Introduction
Inflammation is a biological defense mechanism in
response to microbes and risk factors such as harmful stimuli and
pathogens. The inflammatory response involves inflammatory cells,
complement mediators and molecular mediators. In particular,
macrophages have an important role in the immediate responses to
external stimuli such as lipopolysaccharides (LPS) (1). LPS are extracellular components of
Gram-negative bacteria, which cause various inflammatory reactions.
Toll-like receptor 4 (TLR4) is a class of proteins expressed on the
cell surface to recognize LPS, which leads to signal transmission
and macrophage cells activation (2,3).
TLR4-mediated signaling activates inflammatory pathways such as the
mitogen-activated protein kinase (MAPK) pathways and nuclear
factor-κB (NF-κB) signaling (4).
The transcription of NF-κB and activator protein-1
(AP-1) in inflammatory pathways produces inflammatory mediators,
cytokines and chemokines (2).
Particularly nitric oxide (NO), an inflammatory mediator, has an
important role in the pathogenesis of inflammation-associated
diseases (5). In LPS-stimulated
macrophages, NO is significantly increased, and as part of the
early inflammatory response, the secretion of cytokines, including
interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), is also
increased. The increase of these inflammatory mediators and
pro-inflammatory cytokines causes chronic inflammation and may even
develop into an inflammatory disease (6).
In addition, previous studies have suggested that NF
(erythroid-derived 2)-like-2 (Nrf-2), a protective factor against
oxidative stress, is involved in anti-inflammatory processes
(7,8). Nrf-2/heme oxygenase-1 (HO-1)
signaling has been reported to downregulate the overproduction of
inducible NO synthase (iNOS) and pro-inflammatory cytokines
(9). The transcription of HO-1
following Nrf-2 activation inactivates or neutralizes NF-κB
signaling (10). 5′-Adenosine
monophosphate-activated protein kinase (AMPK) has various functions
and acts as a sensor of stress in the cytoplasm. AMPK has been
studied as a target to interfere with inflammatory signaling. The
AMPK pathway indirectly inhibits NF-κB signaling (11) and the phosphorylation of AMPK has
direct or indirect anti-inflammatory effects (12).
Castanea seguinii Dode (CS), also known as
Castanea davidii Dode or Chinese Chinquapin, is a plant
belonging to the Castanea genus of the Fagaceae family. The
fruit of various species of the Castanea genus is known to
be edible, and the honey of Castanea sativa has antioxidant
and antibacterial effects (13),
while its fruit has been used for medicinal purposes to treat
inflammation (14). Leaves,
chestnut burs and the bark of Castanea plants were used for
natural medicine products, which are robust and effective in
treating nutrition issues, including nutrient deprivation as well
as hemostasis, diarrhea, nausea and vomiting. CS is widely used as
a traditional remedy in Asia; however, its biological activity and
the underlying mechanisms have remained to be fully elucidated. The
present study hypothesized that CS methanolic extract (CSME) exerts
its anti-inflammatory effects by directly targeting inflammatory
signaling, while also targeting molecules indirectly associated
with inflammation. These effects were the focus of the present
study.
Materials and methods
Preparation of the plant extract
Leaves and stems of Castanea seguinii (Maoli)
were collected from LinAn, (Hangzhou, China) (15) and identified by S.W. Lee. Dried
Castanea seguinii leaves and stems (221 g) were ground and
extracted with 3 volumes of MeOH followed by sonication several
times for over the course of three days to obtain CSME as a powder
(63 g).
Cell culture
RAW264.7 macrophage cells (ATCC, Manassas, VA, USA)
were subcultured in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
a 1% antibiotic-antimycotic solution and fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere
containing 5% CO2 at 37°C.
Cell viability assay
Cell viability was determined by an MTT assay.
RAW264.7 cells at a density of 1×104 cells/well were
seeded into 96-well plates and stimulated with CSME at various
concentrations (5–40 µg/ml) for 24 h. Subsequently, 5
µl MTT stock (5 mg/ml; Amresco, LLC, Solon, OH, USA) was
added to each well, followed by incubation for 4 h. After removing
the supernatant, the formazan crystals that had formed were
dissolved by addition of dimethyl sulfoxide and agitation for 10
min at room temperature. The absorbance at 570 nm was then measured
to determine the optical density that was proportional to the
amount of viable cells.
NO assay
RAW264.7 cells were seeded in a 96-well culture
plate (SPL, Gyeonggi-do, Korea) at 5×104 cells/well were
incubated for 16 h, followed by pretreatment with CSME for 30 min
and subsequent incubation with LPS (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 0.5 µg/ml for 24 h. NO secretion in
the culture supernatant was determined using the Griess reagent
(Griess reagent I, 1% sulfanilamide; Griess reagent II, 0.1%
N-(1-naphathyl)-ethylenediamine dihydrochloride and 5% phosphoric
acid) and incubated while shaking at room temperature for 10 min.
The concentration of NO was measured at 540 nm.
Enzyme-linked immunosorbent assay
(ELISA)
Pro-inflammatory cytokines in the culture
supernatant were determined using ELISA kits (TNF set, cat. no.
558534; IL-6 set, cat. no. 555240; BD Biosciences, Santa Clara, CA,
USA; and MCP-1 set, cat. no. DY479; R&D Systems, Inc.,
Minneapolis, MN, USA) using. The 96-well microplates used in this
study were coated with a carbonate-bicarbonate buffer (0.05 M; pH
9.6) overnight at 4°C. The plates were incubated with a blocking
buffer (10% FBS in PBS) at room temperature for 1 h and then
washed. Subsequently, CSME and Dex were pretreated for 1 h and then
treated with LPS. After 24 h, the diluted supernatant and a
standard were added to each well, followed by incubation at room
temperature for 2 h. The samples were then washed and incubated
with a horseradish peroxidase (HRP)-conjugated detection antibody
in blocking buffer at room temperature for 1 h. Concentrations were
determined with a substrate solution (BD Biosciences) at room
temperature for 30 min, at which point the reaction was stopped
with H2SO4 and the absorbance read at 450
nm.
Reverse transcription-polymerase chain
reaction analysis (RT-PCR)
Total RNA was harvested at 6 h after a pretreatment
with CSME for 1 h followed by treatment with LPS. The total RNA was
isolated using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The synthesis of complementary DNA was
performed using a QuantiTect reverse transcription kit (cat. no.
