Introduction
Osteosarcoma is the most common type of malignant
bone tumor, and it frequently originates in the metaphysis of the
long bones (1). The prognosis of
patients with osteosarcoma remains poor, as approximately 80% of
patients eventually develop metastatic disease following surgical
treatment (2) and pulmonary
metastasis is the major cause of fatal outcome (3). The further elucidatation of the
molecular mechanisms responsible for the development of
osteosarcoma will not only help us to understand the pathogenesis
and progression of the disease, but may also provided novel targets
for effective therapies.
WW domain-containing oxidoreductase (WWOX; also
known as FOR/WOX1) encodes a 46-kDa protein with a short-chain
dehydrogenase/reductase domain and two WW domains (4–7).
The gene is altered through deletions or translocations in many
types of cancer, including breast, prostate, esophageal, lung,
stomach and pancreatic cancer (5,7–12).
Moreover, WWOX protein is decreased or lost in the majority of
these cancers, suggesting that the deregulation of WWOX expression
may be involved in cancer development (13,14).
The ectopic overexpression of WWOX in cancer cells
lacking endogenous WWOX has been shown to lead to marked growth
inhibition and to prevent the progression of tumors in nude mice
(15,16). The restoration of WWOX expression
in cancer cells has been shown to contribute to caspase-mediated
apoptosis (16) and decreased
WWOX expression is associated with drug resistance (17). WWOX plays a critical role in
determining the aggressive phenotype of osteosarcoma, and it had
been suggested that the restoration of its expression may be an
attractive therapeutic strategy (18). However, the regulatory mechanisms
of WWOX remain to be fully elucidated in osteosarcoma.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs,
20–22 nucleotides in length that have been shown to be involved in
various types of cancer (19–21). miR-214-3p has been shown to be
upregulated and to function as an oncogene in human ovarian cancer
(22), gastric cancer (23), pancreatic cancer (24) and osteosarcoma (25). In addition, the upregulated
expression of miRNA-214 is linked to tumor progression and adverse
prognosis in pediatric osteosarcoma (25), and it has been shown to promote
the proliferation and invasion of osteosarcoma cells through the
direct suppression of leucine zipper, putative tumor suppressor 1
(LZTS1) (26).
In the present study, we aimed to elucidate the
mechanisms of action of WWOX in osteosarcoma. We also aimed to
determine the role of miRNA-214 in the development and progression
of osteosarcoma. In addition, we wished to determine the existence
of an association between WWOX and miRNA-214 in osteosarcoma in an
effort to provide more efficient therapeutic strategies for the
treatment of osteosarcoma and to prevent tumor metastasis.
Materials and methods
Tissue samples and cell lines
Osteosarcoma tissues and adjacent normal tissues
(n=6) were collected from patients at surgery at Linyi People's
Hospital, Linyi, China. The Medical Ethics Committee of Linyi
People's Hospital approved the study, and all patients agreed to
participate in the project and provided written informed consent
prior to obtaining the samples. The human osteosarcoma cell line,
MG63, was purchased from the Cell Bank of the Chinese Academy of
Sciences (Beijing, China). The cells were maintained in RPMI-1640
medium (HyClone Co., Logan, UT, USA) supplemented with 10% fetal
bovine serum and 1% penicillin G/streptomycin.
Cell transfection
All WWOX overexpression plasmids were purchased from
Tiangen (Tianjin, China). The empty vectors were also obtained from
Tiangen. Anti-miR-214-3p/scramble inhibitor was purchased from
Ambion, Inc. (Austin, TX, USA). The cells were seeded into 6-well
plates 24 h prior to transfection. When the MG63 cells reached 80%
confluence, the expression plasmids were transfected into the cells
using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according
to manufacturer's instructions.
Western blot analysis
The cells were collected and lysed using RIPA lysis
buffer. Proteins were separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and then
electroplotted onto polyvinylidene difluoride (PVDF) membranes. The
blots were blocked and incubated with antibodies against WWOX
(ab137726), cyclin D1 (ab134175), p53 (ab62376), p21 (ab109520),
p27 (ab32034), c-myc (ab32072), E-cadherin (E-Cad; ab15148), zinc
finger E-box-binding homeobox 1 (ZEB1; ab203829), vimentin
(ab16700), Slug (ab27568) and β-actin (ab8227) (all from Abcam,
Cambridge, MA, USA) overnight 4°C. After washing, the membranes
were incubated at 37°C for 1 h with goat anti-rabbit IgG H&L
(HRP) secondary antibody (ab6721; Abcam) and visualized using ECL
detection reagent.
MTT cell proliferation assay
Following transfection, the cells were re-seeded at
a density of 2×104 cells/well in 96-well plates
containing 0.2 ml RPMI-1640 (with 10% FBS). Subsequently, 20
μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) were added followed by incubation for a further 4 h
at 37°C. A total of 150 μl of dimethyl sulfoxide was then
added to each well and the absorbance was measured at 570 nm on an
enzyme immunoassay analyzer (Bio-Rad, Hercules, CA, USA).
BrdU assay
Cell proliferation was also assessed using a
colorimetric BrdU proliferation kit by following the manufacturer's
instructions (Roche; Cat. no. 11647229001). Briefly, the cells
treated with the peptides were labeled with BrdU for 3–4 h. The
genomic DNA was then fixed and denatured, then incubated with the
peroxidase-conjugated anti-BrdU antibody for 90 min. The substrate
of the conjugated peroxidase was then added and the reaction
product was measured by the absorbance (A370
nm−A492 nm). The results were then normalized by
the number of total viable cells, which was determined by a
side-by-side cell viability assay as described above.
Migration and invasion assays
Following transfection, the cells were added to the
chamber with the non-coated membrane (BD Biosciences, San Diego,
CA, USA), and cells migrating to the lower sides of the filters
were fixed in 4% paraformaldehyde and stained with crystal violet.
Cell invasion assay was performed as the migration assay except
that the chamber was coated with Matrigel (BD Biosciences) and the
cells were observed under a microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen) according to manufacturer's instructions. The
First-Strand cDNA was generated by reverse transcription with
random hexamer primers and a M-MLV reverse transcriptase kit
(Promega, Madison, WI, USA). Quantitative PCR (qPCR) for WWOX was
carried out using Power SYBR-Green PCR Master Mix (Applied
Biosystems, Carlsbad, CA, USA) according to the manufacturer's
instructions. We used 5′-AGTTCCTGAGCGAGTGGACC-3′ as the forward
primer and 5′-TTACTTTCAAACAGGCCACCAC-3′ as the reverse primer.
GAPDH was used as a loading control. The primer sequences for GAPDH
were as follows: forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′. The thermal cycle profile was as
follows: denaturation for 30 sec at 95°C, annealing for 45 sec at
52–58°C depending on the primers used, and extension for 45 sec at
72°C. qPCR for miR-214-3p was performed using the mirVana qRT-PCR
miRNA detection kit and qRT-PCR Primer Sets, according to the
manufacturer's instructions (Ambion, Inc.).
