Introduction
According to the statistics, almost a quarter of
female patients suffering from cancer are diagnosed with breast
cancer (1). As the most typical
type of cancer affecting women, even in an era with advanced
medical applications, breast cancer remains a serious concernt and
a threat to human health, causing significant morbidity and
mortality (2). Several subtypes
of breast cancer, each requiring different therapeutic regimens,
limit the treatment options. The standard treatment for breast
cancer is chemotherapy and radiotherapy; however, treatment
outcomes are, in the most part, discouraging for patients (3). In this scenario, it is imperative to
explore different alternative therapies or medicines with low
toxicicity for breast cancer treatment.
Due to the significant cytotoxic activities and less
adverse effects, herbal medicines have gradually become good
candidates for cancer therapy (4). It has been proven that Cordyceps
militaris, a folk tonic in Asia, displays pro-apoptotic
properties in cells and tumor xenografts in C57BL/6 mice via
mitochondrial-related pathways (5,6).
As a type of food and medical fungus, Grifola frondosa has
been studied for years, and amino acids, polysaccharides and
amounts of trace elements have been found in its fruitbody. Since
the first study on the anti-tumor effects of Grifola
frondosa polysaccharide (GFP) in 1984, the structure and
function of its polysaccharides have been gradually analyzed
(7). Pharmacological analyses and
clinical trials have demonstrated that the polysaccharide-enriched
extract of Grifola frondosa exhibits various activities,
including anti-tumor, immunomodulatory, and blood glucose and lipid
regulating effects (8–10). A chemically sulfated
polysaccharide purified from Grifola frondosa has also been
shown to induce HepG2 cell apoptosis via the Notch 1-NF-κB pathway
(11). However, few studies to
date have reported the pro-apoptotic activities of GFP on breast
cancer cells and the underlying mechanisms.
Apoptosis, an energy-dependent process, is regulated
by various signals (12). During
this process, cell shrinkage, chromatin condensation and DNA damage
are observed (13). Mitochondrial
apoptosis occurs gradually along with the depolarization of
mitochondrial transmembrane potential (MMP; ΔΨm), the abnormal
expressions of B-cell lymphoma 2 (Bcl-2) family members, cytochrome
c (Cyto c) over-release and caspase-3 activation
(14,15). The initiator caspase (caspase-8)
controls the proteolytic maturation of caspase-3 (16). The accumulation of intracellular
reactive oxygen species (ROS) is capable of inducing apoptosis by
interacting with proteins related to mitochondrial dysfunction. On
the other hand, the activation of AKT and extracellular
signal-regulated kinases (ERKs) contributes to cell proliferation
and apoptosis (17,18).
This study aimed to investigate the anti-breast
cancer effects of GFP in in vitro and in vivo models.
We found that in MCF-7 and MDA-MB-231 cells, GFP induced apoptotic
cell death related to mitochondrial function. GFP also
significantly suppressed the growth of MCF-7 tumor xenografts in
nude mice. Our data support the possible use of Grifola
frondosa as a therapeutic agent for breast cancer therapy.
Materials and methods
Preparation of polysaccharides separated
from Grifola frondosa
Grifola frondosa powder (100 g) was extracted
twice with 10-fold double-distilled water (DD water) at 90°C for 3
h. The protein existing in the extract was removed using Sevag
reagent [v (n-butanol):v (chloroform) = 1:4, 50 ml].
Polysaccharides were collected via the alcohol precipitation method
with 4-fold ethanol. The content of the total polysaccharides
separated from Grifola frondosa was 65.2±1.05 mg/g.
Cell culture
The cell lines, MDA-MB-231 (human breast epithelial
cell line; ATCC no. HTB-26) and MCF-7 (human breast carcinoma cell
line; ATCC no. HTB-22), were maintained in Dulbecco's modified
Eagle's medium (DMEM) medium, supplemented with a 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 g/ml streptomycin under a
humidified atmosphere containing 5%/95% of CO2/air at
37°C. The cultured medium was refreshed every 3 days. Cell culture
reagents were obtained from Invitrogen Life Technologies (Carlsbad,
CA, USA).
MTT cell survival assay
The cells (5,000 cells/100 μl) were seeded
into 96-well plates and incubated with GFPs at concentrations of
25, 50, 100, 200 and 400 μg/ml for 24 or 48 h. Subsequently,
10 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
(0.5 mg/ml) dissolved in phosphate-buffered saline (PBS) were added
to each well. Following a 4-h incubation at 37°C in the dark, the
supernatant was aspirated, and then 100 μl DMSO were added.
The absorbance was measured at a wavelength of 540 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Values were expressed as a percentage of those from the
corresponding controls.
Analysis of lactate dehydrogenase (LDH)
concentration and caspase-3 activation
The cells (5×104) were seeded into 6-well
plates and treated with 50 and 200 μg/ml GFPs for 24 h. The
LDH concentration in the culture medium was detected using a LDH
assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) according to the manufacturer's instructions.
The treated cells were collected and lysed with
radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, St.
Louis, MO, USA), and the protein concentration was examined using
Bio-Rad protein assays. A caspase-3 colorimetric detection kit
(Enzo Life Sciences, Inc., Farmingdale, NY, USA) was applied to
detect caspase-3 activation. Values were expressed as a percentage
of those from the corresponding controls.
Flow cytometric analysis of cell
apoptosis
The cells were seeded into 6-well plates at
5×104/well and treated with 50 and 200 μg/ml GFPs
for 12 h. The treated cells were harvested and washed with PBS 3
times, and then suspended in binding buffer containing with 5
μl Annexin V-FITC (20 μg/ml) and 5 μl
propidium iodide (PI; 50 μg/ml) (BD Biosciences, Franklin
Lakes, NJ, USA). Following a 15-min incubation at room temperature
in the dark, the apoptotic rate was analyzed using a flow cytometer
(FC500; Beckman Coulter, Inc., Brea, CA, USA).
