Introduction
Asthma is a chronic inflammatory disease of the
respiratory airways characterized by recurrent airway obstruction
and wheezing (1). The most common
features of asthma are airway hyper-responsiveness, the
infiltration of eosinophils and T helper 2 (Th2) cells into the
airway, and mucus hypersecretion and airway remodeling (1–3).
Glucocorticoids have traditionally been used to treat this disease;
however, the majority of the drugs administered for its treatment,
including glucocorticoids, exhibit side effects such as
immunodeficiency, adrenal insufficiency and growth failure, and
delayed puberty (4). In addition,
glucocorticoids are also ineffective in certain patients. Other
treatments such as a monoclonal antibody therapy targeting specific
cytokine receptors are being tested in clinical trials, even though
the antigenicity of these antibodies in humans requires
investigation (5). Therefore,
novel treatments based on natural herbs used in traditional
medicine are being investigated for their therapeutic efficacy
against chronic asthma.
Previous studies have been conducted to generate
scientific evidence concerning the anti-inflammatory activity of
extracts from Asparagus cochinchinensis (A. cochinchinensis)
roots. In one of these studies, the production of pro-inflammatory
cytokines and tumor necrosis factor (TNF)-α in lipopolysaccharide
(LPS)- and substance P-stimulated mouse astrocytes was found to be
significantly inhibited by an aqueous extract of A.
cochinchinensis roots (6). In
another study, three compounds from an ethanol extract of A.
cochinchinensis roots decreased the nitric oxide (NO)
concentration in LPS-stimulated BV-2 microglial cells (7). The ethanol extract also greatly
decreased the degree of ectopic edema, ear thickness, cytokine
secretion and myeloperoxidase activity, which are considered
indicators of skin inflammation progression, in a skin inflammation
model of animals treated with 12-O-tetradecanoyl-phorbol-13-acetate
(TPA) (7). Furthermore, a crude
aqueous extract of A. cochinchinensis roots effectively
inhibited TNF-α-induced cytotoxicity (8), and increased the spleen index and
superoxide dismutase activity and decreased the malondialdehyde
levels of aged mice (9).
Inflammation of the skin of IL-4/Luc/CNS-1 transgenic mice induced
by phthalic anhydride treatment was recently successfully
suppressed by a saponin-enriched extract of A.
cochinchinensis (SEAC) (10).
Therefore, the results of previous studies suggest the possibility
that the administration of SEAC may effectively inhibit
inflammation in various tissues, including those of the lung.
A recent study reported the inhibitory role of A.
cochinchinensis in allergic asthma-associated airway
remodeling, although it was used as only a small part of a
combination of complex drugs. In the above study, the standardized
herbal formula PM014, which consists of a mixture of A.
cochinchinensis root and six species of medicinal herbs,
efficiently reduced the number of total cells, eosinophils,
neutrophils, macrophages and lymphocytes in the bronchoalveolar
lavage fluid (BALF) of cockroach allergen-induced mice (11). However, to the best of our
knowledge, no studies have shown SEAC to exhibit anti-inflammatory
effects in the ovalbumin (OVA)-induced asthma model. Therefore, in
the present study, the possibility that SEAC is able to relieve
pathological phenotypes including airway inflammation and
remodeling was investigated in an OVA-induced asthma model.
Materials and methods
Preparation of SEAC
The roots of A. cochinchinensis used in this
study were obtained from the National Agricultural Cooperation
Federation (Gochang, South Korea) and dried in a drying machine
(FD5510S-FD5520S; Ilshin Biobase Co., Ltd., Seoul, South Korea) at
60°C. Voucher specimens of A. cochinchinensis roots
(WPC-14-003) were deposited in the functional materials bank of the
Wellbeing RIS Center at Pusan National University (Pusan, South
Korea). Dried roots were reduced to a powder using a pulverizer
(MF-3100S; Hanil Electric Co., Ltd., Seoul, Korea), after which the
SEAC was obtained by extraction at 50°C for 24 h using a fixed
liquor ratio (ratio of solid powdered A. cochinchinensis to
ethyl acetate solvent, 1:10) using circulating extraction equipment
(SHWB-30/45; Woori Science Instrument Co., Pocheon, South Korea).
These extraction mixtures were subsequently passed through a
0.4-μm filter, after which they were concentrated by vacuum
evaporation and lyophilization using circulating extraction
equipment (IKA Labortechnik, Staufen, Germany). Finally, the
collected SEAC powder was dissolved in 0.5% Tween-20 solution in
distilled water (dH2O) to 400 mg/kg, and then further
diluted to the required concentration.
In addition, an aqueous extract of Platycodon
grandifloras (AePG; Jangsaeng Doraji Co., Jinju-si, Korea) and
dexamethasone (Dex) were used as positive controls. The AePG was
obtained by extraction of P. grandifloras at 120°C for 45
min using a fixed liquor ratio (solid powdered P.
grandifloras:dH2O ratio, 75 g:500 ml) using
circulating extraction equipment (SHWB-30/45). Dex was purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Determination of total saponin, phenolic
and flavonoid contents
The total phenolic contents of the SEAC were
determined using the Folin-Ciocalteu method as previously described
(12). Briefly, a mixture of SEAC
solution (1 ml) and Folin-Ciocalteu reagent (5 ml; Sigma-Aldrich;
Merck KGaA) was incubated at room temperature for 5 min. This
mixture was subsequently added to 15 ml 20%
Na2CO3 and vortexed for 30 sec, after which
the absorbance was repeatedly measured at 765 nm using a VersaMax
plate reader (molecular Devices, LLC, Sunnyvale, CA, USA). A
standard calibration curve was generated using different
concentrations of gallic acid (Sigma-Aldrich; Merck KGaA), and the
concentration of the total phenolic compounds in the SEAC was
presented as the gallic acid equivalent (mg) of the extract.
The flavonoid contents in the SEAC were measured as
previously described (13). In
brief, 20-μl samples of several different concentrations of
SEAC were mixed with 60 μl 5% NaNO2 and 60
μl 10% AlCl3 (both from Sigma-Aldrich; Merck
KGaA). Following incubation at 25°C for 5 min, the absorbance was
measured using a VersaMax plate reader. A standard calibration
curve was then created using different concentrations of catechin
(Sigma-Aldrich; Merck KGaA). The flavonoid contents of the SEAC are
presented as catechin equivalents (mg) of the extract.
Finally, the total saponin content was determined
using the method previously described by Helaly et al
(14). The SEAC (5 g) was
dissolved in 0.5 ml 80% methanol, and then mixed with 0.5 ml 8%
vanillin in ethanol and 5 ml 72% H2SO4 in
water. These mixtures were placed in a 60°C water bath for 20 min,
and then cooled at 0°C for 5 min, after which the absorbance was
measured at 544 nm using a VersaMax plate reader. The saponin
content was calculated from a calibration curve constructed using a
purified saponin standard (Sigma-Aldrich; Merck KGaA).
Free radical scavenging activity
The scavenging activity was evaluated using
2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals as previously
described (15). In brief, a
100-μl sample comprising one of eleven different
concentrations of SEAC (0, 7.8, 15.6, 31.3, 61.5, 125, 250, 500,
1,000, 1,500 and 2,000 μg/ml) was mixed with 100 μl
DPPH (0.1 mm; Sigma-Aldrich; Merck KGaA) in 95% ethanol solution or
100 μl 95% ethanol solution as a control, and then incubated
for 30 min at room temperature. The absorbance of the reaction
mixture was subsequently measured at 517 nm using a VersaMax plate
reader. The DPPH radical scavenging activity of the SEAC was
expressed as the percentage reduction in absorbance relative to the
control. The half maximal inhibitory concentration
(IC50) was calculated as the concentration of substrate
that caused a 50% loss in DPPH activity.
In vitro assay using RAW264.7 cells
The RAW264.7 cell line used in the present study is
an Abelson murine leukemia virustransformed macrophage cell line.
The RAW264.7 cells were provided by the Korean Cell Line Bank
(Seoul, Korea). The RAW264.7 cells were cultured in Dulbecco's
modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) containing 10% fetal bovine serum (cat. no. S001-01;
Welgene Inc., Gyeongsan, South Korea), L-glutamine, penicillin and
streptomycin (Thermo Fisher Scientific, Inc.) in a humidified
incubator at 37°C with 5% CO2 and 95% air.
The accumulation of NO was measured in the culture
medium of cells pretreated with the vehicle or SEAC (100 or 200
μg/ml) for 2 h at room temperature followed by LPS (1
μg/ml) for 24 h using Griess reagent [1% sulfanilamide, 5%
phosphoric acid and 0.1% N-(1-naphthyl) ethylenediamine
dihydrochloride; Sigma-Aldrich; Merck KGaA] as described previously
(16).
The reactive oxygen species (ROS) levels of cells
pretreated with the vehicle or SEAC (100 or 200 μg/ml) for 2
h followed by LPS (1 μg/ml) for 24 h were measured by
staining with 2′,7′-dichlorodihydrofluorescein (DCFH) diacetate
(Sigma-Aldrich; Merck KGaA), a cell permeable and nonfluorescent
agent that is deacetylated by intracellular esterases to form
nonfluorescent DCFH, and is converted to highly fluorescent
2′,7′-dichlorofluorescein in the presence of intracellular ROS.
The relative expression of inducible nitric oxide
synthase (iNOS) and cyclooxygenase (COX)-2 mRNA in RAW264.7 cells
that had been pretreated with the vehicle or SEAC (100 or 200
μg/ml) for 2 h followed by LPS (1 μg/ml) for 24 h
were measured by reverse transcription-polymerase chain reaction
(RT-PCR) using specific primers as previously described (17).
Animal experimental protocol
The animal protocols used in the present study were
reviewed and approved for ethical and scientific care procedures by
the Pusan National University-Institutional Animal Care and Use
Committee (approval no. PNU-2015-0779). Six-week-old female BALB/c
mice were purchased from Samtako Bio Korea Co., Ltd. (Osan, South
Korea). Prior to the initiation of the animal experiment, the mice
were allowed ≥1 week to adapt to the experimental environment. All
mice were provided with ad libitum access to a standard
irradiated chow diet (Samtako Bio Korea Co., Ltd.) and water
throughout the 25-day study. During the experiment, mice were
maintained in a specific pathogen-free state under a strict light
cycle (lights on at 08:00 a.m. and off at 20:00 p.m.) at 23±2°C and
50±10% relative humidity. The BALB/c mice were housed in the Pusan
National University-Laboratory Animal Resources Center accredited
by the Korean Ministry of Food and Drug Safety in accordance with
the Laboratory Animal Act (accredited unit no. 000231) and AAALAC
International according to the National Institutes of Health
guidelines (accredited unit no. 001525).
