Introduction
According to the current understanding of the
pathophysiology of allergic diseases, mast cells play a key role in
inflammation, and are well known for their influential effector
functions in allergic disorders and reactions (1,2).
The human mast cell line-1 (HMC-1) originates from a patient with
mastocytosis (3) and these cells
do not express the high-affinity IgE-receptor FcεR1 (4,5).
The lack of FcεR1 in HMC-1 cells has led to the use of
physiological stimuli, such as calcium ionophores and phorbol
esters to activate these cells, by many researchers. Previous
studies have demonstrated that HMC-1 cells express a wide range of
cytokines, which can be synthesized, stored and released after
stimulation (6,7). For example, mast cells release
pro-inflammatory cytokines and chemokines, such as interleukin
(IL)-6, IL-8, granulocyte macrophage colony-stimulating factor
(GM-CSF), tumor necrosis factor-α (TNF-α) and inflammatory
mediators, including histamine, leukotrienes and serotonin
(8,9). The cytokines released from mast
cells transform the terminal microenvironment and attract
neutrophils and basophils (10).
TNF-α is a pleiotropic pro-inflammatory cytokine that plays an
important role in several pathological conditions related to
inflammation and infection; the role of TNF in malignancies and
inflammatory disorders, such as arthritis has been reviewed
extensively (11). The
pro-inflammatory activities of IL-6 include the recruitment of
inflammatory cells, the inhibition of the apoptosis of inflammatory
cells, and the inhibition of regulatory T-cell differentiation
(12). IL-8 is the most potent
chemokine that has been studied thus far and is responsible for
inducing chemotaxis, which is the directed migration of cells to a
site of inflammation (13,14).
The mitogen-activated protein kinases (MAPKs)
include extracellular signal-regulated kinase (ERK), p38 and c-Jun
N-terminal kinase (JNK). Each MAPK signaling pathway consists of at
least three components, a MAP kinase kinase kinase (MAP3K), MAPK
kinase (MAP2K) and MAPK. The MAPK pathways are activated by
numerous extracellular and intracellular stimuli, including peptide
growth factors, cytokines, hormones and various cellular stressors.
Studies on the effects of dominant-interfering or constitutively
activated forms of various components of the JNK, p38 and ERK
signaling pathways have reported that the activation of JNK and p38
and the coincident inhibition of ERK are significant for the
induction of apoptosis. The JNK and p38 signaling pathways are
activated by pro-inflammatory cytokines, such as TNF-α in response
to cellular stresses (15,16).
Over the past decade, the pharmacological activities
have been reported for plants of the Rhodiola genus, such as
antihypoxic, antifatigue, antioxidant and anticancer effects, and
their effects on anti-apoptotic processes in cells have also been
reported (17,18). The Rhodiola rosea L. roots
contain a variety of compounds that may contribute to its
pharmacological effects, including phenols, polyphenols, rosavin,
rosin, rosarin, organic acids, terpenoids, phenolcarbonic acids and
their derivatives, flavonoids, anthraquinones and alkaloids
(19–23). Salidroside
[2-(4-hydroxyphenyl)ethyl β-D-glucopyranoside (SAS)] is a glucoside
of tyrosol found in the plant, Rhodiola rosea L. and it
possesses a number of pharmacological properties, including
anti-aging, anti-fatigue, antioxidant, anticancer and
anti-inflammatory properties (24–26). SAS is a powerful anti-inflammatory
agent in asthma (27,28). However, the potential effects of
SAS against phorbol-12-myristate-13-acetate (PMA) plus
A23187-induced inflammation in HMC-1 cells have not yet been fully
investigated. Thus, in this study, to elucidate the molecular
mechanisms responsible for the pharmacological and biochemical
activities of SAS, we examine the effects of SAS on
pro-inflammatory mediators in HMC-1 cells stimulated with PMA plus
A23187.
Materials and methods
Reagents and antibodies
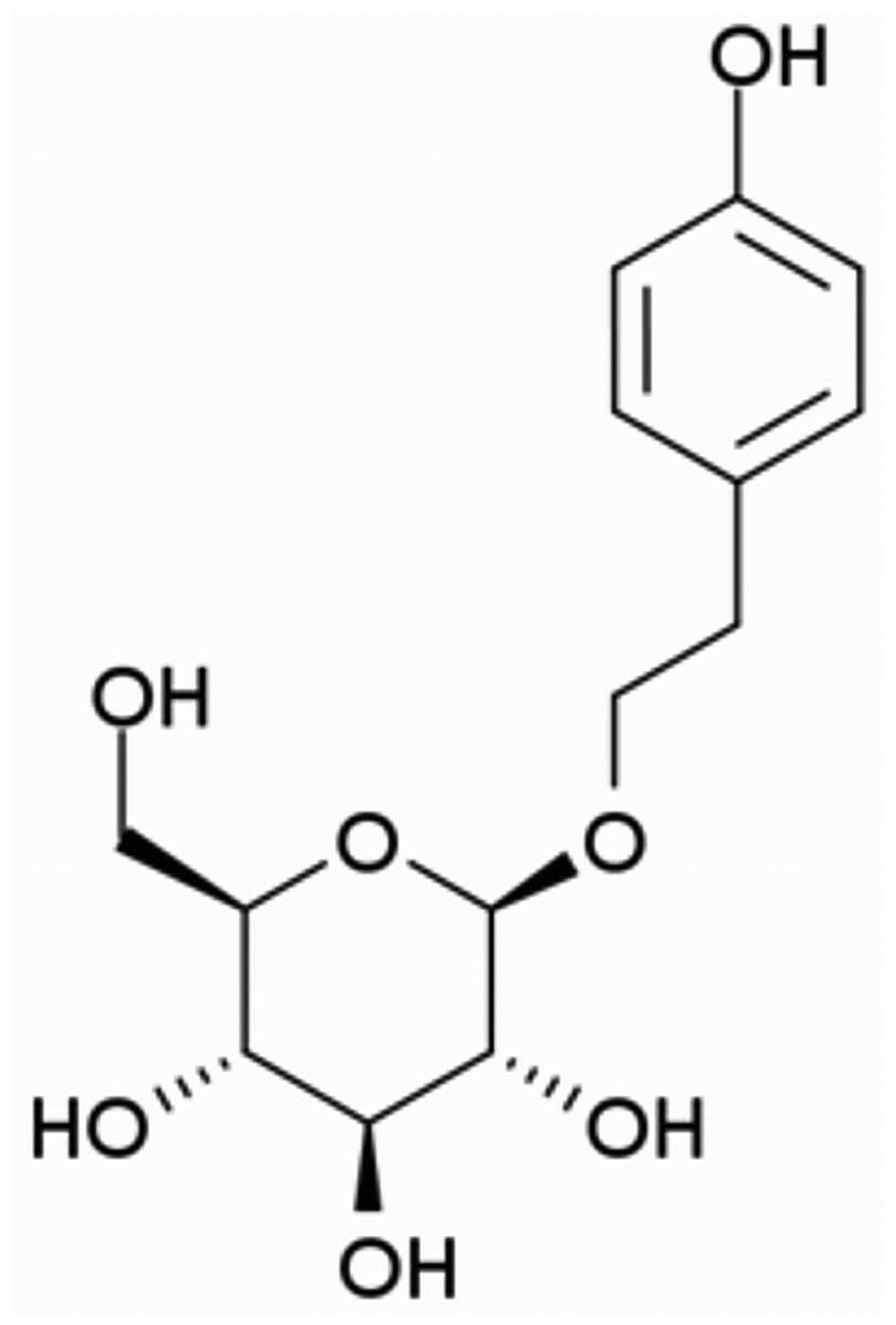
Salidroside (chemical structure shown in Fig. 1) was purchased from Sigma-Aldrich
(St. Louis, MO, USA) and dissolved in dimethyl sulfoxide (DMSO);
the final concentration of DMSO was adjusted to <0.01% (v/v) in
the culture medium. PMA and the calcium ionophore, A23187
(calcymycin;
C29H37N3O6), were
purchased from Sigma-Aldrich. The CellTiter 96® AQueous
One Solution Cell Proliferation assay (MTS) system was purchased
from Promega (Madison, WI, USA). Iscove's modified Dulbecco's
medium (IMDM) was obtained from Welgene (Daegu, Korea). Anti-human
TNF-α (555212), anti-IL-6 (555220) and anti-IL-8 (555244)
antibodies, biotinylated anti-human TNF-α (51-26372E), anti-IL-6
(51-26452E) and IL-8 (51-26542E) antibodies, and recombinant human
TNF-α (51-26376E), IL-6 (51-26456E) and anti-IL-8 (51-26546E)
antibodies were obtained from BD Pharmingen (San Diego, CA, USA).
