Introduction
The incidence of diabetes mellitus (DM) has markedly
increased over the past few decades due to unhealthy diet, lack of
exercise and deteriorating environmental conditions (1,2).
Among the various microvascular complications of the disease,
diabetic nephropathy (DN) is frequently observed and is the leading
cause of end-stage renal disease (3). Podocytes are a key component of the
kidney filtration barrier, and podocyte injury has been indicated
to serve an important role in the pathogenesis of DN, according to
a number of studies (4–8). Podocytes are terminally
differentiated visceral epithelial cells that are located outside
the glomerular capillaries and form the final filtration barrier to
protein loss (9–11). In DN, podocyte injury leads to the
disruption of the filtration barrier and to protein leakage. In
addition, podocyte depletion has been indicated as an important
early pathologic marker of DN (7,12,13). Accumulating evidence also
suggested that the epithelial-mesenchymal transition (EMT) is a
possible cause of podocyte depletion in DN. During EMT, epithelial
cells are transformed to mesenchymal cells in response to injurious
stimuli (10,13,14). Cells lose their original features
when the pathological process of EMT occurs, consequently reducing
cell-to-cell contact, damaging cell polarity and recapturing the
characteristics of the mesenchymal markers, such as α-smooth muscle
actin (α-SMA) and vimentin (15).
Long noncoding RNAs (lncRNAs) are non-protein-coding
transcripts with a length of >200 nucleotides that serve
important roles in tumorigenesis (16,17), RNA transcription (18,19) and mRNA translation (20,21). Furthermore, evidence has suggested
that lncRNAs may be functionally important in DM progression
through the modulation of renal responses to hyperglycemia and the
progression of DN (22,23). Several IncRNAs have been
implicated in diabetic retinopathy, including MALAT1 (24), a potential biomarker for the
prognosis and diagnosis of this disease. Wang et al
(25) reported that a novel
lncRNA, CYP4B1-PS1-001, was significantly downregulated in response
to early DN in vivo and in vitro, and that
overexpression of CYP4B1-PS1-001 inhibited the proliferation and
fibrosis of mesangial cells. In addition, the lncRNAs H19 and HULC
are known to be upregulated by oxidative stress, as well as to
regulate cholangiocarcinoma migration and invasion (26). EMT is a potential pathway leading
to podocyte depletion and proteinuria in diabetic kidney disease
(27). However, the association
of lncRNAs with the EMT in DN remains unclear.
In order to understand the functions of lncRNAs
during the EMT process in streptozocin (STZ)-induced DN, lncRNA
microarrays were initially used in the present study to identify
the lncRNAs with differential expression between normal and DN
rats. The results indicated that ENSRNOG00000037522 was the most
upregulated lncRNA between normal and DN rats, and that the
knockdown of ENSRNOG00000037522 affected the progression of EMT in
DN. The current study provides novel insight into the mechanism
linking lncRNA function with DN, and lncRNA ENSRNOG00000037522 may
serve as a novel target for the development of DN.
Materials and methods
Rat model of diabetes
A total of 24 male Sprague Dawley rats (age, 8
weeks; weight, 180–200 g) were purchased from Forevergen
Biotechnology Co., Ltd. (Guangzhou, China; http://www.forevergen.cn/). All animal studies were
conducted under the review and approval of the Ethics Committee of
Shenzhen Nanshan Hospital (Shenzhen, China). Rats were randomly
allocated into three groups, including the control group, and two
diabetes groups with rats examined at 1 or 6 weeks after STZ
injection (n=8 per group). Each group was subjected to controlled
conditions of 20–22°C, relative humidity of 50–55%, and a 12-h
light/dark cycle. After 24 h of fasting, diabetes in the two groups
was induced by a single intraperitoneal injection of STZ (50 mg/kg)
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) that was dissolved
in 5 mmol/l citrate buffer (pH=4.5) within 30 min, while rats in
the control group received intraperitoneal injection of the same
dose of citrate buffer alone within 30 min (28). Rats were fed with Purina Rat Chow
(Ralston Purina Company, Barcelona, Spain) throughout the
experimental period. All rats had free access to food and water.
Venous blood was collected at 24, 48 or 72 h after STZ injection in
order to assess whether the diabetes model had been successfully
established (blood glucose level of ≥16.7 mmol/l). Rats with
STZ-induced diabetes were treated with 2 U/kg protamine zinc
insulin at 24 h after STZ injection to avoid high glucose-induced
mortality. At the end of 1 and 6 weeks after STZ or citrate buffer
injection, all rats were euthanized with an intraperitoneal
overdose of pentobarbital (200 mg/kg), and the kidneys were rapidly
harvested and stored at −80°C for use in subsequent
experiments.
Cell culture
A total of 8×107 inactivated dynabeads
were suspended in Hank's balanced salt solution (HBSS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Next, the minced
kidney specimens were digested with 1 mg/ml collagenase A at 37°C
for 30 min and gently pressed through a 100-µm cell strainer
using a flattened pestle. Then the kidney lysate was mixed into
HBSS and dynabeads. The mixture containing kidney lysate and
dynabeads was centrifuged at 200 × g for 5 min at 4°C, and the
precipitates were resuspended in 5 ml HBSS. Subsequent to washing
three times with HBSS, the isolated glomeruli containing dynabeads
were gathered by a magnetic particle concentrator and cultured in
Dulbecco's modified Eagle medium (DMEM)/F12 (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C and 5% CO2. After 7 days of
culture, the glomeruli were digested in HBSS containing 1 mg/ml
collagenase A and 0.2 mg/ml deoxyribonuclease I at 37°C for 60 min,
followed by centrifugation for 5 min at 1,500 × g. The podocytes
were then resuspended in DMEM/F12 medium and counted with a blood
cell counter. Finally, the morphology of the podocytes was examined
at day 3, 5 and 7 with a scanning electron microscope (Nikon Corp.,
Tokyo, Japan) at ×100 magnification. In addition, the podocytes
were treated with high glucose (30 mmol/l) at day 7 for 2 h at room
temperature.
Immunohistochemical analysis
All kidney tissues were fixed overnight in formalin
solution, dehydrated in ethanol, embedded in paraffin and then
sectioned at 5 mm. The slides were blocked with 5% normal goat
serum (Gibco, Thermo Fisher Scientific, Inc.) for 15 min at room
temperature and incubated overnight at 4°C with primary antibodies,
as follows: Anti-α-SMA (bs-0189R; 1:200; Bioss Biotechnology Co.,
Ltd., Beijing, China), anti-vimentin (ab137321; 1:100; Abcam,
Cambridge, MA, USA), anti-nephrin (ab216341; 1:100; Abcam), and
anti-podocalyxin-like 1 (PODXL1; ab197769; 1:100; Abcam).
Subsequent to washing with PBS, the slides were incubated with the
rabbit anti-rat horseradish peroxidase (HRP)-conjugated IgG
secondary antibody (ab6734; 1:500; Abcam) at room temperature for
20 min. A DAB kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was used to detect immunohistochemical reactions. The slides were
then examined under a phase contrast light microscope (Eclipse
E600; Nikon Corp.) at ×200 magnification.
ELISA
Blood and urine samples were obtained at 1 and 6
weeks from the rats in each group. Blood was obtained from a tail
vein. Serum was immediately separated by centrifugation at 6,000 ×
g for 20 min and stored at −80°C until required for measurement.
Urine samples were collected using metabolic cages. Kidney injury
was assessed by measuring the levels of serum cystatin C (CysC),
serum β2-microglobulin (β2-MG), blood urea
nitrogen (BUN) and urine microalbumin (UmAlb). ELISA kits were used
to determine the concentration of serum CysC (In-Ra0699; Innova
Biotech Co., Ltd., Beijing, China), serum β2-MG
(48-MICRT-E01; Alpco Diagnostics, Salem, NH, USA) and UmAlb
(MBS9304276; MyBioSource, Inc., San Diego, CA, USA) with the enzyme
immunoassay method according to the manufacturer's protocol. The
content of the samples was then calculated with a spectrophotometer
at 450 nm. BUN concentration was measured using a Urea Assay kit
(ab83362; Abcam) with a spectrophotometer at 570 nm. These
concentrations were quantified against a standard curve calibrated
with known amounts of protein. Measurements were performed in
triplicate.