Detection of ROS
Following treatment with GFPs for 12 h at
concentrations of 50 and 200 μg/ml, the cells were suspended
and incubated with 10 μM dichlorodihydrofluorescein
diacetate (DCFH-DA) for 10 min at 37°C in the dark. After being
washed with PBS 3 times, the intracellular ROS levels were
determined using a flow cytometer (FC500; Beckman Coulter).
Detection of MMP
The cells were seeded into 6-well plates at
5×104/well and treated with 50 and 200 μg/ml GFPs
for 12 h. The cells were further incubated with 2 μM
5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1; Sigma-Aldrich) at 37°C for 10 min. After being washed
with PBS, the changes in fluorescent color were examined using a
fluorescence microscope (x20 magnification; CCD camera, TE2000;
Nikon, Tokyo, Japan).
MCF-7 tumor xenograft model
Six-week-old male BALB/c nude mice purchased from
Weitong Lihua Laboratory Animal Technology Ltd. Co. (Beijing,
China) were used in our in vivo experiments. The protocol
was approved by the Animal Ethics Committee of Jilin University.
The mice were housed in groups 3 per cage and maintained on a 12 h
light/dark cycle at 23±1°C with water and food available ad
libitum.
An amount of 0.1 ml (1×108 cells/ml) of
MCF-7 cells at the mid-log phase was inoculated subcutaneously into
the right flank of BALB/c nude mice. When the diameter of the tumor
reached to 3–5 mm, the mice were divided into 2 groups (n=3 each)
randomly, and orally treated with 0.5 g/kg GFPs or DD water every
other day continuously for 2 weeks. During the GFP administration,
the body weight and tumor dimension were measured. The equation of
length × (width)2 × 0.5 was applied to estimate the
tumor volume (mm3). All the mice were sacrificed via an
injection of 200 mg/kg pentobarbital after the final treatment, and
tumor tissues were dissected.
Western blot analysis
The MCF-7 or MDA-MB-231 (2×105 cells)
were seeded into 6-well plates and exposed vaqrious concentrations
of GFPs for the indicated periods of time. The cells and collected
tumor tissues were lysed by RIPA buffer containing 1% protease
inhibitor cocktail and 2% phenylmethanesulfonyl fluoride (PMSF)
(both from Sigma-Aldrich). The bicinchoninic acid method was
applied to detect the protein concentrations. Protein samples (40
μg) were separated on a 12% SDS-PAGE gel, and then
electroblotted onto nitrocellulose membranes (0.45 μm; Bio
Basic, Inc., Markham, ON, Canada). The membranes were incubated at
4°C overnight with Bcl-2 (MABC573), Bcl-extra large (Bcl-xL;
MAB4625), Bax (AB2915), cleaved caspase-3 (AB3623), cleaved
caspase-8 (AB1879), and phosphorylated (p)-AKT (05–1003) (all from
Merck Millipore, Darmstadt, Germany), total (t)-AKT (ab126811) and
p-glycogen synthase kinase-3β (GSK-3β) (ab75745) (both from Abcam,
Cambrige, UK), T-GSK-3β (PK1111) and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) (ABS16) (both from Merck Millipore) at
dilution of 1:1,000. The membranes were then incubated with
horseradish peroxidase-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 2 h at room
temperature. Band detection was performed using enhanced
chemiluminescence (ECL) detection kits (GE Healthcare Life
Sciences, Chalfont, UK). The intensity of the bands was quantified
using ImageJ software.
Statistical analysis
Data are expressed as the means ± standard deviation
(SD) and analyzed by a one-way analysis of variance (ANOVA)
followed with Dunn's test using SPSS software (SPSS, Inc., Chicago,
IL, USA). The IC50 values are calculated using SPSS 16.0
software (IBM Corporation, Armonk, NY, USA). A value P<0.05 was
considered to indicate a statistically significant difference.
