Introduction
Traditional medical and surgical therapies have been
successful in treating several cardiovascular diseases, however, a
number of patients suffer from heart failure due to the
irreversible loss of cardiomyocytes caused by myocardial ischemia.
Although increasing evidence indicates that cardiac progenitor
cells may be involved in the process of myocardial regeneration in
patients with heart failure (1–3),
the degree of this potential remains controversial (4–7).
Stem cell biology has provided therapeutic approaches for replacing
non-functional cardiac tissue by cell transplantation. In
principle, autologous bone marrow mesenchymal stem cells (BMSCs)
are the ideal cells for use in transplantation. However,
cardiovascular disease occurs more often in middle-aged and elderly
individuals, and the number and function of BMSCs are depressed in
these patient groups (8). This
suggests limitations in the utilization of autologous stem cells
for patients with ischemic cardiomyopathy. Human placenta-derived
mesenchymal stem cells (hPDMSCs) possess the ability of
multi-directional differentiation (9); in addition, hPDMSCs are widely
sourced and easier to isolate, which makes them an attractive
alternative stem cell source, particularly for transplantation
purposes.
The pre-differentiation of stem cells towards a
defined cardiac lineage prior to transplantation may be more
advantageous than transplanting uncommitted stem cells, which may
undergo unanticipated differentiation. Several methods have been
used to promote the cardiomyogenic differentiation of stem cells,
including co-culturing techniques (10,11), demethylating agent treatment
(12), and specific gene
insertions (13). However, the
majority of these methods are unlikely to be clinically applied due
to their low efficacy and potentially harmful effects (14). Salvia miltiorrhiza (SM), a
well-known traditional Chinese medicine, is widely used in China
and neighboring countries to treat patients suffering from ischemic
heart disease with minimal side effects. Our previous study
(15) indicated that SM induced
hPDMSCs to differentiate into cardiomyocytes, and this effect was
superior to that of the co-culture method. The pre-differentiation
of stem cells towards a defined cardiac lineage with SM prior to
transplantation is likely to be clinically applicable. However, the
effective components of SM in promoting cardiomyogenic
differentiation remain to be fully elucidated. Determining the
effective component of SM in promoting cardiomyogenic
differentiation may contribute to investigations aimed at enhancing
the efficiency of cardiomyogenic differentiation and myocardial
regeneration. The present study was performed to investigate the
effective component of SM in promoting cardiomyogenic
differentiation and its possible mechanism.
Materials and methods
hPDMSC isolation and identification
Placentas at term (38–40 weeks gestation; n=10) were
obtained from healthy donor mothers following the provision of
informed consent and according to the procedures of the
institutional review board. The present study was approved by the
Ethics Committee of The First Affiliated Hospital of Jinzhou
Medical University (Jinzhou, China). Briefly, the cells were
isolated using a tissue culture method (15) and cultured in 5% CO2 at
37°C, following which the placenta-derived cells at the fourth
passage were treated with 0.25% trypsin-EDTA, harvested, and washed
twice with phosphate-buffered solution (PBS). The cells were
incubated on ice for 20 min with pre-diluted PE-labeled mouse
anti-human antibodies CD13 (cat. no. 560998), CD73 (cat. no.
561014), CD90 (cat. no. 561970), CD166 (cat. no. 559263), HLA-DR
(cat. no. 555561) and FITC-labeled mouse anti-human antibodies CD14
(cat. no. 555397) (all from BD Biosciences, San Jose, CA, USA),
CD29 (cat. no. 11-0299-41; eBiosiences, San Diego, CA, USA), CD31
(cat. no. 560984), CD44 (cat. no. 560977), CD45 (cat. no. 561865),
CD105 (cat. no. 561443) and HLA-ABC (cat. no. 557348). Control
groups were incubated with FITC-(cat. no. 555786) and PE-conjugated
mouse anti-human IgG (cat. no. 555787) (all from BD Biosciences).
The labeled cells were analyzed using flow cytometry (BD FACSCanto
II; Becton-Dickinson Co., Franklin Lakes, NJ, USA).
Cardiomyogenic differentiation
Briefly, the proliferation of hPDMSCs was measured
using an MTS assay following treatment with SM and its most
commonly examined components, including danshensu (DSS),
salvianolic acid B (SA B), protocatechuic aldehyde (PCAD),
tanshinone I (TS I), tanshinone IIA (TS IIA) and cryptotanshinone
(CTS), at different concentrations for 4 days. As concentrations of
SM <4 mg/l, DSS <10 mg/l, SA B <10 mg/l, PCAD <0.5
mg/l, TS I <1 mg/l, TS IIA <0.1 mg/l and CTS <0.1 mg/l had
minimal effect on cell proliferation (Fig. 2A), 4 mg/l SM, 10 mg/l DSS, 10 mg/l
SA B, 0.5 mg/l PCAD, 1 mg/l TS I, 0.1 mg/l TS IIA and 0.1 mg/l CTS
concentrations were preferred to enable superior assessment of the
effective components of SM in promoting cardiomyogenic
differentiation in experiments. The hPDMSCs were inoculated in
96-well plates, 24-well plates and 10-cm culture dishes at a
density of 1×103 cells/cm2 and cultured in 5%
CO2 at 37°C. The following day, the cells were
respectively treated with 4 mg/l SM, 10 mg/l DSS, 10 mg/l SA B, 0.5
mg/l PCAD, 1 mg/l TS I, 0.1 mg/l TS IIA and 0.1 mg/l T CTS in
complete medium containing Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD,
USA), 1 mmol/l L-glutamine, 0.1 mmol/l, β-mercaptoethanol and 1%
non-essential amino acids for the induction of cardiomyogenic
differentiation. The aforementioned medium was replaced every 4
days. The morphologic characteristics of the cells were analyzed
under a microscope (Leica DMI1; Leica Microsystems Inc; Mannheim,
Germany) every day. As the absorbance values in an MTS assay can
indirectly reflect the number of living cells, with the absorbance
values being positively associated with the number of living cells,
cell proliferation was measured using the MTS assay every 4 days.