Immunofluorescence assay
The cells grown on polylysine-treated slides were
washed with phosphate-buffered saline (PBS) and fixed with 4%
paraformaldehyde on ice, and then blocked with 5% BSA at room
temperature. Anti-WWOX antibody (ab137726; Abcam) were added at 4°C
overnight followed by incubation with goat anti-rabbit (Alexa
Fluor® 488) secondary antibody (ab150077; Abcam) at room
temperature for 30 min. The samples were then washed 3 times with
PBS-Tween-20, and the nuclei were stained with
4,6-diamidino-2-phenylindole (DAPI).
miRNA microarray analysis
Microarray analysis was performed using Affymetrix
GeneChip miRNA 3.0 arrays kit (Affymetrix, Santa Clara, CA, USA)
according to the manufacturer's instructions. After washing and
staining, the arrays were scanned using GeneChip scanner 3000
(Affymetrix).
Northern blot analysis
For northern blot analysis of the cells, all RNA
samples were prepared and separated in a denaturing
urea-polyacrylamid gel and transferred to nylon membranes by
semi-dry electroblotting. The RNA probes were labeled with
[γ-32P] ATP complementary to the target miRNAs. U6 was
used as a loading control.
Bioinformatics analysis
The analysis of potential microRNA target site using
the commonly used prediction algorithms - miRanda (http://www.microrna.org/).
Statistical analysis
Data are expressed as the means ± SD, from at least
3 separate experiments and were analyzed using one-way ANOVA or the
Student's t-test. A value of p<0.05 was considered to indicate a
statistically significant difference.
Results
WWOX regulates cell proliferation and
epithelial to mesenchymal transition (EMT)-associated gene
expression and its expression is downregulated in osteosarcoma
In an attempt to determine the role of WWOX in
regulating cell proliferation and EMT in osteosarcoma, the MG63
cells were transfected with WWOX overexpression plasmids or empty
vectors. Following stable transfection, WWOX expression was
detected by western blot analysis and 9 proliferation- and
EMT-associated markers were also examined by western blot analysis
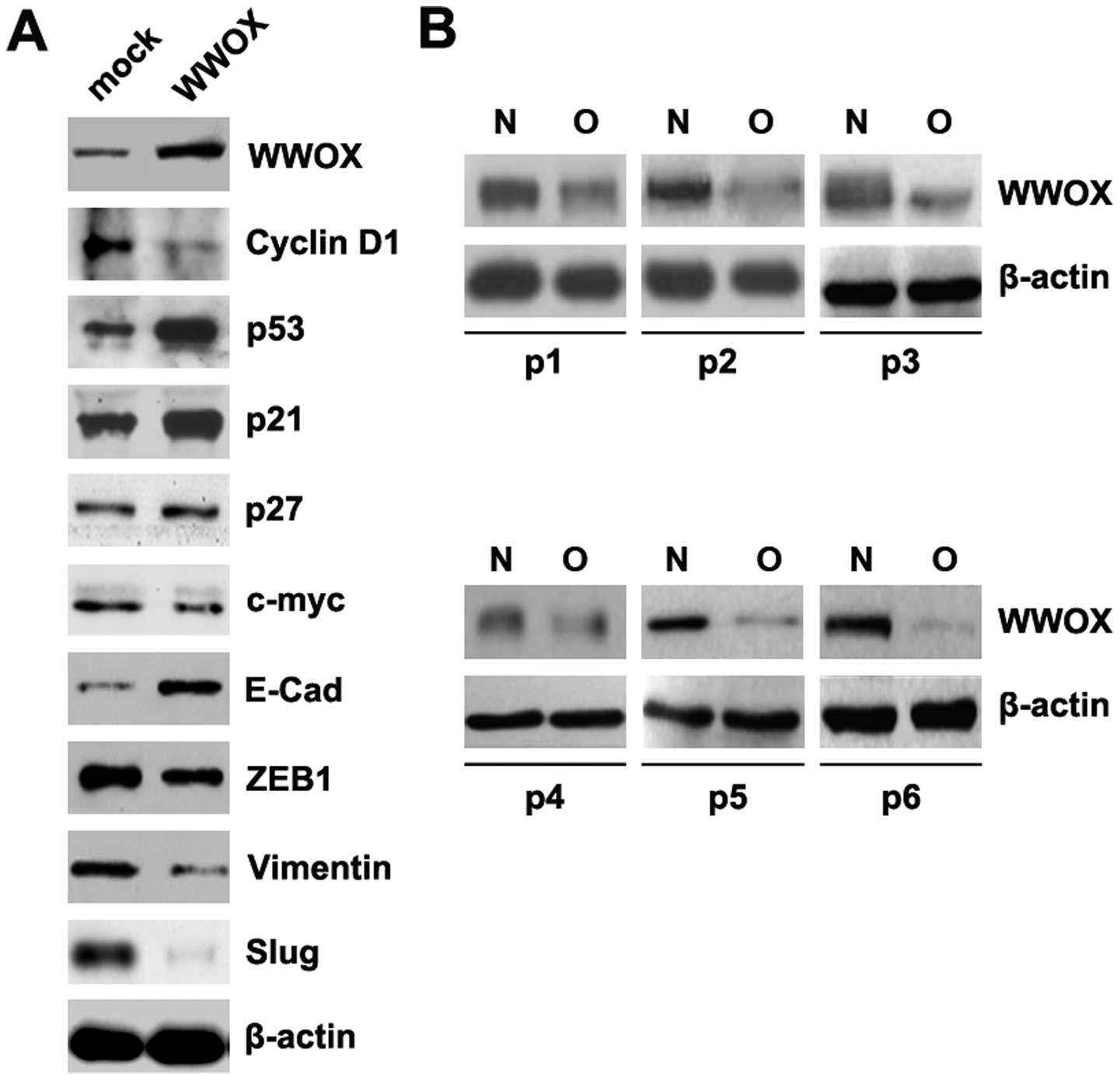
in the MG63 cells. The results revealed that transfection with WWOX
overexpression plasmids markedly increased WWOX protein expression.
It also promoted p53, p21 and E-Cad expression and suppressed
cyclin D1, ZEB1, vimentin and Slug expression in the MG63 cells
(Fig. 1A). To assess the
expression of WWOX in osteosarcoma, western blot analysis was
conducted on 6 pairs of osteosarcoma tissues and matched adjacent
normal tissue samples. The expression of WWOX was consistently
lower in the osteosarcoma tissues compared with the normal tissues
(Fig. 1B).