Western blot analysis
To detect the glomerular proteins, western blot
analysis was conducted. Briefly, cells were harvested and lysed in
radioimmunoprecipitation assay buffer (Gibco, Thermo Fisher
Scientific, Inc.) [containing 0.6 g Tris in 100 ml ddH2O
at pH 7.5, 0.88 g NaCl, 0.1 g sodium dodecyl sulphate (SDS), 0.5 g
sodium deoxycloate and 1.0 g NP-40 Tergitol] and a protease
inhibitor mixture (Sigma-Aldrich; Merck KGaA). Next, the podocyte
extracts were centrifuged at 4°C for 15 min at 1,000 × g, and the
supernatant was collected. The protein concentration was then
determined with a bicinchoninic acid protein assay kit (Sangon
Biotech Co., Ltd., Shanghai, China). Subsequently, 20 µg
protein was separated by 10% SDS-polyacrylamide gel electrophoresis
and transferred to a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc., Shanghai, China). After blocking in 1% blocking
reagent in Tris-buffered saline [10 mM Tris (pH 7.5), 150 mM NaCl]
(Gibco, Thermo Fisher Scientific, Inc.) for 1 h at room
temperature, the protein samples were then incubated overnight at
4°C with primary antibodies for anti-α-SMA (no. 14968, 1:500; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-vimentin (no.
ab92547, 1:500; Abcam), anti-PODXL1 (no. SAB2500809, 1: 500;
Sigma-Aldrich; Merck KGaA), anti-nephrin (no. ab136927, 1:500;
Abcam) and GAPDH (ab9485, 1:500; Abcam). This was followed by
incubation with a HRP-labeled goat anti-rabbit secondary antibody
(1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h
at room temperature. Finally, the proteins were visualized with an
enhanced chemiluminescence detection kit (Thermo Fisher Scientific,
Inc.) and analyzed using ImageJ software, version 14.8 (National
Institutes of Health, Bethesda, MD, USA).
Microarray lncRNA profiling
The lncRNA target sequences were merged from
multiple databases, including RefSeq Build 37 (https://www.ncbi.nlm.nih.gov/refseq/),
Ensembl Release 55 (http://www.ensembl.org), Unigene Build 176 (https://www.ncbi.nlm.nih.gov/unigene),
GenBank (https://www.ncbi.nlm.nih.gov/gene) and RIKEN 3
(http://www.riken.jp). After 6 weeks, the
podocytes were obtained from control and STZ-treated diabetic rats.
Total RNA was extracted using TRIzol reagent (Takara Bio, Inc.,
Kusatsu, Japan), the concentration was measured using a NanoDrop
spectrophotometer ND-1000 (Thermo Fisher Scientific, Inc.). Total
RNA was converted into cDNA using a Takara reverse transcription
kit (Takara Bio, Inc.), after which cRNA was synthesized from ~2
µg total RNA using an mMessage mMachine kit (Ambion, Thermo
Fisher Scientific, Inc.) according to the manufacturer's standard
protocols. Next, cRNA was hybridized to the microarray overnight,
and microarray profiling was performed using the RiboArray™ Rat
lncRNA Array (http://www.ribobio.com/sitecn/Service2.aspx?id=104;
RiboBio Co., Ltd. (Guangzhou, China) following the manufacturer's
protocol. The microarray after hybridization were washed with a
Gene Expression Wash Buffer kit (Agilent Technologies, Inc., Santa
Clara, CA, USA), then scanned in a microarry scanner using Agilent
scan control software version 8.1 (Agilent Technologies, Inc.,
Santa Clara, CA, USA), and data were extracted with Feature
Extraction software (version 10.7; Agilent Technologies, Inc.). For
advanced data analysis, all biological replicates were pooled and
calculated to identify differentially expressed lncRNAs, based on
the threshold of a fold change of ≥2 and P≤0.05. A hierarchical
clustering heat map of the lncRNA expression profile was produced
using fold changes in lncRNA expression. In addition, in order to
determine the potential roles of differentially expressed lncRNAs,
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analyses were both applied. Enrichment for GO terms
was analyzed using GOEAST software 20 (http://omicslab.genetics.ac.cn/GOEAST/). Enrichment
for KEGG pathways using the KOBAS 3.0 Annotation System (http://kobas.cbi.pku.edu.cn).
LncRNA ENSRNOG00000037522 was identified and then
its role was investigated using small interfering (si)RNA
transfection experiments. The primary podocytes were transfected
with siRNA targeting ENSRNOG00000037522. The negative control siRNA
and two siRNAs against lncRNA ENSRNOG00000037522, namely siRNA1 and
siRNA2, were designed by RiboBio Co., Ltd.. The sequences were as
follows: siRNA1 sense, 5′-CCCAGCAUUCCAGCAUCUU-3′, and anti-sense,
5′-AAGAUGCUGGAAUGCUGGG-3′; siRNA2 sense, 5′-GCAUUCCAGCAUCUUACCU-3′,
and antisense, 5′-AGGUAAGAUGCUGGAAUGC-3′. Briefly, podocytes were
plated into 24-well plates at a density of 1×106 cells
per well and grown overnight at room temperature, followed by
addition of DMEM/F12 medium (Gibco; Thermo Fisher Scientific, Inc.)
the following day. The podocytes were then transfected with control
or ENSRNOG00000037522 siRNAs for 24 h, using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Subsequent to transfection, the podocytes
were grown for 72 h at 37°C and washed three times with PBS. The
cells were then recovered in DMEM/F12 medium (Gibco; Thermo Fisher
Scientific, Inc.) for 2 h and collected for use in subsequent
experiments. The silencing efficiency was confirmed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), and
all reactions were performed in triplicate.
RNA extraction and RT-qPCR analysis
Total RNA was isolated from podocytes using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and the
quantity and purity of the RNA were determined by optical density
measurements at an A260/A280 ratio of ≥1.8 using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). Next, cDNA was
synthesized using HiScript Q RT SuperMix for qPCR (Vazyme Biotech
Co., Ltd., Nanjing, China) according to the manufacturer's
protocol. The SYBR Green-based qPCR reaction system consisted of a
final volume of 20 µl, containing 2 µl cDNA, 10
µl SYBR-Green Mix (Takara Bio, Inc., Otsu, Japan), 4
µl primer mix and 4 µl ddH2O. The qPCR
reaction was performed at 95°C for 5 min, followed by 30 cycles of
95°C for 30 sec and 50°C for 30 sec. Primers targeting lncRNAs were
designed and synthetized by Thermo Fisher Scientific, Inc., and the
sequences of the primers used are listed in Table I. The lncRNA expression levels
were quantified using the 2−ΔΔCq method (29), with GAPDH serving as the
endogenous reference gene.
 | Table ISequences of the primers used in
quantitative polymerase chain reaction. |
Table I
Sequences of the primers used in
quantitative polymerase chain reaction.
| Name | Sequences
(5′–3′) |
|---|
|
ENSROG00000011753 | F:
GGTGTTACGCTGGTCTTCCA |
| R:
ACCTGTCCTCATCCAAACCC |
|
ENSROG00000050935 | F:
CGCAGAAGGTAACCTCCGTA |
| R:
TTTTTGGACCCGTCGCTTCT |
| LOC498759 | F:
ATGGAAGTGTGCAAGTCCTCA |
| R:
TCAGGCAAACGAGCACTCAC |
|
ENSROG00000031644 | F:
CTTAAGGATGCCTGGGCGAA |
| R:
CACATCAGCCACTGGGTAGT |
|
ENSROG00000048366 | F:
CAATGGACGGCCATCGTTCT |
| R:
AGGAAAGACCTTGTGAGGCAC |
|
ENSROG00000039080 | F:
CGGCAAGGGAAGGTCAATCA |
| R:
GAAGGCCTCAGTGGGTTTGT |
|
ENSROG00000037522 | F:
CTCCCCAATCACCCAGCATTC |
| R:
AGCGGGCTTTCTCTTTAATATGCT |
| GAPDH | F:
GCAAGAGAGAGGCCCTCAG |
| R:
TGTGAGGGAGATGCTCAGTG |
Statistical analysis
Each experiment was performed three times. Student's
t-test or one-way analysis of variance was used to analyze the data
using SPSS version 19.0 software (IBM Corp., Armonk, NY, USA). All
results were summarized and are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a difference that
was statistically significant.
Results
Diabetes promotes EMT in rat kidneys
Rat blood glucose was monitored at 24, 48 and 72 h
after STZ injection, and the results are shown in Table II. STZ-injected rats with blood
glucose levels of ≥16.7 mmol/l were considered to be diabetic.
Analysis at 24 h after STZ injection indicated that the blood
glucose level had increased significantly as compared with the
control rats, and continued to be elevated throughout the
measurement period, which indicated that diabetes was established
successfully in the STZ-injected rats.
 | Table IIBlood glucose (mmol/l) in different
groups of Sprague Dawley rats (n=12). |
Table II
Blood glucose (mmol/l) in different
groups of Sprague Dawley rats (n=12).
| Group | 24 h | 48 h | 72 h |
|---|
| Control | 6.77±0.25 | 6.77±0.09 | 6.73±0.33 |
| STZ-injected | 23.53±1.06a | 25.03±1.46a | 25.30±1.22a |
In order to investigate the effects of diabetes on
the EMT and the expression of PODXL1 in kidney tissues, an
immunohistochemical assay was used to determine the expression
levels of the mesenchymal phenotypic markers α-SMA and vimentin,
the epithelial marker nephrin and the podocyte-specific marker
PODXL1. Yellow or brown staining was observed in cells that were
positive for α-SMA, vimentin, nephrin or PODXL1, while the nuclei
were stained blue. The immunohistochemistry results demonstrated
that expression of α-SMA and vimentin was almost absent in the
control tissues, whereas these markers were highly expressed in
diabetic kidneys. By contrast, PODXL1 and nephrin were intensely
expressed in the control rats, whereas they were markedly
downregulated in the kidney tissues following STZ injection
(Fig. 1).
