Introduction
Hypoxic pulmonary hypertension (HPH) is common in
patients with pulmonary diseases (1,2).
The leading cause of HPH is alveolar hypoxia due to chronic lung
disease (3). Hypoxia may produce
mild to moderate pulmonary vascular remodeling, which may lead to a
detrimental increase in pulmonary artery pressure and dysfunction
of the pulmonary vascular regulatory mechanisms. Hypoxia-induced
pulmonary changes may contribute to the development of sustained
HPH (4,5). Pulmonary vascular remodeling is a
complicated pathological process that involves all layers of the
vascular wall (6). Previous
research has identified the roles of pulmonary arterial fibroblasts
(PAFs) within the lungs, and established the primary cellular
constituents of the vascular adventitia (7–10).
PAFs have been reported to undergo the earliest and most
significant proliferation in all layers of the vascular wall in HPH
models (10). They also migrate
to the intima and may be associated with neointima formation
(10–12). The activation of PAFs by a variety
of stimuli triggers their differentiation into myofibroblasts,
which have been implicated as key participants in tissue remodeling
(7,13).
However, the mechanisms underlying the
hypoxia-induced proliferation, migration and differentiation of
PAFs remain unclear (14).
Previous studies revealed that hypoxia inducible factor-1, mitogen
activated protein kinase, transforming growth factor-β (TGF-β),
fibroblast grow factor-2 (FGF2), osteopontin, macrophage migration
inhibitory factor and 15-lipoxygenase (15-LO) are associated with
the hypoxia-induced proliferation, migration, protein synthesis and
phenotypic change behav-iors of PAFs (9,15–22). The platelet-derived growth factor
β-receptor/c-Jun N-terminal kinase (JNK), TGF-β1/FGF2 and
JNK/15-LO/p27kipl signaling pathways have been reported
to participate in the regulation of hypoxia-induced PAF
proliferation and differentiation (23). Further research is required to
investigate the in-depth mechanisms underlying hypoxia-induced
alterations in PAFs.
Previous studies demonstrated that the
phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin
(mTOR) signaling pathway serves an important role in cell
proliferation, metabolism, protein synthesis and angiogenesis
(24,25). PI3K may be triggered by a variety
of upstream signals and regulates the activity of various
downstream effectors, including protein kinase B (Akt), mTOR and
p70 ribosomal protein S6 kinase (p70S6K), through its
phosphorylation activity (26).
Negative feedback regulation of the Akt signaling pathway has been
hypothesized to exist, but is not fully understood (26). Under hypoxic conditions, certain
cells, including pulmonary arterial smooth muscle, A549 and porcine
coronary artery endothelial cells and rat hepatocytes may exhibit
activation of the PI3K/Akt signaling pathway, whereas others,
including 293T, PC-3 and COS-7 cells, do not exhibit increased
phosphorylation levels of Akt (25,27). Previous studies have demonstrated
that the PI3K/Akt/p70S6K signaling pathway is associated with the
progression of HPH and the activities of cells in the pulmonary
arteries (25,27). The PI3K/Akt/p70S6K signaling
pathway may be associated with the regulation of PAF proliferation,
migration and differentiation under hypoxia (28). Previous studies revealed that PAFs
from young cows had transiently upregulated PI3K expression when
exposed to hypoxia, and demonstrated that the moderate
hypoxia-induced (3% O2) proliferation of fetal bovine
PAFs required activation of the PI3K/Akt signaling pathway
(28,29). However, the underlying mechanism
by which the PI3K/Akt/p70S6K signaling pathway contributes to the
hypoxia-induced proliferation, migration, differentiation and
pulmonary vascular remodeling in rat PAFs has not been identified.
Therefore, the present study was undertaken to investigate whether
the PI3K/Akt/p70S6K signaling pathway is associated with the
proliferation, migration, differentiation and pulmonary vascular
remodeling of PAFs under hypoxic conditions.
Materials and methods
Animal care and ethics
Experiments were performed using specific
pathogen-free male Sprague-Dawley (SD) rats (Vital River; Charles
River Laboratories International, Inc., Wilmington, MA, USA). The
Peking University First Hospital Ethical Review Committee for
Animal Experiments approved all animal protocols used in the
present study (permit number, J201533). Applicable international
and national institutional guidelines for the care and use of
animals were followed. Procedures were consistent with the Guide
for the Care and Use of Laboratory Animals (30). The procedures performed in the
present study involving animals were in accordance with the ethical
standards of the institution at which the studies were conducted.
All efforts were made to ensure the comfort of the animals, and to
minimize the number of animals used and their suffering.
Cell culture
Primary PAFs were isolated from the pulmonary
arteries adventitia of male SD rats (100±20 g, aged 4–6 weeks, ≥2
rats per primary PAF culture) using a dissecting microscope. Cells
were cultured in high glucose Dulbecco's modified Eagle's medium
(DMEM; HyClone; GE Healthcare Life Sciences, Logan, UT, USA),
supplemented with 100 U/ml penicillin, 100 g/ml streptomycin (both
Beyotime Institute of Biotechnology, Haimen, China) and 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), in a humidified incubator with 5% CO2 at 37°C.
Cells were purified and identified using the fibroblast marker
vimentin (cat. no. 5741; Cell Signaling Technology, Inc., Danvers,
MA, USA), which was detected via immunofluorescence after culturing
for 3 passages; only passages 3–6 were used in the experiments. For
all assays, unless otherwise specified, cells were plated at a
density of 5×105 cells/cm2, cultured to 80%
confluence, starved and growth arrested in DMEM without serum for
24 h and subsequently used in the experiments. Pretreatment with
inhibitors, NVP-BEZ235 as a dual PI3K/mTOR inhibitor, LY294002 as a
PI3K inhibitor and rapamycin as an mTOR inhibitor (all from Selleck
Chemicals, Houston, TX, USA), was achieved by adding the inhibitor
1 h prior to stimulation in the absence or presence of hypoxia. For
hypoxic experiments the PAFs were exposed to either normoxia (21%
O2) or hypoxia (1% O2) in a cell incubation
chamber (Don Whitley Scientific Ltd., Shipley, UK) for the
indicated time period.
Cell counting kit-8 (CCK8) assay
Cell viability and proliferation were assayed using
CCK8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan)
according to the manufacturer's protocol. PAFs were seeded in
96-well plates for 24 h and then starved for another 24 h. The PAFs
were subsequently treated with NVP-BEZ235 (10 nM), rapamycin (10
nM), LY294002 (10 µM) or 0.1% dimethyl sulfoxide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as the control
group 1 h prior to exposure to specific oxygen conditions for the
indicated time periods. The PAFs were incubated with water-soluble
tetrazolium salt-8 for 2 h at 38°C. The reaction product was then
quantified by measuring the absorbance at 450 nm using a
spectrophotometer.
Wound healing and Transwell assays
The PAF wound healing assay was performed according
to a previously published protocol (31). Briefly, the cells were seeded at
5×106 cells/well in 6-well cell culture plates. After 24
h, three straight scratches were made in each well with the tip of
a 200-µl pipette. The wells were rinsed with PBS, which was
replaced with DMEM media containing 0.5% FBS and the cells were
incubated in normoxic or hypoxic conditions for the indicated time
periods. Cell migration was captured at ×200 magnification using an
optical microscope.
The Transwell migration assay was performed using
Transwell inserts with an 8-µm pore size (Corning Inc.,
Corning, NY, USA). The cells were trypsinized (0.5%) for 1–2 min at
room temperature, counted and then seeded into the upper insert at
a density of 1×106 cells/well in DMEM with 1% serum.
DMEM containing 10% FBS was added to the lower chamber. The cells
were incubated at 37°C for the indicated time periods under
normoxic or hypoxic conditions. The counting of the migrated cells
was performed by eye, following fixation with 90% ethanol for 30
min and 0.1% crystal violet staining of the cells for 10 min at
room temperature.
Immunofluorescence assay
An immunofluorescence assay was performed to detect
the differentiation activity of the PAFs. The PAFs were fixed with
4% paraformaldehyde for 15 min at room temperature, and then
blocked with 1% bovine serum albumin (cat. no. B2064;
Sigma-Aldrich, Merck KGaA) for 10 min at room temperature. The
cells were incubated overnight at 4°C with antibodies directed
against α-smooth muscle actin (α-SMA; cat. no. ab5694; dilution
1:200; Abcam, Cambridge, UK). Antibodies directed against GAPDH
(cat. no. bs-2188R; dilution 1:500; Biosynthesis Biotechnology Co.,
Ltd., Beijing, China) were used as a reference. The cells were then
washed with PBS for 15 min at room temperature. The cells were
subsequently incubated with the relevant secondary antibodies,
Alexa Fluor 488 immunoglobulin (Ig)G (cat. no. ZF-0511; dilution
1:500) or Alexa Fluor 594 IgG (cat. no. ZF-0513; dilution 1:500)
(both OriGene Technologies, Inc., Beijing, China) for 60 min at
room temperature. This was followed by washing with PBS for 15 min.
The cells in the target sections were incubated with DAPI (dilution
1:5,000; Sigma-Aldrich; Merck KGaA) for 10 min at room temperature
in the dark. The results were observed through an
immunofluorescence BX-60 microscope at ×400 magnification (Olympus
Corp., Tokyo, Japan). The fluorescence intensities of α-SMA
corresponding to GAPDH were analyzed using ImageJ 2× software
(version 2.1.4.7; National Institutes of Health, Bethesda, MD,
USA).
