Arrestins are a small family of proteins that
regulate signal transduction at G protein-coupled receptors
(1). β-arrestins are ubiquitous
scaffolding proteins initially identified during the purification
of the β-adrenergic receptor kinase (2). β-arrestins are involved in various
physiological and pathological processes, including G
protein-coupled receptor (GPCR) desensitization, sequestration and
vesicle trafficking (3). Four
members of the arrestin family have been identified so far,
including arrestins 1, 2, 3 and 4 (4). Arrestin1 and arrestin4 are visual
arrestins, while arrestin2 (β-arrestin1) and arrestin 3
(β-arrestin2) are non-visual (5).
Arrestin1 is localized in rods and cones, whereas arrestin4 is
localized exclusively to the latter. β-arrestin1 and β-arrestin2
mediate GPCR desensitization and internalization, and are widely
distributed throughout various tissues and cells (6). β-arrestin1 and β-arrestin2
accumulate in the cytoplasm of cells, however β-arrestin1 also
accumulates in the nucleus (7).
β-arrestins serve a role as signal transducers by
acting as multifunctional scaffolds, as downstream targets of
various types of receptor or by participating in
receptor-independent mechanisms (8). In addition, β-arrestin1 is recruited
into the nucleus to mediate the transactivation of the epidermal
growth factor receptor (EGFR) (9)
and the vascular endothelial growth factor receptors-2 and -3
(10,11). The present review assessed the
role of β-arrestins in the invasion and metastasis of cancer by
interacting with certain signaling pathways, including the
mitogen-activated protein kinase (MAPK), extracellular signal
regulated kinase (ERK), Akt, Wnt and nuclear factor (NF)-κB
pathways (12–16).
There are two types of β-arrestins: β-arrestin1 (53
kDa) and β-arrestin2 (46 kDa), located on chromosomes 7 and 11,
respectively (17,18). The amino acid sequences of
β-arrestin1 and β-arrestin2 are 70% identical (5) and sequence similarity between
β-arrestins is highly conserved across vertebrate and invertebrate
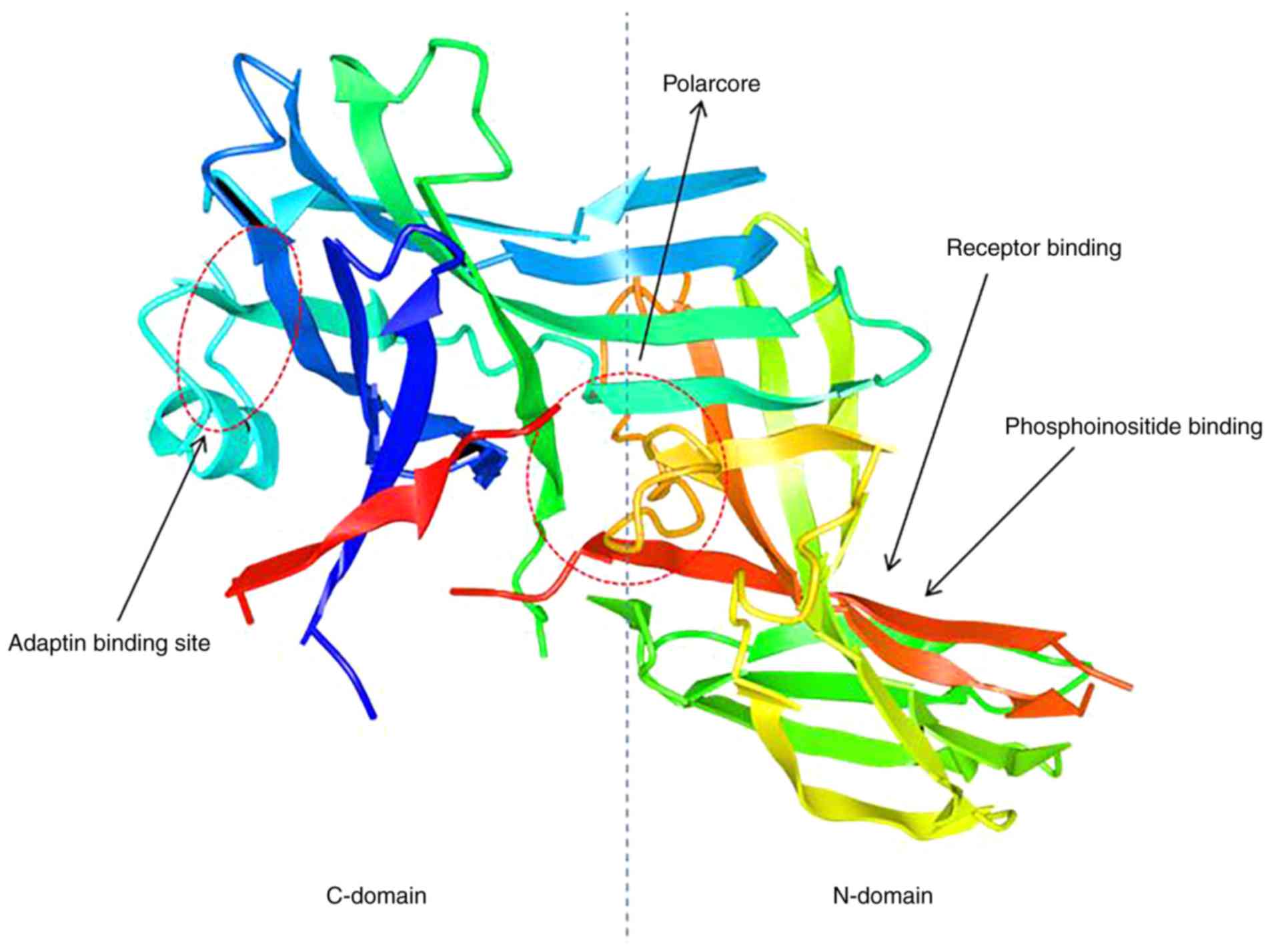
species, including humans, mice, rats and frog (19,20). At rest, β-arrestins exist as long
chained molecules that contain two concave lobes (an N-terminal
domain and a C-terminal domain), which are folded by two layers of
antiparallel β-sheets (Fig. 1).
The convex N-terminal domain contains a short α-helix and is linked
to the C-terminal domain via a polarized core, which is formed
through charged residues of salt bridge constitutes and functions
to maintain its correct position (21,22). β-arrestin1 contains an additional
cationic amphipathic helix that serves as a reversible membrane
anchor (23). When inactive, the
polarization core of β-arrestins relocates to the junction between
the N- and C-terminal domains and the carboxyl tail of the
C-terminus approaches the binding region. Following activation and
subsequent polarization, the β-arrestin core is destroyed, the
C-terminus carboxyl tail is released and the binding regions of
clathrin and adaptin protein-2 (24,25), c-Jun N-terminal kinase (JNK)3
(26) and ERK1/2 (27) are exposed.