205310; Qiagen, Hilden, Germany) with 1 µg of RNA. For PCR
amplification, the primer sequences used were as follows: β-actin
sense, 5′-TGT TTG AGA CCT TCA ACA CC-3′ and antisense, 5′-CGC TCA
TTG CCG ATA GTG AT-3′; iNOS sense, 5′-CAA GAG TTT GAC CAG AGG
ACC-3′ and antisense, 5′-TGG AAC CAC TCG TAG TTG GGA-3′. The PCR
conditions were as follows: 94°C for 5 min followed by 30 cycles of
94°C for 30 sec, 60°C for 30 sec, and 72°C for 45 sec and a final
extension at 72°C for 10 min. The GoTaq® G2 Green Master
Mix (cat. no. M7823; Promega, Madison, USA) was used. PCR products
were separated on a 1.5% agarose gel with RedSafe™ kits (Intron
Biotechnology, Inc., Gyeonggi-do, Korea). Images of the gels were
captured with an Olympus C4000 zoom camera system (Olympus, Tokyo,
Japan) and were analyzed by ImageJ software (version 1.50e;
National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
The RAW264.7 cells were pretreated with CSME for 1
h. NP40 (cat. no. EBA-1049; Elpis Biotech, Inc., Daejeon, Korea)
and NE-PER Nuclear and Cytoplasmic Extraction Reagents (cat. no.
78833; Thermo Fisher Scientific, Inc.) were used for protein
extraction. A Pierce™ BCA Protein assay kit (cat. no. 23225; Thermo
Fisher Scientific, Inc.) was used to quantify the extracted
protein. Total protein, cytosolic protein and nuclear protein were
separated by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) (20 µg) and the samples were
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skimmed
milk, followed by incubation with primary antibodies overnight at
4°C. The following primary antibodies and dilutions were used:
Anti-β-actin (cat. no. 4967), anti-phosphorylated (p)-extracellular
signal-regulated kinase (ERK; cat. no. 4370), anti-p-p38 (cat. no.
9211), anti-p-AMPK (cat. no. 2535), anti-inhibitor of NF-κB (IκB-α;
cat. no. 2859), anti-c-Fos (cat. no. 2250), anti-c-Jun (cat. no.
9165) (1:1,000 dilution; Cell Signaling Technology, Inc., Danvers,
MA, USA), anti-iNOS (cat. no. ADI-905-431, 1:1,000 dilution; Enzo
Life Science, Farmingdale, NY, USA), anti-p-c-Jun N-terminal kinase
(JNK) (cat. no. sc-6254), anti-JNK (cat. no. sc-474), anti-ERK
(cat. no. sc-154), anti-p38 (cat. no. sc-7149), anti-Nrf-2 (cat.
no. sc-722), anti-AMPK (cat. no. sc-25792) and anti-proliferating
cell nuclear antigen (PCNA; cat. no. sc-56, 1:1,000 dilution; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The membranes with the
primary antibodies were washed three times with Tris-buffered
saline containing 0.1% Tween-20 (TBS-T) for 10 min. Finally, the
membranes were incubated with HRP-conjugated secondary antibodies
(anti-mouse, cat. no. sc-2005; anti-rabbit, cat. no. sc-2030;
1:5,000 dilution; Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h. The membranes were washed three times with
TBS-T for 10 min and an enhanced chemiluminescence kit (cat. no.
32106; Thermo Fisher Scientific, Inc.) was used to visualize the
