Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-related mortality worldwide and the
incidence of HCC is increasing (1). Despite advances in surgical
techniques and chemotherapeutic methods, the survival rate of
patients with HCC remains poor. Recent studies have identified a
number of molecules and signaling pathways that influence the
malignant biological behavior of HCC (2–4).
The identification of new molecules targeting these signaling
pathways to inhibit cancer cell proliferation and metastasis may
lead to the development of novel therapeutic strategies for
HCC.
Wogonin belongs to the family of flavonoids, and is
derived from the Chinese herb, Scutellaria baicalensis
Georgi. It has been reported that wogonin exerts antioxidant,
anti-thrombotic and anti-inflammatory effects (5–8).
The inhibitory effects of wogonin on cancer cell growth and
survival have been reported in several cancer cells, such as lung,
cervical, leukemia and breast cancer cells (9–13).
These studies also demonstrated some of the mechanisms through
which wogonin exerts its inhibitory effects on cancer cell growth.
For example, it was demonstrated that wogonin inhibited phorbol
12-myristate 13-acetate (PMA)-induced cyclooxygenase-2 (COX-2)
protein and mRNA expression in human lung epithelial cancer cells
(9). The authors also found that
the mitogen-activated protein kinase kinase 1/2 (MEK1/2) inhibitor,
U0126, also inhibited PMA-induced COX-2 expression. In addition,
the activity of the AP-1-driven promoter, but not that of nuclear
factor-κB (NF-κB), was inhibited by U0126. In that study, the
authors suggested that wogonin inhibited PMA-induced COX-2 mRNA
expression by inhibiting c-Jun expression and AP-1 activation in
lung cancer cells (9). Another
study demonstrated that wogonin induced the apoptosis of lung
cancer cells by promoting the generation of reactive oxygen species
(ROS) (12). It has also been
previously demonstrated that wogonin induces the apoptosis of
breast cancer cells by modulating the PI3K/AKT pathway (13). Thus, these studies demonstrate
that wogonin inhibits cell cycle progression, regulates the p21,
p27 and p53 status, promotes the generation of ROS, and
downregulates the expression of the anti-apoptotic protein, Bcl-2
(14).
EGFR, which is overexpressed in various
malignancies, plays a central role in essential cellular functions,
including proliferation, apoptosis and differentiation, making it
an important target in cancer therapy (15,16). It has been demonstrated that the
ERK pathway, which can induce pro-matrix metalloproteinase 2
(MMP-2) activation, is a downstream target of EGFR (30). EGFR also regulates AKT signaling.
The PI3K/AKT pathway is a well-known signaling pathway, which plays
an important role in cell growth, metabolism, proliferation,
migration and apoptosis (17,18). In addition, EGFR is an effective
target of anti-HCC drugs (19).
However, to date, the molecular mechanisms of action
of wogonin in HCC are not yet fully understood. In this study, we
examined the effects of wogonin on NF-κB and epidermal growth
factor receptor (EGFR) signaling. Importantly, we confirmed that
wogonin promoted HCC cell apoptosis through the inhibition of
NF-κB-induced Bcl-2 expression, and suppressed HCC cell
proliferation and invasion through the inhibition of the EGFR
(Tyr845)/ERK/AKT-induced activation of cyclin D1 and MMP2.
Materials and methods
Cell culture and transfection with small
interfering RNA (siRNA)
The Bel7402 and HepG2 HCC cell lines were obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were cultured in DMEM (Invitrogen, Carlsbad, CA,
USA) containing 10% fetal calf serum. Wogonin was purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and dissolved
at a 500 mM concentration in dimethyl sulfoxide (DMSO) as a stock
solution which was stored at −20°C. The cells were treated with
various concentrations of wogonin (0, 25, 50 and 100 μM) for
24 h. Cells not treated with wogonin were used as controls. In
addition, the cells were treated with the NF-κB inhibitor, Bay
11-7082 (5 μM for 12 h; Sigma-Aldrich, St. Louis, MO, USA).
siRNA targeting EGFR were purchased from Dharmacon (Thermo Fisher
Scientific, Inc., Beijing, China). The sequence of the siRNA
against EGFR was CACAGUGGAGCGAAUUCCU. The control non-targeting
siRNA sequence was GGACUUGGAUGAAGAAAUC. The cells were transfected
with the siRNA using DharmaFECT 1 transfection reagent (Thermo
Fisher Scientific, Waltham, MA, USA) according to the
manufacturer's instructions. The transfection efficiency was
determined by western blot analysis.
Cell counting kit-8 (CCK-8) cell
proliferation assay
Cell proliferation assay was performed using CCK-8
solution (Dojindo, Kumamoto, Japan) according to the manufacturer's
instructions. Cell proliferation was examined on days 1, 2 and 3
following treatment with wogonin. The cells were seeded at
approximately 5×103 cells each well in 96-well plates
and incubated with 10 μl CCK-8 solution for approximately 4
h. The optical density of the wells was measured at 450 nm using a
Tecan F50 microplate reader (Tecan, Männedorf, Switzerland).
Quantitative (real-time) PCR (qPCR)
qPCR was performed using the SYBR-Green master mix
kit (Applied Biosystems, Foster City, CA, USA). PCR was performed
using the 7500 Real-time PCR system (Applied Biosystems). β-actin
was used as the reference gene. The relative expression of target
genes were calculated as ΔCt = Ct gene − Ct reference, and the fold
change of target gene expression was calculated using the
2−ΔΔCt method. All PCR experiments in this study were
repeated in triplicate. The sequences of the primers were as
follows: cyclin D1 forward, 5′-GCTGGAGGTCTGCGAGGA-3′ and reverse,
5′-ACAGGAAGCGGTCCAGGTAGT-3′; cyclin E forward,
5′-AGCCAGCCTTGGGACAATAAT-3′ and reverse,
5′-GAGCCTCTGGATGGTGCAAT-3′; Bcl-2 forward,
5′-ACGGTGGTGGAGGAGCTCTT-3′ and reverse, 5′-CGGTTGACGCTCTCCACAC-3′;
MMP2 forward, 5′-TGTGTTCTTTGCAGGGAATGAAT-3′ and reverse,
5′-TGTCTTCTTGTTTTTGCTCCAGTTA-3′; β-actin forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′.
