Introduction
Since Takahashi and Yamanaka (1) demonstrated that a simple combination
of four genes could reprogram somatic cells, direct lineage
conversion has provided a rich source of somatic cell types for use
in translational medicine. As the most commonly used somatic cells
for direct conversion, fibroblasts can be directly converted into
diverse functional cell types by introduction of known
cell-fate-determining transcription factors or microRNAs (2–5).
However, a major limitation related to current methods is the
required ectopic expression of key developmental genes. Since the
target genes often have to be stably integrated into the genome,
the genetic modifications may have undesired effects.
Chemical-based conversion strategies are gene-free, allowing the
generation of cells without genetic modifications, and could be
very tightly controlled.
Small molecules specifically modifying key signaling
pathways provide a powerful tool to enhance conversion or even
replace reprogramming genes. Indeed, a previous study showed that
neural progenitor cells could be induced from mouse fibroblasts or
human urinary cells with the appropriate chemical cocktail
(6). Recently, chemical-promoted
transdifferentiation from mouse embryonic fibroblasts or human
foreskin fibroblasts to neuronal cells has been reported (7,8).
However, it is invasive and difficult to obtain these cells. The
MRC-5 cell line was derived from the normal lung tissue of a
14-week-old male fetus (9). The
aim of the current study was to convert MRC-5 cells into neuronal
cells using a cocktail of small molecules.
Materials and methods
Materials and reagents
The information of the key reagents used in this
study is presented in Table I.
Small molecules and reagents included: Valproic acid (VPA; V);
CHIR99021 (C); Repsox (R); forskolin (F); Y-27632 (Y); SP600125
(S); DMH1 (H); cyclic adenosine monophosphate (cAMP), 100
μM; brain-derived neurotrophic factor (BDNF), 20 ng/ml;
glial cell-derived neurotrophic factor (GDNF), 20 ng/ml;
neurotrophin-3 (NT3), 20 ng/ml. Induction medium was composed of a
1:1 ratio of Dulbecco's modified Eagle's medium (DMEM)/F12 (cat.
no. 11330032) and Neurobasal medium (cat. no. 21103049) with 0.5%
N-2 supplement (cat. .no. 17502048), 1% B-27 supplement (cat. no.
17504044; all from Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 100 μM cAMP. Maturation medium was composed of
DMEM/F12:Neurobasal medium (1:1), 0.5% N-2 supplement, 1% B-27
supplement, 100 μM cAMP, 20 ng/ml BDNF, 20 ng/ml GDNF and 20
ng/ml NT3.
 | Table IReagents used in the current
study. |
Table I
Reagents used in the current
study.
| Product code | Product name | Manufacturer |
|---|
| R0158-5MG | Respox | Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany) |
| Y0503 | Y-27632 | Sigma-Aldrich; Merck
KGaA |
| SML1046-5MG | CHIR99021 | Sigma-Aldrich; Merck
KGaA |
| D8946-5MG | DMH1 | Sigma-Aldrich; Merck
KGaA |
| F6886-10MG | Forskolin | Sigma-Aldrich; Merck
KGaA |
| S7067-5MG | SB 202190 | Sigma-Aldrich; Merck
KGaA |
| D0260-25MG | cAMP | Sigma-Aldrich; Merck
KGaA |
| 450-02-10 | Human BDNF | PeproTech, Inc.
