Introduction
China is facing critical issues caused by cancer
with nearly 2 million cancer deaths and more than 3 million cancer
incidences annually (1).
Currently, chemotherapy, surgery, radiotherapy, biotherapy and
other adjuvant therapy are common treatments for tumor. However,
chemotherapy is the preferred and the most common treatment for
patients in clinical practice (2). Yet, it is still lacks effective
antitumor drugs, while some patients easily acquire resistance to
many new drugs after a period of treatment. For example, the
mutation of T790M confers resistance to first-generation EGFR TKIs.
Therefore, there is an urgent need to find new and effective
antitumor drugs (3). Moreover,
many chemotherapy drugs in clinical practice are from natural
medicine, such as paclitaxel, vincristine, oxymatrine, and
podophyllotoxin (4). An
increasing number of researchers (5–7)
from all over the world have been focusing on natural medicines to
find new antitumor drugs.
Camellia nitidissima (C. nitidissima)
Chi (Theaceae family) is an evergreen tree, grown mainly in
Guangxi, China. It is a popular commercial and ornamental plant
with golden-yellow flowers. C. nitidissima has also been
classified as one of the rarest plants in China, and is known as
'flora panda' and 'camellia queen'. The tree contains saponins,
flavonoids, polyphenols, vitamins, amino acids and other nutrients
for the organism (8). Therefore,
locally C. nitidissima plays an important role in human
health. Moreover, several studies (8–10)
showed that C. nitidissima could reduce blood-lipid,
decrease blood pressure, resist oxidation, prevent carcinogenesis
and inhibit tumor growth. However, most of the pharmacodynamics
studies of C. nitidissima are based on the crude extracts
from the camellia. Therefore, it is necessary to investigate
bioactive phytochemicals in C. nitidissima.
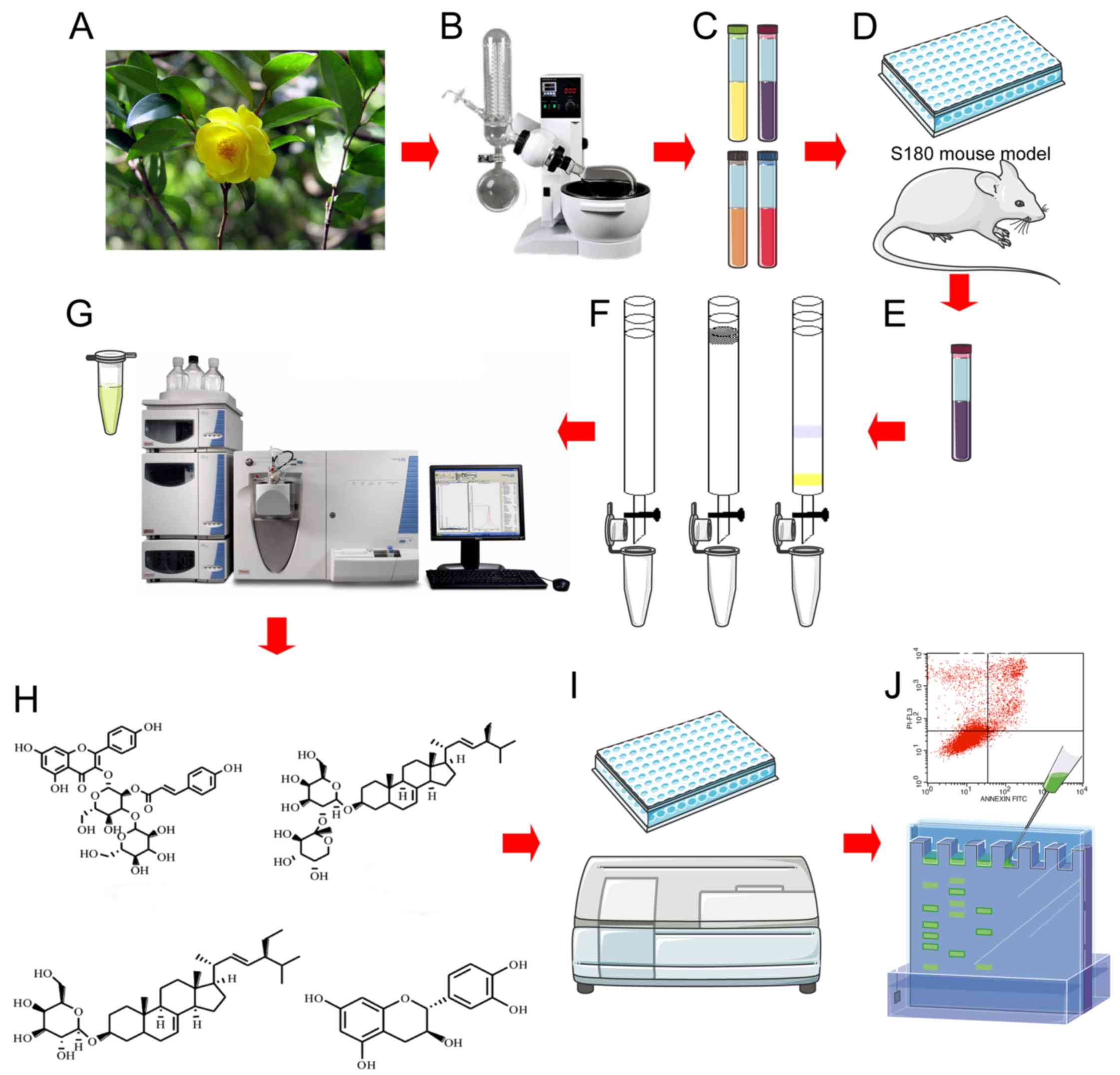
In this study, 16 phytochemicals were isolated from
the leaves of C. nitidissima by undergoing purification via
repeated silica gel chromatography (Sephadex LH-20, MCI gel
columns), recrystallization, and semi-preparative HPLC techniques.
The chemical structures were identified on the basis of spectral
data including NMR and MS. Subsequently, the antitumor activity
screening of the chemical constituents on 4 common cancer cell
lines were detected by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays. In further experiments, we evaluated the IC50 of
compound 3 on the NCI-H1975 and detected the apoptosis effect by
Annexin V/PI double staining to explore the potential mechanism.
Finally, the apoptosis effect was confirmed by western blot
analysis.
In brief, 16 chemical constituents were isolated
from the leaves of C. nitidissima, and 6 phytochemicals
(compound 2, 3, 5, 6, 7 and 10) were first reported in this plant
in this study, while other 8 phytochemicals were first reported in
this plant in our previous published study (11). Furthermore, we evaluated the
antitumor activity of 16 phytochemicals on 4 different cancer cell
lines (3 different cancer types). To our knowledge, it is the first
study that compound 3 has the most potent antitumor effect, based
on the screening data of 4 common malignant cancer cells, showing
potential for antitumor drug development.
Materials and methods
Preparation of chemical constituents
The leaves from C. nitidissima were collected
from Fangchenggang, China, and authenticated by Mr. Ji-zhu Yang
(where?). The leaves were air-dried and coarsely powdered.
