Introduction
Pulmonary arterial hypertension (PAH), whether
idiopathic or of other varied etiologies, is a common clinical
syndrome characterized by the sustained elevation of pulmonary
vascular resistance, inflammatory cell infiltration, vascular
remodeling and the occlusion of vessels with thrombi, leading to
right ventricular hypertrophy or failure and, ultimately, death
(1–4). Although the pharmacotherapy
currently available for PAH can improve the quality of life of
patients to a certain degree, the mortality rate remains high
(5). Recently, mesenchymal stem
cell (MSCs) and gene therapies have emerged as novel methods for
the treatment of PAH, including adipose tissue-derived MSCs, bone
marrow-derived MSCs (BM-MSCs), hematopoietic stem cells and
endothelial progenitor cells (6).
MSCs not only have several favorable features, such as the ease of
isolation, expansion in culture and their capacity to differentiate
into multiple lineages, but they also migrate to sites of injury
and generate prominent paracrine effects through key interactions
with the immune system (7).
Importantly, stem cell-mediated gene therapy appears to be more
advantageous compared with sole stem cell therapy and gene therapy
in PAH (8). Nevertheless, the
mechanisms underlying their function are not yet completely clear
and require further investigation.
The insulin-like growth factor binding protein
(IGFBP) family inhibit cell proliferation, migration and survival
and also play an important role in the stability of vascular
remodeling (9,10). IGFBP-3, a member of the IGFBP
family, is a predominantly secreted protein (11) and has been shown to regulate the
insulin-like growth factor (IGF) signaling pathway by restricting
the access of IGFs to IGF receptors, consequently inhibiting their
proliferative and anti-apoptotic actions at the extracellular level
(12). In addition, IGFBP-3
inhibits growth and enhances apoptosis in an IGF-independent manner
in several mammalian cells (13).
In contrast to its anti-growth and apoptotic roles, IGFBP-3
promotes vascular regrowth and has cytoprotective properties in
response to a range of cellular conditions (14). The ability of IGFBP-3 to pivot
cell fate to either death or survival is associated with the
cellular microenvironment, including the presence of IGFBP-3
binding partners and growth factor receptors (12).
The excessive proliferation of pulmonary artery
smooth muscle cells (PASMCs) plays an essential role in the
pathogenesis of vascular remodeling in PAH (15). It has been demonstrated that
calcitonin gene-related peptide (CGRP)-modified MSCs secrete
CGRP protein and inhibit the proliferation and migration of
vascular smooth muscle cells (VSMCs) (16). It has also been demonstrated that
IGFBP-3 expression is found in BM-MSCs and PASMCs isolated from
PAH-afflicted rats (17), but its
functional role in PASMCs remains unknown. Whether IGFBP-3
overexpression and human IGFBP-3-modified hBM-MSCs can
suppress the proliferation of hPASMCs is also currently unknown.
Therefore, in this study, we investigated the growth-inhibitory
effects of IGFBP-3-modified hBM-MSCs on hPASMCs and also
aimed to elucidate the underlying mechanisms.
Materials and methods
Cell culture
The hBM-MSCs (PCS-500-012™) and hPASMCs
(PCS-100-023™) were purchased from the American Type Culture
Collection (ATCC, Manassas, VA, USA). The hBM-MSCs used were
clonally derived and the hPASMCs were used from passages 4 to 7.
The cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine
serum (FBS) (HyClone; Logan, Utah, USA), 1% L-glutamine, 100 U/ml
penicillin and 100 mg/ml streptomycin (Invitrogen, Burlington, ON,
USA). Angiotensin II (Ang II) (Sigma-Aldrich, St. Louis, MO, USA)
was used to stimulate hPASMC proliferation. These cells were grown
in a humidified incubator with 5% CO2 at 37°C.
Construction of pcDNA4-IGFBP-3 vector and
transfection of hBM-MSCs
The recombinant plasmid, pcDNA4-IGFBP-3, was
constructed as previously described (19), verified and reproduced. The
hBM-MSCs were seeded at 1×106 cells/well in 6-well
plates for 24 h and transfected with the pcDNA4-IGFBP-3 plasmid or
pcDNA4 empty vector using Lipofectamine 2000 (Invitrogen, Carlsbad,
CA, USA), according to the manufacturer's instructions. Following
48 h of transfection, the supernatants were harvested for
enzyme-linked immunosorbent assay (ELISA).
ELISA for IGFBP-3
Following 48 h of co-culture of the hBM-MSCs and
hPASMCs on cell culture inserts, the supernatants of hPASMCs on the
bottom chamber were collected and the production of IGFBP-3 was
measured using the Quantikine human IGFBP-3 immunoassay (R&D
Systems, Minneapolis, MN, USA), according to the manufacturer's
instructions.
Co-culture of hBM-MSCs and hPASMCs on
cell culture inserts
An indirect co-culture system was assembled using
Costar Transwell membranes (12 mm diameter, 0.4 µm pore;
Corning Inc., Corning, NY, USA). As described previously (20), the hPASMCs were first seeded on
the bottom chamber in DMEM containing Ang II and hBM-MSCs
transfected with pcDNA4-IGFBP-3 or pcDNA4 empty vector and then
seeded on a 0.4 µm Transwell membrane (upper chamber) and
cultured in DMEM. Single cultures of hBM-MSCs or hPASMCs were used
as controls. Co-cultures were maintained for 48 h. This assay was
performed at least 3 times.
CCK-8 assay
The proliferation of the hPASMC was evaluated by
Cell Counting kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan)
according to the manufacturer's instructions. A viability >100%
indicated cell proliferation, whereas a viability of <100%
indicated cell damage, as previously described (21). Briefly, the hPASMCs were plated in
96-well plates at 5,500 cells/well and 1.5 µM/l Ang II was
added to each well. Following 48 h of incubation at 37°C, CCK-8
solution was added to each pore and incubation was continued for 3
h. The absorbance value (450 nm) of each pore was analyzed using an
enzyme symbolized meter (Bio-Rad Laboratories Inc., Hercules, CA,
USA). This assay was performed at least 3 times.
Bromodeoxyuridine (BrdU) incorporation
assay
The hBM-MSCs and hPASMCs were co-cultured for 48 h
on cell culture inserts and the DNA synthesis ability of the
hPASMCs was determined by a BrdU incorporation assay. Briefly, the
hPASMCs were incubated with BrdU (Sigma) (20 µl/well) for 12
h and the Fixing/Denaturing solution (200 µl/well) was then
added followed by incubation for 30 min. The BrdU detection
antibody solution (200 µl/well) was then added and the cells
were incubated for 1 h. Subsequently, the peroxidase-labeled sheep
anti-mouse IgG solution (200 µl/well) was added followed by
incubation for 30 min. Finally, tetramethylbenzidine substrate (100
µl) was added followed by incubation for 30 min in the dark.
