Introduction
Osteoporosis is a metabolic disease of bone,
characterized by bone mass decrease, bone microarchitecture
deterioration and increasing risk of fracture, since the bone
resorption by osteoclasts surpasses the bone formation by
osteoblasts. A variety of local and circulating hormones, cytokines
and intermediary metabolites may affect the number and activity of
both bone-forming osteoblasts and bone resorption osteoclasts
(1,2). Currently, there is a growing body of
study demonstrating that oxidative stress plays a crucial role in
the pathophysiology of osteoporosis (1,3–7).
Here, oxidative stress is caused when the production of reactive
oxygen species (ROS) exceeds the antioxidant defense capacity
(8). Abundant evidence has
demonstrated that ROS can elevate the receptor activator of nuclear
factor-κB ligand (RANKL) expression in mouse osteoblasts and in
human MG63 cells, and then stimulate the differentiation of
osteoclasts from its precursor and therefore result in increased
bone resorption (9–11). Accordingly, in the environment of
the bone, the elevated level of ROS leads to increased bone
resorption and impaired osteoblast function. That is, oxidative
stress indeed takes part in the regulation of bone mass and is the
chief culprit of osteoporosis (12).
In addition, ROS can trigger activation of specific
physiologic signaling pathways. Cells protect themselves against
the adverse effect of ROS by upregulating enzymatic scavengers or
expression of DNA damage repair genes. This reaction involves
dephosphorylation and subsequent activation of a small family of
ubiquitous transcription factors known as Forkhead box O (FoxOs)
(13–15). FoxOs, as important antioxidant
defense factors, promote oxidative stress resistance in mammals
(16,17). Rached et al reported that,
among the 4 FoxO proteins (FoxO1, FoxO3, FoxO4 and FoxO6), only
FoxO1 is required for proliferation and redox balance in
osteoblasts through cell-specific deletion and molecular analyses,
and as a result FoxO1 controls bone formation (12). FoxO1 activation is normally
restrained by the phosphatidylinositol-3-kinase (PI3K)/AKT
signaling pathway, which prevents FoxO1 translocation into the
nucleus, and it counteracts the generation of ROS by upregulating
the expression of antioxidant enzymes [glutathione peroxidase
(GSH-PX)] and superoxide dismutase (SOD) (18–20). In addition, the oxidative stress
induced by homocysteine deranges insulin-sensitive FoxO1 and MAP
kinase signaling cascades to decrease osteoproprotegrin (OPG) and
increase RANKL synthesis in osteoblast cultures, leading to the
stimulation of the osteoclast formation (21). Furthermore, Bartell et al
elucidated that macrophage colony-stimulating factor (M-CSF) and
RANKL promote the accumulation of H2O2 in
osteoclasts along with their progenitors via an AKT-mediated
repression of FoxO transcription which lowers catalase protein
level.
Resveratrol (3,5,4-trihydroxystilbene, RES), a
natural polyphenolic component extracted from red grapes, peanuts
and other plants, is known to exert many beneficial pharmacological
effects such as antitumor, scavenging free radicals,
anti-inflammation, cardioprotection and vasoprotection activities
(22–24). Clinical and experimental studies
have shown that RES prevents bone loss by attenuating the damage
caused by oxidative stress. RES can induce the production of major
cellular antioxidant enzymes, such as GSH-PX, heme oxygenase and
SOD, resulting in marked attenuation of oxida-tive stress (25,26). Significantly, RES was also shown
to inhibit mitochondrial production of ROS in the vasculature
(27). Furthermore, a previous
study confirmed that RES intake relieved alveolar bone resorption
and improved systemic oxidative stress in a rat periodontitis model
(28). Bhattarai et al
also demonstrated that RES treatment exhibited multiple beneficial
effects for suppressing alveolar bone loss and these effects were
the result of its antioxidative, anti-inflammatory, bone formation
stimulating, and anti-osteoclastogenic activity (29). It is believed that the bone
protective effect of RES is primarily due to its antioxidant
activity. More importantly, RES exhibited no toxic effects, and
therefore RES can be safely used for the treatment and/or
prevention of osteoporosis, even if used for a long time (30). However, the explicit molecular
mechanisms of how RES, as a natural antioxidant, plays a major role
in preventing bone loss, have not yet been well understood.
Additionally, our previous research revealed that
the effects of the FoxO1/β-catenin signaling pathway on the
proliferation and differentiation of osteoblasts are achieved
through its regulation of redox balance via the upregulation of
antioxidant enzyme levels as well as the expression of DNA damage
repair-related gene Gadd45, while inhibiting the expression of
apoptosis-related gene Bim along with the osteoblast inhibition
gene peroxisome proliferator-activated receptor-γ (PPAR-γ). On the
other hand, RES regulates the expression of FoxO1 by inhibiting the
PI3K/AKT signaling pathway to control white adipose tissue and then
ameliorate an overweight condition induced by a high fat diet.
Based on the above studies, we speculate that RES regulates FoxO1
transcriptional activity via the PI3K/AKT signaling pathway to
protect against oxidative damage in osteoporosis. In order to
further confirm this speculation, in the present study, we
established an ovariectomized (OVX) rat model of osteoporosis in
vivo and an osteoclast oxidative stress model induced by RANKL
and H2O2 in RAW 264.7 cells in vitro
to explore the underlying molecular mechanisms of how RES exhibits
an antioxidant effect and prevents bone loss. Implementation of
this project revealed the effect of RES against oxidative stress
damage together with its molecular mechanisms, and also provides a
new scientific basis for the clinical application of RES in the
prevention and/or treatment of osteoporosis.
Materials and methods
Ovariectomy and administration of
RES
Thirty female Sprague-Dawley (SD) rats, aged 3
months, body weight 220±19.27 g, were purchased from Gansu
University of Chinese Medicine and were equally divided into three
groups randomly. Ten rats were administered sham operation (control
rats; Sham group) and 20 rats underwent bilateral OVX under 10%
chloral hydrate anesthesia (OVX group). One week postoperative, all
rats were administered the medicine by subcutaneous injection: the
Sham group and OVX group received equal amount of sesame oil
(vehicle; Sigma-Aldrich, St. Louis, MO, USA); the third group
(OVX+RES group) received RES solution (40 mg/kg body weight, once
daily; Sigma-Aldrich). After treatment for 10 weeks, the rats were
sacrificed, and the blood along with the bone were collected for
further analyses. All experiments performed involving SD rats were
approved by the Animal Experimental Ethical Inspection of Gansu
University of TCM (permission code 2015–060).
Bone μ-CT scanning
We dissected the femurs, cleaned the soft tissues,
fixed them in 10% neutral formalin for 48 h, and then immersed them
into 70% ethanol. Bone microarchitecture in the middle and distal
femur was scanned by using micro-computed tomography (μ-CT;
VivaCT 40; Scanco, Brüttisellen, Switzerland) with 15 μm
resolution, tube voltage of 70 kV and tube current of 114
μA. The reconstruction and 3D quantitative analyses were
performed using software provided by a desktop μ-CT system
(VivaCT 40; Scanco). In the femur, cortical bone volume (Ct.Vo) and
cortical thickness (Ct.Th) were analyzed with the help of the
reference point at the mid-diaphysis, with whole cortical bone,
where the region of interest (ROI) was 1.0 mm. Trabecular bone
region reference point was at the growth plate joining, offset 2.0
mm, ROI 2.0 mm. The following 3D parameters in the defined ROI were
analyzed, including the relative bone volume over total volume
(BV/TV, %), trabecular number (Tb.N), trabecular thickness (Tb.Th),
trabecular separation (Tb.Sp), connectivity density (Conn.D),
structure model index (SMI) and bone mineral density (BMD).
Bone histomorphometric analysis
The right isolated tibias maintained in 70% alcohol
were used for the bone shape analysis. The processes of alcohol
dehydration, xylene transparency undecalcified plastic embedding,
sectioning (thickness of 30 μm) followed by Van Gieson
staining were included. Bone morphological measurement indices were
analyzed using IPP 6.0 analysis software.
Enzyme-linked immunosorbent assay (ELISA)
measurement
Rat OPG, RANKL and tartrate-resistant acid
phosphatase-5b (TRAP-5b) ELISA kits as well as an ROS ELISA kit
were purchased from Myhalic Biotechnology Co., Ltd. (http://www.myhalic.com/; Wuhan, China). MDA, SOD and
GSH-PX were measured by employing specific kits (Jiancheng,
Nanjing, China) following the manufacturer's instructions.
Cell culture
The mouse macrophage cell line RAW 264.7, purchased
from the American Type Culture Collection (ATCC, TIB-71™; Manassas,
VA, USA), was cultured in high-glucose Dulbecco's modified Eagle's
medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal
bovine serum (FBS; TAN, South America) in a 25 cm2
culture flask at 37°C in a 5% CO2 incubator. Culture
media were changed every other day. Cells were incubated in an
osteoclastic induction medium supplemented with 100 ng/ml RANKL
(315–11; PeproTech, Rocky Hill, NJ, USA) for 7 days. This cell line
has been shown to express receptor activator of NF-κB (RANK) and
differentiate into TRAP-positive, functional osteoclasts when
co-cultured with soluble RANKL (31,32). The RAW 264.7 cells were cultured
to 80% confluence, and the medium was replaced according to the
specific group. The cultured cells were randomly divided into four
groups: the RANKL control group, 10−4 M
H2O2 group, 10−5 M RES group, and
10−4 M H2O2 plus 10−5 M
RES group. We would like to stress that in the latter three groups,
the cells were also incubated in an osteoclastic induction medium
supplemented with 100 ng/ml RANKL. H2O2 was
added 1 h prior to treatment with RES. All of the experiments were
repeated 3 or 5 times.