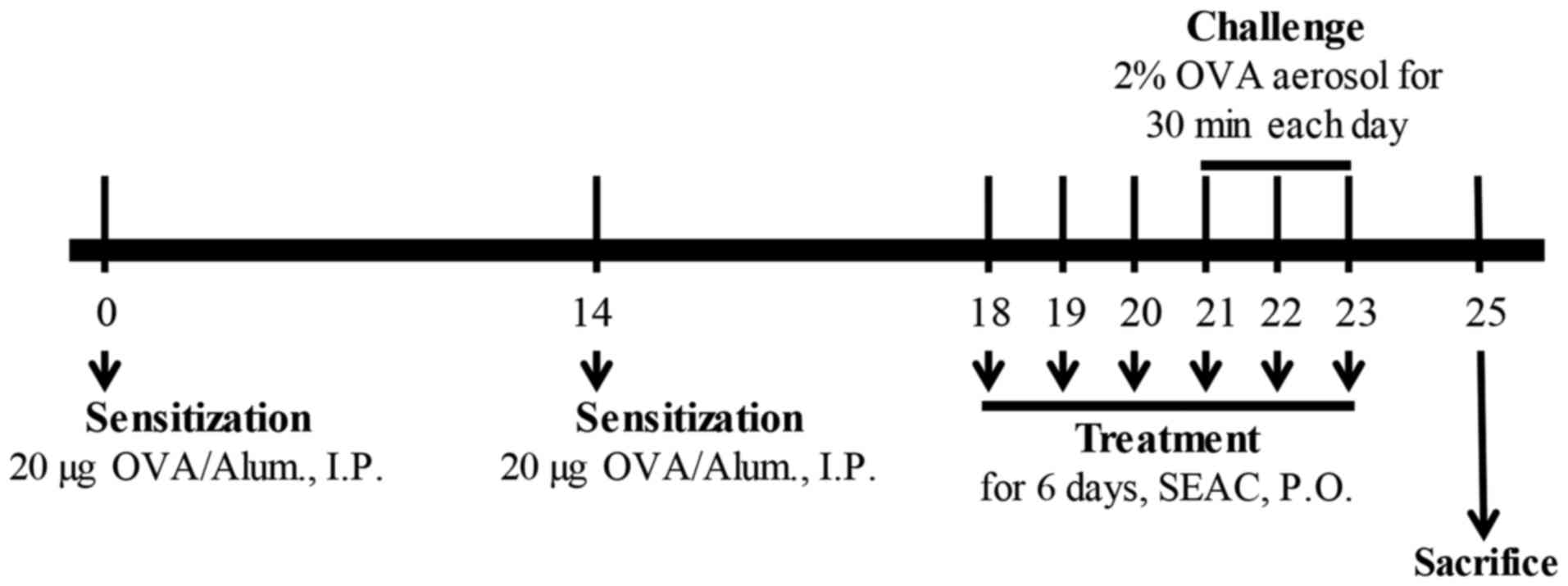
The airway challenge of the BALB/c mice was induced
as previously described (18,19). In brief, 6-week-old Balb/c mice
(female; n=48) were assigned to either an untreated group (n=8) or
an OVA-treated group (n=40). The untreated group did not receive
any treatment during the experimental period. In the other group,
OVA-induced asthma was generated by sensitization for 20 days and
challenge for 3 days. On days 0 and 14, the mice were sensitized by
the intraperitoneal injection of OVA (albumin from chicken; 20
μg) emulsified with aluminum hydroxide (alum; both from
Sigma-Aldrich; Merck KGaA) in 200 μl 1× PBS solution. On
days 21–23, the mice were subjected to a 30-min airway challenge
with 2% OVA in 1× PBS by inhalation through a nebulizer (NE-C28;
Omron, Tokyo, Japan; Fig. 1).
Additionally, the OVA-induced asthma group was further divided into
a vehicle-treated group (OVA + vehicle; n=8), Dex-treated group
(OVA + Dex; n=8), AePG-treated group (OVA + AePG; n=8), low dose
SEAC-treated group (OVA + SEACL; n=8), and high dose SEAC-treated
group (OVA + SEACH, n=8). The two OVA+SEAC-treated groups received
250 and 500 mg/kg body weight of SEAC, respectively, while the OVA
+ vehicle group received the same volume (200 μl) of 0.5%
Tween-20 solution for 6 days. The therapeutic concentration of SEAC
was determined based on the results from previous studies of skin
inflammation (10). Furthermore,
the OVA + Dex and OVA + AePG groups received 3 mg/kg body weight of
Dex solution and 100 mg/kg body weight of AePG, respectively. At 48
h after the final treatment, all animals were euthanized using
CO2 gas, and tissue samples were acquired and stored in
Eppendorf tubes at −70°C until assay.
Enumeration of total cells in BALF
Following anesthesia of the mice with
Alfaxan® (alfaxalone; Jurox Pty Ltd., Rutherford,
Australia), BALF was obtained (yield, 80%; total volume, 0.8 ml)
from the mice in each group via tracheal cannulation with cold 1×
PBS. Total cells were then harvested from the BALF by
centrifugation (2,000 × g) at 4°C for 5 min, after which they were
resuspended in 100 μl hematoxylin. The eosinophils,
macrophages and total cells in 1 mm2 were counted on
glass slides using the Leica Application Suite (Leica Microsystems,
Heerbrugg, Switzerland).
Measurement of body weight and immune
organ weight
The body weights of all mice in each group
throughout the experimental period were measured using an
electronic balance (Mettler Toledo Gmbh, Greifensee, Switzerland).
The weights of the spleen and thymus were also determined following
sacrifice using the electronic balance.
Enzyme-linked immunosorbent assay (ELISA)
for interleukin (IL)-4 in the BALF
The concentration of IL-4 in the BALF was determined
using an IL-4 ELISA kit (cat. no. 431107; BioLegend, Inc., San
Diego, CA, USA) according to the manufacturer's protocol. In brief,
following collection of the supernatant from the BALF, the assay
buffer (50 μl) and the supernatant (50 μl) were added
to a 96-well plate coated with anti-IL-4 antibody, and incubated
for 2 h at room temperature. Following removal of unbound proteins
with wash solution (0.05% Tween-20 in PBS, pH 7.4), 100 μl
detection antibody solution was added and the samples were
incubated for 1 h. After washing, 100 μl avidin-horseradish
peroxidase (HRP) D solution was added to each well, and the plates
were allowed to bind for 30 min. Next, the plate was washed and
reacted with 100 μl substrate solution for 15 min. The
reaction was then stopped by the addition of 100 μl blocking
solution, after which the absorbance at 450 nm was determined with
a VersaMax plate reader.
Detection of OVA-specific immunoglobulin
E (IgE) concentration
The OVA-specific IgE concentration in the serum of
the mice was measured using an ELISA kit (cat. no. 439807;
BioLegend, Inc.) according to the manufacturer's protocol. Briefly,
wells were washed with washing solution (50 mM Tris, 0.14 M NaCl,
0.05% Tween-20, pH 8.0) four times, after which assay buffer (50
μl) and serum samples (50 μl) were added to wells
coated with antibody and the plate was incubated with shaking for 2
h at room temperature. Next, the wells were washed with washing
solution, after which 100 μl detection antibody solution was
added and the samples were incubated for 1 h with shaking. After
washing the wells, Avidin-HRP D solution (100 μl) was added
to each well. The plates were then incubated at room temperature
for 30 min, after which the wells were washed and an enzyme
reaction was initiated by adding substrate solution and incubating
the plates at room temperature in the dark for 15 min. Finally, the
reaction was terminated by adding 2 M H2SO4
solution and the absorbance was measured at 450 nm using a VersaMax
plate reader.
Histopathological analysis
Lung tissues were collected from all mice of the
various groups, fixed in 10% neutral buffered formalin, embedded in
paraffin wax, routinely processed, and then sectioned into
4-μm slices. The tissue sections on a slide glass were
stained with hematoxylin and eosin (H&E; IHC World, Woodstock,
MD, USA) after which they were examined using light microscopy to
observe the infiltration of inflammatory cells into the
peribronchial region of the lung at ×400 magnification. The
epithelial thickness and smooth muscle thickness of the bronchial
tube were also measured using the Leica Application Suite (Leica
Microsystems).
Hyperplasia of goblet cells for mucus production was
detected by staining with periodic acid-Schiff. Following
deparaffinization and dehydration of lung sections, samples were
oxidized in periodic acid solution for 5 min at room temperature.
The lung sections were then washed at warm water and placed in
Schiff reagent for 5 min at room temperature. The sections were
then washed with warm tap water, stained with hematoxylin solution
(IHC world) for 30 sec, and examined by light microscopy to detect
goblet cell hyperplasia and subepithelial fibrosis at ×400
magnification. The mucus score was then determined by four
independent investigators in a single-blind analysis based on four
different random locations using a microscope. The mucus scores
were: 0, no mucus; 1, <5% of the epithelium; 2, 5–10% of the
epithelium; 3, 10–20% of the epithelium; 4, 20–30% of the
epithelium; 5, 30–40% of the epithelium
RT-PCR analysis of cytokine mRNA
expression
The relative quantities of IL-4 and IL-13 mRNA were
measured using RT-PCR. In brief, frozen lung tissue was chopped
with scissors and homogenized in RNA-Bee solution (Tel-Test, Inc.,
Friendswood, TX, USA). Total RNA was then isolated by
centrifugation at 15,000 × g for 15 min, after which the RNA
concentration was measured using UV spectroscopy. The expression of
the target genes was assessed using RT-PCR with 3 μg total
RNA from the tissue of each group. The RNA was annealed with 500 ng
oligo(dT) 12–18 primer (Invitrogen; Thermo Fisher Scientific, Inc.)
at 70°C for 10 min. Complementary DNA, which was used as the
template for further amplification, was synthesized from the RNA by
the addition of 2 mM dNTPs (Deoxynucleoside Triphosphate set; Roche
Diagnostics, Basel, Switzerland) with 200 units of SuperScript™ II
reverse transcriptase (200 U/μl; Invitrogen; Thermo Fisher
Scientific, Inc.). PCR was then conducted with the mixture of 2.5
μl cDNA, 10 pmol sense and antisense primers (2.5
μl), Taq DNA polymerase (0.2 μl; Roche Diagnostics),
2 mM dNTP (2.5 μl; Deoxynucleoside Triphosphate set; Roche
Diagnostics), and 10× reaction buffer containing 15 mM MgCl2 (2.5
μl), and the reaction mixture was subjected to 28–32 cycles
of amplification in a thermal cycler (PerkinElmer, Inc., Waltham,
MA, USA). The following temperature cycle was used for PCR: 30 sec
at 94°C, 30 sec at 62°C and 42 sec at 72°C. The primer sequences
used were as follows: IL-4 sense, 5′-CCA GCT AGT TGT CAT CCT GCT
CTT C-3′ and antisense, 5′-GTG ATG TGG ACT TGG ACT CAT TCA TGG-3′;
IL-5 sense, 5′-GAG AAG GAT GCT TCT GCA CTT GAG-3′ and antisense,
5′-CCA CTC TGT ACT CAT CAC ACC AAG G-3′; IL-13 sense, 5′-CCT TAA
GGA GCT TAT TGA GGA GCT GAG-3′ and antisense, 5′-CAG TTG CTT TGT
GTA GCT GAG CAG-3′; β-actin sense, 5′-TGG AAT CCT GTG GCA TCC ATG
AAA C-3′ and antisense, 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′.