WWOX inhibits the proliferation of MG63
osteosarcoma cells
In order to determine the role of WWOX in regulating
the proliferation of MG63 cells, the proliferation rates of MG63
cells transfected with WWOX overexpression plasmids or empty
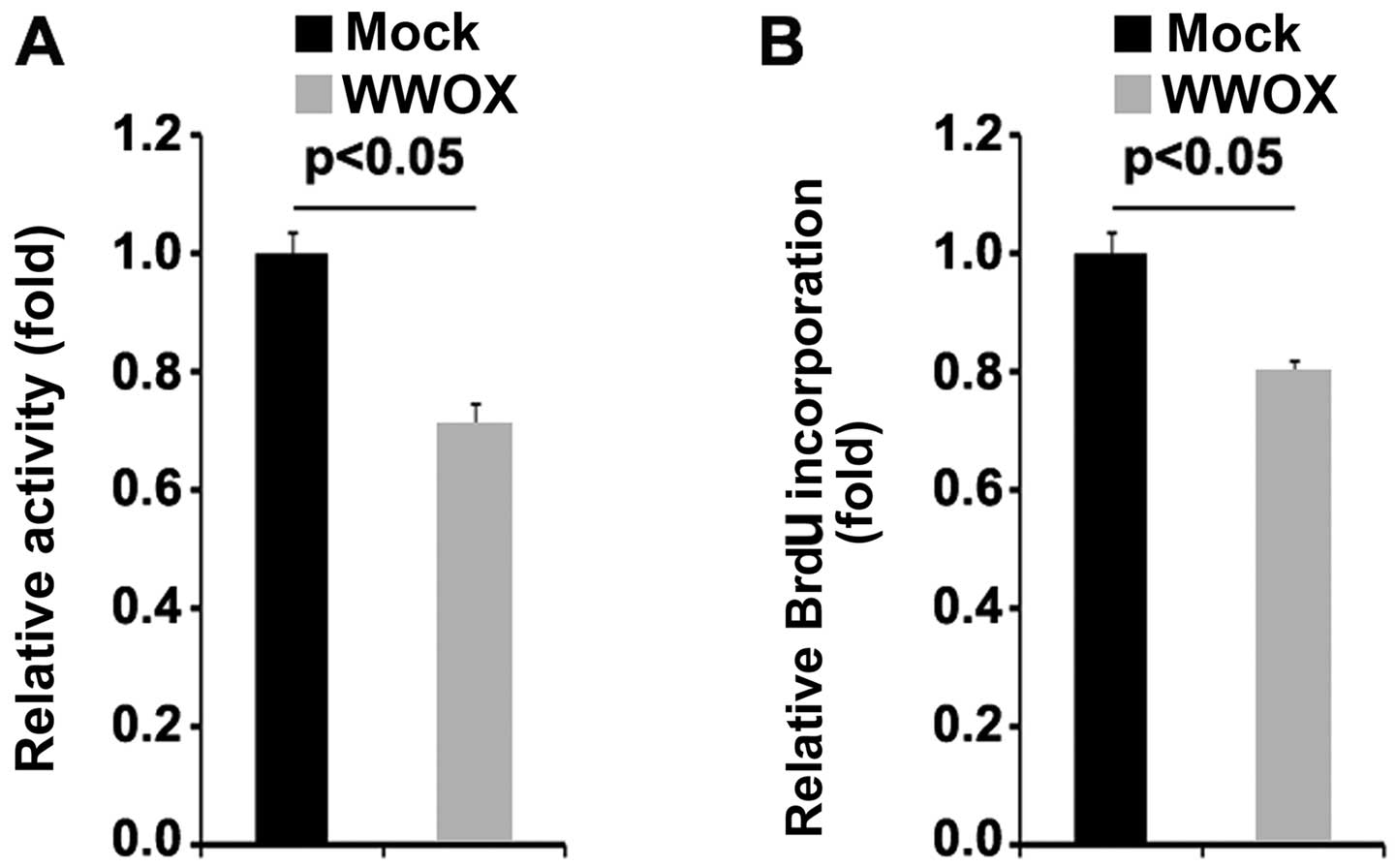
vectors were examined by MTT assay. The results revealed that the
overexpression of WWOX significantly suppressed the proliferation
of MG63 cells (Fig. 2A). This was
confirmed by BrdU incorporation assay which revealed that
transfection with WWOX overexpression plasmids resulted in
decreased DNA synthesis per viable MG63 cell (Fig. 2B).
WWOX inhibits the migration and invasion
of osteosarcoma MG63 cells
E-Cad, ZEB1, Vimentin and slug are EMT associated
markers and they are associated with invasion and migration in
cancer (27–31). Given that WWOX markedly inhibited
the expression of EMT-associated markers and that of markers
associated with invasion and migration (32,33), we then sought to determine whether
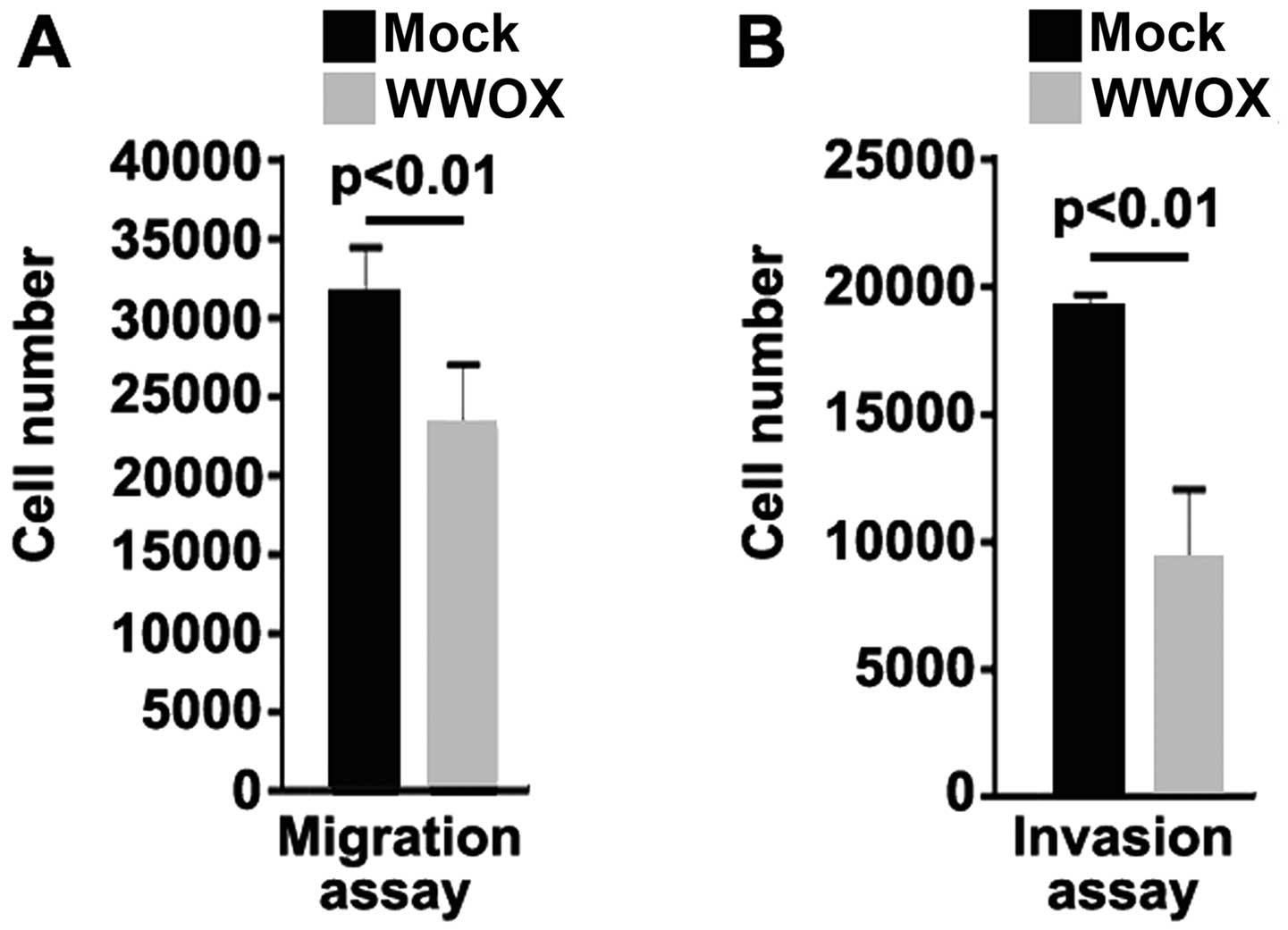
WWOX has an effect on the migration and invasion of MG63 cells. The
results from migration and invasion assay indicated that the
overexpression of WWOX not only inhibited the migration of the MG63
cells, but also suppressed the invasion of these cells (Fig. 3).