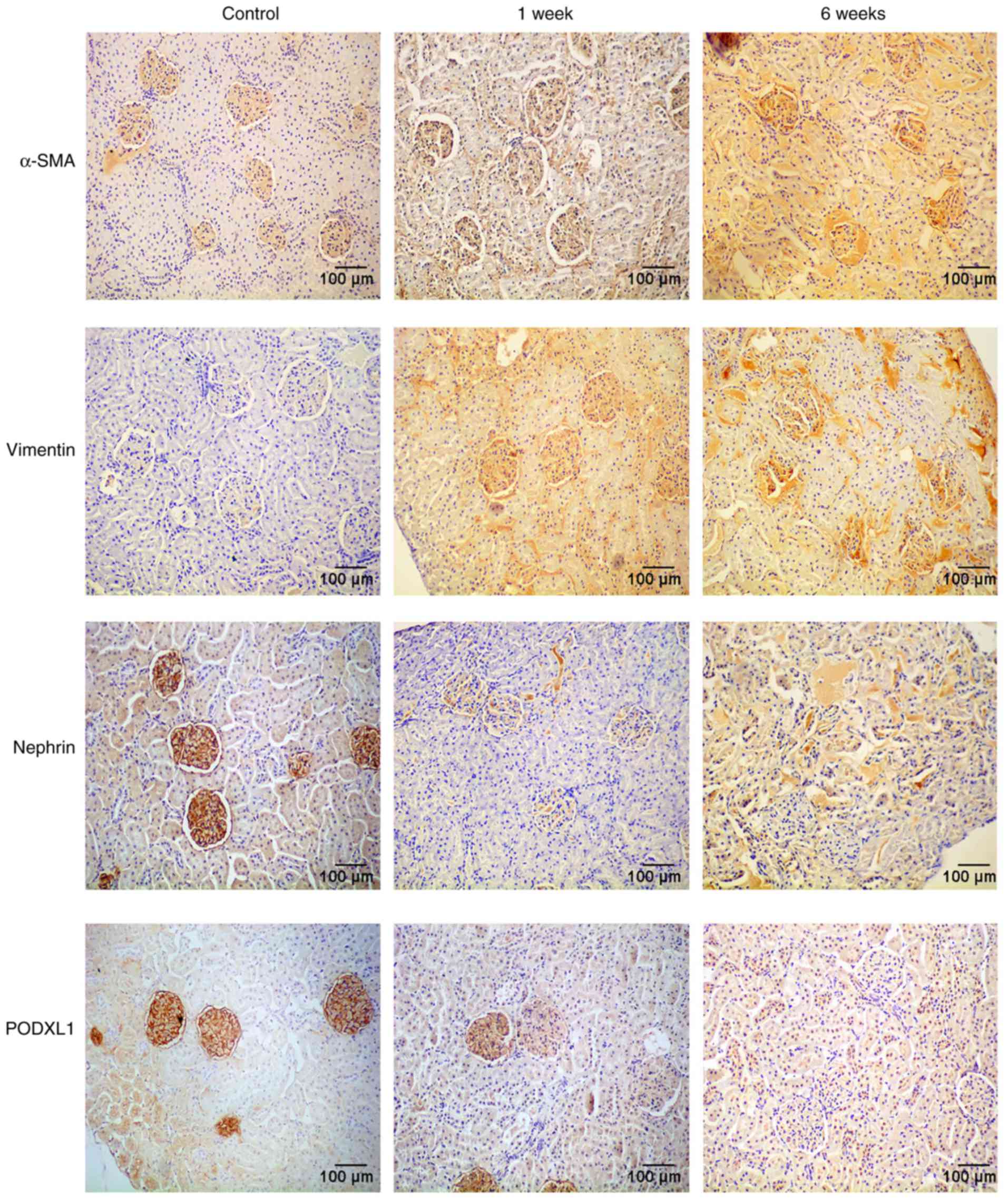 | Figure 1Protein expression of α-SMA,
vimentin, PODXL1 and nephrin, as examined by immunohistochemical
assay. The α-SMA and vimentin expression levels were almost absent
in the control group, but were highly expressed in diabetic kidneys
at 1 and 6 weeks. However, PODXL1 and nephrin were intensely
expressed in the control rats, but markedly downregulated in the
kidneys following STZ injection. All experiments were performed in
triplicate, and representative immunohistochemistry images are
shown. α-SMA, α-smooth muscle actin; PODXL1, podocalyxin-like 1;
STZ, streptozocin. |
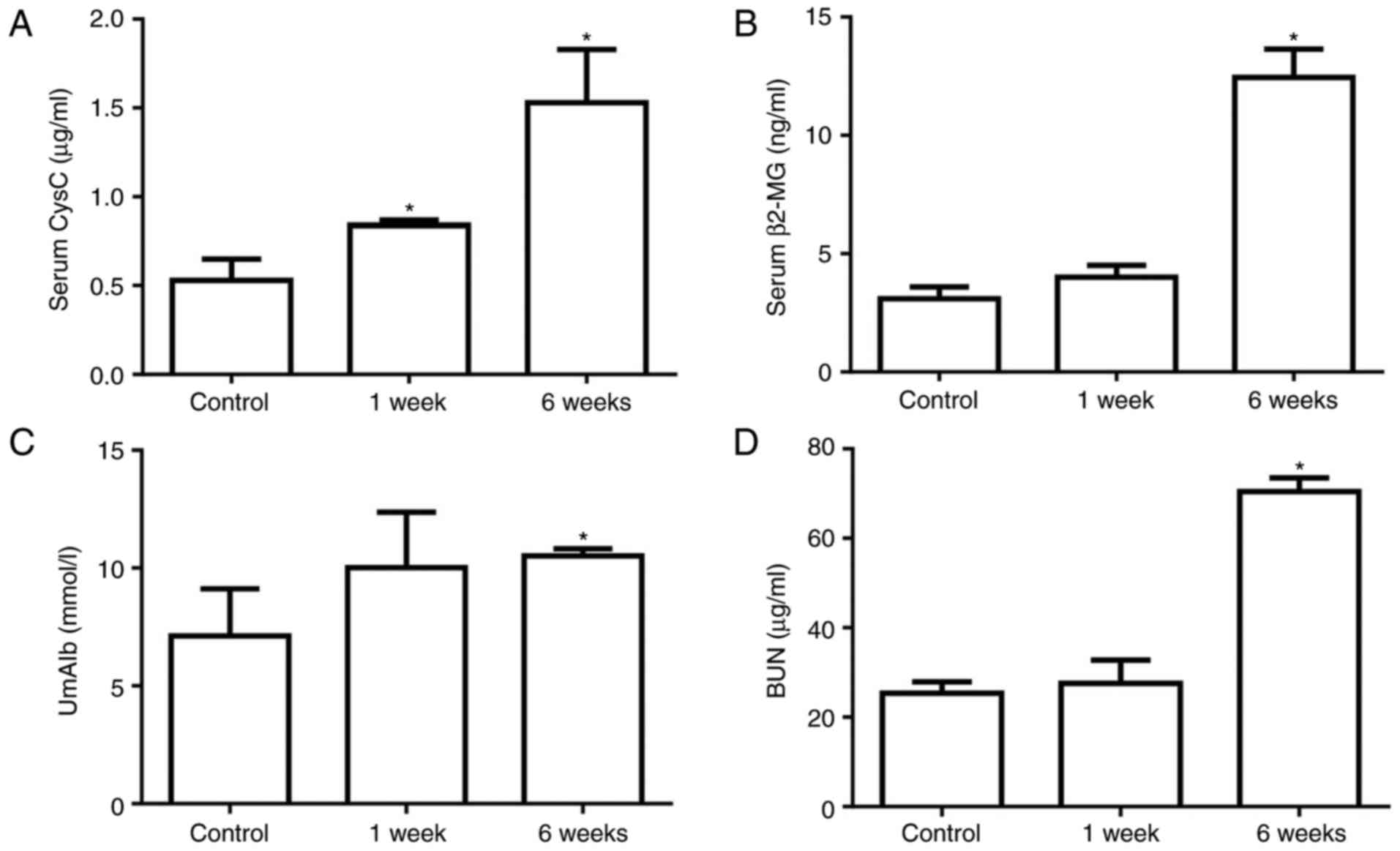
Diabetes promotes kidney injury
At 1 or 6 weeks after STZ injection, the kidney
function and injury were assessed by measuring the serum CysC,
serum β2-MG, UmAlb and BUN levels. The serum CysC level
was 0.53±0.21 µg/ml in the control group, which was
significantly increased to 0.76±0.04 µg/ml at 1 week after
STZ injection and continued to increase after 6 weeks in the
diabetic group (Fig. 2A). The
serum β2-MG, UmAlb and BUN levels were not significantly
different between the control and 1 week diabetic groups, but were
significantly increased in diabetic rats at 6 weeks after STZ
injection (Fig. 2B–D). These data
indicated that kidney injury was induced by STZ stimulation and was
exacerbated in diabetic rats after 6 weeks.
