Introduction
Dental pulp (DP) tissue, termed as the
'ectomesenchyme', is derived from ectodermal cells that grow on the
periphery of the neural tube, migrate to the oral position and then
differentiate into cells of the mesenchymal phenotype (1). Epithelial cells form ameloblasts and
odontoblasts, DP and periodontal ligament (PDL) (2). DP is responsible for the maintenance
and repair of the periodontal tissue and its related immune system,
and it has a high regenerative potency and responds to various
types of damage (3). PDL connects
the tooth and the alveolar jaw bone in the area surrounding the
root surface, and functions as continuous support, attachment,
proprioception and physical protection for the teeth, and minimizes
tissue damage arising from trauma and infection (4). Owing to the anatomical and
functional differences between human DP and PDL, it is reasonable
to assume that there are also differences in the gene expression
profiles of these tissues.
Previous studies have indicated that the gene
expression patterns of mesenchymal stem cells (MSCs) derived from
dental tissues are different from those of other tissue by
employing genome-wide gene expression profiling and gene ontology
analysis (5). Differentially
expressed proteins have also been demonstrated between dental and
non-dental ovine MSC populations from the same donor, which may
attribute to their unique growth and capacity to generate
structures resembling the specific microenvironments from which
they were derived in vivo (6,7).
Recent findings suggest that human DP-derived stem
cells (DPSCs) and PDL-derived stem cells (PDLSCs) have the ability
to regenerate a dentin/pulp-like and cementum/PDL-like complex,
respectively when they are transplanted into the subcutaneous space
of immunocompromised mice (8–11).
Stem cell-based dentistry has emerged as a promising alternative
for the development of regenerative therapies, which have
unavoidable limitations and the effects of which have not yet been
fully determined (12).
The cDNA microarray technique can provide global
profiles of gene expression and facilitate the evaluation of
large-scale genes simultaneously. This method has been used in
dental studies to compare differentially expressed genes (DEGs)
among various types of stem cells (13), or tissues (14–16), or diseases (17–19). However, the differences in gene
expression profiles between DP and PDL tissue have not yet been
fully elucidated. The use of tissue samples provides more
information of the actual situation as the interactions between
different cell types can be important for the function of
tissues.
Therefore, the present study aimed to evaluate and
compare the gene expression patterns in DP and PDL tissues from
human permanent teeth, and to identify their molecular biological
differences and functions. Furthermore, the results may provide
insight into the potential molecular mechanisms of dental tissue
regeneration.
Materials and methods
Gene expression data
A gene expression data set (accession no. GSE50639),
which included 3 DP and PDL tissues, was downloaded from the Gene
Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo). Gene expression
levels were measured through the Affymetrix Human Gene 1.0 ST Array
beadchip platform (Affymetrix, Santa Clara, CA, USA) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL6244).
Platform annotation files were also acquired.
DP and PDL samples
Tissues were obtained from healthy permanent
premolars (n=16; from 8 males and 8 females, aged 11–14 years)
extracted for orthodontic purposes under approved guidelines set by
Nanjing Children's Hospital, Nanjing, China. Written informed
consent was obtained from all the respective parents or legal
guardians.
The DP and PDL samples used for the experiment were
collected according to a previously described procedure (8,9).
Briefly, tooth surfaces were cleaned with sterile water, and PDL
tissues were gently separated from the middle-third of root with a
scalpel. The root was then cut around the cementum-enamel junction
using sterilized dental fissure burs, and fractured off along the
cutting line with sharp-edged pliers to reveal the pulp chamber.
The DP tissues were carefully removed from the crown and root. The
extracted PD and PDL samples were then immediately frozen and
stored in liquid nitrogen.
Microarray data analysis
The expression data were generated using Affymetrix
Expression Console software version 1.4 (Affymetrix). The Robust
Multi-array Average (RMA) algorithm implemented through the
Affymetrix Expression Console software was used to normalize the
data. A one-way ANOVA was performed on the RMA expression values to
determine whether genes were differentially expressed between DP
and PDL groups. A multiple-testing correction was applied to the
p-values of the F-statistics to adjust the false discovery rate
(20). Genes with adjusted
F-statistic p-values of <0.05 were extracted. Microarray
analysis identified 1,405 genes with differences in expression of
≥2-fold, 920 and 485 of which were more abundant in the DP and PDL
tissue, respectively. However, only strongly expressed genes in the
DP or PDL tissue, which differed by >4- or 2.5-fold from the
signal of the DP and PDL tissues, respectively, were selected for
further analysis.. In order to classify the co-expression gene
group with a similar expression pattern, hierarchical clustering
analysis was conducted using Affymetrix Transcriptome Analysis
Console (TAC) software. The WEB-based Gene Set AnaLysis Toolkit was
performed for the biological interpretation of DEGs (21,22). WebGestalt is a system that
facilitates the analysis of sets of genes that can be visualized
and organized by a user-selected method. These genes were
classified based on data on gene function in the gene ontology of
the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
database. Protein-protein interaction (PPI) networks represents a
significant step in the elucidation of the underlying molecular
mechanisms. In our study, PPI networks were constructed for the
protein products using information from the Search Tool for the
Retrieval of Interacting Genes/Proteins (STRING, version 9.1;
http://string-db.org/) (23). Interactions with a score (i.e.,
required confidence) >0.4 were retained in the network.
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated from the DP and PDL tissues using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions. To determine the number of cDNA
molecules in the reverse-transcribed samples, qPCR analyses were
performed using the LightCycler system (Roche, Indianapolis, IN,
USA). PCR was conducted using 2 µl LightCycler DNA Master
SYBR-Green I (Roche), 12.5 µl of reaction mixture, 2
µl of each 5′ and 3′ primer, 2 µl samples.
H2O was then added to a final volume of 25 µl.
The samples were denatured at 95°C for 10 sec, with a temperature
transition rate of 20°C/sec. Four steps were carried out in
amplification and fluorescence determination: denaturation at 95°C
for 1 sec, with a temperature transition rate of 20°C/sec;
annealing for 5 sec, with a temperature transition rate of 8°C/sec;
extension at 72°C for 20 sec, with a temperature transition rate of
4°C/sec; and detection of SYBR-Green fluorescence, which reflects
the amount of double-stranded DNA, at 86°C for 3 sec. The
amplification cycle number was 35. To discriminate specific from
non-specific cDNA products, a melting curve was obtained at the end
of each run. Products were denatured at 95°C for 3 sec, and the
temperature then decreased to 58°C for 15 sec and increased slowly
from 58 to 95°C using a temperature transition rate of 0.1°C/sec.
To determine the number of copies of the targeted DNA in the
samples, purified PCR fragments of known concentrations were
serially diluted and served as external standards that were
measured in each experiment. Data were normalized to glyceraldehyde
3-phosphate dehydrogenase (GAPDH) levels in the samples. The primer
sequences used for PCR are listed in Table I.
 | Table ISpecific primer sequences used for
RT-qPCR. |
Table I
Specific primer sequences used for
RT-qPCR.
| Name | Primers sequences
(5′→3′) | Product size
(bp) |
|---|
| ITGA4 | F:
CACAACACGCTGTTCGGCTA | |
| R:
CGATCCTGCATCTGTAAATCGC | 139 |
| ITGA8 | F:
AGAATGGAGACCTTATTGTGGGA | |
| R:
GAGCCACTTCCGTCTGCTTT | 147 |
| NRXN1 | F:
TAAGTGGCCTCCTAATGACCG | |
| R:
TCGCACCAATACGGCTTCTTT | 91 |
| CNTN1 | F:
CAGCCCTTTCCCGGTTTACAA | |
| R:
TGCTTCTGACCATCCCGTAGT | 170 |
| ACAN | F:
GTGCCTATCAGGACAAGGTCT | |
| R:
GATGCCTTTCACCACGACTTC | 167 |
| COL11A1 | F:
ACCCTCGCATTGACCTTCC | |
| R:
TTTGTGCAAAATCCCGTTGTTT | 128 |
| COL6A1 | F:
ACAGTGACGAGGTGGAGATCA | |
| R:
GATAGCGCAGTCGGTGTAGG | 122 |
| CHAD | F:
CGCGGCCTCAAGCAACTTA | |
| R:
TAGGTCAGCTCGGTCAGGTC | 95 |
| LAMC2 | F:
CAAAGGTTCTCTTAGTGCTCGAT | |
| R:
CACTTGGAGTCTAGCAGTCTCT | 153 |
| LAMA3 | F:
CACCGGGATATTTCGGGAATC | |
| R:
AGCTGTCGCAATCATCACATT | 165 |
| GAPDH | F:
GGAGCGAGATCCCTCCAAAAT | |
| R:
GGCTGTTGTCATACTTCTCATGG | 197 |
Statistical analysis
Data from image analysis are presented as the means
± SEM. Statistical comparisons were made using a two-way ANOVA. A
value of p<0.05 was considered to indicate a statistically
significant difference.
