Introduction
Obesity is the most common metabolic disease
worldwide and represents a serious human health issue (1–3).
It is a state of energy imbalance caused by excessive energy
storage and insufficient energy expenditure (4,5),
and is closely associated with a high-calorie diet, high blood
pressure, cardiovascular disease, atherosclerosis, osteoarthritis,
nonalcoholic fatty liver disease (NAFLD) and metabolic syndrome
(6–8). Besides increased morbidity rates,
obesity is also associated with a variety of metabolic syndromes,
including type 2 diabetes, insulin resistance, hyperlipidemia,
hypertension, stroke, cardiac injury, heart disease and cancer
(9,10).
Exercise and dietary control are effective
therapeutic strategies for obesity but pharmacotherapy is another
important option for direct treatment. A variety of drugs that
provide appetite inhibition, heat production, satiety enhancement
and inhibition of fat absorption have been developed to treat
obesity (11,12). Currently available therapeutic
agents include orlistat, phentermine and phendimetrazine. However,
these drugs have side effects, including abdominal distension,
increased bowel movements, diarrhea, fever, anorexia, nasal
congestion (orlistat), insomnia, cardiovascular disease, extreme
fatigue (phendimetrazine) and depression (phentermine and
phendimetrazine) (13,14). In fact, due to the adverse effects
of these types of pharmaceutical approaches, certain anti-obesity
medicinal products have been withdrawn from the market following
their approval (15). Thus, the
development of safe and effective anti-obesity drugs is of utmost
importance. In addition, dietary supplements and natural herbal
components are increasingly being recognized as viable alternative
therapies. However, there remains a requirement for functional
agents derived from safe and natural sources that are effective for
obesity control with minimal side effects relative to those of
artificially synthesized drugs.
Several studies have attempted to derive
anti-obesity agents from seaweeds that contain a variety of
vitamins and dietary fibre with low energy content. The development
of functional foods from seaweed has led to additional research
demonstrating improvements in hyperlipidemia, suppression of
cholesterol accumulation, improvement of bowel activity and release
of heavy metals (16–18).
Brown algae have potential therapeutic value due to
the abundance of bioactive substances, including sulfated
polysaccharides, proteins, dietary fibres, carotenoids, alginates,
fucoidans and phlorotannines, contained in them (19–25). Furthermore, polysaccharides,
including alginate and fucoidan, reduce serum cholesterol and
triglycerides (TG) (26–29). Therefore, components of seaweed
are likely to serve as safe anti-obesity agents that lack adverse
side effects, and which may be ingested for long periods of time.
Ecklonia cava is an edible species of brown algae found in
the ocean off the coasts of Japan and Korea (30). Phlorotannins, which are polyphenol
components of E. cava, have been isolated and demonstrated
to include fucodiphlorethol G, eckol, 8-8′-bieckol, dieckol,
eckstolonol, phlorofucofuroeckol A, phloroglucinol and
dioxinodehydroeckol (31–33). Studies on E. cava have
demonstrated its anti-inflammatory (24,34,35), anti-oxidative (22–25,32,36–39), anti-bacterial (40,41), anti-cancer (42-44) and hair growth (45,46) effects as well as its actions
against Alzheimer's disease (47,48).
Studies on the anti-obesity effects of E.
cava have been performed in zebrafish (49,50), mice (51–53) and cell cultures (54–57), with most of these studies focusing
on identifying a method to effectively extract phlorotannins (e.g.,
eckol, dieckol and phlorofucofuroeckol-A). For this purpose, a
previous study by our group investigated a variety of methods,
including hot-water extraction, ethanol extraction and enzyme
extraction, and assessed the yield efficiency and economic
efficiency (58). Hot water
treatments (60°C and 90°C), ethanol treatments (60 and 80%) and
enzymatic treatments (Protex 6L, an endo-type protease; Rapidase
press L, a pectinase cellulase/hemicellulase enzyme complex;
Rohament CL, a cellulase β glucanase/hemicellulase enzyme complex;
and a Rapidase press L + Rohament CL complex) were performed to
determine the optimal conditions for processing E. cava. Of
these treatments, the yields were highest for the 90°C hot water
treatment (27.75%), 60% ethanol treatment (11.42%) and Rapidase
press L + Rohament CL enzymatic treatment (21.87%). The total
polyphenol and phlorotannin contents of the raw materials were
1,708.01 and 1,031.74 mg/g, respectively, for the 90°C hot water
treatment; 1,059.54 and 575.57 mg/g, respectively, for the 60%
ethanol treatment and 1,120.83 and 847.03 mg/g, respectively, for
the Rapidase press L + Rohament CL enzymatic treatment.
These results indicated that the hot water treatment
extract produced higher total polyphenol and phlorotannin contents
than the enzymatic treatments; however, enzymatic treatment was
more efficient in terms of stability and economy. The EEc used in
the present study included three components (eckol, dieckol and
phlorofucofuroeckol A) with 17.5 mg/g of dieckol as an indicator
substance, and high yields and high concentrations of polyphenols
were obtained from E. cava. The previous study also
investigated the inhibitory effects of EEc treatment on 3T3-L1
adipocyte differentiation and adipogenesis-associated gene
expression (58). The expression
levels of CCAAT/enhancer-binding protein α (C/EBPα), and the
adipogenesis-associated genes sterol regulatory element-binding
protein-1c (SREBP-1c), adipose fatty acid-binding protein (A-FABP)
and fatty acid synthase (FAS) significantly decreased following
treatment, which indicates that EEc may be a potential agent for
the prevention of obesity at the cellular level.
In addition, Garcinia cambogia extract was
used as a positive control, as the inhibitory effect of EEc on
3T3-L1 adipocyte differentiation and adipogenesis-associated gene
expression have been demonstrated (57,59). Based on these data, the present
study aimed to investigate the anti-obesity effects of EEc in
C57BL/6N mice with high-fat diet (HFD)-induced obesity in
vivo.
Materials and methods
Preparation of enzyme-treated EEc
E. cava was purchased in 2012 from
Taekyug-nongsan (Jeju-do, Korea). For the present study, E.
cava chips of ~5 cm in size were prepared by cutting the
leaves and removing the stems and roots of the algae. Next, the EEc
was prepared by placing 30 kg of E. cava chips in 750
l distilled water with enzymes (300 g Rapidase X-Press L and 300 g
Rohament CL; BISION Co., Gyeonggi-do, Korea), stirring the
suspension at 50°C for 24 h, centrifuging the solution at 3,000 × g
and 4°C for 20 min, vacuum-filtering it, and then adding three
volumes of 60% ethanol. After 18 h of stirring, the solution was
filtered and concentrated using rotary evaporation to 6° Brix. The
concentrated solution was made into a powder using a spray dryer;
the final extracted material had a weight of 3.65 kg, which
represents a yield of 12.2% (EEc; product no. JY202-MM130426R). The
G. cambogia extract powder (main ingredient, hydroxycitric
acid) was purchased from ES Ingredients (Gyeonggi-do, Korea).
Animal care and experimental design
The doses of EEc administered in the animal
experiments were determined based on the concentration of EEc that
was effective at the cellular level in preliminary experiments. The
C57BL/6 mouse strain is the most studied experimental model of
diet-induced obesity, as it is sensitive to HFD-induced weight gain
(60). HFD intake promotes
increases in body weight, adipose tissue weight, hyperlipidemia,
and hyperglycemia in rodents (61). Therefore, for the present study,
60 male C57BL/6N TacSam mice (age, 4 weeks; weight, 17.7±0.73 g)
were purchased from Samtako Bio Korea Co. (Gyeonggi-do, Korea),
housed in standard cages under a 12-h light-dark cycle, and
maintained in a room at 23±3°C with a relative humidity of 55±5%.
All mice consumed a commercial diet ad libitum and had ad
libitum access to tap water for 1 week prior to the start of
the experiments. The mice were randomly divided into six groups
(n=10/group) as follows: Mice receiving a normal diet (ND group),
mice receiving a HFD (HFD group), mice fed a HFD with 25 mg/kg
G. cambogia extract (GHD group), mice fed a HFD with 5
mg/kg/day EEc (EHD5 group), mice fed a HFD with 25 mg/kg/day EEc
(EHD25 group) and mice fed a HFD with 150 mg/kg/day EEc (EHD150
group).
The ND group was fed a purified diet with added corn
oil that was based on the composition of the AIN-76 semi-purified
diet (MP0290545220; MP Biomedicals, LLC, Solon, OH, USA). The HFD
was identical to the ND, except that it contained 220 g fat/kg (170
g lard and 50 g corn oil) and 1% cholesterol, which was intended to
induce obesity in 10 weeks. Therefore, the HFD was more
calorie-dense than the ND (5,380 vs. 3,850 kcal/kg). The diet of
the EHD groups was identical to that of the HFD but with the
addition of 5, 25 or 150 mg/kg/day of EEc to the diet. The mice
were weighed every 7 days and their food intake was recorded daily
during the feeding period. At the end of the experimental period,
the mice were fasted for 12 h, blood was collected from the
abdominal vena cava, and the white adipose tissue and liver were
removed, weighed and frozen in liquid nitrogen. The feed efficiency
ratio (FER) was calculated as the ratio between weight gain and
total feed intake.
The animal protocol of the present study was
approved by the Institutional Animal Care and Use Committee of
Pukyong National University (Busan, Republic of Korea; approval no.
2012–02).
Biochemical analysis
Blood was collected from the abdominal vena cava and
serum was obtained by centrifuging the blood at 2,500 × g for 15
min at 4°C. Serum samples were stored at −70°C and subsequently,
serum concentrations of total cholesterol (TC; cat. no. AM202),
high-density lipoprotein cholesterol (HDL-C; cat. no. AM203), TG
(cat. no. AM157), glutamic oxaloacetic transaminase (GOT; cat. no.
AM103), glutamic pyruvic transaminase (GPT; cat. no. AM102),
glucose (cat. no. AM201; Asan Pharmaceutical Co., Ltd.,
Gyeonggi-do, Korea), insulin (cat. no. 80-INSMS-E01; Alpco
Diagnostics, Windham, NH, USA) and leptin (cat. no. ADI-900-019A;
Enzo Life Sciences, Inc., Farmingdale, NY, USA) were determined
enzymatically using commercial kits. The liver tissue samples (0.2
g) were homogenized in 1 ml PBS, centrifuged at 2,500 × g for 15
min at 4°C and stored at −70°C. All serum sample levels were
measured using enzyme kits according to the manufacturer's
instructions.
Western blot analysis
The liver tissue was washed with PBS and lysed with
extraction buffer (1% Nonidet P-40, 1 mM EDTA, 50 mM Tris, pH 7.4,
0.25% Na-deoxycholate, 150 mM NaCl, 1 mM sodium orthovanadate, 1
µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml
pepstatin A, 1 mM NaF and 1 mM phenylmethane sulfonyl fluoride).
