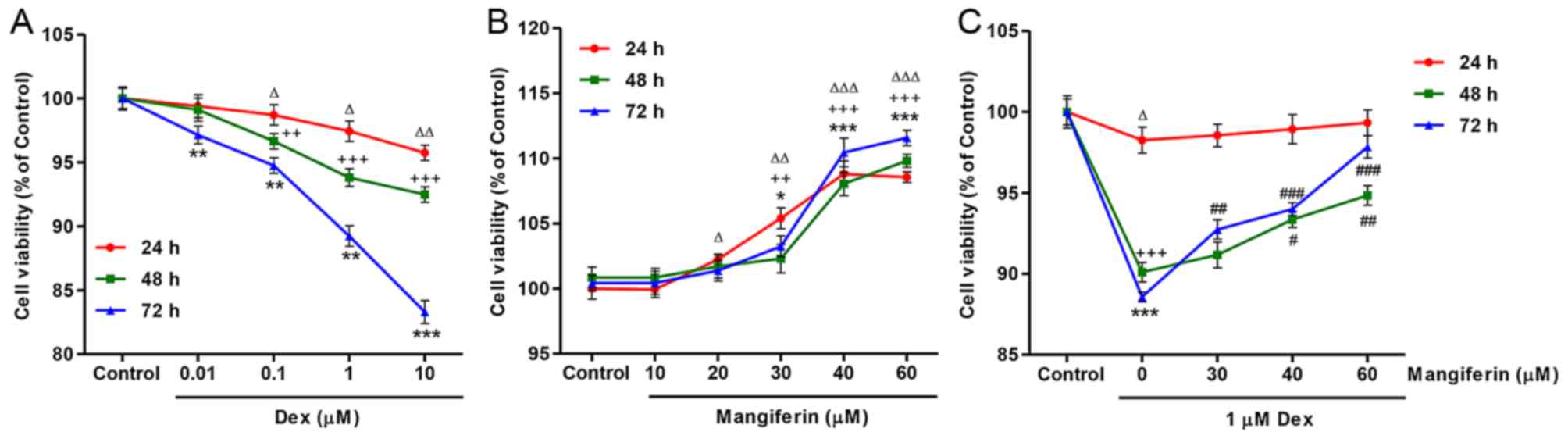
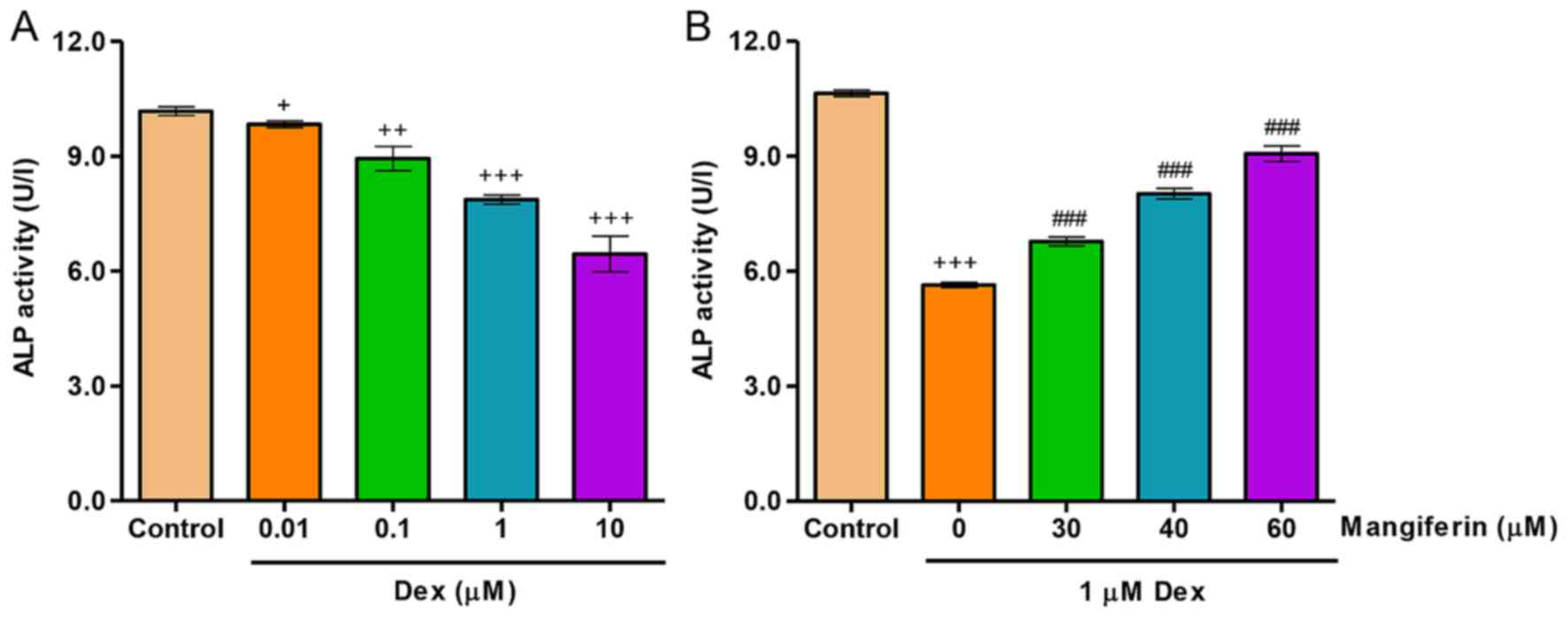
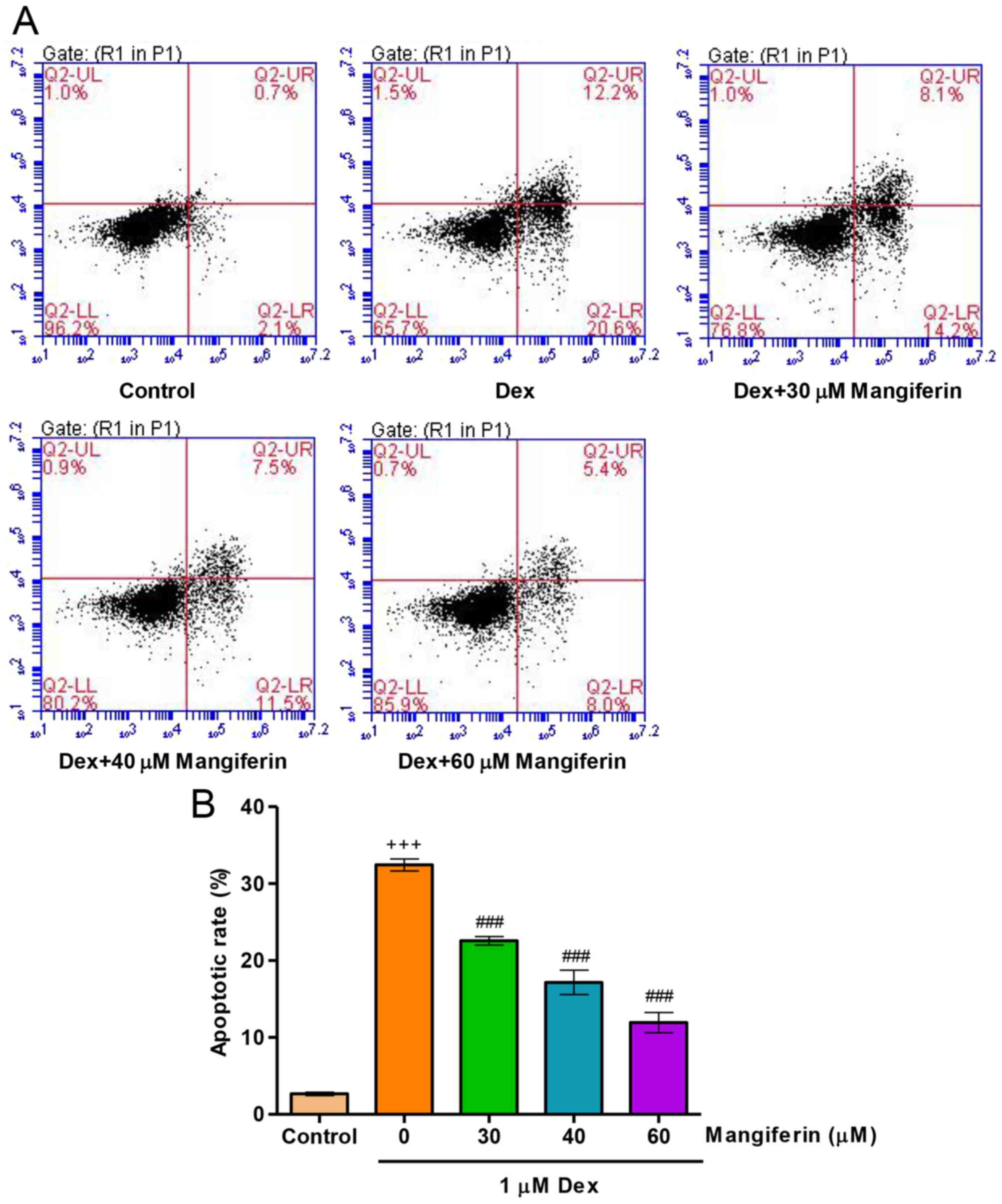
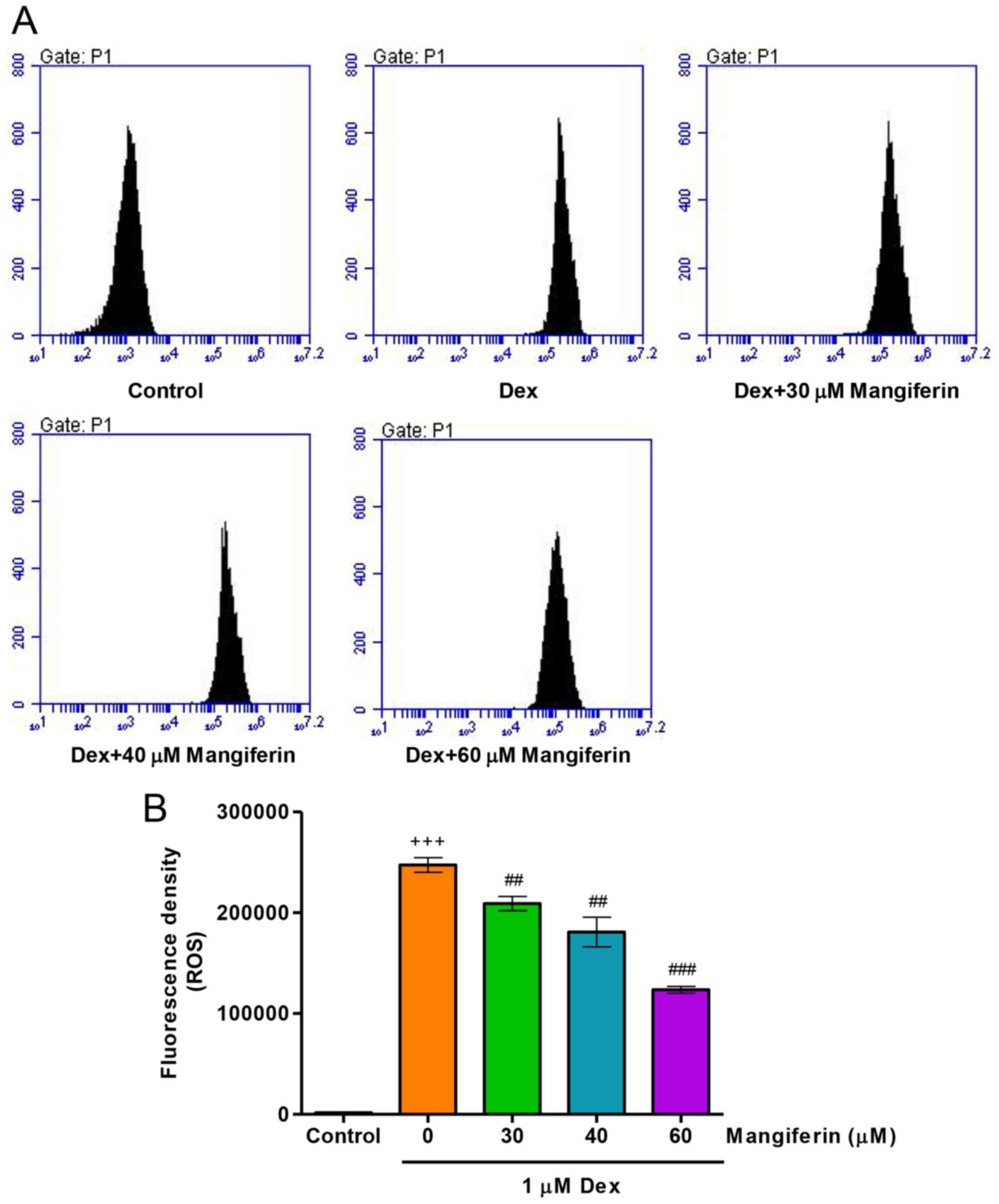
|
1
|
Ang E, Liu Q, Qi M, Liu HG, Yang X, Chen
H, Zheng MH and Xu J: Mangiferin attenuates osteoclastogenesis,
bone resorption, and RANKL-induced activation of NF-κB and ERK. J
Cell Biochem. 112:89–97. 2011. View Article : Google Scholar
|
|
2
|
Bar-Shavit Z: The osteoclast: a
multinucleated, hematopoietic-origin, bone-resorbing osteoimmune
cell. J Cell Biochem. 102:1130–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whittier X and Saag KG:
Glucocorticoid-induced osteoporosis. Rheum Dis Clin North Am.