The powdered leaves of C. nitidissima (6.3 kg) were
extracted with 95% ethanol three times (3, 2 and 1 h) under reflux.
The ethanolic extracts were combined and concentrated under reduced
pressure. The concentrated extract was suspended in water,
partitioned with ethyl acetate (EtOAc, Shuangling Chemical Reagent
Co. 295 g), and n-butanol (n-BuOH, Shuangling Chemical Reagent Co.
137 g) successively. The EtOAc fraction (285 g) was subjected to
silica gel column chromatography and eluted with a petroleum
ether-acetone (PE-CP, 10:1→0:1) gradient system to yield six
fractions (Frs. 1–6) on the basis of TLC analysis. Fr. 1 was
further subjected to silica gel column chromatography and eluted
with PE-CP (5:1) to obtain the compounds; compound 1, compound 2,
compound 3, compound 15, and compound 16. Compound 5, compound 6,
and compound 7 were obtained from Fr. 4. Seven sub-fractions from
Fr. 1 were subjected to repeated silica gel chromatography,
including C18 silica gel chromatography, Sephadex LH-20
chromatography, and MCI gel column chromatography, and a
semi-preparative HPLC technique (Agilent HPLC with Hypersil ODS
column (150×4.6 mm), respectively. compound 4 was obtained from
sub-fraction 1; compound 8 and compound 12 were obtained from
sub-fraction 3; and compound 13, compound 4, and compound 11 were
obtained finally from sub-fraction 4; Compound 13 was obtained from
Fr. 5; compound 14 was recrystallized from Fr. 6
(CH2Cl2-MeOH 10:1). Compounds 9 and 10 were
obtained by further silica gel column chromatography
(CH2Cl2-MeOH 10:1) of this fraction.
Cell culture
The human lung cancer cell lines, A549 and NCI-H1975
were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA). The
human gastric cancer cell line, HGC-27 was cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco). The colon cancer cell line,
SW620 was cultured in L-15 (Gibco). All the cell lines were
obtained from the Cell Bank of the Institute of Biochemistry and
Cell Biology, Chinese Academy of Sciences (Shanghai, China). The
media were supplemented with 10% fetal bovine serum (FBS; Gibco),
100 U/ml penicillin and 100 U/ml streptomycin. The cells were
incubated in a humidified atmosphere with 95% air and 5%
CO2 at 37°C.
Cell proliferation assays
Cell proliferation assays were performed by MTT as
previously described. Briefly, cells were harvested, washed with
phosphate-buffered saline (PBS) and digested with trypsin, then
plated in 96-well plates, at a density of ~2×103 cells
in each well. Twelve hours later, cells were treated with chemical
constituent at specified concentrations for additional 48 h. Then
cells were treated with MTT for 4 h. After the media was removed,
the newly formed formazan was dissolved and measured by
enzyme-linked immunosorbent assay (ELISA) reader at a wavelength of
570 nm, and the results are presented as mean standard deviation
(SD). In addition, triplicate experiments were performed in
parallel.
Annexin V/PI double staining
The apoptosis effect was detected by the Annexin
V/PI double staining. After treatment with chemical constituents
for 48 h, the apoptotic cells were stained by the Annexin V-FITC
Apoptosis Detection kit (Vazyme Biotech, Jiangsu, China). Then, the
stained cells were immediately analysed by flow cytometry. Data
acquisition and analysis were performed with a Becton-Dickinson
FACSCalibur flow cytometer via the Cell Quest software
(Becton-Dickinson, Franklin Lakes, NJ, USA).
Western blot analysis
Total cell lysates were extracted from the untreated
and treated cells, adding phosphatase and protease inhibitors. The
proteins were fractionated by 6–15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electroblotted onto PVDF membrane (Millipore, Billerica, MA, USA).
Subsequently, the membranes were blocked with 5% non-fat milk and
incubated with primary antibodies (diluted in 1% BSA-TBST) for 18 h
at 4°C. Next, they were probed with secondary antibodies for 1 h at
room temperature. Subsequently, the expression of the target
proteins was detected by Immobilon Western Chemiluminescent HRP
Substrate (Millipore).
Statistical analysis
The results were expressed as the means ± standard
deviation (SD). GraphPad Prism 5.0 and Student's t-test were used
to determine the level of significance. Only the P-value of
<0.05 was considered as statistically significant.
Results
Sixteen chemical constituents were
isolated and identified from the leaves of C. nitidissima
Based on a previous study (9), we explored the phytochemicals
derived from the extractive fractions of C. nitidissima with
antitumor effect, according to the flow diagram shown in Fig. 1. Finally, 16 compounds, including
flavonoids, C-27 steroidal saponins, monoterpenes, and triterpenes
were identified as follows: compound 1: 3β-acetoxy-20-lupanol
(11); compound 2:
3β,6α,13β-trihydroxyolean-7-one (12); compound 3:
A1-barrigenol-22a-angelate (13);
compound 4: (3R, 6R, 7E)-3-hydroxy-4,7-megastigmadien-9-one
(11); compound 5:
β-D-glucopyranoside,
3-[(1-oxo-9,12,15-octadecadienyl)oxy]-2-[(1-oxo-9,12,15-octadecatrienyl)oxy
(14); compound 6:
β-D-glucopyranoside,
2-[[(9Z,12Z,15Z)-1-oxo-9,12,15-octadecatrien-1-yl]oxy]-3-[(1-oxooctyl)oxy]propyl
(15); compound 7:
β-D-Glucopyranoside,3-[(1-oxo-9,12-octade-cadienyl)oxy]-2-[(1-oxo-9,12,15-octadecatrienyl)oxy]propyl
(16); compound 8:
kaempferol3-O-[2-O-(trans-p-coumaroyl)-3-O-α-D-glucopyranosyl]-α-D-glucopyranoside
(11); compound 9:
stigmasta-7,22-diene-3-O-[α-L-arabinopyranosyl(1→2)]-β-D-gala-ctopyranoside
(11); compound 10: β-D-galactopy
ranoside,
(2S)-2-(acetyloxy)-3-[[(9Z)-1-oxo-9-octadecen-1-yl]oxy]propyl
(17); compound 11:
aromadendrin(11); compound 12:
α-spinasteryl-β-D-glucopyranoside (11); compound 13: catechin (11); compound 14:
phlorizin4′-O-β-D-glucopyranoside (11); compound 15:
3β,6α,13β-trihydroxyolean-7-one (11); compound 16: dodecanoic acid
(11).