Western blot analysis
Whole cell extracts were prepared in cell lysis
buffer (Pierce, Rockford, IL, USA) and quantified using the
Bradford method. A total of 40 μg protein was separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE). Following electrophoresis, the proteins were
transferred onto PVDF membranes (Millipore, Billerica, MA, USA) and
blocked using non-fat milk. The membranes were incubated overnight
at 4°C with antibodies against p-ERK (4376), p-AKT (4060), p-EGFR
(Tyr845; 6963), cyclin D1 (2978), MMP2 (4022), cyclin E (4129),
Bcl-2 (15071), CDK4 (12790), CDK6 (3136), cleaved caspase-3 (9661)
and cleaved caspase-9 (7237) at a 1:1,000 dilution (all from Cell
Signaling Technology, Danvers, MA, USA) and glyceraldehyde
3-phosphate dehydrogenase (GAPDH; 5174) at a 1:2,000 dilution (Cell
Signaling Technology). This was followed by incubation with
HRP-conjugated IgG antibody (1:2,000 dilution) (Cell Signaling
Technology). All PVDF membranes were visualized using an enhanced
chemiluminescence (ECL) kit (Pierce). Quantitative analysis of the
western blots was performed using ImageJ software by assessing the
grey value of the western blot bands.
Matrigel invasion assay and migration
assay
Matrigel invasion assay was performed using a
24-well Transwell chamber (Costar, Cambridge, MA, USA). The inserts
were coated with 20 μl Matrigel (1:5 dilution; BD
Bioscience, San Jose, CA, USA). Following treatment, the HepG2 and
Bel7402 cells were suspended in 100 μl of medium without
serum and were transferred to the upper Transwell chambers.
Approximately 600 μl of medium containing 10% fetal bovine
serum (FBS) was added to the lower chamber. Following 16 h of
incubation, the non-invaded cells on the upper membrane surface
were removed using a cotton tip, and the cells that had passed
through the filter were stained using hematoxylin (Sigma-Aldrich).
The invading cell number was counted under a microscope (BX53;
Olympus, Tokyo, Japan).
Cell cycle analysis and apoptosis by flow
cytometry
Following incubation with wogonin, the cells were
washed with phosphate-buffered saline (PBS) and suspended in a
propidium iodide (PI) buffer (10 μg/ml PI, 0.5% Tween-20,
