Introduction
Preeclampsia is a common complication during
pregnancy that can occur after 20 weeks of gestation. The clinical
manifestations of preeclampsia include hypertension, proteinuria
and edema. This complication triggers systemic disorders of
multiple organs, including the brain, eyes, liver and kidneys
(1), which greatly affect the
health of the mother and fetus. Preeclampsia can cause multisystem
damage, is closely associated with adverse pregnancy outcomes and
has a high morbidity of 5–7% (2).
According to the complex multifactorial pathogenesis
of preeclampsia, symptomatic treatment remains the principle
therapeutic strategy, rather than etiological treatment. Currently,
the most effective treatment remains pregnancy termination
(3). The pathological mechanism
involves insufficient vascular remodeling of the placental bed,
placental ischemia and anoxia, and secondary systemic endothelial
injury and/or activation (4). The
patient improves following delivery of the placenta, which may
indicate that detrimental factors generated in the placenta have an
important role in inducing the associated endothelial cell damage
(5). These detrimental factors
may directly or indirectly damage vascular epithelial cells and
cause organ disorders in the mother.
Endogenous digitalis-like factor (EDLF) is a
micromolecular polypeptide, most of which binds non-covalently with
plasma protein. It has digitalis-like activity, and inhibits the
specific binding of ATPase to its receptor, and also suppresses the
cell membrane sodium pump in cardiac tissue, the vascular wall, the
central nervous system, the renal tubular epithelium and others.
Thus, EDLF regulates water and salt metabolism, and vascular wall
tension, and thus has cardiac, natriuretic and vasoconstrictive
roles (6). EDLF is associated
with the development of various heart, vascular, kidney and liver
diseases (7). Organ damage in
preeclampsia predominantly involves the cardiovascular system,
liver and kidneys, indicating the potential role of EDLF in the
development of preeclampsia. EDLF biosynthesis is closely
associated with cholesterol side-chain cleavage in the metabolism
of progesterone and pregnenolone (8). Progesterone is a hormone produced by
the placenta during gestation. Another study reported that nuclear
factor-κB (NF-κB) is activated in the placenta in preeclampsia;
NF-κB participates in local inflammatory reactions in the placenta
and the development of preeclampsia (9).
Activated NF-κB participates in initiating and
regulating the transcription of multiple genes associated with
immunity, inflammation, apoptosis and cell proliferation (10,11). Cellular proliferation and
differentiation are involved in embryo implantation and development
(12). Multiple inflammatory
factors are involved in various physical and pathological
processes, including premature delivery and the initiation of
parturition (13). NF-κB p65, the
most explored subunit of NF-κB, has a more crucial role than the
other subunits. NF-κB p65 is involved in physical processes
including embryo implantation, pregnancy maintenance and the
initiation of parturition (14),
and the development and progression of preeclampsia (15–18). Nitric oxide (NO) is an effective
vasodilator that regulates angiostasis and tissue blood flow; in
addition, NO can regulate arterial blood pressure, maternal organ
perfusion, the fetal-placental vascular response and placental
blood flow (19). The widely
accepted pathogenesis of preeclampsia includes epithelial cell
damage, a subsequent decrease in NO production and several further
pathological changes. In the blood vessel endothelium, NO synthase
(NOS) is the rate-limiting enzyme in NO synthesis, and NOS
expression is dependent on NF-κB regulation (20). These data suggest that NF-κB
facilitates the important role of NO in preeclampsia by regulating
NOS.
Although EDLF is closely associated with vascular
epithelial damage and is a natural inhibitor of sodium-potassium
ATPase, it affects the blood vessel endothelium through other
pathways. Various effects of EDLF on blood vessel endothelial cells
were revealed by differences in protein expression, and certain
potential key target proteins were discovered. Annexin A2 is an
important target of EDLS (via alteration of the expression of
Annexin A2 to exert influence on the functions of Annexin A2);
Annexin A2 is closely associated with the blood vessel endothelium
(21). A study on the association
between the Annexin A family and preeclampsia demonstrated that
decreased Annexin A2 production may be associated with the
development of preeclampsia (22).
Based on the aforementioned data, we hypothesize
that EDLF may be a detrimental factor originating from the placenta
that contributes to the development of preeclampsia. Furthermore,
by regulating EDLF expression in hypoxic trophocytes, NF-κB p65 may
affect the production of Annexin A2 (an EDLF target protein), thus
decreasing NO production and resulting in the development of
preeclampsia. This is a potential mechanism underlying vascular
endothelial cell damage in preeclampsia. To evaluate this
hypothesis, hypoxic trophocytes and human umbilical vein
endothelial cells (HUVECs) were co-cultured, and changes in EDLF
production by hypoxic trophocytes and the influence of EDLF on
HUVEC functions were determined. Subsequently, the potential
mechanism underlying the effect of EDLF generated by hypoxic
trophocytes on the biofunctions of HUVECs was explored.
Materials and methods
Patients
Among the singleton pregnancy outpatient cases in
the Second Xiangya Hospital of Central South University (Changsha,
China) from July 2014 to July 2015, 60 cases of severe preeclampsia
(age, 33.73±5.49 years) were randomly selected and included in the
severe preeclampsia group. The diagnostic criteria were based on
those reported in the literature (23): i) Systolic pressure ≥160 mmHg
and/or diastolic pressure ≥110 mmHg; ii) proteinuria ≥5,000 mg/24 h
or random urine protein (+++); iii) persistent headache, vision
disorder or other cranial nerve symptoms; iv) persistent epigastric
pain, subcapsular hematoma of the liver or hepatorrhexis symptoms;
v) hepatosis (increased serum ALT or AST); vi) abnormal renal
function (oliguria: 24 h urine volume <400 ml or urine volume
per hour <17 ml; or serum creatinine >106 ng/l); vii)
hypoproteinemia, hydrothorax or seroperitoneum; viii) blood system
disorders: microangiopathy hemolysis or blood platelet count
<100×109/l; ix) cardiac failure and pulmonary edema; x) fetal
growth restriction or oligohydramnios; or xi) early-onset
preeclampsia before 34 weeks of pregnancy. The exclusion criteria
included the following: i) Chronic hypertension; severe diseases of
the heart, lungs, kidneys, liver, or thyroid, or other endocrine
diseases experienced before pregnancy; ii) obstetric complications,
including placental abruption, gestational diabetes mellitus,
premature rupture of membranes, maternal-fetal blood group
incompatibility, or placenta previa; iii) the use of assisted
reproductive technology; and iv) administration of abortion
prevention medicine.
The 60 singleton pregnancy cases with a prenatal
examination or inpatient delivery and normal blood pressure during
the same period were included in the control group (age, 32.73±4.36
years). Patient information, such as age, blood pressure, height,
weight, gestational weeks, and parity and gravidity, was collected
and recorded. Peripheral venous blood samples were centrifuged to
collect sera. EDLF concentrations were determined using EDLF
radioimmunoassay kit (cat. no. FM0063; Beijing North Institute of
Biological Technology, Beijing, China; used in the in vitro
experiments as well). As numerous factors can influence the outcome
of this radioimmunoassay (such as cross reactivity with digoxin and
ouabain and experimental conditions) (24–26), samples were collected strictly
according to the inclusion criteria, the blood collection time and
conditions were highly standardized, and the experimental
procedures (such as incubation and centrifugation conditions) were
performed in strict accordance with the instructions provided with
the kit.
This study was approved by the Ethics Committee of
the Second Xiangya Hospital of Central South University. Informed
consent was obtained from all participants.
Experimental cells
Human BeWo placental choriocarcinoma trophocyte
cells (Shanghai Sixin Biotechnology, Ltd., Shanghai, China) were
used in this study (27). HUVECs
were purchased from the Physical Laboratory of Xiangya School of
Medicine, South Central University.
Trophocyte and HUVEC cultures
HUVECs were cultured in Dulbecco's modified Eagle's
medium (Hyclone, Logan, UT, USA), and BeWo cells were cultured in
F12K medium (Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA); both cultures were supplemented with fetal
bovine serum (FBS; Gibco, Invitrogen, Grand Island, NY, USA) and
incubated in saturated humidity and 5% CO2 at 37°C.
Culture media were changed every 2 days. Cells were passaged with
0.25% trypsin upon reaching 80% confluence.