Silencing of miR-214-3p upregulates WWOX
protein expression in MG63 osteosarcoma cells
Having demonstrated that WWOX suppresses EMT, as
well as the proliferation, migration and invasion of osteosarcoma
cells, we then wished to determine the mechanisms regulating WWOX
expression in the disease. miRNAs are a class of small non-coding
RNAs (approximately 22 nucleotides in lenght) that negatively
regulate protein-coding gene expression by targeting mRNA
degradation or translation inhibition (34,35). To further confirm whether WWOX is
regulated by miRNAs, we used the commonly used prediction
algorithm, miRanda (http://www.microrna.org/microrna/home.do), to analyze
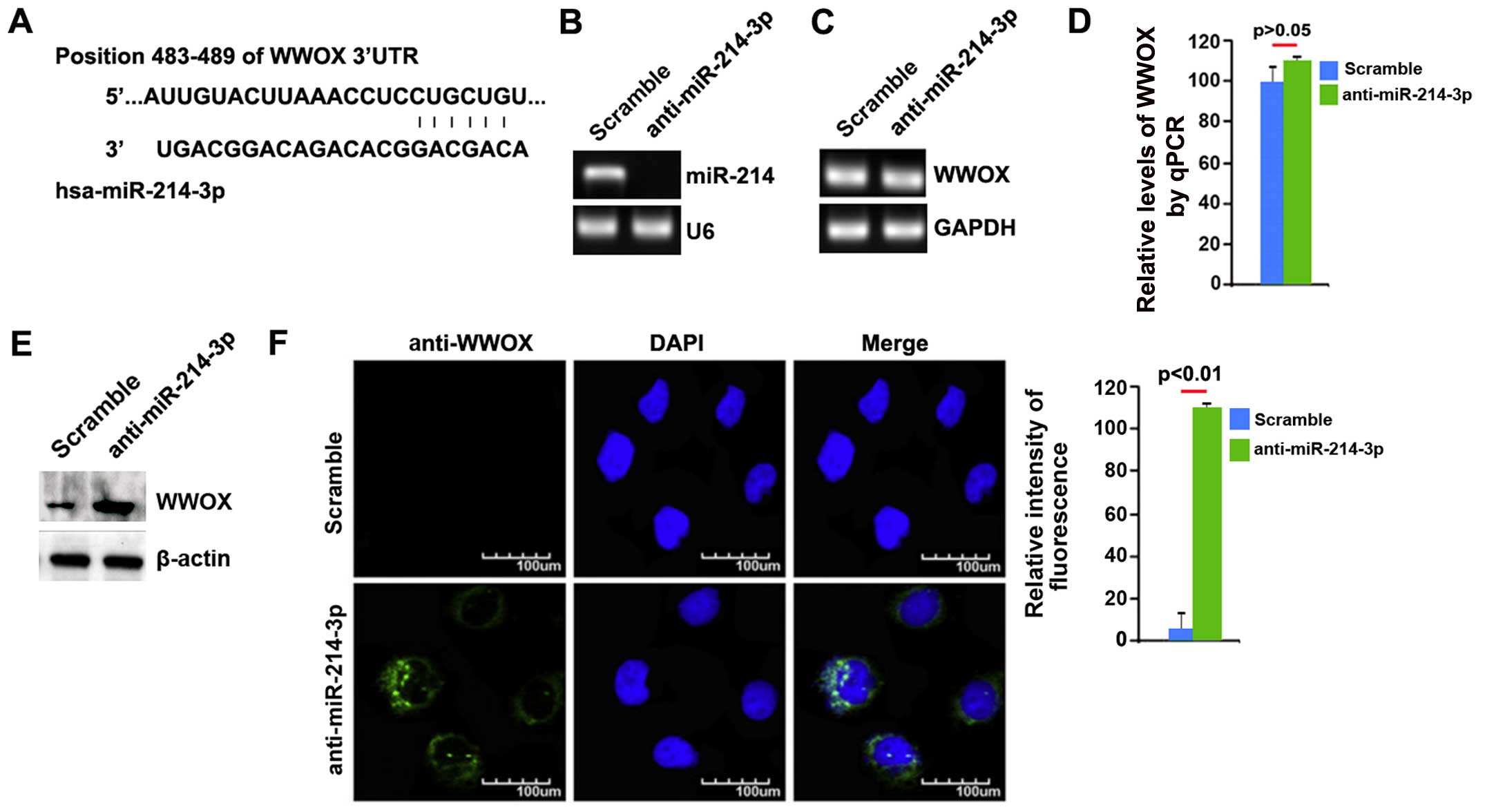
the 3′UTR of WWOX. A dozen miRNAs were found by the algorithm.
However, we were interested in miR-214-3p, as the expression of
miR-214-3p has been confirmed to be significantly higher in
osteosarcoma tissues than in normal tissues (25). It seems to play an important role
in osteosarcoma. The target sites on the 3′UTR of WWOX are shown in
Fig. 4A. We reasoned that
miR-214-3p downregulates WWOX expression by targeting its 3′UTR and
that WWOX protein is suppressed due to the overexpression of
miR-214-3p in osteosarcoma. In an attempt to determine the role of
miR-214-3p in regulating WWOX expression in osteosarcoma cells, the
MG63 cells were transfected with anti-miR-214-3p and scramble
inhibitor. Following transfection, miR-214-3p expression was
detected by qPCR and the results revealed that miR-214-3p
expression was significantly decreased by transfection of the cells
with anti-miR-214-3p (Fig. 4B).
We then performed RT-qPCR to detect WWOX mRNA expression in the
MG63 cells transfected with anti-miR-214-3p or scramble inhibitor.
