Introduction
Granulomatous lobular mastitis (GLM), first defined
by Kessler and Woollock in 1972, is a rare, benign and chronic
inflammatory lesion of the breast (1). The condition is characterized by
painful breast masses, abscesses, draining sinuses and scarring
(2). Dermatitis contusiformis is
also reported to be a common clinical symptom in the majority of
cases (3). Various factors have
been reported to be associated with the occurrence of GLM,
including breast feeding, microbiological infection, autoimmune
diseases, smoking and a high level of prolactin (4). The natural course of GLM is
relatively long. If no treatment is administrated, the mean
duration of spontaneous healing is 14.5 months in 50% of patients,
and common sequelae include scarring, retraction of the skin and
nipple, and shrinkage of the whole breast. Meanwhile, ~50% of
patients experience recurrence after systematic therapy (1).
Clinically, it is difficult to differentiate GLM
from other mammary diseases, including breast cancer, subareolar
abscesses, plasma cell mastitis, mammary tuberculosis, fat necrosis
and sarcoidosis (5). Histological
examination is the only golden criteria able to make a diagnosis.
In the perspective of histology, GLM is characterized by lobular
granuloma consisting of macrophages, neutrophils, lymphocytes,
monocytes and lesions of non-cheese-like necrosis (6). Since granuloma is induced by
macrophage infiltration and proliferation, macrophage activation is
considered to be the central pathogenesis of GLM. Therapeutically,
no standard regimen or guideline exists for GLM, but systematic
corticosteroids and methitrexate have been used as primary
treatments subsequent to surgery to prevent recurrence (6). Antibiotics or anti-tuberculotics
have also been recommended for GLM therapy in certain patients
(7). However, side effects,
including antibiotic resistance, immunosuppression, hormone-induced
concentric obesity and metabolic disorders, greatly limit the
chronic application of current strategies. Meanwhile, breast
reconstruction is usually necessary after surgery in severe GLM
cases. Therefore, it is a necessary and urgent requirement to find
natural anti-inflammatory agents with low toxicity for GLM
therapy.
Macrophages serve important roles in the innate and
adaptive immune responses by releasing various factors, including
interleukin-6 (IL-6), IL-1β, cyclooxygenase-2 (COX-2) and inducible
nitric oxide synthase (iNOS). All these mediators contribute to the
inflammatory disease pathogenesis and are recognized biomarkers for
evaluating the severity of inflammation. Nuclear factor-κB (NF-κB)
and mitogen-activated protein kinase (MAPK) pathways are considered
as the upstream signaling controlling the transcription of the
aforementioned inflammatory factors (8,9).
p50/p65 heterodimer is the most highly characterized NF-κB complex,
which is kept inactive in the cytoplasm of resting cells. At NF-κB
activation, its translocation to the nucleus results in the
promotion of downstream gene transcription. MAPK family members,
including p38 MAPK, c-Jun N-terminal kinase (JNK) and extracellular
signal-regulated kinase (ERK), become activated following
lipopolysaccharide (LPS) stimulation, and MAPK suppression results
in the blockage of the secretion of various inflammatory cytokines.
Meanwhile, MAPKs and NF-κB can collaborate synergistically to
induce the expression and secretion of pro-inflammation cytokines
(10). Therefore, therapies
targeting NF-κB and MAPKs have become an important strategy for
curing inflammatory diseases.
Traditional Chinese medicine (TCM) has a unique
focus on in treating chronic inflammatory wounds and diseases. A
number of studies have demonstrated that numerous Chinese herbs,
including Tripterygium wilfordii, honeysuckle and dandelion,
could exert anti-inflammatory effects via blocking reactive oxygen
species (ROS) burst, balancing dysregulated immune cells or
cytokines, inhibiting NF-κB signaling and interrupting nitric oxide
(NO) synthesis (11,12). Broadleaf Mahonia (BM) is a
traditional Chinese herb that is widely distributed in the majority
of regions worldwide. Although it is recorded that BM is capable of
fighting against chronic inflammation and bacterial infection in
ancient books, little research has been performed to investigate
its anti-inflammatory effects and underlying mechanisms.
The present study investigated the effects of BM
aqueous extracts on the production of inflammatory mediators in
LPS-induced RAW264.7 cells. Meanwhile, the effects of BM extracts
on the cytokine secretion of GLM samples were also studied. To the
best of our knowledge, the study demonstrated the anti-inflammatory
effects of BM in vitro and ex vivo for the first
time, and revealed the involvement of NF-κB/MAPKs and chemokine
(C-C motif) ligand (CCL)-5 signaling.
Materials and methods
Preparation and quality control of BM
extracts
BM leaves (100 g) were cut into small pieces and
immersed in distilled water. The mixture was treated with
ultrasound for 1 h followed by heating at 100°C for 30 min twice.
The supernatant was concentrated by rotary evaporation and kept at
−80°C overnight. The freezing supernatant was then placed in a
freeze dryer for 48 h to obtain the raw aqueous extract powder. The
production ratio of BM was 12.3–13.6%. High-performance liquid
chromatography (HPLC) analysis of BM aqueous extract was conducted
on an Ultimate AQ-C18 column (250×4.6 mm; 5 μm). The mobile
phase consisted of acetonitrile (A) and water (B), using a gradient
elution of 5–8% A at 0–5 min, 8–14% A at 5–30 min, 14–50% A at
30–55 min and 50–90% A at 55–60 min. The solvent flow rate was 1.0
ml/min and the column temperature was ambient. The detection
wavelength was set at 250 nm. The berberine concentration in the
extracts was 0.014–0.018 mg/ml [berberine was recommended as a
representative compound in BM by Chinese Pharmacopeia (13)] based on the absorbance at 250 nm
assessed by ultraviolet-visible spectrophotometry (Fig. 1A). The powder was dissolved in
phosphate-buffered solution (PBS) and passed through a
0.45-μm filter for later use.
Cell culture
Mouse macrophage RAW264.7 cells were obtained from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) at 37°C in a humidified incubator with 5%
CO2. Human GLM samples were collected and cut into small
pieces. The shredded tissues were filtered with 200-μm nylon
mesh and suspended in PBS solution. The filtered cells were
centrifuged at 800 rpm for 5 min at room temperature and washed
three times with PBS, and the precipitates were suspended and
cultured in RPMI-1640 medium (Gibco, Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% FBS (Gibco, Thermo Fisher
Scientific, Inc.) and 1% penicillin and streptomycin.
Cell proliferation assay
The effect of BM on cell proliferation was studied
by cell growth assay using
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
as described previously (14).
Briefly, the cells were seeded onto a 96-well plate at a density of
4×103 cells/well. Following serum starvation, different
concentrations of BM (2.5, 5, 10, 20, 40, 60, 80 and 100
μg/ml) were added to the wells, with 6 repeats for each
concentration. LPS was added at 1 μg/ml to simulate the
inflammation status (15). The
cell growth rate was detected using a cell proliferation MTT kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) according to the
manufacturer's instructions. The purple formazan was dissovled with
Dimethyl sulfoxide (Sigma, St. Louis, MO, USA) and detected at a
wavelength of 540 nm. Triplicate independent experiments were
performed.
Flow cytometry analysis
RAW264.7 cells (1×106/well) were
synchronized in G1 phase by serum deprivation, and different
concentrations of BM (25, 50 and 100 μg/ml) and 1
μg/ml LPS were then added. Cells were harvested after 48 h.
Propidium iodide (PI)-stained single-cell suspension was analyzed
on a FC5000 cytometer (BD Biosciences, Franklin Lakes, NJ, USA)
using CXP software version 2.0 (Beckman Coulter, Fullerton, CA,
USA) for data analysis. The experiment was repeated three times.
For apoptosis analysis, following BM treatment (25, 50 and 100
μg/ml) for 48 h, cells were stained with Annexin V and PI
(Clontech Laboratories, Inc., Mountainview, CA, USA) for 15 min at
room temperature. The apoptotic index was determined by flow
cytometry with Flowjo software. 2′7′-Dichlorofluorescein diacetate
(DCFH-DA; Sigma-Aldrich; Merck KGaA) was used to measure ROS
formation. After exposure to different concentrations of BM (25, 50
and 100 μg/ml) for 48 h, the cells were then incubated in
DCFH-DA-containing medium (final concentration, 10 μM) at
37°C for 20 min. The cells were washed with PBS three times to
remove DCFH-DA that had not entered into the cells. The
intracellular concentration of DCFH-DA was then measured by flow
cytometry.
NO detection
NO levels in the culture media were determined by
the Griess Reagent system according to the manufacturer's protocols
(Promega Corporation, Madison, WI, USA). Briefly, 50 μl cell
culture supernatant was transferred into a 96-well plate and mixed
with 100 μl Griess reagent (equal volumes of 1% w/v
sulfanilamide in 5% v/v phosphoric acid and 0.1% w/v
naphthylethylenediamine-HCl). Next, the plate was incubated for
5–10 min at room temperature, and absorbance was measured at 550 nm
using a microplate reader. The amount of NO was calculated
according to the standard sodium nitrite curve.