HUVEC confluence
HUVECs in the logarithmic growth phase were diluted
to a concentration of 1×105/l, seeded into 5 wells of a
12-well plate at 1 ml/well and incubated in saturated humidity and
5% CO2 at 37°C. Cell growth and confluence were observed
regularly and frequently by light microscopy (BX53; Olympus
Corporation, Tokyo, Japan). The basic features of healthy cells
included the following: Proper transparency, low intracytoplasmic
granular substance level, small cytoplasmic volume and no
hypertrophy. In addition, the healthy cells adhered well and grew
evenly, with no or few cells floating in the culture medium.
Healthy cells were co-cultured with hypoxic trophocytes.
Treatment of BeWo trophocytes with
hypoxia
Following trypsinization (0.25%), centrifugation (at
350 x g at room temperature for 5 min) and resuspension (in
complete medium containing 10% FBS), BeWo cells in the logarithmic
growth phase were diluted to a concentration of 4×105/l,
seeded into 5 wells of a 12-well plate at 1 ml per well and
cultured overnight for adherence in saturated humidity and 5%
CO2 at 37°C. A sterile stock solution of
CoCl2 (1 mol/l) was prepared and diluted in complete
culture medium to a concentration of 10 mmol/l to form the working
solution. The medium was replaced with 1 ml of fresh medium. Then,
30 µl of the working CoCl2 solution was added for
a final concentration of 300 µmol/l. For the hypoxia
treatment, these cells were incubated in saturated humidity and 5%
CO2 at 37°C for 24 h.
Transfection of hypoxic trophocytes with
the NF-κB plasmid
Hypoxia-treated BeWo cells were divided into 3
groups, and culture media were replaced with antibiotic-free F12K
medium (2 ml/bottle). The NF-κBsi-2 plasmid (Shanghai GeneChem Co.,
Ltd., Shanghai, China) was transfected into these cells as follows.
NF-κBsi-2 (20 µl) was added to a sterile Eppendorf tube, and
Lipofectamine® 2000 transfection reagent (5 µl;
GenStar, Beijing, China) was added to another sterile Eppendorf
tube. The sequences of si-1, si-2 and si-3 were
CCGGACUUGUUUACAAAGU, CUACUGUAGGUCUAAGCUA, and UCUACUGUAGGUCUAAGCU,
respectively. Serum-free F12 medium (200 µl) was added to
these tubes, which were then allowed to stand for 5 min at 26°C.
Following gentle mixing, the tubes were allowed to stand for
another 20 min. The mixtures were then added to culture medium, and
the final concentrations were adjusted to 100 pmol/ml.
Untransfected cells were used as the mock control. Scramble control
was used (ACGUGACACGUUCGGUATT). siRNA naked fragment
(FTIC-conjugated) conferred fluorescence to the cells.
Co-culture of transfected trophocytes and
HUVECs
HUVECs were placed in the upper chamber of a
co-culture chamber (co-culture for 48 h), and NF-κBsi-2-transfected
trophocytes were placed in the lower chamber; these cells were
co-cultured for 24 h. Then, the culture medium in both chambers was
refreshed, and fluorescein isothiocyanate (FITC)-labeled glucan (10
ng/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to
the upper chamber. The cultures were incubated for an additional
12, 24, 48, 72, 96 or 120 h. Co-cultures of HUVECs and mock control
trophocytes were prepared, cultured and analyzed in parallel.
Evaluation of HUVEC barrier function
The culture supernatant in the lower chamber was
harvested (500 µl) at the designated time-points, and a
fluorospectrophotometer (excitation wavelength, 488 nm;
sensitivity, 50) was used to determine the fluorescence intensity
of FITC. All the opera- tions were performed in the dark.
MTT assay to evaluate HUVEC
proliferation
HUVEC proliferation was evaluated using MTT assays,
which were performed in accordance with the manufacturer's
instructions (Sigma-Aldrich; Merck KGaA). The endothelial cells in
the upper chamber were treated with trypsin (0.25%), resuspended,
seeded into a 96-well plate at a concentration of 1×104
cells/well and incubated for 48 h. Then, 20 µl MTT solution
(5 mg/ml) was added to each well, and the medium was discarded
after 4 h of incubation. Subsequently, dimethyl sulfoxide (150
µl) was added, and after 10 min of incubation at room
temperature, 100 µl from each well was transferred into the
coated wells of a 96-well plate. Absorbance values were determined
at a wavelength of 490 nm measured using a 960PRO
fluorospectrophotometer (INESA; Shanghai, China; http://en.inesa-instrument.com/index.html). The other
groups were analyzed via the procedures described above.
Nitrate reductase activity assay to
determine the NO concentration
The NO level in the HUVEC culture supernatant was
detected using nitrate reductase activity assays (NO detection kit;
cat. no. A012; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). Endothelial cell culture supernatants from the upper
chambers were harvested at the designated time-points (500
µl/group), and the reagents were mixed well with the
specimens according to the manufacturer's instructions (Nanjing
Jiancheng Bioengineering Institute) and incubated at room
temperature for 10 min. Then, the absorbance values were determined
(550 nm wavelength and 0.5 cm optical path) and normalized to that
of double-distilled water as the blank.
Western blot analysis of Annexin A2
expression in HUVECs
Cells were suspended (20 µl) and treated with
radioimmunoprecipitation lysis buffer (100 µl; Beyotime,
Shanghai, China) containing 1 µl each of
phenylmethylsulfonyl fluoride and protease and protein tyrosine
phosphatase inhibitors. Then, the cells were incubated on ice for
30 min and centrifuged at 350 x g at 4°C for 5 min. The supernatant
was harvested and stored at −20°C; the Laemmli system was used. The
concentrations of the spacer and separation gels were 5 and 12%,
respectively. The prepared separation gel was gently infused into
the space between the fixed double-paned glass, and isopropanol was
used to seal the top; the gels were allowed to set for 30 min at
room temperature. Protein levels were determined using a
bicinchoninic acid protein determination kit (Wellbio, Changsha,
China). A total of 25 µg protein was loaded per lane, with
15% gel. NC membranes were used for protein transfer. TBST
containing 5% skim milk was used for blocking. After immersing, the
membranes were placed at room temperature for 1 h. The following
procedures included primary antibody incubation, secondary antibody
incubation, development (or staining) and exposure (28). The primary antibodies were as
follows: NF-κB (cat. no. 6956S; source, mouse; dilution, 1,000;
molecular weight, 65 kDa; Cell Signaling Technology, Beverly, MA,
USA), Annexin A2 (cat. no. 11256-1-AP; source, rabbit; dilution,
1,000; molecular weight, 36 kDa; Proteintech, Rosemont, IL, USA),
Actin (cat. no. 60008-1-Ig; source, mouse; dilution, 4,000;
molecular weight, 42 kDa; Proteintech). The membranes were
incubated with the primarily antibodies at 4°C overnight. After
incubation, they were washed thrice with TBS-T, for 15 min per
wash. HRP-conjugated goat anti-rabbit IgG was used as the secondary
antibody (dilution, 1: 3,000; Proteintech) (incubation for 45–60
min). The samples were washed thrice, for 15 min per wash. The
exposure was performed using superECL Plus (Thermo Pierce,
Rockford, IL, USA). Western blot analysis was performed using
Quantity One software (4.6.2; Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In addition, Annexin a2mRNA was detected using a
reverse transcription kit [Fermentas (Thermo Fisher Scientific,
Inc., Pittsburgh, PA, USA)]. Annexin A2 mRNA levels in HUVECs were
determined by RT-qPCR. Total RNA was extracted with TRIzol reagent
(Life Technologies; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. The purity and concentration of
total RNA were determined by ultraviolet spectrophotometry. Actin
was used as a normalization control. Reverse transcription was
performed with a kit from Fermentas. Primers were designed with
Primer 5.0 software (PREMIER Biosoft, Vancouver, Canada) and
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The
extension length of the Annexin A2 primers (forward,
5′-GCCCTGGCAAAGGGTAGA-3′ and reverse, 5′-ACGCTCCGCTCGGTCAT-3′)
generated a product of 149 bp. The actin primers (forward,
5′-CATCCTGCGTCTGGACCTGG-3′ and reverse 5′-TAATGTCACGCACGATTTCC-3′)
generated a product of 107 bp. One sample was analyzed in three
parallel experimental wells. The total reaction system of 30
µl contained RT product as a template (1 µl), forward
primer (10 µM, 0.5 µl), reverse primer (10 µM,
0.5 µl), PCR-grade H2O (13 µl) and 2X
SYBR-Green PCR Master Mix (15 µl; Applied Biosystems, Foster
City, CA, USA). The qPCR amplification conditions were as follows:
95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C
for 50 sec. The melting curves were collected at 60–95°C. The qPCR
(5 µl) products were analyzed via 2% agarose gel
electrophoresis. Annexin A2 mRNA was detected with a Gel Doc XR
gel-imaging system (Bio-Rad Laboratories, Inc.). Data were analyzed
using the qPCR analytical tool, and quantification was performed
using the 2−ΔΔCt method.
