Introduction
Nephrotic syndrome is basically characterized by
massive proteinuria. Reducing urinary protein is not only one of
the key goals in the treatment of nephrotic syndrome, but also the
decisive factor in improving the prognosis. To date, glucocorticoid
(GC) therapy still remains the first-line treatment for proteinuria
glomerular nephropathy and is widely used in nephrotic syndrome
including membranous nephropathy (MN), minimal-change disease
(MCD), focal segmental glomerulosclerosis (FSGS), and lupus
nephritis (LN), which are all characterized by podocyte injury and
proteinuria (1–3).
It is well-known that podocytes play an important
role in maintaining normal glomerular structure and function.
Increasing number of studies has shown that podocyte damage or loss
is the key factor which affects the glomerular filtration barrier
and causes proteinuria (4,5).
Present studies indicate that GCs can directly act on podocytes. It
has been demonstrated that there are functional receptors for GCs
on glomerular podocytes (6). Many
in vitro experiments have demonstrated that GCs could
protect or promote repair of podocytes from injury (7–13).
Although GC therapy has shown prominent efficacy in nephrotic
syndrome and thereby improves the survival of patients,
unfortunately, the serious side effects of GCs such as bone
mobilization, muscle mass loss, immunosuppression, and metabolic
alterations have limited their clinical application. Thus, it is
clear that alternative therapies with greater efficacy and/or fewer
side effects are crucially needed.
Nanoparticle-based delivery has become an important
strategy by which to target GCs to specific locations. To achieve
targeted delivery, a ligand on the delivery system binds to its
receptor on the surface of diseased cells and then undergoes
receptor-mediated endocytosis, so as to overcome wide systemic side
effects when GCs are administered in the free form. Many approaches
have been undertaken to obtain such a goal in different fields. For
example, milatuzumab, a humanized monoclonal antibody directed
against CD74, was conjugated to liposomes as a targeted
dexamethasone carrier for therapeutic delivery in CD74+
B-cell malignancies (14). An
anti-CD163 antibody-dexamethasone conjugate was developed in order
to specifically target GC to the hemoglobin scavenger receptor
CD163 in macrophages (15).
Targeted GCs have also been used in renal diseases. Dexamethasone
was encapsulated in liposome modified with monoclonal antibodies
against E-selectin, which is specifically expressed on activated
glomerular endothelium in glomerulonephritis (16,17).
There is a close link between podocyte injury and
protein-urea. However, research concerning the targeted delivery
toward podocytes has not been reported. The key goal is to identify
a specific receptor expressed on podocytes. The so-called neonatal
Fc receptor (FcRn) is a cell surface glycoprotein which is a major
IgG Fc receptor capable of facilitating the translocation of IgG.
Recent evidence suggests that FcRn is a key and promising target in
IgG-related drug delivery and disease therapy (18). Besides acting as the receptor of
IgG, FcRn is also a natural receptor of albumin. Eyre et al
found that mouse and human podocytes are able to specifically
endocytose albumin (19). Haymann
demonstrated the presence of FcRn on glomerular podocytes as well
as in the brush border of proximal tubular cells (20). In the present study, it was found
that FcRn is abundantly expressed in human podocytes in renal
biopsy specimens with nephritic syndrome, including MCD, FSGS, MN,
LN and diabetic nephropathy (DN).
It is well-known that the trapped molecular size of
the normal glomerular filtration barrier is approximately 7 nm,
which happens to be the same as the diameter of the albumin
molecule. Under normal circumstances, only little albumin can be
transported across the glomerular filtration barrier. However, when
nephritic syndrome occurs, the glomerular permeability is
significantly increased and a large amount of albumin passes
through the basement membrane to reach the podocyte side. Based on
the above understanding, we designed a nanoparticle consisted of
albumin and GC molecules. Such a delivery system may have
dual-targeting effects. Firstly, the albumin-carrying drug can pass
through the diseased glomerular filtration barrier. Secondly,
albumin can be recognized by FcRn expressed on podocytes, where GC
molecules can be released and exert their active effect.
Materials and methods
Localization of FcRn in human
glomeruli
Immunofluorescence was performed to study the
presence of FcRn using renal biopsy specimens with nephrotic
syndrome in different pathological types including MCD, FSGS, MN,
LN, mesangial proliferative glomerulonephritis (MsPGN) and DN. The
present study was approved by the Ethics Committee of The First
Affiliated Hospital of Nanjing Medical University (Nanjing, China).
The rabbit anti-FcRn polyclonal antibody (Cat. no. HPA012122) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). Briefly, 3
μm-thick cryostat sections were fixed in 4% paraformaldehyde
for 10 min and washed in PBS. The sections were incubated with
anti-FcRn antibody at a dilution of 1:400 overnight at 4°C, washed
with PBS, and incubated with FITC-labeled anti-rabbit IgG (Cat. no.
ZF-0311; ZSGB-Bio, Beijing, China) for 3 h at 37°C. A rabbit
isotype IgG was used as the negative control to confirm FcRn
specificity. Double staining was performed using a mouse monoclonal
antibody to synaptopodin (Cat. no. 03-61094) at a dilution of 1:30
(American Research Products Inc., Waltham, MA, USA) a podocyte
marker, and a Texas red-labeled anti-mouse IgG (Cat. no. ZF-0513;
ZSGB-Bio) for detection. The fluorescence intensity level was
judged by a renal pathologist under single-blind situation. Images
were captured using fluorescence microscopy.
Synthesis of albumin-based
nanoconjugates
The overall strategy was to covalently conjugate the
GC methylprednisolone (MP) to the albumin carrier which is the
ligand of FcRn. Firstly, bovine serum albumin (BSA; Sigma-aldrich)
was labeled with a fluorescent dye at Cys-34 by reacting it with
Alexa Fluor 633 C5 Maleimide (Gibco Life Technologies, Carlsbad,
CA, USA) at a 1:2 molar ratio of protein to dye in PBS supplemented
with 1 mM EDTA (pH 7.0) for 2 h at room temperature. The labeled
BSA was purified by gel filtration using a G-25 Desalting Column
(GE Healthcare, Piscataway, NJ, USA). The protein concentration of
BSA633 was measured by BCA assay. Secondly, the drug that will be
conjugated to BSA was activated. Briefly, MP-hemisuccinate 9.5 mg,
20 μmol (Wuhan E-ternity Technologies Co., Ltd, China) was
dissolved in 100 μl anhydrous DMSO to form a 200 mM
concentrated solution. An amount of 2 mg Sulfo-N-hydroxysuccinimide
(Sulfo-NHS; Thermo Fisher Scientific, Waltham, MA, USA) was
dissolved in 50 μl H2O and 2 mg
N-ethyl-N′-(3-dimethylaminopropyl) carbodiimide (EDC; Thermo Fisher
Scientific) in 40 μl H2O to form a solution of
200 mM (EDC) and 230 mM (Sulfo-NHS), respectively. An amount 1
μl of 200 mM MP-hemisuccinate solution was added to 1 ml MES
solution (0.1M MES, 0.5 M NaCl, pH 6.0), and 10 μl of 200 mM
EDC solution was added to give ~10 moles of EDC for each mole of
drug. An amount 22 μl of 230 mM Sulfo-NHS solution was added
to the above solution. The reaction was allowed to proceed for 15
min at room temperature. EDC was quenched by 1.4 μl of
2-mercaptoethanol (final concentration of 20 mM). The pH was
increased to 7.2 with concentrated phosphate buffer immediately
before reaction to protein. Lastly, purified BSA633 was added to
the activated drug solution at a molar ratio of 1:30. The mixture
was allowed to remain at room temperature for 2 h. The final
product was subsequently diafiltered using spin filters
(Amicon-Ultra, 30K; EMD Millipore, Billerica, MA, USA) into PBS pH
7.4 to remove the excess raw materials and other byproducts.
Samples were stored at 4°C for future analysis.
Characterization of BSA633-MP
In order to observe the integrity and polymerization
of the final products, a non-reduced SDS-PAGE was carried out using
12% polyacrylamide gel without 2-mercaptoethanol. After
electrophoresis, the gel was stained with Coomassie Blue R-250 and
destained in 45:10:45 (methanol:glacial acetic acid:water).
