Introduction
Chronic obstructive pulmonary disease (COPD) is a
global public health concern and a major cause of morbidity and
mortality worldwide (1). COPD is
characterized by oxidative damage and chronic airway inflammation
that results in airflow obstruction and emphysema (2). The exposure to cigarette smoke (CS)
is the main cause of COPD, as it alters the inflammatory mechanisms
by increasing the influx of inflammatory cells, including
neutrophils, and the production of inflammatory molecules (3). Recent studies reported that CS
contains harmful particles and trace amounts of microbial cell
components, including bacterial lipopolysaccharide (LPS), which
play an important role in lung diseases and respiratory infections
(4,5).
Airway inflammation is one of the major
characteristics of COPD and is associated with an increase in
inflammatory cell recruitment (6). Neutrophil influx is a major
pathophysiological characteristic of COPD, and persistent
activation of neutrophils leads to lung tissue damage by increasing
the production of reactive oxygen species (ROS) and neutrophil
elastase (7). Macrophages affect
airway inflammation via production of inflammatory cytokines and
chemokines in the pathogenesis of COPD (8). High levels of tumor necrosis
factor-α (TNF-α) and interleukin-6 (IL-6) were detected in COPD
patients and CS exposure animal models (9,10).
Monocyte chemoattractant protein-1 (MCP-1), a member of the C-C
chemokine subfamily, is produced by macrophages and airway
epithelial cells, and affects the influx of inflammatory cells,
including neutrophils, in airway inflammatory response (11,12). The administration of antioxidants
effectively ameliorated the airway inflammation with MCP-1
inhibition (13). Downregulation
of extracellular signal-regulated kinase (ERK) reduces the
production of inflammatory cytokines and chemokines in CS extract
(CSE)-stimulated pulmonary epithelial cells and in animal models
with CS-induced airway inflammation (13,14). The antioxidant defense protein
heme oxygenase-1 (HO-1) exerts a protective effect against
CS-induced lung inflammation (15). The levels of inflammatory cell
influx and inflammatory molecules are decreased by HO-1 induction
in animal models of CS-induced airway inflammation (16).
Cape gooseberry [Physalis peruviana L. (PP)]
is a species within the Solanaceae family, which has been widely
used in folk medicine (17). PP
displays a wide range of biological properties, including
neuroprotective, antioxidant and anti-inflammatory properties
(18–20). However, the protective effect of
PP against airway inflammation induced by CS and LPS has not been
extensively investigated.
Materials and methods
Preparation of PP
PP was collected from the Katu Village, Lore Lindu
National Park, Central Sulawesi, Indonesia. Plant samples were
collected and identified by the Center for Pharmaceutical and
Medical Technology (Tangerang, Indonesia) and verified by Herbarium
Bogoriense (Bogor, Indonesia). Voucher specimens were recorded as
KRIB 0049496 and PMT 1884 have been deposited at the herbarium of
the Korea Research Institute of Bioscence and Biotechnolgy and at
the Center for Pharmaceutical and Medical Technology and Herbarium
Bogoriense. After drying and grinding the leaves of PP, 150 g of
powder was added to 150 ml of methanol and extraction was performed
by maceration at room temperature for 18 h. The extract was
filtered and concentrated by a rotary evaporator (Laborota 4000;
Heidolph, Jakarta, Indonesia) under reduced pressure, thereby
obtaining 7.05 g of PP methanolic extract. In the following
experiment, the extract was dissolved in dimethyl sulfoxide (DMSO)
to a concentration of 20 mg/ml, and then diluted to various
concentrations before use.
Mouse models of airway inflammation
induced by CS and LPS
Male 6-week-old C57BL/6N mice were purchased from
Koatech Co. (Pyeongtaek, Korea) and used after 1 week of quarantine
and acclimatization in a specific pathogen-free system. The mice
were randomly divided into 4 groups (n=7 per group) as follows: i)
The normal control (NC) group; ii) the CS + LPS group; iii) the
roflumilast (ROF; 10 mg/kg) group (used as a positive control); and
iv) the PP group (administered 10 and 20 mg/kg PP). CS exposure was
applied as previously described (7). In brief, the mice were whole-body
exposed to room air or CS of 8 cigarettes for 1 h a day for 10
days. CS exposure was generated by 3R4F research cigarette,
containing 11.0 mg of total particulate matter, 9.4 mg of tar, and
0.76 mg of nicotine/cigarette (Tobacco and Health Research
Institute, University of Kentucky, Lexington, KY, USA). ROF and PP
were dissolved with 1% DMSO + 1% Tween-20 in phosphate-buffered
saline (PBS), and were administered orally on days 0–9. The mice
were administered LPS (5 μg dissolved in 30 μl
distilled water) intranasally 1 h after the final ROF and PP
treatment. All the experimental procedures were performed in
accordance with the procedures approved by the Institutional Animal
Care and Use Committee of the Korea Research Institute of
Bioscience and Biotechnology, and in compliance with the National
Institutes of Health Guidelines for the Care and Use of Laboratory
Animals and Korean National Laws for Animal Welfare.
Collection of bronchoalveolar lavage
fluid (BALF) and determination of inflammatory cell counts
The collection of the BALF and determination of
inflammatory cell counts were performed as previously described
(13). In brief, ice-cold PBS
(700 μl) was infused into the lungs via tracheal cannulation
and extraction was performed twice (total volume, 1,400 μl).
In order to count the number of different cells, 100 μl BALF
was centrifuged onto glass slides for 5 min at 264 × g, and the
slides were dried and stained using Diff-Quik® staining
reagent according to the manufacturer's protocol (IMEB Inc.,
Deerfield, IL, USA).
Measurement of ROS and pro-inflammatory
cytokines in the BALF
The intracellular levels of ROS were determined
using 2′,7′-dichlorofluorescein diacetate (DCF-DA; Sigma-Aldrich,
St. Louis, MO, USA) as previously described (21). Briefly, BALF cells were washed
with PBS and incubated with 20 μM DCF-DA for 10 min at 37°C.
Subsequently, the intracellular ROS levels were detected under
fluorescence at 488 nm excitation and 525 nm emission on a
fluorescence plate reader (Perkin-Elmer, Waltham, MA, USA). The
levels of pro-inflammatory cytokines (TNF-α, IL-6 and MCP-1) were
determined using enzyme-linked immunosorbent assay (ELISA) kits
according to the manufacturer's protocol (R&D Systems,
Minneapolis, MN, USA). The absorbance was measured at 450 nm using
a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Western blot analysis
The lung tissues were collected at 6 or 24 h after
the last administration of PP. The levels of ERK and nuclear factor
erythroid 2-related factor 2 (Nrf2) activation were evaluated using
lung tissues that were obtained 6 h after the last administration
of PP. The expression of MCP-1, inducible nitric oxide synthase
(iNOS), cyclooxygenase-2 (COX-2) and HO-1 were evaluated using lung
tissues that were collected 24 h after the last administration of
PP. The lung tissues were homogenized (1/10, w/v) in tissue
lysis/extraction reagent, containing a protease inhibitor cocktail
(Sigma-Aldrich). Equal amounts of the total cellular protein were
resolved by 8–12% SDS-polyacrylamide gels and transferred to Hybond
PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA). The
membranes were incubated in blocking solution [5% skimmed milk in
Tris-buffered saline + 0.1% Tween-20 (TBST)] for 1 h and incubated
overnight at 4°C with the appropriate primary antibody. A rabbit
polyclonal MCP-1 antibody (sc-28879, 1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), a rabbit polyclonal
p-ERK antibody (cat. no. 9101, 1:1,000; Cell Signaling Technology
Inc., Danvers, MA, USA), a rabbit polyclonal iNOS antibody (SC-651,
1:1,000; Santa Cruz Biotechnology, Inc.), a goat polyclonal COX-2
antibody (SC-1747, 1:1,000), a rabbit polyclonal ERK antibody
(sc-154, 1:1,000), a rabbit polyclonal Nrf2 antibody (sc-722,
1:1,000), a rabbit polyclonal p-Nrf2 antibody (ab76026, 1:1,000;
Abcam, Cambridge, UK), a rabbit polyclonal HO-1 antibody (cat. no.