protein bands (LAS-4000 luminescent image analyzer; Fujifilm,
Tokyo, Japan). For quantification, band density values were
assessed using Fuji Multi Gauge version 3.0 (Fujifilm).
Immunohistochemistry assay
RAW264.7 cells were seeded into Chamber Slides
(Thermo Fisher Scientific, Inc.) incubated overnight and then
washed. Subsequently, they were fixed with 4% paraformaldehyde and
treated with 0.1% Triton X-100 (Bio-Rad Laboratories, Inc.) to
permeabilize the membrane. Subsequently, samples were incubated
overnight at 4°C with the first antibody to Nrf-2 (cat. no. sc-722,
1:200 dilution; Santa Cruz Biotechnology, Inc.). Subsequently,
slides were treated with Alexa Fluor 488-conjugated secondary
antibody (cat. no. A-11034; Thermo Fisher Scientific, Inc.) for 1 h
at room temperature for nuclear staining, Hoechst 33342 was added,
followed by washing and mounting with Vectashield®
medium (Vector Laboratories, Inc., Burlingame, CA, USA). The cells
were observed and images were captured with an LSM 510 Meta system
(Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical significance was determined by analysis of
two-group for Student's t-test (Excel 2013; Microsoft Corp.,
Redmond, WA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
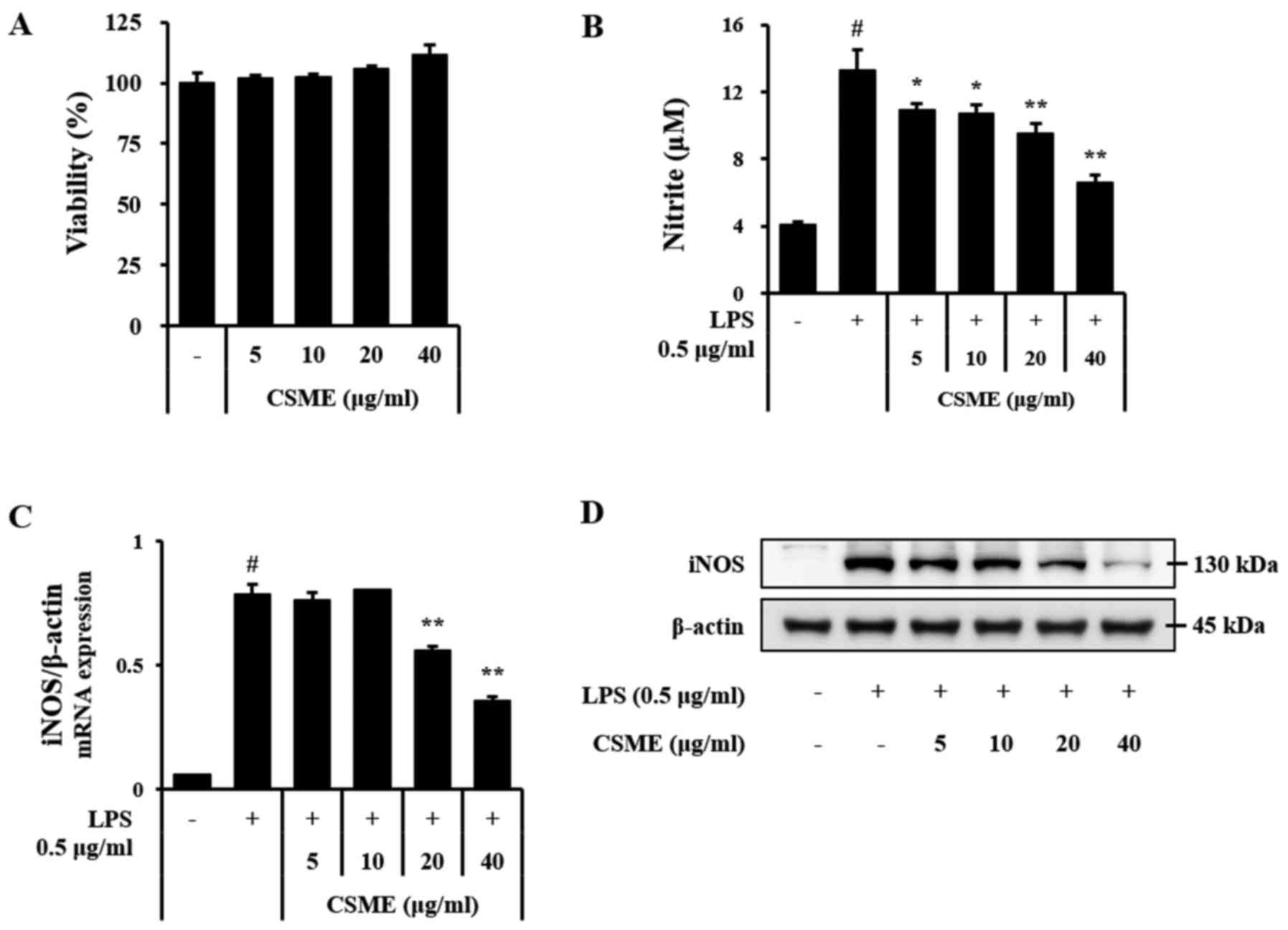
Inhibitory effect of CSME on LPS-induced
NO production
To assess the effect of CSME on cell viability,
RAW264.7 cells were treated with CSME for 24 h and subjected to the
MTT assay. The results indicated cell viability exceeded 95% of
CSME at a concentration of 5–40 µg/ml (Fig. 1A). Based on this outcome, an NO
assay was performed in order to determine the inhibitory effects of
CSME on inflammation. The LPS-induced production of NO in RAW264.7
cells was clearly decreased by pretreatment with CSME (Fig. 1B). The effects of CSME on the mRNA
and protein levels of iNOS were also assessed. In agreement with
the above results, the LPS-induced expression of iNOS was inhibited
by pretreatment with CSME in a dose-dependent manner (Fig. 1C and D).
Inhibitory effects of CSME on LPS-induced
pro-inflammatory cytokine and chemokine secretion
ELISAs were performed to determine whether CSME
affected the expression levels of pro-inflammatory cytokines and
chemokines. The results demonstrated that the increased expression
of IL-6 and TNF-α in LPS-treated RAW264.7 cells was inhibited by
pretreatment with CSME (Fig. 2A and
B) and that the secretion of monocyte chemoattractant protein-1
(MCP-1) was also markedly decreased (Fig. 2C).
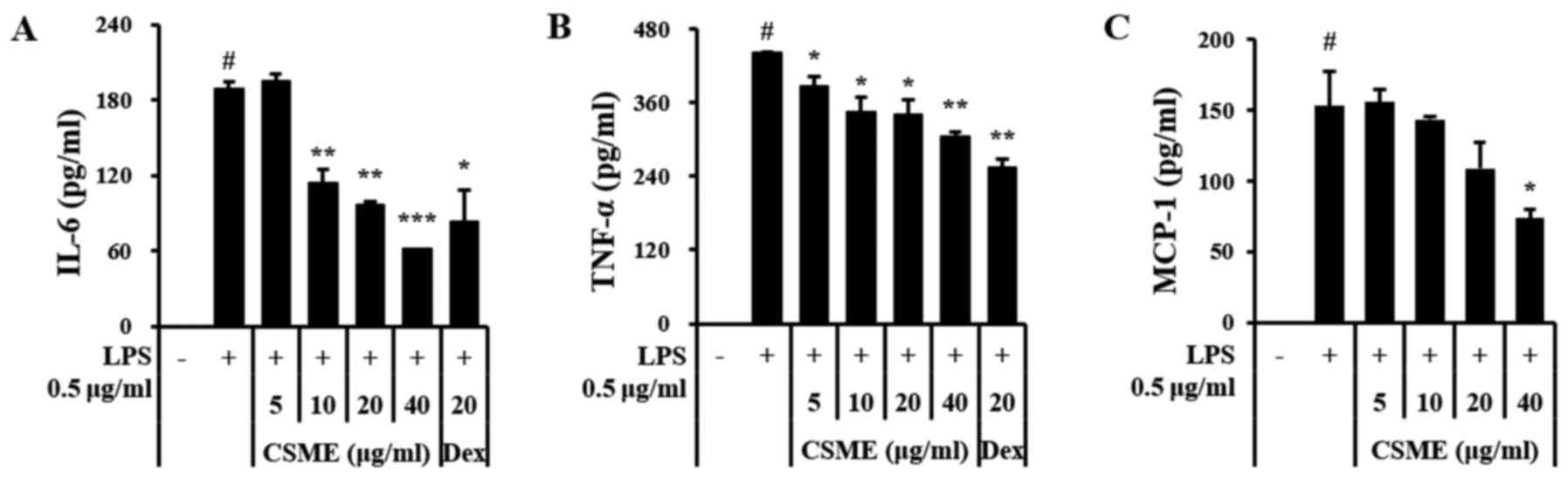 | Figure 2Effects of CSME on pro-inflammatory
cytokines in LPS-induced RAW264.7 cells. Cells were pretreated with
CSME (5–40 µg/ml) for 1 h, followed by a treatment with LPS
(0.5 µg/ml) for 24 h and measurement of (A) IL-6, (B) TNF-α
and (C) MCP-1 by ELISA. #P<0.05 vs. control;
*P<0.05, **P<0.01 and ***P<0.001 vs.