Statistical analysis
The data were analyzed using SPSS 19.0, and data are
presented as the mean ± standard deviation. Normality tests were
performed with the Kolmogorov-Smirnov and Shapiro-Wilk tests. The
homogeneity of the variance between groups was assessed via
Levene's method. The inter-group differences of paired data were
analyzed using the t-test. Pairwise comparisons of differences
among multiple groups were performed using the
Student-Newman-Keuls-q test. P<0.05 was considered to indicate a
statistically significant difference.
Results
EDLF levels
Serum EDLF levels in the severe preeclampsia
patients and the normal pregnancy controls are presented in
Table I. No significant
differences were identified in gestational weeks, age, weight,
height, or parity and gravidity between the two groups (P>0.05).
Serum EDLF levels were significantly higher in severe preeclampsia
cases compared with normal pregnancy cases (P<0.05).
 | Table IComparison of serum EDLS levels
between the two groups. |
Table I
Comparison of serum EDLS levels
between the two groups.
| Group | Serum EDLS
(ng/l) | P-valuea |
|---|
| Control | 66.49±10.36 | P<0.01 |
| Severe
preeclampsia | 125.09±14.33 | |
HUVEC morphology
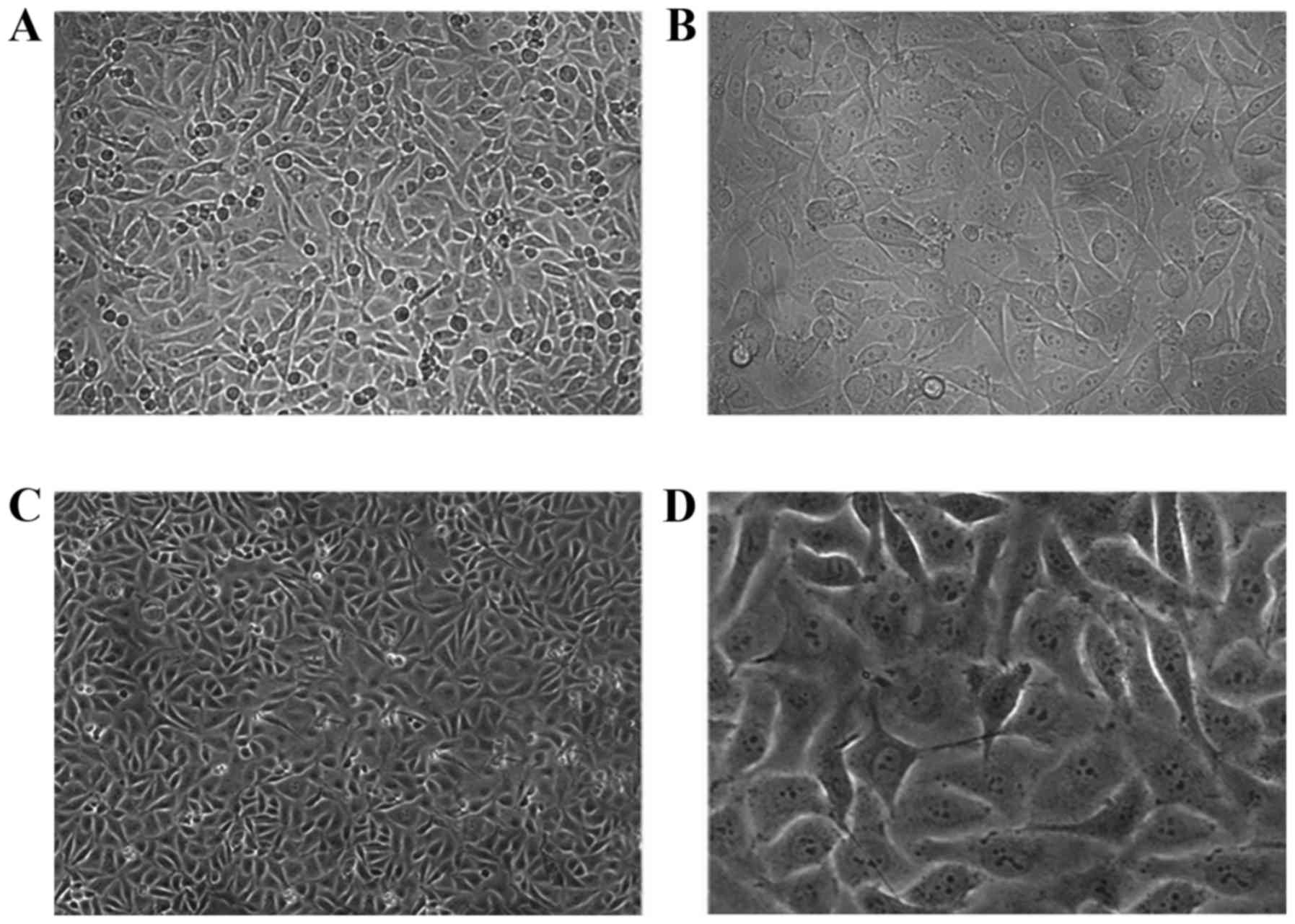
HUVEC morphology was observed by light microscopy
(Fig. 1). The cells grew well to
confluency and exhibited a cobblestone-like arrangement, sharp
edges, abundant cytoplasm, elliptical shape, centralized nuclei and
clear nucleoli.
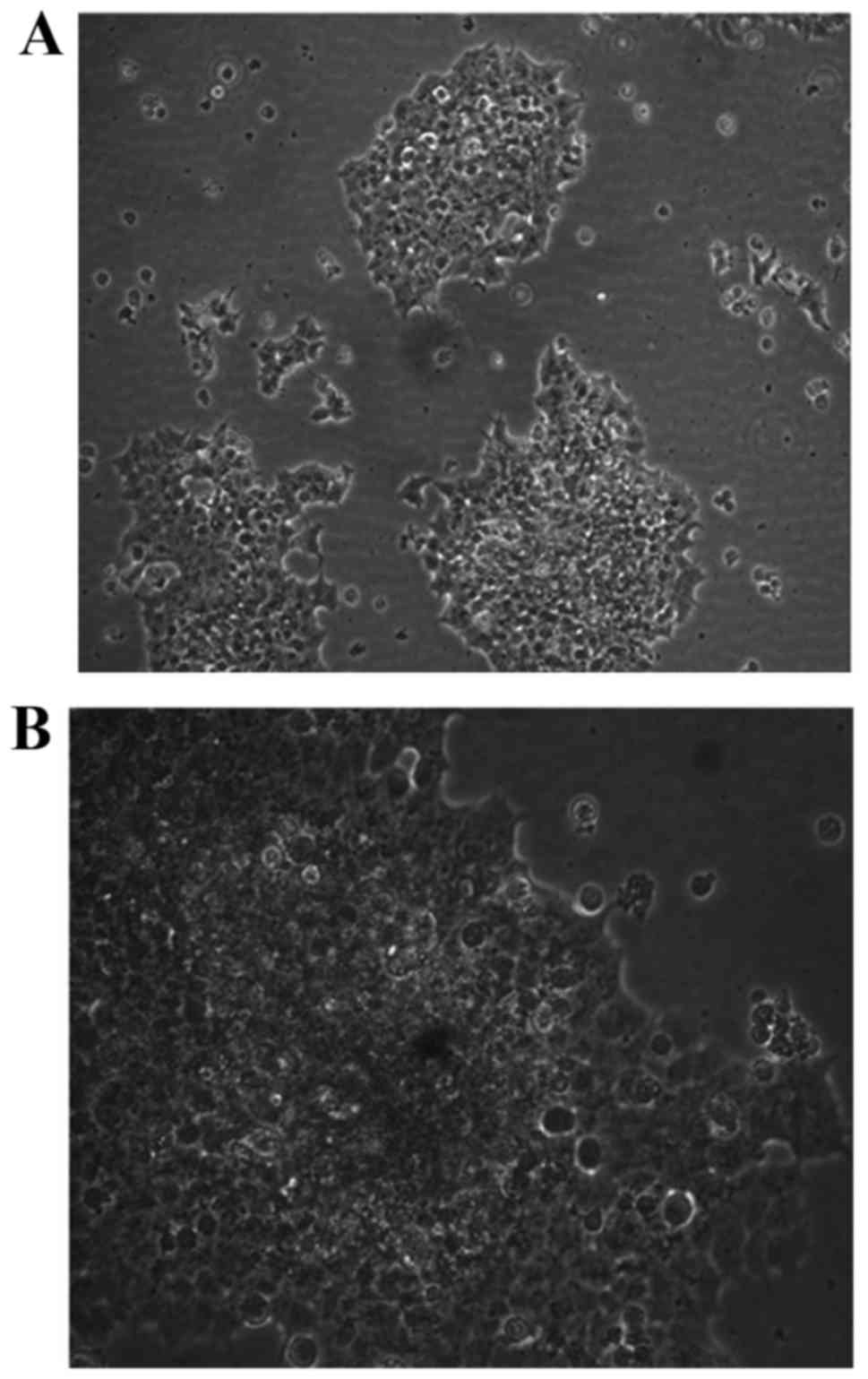
Morphological changes in trophocytes
following transfection
BeWo cells exhibiting the typical characteristics
were passaged after 3 days of culture and observed by light
microscopy after an additional 2 days (Fig. 2). The cells showed healthy and
compact growth, and had formed multiple layers.