(Rocky Hill, NJ, USA) |
| 450-10-10 | Human GDNF | PeproTech, Inc. |
| 450-03-10 | Human NT-3 | PeproTech, Inc. |
| S5567 | SP600125 | Sigma-Aldrich (Merck
KGaA) |
| PHR1061-1G | Valproic acid | Sigma-Aldrich (Merck
KGaA) |
| 845501 | Purified anti-tubulin
β3 | BioLegend, Inc. (San
Diego, CA, USA) |
| ab5392 | Anti-Map2
antibody | Abcam (Cambridge,
UK) |
| ab177487 | Anti-NeuN antibody
(EPR12763) neuronal marker | Abcam |
| ab80579 | Anti-Tau antibody
(TAU-5) | Abcam |
| D9542 | DAPI | Sigma-Aldrich (Merck
KGaA) |
| 74104 | RNeasy mini kit
(50) | Qiagen GmbH (Hilden,
Germany) |
| 33109ES60 | Rhodamine (TRITC)
AffiniPure goat anti-rabbit IgG (H+L) | Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA) |
| 703-545-155 | Alexa Fluor
488-AffiniPure donkey anti-chicken IgY (IgG) (H+L) | Jackson
ImmunoResearch Laboratories, Inc. |
Generation of chemical
induced-neurons
The MRC-5 cells (American Type Culture Collection,
Manassas, VA, USA) were seeded onto 6-well culture plates and
cultured in Eagle's minimum essential medium (Gibco, Carlsbad, CA,
USA) containing 10% fetal bovine serum (Gibco) at 37°C with 5%
CO2 in a humidified atmosphere for 48 h. The induction
medium with the chemical cocktail VCHRFYS was used to induce these
primary cells when the cell confluence was 50–70%. The
concentration of chemicals used was as follows: VPA, 1 mM;
CHIR99021, 3 μM; R, Repsox, 1 μM; forskolin, 10
μM; Y-27632, 5 μM; SP600125, 10 μM; DAPT, 5
μM and DMH1, 2 μM. Medium containing chemical
compounds was half-changed every three days. After 5 days, cells
were switched to maturation medium with CFS (CHIR99021, 3
μM; forskolin, 10 μM; and SP600125, 10 μM) for
7 days. Maturation medium was half-changed every 2 day. Conversion
efficiency was calculated as previously described (4,8).
Briefly, 10 fields of view were randomly selected for each sample
on a Nikon Ti-E microscope (Nikon Corporation, Tokyo, Japan) at
indicated time points, and the total cell number of tubulin β 3
class III (Tuj1), Tuj1+/microtubule-associated protein 2
(Map2)+, Map2+/RNA binding fox-1 homolog 3
(NeuN)+, or Tuj1+/Tau+ cells with
neuron morphology were counted following immunofluorescence
staining. The conversion efficiency was calculated as the ratio of
Tuj1, Tuj1+/Map2+,
Map2+/NeuN+, or
Tuj1+/Tau+ cells to the initial seeding cells
in each visual field. The purity represented the percentage of
induced Tuj1, Tuj1+/Map2+,
Map2+/NeuN+, or
Tuj1+/Tau+ neuronal cells in the total number
of cells stained with DAPI. Quantitative data are presented as the
mean ± standard error of at least three independent
experiments.
Immunofluorescence staining
Immunostaining of cells were performed as previously
described (10). Briefly, cells
were washed with PBS twice and then fixed with 4% paraformaldehyde
for 10 min at room temperature. Then cells were blocked with 5%
bovine serum albumin (MP Biomedicals, Auckland, New Zealand)
containing 0.5% Triton X-100 for 30 min at room temperature.
Subsequently, samples were incubated with primary antibodies
(dilutions 1:100) at 4°C overnight and then with appropriate
fluorescent probe-conjugated secondary antibodies for 1 h at room
temperature. Nuclei were counter-stained with DAPI. Images were
captured using a fluorescence microscope (DM6000; Leica
Microsystems GmbH, Wetzlar, Germany). The primary antibodies were
used at the following dilutions: Tuj1, 1:100; Map2, 1:100; NeuN,
1:50; and Tau, 1:200.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted with the RNeasy mini kit (cat. no.
74104; Qiagen GmbH Hilden, Germany) according to the manufacturer's
protocol. For each reaction, 1 μg RNA was
reverse-transcribed to cDNA using a High Capacity cDNA RT kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was
subsequently conducted with specific primers and SYBR-Green
Universal Master Mix kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in a Pikoreal PCR machine (Thermo Fisher
Scientific, Inc.). The relative expression levels were normalized
against hypoxanthine phosphoribosyltransferase as the internal
control. All reactions were repeated three times. The primers used
are listed in Table II.