cells treated with LPS alone. LPS, lipopolysaccharide; CSME,
Castanea seguinii Dode methanolic extract; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α; MCP-1, monocyte
chemoattractant protein-1; Dex, dexamethasone; ELISA, enzyme-linked
immunosorbent assay. |
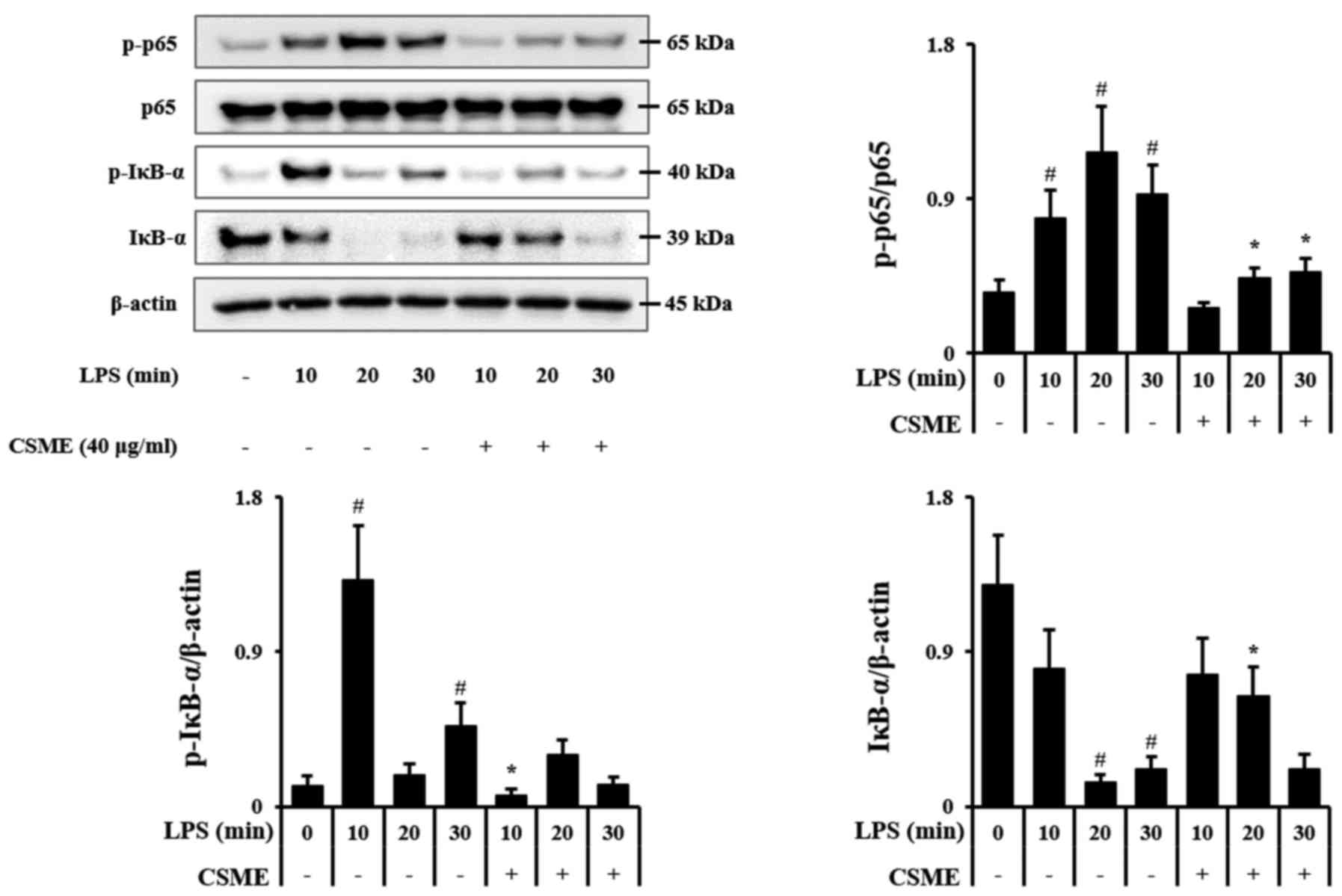
Effects of CSME on LPS-induced
upregulation of NF-κB and MAPK signaling
In order to elucidate the molecular mechanisms of
action of CSME, RAW264.7 cells were treated with LPS for 10, 20 and
30 min with or without pretreatment by CSME, and the
phosphorylation outcomes of p65 and IκB-α along with the
degradation of IκB-α were assessed by western blot analysis. The
rapid increase of p-IκB-α after stimulation with LPS was inhibited
by pretreatment with CSME, and the degradation of IκB-α was also
decreased. LPS-induced phosphorylation of p65 was also decreased by
CSME (Fig. 3).
The present study also investigated the effects of
CSME on MAPK signaling proteins in order to determine which
molecular signaling pathways of inflammation it interferes with.
RAW264.7 cells were pretreated with or without CSME and then
stimulated with LPS for 10, 20 or 30 min, followed by assessment of
the phosphorylation of the MAPKs by western blot analysis. The
results demonstrated that the phosphorylation levels of ERK, JNK
and p38 were increased in the LPS-treated RAW264.7 cells (the
phosphorylation of JNK and ERK peaked at 30 min, while that of p38
peaked at 20 min). However, these LPS-induced increases of p-JNK,
p-ERK and p-p38 in RAW264.7 cells were significantly inhibited by
pretreatment with CSME (Fig. 4A).
In the same manner, involvement of the AP-1 signaling pathway was
confirmed. Nuclear translocation of c-Fos and c-Jun was increased
in RAW264.7 cells after treatment with LPS for 10, 30 and 60 min in
a time-dependent manner. Of note, the translocation of c-Fos and
c-Jun was significantly reduced in the cells pretreated with CSME
(Fig. 4B).
