1. Introduction
Angiogenesis refers to the process through which new
blood vessels form from the pre-existing vasculature (1–8).
It requires a complex interplay between angiogenic stimuli and
angiogenic repressors, which leads to the controlled activation,
proliferation, and migration of endothelial cells (ECs) (9–11).
Normal angiogenesis plays a vital role in growth, development and
wound healing (9,10). However, angiogenesis is also
initiated in a number of diseases due to the imbalanced production
of angiogenic regulators (12).
Pathological angiogenesis results in an aberrant vasculature which
accelerates disease progression (9,11).
To elaborate, elevated levels of vascular endothelial growth factor
(VEGF) are responsible for angiogenesis in cancer, a process that
provides blood supply for tumor growth and metastasis (9,13–15). Likewise, the increased retinal
expression of VEGF in response to hyperglycemic damage leads to
ocular neovascularization which increases the risk of vision loss
in diabetic retinopathy (16,17). Similar observations also present
in rheumatoid arthritis (RA) and macular degeneration (9,14,18,19). In view of the pivotal role of
angiogenesis in the pathogeneses of numerous diseases, the
inhibition of angiogenesis by the use of anti-angiogenic agents has
become an important therapeutic approach (20).
Artemisinin is extracted from the traditional
Chinese medicine 'qinghao' (Artemisia Annua L.) (9,18).
Its derivatives are renowned for their potent anti-malarial effects
and reliable safety records (9,18).
During the past decade, emerging evidence has indicated that
artemisinins also serve as effective treatments for cancer
(9,13–15). The effectiveness of artemisinins
in cancer at least in part relies on the inhibition of tumor
angiogenesis (9,15). For example, the daily injection of
dihydroartemisinin (DHA), a semi-synthetic derivative of
artemisinin, reduces the density of the tumor vasculature and
consequently impairs tumor growth in mouse models (21). Moreover, other derivatives, such
as artesunate (ART) and artemether display similar
anti-angiogenenic properties (15,18,22). Hence, artemisinins demonstrate
auspicious potential for use as novel treatments for a wider
variety of angiogenesis related diseases (23). There is evidence to suggest that
several signaling pathways, including the mitogen-activated protein
kinase (MAPK) pathway, the nuclear factor-κB (NF-κB) pathway, and
the phosphatidylinositide 3-kinase (PI3K)/protein kinase B
(Akt)/mammalian target of rapamycin (mTOR) pathway, may mediate the
inhibitory effect of artemisinin derivatives on angiogenesis
(1,2,18,24,25). Beginning by describing the
characteristics of artemisinin derivatives, this review provides a
comprehensive explanation of the current literature regarding the
molecular mechanisms underlying the effects of artemisinins on
angiogenesis.
2. Derivatives of artemisinin and their
characteristics
Following the isolation of artemisinin, the parent
compound, semi-synthetic derivatives, such as artemether, arteether
and ART have been developed with improved pharmacokinetics
(26–28). The lipid-based derivatives,
artemether and arteether, are highly lipophilic (15,29). While possessing longer half-lives
than more hydrophilic artemisinins, both compounds are better at
transpassing the blood-brain barrier (15,29). Coupled with their anticancer
activity, they may be exceptionally efficient in treating brain
tumors (15,29). Due to the addition of a
hemisuc-cinate group, out of all the artemisinins, ART has the best
water solubility and bioavailability (9). Experiments such as human umbilical
vein endothelial cell (HUVEC) migration assays have confirmed that
ART successfully inhibits angiogenesis induced by human melanoma
cells with a much lower concentration (3,30).
Nevertheless, artemisone and artemiside, two relatively newer
10-alkylaminoartemisinin derivatives, seem to have superior
efficacy compared to ART (31,32). Although artemiside still has to
undergo a toxicological evaluation, it has been well-established
that artemisone has negligible toxicity, particularly neurotoxicity
owing to its low lipophilicity (31–33).
Despite the variability in the structure and
biological properties of artemisinin derivatives, all compounds are
metabolized into DHA after being administered into the body
(9). DHA can also be synthesized
artificially and possesses additional water solubility; hence, it
is the most studied artemisinin analog other than ART (3,9).
On the other hand, novel derivatives of artemisinins are constantly
being developed with further refined pharmacological properties
(34). As argued by Jung et
al, non-acetal-based artemisinins have lower neurotoxicity than
the above-mentioned acetal-based artemisinins (34). For example,
dexoartemisinin-C60 conjugate, which comprises of a
dexoartemisinin dimer and a fullerene cage demonstrates potent
anti-angiogenic activity in chorioallantoic membrane assay with
enhanced efficacy and expected lower toxicity (34).