 | Table IIPrimers for reverse transcription
quantitative polymerase chain reaction. |
Table II
Primers for reverse transcription
quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| Ascl1 |
5′-CAAGAGAGCGCAGCCTTAG-3′ |
5′-GCAAAAGTCAGTGCTGAACG-3′ |
| Brn2 |
5′-AATAAGGCAAAAGGAAAGCAACT-3′ |
5′-CAAAACACATCATTACACCTGCT-3′ |
| Myt1l |
5′-CAATGGAAAGGGATTTTAAGCA-3′ |
5′-TTTGAGATTATGTACCAACGTTAGATG-3′ |
| Ngn2 |
5′-TCAGACATGGACTATTGGCAG-3′ |
5′-GGGACAGGAAAGGGAACC-3′ |
| NeuroD1 |
5′-GTTATTGTGTTGCCTTAGCACTTC-3′ |
5′-AGTGAAATGAATTGCTCAAATTGT-3′ |
| Foxa2 |
5′-AGCAGAGCCCCAACAAGATG-3′ |
5′-TCTGCCGGTAGAAGGGGAAGA-3′ |
| Sox2 |
5′-CAAGATGCACAACTCGGAGA-3′ |
5′-CGGGGCCGGTATTTATAATC-3′ |
| Foxg1 |
5′-AGAAGAACGGCAAGTACGAGA-3′ |
5′-TGTTGAGGGACAGATTGTGGC-3′ |
| Pax6 |
5′-GGCAACCTACGCAAGATGGC-3′ |
5′-TGAGGGCTGTGTCTGTTCGG-3′ |
| Thy1 |
5′-ATCGCTCTCCTGCTAACAGTC-3′ |
5′-CTCGTACTGGATGGGTGAACT-3′ |
| Ctgf |
5′-CATCTCCACCCGGGTTACCAA-3′ |
5′-AGTACGGATGCACTTTTTGC-3′ |
| Col1a1 |
5′-GAGGGCCAAGACGAAGACATC-3′ |
5′-CAGATCACGTCATCGCACAAC-3′ |
| HPRT |
5′-CCTGGCGTCGTGATTAGTGAT-3′ |
5′-AGACGTTCAGTCCTGTCCATAA-3′ |
Statistical analysis
All quantified data were statistically analyzed and
presented as the mean ± standard error. For comparisons between two
groups, a Student's t-test was used. For multiple group
comparisons, one-way analysis of variance (ANOVA) was used to
calculate statistical significance followed by Fisher's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Generation of neuronal cells from human
MRC-5 lung fibro-blasts using small molecules
Previous studies reported that a combination of
small chemicals, VCRF, had important effects on the neural
differentiation of mouse and human somatic cells, and neuronal cell
