Introduction
Obesity is the most common metabolic disease
worldwide and is a major public health issue, particularly in
developed countries (1). Obesity
occurs by a complex interaction between genetic and environmental
factors (2,3), and is characterized by excessive fat
cell size (hypertrophic obesity) or cell number (hyperplasic
obesity). It is often associated with a high-calorie diet, type 2
diabetes, high blood pressure, cardiovascular disease, and/or
metabolic complications (4–9).
In addition to the associated morbidity, many metabolic
complications, including type 2 diabetes, insulin resistance,
hyperlipidemia, hypertension, stroke, coronary heart disease and
cancer, have been linked to obesity (10–12). These complications result in
higher mortality rates in obese patients compared to lean
patients.
Adipogenesis, the process of pre-adipocyte
differentiation into adipocytes, is the result of excess energy
intake and lack of activity. Adipogenesis contributes to the
deposition of excess fat in adipocytes during differentiation from
pre-adipocytes. Multiple processes regulate adipogenesis, including
pre-adipocyte proliferation, differentiation, and fatty acid
oxidation and synthesis, and these processes are controlled by a
number of factors. Adipogenesis includes concerted transcriptional
and cellular events, including growth arrest, re-entry into the
cell cycle for mitotic clonal expansion, and the initiation of
transcription during differentiation (13–15). A number of genes have been shown
to be involved in the development of obesity, including peroxisome
proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding
protein (C/EBP)α, C/EBPβ and C/EBPδ (16–19). The expression levels of
adipocyte-related proteins such as sterol regulatory element
binding protein-1c (SREBP-1c), adiponectin, fatty acid synthase
(FAS), adipocyte fatty acid binding protein-4 (A-FABP4), glucose
transporter (GLUT4), lipoprotein lipase (LPL) and stearoyl-CoA
desaturase-1 (SCD-1) (20–24)
are also induced.
A number of drugs have been developed for the
treatment of obesity that target appetite regulation, fat
absorption and fat oxidation (25,26). A number of these drugs have been
withdrawn from the market because of low efficacy and side effects,
thus only a few drugs remain (27,28). For example, the anti-obesity drugs
orlistat (Xenical®) and sibutramine are commonly
prescribed, although they are associated with significant side
effects such as bladder pain, diarrhea, fever, loss of appetite,
nasal congestion and difficulty in sleeping (orlistat), and
headache, insomnia, increased appetite, asthenia, nausea and
anorexia (sibutramine) (29–31). Thus, there is clearly a need to
develop safer and more effective anti-obesity drugs.
Brown algae may represent a renewable natural
material for use as novel therapeutic agents because they are rich
in bioactive substances, including sulfated polysaccharides,
proteins, dietary fibers and carotenoids (32–40). Ecklonia cava (E. cava) is
an edible marine brown algal species that mainly inhabits coastal
Japan and Korea (41). It has
rarely been used for food but has been widely utilized in the
aquaculture of abalone and seashells. Studies of E. cava
have reported its antioxidant (42–46), anti-inflammatory (47–50), anticancer (51,52), and antibacterial (53,54) properties.
Recently, studies have examined the physiological
activity of E. cava because of its polyphenol components,
which include phlorotannins (55–61). E. cava polyphenols exhibit
antioxidant (55,56) and anticancer (51,52,59–61) properties as well as contribute to
hair growth (57,58). Polyphenol extracts of E.
cava have the potential to treat Alzheimer's disease (62) and the polyphenolic extract Seanol
affects lipid and glucose metabolism (63).
Previous studies have investigated the anti-obesity
properties of E. cava extracts using zebrafish, mice and
cell cultures (64–70). In the present study,
enzyme-treated Ecklonia cava (EEc) extract was prepared
using the digestive enzymes pectinase (Rapidase® X-Press
L) and cellulase (Rohament® CL) for separation and
purification of the effective components in E. cava. We
examined the inhibitory effects of the EEc extract on adipocyte
differentiation and adipogenesis-related gene expression in
vitro using 3T3-L1 adipocytes. Garcinia cambogia (Gar)
extracts are used as natural supplements and are known to suppress
appetite and lower body fat by blocking the lipid synthesis pathway
(71–73). A Gar extract was used as a
positive control.
Materials and methods
Preparation of enzyme-treated E. cava
extract
E. cava was purchased in 2013 from
Taekyug-nongsan (Jeju-do, Korea). E. cava chips of
approximately 5 cm were prepared by cutting the leaves and removing
the stem and roots of the algae. The extract was prepared by
placing 30 kg E. cava chips in 750 liters of distilled water
with the added enzymes (300 g pectinase, Rapidase X-Press L and 300
g cellulase, Rohament CL). The suspension was stirred for 24 h at
50°C, centrifuged at 3,000 × g at 4°C for 20 min, vacuum filtered,
and then three volumes of 60% ethanol were added. After 18 h, the
solution was filtered and concentrated using rotary evaporation to
6° Bx. The concentrated solution was made into a powder using a
spray dryer. The final extract weighed 3.56 kg, representing a
yield of 10.7% (EEc; JY202-MM130126R). Garcinia cambogia
powder extract was purchased from ES Ingredient Co., Ltd.
(Gyeonggi-do, Korea).
Cell culture
3T3-L1 mouse fibroblast cells (CL-173) were obtained
from the American Type Culture Collection (ATCC) (Rockvile, MD,
USA), and were cultured at 37°C with 5% CO2 in
Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10%
newborn calf serum (NBCS; Gibco, Life Technologies Corp., Auckland,
New Zealand) containing 50 µg/ml penicillin, 25 µg/ml
amphotericin B and 50 µg/ml streptomycin. At 70% confluency,
the cells were harvested by trypsinization and seeded in 6-well
plates in pre-adipocyte expansion medium (DMEM supplemented with
10% NBCS). When 100% confluency was reached, the cells were fed
differentiation medium [DMEM supplemented with 10% fetal bovine
serum (FBS) containing 0.25 µM dexamethasone, 0.5 mM
3-isobutyl-1-methylxanthine (IBMX) and 10 µg/ml insulin] for
48 h. The medium was then replaced with adipocyte maintenance
medium (DMEM supplemented with 10 µg/ml insulin) and changed
every 48 h for 8 days.
Cell viability assay
Cell viability was estimated using a Cyto X cell
viability assay kit (LPS solution, Daejeon, Korea). Cells were
seeded in 96-well plates at 2×104 cells/well in 100
µl medium and allowed to attach for 24 h. Attached cells
were treated with 12.5, 50, or 200 µg/ml EEc extract or Gar
extract in serum-free medium (SFM) for 24 h. Cyto X solution was
added to the cells and incubation was carried out for 1 h, and the
absorbance of each well was measured at 450 nm using a FilterMAX F5
microplate reader (Molecular Devices LLC, Sunnyvale, CA, USA).
Glucose utilization assay
Cells were seeded in 6-well plates and
differentiation was induced. Following differentiation, the cells
were treated with 12.5, 50 or 200 µg/ml EEc extract or Gar
extract for 24 h. Glucose utilization was performed with a glucose
assay kit (ASAN glucose kit; Asan Pharm Co., Gyeonggi-do, Korea),
as per the manufacturer's instructions. Briefly, glucose in the
cell medium of each well was allowed to react with the glucose
assay reagent for 5 min; then the contents were transferred to a
96-well plate, and the absorbance was measured at 500 nm using a
microplate reader (Gen 5; Epoch BioTek Instrument, Inc., Winooski,
VT, USA).
Triglyceride (TG) accumulation assay
Following differentiation, the cells were washed
with phosphate-buffered saline (PBS), harvested in ice-cold PBS,
and sonicated for 1 min. To measure TG content, TG assay reagent
was added to the cell lysate according to the manufacturer's
instructions (Cleantech TG-S; Asan Pharm Co.). Briefly, each cell
lysate (30 µl) was reacted with the TG assay reagent
solution for 10 min, transferred to a 96-well plate, and then the
absorbance was measured at 550 nm.