The size of the nanoconjugates and BSA was also
confirmed with transmission electron microscopy (TEM). In this
experiment, the nanoconjugates were diluted to 0.05 mg/ml and
dropped on 200 mesh carbon-coated copper grids (Ted Pella Inc.,
Redding CA, USA) and allowed to attach for 2 min. A concentration
of 2% phosphotungstic acid was used to counterstain the
nanoparticles. Samples were viewed using a TECNAI G2 F30
transmission electron microscope (FEI, Eindhoven, The
Netherlands).
The number of MP linked to BSA was further
determined by matrix-assisted laser desorption ionization (MALDI)
mass spectrometry with time-of-fight (TOF) using 5800 MALDI-TOF-TOF
MS-MS instrument (AB SCIEX, Foster City, CA, USA). An aliquot (1
μl) of the sample solution was mixed with an equal aliquot
of the matrix solution [2,5-dihydroxybenzoic acid (DHB) in 30%
acetonitrile and 0.1% trifluoroacetic acid] and 1 μl of the
mixed solution was spotted onto the target plate and evaporated
under a gentle stream of warm air. Mass spectra were acquired in
positive reflector mode. The accelerating voltage was 25 kV.
The purity of BSA633-MP was determined at 254 nm
with an analytical HPLC-Gel-Filtration column (TSKgel G3000PWXL,
300×7.8 mm; TOSOH Corporation, Tokyo, Japan) according to the
manufacturer's instructions (mobile phase: 0.15 M NaCl, 0.01 M
NaH2PO4, 5% CH3CN, pH 7.0).
PH-dependent stability of BSA633-MP
The amide bond between MP and BSA was sensitive to
strong acid and alkali. Our previous study confirmed this
conclusion in which the conjugates formed in the amide bond
underwent hydrolysis over 60% under pH 4.0 for 48 h (21). In this study, conjugates were
incubated in PBS at pH 7.4 and acetate buffer at pH 4.0 for 48 h at
37°C. A time course release assay (1, 4, 8, 12, 24 and 48 h) was
also carried out at pH 4 and 37°C. After incubation, samples were
eluted in a column with Sephadex G-100 gel (Sigma-Aldrich). The MP
contents in the fractions were then detected by OD240 (with
subtraction of albumin itself), while the albumin was detected by
Alexa Fluor 633.
Cell culture
Conditionally immortalized human podocytes were
kindly provided by Dr Moin A. Saleem (University of Bristol,
Southmead Hospital, Bristol, UK). The human podocytes were cultured
in RPMI-1640 medium (Life Technologies, Grand Island, NY, USA, USA)
supplemented with 10% FBS (Gibco, Grand Island, NY, USA) at a
permissive temperature (33°C). When the podocytes reached ~70–80%
confluence at 33°C, the podocytes were transferred at 37°C for
differentiation. Renal tubular cell line HK-2, with high expression
of FcRn, was maintained in F12 medium containing 10% FBS. Vascular
smooth muscle cells (VSMCs), which lack FcRn expression, were used
as the negative control. VSMCs were cultured in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% FBS.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was isolated from human podocytes as well
as HK-2 cells and VSMCs with Trizol (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manual. cDNA was synthesized
from 1 μg of total RNA using a reverse transcription kit
(Thermo Fisher Scientific). 18S rRNA served as an internal control.
Primer sequences were as follows: FcRn forward,
5′-CTGAGAACGGAAATCGTTGCTAA-3′ and reverse,
5′-TTAGCAGGAACTCGCTCTCCTT-3′; 18S forward,
5′-CATGATTAAGAGGGACGGC-3′ and reverse, 5′-TTC
AGCTTTGCAACCATACTC-3′. qPCR was performed in duplicate with 0.2
μM primers, 1 μl cDNA and SYBR-Green Real-Time PCR
Master Mix (Roche Applied Science, Mannheim, Germany) in a total
volume of 20 μl. Reactions were run at 95°C for 60 sec,
followed by 40 cycles of 15 sec at 95°C, 15 sec at 60°C and 45 sec
at 72°C. The StepOnePlus™ Real-Time PCR system (Applied Biosystems
Life Technologies, Foster City, CA, USA) was used for analysis.
Results are expressed as cycle threshold (Ct) and calculated as
∆Ct, which were normalized to endogenous control 18S rRNA.
Cellular uptake
Total cellular uptake of the Alexa Fluor 633-labeled
nanoconjugate was measured by flow cytometry using a BD FACSCalibur
flow cytometer (Becton-Dickinson, San Jose, CA, USA) and data were
analyzed using CellQuest software (Becton-Dickinson). Before
treatment, cell medium was changed and cells were washed 3 times
with PBS, and then the conjugate in serum-free Opti-MEM was added
to the wells. After treatment with the labeled BSA633-MP for 6 h,
the cells were trypsinized and were analyzed by flow cytometry,
with a 639 nm laser coupled with a 675-20 emission filter for Alexa
Fluor 633.
Confocal fluorescence microscopy
Confocal microscopy was performed to examine the
subcellular distribution of the targeted nanoconjugates. The
differentiated human podocytes were incubated with BSA633-MP in
Opti-MEM® media for 4 h. Thereafter, the cells were
washed with PBS and treated with Lysotracker Green DND-26 (75 nM)
which was used to track lysosomes for 2 h. Cells were imaged with
confocal microscope (Olympus FV1000; Olympus Corp., Tokyo, Japan)
using 488 and 633 nm laser and merged to determine the
intracellular localization of the BSA633-MP nanoconjugates.
Cell viability assay
The cytoprotective ability of the nanoconjugates was
measured with the CCK-8 assay (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). In brief, human podocytes were seeded in
96-well plates at 3,000 cells/well in a 100 μl volume
overnight. Then medium was replaced and exposed to different
concentrations of puromycin aminonucleoside (PAN) for 48 h. After a
suitable dose was determined, dosing for different periods (12, 24
or 48 h) was performed. Then PAN at optimal concentration and
period was administered to podocytes with or without the free MP or
BSA633-MP nanoconjugates. CCK-8 solution (10 μl) was added
and incubated at 37°C for 4 h. The optical density (OD) of the
plates was read at 450 nm using a plate reader.
In vivo and ex vivo imaging
Female BALB/c mice were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd., Shanghai, China. Mice were maintained
under a 12-h light-dark cycle and had access to food and water
ad libitum. All experiments were approved by the
Institutional Animal Care and Use Committee of Nanjing Medical
University.
The near infrared dye Alexa Fluor 633 was conjugated
to the BSA carrier to monitor the biodistribution and targeting
efficacy of the nanoconjugates. The mice were intravenously
injected with 200 μl of BSA633-MP nanoconjugates in PBS at
10 μM in terms of BSA (n=3). After 24 h, the mice were
anesthetized with 2% isoflurane. Whole body optical imaging was
carried out using a small-animal optical molecular imaging system
(VIS Imaging Spectrum System; Caliper Life Sciences, Hopkinton, MA,
USA) equipped with fluorescent filter sets (excitation-emission,
633/647 nm). Fluorescence images were normalized and reported as
counts per second (count/sec). In order to observe more concisely,
mice were then sacrificed and organs including the heart, liver,
spleen, lung and kidney were obtained for fluorescence imaging.