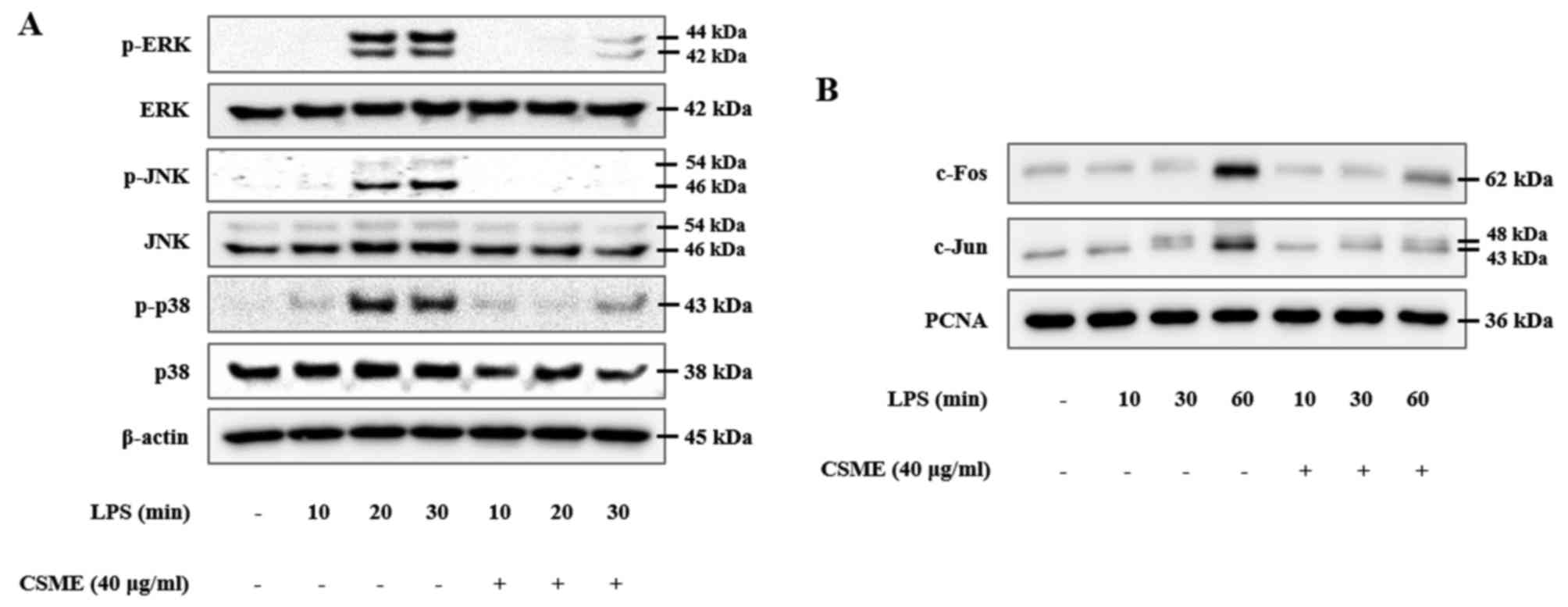 | Figure 4Downregulation of activator protein-1
transcription factor components through inhibition of
mitogen-activated protein kinases by CSME in LPS-induced RAW264.7
cells. (A) Cells were pretreated with or without CSME (5-40
µg/ml) for 1 h and then treated with LPS (0.5 µg/ml)
for 10, 20 or 30 min, after which the phosphorylation of JNK, ERK
and p38 were compared by western blot analysis. (B) Furthermore,
the nuclear protein fraction was isolated, in which c-Fos and c-Jun
were subsequently detected by western blot analysis. CSME,
Castanea seguinii Dode methanolic extract; LPS,
lipopolysaccharide; p-ERK, phosphorylated extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; PCNA,
proliferating cell nuclear antigen. |
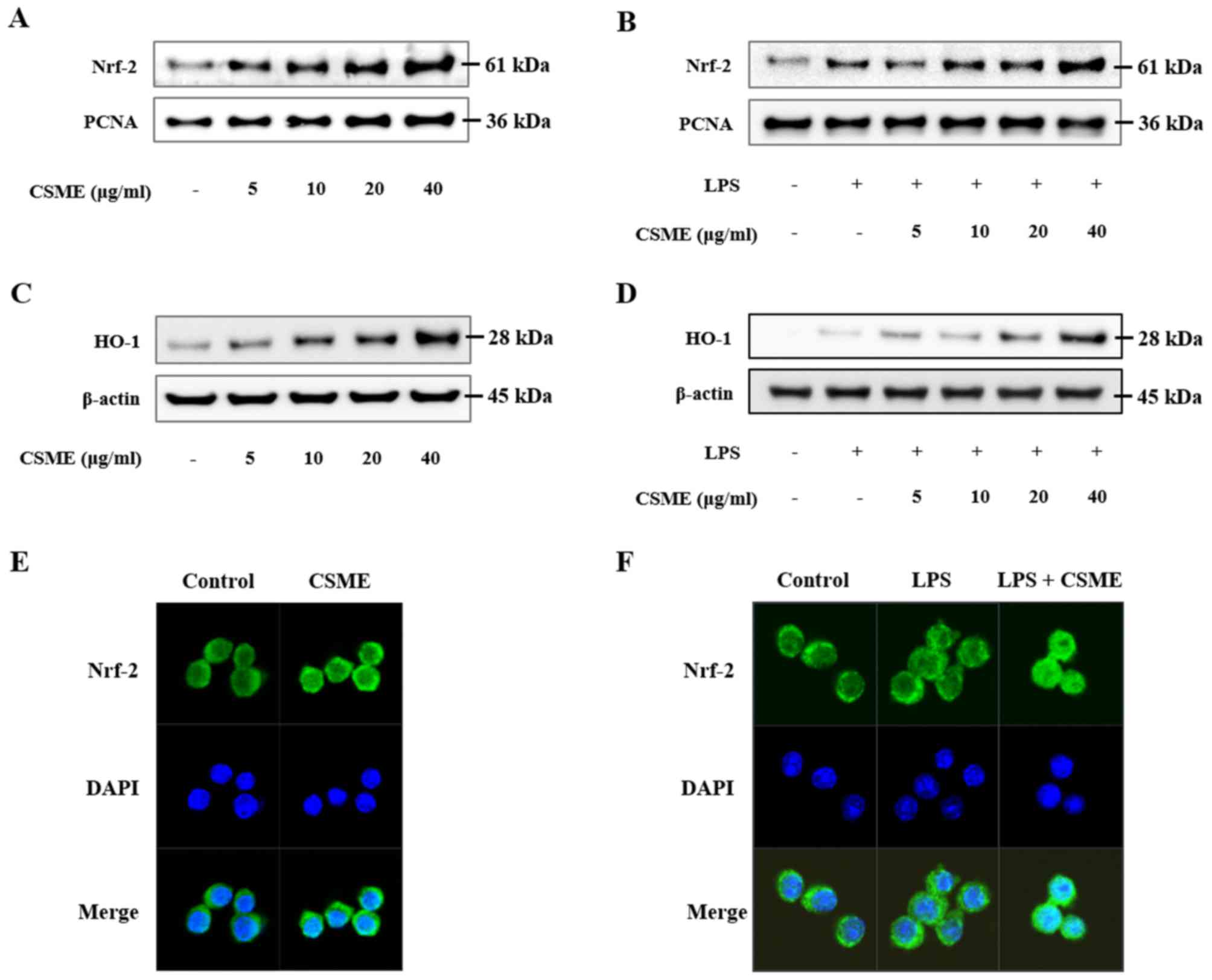
Effect of CSME on Nrf-2 and AMPK
Nuclear protein was isolated and Nrf-2, a
transcription factor associated with anti-oxidative processes, was
assessed by western blot analysis (Fig. 5). Nuclear Nrf-2 was increased by
CSME in a dose-dependent manner (Fig.
5A). CSME also increased trans-location of Nrf-2 in LPS-induced
RAW264.7 cells (Fig. 5B). In
order to confirm the translocation of Nrf-2 into the nucleus, an
immunohistochemistry assay was performed. The results demonstrated
that the translocation of Nrf-2 in RAW264.7 cells was increased by
CSME with or without LPS (Fig. 5E and
F). In addition, the production of HO-1 was increased by CSME
in a dose-dependent manner in RAW264.7 cells with or without LPS
treatment (Fig. 5C and D).