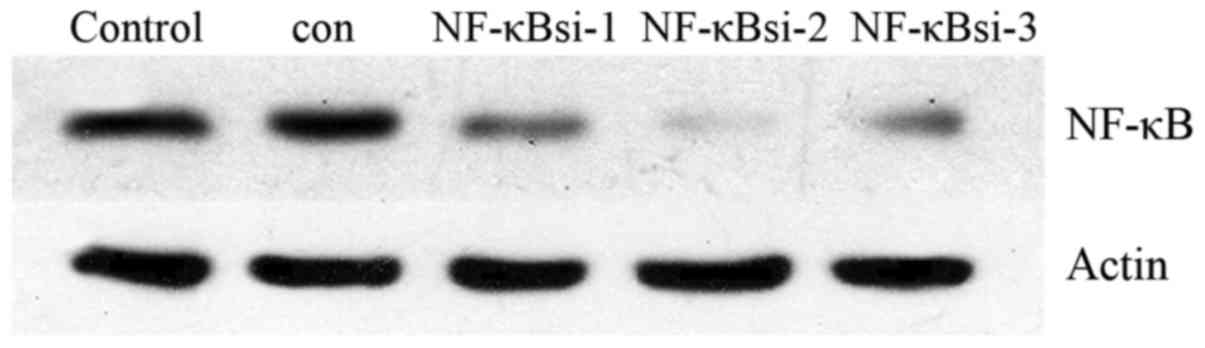
Silencing efficiency of NF-κB siRNA
The gene silencing efficiency of NF-κB siRNA was
evaluated by western blot analysis (Fig. 3 and Table II). NF-κBsi-2 exerted the best
gene-silencing effect and was therefore used in the subsequent
experiments.
 | Table IIGene silencing by NF-κBsi as
determined by western blot analysis. |
Table II
Gene silencing by NF-κBsi as
determined by western blot analysis.
| Mock | Control | NF-κBsi-1 | NF-κBsi-2 | NF-κBsi-3 |
|---|
| NF-κB | 67.68 | 71.52 | 43.91 | 20.9 | 35.5 |
| Actin | 104.97 | 102.43 | 102.54 | 112.02 | 98.94 |
| N/actin | 0.645 | 0.698 | 0.428 | 0.187 | 0.359 |
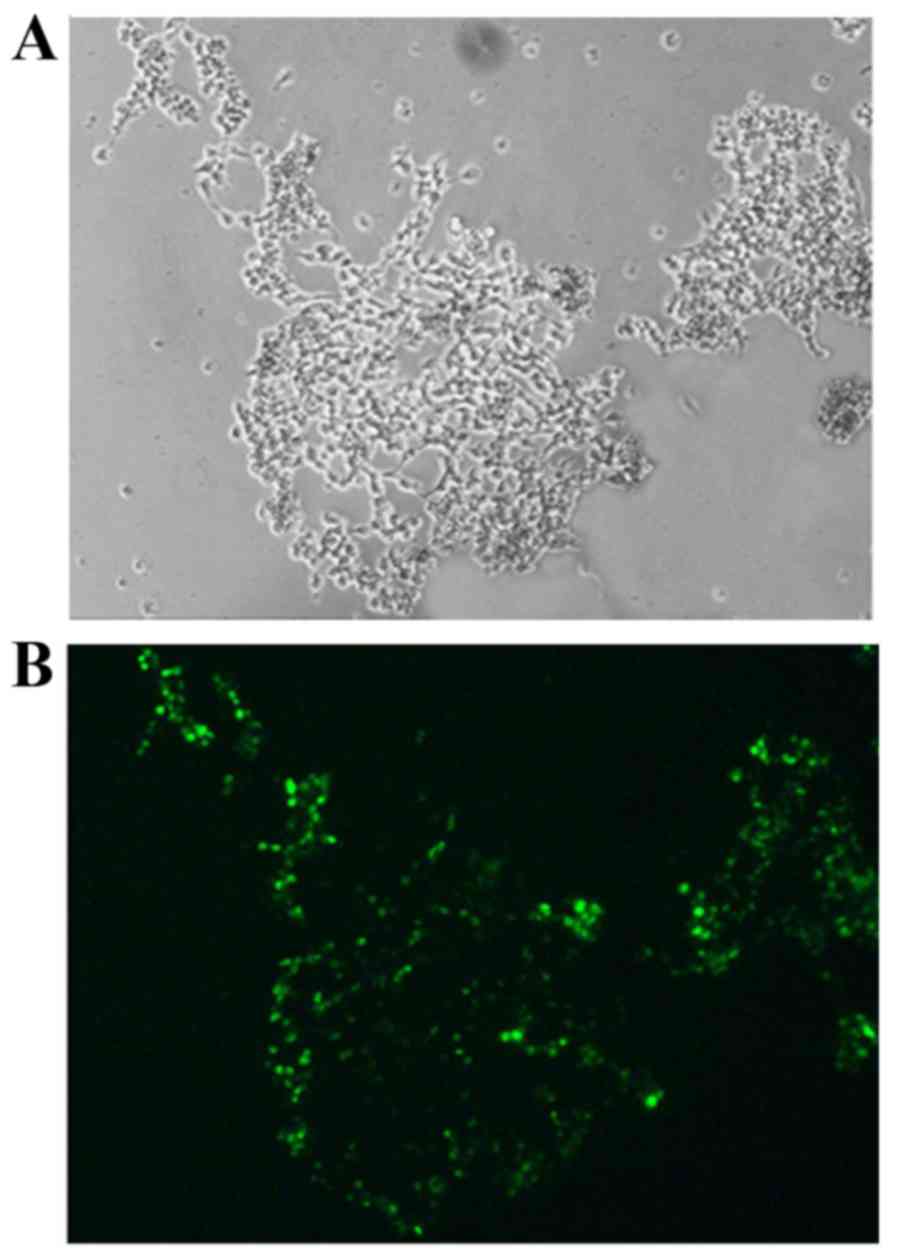
Morphology of trophocytes following
transfection
Fluorescently-labeled siRNA-transfected trophocytes
were observed by fluorescence microscopy. Green fluorescence was
successfully expressed in the trophocytes (Fig. 4).