Data analysis
Data are presented as mean ± standard error of the
mean from at least three experiments. Statistical significance was
evaluated using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
FcRn expression in human glomeruli and
cultured human podocytes
A total of 105 renal biopsy specimens with nephrotic
syndrome of different pathological types including MCD, FSGS, MN,
MsPGN, LN and DN were subjected to immunofluorescence to identify
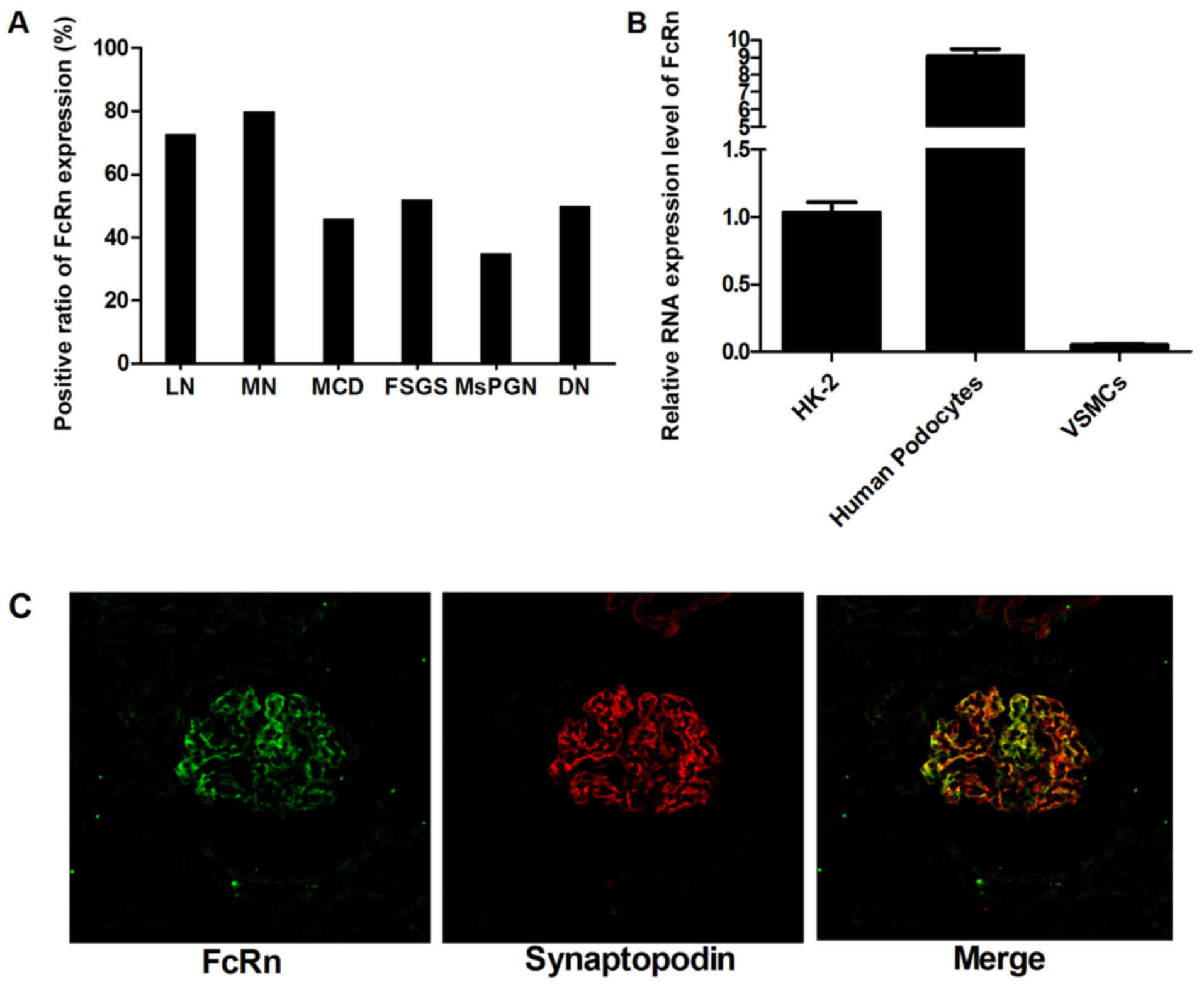
the expression of FcRn. The detailed positive ratio is presented in
Fig. 1A. It was observed that
FcRn was expressed in all the pathological types. Among these, MN
and LN samples showed a relatively higher positive rate for FcRn
staining. Fig 1C shows double
staining which was performed using the synaptopodin and FcRn
antibodies simultaneously, in which FcRn and synaptopodin
expression had obvious coincident sites. As synaptopodin is a
molecular marker of podocytes, we regarded that the expression of
FcRn in human glomeruli was attributed to the podocytes. Notably, a
negative result was obtained when a rabbit isotype IgG was used as
a negative control antibody instead of the anti-FcRn antibody,
which confirmed the specificity of the anti-FcRn antibody. As the
negative result was almost black, it was not presented here. Then
the RNA level of FcRn expressed in human podocytes was assayed by
RT-PCR analysis. The HK-2 cell line was used as positive control
and VSMCs as a negative control. As shown in Fig 1B, the RNA level of FcRn expression
was ~8-fold higher in the human podocytes than it was in the HK-2
cells and was ~400-fold higher than the level in the negative VSMC
control. This was consistent with the previous immunofluorescent
results, confirming FcRn as a suitable target for albumin-mediated
drug delivery.
Synthesis and characterization of the
BSA633-MP nanoconjugates
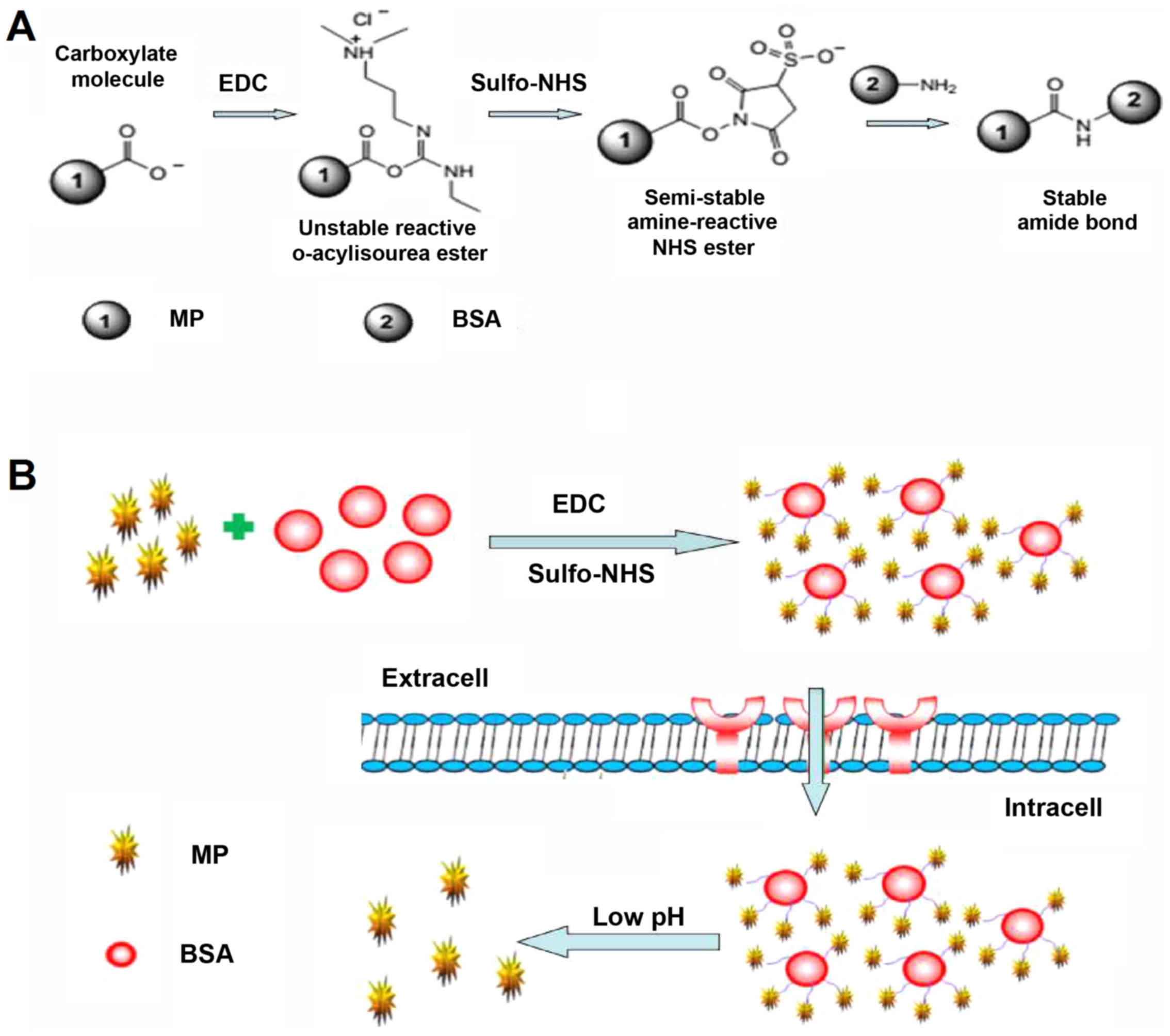
The overall strategy for preparation of the
nanoconjugates is outlined in Fig.
2. First, the single sulfhydryl group on BSA was labeled with
the far red fluorophore Alexa Fluor 633. Subsequently,
MP-hemisuccinate was reacted to Sulfo-NHS in the presence of EDC.
The activated Sulfo-NHS ester was then reacted with the surface
amino groups of BSA to form amide crosslink. The targeted
nanoconjugates were then termed BSA633-MP.
