Introduction
Increasing evidence has demonstrated the association
between air pollution and sebaceous gland-associated diseases
(1–4). Recently, studies regarding
environmental pollutants indicated that dioxins inhibit lipid
synthesis and induce differentiation of human sebaceous cells into
keratinocytes, leading to the development of clinical chlorine acne
(5). Hu et al (6) also identified that cigarette smoke
extract benzo(a)pyrene regulates the synthesis of lipids and induce
pro-inflammatory effects in human sebocytes, as evidenced by
promoting the release of pro-inflammatory cytokines [interleukin-1α
(IL-1α), IL-6 and IL-8] (6). The
above-mentioned studies demonstrate that environmental pollution is
closely associated with the synthesis and secretion of sebaceous
glands, and potentially involved in the development of sebaceous
gland-associated diseases (7).
Particulate matter (PM)2.5, also termed fine
particles, were identified as PM, which are <2.5 µm in
diameter (8). Due to the small
particle size and specific surface area, PM2.5 easily penetrate the
alveoli or even the blood, which increases the risk of human health
problems (9,10). A recent study demonstrated that
ambient PM2.5 increases the risk of eczema and other skin diseases
(11). However, to the best of
our knowledge, the effects of PM2.5 on the function of human
sebaceous glands have not yet been elucidated.
The key molecular signaling pathway involved in the
response of skin cells against environment pollutants is the
aromatic hydrocarbon receptor (AhR) pathway (12,13). AhR, also termed dioxin receptor,
is a ligand-activated transcription factor expressed in all skin
cells, including keratinocytes (14), fibroblasts (15) and SZ95 sebocytes cells (16) in vitro and in human skin
in vivo (17). The
formation of the AhR/AhR nuclear translocator (ARNT) heterodimer
activates the cytoplasmic cytochrome P4501 A1 (CYP1A1) (18,19). Furthermore, increasing evidence
has highlighted that AhR is significant in cell growth,
proliferation, cell cycle progression (20), cell differentiation and
inflammatory responses (21) in
the absence of external ligands. Recently, Kakimoto et al
(22) identified that PM2.5 has
the highest AhR ligand activity among all of the particle sizes,
which supports the possible involvement of AhR signaling in the
mechanism of PM2.5-induced skin diseases. However, whether the
PM2.5 effects on SZ95 sebocytes are associated with AhR/CYP1A1
signaling remains unclear.
Therefore, the present study aimed to investigate
the influences of PM2.5 on human SZ95 sebocytes, and to investigate
the relevant mechanisms. The effects of water-soluble extract
(W-PM2.5) and non-water-soluble extract (NW-PM2.5) exposure on cell
proliferation, cell cycle progression, lipid synthesis and the
inflammatory response were investigated in human SZ95 sebocytes.
Furthermore, whether the AhR/CYP1A1 signaling pathway is involved
in these effects was investigated.
Materials and methods
Collection and extraction of PM2.5
PM2.5 was collected by TH-150C Medium flow air total
suspended particulate sampler (Wuhan TianHong Environmental
Protection Industry Co., Ltd., Wuhan, China) (100 1/min) in Wuhan
urban air during July 2015. The sample was set on the rooftop of a
building of the Institute of Atmospheric Research, School of
Environmental Studies, China University of Geosciences (Wuhan,
China), ~6 m above the ground. The filter was changed every 24 h
using the air total particle sampler. Subsequent to sampling, the
PM2.5 filter was placed under an ultraviolet lamp for 30 min and
cut into two equal size pieces. The W-PM2.5 and NW-PM2.5 were
isolated and prepared as previously described (23). The samples were collected and
stored at −80°C until later use.
Cell culture and exposure
Immortalized human SZ95 sebaceous gland cells,
(patented cell, gifted by Professor Christos C. Zouboulis,
Department of Dermatology, Venereology, Allergology and Immunology,
Dessau Medical Center, Theodore Fontane Medical University of
Brandenburg, Germany) at passages 30–40 were cultured at 37°C in
Sebomed® basal medium (Biochrom GmbH, Berlin, Germany)
supplemented with 10% fetal calf serum, Gibco 5 ng/ml human
epidermal growth factor (Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml penicillin and 100 g/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.). Various concentrations (1, 10, 100
and 250 µg/ml) of PM2.5 were prepared. For the exposure,
cells were exposed to a W-PM2.5 or NW-PM2.5 for 48 h.
Cell counting kit-8 (CCK-8) assay
Following exposure for 48 h, cell viability was
determined using CCK-8 assay kits (C0038; Shanghai Beyotime
Biotechnology Co., Ltd., Shanghai, China) according to the
manufacturer's instructions. CCK-8 solution (10 µl) was
added to each well, followed by an incubation for 1–4 h. Absorbance
was measured using a microplate reader at a wavelength of 450 nm.
The cells treated with the solvent served as a control group and
wells without cells served as the blank group. Cell viability was
calculated according to the following formula: cell proliferation
(%) = optical density (OD) of the experimental group/OD of the
control group ×100.
Cellular toxicity
SZ95 sebocytes were seeded in 96-well plates.
Subsequent to exposure for 48 h, lactate dehydrogenase (LDH)
activity was detected using a Pierce LDH cytotoxicity assay kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Cell cycle assay
SZ95 sebocytes were seeded into 6-well plates.
Following exposure for 48 h with different concentrations of PM2.5
suspension, cells were collected using 0.25% trypsin and
phosphate-buffered saline (PBS). Also, culture was centrifuged at
300 × g for 5 min at room temperature twice. After that precooled
90% ethanol was added. Then cells were resuspended at 4°C for 20
min and centrifuged at 300 × g for 5 min at room temperature. Cells
were incubated with propidium iodide (PI) buffer [50 mg/ml
containing ribonuclease A (50 ng/ml)] at room temperature and
stained in the dark for 20 min at room temperature. Cell cycle
distribution was analyzed using a BD Biosciences Flow Cytometer (BD
Biosciences, San Jose, CA, USA).
Oil Red O staining
SZ95 sebocytes were seeded in 24-well plates
(5×104 cells/well). After a 48-h exposure with different
concentrations of PM2.5 suspension, cells were washed with PBS and
fixed in 10% neutral formaldehyde for 30 min. The cells were then
washed twice in distilled water for 2 min, stained with Oil Red O
dye (AS1083; Wuhan Aspen Biotechnology Co., Ltd., Wuhan, China)
(0.5% Oil Red dissolved in isopropanol, then diluted with distilled
water at a ratio of 6:4) at room temperature in the dark for 10 min
and washed in distilled water for 1 min. Subsequently, cells were
counterstained with hematoxylin, and then sealed by glycerol
gelatin for long-term preservation, and observed under an inverted
Olympus microscope.
