Introduction
The adverse effects of aminoglycosides prominently
target the kidneys, vestibular and auditory organs, and
neuromuscular junction. Nephrotoxicity is reversible and can be
clinically managed with hydration therapy so that patients
generally recover normal renal function once treatment with
aminoglycosides is discontinued (1). By contrast, ototoxicity may be
initially overlooked, as it can occur after the end of drug
treatment and develops only slowly thereafter. Gentamicin (GM), an
aminoglycoside antibiotic, is clinically used in the treatment of
infectious diseases caused by Gram-negative or Gram-positive
organisms, including Pseudomonas, Proteus,
Serratia and Staphylococcus species (2), as well as severe diseases, such as
Meniere's disease and tuberculosis (3). The incidence of hearing loss ranges
from a very low percentages up to 33%, and vestibular toxicity
occurs in approximately 15% of patients who receive aminoglycoside
antibiotics (4). However, GM
remains widely used in developing countries as it is cost-effective
and not subject to strict regulations by prescription. Therefore,
developing otoprotective strategies is a primary and urgent goal
for the prevention of GM-induced ototoxicity.
GM-induced cell death is thought be mediated by
reactive oxygen species (ROS) (5–9),
and several agents that scavenge ROS or block their formation have
been proposed to protect the inner ears (10–14). To protect against the destructive
effects of ROS, living cells have developed various defense
systems, including enzymatic antioxidants, such as catalase,
superoxide dismutases (SODs), glutathione peroxidase and heme
oxygenase-1 (HO-1). Particularly, O2•− is
converted to less reactive H2O2 and
O2 by SODs, and H2O2 is further
converted to H2O and O2 by either the
catalase located in the peroxisomes or by glutathione peroxidase
located in the mitochondria and cytoplasm (15). The enhanced expression of SOD-1
has been shown to exert protective effects against diverse types of
tissue injury, such as ischemic and reperfusion injury, hypoxic
lung injury, brain trauma, various chemicals and drugs (16–19). Similarly, HO-1 induced by various
oxidative agents as a stress-responsive protein plays versatile
roles in the protection of cells from various oxidative stresses
(20–23).
Peroxisome proliferator-activated receptors (PPARs)
are ligand-activated transcription factors that belong to the
nuclear receptor superfamily (24). The activation of PPARs by their
ligands reduces inflammation by decreasing cytokines, adhesion
molecules and nitric oxide synthase 2, and reduces oxidative stress
by increasing antioxidant enzymes in different experimental models
(25–30). PPAR-α, a member of the PPAR
family, plays a critical role in important physiological processes,
such as the regulation of lipoproteins, lipid metabolism and
glucose homeostasis, and has been implicated in relieving oxidative
stress (31). Accordingly, a
PPAR-α-specific binding site was identified in the promoter regions
of catalase and SOD-1, suggesting that PPAR-α may directly regulate
the expression of these genes (32). Recently, fenofibrate, a PPAR-α
agonist that belongs to the fibrate class, has been shown to
protect the kidneys by suppressing oxidative stress (33); however, its otoprotective effects
against ROS have not been reported to date, at least to the best of
our knowledge. In this study, we investigated the protective
effects of fenofibrate on the GM-induced death of sensory hair
cells in both cochlea explant cultures of rats, and in an in
vivo zebrafish model.
Materials and methods
Reagents
Fenofibrate, GM, tin protoporphyrin IX (SnPPIX),
phalloidin-tetramethylrhodamine isothiocyanate (TRITC), Triton
X-100 and gelatin were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Plastic culture dishes were obtained from BD Biosciences
(Franklin Lakes, NJ, USA). Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), 2′,7′-dichlorofluorescein
diacetate (DCFH-DA) and Yo-Pro1 were obtained all from Invitrogen
Life Technologies (Carlsbad, CA, USA). Antibodies, including
anti-PPAR-α (sc-1985), anti-catalase (sc-34285), anti-SOD-1
(sc-11407), anti-HO-1 (sc-1796) and anti-β-actin (sc-47778), were
purchased all from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA,
USA).
Animals
Sprague-Dawley (SD) rats (n=30, 15 male and 15
female) were purchased from Orient Bio, Inc. (Gyeonggi-do, Korea).
The SD rats were fed a standard commercial diet and were housed at
an ambient temperature of 20–22°C and relative humidity of 50±5%
under a 12-h light/12-h dark cycle in a specific pathogen-free
facility. Experiments were performed using in-house born 3-week-old
SD rats weighing between 30 and 35 g, and all rats were age-matched
to within 3 days. For each experiment, 40 rats were divided into 4
different treatment groups (n=10 per group): saline group
(control), intraperitoneally-injected with 200 mg/kg GM for 4 days
(GM), 100 mg/kg fenofibrate (FF) for 10 days followed by GM (GM +
FF), and fenofibrate alone (FF). At the end of the treatment, the
rats were anesthetized for measuring auditory brainstem response
(ABR) and sacrificed for conducting immunohistochemistry to detect
expression of antioxidant enzymes. Zebrafish (Danio rerio)
were bred through the paired mating of wild-type fish. They were
maintained at 28.5°C on a 14-h light/10-h dark cycle. Zebrafish
were tested at the 5th day post-fertilization and maintained in an
incubator at 28.5°C during treatment. Larvae were immersed in a
Petri dish containing 15 ml embryo medium (EM) [13.7 mM NaCl, 540
µM KCl (pH 7.4), 25 µM Na2HPO4,
44 µM KH2PO4, 300 µM
CaCl2, 100 µM MgSO4 and 420 µM
NaHCO3 (pH 7.4)]. Larvae were treated with
fenofibrate-containing EM for 30 min prior to the addition of GM
(in EM) for 1 h, and then rinsed 4 times in EM. Hair cell survival
was assessed by Yo-Pro1 labeling. All animal experiments were
approved by the Institutional Animal Care and Use Committee at
Wonkwang University (WKU16-2; Iksan, Korea).
Organotypic cultures of Corti organ
explants
For ex vivo explant cultures, we sacrificed
in-house born rats on post-natal day 3, and the temporal bones were
isolated in a sterile manner. After placing the tissue in a 6-cm
dish with ice-cold phosphate-buffered saline (PBS; pH 7.4), the
cochlear capsule was peeled away and the membranous labyrinth was
exposed. The spiral ligament and stria vascularis (SV) were removed
and the organ of Corti was dissected under a microscope. Each
explant was placed onto a 0.1% gelatin-coated glass coverslip in a
4-well dish containing DMEM supplemented with 10% FBS. Culture
wells, each containing 500 µl of medium, were maintained in
an incubator at 37°C for 16 h with 5% CO2 and 95%
humidity. For each experiment, four 3-day-old rats were equally
divided into 4 different treatment groups (n=2 ears per group):
control, GM, GM + FF, and FF.
Phalloidin staining
The cochlear explants were fixed with 4%
paraformaldehyde in PBS at room temperature for 15 min, washed with
PBS (pH 7.4), and incubated with 0.1% Triton X-100 at room
temperature for 15 min. The expl ants were stained with
TRITC-labeled phalloidin (1:1,000) in PBS for 30 min in the dark,
and washed 3 times with PBS. The cochlear explants were observed
using a fluorescence microscope (IX71; Olympus, Tokyo, Japan)
equipped with a digital camera (DP70; Olympus, Tokyo, Japan).
Morphologically, intact hair cells were counted in a section
corresponding to 10 inner hair cells at three different zones
located on the basal turn of explants.
Measurement of intracellular ROS
levels
Intracellular ROS levels were measured using the
fluorescent dye, DCFH-DA. In the presence of an oxidant, DCFH-DA is
converted into highly fluorescent 2′,7′-dichlorofluorescein (DCF).
The cochlear explants were pre-treated with 100 µM
fenofibrate for 4 h and then exposed to 300 µM GM for 12 h.
Following incubation, the samples were incubated with 10 µM
DCFH-DA for 30 min. The fluorescence was detected under a
fluorescence microscope. A microplate reader was used to quantify
the ROS levels. Cochlear explants were plated in 96-well plates
overnight and were pre-treated with 100 µM fenofibrate for 4
h and then exposed to 300 µM GM for 12 h. After washing with
PBS, serum-free DMEM containing 10 µM DCFH-DA was added to
each well and the plates were incubated at 37°C for 1 h. ROS
production was measured using a microplate reader equipped with a
spectrofluorometer (SpectraMax M3; Molecular Devices, Sunnyvale,
CA, USA) at an emission wavelength of 538 nm and an excitation
wavelength of 485 nm. Relative ROS production was expressed as the
change in fluorescence of experimental groups compared with that of
the appropriate controls (100%).