The structures of these 16 chemical constituents are
shown in Fig. 2, and the spectra
are described as follows: Compound 1: the spectrum was reported
previously (11). Compound 2:
C32 H50O5 1H-NMR (400
MHz, CDCl3-d1) δ:1.14 (2H, H-1α), 2.60 (2H, ddd,
J=14.00, 3.24, 3.24, H-1β), 1.61 (2H, H-2), 4.49 (1H, dd, J=12.3,
8.0, H-3α), 1.54 (1H, H-5), 4.92 (1H, d, J=12.03, H-6β), 0.83 (1H,
H-9), 1.57 (2H, H-11), 1.21 (2H, H-12), 1.00 (2H, H-15α), 2.11 (2H,
ddd, J=13.74, 13.6, 4.2, H-15β), 1.82 (2H, ddd, J=13.48, 12.70,
3.97, H-16α), 1.19 (2H, H-16β), 1.51 (1H, H-18), 1.98 (2H, dd,
J=12.74, H-19α), 1.25 (2H, H-19β), 1.47 (2H, H-21a), 1.33 (2H,
H-21b), 1.39 (2H, H-22a), 1.10 (2H, H-22b), 0.87 (3H, s, H-23),
0.90 (3H, s, H-24), 1.19 (3H, s, H-25), 1.37 (3H, s, H-26), 0.92
(3H, s, H-27), 1.23 (3H, s, H-28), 0.95 (3H, s, H-29), 0.89 (3H, s,
H-30), 2.04 (3H, H-2′) 13C-NMR (100 MHz,
CDCl3-d1) δ: 39.9 (C-1), 23.8 (C-2), 80.5 (C-3), 39.3
(C-4), 56.7 (C-5), 72.2 (C-6), 211.2 (C-7), 43.3 (C-8), 55.3 (C-9),
38.2 (C-10), 17.5 (C-11), 33.9 (C-12), 82.3 (C-13), 45.0 (C-14),
22.5 (C-15), 29.7 (C-16), 33.5 (C-17), 49.1 (C-18), 38.5 (C-19),
31.3 (C-20), 34.0 (C-21), 39.0 (C-22), 28.1 (C-23), 16.4 (C-24),
16.0 (C-25), 20.5 (C-26), 18.4 (C-27), 31.3 (C-28), 31.8 (C-29),
25.0 (C-30), 171.0 (C-1′), 21.3 (C-2′). Compound 3:
C35H52O6 1H-NMR (600
MHz, MeOH-d4) δ: 3.17 (1H, dd, J=4.2, 11.4, H-3), 5.26 (1H, t,
J=3.6, H-12), 3.79 (1H, d, J=10.2, H-15), 3.92 (1H, d, J=4.2,
H-16), 2.54 (1H, dd, J=4.2, 14.4, H-18), 5.45 (1H, dd, J=6.0, 12.0,
H-22), 0.98 (3H, s, H-23), 0.78 (3H, s, H-24), 0.95 (3H, s, H-25),
1.02 (3H, s, H-26), 1.40 (3H, s, H-27), 3.09, 3.22 (2H, d, J=10.8,
H-28), 0.90 (3H, s, H-29), 1.04 (3H, s, H-30), 6.03 (1H, dq, J=1.2,
7.2, H-3′), 1.99 (3H, dd, J=1.8, 7.2, H-4′), 1.90 (3H, br t, J=1.8,
H-5′) 13C-NMR (150 MHz, MeOH-d4) δ: 39.8 (C-1), 27.9
(C-2), 79.7 (C-3), 38.2 (C-4), 56.6 (C-5), 19.7 (C-6), 37.2 (C-7),
41.9 (C-8), 48.2 (C-9), 33.6 (C-10), 24.7 (C-11), 123.4 (C-12),
144.5 (C-13), 45.7 (C-14), 68.5 (C-15), 75.4 (C-16), 41.9 (C-17),
42.9 (C-18), 47.6 (C-19), 33.6 (C-20) 42.3 (C-21), 73.3 (C-22),
28.7 (C-23), 16.2 (C-24), 15.9 (C-25), 17.8 (C-26), 20.9 (C-27),
63.8 (C-28), 32.4 (C-29), 25.1 (C-30), 169.5 (C-1′), 130.0 (C-2′),
138.1 (C-3′), 16.3 (C-4′), 21.1 (C-5′). Compound 4: the spectrum
was reported previously (11).
Compound 5: C41H66O10
1H-NMR (600 MHz, MeOH-d4) δ: 3.97 (2H, dd, J=5.410.8,
H-1), 4.22 (2H, d, J=7.2, H-3) 13C-NMR (150 MHz,
MeOH-d4) δ: 68.7 (C-1), 70.2 (C-2), 64.0 (C-3), 105.4 (C-1′), 72.4
(C-2′), 74.9 (C-3′), 71.8 (C-4′), 76.8 (C-5′), 62.5 (C-6′), 175.0
(C-1″), 174.7 (C-1‴), 23.6–35.1 (C-2″-C-6″, C-9″, C-12″, C-15″,
C-2‴-C-6‴, C-9‴, C-12‴, C-15‴), 129.1–133.8 (C-7″-C-8″,
C-10″-C-11″, C-13″-C-14″, C-7‴-C-8‴, C-10‴-C-11‴, C-13‴-C-14‴),
14.5 (C-16″), 14.5 (C-16‴). Compound 6:
C35H60O10 1H-NMR (600
MHz, MeOH-d4) δ:4.42 (1H, dd, J=3,12, H-6′), 5.19 (1H, m, C=CH)
13C-NMR (150 MHz, MeOH-d4) δ: 68.7 (C-1), 71.8 (C-2),
64.0 (C-3), 105.4 (C-1′), 72.4 (C-2′), 74.9 (C-3′), 70.2 (C-4′),
76.8 (C-5′), 62.5 (C-6′), 175.1 (C-1″), 174.8 (C-1‴), 129.0–133.8
(C-9‴-C-10‴, C-12‴-C-13‴, C-15‴-C-16‴), 26.0–35.1 (C-2″-C-8″,
C-11‴, C-14‴, C-17‴), 14.0 (C-8″), 14.4 (C-18″). Compound 7:
C45H76O10 1H-NMR (600
MHz, MeOH-d4) δ: 5.32 (1H, m, C=CH), 0.06 (3H, s, H-18″, H-18‴),
4.42 (1H, dd, J=3, 12, H-6′) 13C-NMR (150 MHz, MeOH-d4)
δ: 68.7 (C-1), 70.2 (C-2), 64.0 (C-3), 105.4 (C-1′), 72.4 (C-2′),
74.9 (C-3′), 71.8 (C-4′), 76.8 (C-5′), 62.5 (C-6′), 175.0 (C-1″),
174.7 (C-1‴), 128.2–132.7 (C-9″-C-10″, C-12″-C-13″, C-15‴-C-16‴,
C-9‴-C-10‴, C-12‴-C-13‴), 21.5–35.1 (C-2″-C-8″, C-11″, C-14″-C-17″,
C-2‴-C-8‴, C-11‴, C-16‴, C-17‴). Compound 8: the spectrum was
reported previously (11).
