Introduction
Non-alcoholic fatty liver disease (NAFLD), which is
characterized by excessive triglyceride (TG) accumulation in
hepatocytes, has become a major public health concern worldwide,
with an estimated prevalence range of 24–42% in Western countries
and 5–40% in Asian countries (1,2).
NAFLD may progress to steatohepatitis and fibrosis, or even
cirrhosis, liver cancer and liver failure over time (3). The mechanisms underlying the
development of NAFLD have not yet been fully elucidated. Since
obesity and diabetes are considered to be major risk factors for
the development and progression of NAFLD, current therapies for
NAFLD are mainly aimed at reducing body weight and improving
insulin sensitivity (4).
AMP-activated protein kinase (AMPK) plays an
important role in regulating hepatic lipogenesis (5). In the liver, activation of AMPK by
phosphorylation of threonine 172 induces the phosphorylation and
inactivation of acetyl-CoA carboxylase (ACC), subsequently leading
to the suppression of fatty acid synthesis (6). In addition, AMPK is a negative
sterol regulatory element-binding protein-1c (SREBP-1c), a key
regulator of TG metabolism (7).
Phosphorylation of AMPK downregulates the activity of SREBP-1c
(8,9). However, the activity of AMPK is
dysregulated in patients with metabolic syndromes, such as diabetes
and obesity (10,11). Thus, stimulation of AMPK
activation, which thereby suppresses ACC and SREBP-1c activity, may
alleviate hepatic accumulation of TG.
Our institute has expressed a continuous interest in
the prevention and treatment of chronic liver diseases with
traditional Chinese medicine. Diosgenin, which is abundant in
Chinese yam (Dioscorea villosa) and Rhizoma Dioscoreae
Nipponicae, has been shown to lower increased plasma glucose
levels and improve the distorted tissue lipid profile in high-fat
diet (HFD)-streptozotocin-induced diabetic rats (12). Furthermore, diosgenin was able to
induce AMPK phosphorylation under basal as well as inflammatory
conditions in perivascular adipose tissue (13). These results indicate that
diosgenin may be an effective treatment for NAFLD. Therefore, the
effect of diosgenin on HFD-induced rat NAFLD was observed, and the
underlying mechanisms were investigated in the present study.
Materials and methods
Cell culture
The human liver cancer HepG2 cell line was obtained
from the CellBank of Chinese Academy of Sciences and cultured in
Dulbecco's modified Eagle's medium (DMEM) containing normal glucose
[5.5 mM, D-glucose; low glucose (LG)] supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin and 0.1 mg/ml streptomycin
at 37°C in a humidified atmosphere of 5% CO2. To examine
the accumulation of TG, HepG2 cells were maintained in serum-free
DMEM overnight, as previously described (14), and the cells were then treated
with indicated concentrations of diosgenin in DMEM containing a
high concentration of glucose [30 mmol/l; high glucose (HG)] for
another 24 h, as previously described (14,15). The cells were then lysed and TG
levels were determined using a commercial kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (PCR)
Total RNA was extracted from HepG2 cells after
diosgenin treatment with TRIzol reagent (Invitrogen Life
Technologies; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA)
according to the manufacturer's instructions. Total RNA (500 ng)
was reverse-transcribed into cDNA using a First-Strand cDNA
synthesis kit (FSK-100; Toyobo, Osaka, Japan). The amplification of
the cDNA was accomplished in triplicate using SYBR-Green PCR Master
Mix (Toyobo). The cDNA was amplified under the following
conditions: 95°C for 5 min for denaturation, followed by 40 cycles
at 95°C for 10 sec, 60°C for 20 sec and 72°C for 25 sec. The
primers used in the present study were as follows: SREBP-1c
forward, 5′-ACC GAC ATC GAA GGT GAA GT-3′ and reverse, CCA GCA TAG
GGT GGG TCA AA; LXRα forward, 5′-GGA CCA GCT CCA GGT AGA GA-3′ and
reverse, 5′-ACA CTT GCT CTG AGT GGA CG-3′; and β-actin forward,
5′-AGC GGG AAA TCG TGC GTG-3′ and reverse, 5′-CAG GGT ACA TGG TGG
TGC C-3′. The relative expression level of mRNA in each sample was
normalized to its β-actin content. The relative expression levels
of mRNA were calculated with the 2−ΔΔCq method.
Western blot analysis
Total protein was extracted as previously described
(16). The protein concentration
was determined by the bicinchoninic acid method. Equal quantities
of proteins were separated by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and transferred by electroblotting to a
nitrocellulose membrane. The membrane was blocked with 5% BSA in
TBST buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl and 0.1% Tween-20)
overnight at 4°C. Subsequently, the membrane was incubated with
specific primary antibodies [rabbit monoclonal antibodies (1:1,000)
AMPK (no. 2603), p-AMPK (no. 2535), ACC (no. 3676), and rabbit
polyclonal antibody (1:1,000) pACC (no. 3661) (all from Cell
Signaling Technology, Inc., Danvers, MA, USA), goat polyclonal
antibody LXRα (1:200; sc-1202; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), rabbit monoclonal antibody β-actin (1:5,000;
no. 4970; Cell Signaling Technology, Inc.)] overnight at 4°C,
followed by incubation with a secondary antibody [anti-rabbit IgG
(no. 7074; Cell Signaling Technology, Inc.) or anti-goat IgG
(sc-2354; Santa Cruz Biotechnology, Inc.)] for 1 h. The signal was
visualized with an enhanced chemiluminescence (ECL) kit (Thermo
Fisher Scientific, Inc., Carlsbad, CA, USA).
Animals
A total of 40 male Sprague-Dawley rats (6–8 weeks
old, weighing 250±20 g) were purchased from the Shanghai Laboratory
Animal Center and kept at the Second Military Medical University
Laboratory Animal Center. The animals were maintained on a 12-h
light/dark cycle (7:00 a.m.–19:00 p.m.), at 22±2°C, with food and
water available ad libitum. To avoid the effect of
environmental changes, all the animals were housed for 1 week in
the controlled environment prior to use in the experiment. All the
procedures were performed in accordance with the institutional
guidelines for animal research. The present study was approved by
the Committee on Ethics of Biomedicine, the Second Military Medical
University.