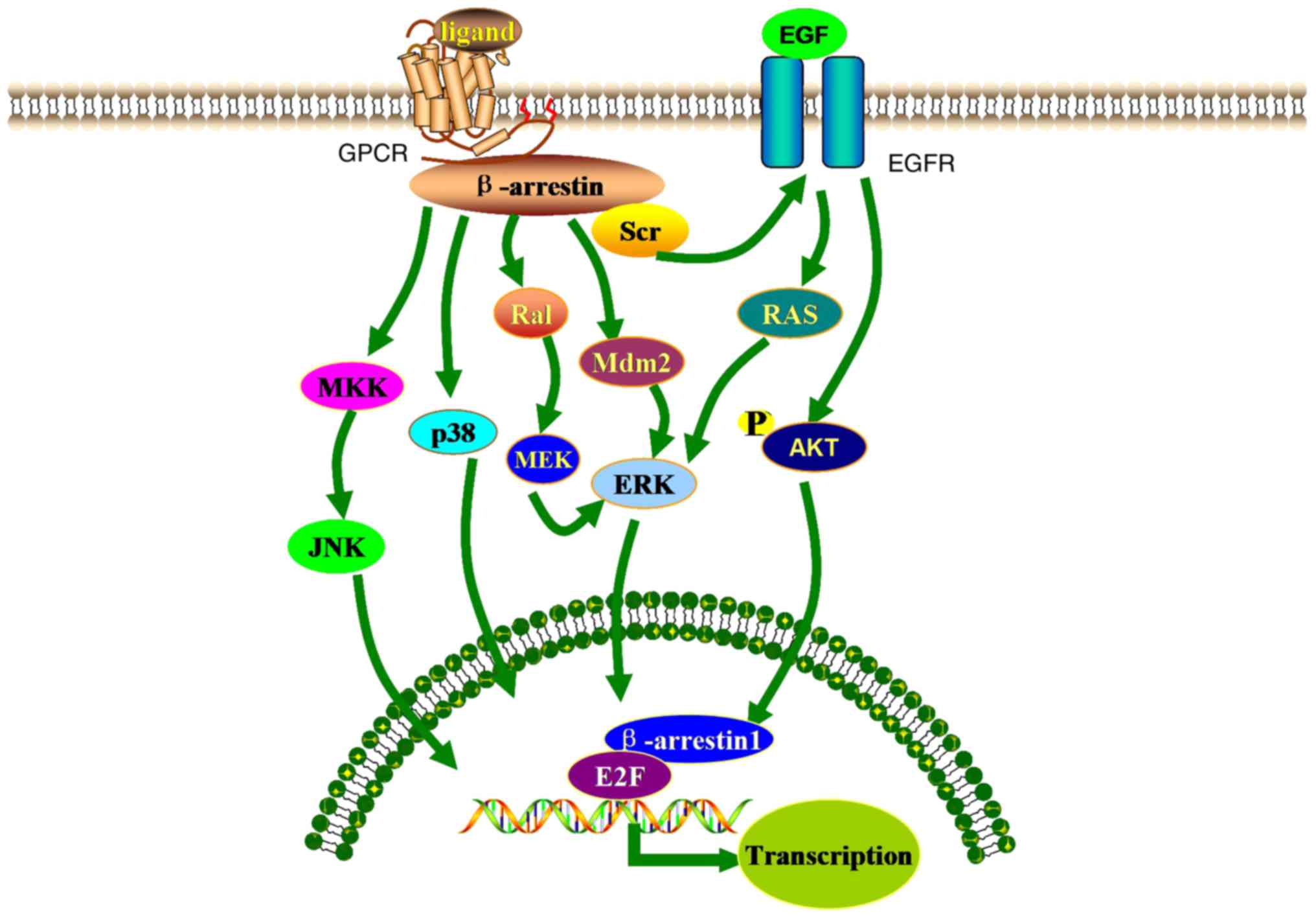
The MAPK pathway serves an important role in
regulating the various physiopathological processes involved in
tumorigenesis and the development of cancer (28). There are three main families of
MAPKs: ERKs, JNKs and stress-activated protein kinases (p38/SAPKs)
(Fig. 2) (29). The MAPK/ERK signaling pathway
regulates the proliferation, migration and invasion of tumor cells,
and is activated by various cell membrane receptors, including
receptor tyrosine kinases, GPCRs and cytokine receptors (30,31). MAPK/ERK overexpression has been
demonstrated to promote the epithelial-mesenchymal transition (EMT)
(32–35) and the expression of matrix
metalloproteases (MMPs) (36–38). Inhibiting the MAPK/ERK signaling
pathway may therefore suppress tumor cell invasion and migration
(39). β-arrestins, as scaffold
proteins, are associated with certain components of the MAPK
cascade and downstream targets of various GPCRs, which promote the
progression of cancer (40).
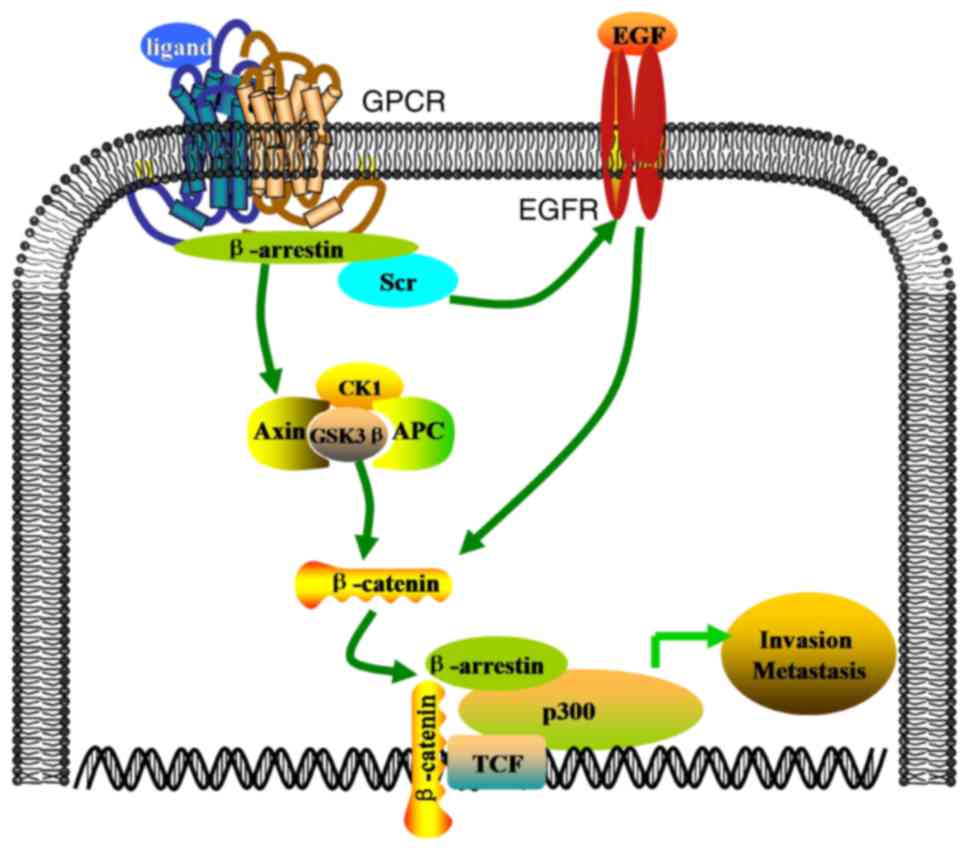
The Wnt family of secreted glycoproteins mediates
the proliferation, invasion and migration of cells through
β-arrestin-dependent (56)
canonical and noncanonical signaling, which involves cell division
cycle protein 42 (57), JNK
(58) and the small G proteins
RhoA and Rac (59). Wnt/β-catenin
signaling serves a fundamental role in various cellular processes.
