Introduction
Recently, various types of biomaterials have been
developed for tissue engineering aims. Used as scaffolds,
biomaterials should provide a suitable microenvironment for the
cells to survive, proliferate, differentiate and migrate (1). Ideal biomimetic scaffolds are
required to possess a series of characteristics: excellent
biocompatibility, low immunogenicity, appropriate porosity,
permeability and mechanical elasticity, and they need to be easily
synthesized (2). Hydrogels, as
scaffolding biomaterials, are water-swollen polymeric networks, but
they cannot dissolve in water. The attractive features to swell
under physiological conditions make them ideal candidates for
tissue engineering applications (3–7).
Hydrogels possess a 3D network structure with physical or chemical
cross-linking. This insoluble cross-linked gel-network possesses
the ability to release active agents and biomolecules effectively.
Due to the high water content, hydrogels generally exhibit good
biocompatibility and high permeability for oxygen, nutrition
supply, bioactive factors and waste products exchange from cells.
That is why hydrogels are regarded as ideal scaffolds for use in 3D
cell culture (5–9). In addition, most hydrogels can be
used as injectable scaffolds for a variety of applications
(10–12). Based on the polymer origin,
hydrogels can be classified into three major types: natural,
synthetic and synthetic/natural hybrid hydrogels. As important
natural hydrogel biomaterials, self-assembling peptides (SAPs) have
emerged as promising scaffolds for tissue engineering applications
over the past two decades.
Among the SAPs, the most widely studied is RADA16
which consists of 16 alternating hydrophobic and hydrophilic amino
acids. RADA16 spontaneously assembles into interwoven nanofiber
scaffolds in water and subsequently forms hydrogel (13,14). Therefore, RADA16 supports a true
3D culture environment with high aspect ratio nanofibers that mimic
the extracellular matrix (ECM) for cell growth, migration and
differentiation (15–17). Moreover, RADA16 is easy to
functionalize using biologically active epitopes and enhances the
bioactivity (18–22). The functionalized RADA16 scaffolds
have been found to be versatile in bone, cartilage, neural and
heart tissue regeneration, wound healing, angiogenesis, osteosis
and hemostasis (23–28).
Despite the advantages of RADA16 mentioned, the
biomaterial scaffolds made of L-amino acids may degrade more
rapidly in vivo by proteases than desired, and such an
instability limits its range of applications for achieving
long-term biostability (29).
Recently, active efforts have been made to maintain the stability
of SAP from enzymatic decomposition in vivo, and D-amino
acid possesses remarkable potentiality to address this issue
(30–32). However, preliminary studies have
suggested that D-amino acid in the peptides backbone causes
conformational changes of the peptides, evidently disrupting the
self-assembly process, and decreasing the flexibility of the
hydrogels (29). We hereby
designed a SAP, D-RADA16, which was made of only D-amino acids to
avoid the disruption of supramolecular self-assembly.
The present study focused on evaluating whether
D-RADA16 forms stable β-sheet structure in water and further
self-assembles into interweaving nanofiber scaffolds, providing a
similar 3D microenvironment with which to promote the proliferation
and migration of rat bone marrow-derived mesenchymal stem cells
(BMSCs), similar to L-RADA16. Additionally, the bioactivity and
biostability of D-RADA16 was assessed. We first confirmed the
self-assembling ability of D-RADA16 by circular dichroism (CD)
spectroscopy and transmission electron microscopy (TEM). Various
concentrations of both peptides were examined by MTT assay to
investigate the optimal concentration for BMSC proliferation in
vitro. BMSCs were cultured in the SAP hydrogels for
osteoblastic differentiation and migration observation. Lastly, we
appraised the enzymatic stability of the SAPs with proteinase K.
Our results suggested that D-RADA16 hydrogel scaffolds exhibited
satisfactory bioactivity and biostability, which may ultimately
broaden the scope of long-term applications in vivo.
Materials and methods
Peptide synthesis and purification
The SAP scaffolds, RADA16
(AcN-RADARADARADARADA-CONH2), were custom-synthesized (Shanghai
Bootech Bioscience and Technology Corp., Ltd., Shanghai, China)
through direct solid phase synthesis, purified by high-performance
liquid chromatography (HPLC) (Thermo Electron Corp., Waltham, MA,
USA), and characterized by mass spectroscopy (Waters Corp. Milford,
MA, USA). The purity of D-RADA16 and L-RADA16 was 95.90 and 95.54%,
respectively. The peptide D-RADA16 sequence contained all D-amino
acids, and the L-RADA16 sequence contained all L-amino acids.
Solutions of the peptides were prepared at concentrations of
1.0–10.0 mg/ml (0.1–1.0%, w/v) in water (18 MΩ•cm; Millipore
Milli-Q System, Billerica, MA, USA) and stored at 4°C before
use.
Reagents
Dulbecco's modified Eagle's medium/F12 (DMEM/F12),
fetal bovine serum (FBS) and 0.25% trypsin/EDTA were purchased from
HyClone (Logan, UT, USA). Penicillin/streptomycin aqueous solution,
MTT assay, β-glycerophosphate, dexamethasone, ascorbic acid and
proteinase K were all obtained from Sigma Chemical Co. (St. Louis,
MO, USA). The antibodies used for western blot analysis were all
purchased from Abcam PLC (Cambridge, UK). Calcein-AM was purchased
from Enzo Life Sciences, Inc. (Farmingdale, NY, USA).
Molecular models and chemical
structures
Molecular models and chemical structures of the
chiral peptides were constructed using free modeling software
(Hyperchem professional version 7.5, http://www.hyper.com) and chemical structure drawing
software (ChemDraw Ultra 14.0) respectively.
CD spectroscopy
The samples consisted of 1.0 mg/ml aqueous stock
solutions of peptide and were adjusted to 0.17 mg/ml in solution in
water. A sample of 400 µl was added in a CD cuvette with a 2
mm path-length. Measurements were carried out on an J-810 CD
spectrometer (JASCO International Co., Tokyo, Japan). The samples
were incubated at 20°C, equilibrated for 30 sec, measured from 190
to 260 nm, averaged over 3 sec through the entire wavelength range,
and took the 190–250 nm analysis.
TEM
TEM samples (2.0 mg/ml) were prepared at 25°C. A
micropipet was used to load an aliquot of 5 µl of peptide
solution to a carbon coated copper grid. The excess solution was
removed by a piece of filter paper. The samples were dyed by 10
µl uranyl acetate for 30 sec and dried overnight in a
desiccator and then conducted on a Tecnai G2 F20 system (FEI
Company, Hillsboro, OR, USA) operating at 200 kV.
Preparation and culture of BMSCs
A total of 18 male Sprague-Dawley rats, 4 weeks of
age, were used in this study. The rats were obtained from the
Laboratory Animal Center of Chongqing Medical University,
Chongqing, China. BMSCs were isolated from the bone shaft of the
rats according to the technique reported by Lennon et al
(33) and the protocol was
approved by the Ethics Committee of the First Affiliated Hospital
of Chongqing Medical University (permit no. 2014-201058). Briefly,
the rats were sacrificed by an overdose of isoflurane. The bone
marrow was flushed out from the femurs by a syringe (21-gauge
needle) with 5 ml of DMEM/F12 containing 10% FBS and 1%
penicillin/streptomycin (200 U/ml). The cell suspension was placed
into two T-25 flasks (Nest Biotechnology Co., Jiangsu, China) and
cultured at 37°C in an atmosphere with 95% humidity and 5%
CO2. The medium was changed on the second day of culture
and every 3 days thereafter. When the cells became subconfluent,
they were detached from the flask by treatment with an aqueous
solution of 0.25% trypsin/EDTA for 3 min at 37°C. The cells were
normally passaged at a density of 2×104
cells/cm2. Cells at the third passage at subconfluence
were used in all the experiments.
Three-dimensional cell culture technique
using the chiral RADA16
In the case of cell viability assay, the chiral
scaffolds at various concentrations (1.25, 2.5, 5.0 and 10.0 mg/ml)
were prepared as L-RADA16 and D-RADA16. Each of the solution was
sonicated for 30 min and loaded (5 µl) in the bottom of
96-well culture plates (Nest Biotechnology Co.). Subsequently, 50
µl medium were slowly added to the solution to induce
gelation. The hydrogel formed in several minutes, and was rinsed
twice with medium to equilibrate the gel to physiological pH. The
hydrogels were thyen incubated overnight at 37°C with 5%
CO2 until cell seeding. Lastly, 50 µl of BMSCs
cells (4×104 cells/ml) in DMEM/F12 mixture were seeded
in each well on top of the various hydrogels in 96-well culture
plates, where they were able to settle into the nanofiber
scaffolds. The 3D cell cultures were maintained in an incubator at
37°C with 5% CO2 for 3, 5 or 7 days and the medium was
changed every 2–3 days if necessary.