Western blot analysis
Cells were lysed in RIPA buffer (Sigma) containing a
protease inhibitor mixture (Roche Diagnostics, GmbH, Mannheim,
Germany). The protein concentration was determined with the
bicinchoninic acid assay (Thermo Fisher Scientific, Inc., Bonn,
Germany). Quantified protein lysates (15 μg) were resolved
by 10% sodium dodecyl sulfate - 12% polyacrylamide gel
electrophoresis. The proteins were then transferred onto PVDF
membranes (Millipore, Billerica, MA, USA). Each membrane was
blocked for 2 h with 2% bovine serum albumin (Sigma) and then
incubated overnight at 4°C with 1 μg/ml primary antibody
(dilution, 1:1,000). The antibodies COX-2 (A5787), NF-κB (A2574),
p-NF-κB (AP0475), IκB kinase (IKK)α (A2062), IKKβ (A2087), ERK
(A0229), p-ERK (AP0472), JNK (A6117), p-JNK (AP0276), p38 (A0227),
p-p38 (AP0056) were obtained from ABclonal (Boston, MA, USA). The
antibodies p-IKKα/β (2697), IKBα (4814) p-IKBα (2859) and β-actin
(4970s) were purchased from Cell Signaling Technology (Danvers, MA,
USA). iNOS (bs-0162R) was provided by BIOSS (Beijing, China). After
3 washes with Tris-buffered saline with 0.05% Tween-20, the
membranes were incubated with HRP-conjugated anti-rabbit (7074s) or
anti-mouse (7076) secondary antibodies (dilution, 1:2,000; Cell
Signaling Technology) for 2 h at room temperature. The signals were
visualized using the ECL Advance reagent (GE Healthcare) and
quantified using Quantity One software (version 4.5; Bio-Rad
Laboratories, Hercules, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the cells was extracted using TRIzol
reagent (Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), and RT was performed using a first strand CDNA
synthesis kit (Roche Applied Science, Penzberg, Germany) according
to the manufacturer's protocols. PCR amplification conditions
consisted of an initial denaturation step at 95°C for 2 min,
followed by 35 cycles of denaturation at 95°C for 30 sec, annealing
at 50°C for 30 sec and extension at 72°C for 45 sec; a final
extension period at 72°C for 5 min completed the amplification.
qPCR analysis was performed using a SYBR-Green kit (Roche
Diagnostics, Basel, Switzerland) on a Roche lightcycler 480
detector (Roche Diagnostics, GmbH, Manheim, Germany). The primers
for iNOS, COX-2, IL-1β, IL-6, CCL-2, CCL-3, CCL-5, secreted tumor
necrosis factor receptor 1 (sTNFR1) and glyceraldehyde 3-phosphate
dehydrogenase are listed in Table
I. Cq value was measured during the exponential amplification
phase. The relative expression level (defined as fold-change) of
the target gene was given by 2−ΔΔCq and normalized to
the internal control.
 | Table IPrimer sequence for target genes. |
Table I
Primer sequence for target genes.
| Gene (Mus
musculus) | Primer sequence
(5′-3′) |
|---|
| iNOS | F:
GCTACCAAACTGGATATAATCAGGA |
| R:
CCAGGTAGCTATGGTACTCCAGAA |
| COX-2 | F:
GCTACCAAACTGGATATAATCAGGA |
| R:
CCAGGTAGCTATGGTACTCCAGAA |
| IL-1β | F:
GCTACCAAACTGGATATAATCAGGA |
| R:
CCAGGTAGCTATGGTACTCCAGAA |
| IL-6 | F:
GCTACCAAACTGGATATAATCAGGA |
| R:
CCAGGTAGCTATGGTACTCCAGAA |
| CCL-2 | F:
CATCCACGTGTTGGCTCA |
| R:
GATCATCTTGCTGGTGAATGAGT |
| CCL-3 | F:
TGCCCTTGCTGTTCTTCTCT |
| R:
GTGGAATCTTCCGGCTGTAG |
| CCL-5 | F:
TGCAGAGGACTCTGAGACAGC |
| R:
GAGTGGTGTCCGAGCCATA |
| sTNFR1 | F:
TCTTCTCATTCCTGCTTGTGG |
| R:
GGTCTGGGCCATAGAACTGA |
| GAPDH | F:
GGGTTCCTATAAATACGGACTGC |
| R:
CCATTTTGTCTACGGGACGA |
Luciferase reporter analysis
The pathway reporter plasmids of phosphorylated
(p)NF-κB-Luc and phosphorylated activated protein 1 (pAP-1)-Luc,
which contain NF-κB or AP-1 binding sites in the promoter region,
were purchased from Clontech Laboratories, Inc. Overnight cultured
RAW264.7 cells in 96-well plates were transfected with 100 ng
pNFκB-Luc or pAP-1-Luc reporter vector and 5 ng phRL-TK
Renilla luciferase reporter (Promega Corporation) using
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). At 16 h post-transfection, the cells were
treated with various concentrations of BM and LPS (for NF-κB
reporter). After another 24 h, the cells were lysed for
Firefly/Renilla luciferase activity assay using a
dual-luciferase assay kit (Promega Corporation). The relative
luciferase activities were normalized by LPS/IFN-c or PMA
control.
Immunofluorescence analysis
RAW264.7 cells (3×105/well) were seeded
in 24-well plates containing coverslips. Following LPS or BM
treatment for 24 h, coverslips were then fixed in 4%
paraformaldehyde for 10 min and permeabilized with 0.2% Triton
X-100. After blocking in 10% goat serum (DAKO, Glostrup, Denmark)
for 1 h at room temperature, the coverslips were incubated with
primary NF-κB antibody (1:100; A2574; ABclonal) overnight at 4°C
and then with secondary Alexa Fluor 555-conjugated antibody (1:200;
4413s; Cell Signaling Technology) for 2 h at room temperature.
Finally, the samples were incubated with 1 μg/ml
4′,6-diamidino-2-phenylindole DAPI (Sigma) for 15 min at room
temperature for nuclear staining and detected under a confocal
microscope (LSM710; Carl Zeiss, Jena, Germany).
Cytokine array
Mouse and human inflammatory antibody array C1 kits
were purchased from RayBiotech (Norcross, GA, USA). Briefly, cell
supernatants from RAW264.7 cells or primary cultured GLM samples
prior to and subsequent to BM treatment were collected. Once the
antibody array membranes had been blocked in 5% BSA for 30 min at
room temperature, cell supernatants were cultured with antibody
arrays overnight at 4°C and washed three times. A biotinylated
antibody cocktail was then incubated with the membranes for 2 h,
followed by signal amplification with horseradish
peroxidase-streptavidin. Finally, the signals were detected by
chemiluminescence method with an ECL kit purchased from GE
Healthcare Life Sciences (Little Chalfont, UK).
Ex vivo human GLM sample experiments
All human GLM patients provided written informed
consent for the use of clinical specimens for medical research.
Studies using human tissue were reviewed and approved by the
committee for the ethical review of research involving human
subjects of Guangzhou University of Chinese Medicine (Guangzhou,
Guangdong, China). The methods for the collection and application
of human specimens were in accordance with International Ethical
Guidelines for Biomedical Research Involving Human Subjects.
Clinical specimens were obtained based on the diagnosis of clinical
syndromes, ultrasound findings and pathological characteristics. A
total of 10 GLM specimens were sliced into small pieces and put
into digestion buffers (StemCell Technologies, Vancouver, Canada)
containing collagenase and hyaluronidase for 2–3 h with agitation.
The cell suspension was then filtered through a 100-μm cell
strainer and the filtered content was then centrifuged at 200 × g
for 8 min at 4°C. The cell pellets were then suspended in 1 ml
RPMI-1640 medium above 5 ml 45% Percoll (GE Healthcare, Uppsala,
Sweden) in the middle and 5 ml 60% Percoll at the bottom in a 15-ml
tube. Mononuclear cells were collected from the cell layer in the
interphase between 45 and 60% Percoll. The cells were then cultured
in RPMI-1640 medium at 37°C and treated with BM. The cells
supernatants were then collected for the cytokine array detection
and the cellular protein lysates were subjected to western blot
assay as stated above.
Statistical analysis
Data are presented as the mean ± standard error of
the mean (SEM). The results were analyzed by SPSS 20.0 software
(IBM Corp., Armonk, NY, USA). Mean values were derived from at
least three independent experiments. Statistical comparisons
between experimental groups were performed by one-way analysis of
variance followed by post hoc Dunnett's test. The level of
statistical significance was set at P<0.05.
Results
Preparation and LC-mass spectrometry (MS)
analysis of BM
As with the majority of TCM formulae, a decoction of
BM used clinically. The pharmacological activity of BM and the
repeatability of the experiments performed using it are dependent
on the controlled quality of the decoction. In the present study,
in order to maintain a consistent quality, BM was extracted with
water and prepared carefully. The preparation procedure is
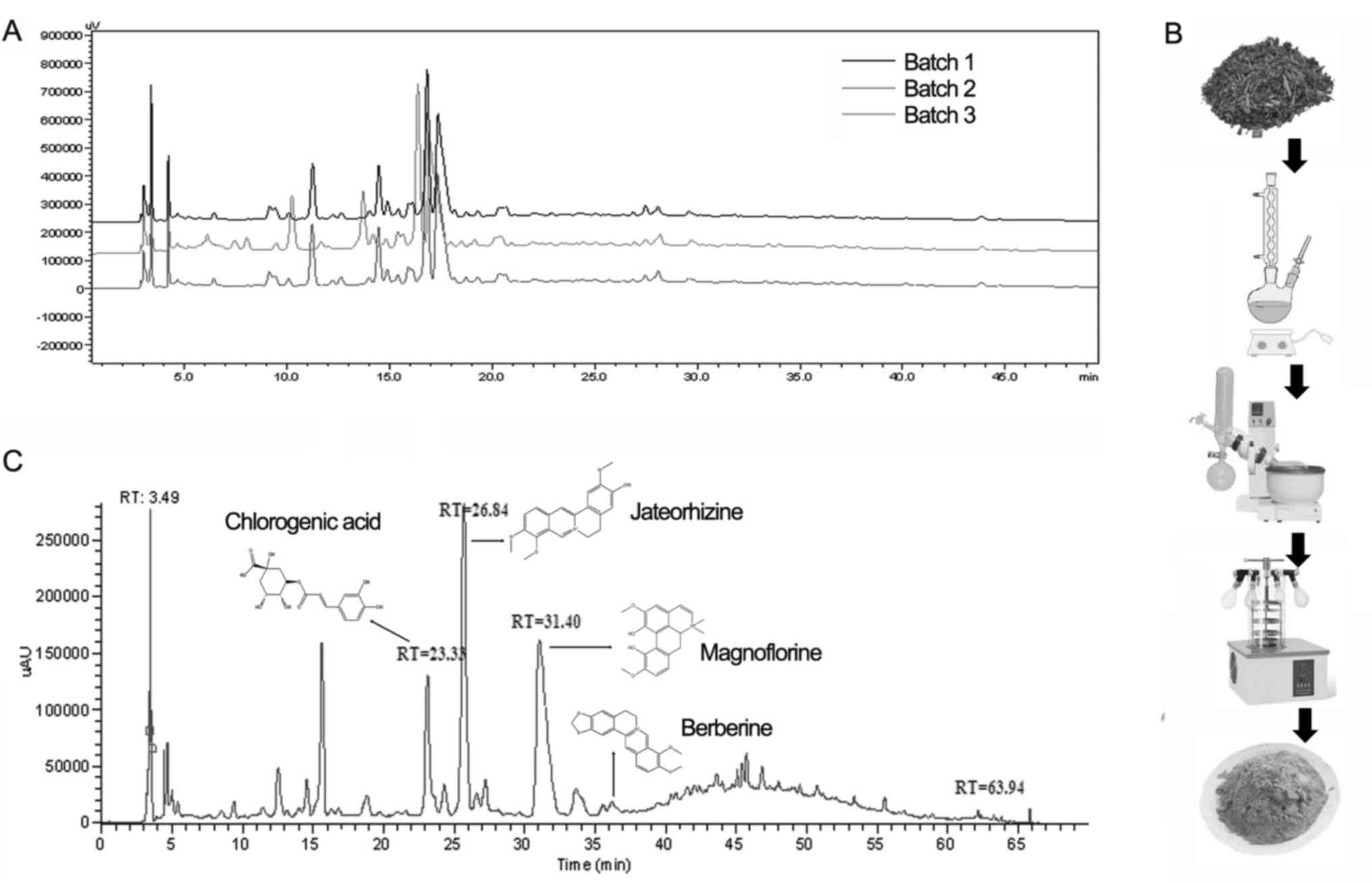
summarized in Fig. 1B. HPLC
analysis further validated that the chemical fingerprints were in
accordance in three different batches (Fig. 1A). Meanwhile, quality analysis of
BM was performed with LC-MS. As shown in Fig. 1C, the MS spectra revealed the
presence of chlorogenic acid, jateorhizine, magnoflorine and
berberine in the BM extract.