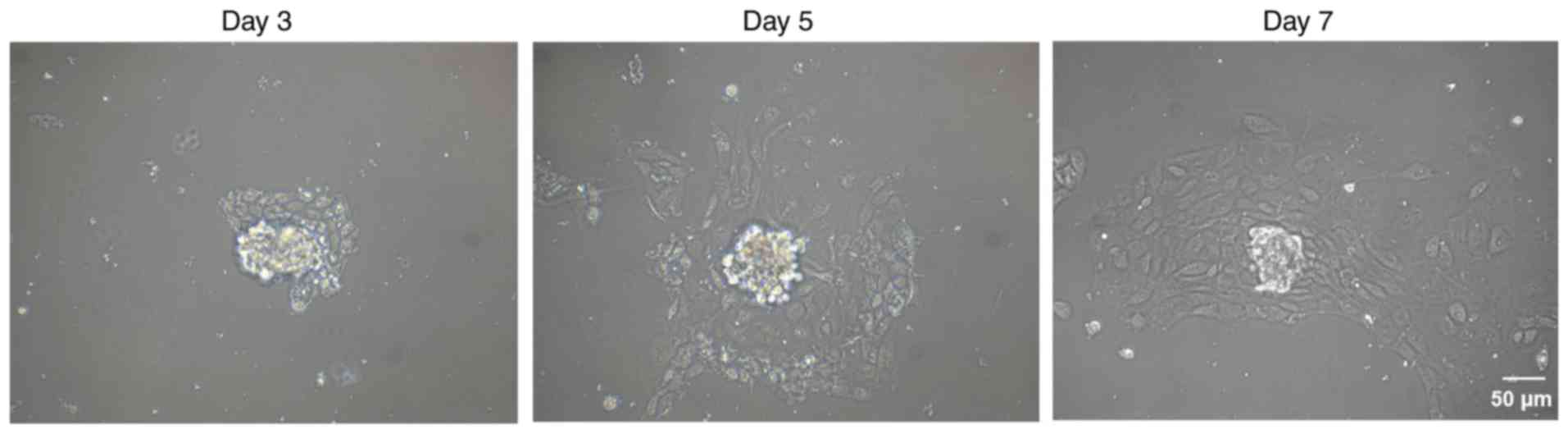
Next, glomeruli were isolated from the kidney
tissues to obtain podocytes. Podocyte growth was detected by cell
morphological observation at day 3, 5 and 7 after culture.
Subsequent to STZ injection, podocytes demonstrated foot process
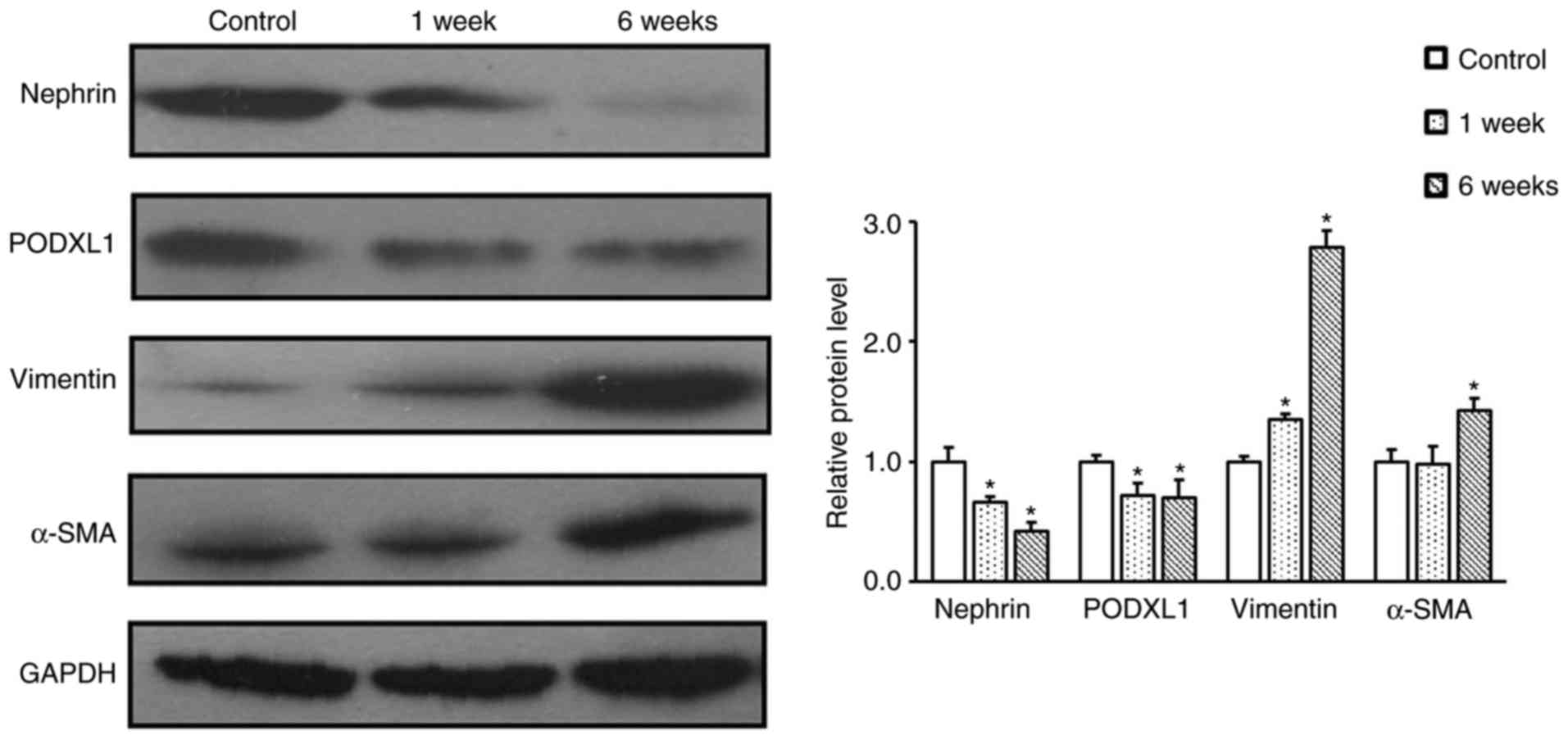
effacement, vacuolar degeneration and detachment (Fig. 3). In order to further confirm the
process of EMT in diabetic rats, western blot analysis was
performed to detect the expression levels of α-SMA, vimentin,
nephrin and PODXL1. The results revealed that the expression levels
of nephrin and POXLD1 were significantly decreased in the podocytes
of diabetic rats when compared with those in the control rats
(P<0.05; Fig. 4). By contrast,
the expression levels of α-SMA and vimentin were markedly
upregulated in the podocytes of diabetic rats, as compared with
those of the control rats (Fig.
4). These findings indicate that STZ-induced podocyte-specific
depletion of nephrin and PODXL1 leads to the aggravation of kidney
injury in diabetic rats.
Differential expression of lncRNA in
diabetic podocytes
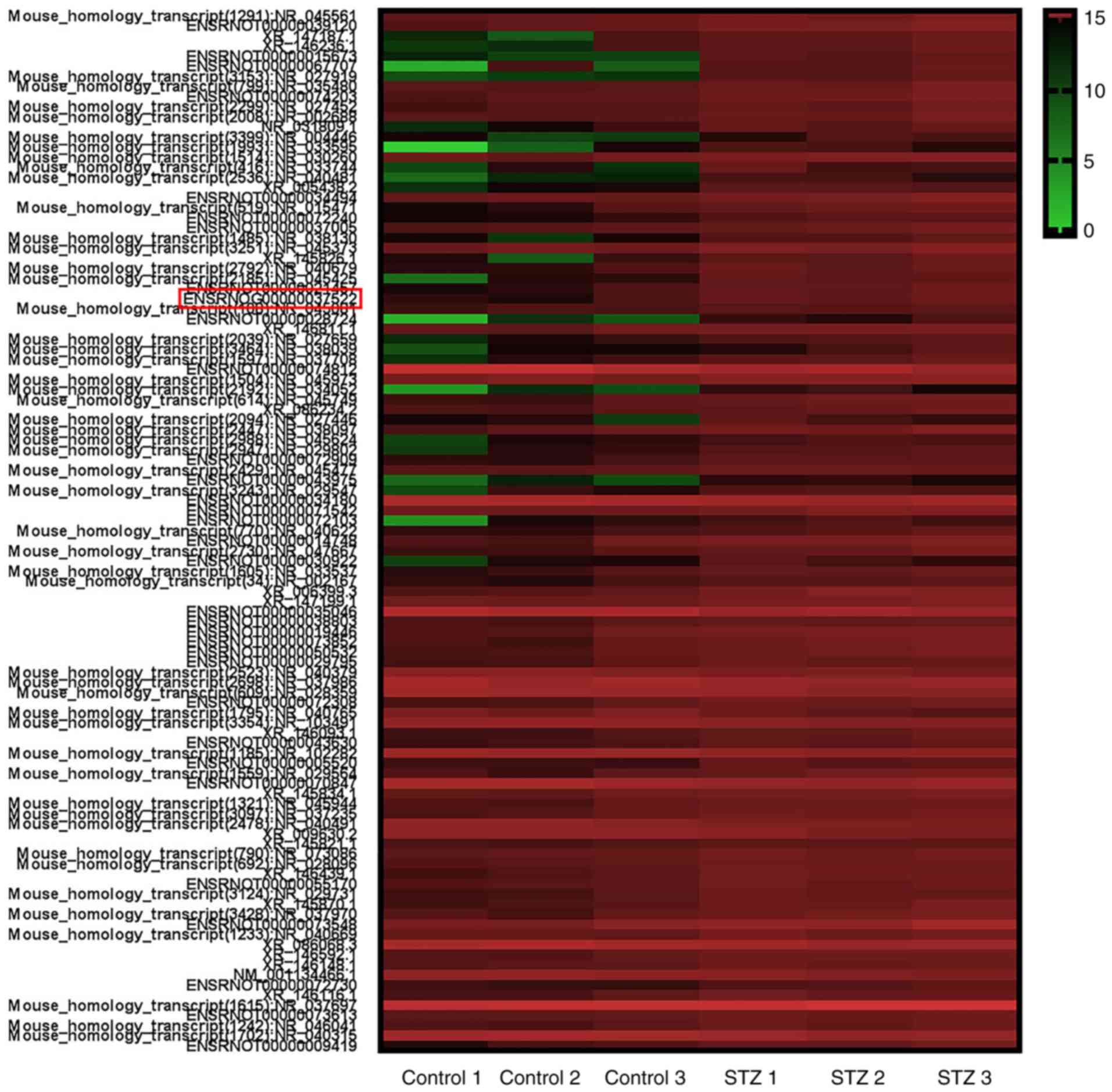
To explore the potential function of lncRNAs during
DN, a microarray analysis of the podocytes obtained from
STZ-induced diabetic and control rats at 6 weeks was conducted. A
total of 88 upregulated and 15 downregulated lncRNAs were
identified between the two groups (data not shown). Heat map
hierarchical clustering revealed distinct lncRNA expression
profiles between the two groups (Fig.
