Introduction
Alzheimer's disease (AD) is a neurodegenerative
disorder, and its pathological lesions often appear in the
neocortex and, particularly, the hippocampus. An estimated ~36
million individuals suffer from AD worldwide. In the UK, AD-related
mortality is ranked no. 6 in the population aged >65 years,
while in the United States it is ranked no. 5 (1). In China, the total number of AD
patients is >5 million (2).
Therefore, the prevention and treatment of AD represent major
challenges for scientists and clinicians. The mechanism of AD
pathogenesis has not yet been fully elucidated. There are numerous
hypotheses, but the most widely accepted hypothesis is the amyloid
cascade reaction. This hypothesis suggests that neurotoxic
β-amyloid (Aβ) protein is deposited in the brain and triggers a
series of pathological alterations, including amyloid plaques and
neurofibrillary tangles (3).
Previous studies confirmed that Aβ neurotoxicity causes formation
of amyloid plaques in the neocortex through a variety of mechanisms
(4); however, the asociation
between the formation of neurofibrillary tangles and cytoskeletal
alterations is not well understood. The cytoskeleton is a fibrous
network mainly composed of microtubules, microfilaments and
intermediate filaments. Previous studies demonstrated that, during
cell apoptosis, the cytoskeleton exhibits major changes (5); therefore, the structure and function
of the neuronal cytoskeleton may play an important role in the
pathogenesis of neurodegenerative diseases (6). In the present study, the neurotoxic
effect of Aβ25-35 on the cytoskeleton were investigated
in a PC12 cell culture, aiming to elucidate the role of the
cytoskeleton in the pathogenesis of AD to provide novel insight and
treatment strategies.
Materials and methods
PC12 cell culture
The PC12 adrenal pheochromocytoma cell line was
purchased from the Institute of Cell Biology, Chinese Academy of
Sciences (Shanghai, China). The present study used differentiated
PC12 cells, as they share several characteristics with nervous
cells and are widely accepted as a neuronal model. In addition,
compared with primary neurons, the procedures of cell culturing are
simple, with efficient cell proliferation. For the cell culture,
PC12 cells removed from liquid nitrogen were thawed at 37°C, and
were then seeded and cultured in high-glucose Dulbecco's modified
Eagle's medium (DMEM; 12100-038) with 10% fetal calf serum (FCS;
140714) (both from Gibco; Thermo Fisher Scientific, Grand Island,
NY, USA) and 1% penicillin and streptomycin. PC12 cells in the
adherent culture started to grow and differentiate and, after 24 h,
spindle-like and polygon-like cells were observed, with clear
boundaries and a neuron-like shape. When cultured PC12 cells
reached a confluence of 70–80%, cell passaging was performed.
Following digestion with 0.25% trypsin, the cells were collected
and seeded for the following experiments. Some cells were
cryopreserved in liquid nitrogen.
Aβ25-35 protein treatment and
experimental grouping
Aβ accumulation in brain is the main event in the
pathogenesis of AD; therefore, instead of toxic oligomers of the
conventional 1–40 or 1–42 subtypes, Aβ has been used as a inducer
for the AD cell model (7).
Naturally occurring Aβ protein exhibits only weak neurotoxicity
in vivo or in vitro; however, the aggregated Aβ
protein and its fragments may be severely neurotoxic. This process
is referred to as 'aging'. According to Pike et al (8), aged Aβ25-35 (A4559;
Sigma-Aldrich; Merck, KGaA, St. Louis, MO, USA) was prepared as
follows: Aβ25-35 protein (1 mg) was dissolved in 1 ml
sterile water (1 mmol/l). Subsequently, 1 mmol/l Aβ25-35
was incubated at 37°C for 4–7 days, and the aged Aβ25-35
was used for further neurotoxicity experiments. In the present
study, cultured PC12 cells were divided into treatment and control
groups. In the control group, the cells were cultured in
high-glucose DMEM with 10% FCS for 48 h. In the experimental
groups, the cells were cultured in the same medium as the control
group, but the medium was supplemented with aged Aβ25-35
at various concentration (10, 50, 90, 180 and 360 µmol/l).
Subsequent experiments were performed in the control and treatment
groups.
Apoptotic assays
MTT assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) reacts with succinate dehydrogenase in mitochondria
and is reduced into water-insoluble blue-violet formazan crystals.
Formazan is deposited in living rather than dead cells; therefore,
formazan colorimetry may be used to evaluate cell viability. After
reaching the logarithmic growth phase, 100 µl PC12 cells at
a concentration of 3×104 cells/ml were seeded in a
96-well culture plate and incubated for 24 h at 37°C with 5%
CO2. After changing the medium (100 µl), PC12
cells in both the control (n=5) and experimental groups (n=5)
continued to be cultured for another 48 h, followed by the addition
of 20 µl MTT (5 mg/ml). The cells were incubated with MTT
for another 4 h, then disrupted with 150 µl dimethyl
sulfoxide. After shaking for 10 min, the absorbance (OD) values of
the sample were read using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a wavelength of 570 nm
(n>3).
4′,6-Diamidino-2-phenylindole (DAPI)
and terminal deoxynucleotidyl transferase dUTP nick-end labeling
(TUNEL) staining
PC12 cells in the logarithmic growth phase
(1×105 cells/ml) were used for DAPI and TUNEL assays.