Three samples were analyzed in duplicate. The final PCR products
were separated on 1% agarose gel, and then visualized by ethidium
bromide staining. The band patterns were detected using a
UV-transilluminator (ATTO, Tokyo, Japan) and analyzed Image saver 6
(ATTO).
Western blot analysis
Lung tissue (50 mg) collected from each group was
homogenized using PRO-PREP™ Solution (Intron Biotechnology, Inc.,
Sungnam, Korea), after which total protein extracts were collected
by centrifugation at 13,000 × g for 5 min at 4°C. The prepared
proteins were subsequently subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis for 2 h at 100 V, after
which they were transferred to a nitrocellulose membrane (GE
Healthcare Life Sciences, Little Chalfont, UK) for 2 h at 40 V in
transfer buffer (25 mM Trizma-base, 192 mM glycine and 20%
methanol). Appropriate dilutions of the primary antibodies
anti-COX-2 (1:1,000; #12282; Cell Signaling Technology, Inc.,
Danvers, MA, USA), anti-vascular endothelial growth factor
(anti-VEGF; 1:1,000; #500-P131; PeproTech, Inc., Rocky Hill, NJ,
USA) and anti-β-actin (1:3,000; #A5316; Sigma-Aldrich) were added
to the membranes and allowed to hybridize overnight at 4°C.
Following removal of the antibodies, the membrane was washed three
times in a solution comprising 10 mM Trizma-base (150 mM NaCl and
0.05% Tween-20) for 10 min. The membrane was subsequently incubated
with 1:3,000 diluted HRP-conjugated goat anti-rabbit IgG (#G21234;
Invitrogen) for 1 h at room temperature, after which it was washed
again as described above and developed using an enhanced
chemiluminescence reagent plus kit (Amersham Biosciences; GE
Healthcare Life Sciences). Finally, the results were quantified
using an image analysis aystem (Fluorchem FC2; ProteinSimple, San
Jose, CA, USA) and expressed as the fold-increase over control
values.
Statistical analysis
One-way analysis of variance was used to identify
significant differences between the untreated and LPS-treated cell
groups or between the untreated and OVA-treated mouse groups.
Additionally, differences between the vehicle + LPS-treated group
and the SEAC + LPS-treated groups, as well between the OVA +
vehicle-treated group and the OVA + Dex, OVA + AePG or OVA +
SEAC-treated groups were evaluated using a post hoc Tukey's test of
the variance and significance levels. All values were expressed as
the mean ± standard deviation. The statistical analysis was
conducted using SPSS for Windows, release 10.10, standard version
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Distribution and antioxidant activity of
the bioactive components of SEAC
As shown Fig. 2A,
SEAC contained high concentrations of three bioactive components
associated with anti-inflammatory activity. Crude saponins were
detected at a high level (57.2 mg/g) in the SEAC. The
concentrations of total phenols and total flavonoids were 88.5 and
102.1 μg/g, respectively. Furthermore, the scavenging
activity of SEAC for DPPH radicals increased in a dose-dependent
manner, with an IC50 of 708.56 μg/ml (Fig. 2B).
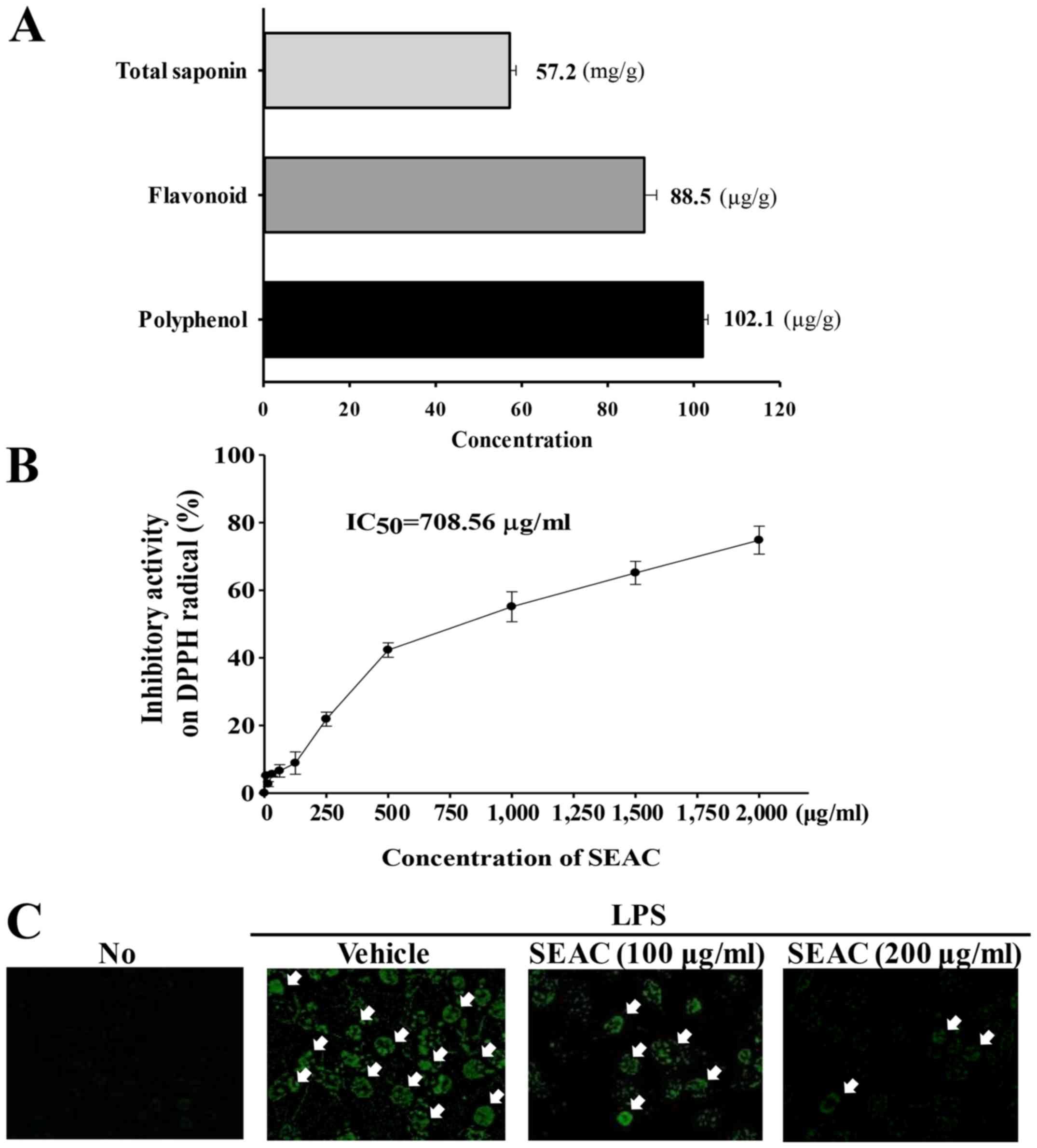 | Figure 2Antioxidant properties of SEAC in
RAW264.7 cells. (A) Total saponin, flavonoid and polyphenol
concentrations in SEAC. (B) Free radical scavenging activity of
SEAC. DPPH radical scavenging activity was assayed in a mixture
containing 0.1 mM DPPH and a range of concentrations of SEAC
(250–2,000 μg/ml). Values are presented as the means ±
standard deviation of three replicates. (C) Green fluorescence
indicating reactive oxygen species in cells was observed using
fluorescence microscopy at ×200 magnification. Arrows indicate
cells stained with DCFH-DA. No, untreated; LPS, lipopolysaccharide;
SEAC, saponin-enriched extract of Asparagus cochinchinensis;
DPPH, 2,2-diphenyl-1-picrylhydrazyl radical; IC50, half
maximum inhibitory concentration; DCFH-DA,
2′,7′-dichlorodihydrofluorescein diacetate. |
Effects of SEAC on NO production, iNOS
and COX-2 expression and intracellular ROS content in RAW264.7
cells
To examine the possibility that SEAC had
anti-inflammatory properties, alterations in NO concentration, iNOS
and COX-2 transcription and intracellular ROS level were measured
in LPS-stimulated RAW264.7 cells following SEAC pretreatment. The
NO concentration, iNOS and COX-2 expression levels (Table I) were significantly increased and
intracellular ROS content was markedly increased in the vehicle +
LPS-treated group compared with the untreated group (Fig. 2C). However, these values decreased
in a dose-dependent manner in the cells pretreated with 100 and 200
μg/ml SEAC, although the iNOS level was maintained at a
constant level in the low dose SEAC-treated group (Table I). These results provide strong
evidence that SEAC pretreatment attenuates various types of
inflammation in specific tissue by inhibiting the increase in NO
concentration, COX-2 and iNOS mRNA expression and intracellular ROS
production.
 | Table IAnti-inflammatory effects of SEAC in
RAW264.7 cells. |
Table I
Anti-inflammatory effects of SEAC in
RAW264.7 cells.
| Variable | No | LPS
|
|---|
| Vehicle | SEAC
(100 μg/ml) | SEAC
(200 μg/ml) |
|---|
| NO concentration
(μM) | 0.032±0.104 |
20.747±0.246a |
18.682±0.722a |
14.701±1.067a,b |
| Relative level of
iNOS expression | 1.000±0.083 | 4.514±0.461a | 4.788±0.358a | 3.061±0.301a,b |
| Relative level of
COX-2 expression | 1±0.061 | 3.217±1.109a | 2.90±0.801a | 2.672±0.724a,b |
Effects of SEAC treatment on whole body
and immune organ weight
To investigate the toxic effects of SEAC on the
whole body and immune-associated organs, the whole body, spleen and
thymus weight were measured following SEAC treatment. The body
weights of the mice in all groups were maintained at a constant
level throughout the experimental period, and no significant
differences between groups were observed. Furthermore, similar
results were detected for the spleen and thymus weights in response
to the inflammatory immune reaction, although insignificant
increases in the spleen weights of the OVA + SEAC-treated groups
were observed (Fig. 3). These
results indicate the absence of toxic effects of SEAC on the whole
body, including the immune organs.