Western blot analysis
Following the indicated treatments, the PAFs were
washed twice with ice-cold PBS and lysed on ice in 0.3 ml lysis
buffer (Tris 50 mM, pH 7.4; NaCl 150 mM; Triton X-100 1%; EDTA 1
mM; and phenylmethane sulfonyl fluoride 2 mM) for 10 min. The
supernatants were collected and the total protein concentrations
were determined using the BCA method (Pierce; Thermo Fisher
Scientific, Inc.). Proteins (20 µg/lane) were separated by
10% SDS-PAGE and transferred onto nitrocellulose microporous
membranes (Pall Life Sciences, Port Washington, NY, USA). The
membranes were blocked with 5% skimmed milk powder for 30 min at
room temperature. The membranes were then incubated with primary
antibodies directed against P110α (cat. no. 4249; dilution
1:1,000), p-Akt (Ser 473; cat. no. 4060; dilution 1:1,000), total
Akt (cat. no. 9272; dilution 1:1,000), p70S6K (cat. no. 2708;
dilution 1:1,000), p-p70S6K (Thr 389; cat. no. 9205; dilution
1:1,000) and β-actin (cat. no. 3700; dilution 1:3,000) (all Cell
Signaling Technology, Inc.) and antibodies directed against
proliferating cell nuclear antigen (PCNA; cat. no. bs2006R;
dilution 1:500; Biosynthesis Biotechnology Co., Ltd.), α-SMA
(dilution 1:1,000) and GAPDH (dilution 1:500) overnight at 4°C. The
membranes were subsequently washed three times with PBS-Tween-20
for 10 min and then incubated with the the goat-anti-mouse (cat.
no. bs2096G; dilution 1:5,000) or goat-anti-rabbit (cat. no.
bs2095G; dilution 1:500) (both from Biosynthesis Biotechnology Co.,
Ltd.) secondary antibody for 1 h at room temperature. The blots
were developed using an enhanced chemiluminescence Western Blotting
Detection system with a ChemiDoc MP Imager (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The densitometry values of the bands were
measured using ImageJ 2× software (version 2.1.4.7).
Hypoxic rat model and grouping
Eight-week old, male SD rats were utilized for the
in vivo experiments. The rats were randomly assigned to a
normoxia group (N group), hypoxia group (H group) or hypoxia plus
NVP-BEZ235 group (HB group; n=6/group). The rats were maintained
under specific conditions for 21 days. Hypobaric hypoxia was
simulated in a laboratory-owned exclusive automatic hypobaric
chamber, which mimicked the gaseous environment at 5,000 m above
sea level with a fraction of inspired oxygen of 0.10. Rats in the
HB group were administered NVP-BEZ235 (in
N-methyl-2-pyrrolidone/PEG300, 1/9 by volume; 35 mg/kg) orally
every other day from day 0 until they were euthanized on day 21,
whereas the rats in the N and H groups were administered 0.9%
sodium chloride water as a control. The N group was placed in the
same pathogen-free room as the H and HB group, but under normoxic
conditions. Rats from all groups were kept under comparable living
conditions (25±2°C; 12 h light/dark cycles and humidity
40±10%).
All rats were anesthetized using 50 mg/kg sodium
pentobarbital, administered by intraperitoneal injection (5% sodium
pentobarbital; 1 ml/kg) at the terminal point of the hypoxia
exposure period. The right ventricular systolic pressure (RVSP) of
the rats was detected via the right heart catheterization technique
by connecting to a BL-420S biological function test system (Chengdu
Techman Software Co., Ltd., Chengdu, China). Blood samples were
taken from abdominal aorta of rats and tissue samples were taken
from chest cavity of the rats. The ratio of the weight of the right
ventricle to the left ventricle with the septum was used to assess
the grade of right ventricular hypertrophy index (RVHI). The ratio
of the weight of the right ventricle to the whole-body weight was
used to assess the right ventricular weight/body weight index
(RV/BW). The hematocrit (HCT) was evaluated by centrifugation of
the blood samples at 112 × g at 4°C for 10 min using a
microcentrifuge (Eppendorf, Hamburg, Germany).
Pathological staining assays
Lung specimens were obtained after blood samples
were taken and saline perfusion was carried out from the abdominal
aorta to reduce the blood cells in the lung tissue. The whole left
and right lung specimens were then taken from chest cavity of the
rats. These were then washed with 1X PBS, fixed with 4%
formaldehyde for 12 h at room temperature and embedded in paraffin
wax prior to the staining of 5-µm sections. Hematoxylin and
eosin (H&E; cat. no. DH0006), Russell Movat (cat. no. DC0080)
and Masson staining kits (cat. no. DC0033) were each purchased from
Beijing Leagene Biotech Co., Ltd. (Beijing, China) and utilized
according to the manufacturer's protocols. The stained specimens
were viewed using an optical micoscope (DP71; Olympus Corp.).
Immunohistochemistry assay
Lung tissues (5-µm sections), prepared as
described above, were tested by immunohistochemistry. An SPlink
Detection kit (Biotin-Streptavidin HRP Detection Systems; cat. no.
SP9000) and DAB Detection kit (cat. no. SP9000D) (both from OriGene
Technologies, Inc.) were used for the immunohistochemistry assay,
according to the manufacturer's protocol. The tissue sections were
incubated overnight at 4°C with primary antibodies, which were
directed against p110α (dilution 1:200), p-Akt (dilution 1:200),
Akt (dilution 1:200), p70S6K (dilution 1:200), fibronectin (cat.
no. 4857R; dilution 1:400; Biosynthesis Biotechnology Co., Ltd.)
and α-SMA (dilution 1:400). The secondary antibodies contained in
the SPlink Detection kits were then incubated with the sections for
1 h at room temperature. The stained specimens were viewed through
an optical micoscope (DP71). Based on the pathology staining and
immunohistochemistry results, the medial thickness (MT), medial
cross-sectional area (MA), adventitial thickness (AT) and collagen
deposition area percentage of the lung specimens of rats were
measured and analyzed (n=15; 5 pulmonary arterials in 3 rat per
group).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Differences between multiple groups were tested by
one-way analysis of variance followed by Tukey's post hoc test and
a two-tailed t-test was used for the comparison of two groups.
P<0.05 was considered to indicate a statistically significant
difference. The plotting and statistical analysis of the data was
performed using GraphPad Prism software, version 5 (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
Hypoxia promotes the proliferation,
migration and differentiation of rat PAFs in vitro
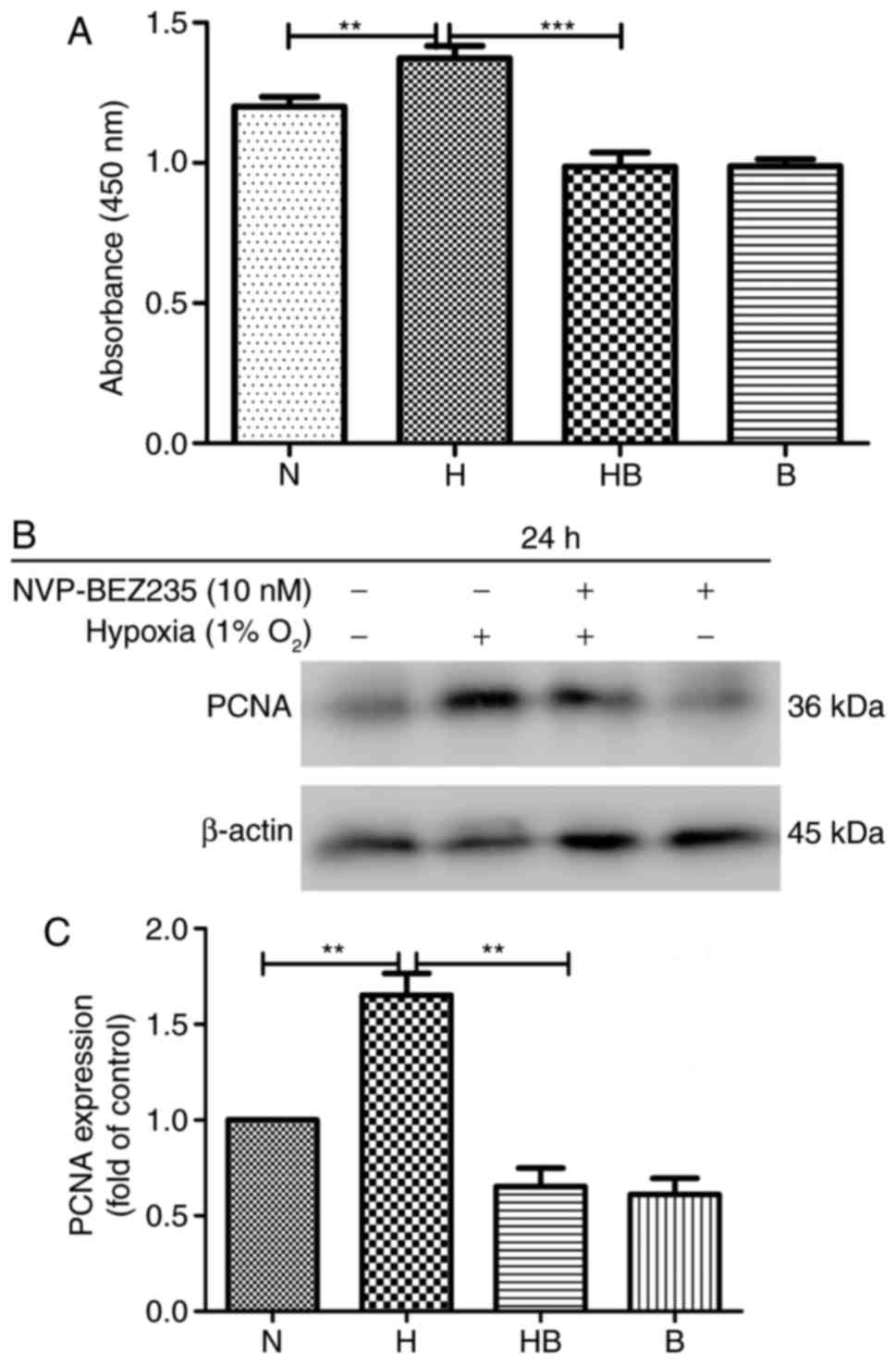
The results of the CCK-8 assay revealed that the
proliferation of PAFs was significantly increased (14%) under
hypoxic conditions compared with normoxic conditions (P=0.008)
(Fig. 1A). Western blot analysis
of the protein samples from cells in each group following culture
for 24 h revealed that the PAFs had a significantly enhanced
expression of PCNA (1.52±0.29 higher) under hypoxic conditions
compared with normoxic conditions (P=0.0032) (Fig. 1B and C).