The stimulation of β-catenin activates certain downstream effector
molecules (60-63) to initiate the transcription of
specific target genes, including MMP9, cyclin D1 and c-Myc
(64) in a variety of tumors
(62,65-67). In addition, the Wnt/β-catenin
pathway may regulate the EMT, which is an important step in the
induction of cell invasion and metastasis (68–70). The EMT involves various critical
mesenchymal markers, including E-cadherin, vimentin, N-cadherin,
zinc finger proteins (Snail/SNAI1 and Slug/SNAI2), twist-related
protein 1 and zinc finger E-box-binding homeobox 1 and 2 (71,72). Previous studies have demonstrated
that β-arrestins modulate the expression of these proteins via the
Wnt signaling pathway (73–75), thereby regulating the EMT. During
the EMT, epithelial cells lose their polarity and a transition
occurs from an epithelial phenotype associated with the basement
membrane, to a mesenchymal phenotype that promotes cell migration
and invasion, the inhibition of apoptosis and degradation of the
extracellular matrix (ECM). Previous studies have determined that
the interaction between β-arrestins and disheveled segment polarity
proteins (DVL) leads to the activation of Wnt signaling and
lymphoid enhancing binding factor (LEF)-mediated transcription
(Fig. 3) (76,77).
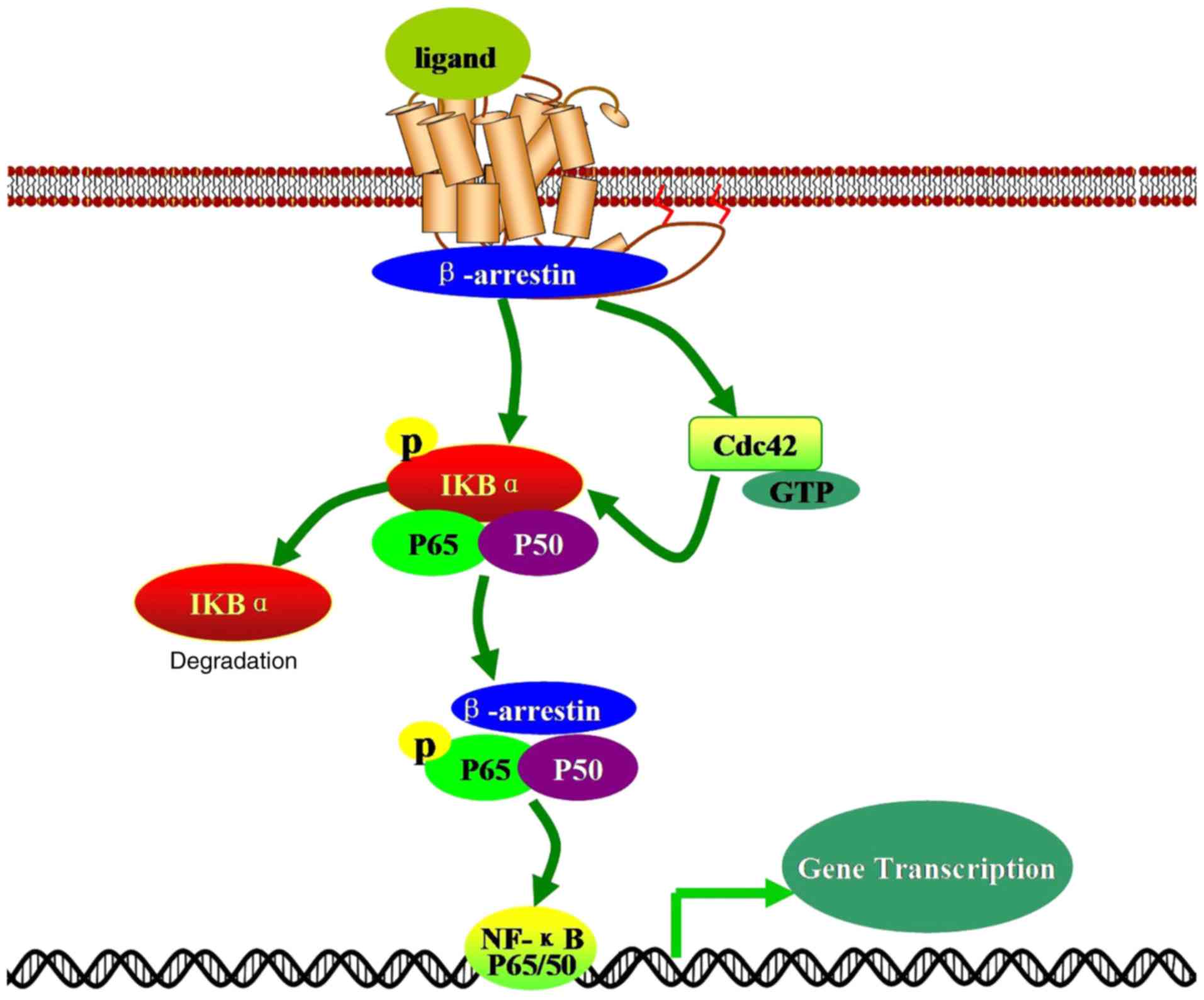
NF-κB is a dimeric transcription factor involved in
immune regulation, cell migration, proliferation, survival,
angiogenesis and apoptosis (82–84). The NF-κB family consists of five
members, including NF-κB1 (p50/105), NF-κB2 (p52/100), RelA (p65),
c-Rel and RelB, which are encoded by NFKB1, NFKB2, RELA, REL and
RELB, respectively. NF-κB is activated in different types of cancer
and serves a vital role in the development and progression of
tumors (85,86). The NF-κB signaling pathway
involves NF-κB, the NF-κB inhibitor (IκB), the IκB kinase (IKK)
complex and IKK upstream kinases (Fig. 4). Following stimulation, the
resulting signal increases the IKK-mediated phosphorylation of
IκBα, resulting in its ubiquitination and degradation (87). This leads to the release of NF-κB,
enabling it to enter the nucleus and regulate multiple downstream
target genes (88). Previous
studies have demonstrated that interfering with NF-κB activation
may regulate cell invasion, migration, proliferation and death
(89,90).