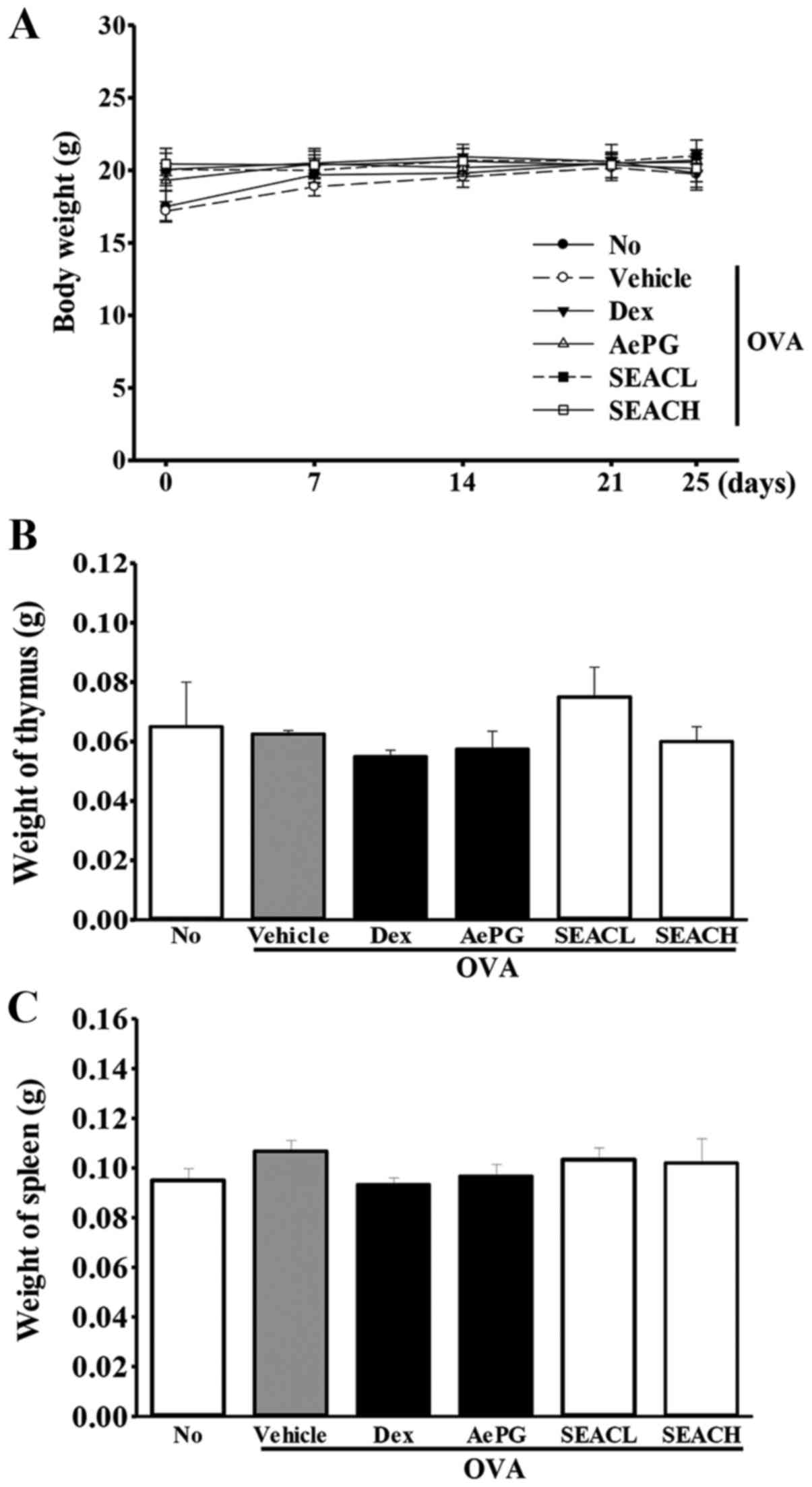 | Figure 3Mouse body and immune organ weights.
(A) Body weights were measured in the morning on days 1, 7, 14, 21
and 25. (B and C) Following sacrifice, the spleens and thymuses
were harvested from the mice and weighed. Data shown are the mean ±
standard deviation (n=5). No, untreated; OVA, ovalbumin; Dex,
dexamethasone; AePG, aqueous extract of Platycodon
grandifloras; SEACL, low dose of saponin-enriched extract of
Asparagus cochinchinensis; SEACH, high dose of
saponin-enriched extract of Asparagus cochinchinensis. |
Effects of SEAC treatment on the number
of eosinophils, macrophages and total cells in the BALF
To examine the suppressive effects of SEAC on the
inflammatory response in the BALF, the total numbers of leukocytes,
eosinophils and macrophages in the BALF were determined. The number
of leukocytes was significantly higher in the OVA + vehicle group
compared with the untreated group, reflecting the challenge of
airway inflammation with OVA. These results imply that OVA antigen
challenge triggers a marked influx of leukocytes into the BALF
(Fig. 4A). Treatment with OVA +
Dex, OVA + AePG or OVA + SEAC induced a significant reduction in
the total number of leukocytes in the BALF when compared with the
OVA + vehicle group (Fig. 4A). A
similar pattern of changes was observed for eosinophils and
macrophages (Fig. 4A). These
findings indicate that SEAC inhibits the inflammatory response of
the BALF to OVA inhalation.
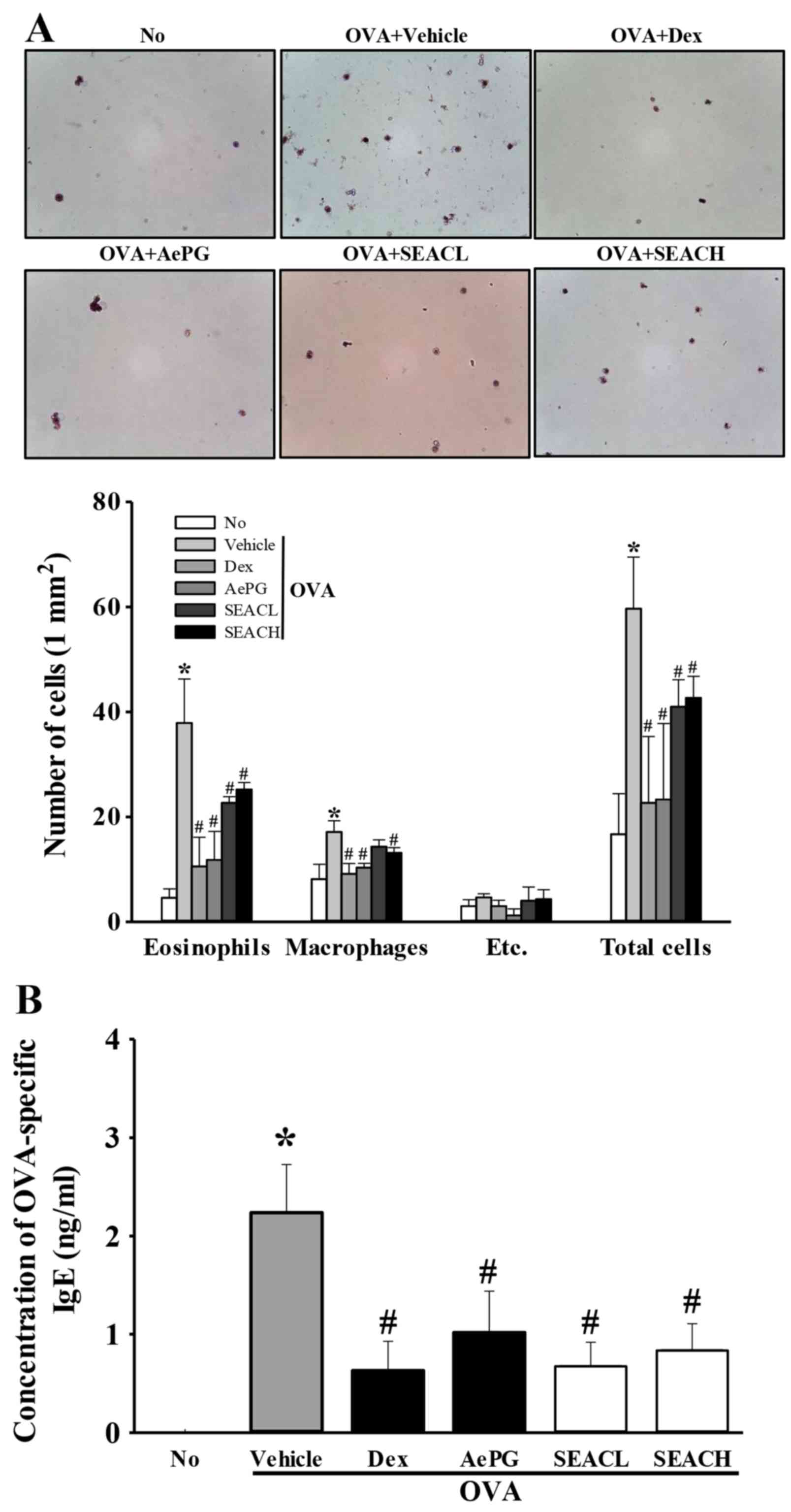 | Figure 4Number of inflammatory cells in BALF
and OVA-specific IgE levels in serum. (A) Cells in the BALF were
isolated by centrifugation, then stained with hematoxylin. The
total number of leukocytes, eosinophils and macrophages was
determined by counting within 1 mm2 under a light
microscope at ×400 magnification. Etc., lymphocytes and
neutrophils. (B) Serum was prepared from blood samples collected
from the abdominal veins of mice. The serum concentration of
OVA-specific IgE was quantified using an enzyme-linked
immunosorbent assay. Data shown are the mean ± standard deviation
(n=5). *P<0.05 vs. the No group;
#P<0.05 vs. the OVA + vehicle-treated group. BALF,
bronchoalveolar lavage fluid; OVA, ovalbumin; IgE, immunoglobulin
E; No, untreated; Dex, dexamethasone; AePG, aqueous extract of
Platycodon grandifloras; SEACL, low dose of saponin-enriched
extract of Asparagus cochinchinensis; SEACH, high dose of
saponin-enriched extract of Asparagus cochinchinensis. |
Effects of SEAC on the OVA-specific IgE
level
The upsurge of inflammatory mediators, including
serum IgE, serves as evidence of the onset of asthma (20,21). Therefore, OVA-specific IgE levels
were measured in the serum of OVA-sensitized mice treated with
vehicle, Dex, AePG or SEAC to evaluate the suppressive effects on
IgE production. The OVA-specific IgE level was increased in the OVA
+ vehicle-treated group compared with the untreated group,
suggesting that OVA induction was successful for creation of the
asthma model (Fig. 4B).
Furthermore, the OVA-specific IgE levels were decreased
significantly in the 250 mg/kg SEAC (OVA + SEACL), 500 mg/kg SEAC
(OVA + SEACH), Dex and AePG treatment groups compared with the OVA
+ vehicle-treated group (Fig.
4B). These results suggest that SEAC treatment successfully
reduced OVA-specific IgE levels in the serum.