3. Mechanisms underlying the anti-angiogenic
effects of artemisinin derivatives
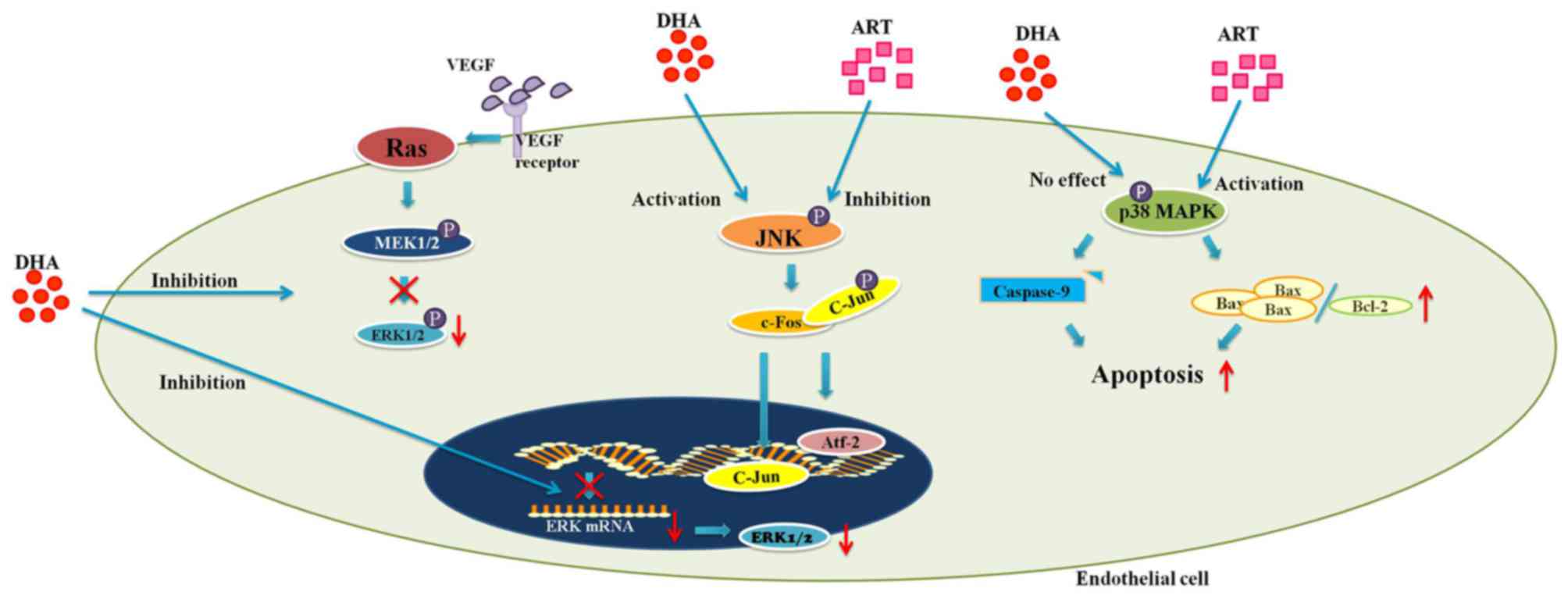
The MAPK pathway
MAPKs, encompassing extracellular signal-regulated
kinases (ERKs), c-Jun N-terminal kinase (JNK) and p38 MAPK, are
involved in a wide range of cellular activities (1,35).
ERKs, which regulate cell proliferation and survival, can be
activated by downstream signals of VEGF (1). The binding of VEGF with its
receptors on ECs stimulates a conformational change of the Ras
protein, which subsequently leads to the phosphorylation of Raf
(1). Activated Raf in turn
phosphorylates MEK1/2, direct activators of ERKs (1). This cascade of signals eventually
results in the promotion of EC proliferation and survival (1). Unlike ERK, JNK and p38 MAPK mediate
both cytoprotective and cytotoxic processes (35–37). JNK is a pro-angiogenic protein
which can be induced by cellular stressors, such as hypoxia or
inflammation (35,36). Upon activation, JNK phosphorylates
the c-jun component of the activator protein-1 (AP-1), which
results in the nuclear translocation of both c-jun and
activating-transcription factor-2 (Atf-2) (36,38). Consequently, the expression levels
of pro-angiogenic stimuli, including VEGF, cyclooxygenase-2 (COX-2)
and matrix metalloproteases (MMPs) are increased (20,36,38–41). p38 MAPK responds to stress-related
extracellular stimuli in a similar manner (37). On the other hand, both JNK and p38
MAPK are key mediators of apoptosis (11,42,43). It has been hypothesized that JNK
is critical for the activation of pro-apoptotic protein Bax
(43). In addition to Bax, p38
MAPK also upregulates the pro-apoptotic Fas, while inhibiting
proteins that promote cell survival (ERK and Akt) (37).
The artemisinin family drugs act upon MAPK signaling
cascades in multiple ways. DHA inhibits HUVEC proliferation by
blocking both the transcription and activation of ERK1/2 (1). The incubation of HUVECs with DHA (20
μM) for up to 12 h was shown to successfully reduce the
expression of ERK1/2 at both the mRNA and protein level (1). Together with the decreased level of
phosphorylated ERK1/2, these results were accompanied by a
dose-dependent decrease in HUVEC proliferation (1) (Fig.
1). Moreover, the addition of PD98059, an inhibitor of MEK1/2,
resulted in a comparable inhibition of HUVEC proliferation
(1). Furthermore, the
co-administration of DHA and PD98059 did not lead to further
reduction in the proportion of proliferating HUVECs (1). Since ERK1/2 are activated by MEK1/2
only, the lack of additive effect between PD98059 and DHA justifies
that DHA restrains angiogenesis by impeding ERK related
cytoprotective activities (1)
(Table I).
 | Table IMechanisms underlying the
anti-angiogenesis effects of artemisinin derivatives. |
Table I
Mechanisms underlying the
anti-angiogenesis effects of artemisinin derivatives.