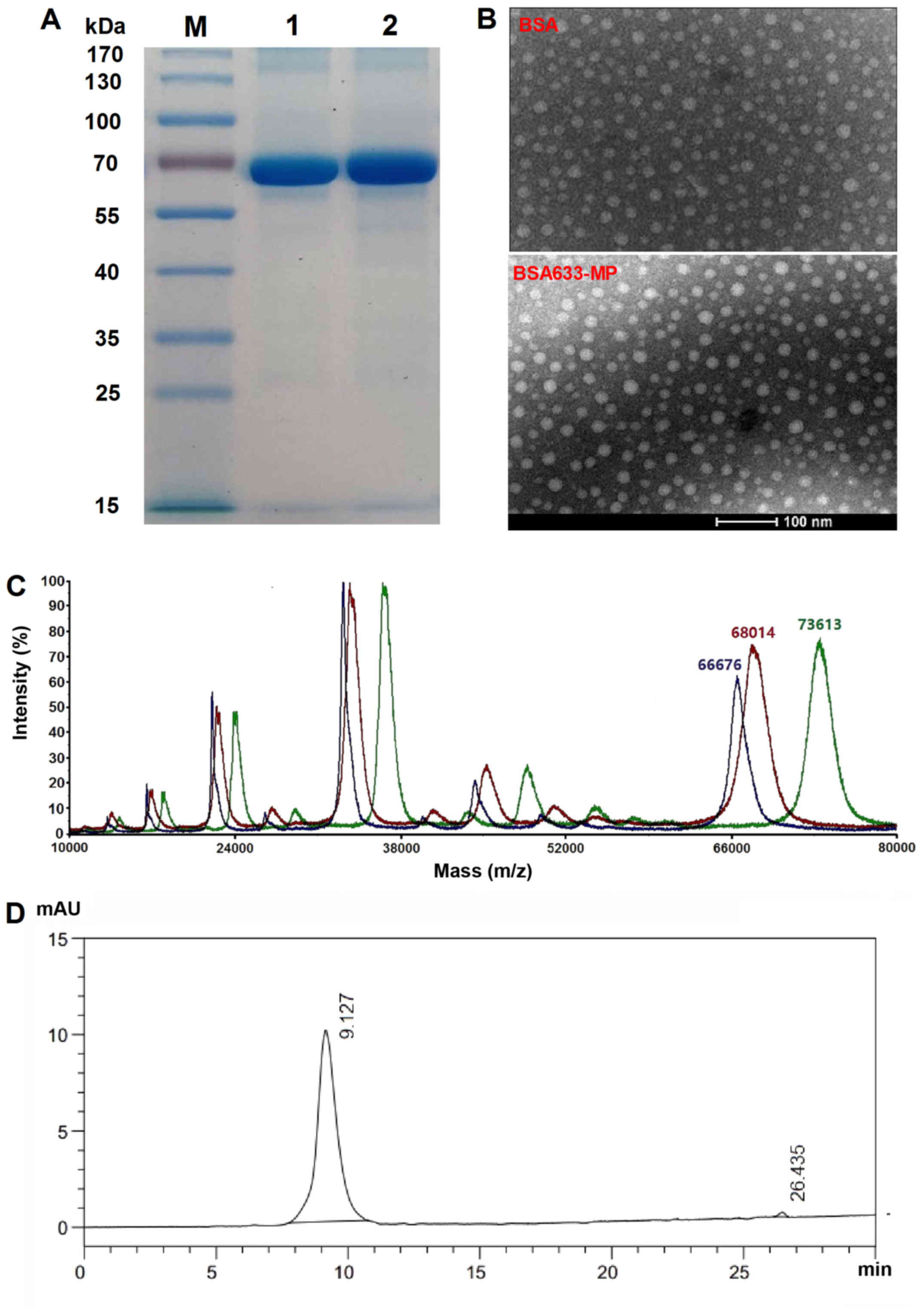
The molecular size of the nanoconjugates
was estimated using non-reducing SDS-PAGE and TEM
The integrity of the synthesized BSA633-MP
conjugates was evaluated by SDS-PAGE. The single band corresponding
to BSA633-MP is shown in Fig. 3A,
suggesting that the conjugate was mostly synthesized as a monomer.
Furthermore, the band representing BSA633-MP was almost at the same
position as BSA itself, without visible molecular mass increase.
The molecular size of the BSA633-MP conjugates was further
confirmed by TEM, which revealed an average diameter of 10 nm,
consistent with the SDS-PAGE result (Fig. 3B). The number of MP linked to BSA
was further determined by MALDI-TOF mass spectrometry. As shown in
Fig 3C, a typical increase
(~5,600 kDa) in the molecular weight of BSA633-MP compared with
BSA633 implied that the amount of drug conjugated was ~12 molecules
per albumin (Mw = 474.54), showing high payloads. To note, other
peaks represented molecules with different charges. For example,
peaks of BSA of ~33,000 and 22,000 kDa indicated that the number of
charge was 2 and 3, respectively.
In order to discern between associated MP (adhered
to BSA) and truly conjugated MP, Gel-Filtration HPLC was applied.
The absorption wavelength of 254 nm was chosen for detection of MP
(Fig. 3D). The main peak at 9.1
min corresponded to the BSA633-MP conjugate, while MP was eluted at
26.4 min. The final product contained <2% MP, suggesting the
successful conjugation of MP to BSA as well as high purity of the
conjugate.
Release profile of BSA633-MP
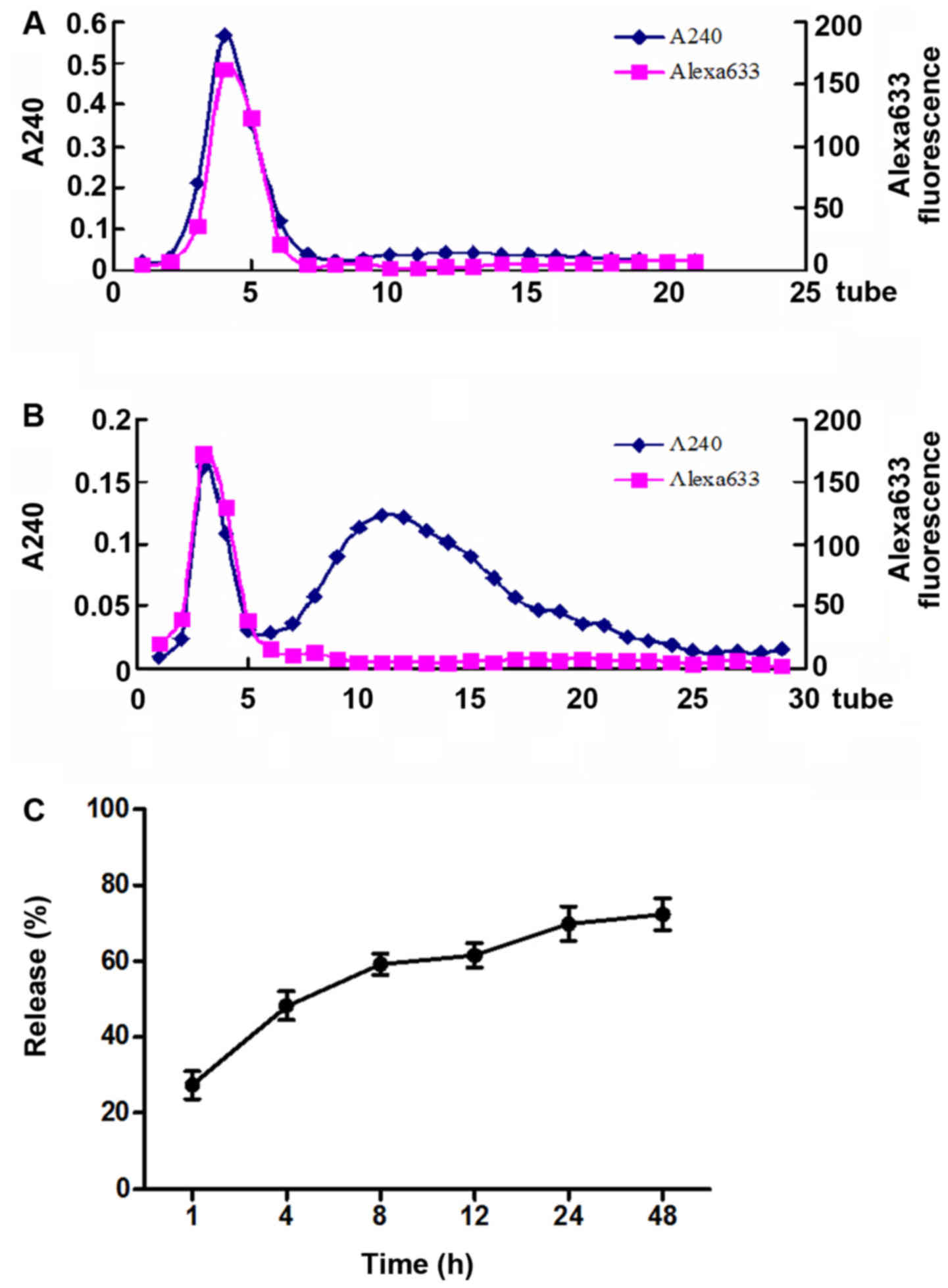
The effects of neutral pH and relatively acidic pH
on the release profile of BSA633-MP conjugate were evaluated. As
shown in Fig. 4A, we clearly see
that under pH 7.4, almost nothing was released from the BSA633-MP
conjugate. However, under pH 4.0, most MP molecules were released,
a small unreleased portion remained which was eluted together with
albumin (Fig. 4B). The OD240
values involved in the second elution peak vs. the total OD240
values (with subtraction of albumin itself) were calculated to
roughly estimate the release percentage. As shown in Fig. 4C, with prolonged incubation, drug
release was increased and at 48 h the release almost reached a
plateau. Under pH 4.0 ~72% of MP drug was released after 48 h.