In the case of differentiation assay, 500 ml of
peptide solution (5 mg/ml) were directly loaded into a 6-well
culture plate (Nest Biotechnology Co.) and then stimulated to
self-assemble following the addition of 1 ml culture medium in each
well. During the following 30 min, the medium was changed twice to
equilibrate the growth environment to physiological pH and then
incubated for 2 h at 37°C with 5% CO2 for gelation.
Subsequently, 200 µl of BMSCs (1×106 cells/ml) in
DMEM/F12 mixture were seeded on top of the hydrogel. The cells were
cultured in the differentiation media consisting of 10 mM
β-glycerophosphate, 10 nM dexamethasone, 50 µg/ml ascorbic
acid. The media were changed every 3 days.
In the case of the migration assay, cell culture
Transwell inserts (Corning Inc., Corning, NY, USA) were used for
peptide hydrogelation as previously reported (20). Briefly, the inserts were placed in
a 24-well culture plate (Nest Biotechnology Co.) with 400 µl
culture medium in the lower chambers of each well. One hundred
microliters of peptide solutions solution (5 mg/ml) were directly
loaded into the upper chamber of each well. The medium was changed
twice and incubated as described in the differentiation assay. To
prevent the drying of the surfaces of the formed hydrogels, 400 ml
of culture medium were gently layered onto the hydrogels and then
incubated overnight at 37°C with 5% CO2. Two hundred
microliters of BMSCs (1×105 cells/ml) in DMEM/F12
mixture were seeded on top of the peptide gel in the inserts. The
cells were cultured in the maintenance medium (DMEM/F12 containing
10% FBS and 1% penicillin/streptomycin) and this was changed every
3 days by removing 400 µl of medium from the lower chambers
and adding 400 µl of fresh medium inside the upper chambers.
The cells were harvested at planned time points for analysis.
In addition, a conventional 2D cell culture method
(tissue culture plate) was used to culture the BMSCs to examine
their proliferation and migration as a control.
Cell viability assay
To determine the extent of the proliferation of the
cells seeded on the chiral scaffolds, a well-characterized
quantitative assat, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl
tetrazolium bromide test (MTT) assay was performed. Briefly, after
the cells were incubated for 3, 5 or 7 days, the medium was removed
and the cells were treated with 20 µl of 5 mg/ml MTT
solution. Follwoing incubation at 37°C for 4 h, the MTT solution
was removed and 150 µl of dimethyl sulfoxide (DMSO) were
added to dissolve the insoluble formanzan crystals. After 20 min,
the absorbance was read at 490 nm using a Multiskan Spectrum
spectrophotometer (Thermo Fisher Scientific, Vantaa, Finland).
Since some hydrogels may dissolve partially when removing the
formanzans, we tested the hydrogels without cells to confirm any
possible bias in the absorbance measurements, and found no
significant differences between any of the tested scaffolds and the
scaffolds without cells (data not shown). In order eliminate dye
absorbance derived from medium, a background group was added to the
analysis groups. Three independent experiments comprising 4
replicates each were performed.
Western blot analysis
At the end of the culture period, the cells were
lysed for 30 min in ice-cold RIPA buffer (BCA; Beyotime, Jiangsu,
China). The cell lysate was centrifuged at 12,000 rpm for 20 min at
4°C. The supernatants were heated at 100°C for 5 min in 5× loading
buffer (Beyotime). Equal aliquots of protein (40 µg) were
loaded onto 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto PVDF membranes
(0.45 µm). The membranes were blocked for 1 h at room
temperature by 5% non-fat milk, washed 3 times and incubated with
primary antibodies [anti-osteopontin (OPN; ab8448, 1:1,000),
anti-runt-related transcription factor 2 (RUNX2) (ab23981, 1:1,000)
and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (ab8245,
1:2,000) (all from Abcam PLC)] at 4°C overnight. The membranes were
then washed in TBST 3 times and incubated with corresponding
horseradish peroxidase conjugated secondary antibody (goat
anti-rabbit) for 1 h at room temperature. The blots were developed
by a chemiluminescence kit (Beyotime) on a Bio-Rad imaging
system.
3D cell migration assay
Cell culture was performed as described above. After
being cultured for 7 days, the cells on the hydrogels were examined
using calcein-AM staining, an indicator of intracellular esterase
activity which stains whole living cells, according to the
manufacturer's instructions. Briefly, cells on the chiral scaffolds
of the inserts were washed twice using phosphate-buffered saline
(PBS). Subsequently, 4 µM calcein-AM solution were added to
the insert and incubated for 30 min within the incubator at 37°C
with 5% CO2. The inserts were rinsed twice with PBS and
multiple images were obtained with confocal laser scanning
microscopy (Nikon A1R; Nikon, Tokyo, Japan) through z-stack
scanning mode with a step size of 3 µm. The fiber diameters
were examined using Image-Pro Plus software (version 6.0) analysis
software. For each image, 200 fibers were measured and
recorded.
Peptide degradation by proteinase K
We dissolved 1 mg of peptide in 5 ml 0.01 M PBS, and
0.25 mg proteinase K was added to the solution, resulting in a 0.2
and 0.05 mg/ml concentration of peptide and proteinase K,
respectively. The samples, consisting of hydrogels and proteinase
K, were incubated at 37°C on shaking tables for 1–24 h and 400
µl aliquots were taken after 1, 2, 4, 6, 8, 12 and 24 h. The
enzymatic reaction was terminated by the addition of
phenylmethanesulfonylfluoride (PMSF) and the concentration of the
intact peptide was analyzed by HPLC (LCMS-2020; Shimadzu, Kyoto,
Japan) using following conditions: column: Venusil XBP C18, 5
µm, 4.6×50 mm (Agela, Tianjin, China); sample injection
volume: 100 µl; gradient elution: A, 0.035% trifluoroacetic
acid (TFA) in water; and B, 0.035% TFA in acetonitrile/water
(80:20, vol/vol). A gradient of 10–100% B in 10 min was conducted.
The experiment was conducted in 3 replicates.
Statistical analysis
Data are presented as the means ± standard deviation
(SD) and compared between any 2 groups using a two-tailed paired
t-test. The differences between multiple group comparisons were
made by one-way ANOVA and followed by multiple pairwise comparisons
using Fisher's least significant difference (LSD) test.
Significance levels were set to P<0.05 for all comparisons.
Results
Hydrogelation of L-RADA16 and
D-RADA16
We observed the formation of hydrogelation after 30
min. Both of the peptide solutions were obtained by dissolving the
peptide powder in water at the concentration of 10 mg/ml. As shown
in Fig. 1A, we found that the
introduction of D-amino acids did not effect on the gelation
behavior of the SAP solution.