Enzyme-linked immunosorbent assay
(ELISA)
SZ95 sebocytes were seeded in 24-well plates
(1×105 cells/well). After 48-h exposure to different
concentrations of PM2.5 suspension, the supernatant was collected
at 4°C, centrifuged for 20 min at 1,000 × g at 4°C, and the
supernatant was collected and stored at −20°C for subsequent
assays. Concentrations of IL-1α, IL-6 and IL-8 were determined
using commercial ELISA kits (E-EL-H0088c, E-EL-H0102c and
E-EL-H0048c; ElabScience Biotechnology Co., Ltd., Wuhan, China)
according to the manufacturer's instructions.
Immunofluorescent cytochemical
analysis
Immunofluorescent cytochemical analysis was
performed using anti-AhR (17840-1-AP), anti-ARNT (14105-1-AP),
anti-CYP1A1 (13241-1-AP) (all from Proteintech Group, Inc., Wuhan,
China) and Cy3-conjugated anti-rabbit IgG antibodies (AS-1109;
Wuhan Aspen Biotechnology Co., Ltd.). SZ95 sebocytes were seeded
into 6-well plates. Following exposure for 48 h to various
concentrations of PM2.5 suspension, coverslips were added to the
plates and incubated with 5% CO2 at 37°C until cells
reached 80% confluence. Media was aspirated from the plates and
washed 3 times with PBS. Cells were fixed with 4% paraformaldehyde
for 30 min at room temperature. We then added Triton X-100 for 10
min and hydrogen peroxide solution at room temperature in the dark
for 20 min; after each step, the cells were washed 3 times with PBS
for 5 min. The cell culture was incubated with primary antibody
overnight at 4°C and washed 3 times for 5 min with PBS. Stained
with conjugated secondary antibody for 30 min at room temperature
for 50 min and washed 3 times for 5 min with PBS. Nuclear staining
was then performed with DAPI. Images of each slide were obtained
using an optical microscope system (Axiomager; Carl Zeiss AG,
Oberkochen, Germany). The protein expression levels of AhR, ARNT
and CYP1A1 were quantified with an image analysis program
(Q-imaging Pro-7; Olympus, Tokyo, Japan) from the microscope
system.
Western blotting
Following exposure for 48 h to various
concentrations of PM2.5 suspension, the adherent cells were washed
with TBS buffer for 3 times. After drying the residual liquid,
protease inhibitor was added for 3 min at room temperature. The
cell total protein extraction reagent was then added for 5 min at
room temperature. Cells were collected to 1.5 ml centrifuge tubes
and centrifuged at 10,000 × g for 5 min at 4°C. The supernatant
which was the total protein solution was collected at 4°C. The
concentration of the proteins was determined using a BCA protein