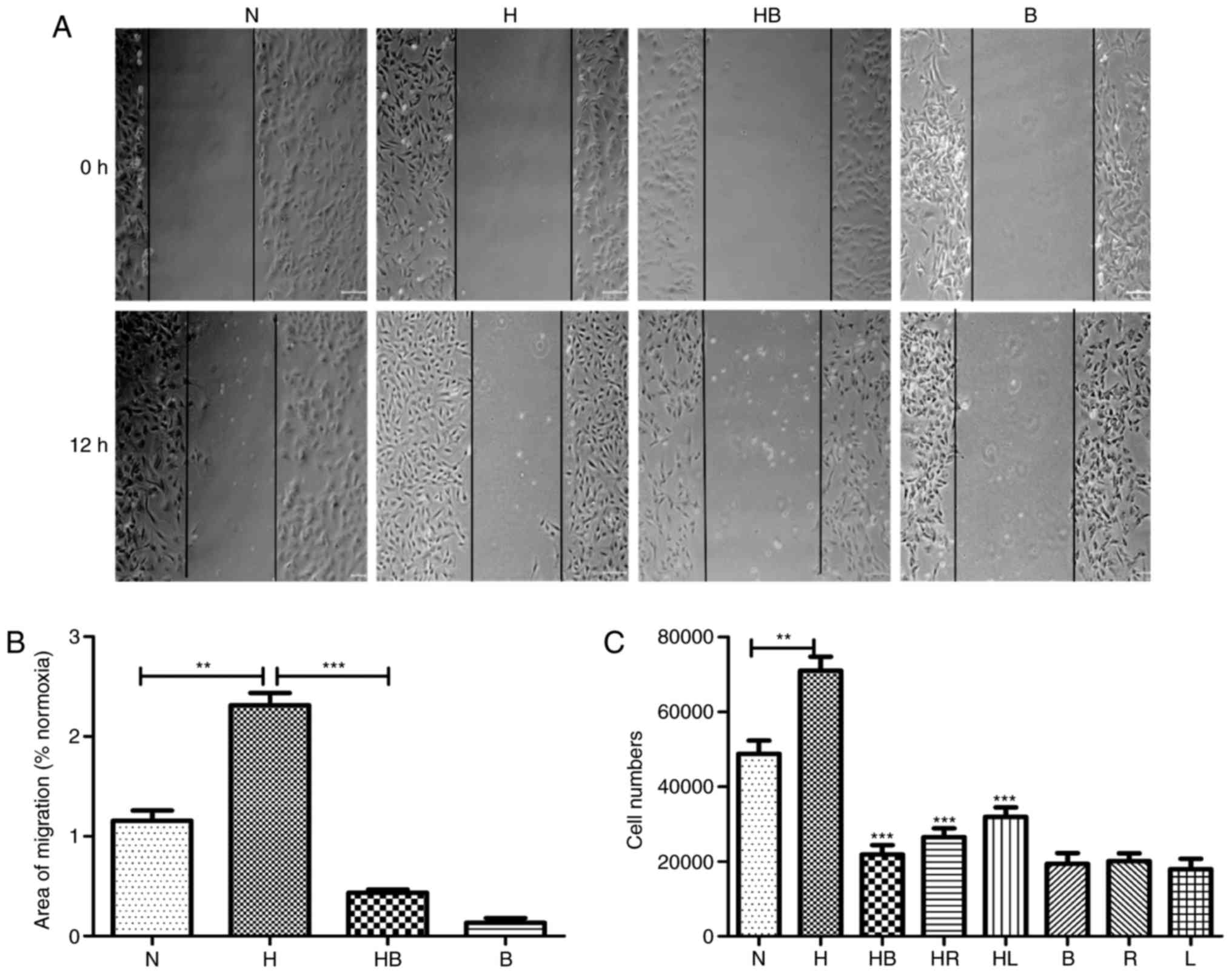
In the wound healing assay, images captured after 12
h demonstrated the migration activity of the groups (Fig. 2A). The migration areas of each
group were measured and the results revealed that hypoxia
significantly promoted the migration activity of PAFs
1.31±0.21-fold compared with that of PAFs cultured in normoxic
conditions (P=0.0018) (Fig. 2B).
In the Transwell migration assay, the stained cell numbers revealed
that hypoxia significantly increased the number of PAFs that
migrated compared with the normoxia group (P=0.008) (Fig. 2C).
Optical microscopy images revealed that PAFs
cultured under hypoxic conditions for 24 and 48 h changed their
cellular morphology from long fusiform to irregular shapes
(Fig. 3A). This was also
demonstrated in the immunofluorescence images (Fig. 3B). α-SMA and GAPDH were stained
green and red, respectively, and the nuclei were stained blue with
DAPI in the merged images. The fluorescence intensities of α-SMA
corresponding to GAPDH were analyzed using ImageJ software
(Fig. 3C). These results revealed
that hypoxia significantly increased α-SMA expression compared with
that in normoxic conditions (P<0.001). Additionally, western
blot analysis also indicated that hypoxia significantly elevated
the expression of α-SMA compared with that in the normoxia group
(P=0.006) (Fig. 3D and E).
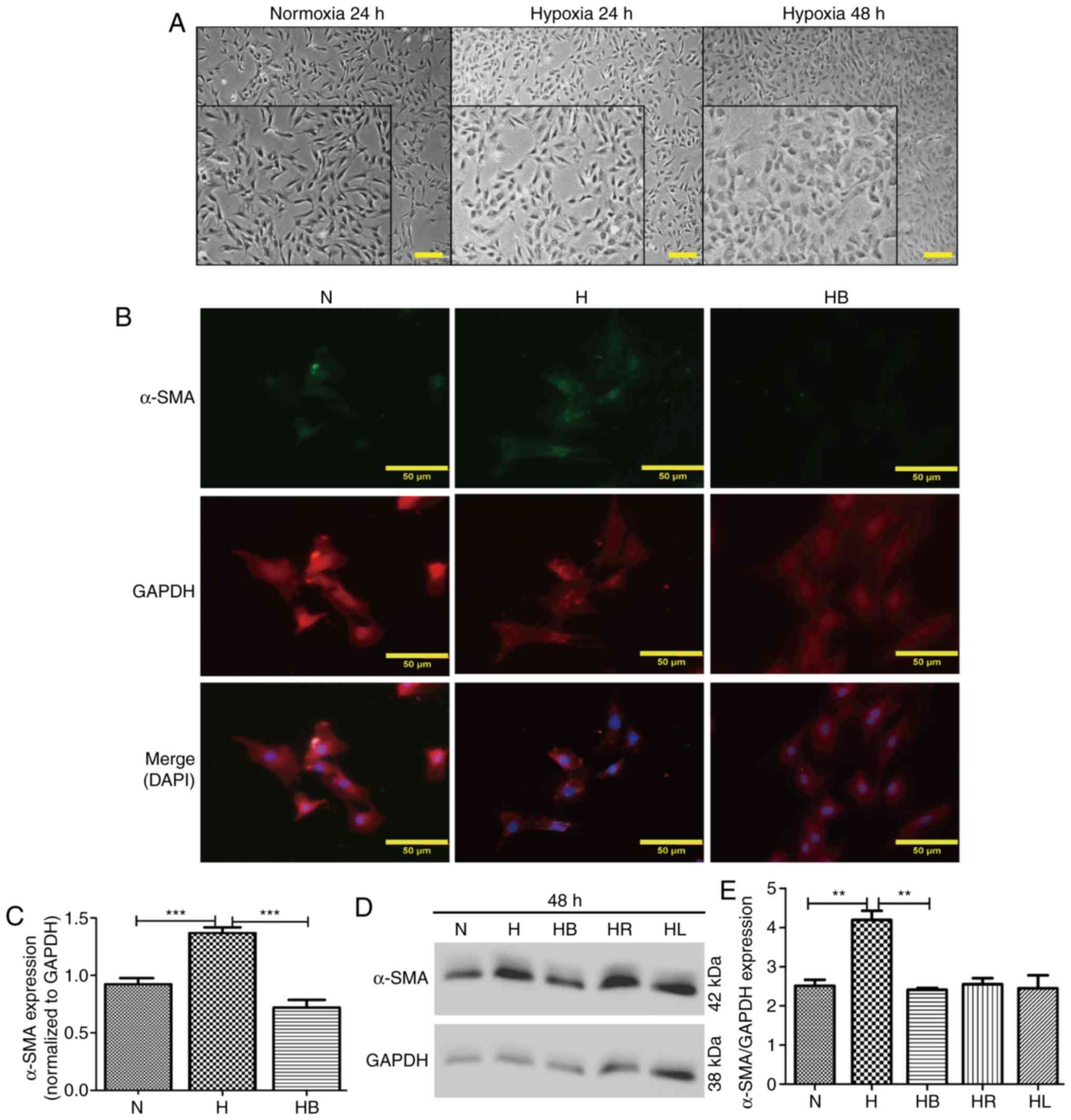 | Figure 3Hypoxia-induced differentiation of
PAFs requires activation of the PI3K/Akt/p70S6K signaling pathway.