Subsequently, the extracts were centrifuged at 9,750 × g for 10 min
and the supernatants were used for western blot analysis.
The total protein (40 µg) was subjected to
8–15% SDS-PAGE and then transferred to a polyvinylidene fluoride
transfer membrane (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 1% bovine serum albumin (BSA; GenDepot
Inc., Barker, TX, USA) in a buffer of 5 mM Tris-HCl, 20 mM sodium
chloride, pH 7.4, and 0.1% Tween-20 (TBS-T) and incubated with
primary antibodies (1:1,000 dilution) in 1% BSA in TBS-T with
gentle agitation overnight at 4°C. Membranes were washed twice for
15 min in TBS-T, incubated with the corresponding horseradish
peroxidase (HRP)-conjugated secondary antibodies (1:10,000
dilution) for 2 h at room temperature and then washed again.
The immunoreactive bands were detected using an
enhanced chemiluminescence substrate (Advansta, Menlo Park, CA,
USA) and visualized using the GeneSys imaging system (SynGene
Synoptics, Ltd., London, UK). The following primary antibodies
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) were
used: Anti-C/EBPα (cat. no. sc-9314; anti-goat), anti-peroxisome
proliferator activated receptor γ (PPARγ; cat. no. sc-1984;
anti-goat), anti-phosphorylated AMP-activated protein kinase
(p-AMPK; cat. no. sc-33524; anti-rabbit), anti-AMPK (cat. no.
sc-74461; anti-mouse), anti-p-acetyl-CoA carboxylase (p-ACC; cat.
no. sc-271965; anti-mouse), anti-ACC (cat. no. sc-30212;
anti-rabbit), anti-SREBP-1c (cat. no. sc-366; anti-rabbit),
anti-A-FABP (cat. no. sc-18661; anti-goat), anti-fatty acid
synthase (FAS; cat. no. sc-55580; anti-mouse), anti-glucose
transporter type 4 (GLUT4; cat. no. sc-1606; anti-rabbit),
anti-adiponectin (cat. no. sc-26497; anti-goat), anti-leptin (cat.
no. sc-842; anti-rabbit) and anti-GAPDH (cat. no. sc-25778;
anti-rabbit). The secondary antibodies used were HRP-conjugated
anti-mouse immunoglobulin (Ig) G (cat. no. sc-2031; Santa Cruz
Biotechnology, Inc.), anti-rabbit IgG (cat. no. A-0545;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and anti-goat IgG
(cat. no. A50-101P; Bethyl Laboratories Inc., Montgomery, TX,
USA).
Statistical analysis
Results were expressed as the mean ± standard
deviation for each group (n=10 animals). Significant differences
among multiple mean values were assessed using one-way analysis of
variance followed by Bonferroni's multiple comparison test using
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
EEc supplementation decreases body
weight, liver weight and adipose tissue weight in C57BL/6N mice
with HDF-induced obesity
Preliminary experiments were conducted to set the
concentrations for the present study. Various concentrations of EEc
(5, 12.5, 25, 50, 150, 200, 250, 500 and 1,000 mg/kg) were
determined by preliminary experiments. Based on the results, the
concentration of the EEc group was set with similar results to the
GHD group. Therefore, in the present study, the concentration of
EEc was set to 5, 25 and 150 mg/kg. After 10 weeks of feeding, the
HFD group had a significantly higher final body weight and more
cumulative body weight gain than the ND group. The EEc-supplemented
groups had significantly lower final body weights than those
observed in the HFD group (HFD, 1.13-; GHD, 1.02-; EHD5, 1.13-;
EHD25, 1.05-; and EHD150, 0.82-fold difference compared with the ND
group), and less body weight gain (HFD, 1.44-; GHD, 1.14-; EHD5,
1.35-; EHD25, 1.17-; EHD150, 0.46-fold difference compared with the
ND group) (Fig. 1A and B).
However, despite the increased body weight of the HFD group, daily
food intake (3.2–3.4 g/day) did not differ among the experimental
groups (data not shown).
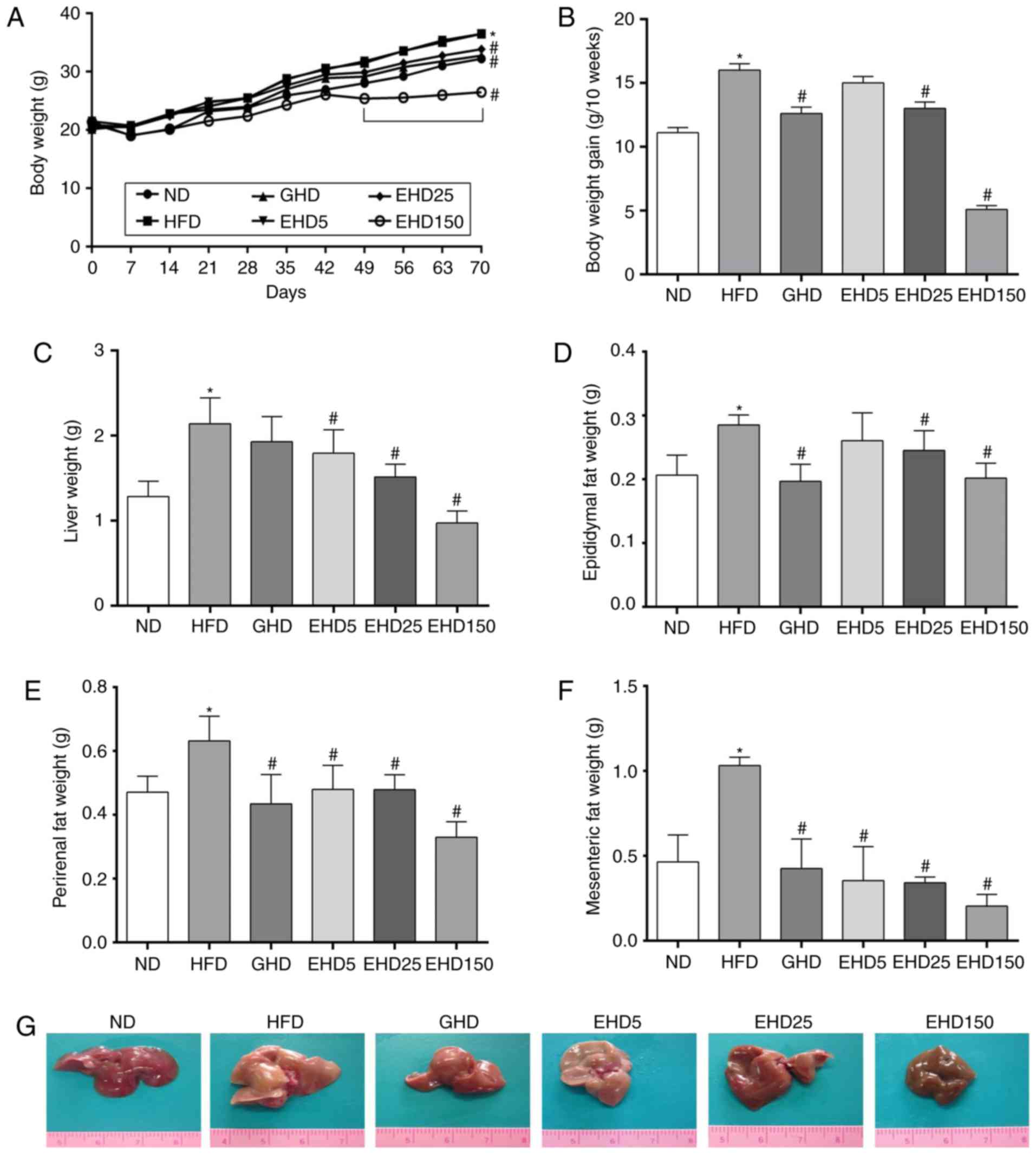 | Figure 1Effects of enzyme-treated E.
cava extracts on the body, liver and adipose tissue weight of
experimental mice. Changes in (A) body weight, (B) body weight
gain, (C) liver weight, (D) epididymal fat weight, (E) perirenal
fat weight and (F) mesenteric fat weight. (G) Representative images
of livers of the ND-, HFD-, GHD- and EHD-fed mice (magnification,
×100). Values are expressed as the mean ± standard deviation
(n=10). Data were analysed using one-way analysis of variance.
*P<0.05 vs. ND between 49–70 days;
#P<0.05 vs. HFD group between 49–70 days. Groups: ND,
normal diet group; HFD, high fat diet group; GHD, mice fed a HFD
and 25 mg/kg/day Garcinia cambogia extract; EHD 5, mice fed
a HFD and 5 mg/kg/day EEc; EHD 25, mice fed a HFD and 25 mg/kg/day
EEc; EHD150, mice fed a HFD and 150 mg/kg/day EEc; EEc,
enzyme-treated Ecklonia cava extract. |
As the HFD was more calorie-dense than the ND (5,380
vs. 3,850 cal/kg), the FER over 10 weeks was 44% greater in the HFD
group than that in the ND group (ND, 4.66±0.48%; HFD, 6.72±0.48%;
GHD 5.29±0.48%; EHD5, 6.30±0.48%; EHD25, 5.46±0.48%; EHD150,
2.28±0.35%). The absolute and relative weights of the livers were
significantly greater in the HFD group than those in the ND group,
while treatment with EEc resulted in a significant reduction in the
absolute liver weight compared with that of the untreated HFD mice
(ND, 1.28±0.18 g; HFD, 2.14±0.31 g; GHD 1.93±0.29 g; EHD5,
1.79±0.28 g; EHD25, 1.52±0.15 g; EHD150, 0.97±0.14 g) (Fig. 1C).
To examine whether the reduced body weight gain in
the EEc-treated groups was associated with decreased fat
accumulation, the epididymal, perirenal and mesenteric white
adipose tissues were weighed; they were significantly reduced in
the EHD group (Fig. 1D–F). The
mean epididymal adipose tissue weights were as follows: ND,
0.21±0.03 g; HFD, 0.29±0.02 g; GHD, 0.20±0.03 g; EHD5, 0.26±0.04 g;
EHD25, 0.25±0.03 g; and EHD150, 0.20±0.02 g (Fig. 1D). The mean perirenal adipose
tissues weights were as follows: ND, 0.47±0.05 g; HFD, 0.63±0.08 g;
GHD, 0.44±0.09 g; EHD5, 0.48±0.08 g; EHD25, 0.48±0.05 g; and
EHD150, 0.33±0.05 g (Fig. 1E).