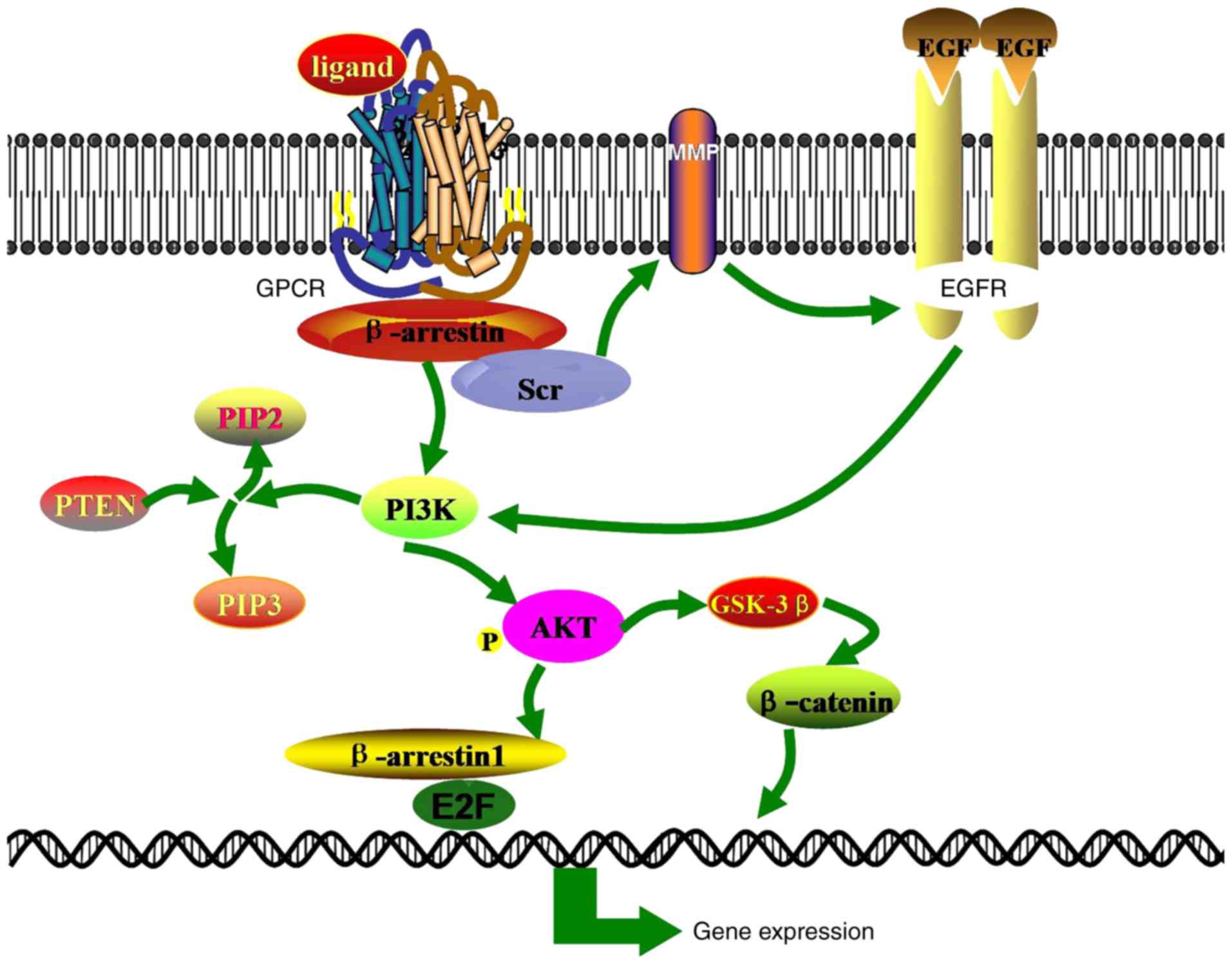
The PI3K signaling pathway serves a primary role in
regulating cell proliferation, differentiation, migration and
trafficking, as well as maintaining glucose homeostasis (97). PI3K expression increases levels of
phosphatidyl-(3,4,5)-trisphosphate (PIP3), which recruits
Akt to the cell membrane by binding to pleckstrin homology domains
(98). Following activation of
PI3K/Akt signaling, E-cadherin levels decrease and the expression
of snail, slug, vimentin and N-cadherin increase (99-101), thereby inducing the EMT and
promoting cell invasion and metastasis (102,103) (Fig. 5).
Cellular migration and invasion are two processes
regarded as the main causes of cancer-associated mortality
(109). Tumor metastasis is a
complex cascade that involves the following stages: Exit from the
primary tumor, cell migration, adherence and invasion via the
basement membrane or ECM, entry into the physical circulatory
system, further invasion into distant secondary organs or tissues,
and the resumption of cellular proliferation (110).
The role of the β-arrestins as primary modulators of
tumor invasion and metastasis is documented in the present review.
β-arrestin1 is primarily localized in the cytoplasm and nucleus of
cells, whereas β-arrestin2 is distributed in the cytoplasm alone
(111). Consequently,
β-arrestin1 and β-arrestin2 exhibit different functions in the
regulation and progression of malignant tumors via various
signaling pathways. β-arrestin1 and β-arrestin2 are involved in
GPCR-mediated signaling pathways but β-arrestin1 may also
participate in GPCR-mediated nuclear signaling. Kang et al
(112) demonstrated that
δ-opioid receptor activation induces the translocation of
β-arrestin1 into the nucleus and stimulates the transcription of
β-arrestin-dependent p27 and c-fos, thereby facilitating histone
acetyltransferase p300 recruitment, resulting in enhanced local
histone H4 acetylation and gene transcription. Furthermore,
β-arrestin1 and β-arrestin2 exert opposite effects in cancer
progression by interacting with different signaling pathways.
β-arrestins serve opposite roles in the development of lung cancer.
EP4/β-arrestin1/c-Src-mediated PGE2 activation induces the
migration of lung cancer cells (113), whilst homology β-arrestin2
exerts the opposite effect (92).
The anti- and pro-cancer effects exerted by β-arrestins in
different types of cancer may depend on the tumor microenvironment
(TME). The TME consists of various cells, including immune cells,
fibroblasts, endothelial cells, perivascular cells, neurons,
adipocytes and components of the ECM. Previous studies have
demonstrated that the TME serves a vital role in tumorigenesis,
tumor invasion and metastasis (114–116).
β-arrestins are scaffolding proteins and are
involved in cancer-associated invasion and metastasis, due to their
interaction with a range of receptor subtypes. A variety of
β-arrestin-biased ligands, which readily associate with β-arrestin,
have been identified, including nicotinic acetylcholine receptors,
EP2- and EP4-receptors, endothelin type A ETARs and transforming
growth factor β (117). Biased
ligands are able to specifically alter the conformation of a
receptor, whereas a specific receptor conformation cannot activate
all of its downstream signals in parallel and can only promoting a
particular downstream signal (118). ZD4054 is an antagonist of
β-arrestin-biased signaling in ETARs. ZD4054 selectively blocks
β-arrestin signals, eliminates the effects of β-arrestins,
decreases Src-EGFR-mediated transfer activation, inhibits the
transcription of β-arrestin genes and prevents β-arrestin-mediated
ovarian cancer cell invasion and metastasis (9). Therefore, the up- or downregulation
of β-arrestins is vital to either promote or inhibit of tumor
invasion and metastasis. Further studies that assess the function
of β-arrestins in tumor invasion and metastasis via different
signaling pathways may elucidate the anti-tumor mechanisms utilized
by β-arrestins and provide a potential therapeutic target for the
treatment of cancer.
The present review was supported by the
International Cooperation Key Project of National Natural Science
Foundation of China (grant no. 81520108031), the National Natural
Science Foundation of China (grant no. 81573749), the Science
Foundation of Shanghai Committee of Science Project (grant no.