The experiment was terminated at day 20.
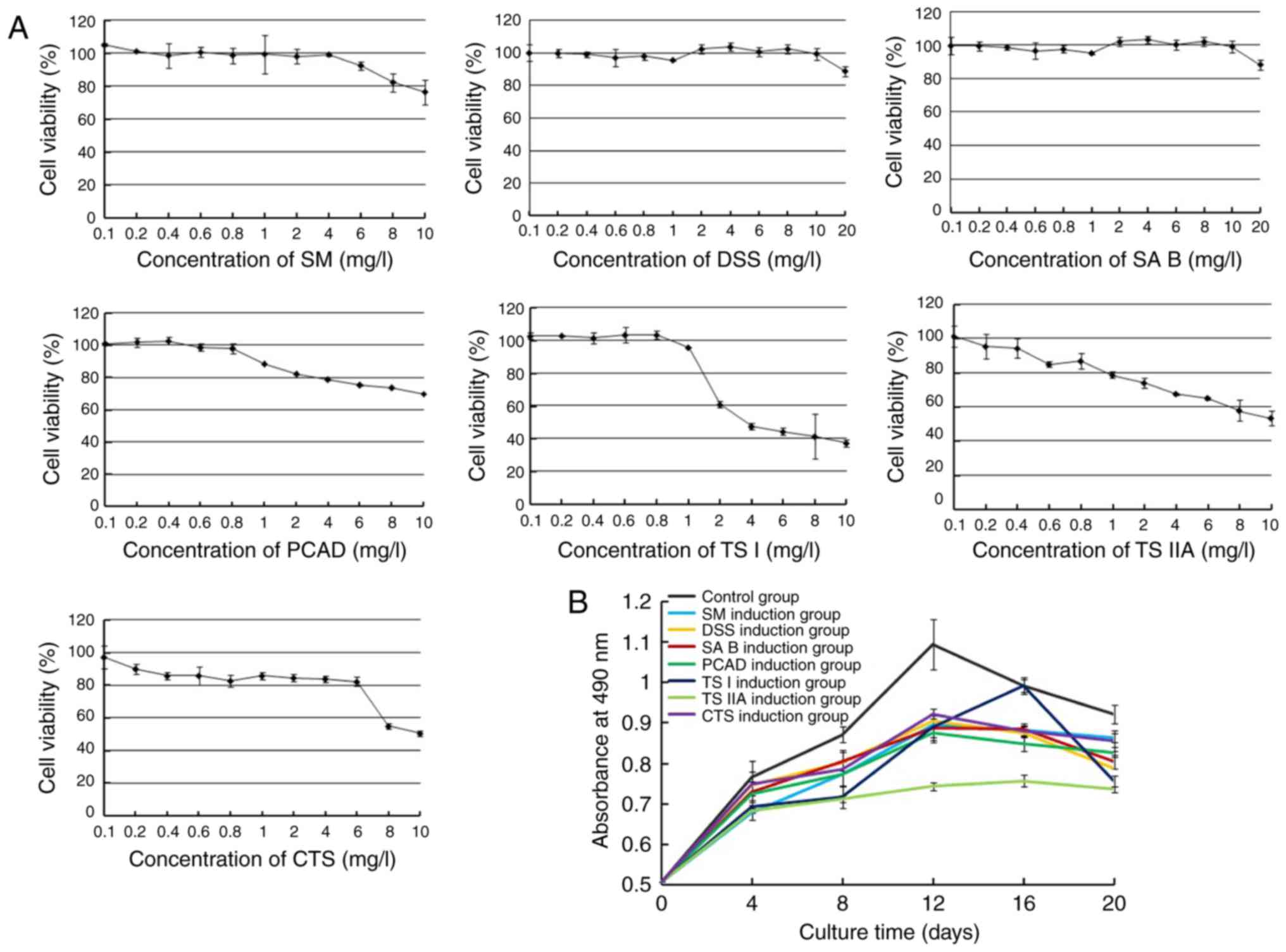 | Figure 2Effects of SM and its active
components on the proliferation of hPDMSCs. (A) Cytotoxicity of SM
and its active components against hPDMSCs. Concentrations of SM
<4 mg/l, DSS <10 mg/l, SA B <10 mg/l, PCAD <0.5 mg/l,
TS I <1 mg/l, TS IIA <0.1 mg/l and CTS <0.1 mg/l had
minimal effect on cell proliferation. (B) Effects of SM and its
active components on cell proliferation. Cells in the control group
maintained a higher growth rate, however, treated cells exhibited
significantly reduced growth rate. TS IIA had the most marked
effect. Data are presented as the mean ± standard deviation of
three independent experiments. hPDMSCs, human placenta-derived
mesenchymal stem cells; SM, Salvia miltiorrhiza; DSS,
danshensu; SA B, salvianolic acid B; PCAD, protocatechuic aldehyde;
TS I, tanshinone I; TS IIA, tanshinone IIA; CTS,
cryptotanshinone. |
Immunocytochemistry
The cells were fixed in PBS containing 4%
paraformaldehyde (PFA) for 20 min, permeabilized in PBS containing
0.2% Triton X-100 for 10 min, and blocked in a serum-free blocking
solution for 5 min at room temperature. The cells were then
incubated with primary antibody against α-SCA (1:100 dilution; cat.
no. ab9465; Abcam, Cambridge, UK) overnight at 4°C. Following
extensive washing with PBS, the cells were incubated with
HRP-conjugated goat anti-rabbit IgG (1:100 dilution; cat. no.
ab6721; Abcam). Finally, the nuclei were stained by incubation with
hematoxylin. The results were analyzed under a microscope (Leica
DMI1; Leica Microsystems Inc.).