Results
Gene expression profiles in human DP and
PDL tissue
Affymetrix Transcriptome Analysis Console software
was applied to analyze the cDNA microarray data set (GSE50639). The
results indicated that the expression of a total of 1,405 genes was
altered by at least 2-fold in one tissue type relative to the
other. In the DP tissue, the expression levels of 920 genes were at
least double those in the PDL tissues, while in the latter, the
expression levels of 485 genes were at least double those in the DP
tissue. Ultimately, only strongly expressed genes (529 genes) in
the DP or PDL tissue whose expression differed by >4- or
2.5-fold from the signal of the DP or PDL tissue were evaluated
further. In the DP tissue, 255 genes were upregulated by at least
4-fold relative to the PDL tissue, while 274 genes were upregulated
by at least 2.5-fold in the PDL tissue relative to the DP tissue.
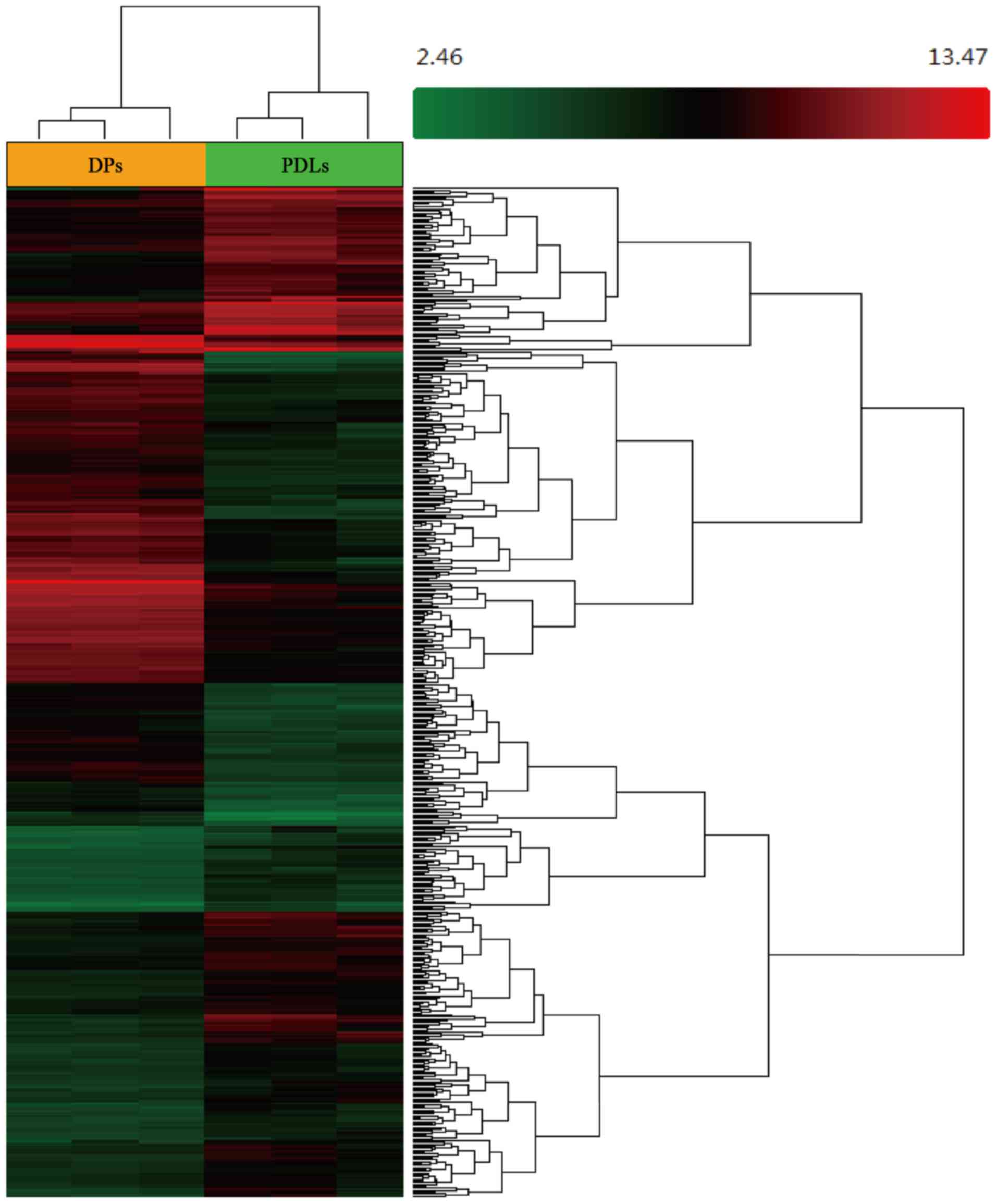
Hierarchical clustering and a heatmap of the DEGs in the DP and PDL
tissue are shown in Fig. 1, and
the up- and downregulated genes in the DP tissues (compared with
the PDL tissues) are shown in Table
II.
 | Table IIUp- and downregulated genes in the DP
tissues (compared with the PDL tissues). |
Table II
Up- and downregulated genes in the DP
tissues (compared with the PDL tissues).
Upregulated genes
in the DP tissues
| Downregulated genes
in the DP tissue
|
|---|
| Name | Gene symbol | Fold change | Name | Gene symbol | Fold change |
|---|
| Anoctamin 1,
calcium activated chloride channel | ANO1 | 6.87 | Cathepsin K | CTSK | −6.62 |
| Scinderin | SCIN | 28.26 | ADAM
metallopeptidase with thrombospondin type 1 motif 14 | ADAMTS14 | −2.64 |
| Bone morphogenetic
protein 7 | BMP7 | 5.71 | Denticleless E3
ubiquitin protein ligase homolog (Drosophila) | DTL | −3.16 |
| Sorbin and SH3
domain containing 2 | SORBS2 | 9.62 | Sushi-repeat
containing protein, X-linked 2 | SRPX2 | −7.94 |
| Leucine-rich repeat
containing G protein-coupled receptor 5 | LGR5 | 43.19 | Extended
synaptotagmin-like protein 3 | ESYT3 | −2.82 |
| Adherens junctions
associated protein 1 | AJAP1 | 10.07 | Fibroblast
activation protein alpha | FAP | −16.58 |
| Transferrin | TF | 53.65 | ATPase, class V,
type 10A | ATP10A | −2.95 |
| Solute carrier
family 16, member 4 | SLC16A4 | 8.58 | Collagen type IV
alpha 1 | COL4A1 | −2.87 |
| Family with
sequence similarity 134, member B | FAM134B | 5.39 | Bone
gamma-carboxyglutamate (gla) protein | BGLAP; PMF1-BGLAP;
PMF1 | −3.84 |
| Homer homolog 2
(Drosophila) | HOMER2 | 4.24 | Lysyl oxidase-like
2 | LOXL2 | −3.11 |
| KN motif and
ankyrin repeat domains 1 | KANK1 | 4.27 | Integrin alpha
11 | ITGA11 | −6.81 |
| Chromosome 10 open
reading frame 107 | C10orf107 | 8.01 | Secreted
phosphoprotein 1 | SPP1 | −14.77 |
| T-cell lymphoma
invasion and metastasis 1 | TIAM1 | 7.23 |
Methylenetetrahydrofolate dehydrogenase
(NADP+ dependent) 2 | MTHFD2 | −3.41 |
| Phosphate
regulating endopeptidase homolog, X-linked | PHEX | 68.57 | Collagen type XVI
alpha1 | COL16A1 | −5.89 |
| Transmembrane
protein 156 | TMEM156 | 21.87 | Retinol binding
protein 4, plasma | RBP4 | −7.29 |
| MOB kinase
activator 3B | MOB3B | 6.42 | G protein-coupled
receptor 1 | GPR1 | −3.2 |
| Ras homolog family
member U | RHOU | 7.76 | Nicotinamide
N-methyltransferase | NNMT;
LOC101928916 | −3.94 |
| Retinoic acid
receptor responder (tazarotene induced) 1 | RARRES1 | 10.38 | Collagen type XVII
alpha 1 | COL17A1 | −2.91 |
| Peroxidasin homolog
(Drosophila)-like | PXDNL | 10.05 |
Methylenetetrahydrofolate dehydrogenase
(NADP+ dependent) 2 | MTHFD2P7;
MTHFD2 | −2.88 |
| KIAA1324 | KIAA1324 | 10.36 | Chondroadherin | CHAD | −4.19 |
| UDP-Gal:betaGlcNAc
beta1,3-galactosyltransferase | B3GALT1 | 10.82 | Semaphorin 7A, GPI
membrane anchor | SEMA7A | −4.13 |
| Unc-80 homolog
(C. elegans) | UNC80 | 8.46 | Dynamin 1 | DNM1 | −2.91 |
| Abhydrolase domain
containing 12B; microRNA 4454 | ABHD12B;
MIR4454 | 33.54 | Carbonic anhydrase
XII | CA12 | −2.85 |
| Clusterin | CLU | 6.84 | Collagen type IV
alpha 2 | COL4A2 | −2.59 |
| WNT1 inducible
signaling pathway protein 1 | WISP1 | 5.12 | Collagen type VI
alpha 1 | COL6A1 | −3.29 |
| Cadherin 12, type 2
(N-cadherin 2) | CDH12 | 56.93 | microRNA 21;
vacuole membrane protein 1 | MIR21; VMP1 | −5.57 |
| Epithelial cell
adhesion molecule | EpCAM | 9.81 | Matrix
metallopeptidase 19 | MMP19 | −3.26 |
| Sema domain,
immunoglobulin domain (Ig), | SEMA3E | 46.94 | Scavenger receptor
class A, member 5 (putative) | SCARA5 | −3.77 |
| EPH receptor
A5 | EPHA5 | 12.41 | MIR155 host gene
(non-protein coding); microRNA 155 | MIR155HG;
MIR155 | −2.97 |
| Charged