The mean mesenteric adipose tissues were as follows: ND, 0.46±0.16
g; HFD, 1.03±0.05 g; GHD, 0.43±0.17 g; EHD5, 0.35±0.20 g; EHD25,
0.34±0.03 g; and EHD150, 0.21±0.08 g (Fig. 1F).
In addition, the livers of the HFD group were
lighter in colour than those of the ND and EHD groups (Fig. 1G); they were enlarged and had a
pinkish colour, which is indicative of liver steatosis. However,
supplementation with EEc reversed these effects as evidenced by the
livers of the EHD groups, which were small and dark red and similar
to those of the ND group. Notably, the liver of the EHD 150 group
was observed to be brownish. The original colour of the EEc powder
used in the present study was dark brown. The discolouration of the
liver may have been due to the powder.
EEc supplementation decreases serum TC
and HDL-C levels in C57BL/6N mice with HFD-induced obesity
To determine whether the effects of EEc
supplementation were associated with changes in the serum levels of
obesity-associated markers, serum TC and HDL-C were measured in
each group. The serum TC levels in the GHD group (59.9±16.6) were
reduced compared with those in the HFD group (vs. 83.6±6.10 mg/dl),
but the TC levels of the EHD groups did not significantly differ
from those in the ND group (48.1±8.36 mg/dl) (Fig. 2A). The EHD-25 and -150 groups had
significantly increased serum HDL-C levels compared with those in
the HFD group (ND, 30.8±4.41; HFD, 38.4±3.87; GHD, 53.3±9.72; EHD5,
40.9±9.39; EHD25, 57.2±11.06; and EHD150, 61.2±7.39 mg/dl; Fig. 2B). In addition, the HDL-C/TC ratio
in the EHD groups increased in a dose-dependent manner (ND, 64.0%;
HFD, 46.0%; GHD, 88.9%; EHD5, 49.4%; EHD25, 74.8%; and EHD 150,
81.9%). The HDL-C/TC ratio in the EHD 150 group was similar to that
in the GHD group (Fig. 2C).
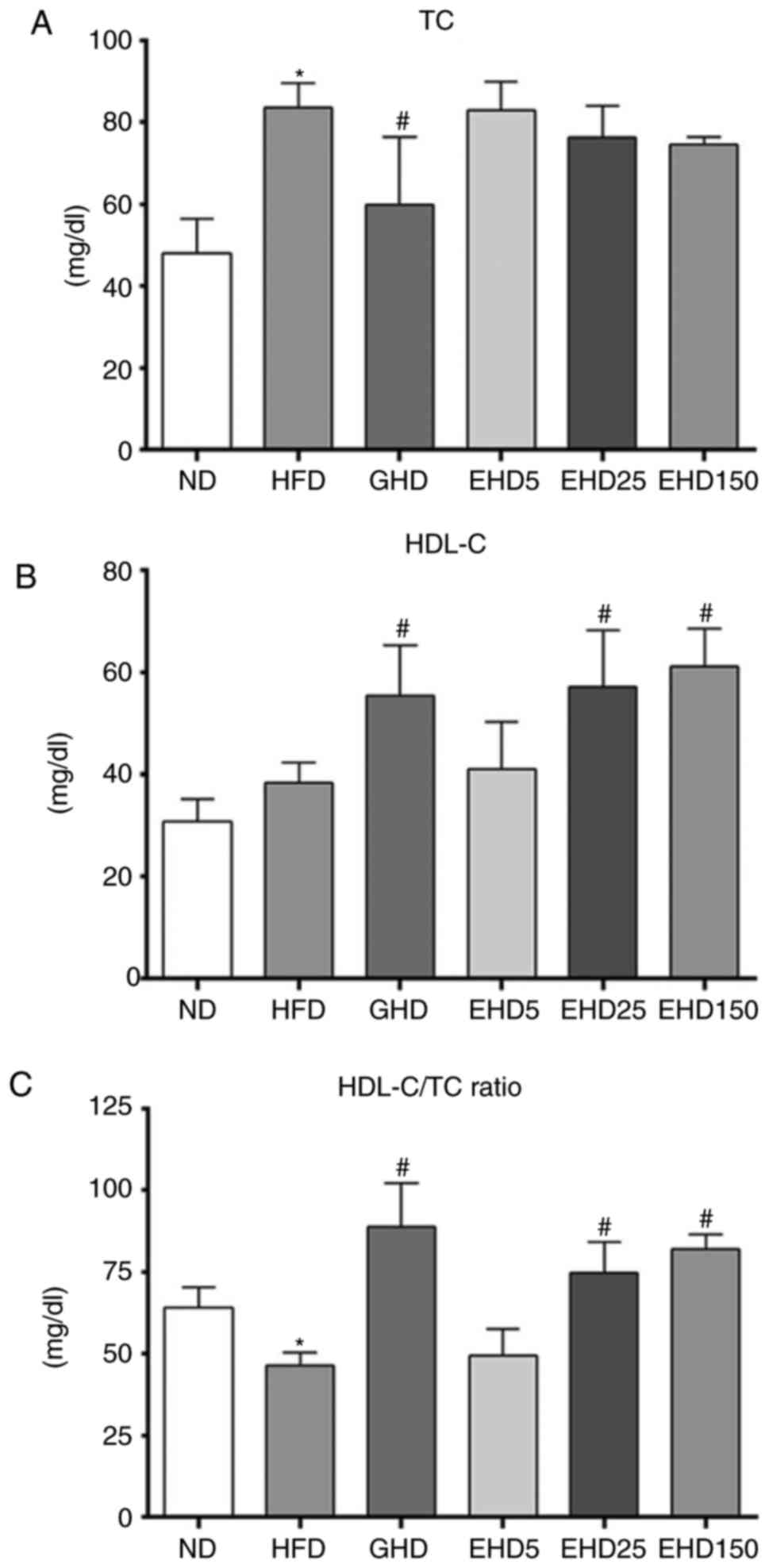 | Figure 2Effects of EEc treatment on serum TC
and HDL-C levels of mice fed a HFD for 10 weeks. (A) Serum TC
levels, (B) serum HDL-C levels and (C) serum HDL-C/TC ratio. Values
are expressed as the mean ± standard deviation (n=10). Data were
analysed using one-way analysis of variance. *P<0.05
vs. ND; #P<0.05 vs. HFD group. Groups: ND, normal
diet group; HFD, high fat diet group; GHD, mice fed a HFD and 25
mg/kg/day Garcinia cambogia extract; EHD 5, mice fed a HFD
and 5 mg/kg/day EEc; EHD 25, mice fed a HFD and 25 mg/kg/day EEc;
EHD150, mice fed a HFD and 150 mg/kg/day EEc. EEc, enzyme-treated
Ecklonia cava extract; TC, total cholesterol; HDL-C,
high-density lipoprotein cholesterol. |
EEc supplementation decreases serum
insulin and leptin levels in C57BL/6N mice with HFD-induced
obesity
A HFD induces NAFLD in animal models as well as
humans, which is important as NAFLD is closely associated with
obesity, diabetes and insulin resistance (62,63). To determine whether the effects of
EEc supplementation were correlated with changes in serum levels of
NAFLD-associated markers, serum glucose, insulin and leptin levels
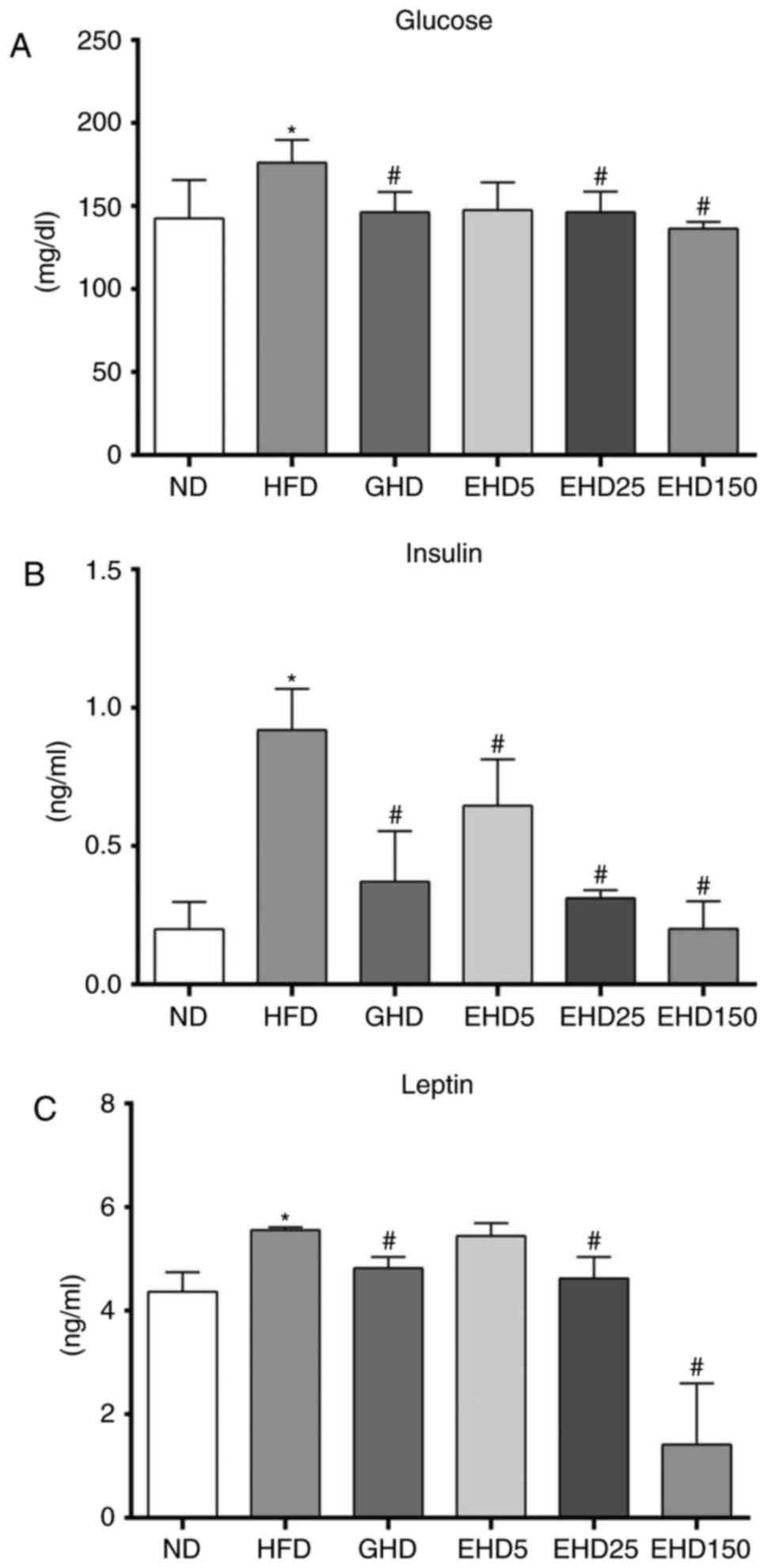
were determined in each group. As demonstrated in Fig. 3, all treatment groups had
decreased blood glucose levels compared with those in the HFD group
(Fig. 3A), but these were not
dose-dependent. The mean blood glucose levels in the HFD group were
176.1±13.9 mg/dl and those of the other groups ranged from
136.3±4.2 to 147.5±16.6 mg/dl.