These results indicated that the BSA633-MP conjugate was
acid-sensitive and very stable in a neutral environment. Actually,
the nanoconjugates were found to be stable for at least 3 months
when stored in buffer at pH 7.4, without manifesting aggregation or
degradation (data not shown).
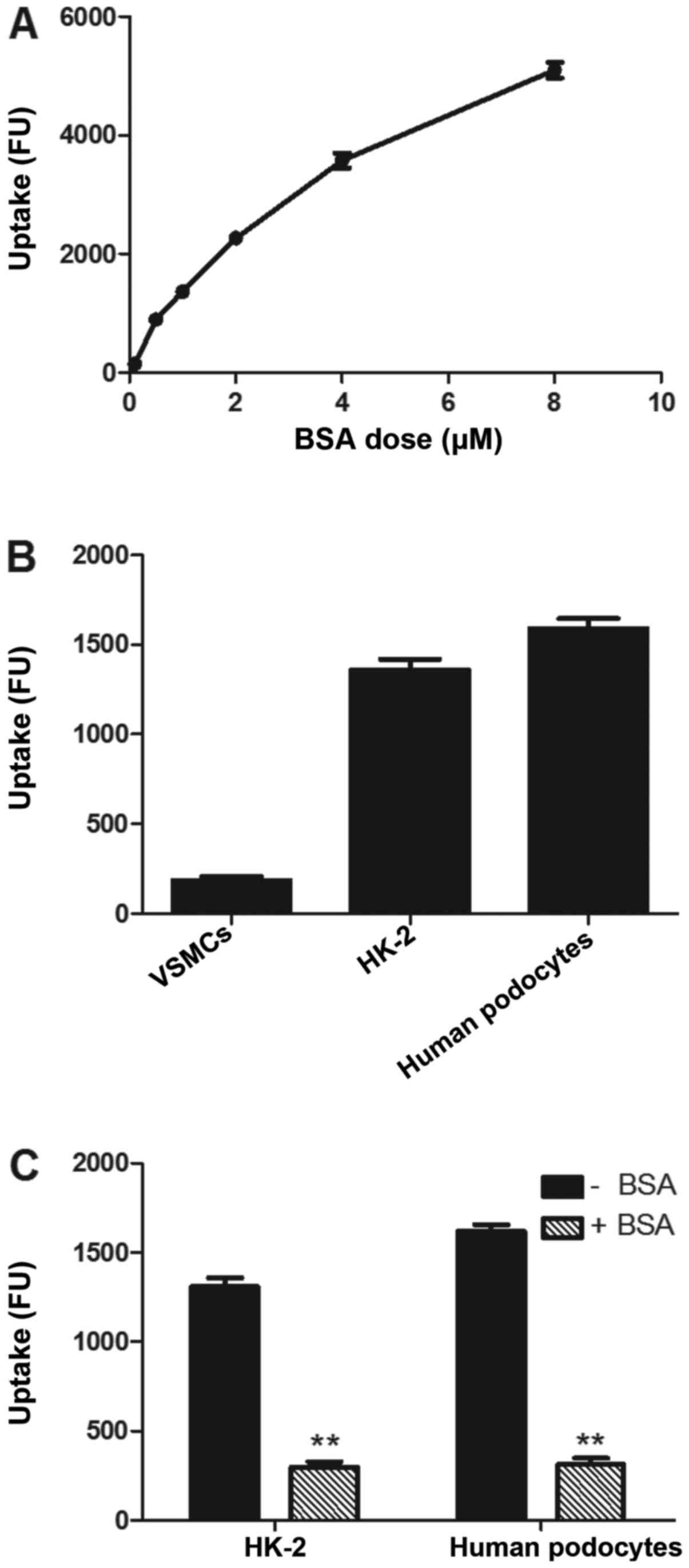
Cellular uptake of BSA633-MP
conjugates
The cellular uptake of the BSA633-MP nanoconjugates
was evaluated by incubating cells with the conjugates for 6 h and
then measuring total cell-associated fluorescence by flow
cytometry. As shown in Fig. 5A,
the uptake of the nanoconjugates was dose-dependent based on BSA
concentration. The uptake of the nanoconjugates was well described
by a classic saturable michaelismenten model, which is consistent
with a saturable, receptor-mediated endocytosis. Uptake of the
targeted nanoconjugates was contrasted in FcRn-positive and
-negative cells. As shown in Fig.
5B, the uptake of nanoconjugates was 36- and 29-fold higher in
the human podocytes and HK-2- positive control cells than that in
the VSMCs which lack the FcRn receptor. Moreover, co-incubation
with excess amounts of free BSA (1.5 mM) led to obvious inhibition
of uptake of the nanoconjugates (Fig.
5C). These observations support the concept that the cellular
uptake of the BSA conjugates depends on FcRn receptor-mediated
endocytosis.