0.1% RNase in PBS). The cell cycle was analyzed using a FACS flow
cytometer (Becton-Dickinson, San Jose, CA, USA).
For the detection of apoptosis, the Annexin V/PI
apoptosis kit (Becton-Dickinson) was used. The cells were washed
twice with PBS and suspended with binding buffer. Subsequently, 5
μl of Annexin V-FITC and 10 μl of PI were added
followed by the incubation of the cells in the dark. The apoptotic
rate was examined using a FACS flow cytometer
(Becton-Dickinson).
Statistical analysis
SPSS version 11 for Windows was used for all
analyses. ANOVA with a post-hoc test was applied to compare the
differences between the control group and the wogonin-treated
group. The Student's t-test was used to compare other data and a
value of p<0.05 was considered to indicate a statistically
significant difference.
Results
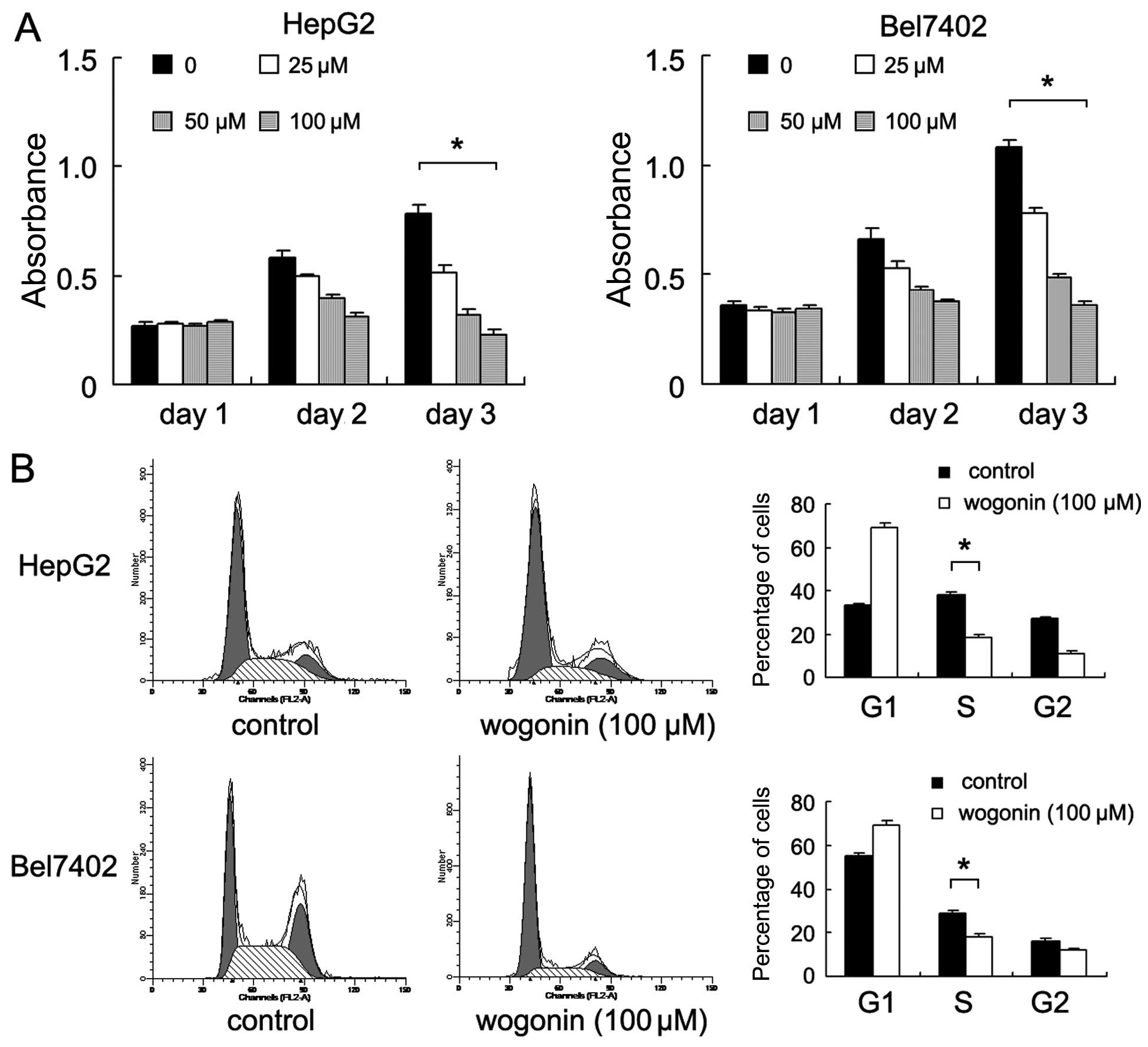
Wogonin inhibits the proliferation of
HepG2 and Bel7402 cells by inhibiting the G1-S phase
transition
The HepG2 and Bel7402 HCC cell lines were used to
examine the growth inhibitory effects of wogonin (0, 25, 50 and 100
μM for 24 h). The results of CCK-8 assay revealed that
treatment with wogonin inhibited the proliferation of both cell
lines in a concentration-dependent manner (day 3: Bel7402,
p<0.001; HepG2, p<0.001; ANOVA test) (Fig. 1A). Further analysis of the cell
cycle revealed that wogonin significantly reduced the percentage of
cells in the S phase and increased the percentage of cells in the
G1 phase compared with the untreated control cells, thus indicating
that wogonin induced arrest cell cycle at the G1-S checkpoint
(Bel7402, p<0.001; HepG2, p<0.001) (Fig. 1B). Wogonin also decreased the
percentage of cells in the G2/M phase in both cell lines
(p<0.05).
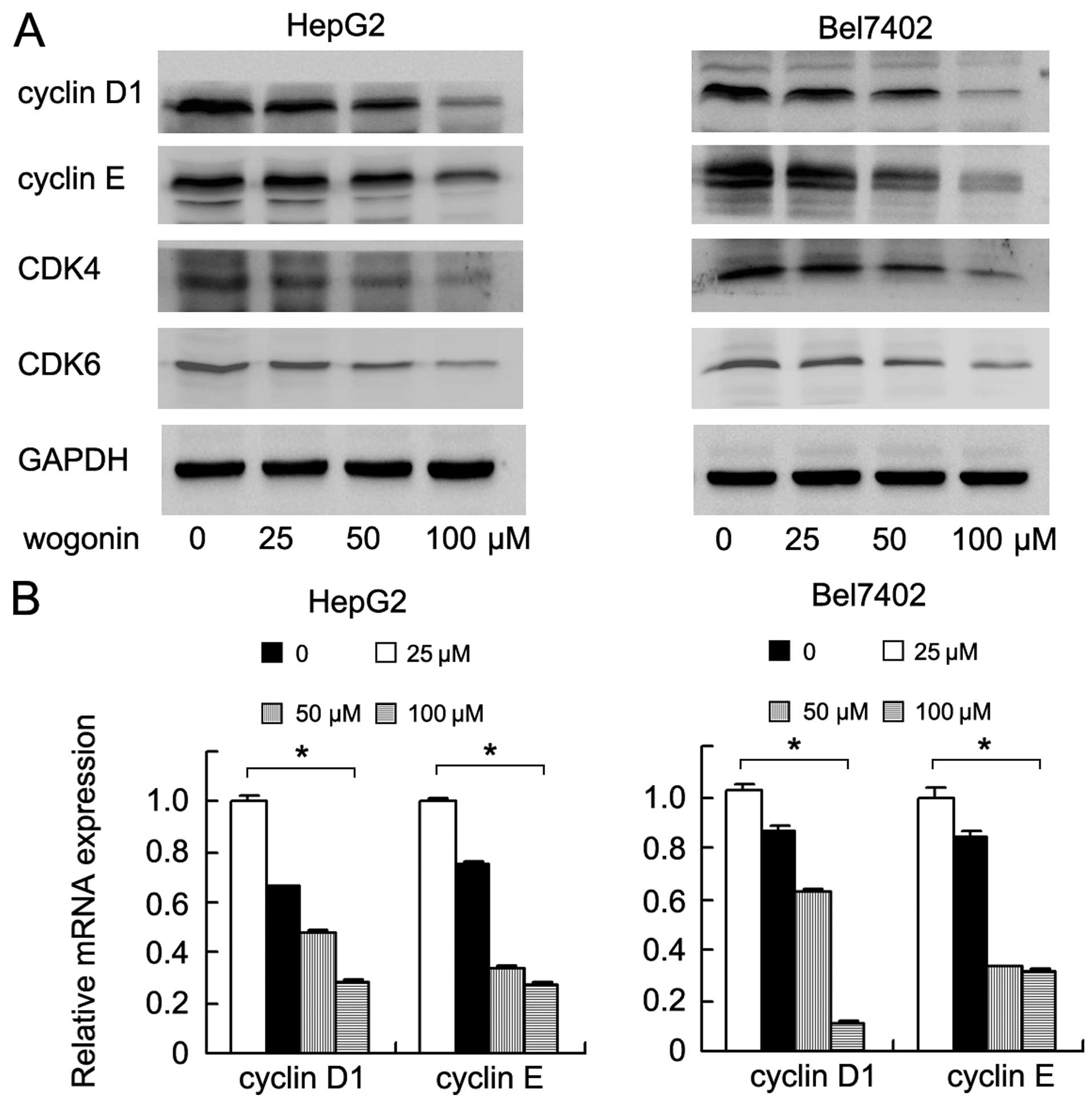
Cyclin D1, cyclin E and CDK4/6 are the key factors
controlling cell cycle progression. Thus, we examined the
expression levels of these proteins in the wogonin-treated HepG2
and Bel7402 cells. By performing western blot analysis, we found
that treatment with wogonin markedly decreased the protein
expression levels of cyclin D1, cyclin E and CDK4/6 in a
concentration dependent manner (Fig.
