1. Introduction
Skeletal development is a multistep process during
which mesenchymal progenitor cells (MSCs) undergo proliferation and
differentiation, giving rise to cartilage and bone cells. Bone is
produced by two distinct processes. In the endochondral
ossification process, which occurs in long bones and vertebrae,
MSC-derived chondrocytes produce a cartilage template, which is
subsequently replaced by a mineralized matrix, deposed by the
bone-making cells, MSC-derived osteoblasts. The intramembranous
ossification process, which occurs in skull bones and clavicle
formation, relies instead on the direct differentiation of
condensed MSCs into osteoblasts. The bone modeling occurring during
development and the life-long process of remodeling are controlled
by several factors, including systemic hormones, bone morphogenetic
proteins (BMPs), fibroblast growth factors (FGFs) and secreted
signaling factors, including Wnt. Various signaling molecules can
trigger intracellular responses by modulating the expression of
transcription factors, which are essential for MSC
chondrogenic/osteogenic commitment; these include Runt-related
transcription factor 2 (RUNX2), SRY-box 9 (SOX9), osterix (OSX) and
activating transcription factor 4 (ATF4). Bone plasticity allows
continuous adjustments to the mechanical demands solicited by
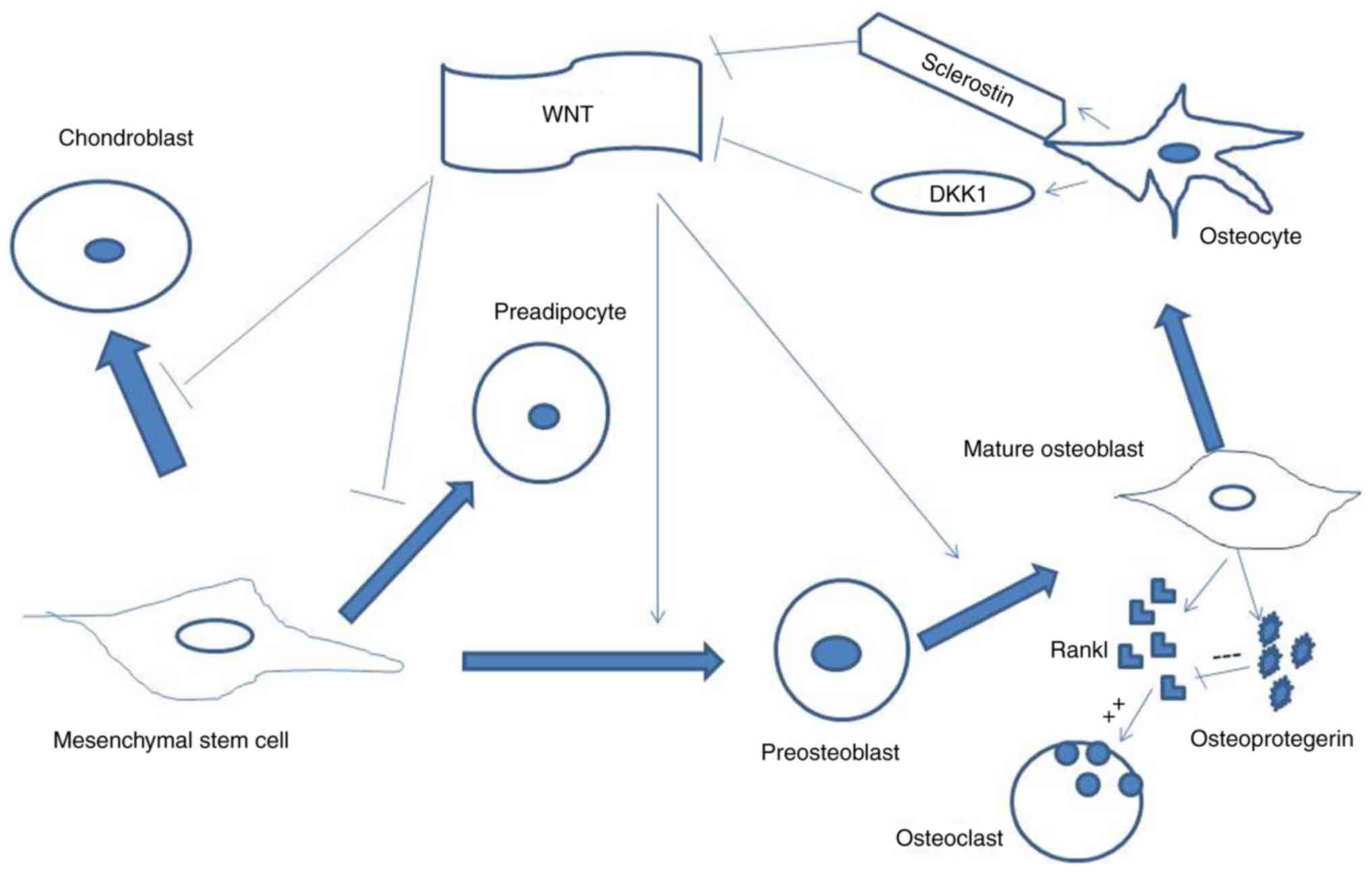
skeletal functions. The leading effectors of bone remodeling are
osteoblasts, osteocytes (mature osteoblasts within the mineralized
matrix) and osteoclasts (large multinucleated cells suited to bone
resorption due to their ability to secrete hydrochloric acid and
proteases which degrade the mineralized matrix). Interactions
occurring between these three cell types contribute to their
reciprocal regulation and determine bone homeostasis in
physiological conditions. Osteoclast differentiation is induced by
the osteoblastic product, receptor activator of nuclear factor-κB
(RANK) ligand (RANKL), which interacts with its osteoclastic
receptor, RANK. Osteoblasts can regulate osteoclast differentiation
by producing osteoprotegerin, a decoy receptor for RANKL, thereby
preventing its interaction with RANK. Osteocytes, far from being
inactive cells trapped in mineralized tissue, control osteoblast
and osteoclast activity, and they are critical in the regulation of
the Wnt signaling pathway. Sclerostin (SOST), a Wnt negative
regulator, is expressed specifically by mature osteocytes (Fig. 1). Mechanical forces and anabolic
agents inhibit the production of SOST. Disturbances in these
complex interactions may cause various bone diseases, including
osteoporosis, osteopetrosis, osteogenesis imperfecta and Paget's
disease (1-3). Epigenetic factors, including
microRNAs (miRNAs) are also essential in bone formation and
homeostasis (4). These are small,
non-coding RNAs, which act as negative regulators of the expression
of specific target mRNAs. A single miRNA can act as a
post-transcriptional repressor by binding to partially
complementary sequences in the 3′UTR sites of various mRNAs. miRNAS
are involved in MSC commitment, osteoblast and osteocyte activity,
osteoclastic maturation and bone cell interactions with their
microenvironment (3-6). Altered patterns in the expression of
miRNAs have been found in in vivo and in vitro models
of bone disorders (7). There is
increasing interest in the promising potential applications of
miRNAs as diagnostic biomarkers and as molecular targets for
therapeutic options in the treatment of bone disorders. miRNAs can
be isolated from cells or bone specimens; they can also be
retrieved from circulatory biofluids, including plasma or serum, as
they are secreted from cells in exosomes or encapsulated within
microvesicles. The present review examines and discusses the role
of selected miRNAs, which have been consistently recognized in
previous studies to affect the activity of various bone cell types
in physiological and pathological conditions (7-9).