The cells in the control and experimental groups were fixed with 4%
paraformaldehyde (pH 7.4) at 4°C for 30 min. After washing 3 times
with 0.01 mol/l phosphate-buffered saline (PBS), the cells were
coverslipped with DAPI-glycerin (DAPI:65% glycerol = 1:1,000) and
examined with a fluorescent microscope (BX61; Olympus, Tokyo,
Japan). With DAPI staining, the cell nuclei and apoptotic bodies
(condensed and fragmented nuclei) were visualized. TUNEL staining
was also performed using a TUNEL assay kit (G3250; Promega,
Madison, WI, USA). Cell collection and fixation were performed as
described above. Subsequently, the cells were treated with 0.2%
Triton X-100 to increase cell transparency. After rinsing with 0.01
mol̸l PBS three times, TUNEL reaction solution was added, followed
by incubation at 37°C for 60 min. The reaction was then stopped
with termination solution. The sections were coverslipped with
DAPI-glycerin and examined with an epifluorescence micro scope
(BX61; Olympus) following excitation with rhodamine or ultraviolet
(UV) light.
Cytoskeleton visualization with
phalloidin staining and immunocytochemistry
Phalloidin staining
F-actin is the main component of microfilaments, and
is capable of binding with phalloidin. Therefore, phalloidin may be
used to stain microfilaments in the cell. Cells in the control and
experimental groups were collected as described above and fixed in
4% paraformaldehyde for 30 min. Subsequently, 0.2 µg/ml
phalloidin (A12380; Invitrogen; Thermo Fisher Scientific; Carlsbad,
CA, USA) was diluted with preparation solution (0.1%
NaN3, 3% BSA, 0.3% Triton X-100 in 0.01 M PBS). After
washing with 0.01 M PBS, the cells were incubated with phalloidin
solution overnight and coverslipped with DAPI-glycerin. The cells
were examined under a fluorescence microscope following rhodamine
or UV excitation.
Immunocytochemistry
The main component of microtubules is β-II-tubulin,
which was used as a marker in the present study to visualize
microtubules. The primary antibody used was rabbit
anti-β-II-tubulin polyclonal antibody (1:100, AB151318; Abcam,
Cambridge, MA, USA). The cells were incubated with the primary
antibody at 4°C overnight. After washing with 0.01 M PBS 3 times,
secondary antibody (Alexa Fluro 488 donkey anti-rabbit IgG, 1:500,
A21206; Invitrogen; Thermo Fisher Scientific) was added and
incubated at room temperature for 3 h. The cells were coverslipped
with DAPI-glycerin, observed and photographed with a fluorescence
microscope.
Measurements and statistical tests
The MTT assay was used to evaluate the viability of
PC12 cells as follows: Cell survival rate (%) = (OD570
in the experimental group − OD570 in the blank
group)/(OD570 in the control group − OD570 in
the blank group) ×100%. The remaining cultured cells were
considered as non-viable, including necrotic and apoptotic cells.
Using the cell survival rate, the ratio of non-viable cells was
calculated using the formula: 1 − cell survival rate (%). ImageJ
1.48 software was used to quantify the apoptotic cells and
disintegrated cytoskeleton as follows: Apoptotic rate (DAPI or
TUNEL staining) = (apoptotic cells/total cells) ×100%. Cytoskeleton
disintegration = (number of cells with disintegrated
cytoskeleton/total cell number) ×100%. In order to exclude the
interference of necrosis, we also estimated the proportional
contribution of apoptosis to the viable reduction according to the
formula: Neurapoptosis/non-viable cells = apoptotic rate/(1 − cell
survival rate) (%).
Statistical analysis
All data were recorded as mean ± standard deviation.
Using SPSS 11.5 statistical software (SPSS, Inc., Chicago, IL,
USA), comparisons of the abovementioned parameters were performed
between the experimental and control groups with one-way analysis
of variance. P<0.05 was considered to indicate statistically
significant differences, and P<0.01 was considered as highly
statistically significant.
Results
Aβ25-35 neurotoxicity and
neuroapoptosis in PC12 cells
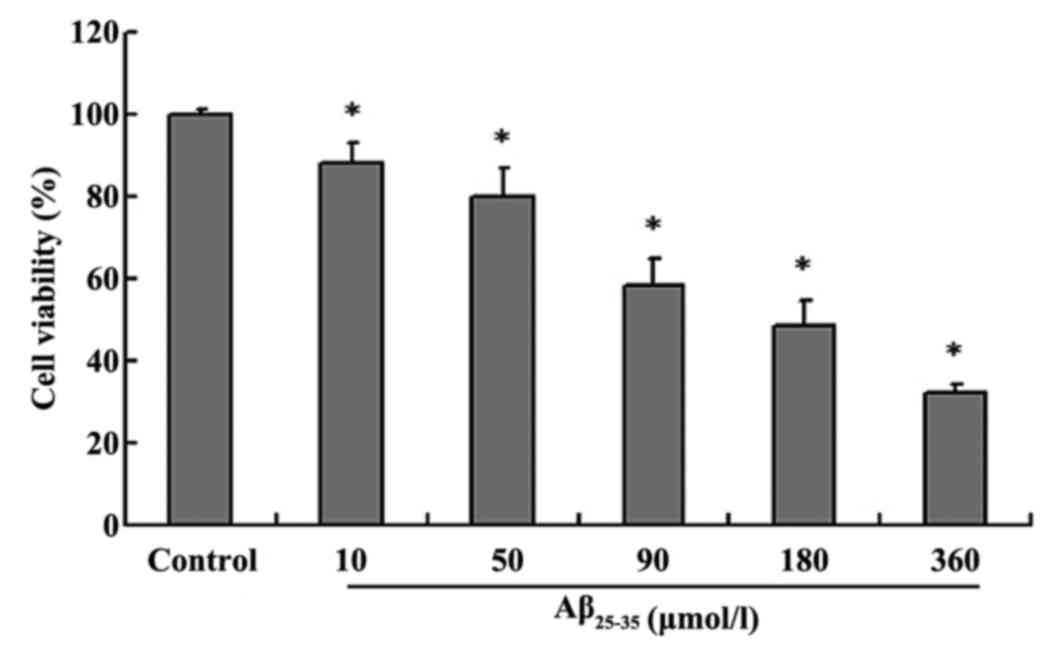
The MTT assay was used to detect cell viability
following exposure to Aβ25-35 toxicity (9). The results revealed that
Aβ25-35 decreased cell viability in a dose-dependent
manner compared with the control group. Cell survival was gradually
reduced with increasing concentrations of Aβ25-35 (10,
50, 90, 180 and 360 µmol/l; P<0.05, Fig. 1).