Anti-phosphorylated (p-)ERK1/2 (sc-7383), anti-p-JNK1/2 (sc-6254),
anti-p-p38 (sc-7973), anti-ERK1/2 (sc-93), anti-JNK1/2 (sc-571),
anti-p38 (sc-535), anti-β-actin (sc-47778), anti-NF-κB (sc-8008)
and anti-rabbit antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The reverse
transcription kit was purchased from Qiagen (Valencia, CA, USA),
and nuclear and cytoplasmic extraction reagents were purchased from
Thermo Scientific (Waltham, MA, USA).
Cell culture
The human leukemic mast cell line, HMC-1, was
obtained from the Korea Research Institute of Bioscience and
Biotechnology (Daejeon, Korea) and grown in IMDM supplemented with
10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100
IU/ml penicillin, 50 μg/ml streptomycin and 1.2 mM
α-thioglycerol at 37°C under 5% CO2 in air.
MTS assay
For the analysis of cell viability by MTS assay, we
used the manufacturer's procedure for the AG Protocol-CellTiter
96® AQueous One Solution Cell Proliferation assay. Cell
aliquots (5×104) were seeded in microplate wells and
treated with SAS (10, 25, 50 and 100 μM) for 30 min. The
following day, the cells were incubated with 20 μl of MTS
solution for 2 h at 37°C under 5% CO2 and 95% air. An
automatic microplate reader (Molecular Devices, Sunnyvale, CA, USA)
was used to read the absorbance of each well at 490 nm.
Enzyme-linked immunosorbent assay
(ELISA)
The HMC-1 cells were treated with various
concentrations of SAS (10, 25 and 50 μM) for 1 h prior to
stimulation with PMA (50 nM) plus A23187 (1 μM). An ELISA
was used to assay the protein levels of IL-6, IL-8 and TNF-8 in the
culture supernatants. To measure the cytokine levels, we used a
modified ELISA. First, we conducted a sandwich ELISA for IL-6, IL-8
and TNF-8 in triplicate in 96-well ELISA plates (Nunc, Roskilde,
Denmark). The supernatant was then transferred to a new
microcentrifuge tube, and the cytokines were quantified using
ELISA. ELISA plates (Falcon; Becton-Dickinson Labware, Franklin
Lakes, NJ, USA) were coated overnight at 4°C with anti-human IL-6,
anti-IL-8 and anti-TNF-α monoclonal antibodies in coating buffer
(0.1 M carbonate, pH 9.5) and then washed 3 imes with
phosphate-buffered saline (PBS) containing 0.05% Tween-20. The
non-specific protein binding sites were blocked with assay diluent
(PBS containing 10% FBS, pH 7.0) for at least 1 h. After washing
the plates again, the test sample or recombinant IL-6, IL-8 and
TNF-α standards were added. Following incubation for 2 h, a working
detector (biotinylated anti-human IL-6, anti-IL-8 and anti-TNF-α
monoclonal antibodies and streptavidin-horseradish peroxidase
reagent) were added followed by incubation for 1 h. The
non-specific protein binding sites were blocked. Subsequently,
substrate solution [tetramethylbenzidine (TMB)] was added to the
wells followed by incubation for 30 min in the dark before the
reaction was terminated by the addition of 1 M
H3PO4. The absorbance was read at 450 nm. All
subsequent steps were carried out at room temperature, and all
standards and samples were assayed in triplicate.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Using an Easy Blue total RNA extraction kit (Intron
Biotechnology, Gyeonggi-do, Korea) we isolated total RNA from the
HMC-1 cells in accordance with the specifications of the
manufacturer. The total RNA was dissolved in DEPC-treated distilled
water. A spectrophotometer (NanoDrop Technologies, Wilmington, DE,
USA) was used to assess RNA purity by measuring the ratio of the
absorbance at 260 and 280 nm; only RNA samples with a value in the
1.6–2.0 range were used. A cDNA synthesis kit (Qiagen, Valencia,
CA, USA) was used for 2 min at 42°C, 30 min at 42°C, and 30 min at
95°C to reverse transcribe each sample into cDNA. The primer
sequences were as follows: IL-6 forward,
5′-GATGGATGCTTCCAATCTGGAT-3′ and reverse,
5′-AGTTCTCCATAGAGAACAACATA-3′; IL-8 forward,
5′-TGTGCTCTCCAAATTTTTTTTACTG-3′ and reverse,
5′-CTCTCTTTCCTCTTTAATGTCCAGC-3; TNF-α forward,
5′-CACCAGCTGGTTATCTCTCAGCTC-3′ and reverse,
5′-CGGGACGTGGAGCTGGCCGAGGAG-3′; glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) forward, 5′CCATGTTCGTCATGGGTGTGAACCA-3′ and
reverse, 5′-GCCAGTAGAGGCAGGGATGATGTTC-3′. Finally, following
electrophoresis on a 2% agarose gel, the expression levels were
confirmed using a UV detector (ImageQuant LAS 500; GE Healthcare
Life Science, Chicago, IL, USA).