BM exhibits few inhibitory effects on
RAW264.7 proliferation, cell cycle progression and apoptosis
induction
To study whether BM could inhibit the proliferation
of RAW264.7 cells, LPS was added as positive inducer of
inflammation and three batches of BM were tested in a dose- and
time-dependent manner, respectively. The results showed that BM
exerted few inhibitory effects on the proliferation of RAW264.7
cells, which not only validated the bioactivity consistency between
three different batches of BM, but also implied that BM had few
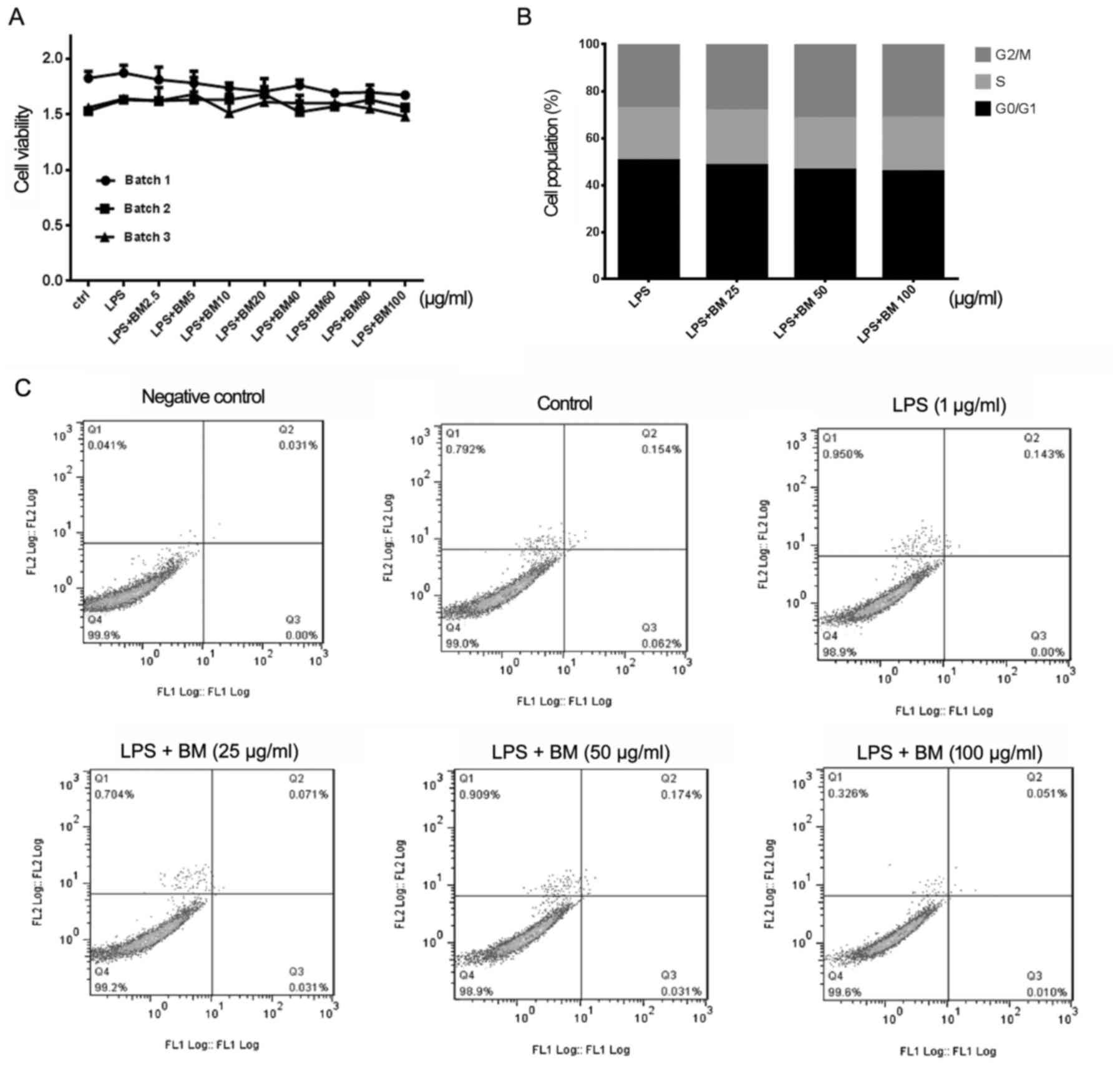
cytotoxic effects (Fig. 2A).
Similarly, cell cycle analysis also indicated that BM had limited
influence on the distribution of the cell cycle (Fig. 2B). Meanwhile, Annexin V-FITC/PI
assay demonstrated that following BM administration, limited
changes in the apoptotic ratio of RAW264.7 cells occurred (Fig. 2C). All these results indicated
that the anti-inflammation activity of BM was not due to its
survival and proliferation inhibitory effects.
BM inhibits ROS generation and NO
synthesis
During inflammation, a significant increase in
oxygen consumption would result in a massive elevation of
intracellular ROS, which acts as an upstream signal to promote the
inflammation process and even destroys cells to induce abscess
formation (16). To determine
whether the LPS-induced intracellular redox state could be
suppressed by BM, ROS production was detected prior to and
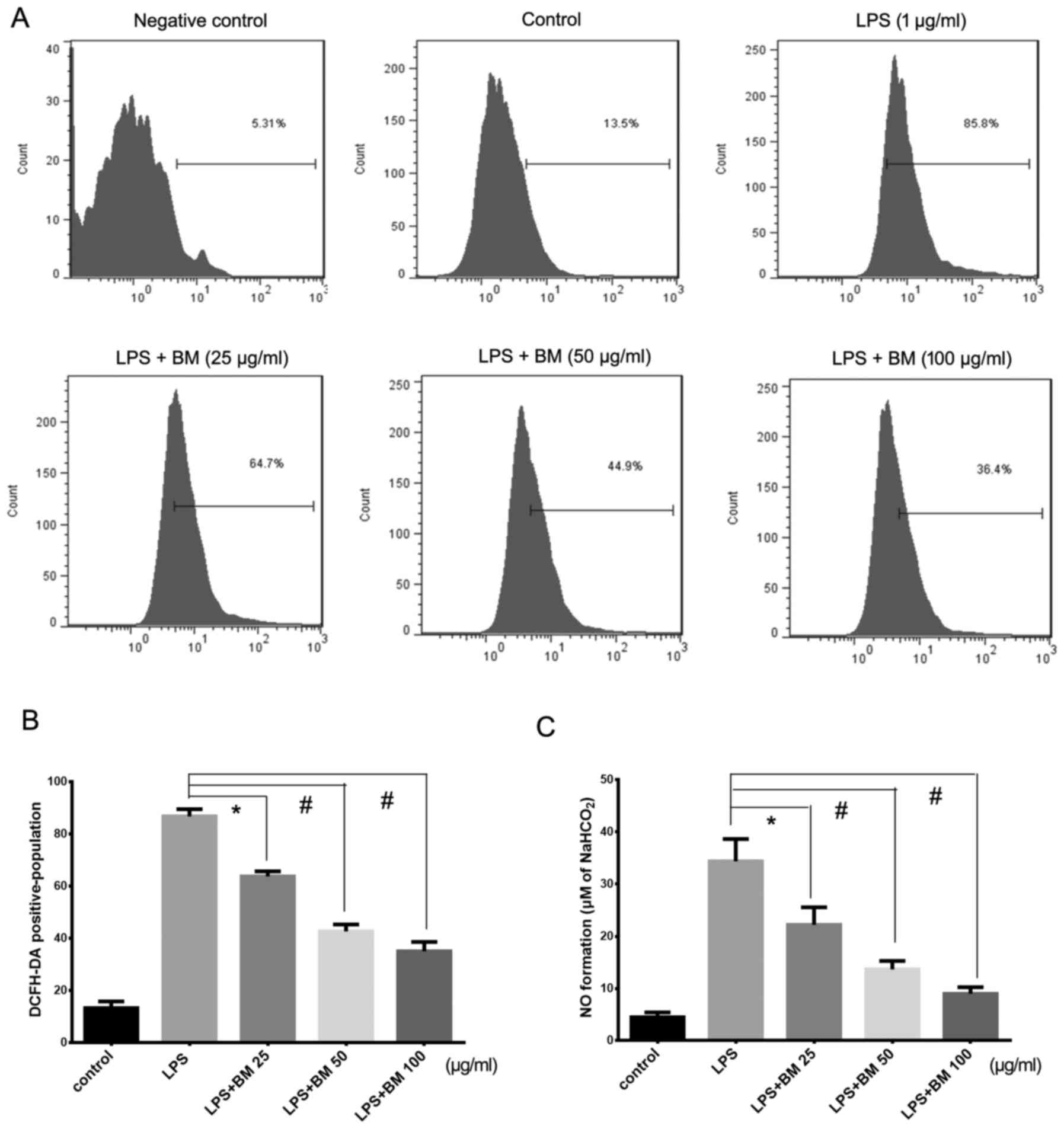
following BM treatment. The results showed that the level of ROS in
response to LPS was significantly higher than that of unstimulated
cells. However, with the increasing dose of BM in the medium, the
ROS production was significantly inhibited in a dose-dependent
manner (Fig. 3A and B).