2. Role of miRNAs in commitment
determination of MSCs
Bone cells progenitors, MSCs, are multipotent stem
cells capable of differentiating into adipocytes, chondrocytes or
osteoblasts. A mutually inhibitory association exists between
osteogenic and adipogenic commitment. Alternative cell fate
decisions are regulated by multiple signaling pathways. Increasing
evidence indicates that miRNAs affect decisions concerning the fate
of bone marrow MSCs. These can operate by silencing components of
the Wnt or BMP signaling pathways at the post-transcriptional
level, and by modulating the expression of key transcription
factors, including RUNX2 (10-12). Chondrocytes arise from mesenchymal
cell aggregation and differentiation. Growth factors, cellular
interactions and extracellular matrix (ECM) elements are involved
in the differentiation process by inducing chondrocyte-specific
gene expression of SOX9, COL2A1, aggrecan, COL10A1 and
parathyroid hormone-related protein (13). In this scenario, miRNAs are
important in chondrocyte differentiation. In particular, miR-30a
has been shown to be significantly upregulated during the
chondrogenic differentiation of rat MSCs; it targets delta-like 4
gene, a ligand of the Notch signaling family (14). miR-140, which targets A
disintegrin and metalloproteinase with thrombospondin motif 5, a
metalloproteinase which degrades aggrecan, is expressed at a high
level during the chondrogenesis of MSCs, in addition to increased
expression levels of SOX9 and COL2A1 (15). By contrast, miR-455-3p has been
shown function as an early activator of chondrogenesis, by
targeting the gene expression of RUNX2 (16). The miRNAs involved in the
adipogenic osteogenic and chondrogenic switch, respectively, in
addition to their targets, are shown in Fig. 2.
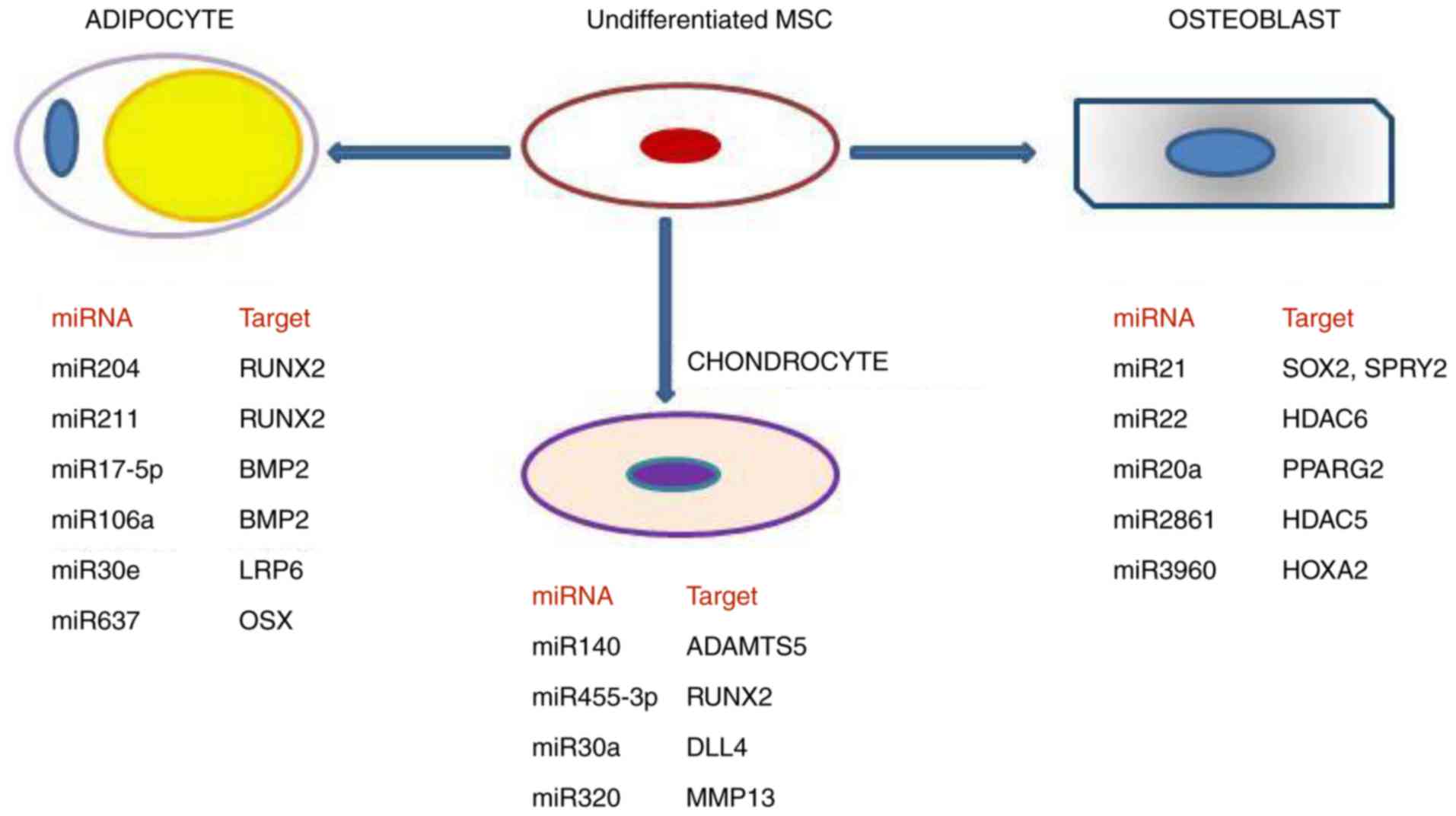 | Figure 2Role of different miRNAs in
contribution to the commitment determination of undifferentiated
MCSc towards adipogenesis, chondrogenesis and osteogenesis,
respectively. MSC, mesenchymal stem cell; miR, microRNA; RUNX2,
Runt-related transcription factor 2; BMP2, bone morphogenetic
protein 2; LRP6, LDL receptor-related protein 6; OSX, osterix;
ADAMTS5, A disintegrin and metalloproteinase with thrombospondin
motif 5; DLL4, delta-like 4; MMP13, matrix metalloproteinase 13;
SOX2, SRY-box 2; SPRY2, sprouty homolog 2; HDAC6, histone
deacetilase 6; PPARG2, peroxisome proliferator activated receptor
γ2; HDAC5, histone deacetilase; HOXA2, homeobox A2. |
3. miRNAs which promote adipogenesis and
inhibit osteogenesis
miR-204 and miR-211 behave as endogenous repressors
of RUNX2, the master regulator of MSC osteogenic commitment
(12). miR-17-p5 and miR-106-a
target BMP2 mRNA, which promotes osteogenic differentiation
(17). miR-30e targets LDL
receptor-related protein 6 (LRP6), a member of the Wnt canonical
pathway co-receptor group (LRP5/LRP6/Frizzled) (18). miR-637 is known to target OSX, a
key regulator of osteoblastic maturation acting downstream of RUNX2
(19).