The serum insulin levels in the EHD groups
(0.64±0.16, 0.31±0.03 and 0.20±0.10 ng/ml for EHD5, −25 and −150,
respectively) were significantly lower than those in the HFD group
(0.92±0.15 ng/ml). The insulin levels in the ND group were
0.20±0.10 ng/ml and those in the GHD group were 0.37±0.18 ng/ml.
The leptin levels in the EHD groups (5.4±0.27, 4.6±0.41 and
1.4±1.17 ng/ml for EHD5, −25 and −150, respectively) were
significantly lower than those in the HFD group (5.6±0.02 ng/ml)
(Fig. 3C), and the leptin levels
in the GHD group were 4.8±0.23 ng/ml. The serum glucose, insulin
and leptin levels in the EHD25 group were similar to those in the
GHD group.
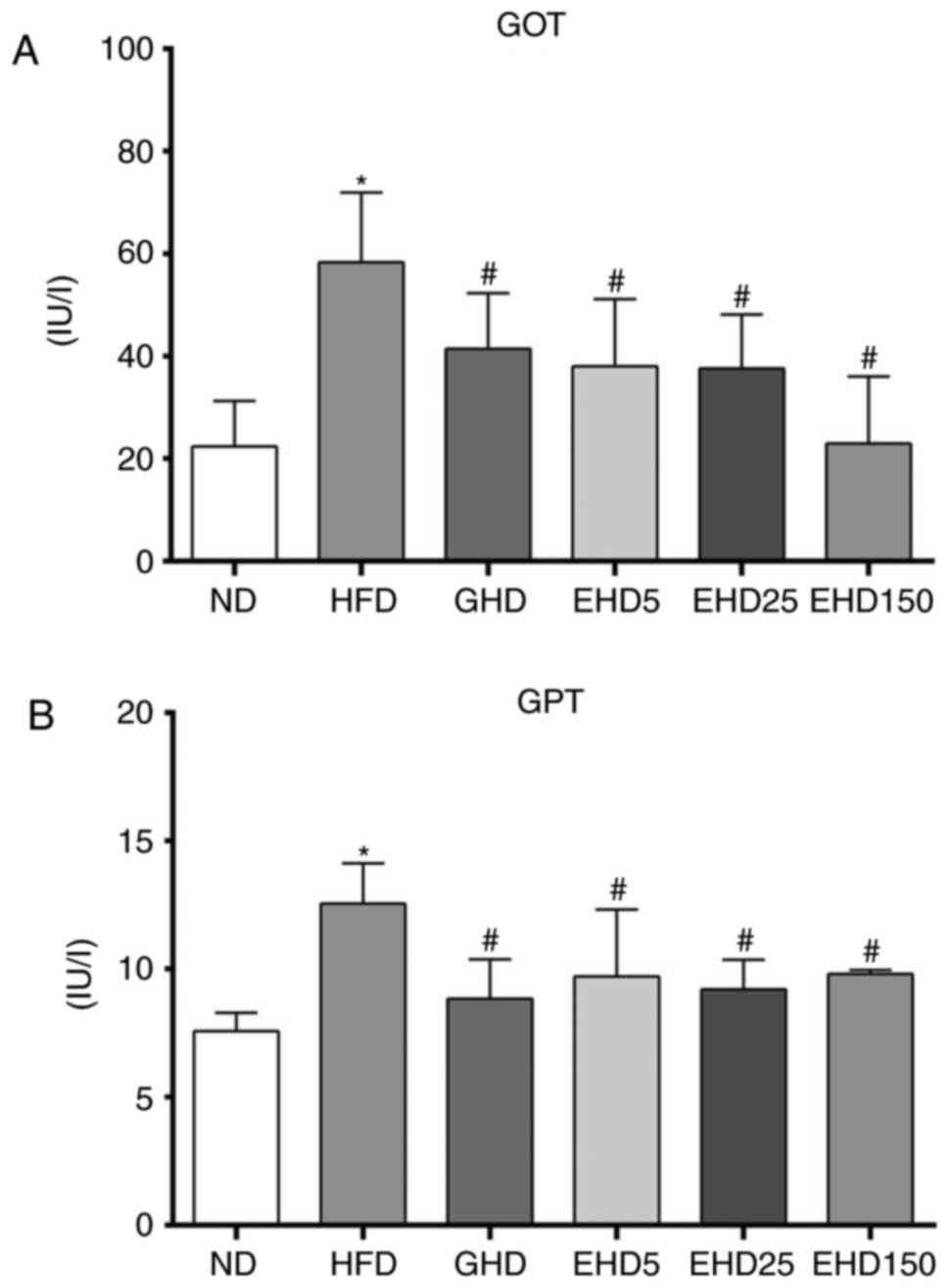
EEc supplementation decreases serum GOT
and GPT levels in C57BL/6N mice with HFD-induced obesity
The consumption of a HFD may induce hepatic
steatosis (64). Thus, to
determine whether EEc supplementation had any effect on the serum
levels of hepatic steatosis-associated markers, the concentrations
of GOT and GPT were measured. All treatment groups exhibited
decreased GOT and GPT levels compared with those in the HFD group
(Fig. 4A and B), but these
differences were not dose-dependent. The GOT and GPT levels in the
HFD group were 58.3±13.6 IU/l and 12.6±1.56 IU/l, respectively. In
the other groups, the GOT levels ranged between 22.3±8.9 and
41.4±10.9 IU/l and the GPT levels ranged between 7.6±0.73 and
9.8±0.16 IU/l. These results supported the protective effect of EEc
against the development of hepatic steatosis.
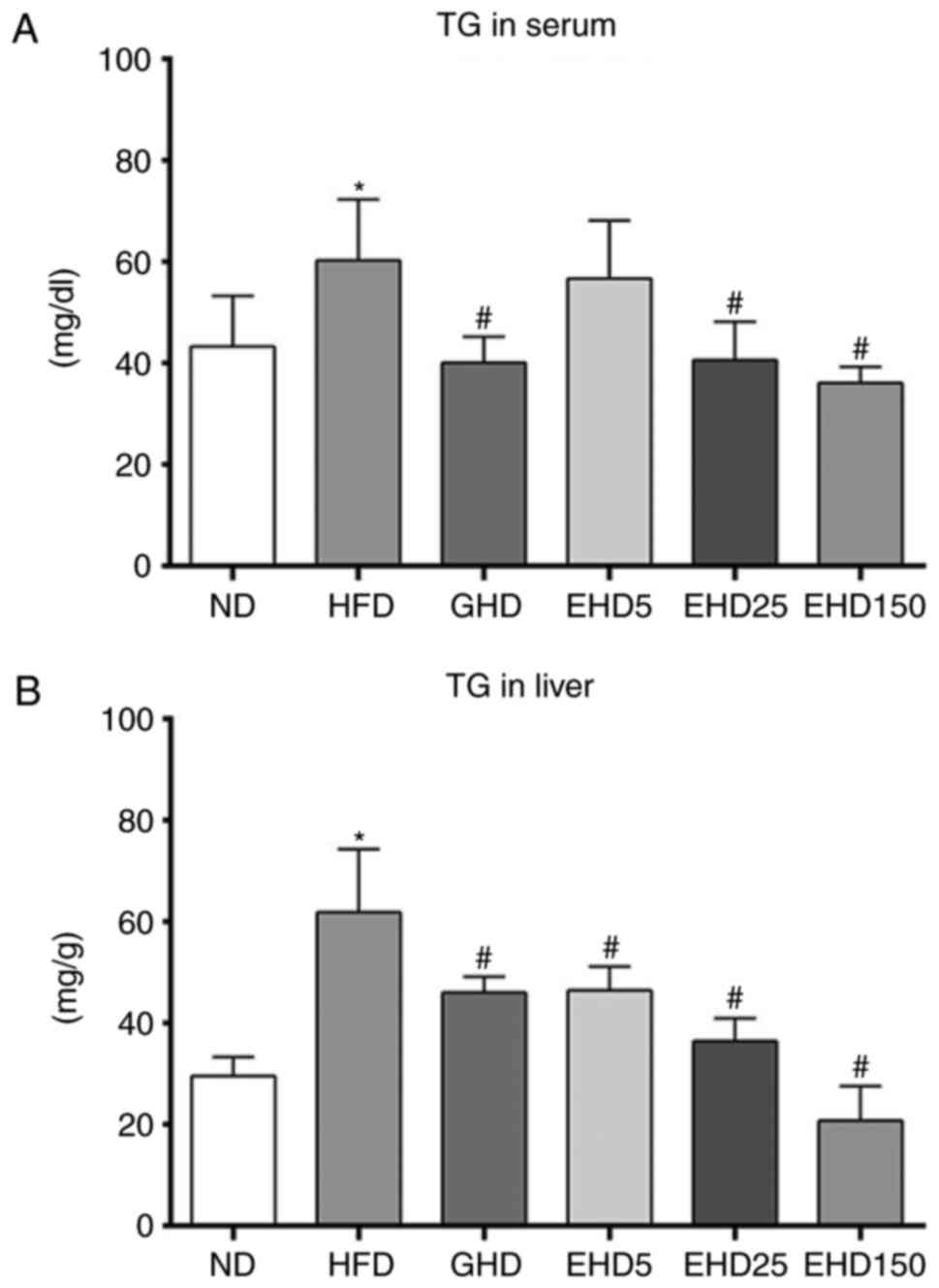
EEc supplementation decreases serum and
hepatic TG in C57BL/6N mice with HFD-induced obesity
To determine whether EEc supplementation affected
the serum and hepatic levels of obesity-associated markers, TG
levels were determined in each group. All treatment groups
exhibited decreased serum TG levels compared with those in the HFD
group and the effects of EHD were dose-dependent (Fig. 5A). The serum TG concentrations in
the ND, HFD, GHD, EHD5, EHD25 and EHD150 groups were 46.4±11.67,
57.9±12.16, 40.1±5.04, 56.7±11.40, 43.4±9.06 and 38.0±5.05 mg/dl,
respectively. The TG levels in the EHD5, EHD25 and EHD150 groups
were 1, 24 and 34% lower than those in the HFD group. The EHD
groups also exhibited significantly lower hepatic TG levels than
the HFD group (Fig. 5B). The
hepatic TG levels in the HFD, ND and GHD groups were 61.9±12.4,
29.6±3.73, and 46.1±3.11 mg/g. Supplementation with EEc resulted in
significant dose-dependent decreases in hepatic TG levels such that
the levels of the EHD5, EHD25 and EHD150 groups were 46.5±4.69,
36.5±4.46 and 20.8±6.79 mg/g, respectively; these levels were 25,
41 and 66% lower, respectively, than those in the HFD group.