Results
Intracellular toxic effects of GFPs on
breast cancer cells
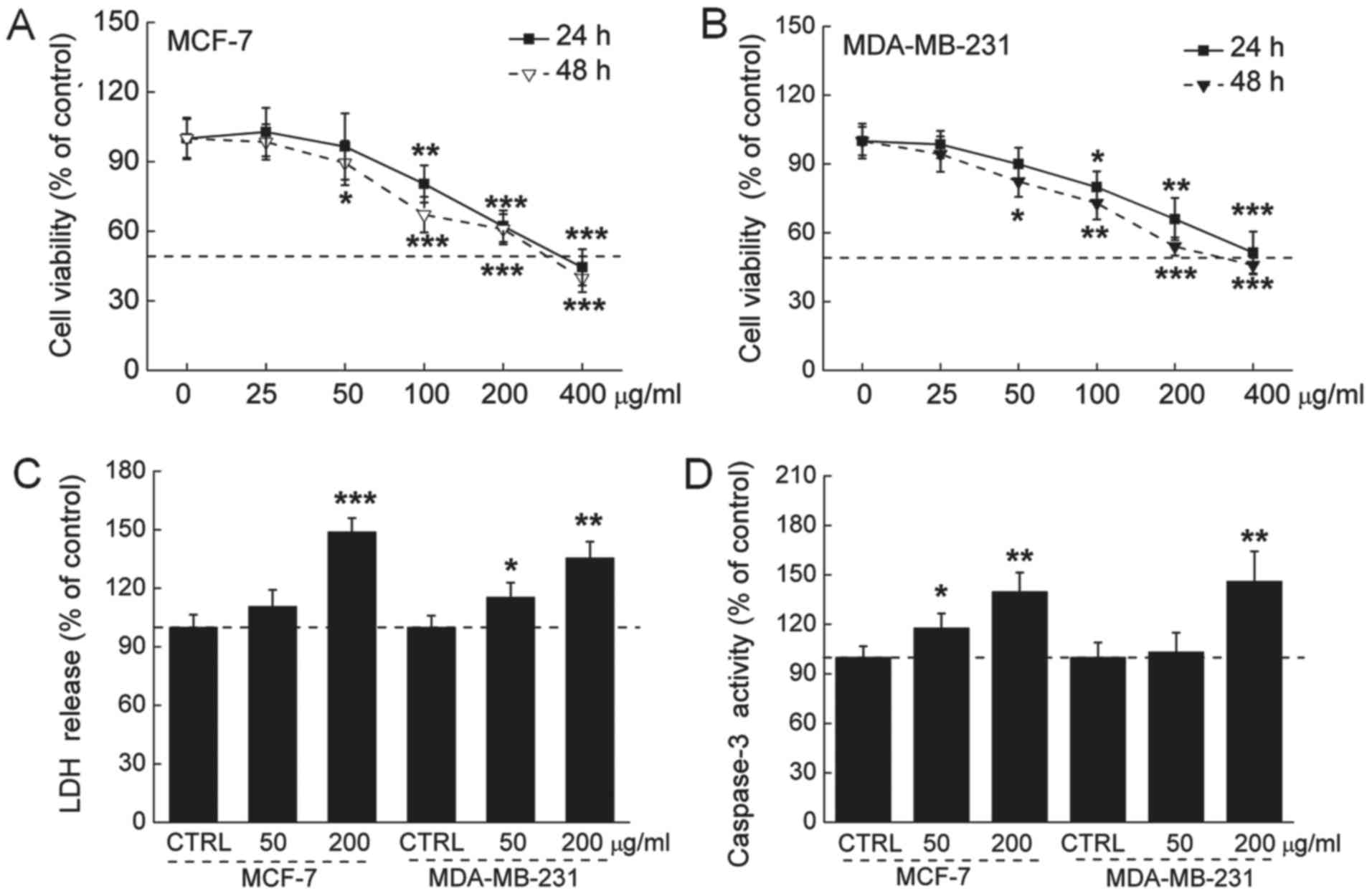
The 24-h IC50 values of the GFPs were 335
and 412 μg/ml, and the 48-h IC50 values of the
GFPs were 295 and 348 µg/ml in the MCF-7 and MDA-MB-231
cells, respectively (Fig. 1A and
B). The release of LDH was increased during cell death. An
approximately 47 and 32% LDH over-release was observed in the 200
µg/ml GFP-treated MCF-7 and MDA-MB-231 cells (P<0.01;
Fig. 1C). The activation of
caspase-3 serves as a marker of cell apoptosis. We found that the
GFPs at 200 µg/ml enhanced caspase-3 activation by almost 35
and 43% in the MCF-7 and MDA-MB-231 cells, respectively (P<0.01;
Fig. 1D).
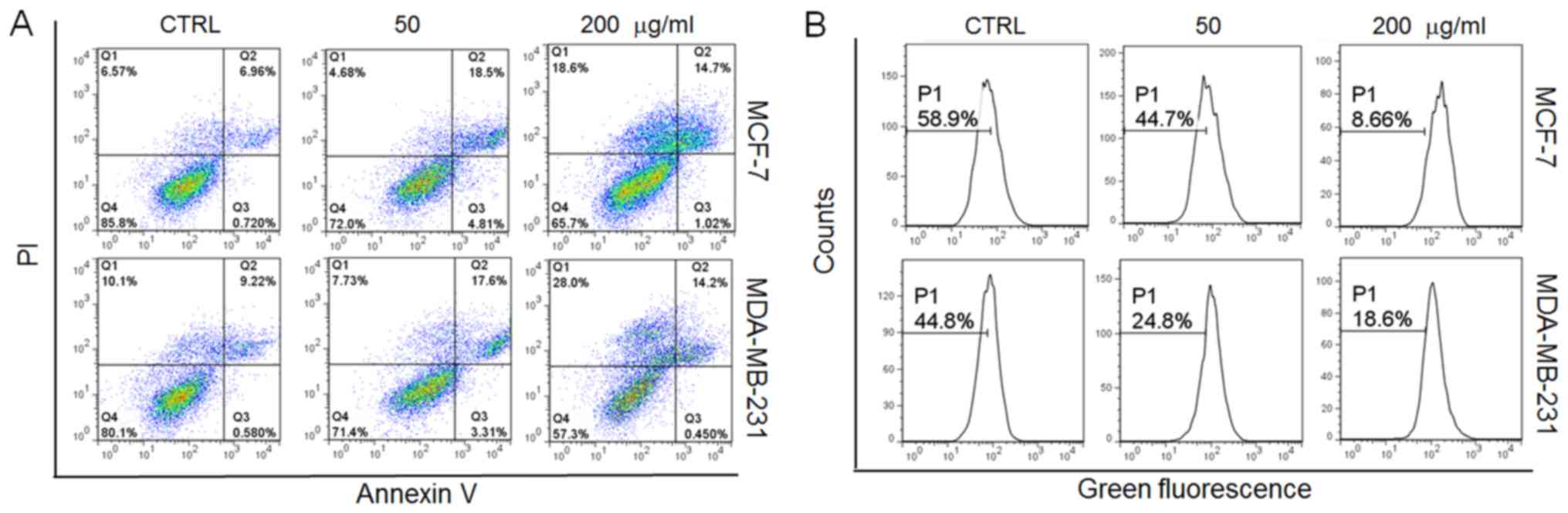
In addition, incubation with the GFPs (50
μg/ml) for 12 h led to approximately 22 and 21% of the MCF-7
and MDA-MB-231 cells, respectively to become apoptotic (Fig. 2A). Furthermore, oxidative stress,
particularly, the overproduction of intracellular ROS, leads to
cellular dysfunction and apoptosis (19). In this study, following incubation
with the GFPs for 12 h at 200 μg/ml, a 50 and 26% increment
in intracellular ROS levels was noted in the MCF-7 and MDA-MB-231
cells, respectively compared with the controls (Fig. 2B). All these data confirmed that
GFPs exerted cytotoxic effects on the MCF-7 and MDA-MB-231
cells.