Oil Red O staining
Following differentiation, the cells were washed
twice with PBS and fixed with 10% formaldehyde for 1 h at room
temperature. After washing with PBS, the cells were stained with
Oil Red O working solution [0.5 g Oil Red O (Sigma-Aldrich, St.
Louis, MO, USA) in 60% isopropanol] for 1 h. After the staining
solution was removed, the lipid droplets were washed with water and
dried. Stained oil droplets in 3T3-L1 cells were imaged with a
light microscope (Eclipse TS100-F; Nikon, Tokyo, Japan). To
quantify the Oil Red O uptake, cells in each well were extracted
with 1 ml 100% isopropanol for 10 min, transferred to a 96-well
plate, and then the absorbance was measured at 540 nm using a
microplate reader.
Western blot analysis
Following differentiation, the cells were incubated
for 24 h in SFM containing 12.5, 50, or 200 µg/ml Gar or EEc
extract. Then the cells were washed with PBS and lysed with
extraction buffer (20 mM Tris, 150 mM NaCl, 10% glycerol, 10 mM
sodium pyrophosphate, 100 µM ammonium molybdate, 1 mM
β-glycerophosphate, 0.1% NP-40, and 0.1% SDS, pH 8.0) containing
protease inhibitors (1 µg/ml aprotinin, 1 µg/ml
leupeptin, 1 µg/ml pepstatin A, 100 µM sodium
orthovanadate and 1 mM PMSF). The extracts were centrifuged at
9,750 × g for 10 min, and the supernatant was used for western blot
analysis.
Total protein (40 µg) was electrophoresed on
an SDS-PAGE gel and transferred to a polyvinylidene fluoride
transfer membrane (Millipore Corp., Billerica, MA, USA). Membranes
were blocked with 1% bovine serum albumin (BSA) in TBS-T (5 mM
Tris-HCl, 20 mM sodium chloride, pH 7.4, and 0.1% Tween-20) and
incubated with primary antibodies (1:1,000) in 1% BSA in TBS-T with
gentle shaking overnight at 4°C. Membranes were washed twice for 15
min in TBS-T, and incubated with the corresponding HRP-conjugated
secondary antibodies (1:10,000) for 2 h at room temperature and
washed again. The immunoreactive bands were detected using an
enhanced chemiluminescence substrate (Advansta, Menlo Park, CA,
USA) and visualized using the GeneSys imaging system (SynGene
Synoptics, Ltd., London, UK). The following primary antibodies were
used: anti-C/EBPα (sc-9314, anti-goat), anti-C/EBPβ (sc-150,
anti-rabbit), anti-C/EBPδ (sc-151, anti-rabbit), anti-PPARγ
(sc-1984, anti-goat), anti-SREBP-1c (sc-366, anti-rabbit),
anti-A-FABP (sc-18661, anti-goat), anti-FAS (sc-55580, anti-mouse),
anti-GLUT4 (sc-1606, anti-rabbit), anti-adiponectin (sc-26497,
anti-goat), anti-leptin (sc-842, anti-rabbit), and
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-25778,
anti-rabbit) (all from Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). The secondary antibodies used were HRP-conjugated
anti-mouse IgG (sc-2031, Santa Cruz Biotechnology, Inc.),
anti-rabbit (A-0545, Sigma-Aldrich), and anti-goat (A50-101P,
Bethyl Laboratories Inc., Montgomery, TX, USA).
Statistical analysis
The results are presented as mean ± standard
deviation of at least three independent experiments (P<0.05).
Significant differences among multiple mean values were assessed by
analysis of variance (ANOVA) followed by the Duncan's multiple
range test using PASW statistics 18 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
EEc extract treatment does not exert
cytotoxic effects on 3T3-L1 pre-adipocytes
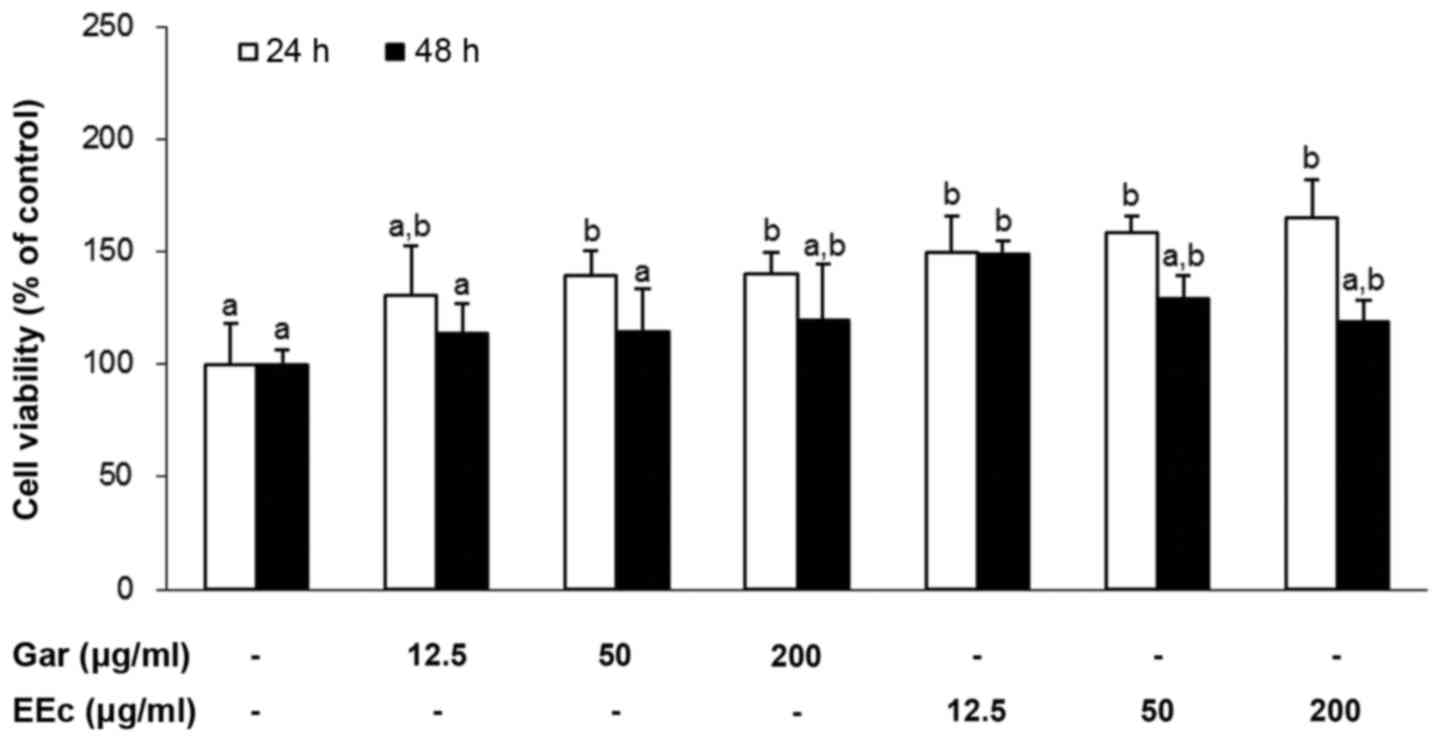
To determine the effects of Gar or EEc extract
treatment on the viability of the 3T3-L1 pre-adipocytes, the cells
were treated with 12.5, 50 or 200 µg/ml Gar or EEc extract
for 24 or 48 h. As shown in Fig.