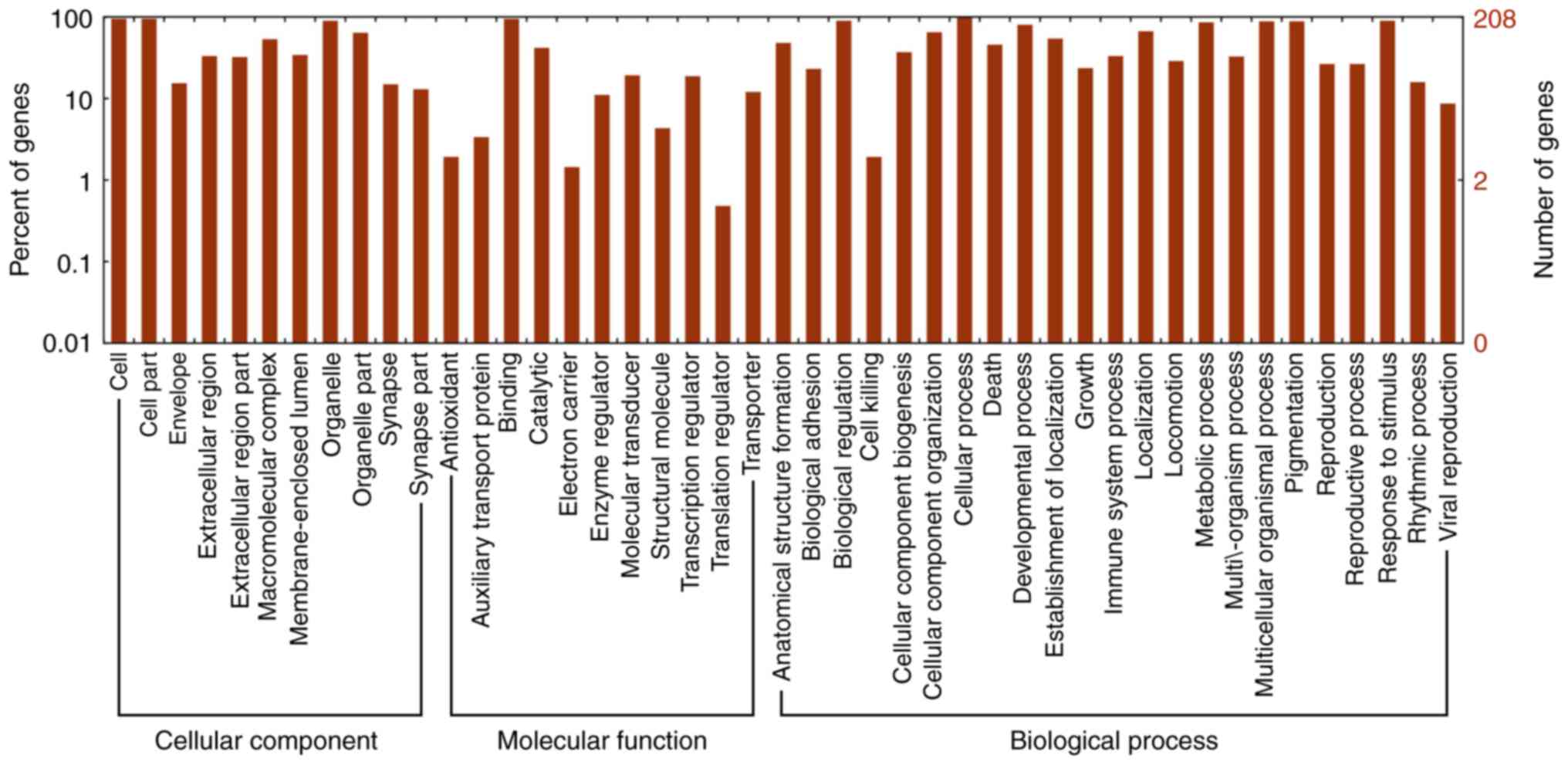
5). Furthermore, the GO categories comprised three structured
networks, including biological processes, the cellular component
and the molecular function (Fig.
6). The GO analysis also indicated that differentially
expressed lncRNAs were mainly involved in the cell, cell part,
binding and cellular process (Fig.
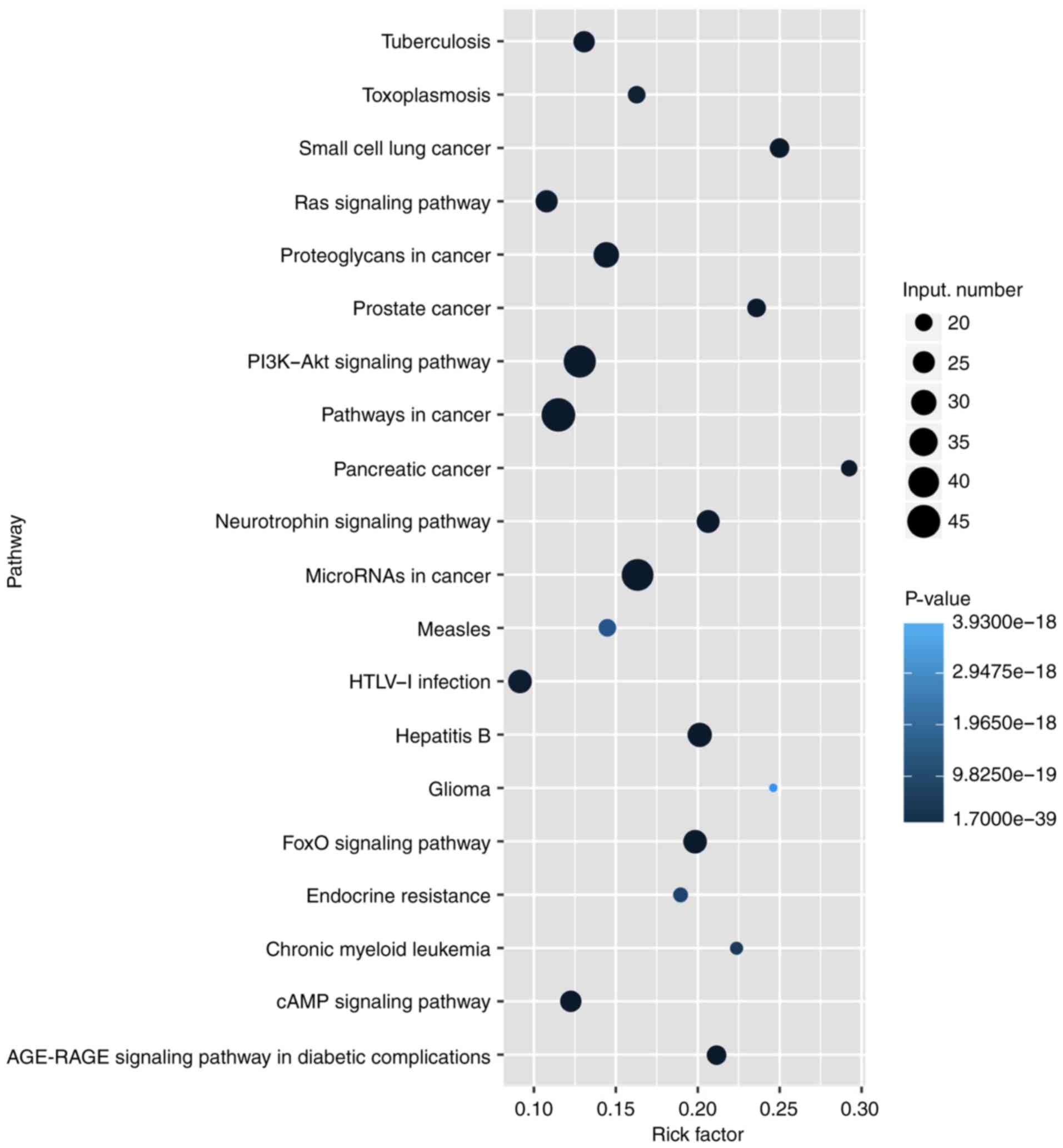
6). Subsequently, KEGG pathway analysis was performed to
ascertain the functions of the differentially expressed lncRNAs.
