Introduction
Renal cell carcinoma (RCC) is the most common
space-occupying lesion in adult kidneys and accounts for ~3% of all
tumors. In addition, the mortality rate of RCC is ≤40% (1). RCC is derived from renal proximal
tubule cells, and clear cell carcinoma is the most common subtype,
which accounts for 80–85% of metastatic RCC (2). With regards to treatment, RCC is not
sensitive to radiotherapy or chemotherapy. Although surgical tumor
resection is the optimal treatment strategy at present, it is
associated with 20–40% postoperative relapse. Since RCC is
resistant to radiotherapy and chemotherapy, effective postoperative
adjuvant treatment for RCC is lacking (3). In addition, the lack of biochemical
markers for the early diagnosis of RCC, as well as follow-up data,
hinders the timely diagnosis of RCC. Therefore, there is an urgent
need to understand the development of RCC at molecular and genetic
levels, and to identify biochemical markers that can
postoperatively predict the early metastasis of RCC (4).
MicroRNA (miRNA) expression profile screening is one
of the most advanced methods used to research tumor-associated
molecules (5). miRNAs are
endogenous, non-coding, single-stranded RNAs. The mechanism of
action of miRNAs is one kind of the epigenetic regulation and
control mechanism, and miRNAs serve important roles in the
regulation of gene expression (6). It has been estimated that one miRNA
can regulate the expression of ~100 target genes, and mRNAs of
>10,000 genes are directly regulated by miRNAs (7). miRNAs are highly conserved among
various species and regulate numerous biological functions
(6). In addition, miRNAs are able
to simultaneously regulate the expression of oncogenes and tumor
suppressor genes through regulating critical cellular activities,
including metabolism, differentiation, development and apoptosis.
It has previously been reported that miRNAs serve a crucial
regulatory role in carcinogenesis of prostate cancer, lung cancer,
breast cancer and colon cancer, and miRNAs are also considered to
be involved in the pathogenesis of RCC (8,9).
Numerous RCC-specific miRNAs have been identified through the
screening of miRNA expression profiles, the majority of which exert
their functions by disrupting the balance between oncogene and
tumor suppressor gene expression (8). A recent study indicated that the
regulatory mechanisms of miRNAs in RCC are complex, and are
associated with numerous biological mechanisms, including
mutations, epigenetic alterations, chromosomal abnormalities and
deletions (10).
miRNA-30a-5p has been reported to inhibit metadherin
(MTDH) expression, which serves an important role in tumor
development. The MTDH gene is located in chromosome 8q22, and
exhibits high expression in numerous tumor types, including breast
cancer, prostate cancer, neuroblastoma and astrocytoma, where it is
associated with poor prognosis (11). MTDH mediates several forms of
chemotherapy resistance, and it has previously been demonstrated
that the activation of MTDH expression can increase the death of
tumor cells mediated by drugs, including cisplatin and doxorubicin
(7). In addition, MTDH can
enhance the invasion of tumor cells and increase the expression
levels of relevant adhesion molecules via the nuclear factor-κB
pathway (12). MTDH is the
downstream gene of Ha-ras, which induces upregulation of MTDH via
the phosphoinositide 3-kinase (P13K)/protein kinase B
(AKT)/glycogen synthase kinase 3β/c-Myc pathway (13).
Among the numerous signal transduction pathways that
mediate tumor cell apoptosis, the PI3K/phosphatase and tensin
homolog (PTEN)/AKT signal transduction pathway is essential for
growth regulation, and has an important role in the regulation of
apoptosis. Activation of the PI3K/AKT signal transduction pathway
can inhibit apoptosis induced by numerous stimuli and promote cell
cycle progression, thus promoting cell survival and proliferation.
Furthermore, this pathway participates in angiogenesis, serves an
important role in tumor formation, and participates in tumor
invasion and metastasis (14). As
well as being able to induce the survival and differentiation of
tumor cells, angiogenesis, and malignant development, the PI3K/AKT
signal transduction pathway is associated with therapeutic
resistance (15). At present,
tumor treatments that target this pathway have gained extensive
attention and abnormal activation of the PI3K/PTEN/AKT signaling
pathway has been detected in several human tumor types, including
lung cancer, pancreatic cancer, leukemia, liver cancer, multiple
myeloma, prostate cancer and RCC (16). The present study aimed to
investigate the effects of miRNA-30a-5p on tumor proliferation and
to seek a potential therapeutic target for the treatment of human
renal cancer.
Materials and methods
Tissue samples
A total of 229 patients with RCC from the Second
Hospital of Tianjin Medical University (Tianjin, China) were
included in the present study from March 2004 to October 2005;
detailed clinicopathological characteristics of the patients are
summarized in Table I. Renal
parenchyma (RP) samples (5 cm) and RCC samples were collected from
the patients. The RCC samples were divided into the following
categories: TNM stage I + II, TNM stage III + IV and metastatic.
The present study was approved by the Ethics Committee of The
Second Hospital of Tianjin Medical University, Tianjin Medical
University (Tianjin, China) and informed consent was obtained from
all participants.
 | Table IClinical characteristics of the
patients. |
Table I
Clinical characteristics of the
patients.
| Characteristic | RCC (n=219) |
|---|
| Gender |
| Male | 122 |
| Female | 97 |
| Age at surgery [mean
(range)] | 64 (33–79) |
| TNM stage, n
(%) |
| I | 76 (34.70) |
| II | 27 (12.33) |
| III | 99 (45.20) |
| IV | 8 (3.65) |
| Not available | 9 (4.11) |
| Metastasis, n
(%) |
| M0 | 191 (87.21) |
| M1 | 3 (1.37) |
| NA | 25 (11.42) |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cancer samples and
RP using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Total RNA (500 ng) was reverse transcribed
into cDNA using the PrimeScript RT reagent kit (Takara Bio, Inc.,
Otsu, Japan) according to the manufacturer's protocol. The
miRNA-30a-5p, si-MTDH and negative plasmids were obtained from
Tianjin Sainie Biologineering Technology Co., Ltd. (Tianjin,
China). The sequence of miRNA-30a-5p was: miR-30a-5p,
UGUAAACAUCCUCGACUGGAAG. qPCR was performed on an Applied Biosystems
PCR7900 system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using SYBR-Green PCR Master Mix reagent kit (Takara Bio, Inc.). U6
was used as an internal control. The qPCR thermocycling conditions
were as follows: 95°C for 5 min, followed by 40 cycles at 95°C for
15 sec, 57°C for 60 sec and 72°C for 30 sec. Relative miRNA-30a-5p
expression levels were calculated using the 2−ΔΔCq
method (17).
Cell culture and transfection
Caki-2 cells, purchased from Shanghai Cell Bank of
Chinese Academy of Sciences (Shanghai, China), were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum and 1% penicillin/streptomycin (all Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a humidified atmosphere
containing 5% CO2 and 95% air. The miRNA-30a-5p and
negative plasmids were obtained from Tianjin Sainie Biologineering
Technology Co., Ltd. Caki-2 cells (1×106) were seeded in
a 6-well plate and were transfected with 100 ng miRNA-30a-5p and
100 ng negative plasmids using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Transfected cells were maintained
at 37°C in a 5% CO2 atmosphere for 4 h, and then old
DMEM was removed and new DMEM was added into the cells at 37°C in a
5% CO2 atmosphere.
MTT assay
Post-transfection for 24 h, Caki-2 cells were
adjusted to l×104 cells/ml, inoculated in a 96-well
plate and cultured for an additional 48 h. Subsequently, 10
µl MTT was added to each well for 4 h, after which dimethyl
sulfoxide was added to each well for 20 min. The absorbance of each
well was detected at 490 nm using an Orion II microplate
luminometer (Berthold Technologies GmbH & Co. KG, Bad Wildbad,
Germany). The overexpression of miR-30a-5p signifcantly suppressed
cell proliferation and induced apoptosis of Caki-2 cells at 48
h.