2A). In addition, qPCR yielded similar results. The mRNA
expression levels of cyclin D1 and cyclin E decreased in the cells
following treatment with wogonin (Bel7402: cyclin D1 and cyclin E,
p<0.001; HepG2: cyclin D1 and cyclin E, p<0.001; ANOVA test)
(Fig. 2B).
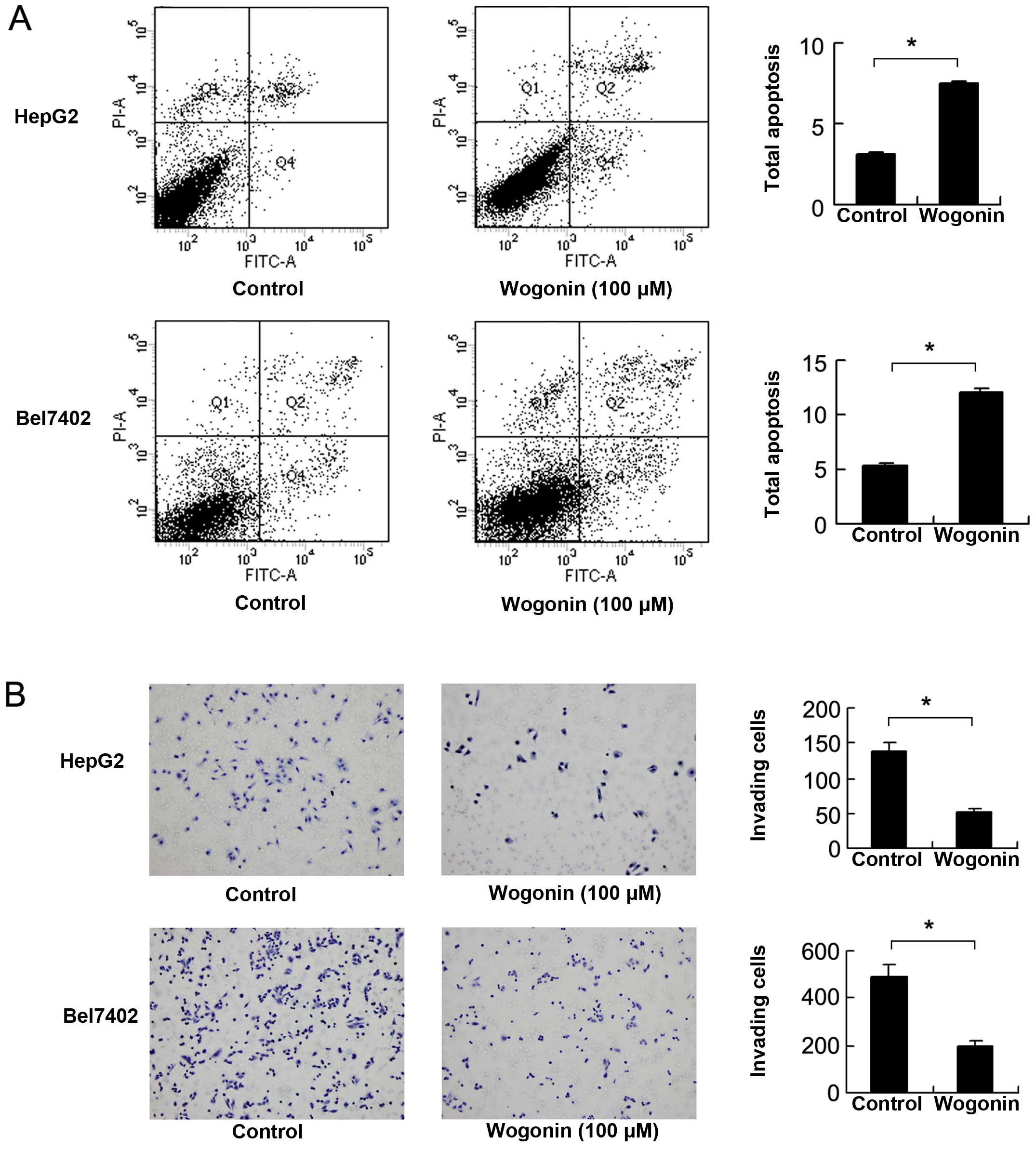
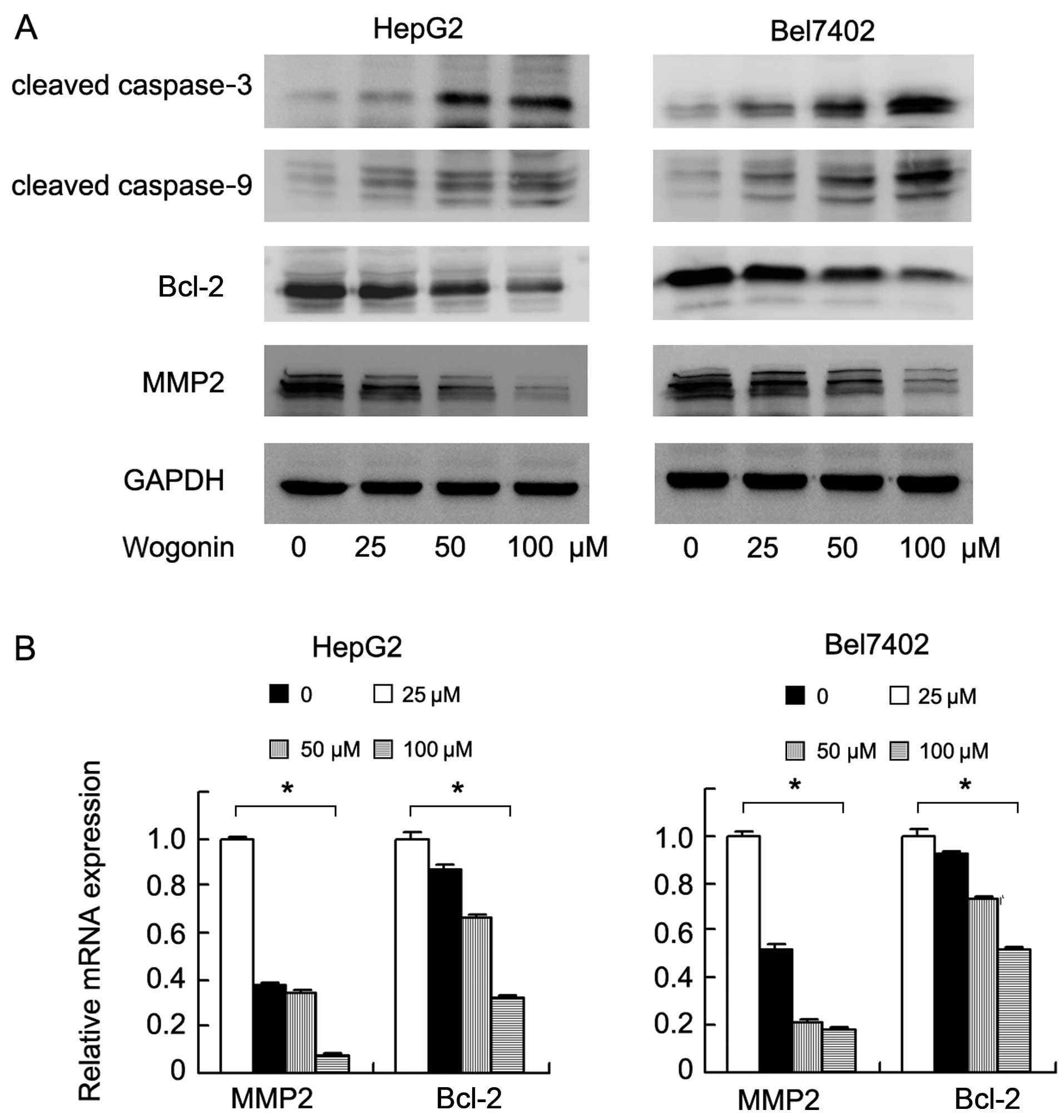
Wogonin induces the apoptosis and
suppresses the invasion of HCC cells
We detected apoptosis using Annexin V/PI staining.
As shown in Fig. 3A, treatment
with wogonin significantly increased the percentage of apoptotic
cells compared with the untreated controls. We also examined the
levels of cleaved caspase-3 and caspase-9. The results of western
blot analysis revealed that wogonin increased the expression levels
of cleaved caspase-3 and caspase-9. We also examined changes in the
levels of apoptosis-regulating proteins and found that the
expression of Bcl-2 was markedly decreased following treatment with
wogonin (Fig. 4A). To examine the
effects of wogonin on the invasive ability of the HepG2 and Bel7402
cells, Matrigel invasion assay was carried out with the
wogonin-treated cells using a Transwell chamber. As shown in
Fig. 3B, treatment with wogonin
for 24 h significantly decreased the number of invading HepG2 and
Bel7402 cells (control vs. treatment: Bel7402, 492±31.5 vs.
198±16.5, p<0.001; HepG2, 137±10.5 vs. 51±6.5, p<0.001). In