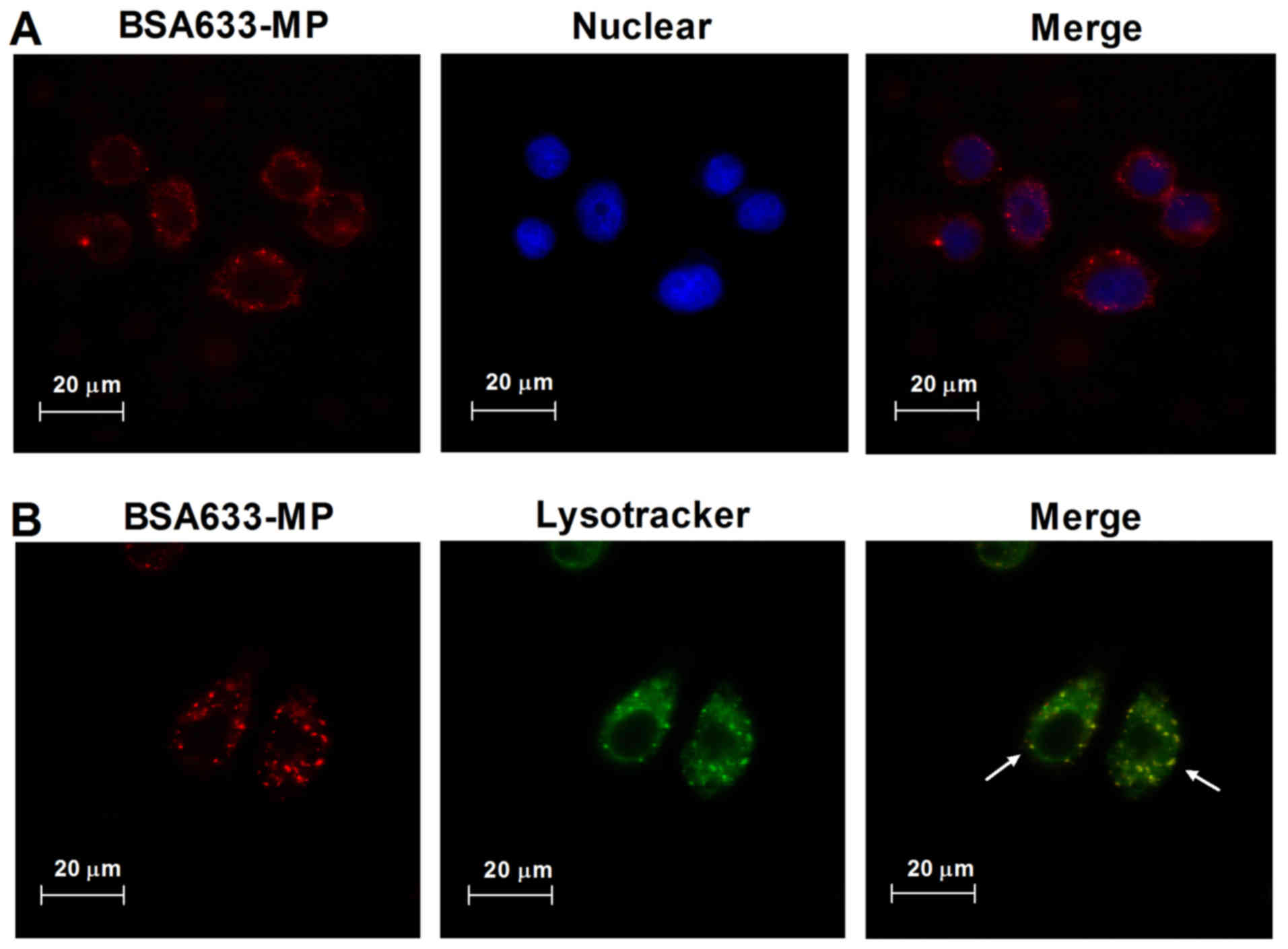
Intracellular trafficking of BSA633-MP
conjugates
Through confocal microscopy, we observed the
subcellular distribution of BSA633-MP conjugates. As shown in
Fig. 6A, human podocytes treated
with BSA633-MP conjugates for 4 h displayed intracellular red
fluorescence. Lysotracker was utilized to further assess the
cellular uptake and trafficking of the BSA633-MP conjugates. As
shown in Fig. 6B, we observed
onsiderable co-localization of conjugates and Lysotracker, which is
a marker for lysosomes. These results indicated that the
nanoconjugates were endocytosed and transported through the
lysosome pathway.
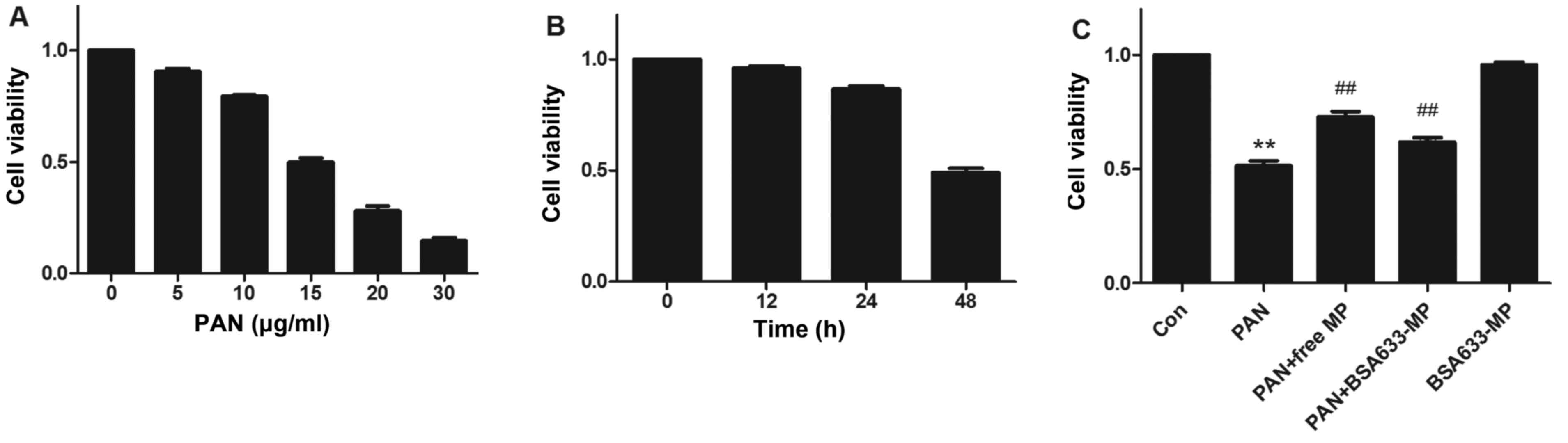
Cytoprotective ability of the BSA633-MP
conjugates
We first determined the percentage of apoptosis
cells with different doses of PAN during a 48-h period. A
dose-dependent decrease in cell viability was observed (Fig. 7A) and 15 μg/ml of PAN was
used in the followed experiments. PAN induced podocyte apoptosis in
a time-dependent manner (Fig.
7B). Compared with control cells, there was almost 50%
apoptosis after 48 h of exposure to PAN. We next measured the
podocyte apoptosis after PAN exposure in the presence of free MP or
BSA633-MP conjugate at an equal drug concentration of 1 μM.
As shown in Fig. 7C, MP
significantly enhanced cell viability at 48 h by 41.36% (72.69±1.39
vs. 51.42±1.29% without MP, P<0.01). However, the BSA633-MP
conjugate showed a relatively weaker cytoprotective ability against
PAN-induced apoptosis with an increase in cell viability by 20.07%
(61.74±1.13 vs. 51.42±1.29% without BSA633-MP, P<0.01). The
toxicity of the conjugates itself was also examined. There was no
significant difference in the viability of cells treated with 1
μM equivalent MP in conjugates compared with control cells.
In addition, the cells treated with the nanoconjugates maintained
similar normal morphology as the control cells.
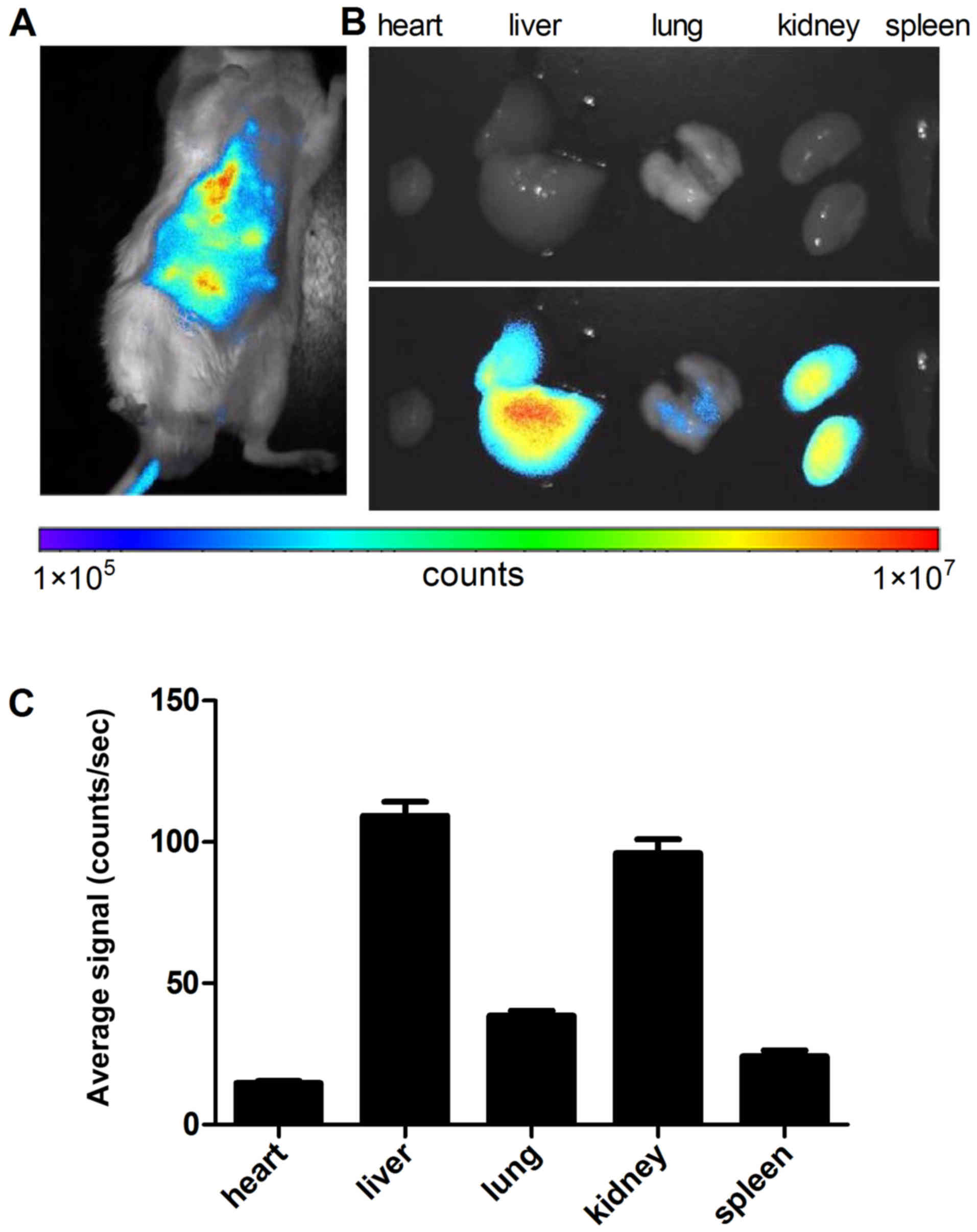
In vivo biodistribution
To evaluate whether BSA carrying nanoconjugates can
reach the kidney in vivo, the infrared dye Alexa Fluor
633-linked BSA nanoconjugate was administered intravenously to
normal mice and its in vivo biodistribution was estimated by
fluorescence imaging. Once 24 h had passed after intravenous
injection of BSA633-MP, NIR fluorescence imaging of the intact mice
was acquired (Fig. 8A). As
expected, the nanoconjugate exhibited strong liver and kidney
targeting and relative weaker signals in other organs with low
background. The ex vivo fluorescence of the dissected organs
further confirmed the preferential accumulation of BSA633-MP
nanoconjugates in the liver and kidney (Fig. 8B and C). These results indicated
that BSA633-MP nanoconjugates possess kidney-targeting ability,
which is favorable for improving targeted kidney administration
efficacy in vivo.