Western blot analysis
Each sample consisted of an apex, middle and base.
The explants were collected from the media, washed with ice-cold
PBS, centrifuged at 3,000 rpm for 3 min at 4°C, lysed with 30
µl lysis buffer [50 mM Tris, pH 6.8, 10% glycerol, 2% sodium
dodecyl sulfate (SDS), 0.005% bromophenol blue, 100 mM
dithiothreitol, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 1X
proteinase inhibitor and 1 mM phenylmethylsulfonyl fluoride], and
boiled for 15 min to denature the proteins. Following
centrifugation at 13,000 rpm for 10 min at 4°C, the supernatants
were collected and loaded for SDS-polyacrylamide gel
electrophoresis (SDS-PAGE). Equal volumes (15 µl) of these
supernatants were separated by 10% SDS-PAGE, and electrotransferred
onto nitrocellulose membranes. The nitrocellulose membranes were
then blocked with 5% non-fat dried milk in TBS-T (50 mM Tris-HCl,
pH 7.4, 150 mM NaCl and 0.1% Tween-20) for 60 min at room
temperature. The blots were incubated overnight at 4°C with primary
antibodies (1:1,000) in 3% non-fat dried milk in TBS-T, washed
extensively with TBS-T, and incubated with a horseradish peroxidase
(HRP)-conjugated anti-rabbit (A120-101p; Bethyl Laboratories,
Montgomery, TX, USA) or anti-goat (P0449; DAKO, Glostrup, Denmark)
IgG antibody (1:2,000) for 1 h. The immunoreactive signal was
detected using an enhanced chemiluminescence detection system. To
quantify band intensity, the images of immunoblot films were
scanned; band intensity was quantified using the Gel-Pro Analyzer
4.0 software program and presented as the indicated ratio compared
to the control expression level (expression level of the control
was regarded as 1-fold). The protein expression levels of each
enzyme were normalized to those of β-actin.
Yo-Pro1 staining
To assess ototoxicity, 5-day-old zebrafish were
treated with GM added directly to the EM. Twenty embryos were used
for each treatment. Additionally, at 5 days post-fertilization,
zebrafish larvae were exposed to either 50 µM GM, 10
µM fenofibrate and 50 µM GM, 10 µM
fenofibrate, or SnPP, fenofibrate and GM. The hair cell lateral
line neuromasts were labeled with 2.5 µM Yo-Pro1 (Molecular
Probes, Eugene, OR, USA) for 30 min, followed by washing 3 times.
The zebrafish were then rinsed 3 times (5 min/wash) with EM and
anesthetized with 8 µg/ml MS-222 (Sigma-Aldrich). The
zebrafish were mounted with methylcellulose on a depression slide
for observation under a fluorescence microscope.
Auditory brainstem response
Auditory brainstem response (ABR) was measured using
System 3 hardware and software (Tucker Davis Technologies, Alachua,
FL, USA), with 1,000 stimulus repetitions/record. Three-week-old SD
rats were anesthetized using a mixture of ketamine (40 mg/kg) and
xylazine (10 mg/kg) and kept warm with a heating pad during ABR
recording. A subdermal needle electrode was inserted at the vertex,
while ground and reference electrodes were inserted subdermally
into the loose skin beneath the pinnae of opposite ears. Tone
bursts of 4-msec durations and a rise-fall time of 1 msec at
frequencies of 4, 8, 16 and 32 kHz were presented to the right ear
and left ear through an insert speculum in the external auditory
meatus. Sound intensity was varied at 5-dB intervals near the
threshold. Judgment of the threshold was made off-line, based on
the ABR records, by two independent, experimentally blinded
observers.
Cochlea immunohistochemical analysis
For immunohistochemical analysis, a Dako
immunohistochemistry kit (LSAB Universal K680; Dako, Carpenteria,
CA, USA) was used according to the manufacturer's instructions. The
removed temporal bone was fixed in 4% paraformaldehyde for 16 h,
and then decalcified with 10% EDTA in PBS for 2 weeks, dehydrated,
and embedded in paraffin wax. Sections (4-µm-thick) were
deparaffinized in xylene and rehydrated in increasing ethanol
concentrations. Endogenous peroxidase was blocked with 3% hydrogen
peroxide for 5 min at room temperature following PBS washing.
Non-specific binding was blocked with 1% bovine serum albumin (BSA)
for 1 h. Subsequently, each antibody was added to the slides and
incubated for 1 h. Following repeated washes with PBS, the sections
were incubated with a biotinylated secondary antibody in the kit
for 1 h and covered for 30 min with streptavidin-peroxidase.
Finally, the sections were stained in freshly prepared substrate
solution (3 mg of 3-amino-9-ethylcarbazole in 10 ml of sodium
acetate buffer pH 4.9, 500 µl of dimethylformamide, 0.03%
hydrogen peroxide) for 10 min. The nuclei of immunostained cells
were counterstained with Mayer's hematoxylin (Sigma-Aldrich).
Statistical analysis
Each experiment was performed independently at least
3 times, and all values represent the means ± standard deviation
(SD) of triplicate experiments. One-way analysis of variance
(ANOVA) was used to analyze the statistical significance of the
results. Reported error bars are one SD from the mean. p-values
≤0.005 were considered to indicate statistically significant
differences.
Results
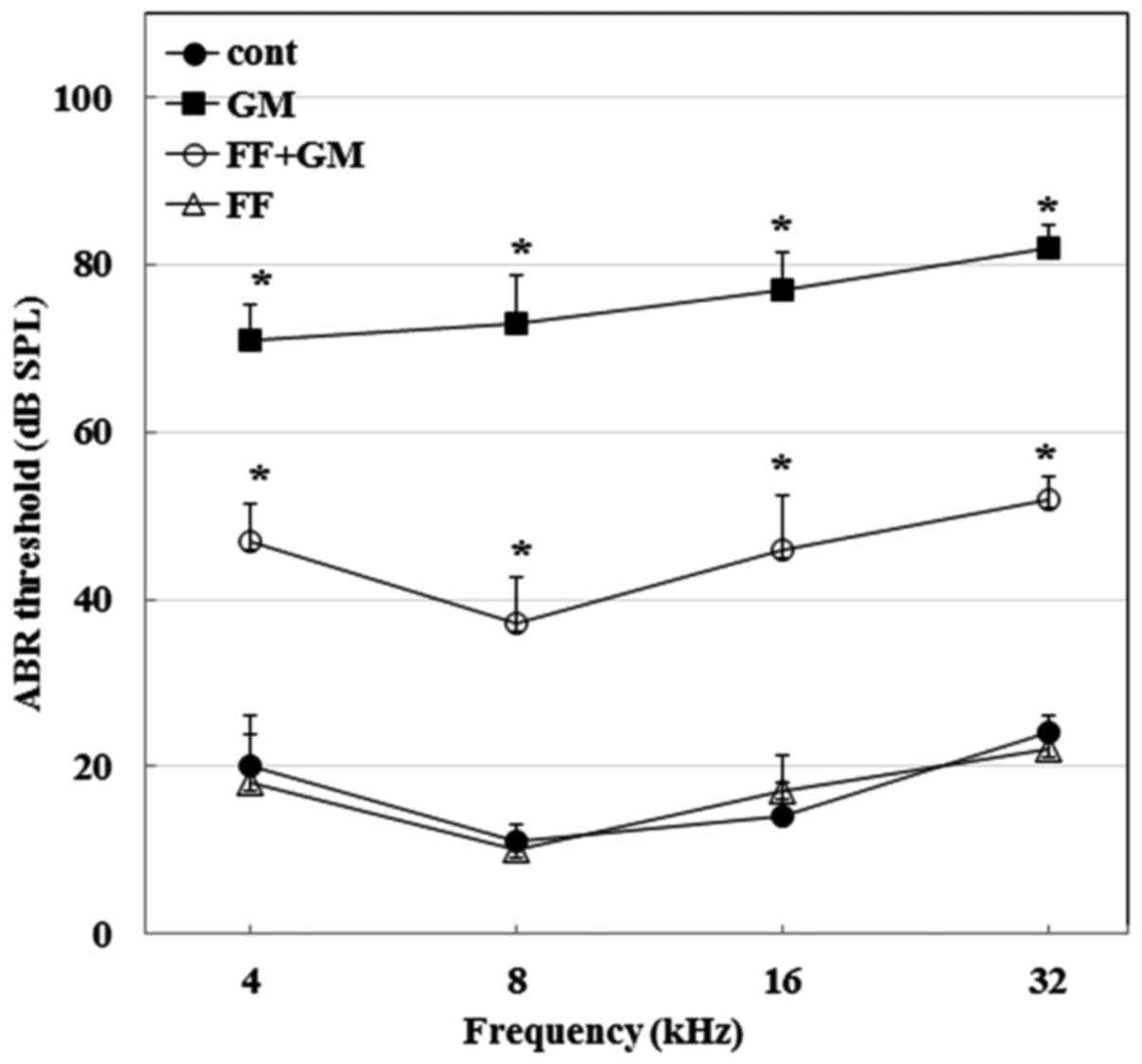
Fenofibrate prevents GM-induced hearing
loss in rats
To examine the preventive effects of fenofibrate
against GM-induced hearing loss in vivo, we first compared
the ABR thresholds of four different treatment groups: saline group
(control), intraperitoneally injected with 200 mg/kg GM for 4 days
(GM group), 100 mg/kg fenofibrate for 10 days followed by GM (GM +
fenofibrate group) and fenofibrate alone (FF group). As shown in
Fig. 1, at the end of drug
treatment on day 14, the average ABR thresholds at all frequencies
in the GM group were significantly higher than those in the control
group (p≤0.0005, n=10), confirming that GM induces hearing loss in
rats. However, the administration of fenofibrate (GM + fenofibrate
group) significantly reduced tone burst ABR as compared to GM
alone. Fenofibrate alone did not affect hearing sensitivity.