EEc supplementation modulates the
expression of genes involved in lipid metabolism and activates AMPK
in mice
Adipogenesis and lipogenesis are accompanied by
changes in the sequential activation of several pro-adipogenic
transcription factors, including C/EBPα/β/δ and PPARγ. Thus, the
present study examined whether the observed reductions in hepatic
lipid accumulation were due to the downregulation of these
transcription factors. The expression of C/EBPα and PPARγ in the
liver tissues obtained from the EEc-supplemented groups (EHD25 and
−150) exhibited a decrease compared with those in the HFD group
(Fig. 6A and B). Among them, all
treatment groups had decreased C/EBPα expression levels compared
with those in the HFD group, apart from those in the EHD5 group
(Fig. 6A and B); however, these
decreases were not dose-dependent. The C/EBPα expression levels in
the HFD, GHD, EHD25 and EHD150 groups were 2.25-, 1.3-, 1.4- and
1.7-fold higher, respectively, than those in the ND group.
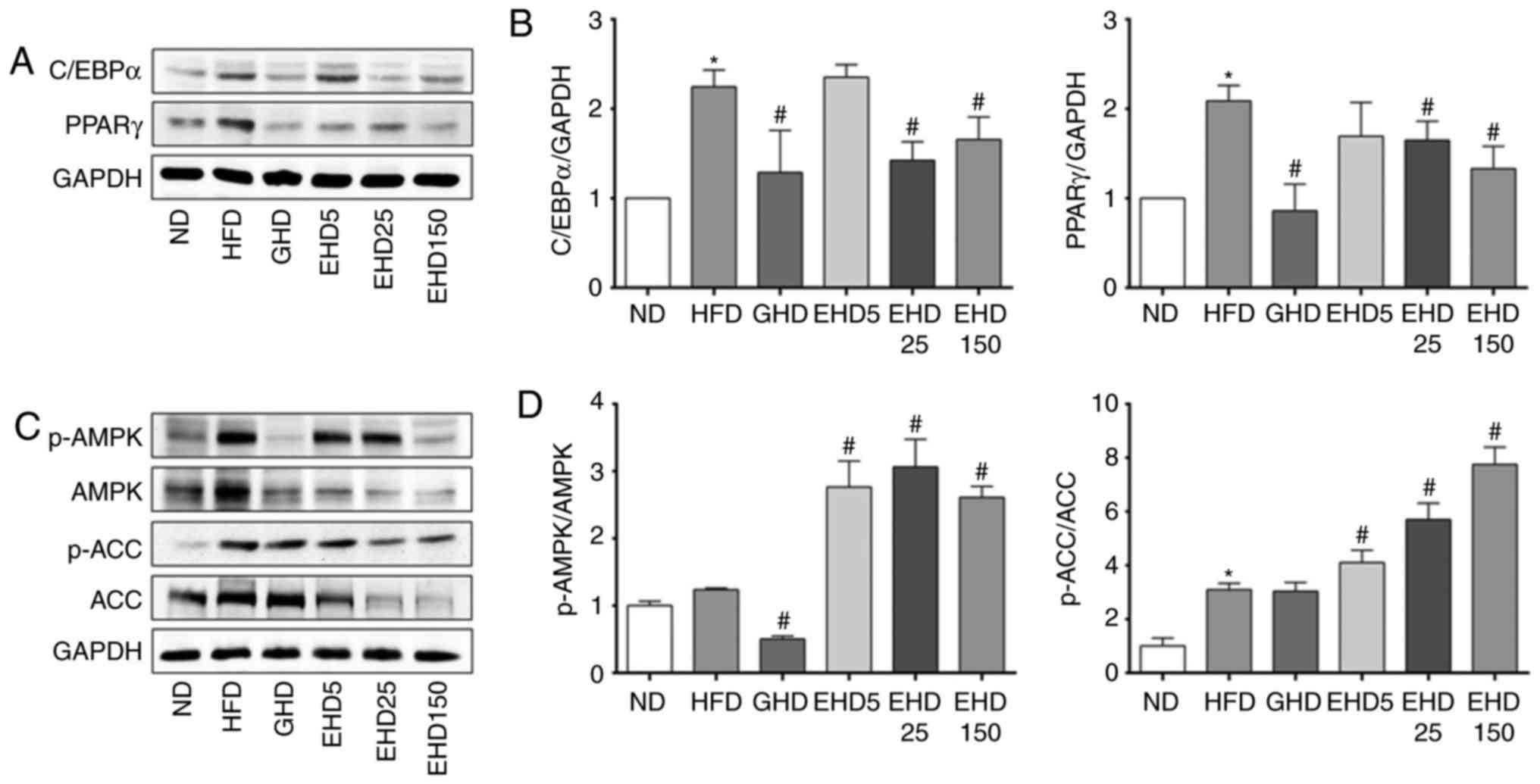 | Figure 6Effects of dietary EEc
supplementation on the hepatic expression of AMPK and the
regulation of lipid metabolism toward lipid catabolism in HFD-fed
mice. (A) The protein expression levels of C/EBPα and PPARγ in the
livers of the experimental mice at 10 weeks were measured by
western blotting. (B) Protein bands were quantified with
normalization to the internal control GAPDH and the relative
expression levels were determined with the ND group set as 1. (C)
Protein expression levels of p-AMPK, AMPK, p-ACC and ACC in the
livers of the experimental mice at 10 weeks were examined using
western blot analysis. (D) Protein bands were quantified with
normalization to the internal control GAPDH and the relative
expression levels were determined with the ND group set as 1. The
ratios of p-AMPK/AMPK and p-ACC/ACC are presented. Values are
expressed as the mean ± standard deviation. Data were analysed
using one-way analysis of variance. *P<0.05 vs. ND;
#P<0.05 vs. HFD group. Groups: ND, normal diet group;
HFD, high fat diet group; GHD, mice fed a HFD and 25 mg/kg/day
Garcinia cambogia extract; EHD 5, mice fed a HFD and 5
mg/kg/day EEc; EHD 25, mice fed a HFD and 25 mg/kg/day EEc; EHD150,
mice fed a HFD and 150 mg/kg/day EEc; EEc, enzyme-treated
Ecklonia cava extract; p-AMPK, phosphorylated AMP-activated
protein kinase; ACC, acetyl-CoA carboxylase; C/EBPα,
CCAAT/enhancer-binding protein α; PPARγ, peroxisome proliferator
activated receptor γ. |
Activated AMPK is able to phosphorylate and regulate
hydroxymethylglutaryl-CoA reductase and ACC in vivo; these
enzymes are key regulatory factors involved in sterol synthesis and
fatty acid synthesis, respectively. The p-AMPK/AMPK and p-ACC/ACC
ratios were significantly increased in liver tissue obtained from
the EHD5, −25, and −150 groups compared with those in the HFD group
(Fig. 6C and D). The hepatic
p-AMPK/AMPK ratio in the HFD, GHD, EHD5, EHD25 and EHD150 groups
was increased by 1.25-, 0.51-, 2.77-, 3.01- and 2.61-fold,
respectively, compared with that in the ND group. The p-ACC/ACC
ratio in the HFD, GHD, EHD5, EHD25 and EHD150 groups was increased
by 3.09-, 3.04-, 4.11-, 6.17- and 7.76-fold of that in the ND
group. Thus, EEc supplementation resulted in a restoration of AMPK
activity as evidenced by the inhibition of HFD-induced increases in
the phosphorylation of AMPK and its target ACC.
EEc supplementation reduces the
expression of proteins involved in adipogenesis in mice
To examine the effects of EEc on lipid metabolism,
the levels of adipogenesis-associated proteins in the liver tissues
of the mice were measured. EEc supplementation inhibited the
expression of several hepatic adipogenesis-associated transcription
factors. The HFD group exhibited a significantly increased
expression of the target genes of PPARγ involved in the
adipogenesis pathway, including A-FABP, FAS and leptin (Fig. 7). By contrast, EEc supplementation
significantly decreased the protein levels of SREBP-1c, A-FABP,
FAS, and leptin compared with those in the HFD group. The GLUT4
protein levels increased significantly in liver tissue obtained
from the EHD5, −25 and −150 groups compared with those in the HFD
group (Fig. 7). The GLUT4 protein
levels in the HFD, GHD, EHD5, EHD25 and EHD150 groups increased by
1.22-, 1.33-, 2.49-, 2.99- and 3.42-fold, respectively, compared
with those in the ND group. The adiponectin protein levels
increased significantly in liver tissue obtained from the EHD-25
and -150 groups compared with those in the HFD group.