42:177–189. 2016. View Article : Google Scholar
|
|
4
|
Wang Y, Liu J, Pang Q and Tao D:
Alpinumisoflavone protects against glucocorticoid-induced
osteoporosis through suppressing the apoptosis of osteoblastic and
osteocytic cells. Biomed Pharmacother. 96:993–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujita K and Janz S: Attenuation of WNT
signaling by DKK-1 and -2 regulates BMP2-induced osteoblast
differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer.
6:712007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross FP: M-CSF, c-Fms, and signaling in
osteoclasts and their precursors. Ann N Y Acad Sci. 1068:110–116.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaguchi Y, Sakai E, Sakamoto H, Fumimoto
R, Fukuma Y, Nishishita K, Okamoto K and Tsukuba T: Inhibitory
effects of tert-butylhydroquinone on osteoclast differentiation via
up-regulation of heme oxygenase-1 and down-regulation of HMGB1
release and NFATc1 expression. J Appl Toxicol. 34:49–56. 2014.
View Article : Google Scholar
|
|
9
|
Boyce BF and Xing L: Biology of RANK,
RANKL, and osteoprotegerin. Arthritis Res Ther. 9(Suppl 1): S12007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xue L, Jiao L, Wang Y, Nie Y, Han T, Jiang
Y, Rahman K, Zhang Q and Qin L: Effects and interaction of icariin,
curculigoside, and berberine in er-xian decoction, a traditional
chinese medicinal formula, on osteoclastic bone resorption. Evid
Based Complement Alternat Med. 2012:4908432012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cancedda R, Giannoni P and Mastrogiacomo
M: A tissue engineering approach to bone repair in large animal
models and in clinical practice. Biomaterials. 28:4240–4250. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bishop GB and Einhorn TA: Current and
future clinical applications of bone morphogenetic proteins in
orthopaedic trauma surgery. Int Orthop. 31:721–727. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Langenfeld EM, Kong Y and Langenfeld J:
Bone morphogenetic protein 2 stimulation of tumor growth involves
the activation of Smad-1/5. Oncogene. 25:685–692. 2006. View Article : Google Scholar
|
|
14
|
Tang CH, Yang RS, Chien MY, Chen CC and Fu
WM: Enhancement of bone morphogenetic protein-2 expression and bone
formation by coumarin derivatives via p38 and ERK-dependent pathway
in osteoblasts. Eur J Pharmacol. 579:40–49. 2008. View Article : Google Scholar
|
|
15
|
Wilkinson AS, Monteith GR, Shaw PN, Lin
C-N, Gidley MJ and Roberts-Thomson SJ: Effects of the mango
components mangiferin and quercetin and the putative mangiferin
metabolite norathyriol on the transactivation of peroxisome
proliferator-activated receptor isoforms. J Agric Food Chem.
56:3037–3042. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campos-Esparza MR, Sánchez-Gómez MV and
Matute C: Molecular mechanisms of neuroprotection by two natural
antioxidant polyphenols. Cell Calcium. 45:358–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajendran P, Ekambaram G and Sakthisekaran
D: Protective role of mangiferin against benzo(a)pyrene induced
lung carcinogenesis in experimental animals. Biol Pharm Bull.
31:1053–1058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo Y, Fu C, Wang Z, Zhang Z, Wang H and
Liu Y: Mangiferin attenuates contusive spinal cord injury in rats
through the regulation of oxidative stress, inflammation and the
Bcl-2 and Bax pathway. Mol Med Rep. 12:7132–7138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang YH, Wang Y, Yusufali AH, Ashby F,
Zhang D, Yin ZF, Aslanidi GV, Srivastava A, Ling CQ and Ling C:
Cytotoxic genes from traditional Chinese medicine inhibit tumor
growth both in vitro and in vivo. J Integr Med. 12:483–494. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Owen TA, Aronow M, Shalhoub V, Barone LM,
Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB and Stein
GS: Progressive development of the rat osteoblast phenotype in
vitro: reciprocal relationships in expression of genes associated
with osteoblast proliferation and differentiation during formation
of the bone extracellular matrix. J Cell Physiol. 143:420–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun M, Xia R, Jin F, Xu T, Liu Z, De W and
Liu X: Downregulated long noncoding RNA MEG3 is associated with
poor prognosis and promotes cell proliferation in gastric cancer.