14430722900) and the Program for Outstanding Medical Academic
Leader and Shanghai Academic Research Leader (grant no.
16XD1403600).
|
1
|
Gimenez LE, Kook S, Vishnivetskiy SA,
Ahmed MR, Gurevich EV and Gurevich VV: Role of receptor-attached
phosphates in binding of visual and non-visual arrestins to G
protein-coupled receptors. J Biol Chem. 287:9028–9040. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma D and Parameswaran N: Multifaceted
role of β-arrestins in inflammation and disease. Genes Immun.
16:5762015. View Article : Google Scholar
|
|
3
|
Smith JS and Rajagopal S: The β-arrestins:
Multifunctional regulators of G protein-coupled receptors. J Biol
Chem. 291:8969–8977. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu S, Wang D, Wu J, Jin J, Wei W and Sun
W: Involvement of β-arrestins in cancer progression. Mol Biol Rep.
40:1065–1071. 2013. View Article : Google Scholar
|
|
5
|
Gurevich EV and Gurevich VV: Arrestins:
Ubiquitous regulators of cellular signaling pathways. Genome Biol.
7:2362006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ranjan R, Gupta P and Shukla AK: Gpcr
signaling: β-arrestins kiss and remember. Curr Biol. 26:R285–R288.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohout TA, Lin FS, Perry SJ, Conner DA and
Lefkowitz RJ: Beta-arrestin 1 and 2 differentially regulate
heptahelical receptor signaling and trafficking. Proc Natl Acad Sci
USA. 98:1601–1606. 2001.PubMed/NCBI
|
|
8
|
Enslen H, Lima-Fernandes E and Scott MG:
Arrestins as regulatory hubs in cancer signalling pathways. Handb
Exp Pharmacol. 219:405–425. 2014. View Article : Google Scholar
|
|
9
|
Rosanò L, Cianfrocca R, Masi S, Spinella
F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG and
Bagnato A: Beta-arrestin links endothelin a receptor to
beta-catenin signaling to induce ovarian cancer cell invasion and
metastasis. Proc Natl Acad Sci USA. 106:2806–2811. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosanò L, Cianfrocca R, Tocci P, Spinella
F, Di Castro V, Caprara V, Semprucci E, Ferrandina G, Natali PG and
Bagnato A: Endothelin a receptor/β-arrestin signaling to the wnt
pathway renders ovarian cancer cells resistant to chemotherapy.
Cancer Res. 74:7453–7464. 2014. View Article : Google Scholar
|
|
11
|
Spinella F, Caprara V, Di Castro V, Rosanò
L, Cianfrocca R, Natali PG and Bagnato A: Endothelin-1 induces the
transactivation of vascular endothelial growth factor receptor-3
and modulates cell migration and vasculogenic mimicry in melanoma
cells. J Mol Med (Berl). 91:395–405. 2013. View Article : Google Scholar
|
|
12
|
Eichel K, Jullié D and von Zastrow M:
β-arrestin drives map kinase signalling from clathrin-coated
structures after GPCR dissociation. Nature Cell Biol. 18:303–310.
2016. View
Article : Google Scholar
|
|
13
|
Bourquard T, Landomiel F, Reiter E,
Crépieux P, Ritchie DW, Azé J and Poupon A: Unraveling the
molecular architecture of a G protein-coupled
receptor/β-arrestin/erk module complex. Sci Rep. 5:107602015.
View Article : Google Scholar
|
|
14
|
Sun WY, Hu SS, Wu JJ, Huang Q, Ma Y, Wang
QT, Chen JY and Wei W: Down-regulation of β-arrestin2 promotes
tumour invasion and indicates poor prognosis of hepatocellular
carcinoma. Sci Rep. 6:356092016. View Article : Google Scholar
|
|
15
|
Kim M, Suh YA, Oh JH, Lee BR, Kim J and
Jang SJ: Corrigendum: KIF3A binds to β-arrestin for suppressing
wnt/β-catenin signalling independently of primary cilia in lung
cancer. Sci Rep. 7:467732017. View Article : Google Scholar
|
|
16
|
Lee SU, Ahn KS, Sung MH, Park JW, Ryu HW,
Lee HJ, Hong ST and Oh SR: Indacaterol inhibits tumor cell
invasiveness and mmp-9 expression by suppressing IKK/NF-κB
activation. Mol Cells. 37:585–591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Conner DA, Mathier MA, Mortensen RM,
Christe M, Vatner SF, Seidman CE and Seidman JG: Beta-arrestin1
knockout mice appear normal but demonstrate altered cardiac
responses to beta-adrenergic stimulation. Circ Res. 81:1021–1026.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bohn LM, Lefkowitz RJ, Gainetdinov RR,
Peppel K, Caron MG and Lin FT: Enhanced morphine analgesia in mice
lacking beta-arrestin 2. Science. 286:2495–2498. 1999. View Article : Google Scholar
|
|
19
|
Gu YJ, Sun WY, Zhang S, Wu JJ and Wei W:
The emerging roles of β-arrestins in fibrotic diseases. Acta
Pharmacol Sin. 36:1277–1287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Philipp M, Evron T and Caron MG: The role
of arrestins in development. Prog Mol Biol Transl Sci. 118:225–242.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bayburt TH, Vishnivetskiy SA, McLean MA,
Morizumi T, Huang CC, Tesmer JJ, Ernst OP, Sligar SG and Gurevich
VV: Monomeric rhodopsin is sufficient for normal rhodopsin kinase
(grk1) phosphorylation and arrestin-1 binding. J Biol Chem.
286:1420–1428. 2011. View Article : Google Scholar :
|
|
22
|
Hamdan FF, Rochdi MD, Breton B, Fessart D,
Michaud DE, Charest PG, Laporte SA and Bouvier M: Unraveling G
protein-coupled receptor endocytosis pathways using real-time
monitoring of agonist-promoted interaction between beta-arrestins
and AP-2. J Biol Chem. 282:29089–29100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han M, Gurevich VV, Vishnivetskiy SA,
Sigler PB and Schubert C: Crystal structure of beta-arrestin at 1.9
A: Possible mechanism of receptor binding and membrane
translocation. Structure. 9:869–880. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan H, Liao Y, Tang Q, Liang L and Chen
XY: Role of β-arrestins in the pathogenesis of inflammatory bowel
disease. World Chinese J Digestol. 18:3114–3120. 2010. View Article : Google Scholar
|
|
25
|
Nobles KN, Guan Z, Xiao K, Oas TG and
Lefkowitz RJ: The active conformation of beta-arrestin1: Direct
evidence for the phosphate sensor in the n-domain and
conformational differences in the active states of beta-arrestins1
and -2. J Biol Chem. 282:21370–21381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seo J, Tsakem EL, Breitman M and Gurevich
VV: Identification of arrestin-3-specific residues necessary for
JNK3 kinase activation. J Biol Chem. 286:27894–27901. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin FT, Miller WE, Luttrell LM and
Lefkowitz RJ: Feedback regulation of beta-arrestin1 function by
extracellular signal-regulated kinases. J Biol Chem.