Furthermore, since excessive NO production is a critical marker of
inflammatory diseases, such as rheumatoid arthritis and autoimmune
diseases, the present study detected whether BM also led to a
decrease in NO levels. RAW264.7 cells were treated with different
doses of BM for 2 h prior to stimulation with LPS (1 μg/ml)
(17). NO production is reflected
in the accumulation of nitrite in the cell culture medium. The
results revealed that LPS stimulation caused a significant increase
of nitrite concentration, while BM administration inhibited NO
release in a dose-dependent manner, with >50% inhibition at a
concentration of 100 μg/ml (Fig. 3C). These results suggested that BM
was capable of scavenging ROS level and thereby alleviating
inflammation severity.
BM inhibits iNOS/COX-2 expression and
pro-inflammatory cytokine secretion
The iNOS gene is known to be the primary regulator
of NO production in macrophages, and the COX-2 gene is usually
increased under inflammation and malignant conditions (18). To determine the anti-inflammatory
effects of BM, the expression levels of iNOS and COX-2 were
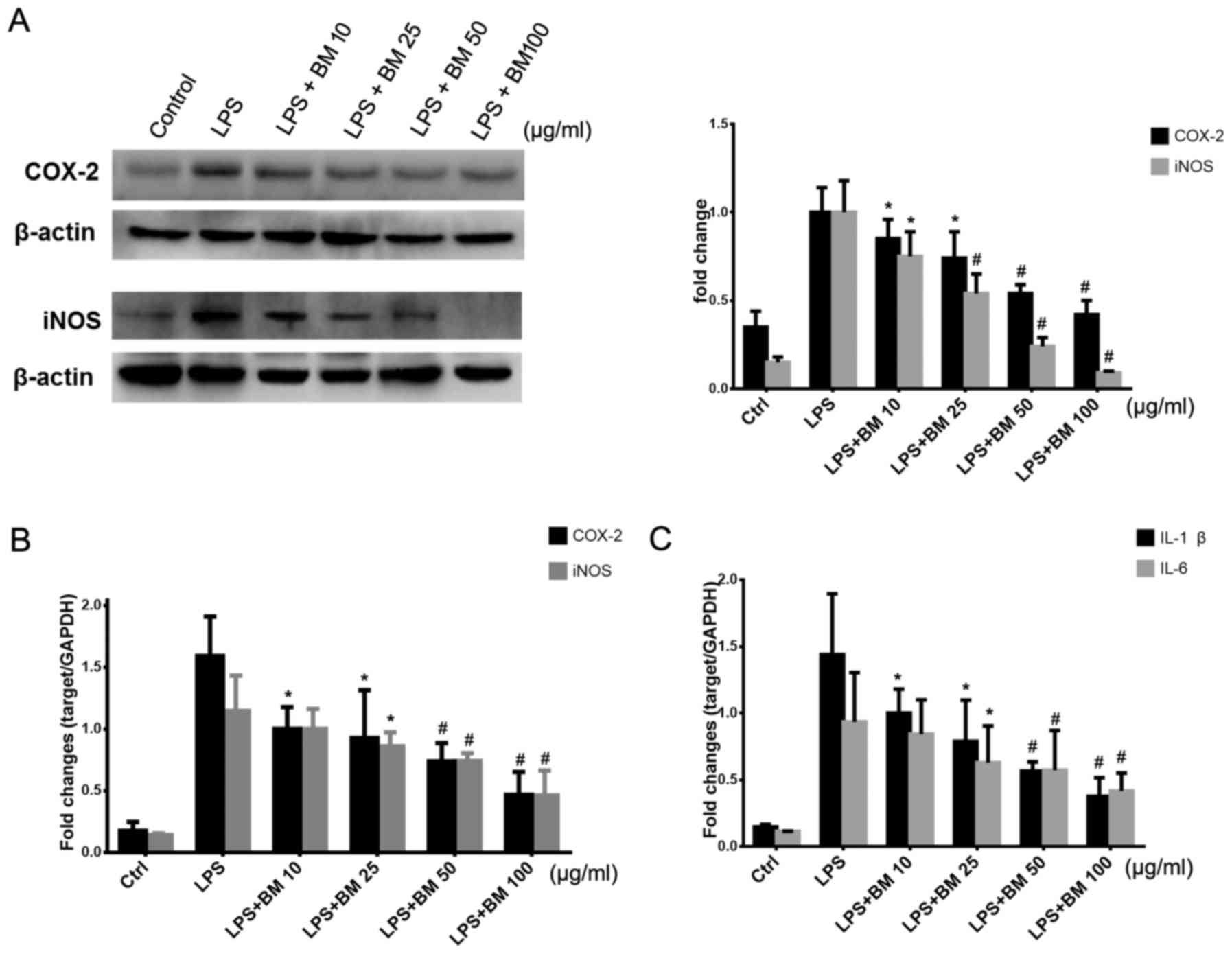
measured by western blotting and qPCR analysis. As shown in
Fig. 4A, the protein expression
levels of iNOS and COX-2 were significantly elevated following LPS
stimulation, but the two proteins displayed a marked downregulated
tendency in response to increasing concentration of BM treatment.
Consistently, the mRNA expression levels of iNOS and COX-2 were
also suppressed by BM (Fig. 4B).
Since NF-κB acts as the common transcription factor of the two
genes, the results indicated that BM may inhibit inflammation via
suppressing NF-κB signaling.
During the progression of inflammation, IL-1β and
IL-6 are known to be pro-inflammatory cytokines, which are
constitutively secreted and activated, mediating macrophage
chemotaxis, angiogenesis and sustained tissue destruction (19,20). qPCR analysis was also applied to
determine the levels of IL-1β and IL-6 prior to and following BM
administration. When the cells were induced by LPS, the expression
levels of IL-1β and IL-6 mRNA were markedly increased. However,
when BM was added to the media, the mRNA levels of the two
cytokines were significantly decreased in a dose-dependent manner
(Fig. 4C). All these results
indicated that BM is able to block the secretion of
pro-inflammatory cytokines.
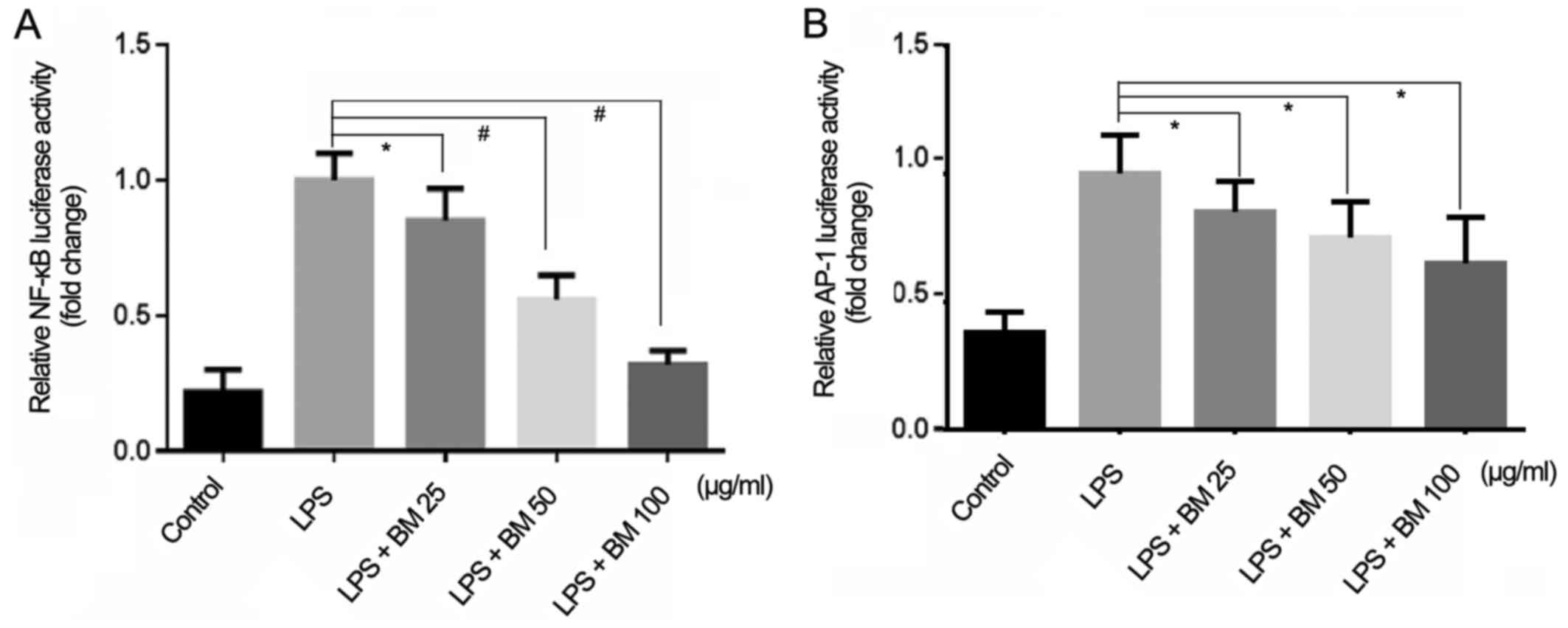
BM blocks the transcriptional activities
of NF-κB and AP-1
Since NF-κB and AP-1 control the transcription of
iNOS/COX-2 and a variety of pro-inflammatory cytokines, the present
study investigated the effect of BM on these signaling pathways
using a luciferase reporter assay. As demonstrated in Fig. 5, the transcriptional activities of
NF-κB and AP-1 were inhibited dose-dependently following BM
treatment, but the inhibition ratio of NF-κB was higher. These
results indicated that BM blocked LPS-induced NF-κB and AP-1
signaling, leading to the inhibition of LPS-induced production of
NO and COX-2.