Experiment design and drug
administration
The animals were randomly assigned into 5 groups
(n=8): Control group (C), model group (M), high-dose diosgenin
group (HDD), low-dose diosgenin group (LDD) and fenofibrate group
(FEN). The rats in the model, HDD, LDD and FEN groups were fed HFD,
HFD mixed with 1% (wt/wt) diosgenin, HFD mixed with 0.5% (wt/wt)
diosgenin and HFD mixed with 0.02% fenofibrate, respectively. The
control group was given the same amount of food as the other
groups. HFD was provided by the Shanghai Laboratory Animal Center.
The energy composition of the HFD consists of 45% fat, 18% protein
and 37% carbohydrate. A regular rat diet (10% fat, 22% protein, 68%
carbohydrate) was used as the maintenance and control diet.
After 16 weeks of feeding according to previous
studies (17,18), blood was collected from the
retro-orbital venous plexus for detecting the content of serum
total cholesterol (TC) and TG and the liver function. The rat liver
was removed and frozen in liquid nitrogen for the following
experiments.
Detection of serum aspartate
aminotransferase (AST) and alanine aminotransferase (ALT)
activity
Serum ALT and AST were determined using biochemical
kits (Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions.
Hematoxylin and eosin (H&E) and Oil
red O staining
Liver tissue was embedded in Tissue-Tek optimum
cutting temperature compound (Sakura Finetek, Torrance, CA, USA),
quickly frozen by immersion in liquid nitrogen, and stained with
H&E. Alternatively, intrahepatic lipids were stained by the Oil
red O method, as previously described (19). Images were obtained using a Leica
inverted fluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
Data are expressed as means ± standard deviation.
One-way analysis of variance followed by Student-Newman-Keuls tests
were used for statistical analysis. Statistical significance was
established at P<0.05.
Results
Diosgenin increases AMPK and ACC
phosphorylation and suppresses HG-induced TG accumulation in HepG2
cells
In view of the important role of AMPK (AMPKα) in
lipid metabolism, the effect of diosgenin on the activation of AMPK
in vitro was first examined by using a specific
anti-phospho-Thr-172 AMPK antibody. Under both LG and HG
conditions, diosgenin treatment for 24 h significantly increased
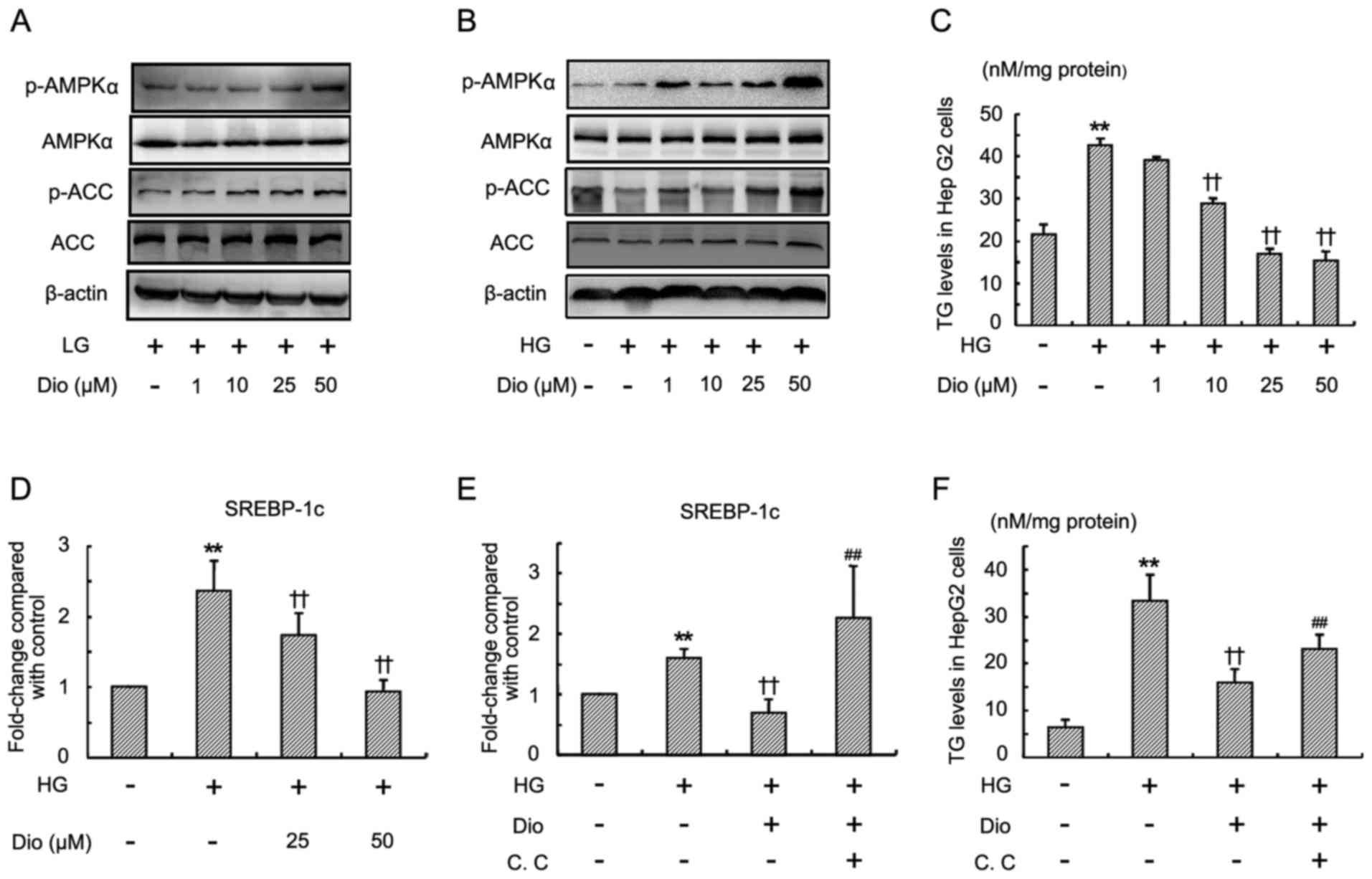
the phosphorylated AMPK levels in a dose-dependent manner (Fig. 1A and B). No changes in endogenous
AMPK protein were observed by immunoblotting in both LG and HG
medium. ACC is a downstream target of AMPK. The phosphorylation of
ACC was next determined. Western blot analysis revealed that
diosgenin obviously induced phosphorylation of ACC in a
dose-dependent manner (Fig. 1A and
B).