survival (6–8). We hypothesized that the combination
of VCRF may facilitate the conversion of human MRC-5 lung
fibroblast cells into neuronal cells. The small molecules Y-27632
[Rho-associated, coiled-coil containing protein kinase (ROCK)
inhibitor; Y) and SP600125 [c-Jun N-terminal kinase (JNK)
inhibitor; S) were previously reported to promote neuronal
conversion (11,12). It was reported that DMH1, a highly
selective small molecule bone morphogenic protein (BMP) inhibitor,
has been demonstrated to promote neurogenesis of human-induced
pluripotent stem cells (13).
Thus, in the current study, MRC-5 cells were treated with a
neuronal induction medium containing VCHRFYS for 7 days (Fig. 1A). VCHRFYS treatment induced a
large fraction of the live cells to exhibit typical neuronal
morphology, and expression of neuronal markers, including Tuj1,
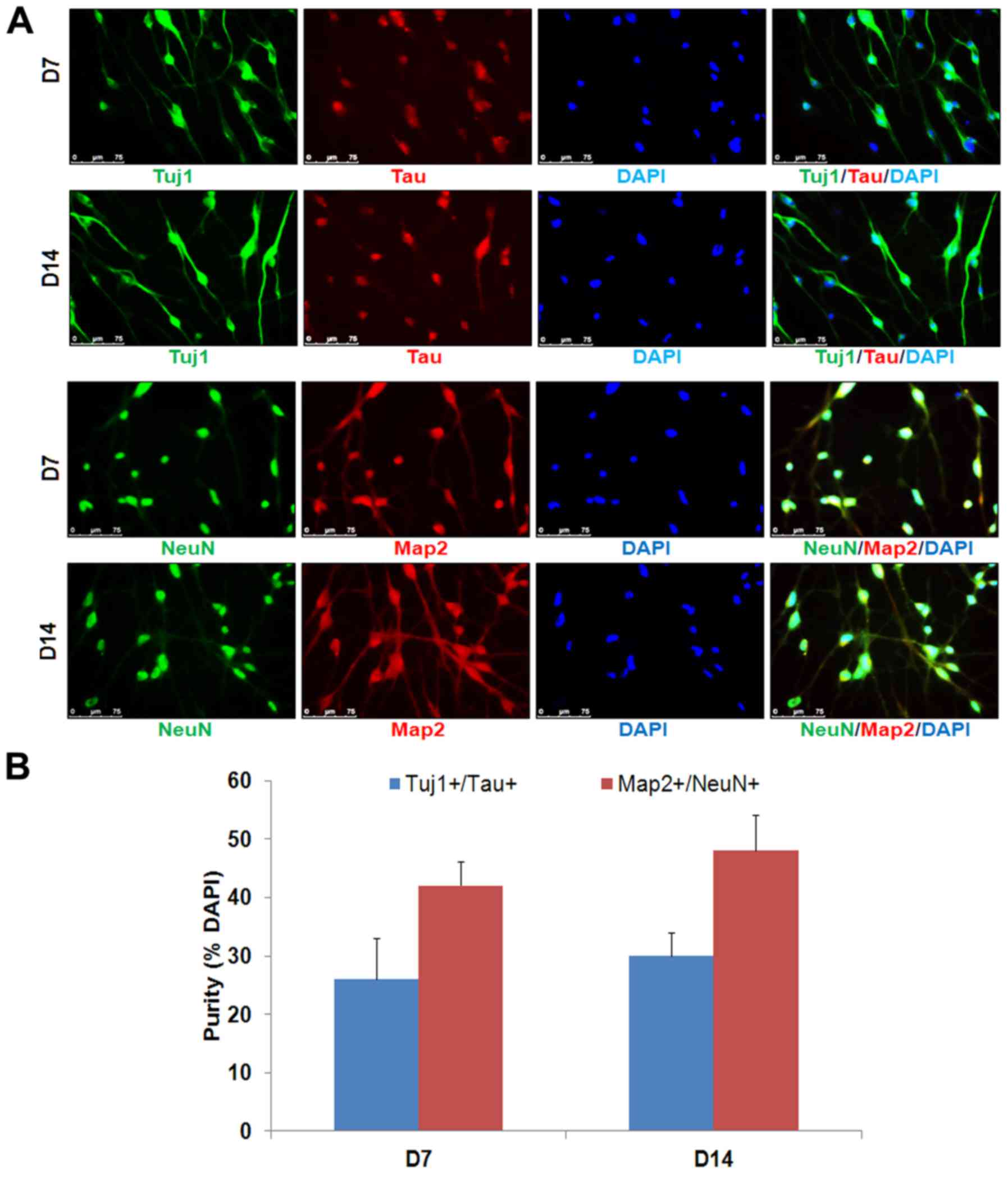
Map2, Tau and NeuN (Figs. 1B–D
and 2). It was observed that the
majority of the induced cells survived until day 8–10, and failed
to become more mature neurons. The CFS chemical cocktail was then
used to promote neuron survival and maturation. Indeed, neuronal
cell survival and maturation were significantly improved after
replacing the induction medium with the neuronal maturation medium
supplemented with CFS and extra neurotropic factors (BDNF, GDNF and
NT3). After 7 days of maturation, the cells were stained positive
for neuronal markers Tau and NeuN (Fig. 2).
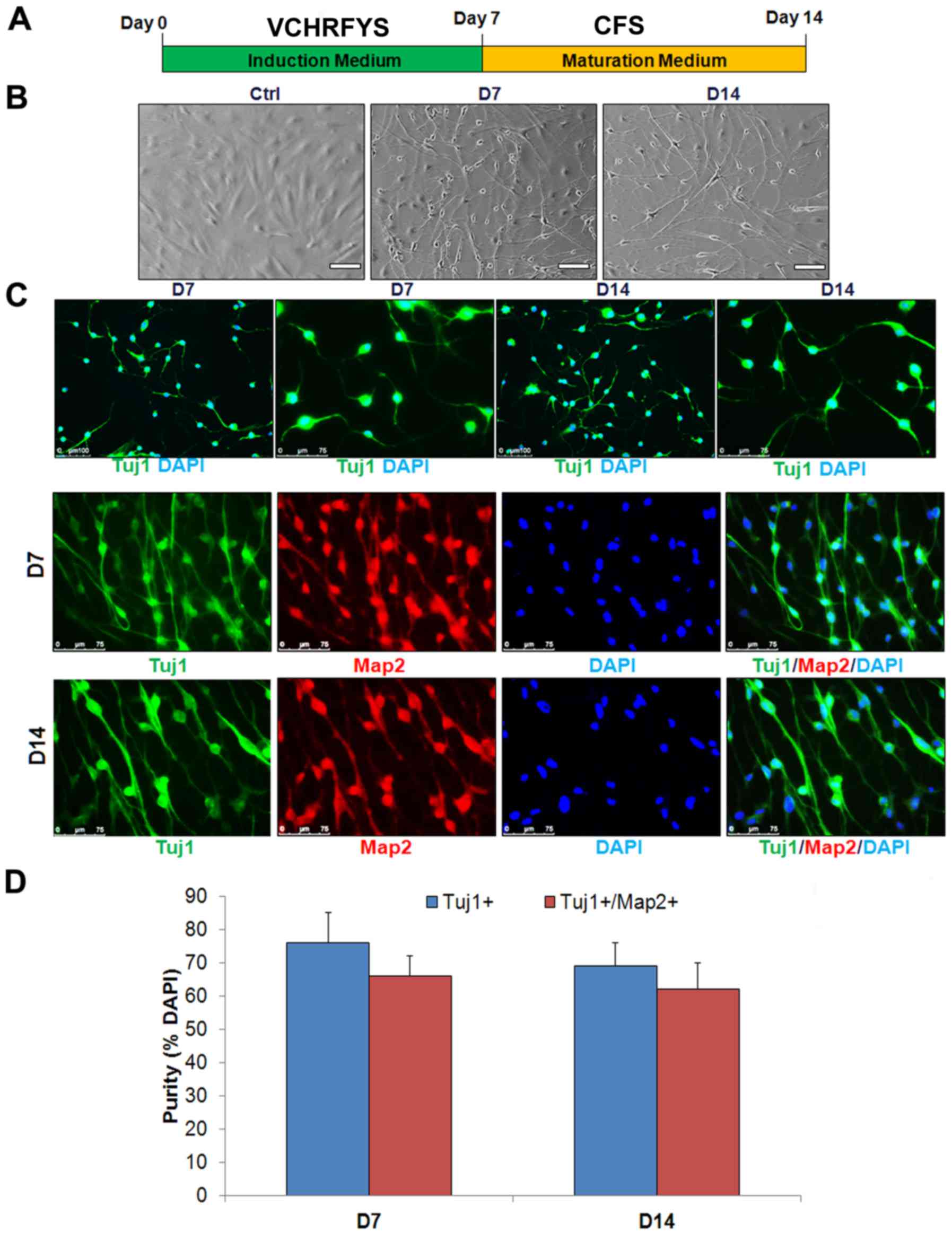 | Figure 1Generation of human neuronal cells by
small molecules. (A) Scheme of induction procedure. (B) The cells
display bipolar neuronal morphologies after 7 days introduction and