4. miRNAs which promote osteogenesis and
inhibit adipogenesis
miR-21 targets SRY-box 2 (SOX2), one of the four
genes which promote induced pluripotent stem cells, and sprouty
homolog 2 (SPRY2), a negative regulator of the extracellular
signal-regulated kinase-mitogen-activated protein kinase signaling
pathway (20,21). In MSCs overexpressing miR-21,
osteogenic markers, including RUNX2 and osteonectin (OCN), are also
overexpressed (22). This
suggests a dual action of miR21 on progenitor cells, namely the
suppression of pluripotency and promotion of osteogenic
differentiation. miR-22 is also present in bone marrow progenitors;
its expression decreases during adipogenic differentiation and
increases during osteogenic differentiation (23). miR-22 and miR-2861 target histone
deacetilase 6 (HDAC6) and histone deacetilase 5 (HDAC5),
respectively. These histone deacetylases act as transcriptional
co-repressors of RUNX2 (24).
miR-3960, which is clustered with miR-2861 and encoded by the same
transcript, targets homeobox A2 gene, another RUNX2 inhibitor
(25). miR-20a promotes
osteogenic differentiation by targeting peroxisome proliferator
activated receptor γ2, a positive regulator of adipocyte
differentiation (26).
5. miRNAs which regulate osteoblast
activity
The miRNAs involved in regulating osteoblast
activity are listed in Table I.
OsteomiRNAs, or bone-regulating miRNAs, can act as stimulators and
repressors of osteogenesis. Among the stimulators, miR322 appears
to increase the expression of OSX by targeting transducer of ERBB2,
2 (TOB2), which belongs to the TOB family of antiproliferative
proteins; it facilitates the deadenylation and degradation of
mRNAs, including OSX (27). By
contrast, miR-181 promotes osteogenesis by targeting mRNAs coding
for members of the transforming growth factor-β (TGF-β) signaling
pathway, namely TGF-β receptor 1 and TGF-β-induced, which
negatively regulate osteoblastogenesis (28). miR-29 exerts a positive regulatory
effect on osteoblast differentiation, enhancing Wnt signaling, and
Dickkopf-related protein 1 (DKK1), a Wnt inhibitor, is among its
targets (10). miR-335-5p also
targets DKK1 (29). miR-26-a
promotes osteogenesis by targeting glycogen synthase kinase 3β, a
member of the β-catenin destruction complex (30). The miRNAs which target RUNX2 and
OSX, respectively, act as negative regulators of osteogenesis;
among others, these include the above-mentioned miR-204 and
miR-211, which downregulate RUNX2, whereas miR-138 and miR-143
target OSX (31,32). A negative regulator of bone
formation is miR-214, which targets ATF4, a transcription factor
modulating the gene expression of osteocalcin (33). miR-483-5p and miR-320-a
downregulate insulin-like growth factor 2 (IGF2) and RUNX2,
respectively, which inhibits osteoblastic function (34). Finally, miR-182 negatively
regulates osteoblast proliferation by targeting Forkhead box
protein O1 (FOXO1), a regulator of bone mass (35); miR-155 has been demonstrated to
inhibit mouse osteoblast differentiation by suppressing the
expression of small mothers against decapentaplegic family member 5
(SMAD5) (36).
 | Table ImiRNAs which regulate osteoblast
activity. |
Table I
miRNAs which regulate osteoblast
activity.
| miRNA | Target gene | Regulatory effect
on osteogenesis |
|---|
| miR-322 | TOB2 | ↑ |
| miR-181 | TGFβR | ↑ |
| TGFβI | |
| miR-29 D | KK1 | ↑ |
| miR-335-5p | | |
| miR-26a | GSK3B | ↑ |
| miR-320-a | RUNX2 | ↓ |
| miR-138 | OSX | ↓ |
| miR-143 | | |
| miR-483-5p | IGF2 | ↓ |
| miR-182 | FOXO1 | ↓ |
| miR-155 | SMAD5 | ↓ |
6. miRNAs which regulate chondrocyte
activity
The miRNAs involved in regulating chondrocyte
activity are listed in Table II.
The differentiation process in chondrocytes is followed by
proliferation, hypertrophy, terminal differentiation,
mineralization and programmed cell death. Of note, miR-140
(mentioned above) is involved in cartilage homeostasis; it has been
shown that miR-140-knockout mice developed osteoarthritis (37). miR-let7, expressed in various
tissues, is required for chondrocytes proliferation in normal
conditions (38). The
downregulation of miR-let7, obtained by Lin28a inhibitor, reduces
chondrocytes proliferation and growth. These effects may be
interpreted as consequences of the overexpression of two miR-let 7
target genes, namely cell division cycle 34 and E2F transcription
factor 5. Therefore, miR-let7, together with miR140, regulates the
skeletal development by acting on chondrocyte proliferation and
homeostasis (38). miR-199a and
miR-214, which are generated from the RNA transcript Dnm3os
and expressed in mesenchymal cells and chondrocytes, contribute to
correct skeletal development. In particular, the upregulation of
miR-199 has been observed upon chondrocytic differentiation
(39). miR-195 can inhibit
chondrocyte proliferation, by acting on the G-protein coupled
receptor kinase interacting protein-1 (GIT1) (40) and promote apoptosis by targeting
hypoxia inducible factor 1α (HIF-1α) (41). miR-138 has been shown to act as a
negative modulator of the chondrocytic phenotype, as it targets
specificity protein 1 and hypoxia-inducible factor 2α (HIF-2α), two
transcription factors necessary for COL2A1 gene expression. The
overexpression of miR-138 has been shown to coincide with loss of
the differentiated phenotype of cultured human articular
chondrocytes (42). Proliferation
and apoptosis are important processes in chondrocyte homeostasis.