BM suppresses LPS-induced activation of
NF-κB and MAPK signaling
Considering the nuclear translocation of the p65
subunit is a critical step determining its downstream
transcriptional activity, the present study investigated whether BM
affects the subcellular compartmentalization of p65. RAW264.7 cells
were pretreated with BM for 12 h and stimulated with LPS for
another 1 h, and the intracellular p65 distribution was then
evaluated by confocal microscopy. As shown in Fig. 6A, the p65 subunit was mainly
distributed in the nucleus following BM treatment. However,
pre-treatment with BM significantly reduced the p65 translocation
compared with that in the control cells with LPS stimulation. In
order to identify the upstream target of BM-mediated NF-κB
suppression, the expression status of IKK and IκBα was
investigated. RAW264.7 cells were stimulated with LPS for 1 h in
the presence or absence of BM. As shown in Fig. 6B–D, the phosphorylated level of
IKKα/β was significantly elevated following LPS stimulation, but
began to decrease gradually with the increasing dose of BM.
However, the expression status of IKKα and IKKβ exhibited limited
changes in response to BM treatment. Notably, the phosphorylation
of IκBα and p65 was also inhibited following BM administration in
the presence of LPS stimulation, respectively. All these results
indicated that BM inhibited NF-κB activation by blocking the
phosphorylation of IκBα and its upstream kinase IKKα/β.
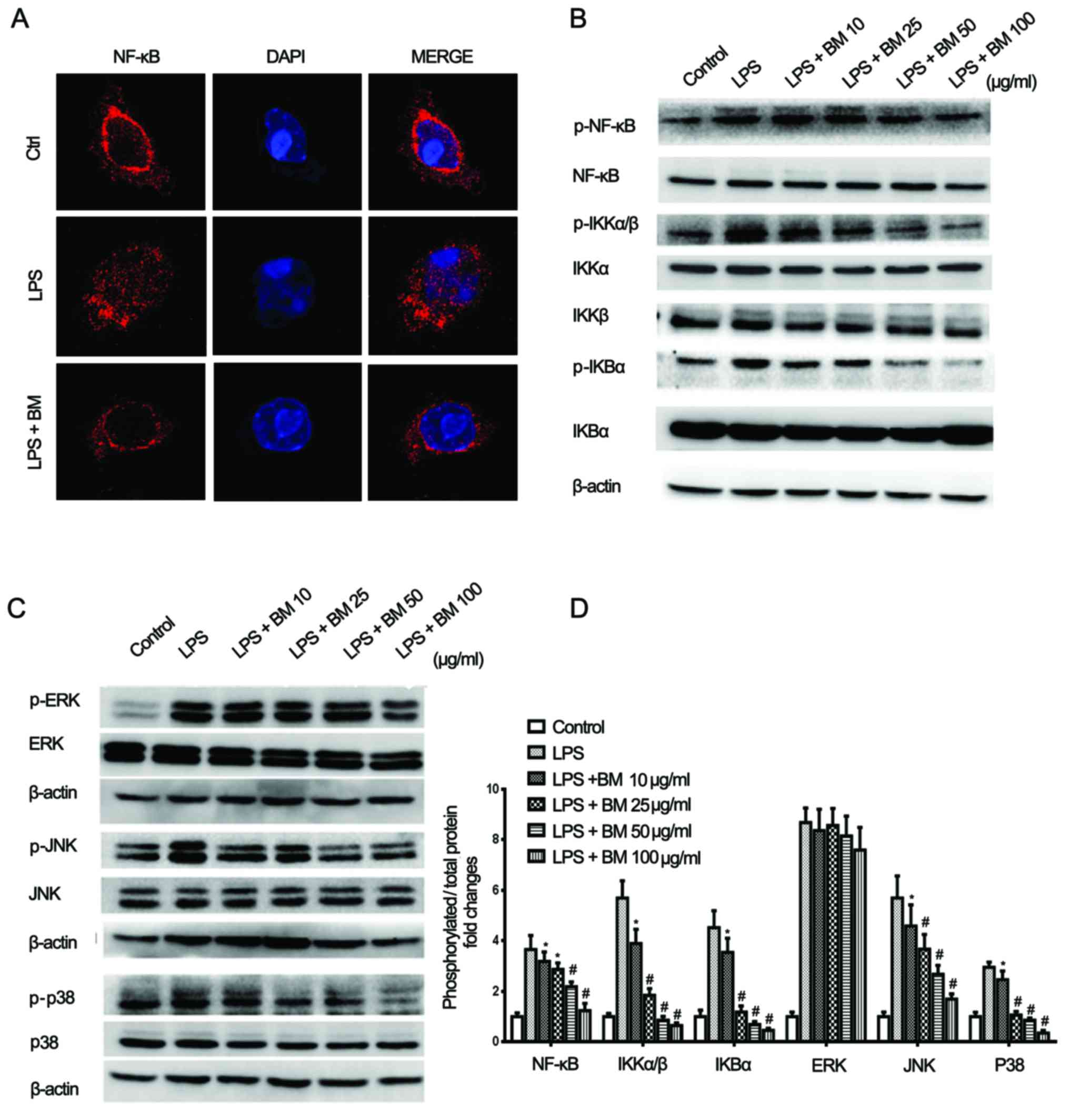 | Figure 6BM inhibits the NF-κB and MAPK
pathways. (A) Confocal fluorescence microscopy showed that BM could
effectively block the nuclear translocation of NF-κB induced by
LPS. (B and C) BM inhibited the NF-κB and MAPK pathways, presenting
as the downregulation of phosphorylated NF-κB, IKKα/β, IKBα, JNK
and p38. (D) Relative quantification of phosphorylated/total
proteins, including NF-κB, IKKα/β, IKBα, ERK, JNK and p38
(*P<0.05 and #P<0.01 vs. LPS group).
BM, broadleaf Mahonia; LPS, lipopolysaccharide; p-,
phosphorylated; NF-κB, nuclear factor-κB; MAPK, mitogen-activated
phosphate kinase; JNK, c-Jun N-terminal kinase; IKK, IκB kinase;
ERK, extracellular signal-regulated kinase. |
Since the MAPK pathway is also involved in
regulating NF-κB and AP-1 signaling transduction, the
phosphorylated levels of ERK1/2, JNK and p38 prior to and following
BM treatment were evaluated. As expected, LPS significantly induced
the phosphorylation of all three after 1 h of incubation. Notably,
BM inhibited the phosphorylation of JNK and p38 in a dose-dependent
manner, but had little effect on the phosphorylation status of
ERK1/2 (Fig. 6C and D). These
findings suggested the ability of BM to suppress JNK and p38
activation, which may be responsible for the inhibition of NF-κB
signaling and pro-inflammatory cytokine expression.
Identification of CCL-5 as the main
target of BM in cell and GLM samples
Although it was demonstrated that BM inhibited the
inflammation via the NF-κB and MAPK pathways, the precise molecular
mechanisms accounting for its anti-inflammation effects remained
unclear. In order to determine the molecular target of BM in
RAW264.7 cells, an inflammation array was applied. The supernatants
of RAW264.7 cells prior to and following BM treatment were
collected and subjected to array test. As shown in Fig. 7A, a total of 40
inflammatory-related cytokines were detected. Following BM
administration, the expression levels of CCL-2 (also known as
monocyte chemotactic protein-1), CCL-3 (also known as macrophage
inflammatory protein-1α), CCL-5 (also known as regulated on
activation, normal T cell expressed and secreted) and sTNFR1 were
significantly inhibited. The results indicated that BM could
inhibit the inflammation via interacting with multiple cytokines.
qPCR further validated that among the 4 cytokines, the inhibited
ratios of CCL-3 and CCL-5 were the most significant, implying that
the anti-inflammatory molecular target of BM may be attributed to
their downregulation (Fig. 7B).
Importantly, western blotting results further validated that the
inhibitory effects of BM on p65 phosphorylation were relieved after
adding CCL-5, indicating that the anti-inflammatory effects of BM
may be partly attributed to CCL-5 suppression (Fig. 7C).