Therefore, these results indicate that GM causes hearing loss,
which may be prevented by the use of fenofibrate in rats.
Pre-treatment with fenofibrate protects
sensory hair cells of rat cochlear explants from GM-induced
toxicity
Since GM induces hearing loss by disrupting sensory
hair cells, we then investigated whether fenofibrate protects
sensory hair cells from GM-induced toxicity in an organotypic
culture of cochlear explants isolated from SD rats at post-natal
day 3. In the control group, sensory hair cells visualized by
TRITC-conjugated phalloidin appeared as three rows of outer hair
cells and a single row of inner hair cells (Fig. 2A). However, exposure to GM
resulted in the destruction of stereocilia bundles and induced a
disordered array of hair cells. By contrast, pre-treatment with
fenofibrate protected the sensory hair cells from the damaging
effects of GM, displaying a well-preserved pattern of layers in
outer hair cells and inner hair cells. Fenofibrate alone did not
induce damage to the cochlear hair cells. We also quantified the
number of cells that survived after each set of drug treatment in
the cochlear explants. We observed a significant reduction in the
survival rate (%) of sensory hair cells following exposure to GM
(Fig. 2B). However, the survival
rate of sensory hair cells in the rat cochlear explants pre-treated
with fenofibrate was significantly higher than that in the
GM-exposed explants. Taken together, our results indicate that
fenofibrate protects auditory hair cells from GM-induced cell
death.
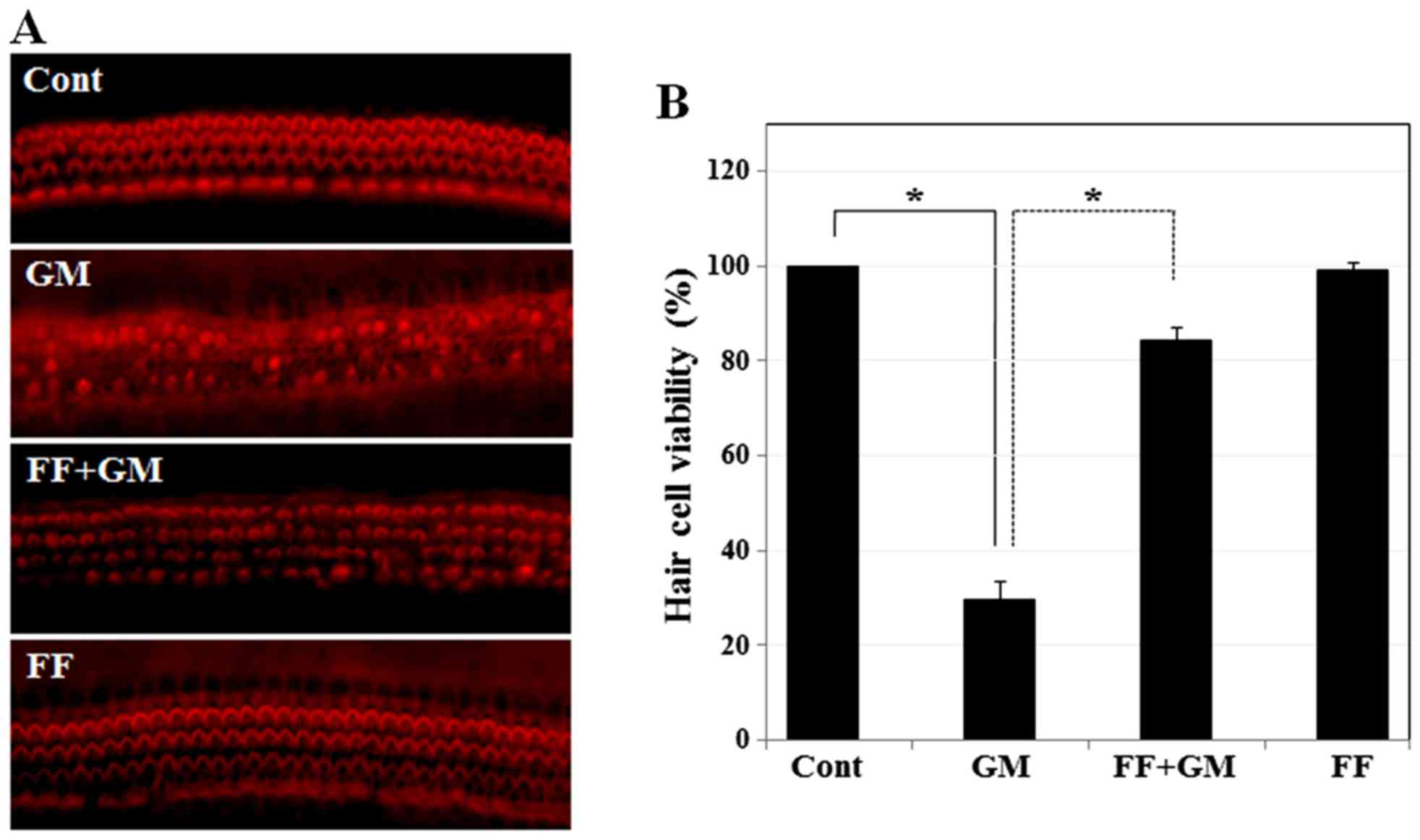 | Figure 2Effect of fenofibrate on GM-induced
hair cell death in rat cochlear explants. (A) Hair cells were
stained with phalloidin-TRITC and observed under a fluorescence
microscope. Cochlear explants were treated with medium alone, GM
(300 µM) for 24 h (29.6±3.78, p≤0.00002), fenofibrate (100
µM) pre-treated for 4 h and then co-treated with GM (300
µM) for 24 h (84.4±2.44, p≤0.0005), or fenofibrate (100
µM) only for 28 h (99.3±1.33, NS). (B) Quantitative analysis
of survival of the sensory hair cells. Histogram shows the mean
viability of the sensory hair cells. The data represent the means ±
SD of 3 independent experiments. *p<0.001 by one-way
ANOVA, compared with the control or GM-treated group. GM,
gentamicin; FF, fenofibrate; TRITC, phalloidin-tetramethylrhodamine
isothiocyanate. |
Fenofibrate reduces GM-induced oxidative
stress in rat cochlear explants
Since the overproduction of ROS is a major cause of
GM-induced sensory hair cell death (34–37), the protective effects of
fenofibrate may be mediated by reducing the ROS levels induced by
GM. We thus quantified the ROS levels in cochlear explants by
staining with DCFH-DA (DCF), a fluorescent probe for measuring
intracellular ROS production. As shown in Fig. 3, GM induced a strong DCF signal,
whereas pre-treatment with fenofibrate significantly reduced the
DCF intensity to a level almost comparable to that of the control,
indicating that fenofibrate prevents GM-induced oxidative stress.
Fenofibrate alone slightly decreased the ROS levels, suggesting
that the drug itself has an antioxidant effect.