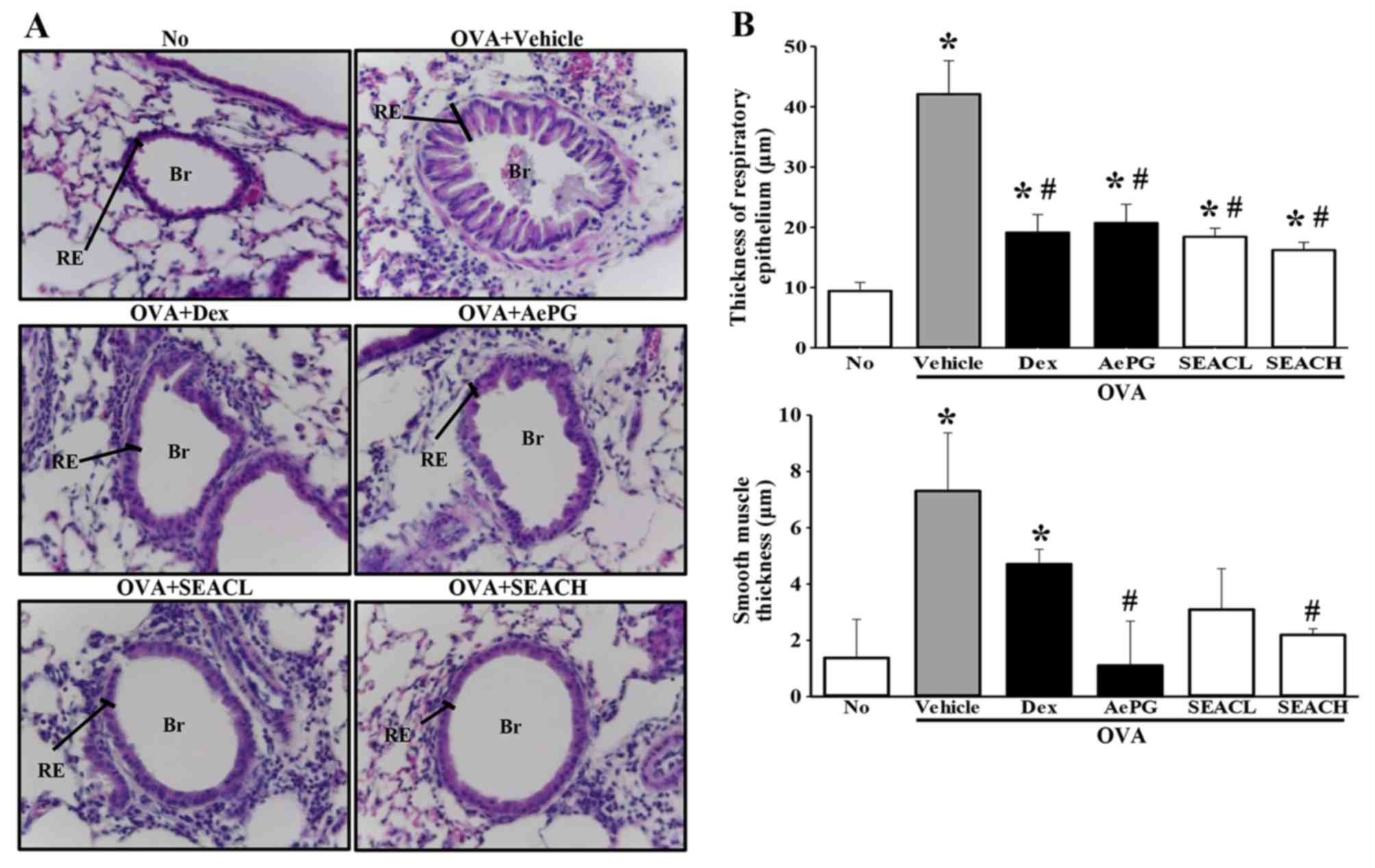
Effects of SEAC treatment on lung
histopathological structure in the OVA asthma model
To discern whether SEAC affects the recovery of
pathological structures in the airways, alterations in the
histopathological structure of the lungs were evaluated in the OVA
+ SEAC-treated group. Lung tissue sections from mice that were
sensitized with OVA (OVA + vehicle-treated group) displayed a
significant expansion in the thickness of the respiratory
epithelium and smooth muscle. However, these thicknesses in the OVA
+ SEACH-treated groups, were significantly decreased compared with
those in the OVA + vehicle-treated group. A similar reduction was
observed in the infiltration of inflammatory cells in the
peribronchiolar region (Fig. 5).
These findings demonstrate that SEAC has the potential to alleviate
the histopathological structural changes, including the increased
thickness of the respiratory epithelium and smooth muscle, in the
airways of the OVA asthma model.
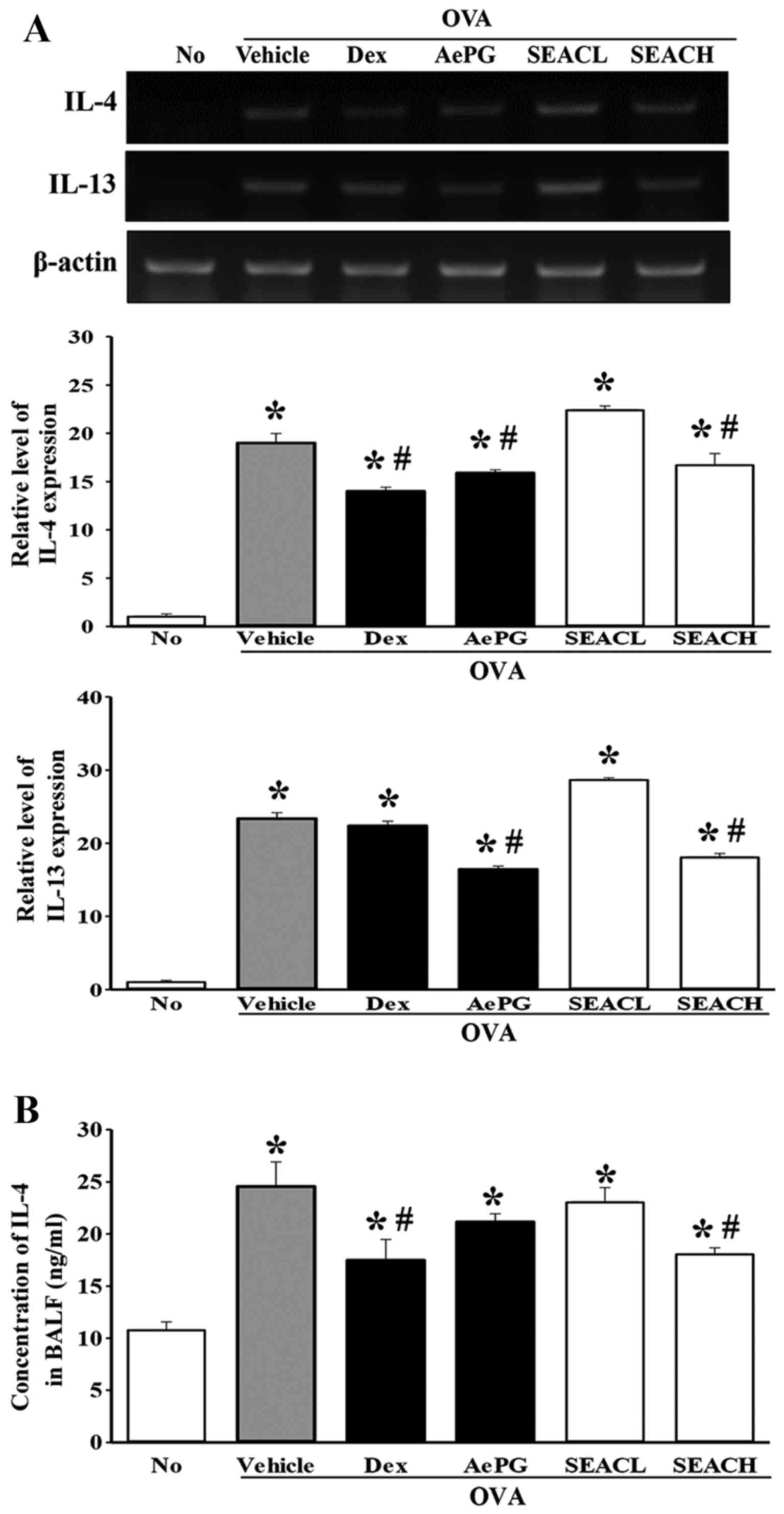
Effects of SEAC on the alteration of
inflammatory mediators in the OVA-induced asthma model
The levels of the Th2 cytokines IL-4, IL-5 and IL-13
in the lung were measured using RT-PCR analysis to evaluate
differences in the expression of inflammatory mediators among the
groups. The transcript levels of these cytokines were higher in the
OVA + vehicle-treated group compared with the untreated group.
However, the mRNA levels of only IL-4 and IL-13 were significantly
decreased in the OVA + SEACH group compared with the OVA +
vehicle-treated group (Fig. 6A),
while the level of IL-5 mRNA was constantly maintained in the same
groups (data not shown). Similar effects were observed in the OVA +
Dex and OVA + AePG-treated groups (Fig. 6A). Furthermore, to verify whether
these changes at the transcriptional level were maintained at the
translational level, IL-4 was selected as one of the key mediators
and the protein level of this protein was measured in the BALF. The
reduction of IL-4 mRNA detected in the OVA + SEACH group was also
observed for IL-4 protein, although the extent of the reduction
differed (Fig. 6B).