Therefore, it was further investigated whether
diosgenin prevented HG-induced lipid accumulation in HepG2 cells.
As shown in Fig. 1C, treatment
with a high concentration of glucose for 24 h significantly
increased the TG level in HepG2 cells. Diosgenin (10, 25 and 50 µM)
significantly inhibited HG-induced TG accumulation in HepG2 cells.
High concentration of glucose also induced a significant increase
of SREBP-1c mRNA (Fig. 1D), while
diosgenin treatment suppressed the increase of SREBP-1c mRNA level.
To further confirm the effect of diosgenin on TG accumulation, and
that SREBP-1c mRNA expression is mediated by the AMPK pathway,
compound C (an inhibitor of AMPK) was applied. As shown in Fig. 1E and F, the inhibition of TG and
SREBP-1c mRNA in HepG2 cells by diosgenin was partially blocked by
compound C.
Diosgenin inhibits LXRα expression in
HepG2 cells
Although activation of LXRα improves glucose
tolerance and insulin resistance, it is also able to regulate the
transcription of several lipid metabolism-related genes, including
SREBP-1c, which lead to hyperlipidemia and hepatic steatosis
(20–22). To further investigate whether the
lipid-lowering effect of diosgenin was associated with LXRα, the
LXRα mRNA expression following diosgenin treatment was examined. HG
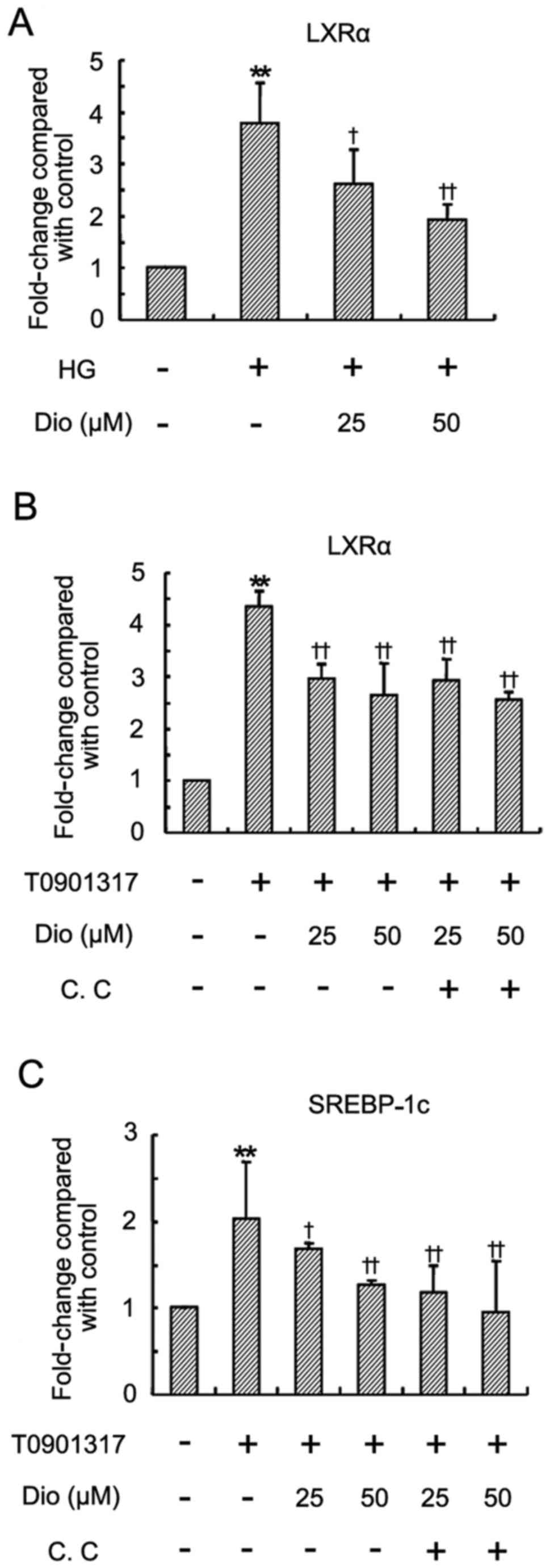
induced a significant upregulation of LXRα mRNA in HepG2 cells
(Fig. 2A). Treatment with
diosgenin significantly lowered the expression of LXRα mRNA.
Diosgenin also significantly inhibited LXRα ligand T0901317-induced
LXRα and SREBP-1c mRNA upregulation (Fig. 2B and C), which was not abolished
by compound C.