5061, 1:1,000), and a rabbit polyclonal anti-β-actin antibody (cat.
no. 4967, 1:2,500) (both from Cell Signaling Technology Inc.) were
diluted in 5% skimmed milk. The membranes were washed in TBST and
incubated with the Peroxidase-AffiniPure goat anti-rabbit IgG (H+L)
(111-035-003, 1:2,000; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA) and the Peroxidase-AffiniPure goat anti-mouse
IgG (H+L) (115-035-003, 1:2,000; Jackson ImmunoResearch
Laboratories, Inc.) for 2 h at room temperature. The membranes were
washed with TBST and developed using an enhanced chemiluminescence
kit (Thermo Fisher Scientific, Inc., Rockford, IL, USA). All bands
were visualized using a LAS-4000 luminescent image analyzer
(Fujifilm, Tokyo, Japan) and quantified by densitometry (Fuji Multi
Gauge software, version 3.0).
CSE-stimulated inflammatory molecules in
human airway epithelial cells
A549 human airway epithelial cells were obtained
from the American Type Culture Collection (Manassas, VA, USA) and
were grown in RPMI-1640 medium (Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA) supplemented with 10% FBS in the presence of
penicillin (100 U/ml) and streptomycin (100 μg/ml), and were
incubated in a 5% CO2 incubator. CSE was purchased from
Kentucky Reference 3R4F research blend cigarettes (University of
Kentucky) and administered as previously described (22). The A549 human lung epithelial
cells were incubated with the indicated concentration of PP prior
to the addition of CSE (10 μg/ml).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The A549 cells were treated with PP in the absence
or presence of CSE (10 μg/ml) for 6 h. Total RNA was
isolated using TRIzol™ reagent and reverse-transcribed into cDNA,
according to the manufacturer's protocol (Thermo Fisher Scientific,
Inc.). The PCR conditions for MCP-1 were as follows: 94°C for 10
min (1 cycle), 94°C for 30 sec, 58°C for 30 sec, and 72°C for 1 min
(25 cycles); for TNF-α, 94°C for 10 min (1 cycle), 94°C for 30 sec,
57°C for 30 sec, and 72°C for 1 min (25 cycles); for β-actin, 94°C
for 10 min (1 cycle), 94°C for 30 sec, 59°C for 30 sec, and 72°C
for 1 min (25 cycles); and then a final extension phase at 72°C for
10 min. The specific forward and reverse primers for TNF-α and
MCP-1 were as follows: TNF-α forward, 5′-TCAACCTCCTCTCTGCCATC-3′
and reverse, 5′-CCTAAGCCCCCAATTCTCTT-3′; MCP-1 forward,
5′-TCTGTGCCTGCTGCTCATAG-3′ and reverse, 5′-CAGATCTCCTTGGCCACAAT-3′;
and β-actin forward, 5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′. The housekeeping gene β-actin was used
for normalization of RT-PCR. The PCR products were separated on
agarose gel.
Histological analysis
To evaluate the protective effect of PP, lung
tissues were obtained at 24 h after PP administration, washed with
PBS and fixed in 10% (v/v) neutral buffered formalin solution. The
lung tissues were then embedded in paraffin, sectioned at 4
μm using a rotary microtome, and stained with hematoxylin
and eosin (H&E; Sigma-Aldrich) to estimate inflammatory
response.
Statistical analysis
The data are expressed as the means ± standard
deviation obtained from at least three independent experiments.
Statistical significance was determined using two-tailed Student's
t-test. A value of P<0.05 was considered to indicate
statistically significant differences.
Results
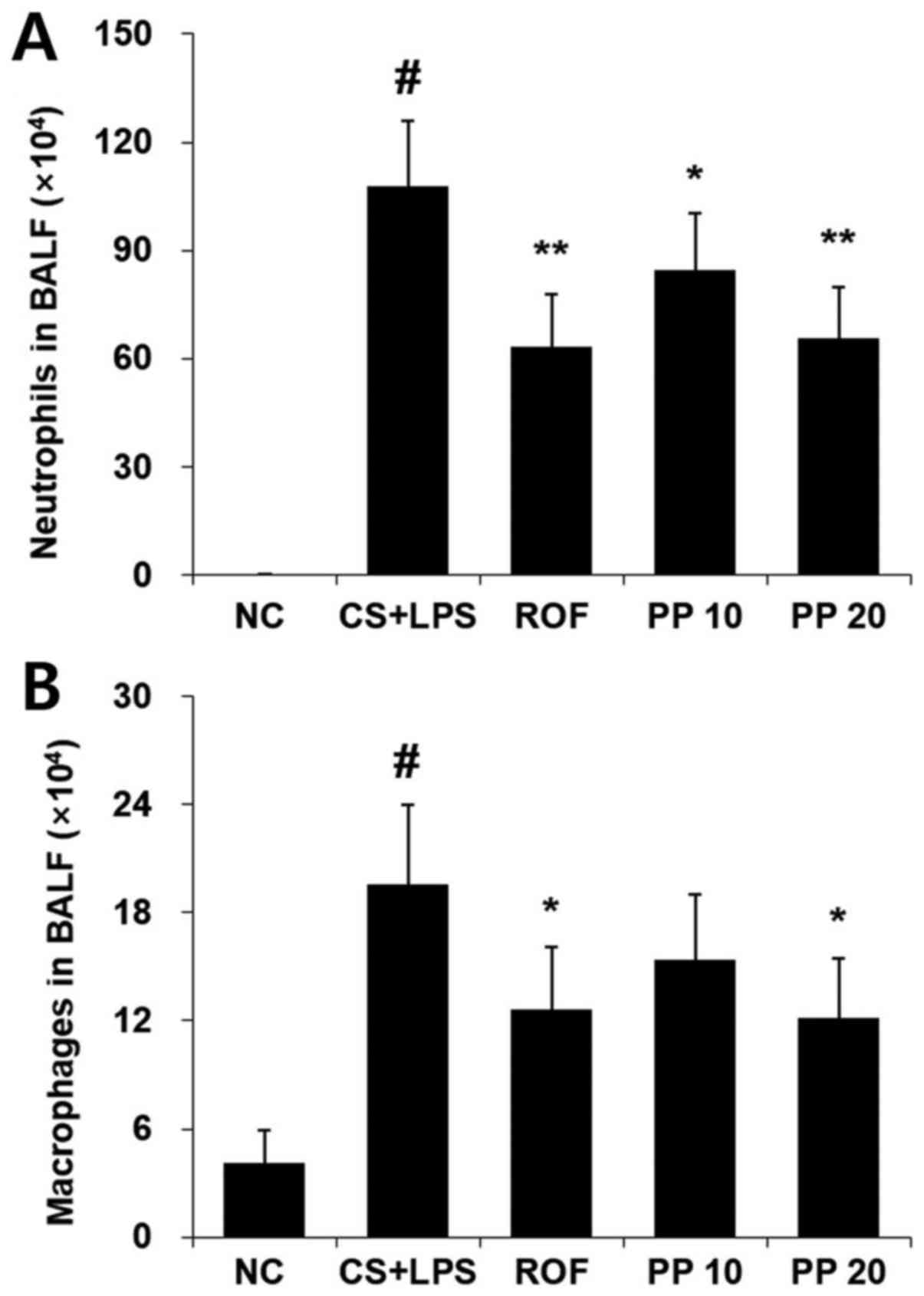
Treatment with PP attenuates the
recruitment of neutrophils in the BALF of CS- and LPS-induced
airway inflammation animal models
The increased number of neutrophils and macrophages
in the BALF is a cornerstone characteristic of CS- and LPS-induced
airway inflammation. Thus, the effect of PP on the infiltration of
these cells was evaluated using Diff-Quik® staining. As
shown in Fig. 1, increased
numbers of neutrophils and macrophages were detected in the CS +
LPS group, whereas decreased numbers of these cells were detected
in the PP group, and this effect was concentration-dependent
(Fig. 1). ROF was used as
positive control. The effect of 20 mg/kg PP was similar to that of
treatment with 10 mg/kg ROF.