Preparation of cytoplasmic and nuclear
extracts
Nuclear extraction reagent (NER) and cytoplasmic
extraction reagent (CER) were used to extract the nucleus and
cytoplasm. A cell volume of 20 μl corresponded to a volume
ratio of CER I:CER II:NER (200:11:100 μl, respectively). A
tube containing CER I was first vortexed vigorously on the highest
setting for 1 sec to fully suspend the cell pellet. The tube was
then incubated on ice for 10 min. Ice-cold CER II was then added to
the tube, and the tube was vortexed for 5 sec on the highest
setting. The tube was then incubated on ice for 1 min before being
vortexed for 5 sec on the highest setting. The tube was then
centrifuged at 13,000 rpm for 5 min on a microcentrifuge
(Centrifuge 5415F; Eppendorf, Hamburg, Germany). The supernatant
(cytoplasmic extract) was then immediately transferred to a clean,
pre-chilled tube and stored until needed. Ice-cold NER was added to
the pellet, and the tube was vortexed on the highest setting for 15
sec. The sample was placed on ice and vortexed for 15 sec every 10
min, for a total of 40 min. The tube was then centrifuged at 13,000
rpm for 5 min on a microcentrifuge. The supernatant (nuclear
extract) was then immediately transferred to a clean, pre-chilled
tube.
Western blot analysis
The HMC-1 cells (5×106 cells/well) were
stimulated with PMA (50 nM) plus A23187 (1 μM). The cell
lysates were prepared in a sample buffer containing sodium dodecyl
sulfate (SDS). The samples were heated at 95°C for 5 min and
briefly cooled on ice. Following centrifugation at 13,000 rpm for 5
min, the proteins in the cell lysates were separated by 12%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred
onto a nitrocellulose membrane. The membrane was then blocked with
5% skim milk in PBS-Tween-20 for 1 h at room temperature and then
incubated with anti-MAPKs, anti-β-actin and anti-NF-κB antibodies
overnight. After washing the blot in Tris-buffered saline and
Tween-20 (TBST) 3 times, it was incubated with a secondary antibody
for 1 h, and then the antibody-specific proteins were visualized
using an ECL™ prime western blotting detection reagent in
accordance with the recommended procedure (Amersham Corp., Newark,
NJ, USA).
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance (ANOVA) followed by Dunnett's t-test for
multiple comparisons and the Student's t-test for single
comparisons. The data from the experiments are presented as the
means ± SEM. The numbers of independent experiments assessed are
provided in the figure legends.
Results
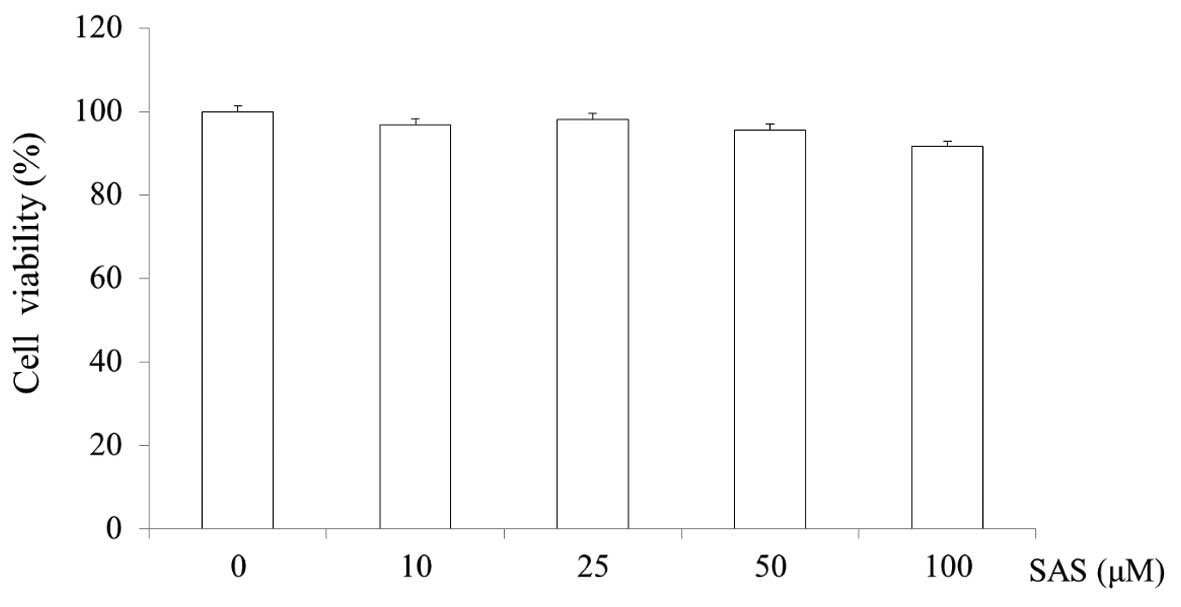
Cytotoxicity of SAS in HMC-1 cells
The cytotoxicity of SAS was evaluated by MTS assay.
SAS was found to not affect the viability of the HMC-1 cells at
concentrations of 10, 25, 50 and 100 μM. The cell viability
of the cells treated with 100 μM SAS was 91.6% (Fig. 2).