Apoptosis assay
Post-transfection for 48 h, Caki-2 cells were
adjusted to l×106 cells/ml and washed with PBS.
Subsequently, the cells were incubated with 5 µl Annexin and
5 µl propidium iodide (both from BD Pharmingen, San Diego,
CA, USA) for 15 min in the dark at room temperature. Flow
cytometric analysis was conducted using a CyAn flow cytometer
(Beckman Coulter, Miami, FL, USA) and the results were analyzed
using ModFit (Verity Software House, Inc., Topsham, ME, USA).
Caspase-3 and caspase-9 activity
assay
Post-transfection for 48 h, Caki-2 cells were
adjusted to l×106 cells/ml, inoculated in a 6-well plate
and proteins were extracted using radioimmunoprecipitation assay
(RIPA) buffer (Beyotime Institute of Biotechnology, Nanjing,
China). Protein concentrations were determined using Micro
Bicinchoninic Acid (BCA) Protein Assay kit (EMD Millipore,
Billerica, MA, USA). Equal amounts of protein (50 µg) were
incubated with reagents from the caspase-3 (C1116) and caspase-9
(C1158) activities kit (Beyotime Institute of Biotechnology,
Haimen, China) for 2 h at 37°C. The absorbance of each well was
detected at 405 nm using an Orion II microplate luminometer
(Berthold Technologies GmbH & Co. KG).
Western blot analysis
Post-transfection for 48 h, Caki-2 cells were
adjusted to l×106 cells/ml, inoculated in a 6-well plate
and proteins were extracted using RIPA buffer. Protein
concentrations were determined using Micro BCA Protein Assay kit
(EMD Millipore). Equal amounts of protein (50 µg) were
separated by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore). After incubation with 5%
non-fat milk in TBST for 1 h at 37°C, the membranes were incubated
with the following specific primary antibodies: B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax; 1:2,000; 14796; Cell Signaling
Technology, Inc., Beverly, MA, USA), Bcl-2 (1:1,000; 3498; Cell
Signaling Technology, Inc.), MTDH (1:1,000; sc-517220; Santa Cruz
Biotechnology, Santa Cruz, CA, USA), PTEN (1:1,000; sc-6817-R;
Santa Cruz Biotechnology), phosphorylated (p)-AKT (1:1,000;
sc-7985-R; Santa Cruz Biotechnology) and GAPDH (1:2,000; 5174; Cell
Signaling Technology, Inc.) at 4°C overnight. Subsequently,
membranes were incubated with an anti-rabbit horseradish
peroxidase-conjugated secondary antibody (1:5,000; 7074; Cell
Signaling Technology, Inc.) for 1 h at 37°C. An enhanced
chemiluminescence western blotting detection system (EMD Millipore)
was used to analyze protein expression levels. An enhanced
chemiluminescence western blotting detection system (EMD Millipore)
was used to analyze protein expression levels and results were
semi-quantified using Image-ProPlus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Immunocytofluorescence
Post-transfection for 12 h, Caki-2 cells
(l×104 cells/ml) were seeded into cell chamber slides
(Corning Life Sciences, Tewksbury, MA, USA) and were fixed with 4%
formaldehyde. Subsequently, cells were incubated with anti-MTDH
overnight at 4°C for 24 h at room temperature, followed by
incubation with the appropriate fluorescent secondary antibodies
(1:500 dilution) at room temperature for 1 h. Cells were also
stained with DAPI at room temperature for 0.5 h. Images were
obtained using an FV1000 confocal microscope (Olympus Corporation,
Center Valley, PA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS 17.0 (n=3). For analyzing significance between the
various groups, one-way analysis of variance by Tukey's post test
(for comparison among 3 groups) or Student's t-test (for comparison
between 2 groups) was conducted. Overall survival (OS) and disease
free survival (DFS) were analyzed using Kaplan-Meier test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miRNA-30a-5p expression
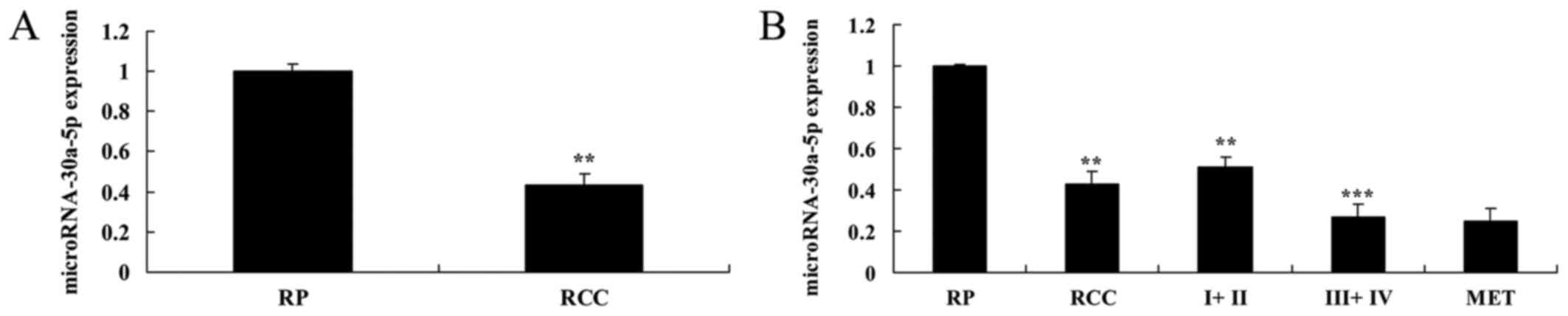
The present study detected the expression levels of
miRNA-30a-5p using RT-qPCR. As presented in Fig. 1A, miRNA-30a-5p expression was
lower in RCC tissue samples compared with in RP tissue samples. In
addition, miRNA-30a-5p expression was much lower in samples from
patients with TNM stage III + IV RCC compared with in the RP tissue
samples (Fig. 1B).
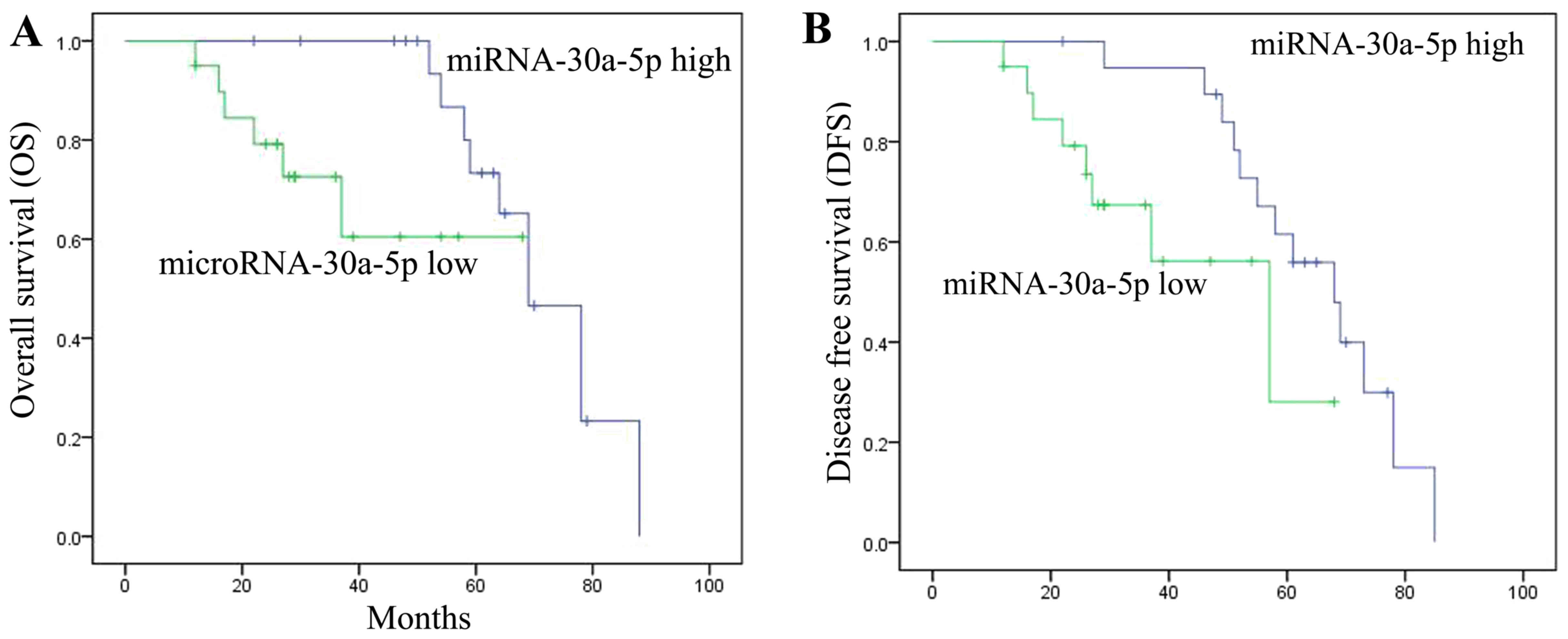
Overall survival (OS) and disease-free
survival (DFS) are associated with miRNA-30a-5p expression
The present study also determined whether OS and DFS
were associated with miRNA-30a-5p expression in patients with RCC.