Immunofluorescence
The cells were washed three times with PBS and fixed
in PBS containing 4% PFA for 30 min. The fixed cells were washed
three times with PBS and permeabilized in PBS containing 0.2%
Triton X-100 for 10 min. The cells were then washed three times
with PBS and blocked in a serum-free blocking solution for 5 min at
room temperature. The blocked cells were incubated with cardiac
troponin-I (cTnI) primary antibody (1:50 dilution; cat. no.
ab47003; Abcam) overnight at 4°C. Following extensive washing with
PBS, the cells were incubated with FITC-conjugated goat anti-rabbit
IgG (1:50 dilution; cat. no. ab6717; Abcam) for 1 h at room
temperature. Following extensive washing with PBS, the nuclei were
stained by incubation with propidium iodide (PI). The results were
analyzed using a fluorescence microscope. The differentiation ratio
of cardiomyocytes was calculated as the fraction of cTnI-positive
cells in total cells, which were in a counterpart visual field. The
rate was calculated as the average of >10 separate fields.
Western blot analysis
The cells were washed twice with PBS and then
collected. Total proteins were extracted with cell lysis buffer
(cat. no. P0013b; Beyotime) according to the manufacturer's
protocol. The protein quantity was determined by BCA assay (BioTek,
Winooski, VT, USA). Equal quantities of protein (30 µg) were
respectively separated with sodium dodecyl sulfate-polyacrylamide
gel electrophoresis on 8 or 10% gel and then transferred onto
polyvinylidene difluoride membranes. The membranes were blocked
with 5% non-fat milk for 1 h and then incubated overnight at 4°C
with the following primary antibodies: GAPDH (1:5,000 dilution;
cat. no. ab9485), GATA-binding protein 4 (GATA4; 1:1,000 dilution;
cat. no. ab84593), atrial natriuretic factor (ANF; 1:1,000
dilution; cat. no. ab14348), cTnI (1:1,000 dilution; cat. no.
ab47003), glycogen synthase kinase-3β (GSK-3β; 1:10,000 dilution;
cat. no. ab32391), phosphorylated (p-)GSK-3β (1:10,000 dilution;
cat. no. ab75814) or β-catenin (1:10,000 dilution; cat. no.
ab10000), all from Abcam. This was followed by incubation with
HRP-conjugated goat anti-rabbit IgG (1:5,000 dilution; cat. no.
ab6721; Abcam) for 2 h at room temperature. Antibody detection was
performed using a chemiluminescence detection kit (Omega Lum G;
Aplegen Inc., Pleasanton CA, USA).
Statistical analysis
Each experiment was repeated at least three times.
All data are presented as the mean ± standard deviation. A one-way
analysis of variance was used for comparisons among the groups.
Statistical analysis was performed using SPSS 13.0 software.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of human
placenta-derived cells
The placenta-derived cells grew to form colonies and
exhibited an adherent fibroblastoid appearance. The results of the
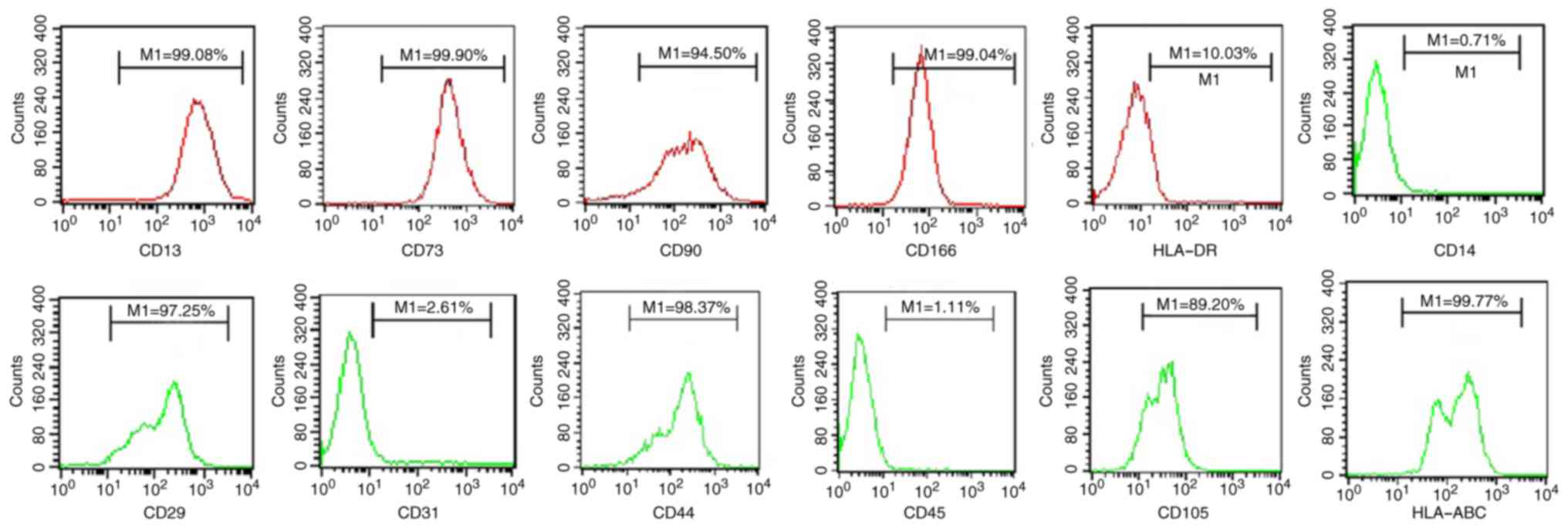
flow cytometric analysis demonstrated that the placenta-derived
cells were positive for CD13, CD29, CD44, CD73, CD90, CD105, CD166
and HLA-ABC, and negative for CD14, CD31, CD45 and HLA-DR (Fig. 1). The surface markers of these
placenta-derived cells were exactly the same as those of previously
reported bone marrow- and cord blood-derived mesodermal cells
(16).