The amount of BrdU incorporation into the cells was measured at 450
nm using a microplate reader (Bio-Rad) according to the
manufacturer's instructions. The experiments were performed in
quintuplicate and repeated 3 times.
Flow cytometric analysis of
apoptosis
Following co-culture of the hBM-MSCs and hPASMCs on
cell culture inserts for 48 h, the apoptosis of the hPASMCs was
detected by BD Annexin V fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining (BD Biosciences, San Jose,
CA, USA) according to the manufacturer's instructions. As
previously described (22), the
hPASMCs were harvested, washed with ice-cold phosphate-buffered
saline (PBS), resuspended in binding buffer and stained with 10
µl of FITC Annexin V buffer at room temperature in the dark
for 15 min. To that, 5 µl of PI was added followed by
incubation for a further 5 min. Finally, the cells were analyzed
using a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
The percentage of cell numbers in each quadrant was calculated
using BD CellQuest software.
Protein extraction and western blot
analysis
Total protein from the hPASMCs was extracted using
RIPA lysis buffer containing phenylmethylsulfonyl fluoride and
using the bicinchoninic acid (BCA) protein assay kit (Boster
Biology Co., Wuhan, China), the protein concentration was then
determined with BSA as the standard. Equal amounts of protein were
separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and
electrotransferred onto polyvinylidene fluoride membranes
(Bio-Rad), followed by incubation at 4°C overnight with primary
antibodies: anti-α-smooth muscle-actin (α-SM-actin; sc-32251),
anti-osteopontin (OPN; sc-21742), anti-B-cell lymphoma-2 (Bcl-2;
sc-492), anti-Bax (sc-493), anti-insulin receptor substrate-1
(IRS-1; sc-559), anti-phosphoinositide 3-kinase (PI3K; sc-1637),
anti-AKT (sc-1618), anti-p38 (sc-535), anti-p-Jun N-terminal kinase
(JNK; sc-7345), anti-extracellular signal-regulated kinase (ERK;
sc-292838) (Santa Cruz Biotechnology, Santa Cruz, CA, USA);
anti-p-IRS-1 (#2381), anti-p-PI3K (#4228), anti-p-AKT (#12694),
anti-p-p38 (#9211), anti-p-JNK (#9251), anti-p-ERK (#4376) (Cell
Signaling Technology, Inc., Beverly, MA, USA). The appropriate
secondary antibody: anti-mouse and anti-rabbit IgG horseradish
peroxidase-linked antibody (Cell Signaling Technology, Inc.), was
applied at room temperature for 1 h. Finally, the protein band of
interest was visualized by a chemiluminescent reaction using an ECL
Detection kit (Pierce Biotechnology, Rockford, IL, USA). Bands were
quantified using Image Lab™ software, version 5.1 (Bio-Rad).
Reverse transcription-quantitative
(real-time) PCR (RT-qPCR)
Total RNA was extracted from the hPASMCs using
TRIzol reagent (Invitrogen) according to the manufacturer's
instructions and cDNA was synthesized using PrimeScript RT Master
Mix Sample kit (Takara, Shiga, Japan) according to the
manufacturer's instructions. Subsequently, qPCR was performed using
SYBR-Green Master Mix and analyzed on a LightCycler 480 instrument
(Roche Diagnostics Ltd., Lewes, UK). The oligonucleotide primers