Compound 9: the spectrum was reported previously (11). Compound 10: C29H52O10
1H-NMR (600 MHz, MeOH-d4) δ: 3.97 (2H, dd, J=5.4, 10.8,
H-1), 5.25 (1H, m, H-2), 4.21 (2H, dd, J=7.2, 12.0, H-3), 4.17 (1H,
d, J=7.8, H-1′), 3.45 (1H, H-2′), 3.40 (1H, dd, J=3.6, 9.6,H-3′),
3.76 (1H, d, J=3.6, H-4′), 3.44 (1H, m, H-5′), 3.67 (2H, H-6′)
13C-NMR (150 MHz, MeOH-d4) δ: 68.7 (C-1), 71.8 (C-2),
64.0 (C-3), 105.4 (C-1′), 72.4 (C-2′), 74.9 (C-3′), 70.2 (C-4′),
76.8 (C-5′) 62.5 (C-6′), 175.0 (C-1″), 174.7 (C-1‴), 26.0–35.1
(C-2″-C-8″, C-11″-C-17″), 129.2 (C-9″), 128.9 (C-10″), 14.0
(C-18″), 14.0 (C-2‴). Compound 11: the spectrum was reported
previously (11). Compound 12:
the spectrum was reported previously (11). Compound 13: the spectrum was
reported previously (11).
Compound 14: the spectrum was reported previously (11). Compound 15: the spectrum was
reported previously (11).
Compound 16: the spectrum was reported previously (11).
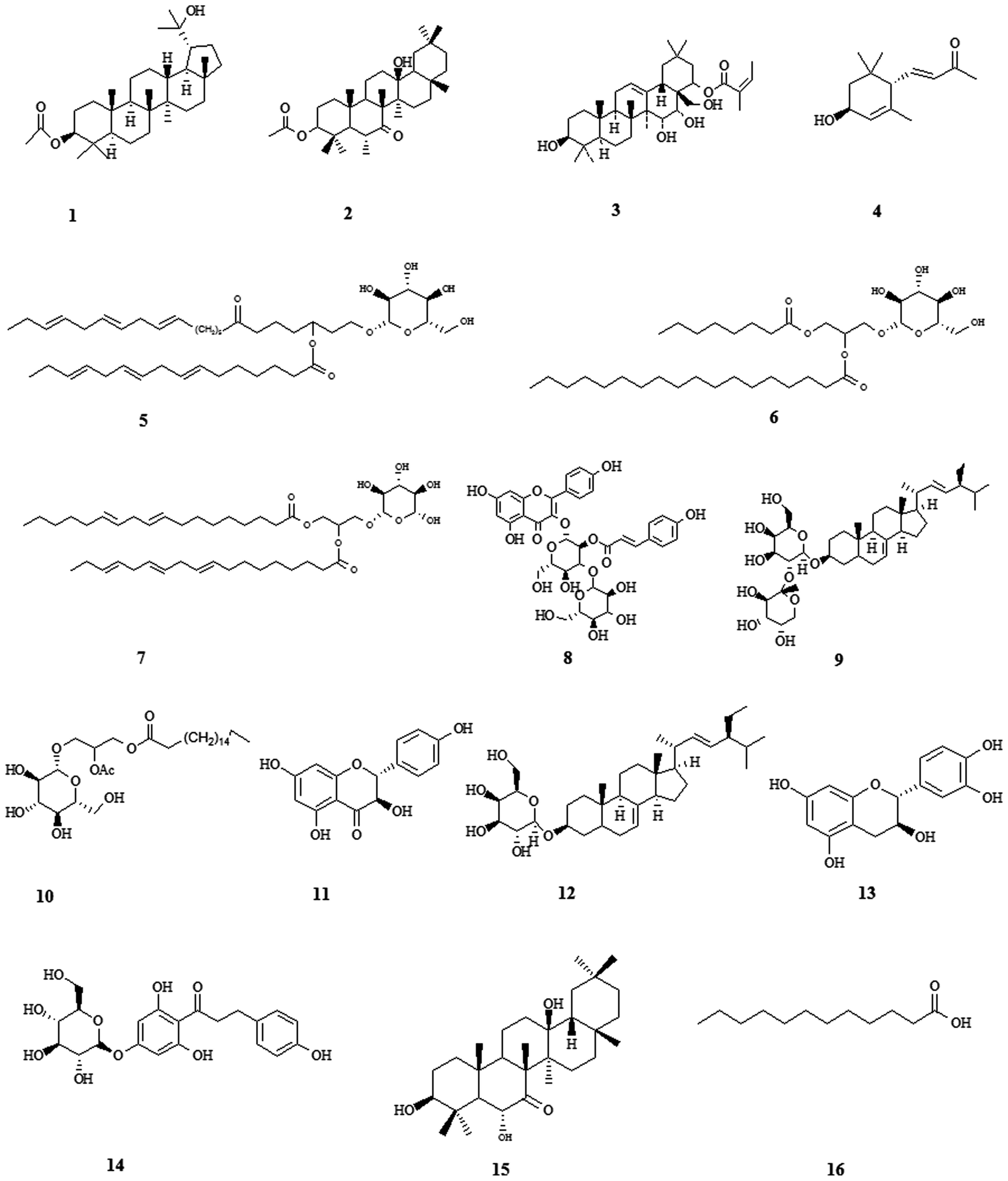 | Figure 2The structures of 16 chemical
constituents. Compound 1: 3β-acetoxy-20-lupanol; compound 2: 3β,
6α, 13β-trihydroxyolean-7-one; compound 3: A1-barrigenol
22a-angelate; compound 4: (3R, 6R,
7E)-3-hydroxy-4,7-megastigmadien-9-one; ψompound 5:
β-D-glucopyranoside,
3-[(1-oxo-9,12,15-octadecadi-enyl)oxy]-2-[(1-oxo-9,12,15-octadecatrienyl)oxy];
compound 6: β-D-glucopyranoside,2-[[(9Z,12Z,
15Z)-1-oxo-9,12,15-octadecatrien-1-yl]oxy]-3-[(1-oxooctyl)
oxy]propyl; compound 7: β-D-glucopyranoside,
3-[(1-oxo-9,12-octadecadienyl)oxy]-2-[(1-oxo-9,12,15-octadecatrienyl)oxy]propyl;
compound 8: kaempferol; compound 9:
stigmasta-7,22-diene-3-O-[α-L-arabinopyranosyl(1→2)]-β-D-galactopyranoside;
compound 10:
β-D-galactopyranoside,(2S)-2-(acetyloxy)-3-[[(9Z)-1-oxo-9-octadecen-1-yl]oxy]propyl;
compound 11: aromadendrin; compound 12:
α-spinasteryl-β-D-glucopyranoside; compound 13: catechin; compound
14: phlorizin 4′-O-β-D-glucopyranoside; compound 15:
3β,6α,13β-trihydroxyolean-7-one; compound 16: dodecanoic acid. The
structures of compound 1, 4, 8, 9, 11, 12, 13, 14, 15 and 16 were
reported in our previous study (11). |
In conclusion, excluding compound 11 and compound 1,
other 14 chemical constituents were first discovered in this plant
by our group. In this study, 6 phytochemicals (compound 2, 3, 5, 6,
7 and 10) were reported for the first time in this plant, whereas
other 8 phytochemicals were first reported in this plant in our
previously published study (11).
The preliminary screening of the extractive fractions for antitumor
activity showed that the extracts of C. nitidissima could
significantly inhibit cancer cell proliferation by MTT assays and
mouse sarcoma 180 subcutaneous graft tumor model in our previous
study (18). However, the
antitumor activity of the 16 specific chemical constituents need to
be evaluated.