addition, we examined the levels of proteins associated with cell
invasion and found that the expression levels of MMP2 and Bcl-2
were significantly downregulated at both the protein and mRNA
levels (Bel7402: MMP2 and Bcl-2, p<0.001; HepG2: MMP2 and Bcl-2,
p<0.001, ANOVA test) (Fig.
4B).
Wogonin induces apoptosis through
NF-κB/Bcl-2 signaling
To explore the potential mechanisms of action of
wogonin in the HepG2 and Bel7402 cell lines, we examined several
signaling pathways which are related to cancer cell proliferation
and invasion. We examined the effects of wogonin on NF-κB
signaling. The results of western blot analysis revealed that the
expression levels of p-IκB and p-p65 were significantly decreased
following treatment with various concentrations of wogonin
(Fig. 5A). Bcl-2 has been
reported as a downstream target of NF-κB signaling (20). We demonstrated that treatment with
the NF-κB inhibitor, Bay 11-7082 (5 μM for 12 h), decreased
the expression of Bcl-2 in both cell lines. In addition, in the Bay
11-7082-treated cells, the suppressive effects of wogonin on Bcl-2
expression were not significant, suggesting that wogonin induced
the apoptosis of HCC cells through the inhibition of NF-κB/Bcl-2
signaling; thus NF-κB signaling is required for the inhibitory
effects of wogonin on Bcl-2 expression and for its promoting
effects on apoptosis (Fig.
5B).