It has been demonstrated that miR-34a reduces the proliferation
rate and induces the apoptosis of primary human chondrocytes by
regulating the sirtuin 1/p53 signaling pathway (43).
 | Table IImiRNAs which regulate chondrocyte
activity. |
Table II
miRNAs which regulate chondrocyte
activity.
| miRNA | Target gene | Regulatory effect
on chondrogenesis |
|---|
| miR-140 | ADAMTS-5 | ↑ |
| miR-let7 CDC | 34 | ↑ |
| E2F5 | |
| miR-199a | SMAD1, COX2 | ↑ |
| miR-214 | OSX, ATF4 | |
| miR-195 | GIT1 | ↓ |
| HIF-1α | |
| miR-138 | SP1 | ↓ |
| HIF-2α | |
| miR-34a | SIRT1 | ↓ |
7. miRNAs which regulate osteoclast
activity
An important function in bone homeostasis is bone
resorption. Various evidence supports the idea that miRNAs are
involved in bone resorption, as they may act on osteoclastic
proliferation, differentiation and survival (Table III). Among the positive
regulators of osteoclastogenesis is miR-223, which targets nuclear
factor IA (NFIA), a negative regulator of macrophage
colony-stimulating factor receptor (44). miR-148-a has been demonstrated to
stimulate osteoclastogenesis by targeting transcription factor
MafB, a transcriptional repressor of RANKL (45). miR-31 is upregulated during
osteoclast differentiation in murine bone marrow cells; its target,
RhoA, is a GTPase involved in cytoskeletal reorganization, which
can affect osteoclast formation (46). Similarly, miR-21 is upregulated in
osteoclast precursors and is identified as a marker of
RANKL-induced osteoclastogenesis, targeting the programmed cell
death 4 (PDCD4) gene (47). By
contrast, miR-503 is a negative regulator of osteoclastogenesis, as
it targets the osteoclastic receptor RANK (48). miR-218 and miR-125-a also act as
negative regulators; miR-218 targets the nuclear factor of
activated T-cells 1 (NFATC1) signaling molecule, which is required
for osteoclastogenesis, and regulates several genes involved in
osteoclast differentiation and function (49). miR-125-a targets TNF
receptor-associated factor 6, an osteoclastogenesis-promoting
factor (50).
 | Table IIImiRNAs which regulate osteoclast
activity. |
Table III
miRNAs which regulate osteoclast
activity.
| miRNA | Target gene | Regulatory effect
on osteogenesis |
|---|
| miR-223 | NF1A | ↑ |
| miR-148a | MAFB | ↑ |
| miR-31 | RhoA | ↑ |
| miR-21-5p | PDCD4 | ↑ |
| miR-503 | RANK | ↓ |
| miR-218 | NFATC1 | ↓ |
| miR-124-3p | | |
| miR-125a | TRAF6 | ↓ |
8. Role of miRNAs in the pathological
osteogenic commitment of progenitor cells
Altered patterns of miRNA expression have been shown
in bone-related pathologies, which may jeopardize progenitor cell
commitment and osteogenic differentiation. miR-29b is known to have
an important regulatory role in osteoblast differentiation, as it
targets COL1A1 and SPARC (osteonectin) mRNAs (51). As osteoblasts mature, miR-29b
attenuates the expression of collagen genes, allowing the
organization of collagen fibrils for subsequent mineralization. Of
note, the downregulation of miR-29b has been reported in two
distinct genetic bone disorders, osteogenesis imperfecta (OI) and
osteopetrosis (OPT). OI is an 'osteoblast disease', characterized
by varying degrees of bone fragility and skeletal deformities.
Kaneto et al (52)
reported that, in patients with OI, heterozygosity for causative
COL1A1 gene mutations led to the reduced mRNA expression of COL1A1,
and the levels of miR-29b were severely reduced. The authors
suggested that the miR-29b control of collagen protein accumulation
in the ECM depends on the levels of its target and hypothesized
that the low mRNA levels of COL1A1 observed in OI cells at various
stages of osteogenic differentiation were not sufficient for the
induction of miR-29b. OPT is considered an 'osteoclast disease'. In
peripheral blood mononuclear cells (PBMCs) from patients with
OPTA2, a survey of aberrantly expressed miRNAs showed that miR-29b
was among the most significantly downregulated (53). A previous study demonstrated that
miR-29b promotes osteoclastogenesis; its knockdown in murine
pre-osteoclasts inhibited differentiation (54). Due to their important effects on
bone marrow progenitor cell differentiation, misregulated miRNAs
may profoundly affect primary and metastatic bone tumors. It has
been demonstrated that the upregulation of miR-135b is involved in
the impaired osteogenic differentiation of MSCs derived from
patients with multiple myeloma (55). The study also demonstrated that
impaired osteogenic differentiation overlapped with a marked
downregulation of osteogenic markers, including BSP, COL1A1 and
OPN. The authors suggested that miR-135b inhibited osteogenic
differentiation by targeting SMAD5 mRNA and possibly other
strategic target mRNAs. Bone metastasis, frequently occurring in
late stages of breast and prostate cancer, disrupts normal bone
remodeling. The involvement of miRNAs in the control and fate of
bone metastasis has been reviewed extensively (56,57). miRNA profiles can be of diagnostic
and prognostic value, and they may become therapeutic agents in the
near future.
Osteosarcoma (OS) is a common malignant bone tumor
in children and young adults. Surgery and chemotherapy failure
occur in certain patients with OS. Therefore, biomarkers for active
disease are required in order to monitor relapses and to predict
prognosis in subjects who have a poor response to multi-agent
chemotherapy. Several studies have investigated the role of
circulating miRNAs as possible biomarkers with various, sometimes
contradictory, findings (58-61). In particular, miR21 (Fig. 2) emerges consistently as a
significant marker for poor prognosis, when its expression levels
in the plasma of patients with OS and cancer cells are compared
with those found in controls (62-64). miR-199a, which has been shown to
be dysregulated in several types of tumor, has been found to be
underexpressed in osteosarcoma cells and patient samples. Keremu
et al (65) demonstrated
that cisplatin-resistant OS cells treated with agomiR199 were
sensitized to cisplatin. Further investigations in this field may
lead to the identification of other miRNA markers for more precise
and timely prognosis, and to miRNAs therapeutic applications for
osteosarcoma.
9. Altered miRNA expression levels in
osteoporosis
Osteoporosis is a common age-related degenerative
disease associated with bone loss and low-traumatic fractures. Bone
fragility in postmenopausal osteoporosis originates from an
imbalance in bone homeostasis, caused by increased osteoclastic
activity and a progressive decline in osteoblastic proliferation
(66). Due to the importance of
miRNAs in the regulation of bone remodeling, several studies have
investigated osteoporosis-related changes in their expression
(Table IV). In several studies
specific miRNAs, selected on the basis of previous reports, were
isolated from serum samples of osteoporotic patients (OP) and
age/sex matched healthy controls, reverse transcribed and then
analyzed using reverse transcription-quantitative polymerase chain
reaction analysis (67-72). In another study, total RNA was
extracted from fresh femoral trabecular bone obtained from OP and
control groups, and hybridized to a miRNA array containing
>1,900 miRNAs (34).