The antitumor activity screening of 16
chemical constituents
Lung cancer, gastric cancer, breast cancer and colon
cancer are the most common malignant tumors worldwide, and
especially in China. Therefore, the 16 chemical constituents from
C. nitidissima were screened for antitumor activity on 3
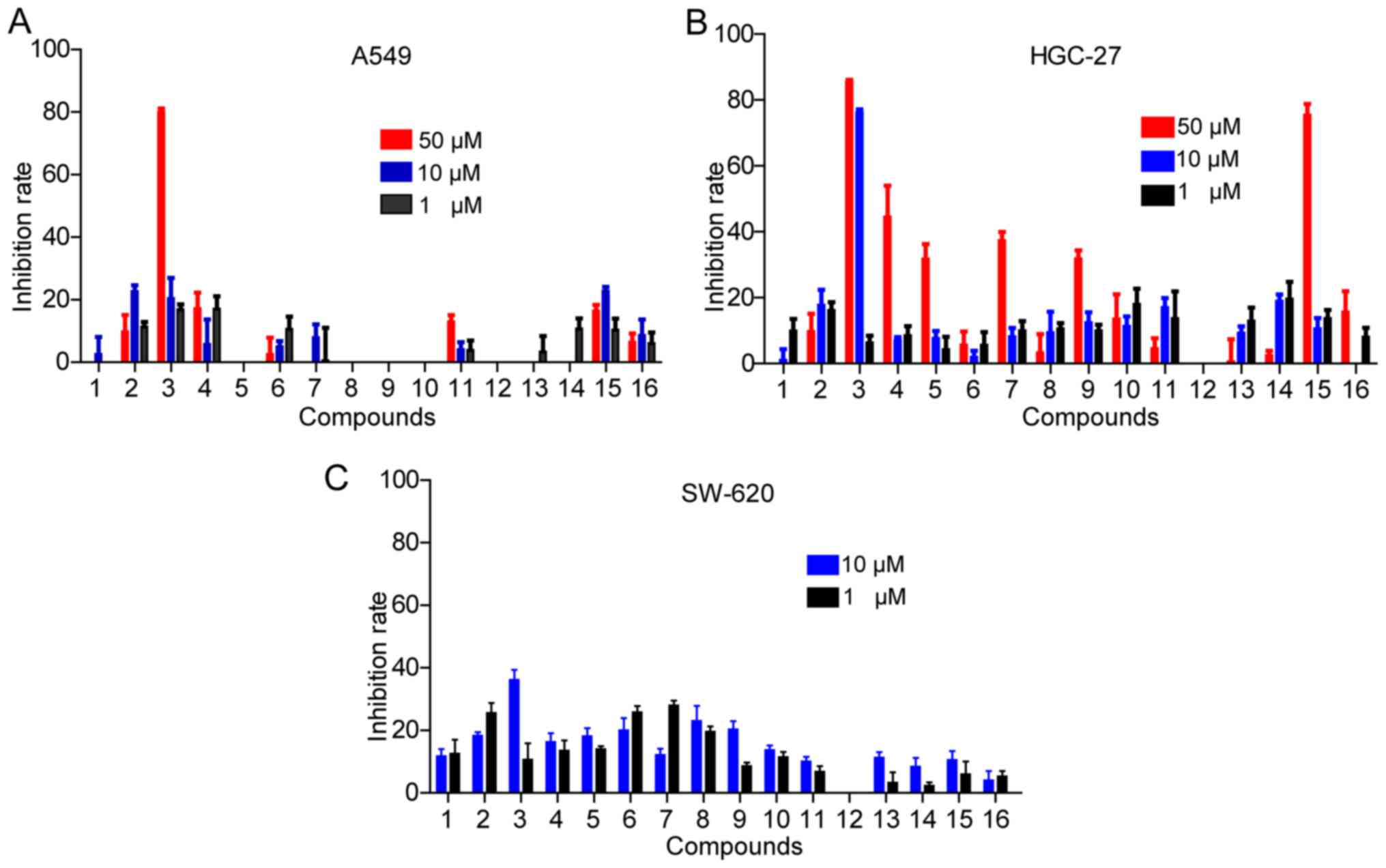
cancer cell lines via MTT assays. As shown in Fig. 3A and B, several natural medicines
exhibited significant antitumor effects on A549 and HGC-27 at a
high concentration (50 µM). However, only compound 3 even
had evident antitumor effects at 10 µM. Furthermore, the 16
chemical constituents exhibited little effect on SW620 (Fig. 3C). Compared to the other chemical
constituents, compound 3 showed superior antitumor effects.
Interestingly, to our knowledge, this is the first study that
compound 3 inhibited cancer cells. In brief, compound 2, 3 and 15
exhibited more significant antitumor effect than others, showing
potential for antitumor drug development.
The antitumor activity of compound 3 on
EGFR-T790M resistant NSCLC cell line NCI-H1975
Our group has focused on EGFR and EGFR inhibitors
for a long time (19). The
recognized EGFR-mutant lung cancer cell line, NCI-H1975 has a point
mutation for T790M in exon 20, which confers resistance to
first-generation EGFR TKIs such as gefitinib and erlotinib
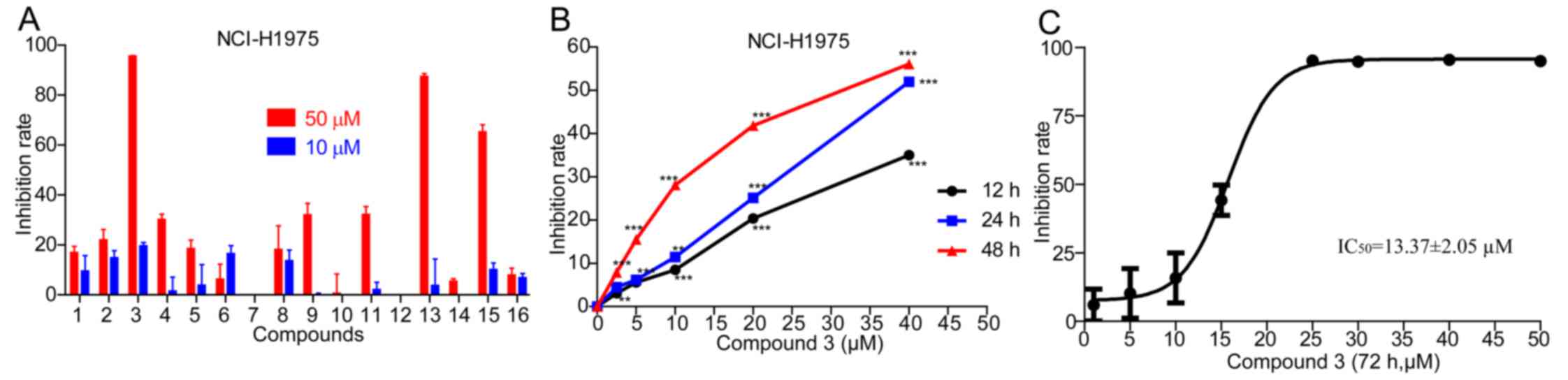
(20). As the screening data in
Fig. 4, only 3 natural medicines
exhibited significant antitumor effect on the NCI-H1975 cell line
(Fig. 4A). Obviously, compound 3
had the most prominent antitumor effect in the 16 natural
medicines, based on the screening data of 4 common malignant cancer
cell lines. Furthermore, compound 3 inhibited the proliferation of
NCI-H1975 in a dose and time-dependent manner (Fig. 4B)with an the IC50 of
13.37±2.05 µM at 48 h treatment (Fig. 4C). Therefore, to our knowledge,
this is the first study showing that compound 3 may help solve the
problem of drug resistance to first-generation EGFR TKIs.