Cytotoxicity assay
The cytotoxic effects of RES or
H2O2 were detected using Cell Counting kit-8
(CCK-8; Jiancheng), according to the manufacturer's instructions.
Briefly, 8,000 cells were seeded per well on a 96-well plate. The
cells were treated with 10−5 M RES or 10−4 M
H2O2 and cultured for 24, 48 and 72 h,
respectively, to measure the toxicity at 450 nm using a microplate
reader.
TRAP staining
After induction of culture for 7 days, TRAP staining
was performed to evaluate TRAP(+)-multinucleated osteoclast
formation. RAW 264.7 cells were cultured on a 24-well plate at a
density of 2×104 cells/well and allowed to adhere
overnight. DMEM was then replaced, and the cells were treated with
new DMEM containing 100 ng/ml RANKL. After induced culture for 7
days, the cells were stained for TRAP expression using an Acid
Phosphatase, Leukocyte (TRAP) kit (387-A; Sigma-Aldrich) following
the manufacturer's instructions.
Assessment of the culture supernatant
MDA, SOD and GSH-PX
We harvested the culture supernatant after the RAW
264.7 cells were cultured as mentioned in the different groups in
the induction medium for 24 h on 96-well plates at a density of
8,000 cells/well, and we assessed the MDA, SOD and GSH-PX by
employing the relevant kits (Jiancheng) according to the
manufacturer's instructions.
Intracellular ROS formation
The generation of ROS was probed with the ROS
fluorescent probe-dihydroethidium (DHE). Cells were plated on
6-well plates at 6×104 cells/well, and were
induced-cultured in terms of the above groups for 24 h. After this
treatment, the cells were washed with PBS and then incubated with
20 μM DHE for 30 min at 37°C. DHE is oxidized by ROS when it
diffuses into the cells, and then the intracellular oxidized DHE
was measured via flow cytometry.
Real-time PCR
Cells were plated in a 25 cm2 flask at
2×105 cells/flask, and induced-cultured in regards to
the above groups for 7 days. Total RNA was extracted from the
cultured cells applying RNAiso™ Plus (cat. no. 9108; Takara, Tokyo,
Japan). The RNA concentration was determined
spectrophotometrically, and only pure RNA (A260:A280 ratio in
1.8–2.1) was used for further analysis. Total RNA (5 μl)
(500 ng) from each sample was reverse-transcribed using
PrimeScript™ RT Master Mix (perfect real-time) (code: RR036A;
Takara). Complementary DNA was mixed with SYBR Premix Ex Taq™ II
(Tli RNaseH Plus) (code: RR820A; Takara) and then was subjected to
PCR amplification using the LightCycler/LightCycler 480 system
real-time PCR. Denaturation was carried out at 95°C for 30 sec
(ramp rate 4.4°C/sec) 1 cycle; PCR (quantification) at 95°C for 5
sec (ramp rate 4.4°C/sec) and 60°C for 30 sec (ramp rate 2.2°C/sec)
40 cycles; melting (melting curves) was performed at 95°C for 5 sec
(ramp rate 4.4°C/sec) followed by 60°C for 60 sec (ramp rate
2.2/sec) and 95°C (ramp rate 0.11°C/sec, acquisition mode:
continuous, acquisitions: 5 per) 1 cycle; and cooling was carried
out at 50°C for 30 sec [ramp rate 2.2°C/sec) 1 cycle]. The primers
used for real-time PCR are listed in Table I.
 | Table IRT-PCR primers employed for
amplification of specific mRNAs. |
Table I
RT-PCR primers employed for
amplification of specific mRNAs.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| Actb |
CATCCGTAAAGACCTCTATGCCAAC |
ATGGAGCCACCGATCCACA |
| MMP-9 |
CCATGCACTGGGCTTAGATCA |
GGCCTTGGGTCAGGCTTAGA |
| TRAP |
GTGCTGGCTGGAAACCATGA |
GTCCAGCATAAAGATGGCCACA |
| Cathepsin K |
CACCCAGTGGGAGCTATGGAA |
GCCTCCAGGTTATGGGCAGA |
Western blot analysis
The cells were plated in a 25 cm2 flask
with 2×105 cells/flask, and induced-culture was carried
out in terms of the above groups for 7 days. Cells were lysed using
a lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1%
Triton X-100, 1% sodium dexycholate, 0:1% SDS, sodium
orthovanadate, sodium fluoride, EDTA, and leupeptin inhibitor (code
P0013B; Beyotime, Shanghai, China). The lysates (20–30 μg)
were separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to nitrocellulose
blotting membranes. After blocking with 5% skim milk (detection of
phosphorylated proteins with BSA) for 1 h at room temperature, the
membranes were incubated with anti-β-actin (1:5,000; ab6276),
anti-AKT (1:500; ab28422), anti-p-AKT (1:500; ab8932), anti-FoxO1
(1:1,000; ab52857), and anti-p-FoxO1 (1:500; ab131339) (all from
Abcam, Cambridge, UK) antibodies overnight at 4°C, and then were
incubated with the secondary antibody (1:1,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 1 h at 37°C.
Analysis of apoptosis
Cells were plated on 6-well plates at
6×104 cells/well, and cultured in terms of the above
groups for 3 days. After this treatment, cells were washed with
phosphate-buffered saline (PBS) and then apoptosis was assessed
using the Annexin V/PI double staining assay via flow
cytometry.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). The values were analyzed by employing a one-way ANOVA of
variance followed by a LSD test for multiple comparisons. The
statistical significance was defined as P<0.05. Error bars in
all figures represent SD.
Results
RES prevents the deterioration of
trabecular bone micro-architecture induced by OVX
One week postoperative, we administered RES (40
mg/kg body weight, once daily) to OVX rats for 10 weeks to examine
its protective effects against bone loss. The animals were all
sacrificed 11 weeks after the operation. When compared with the
Sham group rats, OVX significantly induced deterioration of the
trabecular bone microarchitecture, and reduction in BV/TV, Tb.N,
Tb.Th, Conn.D and BMD (P<0.05 and P<0.01) (Table II). In contrast, other
microstructural parameters such as Tb.Sp and SMI were sharply
increased in response to OVX (P<0.01). However, treatment of OVX
rats with RES at 40 mg/kg body weight markedly reversed changes in
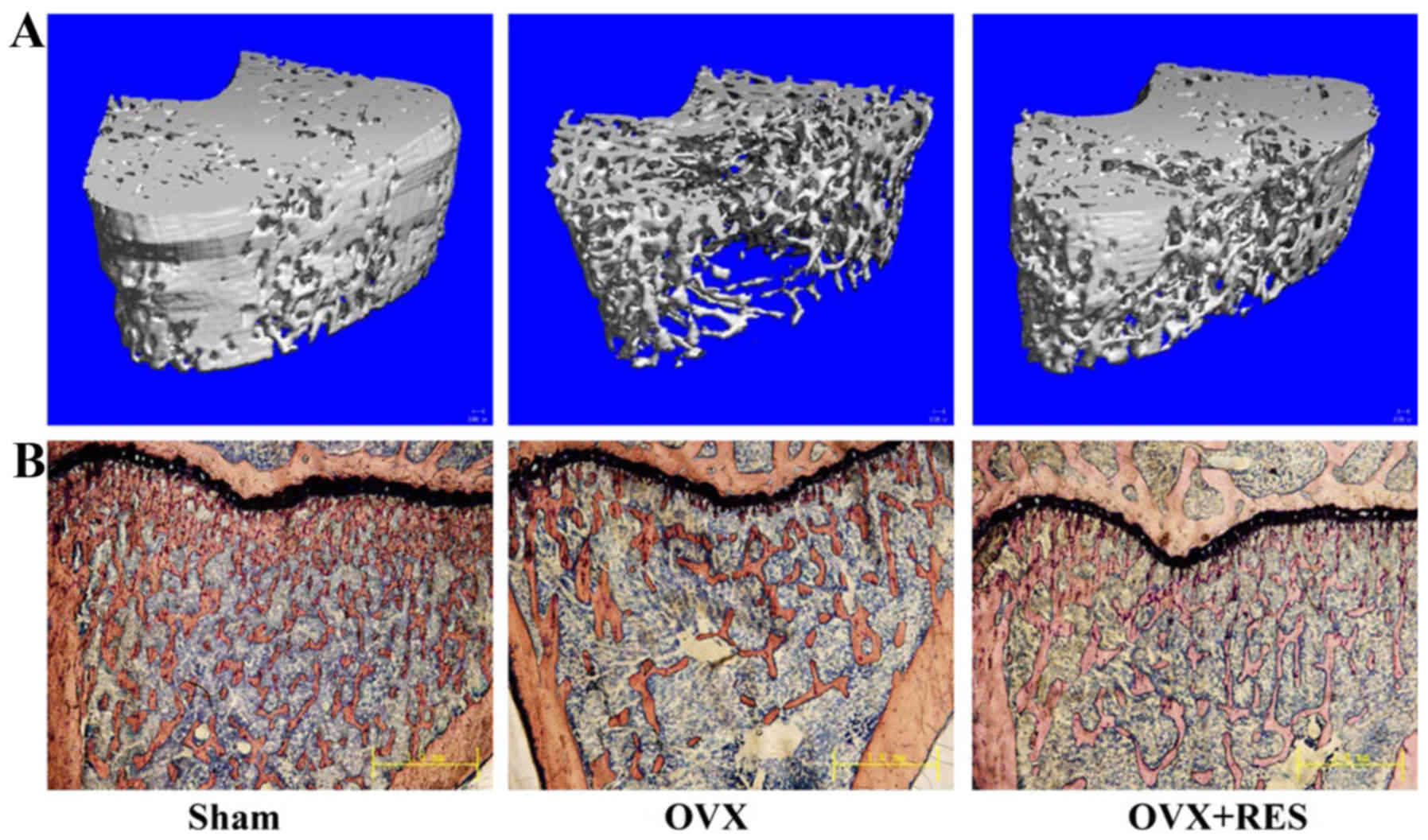
these parameters induced by OVX. In μ-CT images of the
distal femur, these changes in trabecular bone parameters were
readily observed (Fig. 1A). Ct.Vo
and Ct.Th as determined by μ-CT were also analyzed (Table II). OVX did not affect the
cortical bone volume and thickness of the femur mid-diaphysis
(P>0.05). Simultaneously, bone histomorphology of the right
tibias detected using Van Gieson staining were carried out and also
exhibited similar results (Fig.