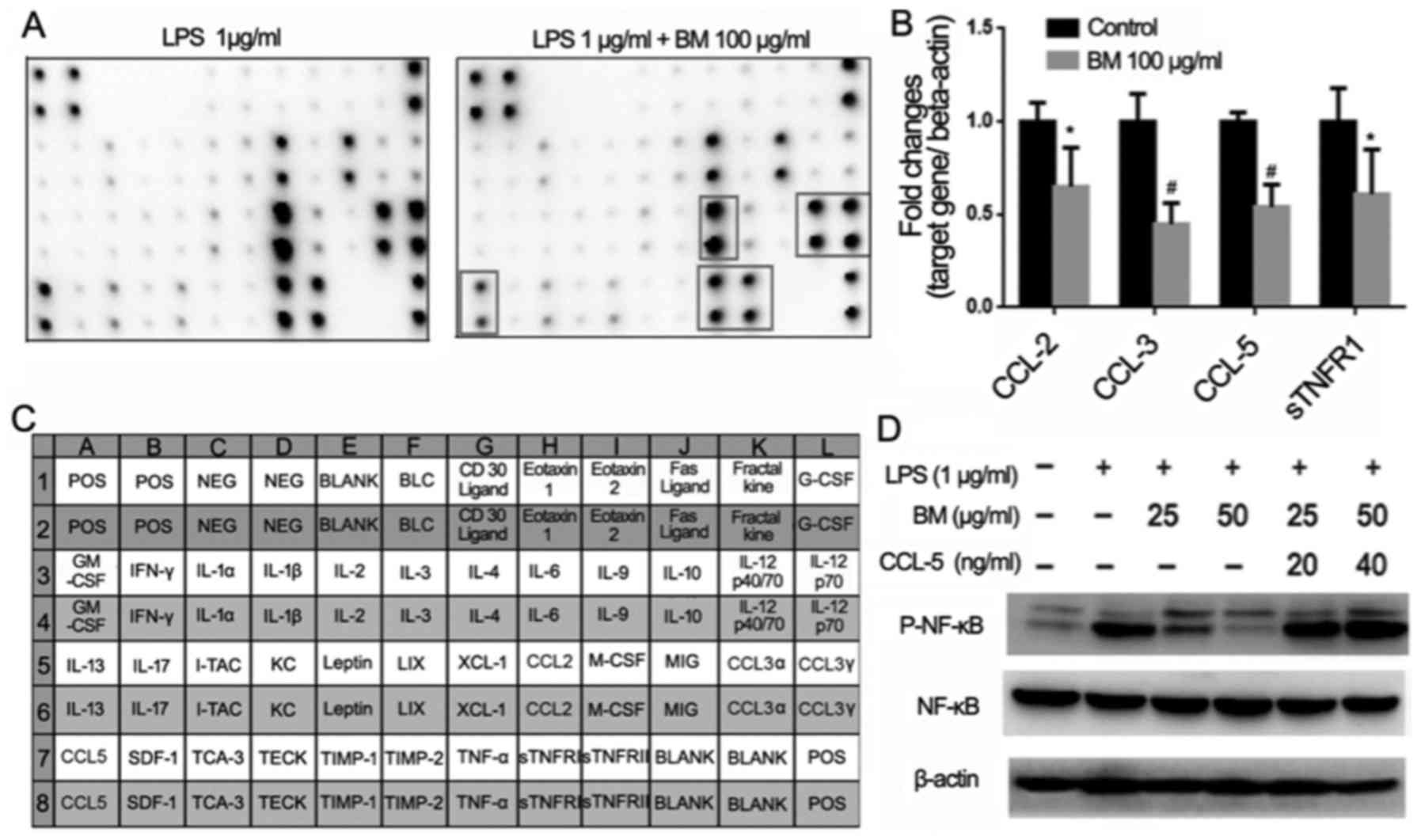 | Figure 7Effects of BM on inflammation-related
cytokine expression. (A) Cytokine array analysis revealed that BM
inhibited the expression of CCL-2, CCL-3, CCL-5 and sTNFR1. (B)
Quantitative polymerase chain reaction validated that among the
four cytokines, BM exhibited the most significant inhibitory
effects on both CCL-3 and CCL-5 (*P<0.05 and
#P<0.01 vs. control group). (C and D) The inhibitory
effects of BM on NF-κB phosphorylation were recovered after adding
CCL-5, implying that CCL-5 may be the main target of BM. BM,
broadleaf Mahonia; LPS, lipopolysaccharide; p-,
phosphorylated; NF-κB, nuclear factor-κB; IL, interleukin; STNFR1,
secreted tumor necrosis factor receptor 1; CCL, chemokine (C-C
motif) ligand; GCSF, granulocyte-colony stimulating factor; GM-CSF,
granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon
gamma; IL, interleukin; I-TAC, interferon-inducible T-cell alpha
chemoattractant; KC, chemokine (C-X-C motif) ligand 1; LIX, C-X-C
motif chemokine 5; XCL-1, chemokine (C motif) ligand; M-CSF,
macrophage colony-stimulating factor; MIG, chemokine (C-X-C motif)
ligand 9; SDF1, stromal cell-derived factor 1; TCA-3, chemokine
(C-C motif) ligand 1; TECK, chemokine (C-C motif) ligand 25; TIMP,
tissue inhibitor of metalloproteinases; TNF-α, tumor necrosis
factor α; sTNFR, soluble tumor necrosis factor receptors; ICAM,
intercellular Adhesion Molecule 1; PDGF, platelet-derived growth
factor. |
Meanwhile, human GLM samples were also collected to
validate the molecular mechanisms of BM (Fig. 8A). Consistent with the in
vitro findings, cytokine array results indicated that the
expression of CCL-2, CCL-3, CCL-5 and sTNFR was inhibited following
BM treatment; the results further confirmed the anti-inflammatory
effects of BM ex vivo (Fig.
8B and Table II). Similar to
the results in in vitro studies, CCL-5 also exhibited the
highest inhibition ratio following BM treatment, and western
blotting results also indicated that the expression of
phosphorylated p65, iNOS and COX-2 was inhibited by BM on GLM
samples (Fig. 8C). All these
results indicated that BM could inhibit inflammation via
suppressing CCL-5 in in vitro and ex vivo models.
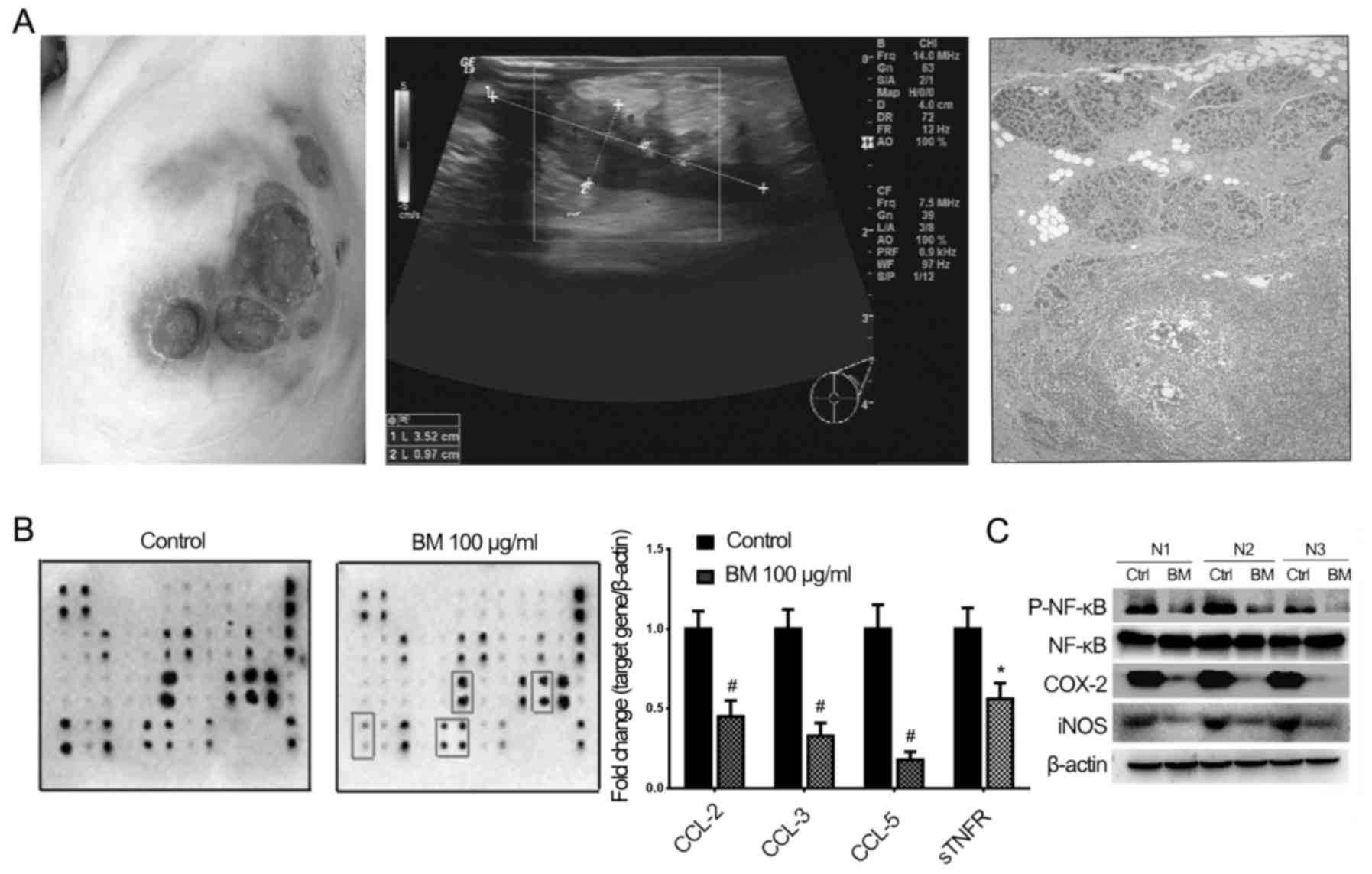 | Figure 8Inflammation inhibition effects of BM
on clinical GLM samples. (A) Representative pictures of GLM
including mammary symptoms, ultrasound image and pathological
diagnosis. (B) Cytokine array and quantitative polymerase chain
reaction analysis revealed that following BM treatment, the
expression of CCL-3, CCL-5 and sTNFR1 was inhibited
(*P<0.05 and #P<0.01 vs. control
group). (C) Western blotting results validated that BM exhibited
inhibitory effects on the expression of p-NF-κB, COX-2 and iNOS.
BM, broadleaf Mahonia; GLM, granulomatous lobular mastitis;
NF-κB, nuclear factor-κB; COX-2, cyclooxygenase-2; iNOS, inducible
nitric oxide synthase; CCL, chemokine (C-C motif) ligand. |
 | Table IIHuman inflammation cytokine
array. |
Table II
Human inflammation cytokine
array.
| No. | A | B | C | D | E | F | G | H | I | J | K | M |
|---|
| 1 | POS | POS | NEG | NEG | Eotaxin-1 | Eotaxin-2 | GCSF | GM-CSF | ICAM-1 | IFN-γ | I-309 | IL-1α |
| 2 | | | | | | | | | | | | |
| 3 | IL-1 | IL-2 | IL-3 | IL-4 | IL-6 | IL-6R | IL-7 | IL-8 | IL-10 | IL-11 | IL-12 P40 | Il-12 P70 |
| 4 | | | | | | | | | | | | |
| 5 | IL-13 | IL-15 | IL-16 | IL-17A | IP-10 | CCL2 | MCP-2 | M-CSF | MIG | CCL3α | CCL3β | MIP-1δ |
| 6 | | | | | | | | | | | | |
| 7 | CCL5 | TGF-β1 | TNF-α | TNF-β | sTNF R1 | sTNF RII | PDGF-BB | TIMP-2 | Blank | Blank | NEG | POS |
| 8 | | | | | | | | | | | | |
Discussion
Chronic inflammation is a complex set of
interactions involving multiple cells and cytokines that can arise
in any tissue in response to traumatic, post-ischemic, infectious,
autoimmune or toxic injuries (21). GLM is considered to be an
autoimmune reaction to the materials secreted from the mammary
ducts, and granuloma formation is the most representative syndrome
of the disease. Since macrophages are the first line of host
defense during the initiation of the innate and adaptive immune
responses and are hypothesized to be the root inducing granuloma
occurrence (22), it is logical
to develop anti-GLM drugs in the perspective of macrophages.