multivesicular body protein 4C | CHMP4C | 8.81 | CUB domain
containing protein 1 | CDCP1 | −2.71 |
| Bone morphogenetic
protein receptor, type IB | BMPR1B | 19.06 | Tumor necrosis
factor receptor superfamily, member 21 | TNFRSF21 | −3.05 |
| Tripartite motif
containing 36 | TRIM36 | 5.13 | Myosin ID | MYO1D | −3.13 |
| Aldehyde
dehydrogenase 1 family, member A3 | ALDH1A3 | 6.01 | Neuroblastoma 1,
DAN family BMP antagonist | NBL1;
MINOS1-NBL1 | −4.74 |
| Nucleic acid
binding protein 1 | NABP1 | −3.17 | Gamma-aminobutyric
acid (GABA) A receptor beta 2 | GABRB2 | −3.03 |
| Uridine-cytidine
kinase 2; microRNA 3658 | UCK2; MIR3658 | 7.34 | RNA, 7SK small
nuclear pseudogene 137 | RN7SKP137 | −4.57 |
| Dystrobrevin
binding protein 1 | DTNBP1 | 6.06 | Matrix
metallopeptidase 3 (stromelysin 1, progelatinase) | MMP3 | −5.72 |
| G protein-coupled
receptor 63 | GPR63 | 13.14 | Coronin 6 | CORO6 | −2.73 |
|
1-Acylglycerol-3-phosphate
O-acyltransferase 4 | AGPAT4 | 4.7 | CD109 molecule | CD109 | −8.66 |
| Distal-less
homeobox 3 | DLX3 | 5.61 | Parathyroid hormone
1 receptor | PTH1R | −3.29 |
| Cytoplasmic FMR1
interacting protein 2 | CYFIP2 | 12.55 | Cadherin-related
23; cadherin-23-like | CDH23;
LOC100653137 | −3.1 |
| Protein tyrosine
phosphatase, receptor type U | PTPRU | 4.67 | Cholinergic
receptor, nicotinic alpha 9 (neuronal) | CHRNA9 | −4.68 |
| Nestin | NES | 8.01 | Acid phosphatase 5,
tartrate resistant | ACP5 | −7.5 |
| Unc-80 homolog
(C. elegans) | UNC80 | 6.22 | Anillin, actin
binding protein | ANLN | −3.76 |
| Solute carrier
family 2 (facilitated glucose transporter), member 13 | SLC2A13 | 6.24 | Aggrecan | ACAN | −5.66 |
| Calpain 6 | CAPN6 | 4.23 | Angiopoietin-like
2 | ANGPTL2 | −7.12 |
|
Dehydrogenase/reductase (SDR family)
member 3 | DHRS3 | 5.68 | Protein tyrosine
phosphatase, non-receptor type 22 (lymphoid) | PTPN22 | −4.17 |
| Meiotic nuclear
divisions 1 homolog (S. cerevisiae) | MND1 | 6.24 | Marker of
proliferation Ki-67 | MKI67 | −4.31 |
| Coiled-coil domain
containing 148 | CCDC148 | 4.97 | Zinc finger protein
385A | ZNF385A | −2.56 |
| Carboxypeptidase
A6 | CPA6 | 22.33 | Matrix
metallopeptidase 9 | MMP9 | −22.27 |
| SH2 domain
containing 4A | SH2D4A | 6.27 | Collagen type VI
alpha 3 | COL6A3 | −8.54 |
| Ameloblastin
(enamel matrix protein) | AMBN | 56.17 | Sushi-repeat
containing protein, X-linked | SRPX | −5.77 |
| Gastrin-releasing
peptide receptor | GRPR | 12.65 | Collagen type XIII
alpha 1 | COL13A1 | −2.61 |
| Family with
sequence similarity 69, member C | FAM69C | 6.29 | Aldo-keto reductase
family 1, member C2 | AKR1C2;
LOC101930400 | −2.9 |
| Scavenger receptor
class A, member 3 | SCARA3 | 7.45 | Collagen type VI
alpha 2 | COL6A2 | −3.05 |
| Dentin
sialophosphoprotein | DSPP | 40.4 | Collagen type XII
alpha 1 | COL12A1 | −34.05 |
| Myosin XVI | MYO16 | 9.15 | V-maf avian
musculoaponeurotic fibrosarcoma oncogene homolog B | MAFB | −2.81 |
| Activating
transcription factor 7 interacting protein 2 | ATF7IP2 | 6.18 | Absent in melanoma
1 | AIM1 | −4.65 |
| Serine/threonine
kinase 33 | STK33 | 6.99 |
Dipeptidyl-peptidase 4 | DPP4 | −5.8 |
| Galactosidase
β1-like 3 | GLB1L3 | 4.97 | Xg blood group | XG; XGY2 | −8.18 |
| Transforming growth
factor-beta 1 | TGFB1 | 2.13 | Laminin alpha
3 | LAMA3 | −3.45 |
| Solute carrier
family 12 (sodium/potassium/chloride transporter), member 2 | SLC12A2 | 6.02 | Cell division cycle
6 | CDC6 | −2.54 |
| Transforming growth
factor-beta 2 | TGFB2 | 3.04 | Laminin gamnma
2 | LAMC2 | −3.29 |
| G protein-coupled
receptor 64 | GPR64 | 11.44 | Paraneoplastic Ma
antigen 2 | PNMA2 | −3.24 |
| Lipid phosphate
phosphatase-related protein type 5 | LPPR5 | 32.35 | Glycoprotein
(transmembrane) nmb | GPNMB | −3.6 |
| Transmembrane
protein 132B | TMEM132B | 6.76 | X-prolyl
aminopeptidase (aminopeptidase P) 2, membrane-bound | XPNPEP2 | −3.15 |
| Sodium channel,
voltage gated, type VIII alpha subunit | SCN8A | 5.21 | Collagen type V
alpha 1 | COL5A1 | −6.45 |
| Gamma-aminobutyric
acid (GABA) A receptor beta 1 | GABRB1 | 18.02 | Asp (abnormal
spindle) homolog, microcephaly associated (Drosophila) | ASPM | −2.9 |
| Long intergenic
non-protein coding RNA 943 | LINC00943 | 7.72 | Solute carrier
family 38, member 5 | SLC38A5 | −2.71 |
| Contactin
associated protein-like 4 | CNTNAP4 | 4.1 | ATPase,
H+ transporting, lysosomal 38 kDa, V0 subunit d2 | ATP6V0D2 | −7.72 |
| Wingless-type MMTV
integration site family, member 5A | WNT5A | 4.44 | Leucine rich repeat
containing 15 | LRRC15 | −14.31 |
| Gamma-aminobutyric
acid (GABA) A receptor gamma 1 | GABRG1 | 11.14 | Fibroblast growth
factor 9 | FGF9 | −3.9 |
| Transmembrane