1B). The trabecular number was decreased and spaces between
trabecules were broader in the OVX group compared with the Sham
group. Nevertheless, treatment with RES significantly inhibited the
OVX-induced deleterious effects, as demonstrated in the OVX+RES
group via an increase in the trabecular number and a decrease in
the trabecular space. The analysis of the properties of trabecular
and cortical bone in the middle and distal femurs indicated that
OVX induces damage of the trabecular bone micro-architecture in
rats, yet RES was able to attenuate the damage effects.
 | Table IITrabecular microstructural and
cortical geometric properties of the right femurs evaluated ex
vivo using μ-CT. |
Table II
Trabecular microstructural and
cortical geometric properties of the right femurs evaluated ex
vivo using μ-CT.
| Parameters | Sham | OVX | OVX+RES |
|---|
| BV/TV (%) | 47.48±6.00 | 20.88±7.53b | 36.14±8.62a,c |
| Tb.N (1/mm) | 4.90±0.61 | 2.94±0.79b | 4.79±0.26d |
| Tb.Th (µm) | 81.43±16.64 | 51.80±3.74a | 78.67±14.76c |
| Tb.Sp (mm) | 0.13±0.03 | 0.24±0.04b | 0.13±0.02d |
| Conn.D
(1/mm3) | 155.59±22.56 |
108.78±18.59b |
144.86±28.12c |
| SMI | 0.42±0.13 | 1.87±0.27b | 1.23±0.20b,d |
| BMD (mg
HA/ccm) | 732.42±15.68 |
693.79±11.46a | 729.66±4.81c |
| Ct.Vo
(mm3) | 4.86±0.64 | 5.18±0.50 | 5.52±0.87 |
| Ct.Th (mm) | 2.82±0.90 | 2.72±0.27 | 2.86±1.17 |
RES suppresses osteoclast activity and
formation
In order to determine the possible regulatory
effects of RES on osteoclast activity and formation, we examined
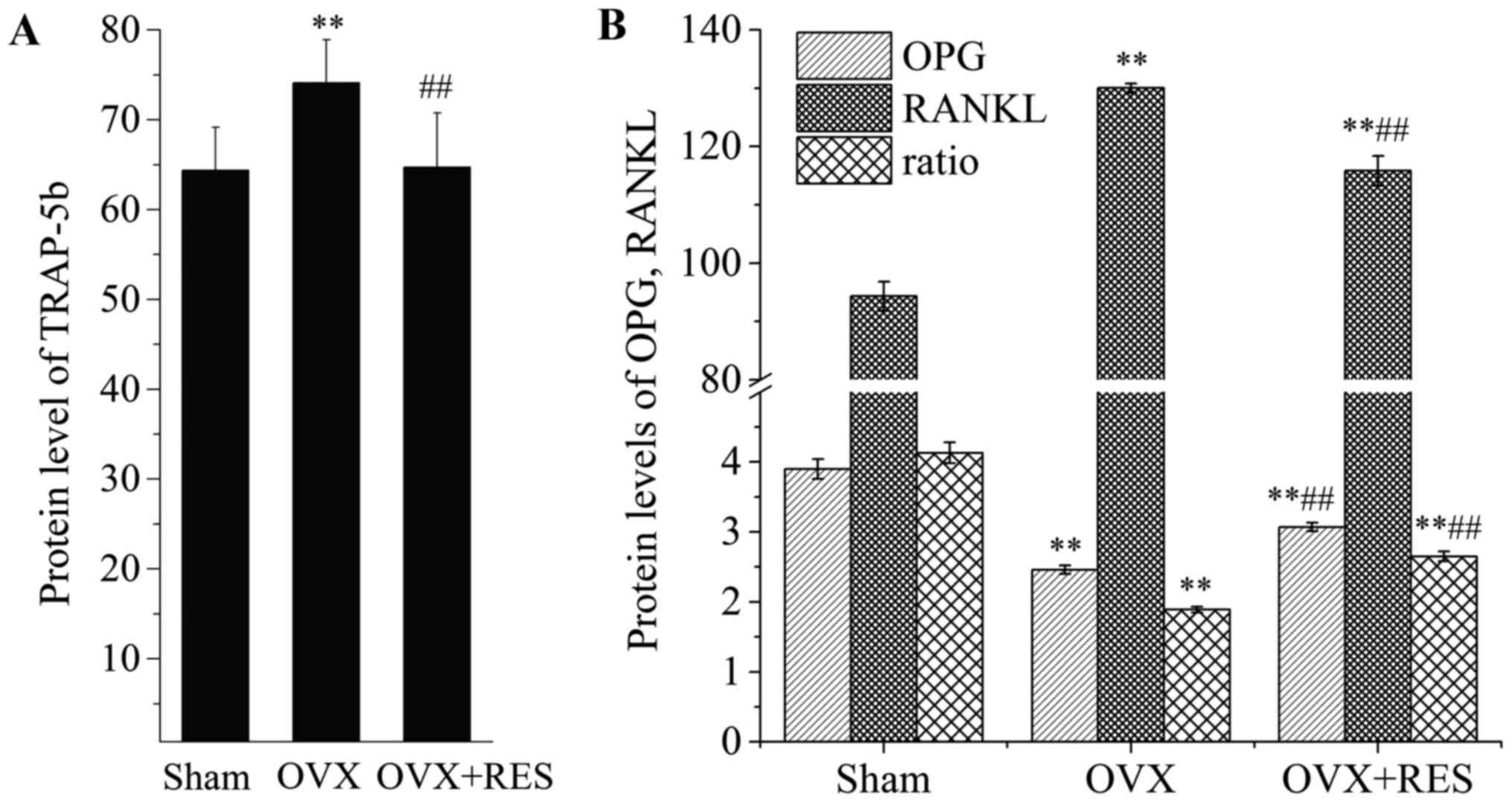
the production of TRAP-5b, OPG and RANKL in the serum of rats.
TRAP-5b is released by osteoclasts, reflecting osteoclast activity
and the bone resorption status. Upon comparison with the Sham
group, the TRAP-5b content in the serum was increased following
OVX. Yet, as shown in Fig. 2A,
RES markedly reversed the change (P<0.01). OPG and RANKL are
primarily released from osteoblasts. Osteoblast-derived RANKL binds
to RANK on osteoclasts, resulting in osteoclast activation. OPG,
osteoclastogenesis inhibitory factor, has also been demonstrated to
compete with RANKL for binding to RANK, leading to suppression of
osteoclast formation. Thus, osteoclast formation is reflected by
the ratio of OPG/RANKL. Compared with the Sham group, as shown in
Fig. 2B, the level of OPG was
reduced, while RANKL was enhanced, and OPG/RANKL was significantly
decreased in response to OVX (P<0.01). However, RES intervention
recovered at least part of the changes (P<0.01) in the
parameters induced by OVX. Our results revealed that, under OVX,
osteoclast activity and formation were enhanced, while RES greatly
weakened the OVX effects.
RES improves the antioxidant power in the
rats
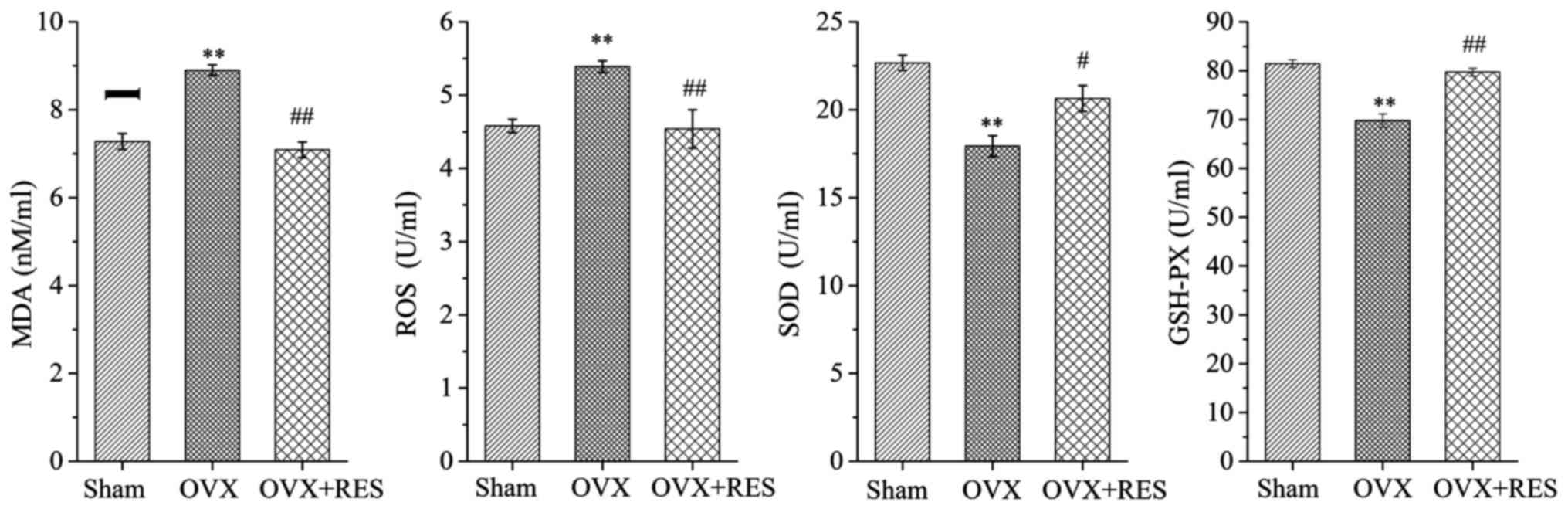
To further confirm the fact that bone protective
effects of the RES are primarily caused by antagonizing the
OVX-induced oxidative stress damage, the levels of MDA, ROS, SOD
and GSHPX in the serum of rats were determined. Analysis of the
oxidative stress status of the experimental rats indicated that the
Sham group also generated traces of MDA and ROS. When rats
underwent the OVX operation, the MDA and ROS concentrations were
increased, while the contents of SOD and GSH-PX were reduced, as
compared with the Sham group. Upon treatment with RES to the OVX
rats, changes were also observed when compared to the OVX group;
that is, MDA and ROS levels were highly decreased while the
antioxidant enzymes such as SOD and GSH-PX were markedly elevated
(P<0.05 and P<0.01) (Fig.