In vitro
experiments
|
|---|
| Analog | Cell type | Effect | Mechanism | (Refs.) |
|---|
| Experiments on
ECs |
| ART | HUVECs | Proliferation
↓ | JNK activation
↓ | (11) |
| Apoptosis ↑ | p38 MAPK activation
↑ | (11) |
| DHA | HUVECs | Proliferation
↓ | ERK signalling
↓ | (1) |
| Migration ↓ | Independent of p38
MAPK activation | (3) |
| Proliferation and
migration ↓ | VEGFR2 expression
↓ | (2) |
| Nuclear
translocation and DNA binding capacity of NF-κB ↓ | (2,21) |
| Experiments on
non-ECs |
| ART | RAFLS | Production of VEGF
and IL-8 ↓ | Akt phosphorylation
↓ | (18) |
| IL-8 production
↓ | Nuclear
translocation and DNA binding capacity of NF-κB ↓ | (52) |
| Akt phosphorylation
↓ | (52) |
| DHA | Rhabdomyosarcoma
cells Ewing sarcoma cells | VEGF production
↓ | Blockade of
mTORC1 | (24) |
In vivo
experiments
|
|---|
| Analog | Animal model | Effect | Mechanism | (Refs.) |
|---|
| ART | Sprague-Dawley
rats | Corneal
neovascularization ↓ | p38 MAPK activation
↑ | (11) |
| DHA | BALB/c nude
mice | Production of
pro-angiogenic cytokines ↓
Tumor microvessel density ↓ | NF-κB activity
↓ | (21) |
| C57BL/6N mice | Retinal
neovascularization ↓ | NF-κB activity
↓ | (2) |
As previously demonstrated, DHA, at a concentration
of 20 μM, significantly increased the level of activated JNK
in HUVECs at 6 h of incubation (44). In addition, the level of activated
JNK plateaued at 12 h-incubation before starting to decline at 24 h
(44). Intriguingly, although the
activation of JNK is also involved in apoptosis, DHA exerted no
effect on HUVEC viability (44).
Nonetheless, beginning from a concentration of 12.5 μM, ART
decreased the level of activated JNK in HUVECs following incubation
for 0.5 h (11) (Fig. 1). Accordingly, the proliferation
of ART-treated HUVECs was also inhibited (11). The results from these two studies
rise controversy regarding the distinctions in effects of different
artemisinin analogs on JNK activation (Table I).
In a previous study, compared to the
phosphate-buffered saline (PBS)-treated controls, ART significantly
increased the proportion of apoptotic HUVECs by inducing p38 MAPK
activation (11). Further
investigation revealed that activated p38 MAPK leads to an increase
in the Bax/Bcl-2 ratio and the cleavage of caspase-9, which
ultimately results in apoptosis via the intrinsic mitochondrial
pathway (11) (Fig. 1). Moreover, pretreatment of HUVECs
with a p38 MAPK inhibitor (SB203850) abolished the ART-induced
activation of p38 MAPK, while it decreased the proportion of
apoptotic cells (11) (Table I). Treatment with ART (25
μM) was also able to reduce rat corneal neovascularization
in response to alkaline burns (11). In addition, TUNEL and CD31 double
staining of those corneal sections revealed a substantially larger
proportion of apoptotic vascular ECs in the ART-treated group
(11) (Table I). Therefore, both in vitro
and in vivo experiments suggest that ART inhibits
angiogenesis by activating p38 MAPK and promoting EC apoptosis
(11). Notably, the pro-apoptotic
effect of ART seems to rely on the formation of reactive oxygen
species (ROS) via the cleavage of the endoperoxide bond by ferrous
iron (9,11,45,46). The reduced phosphorylation of p38
MAPK was observed simultaneously with the inhibition of ROS
generation, whereas the addition of ferrous iron along with ART
facilitated ROS production and increased the proportion of
apoptotic ECs (11).
Intriguingly, although the possession of an endoperoxide bond is a
common feature of all aretemisinin derivatives, DHA (20 μM)
restricts EC proliferation and migration without inducing apoptosis
(1,3,15).
Seeing the role of p38 MAPK in ART-induced EC apoptosis, such a
result provides little evidence for DHA to have a comparable
influence to ART on p38 MAPK signaling (11). Indeed, DHA (20 μM) did not
induce any change in the level of either p38 MAPK or activated p38
MAPK in HUVECs (3) (Fig. 1). Moreover, the blockade of p38
MAPK by SB203850 had no effect on the DHA-suppressed EC migration
(3) (Table I). Therefore, unlike ART, DHA
inhibits EC migration via a mechanism that is independent of p38