In order to determine whether the reduction in cell
survival was caused by cell apoptosis, TUNEL assay with DAPI
counter-staining was performed. DAPI stains cell nuclei with blue
color. The PC12 cells in the control group appeared to grow well,
with uniform brightness, and their nuclei had a smooth outline with
homogeneous chromatin distribution. However, the cells in the
experimental group displayed pyknotic nuclear chromatin, which was
often marginated along the nuclear edge, conferring an appearance
of half moon-like nuclei, with occasional formation of typical
apoptotic bodies (Fig. 2A–D).
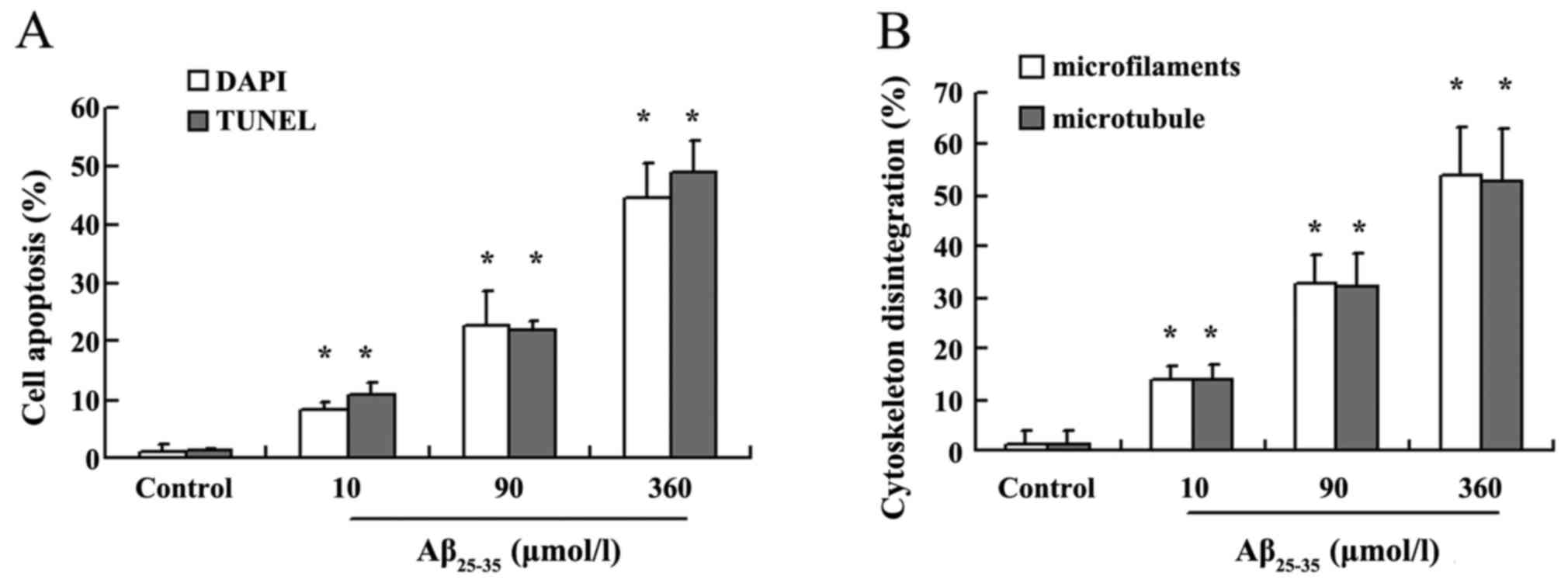
With increasing Aβ25-35 dose, the number of apoptotic
cells gradually increased in a dose-dependent manner (P<0.05;
Fig. 3A). TUNEL assay confirmed
the results of DAPI staining. Apoptotic cells were hardly found in
the control group; however, following Aβ25-35 treatment,
the number of apoptotic cells identified with TUNEL staining
increased in a dose-dependent manner (P<0.05; Fig. 3A). The apoptotic cells observed
with DAPI staining and the TUNEL-positive cells usually overlapped
(Fig. 2A–H). In the present
study, neurapoptosis/non-viable cells were calculated under various
Aβ concentrations; at Aβ25-35 concentrations of 10, 50,
90, 180 and 360 µmol/l, neuroapoptosis/non-viable cells was
95.41, 87.18, 83.61, 82.87 and 84.51%, respectively, suggesting
neuroapoptosis was the main cause of cell death.