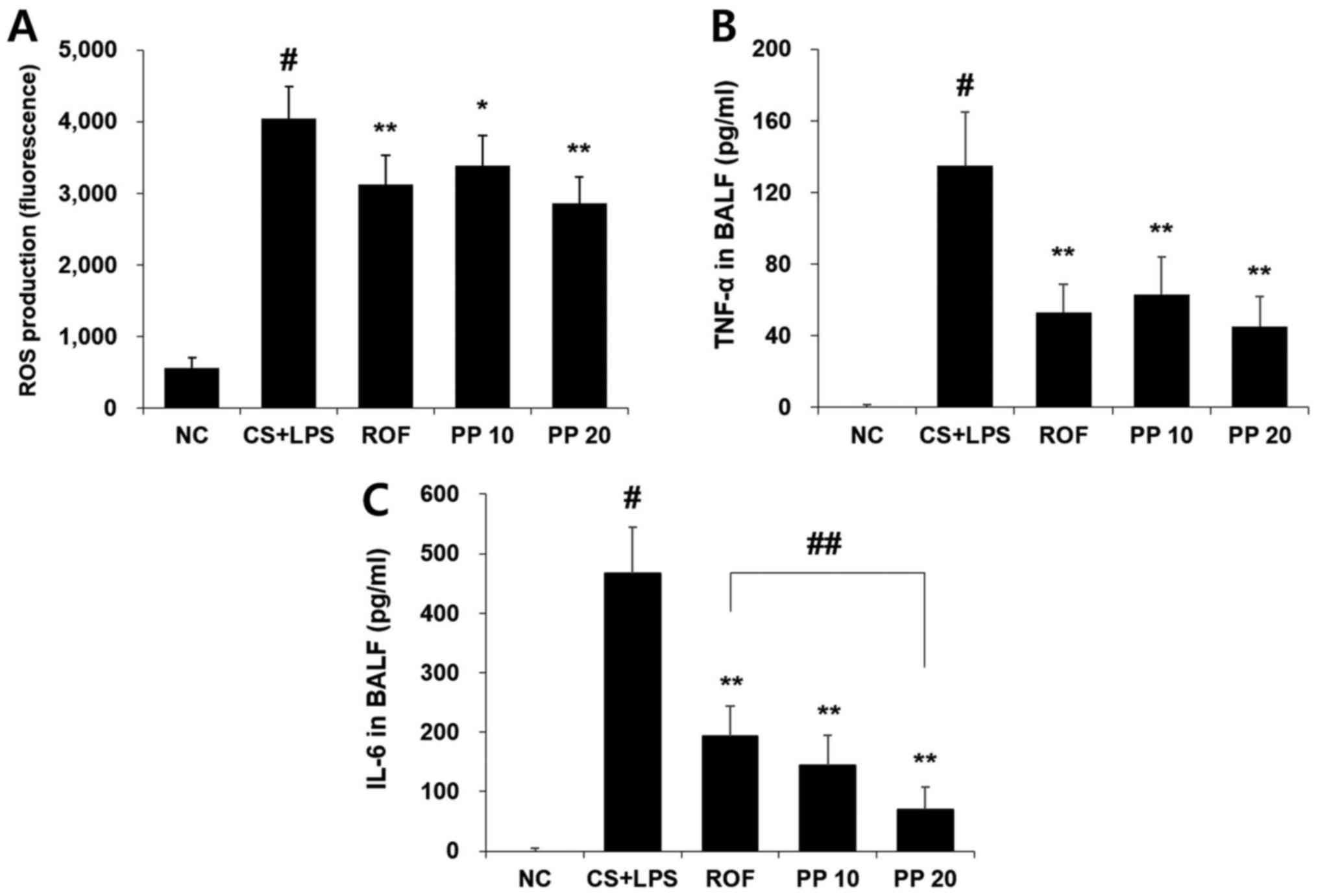
Treatment with PP reduces the levels of
ROS and pro-inflammatory cytokines in the BALF
Intranasal administration of LPS markedly increased
the levels of ROS and inflammatory cytokines, including TNF-α and
IL-6. However, treatment with PP significantly reduced these levels
in a concentration-dependent manner (Fig. 2). In particular, 20 mg/kg PP
attenuated the production of IL-6 more effectively compared with
the ROF or CS + LPS groups (Fig.
2C).