The results suggested that 'pathways in cancer' were significantly
enriched among these lncRNAs, and the three most enriched pathways
included 'pathways in cancer', the 'PI3K-Akt signaling pathway' and
'microRNAs in cancer' (Fig. 7).
Several of these pathways were linked to the EMT, such as the
'PI3K-Akt signaling pathway'. In order to verify the expression of
the dysregulated lncRNAs, seven most upregulated lncRNAs from
differentially expressed lncRNAs were selected for further
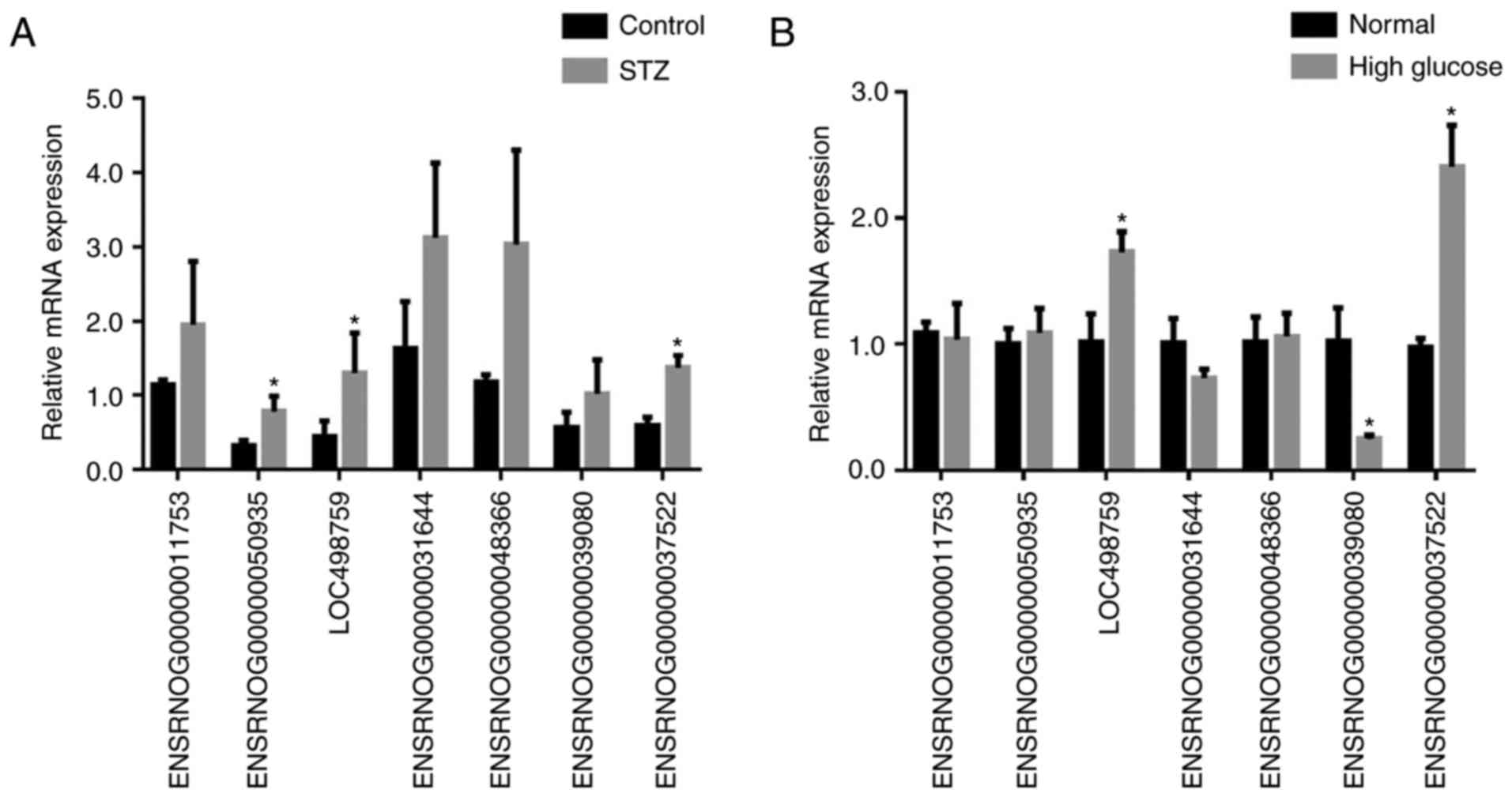
investigation. The RT-qPCR results revealed variations in lncRNA
expression in the podocytes of the STZ group, compared with that of
the control group podocytes. As shown in Fig. 8A, there was a significant
difference in the expression of the lncRNA ENSRNOG00000037522,
ENSRNOG00000050935 and LOC498759 between the two groups.
High glucose induces lncRNA expression in
diabetic podocytes
To confirm the expression of the 7 selected lncRNAs,
high glucose (30 mmol/l) was used to stimulate podocytes.
Subsequently, RT-qPCR was performed to detect the mRNA expression
levels of the 7 lncRNAs in normal podocytes and high
glucose-stimulated podocytes. The results were in accordance with
the microarray data. As shown in Fig.
8B, the lncRNA ENSRNOG00000037522 was the most upregulated
lncRNA in podocytes from the high glucose group compared with those
of the normal group. Consequently, these observations indicated
that high glucose induced the expression of lncRNA
ENSRNOG00000037522 in diabetic podocytes, and this lncRNA was
selected for further investigation.
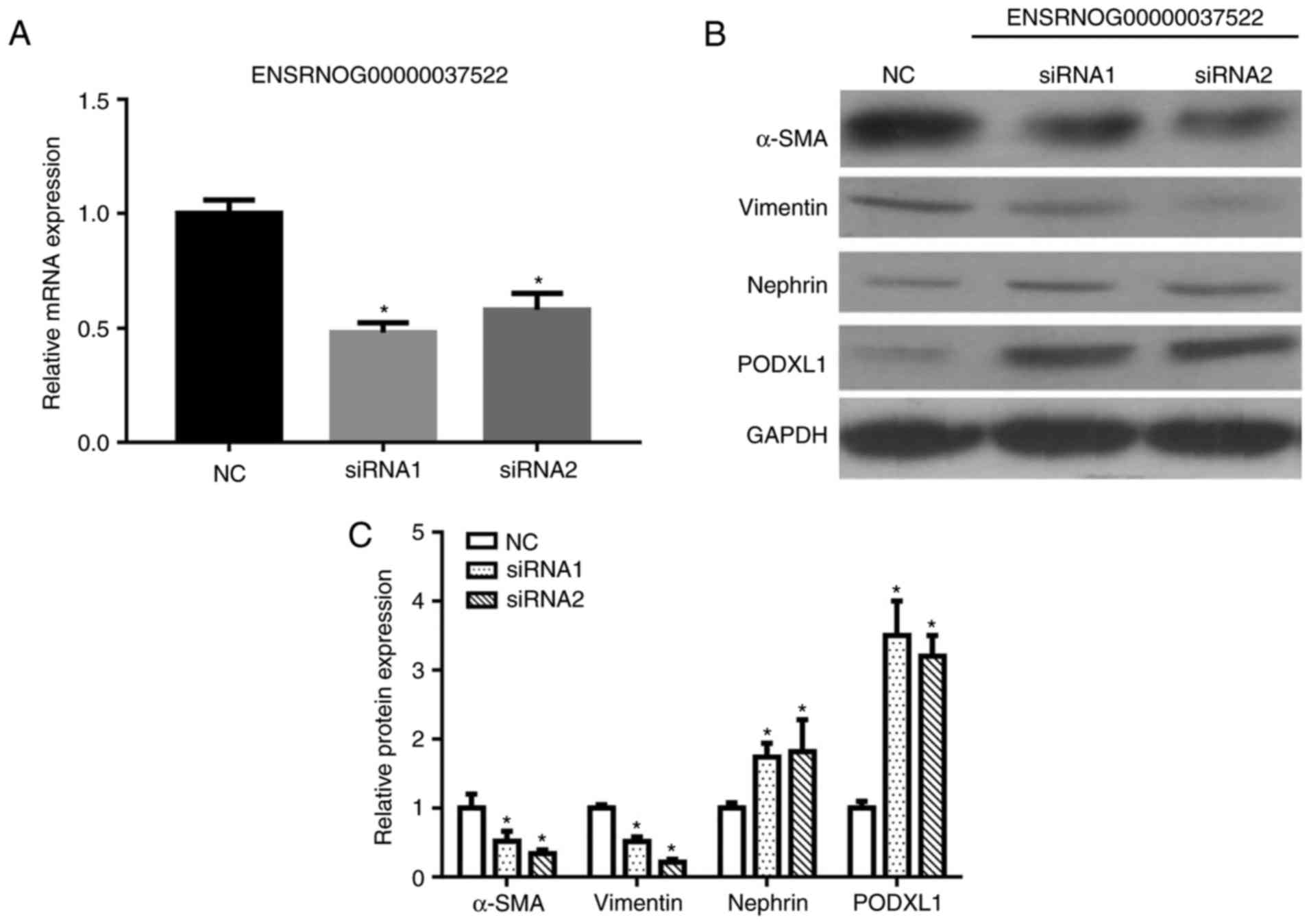
Knockdown of lncRNA ENSRNOG00000037522
inhibits podocyte EMT
Considering that the lncRNA ENSRNOG00000037522 level
was increased after high-glucose stimulation, transfection with two
siRNAs was applied to knockdown lncRNA ENSRNOG00000037522. The
knockdown efficiency was confirmed by RT-qPCR, which revealed a
marked reduction in lncRNA ENSRNOG00000037522 levels subsequent to
siRNA1 and siRNA2 transfection (Fig.