protein 38B | TMEM38B | 5.8 | Syntaxin binding
protein 6 (amisyn) | STXBP6 | −3.91 |
| Vitrin | VIT | 4.77 | Mesenchyme homeobox
1 | MEOX1 | −3.02 |
| Dynein, cytoplasmic
1, intermediate chain 1 | DYNC1I1 | 6.4 | Inhibin beta A | INHBA | −2.59 |
| Sparc/osteonectin,
cwcv and kazal-like domains proteoglycan (testican) 3 | SPOCK3 | 66.65 | Parathyroid
hormone-like hormone | PTHLH | −7.65 |
| EF-hand calcium
binding domain 1 | EFCAB1 | 4.09 | Reticulocalbin 3,
EF-hand calcium binding domain | RCN3 | −2.65 |
| Phosphorylase,
glycogen, liver | PYGL | 5.28 | Coagulation factor
V (proaccelerin, labile factor) | F5 | −3.15 |
| Phospholipase C
delta 4 | PLCD4 | 7.52 | Topoisomerase (DNA)
II alpha 170 kDa | TOP2A | −5.27 |
| Fibulin 2 | FBLN2 | 5.8 | Protease, serine
35 | PRSS35 | −4.54 |
| Ras association
(RalGDS/AF-6) domain family member 2 | RASSF2 | 4.3 | Bone morphogenetic
protein 3 | BMP3 | −15.22 |
|
Hyaluronoglucosaminidase 4 | HYAL4 | 9.39 | B-cell CLL/lymphoma
11B (zinc finger protein) | BCL11B | −2.84 |
| Integrin alpha 4
(antigen CD49D, alpha4 subunit of VLA-4 receptor) | ITGA4 | 6.2 | Collagen type XI
alpha 1 | COL11A1 | −23.93 |
| Integrin alpha
8 | ITGA8 | 5.52 | Wingless-type MMTV
integration site family member 2 | WNT2 | −3.36 |
| Neurexin 1 | NRXN1 | 5.64 | Dickkopf WNT
signaling pathway inhibitor 2 | DKK2 | −5.21 |
| Potassium channel,
subfamily K, member 2 | KCNK2 | 18.13 | Bone morphogenetic
protein 8a | BMP8A | −2.22 |
| Contactin 1 | CNTN1 | 4.73 | Transforming growth
factor beta 3 | TGFB3 | −2.78 |
| Dentin matrix
acidic phosphoprotein 1 | DMP1 | 5.88 | Chemokine (C-X-C
motif) ligand 13 | CXCL13 | −3.51 |
| Integrin alpha 2
(CD49B alpha 2 subunit of VLA-2 receptor) | ITGA2 | 2.35 | Chemokine (C-X-C
motif) ligand 2 | CXCL2 | −2.33 |
| Collagen, type XI,
alpha 2 | COL11A2 | 4.26 | Chemokine (C-X-C
motif) ligand 8 | CXCL8 | −3.19 |
| Reelin | RELN | 8.59 | Chemokine (C-X-C
motif) receptor 1 | CXCR1 | −2.76 |
| Solute carrier
family 1 (neuronal/epithelial high affinity glutamatetransporter,
system Xag), member 1 | SLC1A1 | 4.81 | Collagen, type XI,
alpha 1 | COL11A1 | −23.93 |
| Potassium
intermediate/small conductance calcium-activated channel, subfamily
N, member 4 | KCNN4 | 4.15 | Tenascin N | TNN | −21.91 |
| ATPase,
Na+/K+ transporting, alpha 2 polypeptide | ATP1A2 | 5 | Integrin-binding
sialoprotein | IBSP | −5 |
| MET proto-oncogene,
receptor tyrosine kinase | MET | 6.93 | Carboxypeptidase A3
(mast cell) | CPA3 | −4.09 |
| Nitric oxide
synthase 1 (neuronal) | NOS1 | 4.43 | Insulin-like growth
factor 1 (somatomedin C) | IGF1 | −5.2 |
| Poliovirus
receptor-related 3 | PVRL3 | 5.7 | Interleukin 1β | IL1β | −4.78 |
| Claudin 10 | CLDN10 | 5.11 | Phospholipase C,
beta 1 (phosphoinositide-specific) | PLCB1 | −2.68 |
| Claudin 11 | CLDN11 | 6 | Cystatin SN | CST1 | −5.14 |
| Neuronal growth
regulator 1 | NEGR1 | 6.06 | Cystatin S | CST4 | −2.67 |
| Catenin
(cadherin-associated protein), alpha 2 | CTNNA2 | 9.47 | Lysozyme | LYZ | −2.86 |
| KIT ligand | KITLG | 5.53 | Selectin E | SELE | −2.73 |
| Bone morphogenetic
protein 5 | BMP5 | 3.92 | Peroxisome
proliferator-activated receptor gamma | PPARG | −2.56 |
| Bone morphogenetic
protein 6 | BMP6 | 3.31 | Fas cell surface
death receptor | FAS | −2.75 |
| | | Matrix
metallopeptidase 2 (gelatinase A, 72 kDa gelatinase, 72 kDa type IV
collagenase) | MMP2 | −3.43 |
| | | Cyclin-dependent
kinase 6 | CDK6 | −2.83 |
| | | Interleukin 7 | IL7 | −2.65 |
| | | CD4 molecule | CD4 | −2.91 |
| | | CD1c molecule | CD1C | −2.52 |
| | | Alanyl (membrane)
aminopeptidase | ANPEP | −4.4 |
| | | Leukemia inhibitory
factor | LIF | −3.17 |
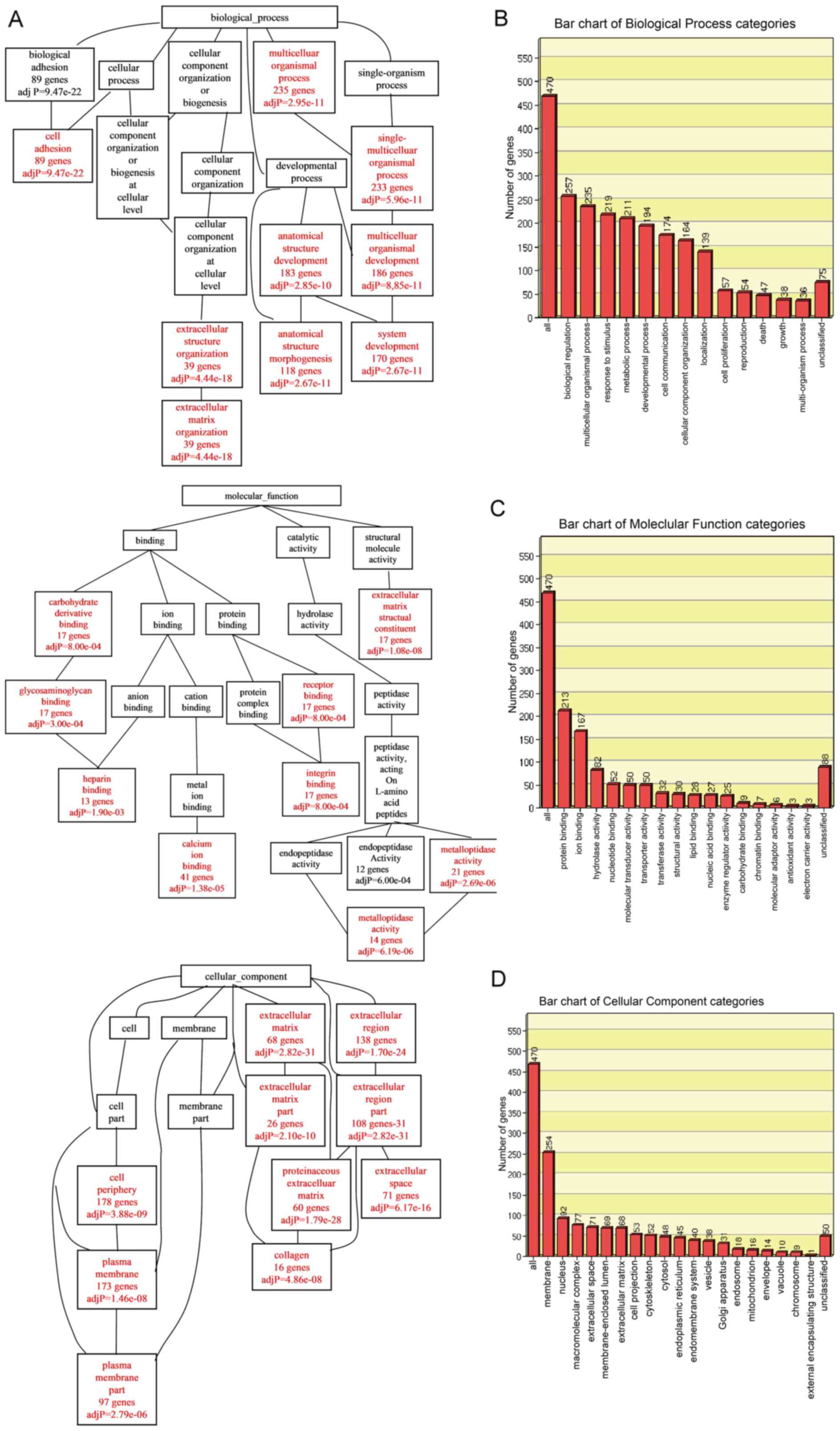
Gene Ontology (GO) analysis
To analyze the specific biological functions and
features of the selected genes, an analysis toolkit (WebGestalt)
was applied for GO annotation and enrichment analysis. The DEGs
were classified according to biological process (BP), molecular
function (MF) or cellular component (CC) using the WebGestalt
software package on the basis of hypergeometric tests. The
Resulting BP, MF and CC networks are shown as directed acyclic
graphs (DAG), which are color-coded (red for p-values <0.05)
(Fig. 2A). Biological process
enrichment was found for genes associated with cell adhesion,
extracellular matrix (ECM) organization, regulation of anatomical
structure, morphogenesis and system development. Molecular function
enrichment was discovered for genes associated with heparin
binding, calcium ion binding, integrin binding and
metalloendopeptidase activity. Cellular component enrichment was
detected for genes associated with the plasma membrane and ECM.