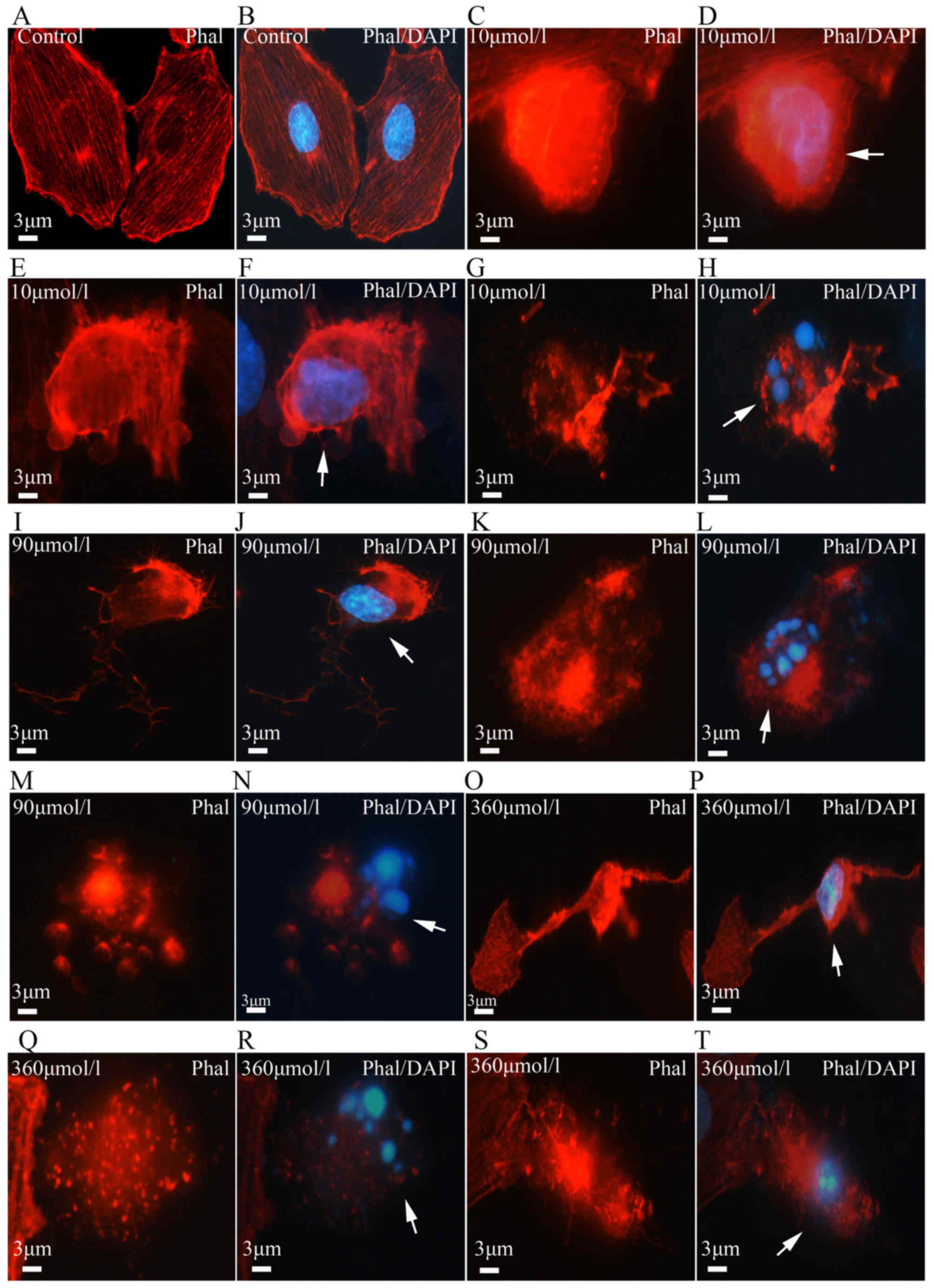
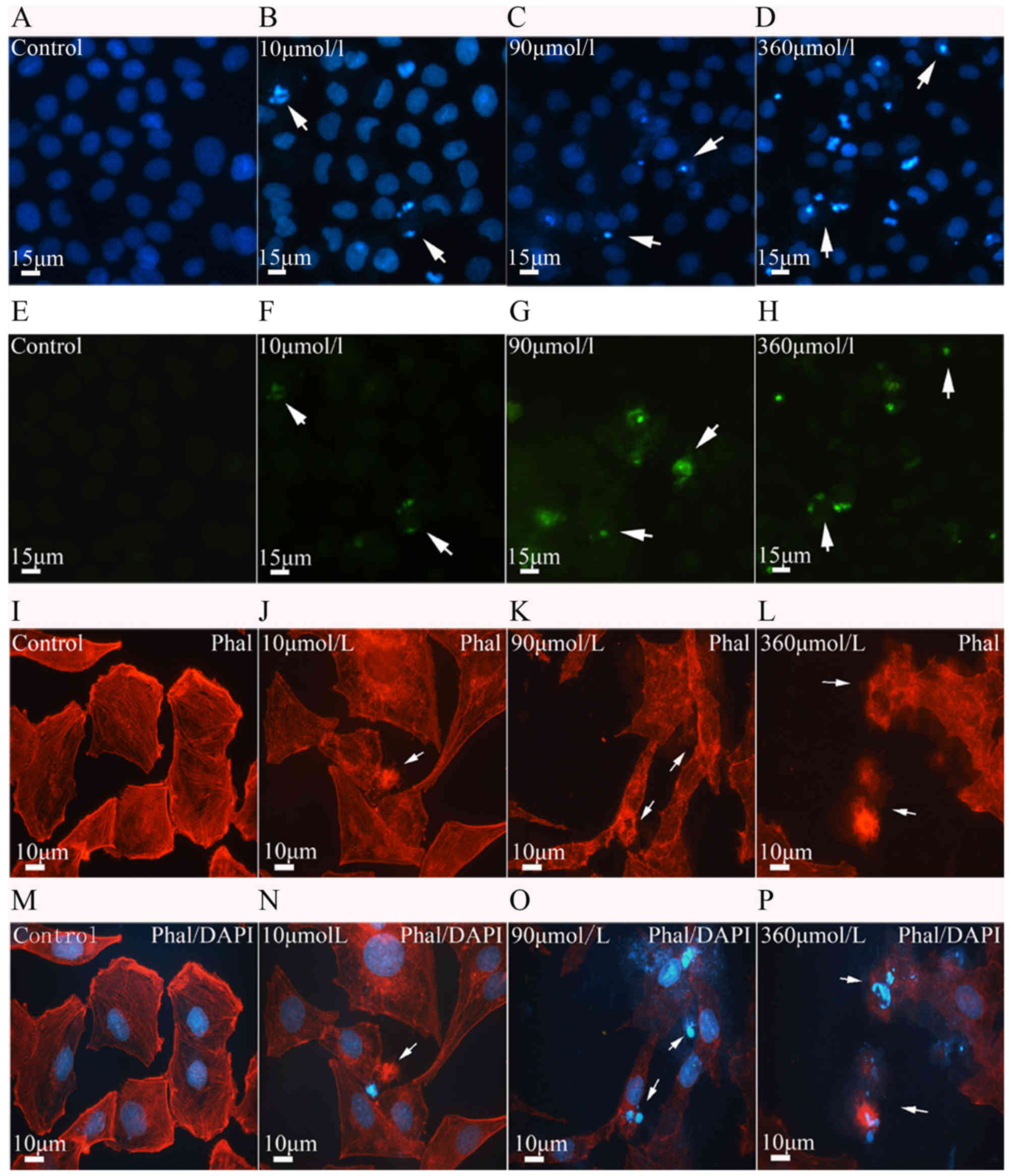 | Figure 2Cell apoptosis and microfilament
alterations following β-amyloid (Aβ)25-35 treatment
(phalloidin staining and DAPI counterstaining). (A-D) Nuclear
condensation and apoptotic bodies (arrows) were visualized with
DAPI staining (blue) in the control and treatment groups. (E-H)
Cell apoptosis determined with the TUNEL assay. Aβ25-35
induced PC12 cell apoptosis (green, arrows) in a dose-dependent
manner. (I-P) Aβ25-35 neurotoxicity and microfilament
alterations (phalloidin staining and DAPI counterstaining).