The results demonstrated that WWOX mRNA expression was not affected
by transfection with anti-miR-214-3p compared with the scramble
inhibitor-transfected groups (Fig. 4C
and D). Moreover, western blot analysis was performed to detect
WWOX protein expression in the MG63 cells transfected with
anti-miR-214-3p or scramble inhibitor. The results revdealed that
WWOX protein (Fig. 4E) was
significantly upregulated in the cells after the silencing of
miR-214-3p. Consistent with the results of western blot analysis,
we performed immunofluorescence assay of the MG63 cells transfected
with anti-miR-214-3p or scramble inhibitor. The results revealed
that WWOX protein expression was evidently upregulated in the cells
transfected with anti-miR-214-3p (Fig. 4F). These data demonstrated that
WWOX protein expression was suppressed by the overexpres-sion of
miR-214-3p in osteosarcoma cells.
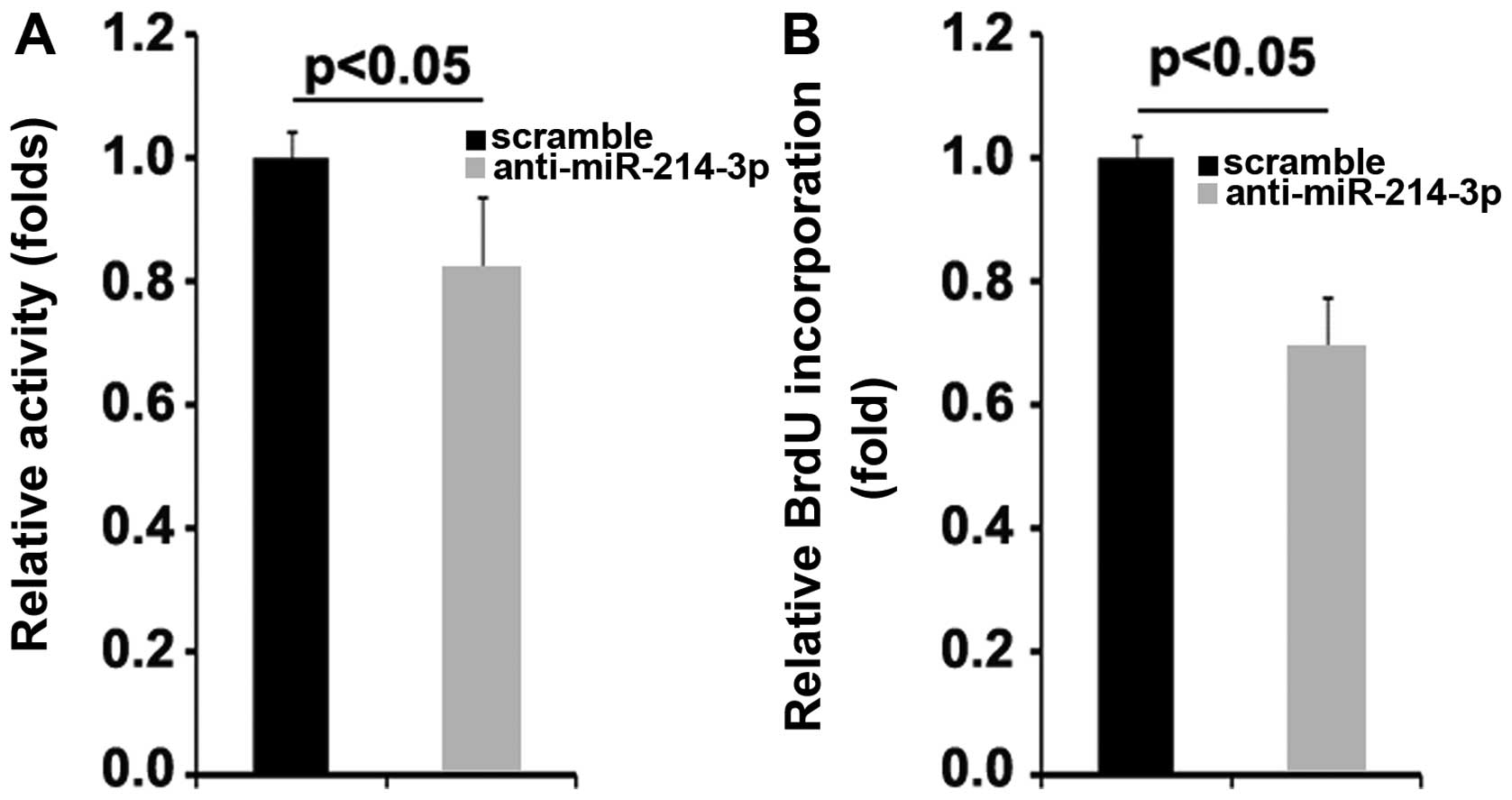
Silencing of miR-214-3p inhibits the
proliferation of MG63 osteosarcoma cells
To determine the role of miR-214-3p in regulating
the proliferation of MG63 cells, the proliferation rates of the
MG63 cells were examined by MTT assay. The results demonstrated
that the silencing of miR-214-3p significantly suppressed the
proliferation of the MG63 cells (Fig.
5A). This was further confirmed by BrdU incorporation assay,
which indicated that transfection with anti-miR-214-3p resulted in
decreased DNA synthesis per viable MG63 cell (Fig. 5B).