7 days maturation. Scale bars, 100 μm. (C) The chemicals
induced-cells display express Tuj1 (green) and
Tuj1+/Map2+. Scale bars, 75 mm. (D) The
percentage of induced Tuj1+ and
Tuj1+/Map2+. Neuronal cells in total cells at
the time of quantification (means ± SD, n=10 random selected 50
fields from triplicate samples). V, VPA; C, CHIR99021; R, Repsox;
F, forskolin; S, SP600625; Y, Y-27632; H, DMH1; S, SP600125; D,
day; Tuj1, tubulin β 3 class III; Map2, microtubule-associated
protein 2. |
Chemical-induced cells exhibit a neuronal
gene expression pattern
To further characterize the induced neuronal cells
phenotype, the expression of nine neuronal genes and three
fibroblastic genes were quantitatively analyzed using RT-qPCR.
Several representative neuronal-specific genes, including
achaetescute family bHLH transcription factor 1, POU class 3
homeobox 2, myelin transcription factor 1 like and neuronal
differentiation 1, were significantly upregulated in
VCHRFYS-treated MRC-5 cells on days 7 (Fig. 3). Notably, these neuronal markers
slightly decreased during the process of maturation under treatment
with CFS chemicals. By contrast, fibroblasts-specific genes,
including collagen type I α 1 chain, Thy-1 cell surface antigen and
connective tissue growth factor, were significant downregulated
during neuronal conversion (Fig.
3). Taken together, the results demonstrated that the VCHRFYS
chemical cocktail reduces fibroblast-specific gene expression in
MRC-5 cells, specifically upregulating neuronal gene expression and
facilitating the neuronal conversion of human lung fibroblasts.
However, the precise regulatory mechanisms of this chemical
cocktail remain to be investigated.