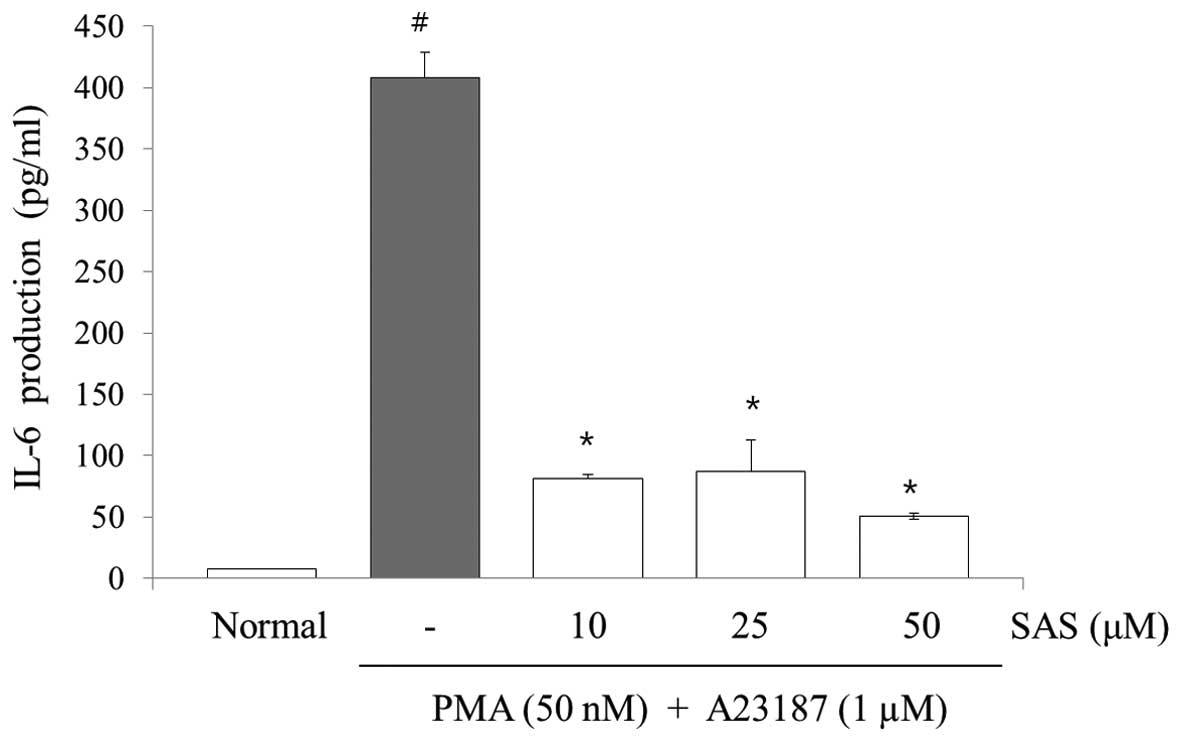
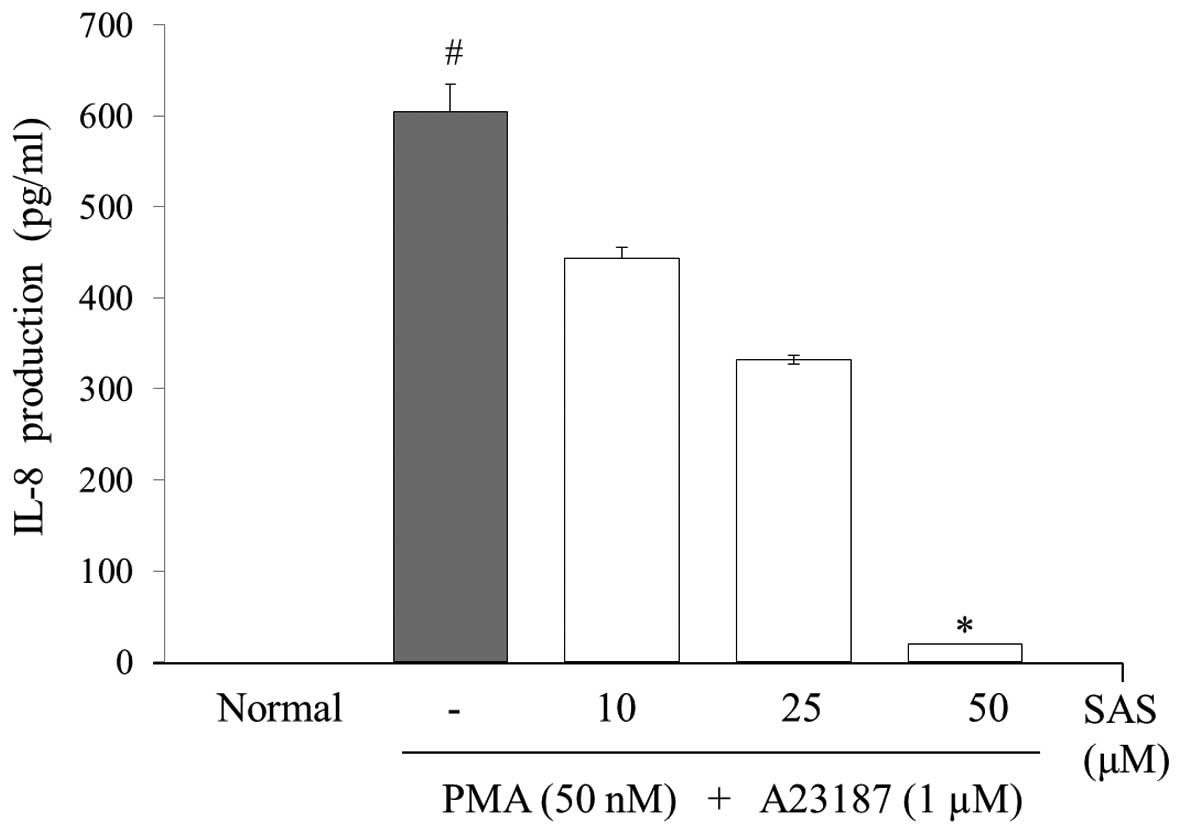
Effect of SAS on the production of IL-6,
IL-8 and TNF-α
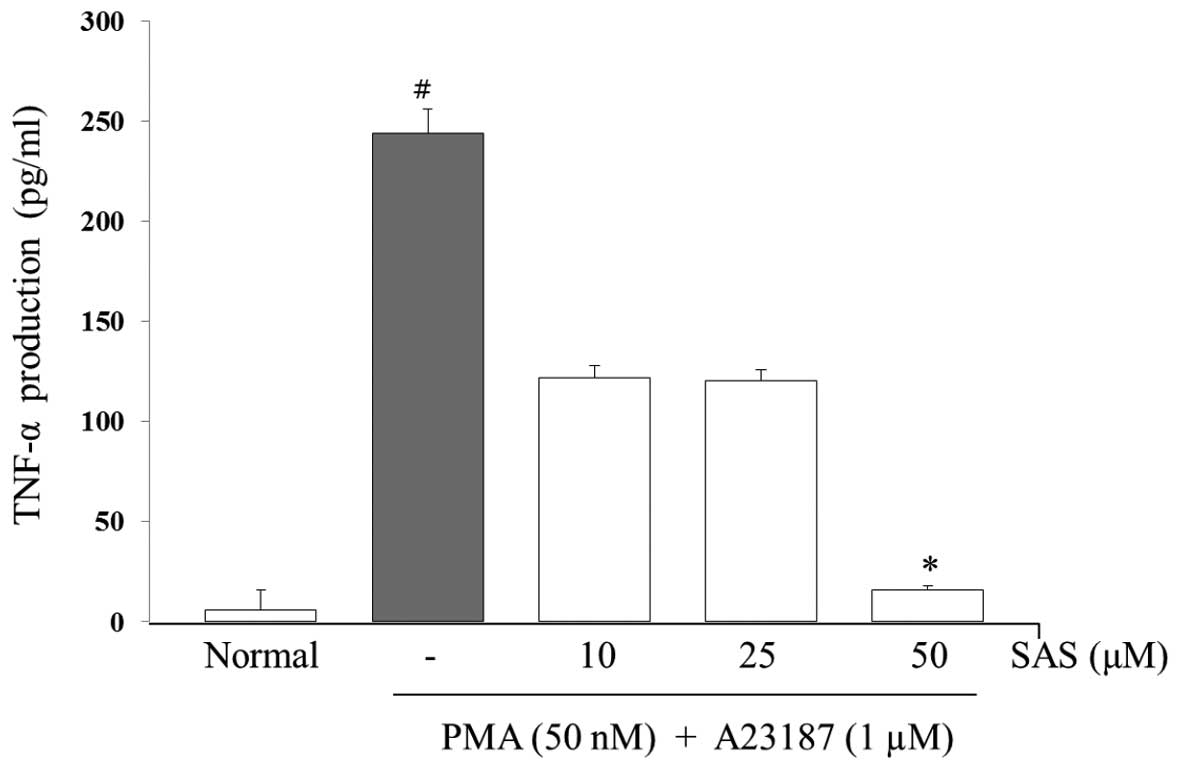
To evaluate the effects of SAS on the production of
IL-6, IL-8 and TNF-α, we treated the cells with SAS (10, 25 and 50
μM) prior to stimulation with PMA (50 nM) plus A23187 (1
μM) for 8 h and analyzed the levels using ELISA. The levels
of IL-6, IL-8, and TNF-α in the HMC-1 cells significantly increased
following stimulation with PMA plus A23187 (Figs. 3Figure 4–5). Pre-treatment of the cells with SAS
(10, 25 and 50 μM) significantly inhibited the increase in
the levels of these cytokines in a concentration-dependent manner.