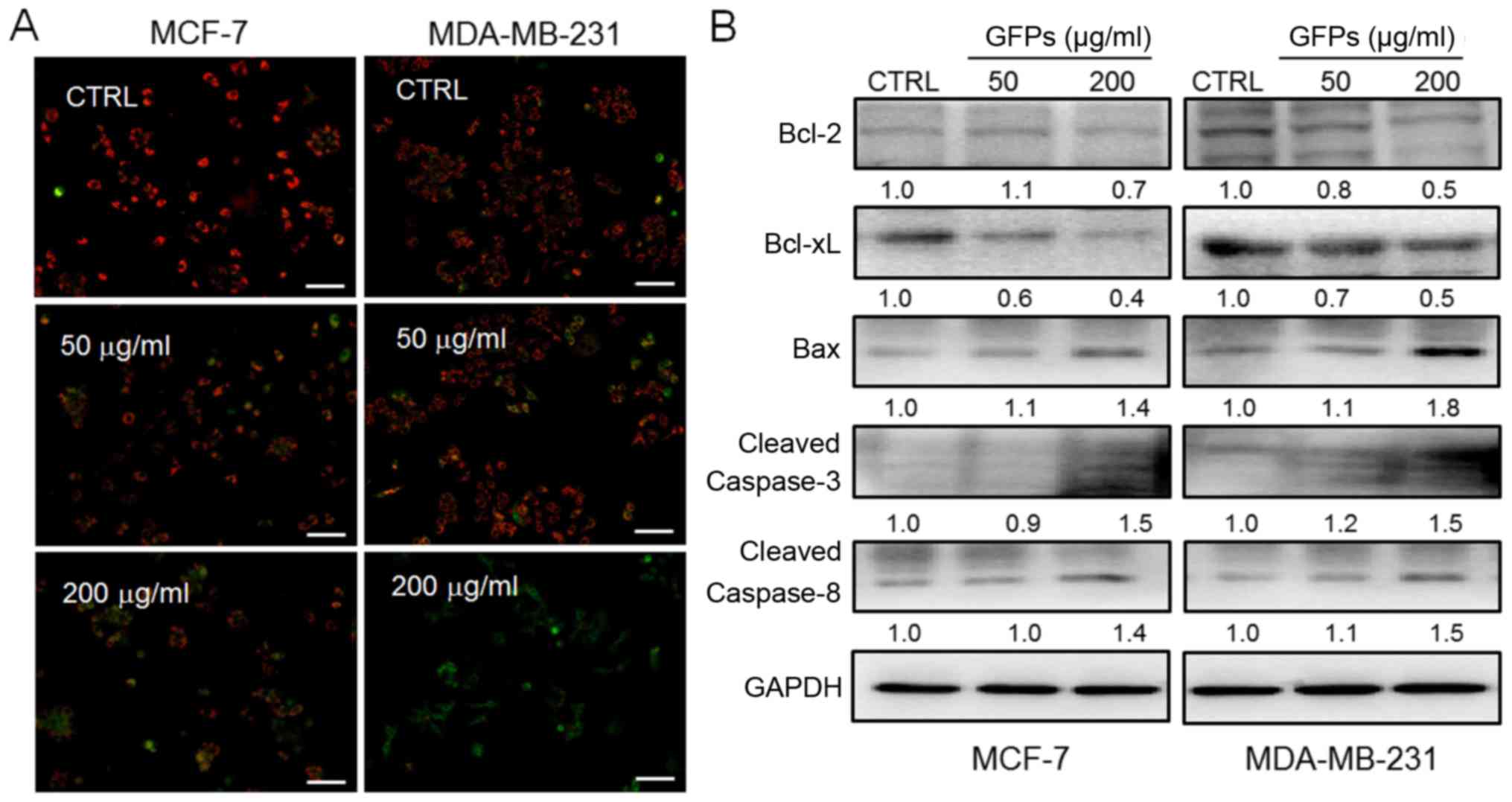
GFPs cause mitochondrial dysfunction
Mitochondrial function plays a central role during
cell apoptosis (20). As
indicated by the reduced ratio of red to green fluorescence by JC-1
staining, treatment with the GFPs for 12 h at concentrations of 50
and 200 μg/ml significantly decreased MMP in the MCF-7 and
MDA-MB-231 cells, compared with untreated cells (Fig. 3A). Furthermore, the increased
expression levels of Bax, cleaved caspase-3 and caspase-8, and the
reduced levels of Bcl-2 and Bcl-xL were observed in the MCF-7 and
MDA-MB-231 cells following incubation with the GFPs for 24 h GFPs
at concentrations of 50 and 200 μg/ml (Fig. 3B).