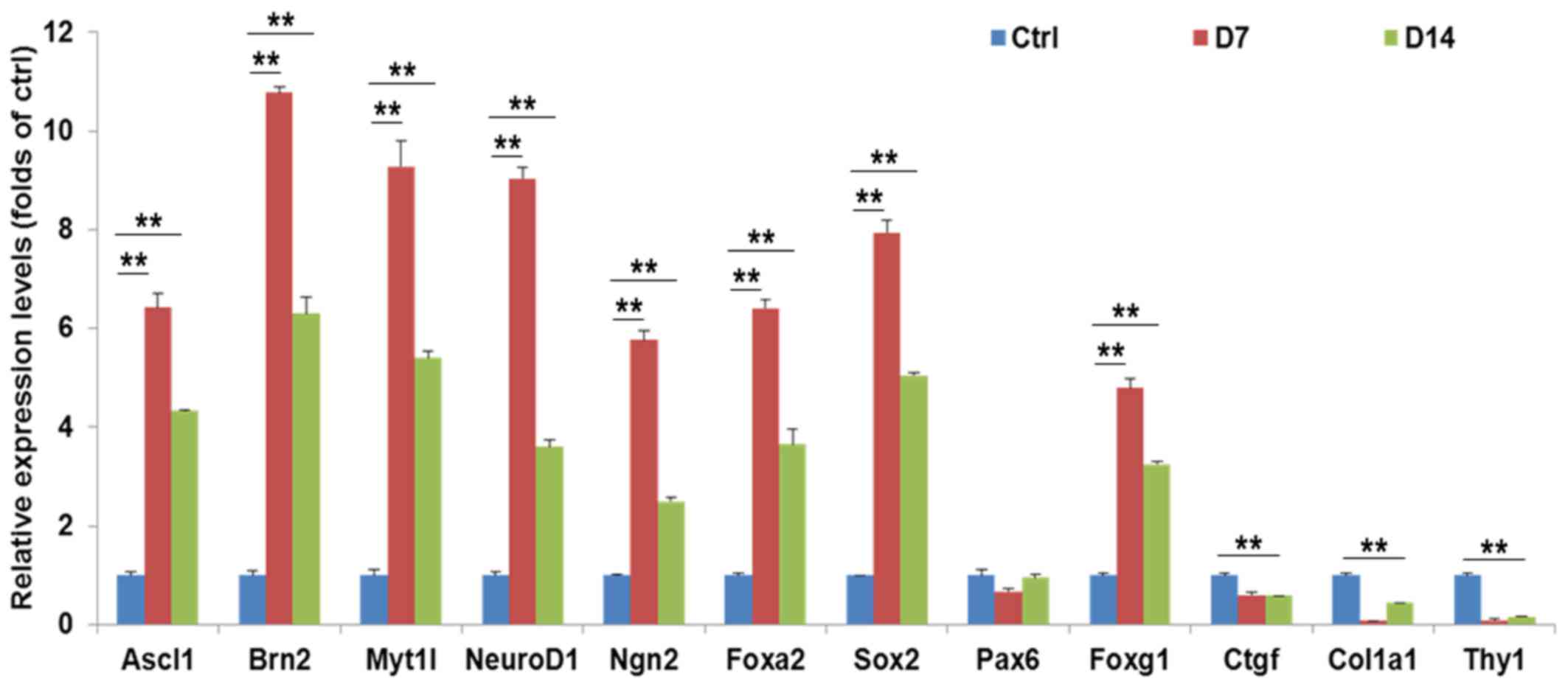 | Figure 3Genes profiles from small
molecule-induced cells. The master neuronal genes were upregulated,
while the genes associated with fibroblasts were downregulated in
the small molecule-induced cells. The data are presented as the
mean ± standard error. Ascl1, achaetescute family bHLH
transcription factor 1; Brn2, POU class 3 homeobox 2; Myt1l, myelin
transcription factor 1 like; NeuroD1, neuronal differentiation 1;
Ngn2, neurogenin 2; Foxa2, forkhead box A2; Sox2, SRY-box 2; Pax6,
paired box 6; Foxg1, forkhead box G1; Ctgf, connective tissue
growth factor; Col1a1, collagen type I α 1 chain; Thy1, Thy-1 cell
surface antigen. |
Discussion
Direct lineage conversion provides a rich source of
somatic cell types for use in regenerative medicine. Direct
conversion of non-neural cells to functional neurons is a promising
strategy for cell-based therapy in the treatment of neurological
disease. In recent years, several studies have demonstrated that
somatic cells, and even terminally differentiated cells, could be
reprogrammed into other cell types. It has been recently reported
that ectopic expression of miR-9/9* and miR-124 (miR-9/9*–124)
could promote direct conversion of human fibroblasts into neurons
using viral vectors (5,20). However, they may have effects on
genetic modifications.