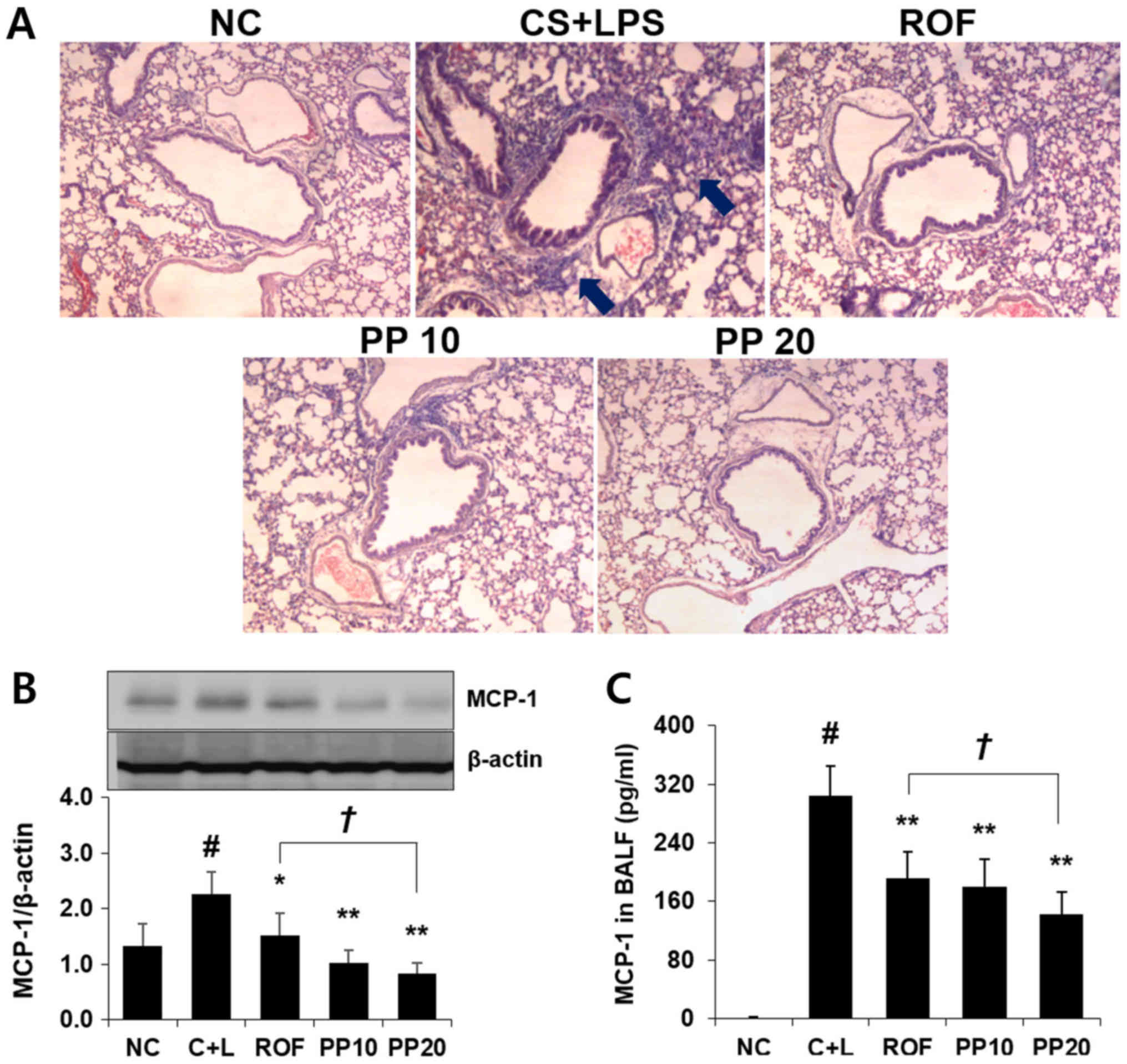
Treatment with PP inhibits the
infiltration of inflammatory cells and the expression of MCP-1 in
the lungs
It was next examined whether PP affects the
infiltration of inflammatory cells and the production of MCP-1 in
the lungs of animal models with CS- and LPS-induced airway
inflammation. As shown in Fig.
3A, increased influx of inflammatory cells was observed around
peribronchial lesions in the CS + LPS group, whereas a significant
reduction of the influx of these cells was detected in the PP
group. Treatment with PP also significantly attenuated the
expression of MCP-1 compared with the ROF or CS + LPS groups
(Fig. 3B). Similar to the results
obtained by PP in the lung, a decreased level of MCP-1 in the BALF
was confirmed following PP administration (Fig. 3C).
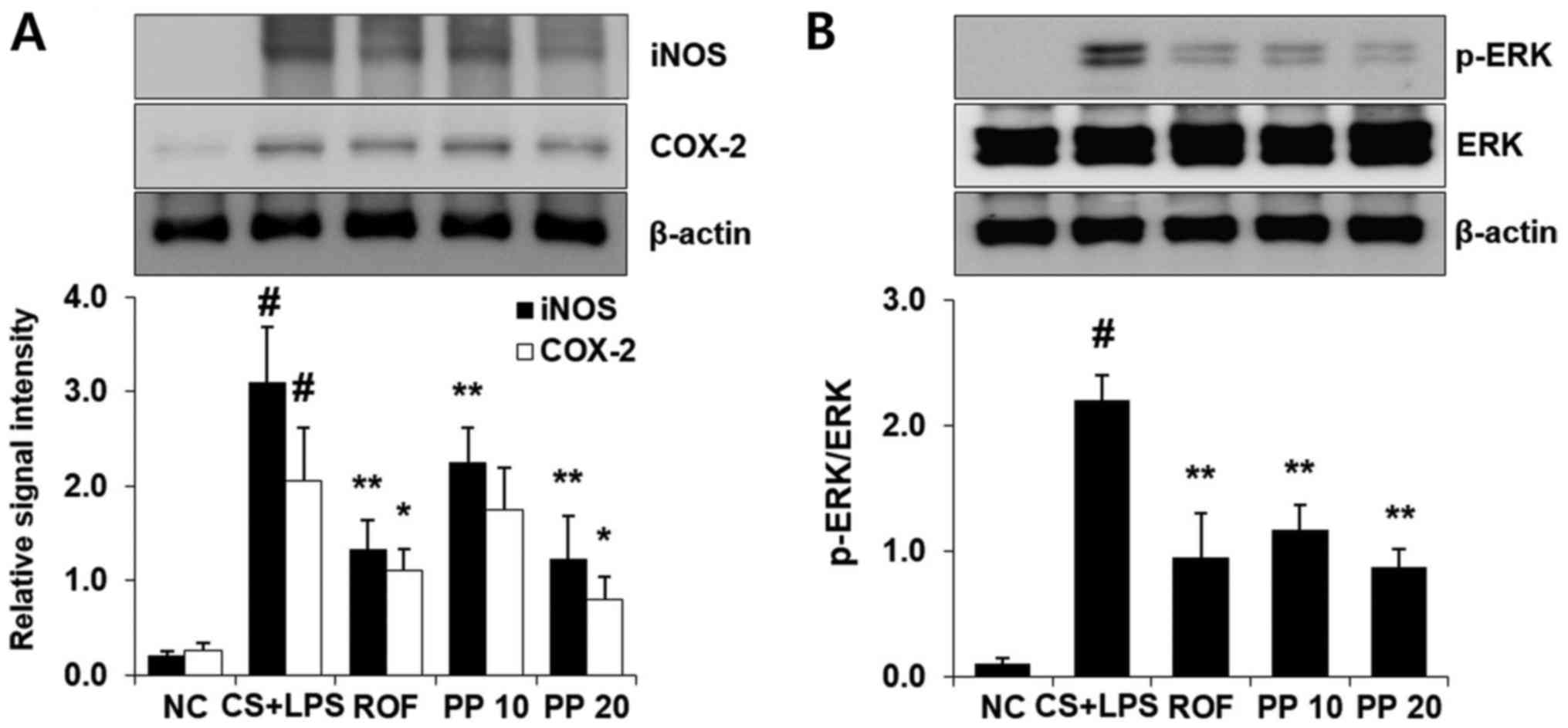
Treatment with PP decreases the
expression of iNOS and COX-2, and reduces the activation of ERK in
the lung
LPS administration markedly increased the expression
of iNOS and COX-2 compared with the NC group (Fig. 4). However, treatment with PP
significantly decreased the levels of iNOS and COX-2 compared with
the CS + LPS group in a concentration-dependent manner (Fig. 4A). Similar to the results for iNOS
and COX-2, ERK phosphorylation was also effectively decreased with
PP administration. In particular, the effect of 20 mg/kg PP was
similar to that of 10 mg/kg ROF (Fig.
4B).