EDLF levels in hypoxic trophocytes at
different time points following NF-κB p65 gene silencing
The culture supernatant of hypoxic trophocytes was
harvested at different time points, and EDLF concentration was
determined by radioimmunoassay. EDLF expression levels were
increased over time in untransfected hypoxic trophocytes
co-cultured with HUVECs. However, in the cells transfected with
NF-κBsi-2 the cells exhibited no significant changes in EDLF
expression over time under the same conditions (Table III).
 | Table IIIComparison of EDLS concentration
expression in hypoxic trophocytes. |
Table III
Comparison of EDLS concentration
expression in hypoxic trophocytes.
| Co-culture
time | EDLS (ng/l)
|
|---|
| Untransfected | NF-κBsi-2 |
|---|
| 12 h | 1.4657±0.069 | 1.4829±0.05730 |
| 24 h | 1.8817±0.0084 |
1.5234±0.05945a |
| 48 h | 2.5149±0.0503 |
1.6196±0.06512b |
| 72 h | 2.8244±0.0445 |
1.6501±0.04676b |
| 96 h | 3.0932±0.0925 |
1.7215±0.09365b |
| 120 h | 3.1506±0.1297 |
1.8380±0.10294b |
Effect of NF-κB p65 silencing in hypoxic
trophocytes on HUVEC functions
Proliferative activity
The proliferation of HUVECs co-cultured with hypoxic
trophocytes changed according to the co-culture time (Table IV). Based on the MTT assay
results, HUVEC proliferation gradually decreased with increasing
time of co-culture with untransfected trophocytes. After
transfection, the proliferation of HUVECs co-cultured with
transfected trophocytes gradually increased, exhibiting significant
differences after 24 h (P<0.05) and 48 h (P<0.01).
 | Table IVChanges in proliferative activity
hypoxic trophocytes. |
Table IV
Changes in proliferative activity
hypoxic trophocytes.
| Co-culture
time | Optical density
|
|---|
| Untransfected | NF-κBsi-2 |
|---|
| 12 h | 0.7748±0.01431 | 0.7888±0.00258 |
| 24 h | 0.7288±0.02148 |
0.8025±0.01108a |
| 48 h | 0.6688±0.01872 |
0.8397±0.02298b |
| 72 h | 0.6168±0.02492 |
0.8514±0.02231b |
| 96 h | 0.5243±0.36065 |
0.8636±0.02731b |
| 120 h | 0.4517±0.01589 |
0.9035±0.00714b |
Barrier function
The barrier function of HUVECs co-cultured with
hypoxic trophocytes changed over time (Table V). FITC-conjugated glucan was
applied to the co-culture with untransfected hypoxic trophocytes
and gradually moved through the HUVEC monolayer from the upper
chamber into the lower chamber, which increased the concentration
of FITC-glucan in the lower chamber over time, and provided an
indicator of the HUVEC barrier function. However, in the culture
using NF-κBsi-2 trophocytes, the amount of FITC-glucan passing
through the HUVEC monolayer decreased gradually, and the barrier
function damage was relatively improved by 24 h of culture
(P<0.05) compared with the untransfected cells at 24 h;
significant differences were also observed after 48 h of co-culture
(P<0.01).
 | Table VChanges in human umbilical vein
endothelial cell barrier function post-transfection. |
Table V
Changes in human umbilical vein
endothelial cell barrier function post-transfection.
| Co-culture
time | Fluorescence
|
|---|
| Untransfected | NF-κBsi-2 |
|---|
| 12 h |
12.6410±0.06003 |
12.0867±0.06027 |
| 24 h |
13.7967±0.56400 |
11.5367±0.43004a |
| 48 h |
15.5517±0.16918 |
8.6600±0.39737b |
| 72 h |
16.3620±0.47820 |
7.5000±0.63379b |
| 96 h |
17.6407±0.51832 |
5.8500±0.6486b |
| 120 h |
18.6810±0.63612 |
4.6867±0.55519b |
NO level
Changes were observed in NO production by HUVECs
co-cultured with hypoxic trophocytes (Table VI). In HUVECs cultured with
untransfected trophocytes, the relative NO level gradually
decreased with increasing co-culture time. However, in HUVECs
cultured with NF-κBsi-2 trophocytes, the relative NO production
increased gradually, and was increased compared with the
untransfected group by 24 h (P<0.05), and significant
differences were also observed after 48 h (P<0.01).
 | Table VIRelative nitric oxide levels in human
umbilical vein endothelial cells. |
Table VI
Relative nitric oxide levels in human
umbilical vein endothelial cells.
| Co-culture
time | Optical density
|
|---|
| Untransfected | NF-κBsi-2 |
|---|
| 12 h |
0.77483±0.01431 | 0.7888±0.00026 |
| 24 h | 0.7288±0.02148 |
0.8025±0.01108a |
| 48 h | 0.6462±0.05151 |
0.8397±0.02298b |
| 72 h | 0.6168±0.02492 |
0.8514±0.02231b |
| 96 h |
0.5243±0.036065 |
0.8636±0.02731b |
| 120 h | 0.4517±0.01589 |
0.9035±0.00714b |
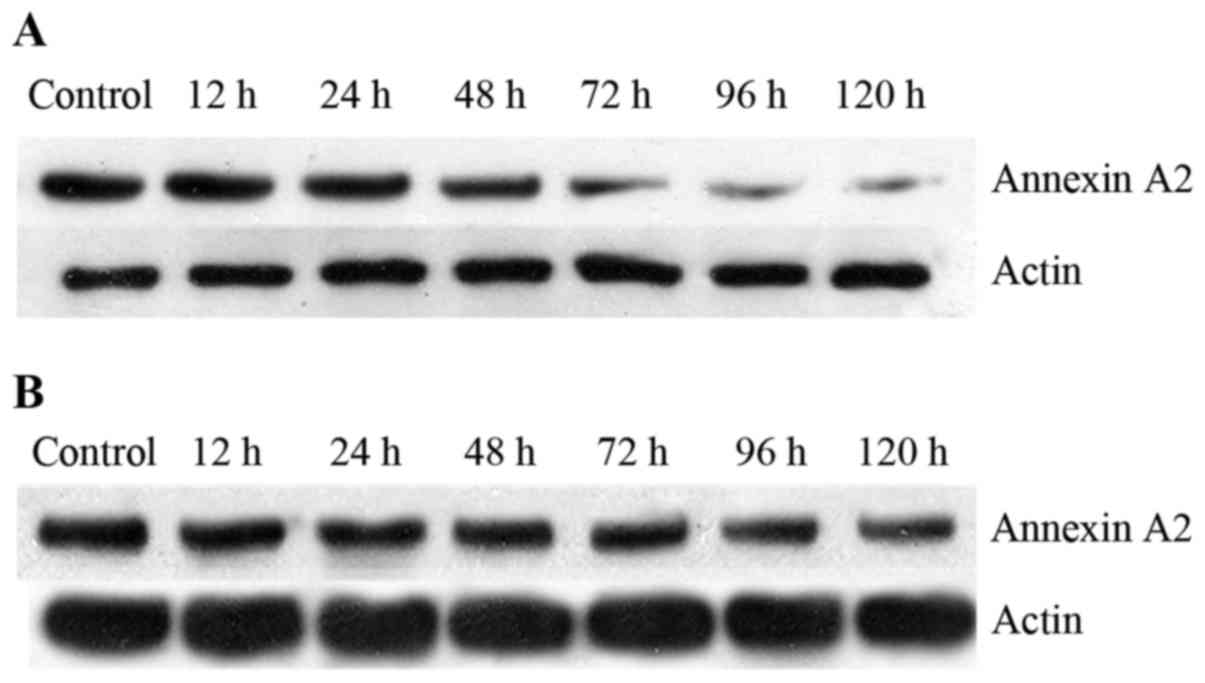
Annexin A2 expression in co-cultured
HUVECs
Annexin A2 expression in HUVECs was determined by
western blot analysis (Fig. 5;
Table VII). In the
untransfected group, Annexin A2 protein expression in co-cultured
HUVECs decreased over time. Following NF-κBsi-2 transfection of the
trophocytes, Annexin A2 levels in HUVECs were increased, beginning
at 24 h, and the differences between the untransfected and
NF-κBsi-2 groups became significant at 72 h (P<0.01).
 | Table VIIRelative Annexin A2 protein
expression levels in human umbilical vein endothelial cells. |
Table VII
Relative Annexin A2 protein
expression levels in human umbilical vein endothelial cells.
| Co-culture
time | Annexin A2
expression
|
|---|
| Untransfected | NF-κBsi-2 |
|---|
| 12 h | 0.5107±0.00856 | 0.5210±0.00897 |
| 24 h | 0.4727±0.00715 |
0.5633±0.00770a |
| 48 h | 0.4180±0.00608 |
0.5855±0.00806a |
| 72 h | 0.3892±0.01639 |
0.5977±0.00190b |
| 96 h | 0.3570±0.01949 |
0.6003±0.01961b |
| 120 h |
0.31176±0.01071 |
0.6257±0.00577b |
Relative Annexin A2 mRNA expression
levels in HUVECs
HUVECs were harvested prior to trophocyte
transfection and following transfection with NF-κBsi-2, and total
RNA was extracted and transcribed into cDNA. Relative Annexin A2
mRNA levels were detected via qPCR, and actin was used for data
normalization (Table VIII).