Following Aβ25-35 treatment, the microfilaments (red) in
PC12 cells appeared to disintegrate (arrows). The disintegrated
microfilaments (J and L, arrows) were often observed in apoptotic
cells with condensed nuclei or apoptotic bodies (N and P, arrows).
With increasing Aβ25-35 dose, the number of cells with
disintegrated microfilaments gradually increased. Scale bars, 15
µm in (A-H) and 10 µm in (I-P). TUNEL, terminal
deoxynucleotidyl transferase dUTP nick-end labeling; DAPI,
4′,6-diamidino-2-phenylindole; Phal, phalloidin. |
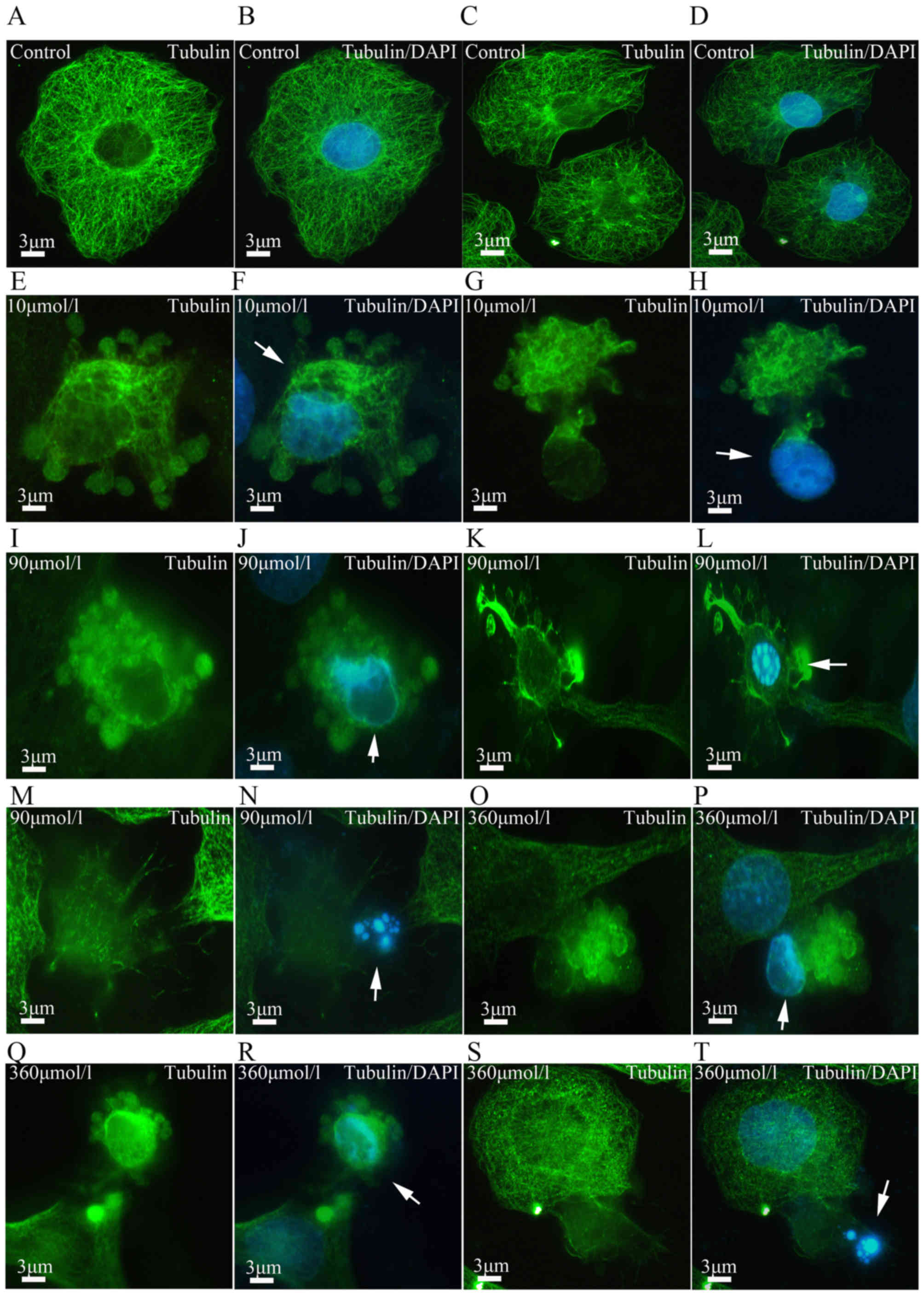
Aβ25-35 neurotoxicity and
cytoskeletal alterations in PC12 cells
The cytoskeleton is an important component of
eukaryotic cells, and it plays a key role in the maintenance of
cell shape, intracellular substance transportation and cell
amoeba-like movement. Recent studies reported that there is a close
association between cytoskeletal abnormalities and cell apoptosis.