The maximal inhibition of IL-6, IL-8 and TNF-α production by SAS
(50 μM) was 88, 94 and 63%, respectively (Figs. 3Figure 4–5).
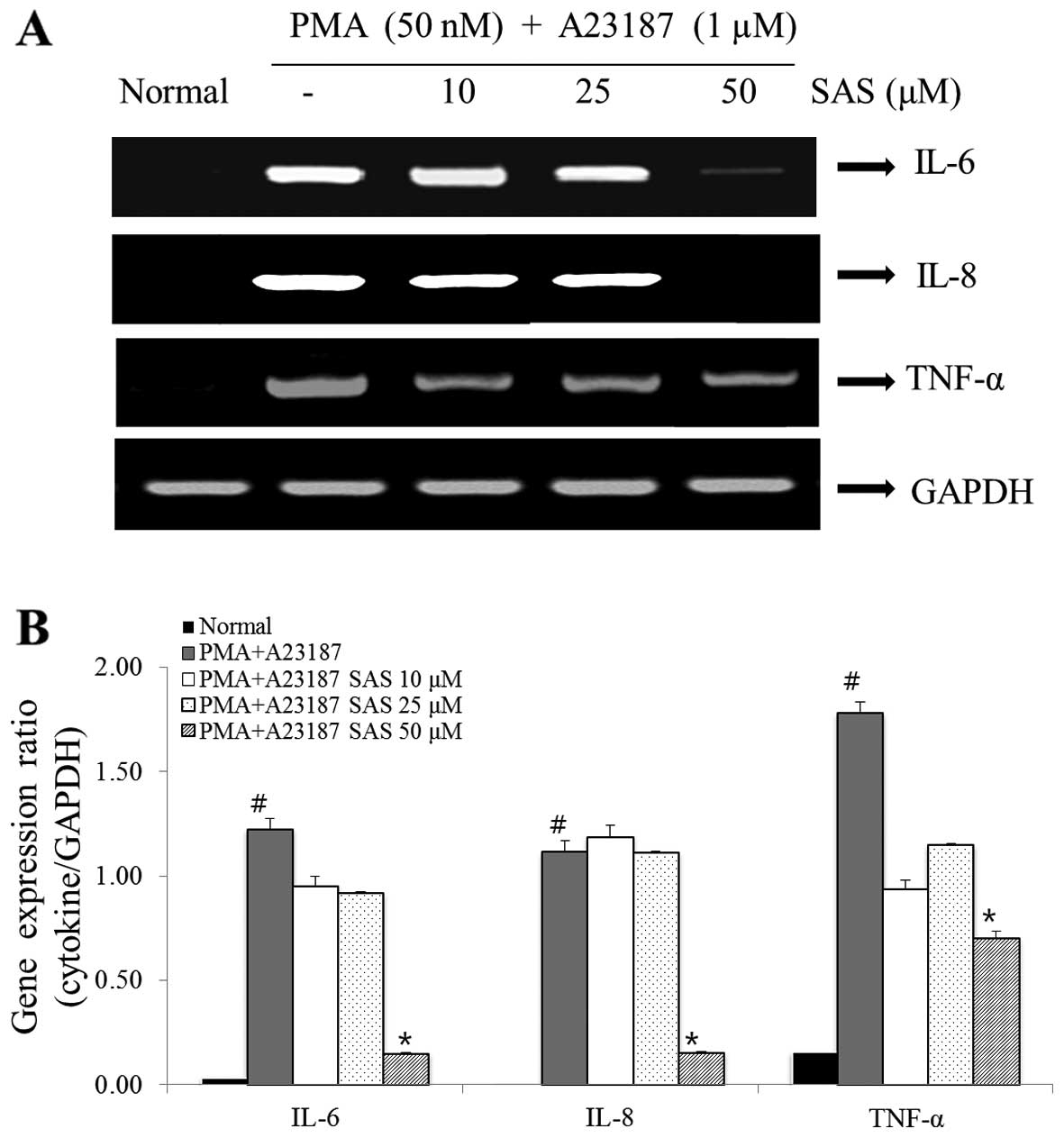
Effect of SAS on IL-6, IL-8 and TNF-α
gene expression
The IL-6, IL-8 and TNF-α gene expression levels were
analyzed by RT-PCR. The increase in the expression of IL-6, IL-8
and TNF-α induced by PMA plus A23187 was inhibited by pre-treatment
of the cells with SAS. In particular, SAS (50 μM)
significantly inhibited the PMA plus A23187-induced increase in the
expression of IL-6 and IL-8 (Fig.
6).