MAPK.
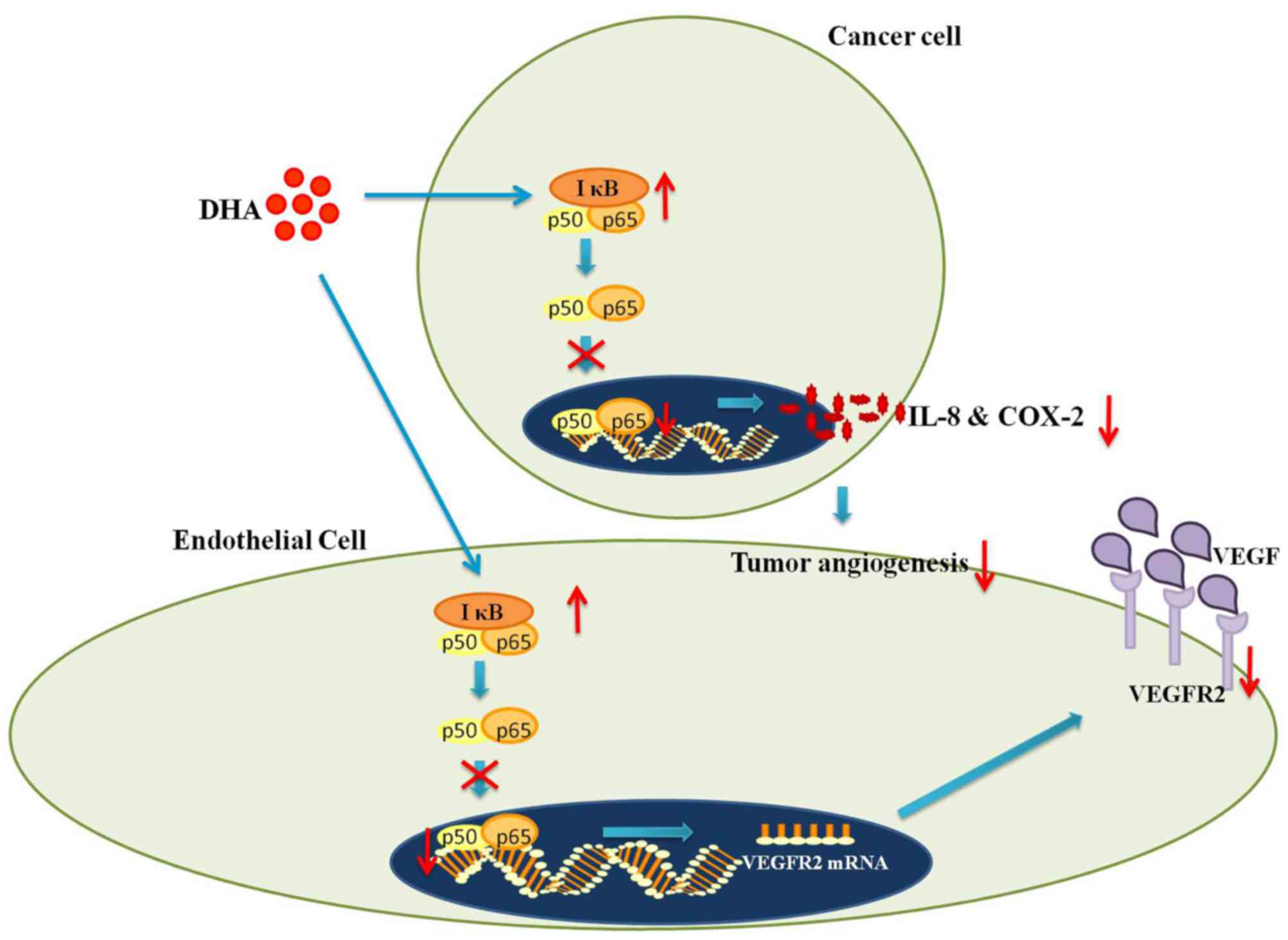
The NF-κB pathway
In addition to its role in innate immunity, NF-κB
regulates the transcription of numerous angiogenesis-related genes
(47). For instance, in
hypoxia-induced angiogenesis, hypoxia inducible factor-α (HIF-α)
becomes stabilized owing to the inhibition of prolyl hydroxylase-1,
which leads to increased degradation of inhibitor κB (IκB)
(48). Since IκB is responsible
for the masking of NF-κB (p65) nuclear localization sequence, its
degradation results in the increased nuclear translocation of NF-κB
and subsequently increased transcriptional expression of vascular
endothelial growth factor receptor 2 (VEGFR2) (2,48).
VEGFR2 is crucial in mediating the VEGF-induced activation of
pro-angiogenic signaling pathways (40). Hence, NF-κB plays an irreplaceable
role in angiogenesis by regulating the production of VEGFR2
(2,40).
On the other hand, the activation of NF-κB by other
pro-inflammatory factors, such as tumor necrosis factor (TNF) may
induce HIF-1α under normoxic conditions (41). Indeed, many inflammation-related
cytokines and chemokines modulated by NF-κB are pro-angiogenic
factors (41). For example, in
vitro experiments with HUVECs have shown that interleukin-8
(IL-8) promotes tube formation as well as EC infiltration (49). Similarly, the incubation of human
appendix ECs with various chemokines has been shown to result in
the formation of pseudovessels (50). Additionally, the activation of
NF-κB by lipopolysaccharides may directly upregulate HIF-2 and thus
induce an increase in nitric oxide (NO) production (41). NO at a high concentration (>1
μM) in turn stabilizes HIF-α expression and consequently
increases the production of both VEGF and its receptors (41,51).
There is extensive evidence to suggest that
interactions between artemisinins and NF-κB signaling inhibit
angiogenesis (2,15,21). In particular, DHA was found to
prevent the nuclear translocation of NF-κB in HUVECs by increasing
the IκB level (2). Consequently,
the production of VEGFR2 was decreased (2) (Fig.
2). Moreover, DHA downregulates the binding of NF-κB p65 to the
promoter region of VEGFR2 (2).
The lack of synergy between DHA and a known NF-κB inhibitor
[pyrrolidine dithiocarbamate (PDTC)] further confirms that DHA
operates by interfering with the NF-κB pathway (2). Considering the aforementioned role
of VEGFR2, suppressed VEGFR2 production conceivably explains the
reduced EC proliferation and migration following treatment with DHA
(2). Moreover, the daily
injection of DHA into the vitreous humor substantially reduced
retinal neovascularization in mice models (2). Moreover, combined treatment with DHA
and PDTC resulted in no further reduction in retinal vessel density
(2) (Table I). Therefore, DHA inhibits
angiogenesis in vitro and in vivo by blocking NF-κB
signaling (2).
In the meantime, artemisinins exert their
anti-angiogenic effects by ameliorating NF-κB-mediated inflammation
(9,21,52). Pretreating human RA
fibroblast-like synoviocytes (RAFLS) with ART (1 μM)
significantly suppressed NF-κB mediated IL-8 production induced by
TNF-α (52). Following the
addition of TNF-α, ART prevented IκB degradation, leading to the
reduced nuclear translocation and weakened DNA-binding capacity of
NF-κB (52). In vitro
experiments using HUVECs treated with DHA produced almost identical
results (21) (Table I). Since IL-8 has long been
recognized as a pro-angiogenic cytokine, it appears that
artemisinins may inhibit angiogenesis by interfering with NF-κB
signaling and consequently inhibiting IL-8 production (49). In addition, the daily injection of
DHA into mice with xenografts of the pancreatic cancer cell line,
BxPC-3, was shown to result in a dose-dependent reduction of VEGF,
IL-8 and COX-2 in tumor cells (21). Moreover, the reduced production of
the above-mentioned pro-angiogenic cytokines was accompanied by
reduced NF-κB activity and decreased tumor microvessel density
(21) (Fig. 2) (Table I). Taken together, artemisinins
inhibit angiogenesis by suppressing the secretion of NF-κB
regulated pro-angiogenic cytokines (21,49).