Diosgenin decreases the body and liver
weight of HFD-fed rats
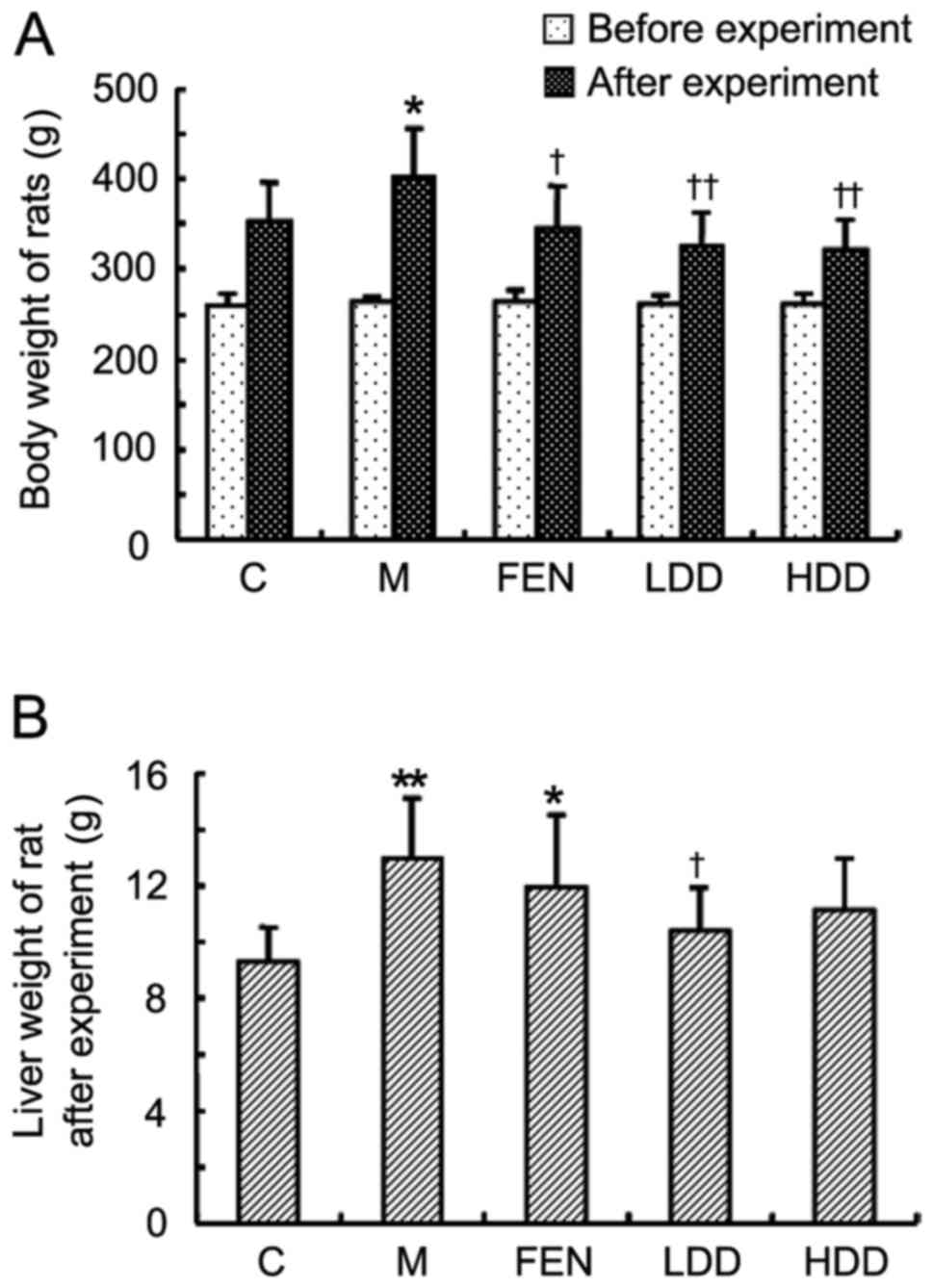
There was no significant difference in the body
weight among the five groups prior to the experiment (Fig. 3A). Following feeding with HFD for
16 weeks, the body weight of the rats in the model group was
significantly increased compared with the control group (Fig. 3A). Diosgenin treatment
significantly suppressed weight gain in HFD-fed rats. The liver
weight of HFD-fed rats was also significantly increased compared
with that of normal diet-fed rats (Fig. 3B). The liver weights of rats in
the HDD and LDD groups were lower compared with those in the model
group, while no significant difference was observed between the
model and FEN groups.
Diosgenin ameliorates hepatic lipid
accumulation in HFD-fed rats
The accumulation of intracellular TG in the liver
parenchyma is the main pathological change observed in NAFLD
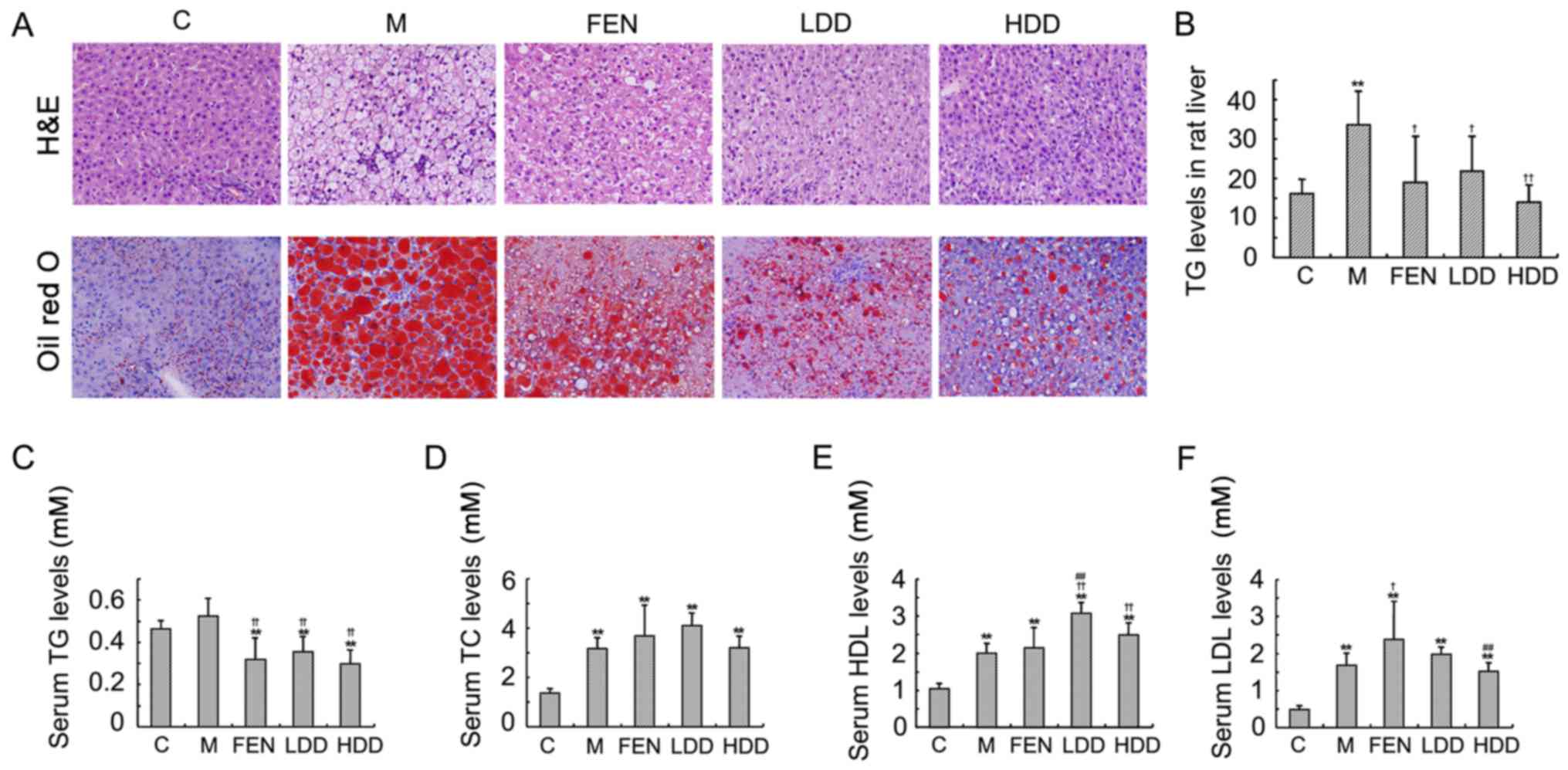
(23). As shown in Fig. 4A, the Oil Red O staining revealed
an obvious lipid accumulation in the liver of HFD-fed rats compared
with normal diet-fed rats. H&E staining demonstrated that the
increase in hepatic adipose infiltration in HFD-fed rats was
significantly reduced by diosgenin and̸or fenofibrate
administration. Both diosgenin and fenofibrate treatment
ameliorated the lipid deposition in the rat liver. The hepatic TG
content in HFD-fed rats was higher compared with that in normal
diet-fed rats (Fig. 4B).