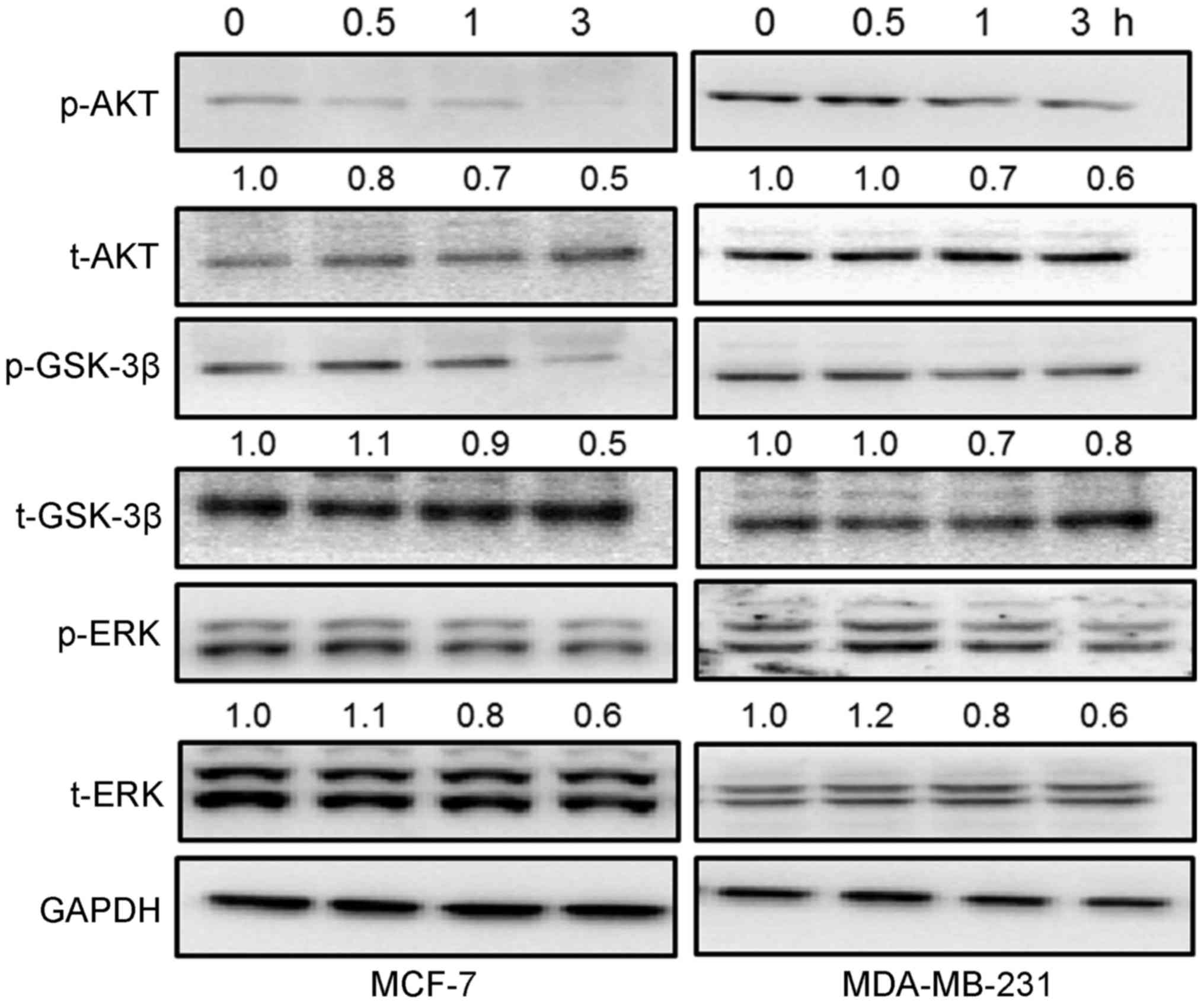
The activation of AKT/GSK-3β and ERK is
involved in GFP-mediated cytotoxicity in breast cancer cells
It has been reported that the activation of
AKT/GSK-3β and ERK participate in cell proliferation, survival and
even apoptosis (21,22). The GFPs time-dependently
suppressed the phosphorylation of AKT and GSK-3β from 0.5 to 3 h in
the breast cancer cells incubated with 200 μg/ml of GFPs,
particularly at 1 and 3 h (Fig.
4). In addition, incubation with 200 μg/ml GFPs
significantly inhibited the activation of ERK from 1 and 3 h in the
MCF-7 and MDA-MB-231 cells (Fig.
4).