OS and DFS were increased in patients with RCC and high
miRNA-30a-5p expression compared with in those with low
miRNA-30a-5p expression. These results indicated that miRNA-30a-5p
expression may affect RCC (Fig.
2).
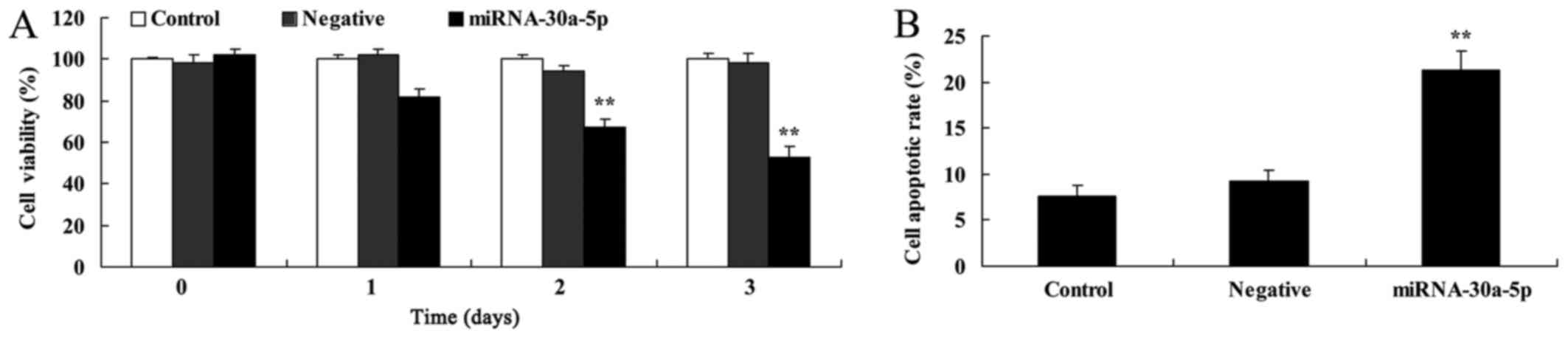
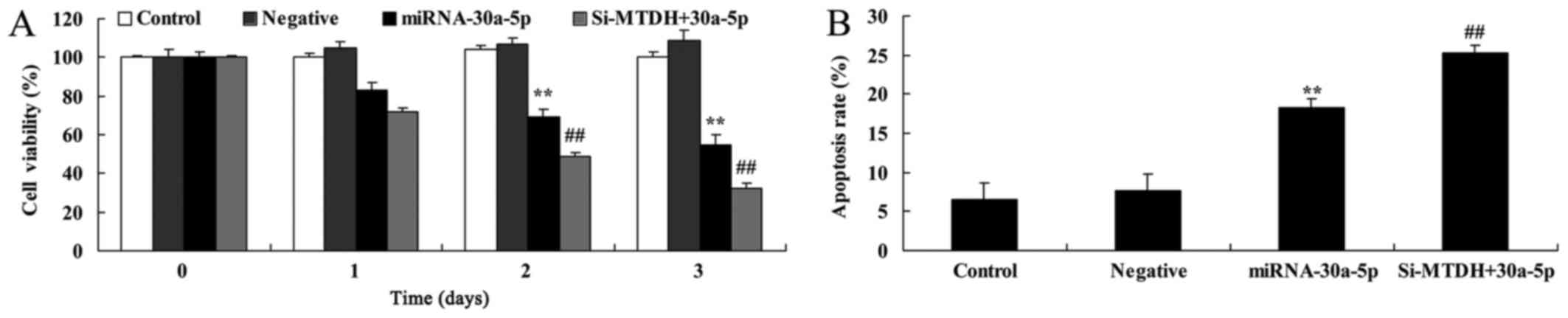
Overexpression of miRNA-30a-5p suppresses
cell proliferation and induces apoptosis of Caki-2 cells
To determine the effects of elevated miRNA-30a-5p
expression on cell proliferation and apoptosis of Caki-2 cells, an
MTT assay and flow cytometry were conducted. As presented in
Fig. 3, overexpression of
miRNA-30a-5p significantly suppressed cell proliferation and
induced apoptosis of Caki-2 cells.