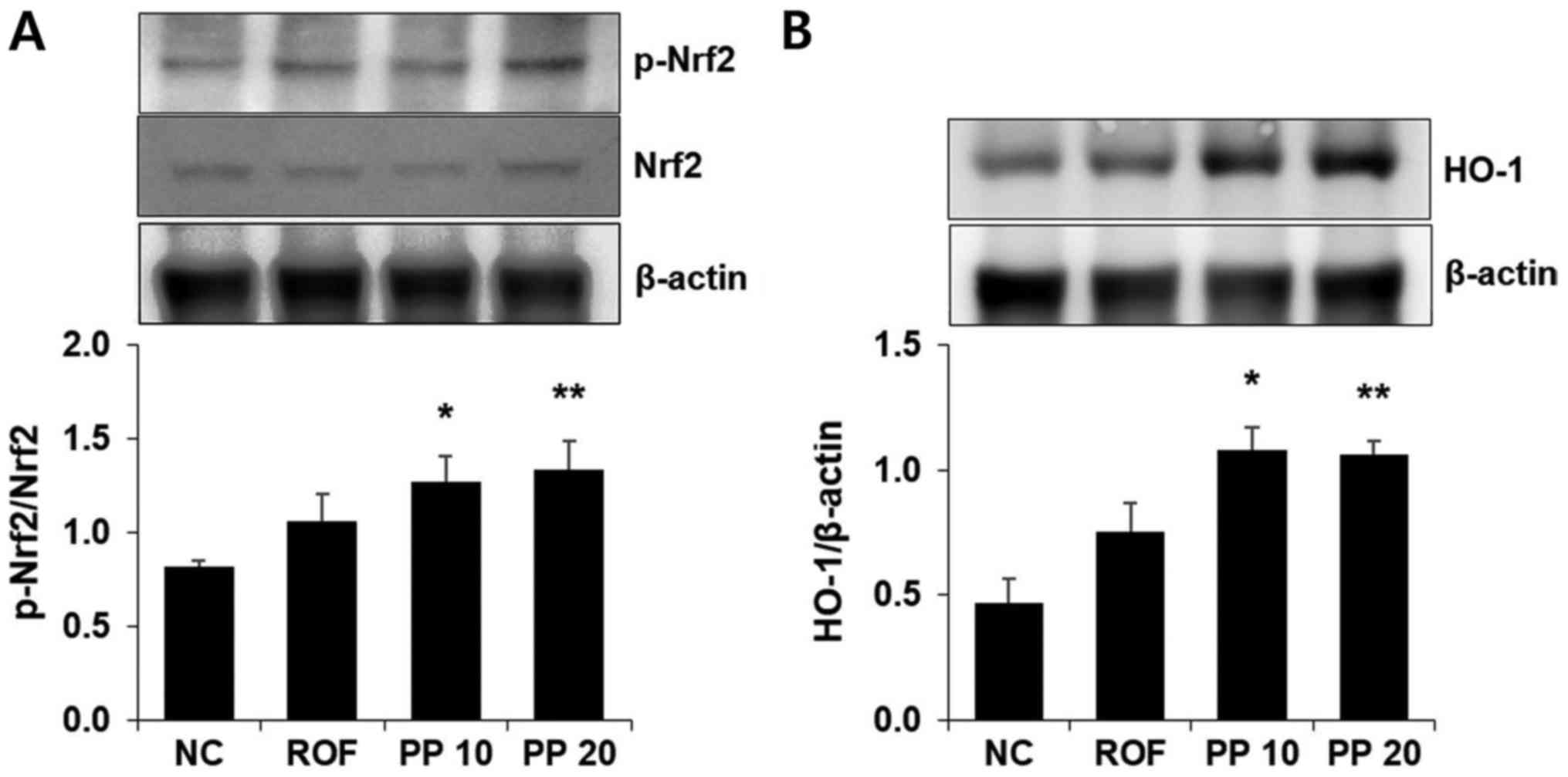
Treatment with PP increases the
expression of HO-1 in the lungs
HO-1 induction exerts a protective effect in airway
inflammatory diseases, including COPD. Thus, western blot analysis
was used to investigate whether PP promotes the expression of HO-1.
There was an increase in HO-1 expression in the PP group compared
with the NC or ROF groups (Fig.
5B). Activation of Nrf2, which is a major transcription factor
of HO-1, was also observed following PP administration (Fig. 5A).
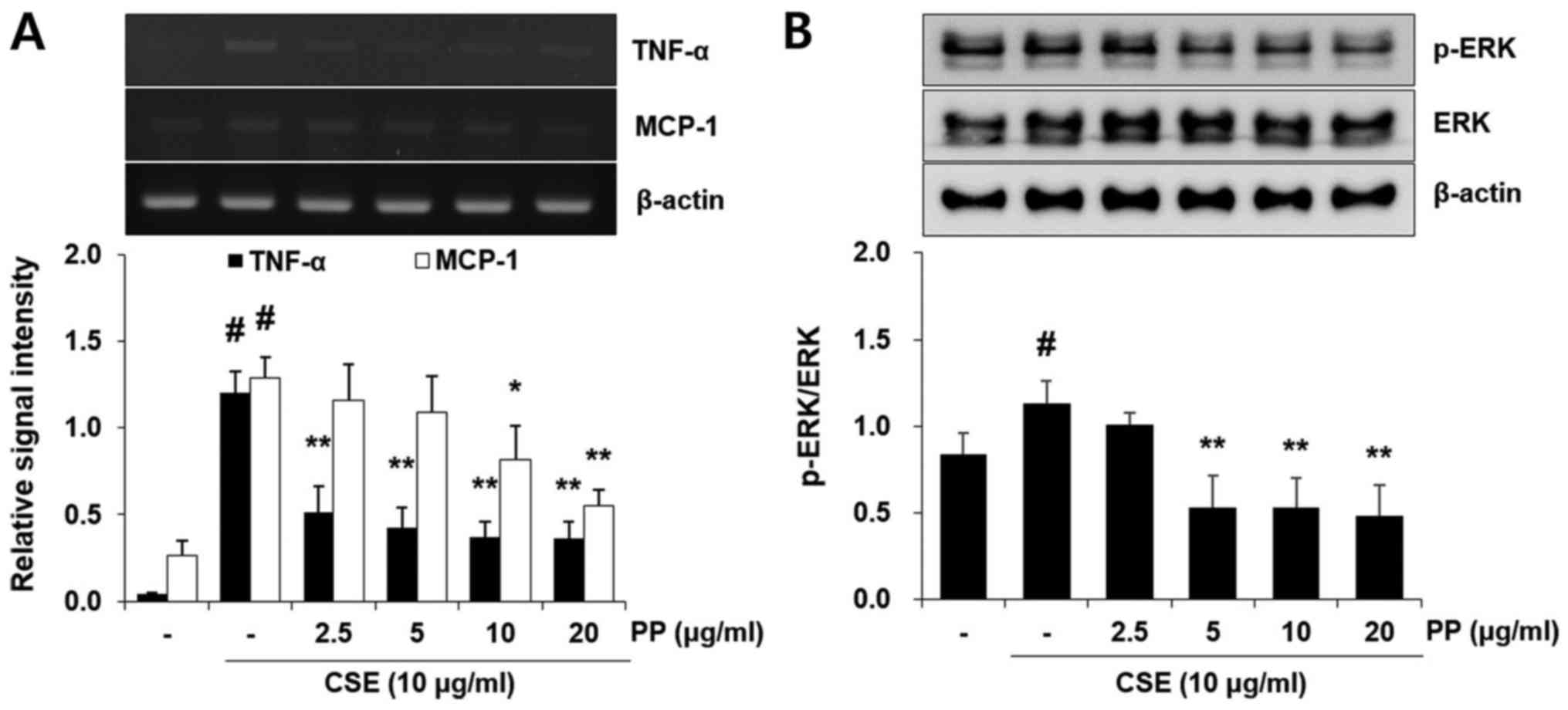
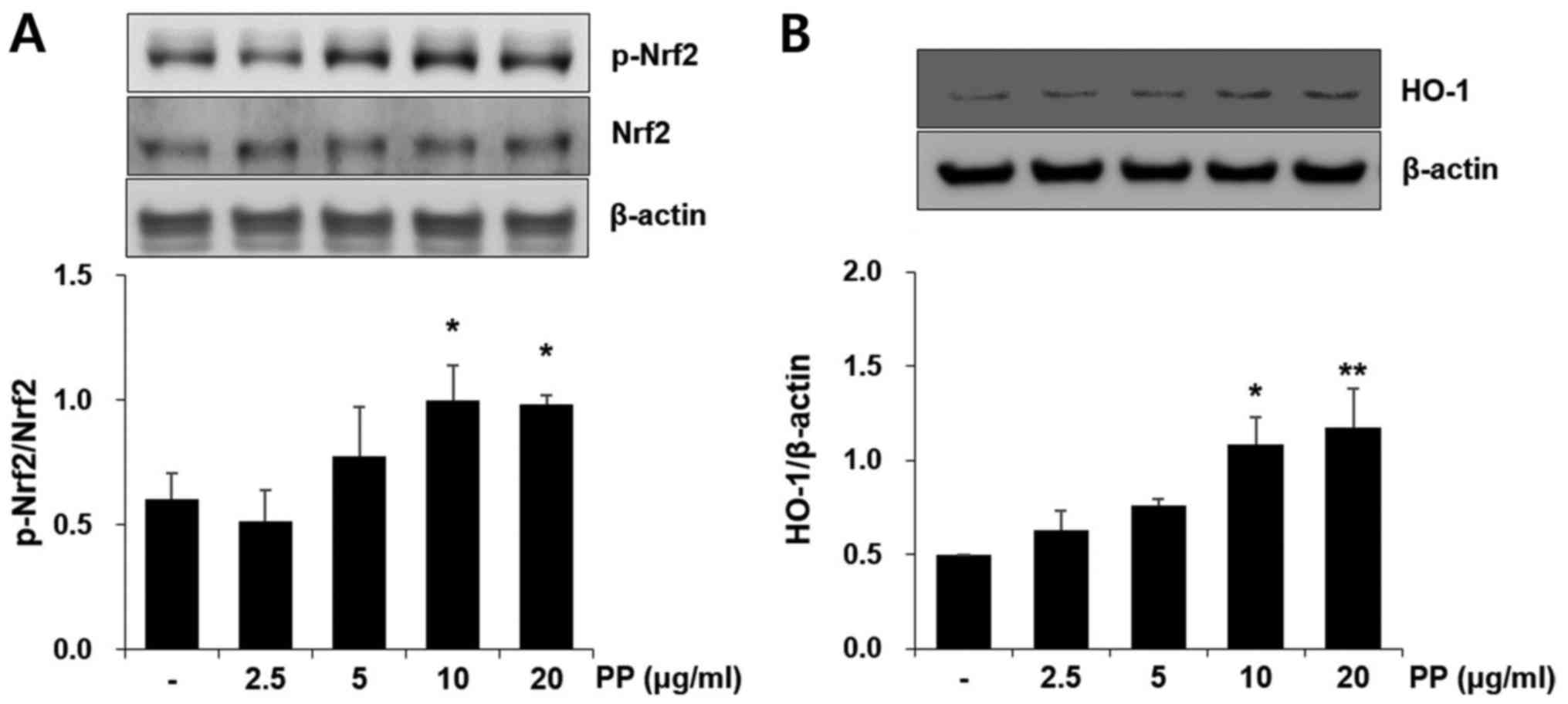
Pretreatment with PP reduces the mRNA
expression of inflammatory molecules and the activation of ERK in
A549 airway epithelial cells
CSE treatment significantly increased the mRNA
expression of TNF-α and MCP-1 in A549 cells. However, these effects
were effectively attenuated by PP pretreatment (Fig. 6A). It was also confirmed that
pretreatment with PP significantly decreased the activation of ERK
in CSE-stimulated A549 cells (Fig.
6B). In addition, PP increased Nrf2 activation and HO-1
expression in A549 cells (Fig.
7).
Discussion
COPD is respiratory condition characterized by
expiratory airway obstruction, the major cause of which is chronic
airway inflammation (23). Given
the importance of airway inflammation in COPD, the
anti-inflammatory activity of PP was investigated using CS- and
LPS-induced airway inflammation animal models. As increased influx
of neutrophils is a cornerstone characteristic of airway
inflammation in COPD, it was investigated whether treatment with PP
attenuates this influx in the airway inflammatory response. As
shown in Fig. 1A, there was a
distinct reduction in the neutrophil influx in the BALF of the PP
group compared with the CS + LPS group. In particular, the effect
of 20 mg/kg PP was similar to that of 10 mg/kg ROF. Oxidative
stress is implicated in airway inflammation and recognized as a one
of the major predicting factors in the pathogenesis of COPD
(24). Increased amounts of ROS
are generated by neutrophils against CS and contribute to oxidative
stress (25). Therefore, it was
investigated whether PP inhibits the increase in the level of ROS.