Annexin A2 mRNA expression in HUVECs decreased gradually over time
when co-cultured with untransfected trophocytes. After 24 h of
co-culture with NF-κBsi-2-transfected trophocytes, Annexin A2 mRNA
levels in HUVECs increased over time compared with the
untransfected group (P<0.05).
 | Table VIIIRelative Annexin A2 mRNA expression
levels in human umbilical vein endothelial cells. |
Table VIII
Relative Annexin A2 mRNA expression
levels in human umbilical vein endothelial cells.
| Co-culture
time | Relative Annexin A2
mRNA
|
|---|
| Untransfected | NF-κBsi-2 |
|---|
| 12 h | 0.4887±0.01553 | 0.5107±0.01723 |
| 24 h | 0.4405±0.01937 |
0.5477±0.00833a |
| 48 h | 0.4201±0.1339 |
0.5503±0.01553a |
| 72 h | 0.3853±0.01079 |
0.5724±0.01716b |
| 96 h | 0.3774±0.01495 |
0.6202±0.02061b |
| 120 h | 0.2953±0.01151 |
0.6645±0.00930b |
Discussion
Preeclampsia is one of the major causes of mortality
in pregnant women and perinatal infants. The fundamental
pathological changes include systemic damage to vascular
endothelial cells, inflammation-associated systemic small vessel
spasms and a hypercoagulable state. These changes can cause various
clinical manifestations, including hypertension, edema and
proteinuria, and subsequent severe ischemia in vital organs,
including the heart, brain, liver and kidneys. Furthermore, in
severe cases, this condition can result in sequelae or mortality in
the mother or infant. Although numerous research efforts have been
directed toward investigating this condition, its pathogenesis
remains unclear (29,30). Therefore, pregnancy termination is
often considered the only feasible strategy because more efficient
treatments have yet to be developed. However, terminating the
pregnancy is not always an acceptable solution. Due to the high
mortality and disability rates in severe preeclampsia cases, early
screening, prevention and diagnosis have more important roles than
active treatment.
The findings of the current study indicated that
serum EDLF levels were significantly higher in patients with severe
preeclampsia patients compared with normal pregnant women, which
indicated a potential association between EDLF and preeclampsia
development. Subsequently, a Transwell system was used to
co-culture hypoxic trophocytes and HUVECs for 12, 24, 48, 72, 96 or
120 h to detect changes in EDLF protein levels. The results
demonstrated that EDLF increased with increasing duration of
co-culture with hypoxic cells, accompanied by a decrease in free
NO. In addition, HUVEC proliferation and cellular monolayer barrier
function, as well as NO production by HUVECs, changed markedly
during co-culture with hypoxic trophocytes, indicating the
potential for cytokine-mediated signaling between trophocytes and
endothelial cells and corresponding effects on endothelial cell
biofunctions. Using RNAi technology, NF-κB p65 gene expression was
silenced in hypoxic trophocytes, which downregulated the expression
of EDLF. This led to upregulation of Annexin A2, an important EDLF
target protein (38), and a
decrease in HUVEC damage. Therefore, it is inferred that EDLF is a
toxic factor originating from the placenta in patients with severe
preeclampsia. NF-κB p65 may regulate EDLF expression in hypoxic
trophocytes, thus influencing Annexin A2 expression and subsequent
NO production. These events cause endothelial cell damage and the
development of preeclampsia.
In patients with severe preeclampsia, hypoxic
trophocytes produce high levels of EDLF, which is regulated by
NF-κB p65, to elicit cytokine secretion by endothelial cells, which
affects vasomotion and cell proliferation (53). Various endothelial cell functions
are realized through the balance of NO and endothelin (ET)
(20). A decrease in NO
production and release may evoke an imbalance in vascular factors,
with subsequent arteriole spasms and increased peripheral
resistance, particularly in the kidneys, uterus and placenta, which
promotes the development of hypertension and preeclampsia (31). A previous study reported that EDLF
levels are significantly higher in newborn umbilical cord blood
than in the peripheral blood of pregnant women (32). In addition, the previous study
reported that EDLF induces the expression and release of ET-1 to
initiate vasoconstriction; this upregulated the sensitivity of the
vasculature to endogenous vasopressin and downregulated the
relaxant effects of NO, prostacyclin and acetylcholine, which may
result in disordered vasodilatation and vasoconstriction during the
development of preeclampsia (32). As a physiological stress,
pregnancy creates tension in sympathetic nerves, which promotes the
development of preeclampsia through exciting sympathetic nerves and
eliciting a series of physiological responses, such as
vasoconstriction and water and sodium retention. A previous study
reported that EDLF evokes a group of sympathetic nerve excitation
reactions via the release of peripheral angiotensin II (Ang II),
noradrenaline and hypophysin (33), which was consistent with our
previous results, namely, that Ang II has an important role in the
development of preeclampsia (34). An intraperitoneal injection of
EDLF increased the blood pressure of normal female rats as a
consequence of upregulated ET levels in the serum and heart tissue
(35). This result indicated that
changes in the ET system may be one of the mechanisms by which EDLF
causes hypertension and target organ damage (35). In another study, a NOS inhibitor
effectively enhanced α-adrenergic vasoconstriction, and EDLF
enhanced the NOS inhibition, which indicated that EDLF increases
the sensitivity to endogenous α-adrenaline as the major mechanism
of EDLF-induced vasoconstriction (36,37).
NF-κB is an essential regulator of endothelial NOS
(eNOS) (38), a rate-limiting
enzyme in NO synthesis that is intricately involved in
physiological activities in the cardiovascular system. NO is a
molecular, chemical signaling moiety that is essential for life; it
has a simple structure and an extremely unstable nature, and it is
produced and secreted by vascular endothelial cells and acts in a
paracrine manner. Thus, NO acts on adjacent smooth muscle cells.
These actions consequently increase the intracellular levels of
cyclic guanosine monophosphate and cause the efflux of calcium, or
promote the conversion of free calcium to bound calcium, resulting
in smooth muscle relaxation and hemangiectasis (39). This is important for maintaining
angiostasis and stable blood pressure. Low NO levels can cause
hypertension and ischemia, resulting in damage and functional
disorders in endothelial cells. Blocking NO synthesis with a NOS
inhibitor in pregnant rats caused eclampsia-like symptoms (40), including hypertension,
proteinuria, intrauterine growth retardation and even a high
incidence of stillbirths and malformations. Thus, certain
researchers recommended NO levels as a predictive index of vascular
endothelial cell damage and preeclampsia (41). NO leads to hemangiectasis,
inhibits the adhesion and aggregation of platelets, and blocks
platelet mitosis and vascular smooth muscle cell proliferation
(42). Decreased NO concentration
and/or activity is considered to be a marker of endothelial damage
and/or activation (43). The
activity of eNOS was reported to be decreased in preeclampsia cases
(44). In another animal
experiment, preeclampsia-like clinical manifestations were observed
in pregnant mice in which NOS activity was inhibited using drugs
(45). Pregnant eNOS-knockout
mice exhibited decreased blood flow in the uterus, shortened spiral
artery extension and insufficient placental oxygen supply (46). The theory of imbalanced
prostacyclin and thromboxane does not account for vascular
disorders in pregnant women. In a recent study, a series of
vascular endothelial cell-produced factors, including NO and ET,
were demonstrated to have the most important roles in regulating
vascular functions in pregnancy (47). Multiple studies have indicated
that NO is an important factor that regulates hemodynamics to adapt
to pregnancy, ensuring sufficient placental blood supply and fetal
nutrition, and oxygen supply (39,48). Furthermore, disorders of vascular
endothelial functions, changes in ET-1 and NO, and an imbalance in
vasoconstrictors and vasodilators are key factors underlying the
pathophysiological changes in preeclampsia (31,49). Various endothelial cell functions
are realized through the balanced secretion of NO and ET. A
previous study (50) reported
that endogenous ouabain, an EDLF, can inhibit the reverse flow of
sodium and calcium in cells, which decreases the outflow of
Ca2+ and increases its inflow. Consequently, ouabain
increases the amount of Ca2+ available to smooth muscle
cells and myocardial cells, thus elevating blood pressure and
aggravating diseases. In pregnant women, high ouabain concentration
can cause vasoconstriction and retention of water and sodium by
increasing the excitation of sympathetic nerves; these humoral
regulatory mechanisms, in combination with a series of signal
transduction pathways, regulate the levels of vasodilators and
vasoconstrictors in vivo. This phenomenon leads to an
imbalance among vascular factors and the formation of a
nervous-humoral regulatory network with ET, NO and
rennin-angiotensin-aldosterone, which are involved in the
development of preeclampsia (51,52).