Compound 3 induces apoptosis of the
NCI-H1975 cell line
The antitumor effect of compound 3 was confirmed in
above results, but the mechanisms relating to the antitumor
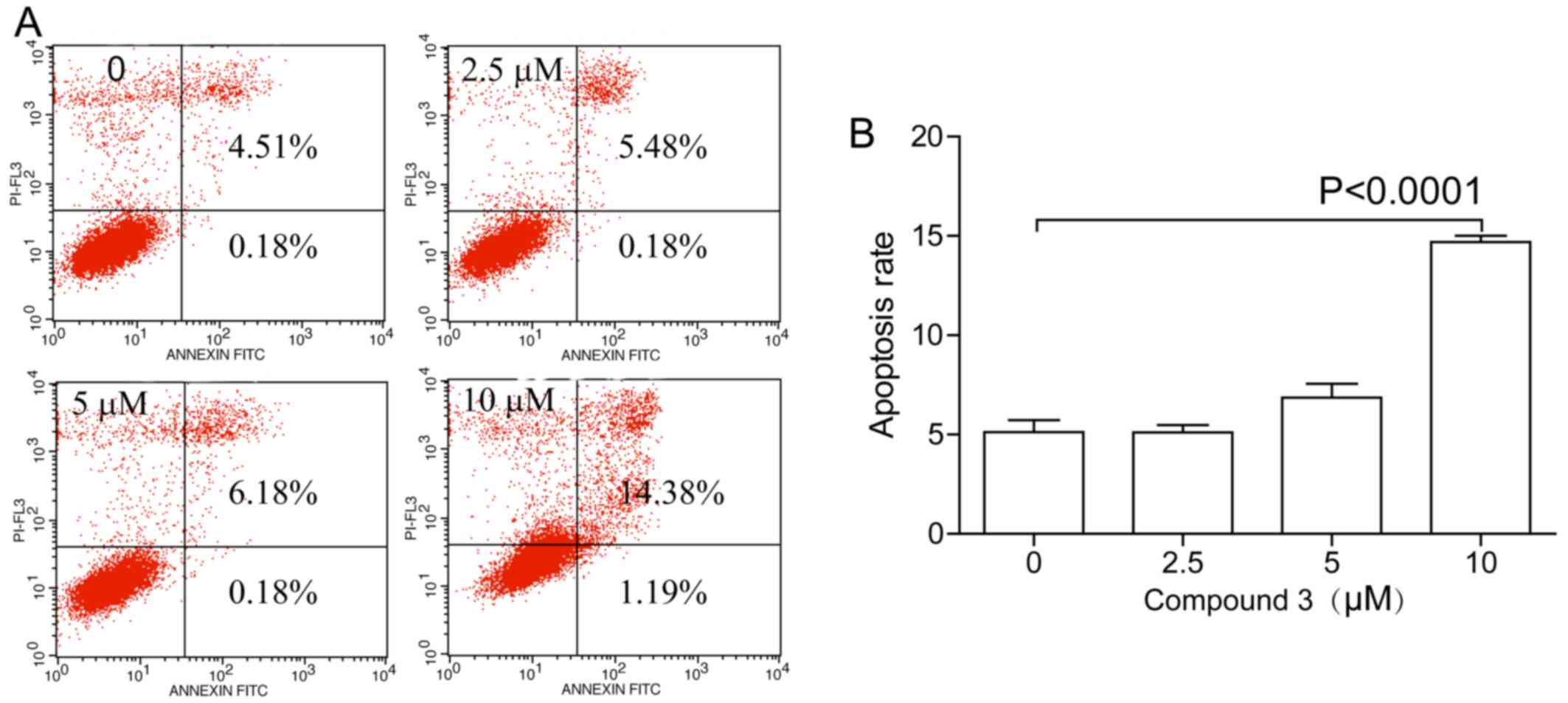
activity remained unknown. Apoptosis is one of the most common
mechanisms of natural medicines (21,22). The Annexin V/PI double staining
showed significant apoptosis effects of compound 3 on NCI-H1975 at
10 µM (Fig. 5).
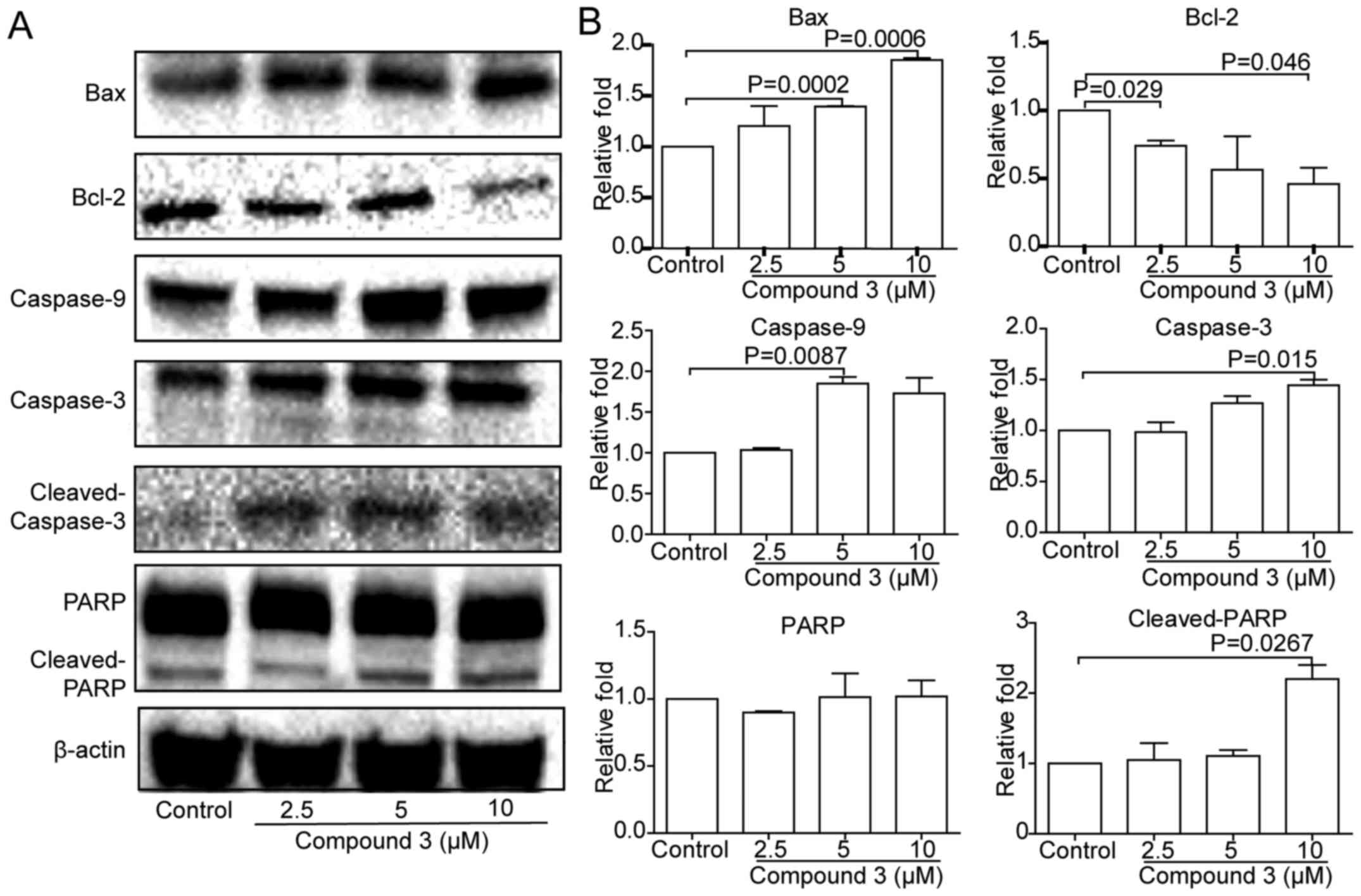
Furthermore, the marker proteins of apoptosis effect
such as cleaved caspase-3, caspase-9 and cleaved PARP, were
upregulated by compound 3 (Fig.