used for RT-qPCR were as follows: α-SMA forward,
5′-CGGGACATCAAGGAGAAACT-3′ and reverse, 5′-CCCATCAGGCAACTCGTAA-3′;
OPN forward, 5′-GCCAGTTGCAGCCTTCTCA-3′ and reverse,
5′-AAAAGCAAATCACTGCAATTCTCA-3′. All real-time reactions were
performed using 50 cycles at 95°C for 10 min, 95°C for 15 sec and
60°C for 1 min. Each sample was run in triplicate and β-actin
served as an internal control. Relative mRNA expression was
normalized to that of β-actin using the equation of
2−ΔΔCT, where CT is threshold cycle. RT-qPCR was
performed at least 3 times.
Statistical analysis
In this study, the results were summarized as the
means ± SD from at least 3 independent experiments. SPSS 17.0
software was used to carry out statistical analyses. The analysis
of the overall effects of the different treatments was evaluated
using one-way analysis of variance (ANOVA). A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
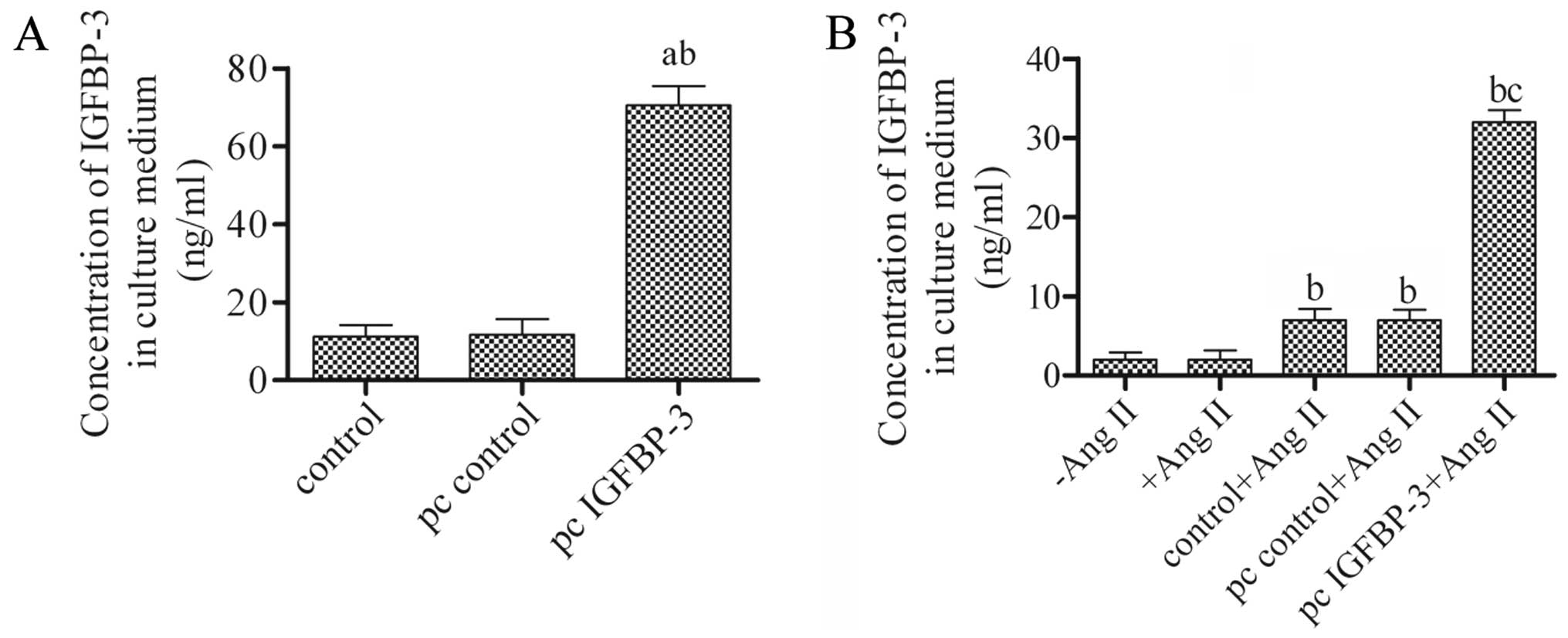
Overexpression and secretion of IGFBP-3
in hBM-MSCs
To investigate the role of IGFBP-3 in hBM-MSCs,
pcDNA4-IGFBP-3 or pcDNA4 empty vector was constructed and
transfected into the hBM-MSCs. The supernatant was harvested after
48 h and the levels of secreted IGFBP-3 were then measured by
ELISA. The results revealed a generally low concentration of
IGFBP-3 either in the untreated hBM-MSCs (control) or in those
transfected with the pcDNA4 empty vector (pc control). However, the
concentration of IGFBP-3 was significantly increased when the
hBM-MSCs were transfected with pcDNA4-IGFBP-3 (pc IGFBP-3)
(Fig. 1A). These data indicated
that the hBM-MSCs transfected with pcDNA4-IGFBP-3 had a high
secretion level of IGFBP-3 and support the notion that IGFBP-3 is
secreted in the supernatant of MSCs (18). Moreover, the concentration of
IGFBP-3 in the culture medium significantly increased compared to
the control + Ang II or pc control + Ang II group, following
co-culture with hBM-MSCs transfected with pcDNA4-IGFBP-3 and Ang
II-stimulated hPASMCs on cell culture inserts (Fig. 1B).
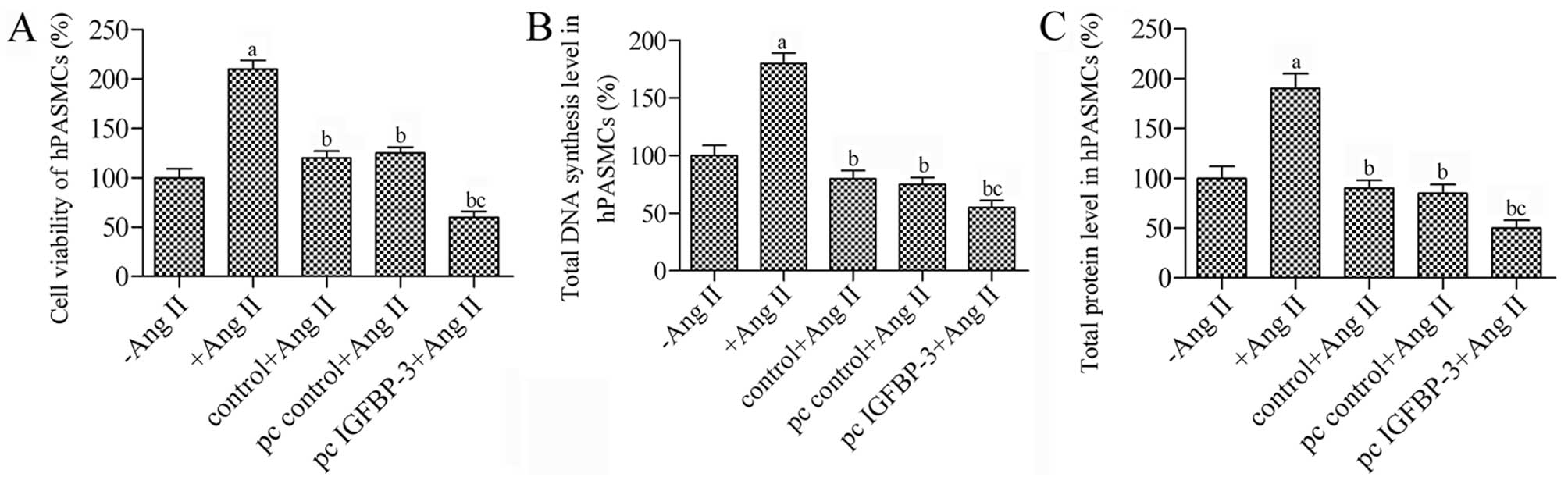
hBM-MSCs modified with IGFBP-3 inhibit
the proliferation of hPASMCs stimulated with Ang II
In order to determine whether IGFBP-3 overexpression
in hBM-MSCs suppresses the proliferation of hPASMCs, an indirect
co-culture system for hBM-MSCs and hPASMCs was used to observe the
proliferation of the hPASMCs. Ang II resulted in a 2.1-fold
increase in the proliferation of hPASMCs (P<0.05 vs. control)
(Fig. 2A). Co-culture of the
untreated hBM-MSCs decreased the survival rate of the hPASMCs by
80% in response to Ang II, while co-culture with the
IGFBP-3-modified hBM-MSCs significantly inhibited the
proliferation of the Ang II-stimulated hPASMCs; we observed a
decrease of 1.33-fold compared to the pc control (P<0.05).
Furthermore, DNA synthesis and the total protein levels in the
hPASMCs in co-culture with the IGFBP-3-modified hBM-MSCs
were decreased almost to 55% (P<0.05) (Fig. 2B and C). Taken together, these
results suggest that co-culture with IGFBP-3-modified
hBM-MSCs is an effective strategy to suppress the proliferation of
hPASMCs.