Biostatistical analyses were performed in all experiments in order
to identify significant (P<0.05) differences in miRNA expression
between cases and controls. Several limitations in these studies do
not allow the obtaining of univocal miRNAs signatures of
osteoporosis. Notably, the patient/control groups in each study
were small and different; in addition, miRNAs were different and
non-randomly selected. However, consistent findings stand out from
the plethora of data. Significant differences in the expression of
specific miRNAs produced either by osteoblast or osteoclast cells,
as described above, and in Tables
I and III, respectively,
appear to be essential in osteoporosis. Table IV illustrates how osteoblastic
miRNAs, which target essential positive effectors of osteogenic
commitment, including miR30e, miR214, miR483-5p, miR182 and
miR320a, are overexpressed in patients with osteoporosis, compared
with matched healthy controls, whereas other miRNAs, including
miR335-5p, which targets DKK1, are underexpressed in OP. Certain
findings conflict with previously reported data. Yavropoulou et
al (72) found serum levels
of miR124-3p, a negative regulator of osteoclastogenesis (Table III) and miR2861, a positive
regulator of osteoblastogenesis (Fig.
2) to be higher in postmenopausal women with a low bone mineral
density (BMD), compared with those in women with a normal BMD. The
authors hypothesized a compensatory mechanism of enhanced
osteogenesis in response to menopause-induced bone loss, in order
to justify their conflicting findings.
 | Table IVmiRNAs as biomarkers of
osteoporosis. |
Table IV
miRNAs as biomarkers of
osteoporosis.
| Cell type | Target gene | miRNAs
differentially expressed in patients with osteoporosis | Expression levels
(compared with controls) |
|---|
| Osteoblast | FOXO1 | miR-182 | ↑ |
| | miR-483-5p | |
| CTNNB1 | miR-320 a | ↑ |
| LRP6 | miR-30e | ↑ |
| HADC5 | miR-2861 | ↑ |
| SPRY, PDCD4 | miR-21-5p | ↓ |
| D | KK1 | miR-335-5p | ↓ |
| Osteoclast | MAFB | miR-148a | ↑ |
| NFATC1 | miR-124-3p | ↑ |
10. miRNAs as potential therapeutic targets
for osteoporosis
As the dysregulation of miRNAs appears to contribute
considerably to bone pathologies, including osteoporosis, several
approaches aiming to correct such dysregulation have been applied
to model systems. One type of approach consists in the delivery of
pro-osteoblastic miRNAs in order to promote osteogenesis; another
is the silencing of endogenous pro-osteoclastic miRNAs. The design
of biologically sTable RNA molecules and their efficient delivery
represent the main requirements in the two approaches. Zhang et
al (73) designed a two-stage
delivery system. A hyperbranched polymer vector containing
miRNA26-a was encapsulated in polylactic-co-glycolic acid (PLGA)
microspheres; these biodegradable PLGA microspheres were attached
to 3D scaffolds, which were then implanted into mice. miRNA26-a had
previously been demonstrated to promote osteogenesis (Table I). Zhang et al (73) showed that long-term miR26-a
delivery locally rescued the osteogenic capacity in osteoporotic
mice. By contrast, Liu et al (74) described a successful strategy to
knock down pro-osteoclastic miRNA148-a (Table III). Downregulation/silencing of
single miRNAs can be achieved by means of antagomiRs, specifically
engineered oligonucleotides complementary to the miRNA target. Liu
et al (74) encapsulated
antagomiR-148 a in (D-Asp8)-modified liposomes, which
favorably bind to bone-resorption surfaces, thus targeting
osteoclasts. The therapeutic effect of antagomiR-148a was assessed
upon delivery to ovariectomized (OVX) mice, which are animal models
for the postmenopausal status. Encouraging results were derived
from bone metabolism marker measurements and the observed
attenuated decrease of BMD in the treatment group. In another
study, Chen et al (48)
demonstrated an opposite effect of antagomiR503 upon injection in
OVX mice. miRNA503 inhibits osteoclastogenesis by targeting RANK,
as described above (Table III)
and the authors found it was dramatically downregulated in
postmenopausal osteoporotic subjects. AntagomiR503-treated animals
showed increased protein expression of RANK and bone resorption,
whereas treatment with exogenous agomiR503 inhibited bone
resorption and prevented bone loss. The above cited studies suggest
that certain miRNAs are important in the pathogenesis of
postmenopausal osteoporosis, due to their various effects on
osteoblast or osteoclast activities. Exogenous agomiRNAs
(potentiators), or exogenous antagomiRNAs (inhibitors) appear to be
novel therapeutic tools against osteoporosis, a disabling disease
which affects ~200 million individuals worldwide (75).
11. Conclusions
The role of miRNAs as modulators of gene expression
and, consequently of physiological and pathological tissue
functions, is attracting increasing attention. Referred to as
'micromanagers of gene expression', miRNAs regulate a substantial
region of the human genome. Experimental in vivo and in
vitro studies have shown how miRNAs complex interactions affect
bone development and homeostasis. Excreted miRNAs, recovered from
blood or other body fluids, represent useful biomarkers for
skeletal disorders; experiments in animal models suggest that
antagomiRNAs or miRNA mimics may function as novel therapeutic
tools. The exploitation of miRNA analysis as a molecular diagnostic
tool is an interesting concept; however, important issues require
consideration, including sample-to-sample biological variability
and modulation of miRNAs in similar phenotypes. Although certain
miRNAs have been observed consistently in different experimental
conditions, their stimulatory or inhibitory effects on osteoblast
and osteoclast differentiation remain to be fully elucidated. For
example, miR-335-5p is upregulated in the commitment of MSCs to the
osteogenic lineage, however, its levels are reduced during
osteoblast maturation (76). As
another example, miR-223 is expressed in mononuclear osteoclast
precursors and has been shown to enhance osteoclast differentiation
(77). By contrast, in PBMCs or
RAW264.7 cells, the overexpression of miR-223 inhibits osteoclast
formation (6). Therefore, these
findings define the role of miRNAs in maintaining bone homeostasis
by modulating osteoblast and osteoclast commitment or maturation;
however, complementary evaluations are advisable in order to
interpret correctly those findings concerning the elevation or
reduction of specific miRNAs. Finally, an awareness of the roles
and functions of miRNAs is a pre-requisite for the development of
promising tools in personalized medicine. A number of issues
remain, including how to optimize miRNA stability and delivery
systems efficiency, and how to reach a specific tissue, specific
cell population and specific mRNA target. As individual miRNAs may
target numerous mRNAs, adverse collateral effects require
consideration. These challenges indicate the requirement for
further basic investigations in the field.