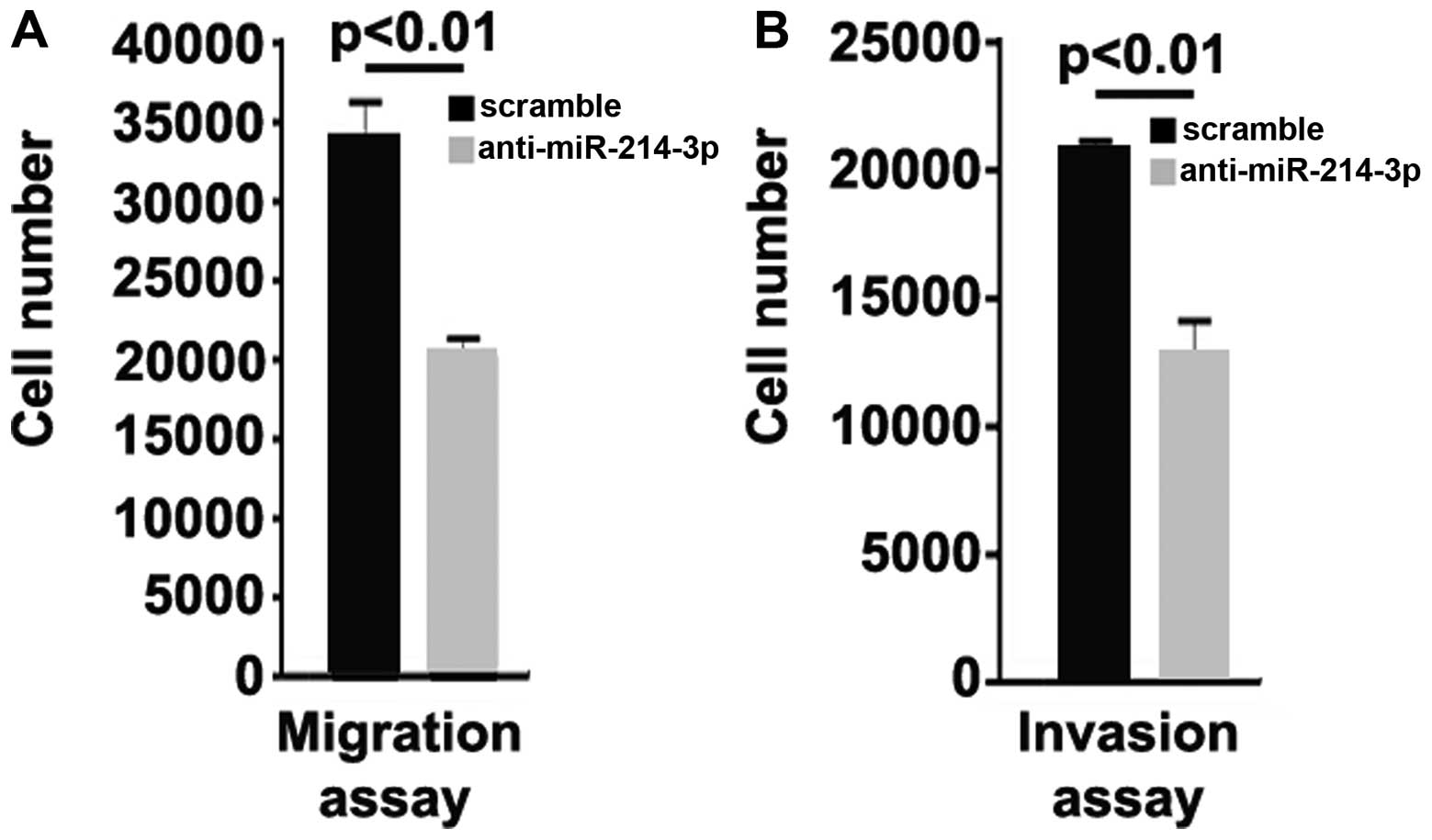
Silencing of miR-214-3p inhibits the
migration and invasion of MG63 osteosarcoma cells
We then sought to determine whether the silencing of
miR-214-3p affects the migration and invasion of MG63 cells. The
results of migration and invasion assay indicated that the
silencing of miR-214-3p inhibited the migration and invasion of
MG63 cells (Fig. 6).
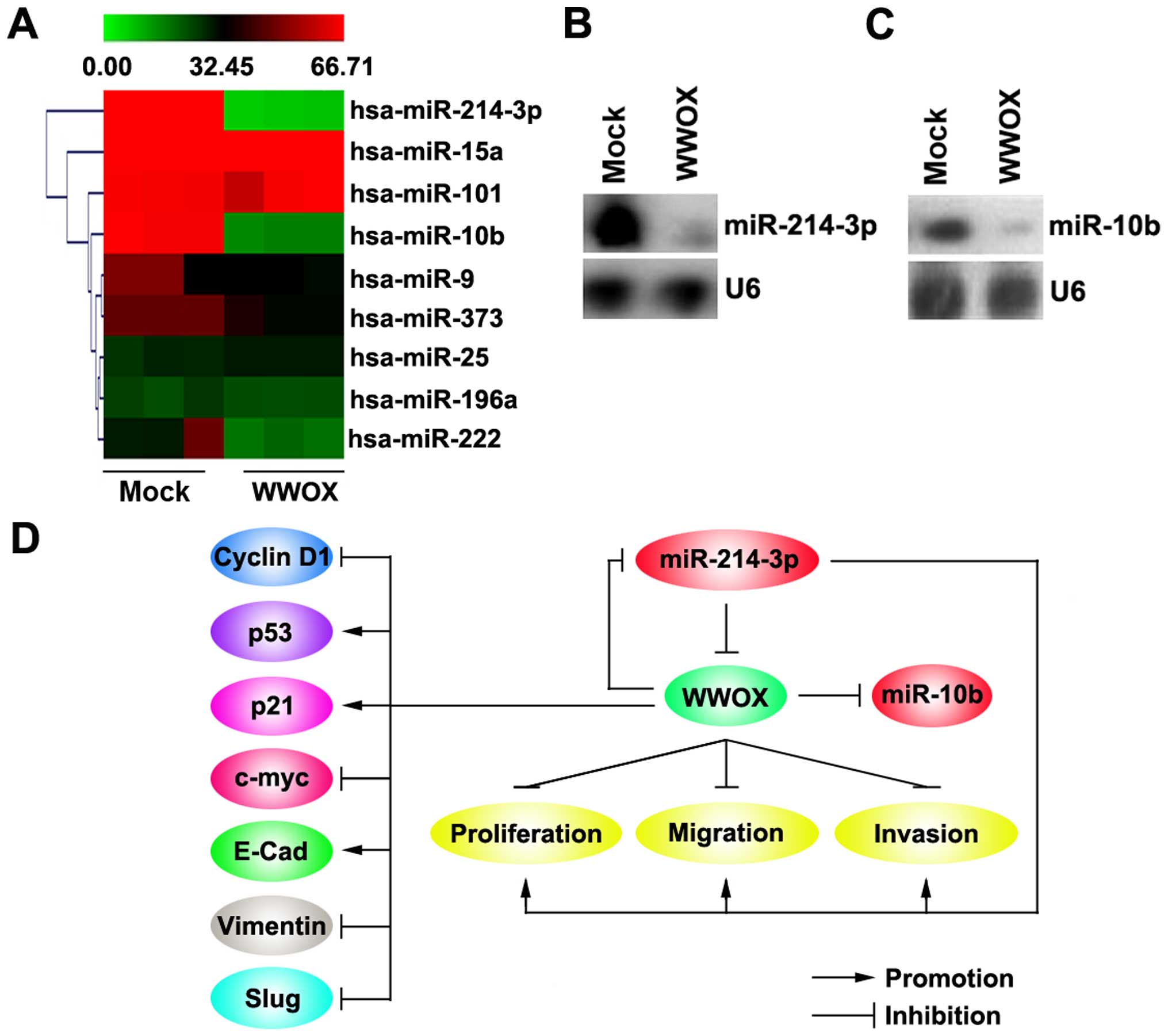
WWOX inhibits miR-214-3p and miR-10b
expression in osteosarcoma MG63 cells
Tumor suppressor genes can exert their effects by
regulating miRNA expression in cancer (36) and miRNAs are known to play a role
in cancer pathogenesis and some of them function as tumor
suppressor genes or oncogenes (37–39). Thus, we hypothesized that WWOX
functions as tumor suppressor by regulating relevant miRNAs. Thus
miRNA microarrays was performed. RNAs isolated from the MG63 cells
were hybridized to a custom miRNA microarray platform. Following
hybridization, quantification, and normalization 3 times, a dozen
miRNAs were foun to be altered in the MG63 cells. However, we were
interested in miR-214-3p and miR-10b, as miR-214-3p and miR-10b
appear to play the roles of oncogenes in various types of cancer
(22,25,40–43). The results of microarray analysis
demonstrated that miR-214-3p and miR-10b expression was decreased
>50-fold in the cells transfected with the WWOX overexpression
plasmids (Fig. 7A). To further
confirm the regulatory role of WWOX, we performed northern blot
analysis to detect the expression of miR-214-3p and miR-10b.