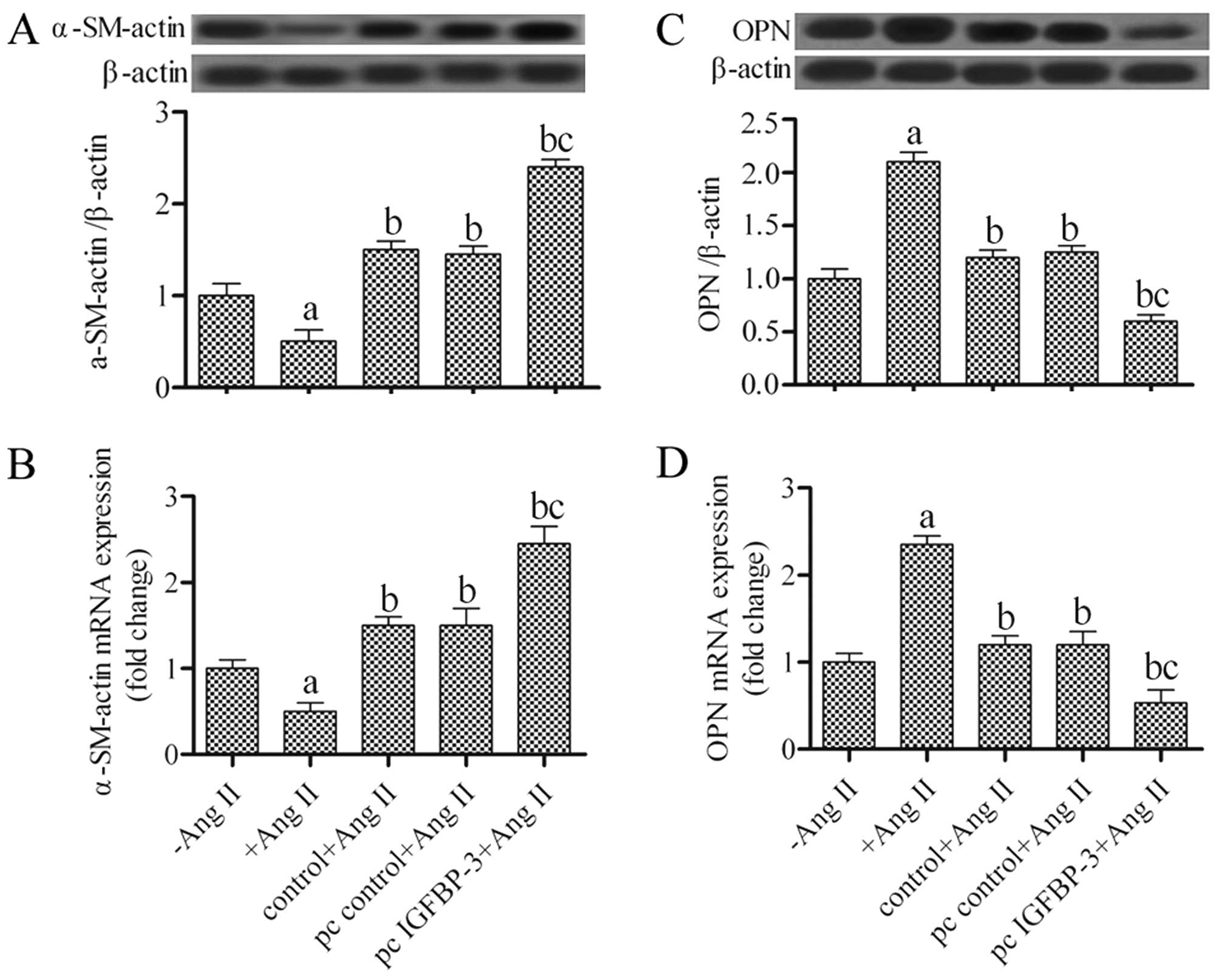
Effects of the upregulation of IGFBP-3 on
α-SM-actin and OPN expression in hPASMCs
The hPASMCs can modulate their phenotype from a
contractile to a synthetic one under certain conditions. To examine
the phenotypic switch of hPASMCs co-cultured with hBM-MSCs, the
α-SM-actin and OPN expression levels were examined by western blot
analysis and RT-qPCR. The results revealed that the hBM-MSCs
transfected with pcDNA4-IGFBP-3 had significantly increased protein
and mRNA expression levels of α-SM-actin in the hPASMCs (P<0.05)
(Fig. 3A and B). Conversely, OPN
protein and mRNA expression decreased by approximately 50% in the
hPASMCs co-cultured with the IGFBP-3-modified hBM-MSCs
(P<0.05) when compared with the untreated hBM-MSCs (Fig. 3C and D). These results suggest
that the hPASMCs underwent a change in phenotype from a synthetic
to a contractile phenotype following co-culture with
IGFBP-3-modified hBM-MSCs.