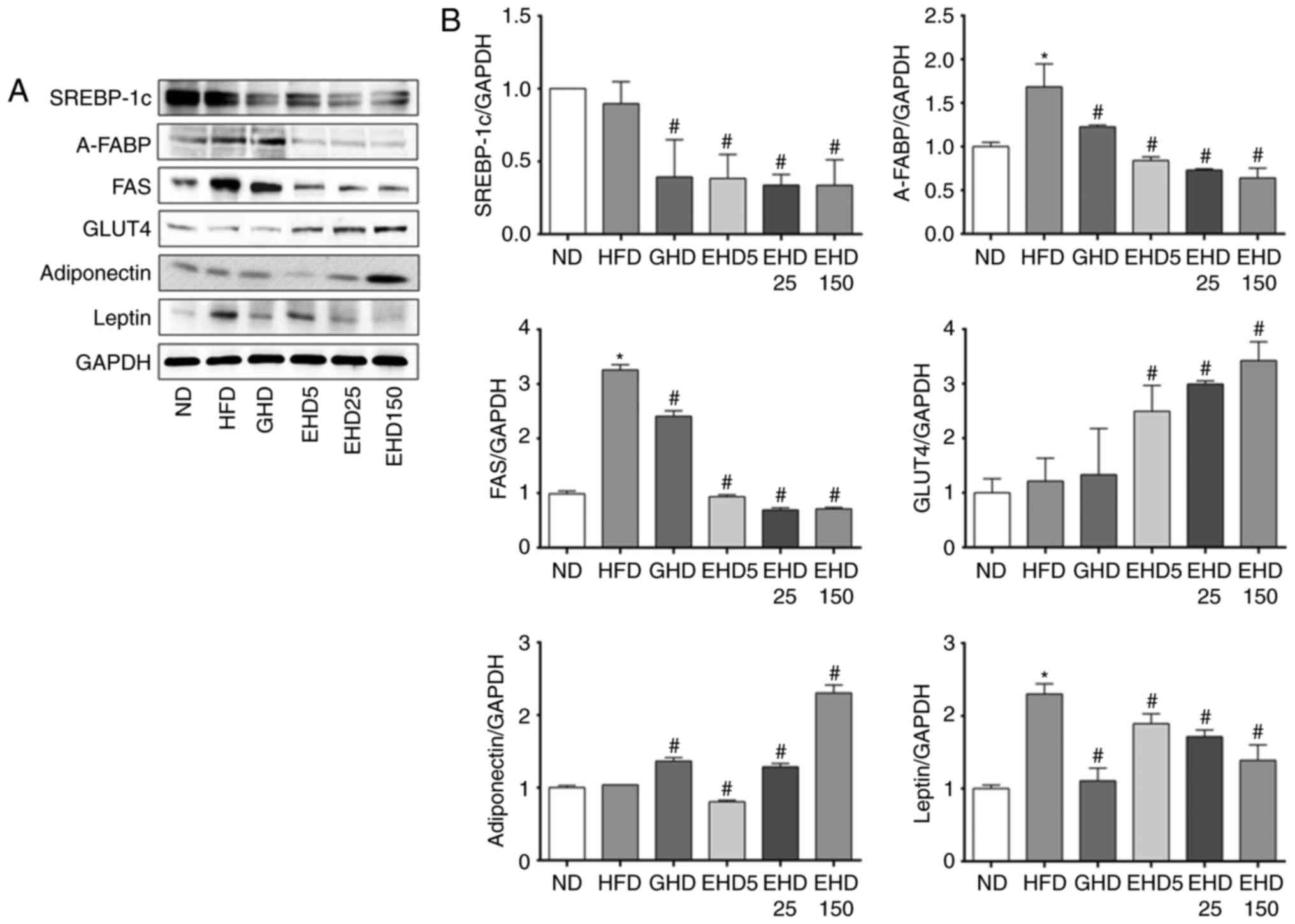 | Figure 7Effects of dietary EEc
supplementation on the hepatic expression of
adipogenesis-associated proteins in HFD-fed mice. (A) Hepatic
protein levels of SREBP-1c, A-FABP, FAS, GLUT4, adiponectin and
leptin in the experimental mice after 10 weeks were examined using
western blot analysis. (B) Bands were normalized to an internal
control (GAPDH) and the relative expression levels were determined
with the ND group set as 1. Values are expressed as the mean ±
standard deviation. Data were analysed using one-way analysis of
variance. *P<0.05 vs. ND; #P<0.05 vs.
HFD group. Groups: ND, normal diet group; HFD, high fat diet group;
GHD, mice fed a HFD and 25 mg/kg/day Garcinia cambogia
extract; EHD 5, mice fed a HFD and 5 mg/kg/day EEc; EHD 25, mice
fed a HFD and 25 mg/kg/day EEc; EHD150, mice fed a HFD and 150
mg/kg/day EEc; EEc, enzyme-treated Ecklonia cava extract;
SREBP-1c, sterol regulatory element-binding protein-1c; A-FABP,
adipose fatty acid-binding protein; FAS, fatty acid synthase;
GLUT4, glucose transporter type 4. |
Discussion
Inhibition of obesity by E. cava extract has
been previously studied (51–53). The phlorofucofuroeckol A (51), polyphenol-rich fraction (52,53) of E. cava extract has been
examined for its anti-obesity effect in a murine model fed on a
high-fat diet. The present study investigated the in vivo
anti-obesity effects of EEc in C57BL/6N mice with HFD-induced
obesity by treating them with doses of 5, 25 and 150 mg/kg/day for
10 weeks. After 10 weeks, HFD-fed mice that received EEc
supplementation exhibited significant decreases in body, liver, as
well as epididymal, perirenal and mesenteric fat weight compared
with the values in HFD-fed mice that did not receive EEc
supplementation. The reduction in body weight was in parallel with
decreased adipose tissue weight. In addition, EEc significantly
decreased the weight of the epididymal, perirenal and mesenteric
adipose tissue, reduced overall body fat stores and lowered the FER
compared with those in the HFD group.
The present study also investigated changes in the
serum levels of obesity-associated factors, including TC, HDL-C,
glucose, insulin, leptin, GOT, GPT and TG. EEc supplementation had
no effect on TC levels but increased the levels of HDL-C. HDL-C and
low-density lipoprotein cholesterol (LDL-C) carry cholesterol in
the body. In particular, LDL-C causes the accumulation of
cholesterol in tissues throughout the body, which results in an
increased risk of cardiovascular disease (65). By contrast, epidemiologic studies
have reported an inverse correlation between HDL-C content and the
risk of cardiovascular disease (66). HDL-C removes cholesterol from the
bloodstream and relocates it to liver tissues, where it is
eliminated. High levels of HDL-C appear to protect against
cardiovascular disease, whereas low levels of HDL-C are an
important risk factor for cardiovascular disease. In addition,
epidemiological and clinical studies have reported that low HDL-C
levels are linked to coronary events (65,66). In the present study, treatment
with EEc was expected to reduce the risk of obesity-induced
hyperlipidemia and cardiovascular disease by increasing HDL-C
levels, regardless of its effects on TC.
EEc supplementation also significantly decreased
insulin and leptin levels. The serum insulin levels in the HFD
group were significantly higher than those in the EHD groups, which
decreased by 29.8, 66.1 and 78.2% in the EHD5, −25, and −150
groups, respectively, compared with those in the HFD group. The
serum leptin levels in the HFD group (5.6±0.02 ng/ml) were higher
than those in the ND group (4.4±0.38 ng/ml) and all three EHD
groups (5.4±0.27, 4.6±0.41 and 1.4±1.17 ng/ml for the EHD5, −25 and
−150 groups, respectively). Increased insulin levels in the blood
promote lipid synthesis by increasing free fatty acid transfer from
fat cells to the liver while inhibiting lipid oxidation (67). Leptin, which is a hormone produced
primarily by white adipose tissue, is associated with obesity and
metabolic syndrome. Leptin affects body weight, food intake and
energy balance by suppressing appetite and promoting satiety
(68). Leptin levels are closely
associated with body fat mass and thus exhibit an increase in
HFD-fed experimental animals and in obese patients (69). In the present study, serum insulin
and leptin levels were lower in mice treated with EEc and were
proportional to body fat mass. The production levels of insulin and
leptin are stimulated by increased levels of these hormones
(70). Therefore, reductions in
serum insulin and leptin levels in the EEc-supplemented groups were
likely due to the inhibition of body fat accumulation by EEc.
GOT and GPT are enzymes used as indicators of liver
tissue damage. In the present study, GOT and GPT activities
decreased in the EHD groups compared with those in the HFD group.
In the pathologies of HFD-induced fat liver, alcohol-induced fatty
liver or liver injury, GOT and GPT are released into the
bloodstream, which increases their activity (71,72). In the present study, the
activities of GOT and GPT were attenuated by the addition of EEc,
but only the activity of GOT enzyme significantly differed from
that the HFD group. EEc supplementation also significantly
decreased serum and hepatic TG levels. Hyperlipidemia is a
metabolic disorder that may be caused by HFDs and is characterized
by elevated serum TG levels (73,74). In addition, abdominal obesity is
associated with elevated blood levels of TG (75). In the present study, serum TG
levels were elevated in the HFD group, which had the highest body
fat weight.
In the present study, the hepatic expression levels
of the lipid metabolism-associated genes AMPK and ACC were
investigated with western blot analysis. In a previous study, EEc
treatment in 3T3-L1 adipocytes reduced C/EBPα/β/δ and PPARγ levels
(58). In the present study, the
EHD groups had lower expression levels of C/EBPα and PPARγ compared
with those in the HFD group. AMPK is a key enzyme involved in
intracellular energy balance, glucose uptake and lipid metabolism
via its effects on transcriptional factors, lipogenesis and fatty
acid oxidation-associated proteins (76). The phosphorylation of AMPK
inhibits PPARs and C/EBP, which are the main transcription factors
involved in adipocyte differentiation (77). In addition, activated AMPK
inhibits downstream ACC activity and prevents the production of
malonyl-CoA from acetyl-CoA (78). In the present study, the EHD
groups exhibited a decreased body weight and increased protein
expression of p-AMPK protein and p-ACC compared with that in the
HFD group. Similarly, E. cava polyphenol extract induced
anti-obesity effects through the activation of AMPK in HFD-fed mice
(79).
Next, the hepatic expression levels of
adipogenesis-associated proteins, including C/EBPα/β/δ, PPARγ,
SREBP-1c, A-FABP, FAS, GLUT4, adiponectin and leptin were assessed
by western blot analysis. In a previous study, EEc treatment of
3T3-L1 adipocytes reduced C/EBPα/β/δ and PPARγ levels (58). In the present study, the C/EBPα
and PPARγ levels in the EHD groups were lower than those in the HFD
group. Previously, EEc treatment in 3T3-L1 adipocytes was reported
to lower the levels of adipogenesis-associated proteins, including
SREBP-1c, A-FABP, FAS and adiponectin (58). In the present study, EEc treatment
resulted in significantly reduced SREBP-1c, A-FABP, FAS and leptin
levels, but in increased GLUT4 and adiponectin levels. Activation
of C/EBPα promotes the differentiation of pre-adipocytes in
cooperation with PPARγ, which, in turn, causes the trans-activation
of adipogenesis-specific genes including A-FABP and FAS (80,81). In addition, although the liver has
generally been regarded as being void of significant expression of
GLUT-4, a previous study suggested the expression of GLUT-4 mRNA in
porcine liver (82). Various
isoforms of GLUT (GLUT-1-6 and -8-12) were identified to be
expressed in the human liver tissue. Particularly GLUT2 and GLUT4
were identified as the main GLUTs responsible for glucose transport
into hepatocytes. Among them, GLUT4 is the major insulin-dependent
glucose transporter and is known to be involved in the
rate-limiting role of glucose utilization in insulin-sensitive
tissue, including brown and white adipose tissues, as well as
skeletal and cardiac muscles (83). However, GLUT-4 mRNA was decreased
in the liver tissue of model mice with diet-induced obesity. The
present study investigated the expression of GLUT-4 in HFD-induced
liver tissue. The results demonstrated that the expression of
GLUT-4 was increased in the EHD groups compared with that in the
HFD group.
