings of the present study
showed that IL-21 promotes osteoclastogenesis in the PBMCs isolated
from patients with RA in the presence or absence of RANKL-providing
T cells. These results indicate that IL-21 may induce
osteoclastogenesis directly in RA.
Our results were different from those of previous
studies reported by Kwok et al (13). They found that IL-21 alone did not
promote osteoclastogenesis; it markedly potentiated
osteoclastogenesis in the presence of RANKL and M-CSF, both in the
mouse and human samples. This effect was mediated by the JAK/STAT3
signaling pathway (13). By
contrast, in the present study, we demonstrated that IL-21 induced
osteoclastogenesis in RAW264.7 cells directly. To further clarify
the mechanism responsible for the induction of osteoclastogenesis
by IL-21, RAW264.7 cells were pre-treated with various signaling
pathway inhibitors prior to culture with IL-21. The results of this
experiment showed that IL-21 activated the JAK/STAT3 and PI3K/AKT
signaling pathways in RAW264.7 cells. The PI3K/AKT pathway
inhibitor LY294002 significantly suppressed IL-21-induced
osteoclastogenesis, showing no TRAP-positive cells, and the
decreased expression of osteoclast markers including TRAP and
cathepsin K. Thus, these findings suggest that IL-21 may promote
osteoclastogenesis through the PI3K/AKT signaling pathway rather
than the JAK/STAT3 signaling pathway. Notably, although IL-21
stimulated RANK expression in RAW264.7 cells, it did not enhance
the osteoclastogenic potential of RANKL. The interaction between
IL-21 and RANKL in inducing osteoclastogenesis remains to be
studied further.
The limitation of the present study is that RAW264.7
cells cannot represent the macrophages of RA patients. For this
reason, further studies regarding the role of IL-21 in
osteoclastogenesis and bone erosion in patients with RA are
warranted.
In conclusion, the findings of the present study
demonstrate that IL-21 promotes osteoclastogenesis in RAW264.7
cells as well as in PBMCs isolated from patients with RA in the
absence of RANKL or RANKL-providing T cells. Thus, therapy
targeting IL-21 may be of value in preventing bone erosions in
patients with RA.
Abbreviations:
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
RANK
|
receptor activator of NF-κB
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (nos. 81102255, 81273293 and
81571573).
References
|
1
|
Maruotti N, Grano M, Colucci S, d'Onofrio
F and Cantatore FP: Osteoclastogenesis and arthritis. Clin Exp Med.
11:137–145. 2011. View Article : Google Scholar
|
|
2
|
Branimir A and Miroslav M: Pathogenesis of
rheumatoid arthritis. Reumatizam. 61:19–23. 2014.In Croatian.
|
|
3
|
Kawai VK, Stein CM, Perrien DS and Griffin
MR: Effects of anti-tumor necrosis factor α agents on bone. Curr
Opin Rheumatol. 24:576–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bloemen V, Schoenmaker T, de Vries TJ and
Everts V: IL-1β favors osteoclastogenesis via supporting human
periodontal ligament fibroblasts. J Cell Biochem. 112:1890–1897.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parrish-Novak J, Dillon SR, Nelson A,
Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West
J, et al: Interleukin 21 and its receptor are involved in NK cell
expansion and regulation of lymphocyte function. Nature. 408:57–63.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leonard WJ and Spolski R: Interleukin-21:
a modulator of lymphoid proliferation, apoptosis and
differentiation. Nat Rev Immunol. 5:688–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di Fusco D, Izzo R, Figliuzzi MM, Pallone
F and Monteleone G: IL-21 as a therapeutic target in inflammatory
disorders. Expert Opin Ther Targets. 18:1329–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Shen W, Kong K and Liu Z:
Interleukin-21 induces T-cell activation and proinflammatory
cytokine secretion in rheumatoid arthritis. Scand J Immunol.
64:515–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spolski R and Leonard WJ: Interleukin-21:
A double-edged sword with therapeutic potential. Nat Rev Drug
Discov. 13:379–395. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monteleone G, Caruso R, Fina D, Peluso I,
Gioia V, Stolfi C, Fantini MC, Caprioli F, Tersigni R, Alessandroni
L, et al: Control of matrix metalloproteinase production in human
intestinal fibroblasts by interleukin 21. Gut. 55:1774–1780. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rasmussen TK, Andersen T, Hvid M, Hetland
ML, Hørslev-Petersen K, Stengaard-Pedersen K, Holm CK and Deleuran
B: Increased interleukin 21 (IL-21) and IL-23 are associated with
increased disease activity and with radiographic status in patients
with early rheumatoid arthritis. J Rheumatol. 37:2014–2020. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Young DA, Hegen M, Ma HL, Whitters MJ,
Albert LM, Lowe L, Senices M, Wu PW, Sibley B, Leathurby Y, et al:
Blockade of the interleukin-21/interleukin-21 receptor pathway
ameliorates disease in animal models of rheumatoid arthritis.
Arthritis Rheum. 56:1152–1163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwok SK, Cho ML, Park MK, Oh HJ, Park JS,
Her YM, Lee SY, Youn J, Ju JH, Park KS, et al: Interleukin-21
promotes osteoclastogenesis in humans with rheumatoid arthritis and
in mice with collagen-induced arthritis. Arthritis Rheum.
64:740–751. 2012. View Article : Google Scholar
|
|
14
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parrish-Novak J, Foster DC, Holly RD and
Clegg CH: Interleukin-21 and the IL-21 receptor: novel effectors of
NK and T cell responses. J Leukoc Biol. 72:856–863. 2002.PubMed/NCBI
|
|
16
|
Spolski R and Leonard WJ: The Yin and Yang
of interleukin-21 in allergy, autoimmunity and cancer. Curr Opin
Immunol. 20:295–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng R, Spolski R, Casas E, Zhu W, Levy DE
and Leonard WJ: The molecular basis of IL-21-mediated
proliferation. Blood. 109:4135–4142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryu JG, Lee J, Kim EK, Seo HB, Park JS,
Lee SY, Moon YM, Yoo SH, Park YW, Park SH, et al: Treatment of
IL-21R-Fc control autoimmune arthritis via suppression of STAT3
signal pathway mediated regulation of the Th17/Treg balance and
plasma B cells. Immunol Lett. 163:143–150. 2015. View Article : Google Scholar
|
|
19
|
Marijnissen RJ, Roeleveld DM, Young D,
Nickerson-Nutter C, Abdollahi-Roodsaz S, Garcia de Aquino S, van de
Loo FA, van Spriel AB, Boots AM, van den Berg WB and Koenders MI:
Interleukin-21 receptor deficiency increases the initial toll-like
receptor 2 response but protects against joint pathology by
reducing Th1 and Th17 cells during streptococcal cell wall
arthritis. Arthritis Rheumatol. 66:886–895. 2014. View Article : Google Scholar : PubMed/NCBI
|