Significantly enriched GO categories under biological process,
molecular function and cellular component are indicated in Fig. 2B–D. In the biological process
category, the GO terms of biological regulation (257 genes),
multicellular organismal process (235 genes), response to stimulus
(219 genes), related to metabolic process (211 genes) and
developmental process were enriched. In the molecular function
category, GO terms related to protein binding (213 genes) and ion
binding (167 genes) were enriched. In the cellular component
category, GO terms related to membrane (254 genes) were
enriched.
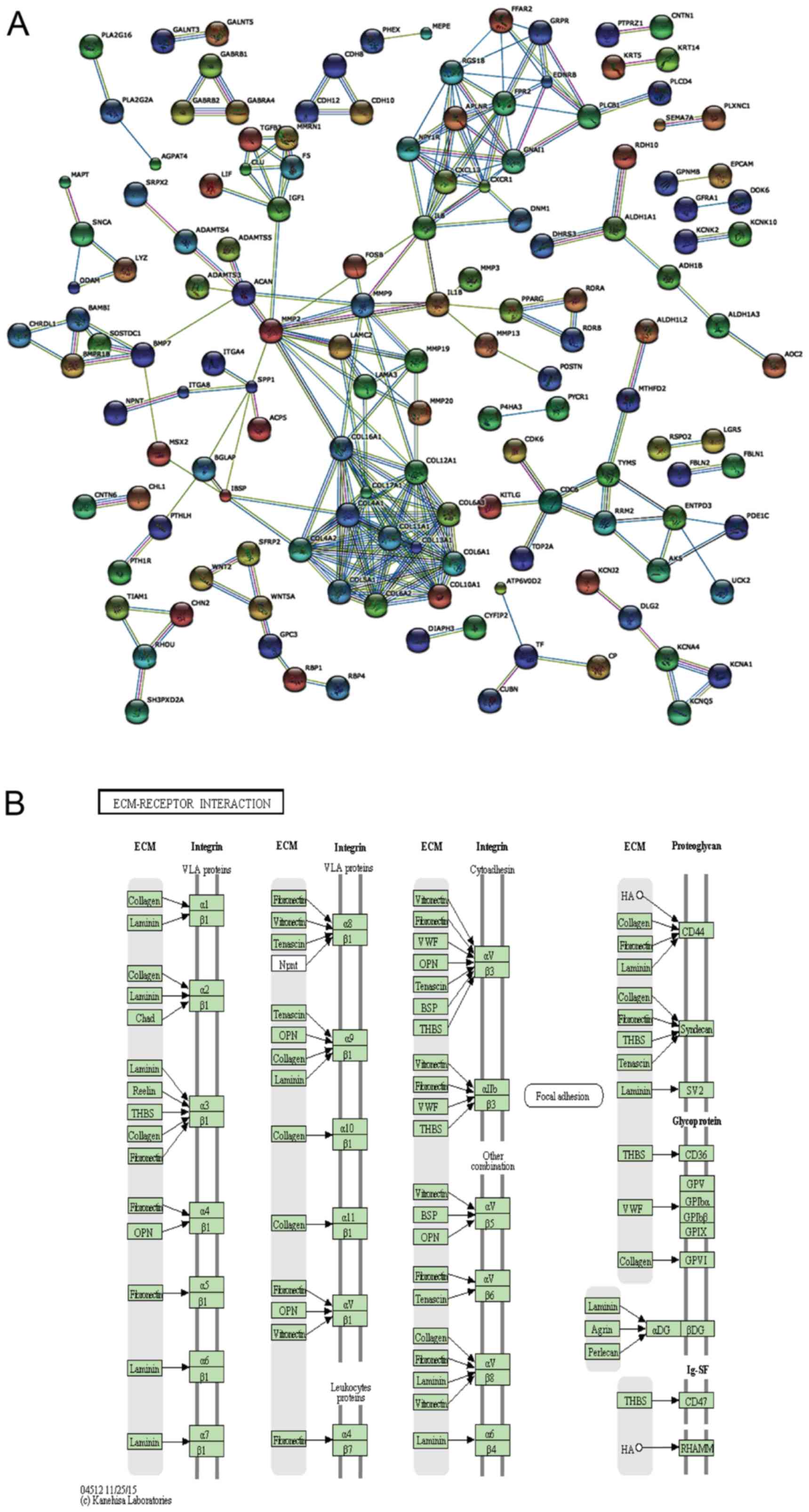
Interaction network and pathway analysis
of DEGs
To further detect the biological function of the
DEGs in the DP and PDL tissue, an interaction network was carried
out using STRING 9.1 (23), as
shown in Fig. 3A. The interaction
network was generated on the basis of experimental and database
knowledge. Markov cluster algorithm (MCL) was used to find group
associations between these DEGs. The inflation factor was set as 1
on a scale of 1–5.
WebGestalt was used to identify the significantly
enriched KEGG pathways. The shared enriched pathways, including
ECM-receptor interaction, protein digestion and absorption, focal
adhesion and cell adhesion molecules (CAMs) (Fig. 3B and Table III) were determined at the
significance levels of p<0.05 in WebGestalt. Among the enriched
pathways, the upregulated pathways in the DP group included CAMs
and salivary secretion pathways and the downregulated pathways in
the DP group included ECM-receptor interaction, protein digestion
and absorption and focal adhesion. Compared with GO analysis, KEGG
pathway analysis provides biological information in a more detailed
and specific manner. Furthermore, good concordance was observed
from the STRING interaction network and the enriched functional
modules identified by the KEGG and GO enrichment analyses.
 | Table IIIList of enriched KEGG pathways of the
differentially expressed proteins. |
Table III
List of enriched KEGG pathways of the
differentially expressed proteins.