It was previously demonstrated that, during cell division,
suppressed microtubule assembly is followed by protein kinase
activation and induction of cell apoptosis (10). In yeast and certain types of
animal cells, such as HL-60 cells, depolymerization of
microfilaments may be induced in apoptotic cells (11). In the present study, major changes
were observed in the cytoskeleton following Aβ25-35
treatment. The cells in the control group contained numerous
microfilaments with intensive fluorescence and a clear outline.
Microfilaments of varying lengths exhibited a rod-like appearance
and were parallelly arranged. Following Aβ25-35
treatment, the microfilaments in the apoptotic cells appeared vague
and started to disintegrate (Figs.
2I–P and 4). Statistical
analysis revealed that the microfilament disintegration rate
increased in Aβ25-35 treatment groups in a
dose-dependent manner (P<0.05; Fig. 3B). Furthermore, a neurotoxic
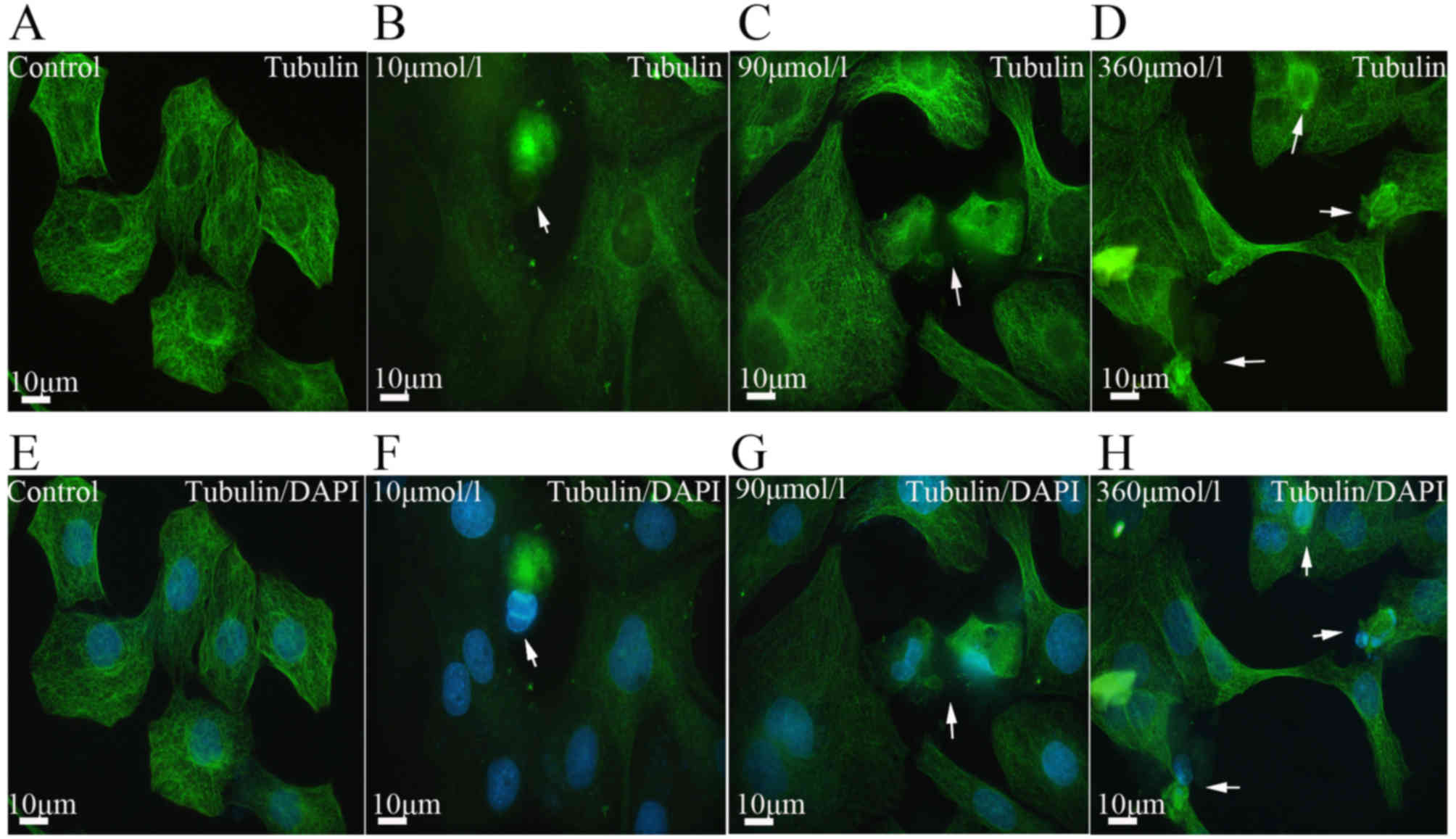
effect of Aβ25-35 on microtubules was also observed. In
the control group, the fibrous microtubules formed a web structure
around the nucleus. In the experimental groups, the microtubules
started to disintegrate and even aggregate in dense lumps (Fig. 5). These pathological alterations
were often observed in apoptotic cells (Fig. 6E–T). Statistical analysis revealed
that Aβ25-35 induced microtubule disintegration in a
dose-dependent manner compared with the control group (P<0.05;
Fig. 3B).