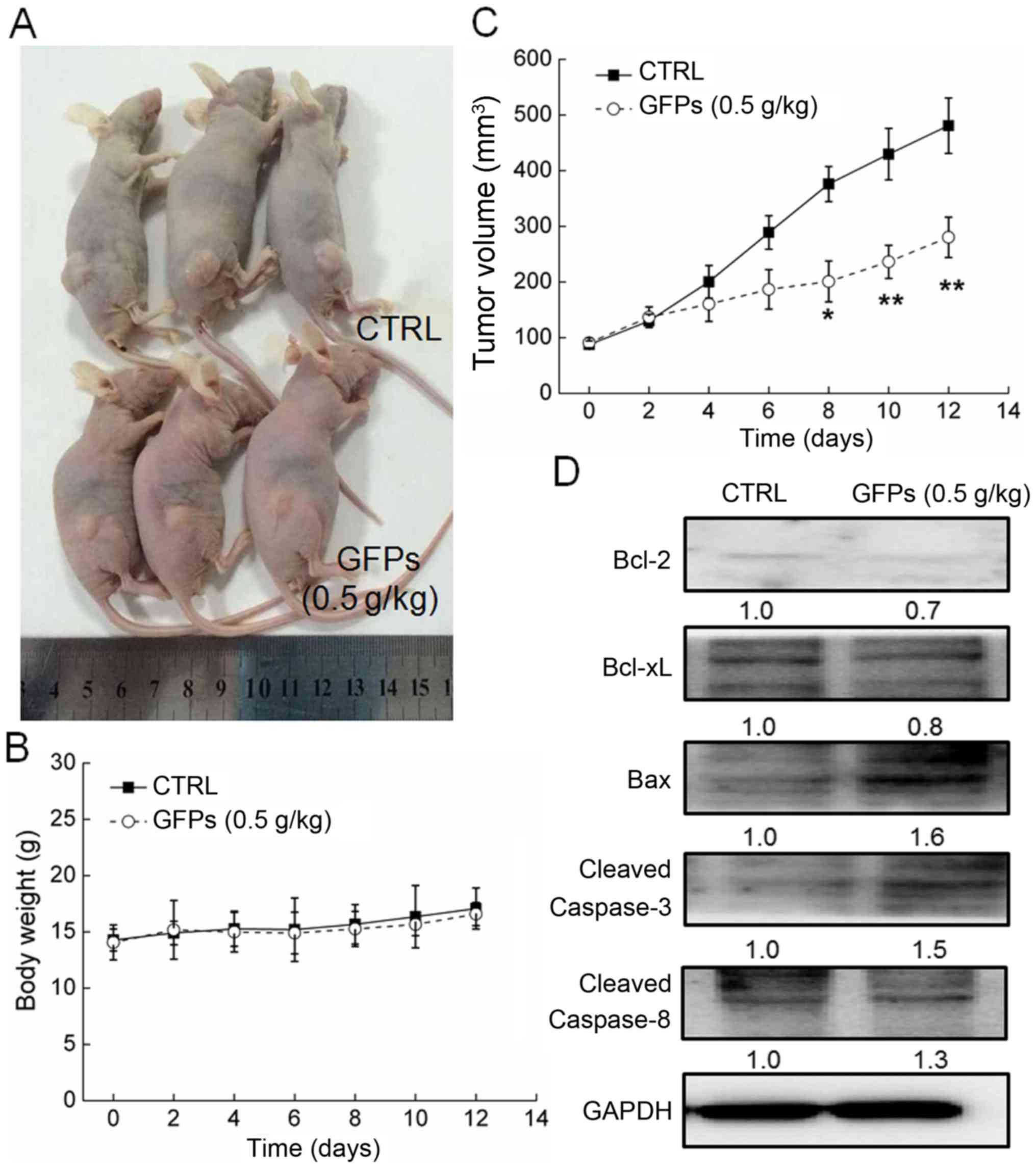
GFPs inhibits the growth of MCF-7 tumor
xenografts
GFP administration at 0.5 g/kg significantly
suppressed the growth of MCF-7 tumor xenografts from the 8th day to
the end of the experiment (P<0.05; Fig. 5A and C). Compared with the
controls, GFPs decreased the tumor size by almost 42% on the 14th
day (P<0.01; Fig. 5C). The
GFPs did not to influence the body weight of the mice compared with
the untreated mice, which suggested limited aggressive side-effects
(Fig. 5B). Additionally, compared
with the controls, in the tumor tissues from the treated mice, GFPs
increased the expression levels of Bax, cleaved caspase-3 and
caspase-8, and suppressed the expression levels of Bcl-2 and Bcl-xL
(Fig. 5D).
Discussion
In the present study, the potential anti-tumor
effects of GPFs on breast cancer were successfully confirmed in
MCF-7 and MDA-MB-231 cells, and tumor-bearing nude mice. The GFPs
exerted cytotoxic effects on the breast cancer cell lines, as
evidenced by a decrease in cell viability, and an increase in LDH
release, ROS accumulation and caspase-3 activation, as well as the
induction of cell apoptosis and mitochondrial apoptotic
alterations. The suppressed phosphorylation of AKT/GSk-3β and ERK,
related to mitochondrial function, revealed the possible mechanisms
involved.
During apoptosis, which is a physiological suicide
process, mitochondrial function plays a central role (23). The functional loss of the
mitochondria is related to the dissipation of MMP (20), which was noted in this study
following incubation with the GFPs for 12 h. The reduced Bcl-2 and
Bcl-xL levels, and enhanced Bax expression levels were also
observed in the GFP-treated cells. The Bcl-2 family, located in the
outer mitochondrial membrane, serves as an important index in
mitochondrial-mediated apoptosis (24). Moreover, in this study, the
accumulation of intracellular ROS was observed in the cells treated
with the GFPs. The overproduction of ROS causes oxidative stress,
further resulting in mitochondrial apoptosis and cellular
dysfunction. It has been reported that ROS accumulation is
responsible for the opening mitochondrial permeability transition
pore (mPTP), which leads to mitochondrial depolarization, matrix
solutes loss and Cyto c release (25). Taken together, the effects of GFPs
on mitochondrial function are involved in its anti-breast cancer
effects.
On the other hand, mitochondria control the
intrinsic pathway of apoptosis, and during this process, MMP
ignites caspases and other catabolic enzyme activation (26). Caspases are considered as inactive
pro-enzymes and will be activated via proteolytic cleavage
(27). Caspase-8, located mostly
in the mitochondria, undergoes dimerization, and then cleaves
itself to its fully activated form (28), which further leads to the
cleavages of effector caspases in the cytosol (caspase-3) (29). Caspase-3, amplifying the
initiation signals from caspase-8, plays a central role in
activating the apoptotic program via regulating other caspases and
some vital proteins (30), and it
is important for cell death in a tissue-, cell type- or death
stimulus-specific manner (31).
In this study, in MCF-7 and MDA-MB-231 cells, and MCF-7 tumor
xenografts, GFPs significantly enhanced the expressions of cleaved
caspase-3 and caspase-8, which revealed that the anticancer
activity of GFPs was associated with the regulation on caspase
activation, which further targets the mitochondria.