Molecular models and chemical structures
of L-RADA16 and D-RADA16
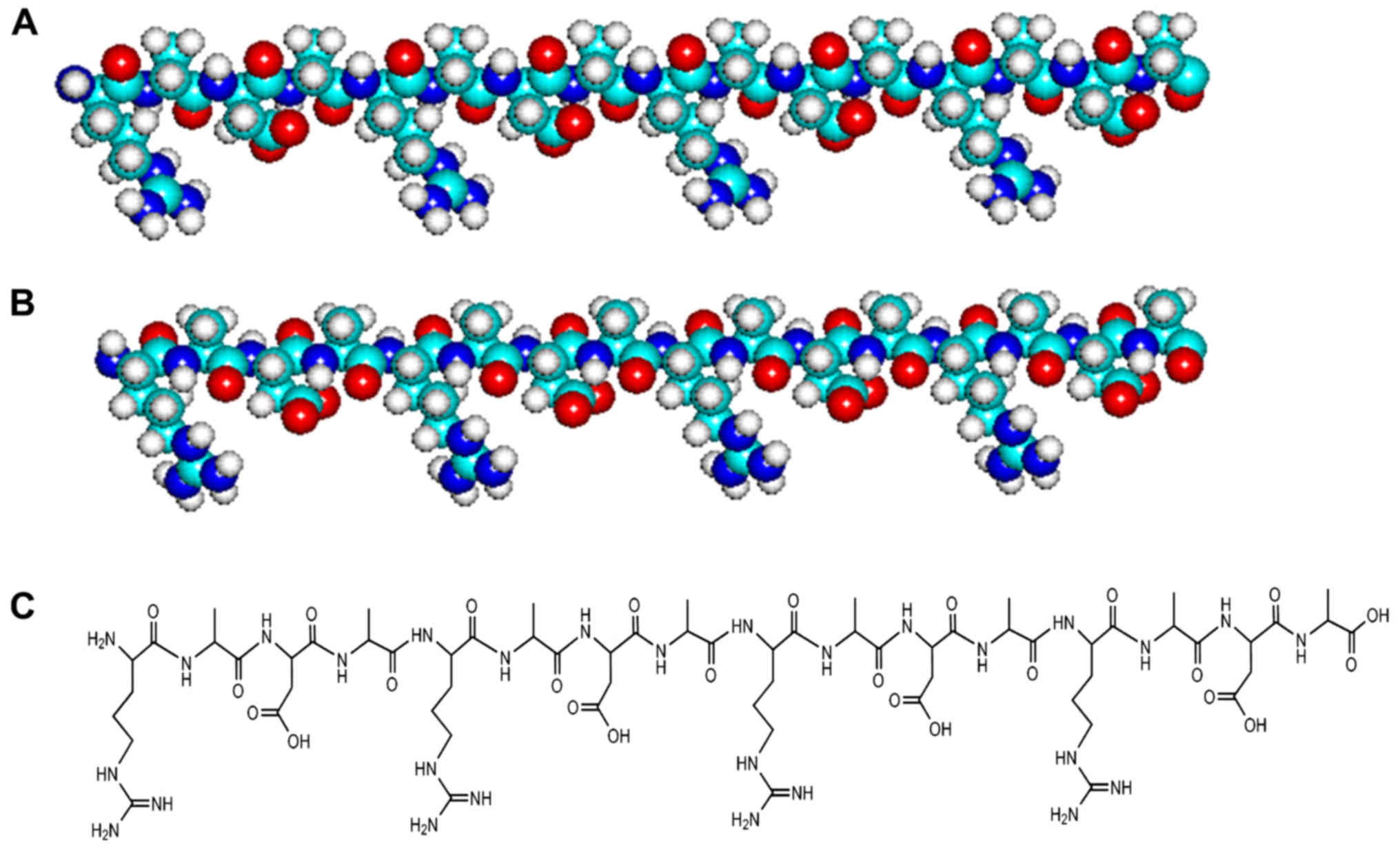
We present two molecular models (Fig. 2A and B) and chemical structures
(Fig. 2C) of L-RADA16 and
D-RADA16 peptides. These peptides have an identical sequence, but
are composed of amino acids of a different chiral form: all L-amino
acids in L-RADA16 and all D-amino acids in D-RADA16. The pair of
RADA16 structures appears similar, but some of their properties are
quite diverse.
Structural characterizations of the
chiral RADA16
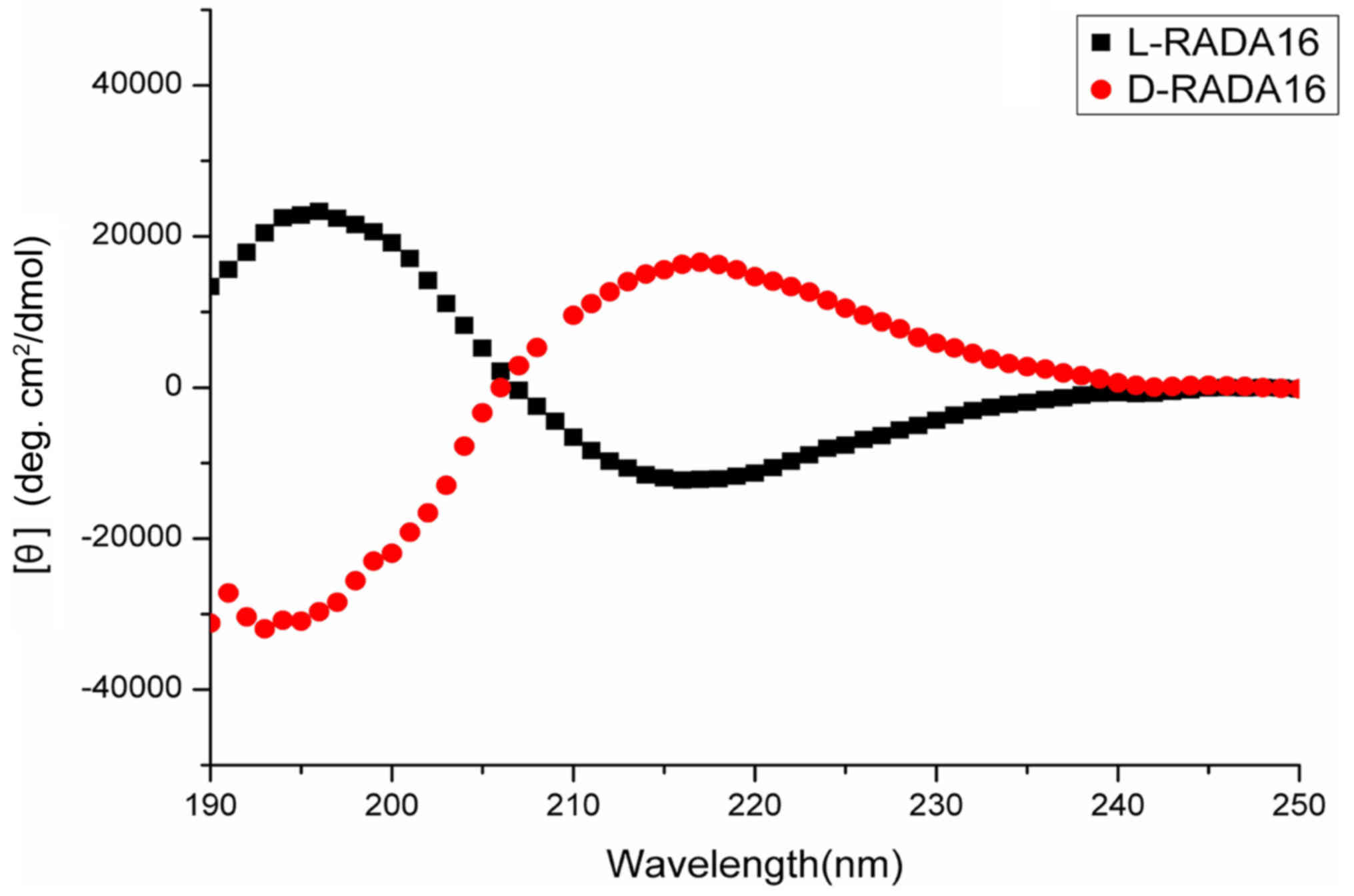
The secondary structures of the chiral RADA16
peptides were examined by CD measurements. From a previous study,
L-RADA16 is known to form a stable β-sheet structure (21). In this study, CD spectroscopy
revealed that the D-RADA16 was not only able to adopt a typical
β-sheet structure, but also had an inverted β-sheet spectrum with a
positive peak at 216.8 nm and a negative peak at 193.1 nm (Fig. 3), which was almost a mirror image
of the L-RADA16 spectrum which had an β-sheet spectrum with a
positive peak at 195.7 nm and a negative peak at 216.2 nm. This
suggests that D-RADA16 is the enantiomer of L-RADA16.