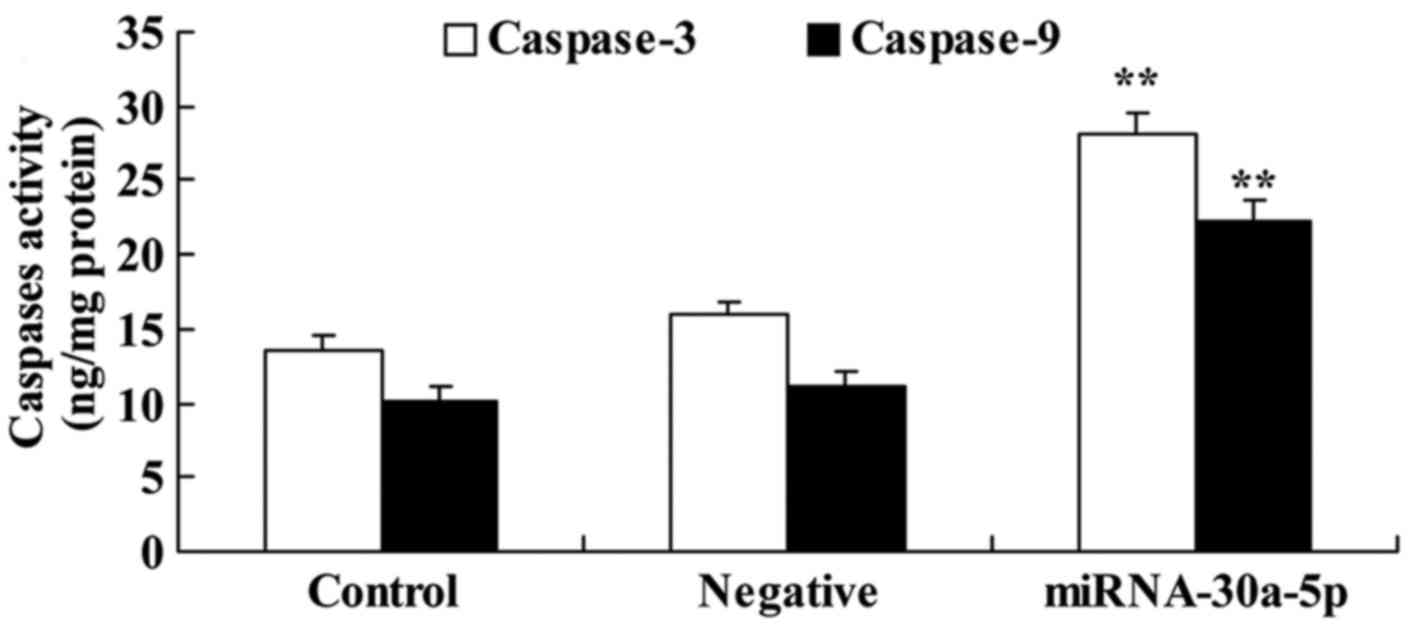
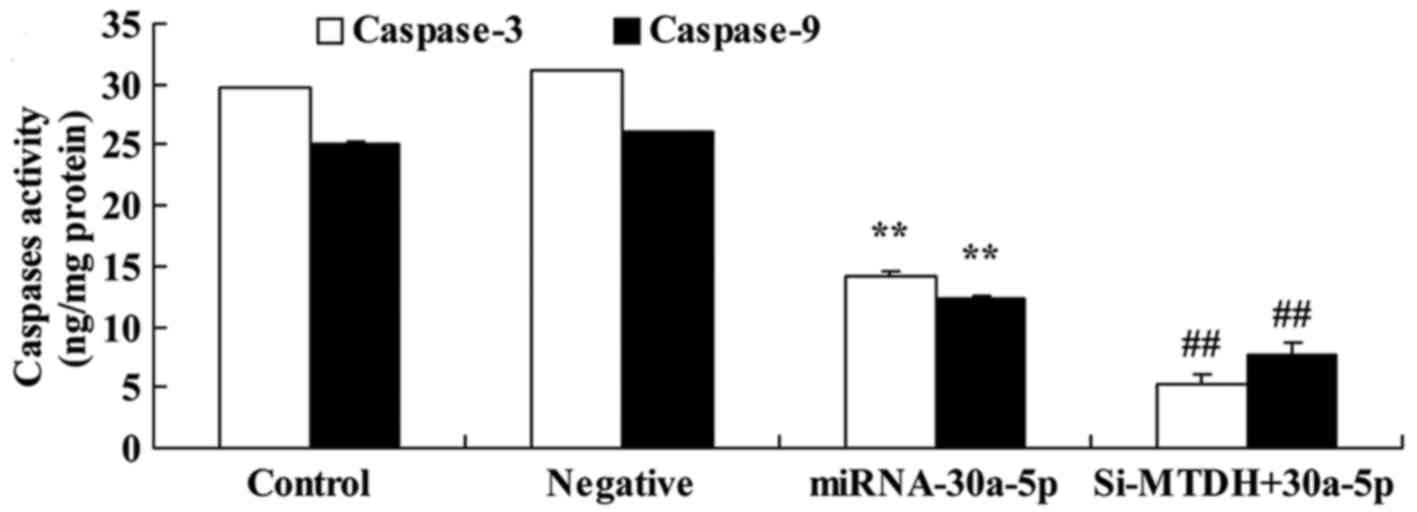
Overexpression of miRNA-30a-5p promotes
caspase-3/9 activities in Caki-2 cells
To identify the potential genes that mediate the
effects of miRNA-30a-5p on apoptosis of Caki-2 cells, caspase-3/9
activities were detected in Caki-2 cells. Caspase-3/9 activities
were significantly increased in Caki-2 cells transfected with
miRNA-30a-5p compared with in the control group (Fig. 4).
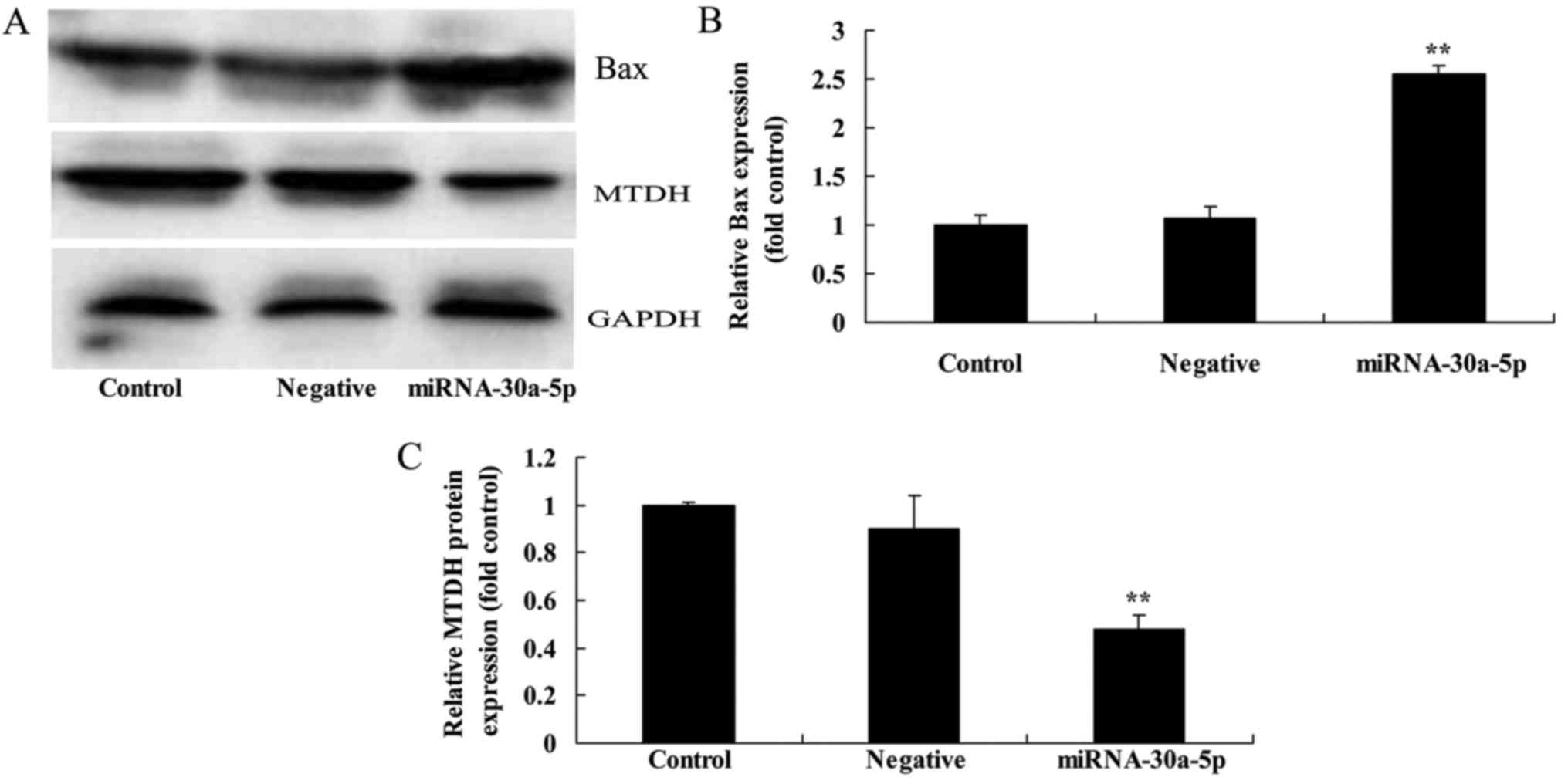
Effects of miRNA-30a-5p overexpression on
Bax and MTDH protein expression levels in Caki-2 cells
To determine whether miRNA-30a-5p expression had an
effect on MTDH and Bax protein expression in Caki-2 cells, Bax and
MTDH protein expression levels were analyzed using western blot
analysis. The results indicated that overexpression of miRNA-30a-5p
increased the protein expression levels of Bax and inhibited MTDH
protein expression in Caki-2 cells (Fig. 5).