Consistent with the findings of microarray analysis, the results of
northern blot analysis revealed that the overexpression of WWOX
significantly downregulated miR-214-3p (Fig. 7B) and miR-10b (Fig. 7C) expression in the MG63
cells.
Discussion
Much research has been carried out in order to
understand the pathogenesis of osteosarcoma and to provide novel
therapeutic targets for the disease (44–46). miRNAs are small non-coding RNAs of
20–22 nucleotides in lenght that have been implicated in the
development of various types of cancer (47). miR-214-3p expression is
deregulated in several human tumors, including melanoma (48), breast cancer (49), ovarian cancer (22), gastric cancer (40), hepatocellular carcinoma (41) and osteosarcoma (25). Its pleiotropic and tumor-specific
role in the formation and progression of various types of cancer is
achieved by targeting its several target genes (22,25,40,41,48,49). miR-214-3p can function as a key
and core regulator by regulating vital signaling networks, such as
β-catenin, PTEN/AKT and the tyrosine kinase receptor pathways
(42,43). Moreover, miR-214-3p can also
regulate the expression of essential cancer-associated genes, for
example, enhancer of zeste homolog 2 (Ezh2; the epigenetic
repressor), p53, transcription factor AP-2 alpha (TFAP2) and
miR-148b (22,48,50). Consistent with its role of
regulating essential cancer-associated genes, miR-214 seems to play
crucial roles in promoting tumor cell proliferation, stemness,
angiogenesis, migration, invasiveness, extravasation,
intravasation, metastasis, resistance to chemotherapy and in the
microenvironment (22,25,40–43).
In the present study, we firstly observed that WWOX
protein was downregulated in osteosarcoma and that it regulated
cell proliferation and EMT-associated gene expression (Fig. 7D). Moreover, WWOX overexpression
suppressed the proliferation, migration and invasion of MG63
osteosarcoma cells. These results, along with the fact that the
deletion of the WWOX gene and the frequent loss of its protein
expression are detected in human osteosarcoma, and the tumor
suppressor, WWOX, inhibits osteosarcoma metastasis by modulating
RUNX2 function (18,51), confirmed that WWOX is a tumor
suppressor in osteosarcoma.
In line with the findings of previous studies
demonstrating that miR-214-3p is an oncogene in various types of
cancer (22,25,40–43), the silencing of miR-214-3p
upregulated WWOX protein expression and inhibited the
proliferation, migration and invasion of osteosarcoma cells. This
finding may partly explain why WWOX protein is downregulated in
osteosarcoma, since miR-214-3p is significantly upregulated in the
disease. When we overexpressed WWOX in MG63 cells, it negatively
regulated miR-214-3p. Thus, we concluded that the deregulation of
microRNA-214-3p expression is involved in a negative feedback loop
with WWOX in osteosarcoma. miR-10b has been proposed as an oncogene
in various types of cancer (52–54). We found that WWOX significantly
suppressed miR-10b expression in MG63 cells, although the role of
miR-10b has not been reported in the disease, at least to the best
of our knowledge.
In conclusion, the deregulation of microRNA-214-3p
expression is involved in a negative feedback loop with WWOX in
osteosarcoma, indicating that miR-214-3p has potential basic and
clinical implications. On the one hand, WWOX may be a powerful
osteosarcoma suppressor by exerting inhibitory effects on cell
proliferation, migration and invasion, and the pharmacological
restoration of WWOX may represent a promising therapeutic strategy.
On the other hand, miR-214-3p as an oncogene is also associated
with the pathogenesis and progression of osteosarcoma, and the
targeting of miR-214-3p may also be apotential therapeutic strategy
for patients with osteosarcoma.
References
|
1
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.
|
|
2
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wada T, Isu K, Takeda N, Usui M, Ishii S
and Yamawaki S: A preliminary report of neoadjuvant chemotherapy
NSH-7 study in osteosarcoma: Preoperative salvage chemotherapy
based on clinical tumor response and the use of granulocyte
colony-stimulating factor. Oncology. 53:221–227. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3–24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
5
|
Ried K, Finnis M, Hobson L, Mangelsdorf M,
Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A,
Venter D, et al: Common chromosomal fragile site FRA16D sequence:
Identification of the FOR gene spanning FRA16D and homozygous
deletions and translocation breakpoints in cancer cells. Hum Mol
Genet. 9:1651–1663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang NS, Pratt N, Heath J, Schultz L,
Sleve D, Carey GB and Zevotek N: Hyaluronidase induction of a WW
domain-containing oxidoreductase that enhances tumor necrosis
factor cytotoxicity. J Biol Chem. 276:3361–3370. 2001. View Article : Google Scholar
|
|
7
|
Paige AJ, Taylor KJ, Taylor C, Hillier SG,
Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H and Watson
JE: WWOX: A candidate tumor suppressor gene involved in multiple
tumor types. Proc Natl Acad Sci USA. 98:11417–11422. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuroki T, Trapasso F, Shiraishi T, Alder
H, Mimori K, Mori M and Croce CM: Genetic alterations of the tumor
suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer
Res. 62:2258–2260. 2002.PubMed/NCBI
|
|
9
|
Driouch K, Prydz H, Monese R, Johansen H,
Lidereau R and Frengen E: Alternative transcripts of the candidate
tumor suppressor gene, WWOX, are expressed at high levels in human
breast tumors. Oncogene. 21:1832–1840. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yendamuri S, Kuroki T, Trapasso F, Henry
AC, Dumon KR, Huebner K, Williams NN, Kaiser LR and Croce CM: WW
domain containing oxidoreductase gene expression is altered in
non-small cell lung cancer. Cancer Res. 63:878–881. 2003.PubMed/NCBI
|
|
11
|
Aqeilan RI, Kuroki T, Pekarsky Y, Albagha
O, Trapasso F, Baffa R, Huebner K, Edmonds P and Croce CM: Loss of
WWOX expression in gastric carcinoma. Clin Cancer Res.
10:3053–3058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuroki T, Yendamuri S, Trapasso F,
Matsuyama A, Aqeilan RI, Alder H, Rattan S, Cesari R, Nolli ML,
Williams NN, et al: The tumor suppressor gene WWOX at FRA16D is
involved in pancreatic carcinogenesis. Clin Cancer Res.
10:2459–2465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SW, Ludes-Meyers J, Zimonjic DB,
Durkin ME, Popescu NC and Aldaz CM: Frequent downregulation and
loss of WWOX gene expression in human hepatocellular carcinoma. Br
J Cancer. 91:753–759. 2004.PubMed/NCBI
|
|
14
|
Pimenta FJ, Gomes DA, Perdigão PF, Barbosa
AA, Romano-Silva MA, Gomez MV, Aldaz CM, De Marco L and Gomez RS:
Characterization of the tumor suppressor gene WWOX in primary human
oral squamous cell carcinomas. Int J Cancer. 118:1154–1158. 2006.