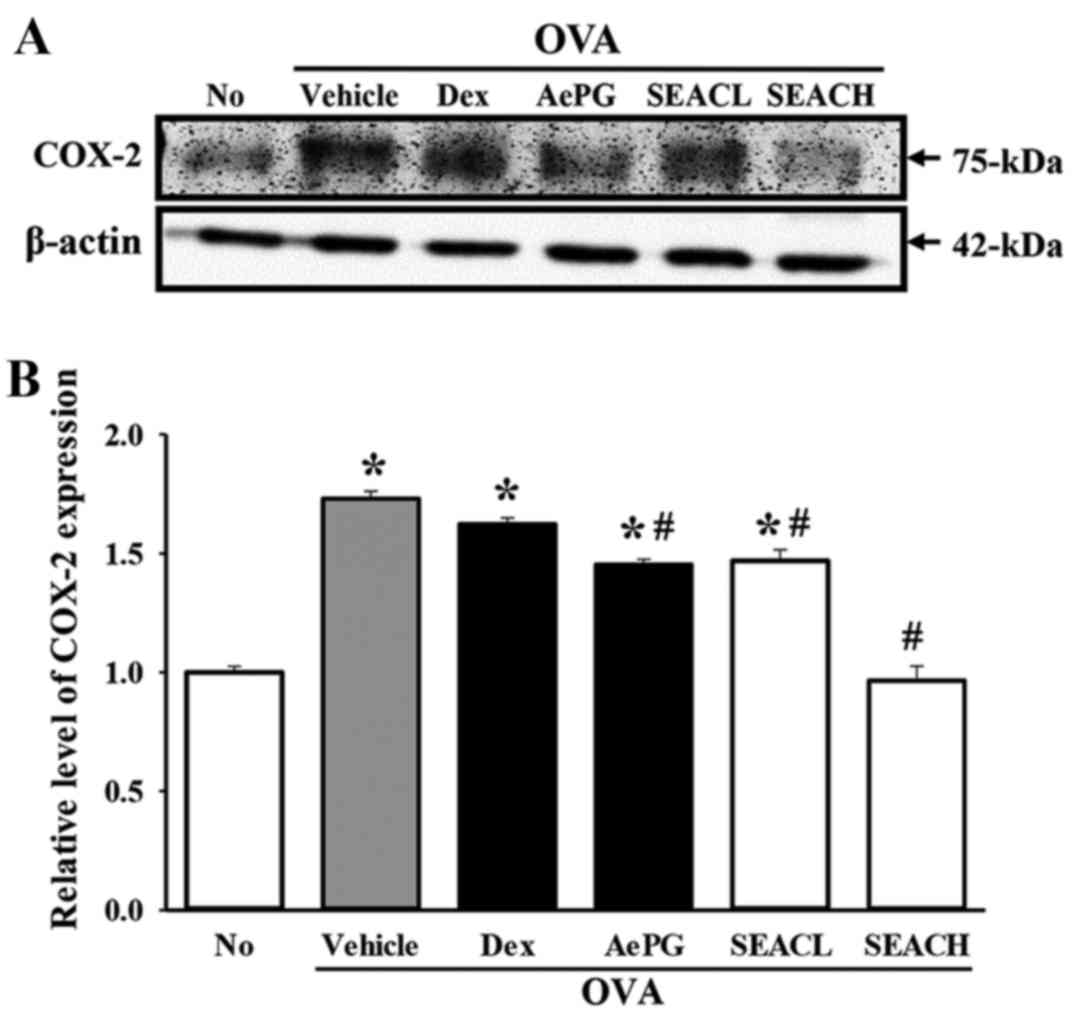
The expression of another inflammatory mediator,
COX-2, was measured in the lung tissue of the OVA + SEAC-treated
group. The level of COX-2 protein in the OVA + vehicle-treated
group was significantly higher than that of the untreated group.
However, COX-2 levels were significantly reduced in the OVA + SEACL
and OVA + SEACH groups compared with the OVA + vehicle-treated
group, and these reductions were slightly dependent on the
concentration of SEAC (Fig. 7).
These results indicate that SEAC is able to inhibit the production
of inflammatory mediators, including several cytokines and COX-2 in
the lung tissue and BALF.
Inhibitory effects of SEAC on airway
remodeling in the OVA-induced asthma model
To determine the effect of SEAC on airway
remodeling, measurements of goblet hyperplasia, the thickness of
the peribronchiolar collagen layer and VEGF expression levels were
made in the OVA-sensitized mice treated with SEAC. A high mucus
score indicative of goblet cell hyperplasia was observed in the
bronchial airways of mice that were sensitized with OVA (OVA +
vehicle-treated group). However, the mucus scores in the OVA +SEACL
and OVA + SEACH-treated groups were significantly lower than that
in the OVA + vehicle-treated group (Fig. 8).
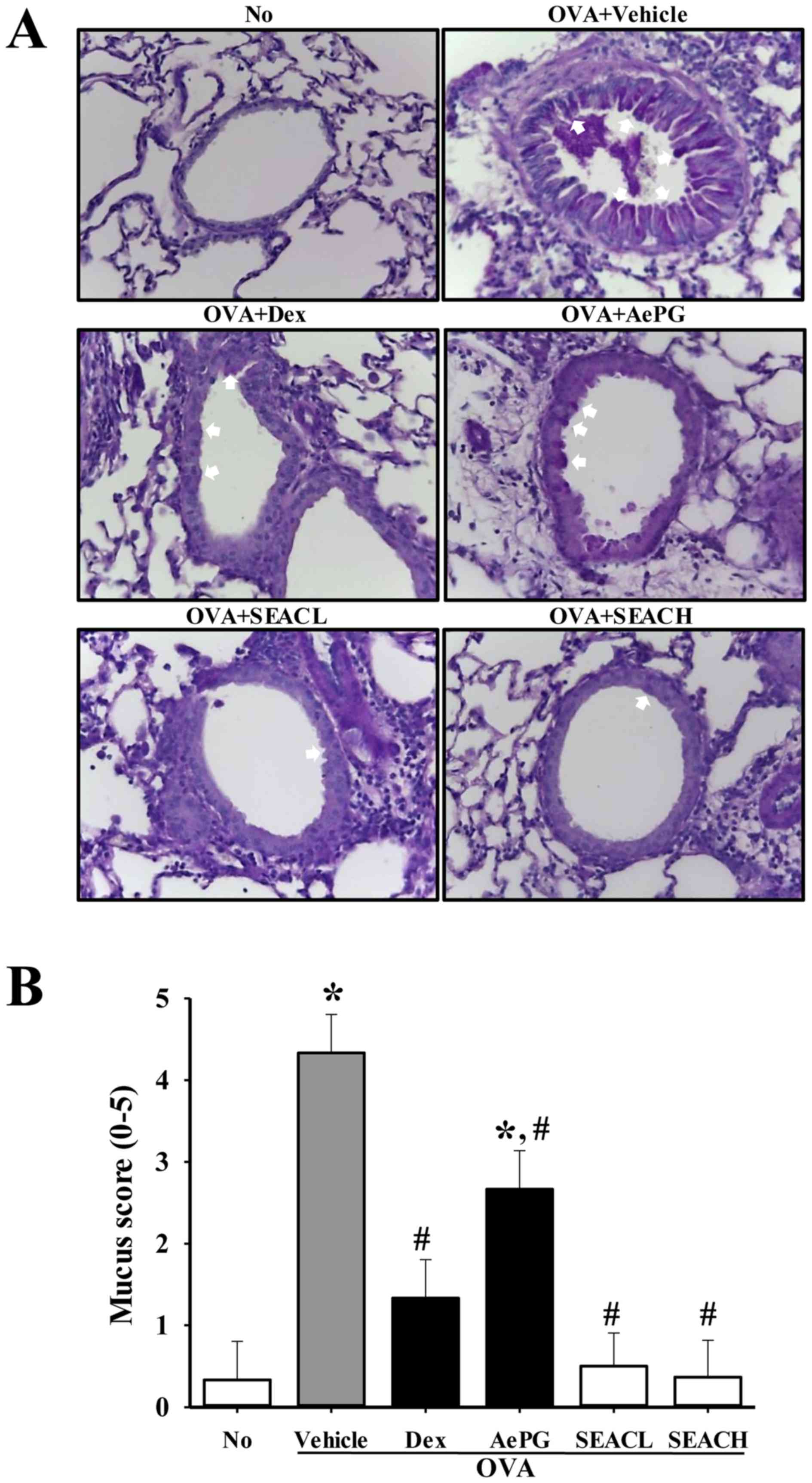 | Figure 8Goblet hyperplasia in lung tissue.
(A) Following staining with periodic acid-Schiff, goblet
hyperplasia was observed in the lung tissue at ×400 magnification.
Arrows represent the areas of mucin secretion. (B) Mucus score was
determined by four independent investigators based on four
different random locations using a microscope. 0, no mucus; 1,
<5% of the epithelium; 2, 5–10% of the epithelium; 3, 10–20% of
the epithelium; 4, 20–30% of the epithelium; 5, 30–40% of the
epithelium. Data shown are the mean ± standard deviation (n=5).
*P<0.05 vs. the No group; #P<0.05 vs.
the OVA + vehicle-treated group. No, untreated; OVA, ovalbumin;
Dex, dexamethasone; AePG, aqueous extract of Platycodon
grandifloras; SEACL, low dose of saponin-enriched extract of
Asparagus cochinchinensis; SEACH, high dose of
saponin-enriched extract of Asparagus cochinchinensis. |
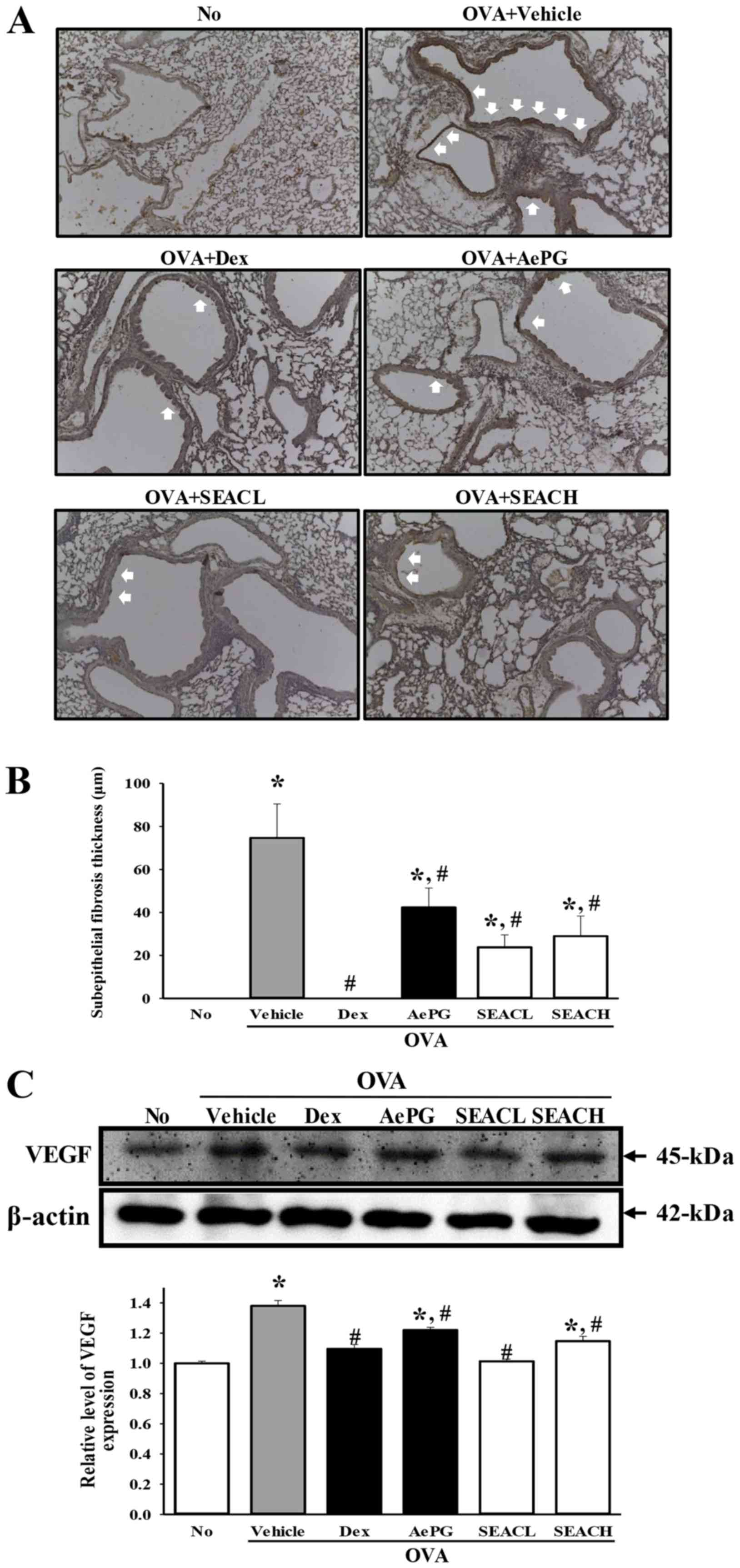
Similar results were observed for the thickness of
the peribronchiolar collagen layer and subepithelial fibrosis, as
may be expected, since collagen deposition in the airways is
associated with severe asthma and a reduction in pulmonary function
(22) (Fig. 9A and B). In addition, the
expression level of VEGF, which is a stimulator of angiogenesis and
structural changes in asthma (23), was significantly decreased in the
OVA + SEAC-treated groups compared with the OVA + vehicle-treated
group (Fig. 9C). These findings
demonstrate that SEAC has the potential to stimulate airway
remodeling through the regulation of goblet hyperplasia,
peribronchiolar collagen layer thickness, subepithelial fibrosis
and VEGF expression in the airways of the mouse model of
OVA-induced asthma.