| KEGG pathway | No. of hits | Expressed gene
participating in the pathway | Statistics for the
enrichment of the pathway |
|---|
| ECM-receptor
interaction | 18 | COL11A2, ITGA8,
ITGA4, RELN, COL11A1, COL6A3, SPP1, CHAD, LAMA3, COL6A1, COL5A1,
ITGA11, COL4A1, TNN, LAMC2, IBSP, COL4A2, COL6A2 | C=82; O=18; E=2.12;
R=8.47; rawP=2.55e-12; adjP=2.63e-10 |
| Protein digestion
and absorption | 16 | COL6A2, COL17A1,
COL4A2, COL11A2, CPA3, COL11A1, SLC1A1, COL12A1, COL6A3, KCNN4,
COL6A1, COL5A1, DPP4, XPNPEP2, COL4A1, ATP1A2 | C=76; O=16; E=1.97;
R=8.12; rawP=8.33e-11; adjP=4.29e-09 |
| Focal adhesion | 20 | COL6A2, COL4A2,
COL11A2, ITGA8, ITGA4, RELN, COL11A1, COL6A3, SPP1, CHAD, LAMA3,
COL6A1, COL5A1, IGF1, ITGA11, COL4A1, TNN, MET, LAMC2, IBSP | C=195; O=20;
E=5.05; R=3.96; rawP=1.80e-07; adjP=6.18e-06 |
| Amoebiasis | 11 | LAMA3, COL4A2,
COL11A2, COL5A1, IL8, TGFB3, COL4A1, COL11A1, PLCB1, IL1B,
LAMC2 | C=104; O=11;
E=2.69; R=4.08; rawP=8.03e-05; adjP=0.0021 |
| Salivary
secretion | 8 | NOS1, CST1, PLCB1,
SLC12A2, ATP1A2, KCNN4, CST4, LYZ | C=78; O=8; E=2.02;
R=3.96; rawP=0.0009; adjP=0.0185 |
| Cell adhesion
molecules (CAMs) | 10 | CD4, ITGA8, ITGA4,
PVRL3, SELE, NRXN1, CLDN10, CLDN11, NEGR1, CNTN1 | C=126; O=10;
E=3.26; R=3.06; rawP=0.0016; adjP=0.0265 |
| Pathways in
cancer | 18 | MMP9, FGF9, COL4A2,
PPARG, WNT2, CTNNA2, WNT5A, FAS, LAMA3, IL8, MMP2, IGF1, TGFB3,
KITLG, COL4A1, MET, LAMC2, CDK6 | C=319; O=18;
E=8.27; R=2.18; rawP=0.0018; adjP=0.0265 |
| Hematopoietic cell
lineage | 7 | IL7, KITLG, CD4,
IL1B, ITGA4, CD1C, ANPEP | C=80; O=7; E=2.07;
R=3.38; rawP=0.0047; adjP=0.0543 |
| Cytokine-cytokine
receptor interaction | 14 | IL7, LIF, CXCL13,
BMP7, TNFRSF21, IL8, TGFB3, KITLG, MET, IL1B, CXCR1, INHBA, BMPR1B,
FAS | C=248; O=14;
E=6.43; R=2.18; rawP=0.0054; adjP=0.0543 |
| Rheumatoid
arthritis | 7 | ATP6V0D2, MMP3,
IL1B, CTSK, IL8, ACP5, TGFB3 | C=82; O=7; E=2.12;
R=3.29; rawP=0.0054; adjP=0.0543 |
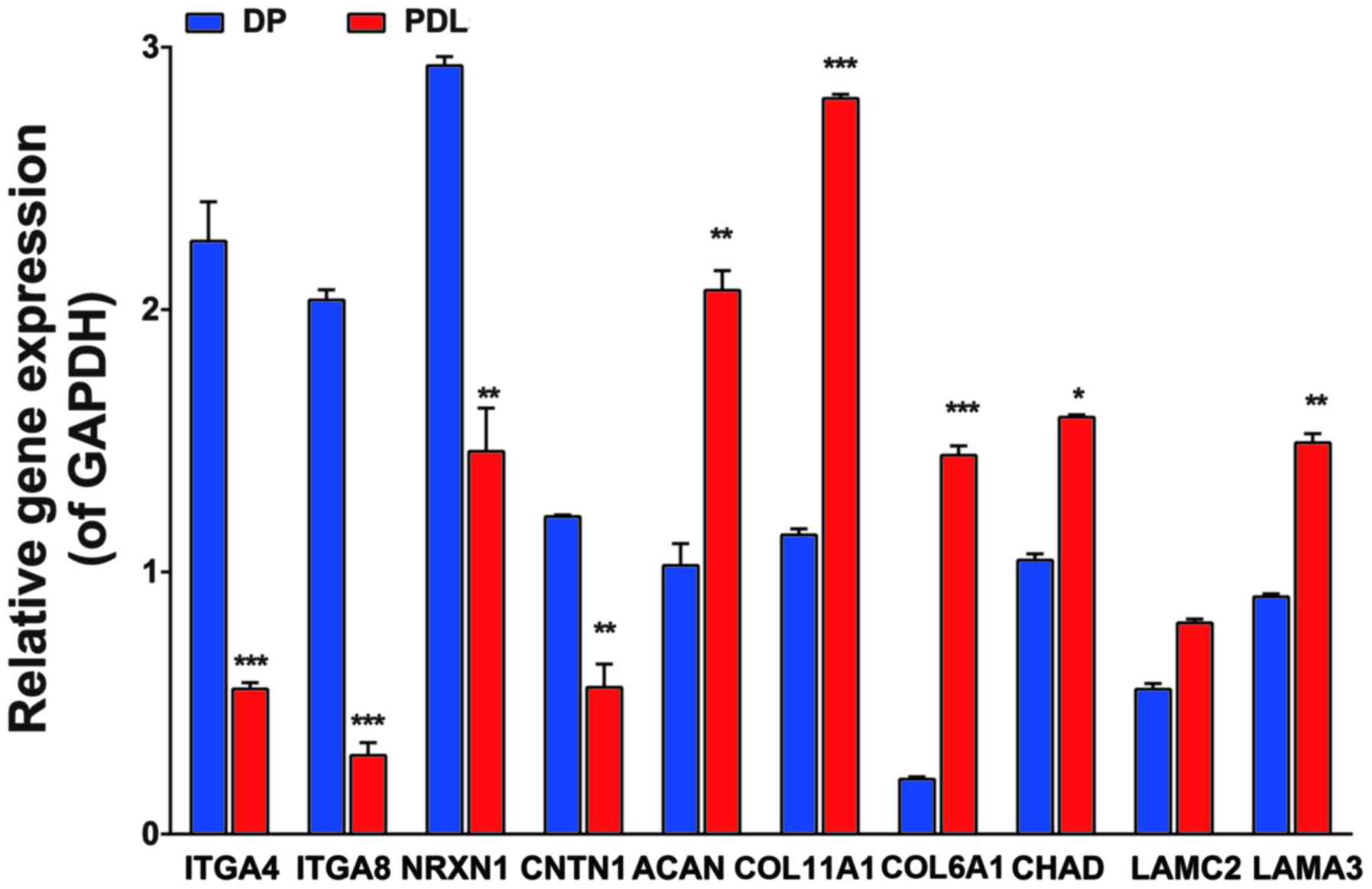
Results of RT-Qpcr
We selected 10 genes [integrin alpha4 (ITGA4),
integrin alpha8 (ITGA8), contactin 1 (CNTN1), neurexin 1 (NRXN1),
laminin alpha3 (LAMA3), laminin gamma2 (LAMC2), collagen type XI
alpha1 (COL11A1), collagen type VI alpha3 (COL6A3), collagen type
VI alpha1 (COL6A1) and chondroadherin (CHAD)] to validate the
expression data from microarray analysis using SYBR-Green based
RT-qPCR. The expression of these genes was significantly altered in
various functional modules, as shown in Table III. The 10 genes with expression
levels differing by at least 2-fold between the DP and PDL tissues
were selected. The results indicated that the mRNA levels of ITGA4,
ITGA8, NRXN1 and CNTN1 were significantly higher in the DP compared
with the PDL tissues. However, the levels of COL11A1, ACAN, COL6A1,
CHAD, LAMC2 and LAMA3 were higher in the PDL tissue compared with
the DP tissue (Fig. 4). The mRNA
expression levels demonstrated a consistent trend as the cDNA
microarray. Taken together, these results suggested that the two
types of tissues have a similar mRNA expression profile,
paralleling the results determined by RT-qPCR.
Discussion
With global gene expression profiling, this study
uncovered DEGs from DP and PDL tissues. The cDNA microarray results
indicated that the expression levels of 1,405 out of 29,096 (4.82%)
genes were altered by at least 2-fold in one tissue type relative
to the other. Lee et al (14) reported that only 490 out of 33,297
(1.49%) genes were differentially expressed between dental follicle
and PDL tissues. This discrepancy may arise from the relative
heterogeneity between DP and PDL tissues.
To further analyze the DEGs, functional enrichment
analyses were conducted using the WebGestalt tool. Our results
revealed that the DEGs were associated with ECM-receptor
interaction, protein digestion and absorption and focal adhesion,
which were the three most enriched terms. The most enriched term
ECM-receptor interaction is a complex network of different
combinations of collagens, proteoglycans, hyaluronic acid, laminin,
fibronectin and many other glycoproteins, including proteolytic
enzymes involved in the degradation and remodeling of the ECM.
Dentin ECM proteins play important roles in the dynamics of
dentinogenesis (24,25). Detailed studies of dentin matrix
proteins may give some insights into unelucidated mechanisms of
dentinogenesis.