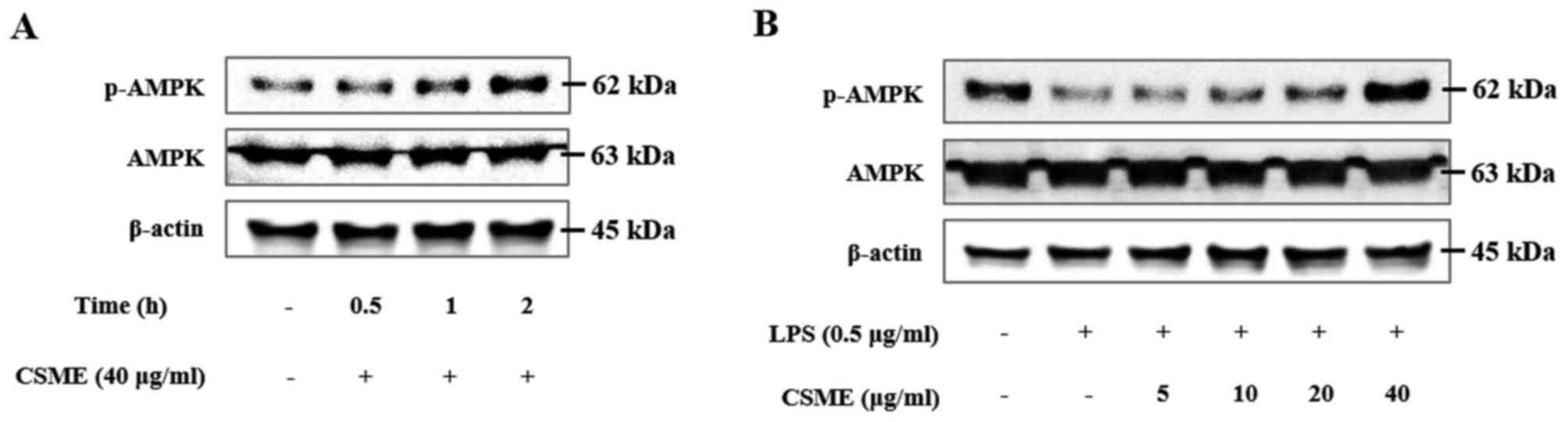
Subsequently, the effect of CSME on the
phosphorylation of AMPK in RAW264.7 cells was examined.
CSME-treated RAW264.7 cells were harvested at intervals of 30 min,
1 h and 2 h. The phosphorylation of AMPK exceeded the basal level
at 1 and 2 h of CSME treatment (Fig.
6A). In addition, RAW264.7 cells were pretreated with CSME and
then stimulated with LPS for 2 h, after which the levels of p-AMPK
were assessed by western blot analysis. In the cells treated with
LPS alone, the levels of p-AMPK were decreased; however, this
effect was inhibited by CSME (Fig.
6B). CSME increased p-AMPK in the presence and in the absence
of LPS in RAW264.7 cells.
Discussion
Macrophages have an important role in inflammation.
Their induction with stimulants such as LPS leads to macrophage
activation by a variety of inflammatory signaling mechanisms,
leading to the secretion of inflammatory mediators and cytokines
(1,16). In particular, TLR signaling by
external stimuli is essential for innate immune responses, and it
results in the activation of inflammatory transcription factors
such as NF-κB and AP-1 (17).
Thus, in the present study, the anti-inflammatory effects of
pretreatment with CSME on LPS-induced inflammatory response in the
RAW264.7 mouse macrophage cell line were observed.
NF-κB, consisting of the p65 and p50 subunits, is a
transcription factor that induces the expression of inflammatory
genes. These subunits block IκB-α in its normal state, but upon
stimulation by the inflammatory response, IκB-α undergoes
phosphorylation and is degraded, while NF-κB is activated (18). LPS induces the activation of
NF-κB, which then stimulates the expression of pro-inflammatory
cytokines, including IL-6 and TNF-α, as well as chemokines
(19). Therefore, the present
study confirmed that CSME suppressed LPS-induced inflammatory
mediators and cytokines, as well as phosphorylation of IκB-α and
p65, in RAW264.7 cells. Therefore, it was demonstrated that CSME
has anti-inflammatory effects through inhibition of NF-κB
signaling.
MAPKs are a class of protein kinases that have the
function of directing cellular responses to external stimuli. MAPK
signaling refers to the activation of ERK, JNK and p38 (20,21). MAPKs also regulate the activity of
the AP-1 transcription factor. AP-1 is a heterodimer formed by the
c-Fos and c-Jun subunits. These are phosphorylated by ERK, JNK and
p38 and then translocate to the nucleus (22,23). AP-1 regulates the expression of
inflammatory cytokines and inflammatory mediators (6). Accordingly, the present study
assessed the effects of the CSME on MAPK signaling. It was revealed
that CSME inhibited the LPS-induced phosphorylation of ERK, JNK and
p38 in RAW264.7 cells. In addition, the effect on the downstream
AP-1 was assessed, which demonstrated that CSME inhibited the
LPS-induced nuclear translocation of c-Fos and c-Jun in RAW264.7
cells. Taken together, CSME was indicated to inhibit the activation
of AP-1 by suppressing the activation of MAPK signaling. These
results indicated that CSME appears to block the upstream kinases
of NF-κB and MAPKs (Fig. 7).