Discussion
Aβ25-35 induces neuroapoptosis in PC12
cells. Apoptosis plays a crucial role during cell differentiation,
organ development and pathogenesis (12,13); it is also physiologically
important for neural pathfinding and synaptogenesis during brain
development. Neuroapoptosis may also occur during cell aging,
neuronal injury and neural degenerative diseases. AD is one of the
most common neurodegenerative diseases, and the mechanism
underlying its pathogenesis is extremely complex. Scientists
believe that Aβ is a key factor in the development of AD. Aβ is
cleaved from amyloid precursor protein by β- or γ-hydrolase. Aβ
consists of 39–43 amino acid residues and it has three types,
namely Aβ25-35, Aβ1-42 and Aβ1-40,
of which Aβ25-35 is considered to be the most toxic. In
the present study, the neurotoxic effect of Aβ25-35 on
PC12 cells was assessed. As Loo et al (14) and Kim et al (15) previously reported, Aβ induces
neuronal apoptosis and necrosis. The present study demonstrated
that Aβ25-35 reduced cell viability, as determined by
the MTT assay. In addition, Aβ25-35 also caused PC12
cell apoptosis in a dose-dependent manner. It was also determined
that apoptosis was the main contributor to cell death in non-viable
cells, indicating that neuropoptosis is a key factor in AD
pathogenesis. The mechanism underlying Aβ neurotoxicity is complex,
and likely involves cell oxidative stress and intracellular calcium
deregulation. Deposition of Aβ triggers the oxidative stress
response in cells, and calcium balance is disturbed. These events
then trigger cell apoptosis through the endoplasmic reticulum and
death receptor pathways (7,16).
In the present study, Aβ clearly induced neuroapoptosis; however,
the findings require confirmation by further studies.
Aβ25-35 induced disintegration of the
cytoskeleton. Microfilaments and microtubules are crucial for cell
functions. If the cytoskeleton is disintegrated, inevitable
pathological alterations will occur. Previous studies reported that
the cytoskeletal malformations during the pathogenesis of
Huntington's chorea, Parkinson's disease and AD (17). As a key risk factor of AD
pathogenesis, Aβ induces neuronal cytoskeleton abnormalities
(18,19). In the present study, following
Aβ25-35 treatment, the cytoskeleton collapsed and
disintegrated in a dose-dependent manner. Cytoskeletal
disintegration was often observed in apoptotic cells. The changes
in the treatment groups were similar to those reported in the brain
of an AD patient, exhibiting nerve inflammation and destruction of
the cytoskeleton caused by Aβ neurotoxicity and Tau protein
hyperphosphorylation (20). It is
known that Aβ may trigger certain signaling pathways and cause
rearrangement of the microfilament and microtubule network, leading
to neuronal function disorders (21,22). The microtubule-associated proteins
regulate the arrangement of the microtubules, and the Tau protein
promotes microtubule assembly; therefore, Aβ neurotoxicity by
cytoskeletal disintegration is probably mediated by the activity of
cytoskeleton-related proteins; however, the detailed mechanism
requires further investigation.
Cytoskeletal disintegration is an important
pathological event in AD. According to the AD cell model
constructed by Ostrovskaya et al (23), the neurotoxicity of
Aβ25-35 was investigated in PC12 cells in an attempt to
elucidate the association between Aβ neurotoxicity and AD
pathogenesis. The results demonstrated that Aβ25-35
induced PC12 cell apoptosis and cytoskeletal disintegration.
Previous studies also demonstrated that cell apoptosis is
accompanied by cytoskeletal destruction (24). In the present study,
Aβ25-35 induced cell apoptosis and cytoskeletal
disintegration, which is likely the main pathological basis of
neurofibrillary tangle formation in AD. According to the amyloid
cascade hypothesis, Aβ may promote abnormal phosphorylation of the
Tau protein (25), cell apoptosis
and cytoskeletal disintegration. The present study suggests that
Aβ25-35 induces not only cell apoptosis, but also
cytoskeletal disintegration.
In conclusion, Aβ25-35 decreased the
viability of PC12 cells and induced cell apoptosis in a
dose-dependent manner. The cytoskeleton is also very sensitive to
the neurotoxic effects of Aβ25-35, leading to
cytoskeletal disintegration. As indicated by the present study,
this Aβ-induced cytoskeletal disintegration is likely an important
event in the pathological base of neurofibrillary tangle formation
during AD pathogenesis. These findings will hopefully provide a new
approach to the prevention and treatment of AD.
Acknowledgments
Not applicable.
Notes
[1]
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. U1204311), Henan
University Youth Training Foundation (grant nos. 0000A40356 and
0000A40475), Henan Postdoctoral Foundation (grant no. 2015051) and
Henan Province Research Program of Basic and Advanced Technology
(grant no. 172102310001).
[2] Availability
of data and materials
All data generated or analyzed during this study are
included in this published article.
[3] Authors'
contributions
LW, JC, ZS and JinD designed the study, and
performed the analyses. WF and HL analyzed data. JinD and JieD were
involved in the conception of the study and wrote the manuscript.
The final version of the manuscript has been read and approved by
all authors.
[4] Ethics
approval and consent to participate
The protocol was approved by the Committee of
Medical Ethics and Welfare for Experimental Animals, Henan
University School of Medicine (HUSOM:2015-87).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Henley DB, Dowsett SA, Chen YF,
Liu-Seifert H, Grill JD, Doody RS, Aisen P, Raman R, Miller DS,
Hake AM, et al: Alzheimer's disease progression by geographical
region in a clinical trial setting. Alzheimers Res Ther. 7:432015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong MJ, Peng B, Lin XT, Zhao J, Zhou YR
and Wang RH: The prevalence of dementia in the People's Republic of
China: a systematic analysis of 1980–2004 studies. Age Ageing.