In most of the experiments of the present study, the
EHD25 group (treated with EEc at 25 mg/kg/day) exhibited results
similar to those of the GHD group (25 mg/kg/day of G.
cambogia extract), including reduction in body weight,
activation of AMPK and ACC, reduction in the levels of
obesity-associated factors in serum and the liver, and alterations
in the expression of lipid metabolism- and adipogenesis-associated
proteins.
The anti-obesity effect observed in present study is
not likely to be due to an enzyme (digestive enzymes pectinase;
Rapidase X-Press L and cellulase; Rohament CL) that may be partly
contained in the EEc. Following the treatment of E. cava
chips with enzymes for 24 h, the supernatant was collected via
centrifugation and immersed in ethanol. Through this process, the
enzymes are mostly filtered out. Taken together, the results of the
present study suggested that EEc supplementation reduces
HFD-induced obesity in C57BL/6N mice. Thus, EEc may prevent and
treat obesity, NAFLD and obesity-associated diseases, and may be a
suitable candidate of dietary supplements and/or anti-obesity
nutraceutical agents that prevent and/or treat obesity-associated
diseases.
Acknowledgments
This study was supported by the Fishery
Commercialization Technology Development Program through iPET
(Korea Institute of Planning and Evaluation for Technology in Food,
Agriculture, Forestry and Fisheries) funded by the Ministry of
Oceans and Fisheries (grant no. 111090-03-3-HD110). This study was
also supported by the Basic Science Research Program through the
National Research Foundation of Korea funded by the Ministry of
Education (grant no. 2012R1A6A1028677).
References
|
1
|
Formiguera X and Cantón A: Obesity:
Epidemiology and clinical aspects. Best Pract Res Clin
Gastroenterol. 18:1125–1146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spieglman BM and Filer JS: Obesity and the
regulation of energy balance. Cell. 104:531–543. 2001. View Article : Google Scholar
|
|
3
|
Bibiloni Mdel M, Pons A and Tur JA:
Prevalence of overweight and obesity in adolescents: A systematic
review. ISRN Obes. 392747:2013.
|
|
4
|
Obici S and Rossetti L: Minireview:
Nutrient sensing and the regulation of insulin action and energy
balance. Endocrinology. 144:5172–5178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gurevich-Panigrahi T, Panigrahi S, Wiechec
E and Los M: Obesity: Pathophysiology and clinical management. Curr
Med Chem. 16:506–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aggoun Y: Obesity, metabolic syndrome, and
cardiovascular disease. Pediatr Res. 61:653–659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Després JP and Lemieux I: Abdominal
obesity and metabolic syndrome. Nature. 444:881–887. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vazzana N, Santilli F, Sestili S,
Cuccurullo C and Davi G: Determinants of increased cardiovascular
disease in obesity and metabolic syndrome. Curr Med Chem.
18:5267–5280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abate N: Obesity and cardiovascular
disease. Pathogenetic role of the metabolic syndrome and
therapeutic implications. J Diabetes Complications. 14:154–174.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bray GA and Tartaglia LA: Medicinal
strategies in the treatment of obesity. Nature. 404:672–677. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alemany M, Remesar X and Fernández-López
JA: Drug strategies for the treatment of obesity. IDrugs.
6:566–572. 2003.PubMed/NCBI
|
|
13
|
Buyukhatipoglu H: A possibly overlooked
side effect of orlistat: Gastroesophageal reflux disease. J Natl
Med Assoc. 100:12072008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Markowitz GS, Tartini A and D'Agati VD:
Acute interstitial nephritis following treatment with anorectic
agents phentermine and phendimetrazine. Clin Nephrol. 50:252–254.
1998.PubMed/NCBI
|
|
15
|
Onakpoya IJ, Heneghan CJ and Aronson JK:
Post-marketing withdrawal of anti-obesity medicinal products
because of adverse drug reactions: A systematic review. BMC Med.
14:1912016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rupérez P: Mineral content of edible
marine seaweeds. Food Chem. 79:23–26. 2002. View Article : Google Scholar
|
|
17
|
Besada V, Andrade JM, Schultze F and
González JJ: Heavy metals in edible seaweeds commercialised for
human consumption. J Mar Syst. 75:305–313. 2009. View Article : Google Scholar
|
|
18
|
Rioux LE, Turgeon SL and Beaulieu M:
Effect of season on the composition of bioactive polysaccharides
from the brown seaweed Saccharina longicruris. Phytochemistry.
70:1069–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cunha L and Grenha A: Sulfated seaweed
polysaccharides as multifunctional materials in drug delivery
applications. Mar Drugs. 14:E422016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gomes DL, Telles CB, Costa MS,
Almeida-Lima J, Costa LS, Keesen TS and Rocha HA: Methanolic
extracts from brown seaweeds Dictyota cilliolata and Dictyota
menstrualis induce apoptosis in human cervical adenocarcinoma HeLa
cells. Molecules. 20:6573–6591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Fu Z and Han C: The potential
applications of marine bioactives against diabetes and obesity. Am
J Mar Sci. 2:1–8. 2014.
|
|
22
|
Kang KA, Lee KH, Chae S, Koh YS, Yoo BS,
Kim JH, Ham YM, Baik JS, Lee NH and Hyun JW: Triphlorethol-A from
Ecklonia cava protects V79.4 lung fibroblast against hydrogen
peroxide induced cell damage. Free Radic Res. 39:883–892. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang KA, Lee KH, Chae S, Zhang R, Jung MS,
Lee Y, Kim SY, Kim HS, Joo HG, Park JW, et al: Eckol isolated from
Ecklonia cava attenuates oxidative stress induced cell damage in
lung fibroblast cells. FEBS Lett. 579:6295–6304. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang K, Hwang HJ, Hong DH, Park Y, Kim SH,
Lee BH and Shin HC: Antioxidant and antiinflammatory activities of
ventol, a phlorotannin-rich natural agent derived from Ecklonia
cava, and its effect on proteoglycan degradation in cartilage
explant culture. Res Commun Mol Pathol Pharmacol. 115–116. 77–95.
2004.
|
|
25
|
Wijesekara I, Yoon NY and Kim SK:
Phlorotannins from Ecklonia cava (Phaeophyceae): Biological
activities and potential health benefits. Biofactors. 36:408–414.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BM, Park JH, Kim DS, Kim YM, Jun JY,
Jeong IH and Chi YM: Effects of the polysaccharide from the
sporophyll of brown alga Undaria Pinnatifida on serum lipid profile
and fat tissue accumulation in rats fed a high-fat diet. J Food
Sci. 81:H1840–H1845. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimura Y, Watanabe K and Okuda H: Effects
of soluble sodium alginate on cholesterol excretion and glucose
tolerance in rats. J Ethnopharmacol. 54:47–54. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hernández-Corona DM, Martínez-Abundis E
and González- Ortiz M: Effect of fucoidan administration on insulin
secretion and insulin resistance in overweight or obese adults. J
Med Food. 17:830–832. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MJ, Jeon J and Lee JS: Fucoidan
prevents high-fat diet-induced obesity in animals by suppression of
fat accumulation. Phytother Res. 28:137–143. 2014. View Article : Google Scholar
|
|
30
|
Wijesinghe WA and Jeon YJ: Exploiting
biological activities of brown seaweed Ecklonia cava for potential
industrial applications: A review. Int J Food Sci Nutr. 63:225–235.
2012. View Article : Google Scholar
|
|
31
|
Ham YM, Baik JS, Hyun JW and Lee NH:
Cheminform Abstract: Isolation of a new phlorotannin,
Fucodiphlorethol G, from a brown alga Ecklonia cava. ChemInform.
39:2008. View Article : Google Scholar
|
|
32
|
Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM and
Kim SK: Chemical components and its antioxidant properties in
vitro: An edible marine brown alga Ecklonia cava. Bioorg Med Chem.
17:1963–1973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH,
Kim TG, Kim SH, Kim NG, Huh H and Kim J: Inhibition of HIV-1
reverse transcriptase and protease by phlorotannins from the brown
alga Ecklonia cava. Biol Pharm Bull. 27:544–547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang YI, Jung SH, Lee KT and Choi JH:
8.8′-Bieckol, isolated from edible brown algae, exerts its
anti-inflammatory effects through inhibition of NF-κB signaling and
ROS production in LPS-stimulated macrophages. Int Immunopharmacol.
23:460–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shin HC, Hwang HJ, Kang KJ and Lee BH: An
anti-oxidative and anti-inflammatory agent for potential treatment
of osteoarthritis from Ecklonia cava. Arch pharm Res. 29:165–171.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Athukorala Y, Kim KN and Jeon YJ:
Antiproliferative and anti- oxidant properties of an enzymatic
hydrolysate from brown alga Ecklonia cava. Food Chem Toxicol.
44:1065–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park MH, Heo SJ, Park PJ, Moon SH, Sung
SH, Jeon BT and Lee SH: 6,6′-bieckol isolated from Ecklonia cava
protects oxidative stress through inhibiting expression of ROS and
proinflammatory enzymes in high-glucose-induced human umbilical
vein endothelial cells. Appl Biochem Biotechnol. 174:632–643. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim KN, Heo SJ, Song CB, Lee J, Heo MS,
Yeo IK, Kang KA, Hyun JW and Jeon YJ: Protective effect of Ecklonia
cava enzymatic extracts on hydrogen peroxide-induced cell damage.
Process Biochem. 41:2393–2401. 2006. View Article : Google Scholar
|
|
39
|
Yang YI, Shin HC, Kim SH, Park WY, Lee KT
and Choi JH: 6,6′-Bieckol, isolated from marine alga Ecklonia cava,
suppressed LPS-induced nitric oxide and PGE2 production and
inflammatory cytokine expression in macrophages: The inhibition of
NFκB. Int Immunopharmacol. 12:510–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee W, Oh JY, Kim EA, Kang N, Kim KN, Ahn
G and Jeon YJ: A prebiotic role of Ecklonia cava improves the
mortality of Edwardsiella tarda-infected zebrafish models via
regulating the growth of lactic acid bacteria and pathogen
bacteria. Fish Shellfish Immunol. 54:620–628. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee W, Ahn G, Oh JY, Kim SM, Kang N, Kim
EA, Kim KN, Jeong JB and Jeon YJ: A prebiotic effect of Ecklonia
cava on the growth and mortality of olive flounder infected with
pathogenic bacteria. Fish Shellfish Immunol. 51:313–320. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahn G, Lee W, Kim KN, Lee JH, Heo SJ, Kang
N, Lee SH, Ahn CB and Jeon YJ: A sulfated polysaccharide of
Ecklonia cava inhibits the growth of colon cancer cells by inducing
apoptosis. EXCLI J. 14:294–306. 2015.PubMed/NCBI
|
|
43
|
Ahn JH, Yang YI, Lee KT and Choi JH:
Dieckol, isolated from the edible brown algae Ecklonia cava,
induces apoptosis of ovarian cancer cells and inhibits tumor
xenograft growth. J Cancer Res Clin Oncol. 141:255–268. 2015.