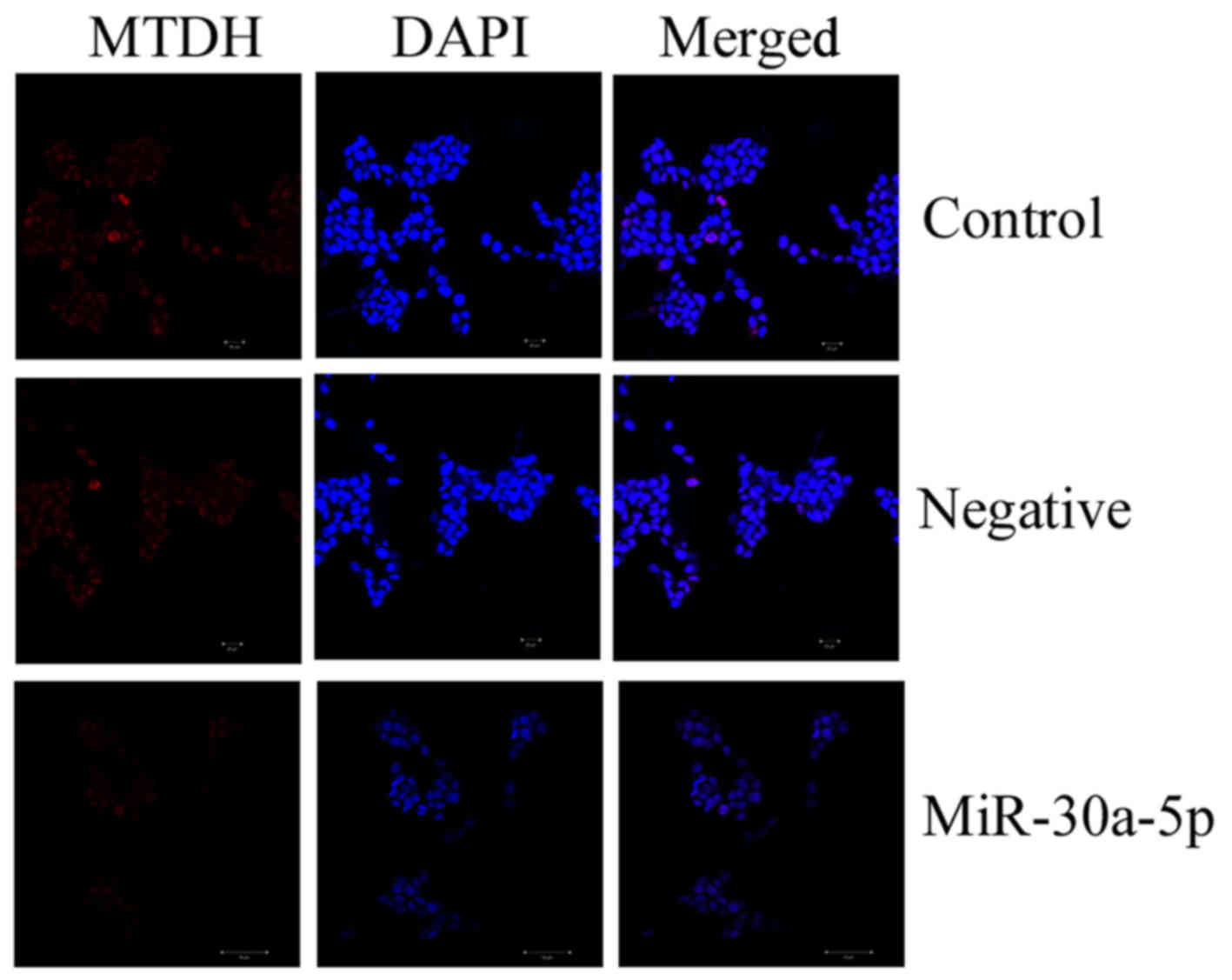
Overexpression of miRNA-30a-5p inhibits
MTDH protein expression in Caki-2 cells
The present study also analyzed whether miRNA-30a-5p
affected MTDH protein expression in Caki-2 cells by
immunocytofluorescence. Overexpression of miRNA-30a-5p markedly
inhibited MTDH protein expression in Caki-2 cells, as determined
using immunocytofluorescence (Fig.
6).
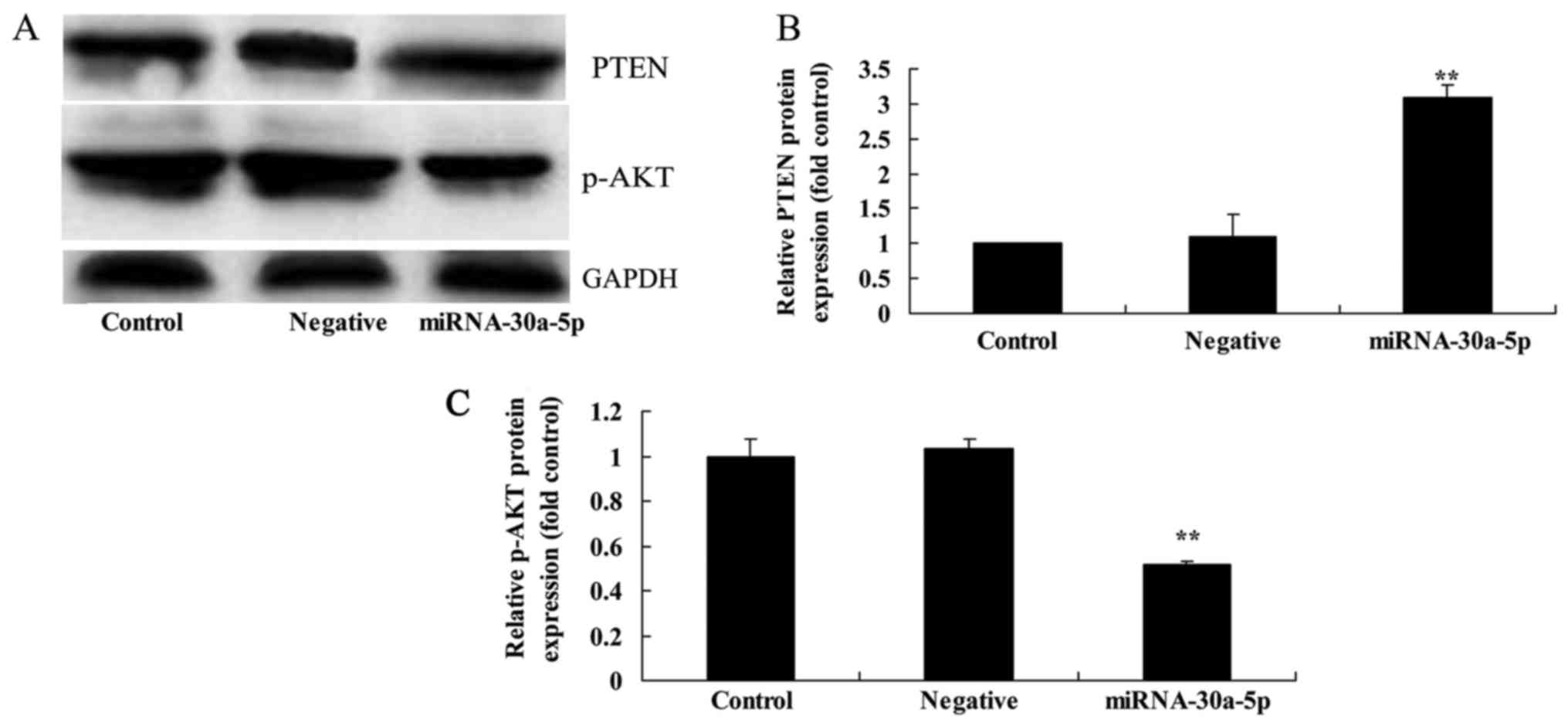
Overexpression of miRNA-30a-5p affects
PTEN and p-AKT protein expression in Caki-2 cells
In order to elucidate whether miRNA-30a-5p affects
p-AKT and PTEN protein expression in Caki-2 cells, western blotting
was conducted. As shown in Fig.