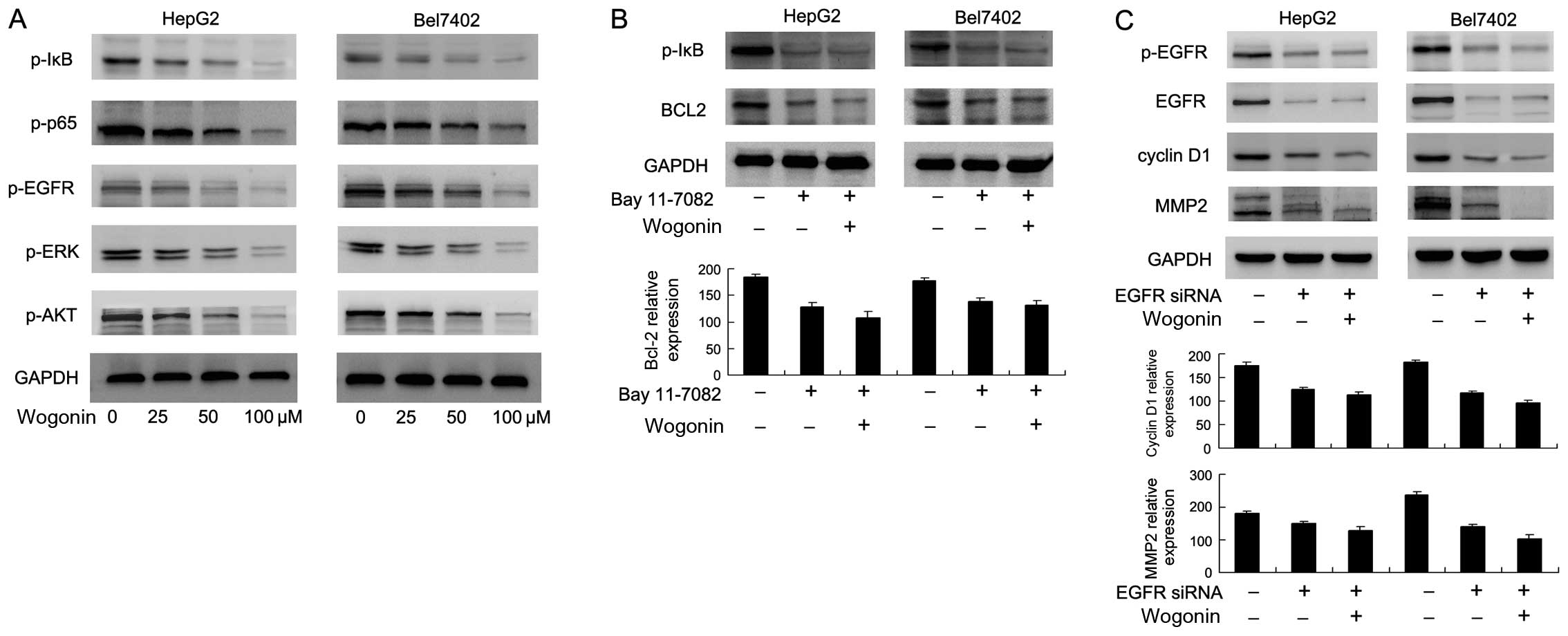 | Figure 5Wogonin regulates nuclear factor-κB
(NF-κB)/Bcl-2 and epidermal growth factor receptor (EGFR)/ERK/AKT
signaling. (A) Western blot analysis revealed that treatment with
wogonin (0, 25, 50 and 100 μM, for 24 h) decreased the
expression levels of p-IκB, p-p65, p-EGFR, p-ERK and p-AKT in a
concentration-dependent manner. (B) Treatment with the NF-κB
inhibitor, Bay 11-7082, decreased p-IκB and Bcl-2 expression. In
the Bay 11-7082-treated cells, the effects of wogonin were not
significant. (C) Transfection with EGFR siRNA significantly
decreased the expression levels of p-EGFR, matrix metalloproteinase
2 (MMP2) and cyclin D1. In the cells transfected with EGFR siRNA,
the effects of wogonin on these proteins were not significant. |
Wogonin suppresses HepG2 and Bel7402 cell
proliferation and invasion through the inhibition of EGFR signaling
and downstream ERK/AKT signaling
EGFR is a tyrosine kinase located at the cell
membrane, which functions as an oncogene, mediating the malignant
growth and invasion of various cancer cells (21–23). Our results revealed that wogonin
inhibited EGFR (Tyr845) phosphorylation. The levels of downstream
factors of EGFR signaling, including p-ERK and p-AKT were also
downregulated following treatment with wogonin (Fig. 5A). To confirm the involvement of
EGFR signaling in the wogonin-induced suppressive effects on MMP2
and cyclin D1 expression, we knocked down EGFR expression in these
cell lines using siRNA. We found that, in the cells transfected
with the siRNA targeting EGFR, the inhibitory effects of wogonin on
cyclin D1 and MMP2 expression were not significant (Fig. 5C). These results suggested that
wogonin inhibited HCC cell proliferation and invasion through the
inhibition of EGFR (Tyr845) activity and that of its downstream
factors, namely that of EGFR/ERK/MMP2, EGFR/AKT and EGFR/cyclin D1
signaling.
Discussion
In this study, we used the HCC cell lines, HepG2 and
Bel7402, to examine the antitumor effects of wogonin. As shown by
CCK-8 assay and Transwell assay, wogonin inhibited the
proliferation and invasion of the HepG2 and Bel7402 cells in a
concentration-dependent manner. In addition, cell cycle progression
was arrested at the G1-S point, with the downregulation of cyclin
family proteins, such as cyclin D1 and cyclin E. Invasion-related
MMP2 expression was also downregulated. When examining the
signaling pathways involved in the wogonin-mediated inhibitory
effects on cell proliferation, we found that ERK and AKT signaling
was significantly inhibited. Of note, we found that wogonin
inactivated EGFR (Tyr845) phosphorylation, which is an upstream
tyrosine kinase of ERK and AKT (24). To confirm the involvement of EGFR
in the anticancer effects of wogonin, we knocked down endogenous
EGFR expression in these cell lines and then examined the effects
of wogonin on EGFR downstream factors, such as cyclin D1 and MMP2
(25,26). In the cells in which EGFR
expression had been depleted, the effects of wogonin on cyclin D1
and MMP2 expression were not significant compared with those of the
normal HepG2 and Bel7402 cells, suggesting wogonin exerts its
anticancer effects through the inhibition of EGFR activity.
EGFR overexpression has been found in many types of
cancer, and it plays important roles in cancer proliferation,
invasion and metastasis, and is also associated with a poor
survival rate (27,28). MMP2 plays a pivotal role in the
invasion of many malignant cancers. EGFR upregulates its downstream
molecule, MMP2, through the phosphorylation of the MEK/ERK/AP1
signaling pathway (29,30). In addition, EGFR activates AKT
signaling, which plays a central role in cancer cell proliferation
and survival (31). Many studies
have demonstrated that targeting EGFR activity inhibits tumor
growth and improves survival (32–34). In this study, we demonstrated that
wogonin targets EGFR phosphorylation to suppress HCC cell
proliferation and invasion, suggesting that wogonin may be used as
a chemotherapeutic agent in the treatment of HCC.