Discussion
To overcome the severe systemic side effects of GCs
used in the therapy of nephrotic syndrome, the present study
described a small non-cytotoxic monomolecular nanoconjugate. In the
present study, albumin, a specific ligand of the FcRn receptor,
which is highly expressed in glomeruli of nephrotic syndrome
specimens and cultured human podocytes (Fig. 1), was selected as the carrier and
targeting moiety. Methylprednisolone (MP) was selected as a
therapeutic entity. Both albumin and MP have been verified as
having adequate safety profiles in clinical applications, enabling
feasibility of this study. BSA is widely used for drug delivery
since it is abundant, has low cost, is biocompatible and easy to
link with drugs. Several albumin nanoparticles have been reported
for drug delivery, such as adsorption of obidoxime onto human serum
albumin (HSA) to form nanoparticles (22), silk fibroin-albumin nanoparticles
towards tumor cells (23), and
albumin-gentamicin microspheres (24). Abraxane®, a
nanoparticulate composite of HSA and paclitaxel, has been approved
by the US Food and Drug Administration (FDA) for cancer treatment
(25). Although the albumin
approach is a well-established method for directing drugs to cancer
cells, to our knowledge this is the first study for using albumin
for directing an anti-inflammatory drug to podocytes. In the
present study, high payloads were realized with multiple MP
molecules displayed on one single albumin. The loading efficiency
was estimated to be approximately 12 MP molecules per molecule of
our nanoconjugates (through MALDI-TOF MS in Fig. 3C).
Through SDS-PAGE and TEM (Fig. 3A and B), the resultant
nanoparticles showed a uniform and mono-dispersed size distribution
with an average diameter of approximately 10 nm, consistent with
their hydrodynamic size. Thus, they are large enough to pass
through renal glomerlular filtration in the pathological condition
of renal syndrome with damaged filtration barrier. We also used
HPLC method to discern between associated drug and conjugated drug.
As shown in Fig. 3D, the amount
of adhered drug was less than 2%, demonstrating that MP was mainly
conjugated to BSA instead of being adhered.
Ideally, a drug delivery system should be stable
enough in circulation so that it can be delivered to targeted sites
successfully. More importantly, to produce pharmacological actions,
the drugs must be released from the delivery system in the target
cells so that they can play a therapeutic role. We utilized an
acid-sensitive amide bond to link GCs to albumin so that in the
acidic environment of cellular lysosomes the linkage can be
potentially broken and the GCs released in the target cells. The
pH-associated stability assays revealed that the targeted
nanoconjugates are stable in neutral pH while the majority of MPs
can be released from the nanoconjugates at pH 4.0 (Fig. 4). During endocytosis, the pH
change can be exploited through acid cleavage of the amide linker
so as to let the drug be released inside the target cells (26).
Uptake assays confirmed the receptor-mediated
transportation of the nanoconjugates. The nanoconjugates
demonstrated a dose-dependent uptake and a 36-fold enhancement in
human podocytes compared to FcRn-negative VSMCs (Fig. 5B). In addition, excess free BSA
markedly inhibited uptake of the nanoconjugates (Fig. 5C). These observations support the
notion that uptake of the BSA-based nanoconjugates depends on the
FcRn receptor-mediated endocytosis. The high FcRn expression in
human podocytes suggests that the receptor is an ideal target
candidate for directing GC drugs to podocytes. Furthermore, the use
of FcRn for GC entry is supported by the abundant expression of
FcRn by our immune fluorescence assay in biopsy specimen (Fig. 2A). It was observed in Fig. 6B that the nanoconjugates were
transported to endosomes. Considering that the fluorescent dye
Alexa Fluor 633 was linked to albumin instead of the drug, we still
could not discern whether the drugs are released from the albumin
carrier. Thus, additional studies are needed to reveal the precise
trafficking pathways of the nanoconjugates.
PAN is characterized by podocyte apoptosis (9,12).
In this study, PAN was used to induce apoptosis in the cultured
human podocytes. It has been widely reported that GCs could prevent
podocytes from being injured by PAN. Our in vitro study
showed a positive effect of BSA633-MP on protection of PAN-induced
apoptosis. However, the free MP drug proved to be more efficient
than the nanoconjugate (Fig. 7C).
This may be due to the fact that when entering cells, large
molecules require recognition by specific receptors and
internalization into the cells, while small free drugs are
accessible to the cells through free diffusion. Additionally, it is
theoretically impossible for the nanoconjugates to release the
linked drugs entirely.
We made an initial investigation of in vivo
distribution of the nanoconjugates using fluorescence imaging
system. Non-invasive molecular imaging is widely recognized as a
tool for disease detection in most organs especially cancers
(27–32). Biological tissues have lower
absorbance and autofluorescence in the near-infrared region (NIR).
NIR dyes, small organic molecules that function in the NIR,
therefore have received increasing attention in the field of tumor
imaging and therapy (33). Our
tracer Alexa Fluor 633 demonstrated a high signal-to-background
ratio. Results (Fig. 8) showed
that the nanoconjugates remained in the kidney 24 h after
intravenous injection. According to our design, when glomerluli
barriers were damaged, the nanoconjugates passed the renal
filtration and entered podocytes through receptor-mediated
endocytosis. It must be mentioned that just like discussions in the
intracellular trafficking, the biodistribution of nanoconjugates
only reflected the situation of the BSA carrier. Yet, the aim of
the present study was to provide a preliminary evaluation on the
passive targeting of the conjugate. We plan to use HPLC to trace
drug distribution in the future when all the prerequisites are
ready.
In conclusion, albumin-mediated drug-targeting to
the FcRn receptor is a new potential approach for the safe therapy
of podocyte-associated kidney diseases. This approach involves
development of a low-dose GC therapy with a specific high-dose
effect on podocytes, thereby enhancing the beneficial GC effects
and reducing severe adverse effects. Although far from clinical
application, FcRn expressed by podocytes may potentially offer a
therapeutic opportunity.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81170660), the Clinical
Science and Technology Projects of Jiangsu Province (nos. BL2012032
and BL2014080) and the Priority Academic Program Development (PAPD)
of Jiangsu Higher Education Institutions.