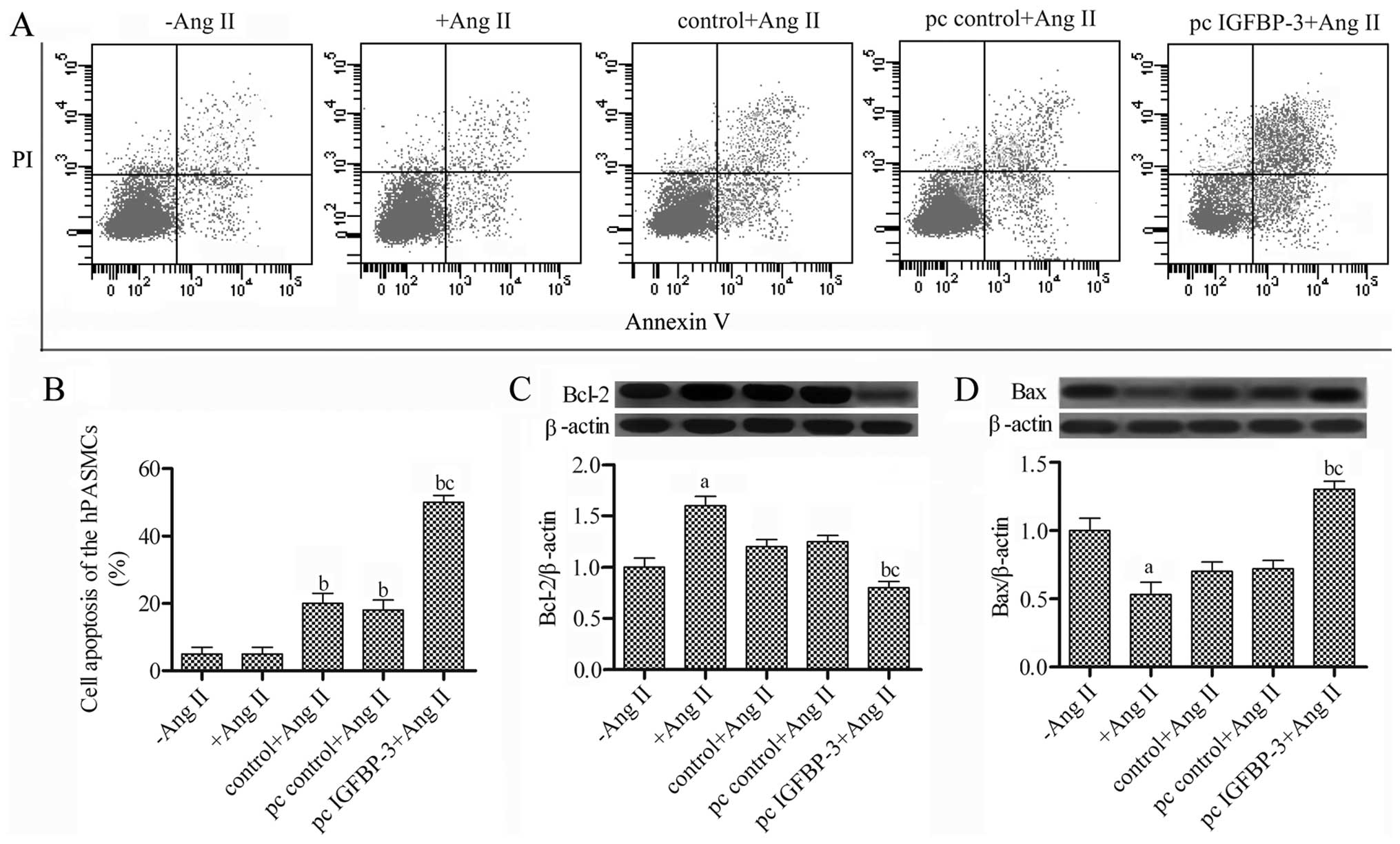
hBM-MSCs modified with IGFBP-3 promote
the apoptosis of hPASMCs
To further investigate the effect of
IGFBP-3-modified hBM-MSCs on hPASMCs, we detected cell
apoptosis using the Annexin V FITC/PI assay, and Bax and Bcl-2
protein expression were examined by western blot analysis.
Following co-culture of the hPASMCs stimulated with Ang II with the
IGFBP-3-modified hBM-MSCs for 48 h, the apoptosis of the
hPASMC increased to 50% (Fig. 4A and
B) compared with the pc control + Ang II group. The Bcl-2
expression levels were decreased, while Bax expression was
increased in the hPASMCs co-cultured with the
IGFBP-3-modified hBM-MSCs (Fig. 4C and D). Taken together, these
results suggest that co-culture with the IGFBP-3-modified
hBM-MSCs promotes the apoptosis of hPASMCs.
Effects of the overexpression of IGFBP-3
on the PI3K-AKT and mitogen-activated protein kinase (MAPK)
pathways in hPASMCs
To determine the underlying mechanisms through which
the IGFBP-3-modified hBM-MSCs regulate hPASMCs, we examined
IRS-1 expression and the expression of related proteins of two
signaling pathways, PI3K-AKT and MAPK, including PI3K, AKT, p38,
JNK and ERK by western blot analysis. The results revealed that
co-culture with the IGFBP-3-modified hBM-MSCs significantly
attenuated the activities of the related proteins compared to those
of the wild-type hBM-MSCs (pc control + Ang II) (Fig. 5). These data indicated that the
IGFBP-3-modified hBM-MSCs markedly downregulated the
expression of related genes in the PI3K-AKT and MAPK pathways in
hPASMCs.
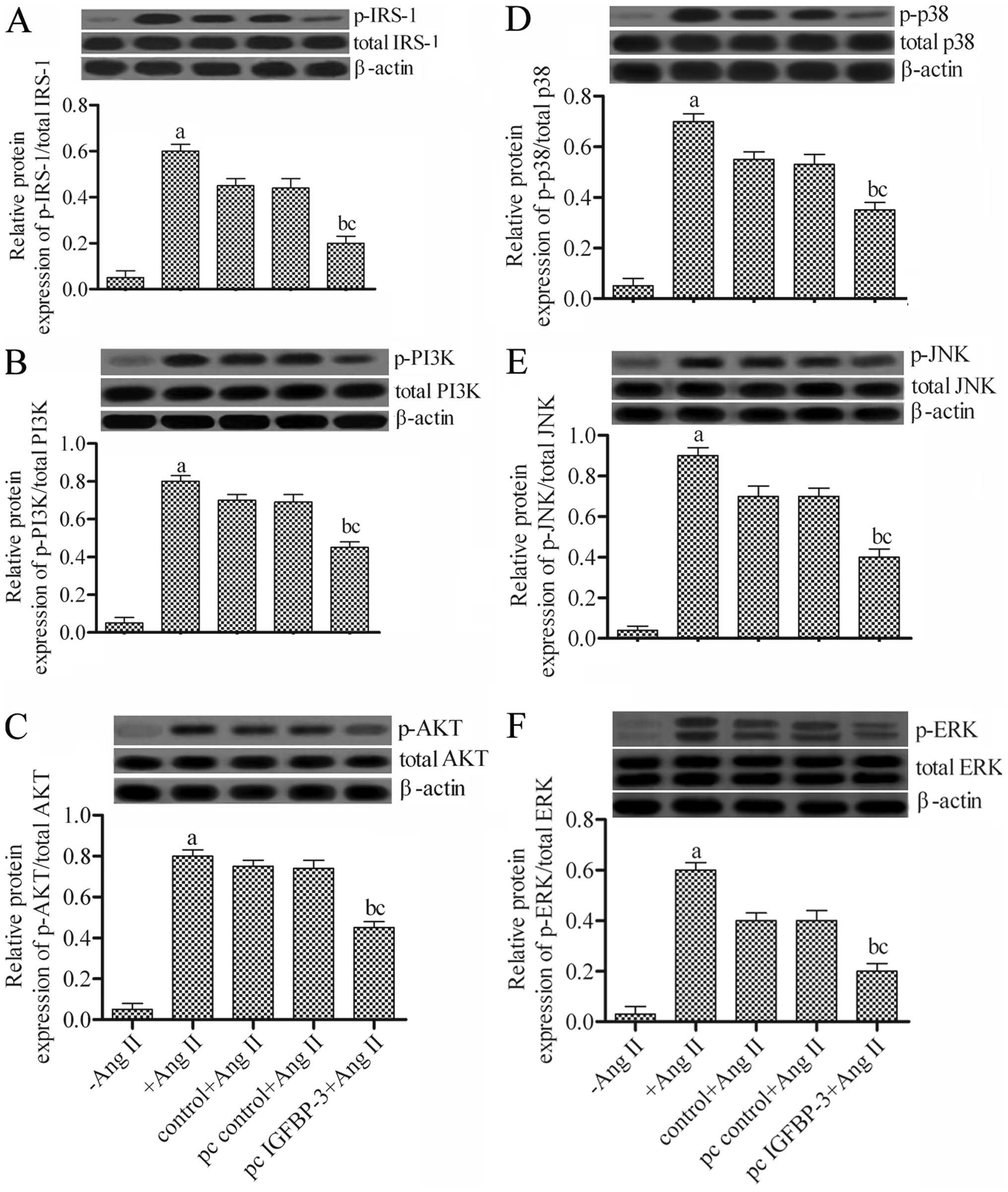 | Figure 5Effects of insulin-like growth factor
binding protein-3 (IGFBP-3) overexpression on P13K-AKT and
mitogen-activated protein kinase (MAPK) pathways in human pulmonary
artery smooth muscle cells (hPASMCs). Protein expression levels of
(A) insulin receptor substrate-1 (IRS-1), (B) PI3K, (C) AKT, (D)
p38, (E) JNK and (F) ERK and their phosphorylated forms as detected
by western blot analysis. aP<0.05 vs. −Ang II;
bP<0.05 vs. +Ang II; cP<0.05 vs. pc
control + Ang II. Ang II, angiotensin II; −Ang II, untreated
hPASMCs; +Ang II, Ang II-stimulated hPASMCs; control + Ang II, Ang
II-stimulated hPASMCs not transfected with any plasmid; pc control
+ Ang II, Ang II-stimulated hPASMCs transfected with empty vector;
pc IGFBP-3 + Ang II, Ang II-stimulated hPASMCs transfected with
IGFBP-3 expression plasmid. |
Discussion
The results of this study are the first to
demonstrate that IGFBP-3-modified hBM-MSCs significantly
inhibit the proliferation and promote the apoptosis of hPASMCs
stimulated with Ang II. We also found that the hPASMCs underwent a
phenotypic transformation from a synthetic to a contractile
phenotupe when in co-culture with IGFBP-3-modified hBM-MSCs.
This study suggests that IGFBP-3-modified hBM-MSCs
downregulate P13K-AKT and MAPK signaling pathways in hPASMCs more
effectively than wild-type hBM-MSCs.
MSCs are multipotent progenitor cells and can be
induced to differentiate into diverse cell lineages, such as
cardiomyocytes, endothelial cells and VSMCs. Currently, MSCs are
one of the cell types being used in clinical trials for the
treatment of various diseases, including hematological diseases,
organ transplantation, inflammatory diseases and autoimmune
diseases (23). In addition,
implanted MSCs promote tissue regeneration via the secretion of a