View Article : Google Scholar
|
|
15
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ and Aldaz CM: WWOX,
the FRA16D gene, behaves as a suppressor of tumor growth. Cancer
Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
16
|
Fabbri M, Iliopoulos D, Trapasso F,
Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D,
Huebner K and Croce CM: WWOX gene restoration prevents lung cancer
growth in vitro and in vivo. Proc Natl Acad Sci USA.
102:15611–15616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schirmer MA, Lüske CM, Roppel S, Schaudinn
A, Zimmer C, Pflüger R, Haubrock M, Rapp J, Güngör C, Bockhorn M,
et al: Relevance of Sp binding site polymorphism in WWOX for
treatment outcome in pancreatic cancer. J Natl Cancer Inst.
108:djv3872016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Del Mare S and Aqeilan RI: Tumor
Suppressor WWOX inhibits osteosarcoma metastasis by modulating
RUNX2 function. Sci Rep. 5:129592015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McManus MT: MicroRNAs and cancer. Semin
Cancer Biol. 13:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu CX, Xu M, Tan L, Yang H, Permuth-Wey J,
Kruk PA, Wenham RM, Nicosia SV, Lancaster JM, Sellers TA and Cheng
JQ: MicroRNA miR-214 regulates ovarian cancer cell stemness by
targeting p53/Nanog. J Biol Chem. 287:34970–34978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang XJ, Ye H, Zeng CW, He B, Zhang H and
Chen YQ: Dysregulation of miR-15a and miR-214 in human pancreatic
cancer. J Hematol Oncol. 3:462010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Z and Cai H, Lin L, Tang M and Cai H:
Upregulated expression of microRNA-214 is linked to tumor
progression and adverse prognosis in pediatric osteosarcoma.
Pediatr Blood Cancer. 61:206–210. 2014. View Article : Google Scholar
|
|
26
|
Xu Z and Wang T: miR-214 promotes the
proliferation and invasion of osteosarcoma cells through direct
suppression of LZTS1. Biochem Biophys Res Commun. 449:190–195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lombaerts M, van Wezel T, Philippo K,
Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de
Water B, Cornelisse CJ and Cleton-Jansen AM: E-cadherin
transcriptional downregulation by promoter methylation but not
mutation is related to epithelial-to-mesenchymal transition in
breast cancer cell lines. Br J Cancer. 94:661–671. 2006.PubMed/NCBI
|
|
28
|
Shen A, Zhang Y, Yang H, Xu R and Huang G:
Overexpression of ZEB1 relates to metastasis and invasion in
osteosarcoma. J Surg Oncol. 105:830–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng CW, Wang HW, Chang CW, Chu HW, Chen
CY, Yu JC, Chao JI, Liu HF, Ding SL and Shen CY: MicroRNA-30a
inhibits cell migration and invasion by downregulating vimentin
expression and is a potential prognostic marker in breast cancer.
Breast Cancer Res Treat. 134:1081–1093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gilles C, Polette M, Zahm JM, Tournier JM,
Volders L, Foidart JM and Birembaut P: Vimentin contributes to
human mammary epithelial cell migration. J Cell Sci. 112:4615–4625.
1999.PubMed/NCBI
|
|
31
|
Uygur B and Wu WS: SLUG promotes prostate
cancer cell migration and invasion via CXCR4/CXCL12 axis. Mol
Cancer. 10:1392011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEB1 and members of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Novakova J, Slaby O, Vyzula R and Michalek
J: MicroRNA involvement in glioblastoma pathogenesis. Biochem
Biophys Res Commun. 386:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sasayama T, Nishihara M, Kondoh T, Hosoda
K and Kohmura E: MicroRNA-10b is overexpressed in malignant glioma
and associated with tumor invasive factors, uPAR and RhoC. Int J
Cancer. 125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guessous F, Zhang Y, Kofman A, Catania A,
Li Y, Schiff D, Purow B and Abounader R: microRNA-34a is tumor
suppressive in brain tumors and glioma stem cells. Cell Cycle.
9:1031–1036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu S, Gao Y, Huang X and Wang X:
Cantharidin exerts anti-hepatocellular carcinoma by miR-214
modulating macrophage polarization. Int J Biol Sci. 10:415–425.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang YS, Wang YH, Xia HP, Zhou SW,
Schmid-Bindert G and Zhou CC: MicroRNA-214 regulates the acquired
resistance to gefitinib via the PTEN/AKT pathway in EGFR-mutant
cell lines. Asian Pac J Cancer Prev. 13:255–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xia H, Ooi LL and Hui KM: MiR-214 targets
β-catenin pathway to suppress invasion, stem-like traits and
recurrence of human hepatocellular carcinoma. PLoS One.
7:e442062012. View Article : Google Scholar
|
|
44
|
Zhang W, Qian JX, Yi HL, Yang ZD, Wang CF,
Chen JY, Wei XZ, Fu Q and Ma H: The microRNA-29 plays a central
role in osteosarcoma pathogenesis and progression. Mol Biol (Mosk).
46:622–627. 2012. View Article : Google Scholar
|
|
45
|
Bao YP, Yi Y, Peng LL, Fang J, Liu KB, Li
WZ and Luo HS: Roles of microRNA-206 in osteosarcoma pathogenesis
and progression. Asian Pac J Cancer Prev. 14:3751–3755. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhou L, Park J, Jang KY, Park HS, Wagle S,
Yang KH, Lee KB, Park BH and Kim JR: The overexpression of BAMBI
and its involvement in the growth and invasion of human
osteosarcoma cells. Oncol Rep. 30:1315–1322. 2013.PubMed/NCBI
|
|
47
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Penna E, Orso F, Cimino D, Vercellino I,
Grassi E, Quaglino E, Turco E and Taverna D: miR-214 coordinates
melanoma progression by upregulating ALCAM through TFAP2 and
miR-148b downmodulation. Cancer Res. 73:4098–4111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Derfoul A, Juan AH, Difilippantonio MJ,
Palanisamy N, Ried T and Sartorelli V: Decreased microRNA-214
levels in breast cancer cells coincides with increased cell
proliferation, invasion and accumulation of the Polycomb Ezh2
methyltransferase. Carcinogenesis. 32:1607–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Juan AH, Kumar RM, Marx JG, Young RA and
Sartorelli V: MiR-14-dependent regulation of the polycomb protein
Ezh2 in skeletal muscle and embryonic stem cells. Mol Cell.
36:61–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang J, Cogdell D, Yang D, Hu L, Li H,
Zheng H, Du X, Pang Y, Trent J, Chen K and Zhang W: Deletion of the
WWOX gene and frequent loss of its protein expression in human
osteosarcoma. Cancer Lett. 291:31–38. 2010. View Article : Google Scholar
|
|
52
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
miR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|
|
54
|
Guessous F, Alvarado-Velez M,
Marcinkiewicz L, Zhang Y, Kim J, Heister S, Kefas B, Godlewski J,
Schiff D, Purow B and Abounader R: Oncogenic effects of miR-10b in
glioblastoma stem cells. J Neurooncol. 112:153–163. 2013.
View Article : Google Scholar : PubMed/NCBI
|