assay kit (AS1086; Wuhan Aspen Biotechnology Co., Ltd.). Total
protein samples (ensure that the total protein amount of each
sample was 40 µg) were separated with 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), then
transferred onto polyvinylidene fluoride membrane (EMD Millipore,
Bedford, MA, USA), blocked with skimmed milk and incubated
overnight at 4°C with primary antibodies against anti-AhR
(dilution, 1:1,000), anti-ARNT (dilution, 1:500), CYP1A1 (dilution,
1:500) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
(dilution, 1:1,000). The membranes were incubated with the
secondary antibody (horseradish peroxidase goat anti rabbit;
dilution, 1:10,000) for 30 min at room temperature, and the blots
were detected using an enhanced chemiluminescence kit (Cell
Signaling Technology, Inc., Danvers, MA, USA). GAPDH served as an
internal control.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Statistical analysis was performed using two-way ANOVA
and Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
PM2.5 exposure inhibits the viability of
SZ95 sebocytes
To evaluate the effects of PM2.5 exposure on the
viability of SZ95 sebocytes, a CCK-8 assay was performed in the
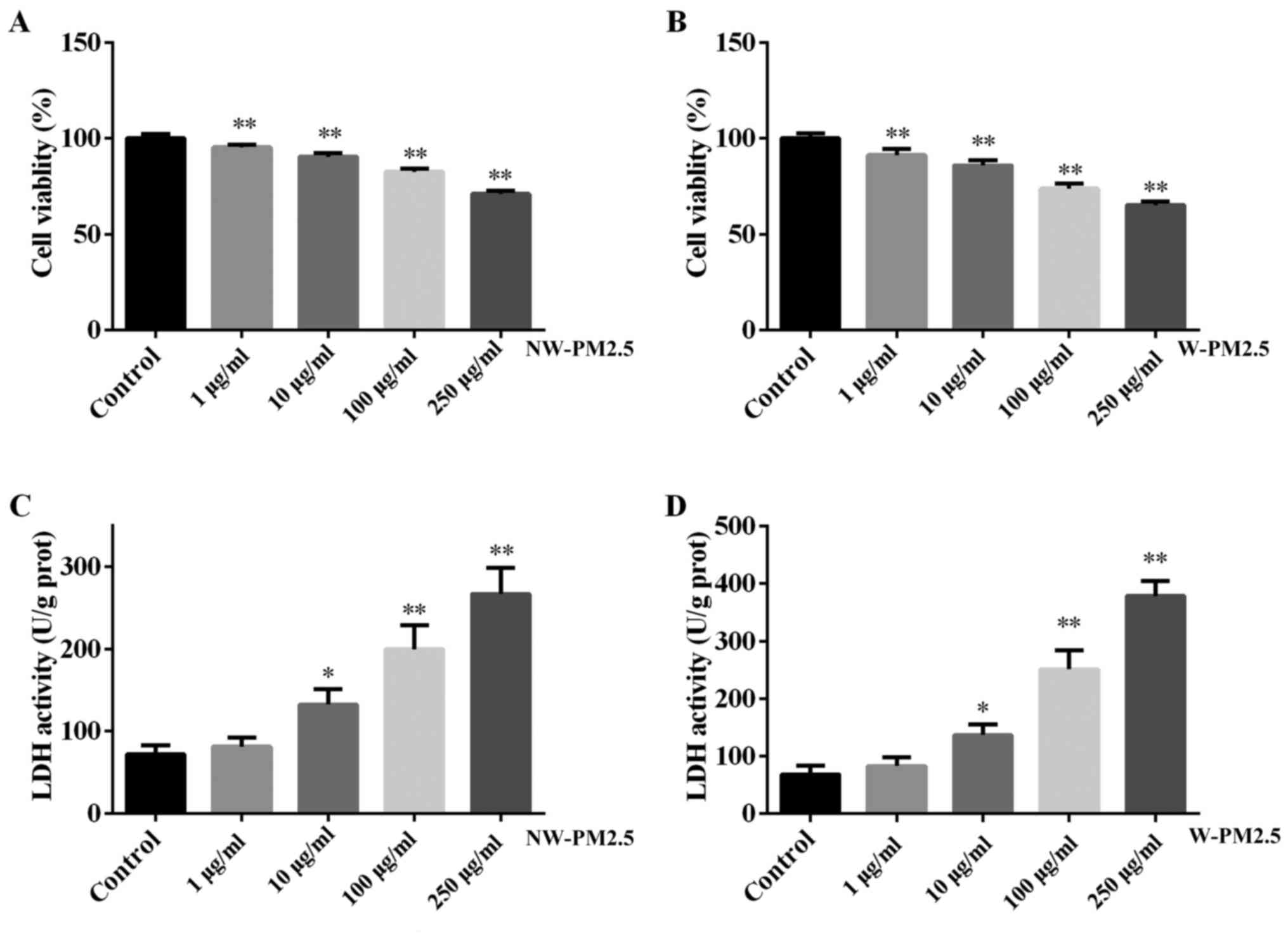
present study and the results are presented in Fig. 1. PM2.5 (NW-PM2.5 and W-PM2.5)
exposure for 48 h significantly induced cell viability when
compared with the control cells (P<0.01) (Fig. 1A and B). No significant difference
was identified between the viability of NW-PM2.5- and
W-PM2.5-exposed SZ95 sebocytes (P>0.05). In addition, the
toxicity of NW-PM2.5 and W-PM2.5 exposure on SZ95 sebocytes was
evaluated using a Pierce LDH cytotoxicity assay kit according to
the manufacturer's instructions. Compared with the control cells,
NW-PM2.5 exposure (10 µg/ml, P<0.05; 100 and 250
µg/ml, P<0.01) significantly increased LDH activity in a
dose-dependent manner (Fig. 1C).
W-PM2.5 exhibited similar effects on SZ95 sebocytes (Fig. 1D).
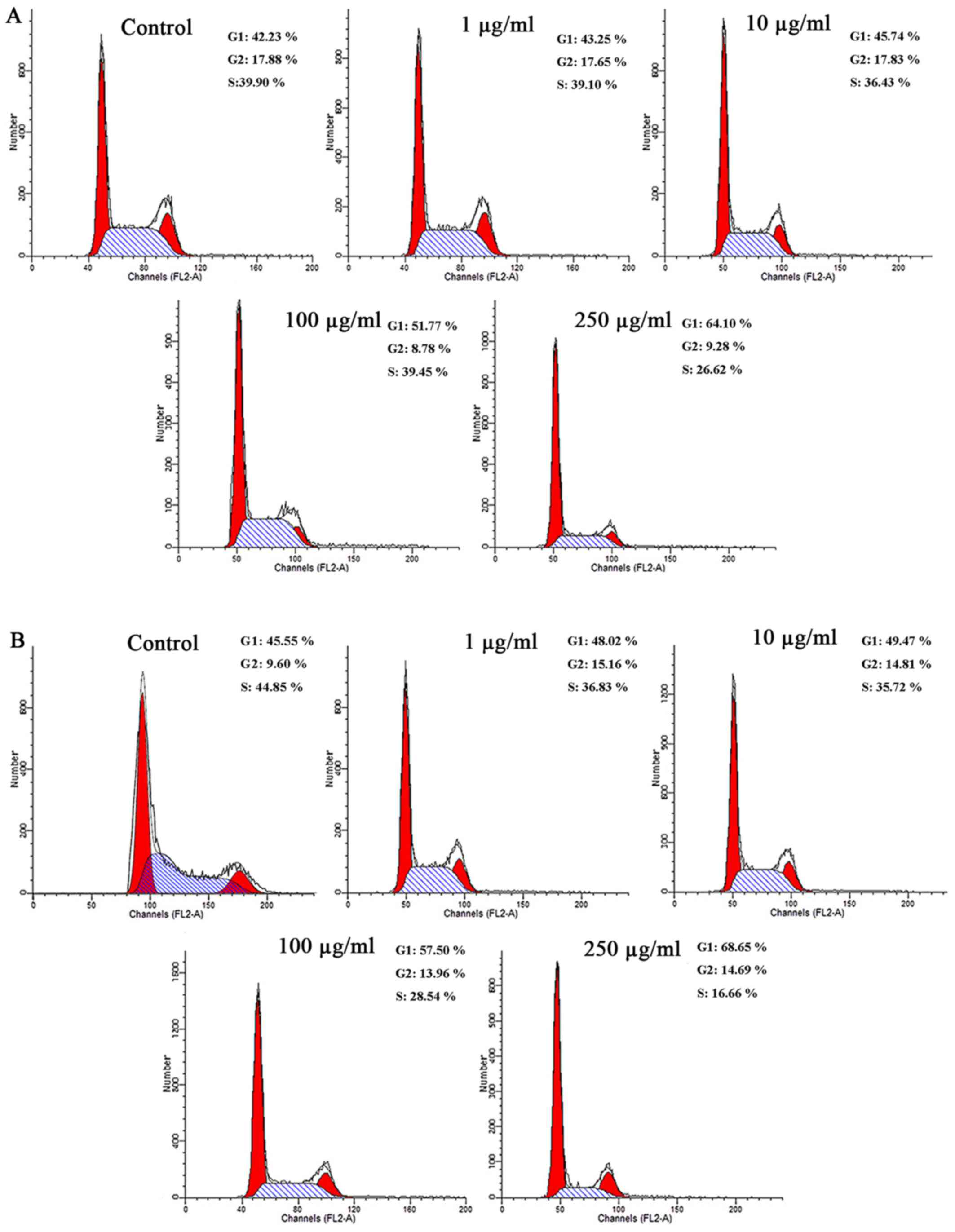
PM2.5 exposure arrested the cell cycle at
the G1 phase in SZ95 sebocytes
The effects of PM2.5 on the cell cycle were
investigated (Fig. 2). Flow
cytometry demonstrated that compared with the unexposed control
cells, the number of cells in the G1 phase were markedly
increased in cells exposed to NW-PM2.5 (Fig. 2A). With increasing NW-PM2.5
concentrations, the cell rates in the G1 phase also
significantly increased. As illustrated in Fig. 2B, compared with the control cells,
W-PM2.5 exposure markedly increased the number of cells in the
G1 phase in a dose-dependent manner.