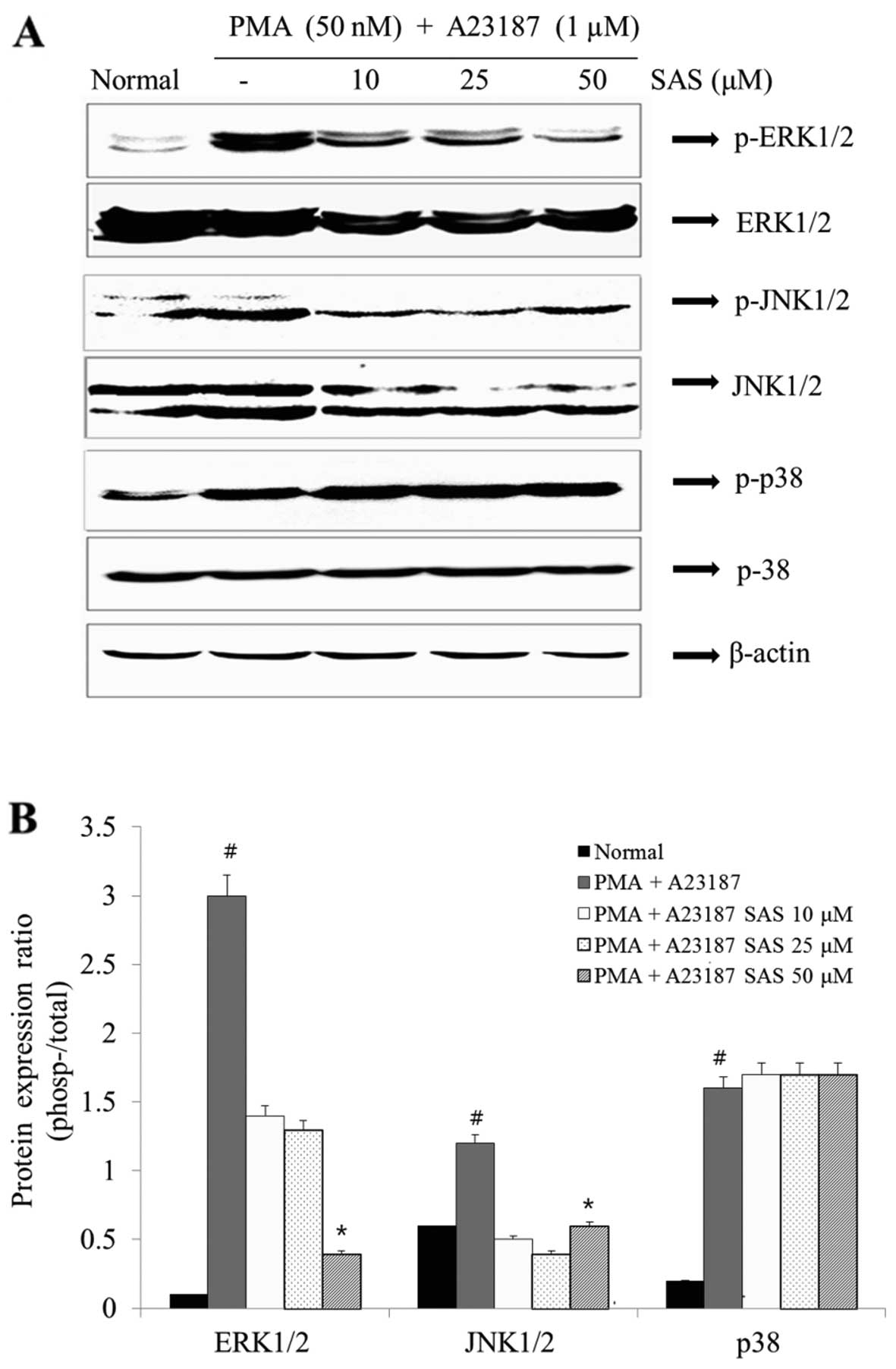
Effect of SAS on the activation of
MAPKs
In order to elucidate the mechanisms underlying the
effect of SAS, we examined the effect of SAS on MAPK activation.
The stimulation of the HMC-1 cells with PMA plus A23187 resulted in
an increased phosphorylation of all three types of MAPKs (ERK, JNK
and p38) after 1 h. SAS reduced the PMA plus A23187-induced
expression of p-ERK1/2 and p-JNK1/2 (Fig. 7). However, SAS had no effect on
the levels of p-p38 (Fig. 7).
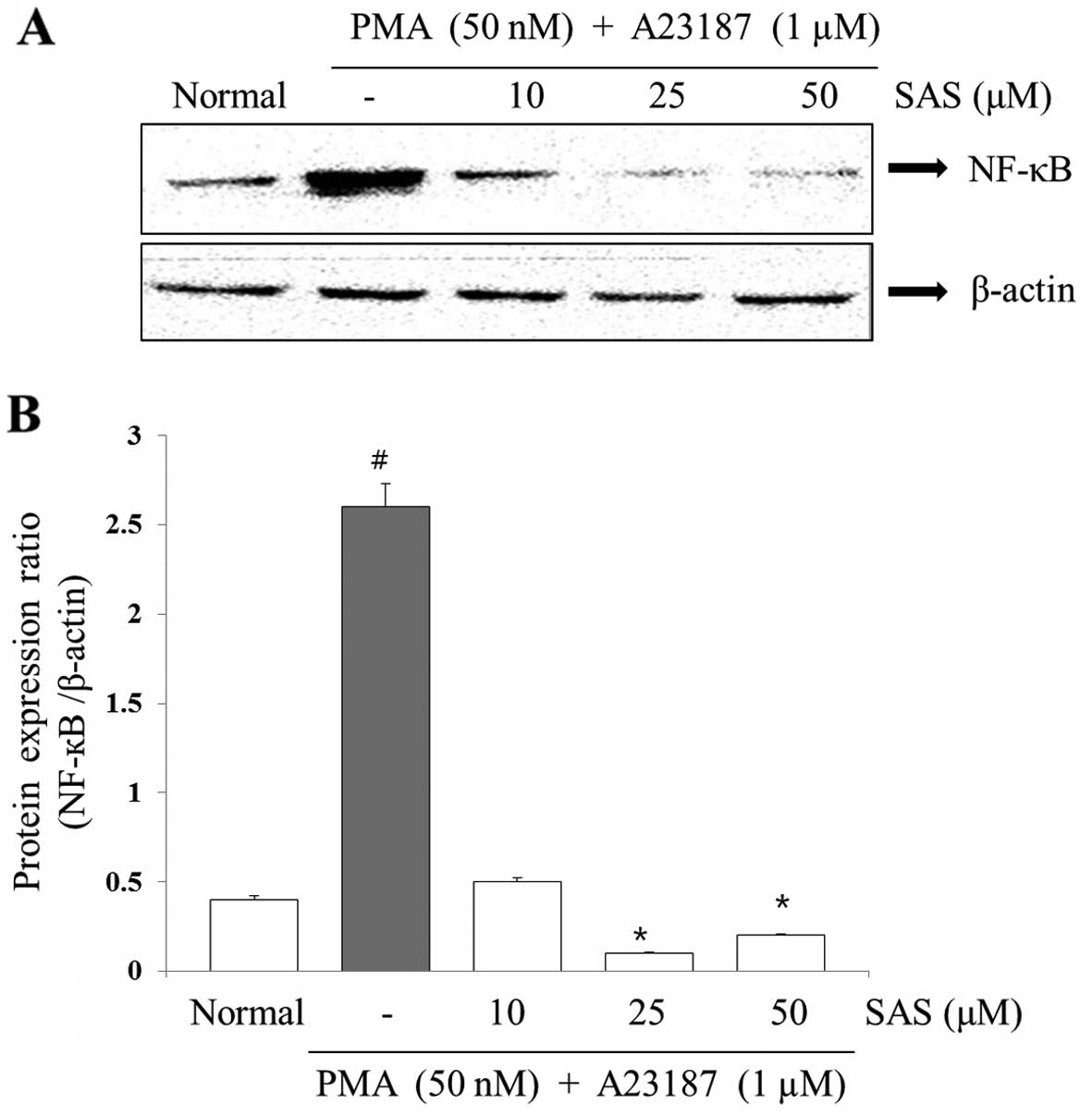
Effect of SAS on the expression of
NF-κB
To evaluate the mechanisms through which SAS
affected the gene expression of pro-inflammatory cytokines, we
examined the effects of SAS on NF-κB expression. The expression of
pro-inflammatory cytokines is regulated by the transcription
factor, NF-κB (8). Stimulation of
the HMC-1 cells with PMA plus A23187 induced the nuclear
translocation of NF-κB p65 after 2 h of incubation (8). SAS inhibited the PMA plus
A23187-initiated nuclear translocation of NF-κB p65 (Fig. 8). In order to confirm the
inhibitory effect of SAS on NF-κB expression, we examined the
effect of SAS on NF-κB-dependent protein expression (Fig. 8).