274:15971–15974. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morrison DK: Map kinase pathways. Cold
Spring Harb Perspect Bio. 4(pii): a0112542012.
|
|
30
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou H, Li XM, Meinkoth J and Pittman RN:
Akt regulates cell survival and apoptosis at a postmitochondrial
level. J Cell Biol. 151:483–494. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okada T, Sinha S, Esposito I, Schiavon G,
López-Lago MA, Su W, Pratilas CA, Abele C, Hernandez JM, Ohara M,
et al: The Rho GTPase Rnd1 suppresses mammary tumorigenesis and EMT
by restraining RAS-MAPK signalling. Nat Cell Biol. 17:81–94. 2015.
View Article : Google Scholar
|
|
33
|
Gu Y, Wang Q, Guo K, Qin W, Liao W, Wang
S, Ding Y and Lin J: TUSC3 promotes colorectal cancer progression
and epithelial-mesenchymal transition (EMT) through WNT/β-catenin
and MAPK signalling. J Pathol. 239:60–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaufhold S and Bonavida B: Central role of
snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mulholland DJ, Kobayashi N, Ruscetti M,
Zhi A, Tran LM, Huang J, Gleave M and Wu H: Pten loss and RAS/MAPK
activation cooperate to promote EMT and metastasis initiated from
prostate cancer stem/progenitor cells. Cancer Res. 72:1878–1889.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou G, Peng F, Zhong Y, Chen Y, Tang M
and Li D: Rhein suppresses matrix metalloproteinase production by
regulating the Rac1/ROS/MAPK/AP-1 pathway in human ovarian
carcinoma cells. Int J Onco. 50:933–941. 2017. View Article : Google Scholar
|
|
37
|
Sangpairoj K, Vivithanaporn P,
Apisawetakan S, Chongthammakun S, Sobhon P and Chaithirayanon K:
RUNX1 regulates migration, invasion, and angiogenesis via 38 MAPK
pathway in human glioblastoma. Cell Mol Neurobiol. 2016.Epub ahead
of print.
|
|
38
|
Cepeda MA, Evered CL, Pelling JJH and
Damjanovski S: Inhibition of MT1-MMP proteolytic function and
ERK1/2 signalling influences cell migration and invasion through
changes in MMP-2 and MMP-9 levels. J Cell Commun Signal.
11:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Suyama K, Shapiro I, Guttman M and Hazan
RB: A signaling pathway leading to metastasis is controlled by
N-cadherin and the FGF receptor. Cancer Cell. 2:301–314. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luttrell LM, Roudabush FL, Choy EW, Miller
WE, Field ME, Pierce KL and Lefkowitz RJ: Activation and targeting
of extracellular signal-regulated kinases by beta-arrestin
scaffolds. Proc Natl Acad Sci USA. 98:2449–2454. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fong AM, Premont RT, Richardson RM, Yu YR,
Lefkowitz RJ and Patel DD: Defective lymphocyte chemotaxis in
beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA.
99:7478–7483. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Décaillot FM, Kazmi MA, Lin Y, Ray-Saha S,
Sakmar TP and Sachdev P: Cxcr7/cxcr4 heterodimer constitutively
recruits beta-arrestin to enhance cell migration. J Biol Chem.
286:32188–32197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu D, Li R, Wu J, Jiang L and Zhong HA:
Drug design targeting the cxcr4/cxcr7/cxcl12 pathway. Curr Top Med
Chem. 16:1441–1451. 2016. View Article : Google Scholar
|
|
44
|
Coggins L, Trakimas D, Chang SL, Ehrlich
A, Ray P, Luker KE, Linderman JJ and Luker GD: Cxcr7 controls
competition for recruitment of β-arrestin 2 in cells expressing
both cxcr4 and cxcr7. PLoS On. 9:e983282014. View Article : Google Scholar
|
|
45
|
Zhang P, He X, Tan J, Zhou X and Zou L:
β-arrestin2 mediates β-2 adrenergic receptor signaling inducing
prostate cancer cell progression. Oncol Rep. 26:1471–1477.
2011.PubMed/NCBI
|
|
46
|
Buchanan FG, Gorden DL, Matta P, Shi Q,
Matrisian LM and DuBois RN: Role of beta-arrestin 1 in the
metastatic progression of colorectal cancer. Proc Natl Acad Sci
USA. 103:1492–1497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lan T, Wang H, Zhang Z, Zhang M, Qu Y,
Zhao Z, Fan X, Zhan Q, Song Y and Yu C: Downregulation of
β-arrestin 1 suppresses glioblastoma cell malignant progression vis
inhibition of src signaling. Exp Cell Res. 357:51–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ge L, Shenoy SK, Lefkowitz RJ and DeFea K:
Constitutive protease-activated receptor-2-mediated migration of
MDA MB-231 breast cancer cells requires both beta-arrestin-1 and
-2. J Biol Chem. 279:55419–55424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Parisis N, Metodieva G and Metodiev MV:
Pseudopodial and β-arrestin-interacting proteomes from migrating
breast cancer cells upon AR2 activation. J Proteomics. 80:91–106.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Girnita L, Shenoy SK, Sehat B, Vasilcanu
R, Vasilcanu D, Girnita A, Lefkowitz RJ and Larsson O:
Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK
activation and cell cycle progression. J Biol Chem.
282:11329–11338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schaal C and Chellappan SP:
Nicotine-mediated cell proliferation and tumor progression in
smoking-related cancers. Mol Cancer Res. 12:14–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu H, Zhang Q, Li K, Gong Z, Liu Z, Xu Y,
Swaney MH, Xiao K and Chen Y: Prognostic significance of USP33 in
advanced colorectal cancer patients: New insights into
β-arrestin-dependent ERK signaling. Oncotarget. 7:81223–81240.
2016.PubMed/NCBI
|
|
53
|
Li XX, Zheng HT, Huang LY, Shi DB, Peng
JJ, Liang L and Cai SJ: Silencing of CXCR7 gene represses growth
and invasion and induces apoptosis in colorectal cancer through ERK
and β-arrestin pathways. Int J Oncol. 45:1649–1657. 2016.
View Article : Google Scholar
|
|
54
|
Goertzen CG, Dragan M, Turley E, Babwah AV
and Bhattacharya M: KISS1R signaling promotes invadopodia formation
in human breast cancer cell via β-arrestin2/ERK. Cell Signal.
28:165–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dasgupta P, Rizwani W, Pillai S, Davis R,
Banerjee S, Hug K, Lloyd M, Coppola D, Haura E and Chellappan SP:
Arrb1-mediated regulation of E2F target genes in nicotine-induced
growth of lung tumors. J Natl Cancer Inst. 103:317–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Korinek V, Barker N, Willert K, Molenaar
M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O and Clevers
H: Two members of the tcf family implicated in wnt/beta-catenin
signaling during embryogenesis in the mouse. Mol Cell Biol.