1, treatment with Gar or EEc extract had no significant effect
on the viability of the 3T3-L1 pre-adipocytes at 48 h. However,
treatment with 12.5, 50 or 200 µg/ml Gar extract
significantly increased cell viability with values of 30.9, 39 and
39.9%, respectively at 24 h. Also, treatment with 12.5, 50, or 200
µg/ml EEc significantly increased cell viability at 49.8,
58.3 and 65.5%, respectively at 24 h, compared to the control
group.
Therefore, we selected the maximum dose of Gar and
EEc (200 µg/ml) for subsequent experiments to rule out the
possibility that EEc-dependent inhibition of adipogenesis may be a
result of its cytotoxic effects on 3T3-L1 cells. In subsequent
experiments, cells were treated with 12.5, 50 or 200 µg/ml
Gar and EEc extract for 24 h without cytotoxicity.
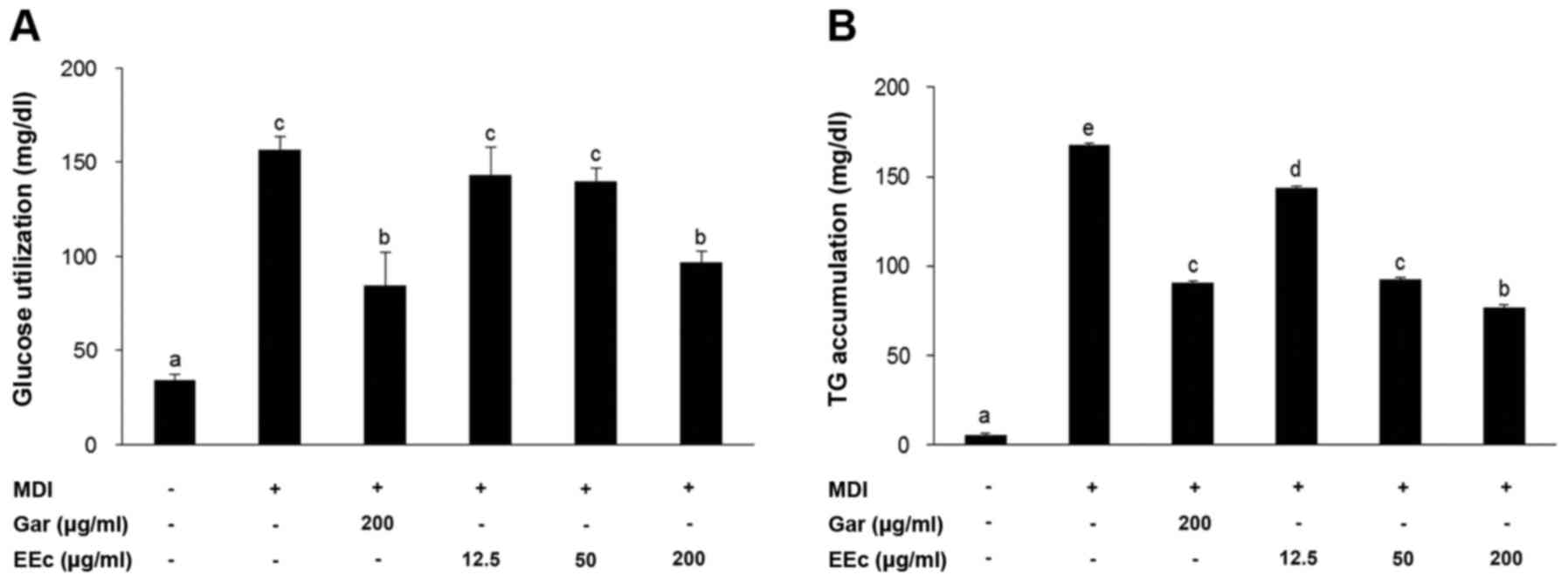
EEc extract treatment inhibits glucose
utilization and TG accumulation in the 3T3-L1 adipocytes
3T3-L1 cells were induced to differentiate for 8
days and were then treated with various concentrations of EEc
extract for 24 h. As shown in Fig.
2A, glucose utilization was assessed following EEc extract
treatment. In the positive control [receiving
3-isobutyl-1-methylxanthine, dexamethasone and insulin (MDI)],
glucose utilization was 156±7.0 mg/dl, while this was reduced to
84.4±17.9 mg/dl in the Gar group. Treatment with 12.5, 50, or 200
µg/ml EEc extract also reduced glucose utilization in a
dose-dependent manner, with values of 143.5±14.6, 139.8±7.3 and
96.7±6.2 mg/dl, respectively.
Next, we examined TG accumulation following EEc
extract treatment of the 3T3-L1 adipocytes. We found that TG
accumulation was 167.9±14.0 mg/dl in the MDI group while only
90.8±3.5 mg/dl in the Gar group (Fig.
2B). Treatment with 12.5, 50, or 200 µg/ml EEc extract
resulted in significantly reduced TG accumulation in a
dose-dependent manner with values of 143.5±8.7, 92.4±1.3 and
77.1±1.3 mg/dl, respectively. Consistent with these results, TG
accumulation in the 3T3-L1 adipocytes treated with 200 µg/ml
EEc extract was lower than that in the Gar group.
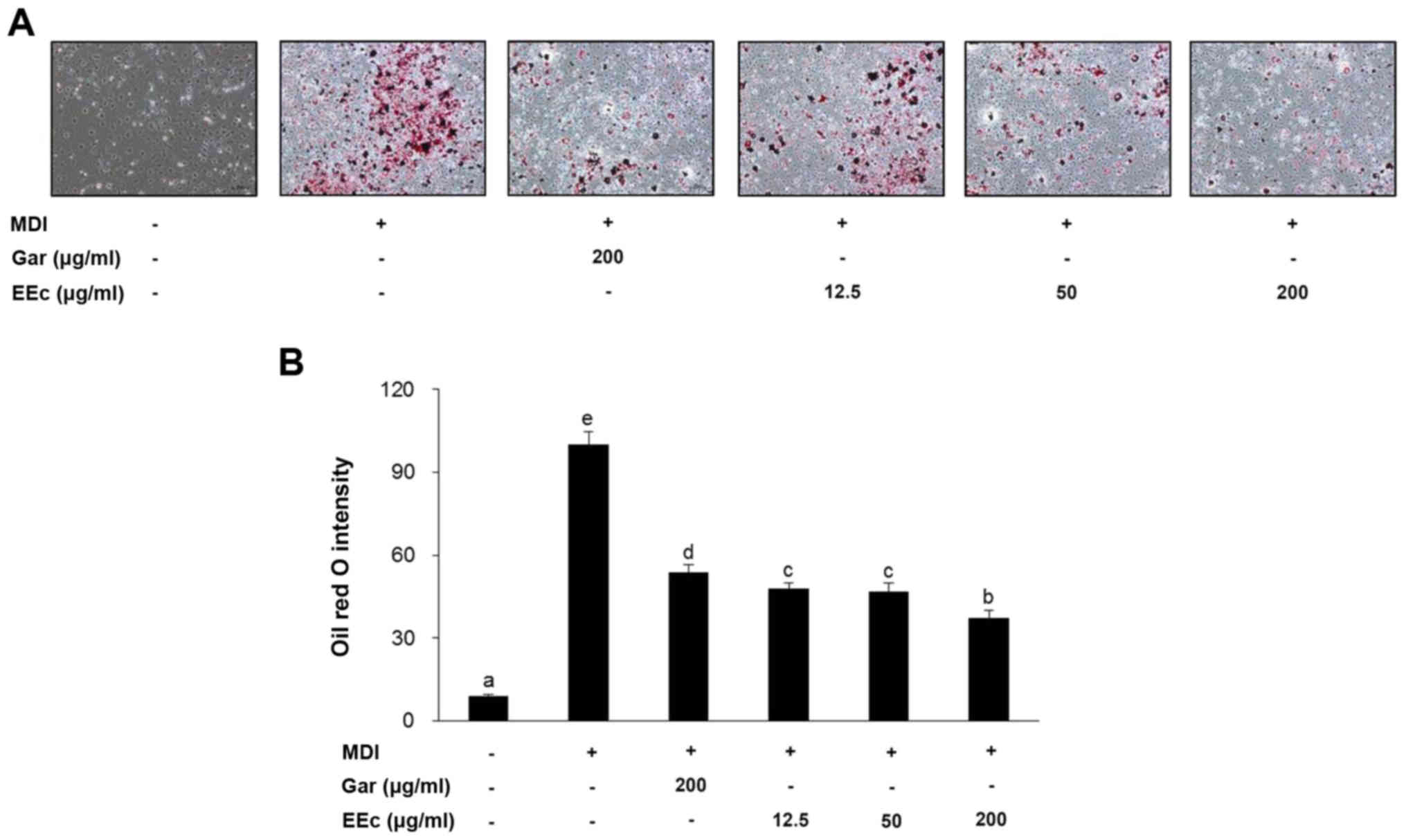
EEc extract treatment increases
adipogenesis in the 3T3-L1 adipocytes
Following the induction of differentiation, 3T3-L1
pre-adipocytes underwent morphological changes, including a
transition from spindle-like features to a round shape and
accumulation of intracellular lipids. Lipid accumulation is a known
indicator of adipogenesis, and thus Oil Red O staining was used to
examine whether EEc treatment influenced lipid accumulation in the
adipocytes. As shown in Fig. 3A,
EEc treatment decreased the intracellular lipid content compared to
that noted in the MDI group as observed in images of the
differentiated 3T3-L1 adipocytes.