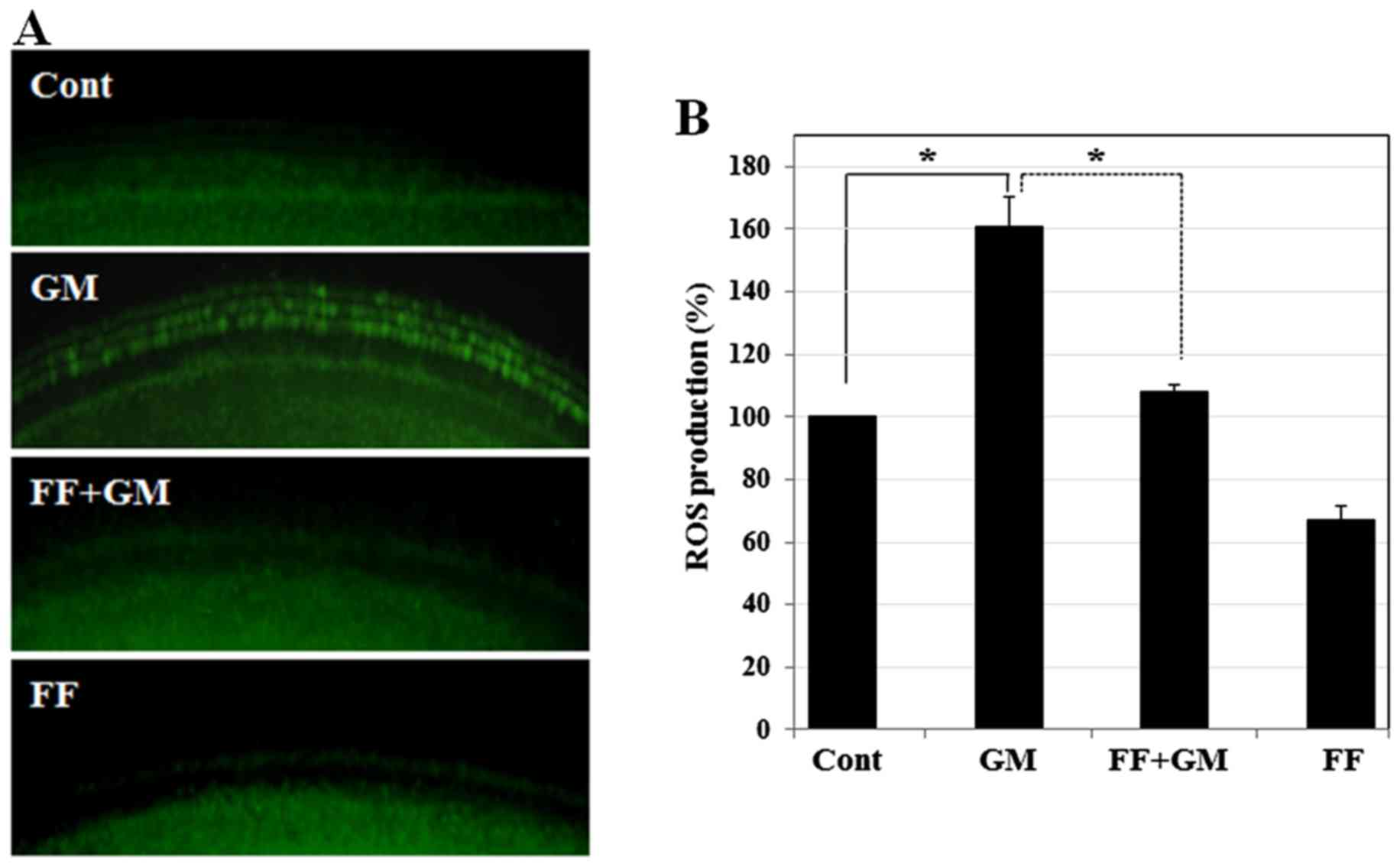 | Figure 3Effect of fenofibrate on GM-induced
oxidative stress in rat cochlear explants. (A) Intracellular ROS
levels in the sensory hair cells were monitored using DCFH-DA under
a fluorescence microscope. Cochlear explants were treated with
medium alone, GM (300 µM) for 12 h (161±9.2, p≤0.0004),
fenofibrate (100 µM) pre-treated for 4 h and then co-treated
with GM (300 µM) for 12 h (108±2.3, p≤0.0004), and
fenofibrate (100 µM) only for 16 h (67±4.3, p≤0.0002). (B)
Intracellular ROS levels in the sensory hair cells were determined
using DCFH-DA under a microplate reader. The histogram shows the
mean ROS production. *p<0.001 by one-way ANOVA,
compared with the control or GM-treated group. GM, gentamicin; FF,
fenofibrate; ROS, reactive oxygen species; DCFH-DA,
2′,7′-dichlorofluorescein diacetate. |
Fenofibrate increases the expression of
antioxidant enzymes in rat cochlear explants
Previously, it has been shown that the activation of
PPAR-α by fenofibrate exerts a protective effect against oxidative
stress in the kidneys (38). In
addition, PPAR-α is known to regulate its own expression (39). To examine whether the
otoprotective effects of fenofibrate are mediated by the regulation
of PPAR-α and antioxidant enzymes, we measured the expression
levels of PPAR-α and antioxidant proteins. Consistent with the
protective effect of fenofibrate previously observed in the
kidneys, pre-treatment with fenofibrate restored the expression of
catalase, SOD-1 and PPAR-α, the levels of which were all
significantly reduced by GM (Fig.
4A). However, the expression of HO-1 seemed to be regulated
differently from the other antioxidant enzymes. In particular, we
found that GM significantly increased the expression of HO-1, which
was barely detectable in the controls. In addition, either
pre-treatment with fenofibrate or fenofibrate alone further induced
the level of HO-1 expression as compared to the GM group,
suggesting a potential role of HO-1 in the otoprotective effects of
fenofibrate.
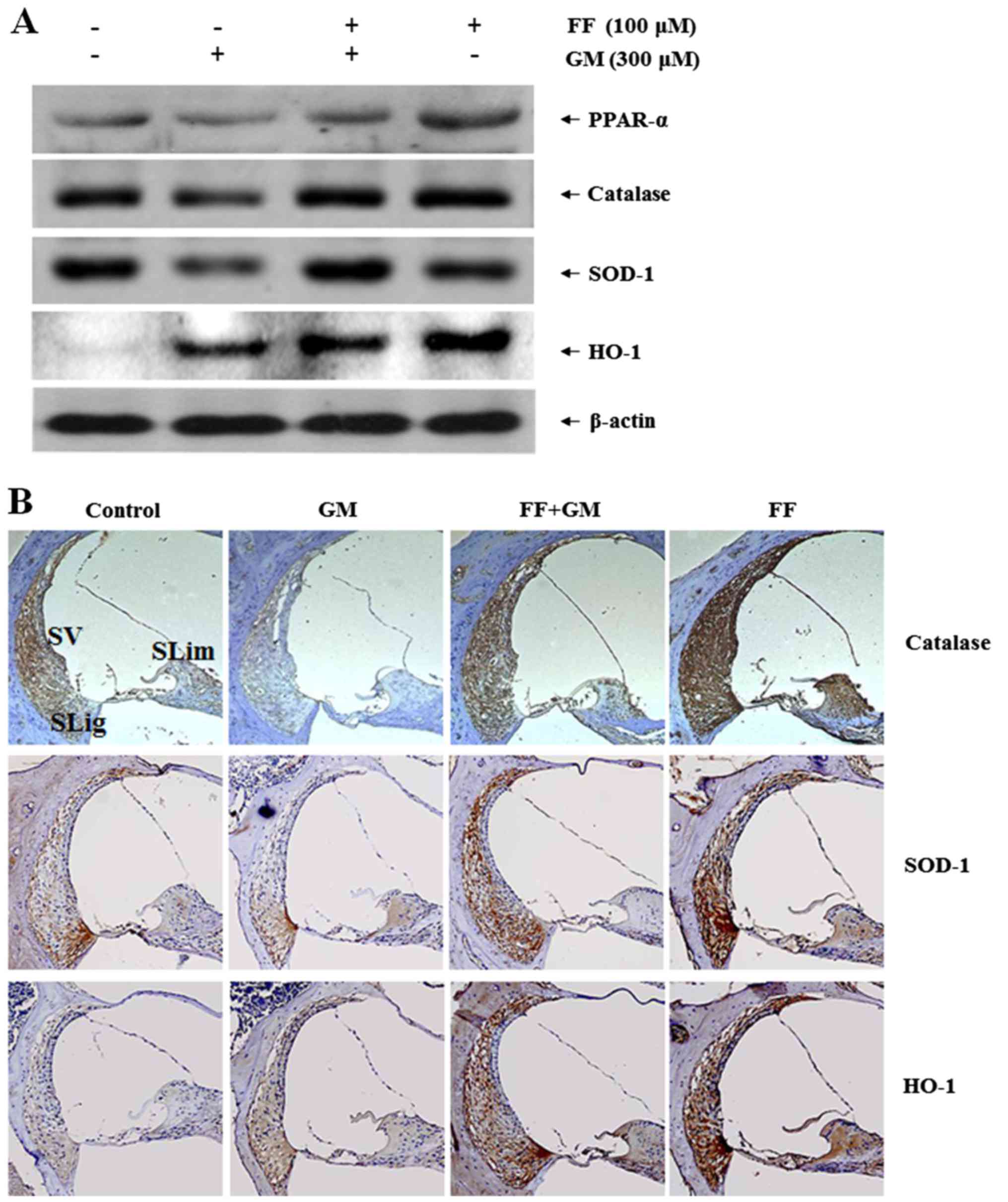 | Figure 4Effect of fenofibrate on the
expression of antioxidant enzymes in rat cochlea. (A) Cochlear
explants were treated with medium alone, GM (300 µM) for 18
h, fenofibrate (100 µM) pre-treated for 4 h and then
co-treated with GM (300 µM) for 18 h, and fenofibrate (100
µM) only for 22 h. The organ of Corti was collected and
proteins were extracted. Total cell lysates were separated by 10%
SDS-PAGE to detect PPAR-α, catalase, SOD-1 and HO-1 proteins. The
protein and mRNA levels of β-actin were determined as controls. (B)
The inner ears from SD rat [saline group (control),
intraperitoneally injected with 200 mg/kg GM for 4 days (GM), 100
mg/kg fenofibrate for 10 days followed by GM (FF + GM) and
fenofibrate alone (FF)] were removed and embedded in paraffin.