A previous study showed that a chemical cocktail VCR
could induce neural progenitor cells from mouse embryonic
fibroblasts and human urinary cells under a hypoxic condition
(6). Another report showed that
chemical VCRFSGY could convert the fibroblasts from human foreskin
or skin into neuronal cells quickly (8). In the present approach, generation
of neuronal cells from MRC-5 cells was induced by sequential
treatment with defined compounds (VCHRFYS) (Table III). Histone deacetylase
inhibitor, VPA, treatment induces cells into a more amenable state
for cell-fate transition through epigenetic modification (21). CHIR99021 (glycogen synthase
kinase-3β inhibitor), Repsox (transforming growth factor-β
inhibitor) and DMH1 (BMP inhibitor) improved the neuronal
conversion of human fibroblasts (22). Forskolin (ROCK2 inhibitor)
promotes migration of neural crest cells, and enables neurogenin 2
to convert human fibroblasts into cholinergic neurons (19). Y-27632 (Rho kinase inhibitor)
enhances the maintenance of pluripotent stem cells and neuron
survival (23). SP600125 (JNK
inhibitor) can facilitate the neuronal conversion of fibroblasts
(24). Thus, the chemical
cocktail VCHRFYS reduces fibroblast-specific gene expression of the
exposed cells, specifically inducing neuronal gene expression and
facilitating the neuronal conversion of MRC-5 lung fibroblasts.
However, the precise regulatory mechanisms of this chemical
cocktail remain to be investigated. Taken together, the results of
the current study demonstrated that MRC-5, a human lung fibroblast
cell line, can be directly converted into neuronal cells by small
compounds, which does not depend on ectopic gene expression, it is
based on chemical modification of defined signaling pathways, such
as TGF-β and SMAD signaling (25,26).
 | Table IIISmall molecule compounds used in this
study reported to affect neural cells reprogramming. |
Table III
Small molecule compounds used in this
study reported to affect neural cells reprogramming.
| Small
molecules | Effects on neural
cells conversion | Refs. |
|---|
| Valproic acid, HDAC
inhibitors | Promote neurons
differentiation and survival of neural progenitor cells | (14,15) |
| CHIR99021 GSK-3
inhibitor | Improve conversion
of human into neurons and induce pluripotent stem cells into
nociceptors | (16,17) |
| Repsox TGF-β
inhibitor | Improve generation
of neural progenitor cells from pluripotent stem cells | (6) |
| Forskolin cAMP
activator | Promote neuronal
conversion of human fibroblasts | (18,19) |
| Y-27632, ROCK
inhibitor | Induce neural
crest-like cells derived from human neural progenitors | (11) |
| SP600125 JNK
inhibitor | Increases survival
of transplanted dopamine neurons in Parkinsonian rats | (12) |
| DMH1, BMP
inhibitor | Increases
neurogenesis of human-induced pluripotent stem cells | (13) |
Acknowledgments
This study is supported by the National Natural
Science Foundation of China (grant no. 81400861). We would like to
thank B.L.G., Y.N.X., P.W. and C.Q.CH for their technical
supports.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu J, Du Y and Deng H: Direct lineage
reprogramming: Strategies, mechanisms, and applications. Cell Stem
Cell. 16:119–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yashar M, Kalani S and Martirosyan N:
Direct conversion of fibroblasts to functional neurons. World
Neurosurg. 77:7–8. 2012. View Article : Google Scholar
|
|
4
|
Vierbuchen T, Ostermeier A, Pang ZP,
Kokubu Y, Südhof TC and Wernig M: Direct conversion of fibroblasts
to functional neurons by defined factors. Nature. 463:1035–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoo AS, Sun AX, Li L, Shcheglovitov A,
Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW and Crabtree
GR: MicroRNA-mediated conversion of human fibroblasts to neurons.
Nature. 476:228–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng L, Hu W, Qiu B, Zhao J, Yu Y, Guan
W, Wang M, Yang W and Pei G: Generation of neural progenitor cells
by chemical cocktails and hypoxia. Cell Res. 25:645–646. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D,
Zhu J, Du X, Xiong L, Du Y, et al: Small-molecule-driven direct
reprogramming of mouse fibroblasts into functional neurons. Cell
Stem Cell. 17:195–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W,
Gao L, Shen L, Huang Y, Xie G, et al: Direct Conversion of normal
and Alzheimer's disease human fibroblasts into neuronal cells by
small molecules. Cell Stem Cell. 17:204–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jacobs JP, Jones CM and Baille JP:
Characteristics of a human diploid cell designated MRC-5. Nature.