(A) Cellular morphology of PAFs under normoxic and hypoxic
conditions. The images were captured using an optical microscope
(magnification, main images, ×200, enlarged images, ×450). A
portion of the photographs was enlarged and displayed at the bottom
left corner. (B) PAFs were treated as indicated for 24 h, then
subjected to immunofluorescence staining. α-SMA was stained green,
GAPDH was stained red and the nuclei were stained blue by DAPI in
the merged images. (C) The expression of α-SMA in the
immunofluorescence photographs was examined (n=3). (D) Western blot
analysis was performed to evaluate the expression of α-SMA and
GAPDH in the PAFs. (E) The expression of α-SMA in the western blot
was quantified. PAFs, pulmonary arterial fibroblasts; N, normoxia;
H, hypoxia; HB, hypoxia with NVP-BEZ235; HR, hypoxia with
rapamycin; HL, hypoxia with LY294002; α-SMA, α-smooth muscle actin.
**P<0.01 and ***P<0.001. |
Hypoxia upregulates the PI3K/Akt/p70S6K
signaling pathway in PAFs in vitro
PAFs were cultured under hypoxia for 24 h and
western blot analysis (Fig. 4A)
was conducted. The results revealed that the expression levels of
p110α (Fig. 4B), p-Akt (Fig. 4C) and p-p70S6K (Fig. 4D) were significantly increased in
the hypoxia group compared with the normoxia group (P<0.01).
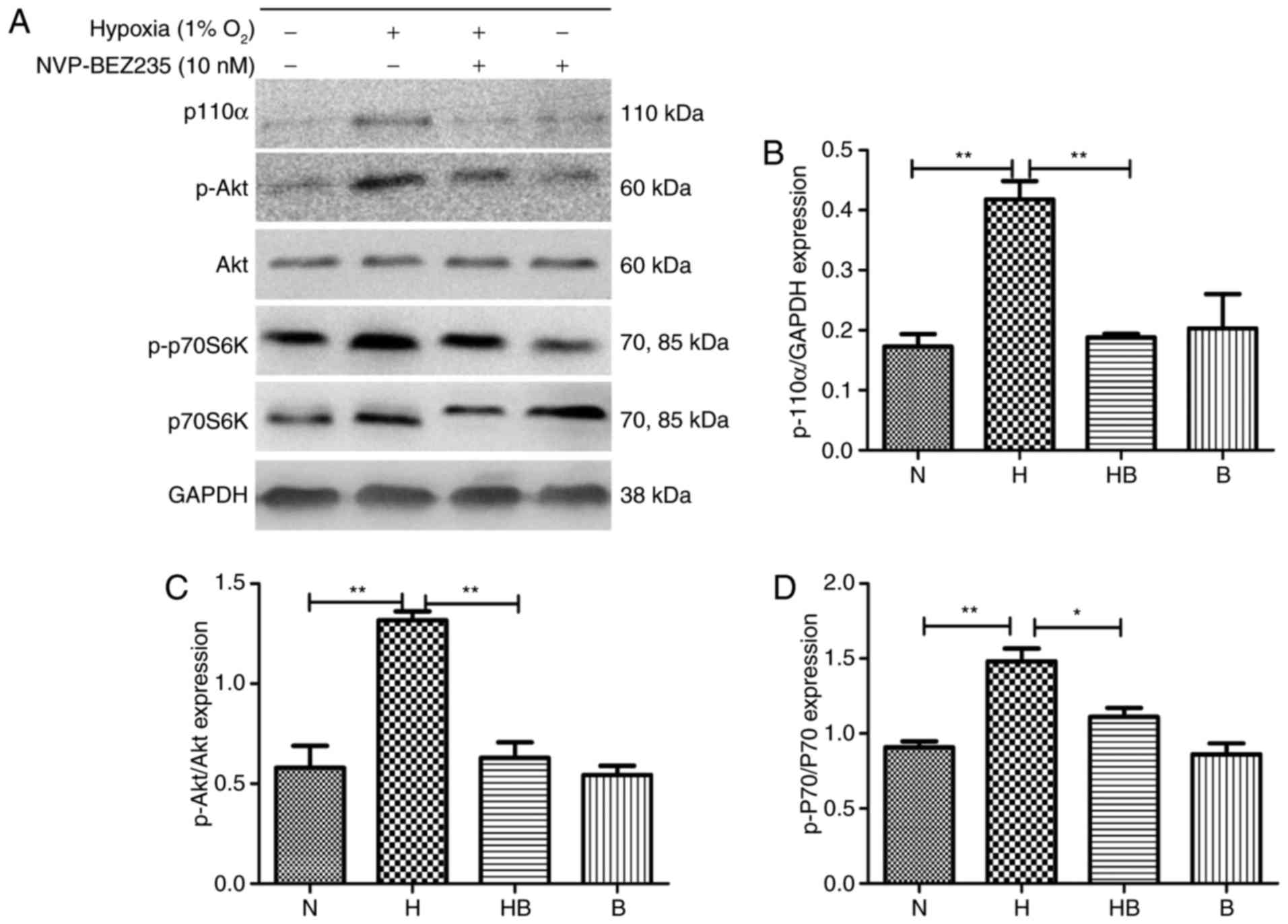 | Figure 4Hypoxia upregulates the
PI3K/Akt/p70S6K signaling pathway in PAFs. (A) Western blot
analysis of the indicated proteins in PAFs following treatment with
NVP-BEZ235 and hypoxia as indicated for 24 h. Quantification of (B)
p110α, (C) p-Akt and (D) p-p70S6K expression in the western blot
assay (n=3). PAFs, pulmonary arterial fibroblasts; PI3K,
phosphatidylinositol-3-kinase; Akt, protein kinase B; p70S6K, p70
ribosomal protein S6 kinase; p, phosphorylated; N, normoxia; H,
hypoxia; HB, hypoxia with NVP-BEZ235; B, NVP-BEZ235.
*P<0.05 and **P<0.01. |
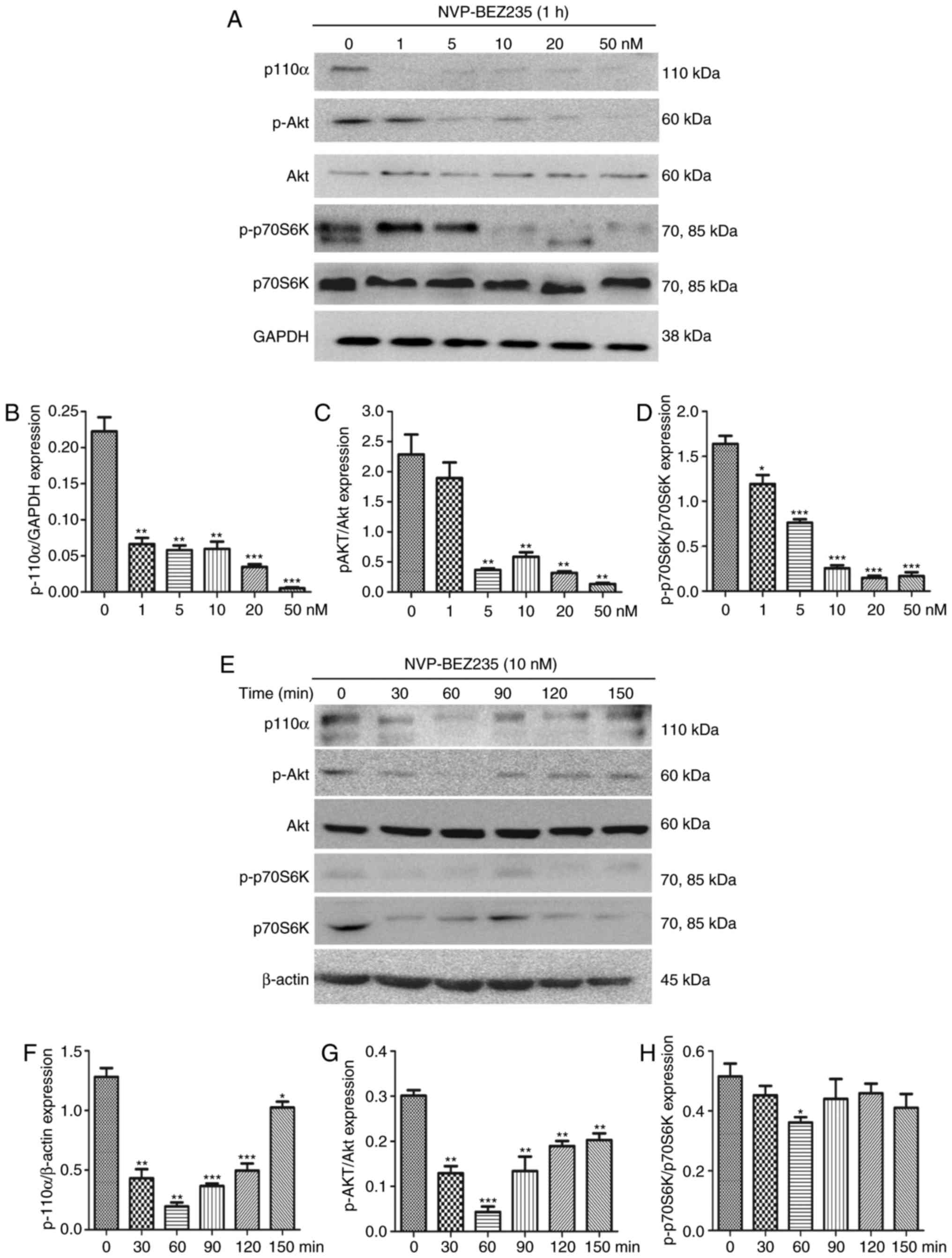
The dual PI3K/mTOR inhibitor NVP-BEZ235 was utilized
to inhibit the PI3K/mTOR signaling pathway. To confirm the
effectiveness of NVP-BEZ235, PAFs were cultured for 1 h with
NVP-BEZ235 at a range of concentrations (2.5–50 nM) and the
expression levels of P110α, p-Akt and p-p70S6K were measured using
western blotting (Fig. 5A–D). The
results demonstrate that low concentrations of NVP-BEZ235 (1–5 nM)
significantly inhibited the expression of p110α (1 nM; P=0.0021),
p-Akt (5 nM; P=0.0043) and p-p70S6K (5 nM; P=0.0065). PAFs were
also treated with 10 nM NVP-BEZ235 for varying time periods (30–150
min; Fig. 5E–H) and the
expression levels of P110α, p-Akt and p-p70S6K were measured using
western blotting. The results reveal that the expression of p110α
(P<0.01) was significantly inhibited at 30 min and the
expression levels of p-Akt (P=0.0013) and p-p70S6K
(P<0.001) were significantly inhibited at 60 min. No significant
differences were identified in the expression of Akt and p70S6K
following treatment with NVP-BEZ235 (data not shown).