References
|
1
|
Hogan J and Radhakrishnan J: The treatment
of idiopathic focal segmental glomerulosclerosis in adults. Adv
Chronic Kidney Dis. 21:434–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andolino TP and Reid Adam J: Nephrotic
syndrome. Pediatr Rev. 36:117–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tran TH, J Hughes G, Greenfeld C and Pham
JT: Overview of current and alternative therapies for idiopathic
membranous nephropathy. Pharmacotherapy. 35:396–411. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rezende GM, Viana VS, Malheiros DM, Borba
EF, Silva NA, Silva C, Leon EP, Noronha IL and Bonfa E: Podocyte
injury in pure membranous and proliferative lupus nephritis:
Distinct underlying mechanisms of proteinuria? Lupus. 23:255–262.
2014. View Article : Google Scholar
|
|
5
|
Akchurin O and Reidy KJ: Genetic causes of
proteinuria and nephrotic syndrome: Impact on podocyte
pathobiology. Pediatr Nephrol. 30:221–233. 2015. View Article : Google Scholar
|
|
6
|
Yan K, Kudo A, Hirano H, Watanabe T,
Tasaka T, Kataoka S, Nakajima N, Nishibori Y, Shibata T, Kohsaka T,
et al: Subcellular localization of glucocorticoid receptor protein
in the human kidney glomerulus. Kidney Int. 56:65–73. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing CY, Saleem MA, Coward RJ, Ni L,
Witherden IR and Mathieson PW: Direct effects of dexamethasone on
human podocytes. Kidney Int. 70:1038–1045. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ransom RF, Lam NG, Hallett MA, Atkinson SJ
and Smoyer WE: Glucocorticoids protect and enhance recovery of
cultured murine podocytes via actin filament stabilization. Kidney
Int. 68:2473–2483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wada T, Pippin JW, Marshall CB, Griffin SV
and Shankland SJ: Dexamethasone prevents podocyte apoptosis induced
by puromycin aminonucleoside: Role of P53 and Bcl-2-related family
proteins. J Am Soc Nephrol. 16:2615–2625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agrawal S, Guess AJ, Benndorf R and Smoyer
WE: Comparison of direct action of thiazolidinediones and
glucocorticoids on renal podocytes: Protection from injury and
molecular effects. Mol Pharmacol. 80:389–399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Gao X, Xu H, Feng C, Kuang X, Li Z
and Zha X: α-Actinin-4 is involved in the process by which
dexamethasone protects actin cytoskeleton stabilization from
adriamycin-induced podocyte injury. Nephrology (Carlton).
17:669–675. 2012. View Article : Google Scholar
|
|
12
|
Yu SY and Qi R: Role of bad in podocyte
apoptosis induced by puromycin aminonucleoside. Transplant Proc.
45:569–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohashi T, Uchida K, Uchida S, Sasaki S and
Nitta K: Dexamethasone increases the phosphorylation of nephrin in
cultured podocytes. Clin Exp Nephrol. 15:688–693. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao Y, Triantafillou G, Hertlein E, Towns
W, Stefanovski M, Mo X, Jarjoura D, Phelps M, Marcucci G, Lee LJ,
et al: Milatuzumab-conjugated liposomes as targeted dexamethasone
carriers for therapeutic delivery in CD74 B-cell malignancies.
Clinical cancer research: Clin Cancer Res. 19:347–356. 2013.
View Article : Google Scholar
|
|
15
|
Graversen JH, Svendsen P, Dagnæs-Hansen F,
Dal J, Anton G, Etzerodt A, Petersen MD, Christensen PA, Møller HJ
and Moestrup SK: Targeting the hemoglobin scavenger receptor CD163
in macrophages highly increases the anti-inflammatory potency of
dexamethasone. Mol Ther. 20:1550–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asgeirsdóttir SA, Kamps JA, Bakker HI,
Zwiers PJ, Heeringa P, van der Weide K, van Goor H, Petersen AH,
Morselt H, Moorlag HE, et al: Site-specific inhibition of
glomerulonephritis progression by targeted delivery of
dexamethasone to glomerular endothelium. Mol Pharmacol. 72:121–131.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asgeirsdóttir SA, Zwiers PJ, Morselt HW,
Moorlag HE, Bakker HI, Heeringa P, Kok JW, Kallenberg CG, Molema G
and Kamps JA: Inhibition of proinflammatory genes in anti-GBM
glomerulonephritis by targeted dexamethasone-loaded AbEsel
liposomes. Am J Physiol Renal Physiol. 294:F554–F561. 2008.
View Article : Google Scholar
|
|
18
|
Wang Y, Tian Z, Thirumalai D and Zhang X:
Neonatal Fc receptor (FcRn): A novel target for therapeutic
antibodies and antibody engineering. J Drug Target. 22:269–278.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eyre J, Ioannou K, Grubb BD, Saleem MA,
Mathieson PW, Brunskill NJ, Christensen EI and Topham PS:
Statin-sensitive endocytosis of albumin by glomerular podocytes. Am
J Physiol Renal Physiol. 292:F674–F681. 2007. View Article : Google Scholar
|
|
20
|
Haymann JP, Levraud JP, Bouet S, Kappes V,
Hagège J, Nguyen G, Xu Y, Rondeau E and Sraer JD: Characterization
and localization of the neonatal Fc receptor in adult human kidney.
J Am Soc Nephrol. 11:632–639. 2000.PubMed/NCBI
|
|
21
|
Wu L, Wu J, Zhou Y, Tang X, Du Y and Hu Y:
Enhanced antitumor efficacy of cisplatin by
tirapazamine-transferrin conjugate. Int J Pharm. 431:190–196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kufleitner J, Wagner S, Worek F, von
Briesen H and Kreuter J: Adsorption of obidoxime onto human serum
albumin nanoparticles: Drug loading, particle size and drug
release. J Microencapsul. 27:506–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Subia B and Kundu SC: Drug loading and
release on tumor cells using silk fibroin-albumin nanoparticles as
carriers. Nanotechnology. 24:0351032013. View Article : Google Scholar
|
|
24
|
Della Porta G, Adami R, Del Gaudio P,
Prota L, Aquino R and Reverchon E: Albumin-gentamicin microspheres
produced by supercritical assisted atomization: Optimization of
size, drug loading and release. J Pharm Sci. 99:4720–4729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kratz F: A clinical update of using
albumin as a drug vehicle - a commentary. J Control Release.
190:331–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rodrigues PC, Roth T, Fiebig HH, Unger C,
Mülhaupt R and Kratz F: Correlation of the acid-sensitivity of
polyethylene glycol daunorubicin conjugates with their in vitro
antiproliferative activity. Bioorg Med Chem. 14:4110–4117. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Du Y, Liu X, Zhang Q, Jing L, Liang
X, Chi C, Dai Z and Tian J: Monitoring tumor targeting and
treatment effects of IRDye 800CW and GX1-conjugated polylactic acid
nanoparticles encapsulating endostar on glioma by optical molecular
imaging. Mol Imaging. 14:356–365. 2015.PubMed/NCBI
|
|
28
|
Francisco AF, Lewis MD, Jayawardhana S,
Taylor MC, Chatelain E and Kelly JM: Limited ability of
posaconazole To cure both acute and chronic Trypanosoma cruzi
infections revealed by highly sensitive in vivo imaging. Antimicrob
Agents Chemother. 59:4653–4661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang C, Liu T, Su Y, Luo S, Zhu Y, Tan X,
Fan S, Zhang L, Zhou Y, Cheng T, et al: A near-infrared fluorescent
heptamethine indocyanine dye with preferential tumor accumulation
for in vivo imaging. Biomaterials. 31:6612–6617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neurath MF: Molecular Endoscopy and in
vivo Imaging in inflammatory bowel diseases. Dig Dis. 33(Suppl 1):
32–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piwnica-Worms D: From the guest editor:
Illuminating cancer in vivo with molecular imaging. Cancer J.
21:150–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan MM, Gray BD, Pak KY and Fong D:
Non-invasive in vivo imaging of arthritis in a collagen-induced
murine model with phosphatidylserine-binding near-infrared (NIR)
dye. Arthritis Res Ther. 17:502015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan A, Wu J, Tang X, Zhao L, Xu F and Hu
Y: Application of near-infrared dyes for tumor imaging,
photothermal, and photo-dynamic therapies. J Pharm Sci. 102:6–28.
2013. View Article : Google Scholar
|