Self-assembling nanofiber morphology of
the chiral peptides
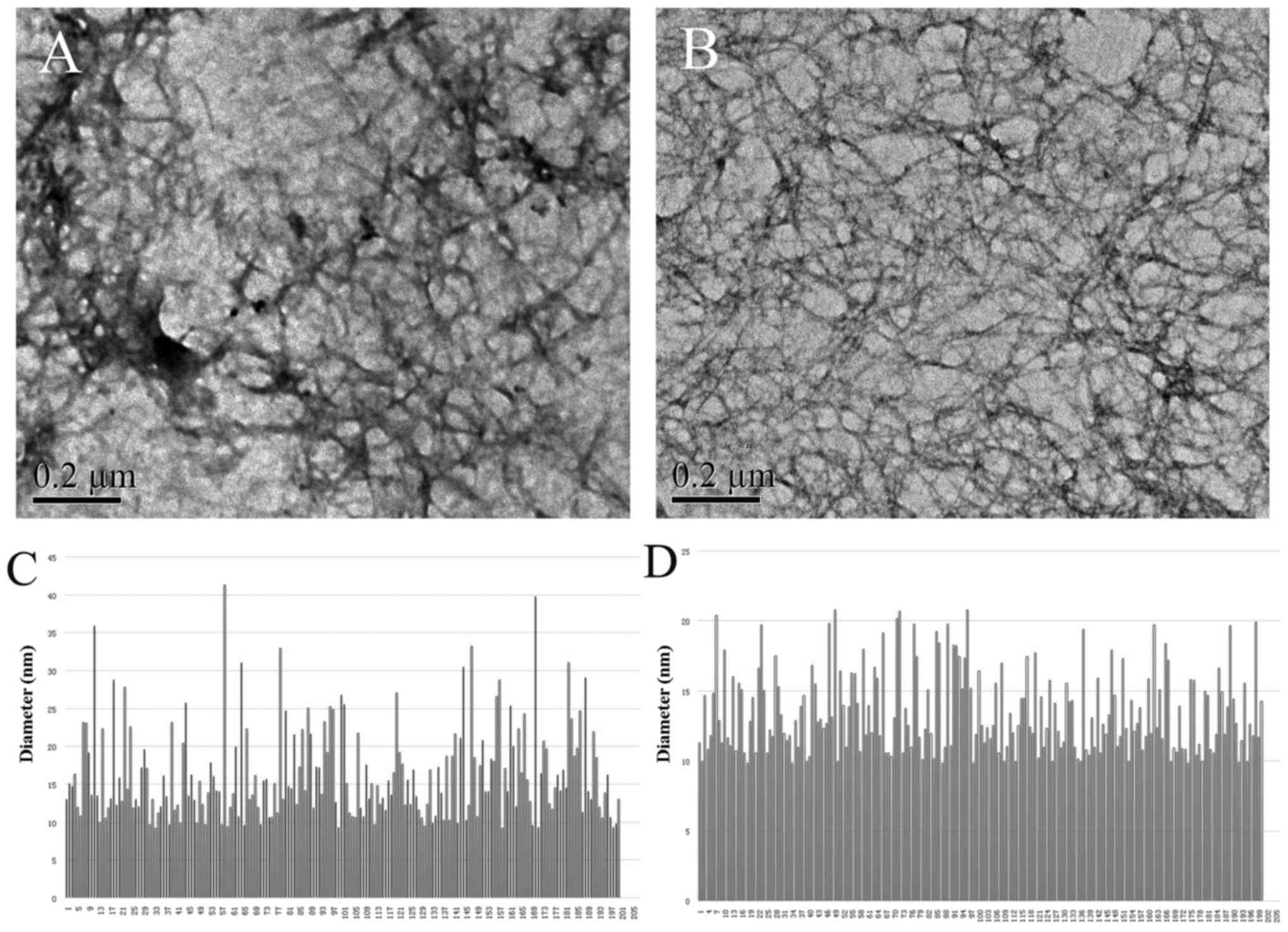
A previous study demonstrated that the peptide
L-RADA16 possesses the ability of self-assembly into interwoven
nanofibers (21). In the present
study, we wished to determine whether D-RADA16 made of D-amino
acids would trigger self-assembly process and form well-ordered
nanofibers. TEM morphological analyses denoted that D-RADA16 indeed
formed ordered nanofibers ranging in length from several hundred
nanometers to a few microns, and the diameters of nanofibers
assembled from L-RADA16 and D-RADA16 were 16.34±6.13 and 13.52±2.94
nm, respectively (Fig. 4). These
observations are in agreement with those of previous findings
(34,35).
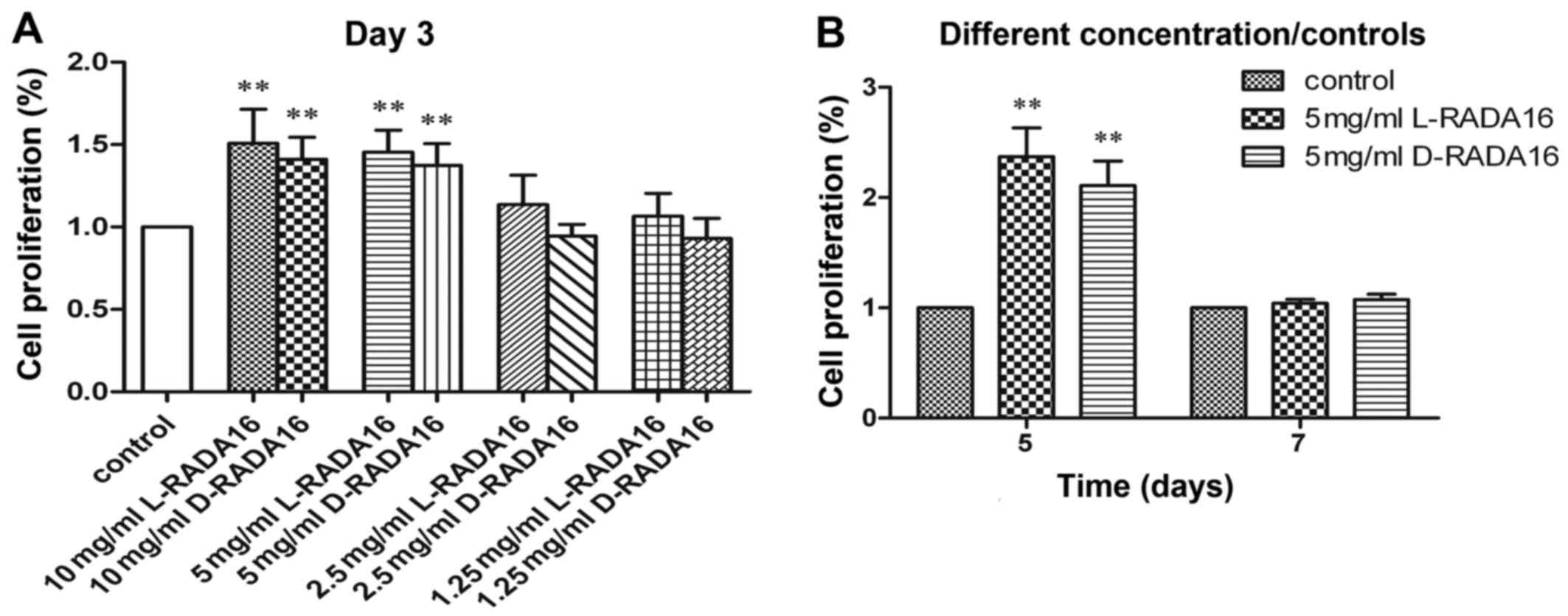
Cell viability
BMSCs were grown on the chiral scaffolds at various
concentrations from 1.25 to 10 mg/ml. The cell proliferation rate
of the 3D cell culture method and the conventional 2D cell culture
method as a control were performed for 3 days (Fig. 5A). We noted that there were no
significant difference in the proliferation rates between the
chiral scaffolds at each concentration after 3 days of culture. The
peptides at low concentrations of 1.25 or 2.5 mg/ml did not lead to
a statistically significant difference in the cell proliferation
rate in comparison to the control. By contrast, the peptides at
high concentrations of 5 or 10 mg/ml led to a statistically
significant difference in the cell proliferation rate in comparison
to the control. These results indicated that the chiral peptide
scaffolds at relatively high concentrations promoted cell
proliferation. However, there was no statistically significant
difference in the cell proliferation rate when the peptide at 5
mg/ml was compared to a higher concentration of the peptide at 10
mg/ml. Therefore, the optimal concentration of the chiral peptides
for cell culture was 5 mg/ml, and this concentration was selected
for use in further experiments.
Subsequently, BMSCs were grown on the chiral
scaffolds at 5 mg/ml and the tissue culure plate for 5 and 7 days
(Fig. 5B). Again, the chiral
scaffolds possessed similar cell-scaffold bioactivity at each time
point. Furthermore, significant differences in the chiral scaffolds
compared to the control were noted after 5 days of culture.
Nonetheless, there were no significant differences in the chiral
scaffolds compared to the control after 7 days of culture, and this
can be explained by the fact that cells stop growing when they
reach confluence. Since L-RADA16 has been proven to be non-toxic
and non-immunogenic (36,37), we hypothesized that D-RADA16
displayed no apparent toxicity in vitro under our present
experimental conditions.