Effects of SM and its active components
on cell proliferation and cell morphology
To better assess whether SM and its active
components inhibited hPDMSC proliferation and stimulated cell
differentiation, cell proliferation was evaluated using an MTS
assay and cell morphological characteristics were analyzed using
microscopy. The cells in the negative control group maintained an
increased growth rate and maintained fibroblast-like morphology.
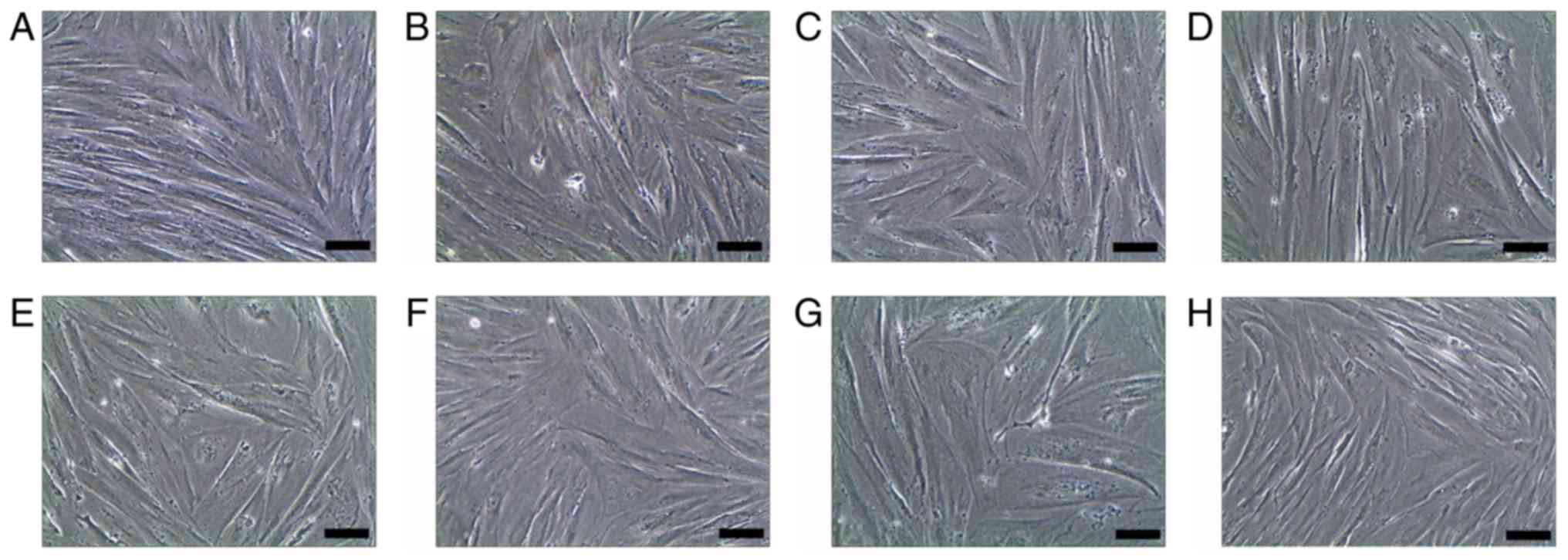
However, the cells treated with SM and its active components
exhibited a slower growth rate, with certain cells beginning to
exhibit altered morphology. These cells gradually increased in size
to form a stick-like appearance. On day 20, the cells became
enlarged and exhibited a number of branches connecting with
adjoining cells (Fig. 2A). Among
the SM components, TS IIA had the most marked effect on the cells
(Figs. 2B and 3).
Effects of SM and its active components
on the cardiomyogenic differentiation of hPDMSCs
In order to corroborate the cardiomyogenic
differentiation of hPDMSCs, immunohistochemistry was performed to
detect α-SCA, and western blot analysis was performed to detect the
protein expression levels of GATA4, ANF and cTnI. The
immunohistochemistry revealed that some of the treated cells
stained positive for α-SCA, and there were more α-SCA-positive
cells in the TS IIA treatment group (Fig. 4A). Compared with the negative
control group, the protein expression levels of GATA4, ANF and cTnI
were increased in the treatment groups, however, the protein
expression levels of GATA4, ANF and cTnI were marginally higher in
the cells treated with TS IIA (Fig.
4B and C).