The inhibitory effect of EEc extract treatment on
lipid accumulation was confirmed by lipid droplet quantification
(Fig 3B). Intracellular lipid
content was markedly decreased to 53.7±2.7 µg/ml when the
3T3-L1 adipocytes were treated with 200 µg/ml Gar extract
(Fig. 3B). Treatment with 12.5,
50, or 200 µg/ml EEc extract reduced lipid content in the
3T3-L1 adipocytes by 52.2, 53.2 and 62.6%, respectively, compared
to these values in the MDI group.
EEc extract treatment decreases the
protein levels of differentiation-related transcription
factors
Adipogenesis is accompanied by a change in the
sequential activation of several pro-adipogenic transcription
factors, including C/EBPα/β/δ and PPARγ. Thus, we examined whether
the reduced lipid accumulation in adipocytes was due to
downregulation of these transcription factors. Compared to the
differentiated control cells, EEc extract treatment significantly
decreased the expression of C/EBPα but not C/EBPβ/δ and PPARγ
(Fig. 4). This suggests that EEc
treatment inhibits adipogenesis by suppressing the expression of
adipogenic transcription factors, particularly C/EBPα.
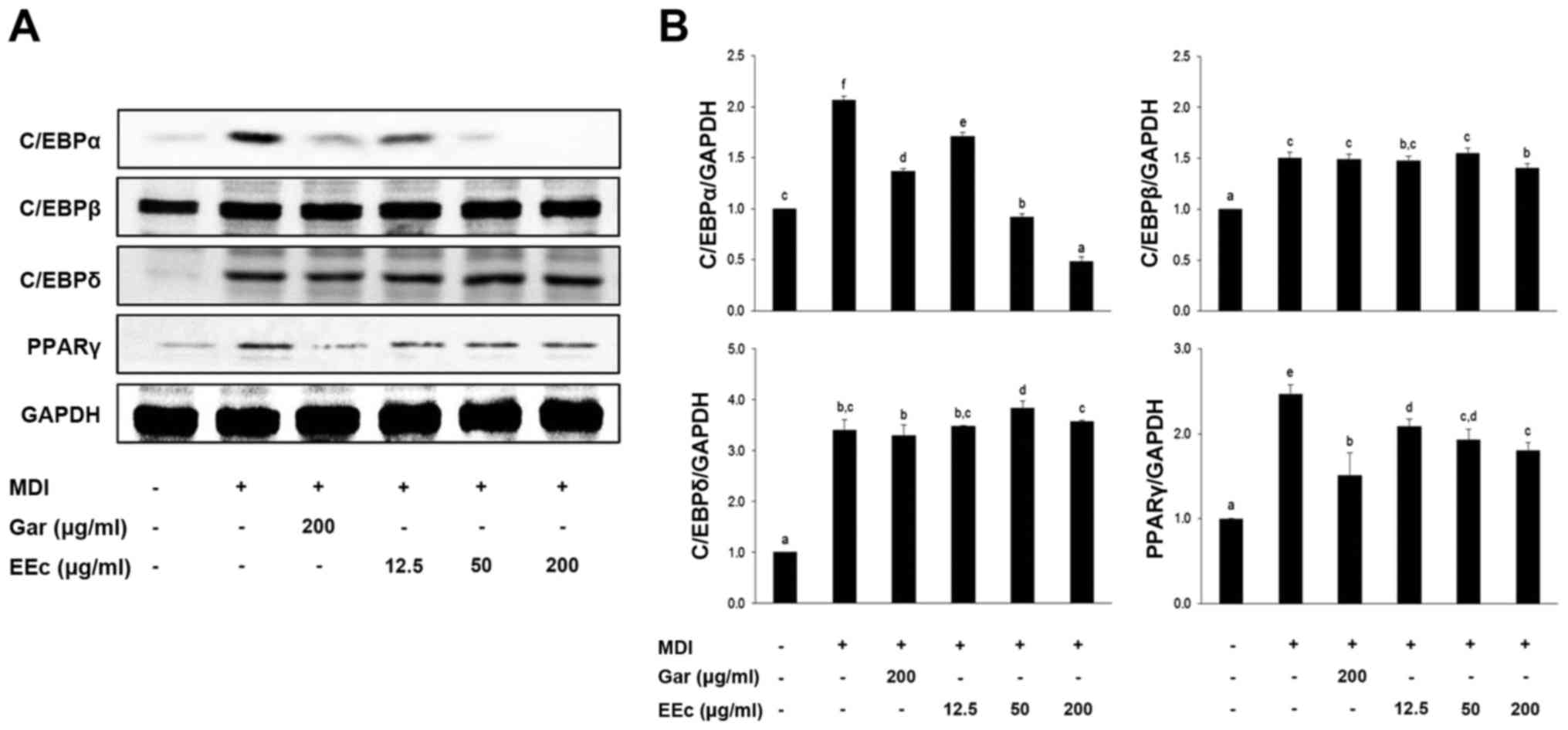 | Figure 4Treatment with the EEc extract
inhibits the expression of differentiation-related transcription
factors. (A) 3T3-L1 adipocytes were incubated with or without
various concentrations of the EEc extract for 24 h. Protein levels
of PPARγ, C/EBPα, C/EBPβ, and C/EBPδ were examined by western
blotting as described in Materials and methods. (B) The bands were
normalized to an internal control (GAPDH), and the relative ratio
is graphed. Data are presented as the means ± standard deviation
(P<0.05) from three independent experiments. C/EBP,
CCAAT/enhancer-binding protein; PPARγ, peroxisome
proliferator-activated receptor γ; Gar, Garcinia cambogia
extract; EEc, enzyme-treated Ecklonia cava extract; MDI,
3-isobutyl-1-methylxanthine, dexamethasone, and insulin. The
different letters at all concentrations represent significant
differences (P<0.05) as determined by Duncan's multiple range
test. |
EEc extract treatment reduces the
expression of adipogenesis-related proteins
To examine the effects of EEc extract treatment on
adipocytes, the expression of adipogenesis-related proteins was
determined in the EEc-treated and untreated 3T3-L1 cells. We also
determined the expression of other adipogenesis-related proteins
involved in lipogenic and fatty acid oxidation and glucose
homeostasis pathways. We found that EEc extract treatment inhibited
the expression of adipogenic differentiation-related transcription
factors in the 3T3-L1 cells. As shown in Fig. 5, differentiated cells exhibited
significantly increased expression of PPARγ target genes in the
adipogenesis pathway, including A-FABP, SREBP-1c, FAS and
adiponectin. In contrast, EEc extract treatment significantly
decreased the protein levels of SREBP-1c, A-FABP, FAS and
adiponectin compared to levels noted in the differentiated control
cells. No significant differences were apparent in regards to GLUT4
and leptin expression.