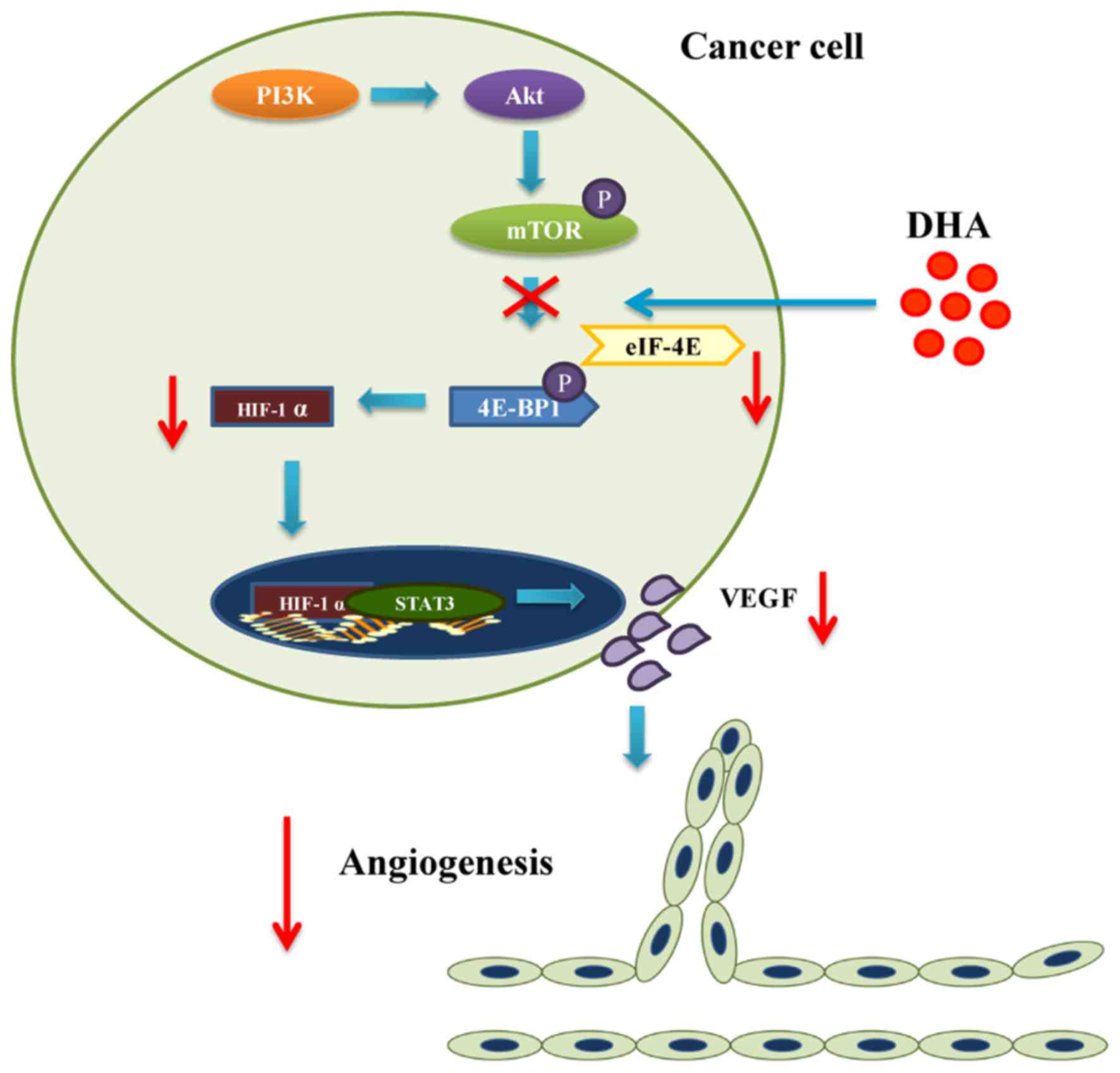
The PI3K/Akt/mTOR pathway
The role of PI3K and its downstream targets Akt/mTOR
in angiogenesis involves the modulation of VEGF expression and
other angiogenic stimuli such as NO and angiopoietins (ANGs)
(53). In mammals, PI3K regulates
the expression of mTOR which phosphorylates the eukaryotic
translation initiation factor 4E binding protein (4E-BP1) (54). The phosphorylation of 4E-BP1
reduces the stability of a complex consisting of eukaryotic
translation initiation factor 4E (eIF-4E) and 4E-BP1 (54). Since the eIF-4E/4E-BP1 complex
inhibits HIF-1α translation, phosphorylated mTOR leads to 4E-BP1
activation which increases the expression of HIF-1α (54). In addition, Akt is able to
activate endothelial NO synthase (eNOS), one of the regulators of
NO synthesis in tumors (53).
Activated eNOS mediates VEGF induced EC migration (53). Meanwhile, ANGs and their receptors
are another class of growth factors facilitating the effect of VEGF
that are related to the PI3K/Akt/mTOR pathway (53).
ART inhibits angiogenesis by preventing Akt
activation. ART reduces the production of pro-inflammatory
cytokines and VEGF in human RAFLS (18). To elaborate, PI3K inhibitor
inhibits the production of several pro-inflammatory cytokines
including the pro-angiogenic IL-8 (52). The inhibition of PI3K also
correlates with reduced expression and nuclear translocation of
HIF-1α (18). Accordingly, the
transcriptional expression of VEGF is decreased (18). There is evidence to suggest that
ART prevents Akt phosphorylation, while hampering the production of
VEGF and IL-8 in a similar manner (18,52) (Table
I). Apart from IL-8 and VEGF, the decreased phosphorylation of
Akt is likely to diminish the effect of eNOS and ANG2 on
angiogenesis. Therefore, inhibited Akt activation by ART leads to
reduced production of angiogenic stimuli (18,52).