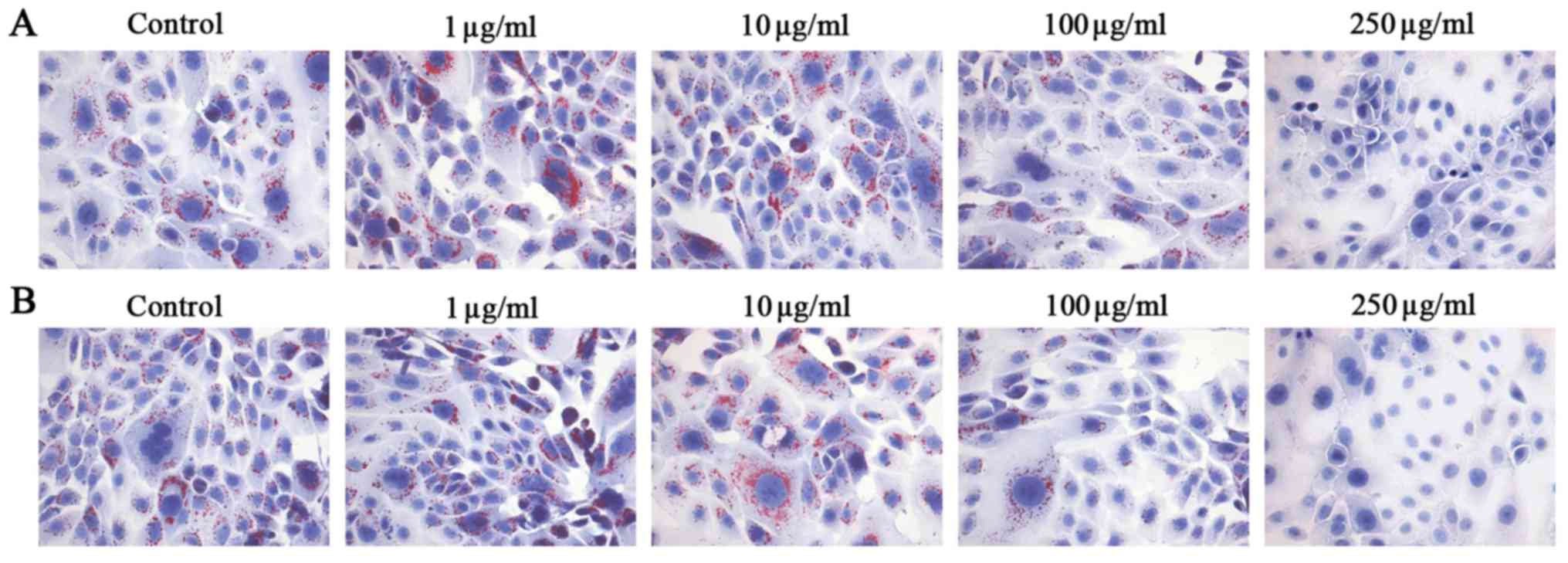
PM2.5 exposure regulated lipid synthesis
in SZ95 sebocytes
As lipids secreted by sebaceous gland cells are
important in the process of skin physiology involving cell
structure, cohesion and desquamation as well as formation and
function of a permeability barrier, the effects of PM2.5 exposure
on lipid synthesis were examined in SZ95 sebocytes. Oil Red O
staining indicated that NW-PM2.5 (Fig. 3A) and W-PM2.5 (Fig. 3B) at low concentrations promoted
lipid synthesis, while high concentrations of NW-PM2.5 and W-PM2.5
markedly inhibited lipid synthesis in SZ95 sebocytes.
PM2.5 exposure regulated pro-inflammatory
cytokine secretion in SZ95 sebocytes
Whether PM2.5 exposure regulated pro-inflammatory
cytokine secretion was investigated by ELISA of pro-inflammatory
cytokines (IL-1α, IL-6 and IL-8). NW-PM2.5 (Fig. 4A, C and E) and W-PM2.5 (Fig. 4B, D and F) exposure
dose-dependently elevated the levels of all the evaluated
pro-inflammatory cytokines that were released by SZ95 sebocytes,
when compared with the unexposed control cells.
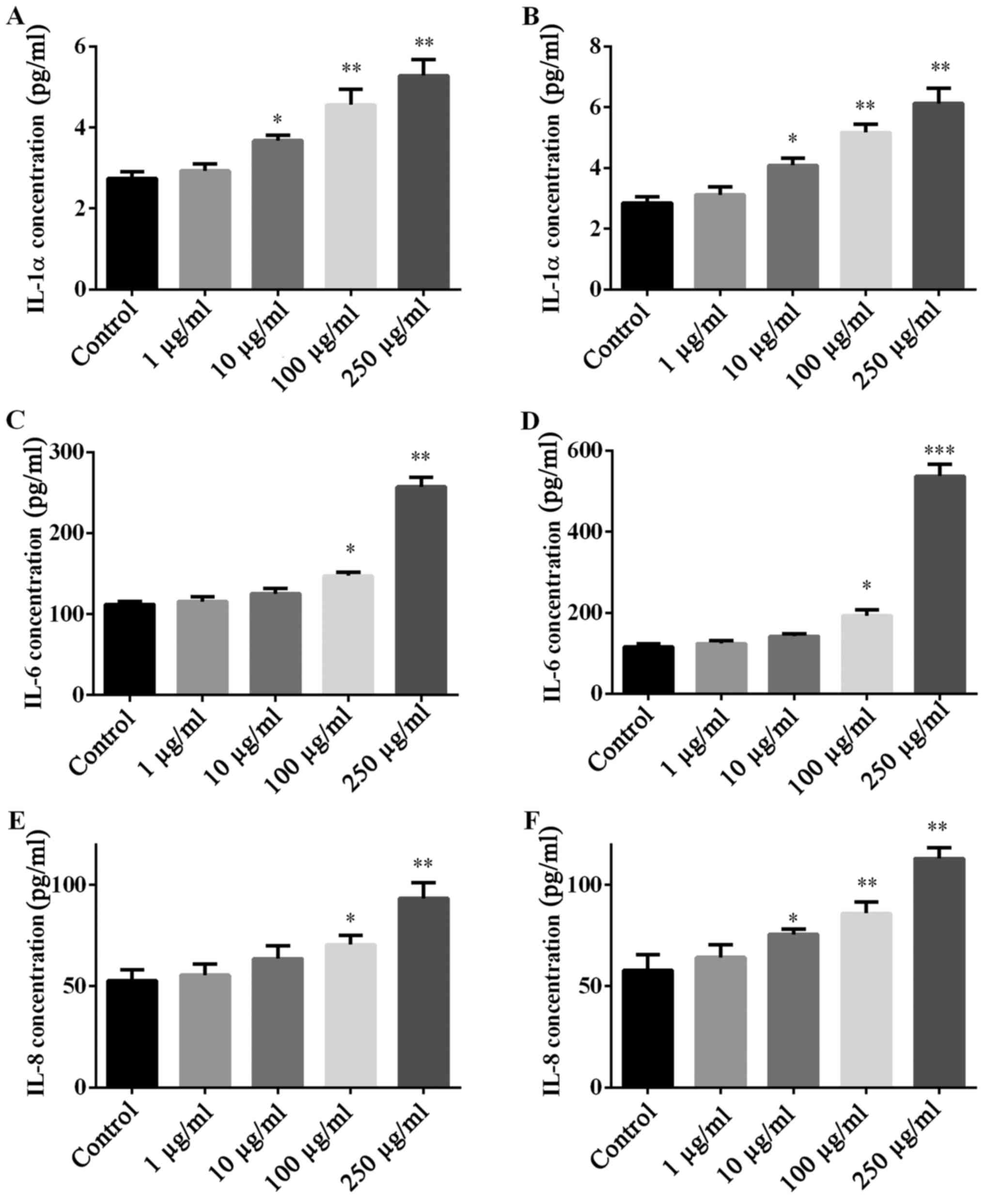 | Figure 4Secretion of IL-1α, IL-6 and IL-8 in