Acknowledgments
This review was supported by Fondo Unico della
Ricerca grants of the University of Verona (Verona, Italy) to
Professor L.D.C. and Professor M.M., respectively.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Valenti MT, Dalle Carbonare L and Mottes
M: Osteogenic differentiation in healthy and pathological
conditions. Int J Mol Sci. 18:E412016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Idolazzi L, Fassio A, Tripi G, Braga V,
Viapiana O, Adami G, Rossini M and Gatti D: Circulating Dickkopf-1
and sclerostin in patients with Paget's disease of bone. Clin
Rheumatol. 36:925–928. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mäkitie RE, Haanpää M, Valta H, Pekkinen
M, Laine CM, Lehesjoki AE, Schalin-Jäntti C and Mäkitie O: Skeletal
characteristics of WNT1 osteoporosis in children and young adults.
J Bone Miner Res. 31:1734–1742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papaioannou G, Mirzamohammadi F and
Kobayashi T: MicroRNAs involved in bone formation. Cell Mol Life
Sci. 71:4747–4761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuan Y, Zhang L, Tong X, Zhang M, Zhao Y,
Guo J, Lei L, Chen X, Tickner J, Xu J and Zou J: Mechanical stress
regulates bone metabolism through MicroRNAs. J Cell Physiol.
232:1239–1245. 2017. View Article : Google Scholar
|
|
6
|
Ji X, Chen X and Yu X: MicroRNAs in
osteoclastogenesis and function: Potential therapeutic targets for
osteoporosis. Int J Mol Sci. 17:3492016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gennari L, Bianciardi S and Merlotti D:
MicroRNAs in bone diseases. Osteoporos Int. 28:1191–1213. 2017.
View Article : Google Scholar
|
|
8
|
Wu C, Tian B, Qu X, Liu F, Tang T, Qin A,
Zhu Z and Dai K: MicroRNAs play a role in chondrogenesis and
osteoarthritis (Review). Int J Mol Med. 34:13–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang C, Geng J and Jiang S: MicroRNAs in
regulation of osteogenic differentiation of mesenchymal stem cells.
Cell Tissue Res. 368:229–238. 2017. View Article : Google Scholar
|
|
10
|
Kapinas K, Kessler C, Ricks T, Gronowicz G
and Delany AM: miR-29 modulates Wnt signaling in human osteoblasts
through a positive feedback loop. J Biol Chem. 285:25221–25231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong Y, Xu F, Zhang L, Qian Y, Chen J,
Huang H and Yu Y: MicroRNA expression signature for Satb2-induced
osteogenic differentiation in bone marrow stromal cells. Mol Cell
Biochem. 387:227–239. 2014. View Article : Google Scholar
|
|
12
|
Huang J, Zhao L, Xing L and Chen D:
MicroRNA-204 regulates Runx2 protein expression and mesenchymal
progenitor cell differentiation. Stem Cells. 28:357–364. 2010.
|
|
13
|
Onyekwelu I, Goldring MB and Hidaka C:
Chondrogenesis, joint formation, and articular cartilage
regeneration. J Cell Biochem. 107:383–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian Y, Guo R, Shi B, Chen L, Yang L and
Fu Q: MicroRNA-30a promotes chondrogenic differentiation of
mesenchymal stem cells through inhibiting Delta-like 4 expression.
Life Sci. 148:220–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyaki S, Nakasa T, Otsuki S, Grogan SP,
Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK and Asahara H:
MicroRNA-140 is expressed in differentiated human articular
chondrocytes and modulates interleukin-1 responses. Arthritis
Rheum. 60:2723–2730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Hou C, Meng F, Zhao X, Zhang Z,
Huang G, Chen W, Fu M and Liao W: MiR-455-3p regulates early
chondrogenic differentiation via inhibiting Runx2. FEBS Lett.
589:3671–3678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Li T, Wang S, Wei J, Fan J, Li J,
Han Q, Liao L, Shao C and Zhao RC: miR-17-5p and miR-106a are
involved in the balance between osteogenic and adipogenic
differentiation of adipose-derived mesenchymal stem cells. Stem
Cell Res. 10:313–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Guan X, Guo F, Zhou J, Chang A,
Sun B, Cai Y, Ma Z, Dai C, Li X and Wang B: miR-30e reciprocally
regulates the differentiation of adipocytes and osteoblasts by
directly targeting low-density lipoprotein receptor-related protein
6. Cell Death Dis. 10:e8452013. View Article : Google Scholar
|
|
19
|
Zhang JF, Fu WM, He ML, Wang H, Wang WM,
Yu SC, Bian XW, Zhou J, Lin MC, Lu G, et al: MiR-637 maintains the
balance between adipocytes and osteoblasts by directly targeting
Osterix. Mol Biol Cell. 22:3955–3961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei Y, Bian C, Li J, Du Z, Zhou H, Yang Z
and Zhao RC: miR-21 modulates the ERK-MAPK signaling pathway by
regulating SPRY2 expression during human mesenchymal stem cell
differentiation. J Cell Biochem. 114:1374–1384. 2013. View Article : Google Scholar
|
|
21
|
Trohatou O, Zagoura D, Bitsika V, Pappa
KI, Antsaklis A, Anagnou NP and Roubelakis MG: Sox2 suppression by
miR-21 governs human mesenchymal stem cell properties. Stem Cells
Transl Med. 3:54–68. 2014. View Article : Google Scholar :
|
|
22
|
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi
S, Cai Y, Cheng S, Wang X, Liu Y, et al: Tumor necrosis factor
alpha suppresses the mesenchymal stem cell osteogenesis promoter
miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner
Res. 28:559–573. 2013. View Article : Google Scholar
|
|
23
|
Huang S, Wang S, Bian C, Yang Z, Zhou H,
Zeng Y, Li H, Han Q and Zhao RC: Upregulation of miR-22 promotes
osteogenic differentiation and inhibits adipogenic differentiation
of human adipose tissue-derived mesenchymal stem cells by
repressing HDAC6 protein expression. Stem Cells Dev. 21:2531–2540.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westendorf JJ: Transcriptional
co-repressors of Runx2. J Cell Biochem. 98:54–64. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu R, Liu W, Li H, Yang L, Chen C, Xia ZY,
Guo LJ, Xie H, Zhou HD, Wu XP and Luo XH: A Runx2/miR-3960/miR-2861
regulatory feedback loop during mouse osteoblast differentiation. J
Biol Chem. 286:12328–12339. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang JF, Fu WM, He ML, Xie WD, Lv Q, Wan
G, Li G, Wang H, Lu G, Hu X, et al: MiRNA-20a promotes osteogenic
differentiation of human mesenchymal stem cells by co-regulating
BMP signaling. RNA Biol. 8:829–838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gámez B, Rodríguez-Carballo E, Bartrons R,
Rosa JL and Ventura F: MicroRNA-322 (miR-322) and its target
protein Tob2 modulate Osterix (Osx) mRNA stability. J Biol Chem.