36:619–624. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barage SH and Sonawane KD: Amyloid cascade
hypothesis: pathogenesis and therapeutic strategies in Alzheimer's
disease. Neuropeptides. 52:1–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sadigh-Eteghad S, Sabermarouf B, Majdi A,
Talebi M, Farhoudi M and Mahmoudi J: Amyloid-beta: a crucial factor
in Alzheimer's disease. Med Princ Pract. 24:1–10. 2015. View Article : Google Scholar
|
|
5
|
Shen ZY, Xu LY, Li EM, Li JT, Chen MH,
Shen J and Zeng Y: Ezrin, actin and cytoskeleton in apoptosis of
esophageal epithelial cells induced by arsenic trioxide. Int J Mol
Med. 12:341–347. 2003.PubMed/NCBI
|
|
6
|
Rudrabhatla P: Regulation of neuronal
cytoskeletal protein phosphorylation in neurodegenerative diseases.
J Alzheimers Dis. 41:671–684. 2014.PubMed/NCBI
|
|
7
|
Kaminsky YG, Marlatt MW, Smith MA and
Kosenko EA: Subcellular and metabolic examination of amyloid-beta
peptides in Alzheimer disease pathogenesis: evidence for
Abeta(25–35). Exp Neurol. 221:26–37. 2010. View Article : Google Scholar
|
|
8
|
Pike CJ, Walencewicz AJ, Glabe CG and
Cotman CW: In vitro aging of beta-amyloid protein causes peptide
aggregation and neurotoxicity. Brain Res. 563:311–314. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatok J, Babusikova E, Matakova T, Mistuna
D, Dobrota D and Racay P: In vitro assays for the evaluation of
drug resistance in tumor cells. Clin Exp Med. 9:1–7. 2009.
View Article : Google Scholar
|
|
10
|
Ndozangue-Touriguine O, Hamelin J and
Bréard J: Cytoskeleton and apoptosis. Biochem Pharmacol. 76:11–18.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gourlay CW, Carpp LN, Timpson P, Winder SJ
and Ayscough KR: A role for the actin cytoskeleton in cell death
and aging in yeast. J Cell Biol. 164:803–809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dekkers MP, Nikoletopoulou V and Barde YA:
Cell biology in neuroscience: death of developing neurons: new
insights and implications for connectivity. J Cell Biol.
203:385–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen A, Xiong LJ, Tong Y and Mao M: The
neuroprotective roles of BDNF in hypoxic ischemic brain injury.
Biomed Rep. 1:167–176. 2013. View Article : Google Scholar
|
|
14
|
Loo DT, Copani A, Pike CJ, Whittemore ER,
Walencewicz AJ and Cotman CW: Apoptosis is induced by beta-amyloid
in cultured central nervous system neurons. Proc Natl Acad Sci USA.
90:7951–7955. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim IK, Lee KJ, Rhee S, Seo SB and Pak JH:
Protective effects of peroxiredoxin 6 overexpression on amyloid
β-induced apoptosis in PC12 cells. Free Radic Res. 10:836–846.
2013. View Article : Google Scholar
|
|
16
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 7:72015. View Article : Google Scholar
|
|
17
|
Leadsham JE, Kotiadis VN, Tarrant DJ and
Gourlay CW: Apoptosis and the yeast actin cytoskeleton. Cell Death
Differ. 17:754–762. 2010. View Article : Google Scholar
|
|
18
|
Hardy J: Has the amyloid cascade
hypothesis for Alzheimer's disease been proved. Curr Alzheimer Res.
3:71–73. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Henriques AG, Vieira SI, da Cruz E Silva
EF and da C ruz E Silva OA: Abeta promotes Alzheimer's
disease-like cytoskeleton abnormalities with consequences to APP
processing in neurons. J Neurochem. 113:761–771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin M, Shepardson N, Yang T, Chen G, Walsh
D and Selkoe DJ: Soluble amyloid beta-protein dimers isolated from
Alzheimer cortex directly induce Tau hyperphosphorylation and
neuritic degeneration. Proc Natl Acad Sci USA. 108:5819–5824. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sohn PD, Tracy TE, Son HI, Zhou Y, Leite
RE, Miller BL, Seeley WW, Grinberg LT and Gan L: Acetylated Tau
destabilizes the cytoskeleton in the axon initial segment and is
mislocalized to the somatodendritic compartment. Mol Neurodegener.
11:472016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng Y, Wei J, Cheng J, Zhong P, Xiong Z,
Liu A, Lin L, Chen S and Yan Z: Partial amelioration of synaptic
and cognitive deficits by inhibiting cofilin dephosphorylation in
an animal model of Alzheimer's disease. J Alzheimers Dis.
53:1419–1432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ostrovskaya RU, Vakhitova YV, Kuzmina US,
Salimgareeva MK, Zainullina LF, Gudasheva TA, Vakhitov VA and
Seredenin SB: Neuroprotective effect of novel cognitive enhancer
noopept on AD-related cellular model involves the attenuation of
apoptosis and Tau hyperphosphorylation. J Biomed Sci. 21:742014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas SG, Huang S, Li S, Staiger CJ and
Franklin-Tong VE: Actin depolymerization is sufficient to induce
programmed cell death in self-incompatible pollen. J Cell Biol.
174:221–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu J, Zhang R, Zuo P, Yang N, Ji C, Liu W,
Wang Y, Wang H, Wu A, Yue Y, et al: Aggravation effect of
isoflurane on Aβ(25–35)-induced apoptosis and Tau
hyperphosphorylation in PC12 cells. Cell Mol Neurobiol.
32:1343–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|