SZ95 sebocytes following PM2.5 exposure. Subsequent to treatment of
SZ95 sebocytes with (A, C and E) NW-PM2.5 and (B, D and F) W-PM2.5,
the production of pro-inflammatory cytokines, IL-1α, IL-6 and IL-8
in culture supernatant were measured by enzyme-linked immunosorbent
assay. Data are expressed as means ± standard error of the mean
(n=3). *P<0.05 and **P<0.01 vs.
control. IL, interleukin; PM, particulate matter; NW-PM2.5,
non-water-soluble extracts; W-PM2.5, water-soluble extracts. |
PM2.5 exposure activated AhR/CYP1A1
signaling in SZ95 sebocytes
In addition, whether AhR/CYP1A1 signaling is
involved in the effect of PM2.5 exposure on SZ95 sebocytes was
evaluated. Western blot analysis revealed that compared with
unexposed control cells, SZ95 sebocyte exposure to NW-PM2.5
dose-dependently induced the elevation of protein expression levels
of AhR, ARNT and CYP1A1. Similarly, the AhR, ARNT and CYP1A1
expression levels were reduced in the unexposed control cells,
whereas the AhR, ARNT and CYP1A1 expression levels in SZ95
sebocytes were markedly increased in a dose-dependent manner by
W-PM2.5 (Fig. 5).
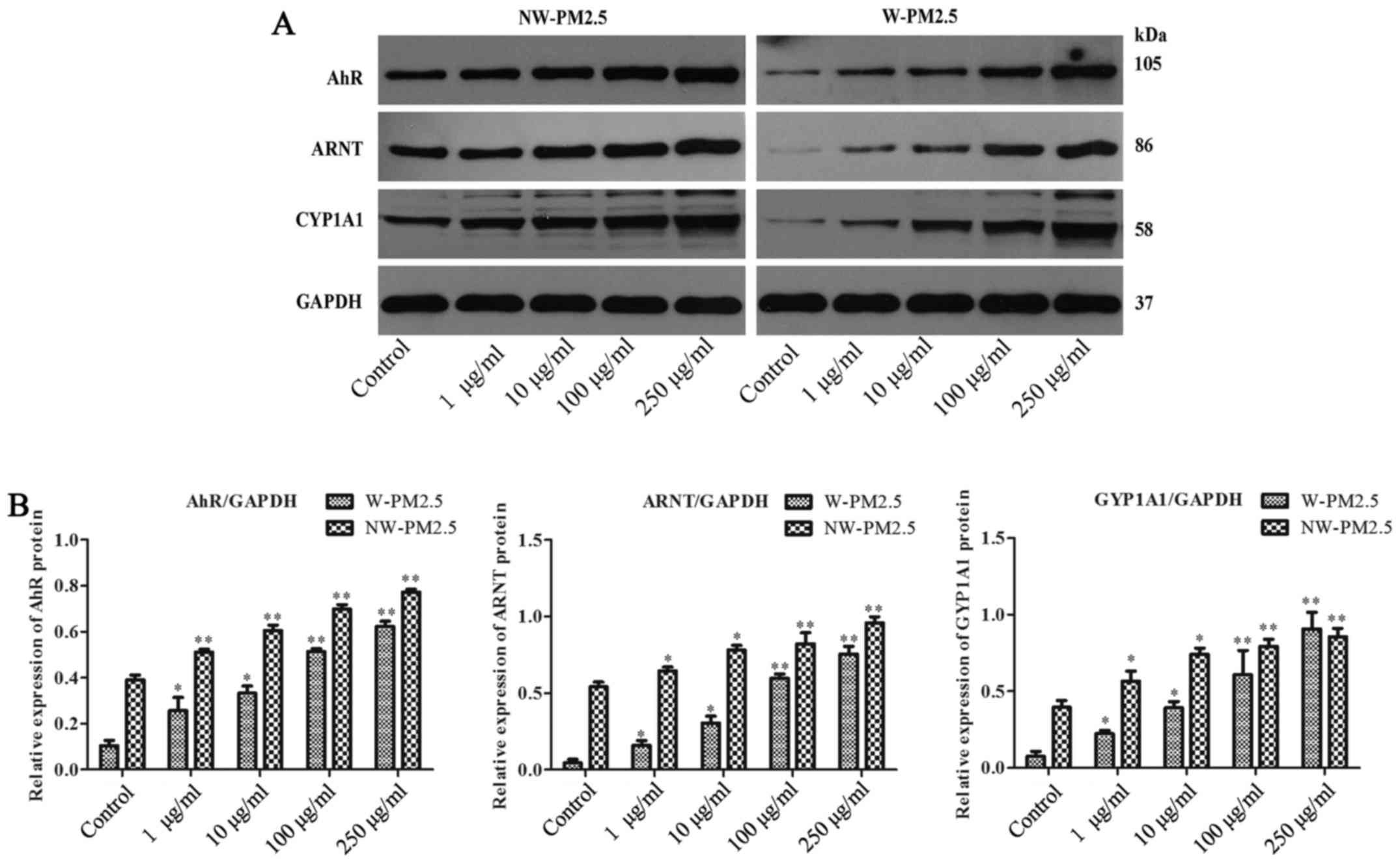 | Figure 5Western blot analysis of AhR, ARNT and