Hypoxia-induced proliferation, migration
and differentiation are reduced following inhibition of the
PI3K/Akt/p70S6K signaling pathway
The proliferation of PAFs under hypoxic conditions
was significantly reduced following the inhibition of the PI3K/mTOR
signaling pathway by NVP-BEZ235 (P<0.001) (Fig. 1A). The expression of PCNA in rat
PAFs was also significantly downregulated by pretreatment with
NVP-BEZ235 prior to hypoxia, compared with that in the hypoxia
group (P=0.0041) (Fig. 1B and
C).
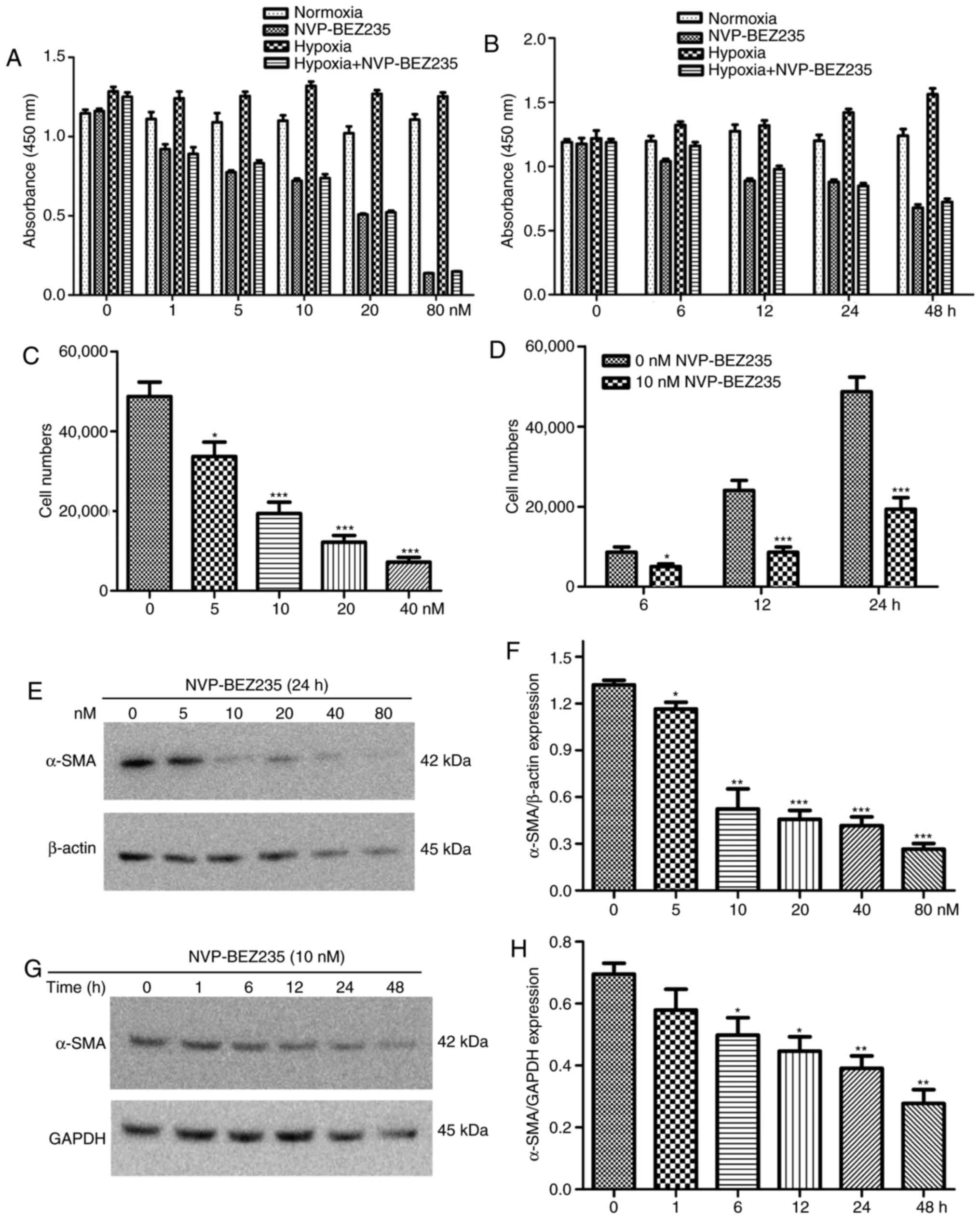
In other experiments, PAFs were incubated with
different concentrations of NVP-BEZ235 (0, 1, 5, 10, 20 and 80 nM)
for 24 h, or they were incubated with 10 nM NVP-BEZ235 for
increasing periods of time (0, 6, 12, 24 and 48 h). The results
indicated that stronger and prolonged inhibition of the PI3K/mTOR
signaling pathway by NVP-BEZ235 influenced the proliferative
activity of PAFs under normoxic and hypoxic conditions (Fig. 6A and B).
Additionally, the wound healing assay revealed that
NVP-BEZ235 significantly reduced the hypoxia-induced migratory
activity of PAFs compared with that in the hypoxia group
(P<0.001) (Fig. 2B).
Furthermore, the Transwell assay revealed that rapamycin, LY294002
and NVP-BEZ235 significantly reduced the migration of rat PAFs
under hypoxic conditions (P<0.001) (Fig. 2C). In other Transwell experiments,
PAFs were incubated with increasing concentrations of NVP-BEZ235
(0, 5, 10, 20 and 40 nM) for 24 h, or were incubated with 10 nM
NVP-BEZ235 for increasing periods of time (0, 6, 12 and 24 h). The
results also indicated that inhibition of the PI3K/mTOR signaling
pathway with NVP-BEZ235 significantly reduced the migratory
activity of PAFs under hypoxia in a dose dependent manner (Fig. 6C) and this significant reduction
in migration was observed at all time periods tested (P<0.05)
(Fig. 6D).
NVP-BEZ235 pretreatment significantly decreased the
hypoxia-induced elevation of α-SMA expression in the
immunofluorescence assay (P<0.001) (Fig. 3C). Western blot analysis confirmed
that NVP-BEZ235 attenuated the expression of α-SMA in PAFs cultured
under hypoxic conditions for 48 h (P=0.017) (Fig. 3E). However, no significant
difference in a-SMA expression was identified in PAFs cultured
under hypoxic conditions following pretreatment with LY294002
(Fig. 3D and E). In addition, it
was observed that treatment of PAFs with ≥10 nM concentrations of
NVP-BEZ235 had a significant inhibitory effect on the expression of
α-SMA compared with the 0 nM control group (P<0.05) (Fig. 6E and F). When PAFs were treated
with NVP-BEZ235 at 10 nM for increasing time periods (1, 6, 12, 24
and 48 h), a significant reduction in α-SMA expression was observed
from 6 h (P<0.05) (Fig. 6G and
H).