18:1248–1256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mythreye K and Blobe GC: The type iii
TGF-beta receptor regulates epithelial and cancer cell migration
through beta-arrestin2-mediated activation of Cdc42. Proc Natl Acad
Sci USA. 106:8221–8226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim GH, Her JH and Han JK: Ryk cooperates
with frizzled 7 to promote wnt11-mediated endocytosis and is
essential for xenopus laevis convergent extension movements. J Cell
Biol. 182:1073–1082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Habas R, Dawid IB and He X: Coactivation
of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate
gastrulation. Genes Dev. 17:295–309. 2008. View Article : Google Scholar
|
|
60
|
Kypta RM and Waxman J: Wnt/β-catenin
signalling in prostate cancer. Nat Rev Urol. 9:418–428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Meng X, Zhu D, Yang S, Wang X, Xiong Z,
Zhang Y, Brachova P and Leslie KK: Cytoplasmic metadherin (MTDH)
provides survival advantage under conditions of stress by acting as
RNA-binding protein. J Biol Chem. 287:4485–4491. 2012. View Article : Google Scholar :
|
|
62
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu Q, Krause M, Samoylenko A and Vainio S:
Wnt signaling in renal cell carcinoma. Cancers (Basel). 8(pii):
E572016. View Article : Google Scholar
|
|
64
|
Chen Z, He X, Jia M, Liu Y, Qu D, Wu D, Wu
P, Ni C, Zhang Z, Ye J, et al: β-catenin overexpression in the
nucleus predicts progress disease and unfavourable survival in
colorectal cancer: A meta-analysis. PLoS One. 8:e638542013.
View Article : Google Scholar
|
|
65
|
Aminuddin A and Ng PY: Promising druggable
target in head and neck squamous cell carcinoma: Wnt signaling.
Front Pharmacol. 7:2442016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liang S, Zhang S, Wang P, Yang C, Shang C,
Yang J and Wang J: Lncrna, TUG1 regulates the oral squamous cell
carcinoma progression possibly via interacting with
Wnt/beta-catenin signaling. Gene. 608:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liang J, Liang L, Ouyang K, Li Z and Yi X:
MALAT1 induces tongue cancer cells' EMT and inhibits apoptosis
through wnt/β-catenin signaling pathway. J Oral Pathol Med.
46:98–105. 2017. View Article : Google Scholar
|
|
68
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Howard S, Deroo T, Fujita Y and Itasaki N:
A positive role of cadherin in Wnt/β-catenin signalling during
epithelial-mesenchymal transition. PLoS On. 6:e238992011.
View Article : Google Scholar
|
|
70
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Felipe Lima J, Nofech-Mozes S, Bayani J
and Bartlett JM: Emt in breast carcinoma-a review. J Clin Me.
5(pii): E652016. View Article : Google Scholar
|
|
72
|
Grant CM and Kyprianou N: Epithelial
mesenchymal transition (EMT) in prostate growth and tumor
progression. Transl Androl Urol. 2:202–211. 2003.
|
|
73
|
Ko CJ, Huang CC, Lin HY, Juan CP, Lan SW,
Shyu HY, Wu SR, Hsiao PW, Huang HP, Shun CT and Lee MS:
Androgen-induced TMPRSS2 activates matriptase and promotes
extracellular matrix degradation, prostate cancer cell invasion,
tumor growth, and metastasis. Cancer Res. 75:2949–2960. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liao X, Thrasher JB, Pelling J,
Holzbeierlein J, Sang QX and Li B: Androgen stimulates matrix
metalloproteinase-2 expression in human prostate cancer.
Endocrinology. 144:1656–1663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang Y, Jiao L, Hou J, Xu C, Wang L, Yu Y,
Li Y, Yang C, Wang X and Sun Y: Dishevelled-2 silencing reduces
androgen-dependent prostate tumor cell proliferation and migration
and expression of Wnt-3a and matrix metalloproteinases. Mol Biol
Rep. 40:4241–4250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sun L, Liu T, Zhang S, Guo K and Liu Y:
Oct4 induces EMT through LEF1/β-catenin dependent WNT signaling
pathway in hepatocellular carcinoma. Oncol Lett. 13:2599–2606.
2017.PubMed/NCBI
|
|
77
|
Zhang Y: Ganodermalucidum (Reishi)
suppresses proliferation and migration of breast cancer cells via
inhibiting Wnt/β-catenin signaling. Biochem Biophys Res Commun.
488:679–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rosanò L, Cianfrocca R, Tocci P, Spinella
F, Di Castro V, Spadaro F, Salvati E, Biroccio AM, Natali PG and
Bagnato A: β-arrestin-1 is a nuclear transcriptional regulator of
endothelin-1-induced β-catenin signaling. Oncogene. 32:5066–5077.
2013. View Article : Google Scholar
|
|
79
|
Turm H, Maoz M, Katz V, Yin YJ, Offermanns
S and Bar-Shavit R: Protease-activated receptor-1 (AR1) acts via a
novel galpha13-dishevelled axis to stabilize beta-catenin levels. J
Biol Chem. 285:15137–15148. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Bonnans C, Flaceliere M, Grillet F, Dantec
C, Desvignes JP, Pannequin J, Severac D, Dubois E, Bibeau F,
Escriou V, et al: Essential requirement for β-arrestin2 in mouse
intestinal tumors with elevated wnt signaling. Proc Natl Acad Sci
USA. 109:3047–3052. 2012. View Article : Google Scholar
|
|
81
|
Duan X, Zhang T, Kong Z, Mai X, Lan C,
Chen D, Liu Y, Zeng Z, Cai C, Deng T, et al: β-arrestin 1 promotes
epithelial-mesenchymal transition via modulating GSK-3β/β-catenin
pathway in prostate cancer cells. Biochem Biophys Res Commun.
479:204–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Witherow DS, Garrison TR, Miller WE and
Lefkowitz RJ: Beta-arrestin inhibits NF-kappaB activity by means of
its interaction with the Nf-KappaB inhibitor IkappaBalpha. Proc
Natl Acad Sci USA. 101:8603–8607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kim YR, Kim IJ, Kang TW, Choi C, Kim KK,
Kim MS, Nam KI and Jung C: HOXB13 downregulates intracellular zinc
and increases NF-κB signaling to promote prostate cancer
metastasis. Oncogene. 33:4558–4567. 2014. View Article : Google Scholar
|
|
84
|
Jiang L, Lin C, Song L, Wu J, Chen B, Ying
Z, Fang L, Yan X, He M, Li J and Li M: Microrna-30e* promotes human
glioma cell invasiveness in an orthotopic xenotransplantation model
by disrupting the NF-κB/IκBα negative feedback loop. J Clin Invest.
122:33–47. 2012. View Article : Google Scholar
|
|
85
|
Karin M: Nuclear factor-kappab in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Karin M, Cao Y, Greten FR and Li ZW:
Nf-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu B, Han M and Wen JK:
Acetylbritannilactone inhibits neointimal hyperplasia after balloon
injury of rat artery by suppressing nuclear factor-{kappa}B
activation. J Pharmacol Exp Ther. 324:292–298. 2008. View Article : Google Scholar
|
|
88
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109(Suppl): S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kong D, Li Y, Wang Z, Banerjee S and
Sarkar FH: Inhibition of angiogenesis and invasion by
3.3′-diindolylmethane is mediated by the nuclear factor-kappaB
downstream target genes MMP-9 and uPA that regulated
bioavailability of vascular endothelial growth factor in prostate
cancer. Cancer Res. 67:3310–3319. 2002. View Article : Google Scholar
|
|
90
|
Liao D, Zhong L, Duan T, Zhang RH, Wang X,
Wang G, Hu K, Lv X and Kang T: Aspirin suppresses the growth and
metastasis of osteosarcoma through the NF-κB pathway. Clin Cancer
Res. 21:5349–5359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Cianfrocca R, Tocci P, Semprucci E,
Spinella F, Di Castro V, Bagnato A and Rosanò L: β-arrestin 1 is
required for endo-thelin-1-induced NF-κB activation in ovarian
cancer cells. Life Sci. 118:179–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Raghuwanshi SK, Nasser MW, Chen X,
Strieter RM and Richardson RM: Depletion of beta-arrestin-2
promotes tumor growth and angiogenesis in a murine model of lung
cancer. J Immunol. 180:5699–5706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B
and Pei G: Identification of beta-arrestin2 as a G protein-coupled
receptor-stimulated regulator of NF-kappaB pathways. Mol Cell.