CYP1A1 in PM2.5-exposed SZ95 sebocytes. Cell extracts were
subjected to 10% SDS-PAGE and western blot analysis with a primary
antibody against AhR, ARNT and CYP1A1, respectively. GAPDH protein
was used as an internal control. (A) Western blot analysis results
of AhR, ARNT and CYP1A1. (B) Quantification of AhR, ARNT and CYP1A1
protein expression. *P<0.05 and
**P<0.01 vs. control. AhR, aryl hydrocarbon receptor;
ARNT, AhR nuclear translocator protein; CYP1A1, cytoplasmic
cytochrome P4501 A1; PM, particulate matter; NW-PM2.5,
non-water-soluble extracts; W-PM2.5, water-soluble extracts; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
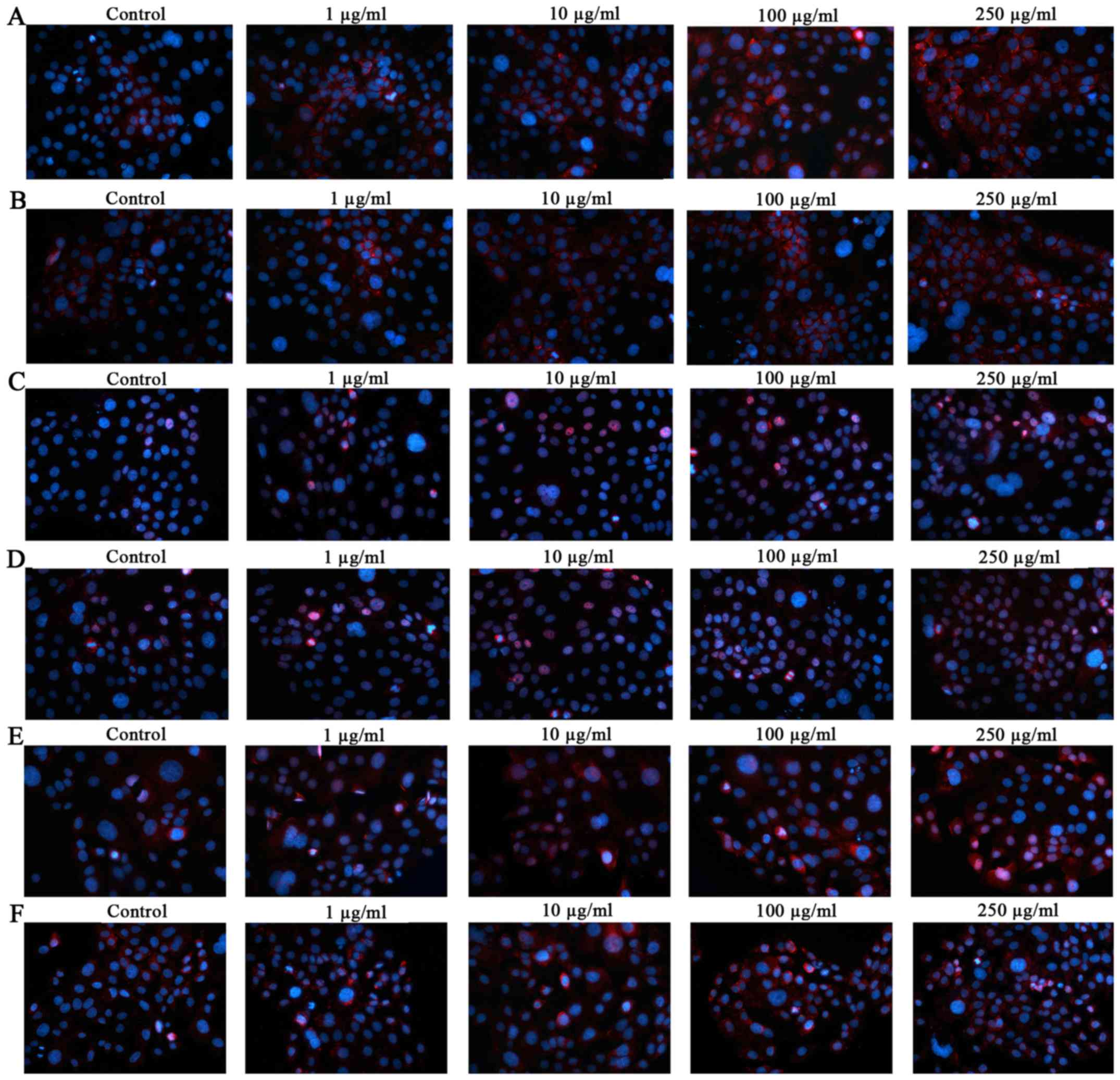
Furthermore, immunocytochemical analysis (Fig. 6) demonstrated the localization of
AhR, ARNT and CYP1A1 in PM2.5-exposed SZ95 sebocytes. Compared with
unexposed control cells, significant AhR induction was observed in
NW-PM2.5- (Fig. 6A) and
W-PM2.5-exposed (Fig. 6B) cells.
Similar to AhR, the staining for ARNT and CYP1A1 was significantly
and dose-dependently increased in NW-PM2.5-exposed [ARNT (Fig. 6C); CYP1A1 (Fig. 6E)] and W-PM2.5-exposed [ARNT
(Fig. 6D); CYP1A1 (Fig. 6F)] SZ95 sebocytes.