Macrophage activation could result in the secretion
of various pro-inflammatory mediators, including IL-1β, IL-6, COX-2
and iNOS (23). Deregulated
overproduction of IL-1β and IL-6 has been demonstrated to serve
pathological roles in chronic inflammatory diseases, including
Castleman's disease, Crohn's disease, rheumatoid arthritis and
juvenile idiopathic arthritis (24,25). Humanized anti-IL-1β or -IL-6
antibodies were also developed into therapeutic agents, such as
tocilizumab and canakinumab, for these diseases, and exhibited
outstanding anti-inflammatory effects in clinical trials (26). Therefore, IL-1β or IL-6 were
selected as parameters to investigate the anti-inflammatory effects
of BM in the present study. It was demonstrated that following BM
treatment, the mRNA expression levels of IL-1β and IL-6 were
downregulated, implying that macrophage activity and the
pro-inflammation process may be inhibited. In particular,
inhibition of IL-1β expression by BM was more enhanced than that of
IL-6, indicating the potential of BM to treat typical
inflammation-related disorders.
Meanwhile, BM also resulted in a significant
reduction of iNOS and COX-2 expression. Of the two isoforms of COX,
COX-1 is suggested to provide a physiological level of
prostaglandins for normal platelet, stomach and kidney function,
whereas COX-2 is highly induced at inflammatory sites in animals,
as well as in patients with inflammatory diseases (27). Since COX-2 is responsible for
prostaglandin E2 production, a critical cytokine considered as one
of the strongest inflammatory mediators, COX-2 is usually believed
to be a therapeutic target for inflammatory diseases, and COX-2
inhibitors, such as celecoxib, are widely applied to fight against
chronic inflammation clinically (28,29). The present data showed that BM
significantly inhibited COX-2 protein expression in a
dose-dependent manner, suggesting that the anti-inflammatory effect
of BM is closely associated with its inhibitory effect on COX-2.
Additionally, since iNOS is responsible for NO synthesis, which
significantly contributes to the initiation and perpetuation of
numerous inflammatory responses (30,31), the NO concentration in the cell
supernatants was detected following BM treatment. The
dose-dependent reduction of NO concentration by BM was consistent
with the decreased expression of iNOS, indicating that the blocking
effect of BM on NO formation may be attributed to the suppressed
iNOS expression. Actually, ROS and reactive nitrogen oxide species
(RNOS), including NO, are oxidants elevated in the inflammatory
process. High levels of ROS and RNOS are capable of damaging
inflammatory cells and tissues, and thereby promoting abscess
formation. ROS could also act as upstream signals to trigger
pro-inflammatory cytokine expression, and ROS scavenging is
considered to be a novel therapeutic strategy to avoid
inflammatory-associated tissue damage (32,33). The present results demonstrated
that BM was capable of reducing LPS-induced ROS accumulation in
RAW264.7 cells, indicating that BM may be active in alleviating
ROS-induced damage during GLM development.
NF-κB and MAPK pathways have been shown to act as
common downstream signaling pathways in mediating the inflammatory
responses of various types of tissues (8,9,34).
The most predominantly characterized NF-κB complex is a p50/p65
heterodimer, which is kept inactive in the cytoplasm of resting
cells by binding with the inhibitory subunit of IκBα (35,36). Upon stimulation, IκBα is
phosphorylated by IKK thereby releasing NF-κB, which subsequently
translocates into the nucleus and activates downstream gene
expression, including that of IL-1β, IL-6, COX-2 and iNOS (37). The present results indicated that
BM extract significantly inhibited NF-κB activation, presenting as
suppressed transcriptional activity, and decreased expression of
p-p65, IκBα and IKK. Additionally, confocal imaging analysis also
demonstrated that the nuclear transport of p65 was also blocked
following BM administration. All these results implied that NF-κB
suppression may be mainly responsible for the anti-inflammatory
effects of BM. MAPKs are also involved in the progress of
inflammation. Previous studies have demonstrated that the
inflammatory process is strongly blocked by inhibiting MAPK family
members, including p38, JNK and ERK (38,39). Meanwhile, MAPKs and NF-κB can
collaborate synergistically to induce pro-inflammatory cytokine
gene products and releases (40,41). The present data showed that
transcriptional activity of AP-1, a MAPK response molecule, was
dose-dependently inhibited subsequent to BM treatment.
Simultaneously, the p-JNK and p38 expression was suppressed,
whereas a limited effect was exhibited on the phosphorylation of
ERK1/2 following BM treatment. These results indicated that the
NF-κB and MAPKs pathways were involved in the anti-inflammatory
activities of BM.
A critical activity of macrophages in the
inflammation process are their ability to migrate in response to
stimuli. The interaction between macrophages and chemoattractants
not only starts a rapid and directed movement, but is also
associated with a complicated range of cellular events, including
ion flux changes, integrin avidity alterations, superoxide
production and lysosomal enzyme secretion (42). Chemokines are an important group
of chemoattractants that can be classified into four categories
based on their cysteine motifs, namely CXC, CC, CX3X and C. In the
present study, a cytokine array was utilized to screen the changes
of the chemokines in response to BM administration. The results
showed that four cytokines, CCL-2, CCL-3, CCL-5 and sTNFR,
exhibited significant reductions in expression after BM treatment,
and that CCL-5 had the highest inhibition ratio. Mounting evidence
records the abilities of CCL-2, 3 and 5 in recruiting macrophages
into inflammation sites (43–45), and the present data also revealed
that the suppressive effects of BM on NF-κB activity were relieved
following the addition of CCL-5, indicating that CCL-5 may be the
main molecular target in the anti-inflammatory activity of BM.
Previous studies demonstrated that the IKK inhibitor repressed the
mRNA levels of CCL-5 by ~81%, and these results indicated that
CCL-5 may also act as an upstream factor enhancing NF-κB activity
(46). The present study also
validated the ability of BM to inhibit the CCL-5 expression and
NF-κB activity in GLM samples; the results not only confirmed the
anti-inflammatory effects of BM ex vivo, but also suggested
that CCL-5 overexpression may contribute to the pathogenesis of
GLM, and that the underlying molecular mechanism is closely
associated with the activation of the NF-κB pathway.
Among the four identified compounds in BM extracts,
chlorogenic acid and berberine are the compounds most reported to
exhibit anti-inflammatory activities. Chlorogenic acid has been
validated to be effective in inhibiting multiple inflammation
models, including atopic dermatitis, liver and kidney injury, gout
and rheumatoid arthritis (47–50). The expression levels of IL-1β,
IL-6, TNF-α and iNOS were downregulated after chlorogenic acid
administration. Meanwhile, chlorogenic acid could also restore the
expression of superoxide dismutase, catalase and malondialdehyde in
the injured liver tissue, whereas it inhibited the transcription of
NF-κB signaling and keratinocyte chemoattractants (49,51). Berberine was also recorded to
exhibit significant anti-inflammatory activities, including
proinflammatory cytokine suppression, NF-κB/MAPK signaling
blockade, oxidative stress reduction and apoptotic induction
(52–56). Therefore, chlorogenic acid and
berberine may synergistically contribute to the anti-inflammatory
activities of BM. However, since one herb may contain hundreds of
bioactive compounds, it remains necessary to investigate the
bioactive compounds in BM using bioactivity-guided fractionation
and high-throughput technologies.
Taken together, the results of the present study
firstly validated the anti-inflammatory activities and mechanisms
of BM for GLM treatment. Meanwhile, novel light was shed on the
potential link between CCL-5 and GLM pathogenesis. However, further
research is required to investigate the bioactive compounds in BM
by targeting CCL-5, and to investigate the clinical significance of
CCL-5 in GLM treatment and model establishment.
Acknowledgments
This study was supported by grants from the National
Natural science Foundation of China (nos. 81402173 and 81573651),
the Pearl River S&T Nova Program of Guangzhou (no.
201506010098) and the Combined Scientific Project Funded by
Guangdong Provincial Science and Technology Agency and Guangdong
Provincial Academy of Traditional Chinese Medicine (no.
2014A020221047).
Glossary
Abbreviations
Abbreviations:
|
GLM
|
granulomatous lobular mastitis
|
|
BM
|
broadleaf Mahonia
|
|
COX-2
|
cyclooxygenase-2
|
|
LPS
|
lipopolysaccharide
|
|
ROS
|
reactive oxygen species
|
|
NO
|
nitric oxide
|
|
sTNFR1
|
secreted tumor necrosis factor
receptor 1
|
|
iNOS
|
inducible nitric oxide synthase
|
|
NF-κB
|
nuclear factor-κB
|
|
MAPK
|
mitogen-activated protein kinase
|
|
JNK
|
c-Jun N-terminal kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
TCM
|
traditional Chinese medicine
|
References
|
1
|
Kessler E and Wolloch Y: Granulomatous
mastitis: a lesion clinically simulating carcinoma. Am J Clin
Pathol. 58:642–646. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hovanessian Larsen LJ, Peyvandi B, Klipfel
N, Grant E and Iyengar G: Granulomatous lobular mastitis: Imaging,
diagnosis, and treatment. AJR Am J Roentgenol. 193:574–581. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mahlab-Guri K, Asher I, Allweis T, Diment
J, Sthoeger ZM and Mavor E: Granulomatous Lobular Mastitis. Isr Med
Assoc J. 17:476–480. 2015.PubMed/NCBI
|
|
4
|
Akcan A, Akyildiz H, Deneme MA, Akgun H
and Aritas Y: Granulomatous lobular mastitis: A complex diagnostic
and therapeutic problem. World J Surg. 30:1403–1409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vingerhoedt NM, Janssen S, Mravunac M,
Wauters CA and Strobbe LJ: Granulomatous lobular mastitis: a benign
abnormality that mimics malignancy. Ned Tijdschr Geneeskd.
152:1052–1056. 2008.In Dutch. PubMed/NCBI
|
|
6
|
Kfoury H and Al Bhlal L: Granulomatous
lobular mastitis: A clinicopathological study of 112 cases. Ann
Saudi Med. 17:43–46. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gurleyik G, Aktekin A, Aker F, Karagulle H
and Saglamc A: Medical and surgical treatment of idiopathic
granulomatous lobular mastitis: A benign inflammatory disease
mimicking invasive carcinoma. J Breast Cancer. 15:119–123. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoesel B and Schmid JA: The complexity of
NF-kappaB signaling in inflammation and cancer. Mol Cancer.