Next, 4-µm-thick sections were prepared. For
immunohistochemistry studies, a commercial kit (LSAB Universal
K680) was used to detect the expression levels of catalase, SOD-1
and HO-1 in the cochlear duct regions. GM, gentamicin; FF,
fenofibrate; PPAR, peroxisome proliferator-activated receptor;
SOD-1, superoxide dismutase-1; HO-1, heme oxygenase-1; SV, stria
vascularis; SLig, spiral limbus; SLim, spiral ligament. |
To confirm the fenofibrate-dependent induction of
antioxidant enzymes, we performed immunohistochemistry using the
rat cochlear samples. In the controls, the expression of both
catalase and SOD-1 was detectable throughout the cochlea, including
the spiral ligament, stria vascularis and spiral limbus, while the
expression of HO-1 was barely detected (Fig. 4B). GM significantly impaired the
expression of catalase and SOD-1, while it increased HO-1
expression. However, pretreatment with fenofibrate restored the
expression of catalase and SOD-1 to a level similar to that of the
control, and further increased the expression of HO-1, as compared
to that in the GM group. Fenofibrate alone also induced the
expression of the antioxidant enzymes to a level similar to, or
even higher than that observed in the fenofibrate- and GM-treated
group. These results were consistent with the results shown in
Fig. 4A, and strongly suggest
that fenofibrate prevents GM-induced hair cell death by
upregulating the expression of antioxidant enzymes in the rat
cochlear explants.
HO-1 inhibitor abolishes the protective
effects of fenofibrate on hair cells
Since the activities of catalase and SOD-1 have well
been documented for mediating the PPAR-α-dependent protective
effects (32,40), we examined whether the strong
induction of HO-1 by fenofibrate is essential for hair cell
survival. Cochlear explants were treated with SnPPIX, a well-known
HO-1 inhibitor, prior to treatment with GM and fenofibrate (SnPPIX
+ FF + GM). As shown in Fig. 5A,
the disruption of sterocilia bundles induced by GM was restored by
pre-treatment with fenofibrate, whereas only a moderate recovery
was observed in the SnPPIX + FF + GM group. Quantitatively, the
number of sensory hair cells in the group pre-treated with
fenofibrate was significantly increased in the rat cochlear
explants compared to GM group (Fig.
6B). However, the inhibition of HO-1 (SnPPIX + FF + GM)
significantly decreased hair cell viability as compared to the
pre-treated with fenofibrate and exposed to GM. These results
strongly suggest that HO-1 plays an indispensable role in the
fenofibrate-mediated protection of sensory hair cells against
GM-induced damage.
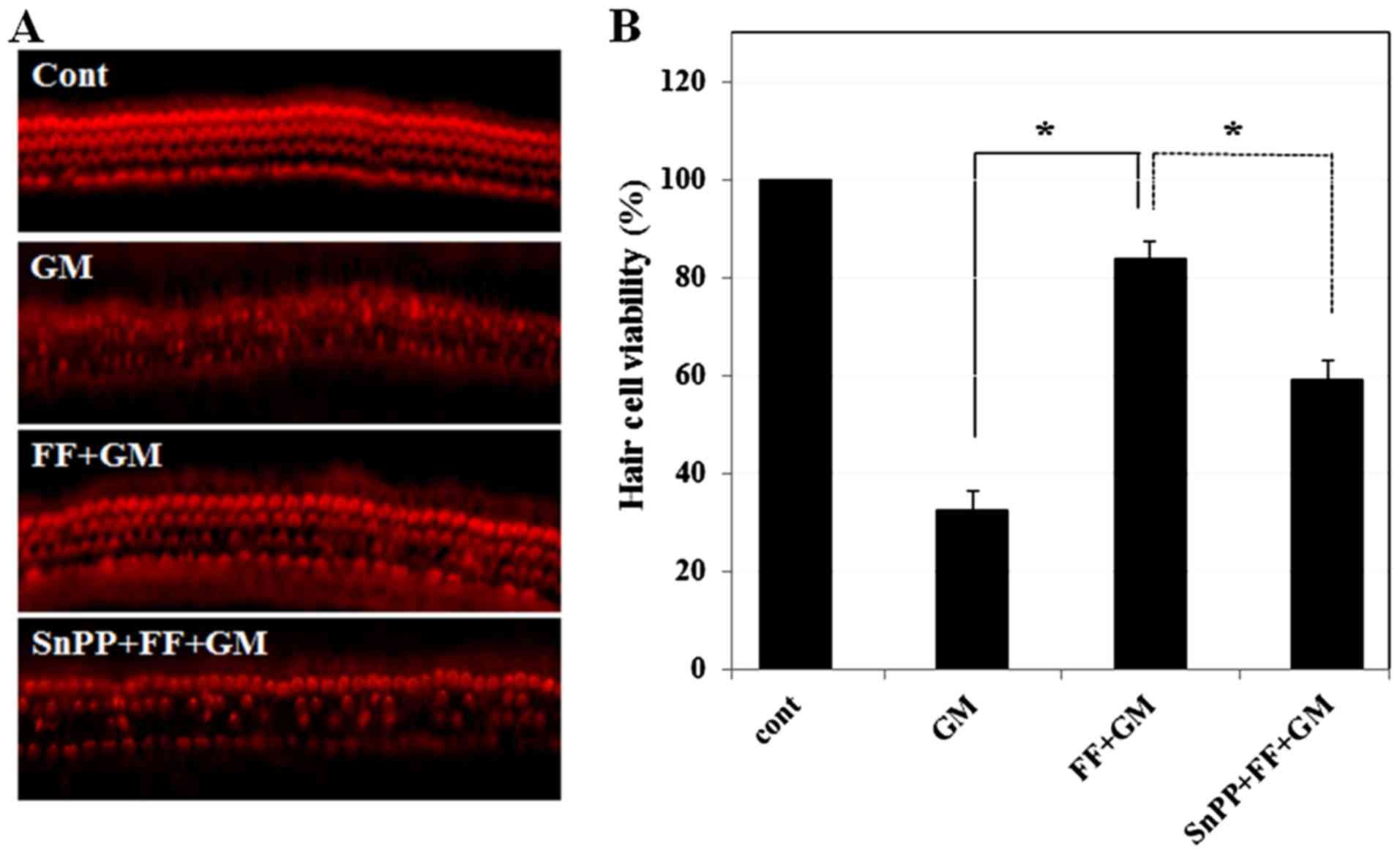 | Figure 5Effect of SnPPIX, an HO-1 inhibitor,
on fenofibrate-mediated protection of the sensory hair cells. (A)
Sensory hair cells were stained with phalloidin-TRITC and observed
under a fluorescence microscope. Cochlear explants were treated
with medium alone, GM (300 µM) for 24 h (32.7±3.78,
p≤0.00001), pre-treated with fenofibrate (100 µM) for 4 h
and then co-treated with GM (300 µM) for 24 h (83.8±3.78,
p≤0.00006), and pre-treated with SnPPIX (10 µM) and
fenofibrate (100 µM) for 4 h and then further incubated with
GM (300 µM) for 24 h (59.3±3.78, p≤0.001). (B) Quantitative
analysis of the survival of sensory hair cells. Histogram
represents mean hair cell viability. *p<0.001 by
one-way ANOVA, compared to GM-treated group or SnPPIX + fenofibrate
+ GM-treated group. The survival rate (%) of sensory hair cells was
calculated using the count method and represented by a bar graph.