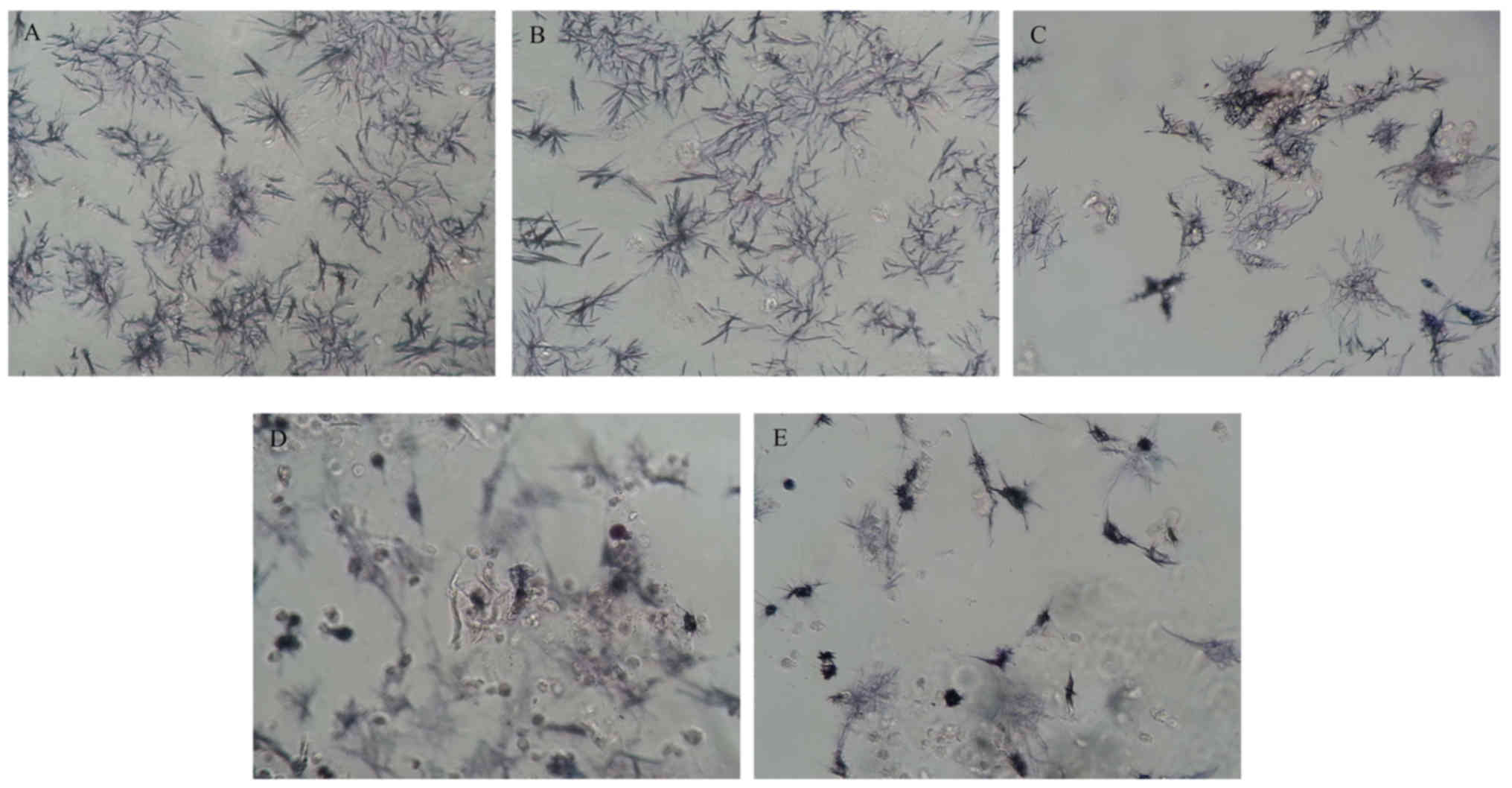
According to the protocol of MTT assay, after the
cells were incubated for a given period of time, MTT solution was
added to each sample and MTT was reduced by metabolically active
cells to insoluble purple formazan dye crystals. We serendipitously
observed the crystals under an inverted phase contrast microscope
(Fig. 6). Of note, the seeded
cell planes were out of focus, overlapping the focused plane,
resulting in relatively fuzzy images when they were grown on
D-RADA16 scaffolds at 5 and 10 mg/ml. This fact suggests that the
formazan exhibits various 3D morphologies at relatively high
concentrations of the D-RADA16 scaffold. By contrast, clear images
can be captured when the cells were cultured in the concentrations
of 0.125 and 2.5 mg/ml, and the control, denoting that formazan
retained 2D morphologies in the control and at low concentrations
of the D-RADA16 scaffolds.
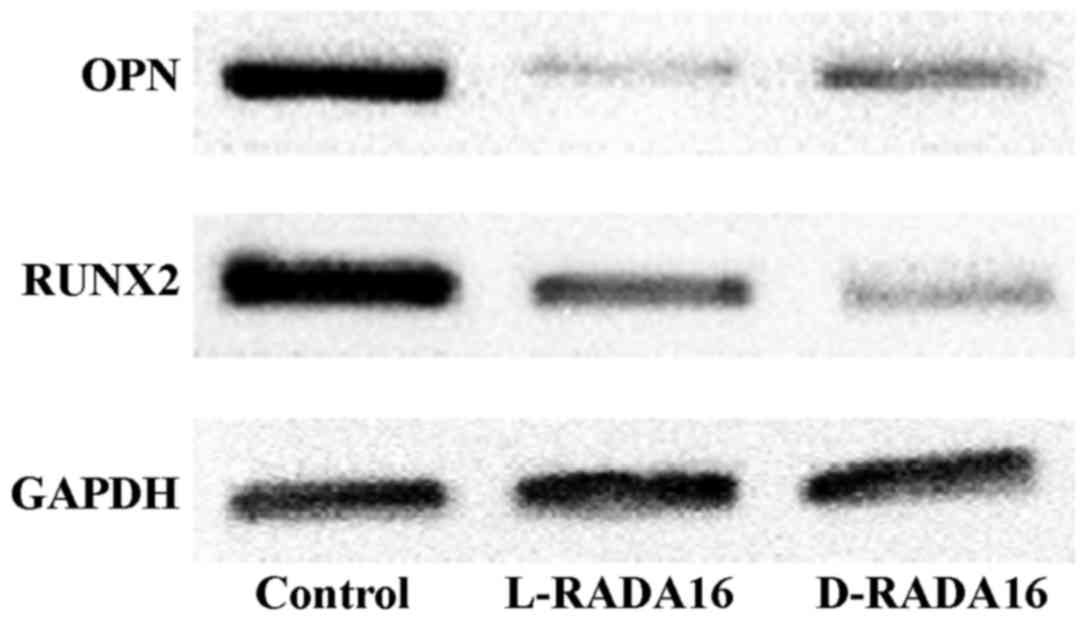
Effects of chiral peptide scaffolds on
the osteogenic differentiation of BMSCs
The BMSCs were cultured in the SAP hydrogels to
evaluate the osteogenic differentiation level at day 7. As a
control, the BMSCs were cultured with the conventional 2D cell
culture method. The relative expression level of RUNX2, osteopontin
(OPN) was examined by western blot analysis. GAPDH was used as an
internal control (n=3). For all proteins, the two 3D scaffold
groups possessed a significantly lower expression than the 2D
culture control group (Fig. 7).
The results indicated that the chiral SAP scaffolds did not promote
the osteogenic differentiation of the BMSCs in vitro under
our present experimental conditions.
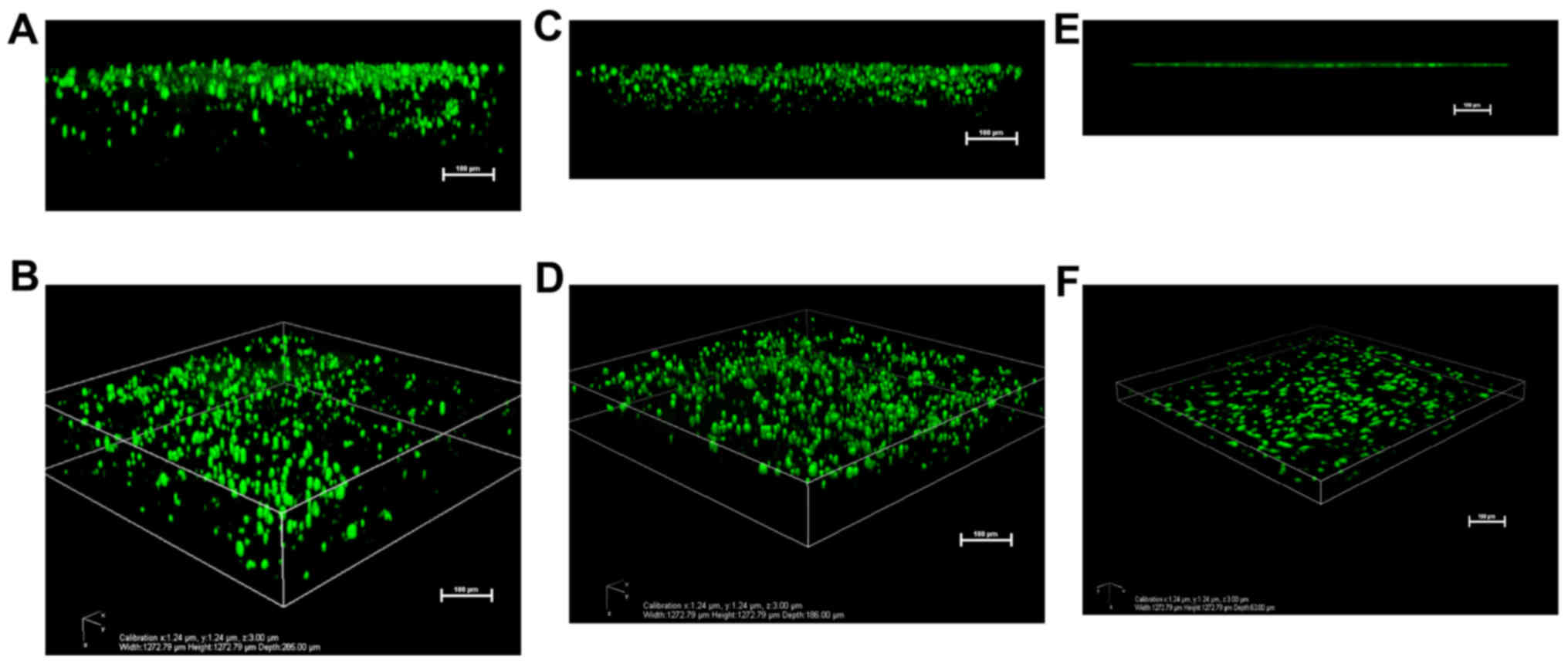
Cell migration into 3D chiral peptide
hydrogel scaffolds
In addition to the cell viability assay, the BMSCs
were seeded in the chiral peptide hydrogels (Fig. 8A–D) and the tissue culture plate
(Fig. 8E and F) to examine their
3D migration using calcein-AM staining. The confocal microscopy 3D
reconstruction images revealed that cells migrated into the
L-RADA16 (Fig. 8A and B) and
D-RADA16 (Fig. 8C and D)
scaffolds at ~285 and ~186 µm, respectively. The results
demonstrated that both of the two chiral peptides promoted cell
migration into the 3D nanofiber scaffolds. They are consistent with
those of a previous study on human adipose stem cells that migrated
into 3D peptide hydrogel scaffolds (20).