AKT signaling is responsible for cell proliferation
and apoptosis, which regulates apoptotic proteins including Bcl-2
family members GSK-3β (21). The
reduced phosphorylation of AKT activates its downstream GSK-3β,
which promotes Bax activation (32). As previously reported, GSK-3β
mediates the release of cytochrome c into the cytosol, and
its activated form helps to open mPTP (33). Via the AKT/GSK-3β- and
ROS-dependent mitochondrial-mediated pathway, 18β-glycyrrhetinic
acid induces the apoptosis of pituitary adenoma cells (23). Furthermore, the ERK pathway has
been reported to be a target for cancer therapy, which is
hyper-acted in human tumors (34,35). p-ERK, an active form, inhibits
pro-apoptotic signals via the modulation of numerous substrates
(22). In our study, the GFPs
strongly suppressed the phosphorylation of AKT/GSK-3β and ERK in
the MCF-7 and MDA-MB-231 cells, and this suppression may be
involved in the GFP-mediated anti-tumor effects. Furthermore, ERK
has been shown to exert positive regulatory effects on Bcl-2 and
Bcl-xL expression, and ERKs/Bcl-2 have been confirmed as potential
targets for cancer cell apoptosis (36,37). Previous studies have indicated
that AKT contributes to the maintenance of mitochondrial integrity,
which also affects Bcl-2 expression (38,39). Collectively, the downregulation of
AKT/GSK-3β and ERK activation contributes to GFP-induced
mitochondrial apoptosis.
The anti-breast cancer effects of GFPs were
successfully confirmed in in vitro and in vivo
experiments. GFPs reduced cell viability, enhanced the apoptotic
rate, increased the ROS and caspase-3 intracellular levels, and
caused LDH over-release, as well as MMP dissipation and the
abnormal expression of pro-apoptotic proteins. The suppressed
activation of ERK and AKT/GSK-3β in the GFP-incubated cells was
responsible for mitochondrial dysfunction. All these findings
reveal that the mitochondrial-dependent apoptotic pathway
contributes to GFP-induced cytotoxicity in the MCF-7 and MDA-MB-231
cells, which provides pharmacological evidence to support the use
of GFPs as a potential chemotherapeutic agent.
Acknowledgments
This study was supported by Jilin Province Science
and Technology Key Problem of China (2014020314YY).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hosseini BA, Pasdaran A, Kazemi T,
Shanehbandi D, Karami H, Orangi M and Baradaran B: Dichloromethane
fractions of Scrophularia oxysepala extract induce apoptosis in
MCF-7 human breast cancer cells. Bosn J Basic Med Sci. 15:26–32.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chang CH, Chen SJ and Liu CY: Adjuvant
treatments of breast cancer increase the risk of depressive
disorders: a population-based study. J Affect Disord. 182:44–49.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vadodkar AS, Suman S, Lakshmanaswamy R and
Damodaran C: Chemoprevention of breast cancer by dietary compounds.
Anticancer Agents Med Chem. 12:1185–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin CY, Kim GY and Choi YH: Induction of
apoptosis by aqueous extract of Cordyceps militaris through
activation of caspases and inactivation of Akt in human breast
cancer MDA-MB-231 cells. J Microbiol Biotechnol. 18:1997–2003.
2008.
|
|
6
|
Yoo HS, Shin JW, Cho JH, Son CG, Lee YW,
Park SY and Cho CK: Effects of Cordyceps militaris extract on
angiogenesis and tumor growth. Acta Pharmacol Sin. 25:657–665.
2004.PubMed/NCBI
|
|
7
|
Ohno N, Suzuki I, Oikawa S, Sato K,
Miyazaki T and Yadomae T: Antitumor activity and structural
characterization of glucans extracted from cultured fruit bodies of
Grifola frondosa. Chem Pharm Bull (Tokyo). 32:1142–1151. 1984.
View Article : Google Scholar
|
|
8
|
Inoue A, Kodama N and Nanba H: Effect of
maitake (Grifola frondosa) D-fraction on the control of the T lymph
node Th-1/Th-2 proportion. Biol Pharm Bull. 25:536–540. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma XL, Meng M, Han LR, Li Z, Cao XH and
Wang CL: Immunomodulatory activity of macromolecular polysaccharide
isolated from Grifola frondosa. Chinese Journal of Natural
Medicines. 13:906–914. 2015. View Article : Google Scholar
|
|
10
|
Cui FJ, Li Y, Xu YY, Liu ZQ, Huang DM,
Zhang ZC and Tao WY: Induction of apoptosis in SGC-7901 cells by
polysaccharide-peptide GFPS1b from the cultured mycelia of Grifola
frondosa GF9801. Toxicol In Vitro. 21:417–427. 2007. View Article : Google Scholar
|
|
11
|
Wang CL, Meng M, Liu SB, Wang LR, Hou LH
and Cao XH: A chemically sulfated polysaccharide from Grifola
frondos induces HepG2 cell apoptosis by notch1-NF-κB pathway.
Carbohydr Polym. 95:282–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakagawa S, Shiraishi T, Kihara S and
Tabuchi K: Detection of DNA strand breaks associated with apoptosis
in human brain tumors. Virchows Arch. 427:175–179. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joselin AP, Schulze-Osthoff K and Schwerk
C: Loss of Acinus inhibits oligonucleosomal DNA fragmentation but
not chromatin condensation during apoptosis. J Biol Chem.
281:12475–12484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen R, Liu S, Piao F, Wang Z, Qi Y, Li S,
Zhang D and Shen J: 2,5-Hexanedione induced apoptosis in
mesenchymal stem cells from rat bone marrow via
mitochondria-dependent caspase-3 pathway. Ind Health. 53:222–235.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Wu Y, Luo K, Liu Y, Zhou M, Yan S,
Shi H and Cai Y: The protective effects of selenium on
cadmium-induced oxidative stress and apoptosis via mitochondria
pathway in mice kidney. Food Chem Toxicol. 58:61–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Q, Wu D, Chen W, Yan Z and Shi Y:
Proteolytic processing of the caspase-9 zymogen is required for
apoptosome-mediated activation of caspase-9. J Biol Chem.