variety of growth factors and cytokines (24). In this study, IGFBP-3 expression
was detected by ELISA in hBM-MSCs (Fig. 1A), which is consistent with the
results obtained that the relative intensity of IGFBP-3 is up to a
value of 55% in the supernatant of MSC culture (18). A recent study suggested that MSCs
significantly ameliorated pulmonary arterial pressure and decreased
right ventricle hypertrophy and pulmonary arteriole remodeling
during the development of PAH in rats (25). In line with these findings, our
data indicated that hBM-MSCs inhibit the proliferation of Ang
II-stimulated hPASMCs following co-culture. We also demonstrated
that DNA synthesis and the total protein levels in hPASMCs in
co-culture were decreased (Fig. 2B
and C).
Moreover, we assessed the pro-apoptotic effects of
hBM-MSCs on hPASMCs (Fig. 4A and
B) and the expression of Bcl-2 was also downregulated (Fig. 4C) and Bax expression was increased
in the hPASMCs co-cultured with IGFBP-3-modified hBM-MSCs
(Fig. 4D), which may have
contributed to the inhibition of the proliferation of hPASMCs. It
has been demonstrated that endogenous IGFBP-3 facilitated the
phosphorylation and nuclear export of orphan nuclear receptor Nur77
to the cytoplasm, where it exerts its apoptotic effect (26). IGFBP-3 also induces apoptosis and
growth inhibition via IGF-1 independent mechanisms in various cell
systems (27,28). Of note, we found that
IGFBP-3-modified hBM-MSCs exerted prominent inhibitory
effects on the proliferation of hPASMCs. Our findings also
indicated that IGFBP-3 was associated with cell apoptosis; however,
further research is required to confirm that this molecular
mechanism is consistent with that of previous research (29), which showed that IGFBP-3 blocked
the type I IGF receptor (IGF1R)/PI3K/Akt survival signaling
pathway, leading to cell apoptosis by sequestering IGF-1 away from
the IGF1R.
In a previous study (30), the knockdown of IGFBP-3 was
associated with only a subtle phenotype under control conditions.
In additional, the idea that phenotype switching of PASMCs from a
contractile to a synthetic phenotype plays a vital role in the
progression of PAH is well established (31). The former type of VSMCs typically
proliferate at a relatively low rate and produce a repertoire of
smooth muscle-specific contractile proteins, such as α-SM-actin.
However, this synthetic phenotype is characterized by
over-proliferation, the secretion of collagen, elastin and
proteoglycans into the extracellular matrix (32,33). Most noteworthy, our findings are
the first to indicate, at least to the best of our knowledge, that
IGFBP-3-modified hBM-MSCs significantly upregulate the level
of α-SM-actin and decrease OPN expression in hPASMCs compared to
the controls following co-culture.
To explore the underlying mechanisms involved in the
inhibition of cell proliferation and the promotion of apoptosis,
and the phenotypic transformation hPASMCs by co-culture with
IGFBP-3-modified hBM-MSCs, we examined IRS-1 expression and
the expression of related proteins of the PI3K-AKT and MAPK
signaling pathways, including p-P13K, p-AKT, p-p38, p-JNK and
p-ERK. IRS-1 is a docking protein critical for mediating signals
from IGF-1 and it has been reported that the overexpression of
IGFBP-3 successfully restores the repressed levels of IRS-1 in
primary human adipocytes (34).