3). On the whole, these findings indicated that OVX induced
oxidative stress, resulting in a lowered OPG level but enhanced
RANKL and TRAP-5b levels, and an increase in osteoclast formation
together with activity, and eventually bone microarchitecture
damage. Notably, RES enhanced the OPG content, lowered the RANKL
and TRAP-5b levels, and finally reduced bone resorption by
improving the oxidative stress status. Subsequently to explore the
hidden molecular mechanisms of the bone protective effects of RES,
we stimulated the RAW 264.7 cells with RANKL to induce osteoclasts
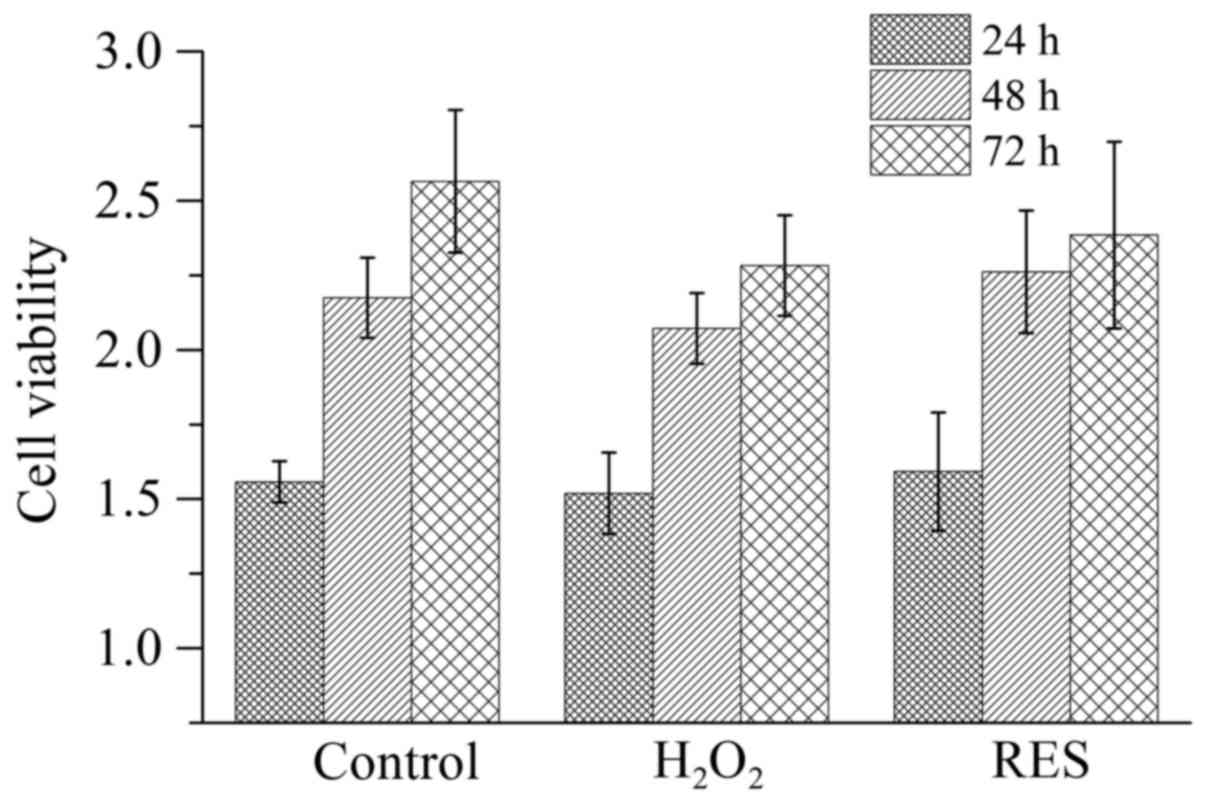
in vitro. Before detecting the effects of
H2O2 and RES on osteoclast activity along
with function, the cell viability (Fig. 4) was determined. The potential
cytotoxicity of these agents was tested since their toxicity would
influence the cell survival and consequently lower the cell number.
Either 10−4 M H2O2 or
10−5 M RES did not have a cytotoxic effect on RAW 264.7
cells (P>0.05).
RES reduces the expression of
osteoclast-related marker enzymes
In order to evaluate the osteoclast activity in
vitro, the expression of osteoclast marker enzymes was assessed
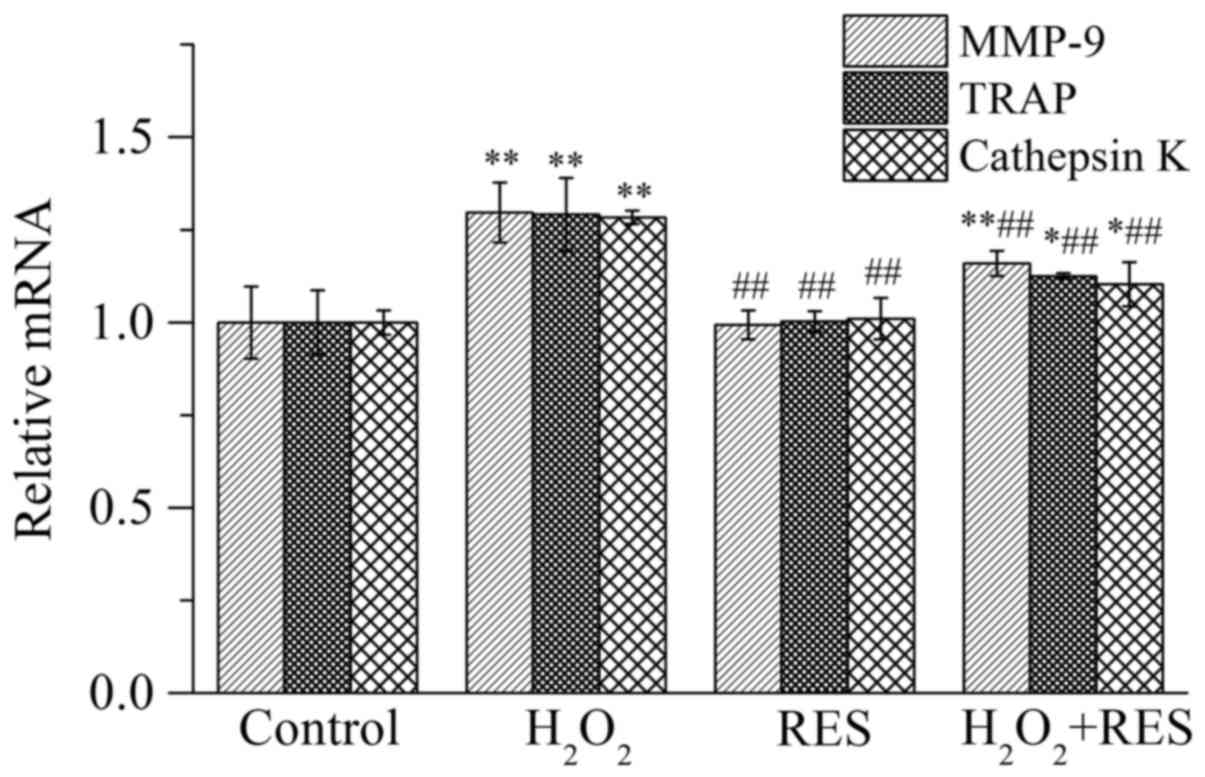
by employing RT-PCR, including matrix metalloproteinase-9 (MMP-9),
TRAP, and cathepsin K. Expressed specifically by osteoclasts, these
three enzymes are important indices which reflect osteoclast
activity as well as bone resorption function. As shown in Fig. 5, compared with the control group,
the mRNA expression levels of MMP-9, TRAP and cathepsin K were
increased (P<0.01) in the presence of 10−4 M
H2O2, indicating that osteoclast activity
along with bone resorption function was facilitated by
H2O2. In contrast, following treatment of the
cells with RES at 10−5 M, the expression levels of the
osteoclast marker enzymes were reduced compared with the
H2O2 group (P<0.01), indicating that RES
inhibited osteoclast activity and bone resorption capacity.