Tumour Biol. 35:1065–1073. 2014. View Article : Google Scholar
|
|
23
|
Walker BR: Glucocorticoids and
cardiovascular disease. Eur J Endocrinol. 157:545–559. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Green KN, Billings LM, Roozendaal B,
McGaugh JL and LaFerla FM: Glucocorticoids increase amyloid-β and
tau pathology in a mouse model of Alzheimer's disease. J Neurosci.
26:9047–9056. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vegiopoulos A and Herzig S:
Glucocorticoids, metabolism and metabolic diseases. Mol Cell
Endocrinol. 275:43–61. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato H, Takahashi T, Sumitani K, Takatsu H
and Urano S: Glucocorticoid generates ROS to induce oxidative
injury in the hippocampus, leading to impairment of cognitive
function of rats. J Clin Biochem Nutr. 47:224–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hurson CJ, Butler JS, Keating DT, Murray
DW, Sadlier DM, O'Byrne JM and Doran PP: Gene expression analysis
in human osteoblasts exposed to dexamethasone identifies altered
developmental pathways as putative drivers of osteoporosis. BMC
Musculoskelet Disord. 8:122007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin H, Lan J, Guan M, Sheng F and Zhang H:
Spectroscopic investigation of interaction between mangiferin and
bovine serum albumin. Spectrochim Acta A Mol Biomol Spectrosc.
73:936–941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holtorf HL, Jansen JA and Mikos AG: Flow
perfusion culture induces the osteoblastic differentiation of
marrow stroma cell-scaffold constructs in the absence of
dexamethasone. J Biomed Mater Res A. 72:326–334. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vali B, Rao LG and El-Sohemy A:
Epigallocatechin-3-gallate increases the formation of mineralized
bone nodules by human osteoblast-like cells. J Nutr Biochem.
18:341–347. 2007. View Article : Google Scholar
|
|
31
|
Iu MF, Kaji H, Sowa H, Naito J, Sugimoto T
and Chihara K: Dexamethasone suppresses Smad3 pathway in
osteoblastic cells. J Endocrinol. 185:131–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mori K, Shioi A, Jono S, Nishizawa Y and
Morii H: Dexamethasone enhances in vitro vascular calcification by
promoting osteoblastic differentiation of vascular smooth muscle
cells. Arterioscler Thromb Vasc Biol. 19:2112–2118. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Yang X, Zhang Y, Dighe A, Li X and
Cui Q: Fullerol antagonizes dexamethasone-induced oxidative stress
and adipogenesis while enhancing osteogenesis in a cloned bone
marrow mesenchymal stem cell. J Orthop Res. 30:1051–1057. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sánchez GM, Re L, Giuliani A, Núñez-Sellés
AJ, Davison GP and León-Fernández OS: Protective effects of
Mangifera indica L. extract, mangiferin and selected antioxidants
against TPA-induced biomolecules oxidation and peritoneal
macrophage activation in mice. Pharmacol Res. 42:565–573. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine
(TNFalpha/IL-1α) induction of human osteoclast formation. J Pathol.
198:220–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kudo O, Sabokbar A, Pocock A, Itonaga I,
Fujikawa Y and Athanasou NA: Interleukin-6 and interleukin-11
support human osteoclast formation by a RANKL-independent
mechanism. Bone. 32:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Styrkarsdottir U, Cazier JB, Kong A,
Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD,
Sigurdardottir MS, Bagger Y, Christiansen C, et al: Linkage of
osteoporosis to chromosome 20p12 and association to BMP2. PLoS
Biol. 1:e692003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen JR, Shankar K, Nagarajan S, Badger TM
and Ronis MJ: Protective effects of estradiol on ethanol-induced
bone loss involve inhibition of reactive oxygen species generation
in osteoblasts and downstream activation of the extracellular
signal-regulated kinase/signal transducer and activator of
transcription 3/receptor activator of nuclear factor-kappaB ligand
signaling cascade. J Pharmacol Exp Ther. 324:50–59. 2008.
View Article : Google Scholar
|