Focal adhesion formation is initiated upon the
binding of adhesion receptors to ECM ligands. The cell adhesion
molecule, EpCAM, CNTN1 and NRXN1 had a higher expression in the DP
tissues, while CHAD, LAMC2 and LAMA3 were upregulated in the PDL
tissues. A recent study presented a close interaction of EpCAM with
other cell-cell contact molecules, such as E-cadherin and claudins
(26). CNTN1, a prototypical
member of the contactin (CNTN) family, is involved, through
cis- and trans-interactions with specific cell
adhesion molecules, in neural cell migration, axon guidance and the
organization of myelin subdomains (27). NRXN functions as synaptic
transmission and maturation of contacts (28,29). CHAD, a leucine rich repeat ECM
protein with functions in cell to matrix interactions, is
associated with both cartilage and bone homeostasis (30). Laminin, one of the major
glycoprotein components, may maintain and regulate odontoblast
differentiation and enamel crystallization (31).
As the ECM receptors in focal adhesions, integrins
are heterodimeric transmembrane proteins that connect the actin
cytoskeleton to the extracellular microenvironment and
bidirectionally mediate signals across cell membrane (32,33). ITGA2, ITGA4 and ITGA8 were
upregulated in the DP compared with the PDL tissues. It has been
have found that ITGA2 is positively expressed in DP cells (34) and ITGA4 was stained in the dental
neural crest-derived progenitor cells (dNC-PCs) (35). Adherent cells can identify laminin
through ITGA2 for adhesion and differentiation (36), and recognize fibronectin via ITGA4
for attachment and migration (37). However, ITGA11 was upregulated in
the DPL compared with the PD tissues. A previous study demonstrated
that the ITGA11 knockout mice were characterized by a disorganized
PDL, which may be attributable to the disturbed matrix
metallopeptidase synthesis, and greatly reduced cell adhesion and
spreading on collagen I (38).
The regulation of matrix metalloproteinase-13 (MMP-13) and
cathepsin K is ITGA11-dependent, which is involved in the
coordinated extracellular and intracellular collagen proteolysis
(39). In addition,
integrin-binding sialoprotein (IBSP), which is a major structural
protein of the bone matrix (40),
was found to be upregulated in the PDL compared with the DP tissue
in this study.
Some of the genes that were relatively strongly
expressed in PDL tissues were associated with the degradation of
the ECM. MMPs, the major players in collagen breakdown, have been
identified in periodontal inflammation (41). The robust expressions of
collagenases cathepsin K (CTSK) and MMP-3, -9 and -19, which were
higher in the PDL tissue than in the DP tissue, are likely to play
a part in the turnover of ECM in normal or pathological processes
(41–43). A disintegrin and metalloproteinase
with thrombospondin motif (ADAMTS14), which is zinc-dependent
metalloproteinase and a member of the ADAMTS family of
extracellular proteases, is involved connective tissue remodeling
and inflammation (44). PDL cells
may play a role in both the production and degradation of versican
through the secretion of ADAMTS1, ADAMTS4 and ADAMTS5 (45).
In this study, the Wnt pathway member, Wnt family
member 2 (WNT2), and Dickkopf-related protein 2 (DKK2), as well as
the transforming growth factor (TGF)β/bone morphogenetic protein
(BMP) pathway member, BMP3, BMP8A and TGFβ3 were upregulated in the
PDL tissues. Cells in the periodontal complex are Wnt responsive,
and removing an important member of the Wnt signaling network gives
rise to a pathological widening of the PDL space (46). However, other BMP family members
(BMP7, BMP6 and BMP5) were upregulated in the DP tissues.
Of note, the PDL expressed more genes associated
with inflammation or immune reaction than did the DP tissues. For
example, CXCL2, 8 and 13, which are associated with chemotaxis,
were upregulated in the PDL tissues. CXCL13, constitutively
expressed in secondary lymphoid tissue, is a potent lymphoid
chemokine (47). Studies have
shown that CXCL13 is associated with B-cell recruitment in chronic
inflammatory periodontal lesions (48). The results of this study suggest
that anti-CXCL13 may be a promising approach to modulate pathogenic
immune responses in PDL tissues.
Dentin sialophosphoprotein (DSPP), the most abundant
non-collagenous protein in dentin, is a marker for DPSC
differentiation into odontoblasts and is essential for the normal
mineralization of dentin (24,25,49,50). DSPP is processed by proteases into
three primary domains: dentin sialoprotein (DSP), dentin
phosphoprotein (DPP) and dentin glycoprotein (DGP). The dentin
matrix proteins (DMPs) lead to tissue calcification due to inherent
calcium binding properties in the ECM (50). Both DSPP and DMP1 were upregulated
in the DP compared with the PDL tissue in this study.
Genes associated with osteogenic [osteopontin (OPN
and osteocalcin (OCN)], osteoclastic [tartrate-resistant acid
phosphatase (TRAP)] and chondrogenic (ACAN) functions were more
strongly expressed in the PDL than in the DP tissue. OPN, indicated
as Spp-1, is a multifunctional sialic acid-rich phosphorylated
glycoprotein. OCN, shown as bone Gla protein, is a major
non-collagenous protein. TRAP, known as ACP5, is involved in
dissolugion of bone mineral though extracellular acidification
(51). ACAN, one of the major
components of the ECM, is mainly responsible for the high
resistance to compression of the load-bearing tissue (52).
RT-qPCR analyses were performed to verify our cDNA
microarray results. ITGA4, ITGA8, NRXN1 and CNTN were upregulated
in the DP relative to the PDL tissue, while COL11A1, ACAN, COL6A1,
CHAD, LAMC2 and LAMA3 were overexpressed in the PDL tissue compared
with the DP tisue. These findings are in line with the microarray
results.
In conclusion, this study compared gene expression
profiles between DP and PDL tissues from human permanent teeth.
Although only the RNA from the entire PD and PDL tissues was
detected in this study, and not from the individual cell that
constitutes these tissues, we consider that our results provide
some novel insight into the characterization of DP and PDL tissues,
and provide the potential molecular mechanisms concerning dental
tissue mineralization and regeneration. The two types of tissue
expressed specific genes related to their functions. The knowledge
generated from this study demonstrated the differences between the
DP and PDL tissues at the molecular biological level and may narrow
down the field of potentially important signaling pathways for
clinically relevant tissue regeneration.
Acknowledgments
We acknowledge financial support from the Nanjing
Medical Science and Technology Development Project (grant no.
YKK15133), and the Maternal and Child Healthcare Project of Jiangsu
Province (grant no. F201557). This work was also funded by the
National Natural Science Foundation of China (grant no. 81230022),
the Priority Academic Program Development of Jiangsu Higher
Education Institutions (grant no. PAPD-2014-37), the Natural
Science Foundation of Jiangsu Province (grant nos. BL2014073 and
15KJA320002) and the Jiangsu Provincial Key Medical Discipline.
References
|
1
|
Chang CC, Chang KC, Tsai SJ, Chang HH and
Lin CP: Neurogenic differentiation of dental pulp stem cells to
neuron-like cells in dopaminergic and motor neuronal inductive
media. J Formos Med Assoc. 113:956–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Volponi AA, Pang Y and Sharpe PT: Stem
cell-based biological tooth repair and regeneration. Trends Cell
Biol. 20:715–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ledesma-Martínez E, Mendoza-Núñez VM and
Santiago-Osorio E: Mesenchymal stem cells derived from dental pulp:
A review. Stem Cells Int. 2016:47095722016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCulloch CA: Proteomics for the
periodontium: Current strategies and future promise. Periodontol
2000. 40:173–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SH, Kim YS, Lee SY, Kim KH, Lee YM,
Kim WK and Lee YK: Gene expression profile in mesenchymal stem
cells derived from dental tissues and bone marrow. J Periodontal
Implant Sci. 41:192–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mrozik KM, Zilm PS, Bagley CJ, Hack S,
Hoffmann P, Gronthos S and Bartold PM: Proteomic characterization
of mesenchymal stem cell-like populations derived from ovine
periodontal ligament, dental pulp, and bone marrow: Analysis of
differentially expressed proteins. Stem Cells Dev. 19:1485–1499.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eleuterio E, Trubiani O, Sulpizio M, Di
Giuseppe F, Pierdomenico L, Marchisio M, Giancola R, Giammaria G,
Miscia S, Caputi S, et al: Proteome of human stem cells from
periodontal ligament and dental pulp. PLoS One. 8:e711012013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multi-potent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cha Y, Jeon M, Lee HS, Kim S, Kim SO, Lee
JH and Song JS: Effects of in vitro osteogenic induction on in vivo
tissue regeneration by dental pulp and periodontal ligament stem
cells. J Endod. 41:1462–1468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang ZH, Zhang XJ, Dang NN, Ma ZF, Xu L,
Wu JJ, Sun YJ, Duan YZ, Lin Z and Jin Y: Apical tooth germ
cell-conditioned medium enhances the differentiation of periodontal
ligament stem cells into cementum/periodontal ligament-like
tissues. J Periodontal Res. 44:199–210. 2009. View Article : Google Scholar
|
|
12
|
Mao JJ and Prockop DJ and Prockop DJ: Stem
cells in the face: Tooth regeneration and beyond. Cell Stem Cell.