Discussion
The adverse effects of PM2.5 on respiratory diseases
have been widely documented (24–26). However, the effects of PM2.5 on
the function of human sebaceous glands have not been fully
elucidated. To the best of our knowledge, this is the first study
to investigate the influence of NW-PM2.5 and W-PM2.5 exposure on
human sebocytes. The present study identified that PM2.5 exposure
significantly inhibits the viability of SZ95 sebocytes, inducing
cell toxicity. Furthermore, a high dose of NW-PM2.5 and W-PM2.5
exposure markedly reduced lipid synthesis in SZ95 sebocytes. In
addition, pro-inflammatory cytokines, including IL-1α, IL-6 and
IL-8, were demonstrated to be markedly elevated in PM2.5-exposed
cells, and PM2.5 exposure induced G1 arrest in SZ95
sebocytes. Finally, the key AhR signaling pathway was activated
following PM2.5 exposure in SZ95 sebocytes. A previous study
demonstrated the cytotoxicity of PM2.5 on human HaCaT keratinocytes
(11). Our study also identified
that PM2.5 significantly inhibited cell viability, inducing
toxicity on SZ95 sebocytes. The effects of PM2.5 exposure on cell
cycle progression are controversial, as certain studies
demonstrated that PM2.5 induced G1 delay (27), while other studies found that
PM2.5 arrested the cell cycle at the G2/M phase
(28). In the present study, flow
cytometry indicated that SZ95 sebocytes exposed to PM2.5 were
arrested at the G1 phase. As G1 is the
preparation phase of DNA synthesis, arresting the cell cycle at the
G1 phase directly inhibits cell growth. Therefore, cell
proliferation suppression induced by PM2.5 is associated with
G1 arrest, and is consistent with the detected LDH
increase.
Reduced lipid synthesis is associated with various
diseases of the sebaceous gland, including chloracne (29,30). In the present study, lipid
synthesis markedly increased at a low PM2.5 concentration, while
increased PM2.5 concentrations rapidly reduced the lipid synthesis
in SZ95 sebocytes. In addition to activating lipid metabolism, SZ95
sebocytes were found to contribute to the inflammatory environment
by stimulating the release of pro-inflammatory cytokines, such as
IL-1α, IL-6 and IL-8. The results of the present study indicated
that PM2.5 dose-dependently elevated the levels of IL-1α, IL-6 and
IL-8, which are involved in the initiation of the pathogenesis of
various types of sebaceous gland-associated disease (31,32).
Previous studies have reported that PM2.5 (33) and extractable organic matter from
PM2.5 (34,35) may activate AhR in human cell
lines. Consistent with these studies, the present study found that
the protein expression level of AhR, ARNT and CYP1A1 was markedly
enhanced in the PM2.5-exposed SZ95 sebocytes, indicating the
activation of AhR signaling following PM2.5 exposure. Additionally,
it has been demonstrated that AhR negatively regulates lipid
synthesis in SZ95 sebocytes (16). Thus, the activated AhR signaling
by PM2.5 exposure may lead to a reduction of lipid synthesis, which
is consistent with the results of the present study. It is likely
that PM2.5 exposure regulates the lipid synthesis in human SZ95
sebocytes by activating AhR/ARNT/CYP1A1 signaling. However, further
studies are required to clarify the molecular mechanism
involved.
In conclusion, the present study investigated the
influences of PM2.5 exposure on the functions of human SZ95
sebocytes. PM2.5 exposure was shown to exhibit inhibitory effects
on cell proliferation, lipid synthesis, and stimulatory effects on
inflammatory cytokine production and AhR signaling activation in
human SZ95 sebocytes. These findings indicate that PM2.5 may
increase the risk for the occurrence of sebaceous gland-associated
diseases, which are dependent on inflammatory responses and lipid
synthesis. While further studies are necessary to fully elucidate
the molecular mechanism, the present study provides a foundation to
better understand the complex interactions involved in extrinsic
triggering of sebaceous gland-associated diseases.
Acknowledgments
The authors would like to thank Professor Qiang Ju,
Dr Tingting Hu (Renji Hospital of Shanghai Jiao Tong University,
Shanghai, China) and Dr Li Meng (Shanghai Dermatology Hospital,
Shanghai, China) for their help in developing this project. The
present study was supported by grants from CMA-L'OREAL China
Skin/Hair Grant Funds (grant no. S2016131419) and the youth funds
of Zhongnan Hospital of Wuhan University (grant no. 2015A014;
Wuhan, China).
References
|
1
|
Kim KE, Cho D and Park HJ: Air pollution
and skin diseases: Adverse effects of airborne particulate matter
on various skin diseases. Life Sci. 152:126–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krutmann J, Liu W, Li L, Pan X, Crawford
M, Sore G and Seite S: Pollution and skin: From epidemiological and
mechanistic studies to clinical implications. J Dermatol Sci.
76:163–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ju Q, Zouboulis CC and Xia L:
Environmental pollution and acne: Chloracne. Dermatoendocrinol.
1:125–128. 2009. View Article : Google Scholar
|
|
4
|
Ju Q and Zouboulis CC:
Endocrine-disrupting chemicals and skin manifestations. Rev Endocr
Metab Disord. 17:449–457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ju Q, Fimmel S, Hinz N, Stahlmann R, Xia L
and Zouboulis CC: 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters
sebaceous gland cell differentiation in vitro. Exp Dermatol.
20:320–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu T, Pan Z, Yu Q, Mo X, Song N, Yan M,
Zouboulis CC, Xia L and Ju Q: Benzo(a)pyrene induces interleukin
(IL)-6 production and reduces lipid synthesis in human SZ95
sebocytes via the aryl hydrocarbon receptor signaling pathway.
Environ Toxicol Pharmacol. 43:54–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ju Q, Yiang K, Zouboulis CC, Ring J and
Chen W: Chloracne: From clinic to research. Zhonghua Pifuke Yixue
Zazhi. 30:2–6. 2012.
|
|
8
|
Díaz RV and Rosa Dominguez E: Health risk
by inhalation of PM2.5 in the metropolitan zone of the City of
Mexico. Ecotoxicol Environ Saf. 72:866–871. 2009. View Article : Google Scholar
|
|
9
|
Dockery DW: Health effects of particulate
air pollution. Ann Epidemiol. 19:257–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weichenthal SA, Godri-Pollitt K and
Villeneuve PJ: PM2.5, oxidant defence and cardiorespiratory health:
A review. Environ Health. 12:402013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Q, Kang Z, Jiang S, Zhao J, Yan S, Xu F
and Xu J: Effects of ambient fine particles PM2.5 on human HaCaT
cells. Int J Environ Res Public Health. 14:E722017. View Article : Google Scholar
|
|
12
|
Sugihara K, Kitamura S, Yamada T, Ohta S,
Yamashita K, Yasuda M and Fujii-Kuriyama Y: Aryl hydrocarbon
receptor (AhR)-mediated induction of xanthine oxidase/xanthine
dehydrogenase activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Biochem Biophys Res Commun. 281:1093–1099. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong CH, Lee CH, Yu HS and Huang SK:
Benzopyrene, a major polyaromatic hydrocarbon in smoke fume,
mobilizes Langerhans cells and polarizes Th2/17 responses in
epicutaneous protein sensitization through the aryl hydrocarbon
receptor. Int Immunopharmacol. 36:111–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jux B, Kadow S and Esser C: Langerhans
cell maturation and contact hypersensitivity are impaired in aryl
hydrocarbon receptor-null mice. J Immunol. 182:6709–6717. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho YC, Zheng W and Jefcoate CR:
Disruption of cell-cell contact maximally but transiently activates
AhR-mediated transcription in 10T1/2 fibroblasts. Toxicol Appl
Pharmacol. 199:220–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu T, Wang D, Yu Q, Li L, Mo X, Pan Z,
Zouboulis CC, Peng L, Xia L and Ju Q: Aryl hydrocarbon receptor
negatively regulates lipid synthesis and involves in cell
differentiation of SZ95 sebocytes in vitro. Chem Biol Interact.