Stability of peptides in proteinase
K
In addition, the chiral peptides were also examined
for enzymatic stability against proteinase K, a potent and highly
non-specific proteolytic enzyme. To avoid the effects of resistance
due to hydrogelation, the concentration of each SAP was set at 0.2
mg/ml, which is markedly lower than the critical gelation
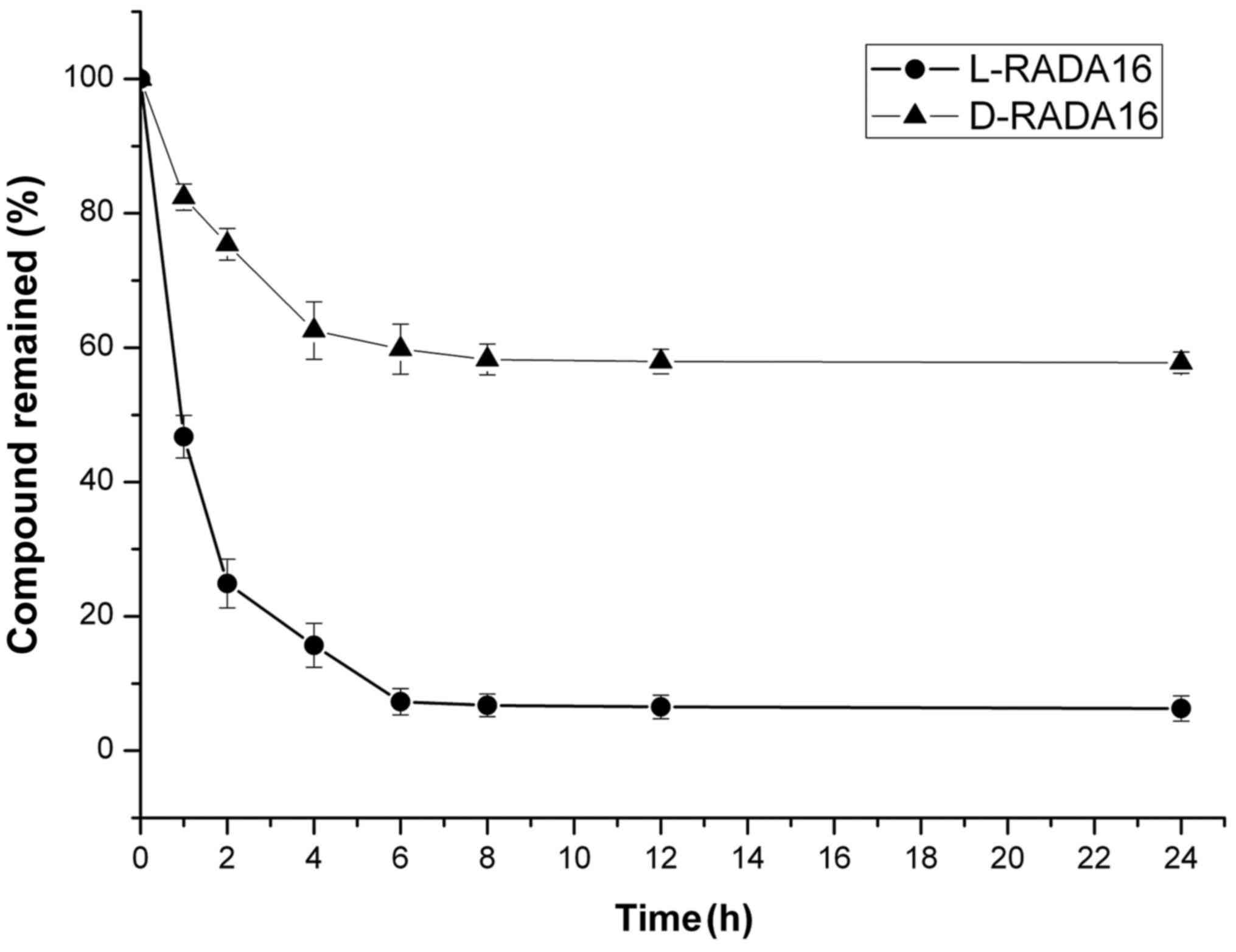
concentration of the hydrogel. As shown in Fig. 9, ~75 and 60% of D-RADA16 remained
after 2 and 6 h of incubation with proteinase K respectively, and
>50% remained after 24 h. As for L-RADA16, <25 and 8% of SAP
remained after 2 and 6 h of incubation respectively. These results
illustrate that D-RADA16 exhibits stronger resistance to proteinase
K digestion.
Discussion
In this study, we devised a SAP D-RADA16 made of
only D-amino acids. It can self-assemble into interweaving
nanofibers in aqueos solution, provide a suitable 3D network which
allows the transport of oxygen, bioactive factors, nutrients and
waste products. It serves further as a medium through which the
diffusion of soluble factors and the migration of cells can occur.
Firstly, CD spectroscopy reveaed that the D-RADA16 not only adopted
a typical β-sheet structure, but was almost also a mirror image of
the L-RADA16 spectrum. This denotes that D-RADA16 and L-RADA16 are
enantiomers. The β-sheet structure is essential for peptides in
tissue engineering, as this structure has the ability of nanofiber
formation and provides support to the cytoskeleton fiber (38). Previous studies have demonstrated
that L-RADA16 can self-assemble into a stable β-sheet structure at
physiologic conditions, forming interwoven nanofiber scaffolds that
mimic the architecture of the ECM (34,39–41). Therefore, it is believable that
D-RADA16 can form nanofiber scafflods as well. We have also
performed a rheology experiment to investigate the mechanical
property of the peptides hydrogelsrheology experiment (unpublished
data). The values of storage moduli (G′) were close to the values
of the loss moduli (G″) when the concentration of L-RADA16 or
D-RADA16 was 2.5 mg/ml. Thus, they exhibited a liquid-like
rheological behavior. When the concentration of the peptides
hydrogels was 5.0 mg/ml, the G′ values of the hydrogels were
greater than the G″ values, suggesting the formation of true gels.
The results revealed that the introduction of D-amino acids do not
affect the gelation behavior of the SAP solution and the minimum
gelation concentration of the peptides is 5.0 mg/ml. Secondly, in
the present study, the formation of uniform nanofibers of D-RADA16
observed by TEM (Fig. 4)
indicated that the self-assembly process had been successfully
attained and the designer SAP D-RADA16 shared the similar fine
structure to L-RADA16. Finally, our study suggests that the
chirality of peptides does not interfere with the molecular
self-assembly nor the formation of well-defined nano-structures.
This is in accordance with the findings of Luo et al
(42), who evaluated the chiral
peptides of EAK16.