Results from numerous studies have indicated that
DHA also functions as a PI3K/Akt/mTOR inhibitor (55–57). Apart from inhibiting Akt activity,
DHA primarily exerts its effect by interacting with mTOR (24,58). DHA effectively blocks mTOR complex
1 (mTORC1) in rhabdomyosarcoma cells and Ewing sarcoma cells in
both a dose- and time-dependent manner (24,25). As a result, binding between 4E-BP1
and eIF-4E is enhanced (24).
Since tumor angiogenesis is powered by the sustained secretion of
VEGF by tumor cells under hypoxic stress, it relies on the
stabilization of HIF-1α induced by degradation of 4E-BP1 eIF-4E
complex (54). Blockade of mTORC1
by DHA impairs the ability of tumor cells to secrete VEGF, which
arguably contributes to the inhibition of tumor angiogenesis
(Fig. 3 and Table I).
The effects of artemisinins on selected signaling
pathways may depend on cell types. For example, as previously
demonstrated, ART activated none of the three MAPKs in TNF-α
stimulated RAFLSs, which is in contrast with the findings using
HUVECs (11,18,52). Likewise, DHA inhibited ERK
signaling in HUVECs but did not alter ERK signaling in cultured T
cells (1,55). Overall, ECs seem to be especially
susceptible to influences of artemisinins, which again signifies
the potential for artemisinin derivatives to be used as
anti-angiogenic agents. Moreover, the results mentioned above
suggest that artemisinin derivatives may have distinct actions in
different disease models. Hence, tailoring treatment schemes
according to these variations may optimize the outcome.
4. Conclusion
Apart from anti-malaria, extensive evidence suggests
that artemisinins inhibit angiogenesis. The effects of artemisinins
on angiogenesis rely on perturbations of MAPK pathway, NF-κB
pathway, and PI3K/Akt/mTOR pathway (1,2,11,21,52). DHA inhibits EC proliferation by
reducing ERK1/2 expression and activation (1,11).
In the meantime, ART and DHA appear to play distinct roles in JNK
and p38 MAPK activation (3,11,44). In addition to decreasing VEGFR2
expression in ECs, artemisinins limit angiogenesis by mitigating
the production of pro-angiogenic cytokines from tumor cells
(2,21,52). Both actions are achieved by the
inhibition of NF-κB activity (2,21,52). Furthermore, since artemisinins
prevent activation of both Akt and mTOR, they are able to interfere
with relevant downstream pro-angiogenic gene transcription to
inhibit angiogenesis (24,25,52,53,55–59).
The pleiotropy of the effects of artemisinins renders them as
potent anti-angiogenic agents. In view of the significance of
angiogenesis in pathogeneses of many diseases, artemisinin and its
derivatives are excellent candidates to be used in novel therapies
(18).
Abbreviations:
|
4E-BP1
|
eukaryotic translation initiation
factor 4E binding protein
|
|
ANG
|
angiopoietin
|
|
AP-1
|
activator protein-1
|
|
ART
|
artesunate
|
|
Atf-2
|
activating transcription factor-2
|
|
COX-2
|
cyclooxygenase-2
|
|
DHA
|
dihydroartemisinin
|
|
EC
|
endothelial cell
|
|
eIF-4E
|
eukaryotic translation initiation
factor 4E
|
|
ERK
|
extracellular signal- regulated
kinase
|
|
HIF
|
hypoxia inducible factor
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
IL-8
|
interleukin-8
|
|
IκB
|
inhibitor κB
|
|
JNK
|
c-Jun N-terminal kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MMP
|
matrix metalloprotease
|
|
mTOR
|
mammalian target of rapamycin
|
|
mTORC1
|
mammalian target of rapamycin complex
1
|
|
NF-κB
|
nuclear factor-κB
|
|
NO
|
nitric oxide
|
|
PDTC
|
pyrrolidine dithio-carbamate
|
|
PI3K
|
phosphatidylinositide 3-kinase
|
|
RA
|
rheumatoid arthritis
|
|
RAFLS
|
rheumatoid arthritis fibroblast-like
synoviocytes
|
|
ROS
|
reactive oxygen species
|
|
TNF
|
tumor necrosis factor
|
|
VEGF
|
vascular endothelial growth
factor
|
|
VEGFR2
|
vascular endothelial growth factor
receptor 2
|
Acknowledgments
This study was supported by grants from the
National Natural Science Foundation of China (no. 81570255), and
the Medical Science and Technology Development Plan of Shandong
Province (no. 2013WS0137). We are grateful for the support from
Shandong Taishan Scholarship (to Ju Liu).
References
|
1
|
Dong F, Tian H, Yan S, Li L, Dong X, Wang
F, Li J, Li C, Cao Z, Liu X, et al: Dihydroartemisinin inhibits
endothelial cell proliferation through the suppression of the ERK
signaling pathway. Int J Mol Med. 35:1381–1387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong F, Zhou X, Li C, Yan S, Deng X, Cao
Z, Li L, Tang B, Allen TD and Liu J: Dihydroartemisinin targets
VEGFR2 via the NF-κB pathway in endothelial cells to inhibit
angiogenesis. Cancer Biol Ther. 15:1479–1488. 2014. View Article : Google Scholar
|
|
3
|
Guo L, Dong F, Hou Y, Cai W, Zhou X, Huang
AL, Yang M, Allen TD and Liu J: Dihydroartemisinin inhibits
vascular endothelial growth factor-induced endothelial cell
migration by a p38 mitogen-activated protein kinase-independent
pathway. Exp Ther Med. 8:1707–1712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh S, Jeong IH, Shin WS and Lee S: Growth
inhibition activity of thioacetal artemisinin derivatives against
human umbilical vein endothelial cells. Bioorg Med Chem Lett.