RES abrogates oxidative stress in
cells
To elucidate whether the inhibitory effects of RES
on osteoclast activity and bone resorption power are linked to its
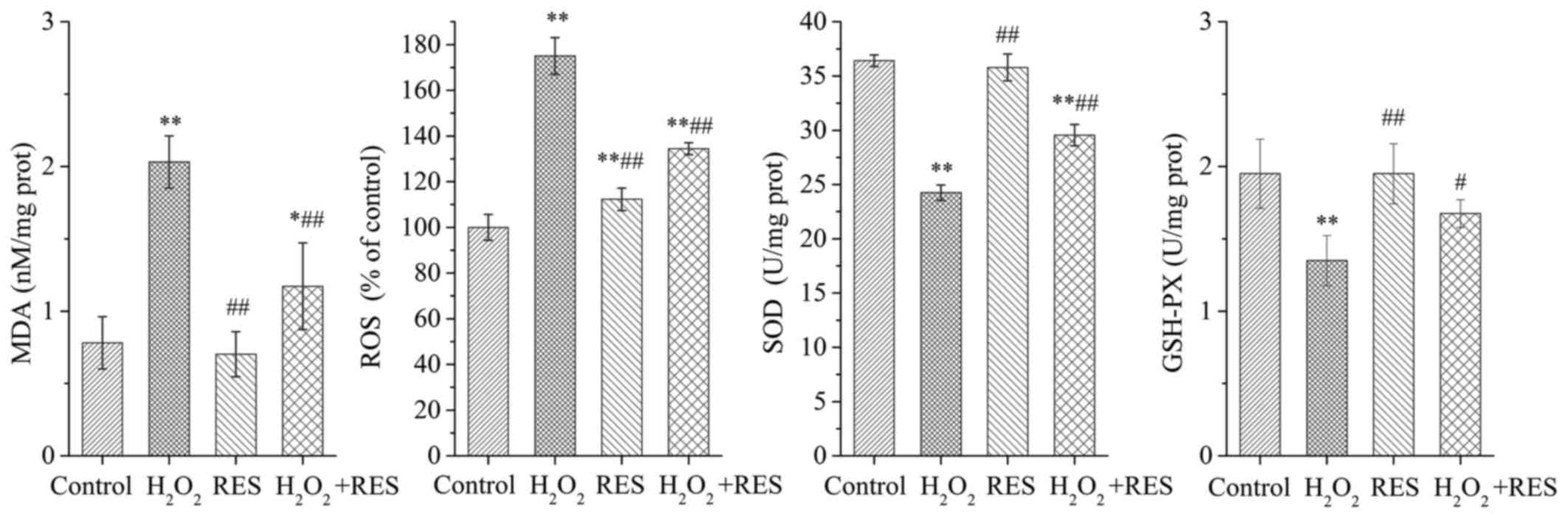
antioxidant properties, the MDA, ROS, SOD and GSH-PX levels were
detected. As shown in Fig. 6,
when RAW 264.7 cells were exposed to 10−4 M
H2O2, the production of MDA and ROS was
strongly enhanced, while the SOD and GSH-PX levels were decreased
(P<0.01). The above results demonstrated that
H2O2 stimulated oxidant generation and led to
oxidative stress in RAW 264.7 cells. However, when the cells were
treated with RES at a concentration of 10−5 M, MDA and
ROS production was inhibited to some degree, while the SOD and
GSH-PX levels were higher compared with the
H2O2 group (P<0.05 and P<0.01). The
study revealed that RES improves the oxidative stress status of the
cells, and the suppressive effects of RES on the expression of
osteoclast marker enzymes are linked to its antioxidant
activity.
RES regulates FoxO1 transcriptional
activity by inhibiting the PI3K/AKT signaling pathway
The PI3K/AKT/FoxO1 signaling pathway plays a crucial
role in osteoclastogenesis. Furthermore, to confirm the
RES-attenuated oxidative stress damage and RES-suppressed
osteoclastogenesis by inhibiting the PI3K/AKT signaling pathway, we
evaluated the effects of RES on the PI3K/AKT/FoxO1 signaling
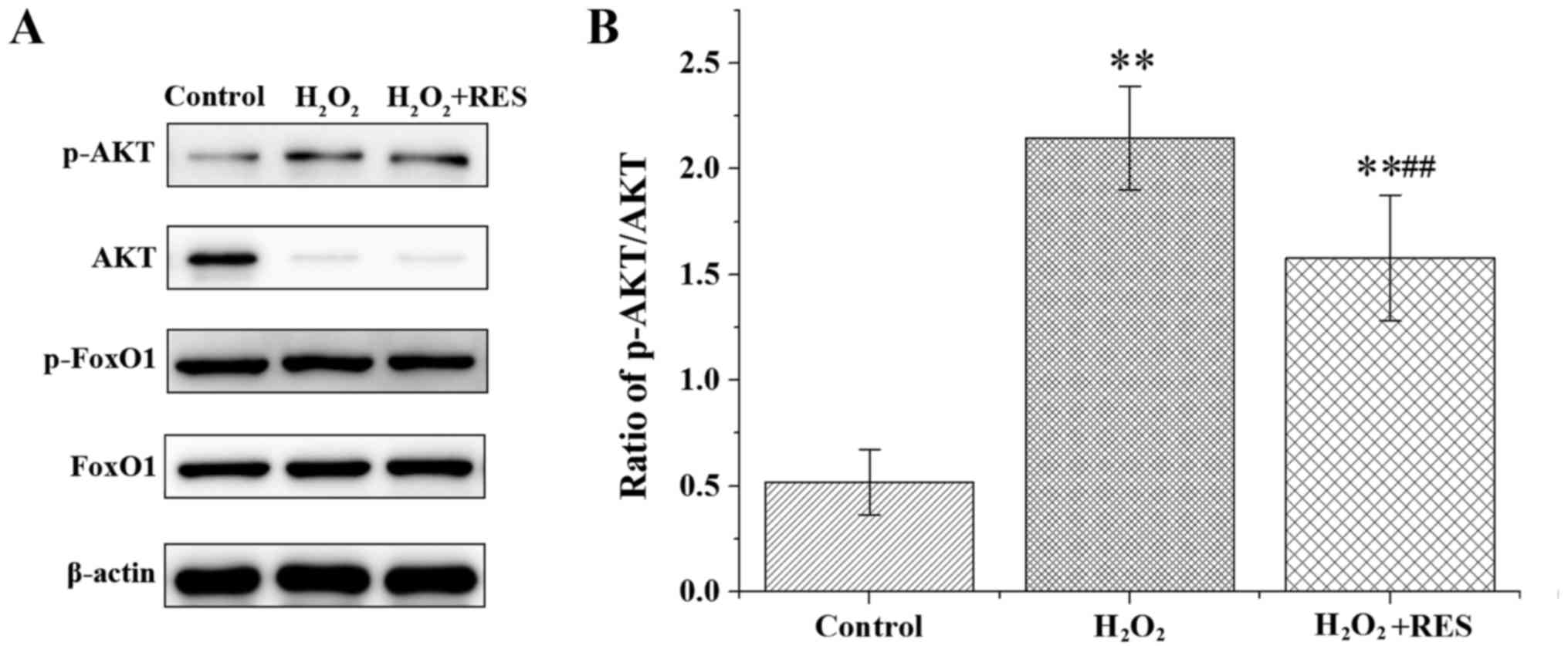
pathway following stimulation with 100 ng/ml RANKL in RAW 264.7
cells. AKT, phosphorylated AKT (p-AKT), FoxO1 and p-FoxO1 were
examined by western blot analysis. Compared with the control group,
H2O2 elevated the ratio of p-AKT/AKT and the
protein content of p-FoxO1, yet the FoxO1 protein level was reduced
(P<0.05 and P<0.01) (Fig.
7). According to these results, FoxO1 transcriptional activity
was suppressed by H2O2. Following treatment
with RES, the above effects of H2O2 were
abolished, at least in part (P<0.05 and P<0.01). These data
indicate the RES was able to upregulate FoxO1 transcriptional
activity by inhibiting the PI3K/AKT signaling pathway. In brief,
our results revealed that RES upregulated FoxO1 transcriptional
activity to achieve attenuation of oxidative stress damage and
inhibition of osteoclastogenesis.