14:303–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang Y, Tang Y, Teng L, Wu Y, Zhao X and
Pei G: Association of beta-arrestin and TRAF6 negatively regulates
toll-like receptor-interleukin 1 receptor signaling. Nat Immunol.
7:139–147. 2006. View
Article : Google Scholar
|
|
95
|
Dranoff G: Cytokines in cancer
pathogenesis and cancer therapy. Nat Rev Cancer. 4:11–22. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Bedini A, Baiula M, Vincelli G, Formaggio
F, Lombardi S, Caprini M and Spampinato S: Nociceptin/orphanin FQ
antagonizes lipopolysaccharide-stimulated proliferation, migration
and inflammatory signaling in human glioblastoma U87 cells. Biochem
Pharmacol. 140:89–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lino MM and Merlo A: I3K inase signaling
in glioblastoma. J Neurooncol. 103:417–427. 2011. View Article : Google Scholar
|
|
98
|
Chalhoub N and Baker SJ: PTEN and the
I3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2017.
View Article : Google Scholar
|
|
99
|
Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y,
Zhang Y, Hua S, Fu Q, Zhao M, et al: Downregulation of FAP
suppresses cell proliferation and metastasis through PTEN/I3K/AKT
and RAS-ERK signaling in oral squamous cell carcinoma. Cell Death
Di. 5:e11552014. View Article : Google Scholar
|
|
100
|
Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y,
Zhao L, Qu H, Fan Y and Wu C: Antagonism of miR-21 reverses
epithelial-mesenchymal transition and cancer stem cell phenotype
through AKT/ERK1/2 inactivation by targeting PTEN. PLoS On.
7:e395202012. View Article : Google Scholar
|
|
101
|
Jensen RL: Brain tumor hypoxia:
Tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a
therapeutic target. J Neurooncol. 92:317–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen B, Zeng X, He Y, Wang X, Liang Z, Liu
J, Zhang P, Zhu H, Xu N and Liang S: STC2 promotes the
epithelial-mesenchymal transition of colorectal cancer cells
through AKT-ERK signaling pathways. Oncotarget. 7:71400–71416.
2016.PubMed/NCBI
|
|
103
|
Wang Z, Qu L, Deng B, Sun X, Wu S, Liao J,
Fan J and Peng Z: Styk1 promotes epithelial-mesenchymal transition
and tumor metastasis in human hepatocellular carcinoma through
Mek/Erk and I3K/AKT signaling. Sci Rep. 6:332052016. View Article : Google Scholar
|
|
104
|
Zhang Y, Yang CQ, Gao Y, Wang C, Zhang CL
and Zhou XH: Knockdown of CXCR7 inhibits proliferation and invasion
of osteosarcoma cells through inhibition of the I3K/AKT and
β-arrestin pathways. Oncol Rep. 32:965–972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zou L, Yang R, Chai J and Pei G: Rapid
xenograft tumor progression in beta-arrestin1 transgenic mice due
to enhanced tumor angiogenesis. FASEB J. 22:355–364. 2008.
View Article : Google Scholar
|
|
106
|
Alvarez CJ, Lodeiro M, Theodoropoulou M,
Camiña JP, Casanueva FF and Pazos Y: Obestatin stimulates
aktsignalling in gastric cancer cells through
beta-arrestin-mediated epidermal growth factor receptor
transactivation. Endocr Relat Cancer. 16:599–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Nawaz Z, Patil V, Paul Y, Hegde AS,
Arivazhagan A, Santosh V and Somasundaram K: Pi3 kinase pathway
regulated mirnome in glioblastoma: Identification of mir-326 as a
tumour suppressor miRNA. Mol Cance. 15:742016. View Article : Google Scholar
|
|
108
|
Lima-Fernandes E, Enslen H, Camand E,
Kotelevets L, Boularan C, Achour L, Benmerah A, Gibson LC, Baillie
GS, Pitcher JA, et al: Distinct functional outputs of PTEN
signalling are controlled by dynamic association with β-arrestins.
EMBO J. 30:2557–2568. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Li Y, Guo G, Song J, Cai Z, Yang J, Chen
Z, Wang Y, Huang Y and Gao Q: B7-H3 promotes the migration and
invasion of human bladder cancer cells via the I3K/AKT/STAT3
signaling pathway. J Cancer. 8:816–824. 2017. View Article : Google Scholar :
|
|
110
|
Tayeh M, Nilwarangoon S, Mahabusarakum W
and Watanapokasin R: Anti-metastatic effect of rhodomyrtone from
rhodomyrtus tomentosa on human skin cancer cells. Int J Oncol.
50:1035–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Scott MG, Le Rouzic E, Périanin A,
Pierotti V, Enslen H, Benichou S, Marullo S and Benmerah A:
Differential nucleo-cytoplasmic shuttling of beta-arrestins.
Characterization of a leucine-rich nuclear export signal in
beta-arrestin2. J Biol Chem. 277:37693–37701. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M,
Zhang M, Bao G, Wang F, Zhang X, et al: A nuclear function of
beta-arrestin1 in GPCR signaling: Regulation of histone acetylation
and gene transcription. Cell. 123:833–847. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kim JI, Lakshmikanthan V, Frilot N and
Daaka Y: Prostaglandin E2 promotes lung cancer cell migration via
EP4-betaArrestin1-c-src signalsome. Mol Cancer Res. 8:569–577.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lin EW, Karakasheva TA, Hicks PD, Bass AJ
and Rustgi AK: The tumor microenvironment in esophageal cancer.
Oncogene. 35:5337–5349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Clark AG and Vignjevic DM: Modes of cancer
cell invasion and the role of the microenvironment. Curr Opin Cell
Biol. 36:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ji RC: Hypoxia and lymphangiogenesis in
tumor microenvironment and metastasis. Cancer Lett. 346:6–16. 2014.
View Article : Google Scholar
|
|
117
|
Whalen EJ, Rajagopal S and Lefkowitz RJ:
Therapeutic potential of β-arrestin- and G protein-biased agonists.
Trends Mol Med. 17:126–139. 2011. View Article : Google Scholar
|
|
118
|
Bologna Z, Teoh JP, Bayoumi AS, Tang Y and
Kim IM: Biased G protein-coupled receptor signaling: New player in
modulating physiology and pathology. Biomol Ther (Seoul). 25:12–25.
2017. View Article : Google Scholar
|