258:52–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ju Q, Yu Q, Song N, Tan Y, Xia L and
Zouboulis CC: Expression of aryl hydrocarbon receptor in human
epidermis, hair follicles and sebaceous glands and its
significance. Zhonghua Pifuke Zazhi. 44:761–764. 2011.
|
|
18
|
Esser C, Bargen I, Weighardt H,
Haarmann-Stemmann T and Krutmann J: Functions of the aryl
hydrocarbon receptor in the skin. Semin Immunopathol. 35:677–691.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu Q, Hu T, Mo X, Zhang C, Xia L,
Zouboulis CC and Ju Q: Effect of tetrachlorodibenzo-p-dioxin on the
expression of cytochrome P4501A1 in human SZ95 sebocytes and its
significance. Zhonghua Pifuke Zazhi. 46:557–560. 2013.
|
|
20
|
Kalmes M, Hennen J, Clemens J and Blömeke
B: Impact of aryl hydrocarbon receptor (AhR) knockdown on cell
cycle progression in human HaCaT keratinocytes. Biol Chem.
392:643–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tauchi M, Hida A, Negishi T, Katsuoka F,
Noda S, Mimura J, Hosoya T, Yanaka A, Aburatani H, Fujii-Kuriyama
Y, et al: Constitutive expression of aryl hydrocarbon receptor in
keratinocytes causes inflammatory skin lesions. Mol Cell Biol.
25:9360–9368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kakimoto K, Nagayoshi H, Konishi Y,
Kajimura K, Ohura T, Nakano T, Hata M, Furuuchi M, Tang N, Hayakawa
K, et al: Size distribution of chlorinated polycyclic aromatic
hydrocarbons in atmospheric particles. Arch Environ Contam Toxicol.
72:58–64. 2017. View Article : Google Scholar
|
|
23
|
Jeong SC, Song MK, Cho Y, Lee E and Ryu
JC: Integrative analysis of mRNA and microRNA expression of a human
alveolar epithelial cell(A549) exposed to water and organic-soluble
extract from particulate matter (PM)2.5. Environ Toxicol.
32:302–310. 2017. View Article : Google Scholar
|
|
24
|
Yang B, Chen D, Zhao H and Xiao C: The
effects for PM2.5 exposure on non-small-cell lung cancer induced
motility and proliferation. Springerplus. 5:20592016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Z, Liu Y, Duan F, Qin M, Wu F, Sheng
W, Yang L, Liu J and He K: Transcriptomic analyses of the
biological effects of airborne PM2.5 exposure on human bronchial
epithelial cells. PLoS One. 10:e01382672015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Q, Zhang J, Peng S, Tian M, Chen J
and Shen H: Effects of water soluble PM2.5 extracts exposure on
human lung epithelial cells (A549): A proteomic study. J Appl
Toxicol. 34:675–687. 2014. View
Article : Google Scholar
|
|
27
|
Zhang J, Ghio AJ, Gao M, Wei K, Rosen GD
and Upadhyay D: Ambient particulate matter induces alveolar
epithelial cell cycle arrest: Role of G cyclins. FEBS Lett.
581:5315–5320. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Longhin E, Holme JA, Gutzkow KB, Arlt VM,
Kucab JE, Camatini M and Gualtieri M: Cell cycle alterations
induced by urban PM2.5 in bronchial epithelial cells:
Characterization of the process and possible mechanisms involved.
Part Fibre Toxicol. 10:632013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Obermayer-Pietsch B, Hong J-B,
Melnik BC, Yamasaki O, Dessinioti C, Ju Q, Liakou AI, Al-Khuzaei S,
Katsambas A, et al: Acne-associated syndromes: Models for better
understanding of acne pathogenesis. J Eur Acad Dermatol Venereol.
25:637–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zouboulis CC, Picardo M, Ju Q, Kurokawa I,
Törőcsik D, Bíró T and Schneider MR: Beyond acne: Current aspects
of sebaceous gland biology and function. Rev Endocr Metab Disord.
17:319–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zouboulis CC, Jourdan E and Picardo M:
Acne is an inflammatory disease and alterations of sebum
composition initiate acne lesions. J Eur Acad Dermatol Venereol.
28:527–532. 2014. View Article : Google Scholar
|
|
32
|
Ganceviciene R, Graziene V, Fimmel S and
Zouboulis CC: Involvement of the corticotropin-releasing hormone
system in the pathogenesis of acne vulgaris. Br J Dermatol.
160:345–352. 2009. View Article : Google Scholar
|
|
33
|
Zhang H, Yao Y, Chen Y, Yue C, Chen J,
Tong J, Jiang Y and Chen T: Crosstalk between AhR and wnt/β-catenin
signal pathways in the cardiac developmental toxicity of PM2.5 in
zebrafish embryos. Toxicology. 355–356:31–38. 2016. View Article : Google Scholar
|
|
34
|
Borgie M, Ledoux F, Verdin A, Cazier F,
Greige H, Shirali P, Courcot D and Dagher Z: Genotoxic and
epigenotoxic effects of fine particulate matter from rural and
urban sites in Lebanon on human bronchial epithelial cells. Environ
Res. 136:352–362. 2015. View Article : Google Scholar
|
|
35
|
Líbalová H, Krčková S, Uhlířová K, Milcová
A, Schmuczerová J, Ciganek M, Kléma J, Machala M, Šrám RJ and
Topinka J: Genotoxicity but not the AhR-mediated activity of PAHs
is inhibited by other components of complex mixtures of ambient air
pollutants. Toxicol Lett. 225:350–357. 2014. View Article : Google Scholar : PubMed/NCBI
|