13:3665–3668. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh S, Jeong IH, Ahn CM, Shin WS and Lee S:
Synthesis and antiangiogenic activity of thioacetal artemisinin
derivatives. Bioorg Med Chem. 12:3783–3790. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oh S, Jeong IH, Shin WS and Lee S:
Synthesis and antiangiogenic activity of exo-olefinated
deoxoartemisinin derivatives. Bioorg Med Chem Lett. 14:3683–3686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci J, Park J, Chung WY, Park KK and
Jung M: Concise synthesis and antiangiogenic activity of
artemisinin-glycolipid hybrids on chorioallantoic membranes. Bioorg
Med Chem Lett. 20:6858–6860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ho WE, Peh HY, Chan TK and Wong WS:
Artemisinins: Pharmacological actions beyond anti-malarial.
Pharmacol Ther. 142:126–139. 2014. View Article : Google Scholar
|
|
10
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng R, Li C, Li C, Wei L, Li L, Zhang Y,
Yao Y, Gu X, Cai W, Yang Z, et al: The artemisinin derivative
artesunate inhibits corneal neovascularization by inducing
ROS-dependent apoptosis in vascular endothelial cells. Invest
Ophthalmol Vis Sci. 54:3400–3409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagy JA, Dvorak AM and Dvorak HF: VEGF-A
and the induction of pathological angiogenesis. Annu Rev Pathol.
2:251–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferrara N: VEGF-A: A critical regulator of
blood vessel growth. Eur Cytokine Netw. 20:158–163. 2009.
|
|
15
|
Crespo-Ortiz MP and Wei MQ: Antitumor
activity of artemisinin and its derivatives: From a well-known
antimalarial agent to a potential anticancer drug. J Biomed
Biotechnol. 2012:2475972012. View Article : Google Scholar
|
|
16
|
Arden GB, Wolf JE and Tsang Y: Does dark
adaptation exacerbate diabetic retinopathy? Evidence and a linking
hypothesis. Vision Res. 38:1723–1729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crawford TN, Alfaro DV III, Kerrison JB
and Jablon EP: Diabetic retinopathy and angiogenesis. Curr Diabetes
Rev. 5:8–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He Y, Fan J, Lin H, Yang X, Ye Y, Liang L,
Zhan Z, Dong X, Sun L and Xu H: The anti-malaria agent artesunate
inhibits expression of vascular endothelial growth factor and
hypoxia-inducible factor-1α in human rheumatoid arthritis
fibroblast-like synoviocyte. Rheumatol Int. 31:53–60. 2011.
View Article : Google Scholar
|
|
19
|
Folkman J: Angiogenesis in cancer,
vascular, rheumatoid and other disease. Nat Med. 1:27–31. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polverini PJ: Angiogenesis in health and
disease: Insights into basic mechanisms and therapeutic
opportunities. J Dent Educ. 66:962–975. 2002.PubMed/NCBI
|
|
21
|
Wang SJ, Sun B, Cheng ZX, Zhou HX, Gao Y,
Kong R, Chen H, Jiang HC, Pan SH, Xue DB, et al: Dihydroartemisinin
inhibits angiogenesis in pancreatic cancer by targeting the NF-κB
pathway. Cancer Chemother Pharmacol. 68:1421–1430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeong E, Song HJ, Lim S, Lee SJ, Lim JE,
Nam DH, Joo KM, Jeong BC, Jeon SS, Choi HY, et al: Repurposing the
anti-malarial drug artesunate as a novel therapeutic agent for
metastatic renal cell carcinoma due to its attenuation of tumor
growth, metastasis, and angiogenesis. Oncotarget. 6:33046–33064.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu XX, Yang L, Li YJ, Zhang D, Chen Y,
Kostecká P, Kmoníčková E and Zídek Z: Effects of sesquiterpene,
flavonoid and coumarin types of compounds from Artemisia annua L.
on production of mediators of angiogenesis. Pharmacol Rep.
65:410–420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Odaka Y, Xu B, Luo Y, Shen T, Shang C, Wu
Y, Zhou H and Huang S: Dihydroartemisinin inhibits the mammalian
target of rapamycin-mediated signaling pathways in tumor cells.
Carcinogenesis. 35:192–200. 2014. View Article : Google Scholar
|
|
25
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Corsello MA and Garg NK: Synthetic
chemistry fuels interdisciplinary approaches to the production of
artemisinin. Nat Prod Rep. 32:359–366. 2015. View Article : Google Scholar
|
|
27
|
Mott BT, He R, Chen X, Fox JM, Civin CI,
Arav-Boger R and Posner GH: Artemisinin-derived dimer phosphate
esters as potent anti-cytomegalovirus (anti-CMV) and anticancer
agents: A structure-activity study. Bioorg Med Chem. 21:3702–3707.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee S: Artemisinin, promising lead natural
product for various drug developments. Mini Rev Med Chem.
7:411–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh NP and Panwar VK: Case report of a
pituitary macroadenoma treated with artemether. Integr Cancer Ther.