View Article : Google Scholar
|
|
44
|
Park SJ, Ahn G, Lee NH, Park JW, Jeon YJ
and Jee Y: Phloroglucinol (PG) purified from Ecklonia cava
attenuates radiation-induced apoptosis in blood lympho cytes and
splenocytes. Food Chem Toxicol. 49:2236–2242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shin H, Cho AR, Kim DY, Munkhbayer S, Choi
SJ, Jang S, Kim SH, Shin HC and Kwon O: Enhancement of human hair
growth using Ecklonia cava polyphenols. Ann Dermatol. 28:15–21.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang JI, Kim SC, Kim MK, Boo HJ, Jeon YJ,
Koh YS, Yoo ES, Kang SM and Kang HK: Effect of dieckol, a component
of Ecklonia cava, on the promotion of hair growth. Int J Mol Sci.
13:6407–6423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Choi BW, Lee HS, Shin HC and Lee BH:
Multifunctional activity of polyphenolic compounds associated with
a potential for Alzheimer's disease therapy from Ecklonia cava.
Phytother Res. 29:549–553. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kang IJ, Jang BG, In S, Choi B, Kim M and
Kim MJ: Phlorotannin-rich Ecklonia cava reduces the production of
beta-amyloid by modulating alpha- and gamma-secretase expression
and activity. Neurotoxicology. 34:16–24. 2013. View Article : Google Scholar
|
|
49
|
Choi HS, Jeon HJ, Lee OH and Lee BY:
Dieckol, a major phlorotannin in Ecklonia cava, suppresses lipid
accumulation in the adipocytes of high-fat diet-fed zebrafish and
mice: Inhibition of early adipogenesis via cell-cycle arrest and
AMPKα activation. Mol Nutr Food Res. 59:1458–1471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang MC, Kim KN, Kang SM, Yang X, Kim EA,
Song CB, Nah JW, Jang MK, Lee JS, Jung WK and Jeon YJ: Protective
effect of dieckol isolated from Ecklonia cava against ethanol
caused damage in vitro and in zebrafish model. Environ Toxicol
Pharmacol. 36:1217–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
You HN, Lee HA, Park MH, Lee JH and Han
JS: Phlorofucofuroeckol A isolated from Ecklonia cava alleviates
postprandial hyperglycemia in diabetic mice. Eur J Pharmacol.
752:92–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Park EY, Choi H, Yoon JY, Lee IY, Seo Y,
Moon HS, Hwang JH and Jun HS: Polyphenol-rich fraction of Ecklonia
cava improves nonalcoholic fatty liver disease in high fat diet-fed
mice. Mar Drugs. 13:6866–6883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Park EY, Kim EH, Kim MH, Seo YW, Lee JI
and Jun HS: Polyphenol-rich fraction of brown alga Ecklonia cava
collected from Gijang, Korea, reduces obesity and glucose levels in
high-fat diet-induced obese mice. Evi Based Complement Alternat
Med. 418912:2012.
|
|
54
|
Lee SH, Min KH, Han JS, Lee DH, Park DB,
Jung WK, Park PJ, Jeon BT, Kim SK and Jeon YJ: Effects of brown
alga, Ecklonia cava on glucose and lipid metabolism in
C57BL/KsJ–db/db mice, a model of type 2 diabetes mellitus. Food
Chem Toxicol. 50:575–582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kang MC, Wijesinghe WA, Lee SH, Kang SM,
Ko SC, Yang X, Kang N, Jeon BT, Kim J, Lee DH and Jeon YJ: Dieckol
isolated from brown seaweed Ecklonia cava attenuates type ІІ
diabetes in db/db mouse model. Food Chem Toxicol. 53:294–298. 2013.
View Article : Google Scholar
|
|
56
|
Kim H, Kong CS, Lee JI, Kim H, Baek S and
Seo Y: Evaluation of inhibitory effect of phlorotannins from
Ecklonia cava on triglyceride accumulation in adipocyte. J Agric
Food Chem. 61:8541–8547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kang C, Jin YB, Lee H, Cha M, Sohn ET,
Moon J, Park C, Chun S, Jung ES, Hong JS, et al: Brown alga
Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt
signaling pathways. Food Chem Toxicol. 48:509–516. 2010. View Article : Google Scholar
|
|
58
|
Kim IH and Nam TJ: Enzyme-treated Ecklonia
cava extracts inhibits adipogenesis through the downregulation of
C/EBPα in 3T3-L1 adipocytes. Int J Mol Med. 39:636–644. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Krishnamoorthy V, Nagappan P, Sereen AK
and Rajendran R: Preliminary phytochemical screening of the fruit
rind of Garcinia cambogia and leaves of Bauhinia variegate-A
comparative study. Int J Curr Microbiol App Sci. 3:479–486.
2014.
|
|
60
|
Rossmeisl M, Rim JS, Koza RA and Kozak LP:
Variation in type 2 diabetes-related traits in mouse strains
susceptible to diet-induced obesity. Diabetes. 52:1958–1966. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bullen JW Jr, Ziotopoulou M, Ungsunan L,
Misra J, Alevizos I, Kokkotou E, Maratos-Flier E, Stephanopoulos G
and Mantzoros CS: Short-term resistance to diet-induced obesity in
A/J mice is not associated with regulation of hypothalamic
neuropeptides. Am J Physiol Endocrinol Metab. 287:E662–E670. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tilg H and Moschen AR: Insulin resistance,
inflammation, and non-alcoholic fatty liver disease. Trends
Endocrinol Metab. 19:371–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
van Herpen NA and Schrauwen-Hinderling VB:
Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol
Behav. 94:231–241. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Toye AA, Dumas ME, Blancher C, Rothwell
AR, Fearnside JF, Wilder SP, Bihoreau MT, Cloarec O, Azzouzi I,
Young S, et al: Subtle metabolic and liver gene transcriptional
changes underlie diet-induced fatty liver susceptibility in
insulin-resistant mice. Diabetologia. 50:1867–1879. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Imano H, Noda H, Kitamura A, Sato S,
Kiyama M, Sankai T, Ohira T, Nakamura M, Yamagishi K, Ikeda A, et
al: Low-density lipoprotein cholesterol and risk of coronary heart
disease among Japanese men and women: The circulatory risk in
communities study (CIRCS). Prev Med. 52:381–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Brewer HB Jr: High-density lipoprotein: A
new potential therapeutic target for the prevention of
cardiovascular disease. Arterioscler Thromb Vasc Biol. 24:387–391.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bonini JA, Colca JR, Dailey C, White M and
Hofmann C: Compensatory alterations for insulin signal transduction
and glucose transport in insulin-resistant diabetes. Am J Physiol.
269:E759–E765. 1995.PubMed/NCBI
|
|
68
|
Park HK and Ahima RS: Physiology of
leptin: Energy homeostasis, neuroendocrine function and metabolism.
Metabolism. 64:24–34. 2015. View Article : Google Scholar
|
|
69
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fasshauer M and Paschke R: Regulation of
adipokines and insulin resistance. Diabetologia. 46:1594–1603.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Chao J, Huo TI, Cheng HY, Tsai JC, Liao
JW, Lee MS, Qin XM, Hsieh MT, Pao LH and Peng WH: Gallic acid
ameliorated impaired glucose and lipid homeostasis in high fat
diet-induced NAFLD mice. PLoS One. 9:e969692014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ye JH, Chao J, Chang ML, Peng WH, Cheng
HY, Liao JW and Pao LH: Pentoxifylline ameliorates non-alcoholic
fatty liver disease in hyperglycaemic and dyslipidaemic mice by
upregulating fatty acid β-oxidation. Sci Rep. 6:331022016.
View Article : Google Scholar
|
|
73
|
Liu X, Xu J, Xue Y, Gao Z, Li Z, Leng K,
Wang J, Xue C and Wang Y: Sea cucumber cerebrosides and long-chain
bases from Acaudina molpadioides protect against high fat
diet-induced metabolic disorders in mice. Food Funct. 6:3428–3436.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kuo YH, Lin CH and Shih CC:
Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and
hyperlipidemia in high-fat-diet treated mice via regulation of
hepatic related genes, glucose transporter 4, and AMP-activated
protein kinase phosphorylation. J Agric Food Chem. 63:2479–2489.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Soret MG, Kupieeki FP and Wyse BM:
Epididymal fat pad alterations in mice with spontaneous obesity and
diabetes and with chemically induced obesity. Diabetologia.
10(Suppl): S639–S648. 1974. View Article : Google Scholar
|
|
76
|
Moffat C and Harper ME: Metabolic
functions of AMPK: Aspects of structure and of natural mutations in
the regulatory gamma subunits. IUBMB Life. 62:739–745. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang T, Sawada K, Yamamoto N and Ashida
H: 4-Hydroxyderricin and xanthoangelol from ashitaba (Angelica
keiskei) suppress differentiation of preadipocytes to adipocytes
via AMPK and MAPK pathways. Mol Nutr Food Res. 57:1729–1740.
2013.PubMed/NCBI
|
|
78
|
Schreurs M, Kuipers F and van der Leij FR:
Regulatory enzymes of mitochondrial beta-oxidation as targets for
treatment of the metabolic syndrome. Obes Rev. 11:380–388. 2010.
View Article : Google Scholar
|
|
79
|
Eo H, Jeon YJ, Lee M and Lim Y: Brown Alga
Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis,
oxidative stress, and inflammation by activation of AMPK and SIRT1
in high-fat diet-induced obese mice. J Agric Food Chem. 63:349–359.
2015. View Article : Google Scholar
|
|
80
|
Farmer SR: Regulation of PPARgamma
activity during adipogenesis. Int J Obes (Lond). 29(Suppl 1):
S13–S16. 2005. View Article : Google Scholar
|
|
81
|
Ha do T, Trung TN, Phuong TT, Yim N, Chen
QC and Bae K: The selected flavonol glycoside derived from Sophorae
Flos improves glucose uptake and inhibits adipocyte differentiation
via activation AMPK in 3T3-L1 cells. Bioorg Med Chem Lett.
20:6076–6081. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Karim S, Adams DH and Lalor PF: Hepatic
expression and cellular distribution of the glucose transporter
family. World J Gastroenterol. 18:6771–6781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kim S, Jung J, Kim H, Heo RW, Yi CO, Lee
JE, Jeon BT, Kim WH, Hahm JR and Roh GS: Exendin-4 improves
nonalcoholic fatty liver disease by regulating glucose transporter
4 expression in ob/ob mice. Korean J Physiol Pharmacol. 18:333–339.
2014. View Article : Google Scholar : PubMed/NCBI
|