Discussion
Previous studies on plant-derived anti-inflammatory
compounds have investigated the potential inhibitory effects of
natural products using in vivo and in vitro methods.
These studies suggest an important role for SAS as a potential
chemoprevention agent due to its anti-inflammatory effects, and
anticancer effects (24–26). The aim of the present study was to
examine the effects of SAS on the production of TNF-α, IL-6 and
IL-8 in PMA plus A23187-stimulated HMC-1 cells, since these
cytokines have potent inflammatory effects.
Mast cells contain potent mediators, including
histamine, heparin, proteinases, leukotrienes and pro-inflammatory
cytokines; all of which potentially contribute to inflammatory
processes (29). Pro-inflammatory
cytokines, particularly TNF-α, IL-6 and IL-8, play critical
biological roles in allergic inflammation. These cytokines are
released as stored cytokines and can be newly synthesized during
mast cell activation (30). TNF-α
promotes inflammation, granuloma formation and tissue fibrosis and
is thought to be an initiator of cytokine-related inflammatory
responses by promoting cytokine production (31). Previous studies have indicated
that reduced amounts of TNF-α and IL-6 released from mast cells is
key to reducing the symptoms of allergic inflammation (32,33). IL-8 released from mast cells acts
on surrounding cells, such as neutrophils and eosinophils, and
induces the migration and activation of inflammatory effector cells
(34). In this study, we found
that SAS reduced the production of TNF-α, IL-6 and IL-8 in PMA plus
A23187-stimulated HMC-1 cells. Increases in levels of intracellular
calcium induce the release of biological mediators, including
TNF-α, IL-8 and IL-6. It has also been reported that the release of
intracellular calcium from internal stores is required for MAPK
activation (35,36). MAPKs have been reported to be
involved in important pathways associated with the differentiation,
activation, proliferation, degranulation and migration of various
immune cells, airway smooth muscle and epithelial cells (37). We also investigated whether
pre-treatment SAS would interfere with the MAPK signaling pathways.
The results from western blot analysis indicated that the
expression of p-ERK, p-JNK, and p-p38 considerably increased in the
PMA plus A23187-stimulated mast cells, as compared to the untreated
mast cells. However, the expression of p-ERK and p-JNK proteins
significantly decreased following treatment with SAS, but no
decreases were observed in p-p38 expression; therefore, it is
thought that SAS is not involved in the p38 pathway. The expression
of inflammatory cytokines requires the phosphorylation of MAPKs. In
this study, no significant changes were found in the expression of
total ERK, JNK and p38 between the different groups. The ERK
pathway is predominantly activated by mitogenic and proliferative
stimuli, whereas the JNK and p38 MAPK pathways respond to
environmental stresses (38).
While the exact nature of the involvement of the ERK1/2 pathway
remains elusive, nuclear retention, dimerization, phosphorylation
and release from cytoplasmic anchors have been shown to play a role
(39). The JNKs are greatly
activated in response to cytokines, growth factor deprivation,
DNA-damaging agents, some G protein-coupled receptors, serum and
growth factors. In mammalian cells, the p38 pathway is strongly
activated by environmental stresses and inflammatory cytokines, but
not appreciably by mitogenic stimuli. Additionally, p38
participates in macrophage and neutrophil functional responses,
including respiratory burst activity, chemotaxis, granular
exocytosis, apoptosis and also mediates T-cell differentiation and
apoptosis by regulating γ-interferon production and regulates the
immune response by stabilizing specific cellular mRNAs (40).
NF-κB is a transcription factor that induces the
transcription of a variety of genes. Many of these genes encode
molecules important in inflammatory processes, such as cytokines
and adhesion molecules. The role of NF-κB, in particular its
regulation of cytokine production, in allergic inflammation has
previously been characterized (41). NF-κB regulates the expression of
multiple inflammatory and immune genes and plays a critical role in
chronic inflammatory diseases (42). To evaluate the mechanisms of the
inhibition of SAS on the expression of pro-inflammatory cytokines,
we examined the effect of SAS on the NF-κB pathway. In this study,
SAS decreased the nuclear translocation of NF-κB p65. These results
indicate that the inhibitory effects of SAS on inflammatory
cytokines were due to the regulation of the NF-κB pathway.
In conclusion, our study demonstrates pre-treatment
with salidroside significantly inhibits the increase in the levels
of TNF-α, IL-6 and IL-8 induced by PMA plus A23187 in mast cells,
suppresses NF-κB p65, ERK1/2 and JNK1/2 expression, and inhibits
the upregulation of pro-inflammatory cytokines. Therefore,
salidroside may prove to be an effective therapeutic agent for the
treatment of inflammation resulting from mast cell-mediated
inflammatory responses.
Acknowledgments
This research was supported by Wonkwang University
in 2015.
References
|
1
|
Bischoff SC: Role of mast cells in
allergic and non-allergic immune responses: Comparison of human and
murine data. Nat Rev Immunol. 7:93–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Metz M and Maurer M: Mast cells–key
effector cells in immune responses. Trends Immunol. 28:234–241.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butterfield JH, Weiler D, Dewald G and
Gleich GJ: Establishment of an immature mast cell line from a
patient with mast cell leukemia. Leuk Res. 12:345–355. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nilsson G, Blom T, Kusche-Gullberg M,
Kjellén L, Butterfield JH, Sundström C, Nilsson K and Hellman L:
Phenotypic characterization of the human mast-cell line HMC-1.