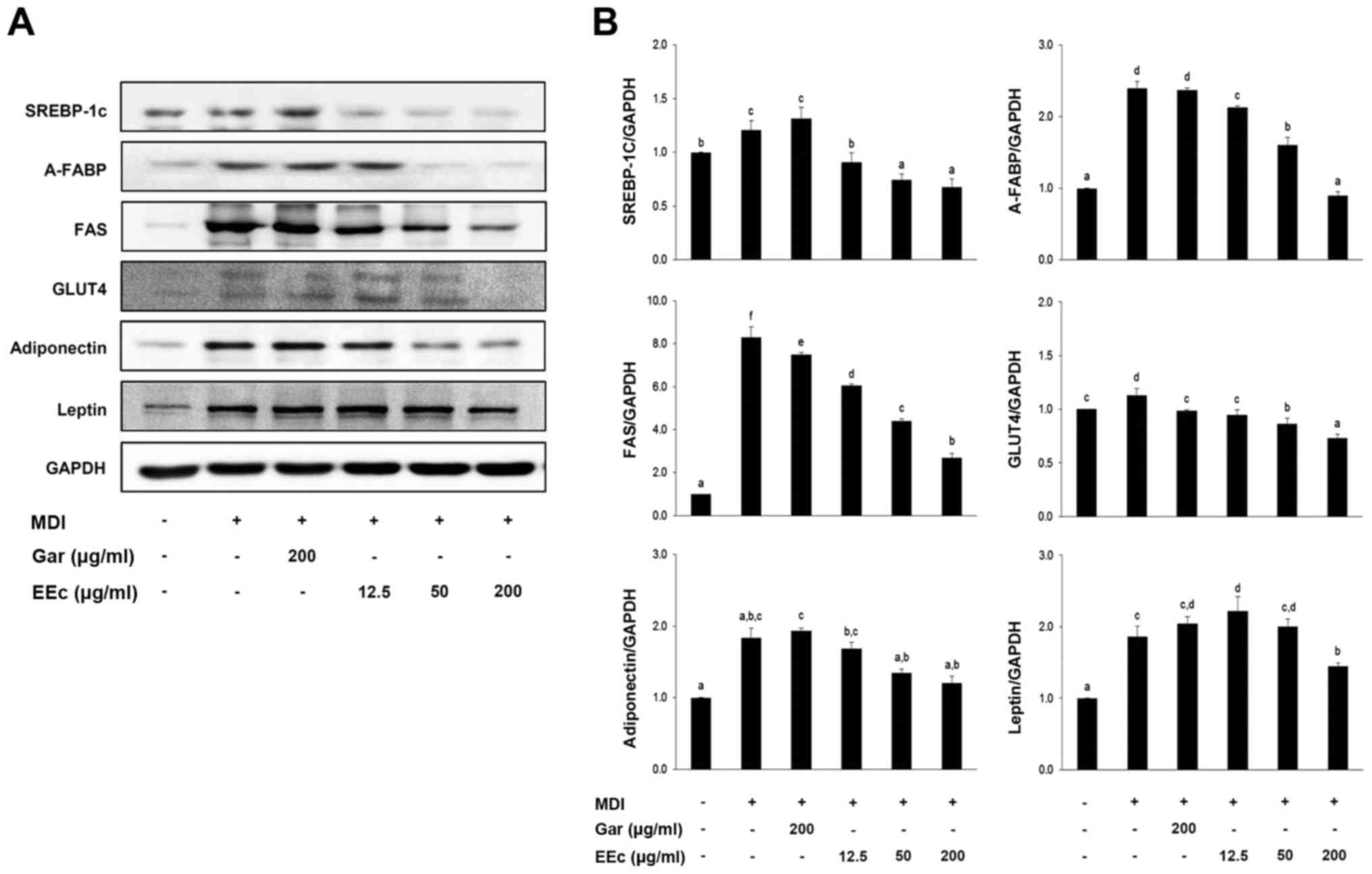 | Figure 5Treatment with the EEc extract
inhibits expression of adipogenesis-related proteins. (A) 3T3-L1
adipocytes were incubated with or without various concentrations of
the EEc extract for 24 h. Protein levels of SREBP-1c, A-FABP, FAS,
GLUT4, adiponectin and leptin were examined by western blotting as
described in Materials and methods. (B) The bands were normalized
to an internal control (GAPDH), and the relative ratio is graphed.
Data are presented as the means ± standard deviation (P<0.05)
from three independent experiments. Gar, Garcinia cambogia
extract; EEc, enzyme-treated Ecklonia cava extract; MDI,
3-isobutyl-1-methylxanthine, dexamethasone, and insulin; SREBP-1c,
sterol regulatory element binding protein-1c; A-FABP, adipocyte
fatty acid binding protein; FAS, fatty acid synthase. The different
letters at all concentrations represent significant differences
(P<0.05) as determined by Duncan's multiple range test. |
Discussion
Studies on E. cava have examined its
antioxidant (42–46), anti-inflammatory (47–50), anticancer (51,52) and antibacterial (53,54) properties. Recently, phlorotannins,
the polyphenol component of E. cava, were examined with
respect to their antioxidant (55,56), anticancer (51,52,59–61) and anti-obesity (64–70) properties, as well as their effects
on hair growth (57,58) and Alzheimer's disease (62). Inhibition of 3T3-L1 cell
differentiation by E. cava extracts has been previously
studied (69,70). The diethyl ether fraction
(69), methanolic extract and its
solvent-partitioned fraction (70) of E. cava extract were
examined for their antiadipogenic effect on 3T3-L1 adipocytes.
Dieckol from the diethyl ether fraction was found to inhibit
adipogenesis (69), and
eckstolonol from the n-BuOH fraction inhibited lipid accumulation
(70).
In the present study, an E. cava extract was
prepared using an enzyme-treatment high-yield extraction method
with a high concentration of polyphenols, and then the effects of
this extract on adipogenesis were determined.
We tested various methods to determine the optimal
conditions for extraction of the extract of E. cava. We
evaluated the following extraction methods: hot water treatment
(60°C, 90°C), ethanol treatment (60%, 80%), and enzymatic treatment
(Protex™ 6L, an endo-type protease; Rapidase® X-Press L,
a pectinase-cellulase-hemicellulase enzyme complex;
Rohament® CL, a cellulase-β-glucanase-hemicellulase
enzyme complex). The resulting yields were as follows: hot water
extraction, 19.31–27.75%; ethanol extraction, 3.24–11.42%; and
enzyme extraction, 19.08–21.87%. Specifically, the enzyme
extraction yields were 21.06% with Protex 6L, 19.08% with Rapidase
X-Press L, 18.66% with Rohament CL, and 21.87% with the Rapidase
X-Press L-Rohament CL complex. Hot water extraction resulted in a
higher yield compared to enzyme extraction; however, we decided
that enzyme extraction was the ideal method. The total polyphenol
content was also measured for each extraction method and ranged
from 206.74 to 812.17 µg/ml, with the 60% ethanol extraction
having the highest content. We decided that this method had low
economic efficiency as raw materials of E. cava were
obtained at a lower yield. Therefore, enzyme extraction using the
Rapidase X-Press L-Rohament CL complex was selected based on a
consideration of both the total yield and cost effectiveness.