7, overexpression of miRNA-30a-5p significantly induced the
protein expression levels of PTEN and suppressed p-AKT protein
expression in Caki-2 cells.
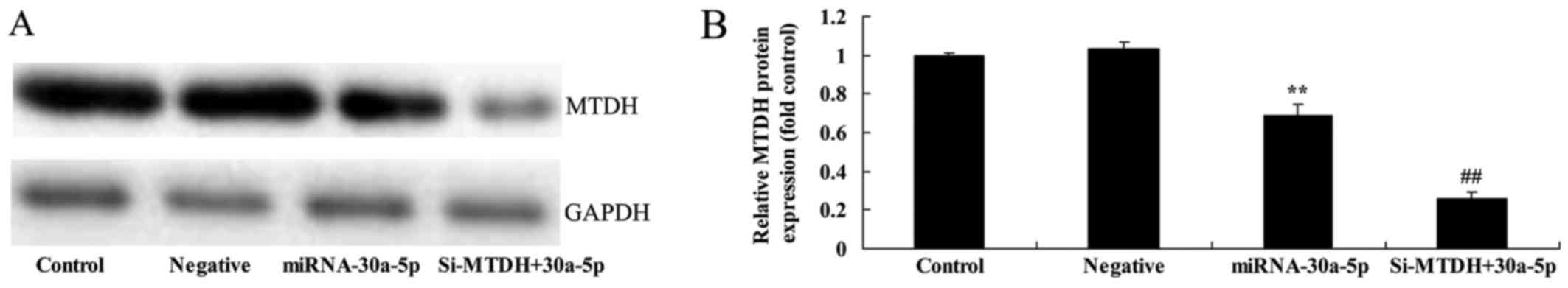
Small interfering RNA (si)-MTDH increases
the effects of miRNA-30a-5p on MTDH protein expression in Caki-2
cells
The present study aimed to determine how MTDH
regulates the effects of miRNA-30a-5p in RCC. As presented in
Fig. 8, knockdown of MTDH,
alongside miRNA-30a-5p overexpression, significantly inhibited MTDH
protein expression in Caki-2 cells compared with in the control
group.
si-MTDH increases the effects of
miRNA-30a-5p on inhibition of cell proliferation and promotion of
apoptosis of Caki-2 cells
The present study aimed to determine whether si-MTDH
affects miRNA-30a-5p-induced inhibition of cell proliferation and
promotion of apoptosis of Caki-2 cells. As presented in Fig. 9, knockdown of MTDH, alongside
miRNA-30a-5p overexpression, inhibited cell proliferation and
increased apoptosis of Caki-2 cells compared with in the control
group.
si-MTDH increases the effects of
miRNA-30a-5p on caspase-3/9 activities of Caki-2 cells
The present study investigated the mechanism
underlying the effects of miRNA-30a-5p on Caki-2 cells; caspase-3/9
activities were analyzed using ELISA kits. Caspase-3/9 activities
were significantly reduced in response to miRNA-30a-5p
overexpression and knockdown of MTDH in Caki-2 cells compared with
in the control group (Fig.
10).
si-MTDH increases the effects of
miRNA-30a-5p on Bax, PTEN and p-AKT protein expression in Caki-2
cells
The present study aimed to determined the effects of
si-MTDH on miRNA-30a-5p-regulated expression of Bax, PTEN and p-AKT
in Caki-2 cells. The protein expression levels of Bax and PTEN were
significantly promoted, whereas p-AKT protein expression was
significantly suppressed in Caki-2 cells in response to
miRNA-30a-5p overexpression and MTDH knockdown compared with in the
control group (Fig. 11).
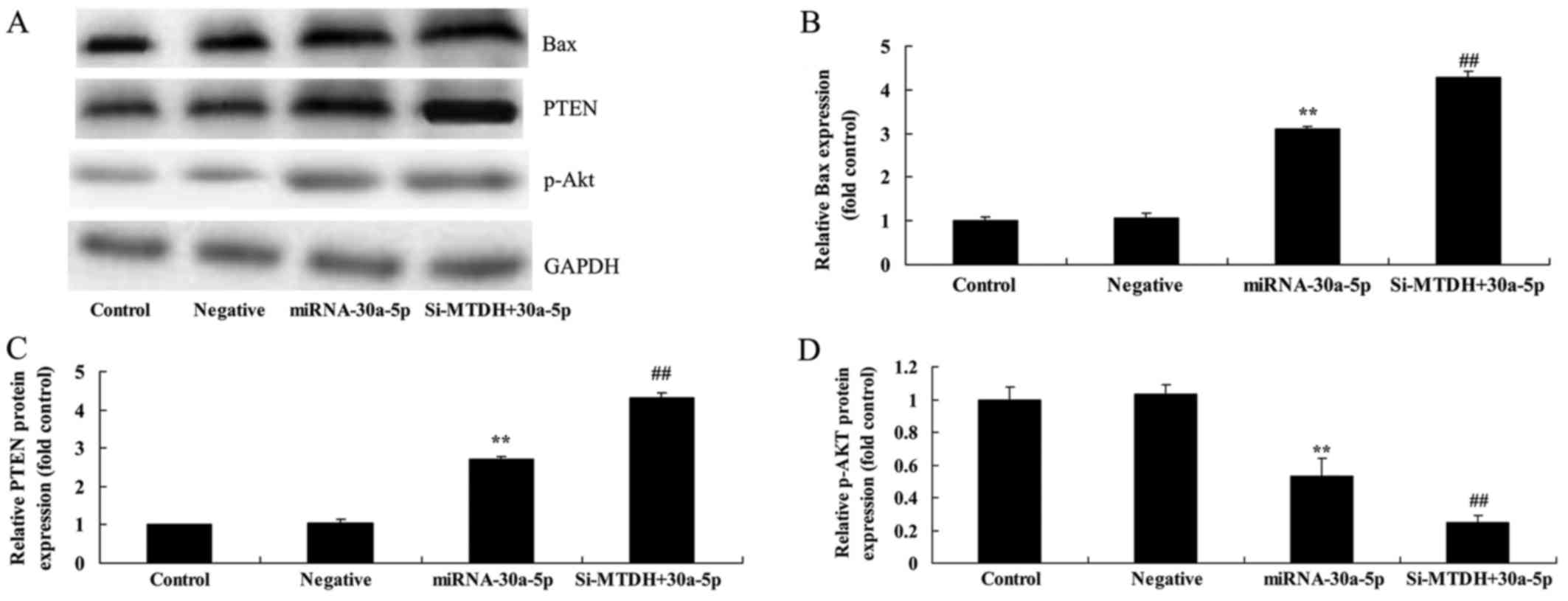 | Figure 11Knockdown of MTDH increases the
effects of miRNA-30a-5p on Bax, PTEN and p-AKT protein expression
in Caki-2 cells. si-MTDH increased the effects of miRNA-30a-5p on
Bax, PTEN and p-AKT protein expression in Caki-2 cells, as
determined using (A) western blot analysis and (B-D) statistical
analysis. **P<0.05 compared with the Control group;
##P<0.05 compared with the miRNA-30a-5p group.