Annexin A2 is an important functional target protein
of EDLF (21). Endothelial cells
that express Annexin A2 may serve as receptors for fibrinolysin,
zymogens and tissue-type plasminogen activator (t-PA). Annexin A2,
as a t-PA cofactor, has an important role in the generation of
plasmin, the release of endothelial adhesion molecules, and the
migration and adhesion of endothelial cells (53,54).
In preeclampsia cases, vascular endothelial cells,
vascular smooth muscle cells and white blood cells are activated
via the NF-κB pathway. Activated NF-κB can upregulate the
expression of various genes, including those for epidermal growth
factor and platelet-derived growth factor. These events are often
followed by vascular smooth muscle cell proliferation,
intramembrane signaling for vascular remodeling, and subsequent
increases in the level of collagenous fibers, the thickness of
vessel walls and the mean pressure in arterioles. These changes
subsequently elicit the clinical manifestations of hypertensive
disorders complicating pregnancy, including hypertension,
proteinuria and edema (18,55).
In the current study, hypoxic trophocytes were
co-cultured with HUVECs. RNAi technology was used to silence the
NF-κB p65 gene in hypoxic trophocytes, which resulted in reduced
EDLF levels. By contrast, the expression of Annexin A2, an EDLF
target protein, was upregulated in HUVECs, and NO production
increased. Furthermore, the monolayer barrier function and HUVEC
proliferation were increased by NF-κB silencing in hypoxic
trophocytes. These results suggested that NF-κB p65 may affect
Annexin A2 expression in HUVECs by regulating EDLF production in
hypoxic trophocytes, which impairs vascular endothelial cell
function and NO secretion.
The results of the current study demonstrated the
effects of EDLF only in terms of vascular endothelial cell damage.
However, EDLF also plays a maintenance role in normal preg- nancy.
The levels of EDLF that lead to positive and negative effects
remain to be determined via analysis of a large number of samples.
The results of such an analysis may lead to the development of a
sensitive and specific marker for convenient, rapid and
reproducible early diagnosis and monitoring, and for assessing
therapeutic effects. EDLF causes vascular endothelial cell damage
through multiple pathways; this study focused on only the NF-κB p65
pathway. Further comprehensive studies of multiple pathways may
contribute more valuable information regarding preeclampsia
pathogenesis.
In conclusion, high levels of EDLF may have an
important role in the multifactorial pathogenesis of preeclampsia,
and NF-κB p65 is a potential key factor in the regulation of EDLF
concentration in preeclampsia. The results of this study may
provide novel strategies for intervening in the development of
preeclampsia in clinical practice.
References
|
1
|
Liu Q and Yang J: Expression and
significance of miR155 and vascular endothelial growth factor in
placenta of rats with preeclampsia. Int J Clin Exp Med.
8:15731–15737. 2015.PubMed/NCBI
|
|
2
|
ACOG Committee on Obstetric Practice: ACOG
practice bulletin. Diagnosis and management of preeclampsia and
eclampsia Number 33, January 2002 American College of Obstetricians
and Gynecologists. Int J Gynaecol Obstet. 77:67–75. 2002.
|
|
3
|
Solomon CG and Seely EW: Preeclampsia -
searching for the cause. N Engl J Med. 350:641–642. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Merviel P, Carbillon L, Challier JC,
Rabreau M, Beaufils M and Uzan S: Pathophysiology of preeclampsia:
Links with implantation disorders. Eur J Obstet Gynecol Reprod
Biol. 115:134–147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YY, Zhou R, Zhou B, Wang T, Zhang L
and Luo D: Overexpression of heparanase is associated with
preeclampsia by inhibiting invasion of trophocytes. Int J Clin Exp
Med. 8:18107–18114. 2015.
|
|
6
|
Stella P, Manunta P, Mallamaci F, Melandri
M, Spotti D, Tripepi G, Hamlyn JM, Malatino LS, Bianchi G and
Zoccali C: Endogenous ouabain and cardiomyopathy in dialysis
patients. J Intern Med. 263:274–280. 2008. View Article : Google Scholar
|
|
7
|
Takahashi H, Yoshika M, Komiyama Y and
Nishimura M: The central mechanism underlying hypertension: A
review of the roles of sodium ions, epithelial sodium channels, the
renin-angiotensin-aldosterone system, oxidative stress and
endogenous digitalis in the brain. Hypertens Res. 34:1147–1160.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv ZR, Sha HJ and Hamillon BP: The
experimental investigation on the biosynthetic pathway of
endogenous ouabain. Chin J Pathophy. 14:9131998.In Chinese.
|
|
9
|
Dechend R, Viedt C, Müller DN, Ugele B,
Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, et al:
AT1 receptor agonistic antibodies from preeclamptic patients
stimulate NADPH oxidase. Circulation. 107:1632–1639. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vento-Tormo R, Rodríguez-Ubreva J, Lisio
LD, Islam AB, Urquiza JM, Hernando H, López-Bigas N, Shannon-Lowe
C, Martínez N, Montes-Moreno S, et al: NF-κB directly mediates
epigenetic deregulation of common microRNAs in Epstein-Barr
virus-mediated transformation of B-cells and in lymphomas. Nucleic
Acids Res. 42:11025–11039. 2014. View Article : Google Scholar :
|
|
11
|
Castri P, Lee YJ, Ponzio T, Maric D, Spatz
M, Bembry J and Hallenbeck J: Poly(ADP-ribose) polymerase-1 and its
cleavage products differentially modulate cellular protection
through NF-kappaB-dependent signaling. Biochim Biophys Acta.
1843:640–651. 2014. View Article : Google Scholar :
|
|
12
|
Hu WT, Huang LL, Li MQ, Jin LP, Li DJ and
Zhu XY: Decidual stromal cell-derived IL-33 contributes to Th2 bias
and inhibits decidual NK cell cytotoxicity through NF-κB signaling
in human early pregnancy. J Reprod Immunol. 109:52–65. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu LJ, Wang B, Parobchak N, Roche N and
Rosen T: STAT3 cooperates with the non-canonical NF-κB signaling to
regulate pro-labor genes in the human placenta. Placenta.
36:581–586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu Y, Wang L, Tang W, Zhang D and Shang T:
RNA interference-mediated knockdown of Notch-1 inhibits migration
and invasion, downregulates matrix metalloproteinases and
suppresses NF-κB signaling pathway in trophoblast cells. Acta
Histochem. 116:911–919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simsek Y, Gul M, Celik O, Aydin NE, Arda
Düz S, Celik E, Ozerol E, Özerol IH and Tanbek K: Nuclear
transcription factor-kappa beta-dependent ultrastructural
alterations within the placenta and systemic inflammatory
activation in pregnant patients with hemolysis, elevated liver
functions and low thrombocyte count (HELLP) syndrome: A
case-control study. Hypertens Pregnancy. 32:281–291. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang LL, Su S, Awale R, Zhang XY, Zhong
LL and Tang H: Expression of anti-inflammatory mediator lipoxin A4
and inflammation responsive transcriptive factors NF-kappa B in
patients with preeclampsia. Clin Exp Obstet Gynecol. 41:561–566.
2014.PubMed/NCBI
|
|
17
|
Xue P, Zheng M, Gong P, Lin C, Zhou J, Li
Y, Shen L, Diao Z, Yan G, Sun H, et al: Single administration of
ultra-low-dose lipopolysaccharide in rat early pregnancy induces
TLR4 activation in the placenta contributing to preeclampsia. PLoS
One. 10:e01240012015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaughan JE and Walsh SW: Activation of
NF-κB in placentas of women with preeclampsia. Hypertens Pregnancy.
31:243–251. 2012. View Article : Google Scholar
|
|
19
|
Craici IM, Wagner SJ, Weissgerber TL,
Grande JP and Garovic VD: Advances in the pathophysiology of
pre-eclampsia and related podocyte injury. Kidney Int. 86:275–285.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai R, Phillips RA, Karpuzoglu E, Khan D
and Ahmed SA: Estrogen regulates transcription factors STAT-1 and
NF-kappaB to promote inducible nitric oxide synthase and
inflammatory responses. J Immunol. 183:6998–7005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu J: Proteomics analysis of effect of
endogeneous digitalis-like substance on human endothelial cells.
PhD Degree Thesis. Shandong University; 2007
|
|
22
|
Wu YJ, Zheng LL and Xin H: Changes and
clinical significance of Annexin A2 and its antibody in patients
with preeclampsia. Chin Gen Pract. 15:1575–1578. 2012.
|
|
23
|
Xie X and Gou WL: Obstetrics and
Gynecology. People's Medical Publishing House; Beijing: pp.