Diosgenin and fenofibrate also significantly reduced the TG content
in the rat liver.
Diosgenin also significantly decreased the serum TG
levels (Fig. 4C), while it had
little effect on the serum TC levels (Fig. 4D). The high-density lipoprotein
(HDL) and low-density lipoprotein cholesterol were both
significantly increased in HFD-fed rats (Fig. 4E and F). The HDL cholesterol
levels in the diosgenin groups were higher compared with those in
the model group. However, the differences between the diosgenin and
model groups were not significant.
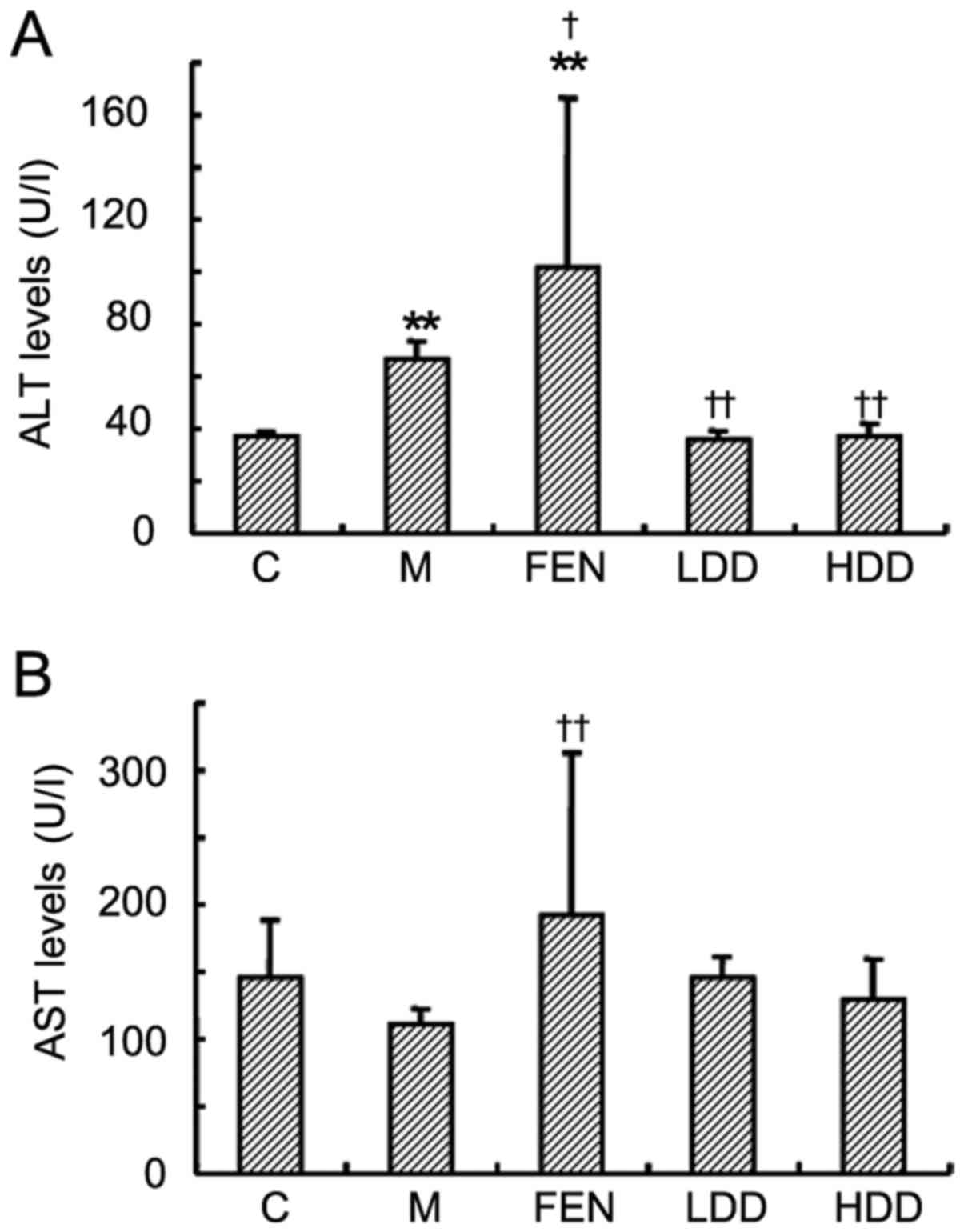
Diosgenin ameliorates hepatic dysfunction
in HFD-fed rats
The ALT level in HFD-fed rats was significantly
increased compared with that in normal diet-fed rats (Fig. 5A). Diosgenin treatment
significantly reduced the ALT levels, whereas fenofibrate further
elevated the level of ALT. The AST level did not significantly
increase in the HFD-fed rats (Fig.
5B). There was no significant difference in the AST levels
between the diosgenin treatment groups and the model group, while
fenofibrate treatment significantly increased the AST level.