High-performance liquid chromatographic analysis of the EEc extract
revealed three components (eckol, dieckol, and
phlorofucofuroeckol-A), and dieckol was the most abundant at 16
mg/g. The hexamer of phloroglucinol and dieckol exhibits various
biological properties, including antioxidant and anti-allergenic
activity, and plays a role in immunomodulation (74–76).
We considered two directions when planning the
present experiment. In other words, we considered whether to treat
the sample during or after completion of differentiation. In many
studies, it was known that sample treatment after the completion of
differentiation inhibited the adipogenesis-related protein
expression levels (64–70). Thus, we examined the inhibitory
effects of the EEc extact treatment upon completion of adipocyte
differentiation and adipogenesis in vitro in the 3T3-L1
adipocytes. First, we determined that treatment of 3T3-L1
pre-adipocytes with 12.5, 50, or 200 µg/ml Gar or EEc
extract did not result in cytotoxicity (Fig. 1). Glucose utilization and TG
accumulation were confirmed following EEc extract treatment in the
3T3-L1 adipocytes. Both the Gar and EEc extract-treated groups
showed similar glucose utilization at 200 µg/ml (96.7±6.2
mg/dl vs. 84.4±17.9 mg/dl), as well as similar TG accumulation at
50 µg/ml (92.4±1.3 mg/dl vs. 90.8±3.5 mg/dl) (Fig. 2). Overall, we found that EEc
extract treatment decreased glucose utilization and TG accumulation
in the 3T3-L1 adipocytes.
Lipid accumulation in adipose tissues occurs at a
late stage in adipogenesis. For this reason, Oil Red O staining was
performed following EEc extract treatment in the 3T3-L1 adipocytes.
We found that the EEc extract-treated group exhibited a marked
decrease in the number of lipid droplets and lipid storage
organelles compared to the MDI-treated group (Fig. 3). To confirm that the decrease in
lipid accumulation shown in Figs.
2 and 3 was due to
downregulation of the differentiation-related transcription factors
C/EBPα/β/δ and PPARγ, we examined their expression levels by
Western blot analysis. Differentiation of pre-adipocytes into
adipocytes is tightly controlled by the sequential activation of
several transcriptional factors, including C/EBPα, C/EBPβ, C/EBPδ
and PPARγ (16,17). Generally, C/EBPβ functions rapidly
following the induction of pre-adipocyte differentiation, followed
by the expression of C/EBPα and PPARγ (18,19). As shown in Fig. 4, treatment with EEc extract
decreased the expression of C/EBPα but did not affect the
expression of C/EBPβ, C/EBPδ, or PPARγ.
Adipogenesis is regulated by a complex
transcriptional cascade, in which members of the C/EBP family and
PPARγ play important roles in the expression of
adipogenesis-related genes, such as SREBP-1c, A-FABP, FAS, GLUT4,
LPL and SCD-1, which are involved in insulin sensitivity,
lipogenesis and lipolysis (16–20). A role for C/EBP in coordinating
transcription during pre-adipocyte differentiation was indicated by
its ability to transactivate the promoters of several
adipogenesis-specific genes (21–24). The activation of C/EBPα promotes
differentiation of pre-adipocytes by cooperating with PPARγ,
resulting in transactivation of adipogenesis-specific genes such as
A-FABP and FAS (26).
SREBP-1 is the first transcription factor involved
in adipocyte differentiation, and it increases the expression of
several lipogenesis-related genes, including LPL, ACC and FAS
(38). To determine the
expression of adipogenesis-related genes, SREBP-1c, A-FABP, FAS,
GLUT4, adiponectin and leptin were examined by Western blotting. As
shown in Fig. 5, EEc extract
treatment decreased the expression of SREBP-1c, A-FABP, FAS and
adiponectin in a dose-dependent manner compared to levels noted in
the MDI-treated group. Our results demonstrated that EEc extract
treatment appears to have an inhibitory effect on adipogenesis by
reducing the expression of differentiation-related transcription
factors and adipogenesis-related proteins in 3T3-L1 adipocytes.
In the present study, most genes examined were early
markers of adipogenesis and related transcription factors. Based on
the present findings, future research will assess markers at
different time points including the early stage of differentiation
using pre-adipocytes.
Our results demonstrated that EEc extract treatment
inhibited adipogenesis in 3T3-L1 adipocytes, shown by the
significant reduction in glucose utilization and TG accumulation
without cytotoxicity. The suppressive effects of EEc extract
treatment may be mediated by downregulation of the expression of
adipogenesis-related genes. Therefore, EEc extract treatment may be
a potential therapeutic agent for the prevention of obesity.
Acknowledgments
This study was supported by the Fishery
Commercialization Technology Development Program through iPET
(Korea Institute of Planning and Evaluation for Technology in Food,
Agriculture, Forestry and Fisheries) funded by the Ministry of
Oceans and Fisheries (MOF) (no. 111090-03-3-HD110). In addition,
this study was supported by the Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Education (grant no. 2012R1A6A1028677).
References
|
1
|
Formiguera X and Cantón A: Obesity:
Epidemiology and clinical aspects. Best Pract Res Clin
Gastroenterol. 18:1125–1146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albu J, Allison D, Boozer CN, Heymsfield
S, Kissileff H, Kretser A, Krumhar K, Leibel R, Nonas C, Pi-Sunyer
X, et al: Obesity solutions: Report of a meeting. Nutr Rev.
55:150–156. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hossain P, Kawar B and El Nahas M: Obesity
and diabetes in the developing world - a growing challenge. N Engl
J Med. 356:213–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caro JF, Dohm LG, Pories WJ and Sinha MK:
Cellular alterations in liver, skeletal muscle, and adipose tissue
responsible for insulin resistance in obesity and type II diabetes.
Diabetes Metab Rev. 5:665–689. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aggoun Y: Obesity, metabolic syndrome, and
cardiovascular disease. Pediatr Res. 61:653–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujioka K: Management of obesity as a
chronic disease: Nonpharmacologic, pharmacologic, and surgical
options. Obes Res. 10(Suppl 2): 116S–123S. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Després JP and Lemieux I: Abdominal
obesity and metabolic syndrome. Nature. 444:881–887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen ED and Spiegelman BM: Adipocytes as
regulators of energy balance and glucose homeostasis. Nature.
444:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J, Eliasson B, Smith U, Cushman SW
and Sherman AS: The size of large adipose cells is a predictor of
insulin resistance in first-degree relatives of type 2 diabetic
patients. Obesity (Silver Spring). 20:932–938. 2012. View Article : Google Scholar
|
|
10
|
Balkau B, Valensi P, Eschwège E and Slama
G: A review of the metabolic syndrome. Diabetes Metab. 33:405–413.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
12
|
Abate N: Obesity and cardiovascular
disease. Pathogenetic role of the metabolic syndrome and
therapeutic implications. J Diabetes Complications. 14:154–174.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JW, Tang QQ, Vinson C and Lane MD:
Dominant-negative C/EBP disrupts mitotic clonal expansion and
differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA.
101:43–47. 2004. View Article : Google Scholar :
|
|
14
|
Kwon JY, Seo SG, Heo YS, Yue S, Cheng JX,
Lee KW and Kim KH: Piceatannol, natural polyphenolic stilbene,
inhibits adipogenesis via modulation of mitotic clonal expansion
and insulin receptor-dependent insulin signaling in early phase of
differentiation. J Biol Chem. 287:11566–11578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang QQ, Otto TC and Lane MD:
CCAAT/enhancer-binding protein beta is required for mitotic clonal
expansion during adipogenesis. Proc Natl Acad Sci USA. 100:850–855.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christy RJ, Kaestner KH, Geiman DE and
Lane MD: CCAAT/enhancer binding protein gene promoter: Binding of
nuclear factors during differentiation of 3T3-L1 preadipocytes.
Proc Natl Acad Sci USA. 88:2593–2597. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farmer SR: Regulation of PPARgamma
activity during adipogenesis. Int J Obes. 29(Suppl 1): S13–S16.