The number of hair cells was 45±0.0 in control, 14.7±1.7 in GM,
37.7±1.7 in fenofibrate + GM and 26.7±1.7 in SnPPIX + fenofibrate +
GM. Note that 10 µM SnPPIX alone did not induce
cytotoxicity. GM, gentamicin; FF, fenofibrate; SnPPIX, tin
protoporphyrin IX; HO-1, heme oxygenase-1; TRITC,
phalloidin-tetramethylrhodamine isothiocyanate. |
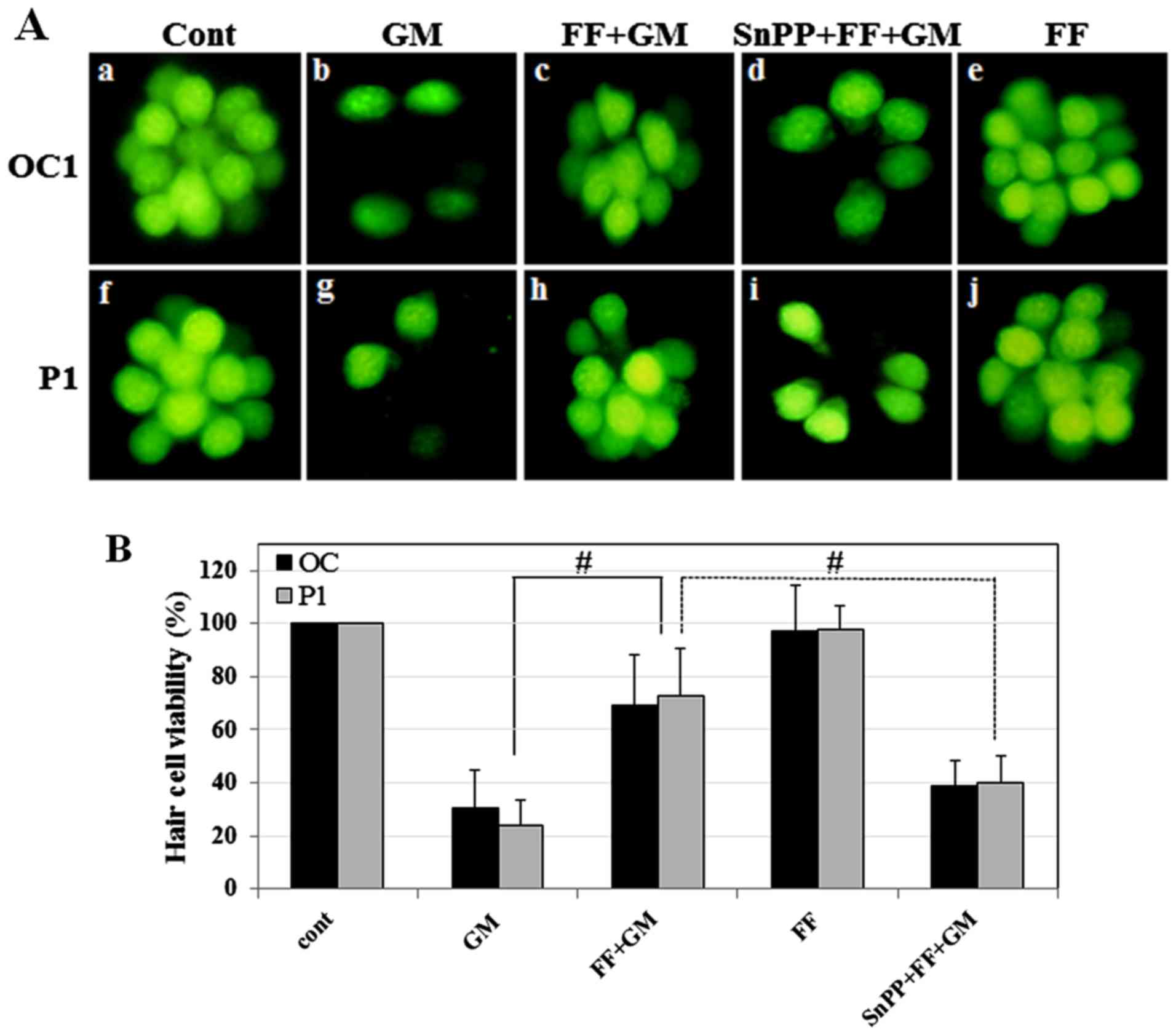 | Figure 6Effect of SnPPIX, a HO-1 inhibitor,
on fenofibrate-mediated protection of zebrafish neuromasts. (A) The
neuromasts of zebrafish were (50 µM) for 1 h (GM, panels b
and g), pre-treated with fenofibrate (10 µM) for 0.5 h and
then co-treated with GM (500 µM) for 1 h (FF + GM, panels c
and h), pre-treated with SnPPIX (10 µM) and fenofibrate (10
µM) for 0.5 h and then co-treated with GM (50 µM) for
1 h (SnPP + FF + GM, panels d and i), and fenofibrate alone (FF,
panels e and j). One of the occipital neuromasts (OC1) is shown in
panels a-e, and a posterior neuromast is (P1) shown in panels f-j.
(B) Quantitative analysis of neuromast survival. Histogram
represents the mean viability of neuromasts. Zebrafish were treated
with medium alone (cont), GM (50 µM) for 1 h (OC and P1:
30.7±14.17, p≤ 0.001 and 24.2±9.29, p≤0.0002, respectively),
pre-treated with fenofibrate (10 µM) for 0.5 h and then
co-treated with GM (500 µM) for 1 h (OC and P1: 69.3±19.2,
p≤0.003 and 72.5±17.86, p≤0.0006, respectively), pre-treated with
SnPPIX (10 µM) and fenofibrate (10 µM) for 0.5 h and
then co-treated with GM (50 µM) for 1 h (OC and P1:
38.6±10.00, p≤0.001 and 40.0±10.00, p≤0.002, respectively), and
fenofibrate alone (FF). #p<0.005 by one-way ANOVA,
compared to GM or SnPPIX + FF + GM. GM, gentamicin; FF,
fenofibrate; SnPPIX, tin protoporphyrin IX; HO-1, heme
oxygenase-1. |
Fenofibrate protects against GM-induced
hair cell death in zebrafish neuromasts
The lateral line of the zebrafish consists of
neuromasts aligned along the animal anteroposterior axis (41). Each neuromast contains a group of
hair cells that functions to detect water currents via movement of
their stereocilia (42) and is
used as an alternative for testing ototoxic drugs due to functional
and morphological similarities to mammalian hair cells (41). Thus, in the present study, we
examined whether the protective effects of fenofibrate are also
observed in this model system. Five-day-old zebrafish larvae were
pre-treated with 10 µM fenofibrate for 0.5 h and then
exposed to 50 µM GM for 1 h. As shown in Fig. 6A, 14 hair cells of the occipital 1
(OC1) and 12 of the posterior 1 (P1) neuromasts were clearly
visible in the controls. However, we found that the administration
of GM alone significantly decreased the number of neuromast hair
cells [OC1, 4.2±2.1 (30±14.17%); P1, 2.9±1.7 (24.2 ±9.29%)],
consistent with a previous study in which GM alone was toxic to
hair cells in the lateral line system (43). By contrast, pre-treatment with
fenofibrate significantly increased the number of neuromast hair
cells [OC1, 10±3.3 (69.3±19.2%); P1, 8.9±2.8 (72.5±17.86%)],
indicating at least a partial rescue. Fenofibrate alone did not
induce changes to the number of neuromast hair cells. Notably, we
found that pre-treatment with SnPPIX and fenofibrate followed by GM
(SnPPIX + FF + GM group) significantly decreased the number of
neuromast hair cells [OC1, 5.4±1.4 (38.6±10.00%); P1, 4.8±1.2
(40.0±10.00%)], which was similar to the effect observed with GM
alone. The survival rate of the neuromast hair cells confirmed that
HO-1 activity was required for the protective effects of
fenofibrate on sensory hair cells in the zebrafish lateral line
(Fig. 6B).
Discussion
GM is one of the most widely used antibiotics.