288:15142–15147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin YL, Wang GJ, Huang CL, Lee YC, Liao
WC, Lai WL, Lin YJ and Huang NK: Ligusticum chuanxiong as a
potential neuroprotectant for preventing serum deprivation-induced
apoptosis in rat pheochromocytoma cells: functional roles of
mitogen-activated protein kinases. J Ethnopharmacol. 122:417–423.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lou H, Fan P, Perez RG and Lou H:
Neuroprotective effects of linarin through activation of the
PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorg
Med Chem. 19:4021–4027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brown DI and Griendling KK: Regulation of
signal transduction by reactive oxygen species in the
cardiovascular system. Circ Res. 116:531–549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hisatomi T, Ishibashi T, Miller JW and
Kroemer G: Pharmacological inhibition of mitochondrial membrane
permeabilization for neuroprotection. Exp Neurol. 218:347–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maurer U, Preiss F, Brauns-Schubert P,
Schlicher L and Charvet C: GSK-3 - at the crossroads of cell death
and survival. J Cell Sci. 127:1369–1378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sweatt JD: The neuronal MAP kinase
cascade: a biochemical signal integration system subserving
synaptic plasticity and memory. J Neurochem. 76:1–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang D, Wong HK, Feng YB and Zhang ZJ:
18beta-glycyrrhetinic acid induces apoptosis in pituitary adenoma
cells via ROS/MAPKs-mediated pathway. J Neurooncol. 116:221–230.
2014. View Article : Google Scholar
|
|
24
|
Chan SL and Yu VC: Proteins of the bcl-2
family in apoptosis signalling: from mechanistic insights to
therapeutic opportunities. Clin Exp Pharmacol Physiol. 31:119–128.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bernardi P and Rasola A: Calcium and cell
death: the mitochondrial connection. Subcell Biochem. 45:481–506.
2007. View Article : Google Scholar
|
|
26
|
Galluzzi L, Vitale I, Kepp O, Séror C,
Hangen E, Perfettini JL, Modjtahedi N and Kroemer G: Methods to
dissect mitochondrial membrane permeabilization in the course of
apoptosis. Methods Enzymol. 442:355–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hippe D, Gais A, Gross U and Lüder CG:
Modulation of caspase activation by Toxoplasma gondii. Methods Mol
Biol. 470:275–288. 2009. View Article : Google Scholar
|
|
28
|
Schug ZT, Gonzalvez F, Houtkooper RH, Vaz
FM and Gottlieb E: BID is cleaved by caspase-8 within a native
complex on the mitochondrial membrane. Cell Death Differ.
18:538–548. 2011. View Article : Google Scholar :
|
|
29
|
Lee KH, Feig C, Tchikov V, Schickel R,
Hallas C, Schütze S, Peter ME and Chan AC: The role of receptor
internalization in CD95 signaling. EMBO J. 25:1009–1023. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Espín R, Roca FJ, Candel S, Sepulcre MP,
González-Rosa JM, Alcaraz-Pérez F, Meseguer J, Cayuela ML, Mercader
N and Mulero V: TNF receptors regulate vascular homeostasis in
zebrafish through a caspase-8, caspase-2 and P53 apoptotic program
that bypasses caspase-3. Dis Model Mech. 6:383–396. 2013.
View Article : Google Scholar :
|
|
31
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang L, Zhang Y and Xing D: LPLI inhibits
apoptosis upstream of Bax translocation via a
GSK-3beta-inactivation mechanism. J Cell Physiol. 224:218–228.
2010.PubMed/NCBI
|
|
33
|
Petit-Paitel A, Brau F, Cazareth J and
Chabry J: Involvment of cytosolic and mitochondrial GSK-3beta in
mitochondrial dysfunction and neuronal cell death of
MPTP/MPP-treated neurons. PLoS One. 4:e54912009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krajarng A, Nakamura Y, Suksamrarn S and
Watanapokasin R: α-Mangostin induces apoptosis in human
chondrosarcoma cells through downregulation of ERK/JNK and Akt
signaling pathway. J Agric Food Chem. 59:5746–5754. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tada K, Kawahara K, Matsushita S,
Hashiguchi T, Maruyama I and Kanekura T: MK615, a Prunus mume Steb.
Et Zucc ('Ume') extract, attenuates the growth of A375 melanoma
cells by inhibiting the ERK1/2-Id-1 pathway. Phytother Res.
26:833–838. 2012. View Article : Google Scholar
|
|
36
|
Zhao Y, Shen S, Guo J, Chen H, Greenblatt
DY, Kleeff J, Liao Q, Chen G, Friess H and Leung PS:
Mitogen-activated protein kinases and chemoresistance in pancreatic
cancer cells. J Surg Res. 136:325–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar
|
|
38
|
Lim JY, Park SI, Oh JH, Kim SM, Jeong CH,
Jun JA, Lee KS, Oh W, Lee JK and Jeun SS: Brain-derived
neurotrophic factor stimulates the neural differentiation of human
umbilical cord blood-derived mesenchymal stem cells and survival of
differentiated cells through MAPK/ERK and PI3K/Akt-dependent
signaling pathways. J Neurosci Res. 86:2168–2178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma R, Xiong N, Huang C, Tang Q, Hu B,
Xiang J and Li G: Erythropoietin protects PC12 cells from
beta-amyloid(25–35)-induced apoptosis via PI3K/Akt signaling
pathway. Neuropharmacology. 56:1027–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|