Hypoxia contributes to the pulmonary
vascular remodeling of hypoxic rats in vivo
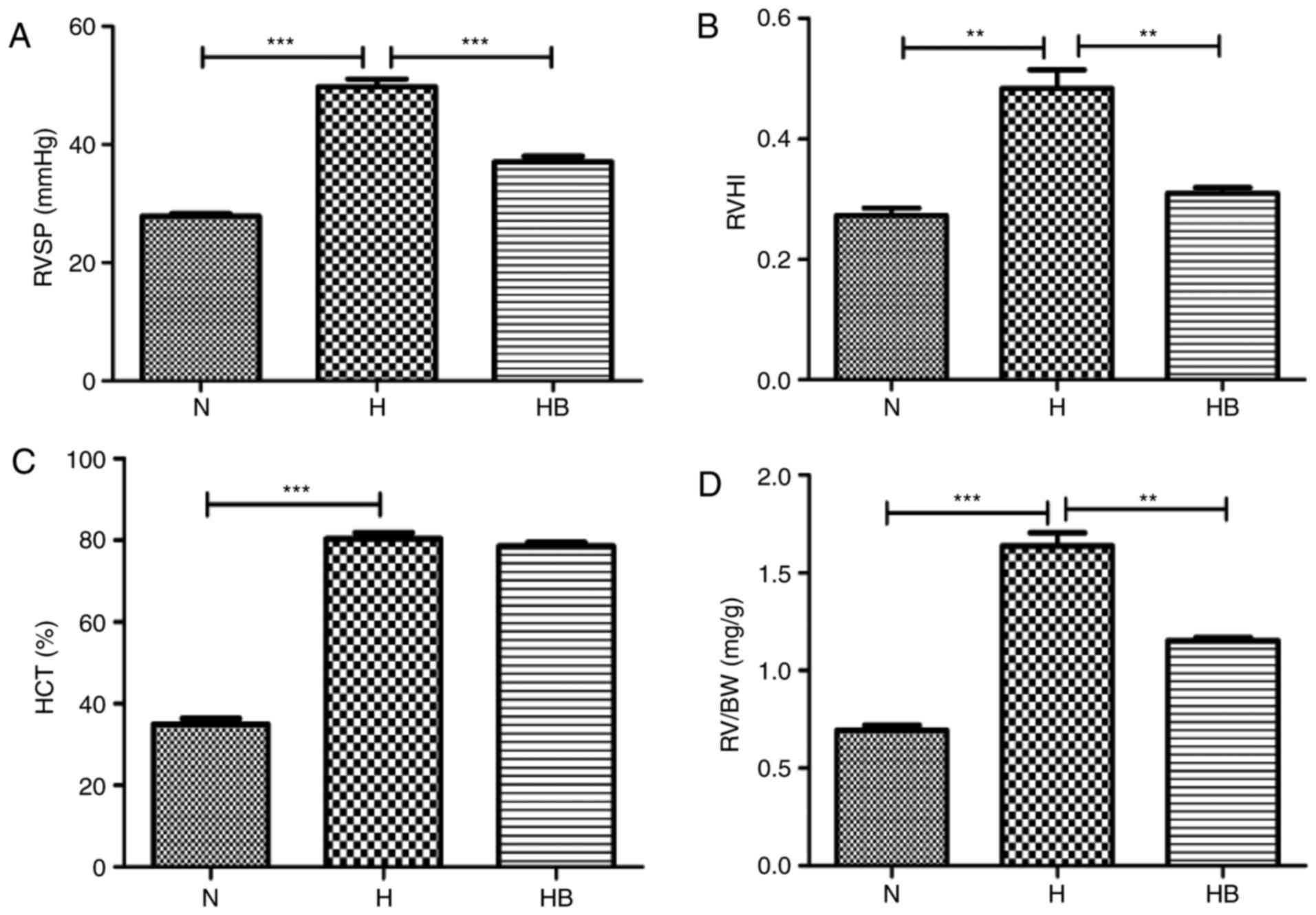
The RVSP in the H group (49.76±1.31 mmHg) was
significantly elevated compared with that in the N group
(27.85±0.42 mmHg; P<0.001) (Fig.
7A). The RVHI reflects the right ventricular remodeling caused
by pulmonary arterial pressure elevation, and the RVHI in the H
group (0.48±0.03) was significantly higher than that in the N group
(0.27±0.01; P=0.0032) (Fig. 7B).
The HCT of the H group (80.38±1.49%) was significantly higher than
that of the N group (34.96±1.40%; P<0.0010 (Fig. 7C). The RV/BW data revealed that
the RV/BW of the H group (1.63±0.07 mg/g) was significantly higher
than that of the N group (0.69±0.02 mg/g; P=0.0002) (Fig. 7D). H&E staining of the rat
lung tissues presented clear views of the arteries (diameter
<200 µm) (Fig. 8A).
Russell Movat staining and Masson staining was conducted to reveal
the collagen deposition around the pulmonary vessels. It was
observed that the H group had an increased thickening of the
pulmonary vessel walls and small vessel lumens compared with the N
group. The same trend was observed for the collagen deposition area
around the vessels. Immunohistochemistry results revealed that the
H group had increased expression of fibronectin and α-SMA compared
with the other groups. The perimeter of the lumen and >5 small
pulmonary vascular vessels in a minimum of 3 rats per group were
measured. The MT, MA, AT and collagen deposition area were
calculated to assess the severity of the pulmonary vascular
remodeling (Fig. 8B–E). The H
group had a significantly higher MT, MA, AT and collagen deposition
area (41.64±1.54, 65.50±1.50, 64.33±7.02 and 64.00±7.81%,
respectively) compared with the N group (28.65±1.91,
P<0.001; 48.67±2.68, P<0.001; 26.00±5.66, P=0.0018;
9.80±2.95%, P<0.001, respectively) (Fig. 8B–E).
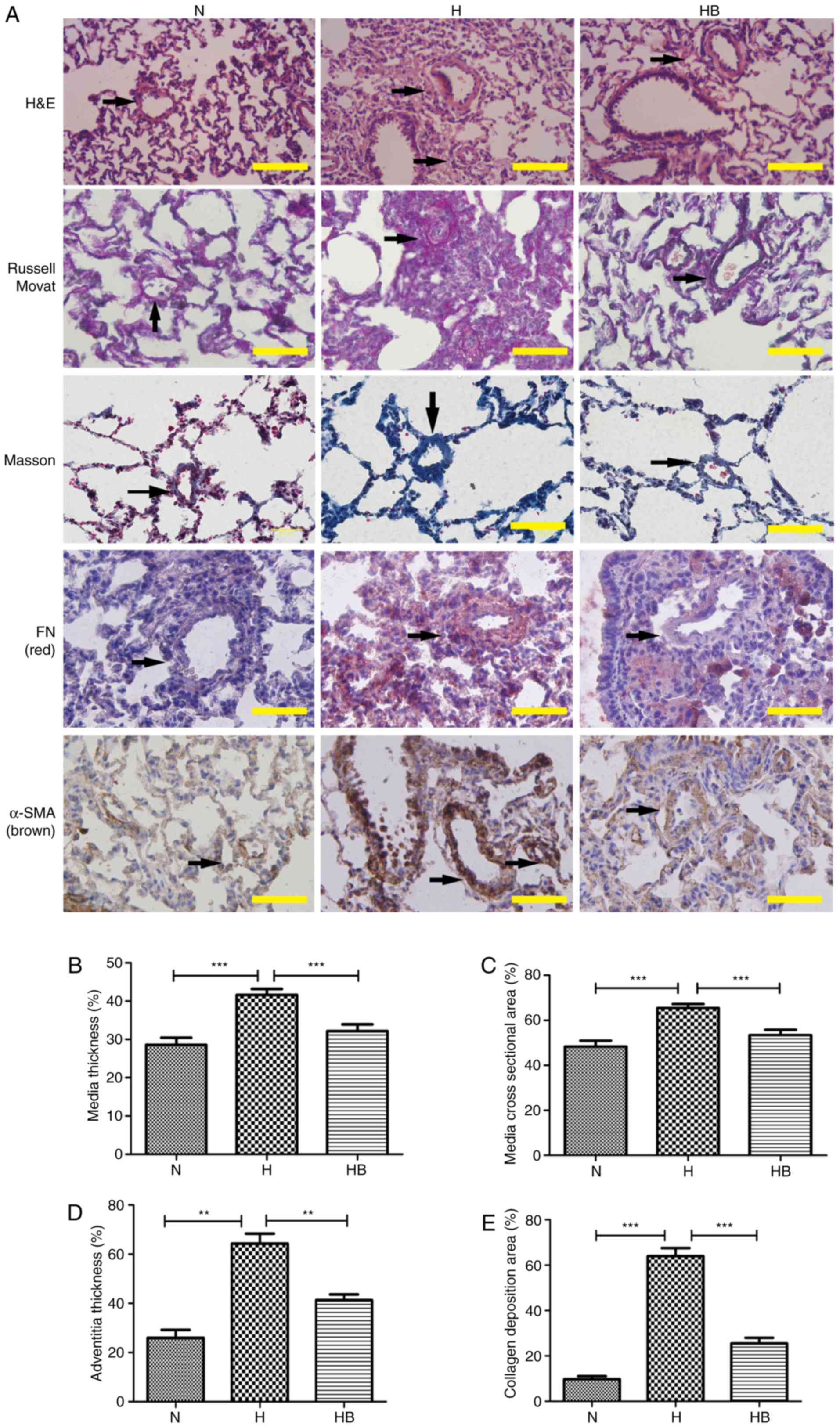 | Figure 8The PI3K/Akt/p70S6K signaling pathway
contributes to the pulmonary vascular remodeling of hypoxic rats.
(A) H&E, Russell Movat, Masson and immunohistochemical staining
of FN and α-SMA in lung tissue isolated from rats in the different
groups. The black arrows indicate the pulmonary vessels. The (B)
medial thickness, (C) medial cross-sectional area, (D) adventitial
thickness and (E) collagen deposition area were calculated. A total
of 3 rats were randomly selected from each group and at least five
pulmonary arterials of each rat were measured. Scale bar, 100
µm. N, normoxia; H, hypoxia; HB, hypoxia with NVP-BEZ235;
FN, fibronectin; H&E, hematoxylin and eosin.