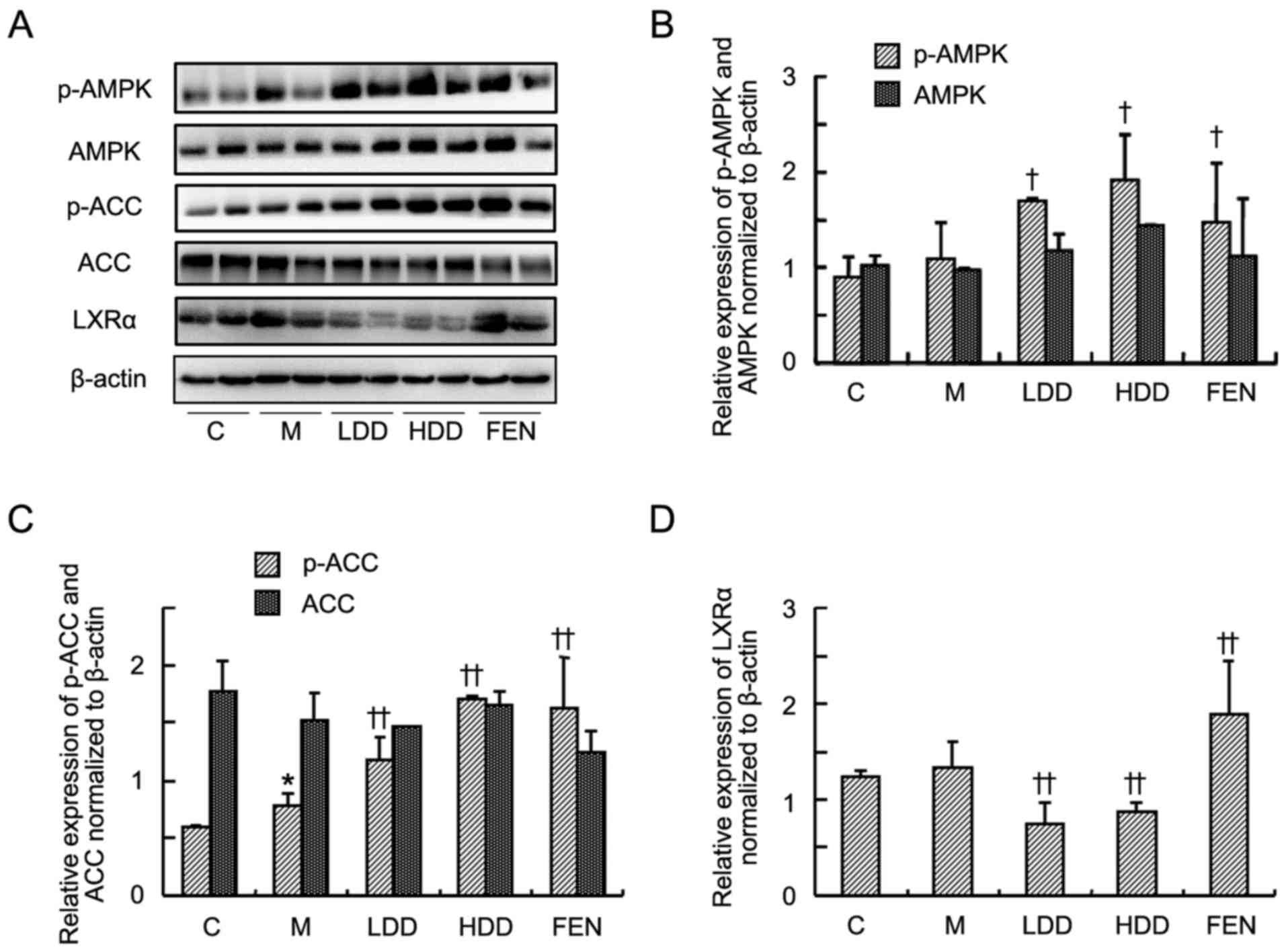
Diosgenin increases AMPK and ACC
phosphorylation and suppresses LXRα in HFD-fed rats liver
Subsequently, the levels of AMPK, ACC and LXRα in
the livers of HFD-fed rats were further investigated by western
blot analysis. As shown in Fig.
6A–C, the levels of p-AMPK and p-ACC were significantly
increased in the diosgenin- and fenofibrate-treated groups compared
with the model group. However, there were no significant
differences in the total AMPK and ACC between the diosgenin- and
fenofibrate-treated groups and the model group. Diosgenin treatment
significantly suppressed the LXRα level compared with the model
group (Fig. 6D). However,
fenofibrate treatment further upregulated the LXRα level compared
with the model group.
Discussion
NAFLD is characterized by increased hepatocellular
lipid accumulation and is frequently associated with
steatohepatitis and liver injury, which may eventually result in
severe liver damage, including hepatic fibrosis and cirrhosis, or
even liver cancer. The data of the present study demonstrated that
diosgenin was able to activate AMPK, thereby inhibiting lipid
accumulation in hepatocytes. Furthermore, the effect of diosgenin
on lipid accumulation was abolished by compound C, an inhibitor of
AMPK, suggesting that the effect of diosgenin on NAFLD is
AMPK-dependent. Moreover, our results indicated that diosgenin may
also inhibit LXRα and LXRα agonist-induced SREBP-1c upregulation
independently of AMPK. In addition, diosgenin also alleviated the
HFD-induced liver function disruption in rats, adding an advantage
over the traditional lipid-lowering medicines.
High glucose concentration is associated with
insulin resistance, which leads to elevated hepatic glucose
production, hyperglycemia and hyperlipidemia (24). Exposure of HepG2 cells to high
concentrations of glucose inhibits the phosphorylation of AMPK and
ACC (25). It has been
demonstrated that the inhibition of AMPK and ACC phosphorylation
contributes to extensive glucose-induced lipid accumulation in
HepG2 cells (14). By contrast,
metformin, an AMPK activator, increases phosphorylation of ACC and
effectively attenuates lipid accumulation in HepG2 cells induced by
high concentration of glucose (14). In the present study, it was first
demonstrated that diosgenin increased the phosphorylation of AMPK
and ACC under conditions of low and high concentrations of glucose,
indicating that diosgenin may be capable of regulating lipid
metabolism in the liver. Therefore, a model of insulin resistance
induced by high concentration of glucose was used to test the
effect of diosgenin on hepatic TG accumulation. As expected,
diosgenin treatment decreased the TG level in HG-treated HepG2
cells. Moreover, the effects of diosgenin on lipid accumulation
were abolished by the inhibitor of AMPK. These data suggest that
diosgenin may be promising for preventing HG-induced lipid
accumulation in the liver through activating the AMPK pathway.