As shown in Fig. 2A, ROS
production was upregulated in the BALF of the CS + LPS group
compared with the NC group (P<0.01), whereas treatment with PP
significantly decreased ROS production in a concentration-dependent
manner, indicating that PP exerts an antioxidant effect in the
airway inflammatory response (Fig.
2A). Similar to the results for ROS, PP effectively attenuated
the levels of TNF-α and IL-6 in the BALF (Fig. 2B and C). In particular, 20 mg/kg
PP inhibited the production of IL-6 more effectively compared with
10 mg/kg ROF (Fig. 2C). The
inhibition rates of IL-6 production were 58.6% (ROF), 69.0% (PP 10
mg/kg) and 84.9% (PP 20 mg/kg). Increased recruitment of
inflammatory cells into the lung is the major pathophysiological
mechanism underlying COPD (26).
Based on this result, H&E staining was used to determine
whether PP exerts an inhibitory effect on the recruitment of
inflammatory cells. As shown in Fig.
3A, exposure to CS and LPS markedly upregulated the recruitment
of inflammatory cells, whereas treatment with PP markedly reduced
this recruitment (Fig. 3A). These
results indicate that PP plays an antioxidant and anti-inflammatory
role in airway inflammation induced by CS and LPS.
Ng et al demonstrated that the inhibition of
MCP-1 may be valuable in the inhibition of CS-induced airway
inflammation (27). Our recent
study also confirmed that antioxidant treatment attenuates airway
inflammation induced by CS through the inhibition of MCP-1
(13). As airway inflammatory
response is accompanied by increased production of MCP-1, it was
investigated whether PP acts as a MCP-1 inhibitor. As shown in
Fig. 3B, treatment with PP
significantly decreased the expression of MCP-1 compared with the
CS + LPS group, in a concentration-dependent manner (P<0.01)
(Fig. 3B). Similar to those
results, the increased level of MCP-1 was effectively reduced with
PP administration (Fig. 3C). In
particular, 20 mg/kg PP decreased these levels more effectively
compared with the ROF or CS + LPS groups (Fig. 3B and C). These results indicate
that PP affects the influx of inflammatory cells as well as the
expression of MCP-1, suggesting that PP may be a useful inhibitor
of MCP-1.