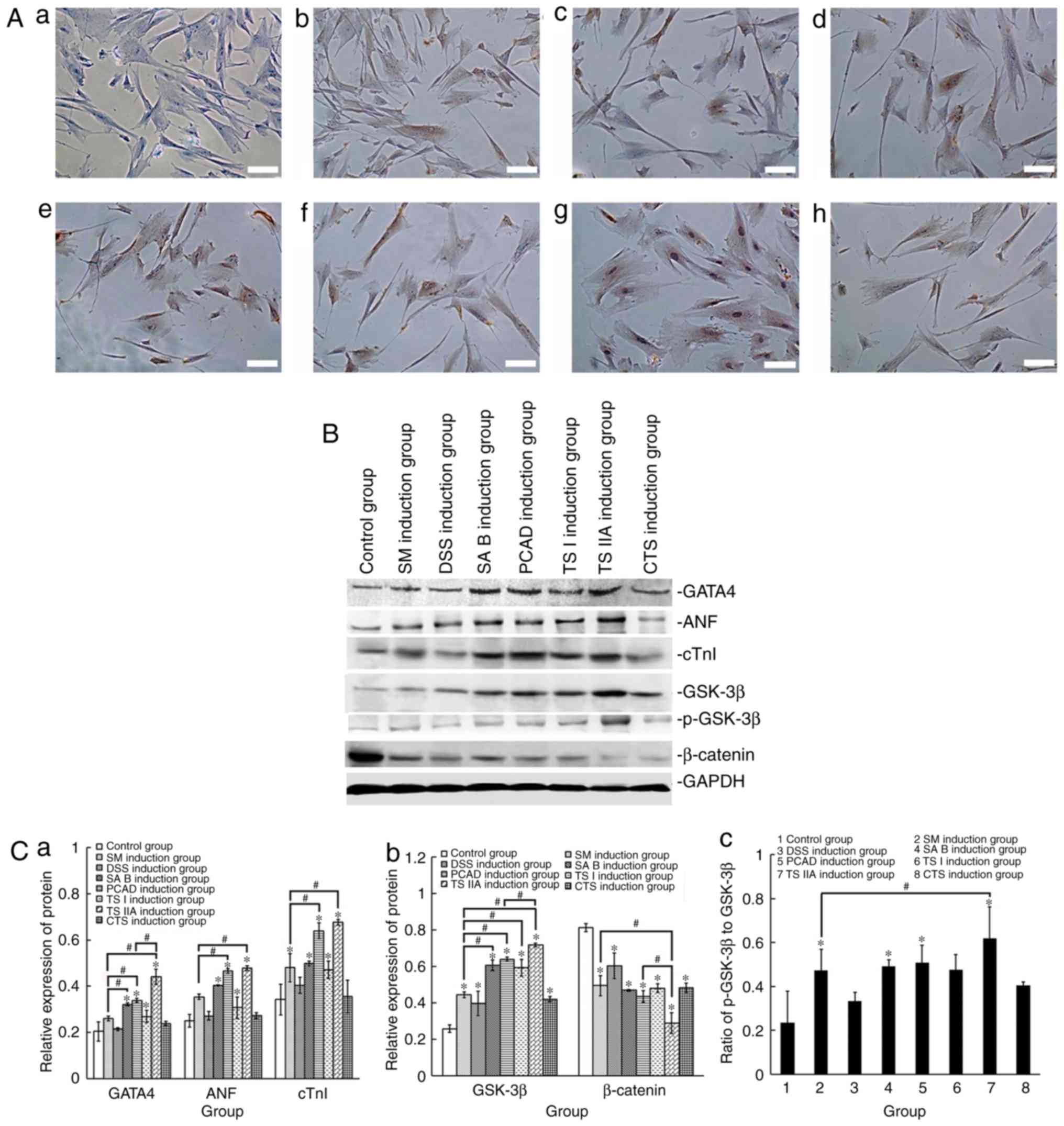 | Figure 4Effects of SM and its active
components on cardiomyogenic differentiation of human
placenta-derived mesenchymal stem cells. (A) Expression of α-SCA;
(a) negative control, cells stained negative for α-SCA; (b) SM
induction group; (c) DSS induction group; (d) SA B induction group;
(e) PCAD induction group; (f) TS I induction group; (g) TS IIA
induction group; (h) CTS induction group, a number of cells stained
positively for α-SCA, with more α-SCA positive cells in TS IIA
induction group. Scale bar, 20 µm. (B) Results of the
western blot analysis of GATA4, ANF, cTnI, GSK-3β, p-GSK-3β and
β-catenin. (C) Relative protein expression levels of (a) GATA4, ANF
and cTnI, and (b) GSK-3β and β-catenin. (c) Ratios of p-GSK-3β to
GSK-3β. Data are presented as the mean ± standard deviation of
three independent experiments. *P<0.05 compared with
the control group; #P<0.05 between the two groups.
SM, Salvia miltiorrhiza; DSS, danshensu; SA B, salvianolic
acid B; PCAD, protocatechuic aldehyde; TS I, tanshinone I; TS IIA,
tanshinone IIA; CTS, cryptotanshinone; ANF, atrial natriuretic
factor; α-SCA, α-sarcomeric actin; cTnI, cardiac troponin-I;
GSK-3β, glycogen synthase kinase-3β; p-GSK-3β, phosphorylated
GSK-3β. |
Effects of Tan IIA on Wnt/β-catenin
signaling
In order to determine whether Wnt/β-catenin
signaling was important in SM and its active components in
promoting the cardiomyo-genic differentiation of hPDMSCs, the
protein expression levels of GSK-3β, p-GSK-3β and β-catenin were
examined. The results showed that the expression levels of GSK-3β
and p-GSK-3β were increased to different degrees, whereas the
expression of β-catenin was decreased to different degrees in the
treatment groups. In addition, the ratios of p-GSK-3β to GSK-3β
were increased to different degrees, particularly in the TS IIA
treatment group (Fig. 4B and
C).
Effects of SM and its active components
on cardiomyogenic differentiation rate
For further analysis of the effective components of
SM in promoting cardiomyogenic differentiation, immunofluorescence
was performed to detect cardiomyogenic differentiation rate. cTnI
was not expressed in the control group. The expression levels of
cTnI in the treatment groups were different, with the
cardiomyogenic induction rate of the TS IIA being marginally higher
(Fig. 5).