Control, control group; Negative, negative control group;
miRNA-30a-5p, microRNA-30a-5p overexpression group; si-MTDH +
30a-5p, si-MTDH + miRNA-30a-5p overexpression group. Bax, B-cell
lymphoma 2-associated X protein; miRNA-30a-5p, microRNA-30a-5p;
MTDH, metadherin; p-AKT, phosphorylated-protein kinase B; PTEN,
phosphatase and tensin homolog; si-MTDH, small interfering
RNA-MTDH. |
Discussion
RCC is the most common type of kidney cancer, which
accounts for ~3% of all body tumors. In addition, the morbidity of
RCC has increased in the past 20 years (18). Surgery remains the main treatment
strategy for RCC; however, ~30% of patients develop recurrence
within 3 years postsurgery. The 5-year survival rate of RCC is
<10% and ~25% of patients have metastasis at the time of
diagnosis (19). Therefore, the
identification of reliable biological markers is of importance for
the early diagnosis of RCC, the judgment of patient prognosis and
the instruction of individualized treatment (20). miRNAs exert their important
functions by influencing tumor proliferation, migration and
invasion (8). The results of the
present study demonstrated that miRNA-30a-5p expression was lower
in tumor samples from patients with RCC compared with in RP tissue
samples. In addition, miRNA-30a-5p expression was much lower in
tumor samples from patients with TNM stage III + IV RCC compared
with in RP tissue samples.
RCC is associated with numerous genes and its
pathogenesis is complex; therefore, the molecular and biological
foundation for its etiology and development remains unclear
(21). However, with in-depth
research regarding the molecular mechanisms underlying tumor cell
growth, proliferation and apoptosis, it has been reported that an
imbalance in the internal environment is an important factor for
tumor development; the main cause of disturbances to the internal
environment is imbalances in numerous intracellular signal pathways
(22). It has previously been
demonstrated that abnormalities in the signal transduction pathways
that control cell proliferation and differentiation results in
cellular growth disorders; in particular, increased signaling of
pathways that promote cell proliferation, or reduced signaling of
pathways that inhibit cell proliferation and promote cell
apoptosis, at the cellular level during tumor formation may lead to
tumor formation or reduced tumor cell apoptosis (23). In the present study, OS and DFS
were increased in patients with RCC and high miRNA-30a-5p
expression compared with in those with low miRNA-30a-5p
expression.
The present study demonstrated that overexpression
of miRNA-30a-5p inhibited MTDH, upregulated PTEN, and suppressed
p-AKT protein expression levels in Caki-2 cells. Our results were
similar to results from previous studies (24–26), which showed that miRNA-30a-5p
regulated MTDH/PTEN/AKT signal pathway to induce renal cancer cell
apoptosis. The present study de monstrated that overexpression of
miRNA-30a-5p inhibited MTDH, upregulated PTEN, and suppressed p-AKT
protein expression levels in Caki-2 cells. The present study
demonstrated that overexpression of miRNA-30a-5p inhibited MTDH,
upregulated PTEN, and suppressed p-AKT protein expression levels in
Caki-2 cells, which showed that miRNA-30a-5p regulates
MTDH/PTEN/AKT pathway to suppress cell growth in human renal
cancer.
A recent study demonstrated that the main function
of AKT is the direct inhibition of cell apoptosis (27). The Bcl-2 family is also closely
associated with cell apoptosis, numerous members of which,
including Bcl-2-associated death promoter (Bad), exert proapoptotic
effects. AKT can phosphorylate the Ser136/Ser112 residue of the Bad
protein, thus resulting in its disaggregation from Bcl-2 or
Bcl-extra large, Bad then binds with the chaperonin 14-3-3, thus
resulting in the upregulation of anti-apoptotic factors, including
Bcl-2, A1, X-linked inhibitor of apoptosis protein and survivin,
and the loss of its proapoptotic effect (28). The present study demonstrated that
overexpression of miRNA-30a-5p may suppress cell proliferation,
induce apoptosis, promote caspase-3/9 activities and increase Bax
protein expression levels in Caki-2 cells.
Activated AKT can directly catalyze and
phosphorylate Ser196 of caspase-9, resulting in its inactivation,
thus reducing its proapoptotic effect. In addition, when the AKT
kinase domain is activated, cells can resist penicillin-induced
apoptosis, suggesting that only AKT with the complete kinase
activity domain can promote the anti-apoptotic effect (29). The present study demonstrated that
si-MTDH increased the effects of miRNA-30a-5p on the inhibition of
cell proliferation and the promotion of apoptosis, caspase-3/9
activities and Bax protein expression in Caki-2 cells. The present
study demonstrated that si-MTDH increased the effects of
miRNA-30a-5p on the inhibition of cell proliferation and the
promotion of apoptosis, caspase-3/9 activities and Bax protein
expression in Caki-2 cells. MTDH is an important role in the
anti-effect of miRNA-30a-5p on human renal cancer.
A previous study indicated that MTDH can activate
numerous signaling pathways, regulate physiological and
pathological cellular processes, and mediate the proliferation,
invasion, metastasis, angiogenesis and chemotherapeutic resistance
of tumor cells (30). MTDH is a
downstream target gene of AKT, as well as an upstream activator of
the PI3K/AKT pathway; PI3K/AKT and c-Myc can induce MTDH
expression, which can further activate PI3K/AKT and upregulate
c-Myc expression, resulting in the expression of N-Myc in a
neuroblastoma cell line, further enhancing oncogenic effects, and
forming a vicious cycle during tumor formation (31). In the present study suppression of
MTDH expression upregulated PTEN and suppressed p-AKT protein
expression levels in Caki-2 cells. In the present study suppression
of MTDH expression upregulated PTEN and suppressed p-AKT protein
expression levels in Caki-2 cells by miRNA-30a-5p. MTDH/PTEN/AKT
pathway participated in the anti-effect of miRNA-30a-5p on human
renal cancer.
In conclusion, the present study demonstrated that
miRNA-30a-5p may suppress human RCC cell proliferation via the
MTDH/PTEN/AKT pathway. Further studies aim to determine whether
miRNAs can be practically applied in the treatment of RCC.
Acknowledgments
The present study was funded by the Application Base
and Frontier Technology Project to T.Z. (grant no. 15JCQNJC10400),
and was supported in part by the National Natural Science
Foundation of China (grant no. 81301949) and the Natural Science
Foundation of Tianjin (grant no. 16JCYBJC26500) to C.L., and the
Natural Science Foundation of Hebei Province (grant no.
H2015209164) to H.L.
References
|
1
|
Rini BI, Melichar B, Fishman MN, Oya M,
Pithavala YK, Chen Y, Bair AH and Grünwald V: Axitinib dose
titration: Analyses of exposure, blood pressure and clinical
response from a randomized phase II study in metastatic renal cell
carcinoma. Ann Oncol. 26:1372–1377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Voss MH, Chen D, Marker M, Hakimi AA, Lee
CH, Hsieh JJ, Knox JJ, Voi M and Motzer RJ: Circulating biomarkers
and outcome from a randomised phase II trial of sunitinib vs
everolimus for patients with metastatic renal cell carcinoma. Br J
Cancer. 114:642–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huijts CM, Santegoets SJ, van den Eertwegh
AJ, Pijpers LS, Haanen JB, de Gruijl TD, Verheul HM and van der
Vliet HJ: Phase I-II study of everolimus and low-dose oral
cyclophosphamide in patients with metastatic renal cell cancer. BMC
Cancer. 11:5052011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Molina AM, Hutson TE, Larkin J, Gold AM,
Wood K, Carter D, Motzer R and Michaelson MD: A phase 1b clinical
trial of the multi-targeted tyrosine kinase inhibitor lenvatinib
(E7080) in combination with everolimus for treatment of metastatic
renal cell carcinoma (RCC). Cancer Chemother Pharmacol. 73:181–189.
2014. View Article : Google Scholar :
|
|
5
|
Li Z, Chen Y, Hu S, Zhang J, Wu J, Ren W,
Shao N and Ying X: Integrative analysis of protein-coding and
non-coding RNAs identifies clinically relevant subtypes of clear
cell renal cell carcinoma. Oncotarget. 7:82671–82685.