9A). The western blot analysis results also demonstrated that
the expression levels of PODXL1 and nephrin were significantly
increased, whereas the α-SMA and vimentin expression levels were
decreased following siRNA transfection (Fig. 9B and C). These aforementioned
observations indicate that the knockdown of lncRNA
ENSRNOG00000037522 influenced the EMT in podocytes by
downregulating vimentin and upregulating nephrin.
Discussion
DN is a common kidney disease caused by
hyperglycemia that can lead to angiopathy of the glomerular
capillaries (30). Glomerular
endothelial cells, the glomerular basement membrane and podocytes
constitute the filtration barrier. Podocytes form the final barrier
to protein loss and podocyte dysfunction allows penetration into
the other slit structures (9,31).
Numerous studies have suggested that podocyte depletion is
responsible for the onset of proteinuria, and the EMT has been
proposed to be a possible engagement for podocyte depletion in DN
(7,13,14,32,33).
In the present study, it was demonstrated that
podocytes were damaged in the kidneys of rats with STZ-induced
diabetes, while the downregulation of PODXL1 and nephrin expression
levels, as well as the upregulation of α-SMA and vimentin
expression levels were observed. Nephrin is the first protein to be
identified at the glomerular podocyte slit diaphragm and its
expression has been described to be transiently increased in the
first 8 weeks subsequent to the establishment of a diabetes animal
model. The downregulation of nephrin expression was identified in
the kidneys of type 1 and type 2 diabetes patients (34). In addition, nephrin is a
podocyte-specific protein; thus, a reduction in its expression may
reflect podocyte loss in diabetic rats, and this loss can lead to a
range of renal diseases, including DN (35). Podocalyxin (PODX) is expressed by
kidney podocytes, vascular endothelia and a subset of neurons
(36). Aberrant expression of
PODX contributes to podocyte-associated renal diseases (37). PODXL1 is an anti-adhesive
transmembrane protein that has been reported to be expressed on the
foot processes of podocytes in the kidney glomerulus, as well as on
the endothelium at certain sites (36). The results of the present study
revealed that PODXL1 expression was decreased in diabetic rats
following STZ injection, which is consistent with the observations
of a previous study (38).
The levels of several serum and urine response
factors, including serum CysC, UmAlb, serum β2-MG and
BUN, were also examined in the present study and were observed to
be increased in diabetic rats. Serum CysC and β2-MG have
been reported to be increased in kidney injury (39,40). Notably, serum CysC is superior to
serum creatinine for the diagnosis of early diabetic nephropathy,
and an elevated serum CysC concentration is a strong predictor of
DN (41). Therefore, in the
present study serum CysC was found to be increased at 1 and 6 weeks
after STZ injection, whereas UmAlb, serum β2-MG and BUN
levels were significantly increased only at 6 weeks after STZ
injection. It is, thus, speculated that the downregulation of
PODXL1 and nephrin expression in podocytes leads to elevated levels
of urine response factors, thereby aggravating kidney injury.
Numerous studies have been published recently on the
functional roles of lncRNAs in DN. For instance, Zhou et al
(42) identified that the lncRNA
MIAT served an essential role in high glucose-induced renal tubular
epithelial injury in a rat model of diabetes. Wang et al
(25) also demonstrated that the
CYP4B1-PS1-001 lncRNA inhibited the proliferation and fibrosis of
mouse mesangial cells during the early stages of DN. In addition,
Beltrami et al (43)
reported that LipcRNAp21 lncRNA was associated with
diabetes-induced vascular complications. Hu et al (44) observed that MALAT1 lncRNA served a
pivotal role in DN and high glucose-induced podocyte damage.
According to the aforementioned findings, a microarray profiling
analysis of the differential expression of lncRNAs in diabetic rats
was conducted in the current study. It was determined that lncRNA
ENSRNOG00000037522 was significantly upregulated in the STZ-induced
DN group compared with the control group. It was further confirmed
that STZ- or high glucose-induced podocyte EMT occurred via the
upregulation of lncRNA ENSRNOG00000037522, and was accompanied by
the downregulation of PODXL1 and nephrin expression and the
upregulation of α-SMA and vimentin expression. In addition, lncRNA
ENSRNOG00000037522 knockdown repaired podocyte damage via the
downregulation of α-SMA and vimentin, and the upregulation of
PODXL1 and nephrin expression levels.
EMT is an important biological process during the
development of DN, and podocytes undergoing EMT lose the phenotypic
characteristics of epithelial cells, exhibiting reduced nephrin and
PODX expression, while they also express phenotypic markers of
mesenchymal cells, including α-SMA and vimentin (10). Nevertheless, the mechanisms that
mediate podocyte EMT remain poorly understood. Given that α-SMA and
vimentin are markers of mesenchymal cells, their expression levels
were observed to be increased in high glucose-induced EMT in
podocytes in the current study, which was consistent with the
findings of a previous study (27). These results indicate that α-SMA
and vimentin are essential for STZ-induced EMT, and it is
speculated that STZ-induced EMT leads to the loss of functional
proteins, which, in turn, damage the function and structure of
podocytes. However, further research is required to investigate
whether influencing the EMT is an efficient treatment strategy for
DN.
In the current study, GO and KEGG pathway analyses
were also used to identify the potential functions of the
differentially expressed lncRNAs. Several terms in the GO results
were involved in the EMT process, including the cellular process
term. The KEGG pathway analysis also revealed that numerous
pathways were associated with the EMT, including the 'PI3K-Akt
signaling pathway'. It has been reported that the PI3K-Akt
signaling pathway is essential in the process of EMT, and
inhibition of this signaling pathway was able to reduce podocytes
damage (45). These results
indicated the general role of ENSRNOG00000037522 in STZ-induced EMT
and podocyte damage.
In conclusion, the expression of the lncRNA
ENSRNOG00000037522 was upregulated in the kidneys of rats with
STZ-induced diabetes. Furthermore, the results indicated that the
inhibitory effects of lncRNA ENSRNOG00000037522 on high
glucose-induced podocyte EMT may provide further evidence for the
prevention and treatment of DN by targeting lncRNA
ENSRNOG00000037522.
Acknowledgments
The present study was supported by the Youth Science
Fund Project of National Natural Science Fund of China (grant no.
81400818) and the Provincial Natural Science Foundation of
Guangdong (grant no. 2017A030313783).
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Forbes JM and Cooper ME: Mechanisms of
diabetic complications. Physiol Rev. 93:137–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guariguata L, Whiting DR, Hambleton I,
Beagley J, Linnenkamp U and Shaw JE: Global estimates of diabetes
prevalence for 2013 and projections for 2035. Diabetes Res Clin
Pract. 103:137–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collins AJ, Foley RN, Chavers B,
Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J,
Peter W St, et al: United States Renal Data System 2011 Annual Data
Report: Atlas of chronic kidney disease & end-stage renal
disease in the United States. Am J Kidney Dis. 59(Suppl 1):
A7e1–420. 2012. View Article : Google Scholar
|
|
4
|
Eid AA, Ford BM, Block K, Kasinath BS,
Gorin Y, Ghosh-Choudhury G, Barnes JL and Abboud HE: AMP-activated
protein kinase (AMPK) negatively regulates Nox4-dependent
activation of p53 and epithelial cell apoptosis in diabetes. J Biol
Chem. 285:37503–37512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eid AA, Gorin Y, Fagg BM, Maalouf R,
Barnes JL, Block K and Abboud HE: Mechanisms of podocyte injury in
diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes.