227:168–170. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao Y, Wang J, Yan W, Zhou Y, Chen Y,
Zhou K, Wen J, Wang Y and Cai W: Dysregulated miR-124 and miR-200
expression contribute to cholangiocyte proliferation in the
cholestatic liver by targeting IL-6/STAT3 signalling. J Hepatol.
62:889–896. 2015. View Article : Google Scholar
|
|
11
|
Hotta R, Pepdjonovic L, Anderson RB, Zhang
D, Bergner AJ, Leung J, Pébay A, Young HM, Newgreen DF and Dottori
M: Small-molecule induction of neural crest-like cells derived from
human neural progenitors. Stem Cells. 27:2896–2905. 2009.PubMed/NCBI
|
|
12
|
Rawal N, Parish C, Castelo-Branco G and
Arenas E: Inhibition of JNK increases survival of transplanted
dopamine neurons in Parkinsonian rats. Cell Death Differ.
14:381–383. 2007. View Article : Google Scholar
|
|
13
|
Neely MD, Litt MJ, Tidball AM, et al:
DMH1, a highly selective small molecule BMP inhibitor promotes
neurogenesis of hiPSCs: comparison of AX6 and SOX1 expression
during neural induction. ACS Chem Neurosci. 3(6): 482–91. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim BW, Yang S, Lee CH and Son H: A
critical time window for the survival of neural progenitor cells by
HDAC inhibitors in the hippocampus. Mol Cells. 31:159–164. 2011.
View Article : Google Scholar
|
|
15
|
Jeong SG, Ohn T, Kim SH and Cho GW:
Valproic acid promotes neuronal differentiation by induction of
neuroprogenitors in human bone-marrow mesenchymal stromal cells.
Neurosci Lett. 554:22–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Liu H, Huang CT, Chen H, Du Z, Liu
Y, Sherafat MA and Zhang SC: Generation of integration-free and
region-specific neural progenitors from primate fibroblasts. Cell
Rep. 3:1580–1591. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chambers SM, Qi Y, Mica Y, Lee G, Zhang
XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, et al: Combined
small-molecule inhibition accelerates developmental timing and
converts human pluripotent stem cells into nociceptors. Nat
Biotechnol. 30:715–720. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Babos K and Ichida JK: Small molecules
take a big step by converting fibroblasts into neurons. Cell Stem
Cell. 17:127–129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu ML, Zang T, Zou Y, Chang JC, Gibson
JR, Huber KM and Zhang CL: Small molecules enable neurogenin 2 to
efficiently convert human fibroblasts into cholinergic neurons. Nat
Commun. 4:21832013.PubMed/NCBI
|
|
20
|
Victor MB, Richner M, Hermanstyne TO, et
al: Generation of human striatal neurons by microRNA-dependent
direct conversion of fibroblasts. Neuron. 84(2): 311–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huangfu D, Maehr R, Guo W, et al:
Induction of pluripotent stem cells by defined factors is greatly
improved by small-molecule compounds. Nat Biotechnol. 26(7): 795–7.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ladewig J, Mertens J, Kesavan J, et al:
Small molecules enable highly efficient neuronal conversion of
human fibroblasts. Nat Methods. (6): 575–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xi G, Hu P, Qu C, et al: Induced neural
stem cells generated from rat fibroblasts. Genomics Proteomics
Bioinformatics. 11(5): 312–9. 2014. View Article : Google Scholar :
|
|
24
|
Zhu S, Ambasudhan R, Sun W, et al: Small
molecules enable OCT4-mediated direct reprogramming into expandable
human neural stem cells. Cell Res. 24(1): 126–9. 2014. View Article : Google Scholar :
|
|
25
|
Ichida JK, Blanchard J, Lam K, et al: A
small-molecule inhibitor of tgf-Beta signaling replaces sox2 in
reprogramming by inducing nanog. Cell Stem Cell. 5:491–503. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chambers SM, Fasanob CA, Papapetrou EP, et
al: Highly efficient neural conversion of human ES and iPS cells by
dual inhibition of SMAD signaling. Nat Biotechnol. 27:275–280.
2009. View
Article : Google Scholar : PubMed/NCBI
|