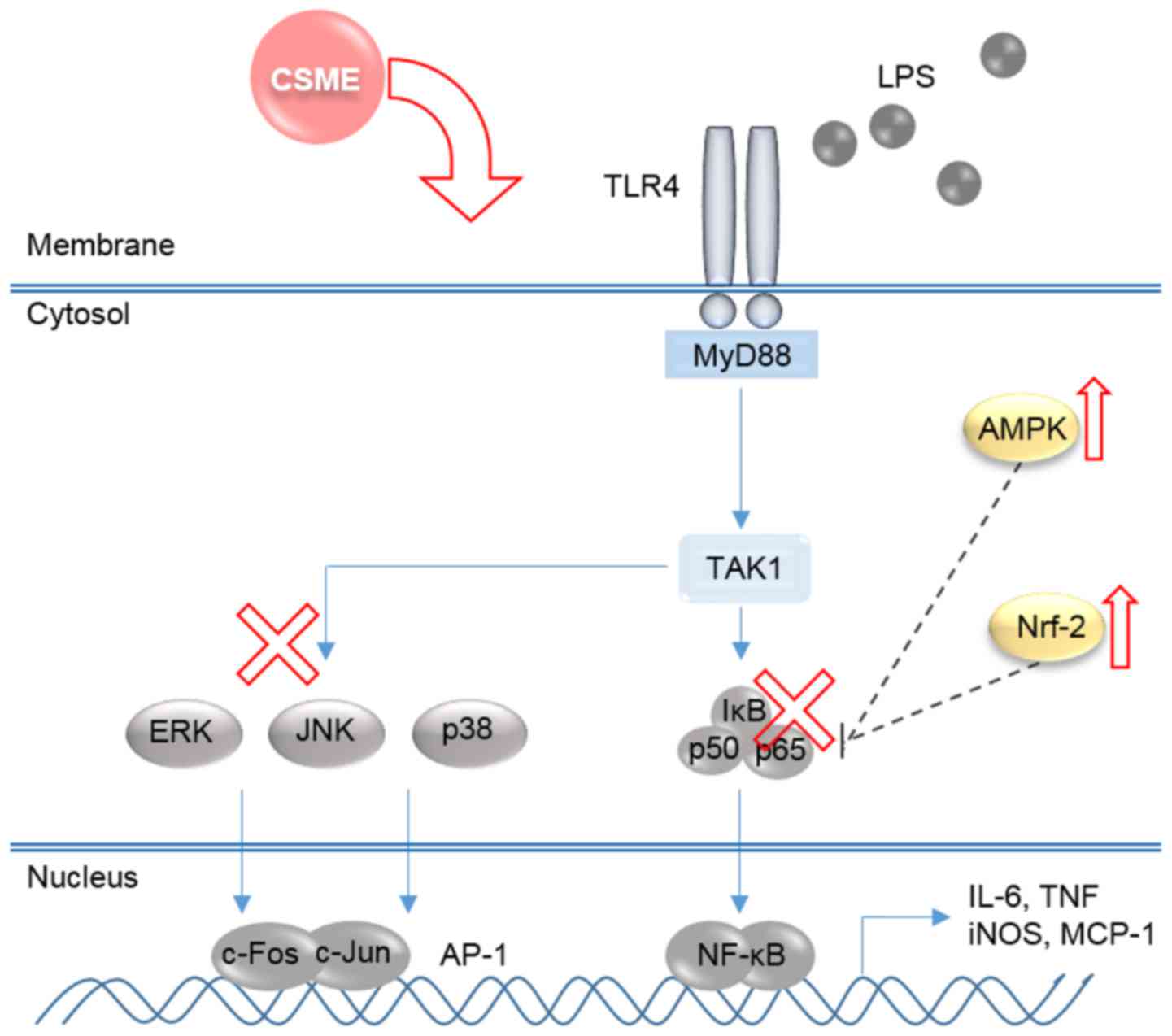 | Figure 7Schematic diagram illustrating the
mechanisms underlying the anti-inflammatory effects of CSME. CSME,
Castanea seguinii Dode methanolic extract; LPS,
lipopolysaccharide; NF-κB, nuclear factor-κB; IκB-α, inhibitor of
NF-κB; AMPK, adenosine monophosphate kinase; AP-1, activator
protein-1; Nrf-2, nuclear factor (erythroid-derived 2)-like-2;
iNOS, inducible nitrogen oxide synthase; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; IL-6,
interleukin-6; TNF-α, tumor necrosis factor-α; MCP-1, monocyte
chemoattractant protein-1; TLR, Toll-like receptor; MyD88, myeloid
differentiation primary response gene 88; TAK1, transforming growth
factor β-activated kinase 1. |
HO-1, an enzyme that catalyzes the degradation of
heme, is expressed following activation of Nrf-2 through oxidative
stress (24). Increased
production of HO-1 was demonstrated to decrease the inflammatory
response in an endotoxin shock model (25). The present study confirmed that
CSME increases HO-1 by activating Nrf-2. CSME also increased the
translocation of Nrf-2 into the nucleus in RAW264.7 cells. The
production of HO-1 in RAW264.7 cells was also increased by CSME.
These results demonstrated that CSME has an antioxidant effect.
High concentrations of LPS induce a large amount of oxidative
stress and Nrf-2 is activated as a defense mechanism. As a result,
CSME activates Nrf-2 and increases the expression of HO-1 without
being affected by LPS in RAW264.7 cells (Fig. 5).
AMPK was reported to be involved in the inflammatory
response. AMPK deficiency increased the expression of LPS-induced
pro-inflammatory cytokines in macrophages (26). The activation of AMPK has been
demonstrated to down-regulate NF-κB, and AMPK therefore also
functions as an anti-inflammatory agent (27). In addition, AMPK/activating
transcription factor 3 signaling inhibits the phosphorylation of
p38, which led to a protective effect of AMPK activation in an
LPS-induced murine endotoxemia model (28). In macrophages, the AMPK/Sirtuin 1
pathway was reported to exert anti-inflammatory effects through
deacetylation of NF-κB (29).
Activation of Nrf-2 was reported to have AMPK-dependent
anti-inflammatory effects, and the functional association between
AMPK and the Nrf-2 pathway has an important role in the suppression
of inflammation (12). The
present study observed the effects of CSME on AMPK, which is
involved in anti-inflammatory responses. CSME had the effect of
activating AMPK reduced by LPS stimulation in RAW264.7 cells. The
results of the present study indicated that CSME has
anti-inflammatory effects via various molecular targets by directly
or indirectly acting on TLR4 signaling stimulated by LPS (Fig. 7). Thus, the present study
confirmed that AMPK and Nrf-2 are involved in anti-inflammatory
responses.
In conclusion, the present study demonstrated that
CSME suppressed the LPS-induced production of inflammatory
mediators and inflammatory cytokines in RAW264.7 macrophage cells
by inhibiting the NF-κB and MAPK signaling pathways, while also
enhancing the anti-inflammatory activity through the activation of
Nrf-2 and AMPK. It is therefore suggested that CSME has therapeutic
potential in inflammation-associated diseases.
Acknowledgments
This study was supported by the National Foundation
for Science and Technology Development (grant no.
NRF-2016K1A1A8A01939075) and the KRIBB of Korea (grant no.
KGM1221713). Some aspects of the present study were reported as
posters at the 8th KRIBB Poster Festival (2016; Daejeon,
Korea).
References
|
1
|
Gordon S: Alternative activation of
macrophages. Nat Rev Immunol. 3:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beutler B: Inferences, questions and
possibilities in Toll-like receptor signalling. Nature.