RES regulates the apoptosis of RAW 264.7
cells
The inhibition of the PI3K/AKT signaling parthway
leads to dephosphorylation and nuclear translocation of active
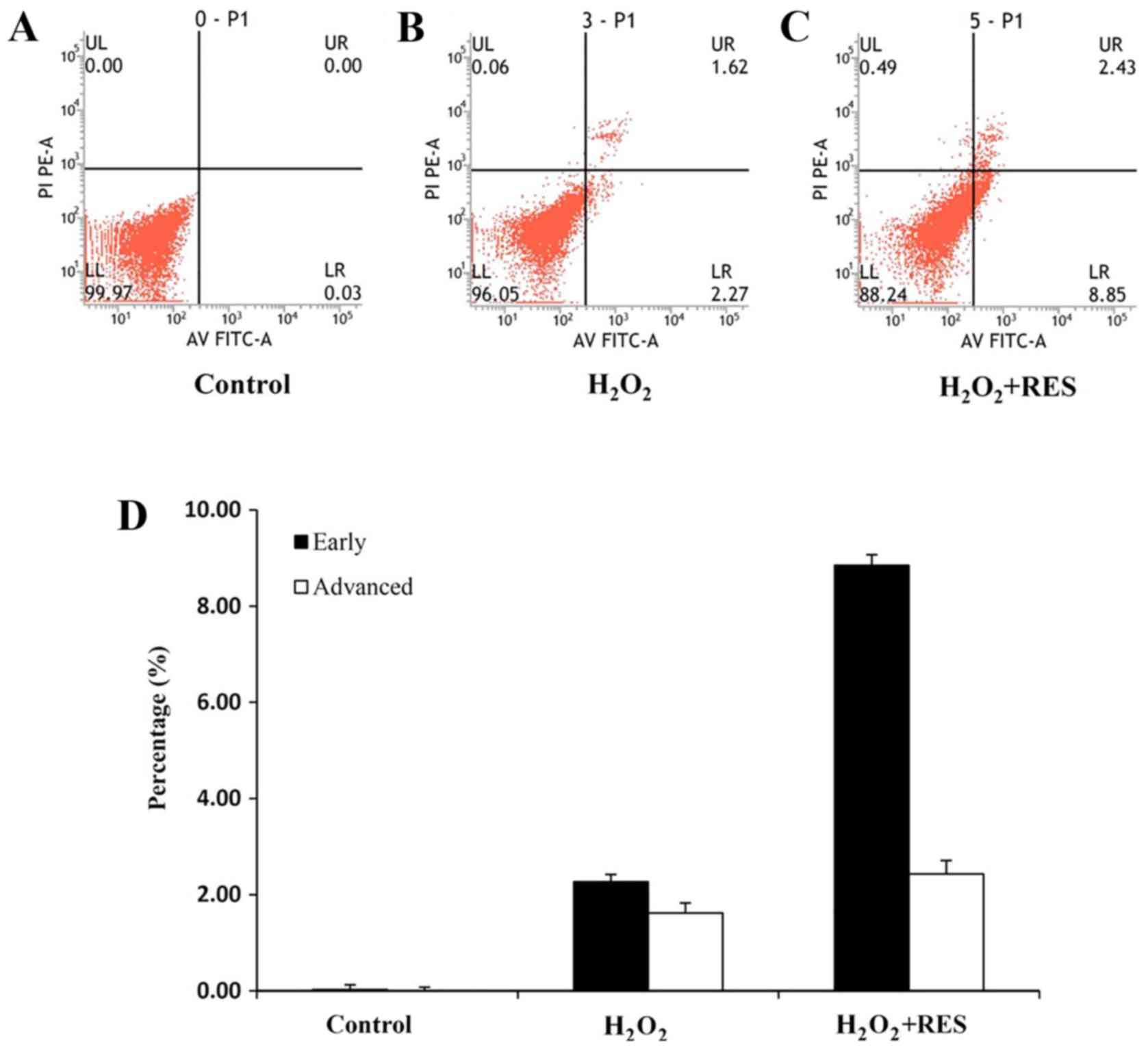
FoxO1, which causes cell cycle arrest and apoptosis (33). Finally, we examined whether RES
induces apoptosis by inhibiting the PI3K/AKT signaling pathway.
Cells were cultured by adding H2O2 or
H2O2+RES for 3 days. After this treatment,
apoptosis was determined. Treatment of RAW 264.7 cells with
10−5 M RES promoted apoptosis (P<0.01) (Fig. 8). Based on the data, it was
revealed that RES induced apoptosis and as a result restrained
osteoclastogenesis via suppressing the PI3K/AKT signaling
pathway.
Discussion
RES, a natural polyphenolic component, is known to
exert numerous beneficial pharmacological effects including
antitumor, scavenging free radical and anti-inflammatory activities
(22–24). Clinical and experimental
investigations suggest that RES prevents bone loss by attenuating
the damage of oxidative stress (25,26). Furthermore, a previous study of
our group demonstrated that OPG production was boosted, whereas
RANKL synthesis was decreased in RES-treated OVX rats, and hence
consequently osteoclast formation and differentiation were
prevented (34). More
importantly, RES had no toxic effect and therefore can be safely
used even for the long-term treatment and/or prevention of
osteoporosis (30). However, the
underlying molecular mechanisms of how RES, a natural antioxidant,
plays a major role in preventing bone loss, has not yet been fully
elucidated. In the present study, we explored the potential
molecular mechanisms of RES against osteoporosis.
Our results demonstrated that, by improving the
oxidative stress status, RES enhanced the ratio of OPG/RANKL
(namely, OPG enhancement vs. RANKL decrease), suppressed
osteoclastogenesis, and thus eventually attenuated bone resorption
and prevented bone loss in vivo. Simultaneously, in
vitro, the activity along with the function of osteoclasts was
facilitated following exposure to 10−4 M
H2O2, but these were inhibited in the
presence of RES owing to its effect of relieving the oxidative
stress damage. Apart from the above findings, RES was able to
induce apoptosis of RAW 264.7 cells and thus restrained
osteoclastogenesis. Moreover, at the molecular level, we confirmed
that RES upregulated the transcriptional activity of FoxO1 by
inhibiting the PI3K/AKT signaling pathway, and caused protection
against oxidative damage and inhibited osteoclastogenesis in
osteoporosis.
Oxygen-derived free radicals are produced as
by-products of aerobic metabolism. This process occurs primarily in
mitochondria due to electron escape passing through the electron
transport chain (35,36), and generates highly reactive and
short-lived superoxide that is rapidly converted to the more stable
and less reactive H2O2 (37–39). H2O2, as the
most abundant form of ROS, diffuses freely through the
mitochondrial membranes into the cytosol (37–39). Oxidative stress is the result of
elevated ROS, which damages protein, lipids, and DNA and eventually
triggers cell death. H2O2 additionally serves
usually as both an extracellular and intercellular signal molecule
(40). In the present study,
10−4 M H2O2 was used to induce
oxidative stress.
RANKL and its receptor, pivotal factors required for
osteoclast differentiation, are fundamental and necessary to
promote osteoclastogenesis (41).
RANKL plays a dominant role in activation of the osteoclast
differentiation program, including the necessary genes required for
bone resorption and for fusion of monocyte progenitor cells
(42). When it binds to RANK, it
triggers several intracellular signaling pathways in osteoclast
precursor cells, ultimately inducing the expression of
osteoclast-specific genes. OPG, as an inhibitor of RANKL, also
binds to RANK to antagonize the effect of RANKL and thereby
regulates osteoclast activity and function in the bone. We here
employed the mouse macrophage cell line, namely, RAW 264.7 cells,
and stimulated the cells with 100 ng/ml RANKL to induce
osteoclastogenesis (32).
There have been several previous studies providing
key evidence that ROS take part in bone regulation. ROS, especially
H2O2, may be involved in the regulation of
osteoclast formation (43), and
has been observed both in vitro and in vivo to be
produced by osteoclasts (44–46). On the other hand,
H2O2 production in differentiated osteoclasts
can also be stimulated by RANKL (47). Bartell et al found that
RANKL promotes the accumulation of H2O2 in
osteoclasts and in their progenitors. In turn,
H2O2 improves osteoclast progenitor
proliferation (11). Furthermore,
Kim et al provided evidence that ROS play an important role
in osteoclast differentiation through NF-κB regulation, while the
antioxidant α-lipoic acid inhibits osteoclast differentiation by
reducing NF-κB DNA binding and has a potential therapeutic effect
against bone erosive diseases (48). Additionally, ROS enhanced the
expression of RANKL in mouse and human MG63 cells (9). Therefore, the increased ROS
(particularly the H2O2) level is a critical
regulatory-step of osteoclastogenesis and hence bone resorption,
which is not a mere epiphenomenon of increased mitochondria number
and/or function required to meet the high-energy demands of
osteoclastic bone resorption (11).
Our findings showed that OVX induced oxidative
stress (ROS and MDA increased, yet SOD and GSH-PX decreased in the
OVX group, compared with the Sham group), and simultaneously
promoted a decrease in the OPG level, an increase in the RANKL
level, increased production of TRAP-5b and damaged bone
microstructure. TRAP-5b in the serum, as a bone resorption marker
enzyme, released by osteoclasts, well reflects the osteoclast
activity directly along with bone resorption status in vivo.
Meanwhile, in vitro, 10−4 M
H2O2 used to induce oxidative stress resulted
in the enhanced expression levels of osteoclast-specific enzymes
(MMP-9, TRAP and cathepsin K), indicating that osteoclast activity
and function were elevated. In contrast, the treatment of the OVX
rats with RES significantly reversed these changes in vivo.
RES, conferring antioxidant power, effectively decreased RANKL
together with the TRAP-5b level, but elevated the OPG level and
attenuated bone microarchitecture damage. Notably, the results
demonstrated that RES, due to its antioxidant effect, suppressed
the RANKL production in the OVX rats. In other words, the increased
ROS level promoted the production of RANKL and then stimulated
osteoclast differentiation and bone resorption. This conclusion is
consistent with previous studies (9,11,47,48). Subsequently, the in vitro
study demonstrated that RES at the concentration of 10−5
M improved the oxidative stress status of cells and inhibited the
expression of osteoclast-specific enzymes. These data indicate that
RES has a significant bone protective effect via antagonizing
oxidative stress to suppress osteoclast formation together with
bone resorption activity both in vitro and in vivo.
Therefore, the reduction of ROS production may be a rational
approach for the treatment of diseases associated with high bone
resorption, including, for example, osteoporosis and arthritis.
FoxO1 activation in osteoclasts could be one valid approach to
achieve this goal. Notably, an important finding of this study is
that the redox regulator FoxO1 is a target of RES resisting
oxidative stress and inhibiting osteoclastogenesis.