Scand J Immunol. 39:489–498. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia HZ, Kepley CL, Sakai K, Chelliah J,
Irani AM and Schwartz LB: Quantitation of tryptase, chymase,
FcεRIα, and FcεRIγ mRNAs in human mast cells and basophils by
competitive reverse transcription-polymerase chain reaction. J
Immunol. 154:5472–5480. 1995.PubMed/NCBI
|
|
6
|
Xia YC, Sun S, Kuek LE, Lopata AL, Hulett
MD and Mackay GA: Human mast cell line-1 (HMC-1) cells transfected
with FcεRIα are sensitive to IgE/antigen-mediated stimulation
demonstrating selectivity towards cytokine production. Int
Immunopharmacol. 11:1002–1011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grützkau A, Krüger-Krasagakes S, Kögel H,
Möller A, Lippert U and Henz BM: Detection of intracellular
interleukin-8 in human mast cells: Flow cytometry as a guide for
immunoelectron microscopy. J Histochem Cytochem. 45:935–945. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang OH, Jang HJ, Chae HS, Oh YC, Choi JG,
Lee YS, Kim JH, Kim YC, Sohn DH and Park H: Anti-inflammatory
mechanisms of resveratrol in activated HMC-1 cells: Pivotal roles
of NF-kappaB and MAPK. Pharmacol Res. 59:330–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP,
Wang J, Zhang Y and Elias JA: Pulmonary expression of
interleukin-13 causes inflammation, mucus hypersecretion,
subepithelial fibrosis, physiologic abnormalities, and eotaxin
production. J Clin Invest. 103:779–788. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HH, Bae Y and Kim SH: Galangin
attenuates mast cell-mediated allergic inflammation. Food Chem
Toxicol. 57:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar A, Abbas W and Herbein G: TNF and
TNF receptor superfamily members in HIV infection: New cellular
targets for therapy? Mediators Inflamm. 2013:4843782013. View Article : Google Scholar
|
|
12
|
Rose-John S: IL-6 trans-signaling via the
soluble IL-6 receptor: importance for the pro-inflammatory
activities of IL-6. Int J Biol Sci. 8:1237–1247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang N, Xu Y, Zhang B, Zhang T, Yang H,
Zhang B, Feng Z and Zhong D: Analysis of interleukin-8 gene
variants reveals their relative importance as genetic
susceptibility factors for chronic periodontitis in the Han
population. PLoS One. 9:e1044362014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Remick DG: Interleukin-8. Crit Care Med.
33(Suppl 12): S466–S467. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mao Y, Li Y and Yao N: Simultaneous
determination of salidroside and tyrosol in extracts of Rhodiola L.
by microwave assisted extraction and high-performance liquid
chromatography. J Pharm Biomed Anal. 45:510–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng W and Wang SY: Antioxidant activity
and phenolic compounds in selected herbs. J Agric Food Chem.
49:5165–5170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peschel W, Prieto JM, Karkour C and
Williamson EM: Effect of provenance, plant part and processing on
extract profiles from cultivated European Rhodiola rosea L. for
medicinal use. Phytochemistry. 86:92–102. 2013. View Article : Google Scholar
|
|
20
|
Perfumi M and Mattioli L: Adaptogenic and
central nervous system effects of single doses of 3% rosavin and 1%
salidroside Rhodiola rosea L. extract in mice. Phytother Res.
21:37–43. 2007. View
Article : Google Scholar
|
|
21
|
Yousef GG, Grace MH, Cheng DM, Belolipov
IV, Raskin I and Lila MA: Comparative phytochemical
characterization of three Rhodiola species. Phytochemistry.
67:2380–2391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ming DS, Hillhouse BJ, Guns ES, Eberding
A, Xie S, Vimalanathan S and Towers GH: Bioactive compounds from
Rhodiola rosea (Crassulaceae). Phytother Res. 19:740–743. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Richard P, Brown MD, Patricia L, Gerbarg
MD and Zakir Ramazanov DS: Rhodiola rosea: A phytomedicinal
overview. HerbalGram. 56:40–52. 2002.
|
|
24
|
Zhu Y, Shi YP, Wu D, Ji YJ, Wang X, Chen
HL, Wu SS, Huang DJ and Jiang W: Salidroside protects against
hydrogen peroxide-induced injury in cardiac H9c2 cells via PI3K-Akt
dependent pathway. DNA Cell Biol. 30:809–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan CB, Gao M, Xu WR, Yang XY, Zhu XM and
Du GH: Protective effects of salidroside on endothelial cell
apoptosis induced by cobalt chloride. Biol Pharm Bull.
32:1359–1363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Zhang Q, Cheng Q and Ding F:
Protective effect of salidroside against
H2O2-induced cell apoptosis in primary
culture of rat hippocampal neurons. Mol Cell Biochem. 332:85–93.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Xiao L, Zhu L, Hu M, Wang Q and
Yan T: The effect of synthetic salidroside on cytokines and airway
inflammation of asthma induced by diisocyanate (TDI) in mice by
regulating GATA3/T-bet. Biochem Biophys Res Commun. 451:79–85.
2014.
|
|
28
|
Yan GH and Choi YH: Salidroside attenuates
allergic airway inflammation through negative regulation of nuclear
factor-kappa B and p38 mitogen-activated protein kinase. J
Pharmacol Sci. 126:126–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bradding P and Holgate ST: Immunopathology
and human mast cell cytokines. Crit Rev Oncol Hematol. 31:119–133.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hide I, Toriu N, Nuibe T, Inoue A, Hide M,
Yamamoto S and Nakata Y: Suppression of TNF-alpha secretion by
azelastine in a rat mast (RBL-2H3) cell line: Evidence for
differential regulation of TNF-alpha release, transcription, and
degranulation. J Immunol. 159:2932–2940. 1997.PubMed/NCBI
|
|
31
|
Vandenabeele P, Declercq W, Van Herreweghe
F and Vanden Berghe T: The role of the kinases RIP1 and RIP3 in
TNF-induced necrosis. Sci Signal. 3:re42010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walczak H: TNF and ubiquitin at the
crossroads of gene activation, cell death, inflammation, and
cancer. Immunol Rev. 244:9–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mican JA, Arora N, Burd PR and Metcalfe
DD: Passive cutaneous anaphylaxis in mouse skin is associated with
local accumulation of interleukin-6 mRNA and immunoreactive
interleukin-6 protein. J Allergy Clin Immunol. 90:815–824. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin TJ, Garduno R, Boudreau RT and
Issekutz AC: Pseudomonas aeruginosa activates human mast cells to
induce neutrophil transendothelial migration via mast cell-derived
IL-1 alpha and beta. J Immunol. 169:4522–4530. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Law M, Morales JL, Mottram LF, Iyer A,
Peterson BR and August A: Structural requirements for the
inhibition of calcium mobilization and mast cell activation by the
pyrazole derivative BTP2. Int J Biochem Cell Biol. 43:1228–1239.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Crossthwaite AJ, Hasan S and Williams RJ:
Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and
JNK in cortical neurones: Dependence on Ca(2+) and PI3-kinase. J
Neurochem. 80:24–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Duan W and Wong WS: Targeting
mitogen-activated protein kinases for asthma. Curr Drug Targets.
7:691–698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mizuno T, Hisamoto N, Terada T, Kondo T,
Adachi M, Nishida E, Kim DH, Ausubel FM and Matsumoto K: The
Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel
JNK-like signaling pathway in stress response. EMBO J.
23:2226–2234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pasparakis M: Role of NF-κB in epithelial
biology. Immunol Rev. 246:346–358. 2012. View Article : Google Scholar : PubMed/NCBI
|