We also found that IGFBP-3-modified hBM-MSCs decreased the
protein expression level of the p-IRS-1 in hPASMCs following
co-culture. Our findings further proved that the protein expression
of detected genes in hPASMCs was significantly downregulated
following co-culture (Fig. 5).
The PI3K/Akt signaling pathway has been shown to mediate VSMC
proliferation, apoptosis and phenotypic transformation (35–37). Moreover, the MAPK pathway is also
related to the proliferation and vascular remodeling of hPASMCs
(38). In the present study, we
demonstrated that the protein expression of p-PI3K, p-Akt, p-p38,
p-JNK and p-ERK in hPASMCs was diminished compared to that of the
controls following co-culture with IGFBP-3-modified
hBM-MSCs, indicating that IGFBP-3 may mediate the proliferation,
apoptosis and phenotypic transformation of hPASMCs via the PI3K/Akt
and MAPK signaling pathways. It has been demonstrated that IGFBP-3
inhibits the IGF1R/PI3K/Akt survival signaling pathway by
competitively binding IGF1R (29). In addition, IGF-1 rapidly induces
the phosphorylation of IGF-1R followed by the activation of the AKT
and MAPK signaling pathways in endometrial cancer lines (39). In addition, Ang II can promote
IGF-1 mRNA expression in human umbilical artery smooth muscle cells
(40). However, the precise
mechanisms through which IGFBP-3 binds IGF1R, inhibits p-IRS-1 and
then hinders the PI3K/Akt and MAPK signaling pathways in hPASMCs
regulated by IGFBP-3-modified hBM-MSCs in the context of PAH
remains unknown.
In conclusion, in this study, and to the best of our
knowledge, we demonstrate for the first time that
IGFBP-3-modified hBM-MSCs inhibit the proliferation and
promote the apoptosis of hPASMCs, and also induced a phenotypic
change to a contractile phenotype in hPASMCs. These effects may be
exerted by acting upon the PI3K/Akt and MAPK signaling pathways.
This study suggests that IGFBP-3-modified hBM-MSCs may be a
promising therapeutic strategy for the treatment of PAH. However,
further research using animal model is required.
Abbreviations:
|
PAH
|
pulmonary arterial hypertension
|
|
hBM-MSCs
|
human bone marrow-derived mesenchymal
stem cells
|
|
hPASMCs
|
human pulmonary artery smooth muscle
cells
|
|
IGFBP-3
|
insulin-like growth factor binding
protein-3
|
References
|
1
|
Ibrahim el-SH and Bajwa AA: Severe
pulmonary arterial hypertension: Comprehensive evaluation by
magnetic resonance imaging. Case Rep Radiol. 2015:946–920.
2015.
|
|
2
|
Nogueira-Ferreira R, Vitorino R, Ferreira
R and Henriques-Coelho T: Exploring the monocrotaline animal model
for the study of pulmonary arterial hypertension: A network
approach. Pulm Pharmacol Ther. 35:8–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perrin S, Chaumais MC, O'Connell C, Amar
D, Savale L, Jaïs X, Montani D, Humbert M, Simonneau G and Sitbon
O: New pharmacotherapy options for pulmonary arterial hypertension.
Expert Opin Pharmacother. 16:2113–2131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weitzenblum E, Chaouat A, Canuet M and
Kessler R: Pulmonary hypertension in chronic obstructive pulmonary
disease and interstitial lung diseases. Semin Respir Crit Care Med.
30:458–470. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao ZC and Liu YB: Treatment advance and
tendency of pulmonary arterial hypertension. Clin Med Engineering.
23:257–260. 2016.In Chinese.
|
|
6
|
Wang CH and An Y: Progress of stem cell
treatment of pulmonary arterial hypertension. Chin J Clin Thorac
Cardiovasc Surg. 23:294–298. 2016.In Chinese.
|
|
7
|
Firth AL, Yao W, Ogawa A, Madani MM, Lin
GY and Yuan JX: Multipotent mesenchymal progenitor cells are
present in endarterectomized tissues from patients with chronic
thromboembolic pulmonary hypertension. Am J Physiol Cell Physiol.
298:C1217–C1225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takemiya K, Kai H, Yasukawa H, Tahara N,
Kato S and Imaizumi T: Mesenchymal stem cell-based prostacyclin
synthase gene therapy for pulmonary hypertension rats. Basic Res
Cardiol. 105:409–417. 2010. View Article : Google Scholar
|
|
9
|
Bach LA: Insulin-like growth factor
binding proteins - an update. Pediatr Endocrinol Rev. 13:521–530.
2015.
|
|
10
|
Kielczewski JL, Jarajapu YP, McFarland EL,
Cai J, Afzal A, Li Calzi S, Chang KH, Lydic T, Shaw LC, Busik J, et
al: Insulin-like growth factor binding protein-3 mediates vascular
repair by enhancing nitric oxide generation. Circ Res. 105:897–905.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moser DR, Lowe WL Jr, Dake BL, Booth BA,
Boes M, Clemmons DR and Bar RS: Endothelial cells express
insulin-like growth factor-binding proteins 2 to 6. Mol Endocrinol.
6:1805–1814. 1992.PubMed/NCBI
|
|
12
|
Johnson MA and Firth SM: IGFBP-3: A cell
fate pivot in cancer and disease. Growth Horm IGF Res. 24:164–173.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valentinis B, Bhala A, DeAngelis T,
Baserga R and Cohen P: The human insulin-like growth factor (IGF)
binding protein-3 inhibits the growth of fibroblasts with a
targeted disruption of the IGF-I receptor gene. Mol Endocrinol.
9:361–367. 1995.PubMed/NCBI
|
|
14
|
Lofqvist C, Chen J, Connor KM, Smith AC,
Aderman CM, Liu N, Pintar JE, Ludwig T, Hellstrom A and Smith LE:
IGFBP3 suppresses retinopathy through suppression of oxygen-induced
vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci
USA. 104:10589–10594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tajsic T and Morrell NW: Smooth muscle
cell hypertrophy, proliferation, migration and apoptosis in
pulmonary hypertension. Compr Physiol. 1:295–317. 2011.PubMed/NCBI
|
|
16
|
Chen PK, Shi B, Long XP, Liu ZJ, Wang ZL
and Wang DM: Effects of rat mesenchymal stem cells modified by CGRP
on proliferation and phenotype transformation of vascular smooth
muscle cells in vitro. Chin J Pathophysiology. 29:1777–1782.