In addition to the inhibitory effects of wogonin on
EGFR-related HCC cell proliferation and invasion, we demonstrated
that wogonin promoted apoptosis, which was in parallel with the
downregulation of Bcl-2 protein and the cleavage of caspase-3 and
caspase-9, both of which contribute to the apoptosis-inducing
effects of wogonin. We also found that wogonin inhibited NF-κB
signaling, which has been reported to be involved in Bcl-2 and the
regulation of apoptosis in various types of cancer (35–37). Our results also revealed that in
the cells treated with the NF-κB inhibitor, the suppressive effects
of wogonin on Bcl-2 were significantly reduced. In cancer cells,
NF-κB is activated either due to mutations in genes encoding the
NF-κB transcription factors themselves,or in genes that control
NF-κB activity. Since the role of NF-κB activation in cancer cell
growth and survival has been reported in HCC (38), we hypothesized that NF-κB
activation may partly suppress the apoptosis-inducing effects of
wogonin. In addition, EGFR has been reported to activate NF-κB
signaling through the CARMA3/Bcl10 complex (39). Since there is a crosstalk between
NF-κB and EGFR signaling, we hypothesized that the effects of
wogonin on NF-κB are partly due to its effects on EGFR
signaling.
In conclusion, in this study, we demonstrated that
wogonin inhibited the malignant biological behavior of the HCC cell
lines, HepG2 and Bel7402, by inhibiting the phosphorylation of EGFR
(Tyr845) and its downstream EGFR/cyclin D1, EGFR/AKT and
EGFR/ERK/MMP2 signaling pathways. Wogonin also inhibited the
activation of the NF-κB/Bcl-2 pathway and induced apoptosis. Thus,
wogonin may serve as a novel therapeutic agent for the treatment of
HCC.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ye H and Liu W: Connectivity-based risk
score for hepatocellular carcinoma prognosis. Hepatology.
58:1191–1192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X,
Liu J, Shi L, Liu C, Wang G, et al: Tim-3/galectin-9 signaling
pathway mediates T-cell dysfunction and predicts poor prognosis in
patients with hepatitis B virus-associated hepatocellular
carcinoma. Hepatology. 56:1342–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu
MY, Xu Y, Song ZJ, Wang ZJ, Wu JC, et al: Role of overexpression of
CD151 and/or c-Met in predicting prognosis of hepatocellular
carcinoma. Hepatology. 49:491–503. 2009. View Article : Google Scholar
|
|
5
|
Ueng YF, Shyu CC, Lin YL, Park SS, Liao JF
and Chen CF: Effects of baicalein and wogonin on drug-metabolizing
enzymes in C57BL/6J mice. Life Sci. 67:2189–2200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakabayashi I and Yasui K: Wogonin
inhibits inducible prostaglandin E(2) production in macrophages.
Eur J Pharmacol. 406:477–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin SJ, Tseng HH, Wen KC and Suen TT:
Determination of gentiopicroside, mangiferin, palmatine, berberine,
baicalin, wogonin and glycyrrhizin in the traditional Chinese
medicinal preparation sann-joong-kuey-jian-tang by high-performance
liquid chromatography. J Chromatogr A. 730:17–23. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin CC and Shieh DE: The anti-inflammatory
activity of Scutellaria rivularis extracts and its active
components, baicalin, baicalein and wogonin. Am J Chin Med.
24:31–36. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen LG, Hung LY, Tsai KW, Pan YS, Tsai
YD, Li YZ and Liu YW: Wogonin, a bioactive flavonoid in herbal tea,
inhibits inflammatory cyclooxygenase-2 gene expression in human
lung epithelial cancer cells. Mol Nutr Food Res. 52:1349–1357.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee E, Enomoto R, Suzuki C, Ohno M, Ohashi
T, Miyauchi A, Tanimoto E, Maeda K, Hirano H, Yokoi T and Sugahara
C: Wogonin, a plant flavone, potentiates etoposide-induced
apoptosis in cancer cells. Ann NY Acad Sci. 1095:521–526. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Himeji M, Ohtsuki T, Fukazawa H, Tanaka M,
Yazaki S, Ui S, Nishio K, Yamamoto H, Tasaka K and Mimura A:
Difference of growth-inhibitory effect of Scutellaria
baicalensis-producing flavonoid wogonin among human cancer cells
and normal diploid cell. Cancer Lett. 245:269–274. 2007. View Article : Google Scholar
|
|
12
|
He F, Wang Q, Zheng XL, Yan JQ, Yang L,
Sun H, Hu LN, Lin Y and Wang X: Wogonin potentiates
cisplatin-induced cancer cell apoptosis through accumulation of
intracellular reactive oxygen species. Oncol Rep. 28:601–605.
2012.PubMed/NCBI
|
|
13
|
Huang KF, Zhang GD, Huang YQ and Diao Y:
Wogonin induces apoptosis and down-regulates survivin in human
breast cancer MCF-7 cells by modulating PI3K-AKT pathway. Int
Immunopharmacol. 12:334–341. 2012. View Article : Google Scholar
|
|
14
|
He L, Lu N, Dai Q, Zhao Y, Zhao L, Wang H,
Li Z, You Q and Guo Q: Wogonin induced G1 cell cycle arrest by
regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in
human colorectal cancer carcinoma cells. Toxicology. 312:36–47.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saif MW: Colorectal cancer in review: the
role of the EGFR pathway. Expert Opin Investig Drugs. 19:357–369.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Navolanic PM, Steelman LS and McCubrey JA:
EGFR family signaling and its association with breast cancer
development and resistance to chemotherapy (Review). Int J Oncol.
22:237–252. 2003.PubMed/NCBI
|
|
17
|
Lee DH, Szczepanski MJ and Lee YJ:
Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt
signaling pathway in human prostate cancer cells. J Cell Biochem.