264–267. 2013
|
|
24
|
Dasgupta A, Kang E and Datta P: The new
enzyme-linked immunosorbent digoxin assay on the ADVIA Integrated
Modular System is virtually free from oleander interference. Ther
Drug Monit. 28:282–285. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amitava D and Meredith AR: Ginseng and
digoxin assays. Discussion. Am J Clin Pathol. 124:229–236.
2005.
|
|
26
|
Reyes MA, Actor JK, Risin SA and Dasgupta
A: Effect of Chinese medicines Chan Su and Lu-Shen-Wan on serum
digoxin measurement by Digoxin III, a new digoxin immunoassay. Ther
Drug Monit. 30:95–99. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benaitreau D, Dos Santos E, Leneveu MC, De
Mazancourt P, Pecquery R and Dieudonné MN: Adiponectin promotes
syncytialisation of BeWo cell line and primary trophoblast cells.
Reprod Biol Endocrinol. 8:1282010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Egger D and Bienz K: Protein (western)
blotting. Mol Biotechnol. 1:289–305. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bilano VL, Ota E, Ganchimeg T, Mori R and
Souza JP: Risk factors of pre-eclampsia/eclampsia and its adverse
outcomes in low- and middle-income countries: A WHO secondary
analysis. PLoS One. 9:e911982014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hund M, Allegranza D, Schoedl M, Dilba P,
Verhagen-Kamerbeek W and Stepan H: Multicenter prospective clinical
study to evaluate the prediction of short-term outcome in pregnant
women with suspected preeclampsia (PROGNOSIS): Study protocol. BMC
Pregnancy Childbirth. 14:3242014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishkaraeva-Yakovleva VV, Fedorova OV,
Solodovnikova NG, Frolova EV, Bzhelyansky AM, Emelyanov IV, Adair
CD, Zazerskaya IE and Bagrov AY: DigiFab interacts with endogenous
cardiotonic steroids and reverses preeclampsia-induced Na/K-ATPase
inhibition. Reprod Sci. 19:1260–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blaustein MP and Hamlyn JM: Signaling
mechanisms that link salt retention to hypertension: Endogenous
ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC
proteins. Biochim Biophys Acta. 1802:1219–1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fedorova OV, Agalakova NI, Talan MI,
Lakatta EG and Bagrov AY: Brain ouabain stimulates peripheral
marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S
rats. J Hypertens. 23:1515–1523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding YL, Yu L and Hu Y: Expression of
angiotensin II type 1 receptor and angiotensinogen in preeclamptic
placenta. Chin J Perina Med. 17:269–271. 2004.
|
|
35
|
Takahashi H: Upregulation of the
Renin-Angiotensin-aldosterone-ouabain system in the brain is the
core mechanism in the genesis of all types of hypertension. Int J
Hypertens. 2012:2427862012. View Article : Google Scholar
|
|
36
|
Xavier FE, Salaices M, Márquez-Rodas I,
Alonso MJ, Rossoni LV, Vassallo DV and Balfagón G: Neurogenic
nitric oxide release increases in mesenteric arteries from ouabain
hypertensive rats. J Hypertens. 22:949–957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xavier FE, Rossoni LV, Alonso MJ, Balfagón
G, Vassallo DV and Salaices M: Ouabain-induced hypertension alters
the participation of endothelial factors in alpha-adrenergic
responses differently in rat resistance and conductance mesenteric
arteries. Br J Pharmacol. 143:215–225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramírez-Vélez R, Bustamante J,
Czerniczyniec A, Aguilar de Plata AC and Lores-Arnaiz S: Effect of
exercise training on eNOS expression, NO production and oxygen
metabolism in human placenta. PLoS One. 8:e802252013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Odibo AO, Zhong Y, Longtine M, Tuuli M,
Odibo L, Cahill AG, Macones GA and Nelson DM: First-trimester serum
analytes, biophysical tests and the association with pathological
morphometry in the placenta of pregnancies with preeclampsia and
fetal growth restriction. Placenta. 32:333–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fernández Celadilla L, Carbajo Rueda M and
Muñoz Rodríguez M: Prolonged inhibition of nitric oxide synthesis
in pregnant rats: Effects on blood pressure, fetal growth and
litter size. Arch Gynecol Obstet. 271:243–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Var A, Yildirim Y, Onur E, Kuscu NK,
Uyanik BS, Goktalay K and Guvenc Y: Endothelial dysfunction in
preeclampsia. Increased homocysteine and decreased nitric oxide
levels. Gynecol Obstet Invest. 56:221–224. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song H, Karashima E, Hamlyn JM and
Blaustein MP: Ouabain-digoxin antagonism in rat arteries and
neurones. J Physiol. 592:941–969. 2014. View Article : Google Scholar :
|
|
43
|
Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M,
Murphy SR and Granger JP: Pathophysiology of hypertension during
preeclampsia: Linking placental ischemia with endothelial
dysfunction. Am J Physiol Heart Circ Physiol. 294:H541–H550. 2008.
View Article : Google Scholar
|
|
44
|
López-Jaramillo P, Arenas WD, García RG,
Rincon MY and López M: The role of the L-arginine-nitric oxide
pathway in preeclampsia. Ther Adv Cardiovasc Dis. 2:261–275. 2008.
View Article : Google Scholar
|
|
45
|
Pisaneschi S, Strigini FA, Sanchez AM,
Begliuomini S, Casarosa E, Ripoli A, Ghirri P, Boldrini A, Fink B,
Genazzani AR, et al: Compensatory fetoplacental upregulation of the
nitric oxide system during fetal growth restriction. PLoS One.
7:e452942012. View Article : Google Scholar
|
|
46
|
Sathishkumar K, Elkins R, Chinnathambi V,
Gao H, Hankins GD and Yallampalli C: Prenatal testosterone-induced
fetal growth restriction is associated with downregulation of rat
placental amino acid transport. Reprod Biol Endocrinol. 9:1102011.
View Article : Google Scholar
|
|
47
|
Johal T, Lees CC, Everett TR and Wilkinson
IB: The nitric oxide pathway and possible therapeutic options in
pre-eclampsia. Br J Clin Pharmacol. 78:244–257. 2014. View Article : Google Scholar :
|
|
48
|
Mangialardi G, Monopoli A, Ongini E,
Spinetti G, Fortunato O, Emanueli C and Madeddu P: Nitric
oxide-donating statin improves multiple functions of circulating
angiogenic cells. Br J Pharmacol. 164:570–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maron BA, Oldham WM, Chan SY, Vargas SO,
Arons E, Zhang YY, Loscalzo J and Leopold JA: Upregulation of
steroidogenic acute regulatory protein by hypoxia stimulates
aldosterone synthesis in pulmonary artery endothelial cells to
promote pulmonary vascular fibrosis. Circulation. 130:168–179.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jacobs BE, Liu Y, Pulina MV, Golovina VA
and Hamlyn JM: Normal pregnancy: Mechanisms underlying the paradox
of a ouabain-resistant state with elevated endogenous ouabain,
suppressed arterial sodium calcium exchange, and low blood
pressure. Am J Physiol Heart Circ Physiol. 302:H1317–H1329. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Saunders R and Scheiner-Bobis G: Ouabain
stimulates endothelin release and expression in human endothelial
cells without inhibiting the sodium pump. Eur J Biochem.
271:1054–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kaur G, Kapoor N, Mohan P, Sri Nageswari
K, Singh MJ and Prasad R: Alteration in ouabain-sensitive sodium
potassium pump of erythrocytes during pregnancy induced
hypertension: A kinetic study. J Biochem Mol Biol Biophys.
6:163–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Harvey KA, Xu Z, Pavlina TM, Zaloga GP and
Siddiqui RA: Modulation of endothelial cell integrity and
inflammatory activation by commercial lipid emulsions. Lipids
Health Dis. 14:92015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bharadwaj A, Bydoun M, Holloway R and
Waisman D: Annexin A2 heterotetramer: Structure and function. Int J
Mol Sci. 14:6259–6305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu YH, Chiu DT, Lin HR, Tang HY, Cheng ML
and Ho HY: Glucose-6-phosphate dehydrogenase enhances antiviral
response through downregulation of NADPH sensor HSCARG and
upregulation of NF-κB signaling. Viruses. 7:6689–6706. 2015.
View Article : Google Scholar : PubMed/NCBI
|