288:14264–14275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bhushan R, Grünhagen J, Becker J, Robinson
PN, Ott CE and Knaus P: miR-181a promotes osteoblastic
differentiation through repression of TGF-β signaling molecules.
Int J Biochem Cell Biol. 45:696–705. 2013. View Article : Google Scholar
|
|
29
|
Zheng L, Tu Q, Meng S, Zhang L, Yu L, Song
J, Hu Y, Sui L, Zhang J, Dard M, et al: Runx2/DICER/miRNA pathway
in regulating osteogenesis. J Cell Physiol. 232:182–191. 2017.
View Article : Google Scholar
|
|
30
|
Li Y, Fan L, Liu S, Liu W, Zhang H, Zhou
T, Wu D, Yang P, Shen L, Chen J and Jin Y: The promotion of bone
regeneration through positive regulation of angiogenicosteogenic
coupling using microRNA-26a. Biomaterials. 34:5048–5058. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eskildsen T, Taipaleenmäki H, Stenvang J,
Abdallah BM, Ditzel N, Nossent AY, Bak M, Kauppinen S and Kassem M:
MicroRNA-138 regulates osteogenic differentiation of human stromal
(mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA.
108:6139–6144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li E, Zhang J, Yuan T and Ma B: MiR-143
suppresses osteogenic differentiation by targeting Osterix. Mol
Cell Biochem. 390:69–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Guo B, Li Q, Peng J, Yang Z, Wang
A, Li D, Hou Z, Lv K, Kan G, et al: miR-214 targets ATF4 to inhibit
bone formation. Nat Med. 19:93–100. 2013. View Article : Google Scholar
|
|
34
|
De-Ugarte L, Yoskovitz G, Balcells S,
Güerri-Fernández R, Martinez-Diaz S, Mellibovsky L, Urreizti R,
Nogués X, Grinberg D, García-Giralt N and Díez-Pérez A: MiRNA
profiling of whole trabecular bone: Identification of
osteoporosis-related changes in MiRNAs in human hip bones. BMC Med
Genomics. 8:752015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin HL, Kim JS, Kim YJ, Kim SJ, Broxmeyer
HE and Kim KS: Dynamic expression of specific miRNAs during
erythroid differentiation of human embryonic stem cells. Mol Cells.
34:177–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu Y, Ma L, Song L, Li X, Chen D and Bai
X: miR-155 inhibits mouse osteoblast differentiation by suppressing
SMAD5 expression. Biomed Res Int. 2017:18935202017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyaki S, Sato T, Inoue A, Otsuki S, Ito
Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, et al:
MicroRNA-140 plays dual roles in both cartilage development and
homeostasis. Genes Dev. 24:1173–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Papaioannou G, Inloes JB, Nakamura Y,
Paltrinieri E and Kobayashi T: let-7 and miR-140 microRNAs
coordinately regulate skeletal development. Proc Natl Acad Sci USA.
110:E3291–E3300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin EA, Kong L, Bai XH, Luan Y and Liu CJ:
miR-199a, a bone morphogenic protein 2-responsive MicroRNA,
regulates chondrogenesis via direct targeting to Smad1. J Biol
Chem. 284:11326–11335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gu YL, Rong XX, Wen LT, Zhu GX and Qian
MQ: miR-195 inhibits the proliferation and migration of
chondrocytes by targeting GIT1. Mol Med Rep. 15:194–200. 2017.
View Article : Google Scholar
|
|
41
|
Bai R, Zhao AQ, Zhao ZQ, Liu WL and Jian
DM: MicroRNA-195 induced apoptosis in hypoxic chondrocytes by
targeting hypoxia-inducible factor 1 alpha. Eur Rev Med Pharmacol
Sci. 19:545–551. 2015.PubMed/NCBI
|
|
42
|
Seidl CI, Martinez-Sanchez A and Murphy
CL: Derepression of MicroRNA-138 contributes to loss of the human
articular chondrocyte phenotype. Arthritis Rheumatol. 68:398–409.
2016. View Article : Google Scholar
|
|
43
|
Yan S, Wang M, Zhao J, Zhang H, Zhou C,
Jin L, Zhang Y, Qiu X, Ma B and Fan Q: MicroRNA-34a affects
chondrocyte apoptosis and proliferation by targeting the SIRT1/p53
signaling pathway during the pathogenesis of osteoarthritis. Int J
Mol Med. 38:201–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lian JB, Stein GS, van Wijnen AJ, Stein
JL, Hassan MQ, Gaur T and Zhang Y: MicroRNA control of bone
formation and homeostasis. Nat Rev Endocrinol. 8:212–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheng P, Chen C, He HB, Hu R, Zhou HD, Xie
H, Zhu W, Dai RC, Wu XP, Liao EY and Luo XH: miR-148a regulates
osteoclastogenesis by targeting V-maf musculoaponeurotic
fibrosarcoma oncogene homolog B. J Bone Miner Res. 28:1180–1190.
2013. View Article : Google Scholar
|
|
46
|
Mizoguchi F, Murakami Y, Saito T, Miyasaka
N and Kohsaka H: miR-31 controls osteoclast formation and bone
resorption by targeting RhoA. Arthritis Res Ther. 15:R1022013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sugatani T, Vacher J and Hruska KA: A
microRNA expression signature of osteoclastogenesis. Blood.
117:3648–3657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen C, Cheng P, Xie H, Zhou HD, Wu XP,
Liao EY and Luo XH: MiR-503 regulates osteoclastogenesis via
targeting RANK. J Bone Miner Res. 29:338–347. 2014. View Article : Google Scholar
|
|
49
|
Qu B, Xia X, Yan M, Gong K, Deng S, Huang
G, Ma Z and Pan X: miR-218 is involved in the negative regulation
of osteoclastogenesis and bone resorption by partial suppression of
p38MAPK-c-Fos-NFATc1 signaling: Potential role for osteopenic
diseases. Exp Cell Res. 338:89–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guo LJ, Liao L, Yang L, Li Y and Jiang TJ:
MiR-125a TNF receptor-associated factor 6 to inhibit
osteoclastogenesis. Exp Cell Res. 321:142–152. 2014. View Article : Google Scholar
|
|
51
|
Laxman N, Rubin CJ, Mallmin H, Nilsson O,
Pastinen T, Grundberg E and Kindmark A: Global miRNA expression and
correlation with mRNA levels in primary human bone cells. RNA.