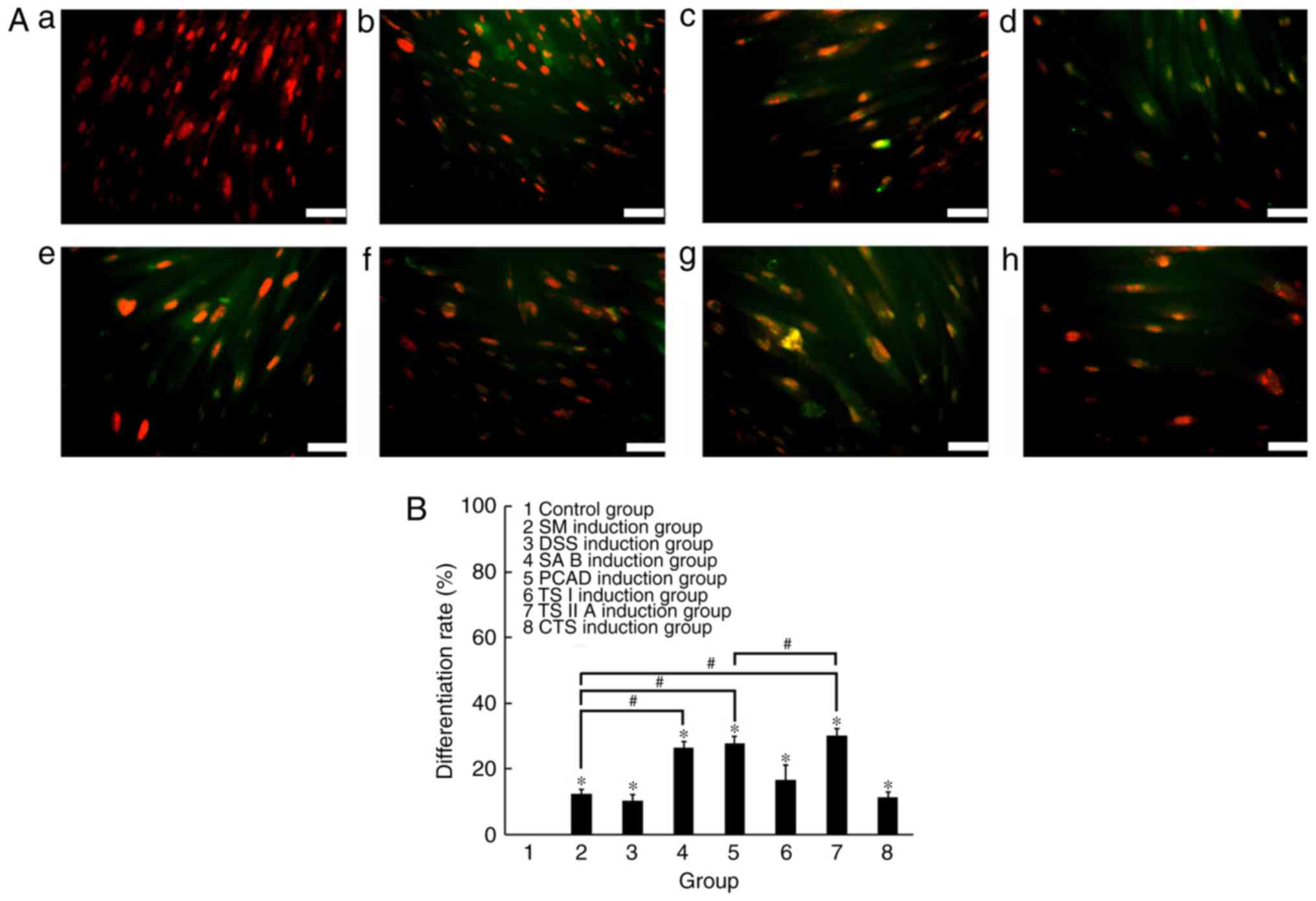 | Figure 5Effects of SM and its active
components on the cardiomyogenic differentiation rate of human
placenta-derived mesenchymal stem cells. (A) Expression of cTnI;
(a) negative control cells stained negative for cTnI; (b) SM
induction group; (c) DSS induction group; (d) SA B induction group;
(e) PCAD induction group; (f) TS I induction group; (g) TS IIA
induction group; (h) CTS induction group, positive staining for
cTnI was observed. The nuclei were stained with propidium iodide
(red) and cTnI antibody was stained using fluorescence
isothiocyanate (green). Scale bar, 20 µm. (B) Cardiomyogenic
differentiation rate. The cardiomyogenic induction rate of TS IIA
was marginally higher, compared with that of SM and its the other
active components. *P<0.05 compared with the control
group; #P<0.05 between the two groups. SM, Salvia
miltiorrhiza; DSS, danshensu; SA B, salvianolic acid B; PCAD,
protocatechuic aldehyde; TS I, tanshinone I; TS IIA, tanshinone
IIA; CTS, cryptotanshinone. |
Discussion
The recovery of cardiac performance following
cellular transplantation in experimental models has been partly
attributed to the transdifferentiation of stem cells leading to
de novo formation of cardiomyocytes (17,18). However, the types and
characteristics of these stem cells remain poorly defined and
widely different transdifferentiation efficiency has been reported
(19). The pre-differentiation of
stem cells into cardiomyocytes may enhance their abilities to
survive and engraft as cardiomyocytes following myocardial
transplantation. In addition, the transplantation of cells with
pre-defined myocardial characteristics may circumvent the
challenges of transdifferentiation and cell fusion in the
generation of cardiomyocytes by undifferentiated stem cells in the
regenerating myocardium. At present, several methods have been used
to promote the cardiomyogenic differentiation of stem cells,
including co-culturing techniques (10,11), treatment with demethylating agents
(12), and specific gene
insertions (13). However, the
majority of these methods are unlikely to be clinically applied due
to their low efficacy and potentially harmful effects (14).