2013.In Chinese.
|
|
17
|
Su XY, Jiang XM and Chen SL: The
expression profile of IGFBP family in pulmonary artery smooth
muscle cells of rats with pulmonary hypertension. Zhonghua
Linchuang Yishi Zazhi. 9:1143–1148. 2015.In Chinese.
|
|
18
|
Schinköthe T, Bloch W and Schmidt A: In
vitro secreting profile of human mesenchymal stem cells. Stem Cells
Dev. 17:199–206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Firth SM, Ganeshprasad U and Baxter RC:
Structural determinants of ligand and cell surface binding of
insulin-like growth factor-binding protein-3. J Biol Chem.
273:2631–2638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia Y, Bhattacharyya A, Roszell EE, Sandig
M and Mequanint K: The role of endothelial cell-bound Jagged1 in
Notch3-induced human coronary artery smooth muscle cell
differentiation. Biomaterials. 33:2462–2472. 2012. View Article : Google Scholar
|
|
21
|
Li Y, Liu G, Cai D, Pan B, Lin Y, Li X, Li
S, Zhu L, Liao X and Wang H: H2S inhibition of chemical
hypoxia-induced proliferation of HPASMCs is mediated by the
upregulation of COX-2/PGI2. Int J Mol Med. 33:359–366. 2014.
|
|
22
|
Liu Y, Tian HY, Yan XL, Fan FL, Wang WP,
Han JL, Zhang JB, Ma Q, Meng Y and Wei F: Serotonin inhibits
apoptosis of pulmonary artery smooth muscle cell by pERK1/2 and PDK
through 5-HT1B receptors and 5-HT transporters. Cardiovasc Pathol.
22:451–457. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Squillaro T, Peluso G and Galderisi U:
Clinical trials with mesenchymal stem cells: An update. Cell
Transplant. 25:829–848. 2016. View Article : Google Scholar
|
|
24
|
Baraniak PR and McDevitt TC: Stem cell
paracrine actions and tissue regeneration. Regen Med. 5:121–143.
2010. View Article : Google Scholar :
|
|
25
|
Chen JY, An R, Liu ZJ, Wang JJ, Chen SZ,
Hong MM, Liu JH, Xiao MY and Chen YF: Therapeutic effects of
mesenchymal stem cell-derived microvesicles on pulmonary arterial
hypertension in rats. Acta Pharmacol Sin. 35:1121–1128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agostini-Dreyer A, Jetzt AE, Stires H and
Cohick WS: Endogenous IGFBP-3 mediates intrinsic apoptosis through
modulation of Nur77 phosphorylation and nuclear export.
Endocrinology. 156:4141–4151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Muzumdar RH, Ma X, Fishman S, Yang X,
Atzmon G, Vuguin P, Einstein FH, Hwang D, Cohen P and Barzilai N:
Central and opposing effects of IGF-I and IGF-binding protein-3 on
systemic insulin action. Diabetes. 55:2788–2796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chan SS, Twigg SM, Firth SM and Baxter RC:
Insulin-like growth factor binding protein-3 leads to insulin
resistance in adipocytes. J Clin Endocrinol Metab. 90:6588–6595.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang RL, Lin JW, Hsieh DJ, Yeh YL, Shen
CY, Day CH, Ho TJ, Viswanadha VP, Kuo WW and Huang CY: Long-term
hypoxia exposure enhanced IGFBP-3 protein synthesis and secretion
resulting in cell apoptosis in H9c2 myocardial cells. Growth
Factors. 33:275–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blouin MJ, Bazile M, Birman E, Zakikhani
M, Florianova L, Aleynikova O, Powell DR and Pollak M: Germ line
knockout of IGFBP-3 reveals influences of the gene on mammary gland
neoplasia. Breast Cancer Res Treat. 149:577–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schermuly RT, Ghofrani HA, Wilkins MR and
Grimminger F: Mechanisms of disease: Pulmonary arterial
hypertension. Nat Rev Cardiol. 8:443–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mandegar M, Fung YC, Huang W, Remillard
CV, Rubin LJ and Yuan JX: Cellular and molecular mechanisms of
pulmonary vascular remodeling: Role in the development of pulmonary
hypertension. Microvasc Res. 68:75–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeffery TK and Morrell NW: Molecular and
cellular basis of pulmonary vascular remodeling in pulmonary
hypertension. Prog Cardiovasc Dis. 45:173–202. 2002. View Article : Google Scholar
|
|
34
|
Mohanraj L, Kim HS, Li W, Cai Q, Kim KE,
Shin HJ, Lee YJ, Lee WJ, Kim JH and Oh Y: IGFBP-3 inhibits
cytokine-induced insulin resistance and early manifestations of
atherosclerosis. PLoS One. 8:e550842013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu J, Yu Z and Su D: BMP4 protects rat
pulmonary arterial smooth muscle cells from apoptosis by
PI3K/AKT/Smad1/5/8 signaling. Int J Mol Sci. 15:13738–13754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kiss T and Kovacs K, Komocsi A, Tornyos A,
Zalan P, Sumegi B, Gallyas F Jr and Kovacs K: Novel mechanisms of
sildenafil in pulmonary hypertension involving
cytokines/chemokines, MAP kinases and Akt. PLoS One. 9:e1048902014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garat CV, Crossno JT Jr, Sullivan TM,
Reusch JE and Klemm DJ: Inhibition of phosphatidylinositol
3-kinase/Akt signaling attenuates hypoxia-induced pulmonary artery
remodeling and suppresses CREB depletion in arterial smooth muscle
cells. J Cardiovasc Pharmacol. 62:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Biasin V, Chwalek K, Wilhelm J, Best J,
Marsh LM, Ghanim B, Klepetko W, Fink L, Schermuly RT, Weissmann N,
et al: Endothelin-1 driven proliferation of pulmonary arterial
smooth muscle cells is c-fos dependent. Int J Biochem Cell Biol.
54:137–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mendivil A, Zhou C, Cantrell LA, Gehrig
PA, Malloy KM, Blok LJ, Burger CW and Bae-Jump VL: AMG 479, a novel
IGF-1-R antibody, inhibits endometrial cancer cell proliferation
through disruption of the PI3K/Akt and MAPK pathways. Reprod Sci.
18:832–841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zha Z, Zhang QH, Jiang ZX, Chen L, Lin H
and Liang XM: Effect of angiotensin II on pregnancy-associated
plasma protein A and insulin-like growth factor 1 gene expression
in human umbilical artery smooth muscle cells. Nan Fang Yi Ke Da
Xue Xue Bao. 29:195–198. 2009.In Chinese. PubMed/NCBI
|