Subsequently, we assessed whether the D-RADA16
scaffold, as the enantiomer of L-RADA16, can provide a suitable
microenvironment for BMSC growth, proliferation, osteogenic
differentiation and migration. We isolated BMSCs from the
Sprague-Dawley rats and successfully cultured them in a 3D
microenvironment on SAP scaffolds. BMSCs were selected as they are
widely regarded as a stem cell for osteoblasts, differentiating
along an osteogenic lineage when properly stimulated (43). Additionally, BMSCs are locally
accessible at the bone microenvironment, being one of the first
major cell types recruited to the surface of implanted bone
biomaterials (44). These
distinguishing properties render BMSCs a promising candidate for
subsequent bone repair in vivo. Subsequently, MTT assay was
carried out to assess the proliferation rate of the BMSCs seeded on
the chiral scaffolds at different SAP concentrations and the
traditional tissue culture plate. The results indicated that the
chiral peptides at both 5.0 and 10.0 mg/ml promoted cell
proliferation in comparison to the concentration of 1.25 and 2.5
mg/ml and the control. The effects of these peptides are mainly due
to their self-assembling ability, which can form scaffolds to mimic
the extracellular environment for cell growth, and the transport of
oxygen, nutrients and waste products to take place in a 3D
environment, while the amino acids of these peptides themselves do
not interact with the cell (14,37,45). We speculated that there was not a
sufficient number of nanofibers to form a 3D environment in the low
concentration of peptide solution, and thus it was similar to the
conventional 2D culture. Conversely, the SAPs with relatively high
concentrations holding enough interwoven nanofibers can form a
stable 3D cell culture microenvironment. This is the reason why
different concentrations of SAPs have differential effects on cell
proliferation. However, no significant differences in the cell
proliferation rate were obtained when the concentration of the
peptide increased from 5.0 to 10.0 mg/ml. It is plausible that
there are enough nanofibers to form a scaffold network, mimicking
3D ECM at a concentration of 5.0 mg/ml. Thereby, this concentration
was selected for further experiments.
An interesting finding in MTT assay was that
formazan crystals were inadvertently observed under an inverted
phase contrast microscope. The formazan crystals, reduced by living
cell enzymes, penetrated long-distances at relatively high
concentrations of D-RADA16 (5.0 and 10.0 mg/ml). We speculated that
the D-RADA16 scaffolds were able to provide suitable 3D culture
environments for BMSC adhesion and migration, which were identified
by an optical microscope at the macro-level.
D-amino acid may be toxic to cells (46). Given that the degradation products
of the D-form SAP consist of D-amino acid which may present complex
toxicity profiles, in this study, cell viability was assessed in a
relatively longer time duration by MTT assay. The results reveaked
that D-RADA16 not only exerted no toxic effects, but also promoted
BMSC growth and proliferation after 7 days of culture. The results
confirmed that the D-RADA16 scaffold demonstrated excellent
cellular biocompatibility in vitro. However, each peptide
must first be screened in vivo carefully, in order to
prevent the immunogenic response of the host animal (13). Biocompatibility and toxicity
examinations are thus required to be carried out in numerous animal
models to assess the safety of D-RADA16.
An ideal tissue engineered scaffold should provide a
porous microstructure to regulate cell adhesion, proliferation and
migration (37,47). The 3D migration assay confirmed
that BMSCs migrated inside the chiral scaffolds after 7 days of
culture. On the contrary, cells remained restricted to the surface
in the 2D control tissue culture plate since the cells could not
migrate. These results indicate that the chiral SAP hydrogel
scaffolds promote cell-cell interactions and provide a porous
microstructure for cell migration, which is vital to facilitate
tissue regeneration in the field of axon extension (13), angiogenesis (26), wound closure and epithelialization
(21) and osteosis (28).
Although L-form SAPs have been successfully used to
culture some cells in vitro (18,20–22,48–51), studies on larger constructs
corresponding to clinical applications are far from being
satisfactory. One of the main reasons is that the L-form peptides
are susceptible to protease degradation, which lead to long-term
instability in vivo (30,52), particularly use for drug delivery
vehicles in pharmaceutical applications, neural repair and induced
osteogenesis. The use of D-amino acids is a potential approach for
protecting biologically active peptides from enzymatic
decomposition. In this study, the chiral scaffolds were explored
for their ability to resist proteinase K degradation. This study
indicated that D-RADA16 scaffolds resisted the enzymatic hydrolysis
catalyzed and exhibited high biostability compared to its L-form
enantiomer. This agrees with the findings of previous studies that
peptide synthesized from D-amino acid can be exceptionally
effective in increasing the resistance towards proteolysis
(29,30,32,42,53).
Despite the hopeful findings posed above, the
relative fragility is a major concern for the D-RADA16 hydrogel. We
are currently exploring the ability of coating D-RADA16 on
hydroxyapatite substrate to increase the stiffness and modifying it
with functional ligands to boost the bioactivity. Moreover, the
more in depth mechanism of self-assembly is incompletely
understood, and it requires under further investigation.
In conclusion, we in this study, we introduced a
designer SAP D-RADA16 and successfully constructed a 3D culture
microenvironment mimicking the ECM which supports rat BMSC growth
and migration. D-RADA16, made of D-amino acid, can undergo
self-assembly to form interweaving nanofiber scaffolds, and it
exhibits high resistance against enzymatic hydrolysis catalyzed by
proteinase K compared to its chiral counterpart peptide, L-RADA16.
Additionally, D-RADA16 and L-RADA16 exhibited similar bioactivity
and biocompatibility in spite of their chiral differences. Beyond
3D cell culture, D-RADA16 hydrogel scaffolds may have potential for
use in long-term clinical applications in controlled drug delivery
and neural repair, and may be able to counteract anatomical defects
in bone regeneration and repair.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (NSFC, no. 81472057).
References
|
1
|
Place ES, Evans ND and Stevens MM:
Complexity in biomaterials for tissue engineering. Nat Mater.
8:457–470. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Owen SC and Shoichet MS: Design of
three-dimensional biomimetic scaffolds. J Biomed Mater Res A.
94:1321–1331. 2010.PubMed/NCBI
|
|
3
|
Oh JK: Engineering of nanometer-sized
cross-linked hydrogels for biomedical applications. Can J Chem.
88:173–184. 2009. View
Article : Google Scholar
|
|
4
|
Lutolf MP: Biomaterials: Spotlight on
hydrogels. Nat Mater. 8:451–453. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu J: Bioactive modification of
poly(ethylene glycol) hydrogels for tissue engineering.
Biomaterials. 31:4639–4656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geckil H, Xu F, Zhang X, Moon S and
Demirci U: Engineering hydrogels as extracellular matrix mimics.
Nanomedicine (Lond). 5:469–484. 2010. View Article : Google Scholar
|
|
7
|
Liu SQ, Tay R, Khan M, Rachel Ee PL,
Hedrick JL and Yang YY: Synthetic hydrogels for controlled stem
cell differentiation. Soft Matter. 6:67–81. 2010. View Article : Google Scholar
|
|
8
|
Hosseinkhani H, Hosseinkhani M, Tian F,
Kobayashi H and Tabata Y: Osteogenic differentiation of mesenchymal
stem cells in self-assembled peptide-amphiphile nanofibers.
Biomaterials. 27:4079–4086. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cunha C, Panseri S, Villa O, Silva D and
Gelain F: 3D culture of adult mouse neural stem cells within
functionalized self-assembling peptide scaffolds. Int J
Nanomedicine. 6:943–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saltzman WM and Olbricht WL: Building drug
delivery into tissue engineering. Nat Rev Drug Discov. 1:177–186.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kretlow JD, Klouda L and Mikos AG:
Injectable matrices and scaffolds for drug delivery in tissue
engineering. Adv Drug Deliv Rev. 59:263–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan H and Marra KG: Injectable,
biodegradable hydrogels for tissue engineering applications.
Materials (Basel). 3:1746–1767. 2010. View Article : Google Scholar
|
|
13
|
Zhang S: Fabrication of novel biomaterials
through molecular self-assembly. Nat Biotechnol. 21:1171–1178.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
He B, Yuan X and Jiang D: Molecular
self-assembly guides the fabrication of peptide nanofiber scaffolds
for nerve repair. RSC Advances. 4:23610–23621. 2014. View Article : Google Scholar
|
|
15
|
Zhang S, Holmes T, Lockshin C and Rich A:
Spontaneous assembly of a self-complementary oligopeptide to form a
stable macroscopic membrane. Proc Natl Acad Sci USA. 90:3334–3338.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holmes TC, de Lacalle S, Su X, Liu G, Rich
A and Zhang S: Extensive neurite outgrowth and active synapse
formation on self-assembling peptide scaffolds. Proc Natl Acad Sci
USA. 97:6728–6733. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamada K, Hirose M, Yamashita T and
Ohgushi H: Spatial distribution of mineralized bone matrix produced
by marrow mesenchymal stem cells in self-assembling peptide
hydrogel scaffold. J Biomed Mater Res A. 84:128–136. 2008.