11:291–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han X and Amar S: Identification of genes
differentially expressed in cultured human periodontal ligament
fibroblasts vs. human gingival fibroblasts by DNA microarray
analysis. J Dent Res. 81:399–405. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HS, Lee J, Kim SO, Song JS, Lee JH,
Lee SI, Jung HS and Choi BJ: Comparative gene-expression analysis
of the dental follicle and periodontal ligament in humans. PLoS
One. 8:e842012013. View Article : Google Scholar :
|
|
15
|
Song JS, Hwang DH, Kim SO, Jeon M, Choi
BJ, Jung HS, Moon SJ, Park W and Choi HJ: Comparative gene
expression analysis of the human periodontal ligament in deciduous
and permanent teeth. PLoS One. 8:e612312013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SW, Jeon M, Lee HS, Song JS, Son HK,
Choi HJ, Jung HS, Moon SJ, Park W and Kim SO: Comparative
gene-expression analysis of periodontal ligament and dental pulp in
the human permanent teeth. J Korean Acad Pediatr Dent (JKAPD).
43:166–175. 2016. View Article : Google Scholar
|
|
17
|
McLachlan JL, Smith AJ, Bujalska IJ and
Cooper PR: Gene expression profiling of pulpal tissue reveals the
molecular complexity of dental caries. Biochim Biophys Acta.
1741:271–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heikinheimo K, Kurppa KJ, Laiho A,
Peltonen S, Berdal A, Bouattour A, Ruhin B, Catón J, Thesleff I,
Leivo I, et al: Early dental epithelial transcription factors
distinguish ameloblastoma from keratocystic odontogenic tumor. J
Dent Res. 94:101–111. 2015. View Article : Google Scholar
|
|
19
|
Heikinheimo K, Jee KJ, Niini T, Aalto Y,
Happonen RP, Leivo I and Knuutila S: Gene expression profiling of
ameloblastoma and human tooth germ by means of a cDNA microarray. J
Dent Res. 81:525–530. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hochberg Y and Benjamini Y: More powerful
procedures for multiple significance testing. Stat Med. 9:811–818.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Duncan D, Shi Z and Zhang B:
WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013.
Nucleic Acids Res. 41:W77–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Kirov S and Snoddy J: WebGestalt:
An integrated system for exploring gene sets in various biological
contexts. Nucleic Acids Res. 33:W741–W748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering
C, et al: STRING v9.1: Protein-protein interaction networks, with
increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar :
|
|
24
|
Butler WT, Brunn JC and Qin C: Dentin
extracellular matrix (ECM) proteins: Comparison to bone ECM and
contribution to dynamics of dentinogenesis. Connect Tissue Res.
44(Suppl 1): 171–178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Butler WT: Dentin extracellular matrix and
dentinogenesis. Oper Dent. (Suppl 5): 18–23. 1992.PubMed/NCBI
|
|
26
|
Martowicz A, Seeber A and Untergasser G:
The role of EpCAM in physiology and pathology of the epithelium.
Histol Histopathol. 31:349–355. 2016.
|
|
27
|
Mohebiany AN, Harroch S and Bouyain S: New
insights into the roles of the contactin cell adhesion molecules in
neural development. Adv Neurobiol. 8:165–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Missler M, Zhang W, Rohlmann A,
Kattenstroth G, Hammer RE, Gottmann K and Südhof TC:
Alpha-neurexins couple Ca2+ channels to synaptic vesicle
exocytosis. Nature. 423:939–948. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Graf ER, Zhang X, Jin SX, Linhoff MW and
Craig AM: Neurexins induce differentiation of GABA and glutamate
post-synaptic specializations via neuroligins. Cell. 119:1013–1026.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hessle L, Stordalen GA, Wenglén C, Petzold
C, Tanner E, Brorson SH, Baekkevold ES, Önnerfjord P, Reinholt FP
and Heinegård D: The skeletal phenotype of chondroadherin deficient
mice. PLoS One. 8:e630802013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fukumoto S and Yamada Y: Review:
Extracellular matrix regulates tooth morphogenesis. Connect Tissue
Res. 46:220–226. 2005. View Article : Google Scholar
|
|
32
|
Iwamoto DV and Calderwood DA: Regulation
of integrin-mediated adhesions. Curr Opin Cell Biol. 36:41–47.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Franceschi N, Hamidi H, Alanko J,
Sahgal P and Ivaska J: Integrin traffic - the update. J Cell Sci.
128:839–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu Q, Safavi KE and Spångberg LS:
Integrin expression in human dental pulp cells and their role in
cell attachment on extracellular matrix proteins. J Endod.
24:641–644. 1998. View Article : Google Scholar
|
|
35
|
Degistirici O, Jaquiery C, Schönebeck B,
Siemonsmeier J, Götz W, Martin I and Thie M: Defining properties of
neural crest-derived progenitor cells from the apex of human
developing tooth. Tissue Eng Part A. 14:317–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mercurio AM: Laminin receptors: Achieving
specificity through cooperation. Trends Cell Biol. 5:419–423. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aota S and Yamada KM: Fibronectin and cell
adhesion: Specificity of integrin-ligand interaction. Adv Enzymol
Relat Areas Mol Biol. 70:1–21. 1995.PubMed/NCBI
|
|
38
|
Popova SN, Barczyk M, Tiger CF, Beertsen
W, Zigrino P, Aszodi A, Miosge N, Forsberg E and Gullberg D:
Alpha11 beta1 integrin-dependent regulation of periodontal ligament
function in the erupting mouse incisor. Mol Cell Biol.
27:4306–4316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Barczyk M, Bolstad AI and Gullberg D: Role
of integrins in the periodontal ligament: Organizers and
facilitators. Periodontol 2000. 63:29–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen J, McCulloch CA and Sodek J: Bone
sialoprotein in developing porcine dental tissues: Cellular
expression and comparison of tissue localization with osteopontin
and osteonectin. Arch Oral Biol. 38:241–249. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hannas AR, Pereira JC, Granjeiro JM and
Tjäderhane L: The role of matrix metalloproteinases in the oral
environment. Acta Odontol Scand. 65:1–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beklen A, Al-Samadi A and Konttinen YT:
Expression of cathepsin K in periodontitis and in gingival
fibroblasts. Oral Dis. 21:163–169. 2015. View Article : Google Scholar
|
|
43
|
Sorsa T, Tjäderhane L, Konttinen YT,
Lauhio A, Salo T, Lee HM, Golub LM, Brown DL and Mäntylä P: Matrix
metalloproteinases: Contribution to pathogenesis, diagnosis and
treatment of periodontal inflammation. Ann Med. 38:306–321. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tang BL: ADAMTS: A novel family of
extracellular matrix proteases. Int J Biochem Cell Biol. 33:33–44.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sone S, Nakamura M, Maruya Y, Takahashi I,
Mizoguchi I, Mayanagi H and Sasano Y: Expression of versican and
ADAMTS during rat tooth eruption. J Mol Histol. 36:281–288. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lim WH, Liu B, Cheng D, Williams BO, Mah
SJ and Helms JA: Wnt signaling regulates homeostasis of the
periodontal ligament. J Periodontal Res. 49:751–759. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cyster JG, Ansel KM, Reif K, Ekland EH,
Hyman PL, Tang HL, Luther SA and Ngo VN: Follicular stromal cells
and lymphocyte homing to follicles. Immunol Rev. 176:181–193. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nakajima T, Amanuma R, Ueki-Maruyama K,
Oda T, Honda T, Ito H and Yamazaki K: CXCL13 expression and
follicular dendritic cells in relation to B-cell infiltration in
periodontal disease tissues. J Periodontal Res. 43:635–641. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu M, Sun Y, Liu Y, Yuan M, Zhang Z and
Hu W: Modulation of the differentiation of dental pulp stem cells
by different concentrations of β-glycerophosphate. Molecules.
17:1219–1232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ravindran S and George A: Dentin matrix
proteins in bone tissue engineering. Adv Exp Med Biol. 881:129–142.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Karlström E, Ek-Rylander B, Wendel M and
Andersson G: Isolation and phenotypic characterization of a
multinucleated tartrate-resistant acid phosphatase-positive bone
marrow macrophage. Exp Hematol. 39:339–350.e3. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Arner EC: Aggrecanase-mediated cartilage
degradation. Curr Opin Pharmacol. 2:322–329. 2002. View Article : Google Scholar : PubMed/NCBI
|