SREBP1 is the most important transcription factor
regulating lipogenesis in the liver, and is primarily responsible
for the regulation of genes involved in fatty acid biosynthesis,
such as FAS. AMPK suppresses SREBP-1c cleavage and nuclear
translocation, and represses SREBP-1c target gene expression in
hepatocytes exposed to HG, leading to reduced lipogenesis and lipid
accumulation (26). In the
present study, diosgenin suppressed HG-induced SREBP-1c mRNA
upregulation, which was partially blocked by the AMPK inhibitor,
suggesting that other pathways may also participate in the
lipid-lowering effect of diosgenin. SREBP1 is also a major target
of LXRα, which upregulates lipogenesis (27). The present study demonstrated that
the HG-induced increase of LXRα mRNA is also suppressed by
diosgenin. Lee et al reported that AMPK activation
suppresses LXRα agonist-induced SREBP-1c mRNA (28). The present study demonstrated that
LXRα agonist increases the expression of LXRα and SREBP-1c mRNA,
which was not blocked by the AMPK inhibitor. These results suggest
that the inhibition of LXRα̸SREBP-1c signaling pathway by diosgenin
is independent of AMPK.
HFD is one of the most important risk factors
associated with the incidence of NAFLD (29,30). Rodents fed a HFD exhibit visceral
adiposity, hyperglycemia, dyslipidemia, hyperinsulinemia and
hepatic steatosis, which are findings similar to those in human
NAFLD (31). In order to
investigate the in vivo effect of diosgenin on NAFLD, a rat
model fed with HFD was used. In accordance with previous studies,
HFD caused obvious increases in rat body and liver weight, which
were significantly decreased by diosgenin. As determined by Oil red
O and H&E staining, diosgenin obviously ameliorated lipid
accumulation in the liver. This result was confirmed by
quantification of liver TG content. Considering the improvement of
lipid accumulation, it is obvious that diosgenin treatment was able
to alleviate NAFLD-induced liver injury.
ALT and AST are common indicators of liver injury in
the majority of liver diseases (32). Under normal conditions, ALT and
AST are mainly localized in the cytoplasm of hepatocytes. When the
hepatocytes are damaged, these enzymes are released from the cells
into the circulation, leading to elevated hepatic and serum ALT
and/or AST levels (33). In the
present study, the elevated serum ALT level in NAFLD rats was
reduced by diosgenin administration. However, fenofibrate treatment
significantly increased the serum ALT and AST levels. Our results
indicate an advantage of diosgenin over fenofibrate in terms of
liver function in NAFLD.
In conclusion, the present study demonstrated that
diosgenin was able to activate the AMPK pathway and suppress LXRα,
thereby ameliorating the hepatic lipid accumulation in vitro
and in vivo. Diosgenin also improved the HFD-induced liver
function disturbance. These data suggest that diosgenin is a
potential agent for preventing NAFLD through the AMPK and LXRα
pathways.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81430101,
81673655 and 81603458) and the E-Institutes of Shanghai Municipal
Education Commission (no. E03008).
References
|
1
|
Caballería L, Auladell MA, Torán P,
Miranda D, Aznar J, Pera G, Gil D, Muñoz L, Planas J, Canut S, et
al: Prevalence and factors associated with the presence of non
alcoholic fatty liver disease in an apparently healthy adult
population in primary care units. BMC Gastroenterol. 7:412007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu X, Huang Y, Bao Z, Wang Y, Shi D, Liu
F, Gao Z and Yu X: Prevalence and factors associated with
nonalcoholic fatty liver disease in Shanghai work-units. BMC
Gastroenterol. 12:1232012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Welsh JA, Karpen S and Vos MB: Increasing
prevalence of nonalcoholic fatty liver disease among United States
adolescents, 1988–1994 to 2007–2010. J Pediatr. 162:496–500. 2013.
View Article : Google Scholar
|
|
4
|
Margini C and Dufour JF: The story of HCC
in NAFLD: from epidemiology, across pathogenesis, to prevention and
treatment. Liver Int. 36:317–324. 2016. View Article : Google Scholar
|
|
5
|
Kahn BB, Alquier T, Carling D and Hardie
DG: AMP-activated protein kinase: ancient energy gauge provides
clues to modern understanding of metabolism. Cell Metab. 1:15–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hardie DG: AMP-activated/SNF1 protein
kinases: conserved guardians of cellular energy. Nat Rev Mol Cell
Biol. 8:774–785. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santamarina AB, Oliveira JL, Silva FP,
Carnier J, Mennitti LV, Santana AA, de Souza GH, Ribeiro EB, Oller
do Nascimento CM, Lira FS, et al: Green tea extract rich in
epigallocatechin-3-gallate prevents fatty liver by AMPK activation
via LKB1 in mice fed a high-fat diet. PLoS One. 10:e01412272015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Min Q, Ouyang C, Lee J, He C, Zou MH
and Xie Z: AMPK activation prevents excess nutrient-induced hepatic
lipid accumulation by inhibiting mTORC1 signaling and endoplasmic
reticulum stress response. Biochim Biophys Acta. 1842:1844–1854.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Li W, Liu Y, Sun Y, Li Y, Yao Q,
Li J, Zhang Q, Gao Y, Gao L, et al: Alpha-lipoic acid improves
high-fat diet-induced hepatic steatosis by modulating the
transcription factors SREBP-1, FoxO1 and Nrf2 via the
SIRT1/LKB1/AMPK pathway. J Nutr Biochem. 25:1207–1217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan H, Weng C, Yang Y, Huang L and Xing
X: Resistin, an adipokine, may affect the improvement of insulin
sensitivity in the metabolic syndrome patient treated with
metformin. Med Hypotheses. 81:969–971. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Mello VD, Kolehmainen M, Pulkkinen L,
Schwab U, Mager U, Laaksonen DE, Niskanen L, Gylling H, Atalay M,
Rauramaa R, et al: Downregulation of genes involved in NFkappaB
activation in peripheral blood mononuclear cells after weight loss
is associated with the improvement of insulin sensitivity in
individuals with the metabolic syndrome: The GENOBIN study.