Discussion
Asthma is a chronic respiratory disease
characterized by airway inflammation and hyperresponsiveness
(1). Although steroids are the
drugs most commonly prescribed for asthma, they have a number of
adverse effects, including growth inhibition in children (24), cataracts and glaucoma,
hypertension, hyperlipidemia, peptic ulcers, myopathy and
immunosuppressive effects (25).
Accordingly, recent studies have focused on the identification of
candidate pharmacologically active ingredients in natural herbs. An
approach using natural herbs for the treatment of chronic asthma
may enable the reduction of unwanted side effects with long-term
drug administration. The present study provides evidence that SEAC
acts as an anti-inflammatory and remodeling agent in a mouse model
of asthma induced by OVA inhalation. A. cochinchinensis is a
perennial herb belonging to the Liliaceae family that is widely
grown in China, Japan and Korea, where its roots have been used as
a herbal medicine for thousands of years (26). Steroidal saponins and sapogenins,
including asparagosides, furostanol oligosides, asparacosin and
sarsasaponen are the major active components of A.
cochinchinensis roots (27–29). Pharmacological studies of A.
cochinchinensis roots have established that they have
therapeutic properties, which include immunostimulant activities
(9), antioxidant, anti-aging
(26,30,31) and antitumor effects (8,32–34) as well as the ability to reduce
blood sugar (35) and ameliorate
cough (8). Furthermore, A.
cochinchinensis is administered in combination with other herbs
as a medicine to treat the lungs, spleen, immune system and aging
(9,11,36). To identify the specific compounds
in SEAC that may exert anti-asthmatic effects, content analysis of
saponins, flavonoids and polyphenols was conducted in the present
study. A high level of saponins in the SEAC (57.2 μg in 1 g)
was detected (Fig. 2B). A number
of studies have reported that saponins, flavonoids and polyphenols
extracted from natural plants exert anti-inflammatory activity
(37).
The results of the present study revealed that SEAC
led to a notable alleviation of lung inflammation in the
OVA-induced mouse model of asthma. These findings were supported by
the observed amelioration of various indicators of inflammation in
the OVA-induced asthma model mice, which included the weights of
immune organs, the number of leukocytes released into the BALF,
serum IgE concentration, thickness of the respiratory epithelium,
mucus score, and Th2 cytokine and COX-2 expression. These
indicators of lung inflammation have been applied in previous
studies to investigate the therapeutic effects of various herbal
medicines, including Phillinus linteus extract (38), Korean ginseng (39) and KOTMIN13 (40), in OVA-induced asthma models.
The anti-inflammatory activity of A.
cochinchinensis ethanol extract (ACE) has previously been
investigated in mouse models of skin inflammation (7). In acute and chronic irritant contact
dermatitis mouse models, ACE markedly reduced the increase of skin
thickness and weight caused by the infiltration of
polymorphonuclear leukocytes into the skin of the ear following TPA
application (7). In the present
study, H&E staining of the lung sections revealed a significant
increase in asthmatic indicators, including the thickness of the
respiratory epithelium by leukocyte infiltration (Fig. 5) and the levels of mucus secretion
(Fig. 8). However, treatment with
SEAC led to a clear reduction in those asthmatic indicators,
highlighting the potential value of this material for the treatment
of asthma.
Alterations in the total cell number of leukocytes,
including eosinophils, macrophages and lymphocytes were also
measured in the present study. The influx of eosinophils into the
BALF is a major cause of allergic airway inflammation associated
with respiratory diseases such as asthma (41). In the present study, SEAC
treatment reduced the total cell number of leukocytes such as
macrophages, eosinophils and lymphocytes in the BALF relative to
the OVA + vehicle-treated group. These results suggest that SEAC
induces inhibitory effects against airway inflammation. These
findings are consistent with the results of previous studies
conducted using PM014, a formulation of 18 herbal medicines
including A. cochinchinensis, in a mouse model of cockroach
allergen-induced asthma (11).
Asthma is a multi-cellular process associated with
Th2 cells and leukocytes (42).
Th2 cells serve a fundamental role in the regulation of allergic
inflammation through the secretion of Th2 cytokines including IL-4,
IL-5 and IL-13 (42,43). Among these, IL-4 has multiple
effects, including the differentiation of naive T cells toward the
Th2 lineage and the induction of B-cell isotype switching to
produce IgE (44). IL-13 is
responsible for various characteristics of Th2 inflammation,
particularly in the asthmatic lung, including goblet cell
hyperplasia, mucus hypersecretion and bronchial hyperresponsiveness
(43). It can also remodel
structural cells, including epithelial cells, in the airway,
stimulating the expression of proinflammatory factors by airway
smooth muscle (45). IL-5 has
been suggested to be important for the recruitment of eosinophils
and basophil from blood vessels into lung tissue during pulmonary
inflammation (46,47). The results of the present study
and previous investigations suggest the possibility that A.
cochinchinensis is able to control the expression of certain
inflammatory cytokines. A prior study clearly demonstrated that ACE
attenuated the increased secretion of the pro-inflammatory
cytokines IL-1β and TNF-α in TPA-induced acute irritant contact
dermatitis (7). In another study
using the PM014 herbal formulation, the levels of Th2 cytokines
IL-4, IL-5 and IL-13 were decreased in the BALF of mice with
cockroach allergen-induced asthma (11). Similarly, the levels of the
inflammatory mediators IL-4, IL-13 and COX-2 were significantly
lower in the lung tissue and BALF of OVA + SEAC-treated mice
compared with OVA + vehicle-treated mice in the present study.
However, the level of IL-5 mRNA was not significantly reduced in
the OVA + SEAC-treated group. It is likely that the detected
difference between these studies can be predominantly attributed to
the composition of the treatment extracts, the administration
conditions and the allergens used to produce the asthma model.
Therefore, additional studies are required to understand what other
factors determine the therapeutic effects of SEAC and the
underlying mechanisms of the asthma model.
Elevated IgE in the serum is characteristic of
asthma (1). In a previous study
of the PM014 herbal formulation, the levels of serum IgE in a mouse
model of cockroach allergen-induced asthma were decreased (11). In the present study, SEAC exerted
prominent inhibitory effects on the levels of serum IgE (Fig. 4B). Accordingly, the administration
of SEAC resulted in a marked reduction in serum IgE levels in the
mouse OVA-induced asthma model (Fig.
4B).
Airway remodeling is characterized by several key
factors, including collagen deposition, smooth muscle hyperplasia
and VEGF expression. In mice subjected to chronic OVA exposure, a
marked increase in collagen deposition and enhancement of the α-SMA
stained area were observed around the airway (48,49). Furthermore, airway remodeling has
been indicated to be promoted by epithelial cell-derived VEGF
(50). Decreased VEGF expression
can lead to a significant reduction in goblet cell hyperplasia and
basement membrane thickness (51). Collagen deposition, smooth muscle
hyperplasia and VEGF expression have been shown to be significantly
inhibited by various natural compounds and extracts, including
proanthocyanidin from grape seed extract (52), Bangpungtongseong-san (53), Astragalus extract (54) and ligustrazine (55). In the present study, the
administration of SEAC significantly reduced goblet cell
hyperplasia, collagen deposition and VEGF expression in the
OVA-induced asthma model, although there were some differences in
the extent to which they were reduced. These results are very
similar to the results reported for various natural products
including Bangpungtongseong-san (53) and Astragalus extract
(54) in several of the
aforementioned previous studies.
The results of the present study suggest that SEAC
is a potential candidate for use in the attenuation of inflammation
in asthma through the inhibition of OVA-specific IgE production,
recovery of histopathological structure and suppression of
inflammatory mediators. To the best of our knowledge, this is the
first study demonstrating that A. cochinchinensis has
anti-inflammatory and remodeling activities in the airway of an
asthma model. However, additional studies are required to advance
our understanding of the effects of SEAC, as well as the mechanisms
accountable for these effects.
Acknowledgments
The present study was supported by a grant to
Professor Dae Youn Hwang from the Korea Institute of Planning
Evaluation for Technology of Food, Agriculture, Forestry and
Fisheries (grant no. 114034-03-1-HD030).
References
|
1
|
Endo Y, Hirahara K, Yagi R, Tumes DJ and
Nakayama T: Pathogenic memory type Th2 cells in allergic
inflammation. Trends Immunol. 35:69–78. 2014. View Article : Google Scholar
|
|
2
|
Rosenberg JL: Antilipid agents may provide
allergy protection. Ann Allergy Asthma Immunol. 110:12013.
View Article : Google Scholar
|
|
3
|
Porter PC, Yang T, Luong A, Delclos GL,
Abramson SL, Kheradmand F and Corry DB: Proteinases as molecular
adjuvants in allergic airway disease. Biochim Biophys Acta.
1810:1059–1065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sousa AR, Lane SJ, Cidlowski JA, Staynov
DZ and Lee TH: Glucocorticoid resistance in asthma is associated
with elevated in vivo expression of the glucocorticoid receptor
β-isoform. J Allergy Clin Immunol. 105:943–950. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Zhang S, Li DW and Jiang SJ:
Efficacy of anti-interleukin-5 therapy with mepolizumab in patients
with asthma: a meta-analysis of randomized placebo-controlled
trials. PLoS One. 8:e598722013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim H, Lee E, Lim T, Jung J and Lyu Y:
Inhibitory effect of Asparagus cochinchinensis on tumor necrosis
factor-alpha secretion from astrocytes. Int J Immunopharmacol.