As important protein resistance to oxidative stress,
FoxOs regulate the expression of antioxidant enzymes, where FoxO1
is a major member of the FoxO family. Through cell-specific
deletion and molecular analyses, among the 4 FoxO proteins, FoxO1
is the only factor required for proliferation and redox balance in
osteoblasts, and as a result FoxO1 controls bone formation
(12). FoxO1 is regulated
predominantly through the PI3K/AKT signaling pathway and the FoxO1
protein is phosphorylated by the PI3K/AKT pathway, leading to
inhibition of FoxO1-dependent transcription and impaired ability of
DNA binding (49). AKT has been
shown to directly phosphorylate and inactivate FoxO1, which results
in cytoplasmic retention, inactivation and inhibition of the
expression levels of FoxO1-regulated genes that control various
processes such as metabolism, cell cycle, cell death and oxidative
stress (50). In contrast,
inhibition of the PI3K/AKT pathway induces dephosphorylation and
nuclear translocation of active FoxO1, and then these processes
enhance FoxO1 transcriptional activity, causing cell cycle arrest
and apoptosis (33). In
hematopoietic stem cells, FoxO1 has been demonstrated to reduce ROS
by upregulating the expression of antioxidant enzymes including
peroxiredoxins, GSH-PX and catalase (19,20). After the deletion of FoxO1 in
cells of the hematopoietic lineage, the number of osteoclast
progenitors is increased in the bone marrow (51). Particularly, Bartell et al
showed that RANKL promotes the accumulation of
H2O2 in osteoclasts and their progenitors via
an AKT-mediated repression of FoxO1 transcription that lowers
catalase protein level. They also demonstrated that as a
consequence of the loss of FoxO1 function, the osteoclast number
and bone resorption are prevented by the systemic administration of
catalase (11). This chain of
events identify that FoxO1 is a major control node of
osteoclastogenesis and bone resorption, both in physiologic or
pathologic conditions. Furthermore, other studies elucidated that
FoxO1 also plays a crucial role in bone metabolism by influencing
osteoblast physiology and function, due to its ability to maintain
redox balance via ROS-dependent or -independent mechanisms
(12,51,52).
In view of the facts that FoxO1 can maintain redox
balance and play a central role in bone metabolism and FoxO1
protein can be phosphorylated by AKT, the inhibition of the
PI3K/AKT pathway and regulation of FoxO1 transcriptional activity
could be regarded as an effective and novel strategy for the
prevention and/or treatment of osteoporosis. Moreover, our previous
studies revealed that the effects of the FoxO1/β-catenin signaling
pathway on the proliferation and differentiation of osteoblasts is
achieved through its regulation of the redox balance (upregulating
antioxidant enzyme levels as well as the expression of DNA
damage-repair-related gene Gadd45, while inhibiting the expression
of apoptosis-related gene Bim along with the osteoblast inhibition
gene PPAR-γ). In contrast, RES regulates the expression of FoxO1 by
inhibiting the PI3K/AKT signaling pathway to control white adipose
tissue and then to ameliorate overweight condition induced by a
high fat diet. Based on the above studies, we speculated that RES
regulates FoxO1 transcriptional activity by the PI3K/AKT signaling
pathway to remedy oxidative damage in osteoporosis. In order to
support this hypothesis, we explored the effect of RES on the
PI3K/AKT pathway. In this study, we revealed that more osteoclasts
were formed, and the osteoclast activity as well as function were
much higher in the presence of 10−4 M
H2O2 used to induce oxidative stress. This
enhancement was confirmed by the higher mRNA levels of
osteoclast-specific enzymes.
Moreover, the p-FoxO1 protein level and the ratio of
p-AKT/AKT were increased in the osteoclasts in response to
H2O2. The results indicated that
10−4 M H2O2 promoted osteoclast
formation via the AKT-mediating inhibition of FoxO1 transcriptional
activity. Taken together based on the findings in vivo,
oxidative stress provokes RANKL production and RANKL promotes the
accumulation of H2O2 in osteoclasts via
AKT-mediated repression of FoxO1 transcription that lowers catalase
protein level (11). Thus, we
concluded that an antioxidant can upregulate the FoxO1
transcriptional activity by lowering the RANKL level. In addition,
FoxO1 transcription activation can elevate the antioxidant enzyme
levels, then lower H2O2 in osteoclasts,
finally restraining osteoclastogenesis. This notion is consistent
with previously studies showing that FoxO1 reduces ROS by boosting
the antioxidant enzyme expression (19,20). N-acetylcysteine, a radical
scavenger, induces FoxO1 nuclear translocation and replenishes the
FoxO1 level in homocysteine-treated osteoblasts (21). Here, the present study
demonstrated that RES, a natural antioxidant, at a concentration of
10−5 M enhancd the FoxO1 protein level together with
transcriptional activity via suppressing AKT phosphorylation. In
contrast, in the presence of RES, the antioxidant power of cells
was enhanced, and RAW 264.7 cell apoptosis was also promoted. It
was confirmed that RES enhanced the FoxO1 protein level and
transcriptional activity to prevent oxidative damage, induction of
apoptosis and inhibition of osteoclastogenesis. Accordingly, FoxO1
may be a novel and effective target of RES to play the role of
resistance to osteoporosis.
RANKL binding to RANK on osteoclast precursors
causes the recruitment of TNF receptor associated factor 6 (TRAF6).
TRAF6 can activate several downstream signaling pathways, including
NF-κB, c-Jun N-terminal protein kinase (JNK), extracellular
signal-regulated kinase (ERK), p38, and PI3K/AKT (53–55). In particular, RANKL-induced
activation of the PI3K/AKT signaling pathway has been shown to
regulate osteoclast survival and differentiation (56). As mentioned above, RANKL-induced
AKT activation downregulates FoxO1 transcription (11). Leonurine hydrochloride negatively
regulates osteoclastogenesis by suppressing the NF-κB and PI3K/AKT
signaling pathway. We here demonstrated that RES provoked FoxO1
dephosphorylation and upregulated FoxO1 transcriptional activity
via suppressing the PI3K/AKT signaling pathway to achieve
protection against oxidative damage, and inhibition of osteoclast
activity, function, and formation. Moreover, our in vivo
results demonstrated that RES inhibited RANKL production in OVX
rats. Based on the above findings, we conclude that inhibition of
the PI3K/AKT signaling pathway by RES was induced by RANKL, which
requires further study to confirm.
In conclusion, the present study provides pivotal
evidence that RES upregulates FoxO1 transcriptional activity by
inhibiting the PI3K/AKT signaling pathway to obtain preventive
effects against oxidative damage and inhibition of
osteoclastogenesis. The study revealed the molecular mechanisms
involved in the prevention of oxidative stress damage and
inhibition of osteoclastogenesis by RES, and provides a new
scientific basis for the clinical application of RES for the
prevention and/or treatment of osteoporosis.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China under grant no. 81370970, and the
Science and Technology Support Program of Gansu Province under
grants no. 144FKCA075.
References
|
1
|
Ozgocmen S, Kaya H, Fadillioglu E and
Yilmaz Z: Effects of calcitonin, risedronate, and raloxifene on
erythrocyte antioxidant enzyme activity, lipid peroxidation, and
nitric oxide in postmenopausal osteoporosis. Arch Med Res.
38:196–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ershler WB, Harman SM and Keller ET:
Immunologic aspects of osteoporosis. Dev Comp Immunol. 21:487–499.
1997. View Article : Google Scholar
|
|
3
|
Manolagas SC: From estrogen-centric to
aging and oxidative stress: A revised perspective of the
pathogenesis of osteoporosis. Endocr Rev. 31:266–300. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muthusami S, Ramachandran I, Muthusamy B,
Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A and Narasimhan
S: Ovariectomy induces oxidative stress and impairs bone
antioxidant system in adult rats. Clin Chim Acta. 360:81–86. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cervellati C, Bonaccorsi G, Cremonini E,
Bergamini CM, Patella A, Castaldini C, Ferrazzini S, Capatti A,
Picarelli V, Pansini FS, et al: Bone mass density selectively
correlates with serum markers of oxidative damage in
post-menopausal women. Clin Chem Lab Med. 51:333–338. 2013.
View Article : Google Scholar
|
|
6
|
Baek KH, Oh KW, Lee WY, Lee SS, Kim MK,
Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, et al: Association of
oxidative stress with postmenopausal osteoporosis and the effects
of hydrogen peroxide on osteoclast formation in human bone marrow
cell cultures. Calcif Tissue Int. 87:226–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yalin S, Bagis S, Polat G, Dogruer N, Cenk
Aksit S, Hatungil R and Erdogan C: Is there a role of free oxygen
radicals in primary male osteoporosis. Clin Exp Rheumatol.
23:689–692. 2005.PubMed/NCBI
|
|
8
|
Halliwell B: Free radicals, antioxidants,
and human disease: Curiosity, cause, or consequence? Lancet.
344:721–724. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou
ZP, Zeng WS, Cheng BL and Luo SQ: Reactive oxygen species
stimulates receptor activator of NF-kappaB ligand expression in
osteoblast. J Biol Chem. 280:17497–17506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee NK, Choi YG, Baik JY, Han SY, Jeong
DW, Bae YS, Kim N and Lee SY: A crucial role for reactive oxygen
species in RANKL-induced osteoclast differentiation. Blood.
106:852–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartell SM, Kim HN, Ambrogini E, Han L,
Iyer S, Serra Ucer S, Rabinovitch P, Jilka RL, Weinstein RS, Zhao
H, et al: FoxO proteins restrain osteoclastogenesis and bone
resorption by attenuating H2O2 accumulation.