High levels of iNOS are associated with a variety of
pathological conditions, including COPD (28). Gupta et al reported that CS
exposure leads to iNOS expression, which results in production of
toxic NO metabolites, leading to severe lung damage (29). The levels of COX-2 are
significantly increased in small airway epithelium of patients with
COPD, and the increase in the levels of this molecule contribute to
airway remodeling and inflammation, which are major characteristics
of COPD (30). Therefore,
inhibition of iNOS and COX-2 may be important for reducing airway
inflammation. In the present study, there was significant increase
in iNOS and COX-2 expression in the CS + LPS group compared with
the NC group (P<0.01). However, treatment with PP significantly
decreased these levels in a concentration-dependent manner
(Fig. 4A and B). The inhibitory
effect of 20 mg/kg PP on these molecules was similar to that of 10
mg/kg ROF.
The ERK signaling cascade is involved in the airway
inflammatory response. Li et al reported that CSE causes an
inflammatory response through the activation of ERK in human
bronchial epithelial cells (31).
In our recent study, it was also confirmed that the increased
levels of inflammatory cytokines, such as TNF-α and IL-6, were
attenuated with downregulation of ERK activation in CSE-stimulated
human airway epithelial cells (22). In an in vivo study in
airway inflammation animal models, treatment with antioxidants
effectively inhibited airway inflammation via inhibition of ERK
activation (13,32). Therefore, controlling the activity
of the ERK pathway may be a valuable therapeutic approach to airway
inflammatory diseases, including COPD. Thus, the effect of PP on
ERK activation was evaluated. As shown in Fig. 4, the phosphorylation of ERK was
markedly increased in the CS + LPS group, whereas decreased
phosphorylation was detected in the PP group. This result indicates
that PP may be a useful inhibitor of ERK activation in airway
inflammation.
Antioxidant enzymes, such as HO-1, NAD(P)H quinone
oxidoreductase 1 and superoxide dismutase exert protective effects
against endotoxin-induced inflammation (33,34). Among those, it is well known that
HO-1 exerts anti-inflammatory effects in inflammatory conditions by
controlling nuclear factor-κB activation and production of
inflammatory molecules (35,36). Treatment with antioxidants
ameliorates the CS-induced neutrophil influx and airway
inflammation through the induction of HO-1 (13). Li et al reported that
antioxidants downregulate the expression of inflammatory cytokines
and upregulate the expression of HO-1 in CSE-stimulated macrophages
and bronchial epithelial cells (15). Therefore, HO-1 induction may be
useful in the treatment of CS-induced airway inflammation. In the
present study, it was confirmed that PP administration upregulated
the expression of HO-1 compared with the CS + LPS group. PP more
effectively increased HO-1 expression compared with the NC group
(P<0.01) (Fig. 5). This result
suggests that PP may be useful in the induction of HO-1 in
CS-induced airway inflammation.
Airway epithelial cells are first-defense cells and
an important source of inflammatory cytokines and chemokines
against CS (12). Similar to the
results obtained by PP in vivo, the increased release of
inflammatory cytokines and chemokines was markedly decreased with
PP administration in CSE-stimulated A549 airway cells (Fig. 6). Nrf2 activation and HO-1
expression were also upregulated with PP treatment in A549 cells
(Fig. 7).
Antioxidants have therapeutic as well as preventive
potential in the airway inflammatory response of COPD and,
therefore, may prove to be a useful therapeutic approach to the
treatment of COPD (37). In
several studies, antioxidants have been reported to protect against
the airway inflammatory response by reducing the influx of
inflammatory cells and the levels of inflammatory molecules, and by
upregulation of HO-1. Therefore, it was evaluated whether PP, which
is a strong antioxidant, exerts a protective effect in airway
inflammation. In the present study, PP effectively attenuated
airway inflammation by inhibition of neutrophil influx and of
inflammatory toxic molecules, such as ROS, which are important
pathophysiological characteristics in COPD. These effects of PP
were accompanied by Nrf2 activation and HO-1 induction, in
vitro as well as in vivo. Therefore, our results suggest
that PP may be a valuable therapeutic adjuvant in airway
inflammatory diseases, including COPD.
Acknowledgments
The present study was supported by grants from the
KRIBB Research Initiative Program (no. KGM 1221713), the
International Biological Material Research Center (no. NRF
2016K1A1A8A01939075) and the Ministry of Health and Welfare (no.
HI14C1277) of the Republic of Korea.
Glossary
Abbreviations
Abbreviations:
|
COPD
|
chronic obstructive pulmonary
disease
|
|
CS
|
cigarette smoke
|
|
LPS
|
lipopolysaccharide
|
|
BALF
|
bronchoalveolar lavage fluid
|
|
ROS
|
reactive oxygen species
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-6
|
interleukin-6
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
HO-1
|
heme oxygenase-1
|
|
PP
|
Physalis peruviana L.
|
References
|
1
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar
|
|
2
|
Wei J, Fan G, Zhao H and Li J: Heme
oxygenase-1 attenuates inflammation and oxidative damage in a rat
model of smoke-induced emphysema. Int J Mol Med. 36:1384–1392.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tamimi A, Serdarevic D and Hanania NA: The
effects of cigarette smoke on airway inflammation in asthma and
COPD: Therapeutic implications. Respir Med. 106:319–328. 2012.
View Article : Google Scholar
|
|
4
|
Lee J, Taneja V and Vassallo R: Cigarette
smoking and inflammation: Cellular and molecular mechanisms. J Dent
Res. 91:142–149. 2012. View Article : Google Scholar :
|
|
5
|
Afonso AS, Verhamme KM, Sturkenboom MC and
Brusselle GG: COPD in the general population: Prevalence, incidence
and survival. Respir Med. 105:1872–1884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Donnell R, Breen D, Wilson S and
Djukanovic R: Inflammatory cells in the airways in COPD. Thorax.
61:448–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JW, Shin NR, Park JW, Park SY, Kwon
OK, Lee HS, Hee Kim J, Lee HJ, Lee J, Zhang ZY, et al: Callicarpa
japonica Thunb. attenuates cigarette smoke-induced neutrophil
inflammation and mucus secretion. J Ethnopharmacol. 175:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee E, Yun N, Jang YP and Kim J: Lilium
lancifolium Thunb. extract attenuates pulmonary inflammation and
air space enlargement in a cigarette smoke-exposed mouse model. J
Ethnopharmacol. 149:148–156. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia-Rio F, Miravitlles M, Soriano JB,
Muñoz L, Duran- Tauleria E, Sánchez G, Sobradillo V and Ancochea J;
EPI-SCAN Steering Committee: Systemic inflammation in chronic
obstructive pulmonary disease: A population-based study. Respir
Res. 11:632010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou R, Luo F, Lei H, Zhang K, Liu J, He
H, Gao J, Chang X, He L, Ji H, et al: Liujunzi Tang, a famous
traditional Chinese medicine, ameliorates cigarette smoke-induced
mouse model of COPD. J Ethnopharmacol. 193:643–651. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jung KH, Beak H, Park S, Shin D, Jung J,
Park S, Kim J and Bae H: The therapeutic effects of tuberostemonine
against cigarette smoke-induced acute lung inflammation in mice.