SM is an important Chinese natural herb used in the
treatment of several diseases, particularly ischemic cardiovascular
diseases. Previous studies on its potential cardioprotective
effects revealed effects in dilating coronary vessels, improving
the microcirculation of ischemic region, inhibiting platelet
aggregation and inflammatory responses, lowering blood lipids, and
inhibiting the development of arteriosclerosis. Our previous study
(15) indicated that SM induced
hPDMSCs to differentiate into cardiomyocytes and the effect was
superior to that of the co-culture method. As is already known, the
active components of SM are generally divided into two major
groups, water-soluble phenolic compounds and lipophilic diterpene
quinines (20). According to the
Chinese Pharmacopeia (21), the
content of SA B in water-soluble phenolic compounds, and the
content of TS I, TS IIA and CTS in lipophilic diterpene quinines
are important indicators in assessing SM quality; in dried products
of SM, the recommended content of SA B is ≥3.0%, and those of TS I,
TS IIA and CTS are ≥0.25% each. According to the active components
of SM produced by 19 major production bases of SM in China, in
which the average content of DSS was 7.15% and the average content
of PCAD was 1.22% Li et al suggested that the Chinese
Pharmacopoeia incorporate DSS and PCAD as indicators of SM quality
assessment (22). Our previous
studies did not determine the effective component of SM in
promoting cardiomyogenic differentiation. However, determining the
effective component of SM in promoting cardiomyogenic
differentiation may contribute to enhancing the efficiency of
cardiomyogenic differentiation and myocardial regeneration. For
improved assessment of the effective components of SM in promoting
cardiomyogenic differentiation, the present study used non-toxic
doses of SM and its active components for experiments. As SM is a
complex of several components, its non-toxic dose is closely
associated with the content of each monomer in SM and the non-toxic
dose of each monomer. The non-toxic doses of SM and its main active
components used in the present study were different (SM, 4 mg/l;
DSS, 10 mg/l; SA B, 10 mg/l; PCAD, 0.5 mg/l; TS I, 1 mg/l; TS IIA,
0.1 mg/l and CTS, 0.1 mg/l), and the statements above may explain
why the non-toxic dose of SM may be higher or lower than the
non-toxic dose of each monomer component. However, this does not
mean that a high concentration of a non-toxic monomer has a
superior effect on cardiomyogenic differentiation. The results of
the present study demonstrated that SM and its effective components
reduced the cell growth rate to different degrees and altered cell
morphology to produce a spindle or irregular shape. The cells
treated with SM and its effective components stained positively for
α-SCA and showed increased cardiac protein expression of GATA4, ANF
and cTnI to different degrees. Among the treatment groups, the
effect of TS IIA was the most marked, and the effect of DSS was
marginally lower, compared with that of SM. These results suggested
that TS IIA was the most effective active component of SM in
inducing hPDMSCs to differentiate into cardiomyocytes. As one of
the effective active ingredients of SM, TS IIA is currently used in
China and other neighboring countries to treat ischemic heart
disease in the clinic (23). It
has been shown to exert beneficial effects on the cardiovascular
system with minimal side effects, including improving the
microcirculation of ischemic regions (24), inhibiting platelet aggregation
(25) and inflammatory responses
(26), lowering blood lipids
(27), and inhibiting the
development of arteriosclerosis (28). Therefore, pretreatment of these
multipotent cells by TS IIA can be used prior to transplantation
investigations.
Previous studies have shown that Wnt/β-catenin
signaling is important in the cardiomyocyte differentiation of
MSCs. GSK-3β is a central component of the canonical Wnt pathway
and negatively regulates β-catenin through
phosphorylation-dependent proteolytic degradation (29). The downregulation of β-catenin is
important in mediating the effect of GSK-3β on the differentiation
of MSCs into cardiomyocytes (30). The results of the present study
demonstrated that the expression of GSK-3β and p-GSK-3β increased
to different degrees, whereas that of β-catenin decreased to
different degrees in the treatment groups. In addition, the ratio
of p-GSK-3β to GSK-3β increased to different degrees, and was
particularly increased the TS IIA treatment group. Therefore, it
was concluded that the Wnt/β-catenin signaling pathway was
inhibited in the hPDMSC differentiation process.
In conclusion, TS IIA was identified as the most
effective active component of the most commonly investigated
compounds of SM, including DSS, SA B, PCAD, TS I, TS IIA and CTS,
in inducing hPDMSCs to differentiate into cardiomyocytes. In the
hPDMSC differentiation process, the Wnt/β-catenin signaling pathway
was inhibited. The pretreatment of multipotent cells by TS IIA can
be used prior to transplantation investigations to ensure that the
differentiation process is directed towards the cardiomyogenic
lineage in the in vivo environment.
Acknowledgments
This study was supported by the National Natural
Science Foundation (grant no. 81303255), the Natural Science
Foundation of Liaoning Province (grant no. 201602291) and the
Principal Foundation of Jinzhou Medical University (grant no.
XZJJ20130217).
Glossary
Abbreviations
Abbreviations:
|
hPDMSCs
|
human placenta-derived mesenchymal
stem cells
|
|
SM
|
Salvia miltiorrhiza
|
|
DSS
|
danshensu
|
|
SA B
|
salvianolic acid B
|
|
PCAD
|
protocatechuic aldehyde
|
|
TS I
|
tanshinone I
|
|
TS IIA
|
tanshinone IIA
|
|
CTS
|
cryptotanshinone
|
|
ANF
|
atrial natriuretic factor
|
|
α-SCA
|
α-sarcomeric actin
|
|
cTnI
|
cardiac troponin-I
|
|
p-GSK-3β
|
phosphorylated glycogen synthase
kinase-3β
|
|
BMSCs
|
bone marrow mesenchymal stem cells
|
|
PBS
|
phosphate buffer solution
|
|
PFA
|
paraformaldehyde
|
|
FITC
|
fluorescence isothiocyanate
|
References
|
1
|
Kajstura J, Urbanek K, Perl S, Hosoda T,
Zheng H, Ogórek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C,
Sanada F, et al: Cardiomyogenesis in the adult human heart. Circ
Res. 107:305–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beltrami AP, Barlucchi L, Torella D, Baker
M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et
al: Adult cardiac stem cells are multipotent and support myocardial
regeneration. Cell. 114:763–776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh H, Bradfute SB, Gallardo TD, Nakamura
T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry
DJ, et al: Cardiac progenitor cells from adult myocardium: Homing,
differentiation, and fusion after infarction. Proc Natl Acad Sci
USA. 100:12313–12318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bergmann O, Bhardwaj RD, Bernard S, Zdunek
S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA,
Druid H, et al: Evidence for cardiomyocyte renewal in humans.
Science. 324:98–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergmann O, Zdunek S, Alkass K, Druid H,
Bernard S and Frisén J: Identification of cardiomyocyte nuclei and
assessment of ploidy for the analysis of cell turnover. Exp Cell
Res. 317:188–194. 2011. View Article : Google Scholar
|
|
6
|
Leri A, Quaini F, Kajstura J and Anversa
P: Myocyte death and myocyte regeneration in the failing human
heart. Ital Heart J. 2(Suppl 3): 12S–14S. 2001.PubMed/NCBI
|
|
7
|
Walsh S, Pontén A, Fleischmann BK and
Jovinge S: Cardiomyocyte cell cycle control and growth estimation
in vivo-an analysis based on cardiomyocyte nuclei. Cardiovasc Res.