Nat Commun. 5:37732014. View Article : Google Scholar
|
|
12
|
Rached MT, Kode A, Xu L, Yoshikawa Y, Paik
JH, Depinho RA and Kousteni S: FoxO1 is a positive regulator of
bone formation by favoring protein synthesis and resistance to
oxidative stress in osteoblasts. Cell Metab. 11:147–160. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu JW, Chandra D, Rudd MD, Butler AP,
Pallotta V, Brown D, Coffer PJ and Tang DG: Induction of
prosurvival molecules by apoptotic stimuli: Involvement of FOXO3a
and ROS. Oncogene. 24:2020–2031. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lehtinen MK, Yuan Z, Boag PR, Yang Y,
Villén J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell
TK, et al: A conserved MST-FOXO signaling pathway mediates
oxidative-stress responses and extends life span. Cell.
125:987–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nemoto S and Finkel T: Redox regulation of
forkhead proteins through a p66shc-dependent signaling pathway.
Science. 295:2450–2452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang H and Tindall DJ: Dynamic FoxO
transcription factors. J Cell Sci. 120:2479–2487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sengupta A, Molkentin JD, Paik JH, DePinho
RA and Yutzey KE: FoxO transcription factors promote cardiomyocyte
survival upon induction of oxidative stress. J Biol Chem.
286:7468–7478. 2011. View Article : Google Scholar :
|
|
18
|
Subauste AR and Burant CF: Role of FoxO1
in FFA-induced oxidative stress in adipocytes. Am J Physiol
Endocrinol Metab. 293:E159–E164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tothova Z, Kollipara R, Huntly BJ, Lee BH,
Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams
IR, Sears C, et al: FoxOs are critical mediators of hematopoietic
stem cell resistance to physiologic oxidative stress. Cell.
128:325–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Keizer PL, Burgering BM and Dansen TB:
Forkhead box o as a sensor, mediator, and regulator of redox
signaling. Antioxid Redox Signal. 14:1093–1106. 2011. View Article : Google Scholar
|
|
21
|
Vijayan V, Khandelwal M, Manglani K, Singh
RR, Gupta S and Surolia A: Homocysteine alters the
osteoprotegerin/RANKL system in the osteoblast to promote bone
loss: Pivotal role of the redox regulator forkhead O1. Free Radic
Biol Med. 61:72–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Csiszar A: Anti-inflammatory effects of
resveratrol: Possible role in prevention of age-related
cardiovascular disease. Ann NY Acad Sci. 1215:117–122. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pearson KJ, Baur JA, Lewis KN, Peshkin L,
Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et
al: Resveratrol delays age-related deterioration and mimics
transcriptional aspects of dietary restriction without extending
life span. Cell Metab. 8:157–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ungvari Z, Orosz Z, Rivera A, Labinskyy N,
Xiangmin Z, Olson S, Podlutsky A and Csiszar A: Resveratrol
increases vascular oxidative stress resistance. Am J Physiol Heart
Circ Physiol. 292:H2417–H2424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ungvari Z, Bagi Z, Feher A, Recchia FA,
Sonntag WE, Pearson K, de Cabo R and Csiszar A: Resveratrol confers
endothelial protection via activation of the antioxidant
transcription factor Nrf2. Am J Physiol Heart Circ Physiol.
299:H18–H24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ungvari Z, Labinskyy N, Mukhopadhyay P,
Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P and Csiszar A:
Resveratrol attenuates mitochondrial oxidative stress in coronary
arterial endothelial cells. Am J Physiol Heart Circ Physiol.
297:H1876–H1881. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamaki N, Cristina Orihuela-Campos R,
Inagaki Y, Fukui M, Nagata T and Ito HO: Resveratrol improves
oxidative stress and prevents the progression of periodontitis via
the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense
pathways in a rat periodontitis model. Free Radic Biol Med.
75:222–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhattarai G, Poudel SB, Kook SH and Lee
JC: Resveratrol prevents alveolar bone loss in an experimental rat
model of periodontitis. Acta Biomater. 29:398–408. 2016. View Article : Google Scholar
|
|
30
|
Cottart CH, Nivet-Antoine V,
Laguillier-Morizot C and Beaudeux JL: Resveratrol bioavailability
and toxicity in humans. Mol Nutr Food Res. 54:7–16. 2010.
View Article : Google Scholar
|
|
31
|
Hsu H, Lacey DL, Dunstan CR, Solovyev I,
Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, et
al: Tumor necrosis factor receptor family member RANK mediates
osteoclast differentiation and activation induced by
osteoprotegerin ligand. Proc Natl Acad Sci USA. 96:3540–3545. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei S, Teitelbaum SL, Wang MW and Ross FP:
Receptor activator of nuclear factor-kappa b ligand activates
nuclear factor-kappaB in osteoclast precursors. Endocrinology.
142:1290–1295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura N, Ramaswamy S, Vazquez F,
Signoretti S, Loda M and Sellers WR: Forkhead transcription factors
are critical effectors of cell death and cell cycle arrest
downstream of PTEN. Mol Cell Biol. 20:8969–8982. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y and Tang XL: Effects of resveratrol
on osteoprotegerin and osteoprotegerin Ligand Expression of Femurs
in Ovariectomized Rats. Chin J Clin Pharmacol Ther. 13:266–270.
2008.
|
|
35
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals. Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: Releasing power for life and unleashing the
machineries of death. Cell. 112:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balaban RS, Nemoto S and Finkel T:
Mitochondria, oxidants, and aging. Cell. 120:483–495. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chance B, Sies H and Boveris A:
Hydroperoxide metabolism in mammalian organs. Physiol Rev.
59:527–605. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
D'Autréaux B and Toledano MB: ROS as
signalling molecules: Mechanisms that generate specificity in ROS
homeostasis. Nat Rev Mol Cell Biol. 8:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Denisova NA, Cantuti-Castelvetri I, Hassan
WN, Paulson KE and Joseph JA: Role of membrane lipids in regulation
of vulnerability to oxidative stress in PC12 cells: Implication for
aging. Free Radic Biol Med. 30:671–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kong YY, Yoshida H, Sarosi I, Tan HL,
Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G,
Itie A, et al: OPGL is a key regulator of osteoclastogenesis,
lymphocyte development and lymph-node organogenesis. Nature.
397:315–323. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Levaot N, Ottolenghi A, Mann M,
Guterman-Ram G, Kam Z and Geiger B: Osteoclast fusion is initiated
by a small subset of RANKL-stimulated monocyte progenitors, which
can fuse to RANKL-unstimulated progenitors. Bone. 79:21–28. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Suda N, Morita I, Kuroda T and Murota S:
Participation of oxidative stress in the process of osteoclast
differentiation. Biochim Biophys Acta. 1157:318–323. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garrett IR, Boyce BF, Oreffo RO, Bonewald
L, Poser J and Mundy GR: Oxygen-derived free radicals stimulate
osteoclastic bone resorption in rodent bone in vitro and in vivo. J
Clin Invest. 85:632–639. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Darden AG, Ries WL, Wolf WC, Rodriguiz RM
and Key LL Jr: Osteoclastic superoxide production and bone
resorption: Stimulation and inhibition by modulators of NADPH
oxidase. J Bone Miner Res. 11:671–675. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Steinbeck MJ, Appel WH Jr, Verhoeven AJ
and Karnovsky MJ: NADPH-oxidase expression and in situ production
of superoxide by osteoclasts actively resorbing bone. J Cell Biol.
126:765–772. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim
HH and Lee ZH: Reactive oxygen species mediate RANK signaling in
osteoclasts. Exp Cell Res. 301:119–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim HJ, Chang EJ, Kim HM, Lee SB, Kim HD,
Kim GS and Kim HH: Antioxidant-α-lipoic acid inhibits osteoclast
differentiation by reducing nuclear factor-κB DNA binding and
prevents in vivo bone resorption induced by receptor activator of
nuclear factor-κB ligand and tumor necrosis factor-α. Free Radic
Biol Med. 40:1483–1493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Van Der Heide LP, Hoekman MF and Smidt MP:
The ins and outs of FoxO shuttling: Mechanisms of FoxO
translocation and transcriptional regulation. Biochem J.
380:297–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Uddin S, Hussain AR, Siraj AK, Manogaran
PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A,
El-Solh H, et al: Role of phosphatidylinositol 3′-kinase/AKT
pathway in diffuse large B-cell lymphoma survival. Blood.
108:4178–4186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ambrogini E, Almeida M, Martin-Millan M,
Paik JH, Depinho RA, Han L, Goellner J, Weinstein RS, Jilka RL,
O'Brien CA, et al: FoxO-mediated defense against oxidative stress
in osteoblasts is indispensable for skeletal homeostasis in mice.
Cell Metab. 11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Iyer S, Ambrogini E, Bartell SM, Han L,
Roberson PK, de Cabo R, Jilka RL, Weinstein RS, O'Brien CA,
Manolagas SC, et al: FOXOs attenuate bone formation by suppressing
Wnt signaling. J Clin Invest. 123:3409–3419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Leibbrandt A and Penninger JM: RANK/RANKL:
Regulators of immune responses and bone physiology. Ann NY Acad
Sci. 1143:123–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar
|
|
55
|
Mandal CC, Ghosh Choudhury G and
Ghosh-Choudhury N: Phosphatidylinositol 3 kinase/Akt signal relay
cooperates with smad in bone morphogenetic protein-2-induced colony
stimulating factor-1 (CSF-1) expression and osteoclast
differentiation. Endocrinology. 150:4989–4998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee
SY and Kim N: Akt induces osteoclast differentiation through
regulating the GSK3β/NFATc1 signaling cascade. J Immunol.
188:163–169. 2012. View Article : Google Scholar
|