58:1201–1211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim DK, Nam BY, Li JJ, Park JT, Lee SH,
Kim DH, Kim JY, Kang HY, Han SH, Yoo TH, et al: Translationally
controlled tumour protein is associated with podocyte hypertrophy
in a mouse model of type 1 diabetes. Diabetologia. 55:1205–1217.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Susztak K, Raff AC, Schiffer M and
Bottinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar
|
|
8
|
Weil EJ, Lemley KV, Mason CC, Yee B, Jones
LI, Blouch K, Lovato T, Richardson M, Myers BD and Nelson RG:
Podocyte detachment and reduced glomerular capillary endothelial
fenestration promote kidney disease in type 2 diabetic nephropathy.
Kidney Int. 82:1010–1017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Faul C, Asanuma K, Yanagida-Asanuma E, Kim
K and Mundel P: Actin up: Regulation of podocyte structure and
function by components of the actin cytoskeleton. Trends Cell Biol.
17:428–437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Kang YS, Dai C, Kiss LP, Wen X and
Liu Y: Epithelial-to-mesenchymal transition is a potential pathway
leading to podocyte dysfunction and proteinuria. Am J Pathol.
172:299–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pavenstädt H, Kriz W and Kretzler M: Cell
biology of the glomerular podocyte. Physiol Rev. 83:253–307. 2003.
View Article : Google Scholar
|
|
12
|
Durvasula RV and Shankland SJ: Podocyte
injury and targeting therapy: An update. Curr Opin Nephrol
Hypertens. 15:1–7. 2006. View Article : Google Scholar
|
|
13
|
Kang YS, Li Y, Dai C, Kiss LP, Wu C and
Liu Y: Inhibition of integrin-linked kinase blocks podocyte
epithelial-mesenchymal transition and ameliorates proteinuria.
Kidney Int. 78:363–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamaguchi Y, Iwano M, Suzuki D, Nakatani
K, Kimura K, Harada K, Kubo A, Akai Y, Toyoda M, Kanauchi M, et al:
Epithelial-mesenchymal transition as a potential explanation for
podocyte depletion in diabetic nephropathy. Am J Kidney Dis.
54:653–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai H, Liu Q and Liu B: Research progress
on mechanism of podocyte depletion in diabetic nephropathy. J
Diabetes Res. 2017:1–10. 2017. View Article : Google Scholar
|
|
16
|
Boon RA, Jaé N, Holdt L and Dimmeler S:
Long Noncoding RNAs: From clinical genetics to therapeutic targets.
J Am Coll Cardiol. 67:1214–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu MT, Hu JW, Yin R and Xu L: Long
noncoding RNA: An emerging paradigm of cancer research. Tumour
Biol. 34:613–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gibb EA, Warren RL, Wilson GW, Brown SD,
Robertson GA, Morin GB and Holt RA: Activation of an endogenous
retrovirus-associated long non-coding RNA in human adenocarcinoma.
Genome Med. 7:222015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Chen Z, Wang X, Huang Z, He Z and
Chen Y: Long non-coding RNA: A new player in cancer. J Hematol
Oncol. 6:372013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amit-Avraham I, Pozner G, Eshar S, Fastman
Y, Kolevzon N, Yavin E and Dzikowski R: Antisense long noncoding
RNAs regulate var gene activation in the malaria parasit Plasmodium
falciparum. Proc Natl Acad Sci USA. 112:E982–E991. 2015. View Article : Google Scholar
|
|
21
|
Hung CL, Wang LY, Yu YL, Chen HW,
Srivastava S, Petrovics G and Kung HJ: A long noncoding RNA
connects c-Myc to tumor metabolism. Proc Natl Acad Sci USA.
111:18697–18702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alvarez ML and Distefano JK: The role of
non-coding RNAs in diabetic nephropathy: Potential applications as
biomarkers for disease development and progression. Diabetes Res
Clin Pract. 99:1–11. 2013. View Article : Google Scholar
|
|
23
|
Ding GL, Wang FF, Shu J, Tian S, Jiang Y,
Zhang D, Wang N, Luo Q, Zhang Y, Jin F, et al: Transgenerational
glucose intolerance with Igf2/H19 epigenetic alterations in mouse
islet induced by intrauterine hyperglycemia. Diabetes.
61:1133–1142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan B, Tao ZF, Li XM, Zhang H, Yao J and
Jiang Q: Aberrant expression of long noncoding RNAs in early
diabetic retinopathy. Invest Ophthalmol Vis Sci. 55:941–951. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang M, Wang S, Yao D, Yan Q and Lu W: A
novel long non-coding RNA CYP4B1-PS1-001 regulates proliferation
and fibrosis in diabetic nephropathy. Mol Cell Endocrinol.
426:136–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang WT, Ye H, Wei PP, Han BW, He B, Chen
ZH and Chen YQ: LncRNAs H19 and HULC, activated by oxidative
stress, promote cell migration and invasion in cholangiocarcinoma
through a ceRNA manner. J Hematol Oncol. 9:1172016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen T, Zheng LY, Xiao W, Gui D, Wang X
and Wang N: Emodin ameliorates high glucose induced-podocyte
epithelia-mesenchymal transition in-vitro and in-vivo. Cell Physiol
Biochem. 35:1425–1436. 2015. View Article : Google Scholar
|
|
28
|
Roy S, Rahaman N, Ahmed F, Metya S and
Sannigrahi S: Naringenin attenuates testicular damage, germ cell
death and oxidative stress in streptozotocin induced diabetic rats:
naringenin prevents diabetic rat testicular damage. J Applied
Biomedicine. 11:195–208. 2013. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔC T method. Methods. 25:402–408.
2001. View Article : Google Scholar
|
|
30
|
Xu X, Luo P, Wang Y, Cui Y and Miao L:
Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) is a novel
therapeutic target for diabetic complications. J Int Med Res.
41:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mundel P and Shankland SJ: Podocyte
biology and response to injury. J Am Soc Nephrol. 13:3005–3015.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wiggins RC: The spectrum of
podocytopathies: A unifying view of glomerular diseases. Kidney
Int. 71:1205–1214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu D, Petermann A, Kunter U, Rong S,
Shankland SJ and Floege J: Urinary podocyte loss is a more specific
marker of ongoing glomerular damage than proteinuria. J Am Soc
Nephrol. 16:1733–1741. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cooper ME, Mundel P and Boner G: Role of
nephrin in renal disease including diabetic nephropathy. Semin
Nephrol. 22:393–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benigni A, Gagliardini E, Tomasoni S,
Abbate M, Ruggenenti P, Kalluri R and Remuzzi G: Selective
impairment of gene expression and assembly of nephrin in human
diabetic nephropathy. Kidney Int. 65:2193–2200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sassetti C, Tangemann K, Singer MS,
Kershaw DB and Rosen SD: Identification of podocalyxin-like protein
as a high endothelial venule ligand for L-selectin: Parallels to
CD34. J Exp Med. 187:1965–1975. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hara M, Yamagata K, Tomino Y, Saito A,
Hirayama Y, Ogasawara S, Kurosawa H, Sekine S and Yan K: Urinary
podocalyxin is an early marker for podocyte injury in patients with
diabetes: Establishment of a highly sensitive ELISA to detect
urinary podocalyxin. Diabetologia. 55:2913–2919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xing Y, Ye S and Chen Y, Hu W and Chen Y:
Hydrochloride pioglitazone protects diabetic rats against podocyte
injury through preserving glomerular podocalyxin expression. Arq
Bras Endocrinol Metabol. 58:630–639. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ataei N, Bazargani B, Ameli S, Madani A,
Javadilarijani F, Moghtaderi M, Abbasi A, Shams S and Ataei F:
Early detection of acute kidney injury by serum cystatin C in
critically ill children. Pediatr Nephrol. 29:133–138. 2014.
View Article : Google Scholar
|
|
40
|
Sharifiaghdas F, Kashi AH and Eshratkhah
R: Evaluating percutaneous nephrolithotomy-induced kidney damage by
measuring urinary concentrations of beta2-microglobulin. Urol J.
8:277–282. 2011.PubMed/NCBI
|
|
41
|
Shimizu A, Horikoshi S, Rinnno H, Kobata
M, Saito K and Tomino Y: Serum cystatin C may predict the early
prognostic stages of patients with type 2 diabetic nephropathy. J
Clin Lab Anal. 17:164–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou L, Xu DY, Sha WG, Shen L, Lu GY and
Yin X: Long non-coding MIAT mediates high glucose-induced renal
tubular epithelial injury. Biochem Biophys Res Commun. 468:726–732.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Beltrami C, Angelini TG and Emanueli C:
Noncoding RNAs in diabetes vascular complications. J Mol Cell
Cardiol. 89:42–50. 2015. View Article : Google Scholar
|
|
44
|
Hu M, Wang R, Li X, Fan M, Lin J, Zhen J,
Chen L and Lv Z: LncRNA MALAT1 is dysregulated in diabetic
nephropathy and involved in high glucose-induced podocyte injury
via its interplay with β-catenin. J Cell Mol Med. 21:2732–2747.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li D, Lu Z, Xu Z, Ji J, Zheng Z, Lin S and
Yan T: Spironolactone promotes autophagy via inhibiting
PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity
damage in podocytes under mechanical stress. Biosci Rep. 36:pii:
362016. 2016. View Article : Google Scholar
|