106:1113–1122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raufman JP, Shant J, Guo CY, Roy S and
Cheng K: Deoxycholyltaurine rescues human colon cancer cells from
apoptosis by activating EGFR-dependent PI3K/Akt signaling. J Cell
Physiol. 215:538–549. 2008. View Article : Google Scholar
|
|
19
|
Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y,
Gao Q and Su C: Matrine derivative WM130 inhibits hepatocellular
carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling
pathways. Cancer Lett. 368:126–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao X, Ning Q, Sun X and Tian D: Pokemon
reduces Bcl-2 expression through NF-κ Bp65: A possible mechanism of
hepatocellular carcinoma. Asian Pac J Trop Med. 4:492–497. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia XF, Li J, Zhao HB, Liu J and Liu JJ:
Correlation of EGFR gene amplification with invasion and metastasis
of non-small cell lung cancer. Genet Mol Res. 14:11006–11012. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mader CC, Oser M, Magalhaes MA,
Bravo-Cordero JJ, Condeelis J, Koleske AJ and Gil-Henn H: An
EGFR-Src-Arg-cortactin pathway mediates functional maturation of
invadopodia and breast cancer cell invasion. Cancer Res.
71:1730–1741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gong C, Zhang J, Zhang L, Wang Y, Ma H, Wu
W, Cui J, Wang Y and Ren Z: Dynamin2 downregulation delays EGFR
endocytic trafficking and promotes EGFR signaling and invasion in
hepatocellular carcinoma. Am J Cancer Res. 5:702–713.
2015.PubMed/NCBI
|
|
24
|
Wang YP, Huang LY, Sun WM, Zhang ZZ, Fang
JZ, Wei BF, Wu BH and Han ZG: Insulin receptor tyrosine kinase
substrate activates EGFR/ERK signalling pathway and promotes cell
proliferation of hepatocellular carcinoma. Cancer Lett. 337:96–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alam S, Pal A, Kumar R, Dwivedi PD, Das M
and Ansari KM: EGFR-mediated Akt and MAPKs signal pathways play a
crucial role in patulin-induced cell proliferation in primary
murine keratinocytes via modulation of Cyclin D1 and COX-2
expression. Mol Carcinog. 53:988–998. 2014.
|
|
26
|
Fiano V, Ghimenti C, Imarisio S, Silengo L
and Schiffer D: PAkt, cyclin D1 and p27/Kip.1 in glioblastomas with
and without EGFR amplification and PTEN mutation. Anticancer Res.
24:2643–2647. 2004.PubMed/NCBI
|
|
27
|
Ezzoukhry Z, Louandre C, Trécherel E,
Godin C, Chauffert B, Dupont S, Diouf M, Barbare JC, Mazière JC and
Galmiche A: EGFR activation is a potential determinant of primary
resistance of hepatocellular carcinoma cells to sorafenib. Int J
Cancer. 131:2961–2969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kannangai R, Sahin F and Torbenson MS:
EGFR is phosphorylated at Ty845 in hepatocellular carcinoma. Mod
Pathol. 19:1456–1461. 2006.PubMed/NCBI
|
|
29
|
Bae GY, Choi SJ, Lee JS, Jo J, Lee J, Kim
J and Cha HJ: Loss of E-cadherin activates EGFR-MEK/ERK signaling,
which promotes invasion via the ZEB1/MMP2 axis in non-small cell
lung cancer. Oncotarget. 4:2512–2522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong QZ, Wang Y, Tang ZP, Fu L, Li QC,
Wang ED and Wang EH: Derlin-1 is overexpressed in non-small cell
lung cancer and promotes cancer cell invasion via EGFR-ERK-mediated
up-regulation of MMP-2 and MMP-9. Am J Pathol. 182:954–964. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim H and Lim HY: Novel EGFR-TK inhibitor
EKB-569 inhibits hepatocellular carcinoma cell proliferation by AKT
and MAPK pathways. J Korean Med Sci. 26:1563–1568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ling Y, Yang X, Li W, Li Z, Yang L, Qiu T,
Guo L, Dong L, Li L, Ying J and Lin D: Overexpression of mutant
EGFR protein indicates a better survival benefit from EGFR-TKI
therapy in non-small cell lung cancer. Oncotarget. July
13–2016.Epub ahead of print. View Article : Google Scholar
|
|
33
|
Shan L, Wang Z, Guo L, Sun H, Qiu T, Ling
Y, Li W, Li L, Liu X and Zheng B: Concurrence of EGFR amplification
and sensitizing mutations indicate a better survival benefit from
EGFR-TKI therapy in lung adenocarcinoma patients. Lung Cancer.
89:337–342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nose N, Uramoto H, Iwata T, Hanagiri T and
Yasumoto K: Expression of estrogen receptor beta predicts a
clinical response and longer progression-free survival after
treatment with EGFR-TKI for adenocarcinoma of the lung. Lung
Cancer. 71:350–355. 2011. View Article : Google Scholar
|
|
35
|
Chen GG, Liang NC, Lee JF, Chan UP, Wang
SH, Leung BC and Leung KL: Over-expression of Bcl-2 against Pteris
semipinnata L-induced apoptosis of human colon cancer cells via a
NF-kappa B-related pathway. Apoptosis. 9:619–627. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kurland JF, Voehringer DW and Meyn RE: The
MEK/ERK pathway acts upstream of NF kappa B1 (p50) homodimer
activity and Bcl-2 expression in a murine B-cell lymphoma cell
line. MEK inhibition restores radiation-induced apoptosis. J Biol
Chem. 278:32465–32470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Herrmann JL, Beham AW, Sarkiss M, Chiao
PJ, Rands MT, Bruckheimer EM, Brisbay S and McDonnell TJ: Bcl-2
suppresses apoptosis resulting from disruption of the NF-kappa B
survival pathway. Exp Cell Res. 237:101–109. 1997. View Article : Google Scholar
|
|
38
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang T, Grabiner B, Zhu Y, Jiang C, Li H,
You Y, Lang J, Hung MC and Lin X: CARMA3 is crucial for
EGFR-Induced activation of NF-kappaB and tumor progression. Cancer
Res. 71:2183–2192. 2011. View Article : Google Scholar : PubMed/NCBI
|