2005. View Article : Google Scholar
|
|
18
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lowe CE, O'Rahilly S and Rochford JJ:
Adipogenesis at a glance. J Cell Sci. 124:2681–2686. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu A, Wang Y, Xu JY, Stejskal D, Tam S,
Zhang J, Wat NM, Wong WK and Lam KS: Adipocyte fatty acid-binding
protein is a plasma biomarker closely associated with obesity and
metabolic syndrome. Clin Chem. 52:405–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hassan M, El Yazidi C, Landrier JF, Lairon
D, Margotat A and Amiot MJ: Phloretin enhances adipocyte
differentiation and adiponectin expression in 3T3-L1 cells. Biochem
Biophys Res Commun. 361:208–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosen ED and MacDougald OA: Adipocyte
differentiation from the inside out. Nat Rev Mol Cell Biol.
7:885–896. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicholson AC, Hajjar DP, Zhou X, He W,
Gotto AM Jr and Han J: Anti-adipogenic action of pitavastatin
occurs through the coordinate regulation of PPARgamma and Pref-1
expression. Br J Pharmacol. 151:807–815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh WC, Cao Z, Classon M and McKnight SL:
Cascade regulation of adipocyte terminal differentiation by three
members of the C/EBP family of leucine zipper proteins. Genes Dev.
15:168–181. 1995. View Article : Google Scholar
|
|
25
|
Bray GA and Tartaglia LA: Medicinal
strategies in the treatment of obesity. Nature. 404:672–677.
2000.PubMed/NCBI
|
|
26
|
Alemany M, Remesar X and Fernández-López
JA: Drug strategies for the treatment of obesity. IDrugs.
6:566–572. 2003.PubMed/NCBI
|
|
27
|
Elangbam CS: Review paper: Current
strategies in the development of anti-obesity drugs and their
safety concerns. Vet Pathol. 46:10–24. 2009. View Article : Google Scholar
|
|
28
|
Rodgers RJ, Tschöp MH and Wilding JP:
Anti-obesity drugs: Past, present and future. Dis Model Mech.
5:621–626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buyukhatipoglu H: A possibly overlooked
side effect of Orlistat: Gastroesophageal reflux disease. J Natl
Med Assoc. 100:12072008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van der Zwaal EM, Janhunen SK, Luijendijk
MC, Baclesanu R, Vanderschuren LJ, Adan RA and La Fleur SE:
Olanzapine and sibutramine have opposing effects on the motivation
for palatable food. Behav Pharmacol. 23:198–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Apfelbaum M, Vague P, Ziegler O, Hanotin
C, Thomas F and Leutenegger E: Long-term maintenance of weight loss
after a very-low-calorie diet: A randomized blinded trial of the
efficacy and tolerability of sibutramine. Am J Med. 106:179–184.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cunha L and Grenha A: Sulfated seaweed
polysaccharides as multifunctional materials in drug delivery
applications. Mar Drugs. 14:1–41. 2016. View Article : Google Scholar
|
|
33
|
Grasa-López A, Miliar-García Á,
Quevedo-Corona L, Paniagua-Castro N, Escalona-Cardoso G,
Reyes-Maldonado E and Jaramillo-Flores ME: Undaria pinnatifida and
fucoxanthin ameliorate lipogenesis and markers of both inflammation
and cardiovascular dysfunction in an animal model of diet-induced
obesity. Mar Drugs. 14:1482016. View Article : Google Scholar :
|
|
34
|
Geisen U, Zenthoefer M, Peipp M, Kerber J,
Plenge J, Managò A, Fuhrmann M, Geyer R, Hennig S, Adam D, et al:
Molecular mechanisms by which a Fucus vesiculosus extract mediates
cell cycle inhibition and cell death in pancreatic cancer cells.
Mar Drugs. 13:4470–4491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gomes DL, Telles CB, Costa MS,
Almeida-Lima J, Costa LS, Keesen TS and Rocha HA: Methanolic
extracts from brown seaweeds Dictyota cilliolata and Dictyota
menstrualis induce apoptosis in human cervical adenocarcinoma HeLa
cells. Molecules. 20:6573–6591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pereira DM, Cheel J, Areche C, San-Martin
A, Rovirosa J, Silva LR, Valentao P and Andrade PB:
Anti-proliferative activity of meroditerpenoids isolated from the
brown alga Stypopodium flabelliforme against several cancer cell
lines. Mar Drugs. 9:852–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Patra S, Muthuraman MS, Prabhu AR,
Priyadharshini RR and Parthiban S: Evaluation of antitumor and
antioxidant activity of Sargassum tenerrimum against Ehrlich
ascites carcinoma in mice. Asian Pac J Cancer Prev. 16:915–921.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moussavou G, Kwak DH, Obiang-Obonou BW,
Maranguy CA, Dinzouna-Boutamba SD, Lee DH, Pissibanganga OG, Ko K,
Seo JI and Choo YK: Anticancer effects of different seaweeds on
human colon and breast cancers. Mar Drugs. 12:4898–4911. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang FW: Bioactive metabolites from
Guignardia sp., an endophytic fungus residing in Undaria
pinnatifida. Chin J Nat Med. 10:72–76. 2012. View Article : Google Scholar
|
|
40
|
Wang H, Fu Z and Han C: The potential
applications of marine bioactives against diabetes and obesity. Am
J Mar Sci. 2:1–8. 2014. View Article : Google Scholar
|
|
41
|
Wijesinghe WA and Jeon YJ: Exploiting
biological activities of brown seaweed Ecklonia cava for potential
industrial applications: A review. Int J Food Sci Nutr. 63:225–235.
2012. View Article : Google Scholar
|
|
42
|
Athukorala Y, Kim KN and Jeon YJ:
Antiproliferative and antioxidant properties of an enzymatic
hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol.
44:1065–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park MH, Heo SJ, Park PJ, Moon SH, Sung
SH, Jeon BT and Lee SH: 6,6′-bieckol isolated from Ecklonia cava
protects oxidative stress through inhibiting expression of ROS and
proinflammatory enzymes in high-glucose-induced human umbilical
vein endothelial cells. Appl Biochem Biotechnol. 174:632–643. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee SH, Park MH, Kang SM, Ko SC, Kang MC,
Cho S, Park PJ, Jeon BT, Kim SK, Han JS, et al: Dieckol isolated
from Ecklonia cava protects against high-glucose induced damage to
rat insulinoma cells by reducing oxidative stress and apoptosis.
Biosci Biotechnol Biochem. 76:1445–1451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang SM, Lee SH, Heo SJ, Kim KN and Jeon
YJ: Evaluation of antioxidant properties of a new compound,
pyrogallol-phloro-glucinol-6,6′-bieckol isolated from brown algae,
Ecklonia cava. Nutr Res Pract. 5:495–502. 2011. View Article : Google Scholar
|
|
46
|
Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM and
Kim SK: Chemical components and its antioxidant properties in
vitro: An edible marine brown alga, Ecklonia cava. Bioorg Med Chem.
17:1963–1973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang YI, Jung SH, Lee KT and Choi JH:
8,8′-Bieckol, isolated from edible brown algae, exerts its
anti-inflammatory effects through inhibition of NF-κB signaling and
ROS production in LPS-stimulated macrophages. Int Immunopharmacol.
23:460–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee SH, Ko CI, Jee Y, Jeong Y, Kim M, Kim
JS and Jeon YJ: Anti-inflammatory effect of fucoidan extracted from
Ecklonia cava in zebrafish model. Carbohydr Polym. 92:84–89. 2013.