View Article : Google Scholar
|
|
18
|
Horii A, Wang X, Gelain F and Zhang S:
Biological designer self-assembling peptide nanofiber scaffolds
significantly enhance osteoblast proliferation, differentiation and
3-D migration. PLoS One. 2:e1902007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koutsopoulos S and Zhang S: Long-term
three-dimensional neural tissue cultures in functionalized
self-assembling peptide hydrogels, matrigel and collagen I. Acta
Biomater. 9:5162–5169. 2013. View Article : Google Scholar
|
|
20
|
Liu X, Wang X, Wang X, Ren H, He J, Qiao L
and Cui FZ: Functionalized self-assembling peptide nanofiber
hydrogels mimic stem cell niche to control human adipose stem cell
behavior in vitro. Acta Biomater. 9:6798–6805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bradshaw M, Ho D, Fear MW, Gelain F, Wood
FM and Iyer KS: Designer self-assembling hydrogel scaffolds can
impact skin cell proliferation and migration. Sci Rep. 4:69032014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang B, Sun C, Shao Z, Yang S, Che B, Wu Q
and Liu J: Designer self-assembling peptide nanofiber scaffolds
containing link protein N-terminal peptide induce chondrogenesis of
rabbit bone marrow stem cells. Biomed Res Int. 2014:4219542014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kisiday J, Jin M, Kurz B, Hung H, Semino
C, Zhang S and Grodzinsky AJ: Self-assembling peptide hydrogel
fosters chondrocyte extracellular matrix production and cell
division: Implications for cartilage tissue repair. Proc Natl Acad
Sci USA. 99:9996–10001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davis ME, Motion JPM, Narmoneva DA,
Takahashi T, Hakuno D, Kamm RD, Zhang S and Lee RT: Injectable
self-assembling peptide nanofibers create intramyocardial
microenvironments for endothelial cells. Circulation. 111:442–450.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang B, Wu Y, Shao Z, Yang S, Che B, Sun
C, Ma Z and Zhang Y: Functionalized self-assembling peptide
nanofiber hydrogel as a scaffold for rabbit nucleus pulposus cells.
J Biomed Mater Res A. 100:646–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Wang X, Horii A, Wang X, Qiao L,
Zhang S and Cui FZ: In vivo studies on angiogenic activity of two
designer self-assembling peptide scaffold hydrogels in the chicken
embryo chorioallantoic membrane. Nanoscale. 4:2720–2727. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soler-Botija C, Bagó JR,
Llucià-Valldeperas A, Vallés-Lluch A, Castells-Sala C,
Martínez-Ramos C, Fernández-Muiños T, Chachques JC, Pradas MM,
Semino CE, et al: Engineered 3D bioimplants using elastomeric
scaffold, self-assembling peptide hydrogel, and adipose
tissue-derived progenitor cells for cardiac regeneration. Am J
Transl Res. 6:291–301. 2014.PubMed/NCBI
|
|
28
|
Wu M, Ye Z, Zhu H and Zhao X:
Self-assembling peptide nanofibrous hydrogel on immediate
hemostasis and accelerative osteosis. Biomacromolecules.
16:3112–3118. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li X, Du X, Li J, Gao Y, Pan Y, Shi J,
Zhou N and Xu B: Introducing D-amino acid or simple glycoside into
small peptides to enable supramolecular hydrogelators to resist
proteolysis. Langmuir. 28:13512–13517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tugyi R, Uray K, Iván D, Fellinger E,
Perkins A and Hudecz F: Partial D-amino acid substitution: Improved
enzymatic stability and preserved Ab recognition of a MUC2 epitope
peptide. Proc Natl Acad Sci USA. 102:413–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Welch BD, VanDemark AP, Heroux A, Hill CP
and Kay MS: Potent D-peptide inhibitors of HIV-1 entry. Proc Natl
Acad Sci USA. 104:16828–16833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang G, Yang Z, Zhang R, Li L, Fan Y,
Kuang Y, Gao Y, Wang T, Lu WW and Xu B: Supramolecular hydrogel of
a D-amino acid dipeptide for controlled drug release in vivo.
Langmuir. 25:8419–8422. 2009. View Article : Google Scholar
|
|
33
|
Lennon DP, Haynesworth SE, Young RG,
Dennis JE and Caplan AI: A chemically defined medium supports in
vitro proliferation and maintains the osteochondral potential of
rat marrow-derived mesenchymal stem cells. Exp Cell Res.
219:211–222. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yokoi H, Kinoshita T and Zhang S: Dynamic
reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci
USA. 102:8414–8419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hauser CAE and Zhang S: Designer
self-assembling peptide nanofiber biological materials. Chem Soc
Rev. 39:2780–2790. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tang C, Shao X, Sun B, Huang W and Zhao X:
The effect of self-assembling peptide RADA16-I on the growth of
human leukemia cells in vitro and in nude mice. Int J Mol Sci.
10:2136–2145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nune M, Kumaraswamy P, Krishnan UM and
Sethuraman S: Self-assembling peptide nanofibrous scaffolds for
tissue engineering: Novel approaches and strategies for effective
functional regeneration. Curr Protein Pept Sci. 14:70–84. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tavakol S, Saber R, Hoveizi E, Aligholi H,
Ai J and Rezayat SM: Chimeric self-assembling nanofiber containing
bone marrow homing peptide's motif induces motor neuron recovery in
animal model of chronic spinal cord injury; An in vitro and in vivo
investigation. Mol Neurobiol. 53:3298–3308. 2016. View Article : Google Scholar
|
|
39
|
Taraballi F, Campione M, Sassella A,
Vescovi A, Paleari A, Hwangc W and Gelain F: Effect of
functionalization on the self-assembling propensity of β-sheet
forming peptides. Soft Matter. 5:660–668. 2009. View Article : Google Scholar
|
|
40
|
Cormier AR, Pang X, Zimmerman MI, Zhou HX
and Paravastu AK: Molecular structure of RADA16-I designer
self-assembling peptide nanofibers. ACS Nano. 7:7562–7572. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ravichandran R, Griffith M and Phopase J:
Applications of self-assembling peptide scaffolds in regenerative
medicine: The way to the clinic. J Mater Chem B Mater Biol Med.
2:8466–8478. 2014. View Article : Google Scholar
|
|
42
|
Luo Z, Yue Y, Zhang Y, Yuan X, Gong J,
Wang L, He B, Liu Z, Sun Y, Liu J, et al: Designer D-form
self-assembling peptide nanofiber scaffolds for 3-dimensional cell
cultures. Biomaterials. 34:4902–4913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anderson JM, Patterson JL, Vines JB, Javed
A, Gilbert SR and Jun HW: Biphasic peptide amphiphile nanomatrix
embedded with hydroxyapatite nanoparticles for stimulated
osteoinductive response. ACS Nano. 5:9463–9479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hosseinkhani H, Hong PD and Yu DS:
Self-assembled proteins and peptides for regenerative medicine.
Chem Rev. 113:4837–4861. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Friedman M: Chemistry, nutrition, and
microbiology of D-amino acids. J Agric Food Chem. 47:3457–3479.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bergmeister H, Schreiber C, Grasl C,
Walter I, Plasenzotti R, Stoiber M, Bernhard D and Schima H:
Healing characteristics of electrospun polyurethane grafts with
various porosities. Acta Biomater. 9:6032–6040. 2013. View Article : Google Scholar
|
|
48
|
Zhang F, Shi GS, Ren LF, Hu FQ, Li SL and
Xie ZJ: Designer self-assembling peptide scaffold stimulates
pre-osteoblast attachment, spreading and proliferation. J Mater Sci
Mater Med. 20:1475–1481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ozeki M, Kuroda S, Kon K and Kasugai S:
Differentiation of bone marrow stromal cells into osteoblasts in a
self-assembling peptide hydrogel: In vitro and in vivo studies. J
Biomater Appl. 25:663–684. 2011. View Article : Google Scholar
|
|
50
|
Ni N, Hu Y, Ren H, Luo C, Li P, Wan JB and
Su H: Self-assembling peptide nanofiber scaffolds enhance
dopaminergic differentiation of mouse pluripotent stem cells in
3-dimensional culture. PLoS One. 8:e845042013. View Article : Google Scholar :
|
|
51
|
Lu T, Chen T, Zhai Y, Ma Y and Xiao Y:
Designer functionalized self-assembling peptide scaffolds for
adhesion, proliferation, and differentiation of MC3T3-E1. Soft
Mater. 12:79–87. 2013. View Article : Google Scholar
|
|
52
|
Nagy KJ, Giano MC, Jin A, Pochan DJ and
Schneider JP: Enhanced mechanical rigidity of hydrogels formed from
enantiomeric peptide assemblies. J Am Chem Soc. 133:14975–14977.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Luo Z, Zhao X and Zhang S:
Self-organization of a chiral D-EAK16 designer peptide into a 3D
nanofiber scaffold. Macromol Biosci. 8:785–791. 2008. View Article : Google Scholar : PubMed/NCBI
|