**P<0.01 and ***P<0.001. |
The PI3K/Akt/p70S6K signaling pathway is
upregulated in the pulmonary vessels of hypoxic rats
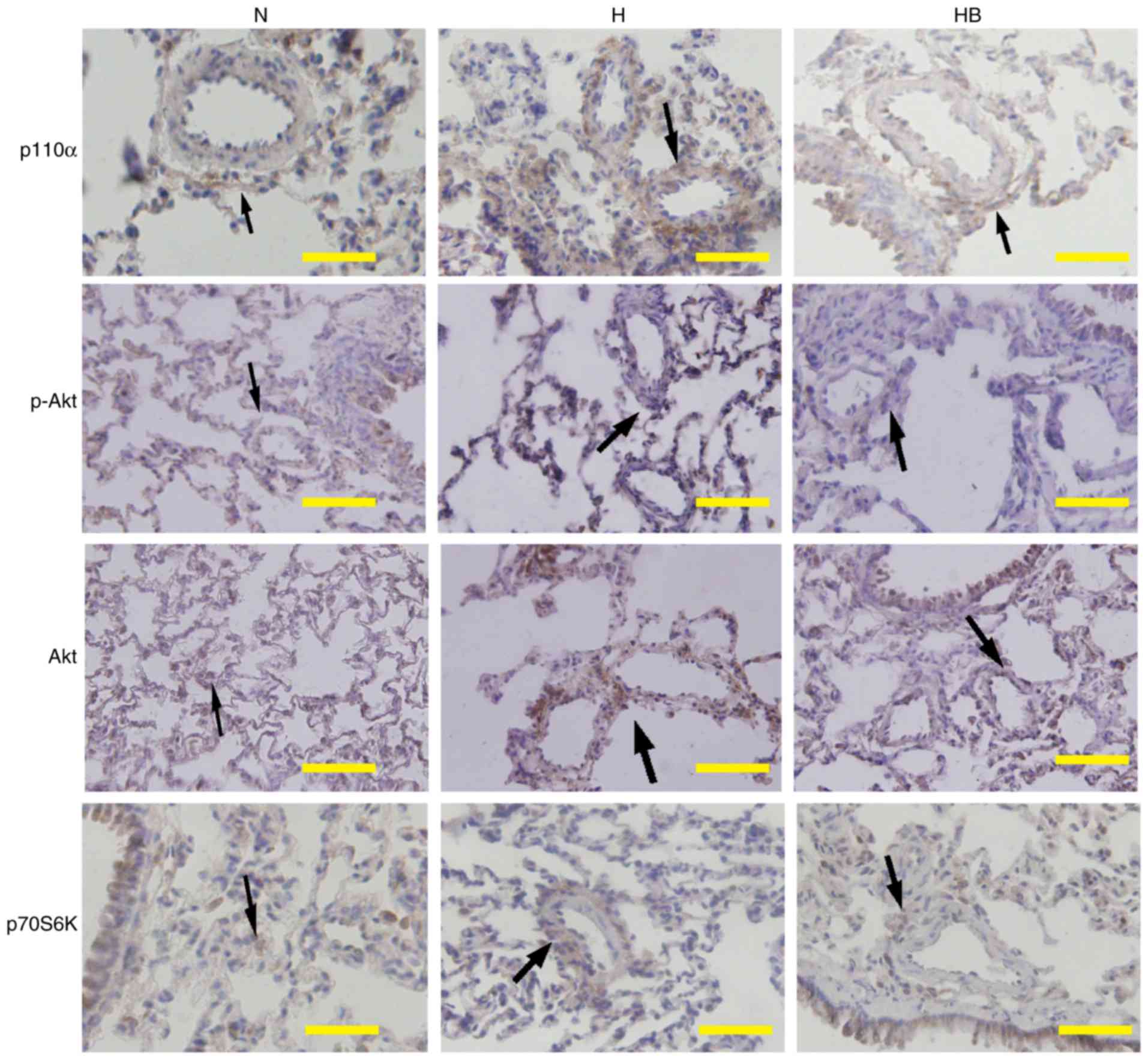
Immunohistochemistry results of lung tissues from
the HPH rats revealed that hypoxia markedly increased the
expression of p110α, p-Akt and p70S6K around the adventitious small
pulmonary vasculature in vivo. Pretreatment with NVP-BEZ235
clearly inhibited the hypoxia-induced elevation of p110α, p-Akt and
p70S6K expression in vivo (Fig. 9).
NVP-BEZ235 attenuates hypoxia-induced
pulmonary vascular remodeling
The RVSP of the HB group (37.10±0.91 mmHg) was
significantly lower than that of the H group (P<0.001)
(Fig. 7A). The RVHI of the HB
group (0.31±0.01) was significantly lower than that of the H group
(P=0.006) (Fig. 7B). The RV/BW
ratio of the HB group (1.15±0.02 mg/g) was also significantly lower
than that of the H group (P=0.0021) (Fig. 7D). No significant difference was
identified in the HCT of the HB group (78.57±0.87%) compared with
the H group (Fig. 7C). These
results indicate that the HB group had less thickening of the
vascular walls compared with the H group. The immunohistochemistry
staining revealed that pretreatment with NVP-BEZ235 (HB group)
significantly attenuated the hypoxia-induced MT, MA, AT and
collagen deposition area (32.17±1.75, 53.42±2.34, 41.33±4.04 and
25.60±5.27, respectively) compared with the H group (P<0.001,
P=0.0003, P=0.0008 and P<0.001, respectively) (Fig. 8B–E).
Discussion
The in vitro experiments in the present study
demonstrated that a hypoxic environment promotes the proliferation,
migration and differentiation of rat PAFs. To investigate the
possible mechanisms underlying this enhanced proliferation,
migration and differentiation, the present study focused on the
PI3K/Akt/p70S6K signaling pathway. PI3K may be activated by growth
factors and hormones, and in turn coordinates the cell cycle, and
the growth, migration and survival of cells. p70S6K is activated by
positive signaling from mTOR, and serves a critical role in the
regulation of migration where it acts in the process of
polymerization (32,33). Akt promotes cell survival by
inhibiting apoptosis, and is also associated with the regulation of
the cell cycle (32–36). Previous studies have indicated
that the PI3K/Akt/p70S6K signaling pathway serves an essential role
in the development and functions of blood vessels (37). The authors of the present study
hypothesized that the PI3K/Akt/p70S6K signaling pathway may be
involved in the hypoxia-induced proliferation, migration and
differentiation behaviors of rat PAFs, and could be associated with
hypoxia-induced pulmonary vascular remodeling in hypoxic rats.
The results of the present study support this
hypothesis. The in vitro experiments demonstrated that the
PI3K/Akt/p70S6K signaling pathway in rat PAFs was significantly
activated by hypoxia (1% O2), although previous studies
have reported that hypoxia triggers the PI3K/mTOR signaling pathway
in a cell-specific manner, different kinds of cells could show
quite different changes of the PI3K/mTOR signaling pathway under
hypoxia. Consistent with the previous studies, the present study
demonstrated that hypoxia upregulates the PI3K/mTOR signaling
pathway of PAFs (10,24,29). In the present study, rat PAFs
exhibited distinct capabilities to proliferate, migrate and
differentiate in response to hypoxia without exogenous growth
factors, which was consistent with the findings of previous studies
(28,37). It was subsequently considered that
the PI3K/Akt/p70S6K signaling pathway may be involved in the
hypoxia-induced proliferation, migration and differentiation
behaviors of rat PAFs. Various inhibitors of the PI3K/Akt/p70S6K
signaling pathway were used to investigate this, and it was
demonstrated that the hypoxia-induced proliferation, migration and
differentiation were notably reduced when the inhibitors were
administered. This strengthened the evidence that the
PI3K/Akt/p70S6K signaling pathway was necessary for the changes in
the hypoxia-induced proliferation, migration and differentiation in
rat PAFs that were observed.
The results of the in vivo experiments
revealed that hypoxia induced pulmonary vascular remodeling with
the elevation of RVSP, RVHI and RV/BW, and the promotion of MA, MT,
AT and the collagen deposition area. This suggests that hypoxia
induced pulmonary vascular remodeling. The results also
demonstrated that the expression of p110α, p-Akt and p70S6K was
significantly increased and the PI3K/Akt/p70S6K signaling pathway
was activated during the hypoxic exposure period. To examine if the
activation of the PI3K/Akt/p70S6K signaling pathway was necessary
for the development of hypoxic pulmonary vascular remodeling,
NVP-BEZ235 was used to block the PI3K/Akt/p70S6K signaling pathway
and the results indicated that the hypoxia-induced pulmonary
vascular remodeling was attenuated. The results of the in
vivo experiments indicate the important roles of the
PI3K/Akt/p70S6K signaling pathway in the hypoxia-induced pulmonary
vascular remodeling process of hypoxic rats.
The results of the present study also indicated that
NVP-BEZ235 significantly inhibited Akt signaling, which is likely
to be due to the inhibition of upstream PI3K and its downstream
feedback as the agent did not inhibit Akt directly (38,39). The HCT results suggest that
erythrocyte production under hypoxia is not directly regulated by
the PI3K/mTOR signaling pathway; further research is required to
explore the detailed mechanisms behind this (40,41).
In conclusion, the findings of the present study
suggest that the PI3K/Akt/p70S6K signaling pathway is essential for
hypoxia-induced PAF proliferation, migration and differentiation,
as well as for pulmonary vascular remodeling in hypoxic rats.
NVP-BEZ235 is able to attenuate the hypoxia-induced pulmonary
vascular remodeling of hypoxic rats in vivo, which suggests
it may be a potential intervention target for HPH.
Acknowledgments
The authors would like to thank Professor Junbao Du
and Professor Hongfang Jin (Pediatric Department of Peking
University First Hospital, Beijing, China) and Dr Yong Wang and Dr
Lei Pan (Department of Geriatrics, Beijing Shijitan Hospital of
Capital Medicine University, Beijing, China) for their technical
assistance, and the Beijing Shijitan Hospital of Capital Medicine
University (Beijing, China) for access to its hypoxia laboratory.
The present study was supported by the National Natural Science
Foundation of China (grant no. 81270114).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Oudiz RJ: Classification of pulmonary
hypertension. Cardiol Clin. 34:359–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Poor HD, Girgis R and Studer SM: World
Health Organization Group III pulmonary hypertension. Prog
Cardiovasc Dis. 55:119–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nathan SD and Hassoun PM: Pulmonary
hypertension due to lung disease and/or hypoxia. Clin Chest Med.