View Article : Google Scholar
|
|
49
|
Lee SH, Ko CI, Ahn G, You S, Kim JS, Heu
MS, Kim J, Jee Y and Jeon YJ: Molecular characteristics and
anti-inflammatory activity of the fucoidan extracted from Ecklonia
cava. Carbohydr Polym. 89:599–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shin HC, Hwang HJ, Kang KJ and Lee BH: An
antioxidative and antiinflammatory agent for potential treatment of
osteoarthritis from Ecklonia cava. Arch Pharm Res. 29:165–171.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ahn G, Lee W, Kim KN, Lee JH, Heo SJ, Kang
N, Lee SH, Ahn CB and Jeon YJ: A sulfated polysaccharide of
Ecklonia cava inhibits the growth of colon cancer cells by inducing
apoptosis. EXCLI J. 14:294–306. 2015.PubMed/NCBI
|
|
52
|
Ahn JH, Yang YI, Lee KT and Choi JH:
Dieckol, isolated from the edible brown algae Ecklonia cava,
induces apoptosis of ovarian cancer cells and inhibits tumor
xenograft growth. J Cancer Res Clin Oncol. 141:255–268. 2015.
View Article : Google Scholar
|
|
53
|
Lee W, Oh JY, Kim EA, Kang N, Kim KN, Ahn
G and Jeon YJ: A prebiotic role of Ecklonia cava improves the
mortality of Edwardsiella tarda-infected zebrafish models via
regulating the growth of lactic acid bacteria and pathogen
bacteria. Fish Shellfish Immunol. 54:620–628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lee W, Ahn G, Oh JY, Kim SM, Kang N, Kim
EA, Kim KN, Jeong JB and Jeon YJ: A prebiotic effect of Ecklonia
cava on the growth and mortality of olive flounder infected with
pathogenic bacteria. Fish Shellfish Immunol. 51:313–320. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang YI, Woo JH, Seo YJ, Lee KT, Lim Y and
Choi JH: Protective effect of brown alga phlorotannins against
hyper-inflammatory responses in lipopolysaccharide-induced sepsis
models. J Agric Food Chem. 64:570–578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Choi YH: The cytoprotective effects of
ethanol extract of Ecklonia cava against oxidative stress are
associated with upregulation of Nrf2-mediated HO-1 and NQO-1
expression through activation of the MAPK pathway. Gen Physiol
Biophys. 35:45–53. 2016.
|
|
57
|
Shin H, Cho AR, Kim DY, Munkhbayer S, Choi
SJ, Jang S, Kim SH, Shin HC and Kwon O: Enhancement of human hair
growth using Ecklonia cava polyphenols. Ann Dermatol. 28:15–21.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chang MY, Han SY, Shin HC, Byun JY, Rah YC
and Park MK: Protective effect of a purified polyphenolic extract
from Ecklonia cava against noise-induced hearing loss: Prevention
of temporary threshold shift. Int J Pediatr Otorhinolaryngol.
87:178–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park SJ and Jeon YJ: Dieckol from Ecklonia
cava suppresses the migration and invasion of HT1080 cells by
inhibiting the focal adhesion kinase pathway downstream of Rac1-ROS
signaling. Mol Cells. 33:141–149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bae MJ, Karadeniz F, Ahn BN and Kong CS:
Evaluation of effective MMP inhibitors from eight different brown
algae in human fibrosarcoma HT1080 cells. Prev Nutr Food Sci.
20:153–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kim EK, Tang Y, Kim YS, Hwang JW, Choi EJ,
Lee JH, Lee SH, Jeon YJ and Park PJ: First evidence that Ecklonia
cava-derived dieckol attenuates MCF-7 human breast carcinoma cell
migration. Mar Drugs. 13:1785–1797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Choi BW, Lee HS, Shin HC and Lee BH:
Multifunctional activity of polyphenolic compounds associated with
a potential for Alzheimer's disease therapy from Ecklonia cava.
Phytother Res. 29:549–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Douglas TE, Dokupil A, Reczyńska K,
Brackman G, Krok-Borkowicz M, Keppler JK, Božič M, Van Der Voort P,
Pietryga K, Samal SK, et al: Enrichment of enzymatically
mineralized gellan gum hydrogels with phlorotannin-rich Ecklonia
cava extract Seanol® to endow antibacterial properties
and promote mineralization. Biomed Mater. 11:0450152016. View Article : Google Scholar
|
|
64
|
Choi HS, Jeon HJ, Lee OH and Lee BY:
Dieckol, a major phlorotannin in Ecklonia cava, suppresses lipid
accumulation in the adipocytes of high-fat diet-fed zebrafish and
mice: Inhibition of early adipogenesis via cell-cycle arrest and
AMPKα activation. Mol Nutr Food Res. 59:1458–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
You HN, Lee HA, Park MH, Lee JH and Han
JS: Phlorofucofuroeckol A isolated from Ecklonia cava alleviates
postprandial hyperglycemia in diabetic mice. Eur J Pharmacol.
752:92–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jeon HJ, Choi HS, Lee YJ, Hwang JH, Lee
OH, Seo MJ, Kim KJ and Lee BY: Seapolynol extracted from Ecklonia
cava inhibits adipocyte differentiation in vitro and decreases fat
accumulation in vivo. Molecules. 20:21715–21731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Park EY, Choi H, Yoon JY, Lee IY, Seo Y,
Moon HS, Hwang JH and Jun HS: Polyphenol-rich fraction of Ecklonia
cava improves nonalcoholic fatty liver disease in high fat diet-fed
mice. Mar Drugs. 13:6866–6883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lee SH and Jeon YJ: Efficacy and safety of
a dieckol-rich extract (AG-dieckol) of brown algae, Ecklonia cava,
in pre-diabetic individuals: A double-blind, randomized,
placebo-controlled clinical trial. Food Funct. 6:853–858. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ko SC, Lee M, Lee JH, Lee SH, Lim Y and
Jeon YJ: Dieckol, a phlorotannin isolated from a brown seaweed,
Ecklonia cava, inhibits adipogenesis through AMP-activated protein
kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ Toxicol
Pharmacol. 36:1253–1260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kim H, Kong CS, Lee JI, Kim H, Baek S and
Seo Y: Evaluation of inhibitory effect of phlorotannins from
Ecklonia cava on triglyceride accumulation in adipocyte. J Agric
Food Chem. 61:8541–8547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hong SY, Park JY, Sohn JS, Kim JH and Kim
MK: Effects of garcinia cambogia extract feeding on body weight and
lipid profiles in rats fed a high-carbohydrate or high-fat diet.
Food Sci Biotechnol. 18:649–654. 2009.
|
|
72
|
Chuah LO, Yeap SK, Ho WY, Beh BK and
Alitheen NB: In vitro and in vivo toxicity of garcinia or
hydroxycitric Acid: A review. Evid Based Complement Alternat Med.
2012:1979202012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Krishnamoorthy V, Nagappan P, Sereen AK
and Rajendran R: Preliminary phytochemical screening of the fruit
rind of Garcinia cambogia and leaves of Bauhinia variegate-A
comparative study. Int J Curr Microbiol App Sci. 3:479–486.
2014.
|
|
74
|
Ahn GN, Kim KN, Cha SH, Song CB, Lee JH,
Heo MS, Yeo IK, Lee NH, Jee YH, Kim JS, et al: Antioxidant
activities of phlorotannins purified from Ecklonia cava on free
radical scavenging using ESR and
H2O2-mediated DNA damage. Eur Food Res
Technol. 226:71–79. 2007. View Article : Google Scholar
|
|
75
|
Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH,
Kim TG, Kim SH, Kim NG, Huh H and Kim J: Inhibition of HIV-1
reverse transcriptase and protease by phlorotannins from the brown
alga Ecklonia cava. Biol Pharm Bull. 27:544–547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Le QT, Li Y, Qian ZJ, Kim MM and Kim SK:
Inhibitory effects of polyphenols isolated from marine alga
Ecklonia cava on histamine release. Process Biochem. 44:168–176.
2008. View Article : Google Scholar
|