However, its use is restricted due to ototoxicity, including
hearing loss and vestibular dysfunction (44,45). The ototoxicity of GM is attributed
to the selective loss and/or death of sensory hair cells in the
inner ear. Hair cell loss in the cochlea results in acquired
permanent hearing loss which, to date, is incurable (46). Therefore, it is critical to
identify agents that provide protective interventions for
aminoglycoside-induced ototoxicity. Several compounds have been
introduced as preventive or protective agents against GM-induced
ototoxicity (11,47,48). We thus hypothesized that
fenofibrate may be a putative preventive therapeutic agent to
protect against GM-induced ototoxicity.
GM-induced ototoxicity
Aminoglycoside causes hair cell damage and thus
induces prevalent and irreversible ototoxicity (2,3,49).
In the present study, we found that GM significantly decreased hair
cell numbers in the organ of Corti explants. In addition, ABR
experiments revealed that GM induced a significant increase in the
hearing threshold in rats. By contrast, fenofibrate prevented hair
cell death induced by GM in cochlear explant tissues and
significantly attenuated the threshold shifts caused by GM in
rats.
Protective effects of fenofibrate are
mediated by antioxidant enzymes, including HO-1
Fenofibrate, a PPAR-α activator, belongs to the
fibrate drug class. It is mainly used to reduce cholesterol levels
in patients at risk of cardiovascular disease (50,51). PPAR-α is one of the three subtypes
of PPARs, which have been implicated in several physiological
processes, such as the regulation of lipoproteins, lipid metabolism
and glucose homeostasis (31).
PPARs are ligand-activated transcription factors that belong to the
nuclear receptor superfamily (24). Upon activation by their ligands,
PPARs regulate gene transcription by binding to PPREs in the
promoter regions of target genes as a heterodimer with the retinoid
X receptor (52). Previous
studies have indicated that PPAR-α activators reduce inflammation
by decreasing cytokines, adhesion molecules and nitric oxide
synthase 2, and also reduce oxidative stress by increasing
antioxidant enzymes in different experimental models (25–30). Furthermore, a PPRE has been
identified in the promoter regions of catalase and SOD-1, which are
key enzymes involved in reducing ROS production (32). It has been suggested that GM
induces the apoptosis of hair cells of the inner ear (53). Since GM-induced cell death is
largely mediated by ROS (5–9),
several agents that scavenge ROS or block their formation have been
proposed to protect the inner ear (10–14). In this study, we found that
fenofibrate significantly induced catalase and SOD-1 expression, as
shown by western blot analysis and immunohistochemistry, which is
consistent with the findings of previous studies (28,40,54). Notably, we found the most
prominent increase in response to fenofibrate to be the level of
HO-1, whose expression was also induced by GM alone.
HO-1 is a rate-limiting enzyme involved in heme
catabolism, which eventually leads to the generation of bilirubin,
free iron and carbon monoxide (55). Various oxidative agents induce
HO-1 as a stress-responsive protein (20), and several groups have recently
reported the versatile functions of HO-1, which protects cells from
various oxidative stresses (21–23). A previous study demonstrated the
induction of HO-1 expression by PPAR-α and PPAR-γ ligands in
cultured vascular cells (56),
suggesting that HO-1 may be directly regulated by PPAR. Our
previous studies demonstrated a protective role of HO-1 against
cisplatin-induced ototoxicity (23,57). In this study, we found that the
expression of HO-1 was significantly increased by GM, possibly due
to GM-induced oxidative stress (20,57). We also found that the expression
of HO-1 was further increased by fenofibrate. Importantly, we
demonstrated that SnPPIX, a well-known HO-1 inhibitor,
significantly reduced the protective effects of fenofibrate against
GM-induced hair cell death in the organ of Corti of adult rats and
zebrafish neuromasts, indicating that HO-1 is essential for the
protective effects of fenofibrate against GM-induced
ototoxicity.
Collectively, our data suggest that the
otoprotective role of fenofibrate is mediated by the induction of
the expression of antioxidant enzymes, including HO-1. Furthermore,
our results strongly suggest that fenofibrate may be used in the
development of therapeutic approaches aimed at preventing the
extent of acquired hearing loss due to aminoglycoside
treatment.
Acknowledgments
This study was supported by National Research
Foundation of Korea (NRF) grants funded by the Korea government
(nos. 2014M3A9D8034463 and 2011-0030130) and by a grant of the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute (KHIDI), funded by the Ministry of
Health and Welfare, Republic of Korea (grant no. HI14C0384).
References
|
1
|
Heller J: Effect of some simple manoeuvres
on the course of acute renal failure after gentamycin treatment in
rats. Int Urol Nephrol. 16:243–251. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jao RL and Jackson GG: Gentamicin sulfate,
new antibiotic against Gram-negative bacilli. Laboratory,
pharmacological, and clinical evaluation. JAMA. 189:817–822. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Assimakopoulos D and Patrikakos G:
Treatment of Ménière's disease by intratympanic gentamicin
application. J Laryngol Otol. 117:10–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Y, Huang WG, Zha DJ, Qiu JH, Wang JL,
Sha SH and Schacht J: Aspirin attenuates gentamicin ototoxicity:
from the laboratory to the clinic. Hear Res. 226:178–182. 2007.
View Article : Google Scholar
|
|
5
|
Heinrich UR, Helling K, Sifferath M,
Brieger J, Li H, Schmidtmann I and Mann WJ: Gentamicin increases
nitric oxide production and induces hearing loss in guinea pigs.
Laryngoscope. 118:1438–1442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
López-González MA, Lucas M, Delgado F and
Diaz P: The production of free oxygen radicals and nitric oxide in
the rat cochlea. Neurochem Int. 33:55–59. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sha SH and Schacht J: Stimulation of free
radical formation by aminoglycoside antibiotics. Hear Res.
128:112–118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takumida M, Popa R and Anniko M: Free
radicals in the guinea pig inner ear following gentamicin exposure.
ORL J Otorhinolaryngol Relat Spec. 61:63–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu WJ, Sha SH and Schacht J: Recent
advances in understanding aminoglycoside ototoxicity and its
prevention. Audiol Neurootol. 7:171–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung HW, Chang SO, Kim CS, Rhee CS and Lim
DH: Effects of Ginkgo biloba extract on the cochlear damage induced
by local gentamicin installation in guinea pigs. J Korean Med Sci.
13:525–528. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sha SH and Schacht J: Antioxidants
attenuate gentamicin-induced free radical formation in vitro and
ototoxicity in vivo: D-methionine is a potential protectant. Hear
Res. 142:34–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McFadden SL, Ding D, Salvemini D and Salvi
RJ: M40403, a superoxide dismutase mimetic, protects cochlear hair
cells from gentamicin, but not cisplatin toxicity. Toxicol Appl
Pharmacol. 186:46–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang AM, Sha SH, Lesniak W and Schacht J:
Tanshinone (Salviae miltiorrhizae extract) preparations attenuate
aminoglycoside-induced free radical formation in vitro and
ototoxicity in vivo. Antimicrob Agents Chemother. 47:1836–1841.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fetoni AR, Sergi B, Ferraresi A, Paludetti
G and Troiani D: alpha-Tocopherol protective effects on gentamicin
ototoxicity: an experimental study. Int J Audiol. 43:166–171. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matés JM: Effects of antioxidant enzymes
in the molecular control of reactive oxygen species toxicology.
Toxicology. 153:83–104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan PH, Yang GY, Chen SF, Carlson E and
Epstein CJ: Cold-induced brain edema and infarction are reduced in
transgenic mice overexpressing CuZn-superoxide dismutase. Ann
Neurol. 29:482–486. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinouchi H, Epstein CJ, Mizui T, Carlson
E, Chen SF and Chan PH: Attenuation of focal cerebral ischemic
injury in transgenic mice overexpressing CuZn superoxide dismutase.