20:153–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee DY, Choo BK, Yoon T, Cheon MS, Lee HW,
Lee AY and Kim HK: Anti-inflammatory effects of Asparagus
cochinchinensis extract in acute and chronic cutaneous
inflammation. J Ethnopharmacol. 121:28–34. 2009. View Article : Google Scholar
|
|
8
|
Luo J, Long QD, Li CX, Li L, Huang NH, Nie
M and Tang PX: Comparison of antitussive, expectorant and
anti-asthmatic effect between ALWB and ACM. Guiyang Yi Xue Yuan Xue
Bao. 23:132–134. 1998.In Chinese.
|
|
9
|
Xiong D, Yu LX, Yan X, Guo C and Xiong Y:
Effects of root and stem extracts of Asparagus cochinchinensis on
biochemical indicators related to aging in the brain and liver of
mice. Am J Chin Med. 39:719–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sung JE, Lee HA, Kim JE, Go J, Seo EJ, Yun
WB, Kim DS, Son HJ, Lee CY, Lee HS, et al: Therapeutic effect of
ethyl acetate extract from Asparagus cochinchinensis on phthalic
anhydrideinduced skin inflammation. Lab Anim Res. 32:34–45. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung KH, Choi HL, Park S, Lee G, Kim M,
Min JK, Min BI and Bae H: The effects of the standardized herbal
formula PM014 on pulmonary inflammation and airway responsiveness
in a murine model of cockroach allergen-induced asthma. J
Ethnopharmacol. 155:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singleton VL and Rossi JA: Colorimetry of
total phenolics with phosphomolybdic-phosphotungstic acid reagents.
Am J Enol Vitic. 16:144–158. 1965.
|
|
13
|
Zhishen J, Mengcheng T and Jianming W: The
determination of flavonoid contents in mulberry and their
scavenging effects on superoxide radicals. Food Chem. 64:555–559.
1999. View Article : Google Scholar
|
|
14
|
Helaly FM, Soliman HSM, Soheir AD and
Ahmed AA: Controlled release of migration of molluscicidal saponin
from different types of polymers containing Calendula officinalis.
Adv Polym Technol. 20:305–311. 2001. View Article : Google Scholar
|
|
15
|
Oh H, Ko EK, Kim DH, Jang KK, Park SE, Lee
HS and Kim YC: Secoiridoid glucosides with free radical scavenging
activity from the leaves of Syringa dilatata. Phytother Res.
17:417–419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jie S, Xueji Z, Mark B and Harry F:
Measurement of nitric oxide production in biological systems by
using Griess reaction assay. Sensors (Basel). 3:276–284. 2003.
View Article : Google Scholar
|
|
17
|
Kim JE, Park SH, Kwak MH, Go J, Koh EK,
Song SH, Sung JE, Lee HS, Hong JT and Hwang DY: Characterization of
changes in global genes expression in the distal colon of
loperamide-induced constipation SD rats in response to the laxative
effects of Liriope platyphylla. PLoS One. 10:e01296642015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung JY, Lee KY, Lee MY, Jung D, Cho ES
and Son HY: Antioxidant and antiasthmatic effects of saucerneol D
in a mouse model of airway inflammation. Int Immunopharmacol.
11:698–705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou E, Fu Y, Wei Z, Yu Y, Zhang X and
Yang Z: Thymol attenuates allergic airway inflammation in ovalbumin
(OVA)- induced mouse asthma. Fitoterapia. 96:131–137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Platts-Mills TA: The role of
immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med.
164:S1–S5. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spina D: Asthma mediators: current views.
J Pharm Pharmacol. 52:125–145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamauchi K and Inoue H: Airway remodeling
in asthma and irreversible airflow limitation - ECM deposition in
airway and possible therapy for remodeling-. Allergol Int.
56:321–329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zha W, Su M, Huang M, Cai J and Du Q:
Administration of pigment epithelium-derived factor inhibits airway
inflammationand remodeling in chronic OVA-induced mice via VEGF
suppression. Allergy Asthma Immunol Res. 8:161–169. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wise J: Corticosteroids for asthma may
suppress growth in children in first year of treatment, researchers
say. BMJ. 349:g46232014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciriaco M, Ventrice P, Russo G,
Scicchitano M, Mazzitello G, Scicchitano F and Russo E:
Corticosteroid-related central nervous system side effects. J
Pharmacol Pharmacother. 4(Suppl 1): S94–S98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qu FY, Wei XD, Li SL, Wang YM and Bai SG:
Experimental study of Asparagus cochinchinensis delay aging. Zhong
Yi Yao Xue Bao. 2:68–70. 1999.In Chinese.
|
|
27
|
Konishi T and Shoji J: Studies on the
constituents asparagi radix. I. On the structures of furostanol
oligosides of Asparagus cochinensis (Loureio) Merrill. Chem Pharm
Bull (Tokyo). 27:3086–3094. 1979. View Article : Google Scholar
|
|
28
|
Yang YC, Huang SY and Shi JG: Two new
furostanol glycosides from Asparagus cochinchinensis. Chin Chem
Lett. 13:1185–1188. 2002.
|
|
29
|
Zhang ZJ: Therapeutic effects of herbal
extracts and constituents in animal models of psychiatric
disorders. Life Sci. 75:1659–1699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li M, Fei Y and Wang JK: Studies on
pharmacologic effects of Radix Asparagi. Shizhen Guo Yi Guo Yao.
16:580–582. 2005.In Chinese.
|
|
31
|
Zhao YJ, Meng XL, Li XL and Qu FY:
Influence of Radix Asparagi nano-pharmaceutics on NOS, NO, LPF of
senile mice. Zhongguo Ye Sheng Zhi Wu Zi Yuan. 24:49–51. 2005.In
Chinese.
|
|
32
|
Wen JY, Li Y, Ding SS and Li QH:
Pharmacological screening of 9 medicinal plants of the genus
Asparagus (Liliaceae) in China. Shanghai Yi Ke Da Xue Xue Bao.
20:107–111. 1993.In Chinese.
|
|
33
|
Koo HN, Jeong HJ, Choi JY, Choi SD, Choi
TJ, Cheon YS, Kim KS, Kang BK, Park ST, Chang CH, et al: Inhibition
of tumor necrosis factor-alpha-induced apoptosis by Asparagus
cochinchinensis in Hep G2 cells. J Ethnopharmacol. 73:137–143.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park M, Cheon MS, Kim SH, Chun JM, Lee AY,
Moon BC, Yoon T and Choo BK: Anticancer activity of Asparagus
cochinchinensis extract and fraction in HepG2 cells. J Korean Soc
Appl Biol Chem. 54:188–193. 2011. View Article : Google Scholar
|
|
35
|
Yu FR, Lian XZ and Guo HY: Effect of Lucid
asparagus extract on the regulation of blood sugar. Zhongguo Lin
Chuang Kang Fu. 10:57–59. 2006.In Chinese.
|
|
36
|
Xiao PG: Modern Chinese Material Medica.
China Press; Beijing: pp. 1502002
|
|
37
|
Huang Y, Cai T, Xia X, Cai Y and Wu XY:
Research advances in the intervention of inflammation and cancer by
active ingredients of traditional Chinese medicine. J Pharm Pharm
Sci. 19:114–126. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yan GH and Choi YH: Phellinus linteus
extract exerts antiasthmatic effects by suppressing NF-κB and p38
MAPK activity in an OVA-induced mouse model of asthma. Immune Netw.
14:107–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lim CY, Moon JM, Kim BY, Lim SH, Lee GS,
Yu HS and Cho SI: Comparative study of Korean White Ginseng and
Korean Red Ginseng on efficacies of OVA-induced asthma model in
mice. J Ginseng Res. 39:38–45. 2015. View Article : Google Scholar
|
|
40
|
Lee E, Kim SG, Park NY, Park HH, Jeong KT,
Choi J, Lee IH, Lee H, Kim KJ and Lee E: KOTMIN13, a Korean herbal
medicine alleviates allergic inflammation in vivo and in vitro. BMC
Complement Altern Med. 16:1692016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kay AB: Asthma and inflammation. J Allergy
Clin Immunol. 87:893–910. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ngoc PL, Gold DR, Tzianabos AO, Weiss ST
and Celedón JC: Cytokines, allergy, and asthma. Curr Opin Allergy
Clin Immunol. 5:161–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Larché M, Robinson DS and Kay AB: The role
of T lymphocytes in the pathogenesis of asthma. J Allergy Clin
Immunol. 111:450–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li-Weber M and Krammer PH: Regulation of
IL4 gene expression by T cells and therapeutic perspectives. Nat
Rev Immunol. 3:534–543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng W and Flavell RA: The transcription
factor GATA-3 is necessary and sufficient for Th2 cytokine gene
expression in CD4 T cells. Cell. 89:587–596. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sedgwick JB, Calhoun WJ, Gleich GJ, Kita
H, Abrams JS, Schwartz LB, Volovitz B, Ben-Yaakov M and Busse WW:
Immediate and late airway response of allergic rhinitis patients to
segmental antigen challenge. Characterization of eosinophil and
mast cell mediators. Am Rev Respir Dis. 144:1274–1281. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Greenfeder S, Umland SP, Cuss FM, Chapman
RW and Egan RW: Th2 cytokines and asthma. The role of interleukin-5
in allergic eosinophilic disease. Respir Res. 2:71–79. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Makinde T, Murphy RF and Agrawal DK: The
regulatory role of TGF-beta in airway remodeling in asthma. Immunol
Cell Biol. 85:348–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Martin JG, Duguet A and Eidelman DH: The
contribution of airway smooth muscle to airway narrowing and airway
hyperresponsivenessin disease. Eur Respir J. 16:349–354. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lopez-Guisa JM, Powers C, File D, Cochrane
E, Jimenez N and Debley JS: Airway epithelial cells from asthmatic
children differentially express proremodeling factors. J Allergy
Clin Immunol. 129:990–7.e6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yuksel H, Yilmaz O, Karaman M, Bagriyanik
HA, Firinci F, Kiray M, Turkeli A and Karaman O: Role of vascular
endothelial growth factor antagonism on airway remodeling in
asthma. Ann Allergy Asthma Immunol. 110:150–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou DY, Fang SR, Zou CF, Zhang Q and Gu
W: Proanthocyanidin from grape seed extract inhibits airway
inflammation and remodeling in a murine model of chronic asthma.
Nat ProdCommun. 10:257–262. 2015.
|
|
53
|
Lee MY, Shin IS, Jeon WY, Shin N and Shin
HK: Bangpungtongseong-san, a traditional herbal medicine,
attenuates chronic asthmatic effects induced by repeated
ovalbuminchallenge. Int J Mol Med. 33:978–986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Qu ZH, Yang ZC, Chen L, Lv ZD, Yi MJ and
Ran N: Inhibition airway remodeling and transforming growth
factor-β1/Smadsignaling pathway by astragalus extract in asthmatic
mice. Int J Mol Med. 29:564–568. 2012. View Article : Google Scholar
|
|
55
|
Wang WJ, Yang L, Wang XH and Li HL: Effect
of ligustrazine on airway remodeling in asthmatic rats. Zhonghua
Jie He He Hu Xi Za Zhi. 27:833–836. 2004.In Chinese.
|