6). The relevant apoptosis pathways included mitochondrial
mediated apoptosis and death receptor mediated apoptosis. The
expression of Bax was upregulated, but the expression of Bcl-2 was
downregulated (Fig. 6),
suggesting apoptosis may be via mitochondrial mediated apoptosis
pathway.
Discussion
The drug development based on natural medicine is
considered to be a more targeted approach (23) than random screening. An increasing
number of researchers all over the world are focusing on natural
medicine to find new antitumor drugs (24,25). In this study, 16 chemical
constituents were isolated from the leaves of C.
nitidissima, and 6 compounds were discovered in this plant for
the first time. Meanwhile, an oleanane-type triterpene, compound 3,
exhibited the most potential antitumor effects, based on a mass of
drug screening studies. To our knowledge, this is the first study
that compound 3 inhibited cancer cells, and compound 3 could
inhibit the EGFR-mutant lung cancer cell line NCI-H1975 via
apoptosis effect. Based on these data, compound 3 showed potential
for antitumor drug development.
Several previous studies (9,26)
have suggested that the crude extracts of C. nitidissima
could inhibit cancer growth, whereas our study also showed the
prevention effects of crude extracts on carcinogenesis. However,
the pharmacodynamics of the chemical constituents derived from
C. nitidissima need to be explored. Until now, only a few
chemical constituents have been isolated and identified from C.
nitidissima (11). Therefore,
6 compounds among the 16 chemical constituents are first reported
in this plant. Furthermore, based on extensive drug screening,
compound 3 was found to be a potential antitumor drug.
Interestingly, to our knowledge, this is the first study of
compound 3 demonstrating an antitumor effect. Consequently, these
data provide the scientific basis for antitumor activity of C.
nitidissima.
Compound 3 is an oleanane-type triterpene which is
widely used in traditional Chinese medicine (TCM), and is the most
common type of terpene (27).
Common triterpenoids can be divided into oleanane type, dammarane
type, ursane type, lupane type and friedelane type. Triterpene
compounds have many beneficial physiological and pharmacological
functions (27), including
antitumor, anti-inflammatory, antiviral, and antibacterial
activities. Therefore, triterpene components can be considered as
potential therapeutic strategy for the prevention and treatment of
tumors in the future (28).
Chemotherapy is the preferred and the most common
treatment for patients in clinical practice (2). Although, the world famous
pharmaceutical companies have been committed to antitumor drugs
development, there is a lack of effective anti-tumor drugs. Because
some patients easily acquire resistance to many new drugs after a
period of treatment. For example the mutation of T790M confers
resistance to first-generation EGFR TKIs (20,29). Our group has been focused on
natural medicines for antitumor effect. And, we have discovered
some effective antitumor drugs from traditional Chinese Medicine
(19,30). It is believed that the drug
development based on natural medicine is a more targeted
approach.
The antitumor effects of compound 3 on lung cancer
cells via apoptosis were significant, in vitro. However, the
detailed regulation mechanisms may need to be further explored.
Similar to many studies on natural medicine (31), the antitumor effects should be
confirmed via orthotopic tumor models, in vivo.
On the basis of these findings, compound 3 showed
the potential to be developed as an antitumor drug, and provided a
scientific basis for the antitumor activity of C.
nitidissima. The study based on natural medicine is a targeted
mode for new drug research and development.
Acknowledgments
The authors would like to thank the Jiangsu Key
Laboratory of Drug Screening and Jiangsu Center for
Pharmacodynamics Research and Evaluation for providing the
facilities to carry out this research.
Notes
[1]
Funding
This study was funded by the National High-tech
Research and Development Projects (863) (no. 2014AA022208), the
National Natural Science Foundation of China (no. 81573456) and the
National Undergraduate Innovation Program in China Pharmaceutical
University (no. 2016-114).
[2] Availability
of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
SY and LS defined the research subject and its aims,
conceived and designed the experiments. XH, HD, RY, JQ and YH
conducted the experiments. SF, YW, SL, ZL and AJ analyzed the data.
XH and HD wrote the paper.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Zhang S, Zhao P, Zeng H,
Zou X and He J: Annual report on status of cancer in China, 2010.
Chin J Cancer Res. 26:48–58. 2014.PubMed/NCBI
|
|
2
|
Moth E, McLachlan SA, Veillard AS, Muljadi
N, Hudson M, Stockler MR and Blinman P: Patients' preferred and
perceived roles in making decisions about adjuvant chemotherapy for
non-small-cell lung cancer. Lung Cancer. 95:8–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tricker EM, Xu CX, Wong KK and Janne PA:
Efficacy of cetuximab and mutant selective EGFR inhibitor WZ4002 in
EGFR T790M and non-T790M models of erlotinib resistant non-small
cell lung cancer. Cancer Res. (Suppl 15)75:28132015. View Article : Google Scholar
|
|
4
|
Konkimalla VB and Efferth T: Anti-cancer
natural product library from traditional Chinese medicine. Comb
Chem High Throughput Screen. 11:7–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du H, Huang Y, Hou X, Yu X, Lin S, Wei X,
Li R, Khan GJ, Yuan S and Sun L: DT-13 inhibits cancer cell
migration by regulating NMIIA indirectly in the tumor
microenvironment. Oncol Rep. 36:721–728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L and Leung PS: Use of herbal medicines
and natural products: An alternative approach to overcoming the
apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol.