5:391–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen H, Shi L, Yang X, Li S, Guo X and Pan
L: Artesunate inhibiting angiogenesis induced by human myeloma
RPMI-8226 cells. Int J Hematol. 92:587–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagelschmitz J, Voith B, Wensing G, Roemer
A, Fugmann B, Haynes RK, Kotecka BM, Rieckmann KH and Edstein MD:
First assessment in humans of the safety, tolerability,
pharmacokinetics, and ex vivo pharmacodynamic antimalarial activity
of the new artemisinin derivative artemisone. Antimicrob Agents
Chemother. 52:3085–3091. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ansari MT, Saify ZS, Sultana N, Ahmad I,
Saeed-Ul-Hassan S, Tariq I and Khanum M: Malaria and artemisinin
derivatives: An updated review. Mini Rev Med Chem. 13:1879–1902.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haynes RK, Fugmann B, Stetter J, Rieckmann
K, Heilmann HD, Chan HW, Cheung MK, Lam WL, Wong HN, Croft SL, et
al: Artemisone - a highly active antimalarial drug of the
artemisinin class. Angew Chem Int Ed Engl. 45:2082–2088. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung M, Tak J, Chung WY and Park KK:
Antiangiogenic activity of deoxoartemisinin derivatives on
chorioallantoic membrane. Bioorg Med Chem Lett. 16:1227–1230. 2006.
View Article : Google Scholar
|
|
35
|
Shen K, Ji L, Lu B and Wang Z: c-Jun
N-terminal kinase mediated VEGFR2 sustained phosphorylation is
critical for VEGFA-induced angiogenesis in vitro and in vivo. Cell
Biochem Biophys. 64:17–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miura S, Matsuo Y and Saku K: Jun
N-terminal kinase inhibitor blocks angiogenesis by blocking VEGF
secretion and an MMP pathway. J Atheroscler Thromb. 15:69–74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Z, Meng D, Li G, Xu J, Tian K and Li Y:
Celecoxib combined with diacerein effectively alleviates
osteoarthritis in rats via regulating JNK and p38MAPK signaling
pathways. Inflammation. 38:1563–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma X, Liu Y, Wang Q, Chen Y, Liu M, Li X,
Xiang R, Wei Y, Duan Y and Han J: Tamoxifen induces the development
of hernia in mice by activating MMP-2 and MMP-13 expression.
Biochim Biophys Acta. 1852:1038–1048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sato Y, Kanno S, Oda N, Abe M, Ito M,
Shitara K and Shibuya M: Properties of two VEGF receptors, Flt-1
and KDR, in signal transduction. Ann NY Acad Sci. 902:201–207.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szade A, Grochot-Przeczek A, Florczyk U,
Jozkowicz A and Dulak J: Cellular and molecular mechanisms of
inflammation-induced angiogenesis. IUBMB Life. 67:145–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta K, Kshirsagar S, Li W, Gui L,
Ramakrishnan S, Gupta P, Law PY and Hebbel RP: VEGF prevents
apoptosis of human microvascular endothelial cells via opposing
effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res.
247:495–504. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Cell Biol. 19:142–149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dong F, Han J, Jing G, Chen X, Yan S, Yue
L, Cao Z, Liu X, Ma G and Liu J: Dihydroartemisinin transiently
activates the JNK/SAPK signaling pathway in endothelial cells.
Oncol Lett. 12:4699–4704. 2016.
|
|
45
|
Firestone GL and Sundar S: Anticancer
activities of artemisinin and its bioactive derivatives. Expert Rev
Mol Med. 11:e322009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Devasagayam TP, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: Current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.
|
|
47
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Oliver KM, Taylor CT and Cummins EP:
Hypoxia. Regulation of NFkappaB signalling during inflammation: The
role of hydroxylases. Arthritis Res Ther. 11:2152009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsiao KY, Chang N, Lin SC, Li YH and Wu
MH: Inhibition of dual specificity phosphatase-2 by hypoxia
promotes interleukin-8-mediated angiogenesis in endometriosis. Hum
Reprod. 29:2747–2755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Suffee N, Richard B, Hlawaty H, Oudar O,
Charnaux N and Sutton A: Angiogenic properties of the chemokine
RANTES/CCL5. Biochem Soc Trans. 39:1649–1653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mateo J, García-Lecea M, Cadenas S,
Hernández C and Moncada S: Regulation of hypoxia-inducible
factor-1alpha by nitric oxide through mitochondria-dependent and
-independent pathways. Biochem J. 376:537–544. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y,
Yang X, Lian F and Sun L: Anti-malarial agent artesunate inhibits
TNF-alpha-induced production of proinflammatory cytokines via
inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human
rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology
(Oxford). 46:920–926. 2007. View Article : Google Scholar
|
|
53
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Masoud GN and Li W: HIF-1α pathway: Role,
regulation and intervention for cancer therapy. Acta Pharm Sin B.
5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao YG, Wang Y, Guo Z, Gu AD, Dan HC,
Baldwin AS, Hao W and Wan YY: Dihydroartemisinin ameliorates
inflammatory disease by its reciprocal effects on Th and regulatory
T cell function via modulating the mammalian target of rapamycin
pathway. J Immunol. 189:4417–4425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chen Q, Chen L, Wu X, Zhang F, Jin H, Lu
C, Shao J, Kong D, Wu L and Zheng S: Dihydroartemisinin prevents
liver fibrosis in bile duct ligated rats by inducing hepatic
stellate cell apoptosis through modulating the PI3K/Akt pathway.
IUBMB Life. 68:220–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Feng X, Li L, Jiang H, Jiang K, Jin Y and
Zheng J: Dihydroartemisinin potentiates the anticancer effect of
cisplatin via mTOR inhibition in cisplatin-resistant ovarian cancer
cells: Involvement of apoptosis and autophagy. Biochem Biophys Res
Commun. 444:376–381. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liao K, Li J and Wang Z:
Dihydroartemisinin inhibits cell proliferation via
AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung
cancer cells. Int J Clin Exp Pathol. 7:8684–8691. 2014.
|
|
59
|
Tan SS, Ong B, Cheng C, Ho WE, Tam JK,
Stewart AG, Harris T, Wong WS and Tran T: The antimalarial drug
artesunate inhibits primary human cultured airway smooth muscle
cell proliferation. Am J Respir Cell Mol Biol. 50:451–458.
2014.
|