Diabetologia. 51:2060–2067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Huang P, Yang B, Wang X and Xia J:
Roles of CXCL5 on migration and invasion of liver cancer cells. J
Transl Med. 12:1932014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Xu X, Zhang Y, Liu K, Huang F, Liu
B and Kou J: Diosgenin regulates adipokine expression in
perivascular adipose tissue and ameliorates endothelial dysfunction
via regulation of AMPK. J Steroid Biochem Mol Biol. 155:155–165.
2016. View Article : Google Scholar
|
|
14
|
Zang M, Zuccollo A, Hou X, Nagata D, Walsh
K, Herscovitz H, Brecher P, Ruderman NB and Cohen RA: AMP-activated
protein kinase is required for the lipid-lowering effect of
metformin in insulin-resistant human HepG2 cells. J Biol Chem.
279:47898–47905. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uemura T, Goto T, Kang MS, Mizoguchi N,
Hirai S, Lee JY, Nakano Y, Shono J, Hoshino S, Taketani K, et al:
Diosgenin, the main aglycon of fenugreek, inhibits LXRα activity in
HepG2 cells and decreases plasma and hepatic triglycerides in obese
diabetic mice. J Nutr. 141:17–23. 2011. View Article : Google Scholar
|
|
16
|
Ghosh S, Sikdar S, Mukherjee A and
Khuda-Bukhsh AR: Evaluation of chemopreventive potentials of
ethanolic extract of Ruta graveolens against A375 skin melanoma
cells in vitro and induced skin cancer in mice in vivo. J Integr
Med. 13:34–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Zhang H, Zheng H and Jiang Y:
Hepatic inflammation scores correlate with common carotid
intima-media thickness in rats with NAFLD induced by a high-fat
diet. BMC Vet Res. 10:1622014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akaslan SB, Degertekin CK, Yilmaz G, Cakir
N, Arslan M and Toruner FB: Effects of sitagliptin on nonalcoholic
fatty liver disease in diet-induced obese rats. Metab Syndr Relat
Disord. 11:243–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park KG, Min AK, Koh EH, Kim HS, Kim MO,
Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS, et al: Alpha-lipoic
acid decreases hepatic lipogenesis through adenosine
monophosphate-activated protein kinase (AMPK)-dependent and
AMPK-independent pathways. Hepatology. 48:1477–1486. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Edwards PA, Kast HR and Anisfeld AM:
BAREing it all: the adoption of LXR and FXR and their roles in
lipid homeostasis. J Lipid Res. 43:2–12. 2002.PubMed/NCBI
|
|
21
|
Gao M and Liu D: The liver X receptor
agonist T0901317 protects mice from high fat diet-induced obesity
and insulin resistance. AAPS J. 15:258–266. 2013. View Article : Google Scholar :
|
|
22
|
Chisholm JW, Hong J, Mills SA and Lawn RM:
The LXR ligand T0901317 induces severe lipogenesis in the db/db
diabetic mouse. J Lipid Res. 44:2039–2048. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jegatheesan P, Beutheu S, Freese K,
Waligora-Dupriet AJ, Nubret E, Butel MJ, Bergheim I and De Bandt
JP: Preventive effects of citrulline on Western diet-induced
non-alcoholic fatty liver disease in rats. Br J Nutr. 116:191–203.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsujimoto S, Kishina M, Koda M, Yamamoto
Y, Tanaka K, Harada Y, Yoshida A and Hisatome I: Nimesulide, a
cyclo-oxygenase-2 selective inhibitor, suppresses obesity-related
non-alcoholic fatty liver disease and hepatic insulin resistance
through the regulation of peroxisome proliferator-activated
receptor γ. Int J Mol Med. 38:721–728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dyck JR, Kudo N, Barr AJ, Davies SP,
Hardie DG and Lopaschuk GD: Phosphorylation control of cardiac
acetyl-CoA carboxylase by cAMP-dependent protein kinase and 5′-AMP
activated protein kinase. Eur J Biochem. 262:184–190. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X,
Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al: AMPK
phosphorylates and inhibits SREBP activity to attenuate hepatic
steatosis and atherosclerosis in diet-induced insulin-resistant
mice. Cell Metab. 13:376–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schultz JR, Tu H, Luk A, Repa JJ, Medina
JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al:
Role of LXRs in control of lipogenesis. Genes Dev. 14:2831–2838.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee J, Hong SW, Park SE, Rhee EJ, Park CY,
Oh KW, Park SW and Lee WY: AMP-activated protein kinase suppresses
the expression of LXR/SREBP-1 signaling-induced ANGPTL8 in HepG2
cells. Mol Cell Endocrinol. 414:148–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cabrera D, Ruiz A, Cabello-Verrugio C,
Brandan E, Estrada L, Pizarro M, Solis N, Torres J, Barrera F and
Arrese M: Diet-induced nonalcoholic fatty liver disease is
associated with sarcopenia and decreased serum insulin-like growth
factor-1. Dig Dis Sci. 61:3190–3198. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang C, Tao Q, Wang X, Wang X and Zhang X:
Impact of high-fat diet on liver genes expression profiles in mice
model of nonalcoholic fatty liver disease. Environ Toxicol
Pharmacol. 45:52–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu ZJ, Fan JG, Ding XD, Qiao L and Wang
GL: Characterization of high-fat, diet-induced, non-alcoholic
steatohepatitis with fibrosis in rats. Dig Dis Sci. 55:931–940.
2010. View Article : Google Scholar :
|
|
32
|
Jiang SL, Hu XD and Liu P:
Immunomodulation and liver protection of Yinchenhao decoction
against concan-avalin A-induced chronic liver injury in mice. J
Integr Med. 13:262–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moghadam AR, Tutunchi S,
Namvaran-Abbas-Abad A, Yazdi M, Bonyadi F, Mohajeri D, Mazani M,
Marzban H, Łos MJ and Ghavami S: Pre-administration of turmeric
prevents methotrexate-induced liver toxicity and oxidative stress.
BMC Complement Altern Med. 15:2462015. View Article : Google Scholar : PubMed/NCBI
|