53:224–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu X, Chen D, Ye B, Zhong F and Chen G:
Curcumin induces apoptosis of non-small cell lung cancer cells
through a calcium signaling pathway. Int J Mol Med. 35:1610–1616.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Liu H, Wang Z, Qi J, Yuan S, Zhang
W, Chen H, Finley JW, Gu L and Jia AQ: Phytochemicals from Camellia
nitidissima Chi inhibited the formation of advanced glycation
end-products by scavenging methylglyoxal. Food Chem. 205:204–211.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dai L, Li JL, Liang XQ, Li L, Feng Y, Liu
HZ, Wei WE, Ning SF and Zhang LT: Flowers of Camellia nitidissima
cause growth inhibition, cell-cycle dysregulation and apoptosis in
a human esophageal squamous cell carcinoma cell line. Mol Med Rep.
14:1117–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oku H, Ogawa Y, Iwaoka E, Yamaguchi Y,
Kagota S, Kazumasa S, Kunitomo M and Ishiguro K: Preventive effects
of the extract of kinka-cha, a folk tea, on a rat model of
metabolic syndrome. J Nat Med. 65:610–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi J, Shi RF, Yu JM, Li Y, Yuan S, Yang J,
Hu J and Jia A: Chemical constituents from leaves of Camellia
nitidissima and their potential cytotoxicity on SGC7901 cells. Chin
Herb Med. 8:80–84. 2016. View Article : Google Scholar
|
|
12
|
Ling TJ, Wan XC, Ling WW, Zhang ZZ, Xia T,
Li DX and Hou RY: New triterpenoids and other constituents from a
special microbial-fermented tea-Fuzhuan brick tea. J Agric Food
Chem. 58:4945–4950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren Y, VanSchoiack A, Chai HB, Goetz M and
Kinghorn AD: Cytotoxic Barrigenol-like triterpenoids from an
extract of Cyrilla racemiflora housed in a repository. J Nat Prod.
78:2440–2446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruns N, Collisi W, Bernecker S, Stadler
M, Richter C, Schwalbe H and Kalesse M: Spirangien derivatives from
the myxobacterium Sorangium cellulosum: Isolation, structure
elucidation, and biological activity. Eur J Org Chem. 2015:847–857.
2015. View Article : Google Scholar
|
|
15
|
Cateni F, Bonivento P, Procida G,
Zacchigna M, Scialino G and Banfi E: Chemoenzymatic synthesis and
in vitro studies on the hydrolysis of antimicrobial monoglycosyl
diglycerides by pancreatic lipase. Bioorg Med Chem Lett.
17:1971–1978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abrous-Belbachir O, De Paepe R,
Trémolières A, Mathieu C, Ad F and Benhassaine-Kesri G: Evidence
that norflurazon affects chloroplast lipid unsaturation in soybean
leaves (Glycine max L.). J Agric Food Chem. 57:11434–11440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janwitayanuchit W, Suwanborirux K,
Patarapanich C, Pummangura S, Lipipun V and Vilaivan T: Synthesis
and anti-herpes simplex viral activity of monoglycosyl
diglycerides. Phytochemistry. 64:1253–1264. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang Y, Hou XY, Guo H, Lin SS, Yuan ST
and Sun L: Anti-tumor effect of Camellia nitidissima Chi extracts
in vitro and in vivo. Asia-Pac Tradit Med Chin. 12:8–11. 2016.
|
|
19
|
Yu XW, Lin S, Du HZ, Zhao RP, Feng SY, Yu
BY, Zhang LY, Li RM, Qian CM, Luo XJ, et al: Synergistic
combination of DT-13 and topotecan inhibits human gastric cancer
via myosin IIA-induced endocytosis of EGF receptor in vitro and in
vivo. Oncotarget. 7:32990–33003. 2016.PubMed/NCBI
|
|
20
|
Sakuma Y, Yamazaki Y, Nakamura Y,
Yoshihara M, Matsukuma S, Nakayama H, Yokose T, Kameda Y, Koizume S
and Miyagi Y: WZ4002, a third-generation EGFR inhibitor, can
overcome anoikis resistance in EGFR-mutant lung adenocarcinomas
more efficiently than Src inhibitors. Lab Invest. 92:371–383. 2012.
View Article : Google Scholar
|
|
21
|
Cao W, Liu Y, Zhang R, Zhang B, Wang T,
Zhu X, Mei L, Chen H, Zhang H, Ming P, et al: Homoharringtonine
induces apoptosis and inhibits STAT3 via IL-6/JAK1/STAT3 signal
pathway in Gefitinib-resistant lung cancer cells. Sci Rep.
5:84772015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang W, Luo J, Liang Y and Li X:
Echinacoside suppresses pancreatic adenocarcinoma cell growth by
inducing apoptosis via the mitogen-activated protein kinase
pathway. Mol Med Rep. 13:2613–2618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan SM and Ye JM: Strategies for the
discovery and development of anti-diabetic drugs from the natural
products of traditional medicines. J Pharm Pharm Sci. 16:207–216.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo X, Yu X, Liu S, Deng Q, Liu X, Peng S,
Li H, Liu J and Cao Y: The role of targeting kinase activity by
natural products in cancer chemoprevention and chemotherapy
(Review). Oncol Rep. 34:547–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song LX, Wang XS, Zheng XQ and Huang DJ:
Polyphenolic antioxidant profiles of yellow camellia. Food Chem.
129:351–357. 2011. View Article : Google Scholar
|
|
27
|
Zhang W, Men X and Lei P: Review on
anti-tumor effect of triterpene acid compounds. J Cancer Res Ther.
10(Suppl 1): 14–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang H, Chen D, Cui QC, Yuan X and Dou QP:
Celastrol, a triterpene extracted from the Chinese 'Thunder of God
Vine' is a potent proteasome inhibitor and suppresses human
prostate cancer growth in nude mice. Cancer Res. 66:4758–4765.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ofuji K, Tada Y, Yoshikawa T, Shimomura M,
Yoshimura M, Saito K, Nakamoto Y and Nakatsura T: A peptide antigen
derived from EGFR T790M is immunogenic in non small cell lung
cancer. Int J Oncol. 46:497–504. 2015. View Article : Google Scholar
|
|
30
|
Fan W, Sun L, Zhou JQ, Zhang C, Qin S,
Tang Y, Liu Y, Lin SS and Yuan ST: Marsdenia tenacissima extract
induces G0/G1 cell cycle arrest in human esophageal carcinoma cells
by inhibiting mitogen-activated protein kinase (MAPK) signaling
pathway. Chin J Nat Med. 13:428–437. 2015.PubMed/NCBI
|
|
31
|
Bai Y, Chen B, Hong W, Liang Y, Zhou M and
Zhou L: Sedum sarmentosum Bunge extract induces apoptosis and
inhibits proliferation in pancreatic cancer cells via the hedgehog
signaling pathway. Oncol Rep. 35:2775–2784. 2016. View Article : Google Scholar : PubMed/NCBI
|