Eur J Pharmacol. 774:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Victoni T, Gleonnec F, Lanzetti M, Tenor
H, Valença S, Porto LC, Lagente V and Boichot E: Roflumilast
N-oxide prevents cytokine secretion induced by cigarette smoke
combined with LPS through JAK/STAT and ERK1/2 inhibition in airway
epithelial cells. PLoS One. 9:e852432014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JW, Park HA, Kwon OK, Jang YG, Kim JY,
Choi BK, Lee HJ, Lee S, Paik JH, Oh SR, et al: Asiatic acid
inhibits pulmonary inflammation induced by cigarette smoke. Int
Immunopharmacol. 39:208–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ge LT, Liu YN, Lin XX, Shen HJ, Jia YL,
Dong XW, Sun Y and Xie QM: Inhalation of ambroxol inhibits
cigarette smoke-induced acute lung injury in a mouse model by
inhibiting the Erk pathway. Int Immunopharmacol. 33:90–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Tong D, Liu J, Chen F and Shen Y:
Oroxylin A attenuates cigarette smoke-induced lung inflammation by
activating Nrf2. Int Immunopharmacol. 40:524–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Li F, Ma R and Hu X:
Forsythiaside inhibits cigarette smoke-induced lung inflammation by
activation of Nrf2 and inhibition of NF-κB. Int Immunopharmacol.
28:494–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Osorio-Guarín JA, Enciso-Rodríguez FE,
González C, Fernández-Pozo N, Mueller LA and Barrero LS:
Association analysis for disease resistance to Fusarium oxysporum
in cape gooseberry (Physalis peruviana L). BMC Genomics.
17:2482016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu SJ, Tsai JY, Chang SP, Lin DL, Wang SS,
Huang SN and Ng LT: Supercritical carbon dioxide extract exhibits
enhanced antioxidant and anti-inflammatory activities of Physalis
peruviana. J Ethnopharmacol. 108:407–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abdel Moneim AE: Prevention of carbon
tetrachloride (CCl4)-induced toxicity in testes of rats treated
with Physalis peruviana L. fruit. Toxicol Ind Health. 32:1064–1073.
2016. View Article : Google Scholar
|
|
20
|
Al-Olayan EM, El-Khadragy MF, Omer SA,
Shata MT, Kassab RB and Abdel Moneim AE: The beneficial effect of
cape gooseberry juice on carbon tetrachloride-induced neuronal
damage. CNS Neurol Disord Drug Targets. 15:344–350. 2016.
View Article : Google Scholar
|
|
21
|
Lee JW, Park JW, Shin NR, Park SY, Kwon
OK, Park HA, Lim Y, Ryu HW, Yuk HJ, Kim JH, et al: Picrasma
quassiodes (D. Don) Benn. attenuates lipopolysaccharide
(LPS)-induced acute lung injury. Int J Mol Med. 38:834–844. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JW, Park JW, Kwon OK, et al: NPS2143
inhibits MUC5AC and proinflammatory mediators in cigarette smoke
extract (CSE)-stimulated human airway epithelial cells.
Inflammation. 2016.PubMed/NCBI
|
|
23
|
Sutherland ER and Martin RJ: Airway
inflammation in chronic obstructive pulmonary disease: Comparisons
with asthma. J Allergy Clin Immunol. 112:819–827; quiz 828. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kirkham PA and Barnes PJ: Oxidative stress
in COPD. Chest. 144:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rahman I: The role of oxidative stress in
the pathogenesis of COPD: Implications for therapy. Treat Respir
Med. 4:175–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnes PJ: Inflammatory mechanisms in
patients with chronic obstructive pulmonary disease. J Allergy Clin
Immunol. 138:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ng DS, Liao W, Tan WS, Chan TK, Loh XY and
Wong WS: Anti-malarial drug artesunate protects against cigarette
smoke-induced lung injury in mice. Phytomedicine. 21:1638–1644.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bodas M, Silverberg D, Walworth K, Brucia
K and Vij N: Augmentation of S-nitrosoglutathione controls
cigarette smoke-induced inflammatory-oxidative stress and chronic
obstructive pulmonary disease-emphysema pathogenesis by restoring
cystic fibrosis transmembrane conductance regulator function.
Antioxid Redox Signal. 27:433–451. 2017. View Article : Google Scholar
|
|
29
|
Gupta I, Ganguly S, Rozanas CR, Stuehr DJ
and Panda K: Ascorbate attenuates pulmonary emphysema by inhibiting
tobacco smoke and Rtp801-triggered lung protein modification and
proteolysis. Proc Natl Acad Sci USA. 113:E4208–E4217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen B, You WJ, Xue S, Qin H, Zhao XJ,
Zhang M, Liu XQ, Zhu SY and Jiang HD: Overexpression of farnesoid X
receptor in small airways contributes to epithelial to mesenchymal
transition and COX-2 expression in chronic obstructive pulmonary
disease. J Thorac Dis. 8:3063–3074. 2016. View Article : Google Scholar
|
|
31
|
Li D, Hu J, Wang T, Zhang X, Liu L, Wang
H, Wu Y, Xu D and Wen F: Silymarin attenuates cigarette smoke
extract-induced inflammation via simultaneous inhibition of
autophagy and ERK/p38 MAPK pathway in human bronchial epithelial
cells. Sci Rep. 6:377512016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li D, Xu D, Wang T, Shen Y, Guo S, Zhang
X, Guo L, Li X, Liu L and Wen F: Silymarin attenuates airway
inflammation induced by cigarette smoke in mice. Inflammation.
38:871–878. 2015. View Article : Google Scholar
|
|
33
|
Wu X, Song M, Rakariyatham K, Zheng J, Guo
S, Tang Z, Zhou S and Xiao H: Anti-inflammatory effects of
4′-demethylnobiletin, a major metabolite of nobiletin. J Funct
Foods. 19:278–287. 2015. View Article : Google Scholar
|
|
34
|
Yao W, Luo G, Zhu G, Chi X, Zhang A, Xia Z
and Hei Z: Propofol activation of the Nrf2 pathway is associated
with amelioration of acute lung injury in a rat liver
transplantation model. Oxid Med Cell Longev. 2014:2585672014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kwon YS,
Lee HJ, Kim SS and Chun W: 3,4,5-Trihydroxycinnamic acid inhibits
lipopolysaccharide (LPS)-induced inflammation by Nrf2 activation in
vitro and improves survival of mice in LPS-induced endotoxemia
model in vivo. Mol Cell Biochem. 390:143–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li RJ, Gao CY, Guo C, Zhou MM, Luo J and
Kong LY: The Anti-inflammatory activities of two major withanolides
from Physalis minima via acting on NF-κB, STAT3, and HO-1 in
LPS-stimulated RAW264.7 cells. Inflammation. 40:401–413. 2017.
View Article : Google Scholar
|
|
37
|
Domej W, Oettl K and Renner W: Oxidative
stress and free radicals in COPD - implications and relevance for
treatment. Int J Chron Obstruct Pulmon Dis. 9:1207–1224. 2014.
View Article : Google Scholar :
|