21:1433–1443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kaneto CM, Lima PS, Zanette DL, Prata KL,
Pina Neto JM, de Paula FJ and Silva WA Jr: COL1A1 and miR-29b show
lower expression levels during osteoblast differentiation of bone
marrow stromal cells from Osteogenesis Imperfecta patients. BMC Med
Genet. 15:1471–2350. 2014. View Article : Google Scholar
|
|
53
|
Ou M, Zhang X, Dai Y, Gao J, Zhu M, Yang
X, Li Y, Yang T and Ding M: Identification of potential
microRNA-target pairs associated with osteopetrosis by deep
sequencing, iTRAQ proteomics and bioinformatics. Eur J Hum Genet.
22:625–632. 2014. View Article : Google Scholar
|
|
54
|
Franceschetti T, Kessler CB, Lee SK and
Delany AM: miR-29 promotes murine osteoclastogenesis by regulating
osteoclast commitment and migration. J Biol Chem. 288:33347–33360.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xu S, Cecilia Santini G, De Veirman K,
Vande Broek I, Leleu X, De Becker A, Van Camp B, Vanderkerken K and
Van Riet I: Upregulation of miR-135b is involved in the impaired
osteogenic differentiation of mesenchymal stem cells derived from
multiple myeloma patients. PLoS One. 8:e797522013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ell B and Kang Y: MicroRNAs as regulators
of bone homeostasis and bone metastasis. Bonekey Rep. 3:5492014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zoni E and van der Pluijm G: The role of
microRNAs in bone metastasis. J Bone Oncol. 5:104–108. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li H, Zhang K, Liu LH, Ouyang Y, Guo HB,
Zhang H, Bu J and Xiao T: MicroRNA screening identifies circulating
microRNAs as potential biomarkers for osteosarcoma. Oncol Lett.
10:1662–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lian F, Cui Y, Zhou C, Gao K and Wu L:
Identification of a plasma four-microRNA panel as potential
noninvasive biomarker for osteosarcoma. PLoS One. 10:e01214992015.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dong J, Liu Y, Liao W, Liu R, Shi P and
Wang L: miRNA-223 is a potential diagnostic and prognostic marker
for osteosarcoma. J Bone Oncol. 5:74–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ma W, Zhang X, Chai J, Chen P, Ren P and
Gong M: Circulating miR-148a is a significant diagnostic and
prognostic biomarker for patients with osteosarcoma. Tumour Biol.
35:12467–12472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ziyan W, Shuhua Y, Xiufang W and Xiaoyun
L: MicroRNA-21 is involved in osteosarcoma cell invasion and
migration. Med Oncol. 28:1469–1474. 2011. View Article : Google Scholar
|
|
63
|
Ren X, Shen Y, Zheng S, Liu J and Jiang X:
miR-21 predicts poor prognosis in patients with osteosarcoma. Br J
Biomed Sci. 73:158–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nakka M, Allen-Rhoades W, Li Y, Kelly AJ,
Shen J, Taylor AM, Barkauskas DA, Yustein JT, Andrulis IL, Wunder
JS, et al: Biomarker significance of plasma and tumor miR-21,
miR-221, and miR-106a in osteosarcoma. Oncotarget. 27:96738–96752.
2017.
|
|
65
|
Keremu A, Aini A, Maimaitirexiati Y, Liang
Z, Aila P, Xierela P, Tusun A, Moming H and Yusufu A: Overcoming
cisplatin resistance in osteosarcoma through the miR-199a-modulated
inhibition of HIF-1α. Biosci Rep. BSR20170080. 2017. View Article : Google Scholar
|
|
66
|
Dalle Carbonare L, Valenti MT, Zanatta M,
Donatelli L and Lo Cascio V: Circulating mesenchymal stem cells
with abnormal osteogenic differentiation in patients with
osteoporosis. Arthritis Rheum. 60:3356–3365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Weilner S, Skalicky S, Salzer B, Keider V,
Wagner M, Hildner F, Gabriel C, Dovjak P, Pietschmann P,
Grillari-Voglauer R, et al: Differentially circulating miRNAs after
recent osteoporotic fractures can influence osteogenic
differentiation. Bone. 79:43–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kocijan R, Muschitz C, Geiger E, Skalicky
S, Baierl A, Dormann R, Plachel F, Feichtinger X, Heimel P,
Fahrleitner-Pammer A, et al: Circulating microRNA signatures in
patients with idiopathic and postmenopausal osteoporosis and
fragility fractures. J Clin Endocrinol Metab. 101:4125–4134. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li H, Wang Z, Fu Q and Zhang J: Plasma
miRNA levels correlate with sensitivity to bone mineral density in
postmenopausal osteoporosis patients. Biomarkers. 19:553–556. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Seeliger C, Karpinski K, Haug AT, Vester
H, Schmitt A, Bauer JS and van Griensven M: Five freely circulating
miRNAs and bone tissue miRNAs are associated with osteoporotic
fractures. J Bone Miner Res. 29:1718–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hackl M, Heilmeier U, Weilner S and
Grillari J: Circulating microRNAs as novel biomarkers for bone
diseases-complex signatures for multifactorial diseases? Mol Cell
Endocrinol. 432:83–95. 2016. View Article : Google Scholar
|
|
72
|
Yavropoulou MP, Anastasilakis AD, Makras
P, Tsalikakis DG, Grammatiki M and Yovos JG: Expression of
microRNAs that regulate bone turnover in the serum of
postmenopausal women with low bone mass and vertebral fractures.
Eur J Endocrinol. 176:169–176. 2017. View Article : Google Scholar
|
|
73
|
Zhang X, Li Y, Chen YE, Chen J and Ma PX:
Cell-free 3D scaffold with two-stage delivery of miRNA-26a to
regenerate critical-sized bone defects. Nat Commun. 7:103762016.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu J, Dang L, Li D, Liang C, He X, Wu H,
Qian A, Yang Z, Au DW, Chiang MW, et al: A delivery system
specifically approaching bone resorption surfaces to facilitate
therapeutic modulation of microRNAs in osteoclasts. Biomaterials.
52:148–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wright NC, Looker AC, Saag KG, Curtis JR,
Delzell ES, Randall S and Dawson-Hughes B: The recent prevalence of
osteoporosis and low bone mass in the United States based on bone
mineral density at the femoral neck or lumbar spine. J Bone Miner
Res. 29:2520–2526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang J, Tu Q, Bonewald LF, He X, Stein G,
Lian J and Chen J: Effects of miR-335-5p in modulating osteogenic
differentiation by specifically downregulating Wnt antagonist DKK1.
J Bone Miner Res. 26:1953–1963. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sugatani T and Hruska KA: Impaired
micro-RNA pathways diminish osteoclast differentiation and
function. J Biol Chem. 284:4667–4678. 2009. View Article : Google Scholar :
|