12:862013. View Article : Google Scholar
|
|
9
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
A 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guma M, Stepniak D, Shaked H, Spehlmann
ME, Shenouda S, Cheroutre H, Vicente-Suarez I, Eckmann L, Kagnoff
MF and Karin M: Constitutive intestinal NF-kappaB does not trigger
destructive inflammation unless accompanied by MAPK activation. J
Exp Med. 208:1889–1900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen KC, Sun MF, Yang SC, Chang SS, Chen
HY, Tsai FJ and Chen CY: Investigation into potent inflammation
inhibitors from traditional Chinese medicine. Chem Biol Drug Des.
78:679–688. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Y, Cai T, Xia X, Cai Y and Wu XY:
Research advances in the intervention of inflammation and cancer by
active ingredients of traditional Chinese medicine. J Pharm Pharm
Sci. 19:114–126. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Pharmacopoeia Committee:
Pharmacopoeia of People's Republic of China [M]. Part 1. Beijing:
China Medical Science Press; pp. 85–86. 2015
|
|
14
|
Wang N, Wang Z, Peng C, You J, Shen J, Han
S and Chen J: Dietary compound isoliquiritigenin targets GRP78 to
chemosensitize breast cancer stem cells via β-catenin/ABCG2
signaling. Carcinogenesis. 35:2544–2554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SY, Hwang JS, Jang M, Lee SH, Park JH
and Han IO: A novel caffeic acid-1-piperonylpiperazine
hybridization compound HBU-47 inhibits LPS-mediated inflammation in
RAW264.7 macrophage cells. Int Immunopharmacol. 19:60–65. 2014.
View Article : Google Scholar
|
|
16
|
Cho EC, Kuo ML, Cheng JH, Cheng YC, Hsieh
YC, Liu YR, Hsieh RH and Yen Y: RRM2B-mediated regulation of
mitochondrial activity and inflammation under oxidative stress.
Mediators Inflamm. 2015:2873452015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maianskiĭ DN: The pathogenesis of chronic
inflammation. Ter Arkh. 64:3–7. 1992.In Russian.
|
|
18
|
Watanabe K, Kawamori T, Nakatsugi S and
Wakabayashi K: COX-2 and iNOS, good targets for chemoprevention of
colon cancer. Biofactors. 12:129–133. 2000. View Article : Google Scholar
|
|
19
|
Dinarello CA, Simon A and van der Meer JW:
Treating inflammation by blocking interleukin-1 in a broad spectrum
of diseases. Nat Rev Drug Discov. 11:633–652. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fonseca JE, Santos MJ, Canhão H and Choy
E: Interleukin-6 as a key player in systemic inflammation and joint
destruction. Autoimmun Rev. 8:538–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kzhyshkowska J, Gudima A, Moganti K,
Gratchev A and Orekhov A: Perspectives for
monocyte/macrophage-based diagnostics of chronic inflammation.
Transfus Med Hemother. 43:66–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schulert GS and Grom AA: Macrophage
activation syndrome and cytokine-directed therapies. Best Pract Res
Clin Rheumatol. 28:277–292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dinarello CA: A clinical perspective of
IL-1beta as the gatekeeper of inflammation. Eur J Immunol.
41:1203–1217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishimoto N and Kishimoto T: Inhibition of
IL-6 for the treatment of inflammatory diseases. Curr Opin
Pharmacol. 4:386–391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rajakariar R, Yaqoob MM and Gilroy DW:
COX-2 in inflammation and resolution. Mol Interv. 6:199–207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kapoor M, Shaw O and Appleton I: Possible
anti-inflammatory role of COX-2-derived prostaglandins:
Implications for inflammation research. Curr Opin Investig Drugs.
6:461–466. 2005.PubMed/NCBI
|
|
28
|
Idanpaan-Heikkila JE, Idanpaan-Heikkila JJ
and Klaukka T: Treatment for inflammation related pain - COX-2
inhibitors knocking on the door. Duodecim. 120:229–234. 2004.In
Finnish.
|
|
29
|
del Zoppo G, Ginis I, Hallenbeck JM,
Iadecola C, Wang X and Feuerstein GZ: Inflammation and stroke:
Putative role for cytokines, adhesion molecules and iNOS in brain
response to ischemia. Brain Pathol. 10:95–112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Predonzani A, Cali B, Agnellini AH and
Molon B: Spotlights on immunological effects of reactive nitrogen
species: When inflammation says nitric oxide. World J Exp Med.
5:64–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
32
|
Harijith A, Ebenezer DL and Natarajan V:
Reactive oxygen species at the crossroads of inflammasome and
inflammation. Front Physiol. 5:3522014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan Y, Mao R and Yang J: NF-kappaB and
STAT3 signaling pathways collaboratively link inflammation to
cancer. Protein Cell. 4:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar
|
|
35
|
Ali S and Mann DA: Signal transduction via
the NF-kappaB pathway: A targeted treatment modality for infection,
inflammation and repair. Cell Biochem Funct. 22:67–79. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gilmore TD: The Rel/NF-kappaB signal
transduction pathway: Introduction. Oncogene. 18:6842–6844. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schieven GL: The biology of p38 kinase: A
central role in inflammation. Curr Top Med Chem. 5:921–928. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ip YT and Davis RJ: Signal transduction by
the c-Jun N-terminal kinase (JNK) - from inflammation to
development. Curr Opin Cell Biol. 10:205–219. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haddad JJ: The role of inflammatory
cytokines and NF-kappaB/MAPK signaling pathways in the evolution of
familial Mediterranean fever: Current clinical perspectives and
potential therapeutic approaches. Cell Immunol. 260:6–13. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saklatvala J: Inflammatory signaling in
cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use
of inhibitors for research into pathogenesis and therapy of
osteoarthritis. Curr Drug Targets. 8:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schulert GS and Grom AA: Pathogenesis of
macrophage activation syndrome and potential for cytokine- directed
therapies. Annu Rev Med. 66:145–159. 2015. View Article : Google Scholar
|
|
42
|
Sierra-Filardi E, Nieto C, Dominguez-Soto
A, Barroso R, Sanchez-Mateos P, Puig-Kroger A, Lopez-Bravo M, Joven
J, Ardavin C, Rodriguez-Fernandez JL, et al: CCL2 shapes macrophage
polarization by GM-CSF and M-CSF: Identification of
CCL2/CCR2-dependent gene expression profile. J Immunol.
192:3858–3867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen S, Jiao J, Jiang D, Wan Z, Li L, Li
K, Xu L, Zhou Z, Xu W and Xiao J: T-box transcription factor
Brachyury in lung cancer cells inhibits macrophage infiltration by
suppressing CCL2 and CCL4 chemokines. Tumour Biol. 36:5881–5890.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Datar I, Qiu X, Ma HZ, Yeung M, Aras S, de
la Serna I, Al-Mulla F, Thiery JP, Trumbly R, Fan X, et al: RKIP
regulates CCL5 expression to inhibit breast cancer invasion and
metastasis by controlling macrophage infiltration. Oncotarget.
6:39050–39061. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsang MS, Jiao D, Chan BC, Hon K-L, Leung
P, Lau C, Wong E, Cheng L, Chan C, Lam C, et al: Anti-inflammatory
activities of pentaherbs formula, berberine, gallic acid and
chlorogenic acid in atopic dermatitis-like skin inflammation.
Molecules. 21:5192016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Washio K, Kobayashi M, Saito N, Amagasa M
and Kitamura H: Propolis ethanol extract stimulates cytokine and
chemokine production through NF-κB activation in C2C12 myoblasts.
Evid Based Complement Alternat Med. 2015:3497512015. View Article : Google Scholar
|
|
47
|
Meng ZQ, Tang ZH, Yan YX, Guo CR, Cao L,
Ding G, Huang WZ, Wang ZZ, Wang KD, Xiao W, et al: Study on the
anti-gout activity of chlorogenic acid: Improvement on
hyperuricemia and gouty inflammation. Am J Chin Med. 42:1471–1483.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng Z, Sheng Y, Lu B and Ji L: The
therapeutic detoxification of chlorogenic acid against
acetaminophen-induced liver injury by ameliorating hepatic
inflammation. Chem Biol Interact. 238:93–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shi H, Dong L, Jiang J, Zhao J, Zhao G,
Dang X, Lu X and Jia M: Chlorogenic acid reduces liver inflammation
and fibrosis through inhibition of toll-like receptor 4 signaling
pathway. Toxicology. 303:107–114. 2013. View Article : Google Scholar
|
|
50
|
Feng Y, Yu YH, Wang ST, Ren J, Camer D,
Hua YZ, Zhang Q, Huang J, Xue DL, Zhang XF, et al: Chlorogenic acid
protects D-galactose-induced liver and kidney injury via
antioxidation and anti-inflammation effects in mice. Pharm Biol.
54:1027–1034. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lin K, Liu S, Shen Y and Li Q: Berberine
attenuates cigarette smoke-induced acute lung inflammation.
Inflammation. 36:1079–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li Z, Zheng J, Zhang N and Li C: Berberine
improves airway inflammation and inhibits NF-kappaB signaling
pathway in an ovalbumin-induced rat model of asthma. J Asthma.
53:999–1005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu L, Li Q, Yu B, Yang Y, Jin Z, Duan W,
Zhao G, Zhai M, Liu L, Yi D, et al: Berberine attenuates myocardial
ischemia/reper-fusion injury by reducing oxidative stress and
inflammation response: Role of silent information regulator 1. Oxid
Med Cell Longev. 2016:16896022016. View Article : Google Scholar
|
|
54
|
Kim BY, Park HR, Jeong HG and Kim SW:
Berberine reduce allergic inflammation in a house dust mite
allergic rhinitis mouse model. Rhinology. 53:353–358.
2015.PubMed/NCBI
|
|
55
|
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan
T, Xu S, Peng J, Xie X and Huang H: Berberine attenuates
lipopolysaccharide-induced extracelluar matrix accumulation and
inflammation in rat mesangial cells: Involvement of NF-kappaB
signaling pathway. Mol Cell Endocrinol. 331:34–40. 2011. View Article : Google Scholar
|
|
56
|
Li H, Zhu L, Yuan G, Heng S, Yi B, Ma C,
Shen J, Tu J, Fu T and Wen J: Fine mapping and candidate gene
analysis of an anthocyanin-rich gene, BnaA.PL1 conferring purple
leaves in Brassica napus L. Molecular genetics and genomics. MGG.
291:1523–1534. 2016. View Article : Google Scholar
|