Proc Natl Acad Sci USA. 88:11158–11162. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White CW, Avraham KB, Shanley PF and
Groner Y: Transgenic mice with expression of elevated levels of
copper-zinc superoxide dismutase in the lungs are resistant to
pulmonary oxygen toxicity. J Clin Invest. 87:2162–2168. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mikawa S, Kinouchi H, Kamii H, Gobbel GT,
Chen SF, Carlson E, Epstein CJ and Chan PH: Attenuation of acute
and chronic damage following traumatic brain injury in copper,
zinc-superoxide dismutase transgenic mice. J Neurosurg. 85:885–891.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alam J and Cook JL: Transcriptional
regulation of the heme oxygenase-1 gene via the stress response
element pathway. Curr Pharm Des. 9:2499–2511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He X, Lin GX, Chen MG, Zhang JX and Ma Q:
Protection against chromium (VI)-induced oxidative stress and
apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting
the nuclear Nrf2/Keap1 association. Toxicol Sci. 98:298–309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li HY, Zhong YF, Wu SY and Shi N: NF-E2
related factor 2 activation and heme oxygenase-1 induction by
tert-butylhydroquinone protect against deltamethrin-mediated
oxidative stress in PC12 cells. Chem Res Toxicol. 20:1242–1251.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SJ, Park C, Han AL, Youn MJ, Lee JH,
Kim Y, Kim ES, Kim HJ, Kim JK, Lee HK, et al: Ebselen attenuates
cisplatin-induced ROS generation through Nrf2 activation in
auditory cells. Hear Res. 251:70–82. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sher T, Yi HF, McBride OW and Gonzalez FJ:
cDNA cloning, chromosomal mapping, and functional characterization
of the human peroxisome proliferator activated receptor.
Biochemistry. 32:5598–5604. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Staels B, Koenig W, Habib A, Merval R,
Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC,
et al: Activation of human aortic smooth-muscle cells is inhibited
by PPARalpha but not by PPARgamma activators. Nature. 393:790–793.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoue I, Goto S, Matsunaga T, Nakajima T,
Awata T, Hokari S, Komoda T and Katayama S: The ligands/activators
for peroxisome proliferator-activated receptor alpha (PPARalpha)
and PPARgamma increase Cu2+, Zn2+-superoxide
dismutase and decrease P22p hox message expressions in primary
endothelial cells. Metabolism. 50:3–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inoue H, Jiang XF, Katayama T, Osada S,
Umesono K and Namura S: Brain protection by resveratrol and
fenofibrate against stroke requires peroxisome
proliferator-activated receptor alpha in mice. Neurosci Lett.
352:203–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toyama T, Nakamura H, Harano Y, Yamauchi
N, Morita A, Kirishima T, Minami M, Itoh Y and Okanoue T: PPARalpha
ligands activate antioxidant enzymes and suppress hepatic fibrosis
in rats. Biochem Biophys Res Commun. 324:697–704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lo Verme J, Fu J, Astarita G, La Rana G,
Russo R, Calignano A and Piomelli D: The nuclear receptor
peroxisome proliferator-activated receptor-alpha mediates the
anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol.
67:15–19. 2005. View Article : Google Scholar
|
|
30
|
Bordet R, Ouk T, Petrault O, Gelé P,
Gautier S, Laprais M, Deplanque D, Duriez P, Staels B, Fruchart JC,
et al: PPAR: a new pharmacological target for neuroprotection in
stroke and neurodegenerative diseases. Biochem Soc Trans.
34:1341–1346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemberger T, Desvergne B and Wahli W:
Peroxisome proliferator-activated receptors: a nuclear receptor
signaling pathway in lipid physiology. Annu Rev Cell Dev Biol.
12:335–363. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Girnun GD, Domann FE, Moore SA and Robbins
ME: Identification of a functional peroxisome
proliferator-activated receptor response element in the rat
catalase promoter. Mol Endocrinol. 16:2793–2801. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hou X, Shen YH, Li C, Wang F, Zhang C, Bu
P and Zhang Y: PPARalpha agonist fenofibrate protects the kidney
from hypertensive injury in spontaneously hypertensive rats via
inhibition of oxidative stress and MAPK activity. Biochem Biophys
Res Commun. 394:653–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choung YH, Taura A, Pak K, Choi SJ, Masuda
M and Ryan AF: Generation of highly-reactive oxygen species is
closely related to hair cell damage in rat organ of Corti treated
with gentamicin. Neuroscience. 161:214–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clerici WJ, Hensley K, DiMartino DL and
Butterfield DA: Direct detection of ototoxicant-induced reactive
oxygen species generation in cochlear explants. Hear Res.
98:116–124. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hirose K, Hockenbery DM and Rubel EW:
Reactive oxygen species in chick hair cells after gentamicin
exposure in vitro. Hear Res. 104:1–14. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sha SH and Schacht J: Formation of
reactive oxygen species following bioactivation of gentamicin. Free
Radic Biol Med. 26:341–347. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tanaka Y, Kume S, Araki S, Isshiki K,
Chin-Kanasaki M, Sakaguchi M, Sugimoto T, Koya D, Haneda M,
Kashiwagi A, et al: Fenofibrate, a PPARα agonist, has
renoprotective effects in mice by enhancing renal lipolysis. Kidney
Int. 79:871–882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Hannafon BN, Zhou J and Ding WQ:
Clofibrate induces heme oxygenase 1 expression through a
PPARα-independent mechanism in human cancer cells. Cell Physiol
Biochem. 32:1255–1264. 2013. View Article : Google Scholar
|
|
40
|
Yoo HY, Chang MS and Rho HM: Induction of
the rat Cu/Zn superoxide dismutase gene through the peroxisome
proliferator-responsive element by arachidonic acid. Gene.
234:87–91. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chiu LL, Cunningham LL, Raible DW, Rubel
EW and Ou HC: Using the zebrafish lateral line to screen for
ototoxicity. J Assoc Res Otolaryngol. 9:178–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Montgomery J, Carton G, Voigt R, Baker C
and Diebel C: Sensory processing of water currents by fishes.
Philos Trans R Soc Lond B Biol Sci. 355:1325–1327. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Van Trump WJ, Coombs S, Duncan K and
McHenry MJ: Gentamicin is ototoxic to all hair cells in the fish
lateral line system. Hear Res. 261:42–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Winkel O, Hansen MM, Kaaber K and Rozarth
K: A prospective study of gentamicin ototoxicity. Acta Otolaryngol.
86:212–216. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Matz GJ: Aminoglycoside cochlear
ototoxicity. Otolaryngol Clin North Am. 26:705–712. 1993.PubMed/NCBI
|
|
46
|
Cotanche DA: Genetic and pharmacological
intervention for treatment/prevention of hearing loss. J Commun
Disord. 41:421–443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garetz SL, Altschuler RA and Schacht J:
Attenuation of gentamicin ototoxicity by glutathione in the guinea
pig in vivo. Hear Res. 77:81–87. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ding D, Stracher A and Salvi RJ: Leupeptin
protects cochlear and vestibular hair cells from gentamicin
ototoxicity. Hear Res. 164:115–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Priuska EM and Schacht J: Formation of
free radicals by gentamicin and iron and evidence for an
iron/gentamicin complex. Biochem Pharmacol. 50:1749–1752. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Forcheron F, Cachefo A, Thevenon S,
Pinteur C and Beylot M: Mechanisms of the triglycerideand
cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2
diabetic patients. Diabetes. 51:3486–3491. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jeong S, Kim M, Han M, Lee H, Ahn J, Kim
M, Song YH, Shin C, Nam KH, Kim TW, et al: Fenofibrate prevents
obesity and hypertriglyceridemia in low-density lipoprotein
receptor-null mice. Metabolism. 53:607–613. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Willson TM, Brown PJ, Sternbach DD and
Henke BR: The PPARs: from orphan receptors to drug discovery. J Med
Chem. 43:527–550. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Forge A and Li L: Apoptotic death of hair
cells in mammalian vestibular sensory epithelia. Hear Res.
139:97–115. 2000. View Article : Google Scholar
|
|
54
|
Olukman M, Sezer ED, Ulker S, Sözmen EY
and Cınar GM: Fenofibrate treatment enhances antioxidant status and
attenuates endothelial dysfunction in streptozotocin-induced
diabetic rats. Exp Diabetes Res. 2010:8285312010. View Article : Google Scholar
|
|
55
|
Kikuchi G, Yoshida T and Noguchi M: Heme
oxygenase and heme degradation. Biochem Biophys Res Commun.
338:558–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Krönke G, Kadl A, Ikonomu E, Blüml S,
Fürnkranz A, Sarembock IJ, Bochkov VN, Exner M, Binder BR and
Leitinger N: Expression of heme oxygenase-1 in human vascular cells
is regulated by peroxisome proliferator-activated receptors.
Arterioscler Thromb Vasc Biol. 27:1276–1282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kim HJ, So HS, Lee JH, Lee JH, Park C,
Park SY, Kim YH, Youn MJ, Kim SJ, Chung SY, et al: Heme oxygenase-1
attenuates the cisplatin-induced apoptosis of auditory cells via
down-regulation of reactive oxygen species generation. Free Radic
Biol Med. 40:1810–1819. 2006. View Article : Google Scholar : PubMed/NCBI
|