86:365–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanawa M, Igarashi A, Ronald VS, Higashi
Y, Kurihara H, Sugiyama M, Saskianti T, Pan H and Kato Y:
Age-dependent decrease in the chondrogenic potential of human bone
marrow mesenchymal stromal cells expanded with fibroblast growth
factor-2. Cytotherapy. 15:1062–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Portmann-Lanz CB, Schoeberlein A, Huber A,
Sager R, Malek A, Holzgreve W and Surbek DV: Placental mesenchymal
stem cells as potential autologous graft for pre- and perinatal
neuroregeneration. Am J Obstet Gynecol. 194:664–673. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuhara S, Tomita S, Yamashiro S,
Morisaki T, Yutani C, Kitamura S and Nakatani T: Direct cell-cell
interaction of cardiomyocytes is key for bone marrow stromal cells
to go into cardiac lineage in vitro. J Thorac Cardiovasc Surg.
125:1470–1480. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rangappa S, Entwistle JW, Wechsler AS and
Kresh JY: Cardiomyocyte-mediated contact programs human mesenchymal
stem cells to express cardiogenic phenotype. J Thorac Cardiovasc
Surg. 126:124–132. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makino S, Fukuda K, Miyoshi S, Konishi F,
Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al:
Cardiomyocytes can be generated from marrow stromal cells in vitro.
J Clin Invest. 103:697–705. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valiunas V, Doronin S, Valiuniene L,
Potapova I, Zuckerman J, Walcott B, Robinson RB, Rosen MR, Brink PR
and Cohen IS: Human mesenchymal stem cells make cardiac connexins
and form functional gap junctions. J Physiol. 555:617–626. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Song J, Liu W, Wan Y, Chen X and Hu
C: Growth and differentiation of rat bone marrow stromal cells:
Does 5-azacytidine trigger their cardiomyogenic differentiation?
Cardiovasc Res. 58:460–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li K, Li SZ, Zhang YL and Wang XZ: The
effects of dan-shen root on cardiomyogenic differentiation of human
placenta-derived mesenchymal stem cells. Biochem Biophys Res
Commun. 415:147–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop DJ and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gojo S, Gojo N, Takeda Y, Mori T, Abe H,
Kyo S, Hata J and Umezawa A: In vivo cardiovasculogenesis by direct
injection of isolated adult mesenchymal stem cells. Exp Cell Res.
288:51–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orlic D, Kajstura J, Chimenti S, Jakoniuk
I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM,
et al: Bone marrow cells regenerate infarcted myocardium. Nature.
410:701–705. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murry CE, Soonpaa MH, Reinecke H, Nakajima
H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH,
Poppa V, et al: Haematopoietic stem cells do not transdifferentiate
into cardiac myocytes in myocardial infarcts. Nature. 428:664–668.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YG, Song L, Liu M, Hu ZB and Wang ZT:
Advancement in analysis of Salviae miltiorrhizae Radix et Rhizoma
(Danshen). J Chromatogr A. 1216:1941–1953. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
The State Pharmacopoeia Commission of
China. Chinese Pharmacopeia. 1:762015.
|
|
22
|
Li CQ, Xu WL, Shen ZG and Wang JZ:
Comparison of the contents of 5 active components in Salvia
miltiorrhiza from different habitats. Proceedings of Chemistry of
Medicinal Plant and Analysis of Active Components of Traditional
Chinese Medicine Seminar; 113–115. 2008.
|
|
23
|
Shang Q, Xu H and Huang L: Tanshinone IIA:
A promising natural cardioprotective agent. Evid Based Complement
Alternat Med. 2012:7164592012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang WD, Yu YZ, Liu WW, Chen YH, Wang YP
and Huang TC: Effects of sodium tanshinone II-A sulfonate and
propranolol on coronary collaterals in acutely infarcted dogs
(author's transl). Zhongguo Yao Li Xue Bao. 2:29–33. 1981.In
Chinese. PubMed/NCBI
|
|
25
|
Li CZ, Yang SC and Zhao FD: Effects of
tanshinone II-A sulfonate on thrombus formation, platelet and blood
coagulation in rats and mice. Zhongguo Yao Li Xue Bao. 5:39–42.
1984.In Chinese. PubMed/NCBI
|
|
26
|
Fan GW, Gao XM, Wang H, Zhu Y, Zhang J, Hu
LM, Su YF, Kang LY and Zhang BL: The anti-inflammatory activities
of Tanshinone IIA, an active component of TCM, are mediated by
estrogen receptor activation and inhibition of iNOS. J Steroid
Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang YJ, Jin UH, Chang HW, Son JK, Lee SH,
Son KH, Chang YC, Lee YC and Kim CH: Inhibition of microsomal
triglyceride transfer protein expression and atherogenic risk
factor apolipoprotein B100 secretion by tanshinone IIA in HepG2
cells. Phytother Res. 22:1640–1645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adams JD, Wang R, Yang J and Lien EJ:
Preclinical and clinical examinations of Salvia miltiorrhiza and
its tanshinones in ischemic conditions. Chin Med. 1:32006.
View Article : Google Scholar
|
|
29
|
Hardt SE and Sadoshima J: Glycogen
synthase kinase-3beta: A novel regulator of cardiac hypertrophy and
development. Circ Res. 90:1055–1063. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho J, Rameshwar P and Sadoshima J:
Distinct roles of glycogen synthase kinase (GSK)-3alpha and
GSK-3beta in mediating cardiomyocyte differentiation in murine bone
marrow-derived mesenchymal stem cells. J Biol Chem.
284:36647–36658. 2009. View Article : Google Scholar : PubMed/NCBI
|