430:257–263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto M, Sato S, Hemmi H, Uematsu S,
Hoshino K, Kaisho T, Takeuchi O, Takeda K and Akira S: TRAM is
specifically involved in the Toll-like receptor 4-mediated
MyD88-independent signaling pathway. Nat Immunol. 4:1144–1150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hong JM, Kwon OK, Shin IS, Jeon CM, Shin
NR, Lee J, Park SH, Bach TT, Hai V, Oh SR, et al: Anti-inflammatory
effects of methanol extract of Canarium lyi C.D. Dai & Yakovlev
in RAW 264.7 macrophages and a murine model of
lipopolysaccharide-induced lung injury. Int J Mol Med.
35:1403–1410. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Islam T, Breton C, Salam MT, McConnell R,
Wenten M, Gauderman WJ, Conti D, Van Den Berg D, Peters JM and
Gilliland FD: Role of inducible nitric oxide synthase in asthma
risk and lung function growth during adolescence. Thorax.
65:139–145. 2010. View Article : Google Scholar
|
|
6
|
Lu Y, Suh SJ, Kwak CH, Kwon KM, Seo CS, Li
Y, Jin Y, Li X, Hwang SL, Kwon O, et al: Saucerneol F, a new
lignan, inhibits iNOS expression via MAPKs, NF-κB and AP-1
inactivation in LPS-induced RAW264.7 cells. Int Immunopharmacol.
12:175–181. 2012. View Article : Google Scholar
|
|
7
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010. View Article : Google Scholar
|
|
8
|
Kobayashi EH, Suzuki T, Funayama R,
Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi
H, Nakayama K, et al: Nrf2 suppresses macrophage inflammatory
response by blocking proinflammatory cytokine transcription. Nat
Commun. 7:116242016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai ZY, Sheng ZX and Yao H: Pachymic acid
ameliorates sepsis-induced acute kidney injury by suppressing
inflammation and activating the Nrf2/HO-1 pathway in rats. Eur Rev
Med Pharmacol Sci. 21:1924–1931. 2017.PubMed/NCBI
|
|
10
|
Lee SH, Sohn DH, Jin XY, Kim SW, Choi SC
and Seo GS: 2′,4′,6′-tris(methoxymethoxy) chalcone protects against
trinitrobenzene sulfonic acid-induced colitis and blocks tumor
necrosis factor-alpha-induced intestinal epithelial inflammation
via heme oxygenase 1-dependent and independent pathways. Biochem
Pharmacol. 74:870–880. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salminen A, Hyttinen JM and Kaarniranta K:
AMP-activated protein kinase inhibits NF-κB signaling and
inflammation: Impact on healthspan and lifespan. J Mol Med (Berl).
89:667–676. 2011. View Article : Google Scholar
|
|
12
|
Mo C, Wang L, Zhang J, Numazawa S, Tang H,
Tang X, Han X, Li J, Yang M, Wang Z, et al: The crosstalk between
Nrf2 and AMPK signal pathways is important for the
anti-inflammatory effect of berberine in LPS-stimulated macrophages
and endotoxin-shocked mice. Antioxid Redox Signal. 20:574–588.
2014. View Article : Google Scholar :
|
|
13
|
Kolayli S, Can Z, Yildiz O, Sahin H and
Karaoglu SA: A comparative study of the antihyaluronidase,
antiurease, antioxidant, antimicrobial and physicochemical
properties of different unifloral degrees of chestnut (Castanea
sativa Mill.) honeys. J Enzyme Inhib Med Chem. 31(Suppl 3): 96–104.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zlatanov MD, Antova GA, Angelova-Romova MJ
and Teneva OT: Lipid composition of Castanea sativa Mill. and
Aesculus hippocastanum fruit oils. J Sci Food Agric. 93:661–666.
2013. View Article : Google Scholar
|
|
15
|
Ahn K: The worldwide trend of using
botanical drugs and strategies for developing global drugs. BMB
Rep. 50:111–116. 2017. View Article : Google Scholar :
|
|
16
|
Chen IT, Hsu PH, Hsu WC, Chen NJ and Tseng
PH: Polyubiquitination of transforming growth factor β-activated
Kinase 1 (TAK1) at lysine 562 residue regulates TLR4-mediated JNK
and p38 MAPK activation. Sci Rep. 5:123002015. View Article : Google Scholar
|
|
17
|
Kueanjinda P, Roytrakul S and Palaga T: A
novel role of numb as a regulator of pro-inflammatory cytokine
production in macrophages in response to Toll-like receptor 4. Sci
Rep. 5:127842015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ajibade AA, Wang HY and Wang RF: Cell
type-specific function of TAK1 in innate immune signaling. Trends
Immunol. 34:307–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JW, Kwon OK, Yuniato P, Marwoto B,
Lee J, Oh SR, Kim JH and Ahn KS: Amelioration of an LPS-induced
inflammatory response using a methanolic extract of Lagerstroemia
ovalifolia to suppress the activation of NF-κB in RAW264.7
macrophages. Int J Mol Med. 38:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arthur JS and Ley SC: Mitogen-activated
protein kinases in innate immunity. Nat Rev Immunol. 13:679–692.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nateri AS, Spencer-Dene B and Behrens A:
Interaction of phosphorylated c-Jun with TCF4 regulates intestinal
cancer development. Nature. 437:281–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitmarsh AJ and Davis RJ: Transcription
factor AP-1 regulation by mitogen-activated protein kinase signal
transduction pathways. J Mol Med (Berl). 74:589–607. 1996.
View Article : Google Scholar
|
|
24
|
Guo RF and Ward PA: Role of oxidants in
lung injury during sepsis. Antioxid Redox Signal. 9:1991–2002.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tamion F, Richard V, Renet S and Thuillez
C: Intestinal preconditioning prevents inflammatory response by
modulating heme oxygenase-1 expression in endotoxic shock model. Am
J Physiol Gastrointest Liver Physiol. 293:G1308–G1314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sag D, Carling D, Stout RD and Suttles J:
Adenosine 5′-mono-phosphate-activated protein kinase promotes
macrophage polarization to an anti-inflammatory functional
phenotype. J Immunol. 181:8633–8641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salminen A and Kaarniranta K:
AMP-activated protein kinase (AMPK) controls the aging process via
an integrated signaling network. Ageing Res Rev. 11:230–241. 2012.
View Article : Google Scholar
|
|
28
|
Liu X, Wang N, Fan S, Zheng X, Yang Y, Zhu
Y, Lu Y, Chen Q, Zhou H and Zheng J: The citrus flavonoid
naringenin confers protection in a murine endotoxaemia model
through AMPK-ATF3-dependent negative regulation of the TLR4
signalling pathway. Sci Rep. 6:397352016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue B, Yang Z, Wang X and Shi H: Omega-3
polyunsaturated fatty acids antagonize macrophage inflammation via
activation of AMPK/SIRT1 pathway. PLoS One. 7:e459902012.
View Article : Google Scholar : PubMed/NCBI
|