34:695–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Veit F, Pak O, Brandes RP and Weissmann N:
Hypoxia-dependent reactive oxygen species signaling in the
pulmonary circulation: Focus on ion channels. Antioxid Redox
Signal. 22:537–552. 2015. View Article : Google Scholar :
|
|
5
|
Humbert M, Montani D, Evgenov OV and
Simonneau G: Definition and classification of pulmonary
hypertension. Handb Exp Pharmacol. 218:3–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Veith C, Schermuly RT, Brandes RP and
Weissmann N: Molecular mechanisms of hypoxia-inducible
factor-induced pulmonary arterial smooth muscle cell alterations in
pulmonary hypertension. J Physiol. 594:1167–1177. 2016. View Article : Google Scholar
|
|
7
|
Stenmark KR, Nozik-Grayck E,
Gerasimovskaya E, Anwar A, Li M, Riddle S and Frid M: The
adventitia: Essential role in pulmonary vascular remodeling. Compr
Physiol. 1:141–161. 2011.PubMed/NCBI
|
|
8
|
Chazova I, Loyd JE, Zhdanov VS, Newman JH,
Belenkov Y and Meyrick B: Pulmonary artery adventitial changes and
venous involvement in primary pulmonary hypertension. Am J Pathol.
146:389–397. 1995.PubMed/NCBI
|
|
9
|
Krick S, Hänze J, Eul B, Savai R, Seay U,
Grimminger F, Lohmeyer J, Klepetko W, Seeger W and Rose F:
Hypoxia-driven proliferation of human pulmonary artery fibroblasts:
Cross-talk between HIF-1alpha and an autocrine angiotensin system.
FASEB J. 19:857–859. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stenmark KR, Davie N, Frid M,
Gerasimovskaya E and Das M: Role of the adventitia in pulmonary
vascular remodeling. Physiology. 21:134–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das M, Bouchey DM, Moore MJ, Hopkins DC,
Nemenoff RA and Stenmark KR: Hypoxia-induced proliferative response
of vascular adventitial fibroblasts is dependent on g
protein-mediated activation of mitogen-activated protein kinases. J
Biol Chem. 276:15631–15640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Si Y, Ren J, Wang P, Rateri DL, Daugherty
A, Shi XD, Kent KC and Liu B: Protein kinase C-delta mediates
adventitial cell migration through regulation of monocyte
chemoattractant protein-1 expression in a rat angioplasty model.
Arterioscler Thromb Vasc Biol. 32:943–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Conte E, Fruciano M, Fagone E, Gili E,
Caraci F, Iemmolo M, Crimi N and Vancheri C: Inhibition of PI3K
prevents the proliferation and differentiation of human lung
fibroblasts into myofibroblasts: The role of class I P110 isoforms.
PLoS One. 6:e246632011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Das M, Dempsey EC, Reeves JT and Stenmark
KR: Selective expansion of fibroblast subpopulations from pulmonary
artery adventitia in response to hypoxia. Am J Physiol Lung Cell
Mol Physiol. 282:L976–L986. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L, Li Y, Liu Y, Wang X, Chen M, Xing
Y and Zhu D: STAT3-mediated MMP-2 expression is required for
15-HETE-induced vascular adventitial fibroblast migration. J
Steroid Biochem Mol Biol. 149:106–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Chen Y, Li G, Chen M, Huang W,
Liu Y and Li Y: TGF-β1/FGF-2 signaling mediates the 15-HETE-induced
differentiation of adventitial fibroblasts into myofibroblasts.
Lipids Health Dis. 15:22016. View Article : Google Scholar
|
|
17
|
Li Y, Zhang L, Wang X, Chen M, Liu Y, Xing
Y, Wang X, Gao S and Zhu D: Elk-1-mediated 15-lipoxygenase
expression is required for hypoxia-induced pulmonary vascular
adventitial fibroblast dynamics. Acta Physiol (Oxf). 218:276–289.
2016. View Article : Google Scholar
|
|
18
|
Zhang Y, Talwar A, Tsang D, Bruchfeld A,
Sadoughi A, Hu M, Omonuwa K, Cheng KF, Al-Abed Y and Miller EJ:
Macrophage migration inhibitory factor mediates hypoxia-induced
pulmonary hypertension. Mol Med. 18:215–223. 2012. View Article : Google Scholar :
|
|
19
|
Anwar A, Li M, Frid MG, Kumar B,
Gerasimovskaya EV, Riddle SR, McKeon BA, Thukaram R, Meyrick BO,
Fini MA and Stenmark KR: Osteopontin is an endogenous modulator of
the constitutively activated phenotype of pulmonary adventitial
fibroblasts in hypoxic pulmonary hypertension. Am J Physiol Lung
Cell Mol Physiol. 303:L1–L11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan W, Liu W, Cai H, Sun X, Yang D, Xu F
and Jin C: SB-431542, a specific inhibitor of the TGF-β type I
receptor inhibits hypoxia-induced proliferation of pulmonary artery
adventitial fibroblasts. Pharmazie. 71:94–100. 2016.PubMed/NCBI
|
|
21
|
Welsh DJ, Scott PH and Peacock AJ: p38 MAP
kinase isoform activity and cell cycle regulators in the
proliferative response of pulmonary and systemic artery fibroblasts
to acute hypoxia. Pulm Pharmacol Ther. 19:128–138. 2006. View Article : Google Scholar
|
|
22
|
Carlin CM, Celnik DF, Pak O, Wadsworth R,
Peacock AJ and Welsh DJ: Low-dose fluvastatin reverses the hypoxic
pulmonary adventitial fibroblast phenotype in experimental
pulmonary hypertension. Am J Respir Cell Mol Biol. 47:140–148.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panzhinskiy E, Zawada WM, Stenmark KR and
Das M: Hypoxia induces unique proliferative response in adventitial
fibroblasts by activating PDGFβ receptor-JNK1 signalling.
Cardiovasc Res. 95:356–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gerasimovskaya EV, Tucker DA and Stenmark
KR: Activation of phosphatidylinositol 3-kinase, Akt, and mammalian
target of rapamycin is necessary for hypoxia-induced pulmonary
artery adventitial fibroblast proliferation. J Appl Physiol (1985).
98:722–731. 2005. View Article : Google Scholar
|
|
25
|
Garat CV, Crossno JT Jr, Sullivan TM,
Reusch JE and Klemm DJ: Inhibition of phosphatidylinositol
3-kinase/Akt signaling attenuates hypoxia-induced pulmonary artery
remodeling and suppresses CREB depletion in arterial smooth muscle
cells. J Cardiovasc Pharmacol. 62:539–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan Z, Li C, Qin C, Xie L, Wang X, Gao Z,
Qiangbacuozhen, Wang T, Yu L and Liu H: Role of the PI3K/AKT
pathway in modulating cytoskeleton rearrangements and phenotype
switching in rat pulmonary arterial vascular smooth muscle cells.
DNA Cell Biol. 33:12–19. 2014. View Article : Google Scholar
|
|
27
|
Woodward HN, Anwar A, Riddle S,
Taraseviciene-Stewart L, Fragoso M, Stenmark KR and Gerasimovskaya
EV: PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP
release and ATP-stimulated angiogenic responses in pulmonary artery
vasa vasorum endothelial cells. Am J Physiol Lung Cell Mol Physiol.
297:L954–L964. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stenmark KR, Tuder RM and El Kasmi KC:
Metabolic reprogramming and inflammation act in concert to control
vascular remodeling in hypoxic pulmonary hypertension. J Appl
Physiol (1985). 119:1164–1172. 2015. View Article : Google Scholar
|
|
29
|
Weichhart T and Säemann MD: The
PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic
applications. Ann Rheum Dis. 67(Suppl 3): iii70–iii74. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals: Guide for the Care and Use
of Laboratory Animals. 8th edition. The National Academies Press;
Washington, DC: pp. 11–151. 2011
|
|
31
|
Xu X, Wang X, Geng J, Li F, Yang T and Dai
H: Rapamycin regulates connective tissue growth factor expression
of lung epithelial cells via phosphoinositide 3-kinase. Exp Biol
Med (Maywood). 238:1082–1094. 2013. View Article : Google Scholar
|
|
32
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fang Y, Vilella-Bach M, Bachmann R,
Flanigan A and Chen J: Phosphatidic acid-mediated mitogenic
activation of mTOR signaling. Science. 294:1942–1945. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gesbert F, Sellers WR, Signoretti S, Loda
M and Griffin JD: BCR/ABL regulates expression of the
cyclin-dependent kinase inhibitor p27Kip1 through the
phosphatidylinositol 3-kinase/AKT pathway. J Biol Chem.
275:39223–39230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu EZ, Kantores C, Ivanovska J, Engelberts
D, Kavanagh BP, McNamara PJ and Jankov RP: Rescue treatment with a
Rho-kinase inhibitor normalizes right ventricular function and
reverses remodeling in juvenile rats with chronic pulmonary
hypertension. Am J Physiol Heart Circ Physiol. 299:H1854–H1864.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berven LA and Crouch MF: Cellular function
of p70S6K: A role in regulating cell motility. Immunol Cell Biol.
78:447–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marinov M, Fischer B and Arcaro A:
Targeting mTOR signaling in lung cancer. Crit Rev Oncol Hematol.
63:172–182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maira SM, Stauffer F, Brueggen J, Furet P,
Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker
K, et al: Identification and characterization of NVP-BEZ235, a new
orally available dual phosphatidylinositol 3-kinase/mammalian
target of rapamycin inhibitor with potent in vivo antitumor
activity. Mol Cancer Ther. 7:1851–1863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cui B, Tao J and Yang Y: Studies on the
expression patterns of class I PI3K catalytic subunits and its
prognostic significance in colorectal cancer. Cell Biochem Biophys.
62:47–54. 2012. View Article : Google Scholar
|
|
40
|
Shimoda LA and Laurie SS: HIF and
pulmonary vascular responses to hypoxia. J Appl Physiol (1985).
116:867–874. 2014. View Article : Google Scholar
|
|
41
|
Rogers SC, Said A, Corcuera D, McLaughlin
D, Kell P and Doctor A: Hypoxia limits antioxidant capacity in red
blood cells by altering glycolytic pathway dominance. FASEB J.
23:3159–3170. 2009. View Article : Google Scholar : PubMed/NCBI
|