2016.PubMed/NCBI
|
|
6
|
Zhang L, Xul B, Chen S, Lu K, Liu C, Wang
Y, Zhao Y, Zhang X, Liu D and Chen M: The complex roles of
microRNAs in the metastasis of renal cell carcinoma. J Nanosci
Nanotechnol. 13:3195–3203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang P, Yin B, Shan L, Zhang H, Cui J,
Zhang M and Song Y: RNA interference-mediated knockdown of
astrocyte elevated gene-1 inhibits growth, induces apoptosis, and
increases the chemosensitivity to 5-fluorouracil in renal cancer
Caki-1 cells. Mol Cells. 37:857–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Ali BM, Ress AL, Gerger A and Pichler
M: MicroRNAs in renal cell carcinoma: Implications for
pathogenesis, diagnosis, prognosis and therapy. Anticancer Res.
32:3727–3732. 2012.PubMed/NCBI
|
|
10
|
Mlcochova H, Machackova T, Rabien A,
Radova L, Fabian P, Iliev R, Slaba K, Poprach A, Kilic E, Stanik M,
et al: Epithelial-mesenchymal transition-associated microRNA/mRNA
signature is linked to metastasis and prognosis in clear-cell renal
cell carcinoma. Sci Rep. 6:318522016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo Z, Hu X, Xiong H, Qiu H, Yuan X, Zhu
F, Wang Y and Zou Y: A polysaccharide from Huaier induced apoptosis
in MCF-7 breast cancer cells via downregulation of MTDH protein.
Carbohydr Polym. 151:1027–1033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du C, Yi X, Liu W, Han T, Liu Z, Ding Z,
Zheng Z, Piao Y, Yuan J, Han Y, et al: MTDH mediates trastuzumab
resistance in HER2 positive breast cancer by decreasing PTEN
expression through an NFκB-dependent pathway. BMC Cancer.
14:8692014. View Article : Google Scholar
|
|
13
|
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke
TF and Fisher PB: Astrocyte elevated gene-1 activates cell survival
pathways through PI3K-Akt signaling. Oncogene. 27:1114–1121. 2008.
View Article : Google Scholar
|
|
14
|
Seo BR, Min KJ, Cho IJ, Kim SC and Kwon
TK: Curcumin significantly enhances dual PI3K/Akt and mTOR
inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma
Caki cells through downregulation of p53-dependent Bcl-2 expression
and inhibition of Mcl-1 protein stability. PLoS One. 9:e955882014.
View Article : Google Scholar
|
|
15
|
Lim W, Jeong W and Song G: Delphinidin
suppresses proliferation and migration of human ovarian clear cell
carcinoma cells through blocking AKT and ERK1/2 MAPK signaling
pathways. Mol Cell Endocrinol. 422:172–181. 2016. View Article : Google Scholar
|
|
16
|
Ribback S, Cigliano A, Kroeger N, Pilo MG,
Terracciano L, Burchardt M, Bannasch P, Calvisi DF and Dombrowski
F: PI3K/AKT/mTOR pathway plays a major pathogenetic role in
glycogen accumulation and tumor development in renal distal tubules
of rats and men. Oncotarget. 6:13036–13048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Fournier LS, Oudard S, Thiam R, Trinquart
L, Banu E, Medioni J, Balvay D, Chatellier G, Frija G and Cuenod
CA: Metastatic renal carcinoma: Evaluation of antiangiogenic
therapy with dynamic contrast-enhanced CT. Radiology. 256:511–518.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bracarda S, Porta C, Boni C, Santoro A,
Mucciarini C, Pazzola A, Cortesi E, Gasparro D, Labianca R, Di
Costanzo F, et al: Could interferon still play a role in metastatic
renal cell carcinoma? A randomized study of two schedules of
sorafenib plus interferon-alpha 2a (RAPSODY). Eur Urol. 63:254–261.
2013. View Article : Google Scholar
|
|
20
|
Li HC, Li JP, Wang ZM, Fu DL, Li ZL, Zhang
D, Gan WM and Chong T: Identification of angiogenesis-related
miRNAs in a population of patients with renal clear cell carcinoma.
Oncol Rep. 32:2061–2069. 2014.PubMed/NCBI
|
|
21
|
Su Z, Ni L, Yu W, Yu Z, Chen D, Zhang E,
Li Y, Wang Y, Li X, Yang S, et al: MicroRNA-451a is associated with
cell proliferation, migration and apoptosis in renal cell
carcinoma. Mol Med Rep. 11:2248–2254. 2015. View Article : Google Scholar
|
|
22
|
Zheng J, Qin W, Jiao D, Ren J, Wei M, Shi
S, Xi W, Wang H, Yang AG, Huan Y, et al: Knockdown of COUP-TFII
inhibits cell proliferation and induces apoptosis through
upregulating BRCA1 in renal cell carcinoma cells. Int J Cancer.
139:1574–1585. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang T, Hu XY, Li YH, Tian BQ, Li ZW and
Fu Q: MicroRNA-21 regulates the proliferation, differentiation, and
apoptosis of human renal cell carcinoma cells by the mTOR-STAT3
signaling pathway. Oncol Res. 24:371–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada T, Horinaka M, Shinnoh M, Yoshioka
T, Miki T and Sakai T: A novel HDAC inhibitor OBP-801 and a PI3K
inhibitor LY294002 synergistically induce apoptosis via the
suppression of survivin and XIAP in renal cell carcinoma. Int J
Oncol. 43:1080–1086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou J, Zhu G, Huang J, Li L, Du Y, Gao Y,
Wu D, Wang X, Hsieh JT, He D, et al: Non-canonical GLI1/2
activation by PI3K/AKT signaling in renal cell carcinoma: A novel
potential therapeutic target. Cancer Lett. 370:313–323. 2016.
View Article : Google Scholar
|
|
26
|
Han X, Yang J, Jia Z, Wei P, Zhang H, Lv
W, Sun J and Huo Q: Knockdown of serine-arginine protein kinase 1
inhibits the growth and migration in 6 renal cell carcinoma cells.
Oncol Res. 25:389–395. 2017. View Article : Google Scholar
|
|
27
|
Papadopoulos EI, Yousef GM and Scorilas A:
Gemcitabine impacts differentially on bladder and kidney cancer
cells: Distinct modulations in the expression patterns of
apoptosis-related microRNAs and BCL2 family genes. Tumour Biol.
36:3197–3207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burke MT, Morais C, Oliver KA, Lambie DL,
Gobe GC, Carroll RP, Staatz CE, Sinnya S, Soyer HP, Winterford C,
et al: Expression of Bcl-xL and Mcl-1 in the nonmelanoma skin
cancers of renal transplant recipients. Am J Clin Pathol.
143:514–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong WF, Wang XH, Pan B, Li F, Kuang L
and Su ZX: Eupatilin induces human renal cancer cell apoptosis via
ROS-mediated MAPK and PI3K/AKT signaling pathways. Oncol Lett.
12:2894–2899. 2016.PubMed/NCBI
|
|
30
|
Zhu GC, Yu CY, She L, Tan HL, Li G, Ren
SL, Su ZW, Wei M, Huang DH, Tian YQ, et al: Metadherin regulation
of vascular endothelial growth factor expression is dependent upon
the PI3K/Akt pathway in squamous cell carcinoma of the head and
neck. Medicine (Baltimore). 94:e5022015. View Article : Google Scholar
|
|
31
|
Li WF, Ou Q, Dai H and Liu CA:
Lentiviral-mediated short hairpin RNA knockdown of MTDH inhibits
cell growth and induces apoptosis by regulating the PTEN/AKT
pathway in hepatocellular carcinoma. Int J Mol Sci. 16:19419–19432.
2015. View Article : Google Scholar : PubMed/NCBI
|